Abstract
Eosinophilia with pulmonary involvement is characterized by the presence of peripheral blood eosinophilia, typically >500 cells/mm3, nonspecific pulmonary symptoms, and radiographic evidence of pulmonary disease. Clinical, laboratory, and radiologic features can be overlapping in these diseases, thus, it is wise to approach eosinophilia with pulmonary involvement systematically to determine the diagnosis and provide definitive treatment for a better outcome. The authors present a case of idiopathic chronic eosinophilic pneumonia in a patient with a long history of chronic obstructive pulmonary disease (COPD) which was resolved by corticosteroid.
Keywords: Eosinophilic pneumonia, Eosinophilia, Bronchoalveolar lavage, Pneumonia, Interstitial lung disease
1. Background
Eosinophilia with pulmonary involvement is defined by the presence of eosinophilia on peripheral blood analysis, mostly >500 cells/mm3, nonspecific respiratory signs and symptoms, and evidence of pulmonary disease on imaging studies, along with compatible histopathologic findings of eosinophilia in a specimen from lung or pleura biopsy and/or increased eosinophils of more than 10% in bronchoalveolar lavage (BAL) fluid. Various etiologies were associated with eosinophilia and lung involvement including infections, drugs, parasites, autoimmune processes, malignancies, and obstructive lung diseases.1 Clinical, laboratory, and radiologic features can be overlapping in these diseases, thus, it is wise to approach eosinophilia with pulmonary involvement systematically to determine the diagnosis and provide definitive treatment for a better outcome. The authors present a case of idiopathic chronic eosinophilic pneumonia in a patient with a long history of chronic obstructive pulmonary disease (COPD) which was resolved by corticosteroid.
2. Case presentation
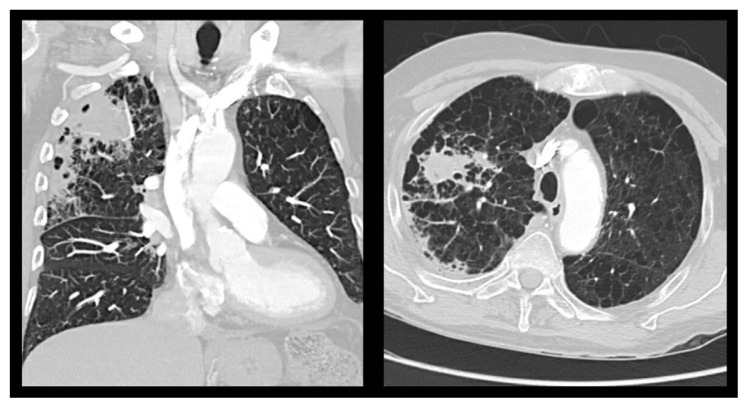
A 71-year-old man, current heavy smoker (47 pack-year) with a history of hypertension, hypothyroidism, COPD with chronic hypoxemic respiratory failure on 2 L/min supplemental oxygen, and coronary and peripheral artery disease status post percutaneous interventions presented to an outpatient clinic with worsening shortness of breath for 10 days. Point-of-care tests for SARS-CoV-2 and group A beta-hemolytic streptococci were negative. A course of oral levofloxacin was previously prescribed empirically for community-acquired pneumonia. However, his oxygen requirement increased to 4 L/min and his productive cough worsened with streaks of blood without fresh blood or clots. He denied a history of atopic dermatitis, urticaria, allergic rhinitis, nasal polyp, or childhood asthma. His medications included albuterol, budesonide-formeterol, aspirin, clopidogrel, levothyroxine, lisinopril, and pantoprazole. He has a history of right upper lobe community-acquired pneumonia which required 3 days of hospitalization and 7 days of antibiotic 3 months ago, initial chest x-ray (CXR) was shown in Fig. 1. Upon evaluation, he was afebrile with a blood pressure of 130/85 mmHg, heart rate of 81 beats per minute, and respiratory rate of 16 per min with oxygen saturation of 98% on a 4 L/min nasal cannula. Initial laboratory work-up was unremarkable except for elevated blood eosinophilia 9.2% with an absolute eosinophil count of 770/μL, elevated erythrocyte sedimentation rate (ESR) 104 mm/Hr, C-reactive protein (CRP) 18.1 mg/dL, and IgE level 848 intl units/mL. CXR showed migratory right upper and middle lung opacities in peripheral distribution (Fig. 2). Computed tomography (CT) chest demonstrated peripheral right upper lobe opacities with a focal 5 × 3.2 cm area of opacities, right hilar lymphadenopathy, emphysematous changes, and a few scattered pulmonary nodules (Fig. 3).
Fig. 1.
Initial CXR 3 months prior to presentation showed right upper lung opacities consistent with pneumonia.
Fig. 2.
CXR at this presentation showed migratory right upper and middle lung opacities in a peripheral distribution.
Fig. 3.
CT chest demonstrated peripheral right upper lobe opacities with a focal 5 × 3.2 cm area of opacities (yellow arrow), right hilar lymphadenopathy (blue arrow), emphysematous changes, and a few scattered pulmonary nodules.
Further laboratory work-up, including viral respiratory panel, an autoimmune panel including ANA, ANCA, blood cultures for bacteria and fungi, beta-(1,3)-D-glucan, Strongyloides IgG, and urine legionella antigen, were all negative. Idiopathic chronic eosinophilic pneumonia was suspected given a history of persistent right upper lobe infiltration for 3 months with an initial diagnosis of pneumonia, symptoms, radiological features, and eosinophilia. Bronchoscopy was not performed at that time since his risks outweighed the benefits given his multiple comorbidities. He was started on oral prednisone 40 mg daily with gradual improvement of his respiratory symptoms. He was discharged and scheduled for a follow-up in an outpatient clinic. Upon evaluation a month after his hospital discharge, he reported marked improvement in his symptoms. His eosinophilia had resolved, and his CT scan revealed the resolution of his right upper lobe opacities, otherwise unchanged.
3. Discussion
Idiopathic chronic eosinophilic pneumonia (ICEP) is a relatively rare diffuse parenchymal lung disorder with the annual incidence of ICEP at 0.23 to 0.53 cases/100,000 population.2,3 It is the most common eosinophilic pneumonia in non-endemic areas to parasitic infection.4 It may affect every age group, especially in their 30s–50s, but is extremely rare in childhood. It affects women twice compared to men and up to half of ICEP patients have a diagnosis of asthma. However, an association between smoking and ICEP is uncommon with less than 10% being active smokers and most patients with ICEP have never smoked.2,5 Symptoms are non-specific and usually include chronic productive cough (60–90% of the cases), shortness of breath (20–50% of the cases), less commonly, elevated temperature, and wheezing. The most recognized imaging features of ICEP consist of ill-defined margins, and bilateral alveolar infiltrates, with peripheral and upper lobes predominance in approximately 25% of patients while the global architecture of the lung remains intact. Spontaneous migration of the opacities was observed in up to 25% of cases with rapid regression on corticosteroid therapy, often within 48 h.6 The current diagnostic criteria are as follows at least 2 weeks of clinical symptoms, compatible findings on radiographic studies, usually more than 25% of eosinophil differential detected in BAL, blood eosinophilia, and/or evidence of eosinophil infiltration in the lungs, and lastly, exclusion of other known eosinophilic pneumonia such as parasitic infection, medication-induced, allergic bronchopulmonary aspergillosis (ABPA), and eosinophilic granulomatosis with polyangiitis (EGPA).2 Regarding eosinophil count in BAL, the percentage of eosinophils may help guide etiology. BAL eosinophils less than 1%, 3–25%, and 40% are found in normal populations, various conditions such as hypersensitivity pneumonitis, autoimmune processes and infections, and chronic eosinophilic pneumonia, respectively.6 Lung biopsy is complementary to BAL but is seldom necessary to confirm the diagnosis of eosinophilic pneumonia, showing characteristic features including tissue eosinophilia but with low sensitivity.2,7 This clinical course is sufficiently suggestive of the diagnosis of ICEP in approximately 75% of cases in the appropriate setting. Leukocytosis with peripheral eosinophilia is found in up to 90% of patients with elevated inflammatory markers. It shows improvement with systemic steroids with rapid resolution of pulmonary and peripheral eosinophilia. Recurrences commonly occur months to years after the initial event, particularly after cessation of therapy.6 In our patient, despite having atypical demographic data including sex and comorbidity, he still had many features suggestive of ICEP. His clinical course was a gradual worsening of sole respiratory symptoms for more than 2 weeks. The presence of eosinophilia on peripheral blood along with migratory lesions of the upper lobe and peripheral alveolar infiltration on chest radiographic studies, further support the diagnosis of ICEP. His extensive workup has ruled out other possible etiologies with a favorable outcome of treatment with corticosteroids.
Other differential diagnoses of eosinophilia with lung involvement, in this case, include, first, cryptogenic organizing pneumonia (COP) which is a term of idiopathic interstitial pneumonia from an unknown cause and is considered a rare disease with an incidence between 1.97 and 7 per 100,000. There is no sex predominance, the median age of manifestation is in the fifth to sixth decades, and it is common in nonsmokers. The radiographic presentations can be similar to CEP however leukocytosis with neutrophilia is seen in approximately 50% of cases.8 Second, acute eosinophilic pneumonia (AEP) can be considered in patients with eosinophilia. However, it usually occurs in a healthy man with a mean age of 30 and a history of cigarette smoking or exposure to smoke, fine sand, dust, or ecigarette presents who presents with the sudden, rapid development of fever, dyspnea, or acute respiratory failure that can closely resemble acute respiratory distress syndrome (ARDS). Blood eosinophil count in most cases is normal at presentation. 9 Third, eosinophilic granulomatosis with polyangiitis is a multisystem disorder, characterized by asthma, eosinophilia, and granulomatous inflammation involving small to medium-sized vessels. Chest x-rays commonly show migratory patchy infiltrates on both lungs, while ground-glass infiltrates and airspace consolidations are characteristic computed tomographic findings. However, this patient did not meet the criteria diagnosis for EGPA as the patient had no extrapulmonary manifestations and no evidence of antineutrophil cytoplasmic antibodies with anti-myeloperoxidase or anti-proteinase-3 specificity.10
4. Conclusions
Clinical, laboratory, and radiologic features can be overlapping in these diseases, thus, it is wise to approach eosinophilia with pulmonary involvement systematically to determine the diagnosis and provide definitive treatment for a better outcome. This case demonstrates an interesting presentation of ICEP with the migratory lesion in a COPD patient which is an uncommon comorbidity, without BAL evaluation. The mainstay treatment is a corticosteroid which will resolve both clinical and radiographic findings. This case indicates that cautious empiric treatment is reasonable and beneficial in suspected ICEP cases with close follow-up.
Footnotes
Verbal and written consent was obtained from the patient to publish his case.
Conflict of interest
All the authors declare no conflict of interest nor any source of support.
References
- 1. Rose DM, Hrncir DE. Primary eosinophilic lung diseases. Allergy Asthma Proc. 2013;34(1):19–25. doi: 10.2500/aap.2013.34.3628. Published 2019 Jan 01. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68(4):413–419. doi: 10.1016/j.alit.2019.05.006. Published 2019 Oct 04. [DOI] [PubMed] [Google Scholar]
- 3. Sveinsson OA, Isaksson HJ, Gudmundsson G. Langvinn eósínófíl lungnabólga á Islandi Faraldsfraedi, klínísk einkenni og yfirlit [Chronic eosinophilic pneumonia in Iceland: clinical features, epidemiology and review] Laeknabladid. 2007;93(2):111–116. [PubMed] [Google Scholar]
- 4. Thomeer MJ, Costabe U, Rizzato G, Poletti V, Demedts M. Comparison of registries of interstitial lung diseases in three European countries. Eur Respir J Suppl. 2001;32:114s–118s. [PubMed] [Google Scholar]
- 5. Marchand E, Cordier JF. Idiopathic chronic eosinophilic pneumonia. Orphanet J Rare Dis. 2006;1:11. doi: 10.1186/1750-1172-1-11. Published 2006 Apr 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cottin V. Eosinophilic lung diseases. Clin Chest Med. 2016;37(3):535–556. doi: 10.1016/j.ccm.2016.04.015. Published 2016 Aug 09. [DOI] [PubMed] [Google Scholar]
- 7. Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 8. Cherian SV, Patel D, Machnicki S, et al. Algorithmic approach to the diagnosis of organizing pneumonia: a correlation of clinical, radiologic, and pathologic features. Chest. 2022;162(1):156–178. doi: 10.1016/j.chest.2021.12.659. Published 2022 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Giacomi F, Vassallo R, Yi ES, Ryu JH. Acute eosinophilic pneumonia. Causes, diagnosis, and management. Am J Respir Crit Care Med. 2018;197(6):728–736. doi: 10.1164/rccm.201710-1967CI. Published 2017 Dec 05. [DOI] [PubMed] [Google Scholar]
- 10. Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68(3):261–273. doi: 10.1111/all.12088. Published 2013 Jan 18. [DOI] [PubMed] [Google Scholar]