Abstract
Myocarditis and pericarditis are rare adverse reactions, more commonly seen in young males after receiving the second dose of an mRNA vaccine. However, the benefits of vaccination heavily outweigh the risk of these side effects. In addition, vaccination boosters are effective against the newest, more infective variants. Therefore we expect more vaccines to be administered in the following years. The objective of this study is to review the current understanding of the mechanism, diagnosis, and treatment of myocarditis and pericarditis. Proposed mechanisms include molecular mimicry against the S protein and hypersensitivity reactions with mRNA vaccines and platelet aggregation and thrombus formation in cardiac blood vessels with adenoviral vaccines. Diagnosis of myocarditis is based on clinical findings, cardiac enzymes, ECG, MRI, and echocardiographic findings. Management includes NSAIDs and cardiovascular support in selected cases with ventricular dysfunction. Most patients have a mild presentation with preservation of cardiac function and recover entirely within seven days; the average hospital stay is three days. Long-term complications are infrequent.
Keywords: COVID-19, Myocarditis, COVID-19 vaccines, Molecular mimicry
1. Introduction
COVID-19 is a viral respiratory infection first detected in China in 2019 that has infected 513,955,910 people and caused 6,249,700 deaths worldwide;1 the estimated excess mortality during the covid pandemic is between 14.63 and 24.77 million.2 COVID-19 was declared a pandemic on March 11, 2020, by the World Health Organization (WHO).3 The rapid spread of the virus after the WHO announcement prompted global efforts toward developing a vaccine. By the end of May 2022, the WHO has approved ten vaccines against COVID-19 for emergency use which use different technologies including mRNA (BNT162b2/COMIRNATY, mRNA-1273), adenoviral vectors (ChAdOx1, Ad26.COV2.S), inactivated viruses (Coronavac, Vero Cell, COVAXIN), and Recombinant nanoparticle (NVX-CoV2373/Nuvaxovid, NVXCoV2373/Covovax).4
As of May 1st, 2022, over 11.5 billion doses of the COVID-19 vaccine have been administered globally5, with 65% of the world’s population having received at least one dose. Currently approved COVID-19 vaccines effectively reduce morbidity and mortality associated with the disease.6,7 Inclusion criteria vary worldwide: vaccination in adults is recommended worldwide, while recommendations for vaccination in children vary with country. Federal agencies like the Center for Diseases (CDC) and European Medical Agency (EMA) now recommend immunization for children aged 6–18. Vaccination for children under five years of age is not recommended due to a lack of safety data.8,9,10 However, no vaccine is free of adverse effects. Myocarditis and pericarditis are rare side effects occurring particularly after mRNA vaccination. The estimated overall incidence per 100,000 persons who had received at least one vaccine dose is 0.57–2.13 cases.11,12 The incidence of this side effect varies with age, sex, time interval between vaccination, and the number of doses received. Young males have the highest incidence of this side effect, with an estimated incidence of 18.52 per 100,000 12–18 years old vaccinated.13 Its low incidence may explain why initial clinical trials, with relatively small sample size, did not detect this side effect. However, more myocarditis and pericarditis cases have been reported as mass vaccination programs developed. As of May 1st, more than four thousand myocarditis, pericarditis, and myopericarditis cases after vaccination against Covid-19 had been reported to the United States Vaccine Adverse Event Reporting System (VAERS) 367 cases have been reported in the EEA from EudraVigilance database.14,15
The original mRNA vaccine scheme consisted of two doses. However, with time, follow-up studies showed a decrease in vaccine effectiveness against COVID-19 infection after six months16,17,18 and new, more infectious variants of the virus created waves of infections. As a result, health agencies worldwide have approved vaccine boosters.19,20,21 Vaccine boosters have shown to be more effective in reducing covid-associated outcomes than two doses alone.22,23,24 mRNA vaccines have also been associated with an increased risk of carditis compared to unvaccinated patients. However, the risk appears to be significantly lower than after the second dose.25,26
The benefits of vaccination heavily outweigh the risks of this rare side effect.27 First, vaccination with mRNA vaccines prevents >2800 hospitalizations per million 2nd doses in patients 18–39 years old, while the incidence of myocarditis after immunization in the same group is < 33 per million 2nd doses.28 Secondly, the incidence of myocarditis in patients with COVID-19 infection is 1000 times higher than myocarditis after vaccinations with the mRNA vaccines. Furthermore, most cases of myocarditis after COVID-19 mRNA vaccination are mild, with survival over 99%. In contrast, COVID-19-associated myocarditis has a 30–80% estimated survival.29
The incidence of myocarditis and pericarditis after vaccination against COVID-19 varies with age, sex, the interval between doses, and the vaccine administered. Shorter vaccine intervals are associated with an increased risk of myocarditis.30 In his article, Walid F. Gellad discusses how this could explain why countries with longer intervals between doses, such as Denmark, where the interval is six weeks, have a lower incidence of myocarditis after COVID-19 vaccines than the US and Israel, where the recommended interval is three weeks.31 Therefore, increasing dose intervals and vaccination with homologous vaccines in patients at greater risk (younger males) might be an alternative recommendation to reduce the risk of myocarditis.
As the virus continues to acquire mutations, producing new, more infectious variants, and immunity from previous vaccines wanes, it is possible that further boosters of the vaccines will be needed. Consequently, we anticipate increased myocarditis and pericarditis reports as more vaccines are administered. Due to the sheer size of vaccination campaigns, more evidence about the association between heart inflammation and vaccination against Covid-19 is needed. This study reviews the association of myocarditis with COVID-19 vaccines, their pathophysiology, diagnosis, and treatment.
2. Mechanism
The COVID-19 vaccine causes myocarditis through immune-mediated, molecular mimicry, and thrombus formation (Fig. 1). SARS-CoV-2 is an enveloped positive-sense single-stranded RNA (ssRNA) virus of the Coronaviridae family. It has four major structural proteins: S (spike glycoprotein), N (nucleocapsid protein), M (membrane protein), and E (envelope protein). The S protein is ubiquitous over the surface of SARS-CoV-2 which makes the S protein the main immunologic target of the COVID-19 vaccines.32 The first mechanism for myocarditis is molecular mimicry against the S protein. Antibodies against SARS-CoV2 can cross-react with host tissue proteins, causing autoimmune reactions against their cells.33 The increased risk of myocarditis in patients with COVID-19 infection34 further supports the molecular mimicry theory. Expression of SARS-CoV-2 surface spike protein on the surface of cardiomyocytes could trigger an immune response. It has been speculated that this surface spike protein is encoded by mRNA vaccines.35
Fig. 1.
Molecular mimicry myocarditis.
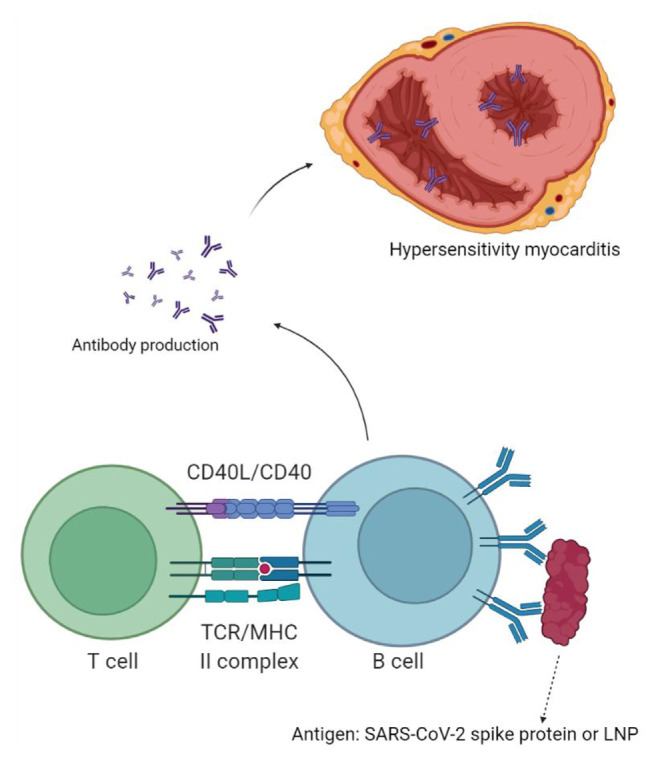
The immune system may register mRNA in the vaccine as an antigen, which leads to the activation of immune-mediated inflammation of the myocardium. Immune-mediated mechanisms are delayed hypersensitivity reaction and Th1 response.29 The first dose sensitizes the immune system and the second dose activates it. This can be caused by a hypersensitivity reaction to vaccine components, such as lipid nanoparticle (LNP) sheath, which encapsulates the mRNA. Polyethylene glycol, a component of LNP sheath, could be responsible for such reactions.36 Most people are sensitized to polyethylene glycol as it is used in ointments, lotions, cosmetics, and dental materials.37 Tsilingiris and collaborators suggested an immune response against LNP as a plausible explanation of the aberrant immune response against own cardiac cells. They support this claim by referring to previous reports of anaphylaxis against components of the LNP sheath in patients receiving mRNA vaccines.36 SARS-CoV-2 virus can sensitize the immune system; therefore, patients previously infected with the virus can develop hypersensitivity myocarditis even after the first dose. RNA in the mRNA vaccines can activate pre-existing auto-reactive immune cells in patients previously infected with SARS-CoV-2.38 Whether hypersensitivity to LNP sheath or mRNA itself, activated immune cells move to the myocardium and release cytokines, causing inflammation and leading to Myocarditis39(Fig. 2).
Fig. 2.
Hypersensitivity myocarditis.
Apart from hypersensitivity reactions, T helper one cell response can also cause myocarditis. For instance, the Moderna vaccine can induce a robust CD4 response involving Th1 cells, causing myocarditis. 40 Immune–genetic background, age, and sex can alter these processes. The major risk factors for developing myocarditis or pericarditis after COVID-19 vaccination are male sex and young age; therefore, higher testosterone levels have been proposed as a culprit of the aberrant immune response against self heart cells. Testosterone promotes aggressive T helper one cell response and suppresses anti-inflammatory, immune cells.
Similarly, estrogen inhibits pro-inflammatory T cells.41 In the immune-mediated mechanisms described above, obesity has a significant impact on causing myocarditis. In a case series done by Puchalski et al., they suggested that obesity may be partially responsible for post-vaccination myocarditis. This is further supported by the fact that obesity is a known risk factor for hyperimmune reactions.42
Lastly, the Spike protein, which is vaccine-encoded, enhances platelet aggregation and granular secretion, promoting thrombus formation.40 This phenomenon primarily affects young women and has been linked with the AstraZeneca and Johnson & Johnson Covid-19 vaccines, both of which are adenoviral vector vaccines.43 The thrombus thus formed can lead to myocardial ischemia, causing myocardial inflammation39
3. Diagnosis and treatment
Myocarditis after COVID-19 vaccines has variable clinical expression, with symptoms like chest pain, dyspnea, and palpitations. These symptoms, along with abnormal tests for example elevated cardiac troponins, key MRI findings such as myocardial edema and late gadolinium enhancement, decreased cardiac function on echo presenting as increased myocardial wall thickness and echogenicity, impaired global systolic function and regional wall motion abnormalities, and electrocardiogram findings consistent with myocarditis for example sinus tachycardia, T wave inversion and diffuse ST-segment elevations play an important role in its diagnosis.44,45 These findings could be confirmed with a biopsy which is not routinely done due to its invasive nature.47
Management of vaccine-induced myocarditis depends on the severity of the disease. Patients presenting with myocarditis should be hospitalized for close monitoring, although supportive care is the mainstay for treating most patients with improving medical tests. Moreover, nonsteroidal anti-inflammatory drugs, colchicine, and corticosteroids should be considered for symptomatic patients.46 More aggressive treatment options are needed for patients with decreased heart function and arrhythmias, where drugs like angiotensin-converting enzyme [ACE] inhibitors and beta-blockers become the cornerstone of treatment.48 Furthermore, restriction from exercise and strenuous activities comprises an important part of management as it provides time for the heart to recover. Hence, treatment of COVID-19 vaccine-induced acute myocarditis involves a combination of rest and supportive care, along with anti-inflammatory and heart failure medications, depending on the extent and severity of the disease.
4. Conclusion
At present, the COVID-19 vaccine seems like the only solution to this problem of the Coronavirus pandemic. However, as more time passes, several studies and the government’s vaccine adverse events monitoring platforms report various adverse effects that may be seen in certain people after getting the COVID-19 vaccine. Due to the global need for developing vaccines against COVID-19 in a record time, few of these side effects might have been missed during initial trials due to the comparatively smaller study sample. Cases of myocarditis and pericarditis, which is the inflammation of layers of the heart, are being reported in the United States and some parts of Europe. They commonly affect young males after the second dose of the vaccine. A booster dose is also shown to affect certain people during the first seven days after receiving the vaccine. The association of the mRNA vaccine with myocarditis and pericarditis is stronger than other kinds of available COVID-19 vaccines. Molecular mimicry between the COVID-19 surface spike protein and cardiac myocytes, the body’s hypersensitive response towards viral lipid nanoparticle (LNP) and polyethylene glycol (PEG), and increased risk of thrombus formation due to spike protein-mediated platelet aggregation are the three possible mechanisms behind vaccine-induced myocarditis and pericarditis. Diagnosis and management are similar to those of the general population with myocarditis without any association with the COVID-19 vaccine. Lastly, as more vaccines are being administered with a few countries considering the fourth dose, cases of this adverse effect should be addressed promptly to prevent a further rise in their incidence.
Footnotes
Disclaimers
This article has not been submitted to any journal.
Conflicts of interest
The authors report no competing interests.
Source(s) of support
None.
References
- 1.Who coronavirus (COVID-19) dashboard. World Health Organization; Jun 10, 2022. [Accessed June 12, 2022]. https://covid19.who.int/ [Google Scholar]
- 2.Coronavirus pandemic (COVID-19) OurWorldInData.org. Jun 7, 2022. [Accessed June 12, 2022]. https://ourworldindata.org/excess-mortality-covid .
- 3.Who director-general’s opening remarks at the media brie fing on COVID-19, 11 march 2020. World Health Organization; Mar 11, 2022. [Accessed June 12, 2022]. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-themedia-briefing-on-covid-19--11-march-2020 . [Google Scholar]
- 4.Covid-19 vaccines. World Health Organization; May 26, 2022. [Accessed June 12, 2022]. https://www.who.int/teams/regulation-prequalification/eul/covid-19 . [Google Scholar]
- 5.Coronavirus (COVID-19) vaccinations. OurWorldInData.org. Jun 12, 2022. [Accessed June 12, 2022]. Retrieved from: https://ourworldindata.org/covid-vaccinations.
- 6. Tan ST, Park HJ, Rodríguez-Barraquer I, et al. Covid-19 vaccination and estimated public health impact in California. JAMA Netw Open. 2022;4:5. doi: 10.1001/jamanetworkopen.2022.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen X, Huang H, Ju J, et al. Impact of vaccination on the COVID-19 pandemic: evidence from U.S. states. SSRN Electron J. 2021 doi: 10.2139/ssrn.3845163. [DOI] [Google Scholar]
- 8.Covid-19 vaccine recommendations for children and teens. Cent Dis Contr Prev. [Accessed June 12, 2022]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccines-children-teens.html .
- 9.EMA. EMA recommends approval of Spikevax for children aged 6 to 11. European Medicines Agency; Feb 24, 2022. [Accessed June 12, 2022]. https://www.ema.europa.eu/en/news/ema-recommends-approval-spikevax-children-aged-6-11 . [Google Scholar]
- 10.EMA. Comirnaty covid-19 vaccine: EMA recommends approval for children aged 5 to 11. European Medicines Agency; Nov 25, 2021. [Accessed June 12, 2022]. https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11 . [Google Scholar]
- 11. Witberg G, Barda N, Hoss S, et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/nejmoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai FT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/m21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li X, Lai FT, Chua GT, et al. Myocarditis following covid-19 BNT162B2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022;176(6):612. doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ema Covid-19 vaccines. Update on ongoing evaluation of myocarditis and pericarditis. Eur Med Agen. May 20, 2022. [Accessed June 12, 2022]. https://www.ema.europa.eu/en/news/covid-19-vaccinesupdate-ongoing-evaluation-myocarditis-pericarditis .
- 15.Vaccine Adverse Event Reporting System (VAERS) wonder.cdc.gov. [Accessed June 12, 2022]. https://wonder.cdc.gov/controller/datarequest/D8 .
- 16. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mrna BNT162B2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/s0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferdinands JM, Fireman B, Thompson MG, et al. Waning 2-dose and 3-dose effectiveness of mrna vaccines against COVID-19–Associated Emergency Department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - vision network, 10 states, August 2021–January 2022. Morb Mortal Wkly Rep. Feb 18, 2022. [Accessed June 12, 2022]. https://www.cdc.gov/mmwr/volumes/71/wr/mm7107e2.htm . [DOI] [PMC free article] [PubMed]
- 18.Science brief. SARS-COV-2 infection-induced and vaccine-induced immunity. Centers for Disease Control and Prevention; Oct 29, 2021. [Accessed June 12, 2022]. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html . [PubMed] [Google Scholar]
- 19.CDC strengthens recommendations and expands eligibility for covid-19 booster shots. Cent Dis Contr Prev. May 19, 2022. [Accessed June 12, 2022]. https://www.cdc.gov/media/releases/2022/s0519-covidbooster-acip.html .
- 20.EMA. Comirnaty and Spikevax: EMA recommendations on extra doses boosters. European Medicines Agency; Oct 6, 2021. [Accessed June 12, 2022]. https://www.ema.europa.eu/en/news/comirnaty-spikevaxema-recommendations-extra-doses-boosters . [Google Scholar]
- 21.EMA. ECDC and EMA issue advice on fourth doses of mrna COVID-19 vaccines. European Medicines Agency; Apr 6, 2022. [Accessed June 12, 2022]. https://www.ema.europa.eu/en/news/ecdc-ema-issueadvice-fourth-doses-mrna-covid-19-vaccines . [Google Scholar]
- 22. Moreira ED, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162B2 covid-19 vaccine. N Engl J Med. 2022;386(20):1910–1921. doi: 10.1056/nejmoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mrna COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg Health - Am. 2022;9:100198. doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162B2 mrna covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603–1614. doi: 10.1056/nejmoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein N, Shimabukuro T.Safety update of 1st booster mRNA COVID-19 vaccination. cdc.gov. Apr 20, 2022. [Accessed June 12, 2022]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-04-20/03-COVID-Klein-Shimabukuro-508.pdf .
- 26.An Advisory Committee Statement (ACS). Guidance on booster COVID-19 vaccine doses in Canada. Canada.ca. Dec 3, 2021. [Accessed June 13, 2022]. https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-onimmunization-naci/guidance-booster-covid-19-vaccinedoses/guidance-booster-covid-19-vaccine-doses.pdf .
- 27.Stecker E, Mullen B.Vaccine-associated myocarditis risk in context: emerging evidence. Am Coll Cardiol. Feb 9, 2022. [Accessed June 12, 2022]. https://www.acc.org/latest-in-cardiology/articles/2022/02/09/12/56/vaccine-associated-myocarditis-risk-in-context .
- 28.Oliver S.Summary and Work Group Interpretation: extended intervals for mRNA COVID-19 vaccines. Cent Dis Contr Prev. Feb 4, 2022. [Accessed June 12, 2022]. https://www.cdc.gov/vaccines/acip/meetings/slides-2022-02-04.html .
- 29. Heymans S, Cooper LT. Myocarditis after COVID-19 mrna vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2021;19(2):75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mrna vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021 December; doi: 10.1101/2021.12.02.21267156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gellad WF. Myocarditis after vaccination against covid-19. BMJ. 2021:375. doi: 10.1136/bmj.n3090. [DOI] [PubMed] [Google Scholar]
- 32. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. JMicrobiol Immunol Infect. 2021;54(2):159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shiravi AA, Ardekani A, Sheikhbahaei E, et al. Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Ther. 2022;11(1):13–21. doi: 10.1007/s40119-021-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. https://www.cdc.gov/mmwr/volumes/70/wr/mm7035e5.htm
- 35. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsilingiris D, Vallianou NG, Karampela I, et al. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabol Open. 2022;13:100159. doi: 10.1016/j.metop.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kounis NG, Koniari I, Mplani V, et al. Hypersensitivity myocarditis and the pathogenetic conundrum of COVID 19 vaccine related myocarditis [published online ahead of print, 2022 Mar 22] Cardiology. 2022 doi: 10.1159/000524224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hassanzadeh S, Sadeghi S, Mirdamadi A, et al. Myocarditis following AstraZeneca (an adenovirus vector vaccine) COVID-19 vaccination: a case report. Clin Case Rep. 2022;10(4):e05744. doi: 10.1002/ccr3.5744. . Published 2022 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ali M, Shiwani HA, Elfaki MY, et al. COVID-19 and myocarditis: a review of literature. Egypt Heart J. 2022;74(1):23. doi: 10.1186/s43044-022-00260-2. . Published 2022 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu R, Pan J, Zhang C, et al. Cardiovascular complications of COVID-19 vaccines. FrontCardiovascMed. 2022;9:840929. doi: 10.3389/fcvm.2022.840929. . Published 2022 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fatima M, Ahmad Cheema H, Ahmed Khan MH, et al. Development of myocarditis and pericarditis after COVID-19 vaccination in adult population: a systematic review. AnnMed Surg (Lond) 2022;76:103486. doi: 10.1016/j.amsu.2022.103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Puchalski M, Kamińska H, Bartoszek M, et al. COVID-19-Vaccination-Induced myocarditis in teenagers: case series with further follow-up. Int J Environ Res Publ Health. 2022;19(6):3456. doi: 10.3390/ijerph19063456. . Published 2022 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez Tijmes F, Zamorano A, Thavendiranathan P, et al. Imaging of myocarditis following mrna COVID-19 booster vaccination. Radiology: Cardiothor Imag. 2022. p. 4. [DOI] [PMC free article] [PubMed]
- 45. Buttà C, Zappia L, Laterra G, et al. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: a comprehensive review. Ann Noninvasive Electrocardiol. 2019;3:25. doi: 10.1111/anec.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gluckman TJ, Bhave NM, Allen LA, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of covid-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-COV-2 infection, and return to play. J Am Coll Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oster M. Overview of myocarditis and pericarditis. ACIP COVID-19 vaccines work group; 2021. [Accessed June 17, 2022]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/02-COVID-Oster-508.pdf . [Google Scholar]
- 48. Haaf P, Kuster GM, Mueller C, et al. The very low risk of myocarditis and pericarditis after mRNA COVID-19 vaccination should not discourage vaccination. Swiss Med Wkly. 2021;151:w30087. doi: 10.4414/smw.2021.w30087. . Published 2021 Oct 19. [DOI] [PubMed] [Google Scholar]