Abstract
Pulmonary embolism (PE) is a serious medical condition that can occur as a result of venous thromboembolism (VTE). COVID-19, also known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC), can potentially lead to PE due to the formation of blood clots in the lungs. This study aims to collate and report trends of PE in patients with long COVID (4–12 weeks since infection) and post-COVID-19 syndrome (>12 weeks since infection). The study adhered to PRISMA Statement 2020 guidelines, and a systematic search was conducted in four databases. In total, nine observational studies were included with a total patient count of 45,825,187. The incidence of PE with long COVID/post-COVID-19 syndrome was seen among 31,885 individuals out of 44,967,887 participants. The incidence rate of PE was observed as 0.07%, given that the studies included matched controls. While we cannot state with certainty that COVID-19 infection in itself leads to higher risks of PE at a later time, this study emphasizes the need for optimized care and longitudinal studies during the COVID-19 era to account for deviations from the norm.
Keywords: Pulmonary embolism, Thromboembolic disorder, Post-COVID-19 syndrome, Long COVID, SARS-CoV-2
1. Background
Pulmonary embolism (PE) is a life-threatening acute medical condition that is a manifestation of venous thromboembolism (VTE).1 PE, a blockage in the arteries of the lungs due to blood clots, is one of the potential complications of long coronavirus disease 19 (COVID-19)2– also known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC); it is a condition where individuals continue to experience symptoms or develop new symptoms after recovering from acute COVID-19 infection. In this study, we present key information pertaining to PE and long COVID or PASC. As the third most common acute cardiovascular syndrome, VTE closely follows myocardial infarction and stroke.1 With COVID-19 repeatedly stunting populations worldwide, the incidence of PE has increased as we progress through the pandemic.3 Nearly 3 years after its inception, studies have found strong correlations between coagulopathy and SARS-CoV-2 infection. During the infectious period, COVID-19-associated coagulopathies (CAC) are reported along with infection-induced coagulation patterns.4,5 With persistent viral replication, hypoxia, inflammation, and endothelial injury leading to organ dysfunction and thrombosis in long COVID, this may explain associated PE incidence.1,4 Based on standardized reports, this study, identifies long COVID as an ongoing symptomatic COVID-19 spanning 4–12 weeks since infection, while post-COVID-19 syndrome spans over 12 weeks since the first infection.6,7
A study of 353,164 COVID-19 patients matched with 1,640,776 controls based in the United States (US) reports that COVID-19 survivors are twice as likely of developing PE or respiratory conditions in the year post-infection.8 The US Centers for Disease Control and Prevention (CDC) also reports that with newer transmissible strains of COVID-19, illness of hematological origin is set to rise.9 In the morbidity and Mortality Weekly Report (MMWR), patients aged between 18 and 64 years and 65 or older experienced the highest risk for respiratory symptoms and PE.8 However, individuals aged 65 and older were also at higher risk of neurological manifestation of disease.8 It is additionally imperative to understand the physiologic mechanisms that lead to post-COVID conditions.10 This study aims to systematically collate and report trends of PE due to long COVID (4–12 weeks since infection) and/or post-COVID-19 syndrome (>12 weeks since infection).
2. Methods
2.1. Search strategy and study selection
This study was reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement 2020 guidelines.11 A systematic search was conducted in four databases including PubMed, Embase, Scopus and Cochrane to identify primary clinical studies until December 28, 2022. Keywords comprised of the following: long covid, post covid, pulmonary, venous, thrombo*, and/or embolism. The inclusion criteria comprised of observational studies, either retrospective or prospective in nature, reporting the incidence of pulmonary embolism among patients with long-COVID/post-COVID-19 syndrome. We did not include case series/reports, previously conducted reviews, or any secondary studies. The software EndNote X9 (Clarivate Analytics, USA) was utilized as the bibliographic management tool. An umbrella search strategy methodology was applied where the reference lists of all screened studies were additionally searched to locate observational studies that met the inclusion criteria.
All investigators screened the titles and abstracts of the studies to reach a consensus for a full-text review of the screened studies. Any disagreements were resolved through active discussion. The authors utilized a modified Delphi approach to determine the best reporting practice for obtained findings.12
2.2. Case definitions
In this study, the following definitions were utilized by corroborating timelines in current literature. 13–15 Although there is no widely accepted definition of the various stages of recovering from COVID-19, the following are proposed by The World Health Organization,16 and by the Centers for Disease Control and Prevention17:
Long COVID: ongoing symptomatic COVID-19 (4–12 weeks since infection);
Post-COVID-19 syndrome (over 12 weeks since infection): broad range of symptoms that continue for three months from the onset of disease, impact the patient’s life and cannot be explained by an alternative diagnosis.
2.3. Data extraction and synthesis
All authors extracted the data onto a shared spreadsheet in the extraction phase. The characteristics and key findings of the obtained studies were reported under the following headings: author, year, title, country, sample size (N), gender, age, comorbidities, the incidence of pulmonary embolism with long COVID, admission to the ICU, mechanical ventilation, duration since COVID infection/discharge, and key findings.
These were thereby reported in a textual and tabulated format. Quantitative outcomes were computed in Statistical Package for Social Sciences (SPSS, IBM). The data were thereby presented as mean, standard deviation and/or proportions (number and percentage). The Cohen’s kappa coefficient (κ) was also computed to measure inter-rater reliability during the study selection process.
Funding role and ethical approval
No funding was obtained for this study. Ethical approval was not required as only secondary clinical data was utilized for this systematic review.
3. Results
3.1. Study selection
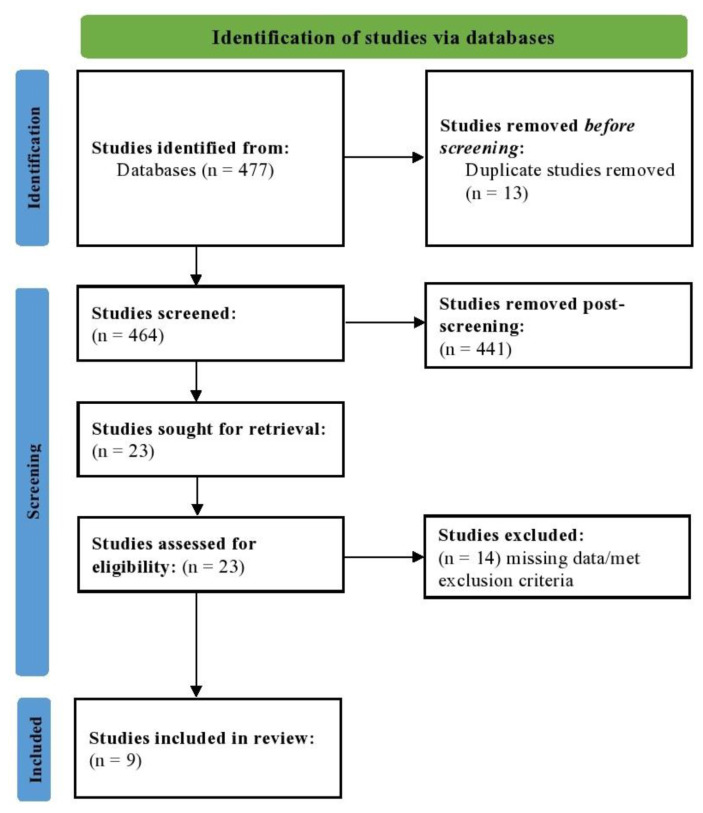
A total of 477 studies were located; of these 13 were duplicates. All other studies (N = 464) were screened for their titles and abstracts. Of these, 23 studies were screened using full-texts; 14 of them were removed as they did not fit the criteria of long COVID or post-COVID-19 syndrome reporting VTE/PE. The PRISMA flowchart is depicted in Fig. 1. The Cohen’s kappa coefficient (κ) was computed to be 0.935, which suggests excellent agreement between the investigators.
Fig. 1.
PRISMA flowchart depicting the study selection process.
3.2. Summary of included studies
In total, 9 studies were included from the Czech Republic, France, Italy, Japan, Russia, Spain, Turkey, and the United Kingdom. The total patient count was 45,825,187. The overall mean age of all participants was 54.38 years. There were 23,439,415 females, which was 51.15% of the total sample. The comorbidities were variable and comprised of hypertension (HTN), coronary artery disease (CAD), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), arterial thrombosis, and smoking. The incidence of PE with long COVID/post-COVID-19 syndrome was seen among 31,885 individuals out of 44,967,887 participants. Given that studies included matched controls the incidence rate of PE was observed as 0.07%. The characteristics of included study findings are enlisted in Table 1.
Table 1.
Baseline characteristics, duration since COVID infection, and incidence of pulmonary embolism enlisted in the included studies (N=9).
| Author, Year | Title | Country | N | Gender (Female) | Age | Comorbidities | Incidence of Pulmonary Embolism with Long COVID | Admitted to the ICU | Mechanical Ventilation | Duration since COVID infection/ Discharge (in days) | Key Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Venturelli, 2021 | Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation | Italy | 767 | 252 (32.9%) | 63 ± 13.6 (Range: 20–92) | HTN=169 (21.7%) CAD=73 (9.5%) DM=57 (7.4%) COPD=36 (4.7%) HF=34 (4.4%) Former/Current Smoker=212 (27.6%) |
13 (6.1%) | 66 (8.6%) | 62 (8.3%) | Since discharge =Median of 81 | Two asymptomatic pulmonary sub-segmental thromboses were discovered at follow-up, by investigating striking D-dimer elevation. |
| Ares-Blanco, 2021 | SARS-CoV-2 pneumonia follow-up and long COVID in primary care: A retrospective observational study in Madrid city | Spain | 155 | 80 (51.6%) | 58.8 ± 16.7 | HTN= 71 (45.8%) HF=6 (3.9%) DM=29 (18.7%) COPD=8 (5.2%) Smoker=12 (8.3%) |
6 (3.9%) | 4 (2.6%) | NR | Since discharge =54 (IQR=42–88) | Embolism was more frequent in patients who persisted symptoms >4 weeks compared to ≤4 weeks (9.1% vs 1.8%, P=0.034). The >4 weeks group was followed up for a longer time (81 vs 50 days, P<0.001); 6.8% (n= 3) still required oxygen at the end of the study. |
| Murata, 2022 | Cardiovascular manifestations identified by multi-modality imaging in patients with long COVID | Japan | 52; Without CVD (n = 38) With CVD (n = 14) | 15 (28.8%) | Without CVD: 56 ± 14 With CVD: 62 ± 10 |
Without CVD: HTN=20 (53%) DM=6 (16%) COPD/Asthma=3 (8%) Smoker=18 (48%) With CVD: HTN=7 (50%) DM=4 (29%) COPD/Asthma= 0 (0%) Smoker=7 (50%) |
4 (8%) | Without CVD=3 (8%) With CVD=5 (36%) |
Without CVD=0% With CVD=3 (21%) |
Since infection= 40 (IQR: 35–52) Since discharge =21 (IQR: 15–29) |
26% (n=10) without CVD and 43% (n=6) with CVD were administered anti coagulation outpatient; long COVID symptoms improved within an average of 12 weeks with no major adverse events |
| Jakubec, 2022 | Pulmonary Complications after COVID-19 | Czech Republic |
98; Ongoing-COVID (n = 56) Post-COVID (n = 42) |
33 (33.7%) | 68 (range: 21–93) | HTN=56 (57.1%) CVD=30 (30.6%) Respiratory disease=41 (41.8%) DM=38 (38.8%) |
5 (11.9%) | NR | 1 (1%) | Since infection=60 (29–252) | Pulmonary embolism occurred in 13% of patients; hypercoagulable states may persist for as long as several weeks after acute infection |
| Motloch, 2022 | Early antithrombotic post-discharge therapy using prophylactic DOAC or dipyridamole improves long-term survival and cardiovascular outcomes in hospitalized COVID-19 survivors | Russia |
1746; A=DOAC (n = 1,002); B=Dipyridamole (n = 304); C=Controls (n = 440) |
1006 (57.6%) | A=59 (IQR: 48–66); B=56 (IQR: 46–65); C=55 (IQR: 43–63) |
HTN: A=391 (39%); B=109 (35.9%); C=135 (30.8%) DM: A=121 (12.1%); B=31 (10.2%); C=47 (10.7%) CVD: A=85 (8.5%); B=26 (8.6%); C=34 (7.7%) HF: A=77 (7.7%); B=24 (7.9%); C=37 (8.4%) COPD: A=29 (2.9%); B=9 (3%); C=17 (3.9%) |
A: 1 (0.1%); B: 0%; C: 3 (0.7%) |
NR | Excluded from study | Overall follow-up: 393 ± 87 | Protective effects of treatment with DOAC (B (SE) = −3.12 (1.42)) or dipyridamole (B (SE) = −17.05 (1.01)) were revealed for pulmonary embolism; all cause survival probability was higher with DOAC (1.1%) and Dipyridamole (1.2%) compared to Control (5.7%) |
| Ekici, 2022 | Pulmonary embolism in patients with dyspnea after COVID-19 infection | Turkey |
105; A= with PE (n=25); B=without PE (n=80) |
A=17 (68%), B=61 (76.2%) | A=55.4 ± 16.4; B=42.7 ± 13.1 | CVD: A=4%, B=1.3% Respiratory disease: A=4%, B=0% Former/Current Smoker: A=20%, B=25% |
25 (23.8%) | A=16%; B=1.3% | NR | Since infection: A=89.9 ± 83.5; B=83.8 ± 74.4 | PE patients had larger pulmonary artery diameter (27.9 ± 2.9 vs. 24.8 ± 3.3), higher uric acid levels (5.1 ± 1.1 vs. 4.6 ± 1.0), higher Pro-BNP levels (243.8 ± 270.2 vs. 78.8 ± 124) and higher glucose level (150.9 ± 103 vs. 106.2 ± 40.1) |
| Rezel-Potts, 2022 | Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK | United Kingdom |
857,300; A=COVID-19 patients (n=428,650); B=matched controls (n=428,650) |
A=238,249 (56%), B=238,249 (56%) |
A=35 (IQR: 22–50); B=35 (IQR:22–50) | High-Very High SBP: A=43,684 (10.2%); B=41,767 (%9.7%) Current/Former Smoker: A=132,299 (30.9%); B= 130,618 (30.5%) |
Risk Ratio: 0.66 (95% CI=0.50 to 0.87)* | NR | Since infection: 13–52 weeks | Long COVID risk of PE was returned to baseline levels or below from 12 weeks to 1 year after infection | |
| Jutant, 2022 | Respiratory symptoms and radiological findings in post-acute COVID-19 syndrome | France | 478 | 201 (42.1%) | 61.0 ± 16.1 | HTN=225 (47.1%) CAD=77 (16.1%) DM=128 (26.8%) COPD=17 (3.6%) Current/Former Smoker=109 (24.1%) |
39 (9.1%) of all patients had PE; 78 patients had new-onset dyspnea with 14 (18%) attributed to PE, whereas 400 patients without newonset dyspnea reported 25 (6.8%) PE | 94 (55.3%) | NR | Since dischatge: 122 days (106–143) | DLCO was decreased in 22% of the patients; no significant differences in radiologic abnormalities (type of lesion and extension) were observed; differences were lower levels of FEV1 (79.3 vs. 94.6%), FVC (73.9 vs. 88.7%) and TLC (68.6 vs. 81.3%) |
| Knight, 2022 | Association of COVID-19 With Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales | England and Wales | 44,964,486; A=Hospitalized (n=118,879); B=Non-Hospitalized (n=1,248,180) |
22961252 (40.45%) | 18–29=8,185,993 30–39=8,088,594 40–49=7,384,405 50–59=7,709,556 60–69=5,920,117 70–79=4,723,368 80–89=2,374,255 >90=578,198 |
Arterial Thrombosis- =1,877,562 (4.18%) Venous Thromboembolism- =629,769 (1.4%) Current/Former Smoker= 18,186,951 (40.45%) |
Total incidence- =31,814, among non-COVID patients=30,021, post-hospitalization COVID=569 (0.48%%), post non-hospitalized COVID=1224 (0.1%) | 22,992 of 118, 879 (19.34%) | Total person- years of follow-up in all= 41,595,372 before COVID-19; 32,471 years after hospitalized COVID-19, and 245,817 years after non-hospitalized COVID-19 | Hazard Ratio (HR): Weeks 5–8, for all=5.55 (95% CI=4.91–6.27); A=14.4 (12.2–17); B=3.07 (2.57–3.67). Weeks 9–12, for all=3.22 (2.66–3.9); A=5.67 (4.23–7.6); B=2.27 (1.76–2.92). Weeks 13–26, for all=2.41 (2.1–2.76); A=2.76 (2.19–3.48); B=2.08 (1.76–2.47). Weeks 27–49, for all=1.61 (1.23–2.12); A=1.40 (0.9–2.18); B=1.6 (1.13–2.27) | |
CAD: Coronary Artery Disease, CI: Confidence Interval, COPD: Chronic Obstructive Pulmonary Disease, COVID: Coronavirus Disease, COVID-19: Coronavirus Disease 2019, CVD: Cardiovascular Disease, DM: Diabetes Mellitus, DLCO: Diffusing Capacity of the Lung for Carbon Monoxide, DOAC: Direct Oral Anticoagulants, FEV1: Forced Expiratory Volume in 1 Second, FVC: Forced Vital Capacity, HF: Heart Failure, HR: Hazard Ratio, HTN: Hypertension, ICU: Intensive Care Unit, IQR: Interquartile Range, N: Number, NR: Not Reported, PE: Pulmonary Embolism, Pro-BNP: Pro B-type Natriuretic Peptide, SBP: Systolic Blood Pressure, SE: Standard Error, TLC: Total Lung Capacity, UK: United Kingdom.
3.3. Study-by-study findings
Venturelli and colleagues implemented a comprehensive post-discharge follow-up program for COVID-19 patients discharged from emergency/inpatient departments in Italy.18 A total of 767 patients participated in the program, with a median follow-up time of 81 days after discharge. Out of these patients, 51.4% reported persistent symptoms, with fatigue and exertional dyspnea being the most commonly reported complaints. Notably, impaired lung diffusion was identified in 19% of the patients, and 17% had D-dimer values that were twice above the threshold for PE diagnosis. Two silent pulmonary thromboses were discovered during the evaluation of the elevated D-dimer levels. Despite the presence of a wide range of symptoms, the underlying pathology was yet to be fully elucidated; a multidisciplinary approach was considered crucial in addressing these diverse issues and finding effective medical solutions.
Ares-Blanco et al. (2021) investigated the management of COVID-19 patients in primary care settings and determining the cut-off points for defining long COVID.19 The study was conducted in Madrid, Spain, during the first wave of the pandemic, and included patients over 18 years of age with a diagnosis of SARS-CoV-2 pneumonia. Data on sociodemographic information, comorbidities, symptoms, complications, laboratory test results, and chest X-ray findings were collected. Descriptive statistics were used to analyze the data, and different cut-off points were applied to define long COVID. The study found that the optimal cutoff points for defining long COVID was between 4 and 12 weeks from the onset of symptoms, with 28.3% of the sample still experiencing symptoms after week 4 compared to 8.3% after week 12. Patients who still had symptoms after more than 4 weeks of follow-up were more likely to experience embolism than those with symptoms lasting ≤4 weeks. Overall, most patients recovered within the first 4 weeks after the onset of infection.
Murata et al. (2022) assessed the prevalence of cardiovascular disorders in 584 long COVID patients between January 2020 and September 2021.20 Of these, 9% (N = 52) of patients were suspected to have cardiovascular long COVID based on symptoms of palpitations, chest pain and dyspnea. In this patient group, 4 of 52 (8%) had PE; overall, they had higher association to severe SARS-CoV-2 infection (P = 0.014) and in-hospital cardiac events (P = 0.002) as compared to those without long COVID. The authors reported that severe conditions during in-hospital care were independent risk factors for thromboembolic disorders in patients with long COVID.
Jakubec et al. (2022) assessed 98 patients who had been hospitalized for over 29 days after the initial diagnosis.21 The first group was hospitalized between weeks 5 and 11, whereas the other group was hospitalized more than 12 weeks after a positive COVID-19 test result. While 77.2% of the cases were hospitalized due to respiratory tract infection specifically pneumonia, other causes included PE, sarcoidosis, and interstitial lung disease. The overall in-hospital mortality was 5.1%.
Motloch et al. (2022) followed-up on the effects of post-discharge antithrombotic treatment on cardiovascular outcomes and all-cause mortality in COVID-19 survivors.22 A total of 1746 participants were enrolled between April and December 2020. The participants received a post-discharge 30-day antithrombotic treatment regimen, which comprised of direct oral prophylactic anticoagulation (DOAC). The study outcomes included mortality, hospitalizations due to PE, stroke and myocardial infarction. The authors reported that dipyridamole and DOAC significantly reduced cardiovascular and all-cause mortality as compared to the control group. Prophylactic DOAC reduced both stroke and PE, whereas dipyridamole reduced PE only. The authors recommended using dipyridamole or prophylactic DOAC in the early post-discharge period of COVID-19 patients.
Ekici et al. (2022) conducted a single-center, prospective observational study to determine the frequency of PE in patients who had new or ongoing dyspnea during their recovery from COVID-19.23 The authors evaluated the radiological and clinical outcomes of patients presenting with dyspnea in outpatient clinics after COVID-19 infection. The patients’ demographic, laboratory, and clinical data were recorded; their dyspnea was computed based on the New York Heart Association (NYHA) classification. The study reported that 23.8% (25/105) of patients with new/ongoing dyspnea post-infection had a pulmonary embolism. The proportion of pulmonary embolisms in patients with NYHA classes I, II, III, and IV was 8.7%, 20%, 30%, and 35.3%, respectively (P = 0.02). The authors reported that patients in NYHA classes III and IV had a higher likelihood of PE as compared to those in NYHA classes I and II. Statistical testing also helped ascertain that the NYHA classes of dyspnea determined the likelihood of PE in long COVID infections.
In 2022, Rezel-Potts et al. conducted an investigation of the prolonged effects of COVID-19 on cardiometabolic outcomes, focusing on the occurrence of new-onset cardiovascular disease (CVD) one year following infection.24 The study utilized electronic health records from 1356 family practices in the UK, with a population of 13.4 million. The cohort comprised 428,650 COVID-19 patients without preexisting CVD or diabetes mellitus, who were matched with controls. The authors observed that acute COVID-19 infection was correlated with an overall increase in the incidence of CVD, primarily attributable to PE, atrial arrhythmias, and venous thromboses. However, the incidence of CVD decreased between 5 and 12 weeks and exhibited a net reduction from 13 to 52 weeks following initial diagnosis. The study’s results indicated that individuals without preexisting CVD who had COVID-19 did not experience a long-term rise in the incidence of thromboembolic disorders. It is important to note that the findings relied on health record data, and exposure and outcome status of participants may have been misclassified.
Jutant et al. (2022) investigated characteristics of patients who were priorly hospitalized with COVID-19 and thereby presented with clinical and radiological pulmonary problems.25 The authors intended to assess the relationship between functional impairment, radiological anomalies and dyspnea in a cohort of 478 survivors. In total, 16.3% participants reported new-onset dyspnea, whereas 4.8% of them presented with new-onset cough. A subgroup of participants had new-onset dyspnea, were younger and presented with more severe COVID-19; this subset of patients had more frequent PE as compared to individuals without dyspnea. Nevertheless, the study has limitations including the lack of a control group and a small sample size. The findings contribute to the current understanding of complications COVID-19 survivors experience.
Knight et al. (2022) studied the incidence of VTE and arterial thromboses following a COVID-19 diagnosis compared to a non-infected control group using electronic health records from England and Wales.26 The authors estimated hazard ratios for first time VTE and arterial thrombosis post COVID-19 diagnosis, which decreased overtime but remained high for up to 49 weeks. The hazard ratios were higher among hospitalized patients and individuals of Black or Asian ethnicity. The increased risk of VTE and arterial thromboses was 0.25% and 0.5% respectively 49 weeks post diagnosis corresponding to 3500 and 7500 additional events based on a total pool of 1.4 million patients with COVID-19. The study highlights the importance of policy implementation to prevent severe disease and manage risk factors while promoting secondary preventative measures among high-risk patients.
4. Discussion
With nearly 46 million participants included in this study and reports of variable frequency for VTE/PE, it is imperative to acknowledge the possibility of events being underreported. While there may be counts of doubt given missed causation or correlation, it is still good practice to see cases with no comorbidities reporting sudden symptoms of PE onset. Medical departments have a well-placed diagnostic strategy for PE.27 Various clinical decisions have been validated including the standardized use of computed tomography pulmonary angiograms.28 The use of pulmonary embolism rule our criteria (PERC) also allows for the exclusion of PE as a probability.29 While these guidelines have been in place since before the COVID-19 pandemic, the current significance of these modalities is still unknown.30 The evidence from the various observational studies we included in this study suggests that patients who have contracted COVID-19 may be at a higher risk of developing a first PE, DVT, and bleeding events. Regardless of the severity of the disease, the risk of PE is particularly high during the post-acute phase.31,32
While the included studies report the significance of PE with long-COVID in select cases and based on the key findings, the current medical management standards may require modulation as we progress into longer stages of the pandemic.33 Patients with no comorbidities and typically low-risk classifications have been diagnosed with PE following COVID-19 infection, highlighting the need of a new risk-based thromboembolic approach. Another aspect in critical care is that D-dimer levels even when low, and with non-imaging studies not pointing towards PE; the diagnosis can be missed if CTPA is not ordered.34 For this reason, this study identifies VTE/PE as a consequence of the ongoing pandemic where a clinical index of suspicion is required even if the patient does not fit a typical profile.35
There is a strong link between SARS-CoV-2 infection and induced coagulopathy found in recent studies, whether due to causation or correlation cannot be stated with certainty.36,37 The distinct coagulation pattern and high incidence of VTE in COVID-19 patients suggest that CAC may involve novel mechanisms beyond those already known.4,38 In COVID-19, thrombosis is primarily caused by the activation of the innate immune system and large-vessel occlusion brought on by thromboembolism. 39,40 This includes immune system dysregulation, leukocyte and platelet activation and aggregation, the cytokine cascade, and activation of the complement system, all of which play a role in the pathogenesis of CAC.41,42 Nonetheless, unspecified pathophysiological mechanisms may contribute to thromboembolic events in the post-infectious period (i.e., 4–12 weeks and beyond).
The International Society for Thrombosis and Haemostasis suggests that a treatment regimen with prophylactic LMWH is recommended among all patients with COVID-19, particularly among those who originally present with derangements in clotting parameters or indications of severe disease.43 During admission with severe disease, a regular monitoring of clotting parameters was also recommended. The British Society of Haematology also recommends using LMWH to manage COVID-1944; patients with disease reportedly have higher incidences of VTE with deranged clotting markers and critical care during admission. The American College of Cardiology reports that implementing extending regiments of thromboprophylaxis among COVID-19 patients with risk factors for VTE, including pre-existing malignancy, comorbidity or reduced mobility. D-dimer, being an imperative demarcation, if twice the upper limit at the time of discharge, has also been suggested as a probing factor for prolonged anticoagulation.45
The purpose of this systematic review was to examine whether post-COVID-19 patients, especially those with mild or asymptomatic disease, require increased awareness of PE. The risk of VTE duration in non-hospitalized patients or those with brief hospital stays is still unknown, despite reports positing that the highest risk of VTE occurs within the first three weeks of hospital admission. Notably, patients’ risks of developing PE are known to rise after contracting COVID-19, and evidence in current literature suggests that this risk continues to post the infection. There is a paucity of clinical data on PE incidence in post-COVID-19 patients. Several terms have been used to describe the prolonged symptoms following COVID-19 infection, including long COVID, post-COVID conditions, postacute sequelae of SARS-CoV-2 infection, chronic COVID- 19, post-COVID syndrome and postacute COVID-19. Whether this constellation is a new syndrome unique to infection or if there is an overlap owing to recovery from similar illness has not been determined in current literature. However, based on the observational data included in this study, it is critical to identify the window of time after COVID-19 resolution during which patients are most at risk of developing PE. Future research should determine the high-risk period following COVID-19 convalescence and look into any potential causal relationships between post-COVID-19 PE and SARS-CoV-2 infection, regardless of the severity of the disease.
5. Conclusion
We cannot state with certainty that COVID-19 infection in itself leads to higher risks of PE at a later time, but we do find that standardized care in the era of COVID-19 must be optimized to account for deviations from the norm. Longitudinal studies are warranted in this area to make more concrete associations along with laboratory testing of molecular changes in the body to either nullify or confirm the incidence of PE due to long COVID.
Acknowledgements
None.
Footnotes
Disclaimers
The study has not been submitted to other publications or presented at conferences earlier.
Conflict of Interest
All authors declare no conflict of interest.
Source(s) of support
No support was acquired for this study.
Role of funding
No funding was obtained for this study.
References
- 1. Li N, Poor H. Venous thromboembolism and pulmonary embolism. Mt Sinai Expert Guid Crit Care. 2021:575–585. Published online. [Google Scholar]
- 2. Sastry S, Cuomo F, Muthusamy J. COVID-19 and thrombosis: the role of hemodynamics. Thromb Res. 2022;212:51–57. doi: 10.1016/j.thromres.2022.02.016. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomidokoro D, Hayama H, Okazaki T, Hara H, Hiroi Y. The effect of the COVID-19 pandemic on incidence and characteristics of pulmonary embolism. Glob Heal Med. 2021;3(2):122–124. doi: 10.35772/ghm.2020.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conway EM, Mackman N, Warren RQ, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(10):639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teimury A, Khameneh MT, Khaledi EM. Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res. 2022;27(1):1–10. doi: 10.1186/s40001-022-00655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanichkachorn G, Newcomb R, Cowl CT, et al. Mayo clinic proceedings. Vol. 96. Elsevier; 2021. Post–COVID-19 syndrome (long haul syndrome): description of a multi-disciplinary clinic at Mayo Clinic and characteristics of the initial patient cohort; pp. 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. Group WHOCCDW. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 Years—United States, march 2020–november 2021. Morb Mortal Wkly Rep. 2022;71(21):713. doi: 10.15585/mmwr.mm7131a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19) Statpearls [internet] 2022 Published online. [PubMed] [Google Scholar]
- 10. Maltezou HC, Pavli A, Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines. 2021;9(5):497. doi: 10.3390/vaccines9050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 2021;11(4):116. doi: 10.5662/wjm.v11.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHS. Long-term effects of coronavirus (long COVID) - NHS. 2022. [Accessed March 11, 2023]. Published https://www.nhs.uk/conditions/coronavirus-covid-19/long-term-effects-of-coronavirus-long-covid/
- 14. Peter RS, Nieters A, Kräusslich H-G, et al. Post-acute sequelae of covid-19 six to 12 months after infection: population based study. BMJ. 2022:379. doi: 10.1136/bmj-2022-071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai C-C, Hsu C-K, Yen M-Y, Lee P-I, Ko W-C, Hsueh P-R. Long COVID: an inevitable sequela of SARS-CoV-2 infection. J Microbiol Immunol Infect. 2022;56:1–9. doi: 10.1016/j.jmii.2022.10.003. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) A clinical case definition of post COVID-19 condition by a Delphi consensus. World Health Organization; october 6, 2021. 2021. [Google Scholar]
- 17.CDC. Post-COVID conditions: information for healthcare providers. 2022. [Accessed March 11, 2023]. Published https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html.
- 18. Venturelli S, Benatti SV, Casati M, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021:149. doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ares-Blanco S, Pérez Álvarez M, Gefaell Larrondo I, et al. SARS-CoV-2 pneumonia follow-up and long COVID in primary care: a retrospective observational study in Madrid city. PLoS One. 2021;16(9):e0257604. doi: 10.1371/journal.pone.0257604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murata N, Yamada A, Fujito H, et al. Cardiovascular manifestations identified by multi-modality imaging in patients with long COVID. Front Cardiovasc Med. 2022:9. doi: 10.3389/fcvm.2022.968584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jakubec P, Fišerová K, Genzor S, Kolář M. Pulmonary complications after COVID-19. Life. 2022;12(3):357. doi: 10.3390/life12030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Motloch LJ, Jirak P, Mirna M, et al. Early antithrombotic post-discharge therapy using prophylactic DOAC or dipyridamole improves long-term survival and cardiovascular outcomes in hospitalized COVID-19 survivors. Front Cardiovasc Med. 2022:9. doi: 10.3389/fcvm.2022.916156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekici A, Ekici M, Baçcõoğlu A, İnanç FA, Aslan H. Pulmonary embolism in patients with dyspnea after COVID-19 infection. Eur Rev Med Pharmacol Sci. 2022;26(10):3751–3759. doi: 10.26355/eurrev_202205_28872. [DOI] [PubMed] [Google Scholar]
- 24. Rezel-Potts E, Douiri A, Sun X, Chowienczyk PJ, Shah AM, Gulliford MC. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022;19(7):e1004052. doi: 10.1371/journal.pmed.1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jutant E-M, Meyrignac O, Beurnier A, et al. Respiratory symptoms and radiological findings in post-acute COVID-19 syndrome. ERJ open Res. 2022;8(2) doi: 10.1183/23120541.00479-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight R, Walker V, Ip S, et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146(12):892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Gal G, Righini M, Roy P-M, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 28. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;95:200463. doi: 10.1148/radiol.2020200463. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freund Y, Drogrey M, Ò Miró, et al. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: the PEPCOV international retrospective study. Acad Emerg Med. 2020;27(9):811–820. doi: 10.1111/acem.14096. [DOI] [PubMed] [Google Scholar]
- 30. Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imag. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elseidy SA, Awad AK, Vorla M, et al. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS) IJC Hear Vasc. 2022;40:101012. doi: 10.1016/j.ijcha.2022.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect. 2021;83(1):1–16. doi: 10.1016/j.jinf.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vadukul P, Sharma DS, Vincent P. Massive pulmonary embolism following recovery from COVID-19 infection: inflammation, thrombosis and the role of extended thromboprophylaxis. BMJ case reports CP. 2020;13(9):e238168. doi: 10.1136/bcr-2020-238168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poissy J, Goutay J, Caplan M, et al. Haemostasis COVID-19 group. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 36. Roy S, Demmer RT. Impaired glucose regulation, SARS-CoV-2 infections and adverse COVID-19 outcomes. Transl Res. 2022;241:52–69. doi: 10.1016/j.trsl.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Gu X, Zhang H, Li W, Yang H, Shang F. Clinical value of platelets and coagulation parameters in predicting the severity of delta variant SARS-CoV-2. Pathobiology. 2023:1–10. doi: 10.1159/000528318. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mouzarou A, Ioannou M, Leonidou E, Chaziri I. Pulmonary embolism in post-CoviD-19 patients, a literature review: red flag for increased awareness? SN Compr ClinMed. 2022;4(1):190. doi: 10.1007/s42399-022-01273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lucas FR, Araújo E, Gigante J, Silva S, Yousafzai Y. Coagulopathy and thromboembolism in covid-19-A review. SRC/JPRR-131 DOI doi org/1047363/JPRR/2022. J Pulmonol Res Rep. 2022;123:2–7. [Google Scholar]
- 40. De Michele M, Kahan J, Berto I, et al. Cerebrovascular complications of COVID-19 and COVID-19 vaccination. Circ Res. 2022;130(8):1187–1203. doi: 10.1161/CIRCRESAHA.122.319954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Subramaniam S, Kothari H, Bosmann M. Tissue factor in COVID-19-associated coagulopathy. Thromb Res. 2022 doi: 10.1016/j.thromres.2022.09.025. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaiafa G, Savopoulos C, Karlafti E, et al. Coagulation profile of COVID-19 patients. Life. 2022;12(10):1658. doi: 10.3390/life12101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemostasis. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hunt B, Retter A, McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. 2020 Published online. [Google Scholar]
- 45. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]