Abstract
Gastrinomas are the most common neuroendocrine tumors worldwide and cause a clinical syndrome known as Zollinger-Ellison Syndrome (ZES). Increased acid production resulting from elevated gastrin levels contributes to symptoms such as abdominal pain, heartburn, and diarrhea. However, the non-specificity and overlap in the symptoms with idiopathic peptic ulcer disease and gastroesophageal reflux disease (GERD) can lead to delayed diagnosis. In this case, we describe a patient with a past medical history of GERD and a perforated gastric ulcer who continued to experience symptoms of dyspepsia and had a subsequent esophageal perforation, despite H. pylori eradication and high-dose proton pump inhibitor (PPI) therapy. Multiple ulcers were visualized in the first portion of the duodenum, and metastatic lesions were demonstrable in the liver. Serum gastrin level was elevated to 433 pg/mL. Histology of liver biopsy showed a well-differentiated neuroendocrine tumor, supporting the diagnosis of ZES. This article underscores the significance of considering ZES in the differential in cases of refractory gastric hyperacidity and the importance of early diagnosis with serum gastrin testing to prevent complications such as gastric obstruction, perforation, hemorrhage, esophageal strictures, or rupture and to minimize the risk of metastasis. It is noteworthy that while perforations in cases of ZES typically occur in the duodenum, this particular case is atypical as it had involved the stomach. Furthermore, it was associated with esophageal perforation, likely resulting from forceful and prolonged vomiting caused by persistent dyspepsia.
Keywords: Gastrinoma, Zollinger-Ellison syndrome, Neuroendocrine tumor, Gastrin, Esophageal perforation
1. Introduction
Zollinger-Ellison Syndrome (ZES) was first described in patients with refractory peptic ulcer disease by two surgeons, RM Zollinger, and EH Ellison, in 1955.1 It is characterized by the presence of gastrinomas, neuroendocrine tumors that secrete gastrin. The duodenum is the most common site, followed by the pancreas. About 80% are sporadic, and 20–25% occur as a part of multiple endocrine neoplasia type-1 (MEN1).2 The annual incidence is 0.5–3 cases per million worldwide. Incidence is more common in men. The median age of onset is between 20 and 50 years of age.3
An elevated gastric acid state is driven by increased gastrin output from the tumor. It results in symptoms such as abdominal pain and heartburn, often indistinguishable from those of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD). While concomitant malabsorption, weight loss, and anorexia points towards ZES, the absence of these symptoms can lead to a diagnostic dilemma. Early diagnosis can be facilitated by having a high index of suspicion for refractory gastric hyper acidemia in cases of ulcers that are large, multiple, located distal to the first portion of the duodenum, or that recur despite maximal medical management.3
If not diagnosed early, ZES may present with complications such as gastric obstruction, perforation, hemorrhage, esophageal strictures, or rupture. Peptic ulcer perforations usually involve the duodenum, although there are rare documented instances of gastric perforation to date.4 In addition, while esophageal complications are being reported increasingly, esophageal perforation is rare and mainly described through case reports.
Here we present an esophageal perforation that ultimately led to the diagnosis of ZES. Notably, the patient had a history of gastric perforation previously and continued to experience symptoms despite H. pylori eradication therapy.
2. Case presentation
A 65-year-old African-American male presented with worsening substernal chest discomfort, nausea, and multiple episodes of non-bloody, bilious vomiting. The vomiting had been ongoing for a day with one episode of coffee ground emesis. He was unable to swallow secretions.
His past medical history was significant for GERD. Two months prior to the presentation, he had a perforated gastric ulcer requiring exploratory laparotomy and Graham patch repair. He had no reported history of NSAID use. H. pylori stool antigen test at that time was positive, and the patient completed an entire course of quadruple eradication therapy with bismuth, pantoprazole, tetracycline, and metronidazole. He had been taking pantoprazole 40 mg two times daily.
He was hypotensive and tachycardic. Laboratory tests were notable for a white blood cell count of 12.5 × 109/L. Lactate was elevated to 7.6 mg/dL. There was no elevation of transaminases, alkaline phosphatase (ALP), or serum lipase. CT chest showed pneumomediastinum and a perforation in the distal esophagus. He was admitted to the medical intensive care unit (ICU) and required intravenous fluid boluses, broad-spectrum antibiotics, and vasopressor support. He was placed on a strict NPO (nothing by mouth). An urgent upper gastrointestinal (GI) endoscopy was performed (Fig. 1). A perforation was noted in the distal esophagus at approximately 38 cm from the incisors (Fig. 1A and B). The entire examined stomach was normal. Multiple ulcers were visualized in the first part of the duodenum (Fig. 1C). A stent was placed across the perforation under fluoroscopic guidance (Fig. 1D). Robotic-assisted drainage of the posterior mediastinum was pursued the following day. A repeat esophagogram on day 3 confirmed the patency of the stent and revealed no leak. Biopsies taken from the stomach showed no evidence of recurrent H. pylori infection.
Fig. 1.
A, B, C and D are images from upper endoscopy. The yellow arrows in images A and B demonstrate a perforation in the distal esophagus with false lumen. Image C shows multiple ulcers (blue arrows) in the first part of the duodenum. Image D was taken after insertion of esophageal stent.
The hospital course was complicated by abdominal distension that required evaluation with a plain radiograph followed by abdominal/pelvis CT with contrast. The abdominal CT did not demonstrate a bowel obstruction but showed an incidental liver mass concerning for a neoplasm (Fig. 2A and B). An MRI that characterized the lesion further revealed multiple enhancing masses in segments I, II, and IVA of the liver with subtle washout and associated periportal adenopathy, most compatible with a metastatic disease process (Fig. 2C, D, and 2E). Further evaluation with fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan showed increased uptake by the left liver lesion and multiple porta hepatis lymphadenopathy, consistent with metastatic disease (Fig. 3A and B). The primary tumor could not be clearly delineated.
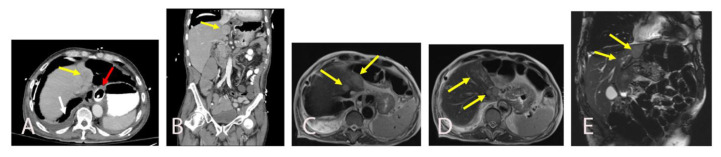
Fig. 2.
A and B are axial and coronal images of CT abdomen and pelvis with intravenous and oral contrast showing a segment II hepatic mass measuring 3.5 × 2.6 × 3 cm (yellow arrow). There is a distal esophageal stent with adjacent pneumomediastinum with oral contrast surrounding the esophageal wall (red arrow) suggests esophageal perforation. A pleural effusion is also seen (white arrow). C, D and E are subsequently obtained abdominal MRI images. Axial (C, D) and coronal T2-weighted (E) abdominal MRI images show round enhancing masses in segment I, II, and IVA as well as additional adjacent sub centimeter satellite hypo enhancing masses (yellow arrows).
Fig. 3.
These are fused PET/CT axial images. A shows a PET-avid segment II lesion corresponding to the metastatic lesion visualized on MRI. B shows PET-avid precaval and periportal lymph nodes that correspond to enlarged lymph nodes on the MRI.
A percutaneous liver biopsy revealed a metastatic, grade 1 well-differentiated neuroendocrine tumor (Fig. 4). Serum gastrin levels were elevated to 433 pg/mL. A diagnosis of Zollinger-Ellison Syndrome was made based on the chronicity of the hyperacid state (long-standing GERD), the presence of refractory peptic ulcer disease despite high dose proton pump inhibitor (PPI) therapy (breakthrough esophageal perforation occurred despite PPI therapy after gastric perforation), elevated serum gastrin levels, endoscopic findings of multiple, duodenal ulcers and presence of metastatic, neuroendocrine tumor on liver biopsy. The absence of family history and normal serum ionized calcium levels pointed towards a sporadic, metastatic case of ZES. The patient was discharged on a high dose of lansoprazole (30 mg) two times daily and monthly long-acting octreotide injectable suspensions. An outpatient appointment for primary tumor localization with somatostatin receptor scintigraphy (SRS) was set up, and an oncology follow-up was provided for definitive treatment. The patient was unable to attend these appointments and succumbed to the disease in the few weeks that followed.
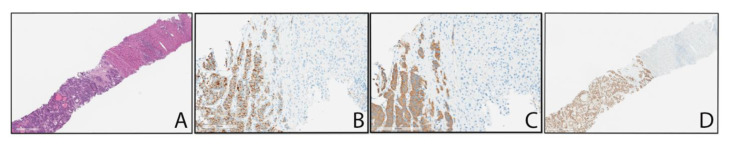
Fig. 4.
A is image of a liver core needle biopsy showing the transition from liver parenchyma (upper right) to the tumor (lower left). The nested pattern of the tumor is suggestive of possible neuroendocrine origin. B and C represent chromogranin and synaptophysin staining respectively. These stains positively highlight the tumor cells (brown) and do not stain the liver parenchyma, confirming neuroendocrine tumor. The lack of pleomorphism, mitotic figures, and low Ki-67 proliferation index (~25, not shown) identify this as a well-differentiated neuroendocrine tumor. D represents CDX-2 immunohistochemical staining that positively highlights the tumor cell nuclei (brown), whereas the liver parenchyma is negative for CDX-2 staining. This suggests the tumor is of gastrointestinal origin.
3. Discussion
Zollinger-Ellison Syndrome (ZES) is a rare cause of PUD, accounting for only 0.1–1% of cases.2 Diagnosis requires a demonstration of inappropriate hypergastrinemia, which means an increase in gastrin levels that is associated with hyperchlorhydria (gastric pH ≤ = 2).5,6 No degree of hypergastrinemia alone can distinguish between physiological (such as atrophic gastritis and PPI therapy) and inappropriate causes (such as ZES) of gastrin elevation. Patients with chronic atrophic gastritis can have elevated fasting serum gastrin (FSG) levels as high as >1000–2000 pg/mL.7 Additionally, smaller gastrinomas may cause lower or equivocal gastrin levels.8 Therefore, to accurately diagnose ZES, it is recommended to assess gastric pH (which is ≤ = 2). Nevertheless, evaluating gastric fluid acidity is not always feasible, and discontinuing PPI therapy to measure gastric pH can be risky.5,6,9 For these reasons, new diagnostic criteria have been proposed, which involve demonstrating fasting hypergastrinemia and repeatedly assessing gastric pH with gradual decrements in the dosage of PPI therapy until pH ≤ = 2 or pursuing alternative diagnostic techniques, such as neuroendocrine tumor (NET)-specific imaging or a positive biopsy for a NET.7,10 Based on these new criteria, the presence of active PUD and a positive biopsy for a NET in our patient strongly support the diagnosis of ZES.
Early diagnosis is essential to prevent further disease complications and the risk of metastasis. Empirical PPI therapy early on in the disease course is standard, resulting in symptom relief but perhaps missed chance for diagnosis. Patients face a high probability of tumor progression and a poor prognosis despite treatment of the hormonal state.5 This comes from the fact that 60%–90% of gastrinomas are malignant and can metastasize to the liver. Metastasis can have implications on overall survival, with a 15% 10-year survival in the presence of liver metastasis and 95% 20-year survival in its absence.4 In addition, delayed diagnosis can result in complications from PUD such as peptic ulcer perforation, bleeding, or penetration (10–15%), or from gastroesophageal reflux such as esophageal stricture, perforation, ulcer (<5%).8 Thus, refractory peptic ulcer disease presentations such as perforation may require serum gastrin level testing to facilitate early diagnosis and treatment.8
Peptic ulcer perforation can occur in 4–6% of patients with ZES. The perforations mostly involve the duodenum, with rare reports of jejunal and gastric perforations.4,11,12 The possibility of ZES was not explored in the prior presentation in our patient due to the gastric location of the ulcer and positive testing for H. pylori. However, in retrospect, ongoing symptoms despite PPI therapy, elevated serum gastrin levels, multiple duodenal ulcers and absence of H. pylori infection on subsequent endoscopy, and presence of metastatic disease and NET on liver biopsy suggest that gastric perforation was dominantly due to ZES.
Esophageal perforations are most commonly iatrogenic in the setting of endoscopies and, less typically, a result of blunt or penetrating trauma, tumors, or infections. Spontaneous perforations are largely barogenic and occur due to increased intraesophageal pressure, as can occur with active vomiting (Booerhave syndrome).13,14 To our knowledge, three cases of esophageal perforation were documented in the setting of ZES. Two were secondary to emesis, with one being spontaneous.15 The patient discussed in this case likely experienced increased intra-esophageal pressure due to forceful emesis, leading to the perforation. This represents the fourth documented case of esophageal perforation associated with ZES.
Esophageal involvement is increasingly being reported in ZES in recent studies. This is a result of chronic gastroesophageal reflux, and the symptoms can often remain masked due to other, more compelling clinical problems.15 The symptoms are more frequent and severe in MEN1, likely from hypercalcemia, which can increase the acid secretory rate leading to antisecretory drug resistance. Hypercalcemia can also alter esophageal motility and decrease lower esophageal sphincter (LES) pressure resulting in increased reflux.16 Miller et al. demonstrated reflux esophagitis in 61% of patients with ZES.17 Three significant positive predictors for the development of esophagitis include lower esophageal sphincter pressure (LESP) 16 mm Hg, vomiting, and obesity.18 Even so, the incidence of complicated esophagitis such as strictures, Barrett's esophagus, and esophageal adenocarcinoma in ZES remains relatively rare.19
Assessment of the primary tumor location and defining the extent of metastases with positron emission tomography-computed tomography (PET/CT) scan or SRS is critical once the diagnosis is established.3 Alleviating gastric hypersecretion and surgical resection for cure or debulking is the cornerstone of treatment.5 The combination of high-dose PPI and somatostatin therapy is reserved for patients with unresectable disease, metastatic disease, numerous gastrinomas, or who are medically inoperable.15
4. Conclusion
Thus, we describe a case of esophageal perforation that resulted in the diagnosis of ZES. This case highlights the importance of having ZES in the differential for complicated peptic ulcer disease presentations, such as a perforation, to facilitate early diagnosis. Early diagnosis with serum gastrin testing is recommended. Treatment with high-dose PPI therapy and surgical resection is critical to positive outcomes and preventing metastatic spread and further complications.
Footnotes
Conflict of interest
We have no potential conflicts of interest or sources of financial support.
References
- 1. Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709–723. discussion, 724–708. [PMC free article] [PubMed] [Google Scholar]
- 2.Cho MS, Kasi A. Zollinger Ellison syndrome. Treasure Island (FL): StatPearls; 2022. [PubMed] [Google Scholar]
- 3. Epelboym I, Mazeh H. Zollinger-Ellison syndrome: classical considerations and current controversies. Oncol. 2014;19:44–50. doi: 10.1634/theoncologist.2013-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen RT. Zollinger–Ellison syndrome Yamada’s textbook of gastroenterology. 2015:1078–1102. doi: 10.1002/9781118512074.ch57. [DOI] [Google Scholar]
- 5. Metz DC. Diagnosis of the Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10:126–130. doi: 10.1016/j.cgh.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 6. Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495–5503. doi: 10.3748/wjg.v18.i39.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metz DC, Cadiot G, Poitras P, Ito T, Jensen RT. Diagnosis of Zollinger-Ellison syndrome in the era of PPIs, faulty gastrin assays, sensitive imaging and limited access to acid secretory testing. Int J Endocr Oncol. 2017;4:167–185. doi: 10.2217/ije-2017-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen RT, Ito T, Gastrinoma Endotext, et al., editors. South Dartmouth (MA) 2000. [Google Scholar]
- 9. Ito T, Igarashi H, Jensen RT. Zollinger-Ellison syndrome: recent advances and controversies. Curr Opin Gastroenterol. 2013;29:650–661. doi: 10.1097/MOG.0b013e328365efb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison syndrome: dangers and consequences of interrupting antisecretory treatment. Clin Gastroenterol Hepatol. 2012;10:199–202. doi: 10.1016/j.cgh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 11. DeBoer JL, James EC. Treating perforated jejunal ulcer, a complication in the Zollinger-Ellison syndrome. Am Surg. 1976;42:196–200. [PubMed] [Google Scholar]
- 12. Watson CG, Moseley RV, Wheeler HB. Perforated jejunal ulcer and the Zollinger-Ellison syndrome. Arch Surg. 1968;96:274–276. doi: 10.1001/archsurg.1968.01330200112023. [DOI] [PubMed] [Google Scholar]
- 13. Jones WG, 2nd, Ginsberg RJ. Esophageal perforation: a continuing challenge. Ann Thorac Surg. 1992;53:534–543. doi: 10.1016/0003-4975(92)90294-e. [DOI] [PubMed] [Google Scholar]
- 14. Nirula R. Esophageal perforation. Surg Clin North Am. 2014;94:35–41. doi: 10.1016/j.suc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 15. Ng T, Maziak DE, Shamji FM. Esophageal perforation: a rare complication of Zollinger-Ellison syndrome. Ann Thorac Surg. 2001;72:592–593. doi: 10.1016/s0003-4975(00)02308-0. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann KM, Gibril F, Entsuah LK, Serrano J, Jensen RT. Patients with multiple endocrine neoplasia type 1 with gastrinomas have an increased risk of severe esophageal disease including stricture and the premalignant condition, Barrett's esophagus. J Clin Endocrinol Metab. 2006;91:204–212. doi: 10.1210/jc.2005-1349. [DOI] [PubMed] [Google Scholar]
- 17. Miller LS, Vinayek R, Frucht H, Gardner JD, Jensen RT, Maton PN. Reflux esophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology. 1990;98:341–346. doi: 10.1016/0016-5085(90)90823-j. [DOI] [PubMed] [Google Scholar]
- 18. Hirschowitz BI, Simmons JL, Johnson LF, Mohnen J. Risk factors for esophagitis in extreme acid hypersecretors with and without Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2004;2:220–229. doi: 10.1016/s1542-3565(04)00009-6. [DOI] [PubMed] [Google Scholar]
- 19. Strader DB, Benjamin SB, Orbuch M, et al. Esophageal function and occurrence of Barrett's esophagus in Zollinger-Ellison syndrome. Digestion. 1995;56:347–356. doi: 10.1159/000201258. [DOI] [PubMed] [Google Scholar]