Abstract
Background
Giant cell arteritis (GCA) is a common inflammatory condition that affects medium and large‐sized arteries and can cause sudden, permanent blindness. At present there is no alternative to early treatment with high‐dose corticosteroids as the recommended standard management. Corticosteroid‐induced side effects can develop and further disease‐related ischaemic complications can still occur. Alternative and adjunctive therapies are sought. Aspirin has been shown to have effects on the immune‐mediated inflammation in GCA, hence it may reduce damage caused in the arterial wall.
Objectives
To assess the safety and effectiveness of low‐dose aspirin, as an adjunctive, in the treatment of giant cell arteritis (GCA).
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2013, Issue 12), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to January 2014), EMBASE (January 1980 to January 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to January 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) and the US Food and Drugs Administration (FDA) web site (www.fda.gov). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 24 January 2014.
Selection criteria
We planned to include only randomised controlled trials (RCTs) comparing outcomes of GCA with and without concurrent adjunctive use of low‐dose aspirin.
Data collection and analysis
Two authors independently assessed the search results for trials identified by the electronic searches. No trials met our inclusion criteria, therefore we undertook no assessment of risk of bias or meta‐analysis.
Main results
We found no RCTs that met the inclusion criteria.
Authors' conclusions
There is currently no evidence from RCTs to determine the safety and efficacy of low‐dose aspirin as an adjunctive treatment in GCA. Clinicians who are considering the use of low‐dose aspirin as an adjunctive treatment in GCA must also recognise the established haemorraghic risks associated with aspirin, especially in the context of concurrent treatment with corticosteroids. There is a clear need for effectiveness trials to guide the management of this life‐threatening condition.
Plain language summary
Aspirin as an additional treatment for giant cell arteritis
Background
Giant cell arteritis (GCA) is a condition where inflammation destroys the wall of arterial blood vessels usually seen in the head. GCA affects people over the age of 50 years and is more common as people get older. Early on people feel tired and unwell; they have loss of appetite and can lose weight. Most people then develop a new headache, which can make it uncomfortable to touch their hair and scalp. Some people find chewing food uncomfortable. GCA can cause sudden blindness in one or both eyes. Other rare complications include double vision and life‐threatening aneurysms and stroke.
Making the diagnosis can be difficult for doctors. Blood tests can help, but not everyone has signs in the blood of raised inflammation. A temporal artery biopsy is recommended. If the biopsy is negative some people still remain on treatment as their clinical story matches the typical disease presentation.
At diagnosis the emergency treatment is with high‐dose steroids (corticosteroids). Corticosteroids are typically reduced slowly over 12 to 18 months, however some people relapse and need long‐term treatment. Corticosteroids have serious side effects such as weight gain, mood changes, stomach bleeds, bone thinning and fractures. Despite best treatment people can still go blind in one or both eyes. A different drug needs to be found to treat this condition to reduce the risk of blindness, other complications and treatment‐related side effects. Aspirin has been shown to have beneficial effects on the type of inflammation that causes damage in GCA and could therefore help to reduce disease‐related complications.
Review question
The review authors searched the medical evidence for low‐dose aspirin used as an additional treatment to corticosteroids in GCA. The purpose was to investigate whether aspirin helps reduce the risk of blindness and other life‐threatening complications. We also wanted to know whether aspirin causes an increase in side effects, particularly stomach bleeds, when used together with corticosteroids.
Key results
The evidence provided by this review is current to January 2014. There were no randomised controlled trials found that met the criteria for inclusion. There is limited medical information on the use of aspirin in GCA.
Conclusions
At the present time there is not enough data to make a comment on whether aspirin is of benefit in GCA. More research is needed.
Background
Description of the condition
Giant cell arteritis (GCA), also known as temporal arteritis, is an immune‐mediated disease where inflammation affects medium to large arteries such as the extracranial branches of the carotid artery, the aorta, the coronary arteries and the renal arteries. People with GCA can complain of a variety of problems, and sometimes complain of no problems prior to blindness occurring. Despite corticosteroid treatment, GCA causes significant complications, including permanent visual loss (Salvarani 2005), dissecting aneurysm (Evans 1995; Robson 2013) and stroke (Nesher 2004a). GCA is associated with increased mortality (Nordborg 1989) and a reduced five‐year survival rate following diagnosis (Crow 2009). The underlying cause of GCA is unknown.
Epidemiology
GCA is more common in white persons older than 50 years (Lawrence 1998) and the incidence increases with age (Machado 1988). The incidence of GCA in the population varies worldwide, with the highest frequencies being reported from Scandinavian countries (Petursdottir 1999) and those with populations of Scandinavian descent (Borchers 2012). There is confirmation of a genetic susceptibility for GCA (Carmona 2013; Serrano 2013).
Socio‐economic deprivation has been reported in association with ischaemic manifestations, which is not mediated by traditional cardiovascular risk factors (Mackie 2011). The authors concluded that this may suggest that the delay between first symptoms and presentation may play a significant role in the development of ischaemic complications.
Presentation
People with GCA can typically complain of the following (Salvarani 2005; Smetana 2002):
New onset unilateral headache
Scalp pain or tenderness
Jaw claudication
Neck pain
Visual complaints such as transient visual obscurations, visual loss or double vision
Constitutional symptoms such as weight loss, loss of appetite, fatigue, fever and myalgia (muscle pain)
There is a dramatic variability in how people present with GCA, which makes it difficult for medical practitioners to diagnose (or dismiss) the disease on clinical grounds alone. It requires that the physician have a high index of suspicion. In one study, over 20% of patients with visual loss from biopsy‐proven GCA presented with no systemic signs (Hayreh 1998). In the literature, polymyalgia rheumatica is thought by some to be closely associated with GCA, with 16% to 21% of polymyalgia rheumatica patients having GCA, and 40% to 60% of GCA patients having polymyalgia rheumatica symptoms (Salvarani 2008). For the purpose of this review polymyalgia rheumatica will not be specifically investigated.
Diagnosis
Early diagnosis is paramount because there is a short window of time during which treatment can prevent serious ischaemic complications, particularly sight loss. Prior to the widespread use of corticosteroid treatment, the rate of sight loss was between 30% and 60% (Birkhead 1957); this has been reduced to between 5% and 20% (Salvarani 2005).
The clinical history and examination, including palpation of the temporal arteries, are key steps in establishing the diagnosis. Blood investigations typically include the erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP) and platelet count. All three tests have a positive correlation with a diagnosis of GCA, but are frequently discordant in individual patients, leading to diagnostic uncertainty.
Temporal artery biopsy (TAB) is the current standard diagnostic investigation for GCA. However a TAB is invasive and when unilateral has a calculated sensitivity of 87.1% (Niederkohr 2007). False negative biopsies occur due to areas of unaffected artery, so called 'skip lesions', sampling errors including sampling of a non‐involved vessel, and differences in how the biopsy is histopathologically assessed (Mahr 2006). These all contribute to a reduced sensitivity. Rare clinical scenarios exist in which the clinical presentation and blood testing make the diagnosis of GCA likely without the need for a TAB. However, empiric steroid therapy (treating without histological confirmation of GCA) is almost never recommended (Niederkohr 2005). Non‐invasive imaging modalities, such as temporal artery colour doppler ultrasound, 3‐Tesla magnetic resonance angiography and F18‐deoxyglucose positron emission tomography, are being investigated and have in some cases been adopted. However, none of these tests has been proven to be as reliable, available and cost‐effective as TAB.
Management
Early intervention with high‐dose corticosteroids is the standard treatment for GCA. Subsequently corticosteroids are tapered according to clinical symptoms, signs and acute‐phase serological markers (ESR and CRP). Typically treatment is required for a prolonged period of time. A randomised controlled trial found that the total cumulative steroid dose was reduced by initiating treatment with three days of pulsed intravenous methylprednisolone (Mazlumzadeh 2006).
Relapse can occur, whether clinical or biochemical. Once treatment is initiated, the risk of recurrent visual loss is as high as 7% at three years (Chan 2005). In those who discontinue corticosteroid therapy within 12 months, the rate of relapse is as high as 77% (Hoffman 2002). Late recurrences have also been reported (Kim 2003). Corticosteroid toxicity remains a concern, especially as it is pronounced in the older age group most affected by GCA. In routine practice gastric and bone prophylaxis are given concurrently. The British Society of Rheumatology Guidelines also recommend the use of low‐dose aspirin (Dasgupta 2010).
Combined therapy with other immunosuppressant drugs is being evaluated: azathioprine has not proven to be effective (De Silva 1986). A meta‐analysis of the adjunctive use of methotrexate reported a significantly reduced risk of primary and secondary relapse, with hazard ratios of 0.65 (95% confidence interval (CI) 0.44 to 0.98, P value = 0.04) and 0.49 (95% CI 0.27 to 0.89, P value = 0.02), respectively. There was a reduction in the cumulative corticosteroid dose at 12 weeks (P value = 0.01), 24 weeks (P value = 0.01), 36 weeks (P value < 0.001) and 48 weeks (P value < 0.001). There was no difference in the corticosteroid‐related side effects between the treatment groups (Mahr 2007). Biological agents such as tumour necrosis factor‐α inhibitors have been investigated and have not proven to be of benefit (Hoffman 2007); the interleukin (IL)‐6 receptor antagonist tocilizumab is currently under trial (Unizony 2013). The role of second‐line steroid‐sparing agents is currently being evaluated by another Cochrane review (Hill 2009), and is therefore beyond the remit of this review.
Description of the intervention
Antiplatelet treatments are drugs that interfere with platelet function: they inhibit thrombosis (clot) formation by decreasing platelet aggregation. Platelet aggregation is a dynamic and complex process where platelets stick to each other at the site of blood vessel injury to form a clot. Aspirin (acetylsalicylic acid; ASA) is an oral antiplatelet drug, which is technically termed a cyclooxygenase (COX) inhibitor. Aspirin irreversibly inhibits the enzyme COX, resulting in reduced platelet production of thromboxane. Low‐dose aspirin is well known to reduce the risk of stroke in other populations (Lee 2006; Weisman 2002).
How the intervention might work
Description of the immunopathogenesis of GCA
GCA is an immune‐mediated primary systemic vasculitis, where the arterial wall is the site of the disease process. Activated immune cells, macrophages and T‐cells in the adventitia (arterial wall) produce high levels of cytokines, such as interferon‐γ (IFN‐γ), interleukin‐2 (IL‐2) and interleukin‐17 (IL‐17). These stimulate macrophages in the media to express metalloproteinases and reactive oxygen species, which break down the internal elastic laminae. A healing response causes proliferation of smooth muscle cells and intimal hyperplasia, which results in vascular stenosis and occlusion (Ly 2010; Weyand 2004; Weyand 2011).
Altering inflammation
Aspirin has been shown to have a wide range of effects on the immune system, including inducing tolerance in dendritic cells and inducing regulatory T cells (Hussain 2011). In addition, aspirin suppresses the transcription of IFN‐γ, a key cytokine in GCA that recruits macrophages in the vessel to produce metalloproteinases and reactive oxygen species that cause destruction of the internal laminae (Weyand 2002). Corticosteroids suppress the production of macrophage‐derived IL‐1, IL‐6 and NOS‐2 and suppress the T cell cytokine IL‐2. They only have a marginal effect on IFN‐γ. Therefore the mechanism of action of aspirin would be complementary to corticosteroids.
Preventing thrombosis formation
Aspirin has an antithrombotic action via its inhibition of thromboxane A2 production and consequent reduction in platelet aggregation. Although there is no clear evidence that the reactive thrombocytosis associated with GCA can cause thrombosis, thrombus formation has been histologically documented in the vertebral arteries of a small case series of GCA patients (Rüegg 2003).
Why it is important to do this review
To assess the safety and effectiveness of using low‐dose aspirin as an adjunctive treatment, in combination with corticosteroids, for treatment of GCA. GCA is associated with significant organ and life‐threatening complications such as:
sight loss (Salvarani 2005);
thoracic aortic aneurysms (Evans 1995);
abdominal aortic aneurysms (Evans 1995); and
stroke (Nesher 2004a; Nesher 2004b).
It is clear that although corticosteroids are the main therapeutic intervention for GCA, adjunctive therapy is required because:
the spectrum of corticosteroid repression of the inflammatory cytokines found in GCA is inadequate;
despite adequate treatment with corticosteroids, there is histopathological evidence that the inflammatory infiltration of the vessel wall persists;
late complications, such as thoracic and abdominal aorta aneurysm, occur; and
corticosteroids do not shorten the natural history of the disease.
There is controversy in the literature over whether antiplatelet therapy should be considered in GCA (Hayreh 2003; Hellmann 2004). Hayreh 2003 points out that although essential thrombocytosis has increased thrombotic morbidity, the thrombocytosis in GCA is reactive and thus an antithrombotic agent is likely to have little effect. There is some evidence suggesting that the risk of cranial ischaemic complications in GCA is reduced by aspirin (Lee 2006; Nesher 2004a). In addition, the presence of atherosclerosis risk factors at the time of diagnosis of GCA may influence the development of cranial ischaemic complications (Gonzalez‐Gay 2004). Thus aspirin therapy needs to be systematically evaluated.
Objectives
To assess the safety and effectiveness of low‐dose aspirin, as an adjunctive, in the treatment of giant cell arteritis (GCA).
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) comparing outcomes of GCA with and without concurrent adjunctive use of low‐dose aspirin.
Types of participants
For this review we planned to included studies that enrolled participants who:
were over the age of 50 years; and
had histological findings of GCA on temporal artery biopsy, such as the presence of inflammatory cell infiltrate, giant cells, intimal thickening and fragmentation of the internal elastic lamina.
We planned to exclude studies where the participant group has GCA diagnosed by clinical criteria alone, or that included juvenile temporal arteritis.
Types of interventions
We planned to include trials where low‐dose aspirin in combination with corticosteroids was compared to placebo or no aspirin.
Types of outcome measures
For the purpose of the review sight loss is defined as any loss of visual acuity or development of a visual field defect in either eye that is attributable to GCA at baseline, 24 weeks and 48 weeks.
Primary outcomes
Risk of sight‐threatening complications at one year, with or without aspirin use, defined as continued (worsening) or recurrent (repeated) sight loss in the affected eye, or the onset of sight loss in the other previously non‐affected eye.
Risk of life‐threatening ischaemic complications at one year, with or without aspirin use, which includes any one of the following: cranial nerve palsy, aneurysm, myocardial infarction, renal infarction or stroke.
Secondary outcomes
Disease relapse
Time‐to‐event outcome of biochemical and/or clinical relapse. We defined relapse as the re‐introduction of corticosteroids, or an increase of corticosteroids, to suppress either inflammatory markers (biochemical relapse) or any GCA‐related clinical symptoms (clinical relapse).
If the included studies did not report biochemical or clinical relapse as time‐to‐event, we planned to analyse the proportion of participants in each group experiencing a biochemical or clinical relapse as defined above at one‐year follow‐up and at other time points as reported in the included studies.
Disease remission
Time‐to‐event outcome of disease remission defined as participants no longer requiring immunosuppression, where inflammatory markers have normalised and there are no signs and symptoms of GCA.
If the included studies did not report time‐to‐event data for disease remission, we planned to analyse the proportion of participants in each group with remission as defined above at one‐year follow‐up and at other time points as reported by included studies.
Mortality
The proportion of patients dying in each treatment arm during the study period.
Adverse outcomes
We planned to record the number of adverse events reported during the study period for each treatment arm. Treatment‐related adverse events include gastrointestinal ulcers, stomach bleeding and tinnitus. In addition, we planned to record the number of patients discontinuing with the study due to drug‐related side effects.
Economic data
We planned to extract any available cost analysis.
Quality of life data
We planned to collect any information on the effects on quality of life. We planned to document Information about patient preference with respect to therapy, including ease of administration, convenience, number of required follow‐up visits and out‐of‐pocket expenses after treatment initiated.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2013, Issue 12), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to January 2014), EMBASE (January 1980 to January 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to January 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en) and the US Food and Drugs Administration (FDA) web site (www.fda.gov). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 24 January 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6), the ICTRP (Appendix 7) and the FDA web site (Appendix 8).
Searching other resources
We searched the bibliographies of retrieved articles for additional references. In addition, we contacted experts within the field where appropriate.
Data collection and analysis
Selection of studies
Two review authors (SPM and NS) independently screened the titles and abstracts identified from the above searches. We obtained a full‐text copy for any study which appeared partially or definitely relevant from this initial assessment and which met the inclusion criteria.
Two review authors (SPM and NS) examined the full‐text articles independently. If any clarification or further details were needed to make a complete assessment of any study, we planned to contact the authors of the study directly. We planned to group studies as included, excluded or unsure. Where a disagreement arose between the two authors another review author (MAB) adjudicated.
Methods for future updates
We will use the following methods to evaluate included studies identified in future updates.
Data extraction and management
Two review authors (SPM and NS) will use an electronic data extraction form to independently extract all data required for the review in relation to study characteristics, primary and secondary outcomes. One review author (SPM) will enter the data into Review Manager (RevMan 2012) and a second review author (NS) will verify all entries. We will record the following:
Methods: study design, allocation, masking, exclusions (especially those after randomisation), patient drop‐out and loss to follow‐up and noncompliance.
Participants: country and setting where participants enrolled, number of patients in study, number of patients randomised (if applicable), age, age range, sex, number of women and number of men, ethnic group, inclusion criteria and exclusion criteria.
Interventions: treatment, control, duration of treatment, dose of treatment.
Outcomes: endpoints on which data will be collected, length of follow‐up, number of relapses and ischaemic complications, and time to disease relapse and remission, source of funding and declaration of interest.
'Summary of findings' table
We will construct 'Summary of findings' tables incorporating the body of evidence included in this review, focusing on patients with biopsy‐confirmed GCA on standard treatment. We will include the following outcomes in each 'Summary of findings' table: 1) sight‐threatening ischaemic events; 2) life‐threatening ischaemic events; 3) disease relapse; 4) disease remission; and 5) mortality. For each outcome, we will use the results from included studies to estimate the absolute risks for the aspirin and comparison groups as well as the relative effect estimates (risk ratio).
We will use the GRADE (Grading of Recommendation Assessment Development and Evaluation) approach as described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions to rate the overall quality of the body of evidence for the five outcomes listed above according to each comparison (Schünemann 2011). Two review authors will evaluate the evidence for the following factors that may decrease the quality of evidence: 1) factors related to design and execution of included studies (risk of bias); 2) indirectness in the population, intervention, control, outcomes; 3) inconsistency or heterogeneity in reported results; 4) imprecision in effect estimates influenced by sample size and confidence intervals; and 5) potential publication bias. If there are insufficient quantitative results, we will provide a narrative 'Summary of findings' table addressing the impact of the intervention on the same outcomes described previously.
Assessment of risk of bias in included studies
We will critically appraise all studies meeting our inclusion criteria in relation to internal study validity with emphasis on selection bias, performance bias, attrition bias and reporting bias. Two review authors (SPM and NS) will assess the risk of bias using the Cochrane 'Risk of bias' tool given in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will make the following judgements for each domain: 'high', 'low', or 'unclear' risk of bias. If a consensus cannot be reached for the final judgement, a third review author (MAB) will adjudicate. Each 'Risk of bias' domain will address the following methodological study characteristics:
Selection bias
Random sequence generation
Allocation concealment
Performance and detection bias
Masking of study participants to their assigned treatment
Masking of study personnel providing care to the study participants
Masking of study personnel assessing treatment outcomes
Attrition bias
Incomplete outcome data
Reporting bias
Selective outcome reporting
Measures of treatment effect
For the primary outcomes, ischaemic complications (sight‐threatening and life‐threatening), and the secondary outcomes, relapse (biochemical and clinical) and disease remission, we will plan to use risk ratios and corresponding variance estimates such as 95% confidence intervals to measure the treatment effect. If the included studies do not measure treatment effects as time‐to‐event outcomes, we will record the proportion of participants in each treatment arm that experience an ischaemic complication, relapse or disease remission at the end of one year of follow‐up and compute risk ratio estimates for each outcome. We will also record the number of deaths for each treatment arm and compute risk ratio estimates for mortality.
Unit of analysis issues
The unit of analysis will be the individual participant. There may be variation in which RCTs record visual outcomes in terms of reporting as unilateral or bilateral.
Dealing with missing data
We will study intention‐to‐treat to assess the number of patients who were assigned to treatment and the number of patients who were actually treated. We will report follow‐up by treatment group and collect data on reason for loss to follow‐up, where possible. We will contact study authors for missing outcome data and allow four weeks for investigator responses. If study investigators do not respond or cannot provide the data that is required we will record this as a potential source of attrition bias, as per the Cochrane 'Risk of bias' reporting tool.
Assessment of heterogeneity
We will evaluate clinical heterogeneity (participants, interventions and outcomes reported) and methodological heterogeneity (study design and risk of bias) across the included studies before conducting a meta‐analysis. We will also examine the size and direction of effect estimates and overlap of 95% confidence intervals. We will use the I² statistic to quantify inconsistency across studies, with a value of 50% or more indicating significant statistical heterogeneity.
Assessment of reporting biases
We will construct and inspect a funnel plot of the available studies for asymmetry to ascertain publication bias. However, if the number of studies is low (fewer than 10), or the sample sizes within the studies are small, this could be unreliable. Asymmetry within the plot may also be due to language and citation bias or poor methodological design of the trial.
Data synthesis
When three or more studies are included in a meta‐analysis, or when noticeable clinical or methodological heterogeneity is detected, we will use a random‐effects model. We will use fixed‐effect models when fewer than three studies are included in a meta‐analysis. We will use the generic inverse variance method in Review Manager 5 to perform meta‐analyses of time‐to‐event outcomes (relapse and disease remission). For dichotomous data (e.g. ischaemic complications and number of deaths per treatment group) we will calculate Mantel‐Haenszel risk ratios with 95% confidence intervals as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Subgroup analysis and investigation of heterogeneity
If multiple interventions are reported, such as high‐dose and low‐dose aspirin, we will perform separate subgroup analyses according to specific treatment regimens.
Sensitivity analysis
We will perform sensitivity analyses to determine the impact on the treatment effects of removing studies judged to have a high risk of bias for incomplete outcome data and selective outcome reporting, industry‐funded studies and unpublished studies (conference abstracts).
Results
Description of studies
Results of the search
The electronic searches yielded a total of 174 records (Figure 1). After deduplication we screened 157 records and excluded 148 records as not being relevant to the review question. We obtained full‐text copies of nine reports for further assessment, however we did not identify any potentially eligible studies for this review.
1.
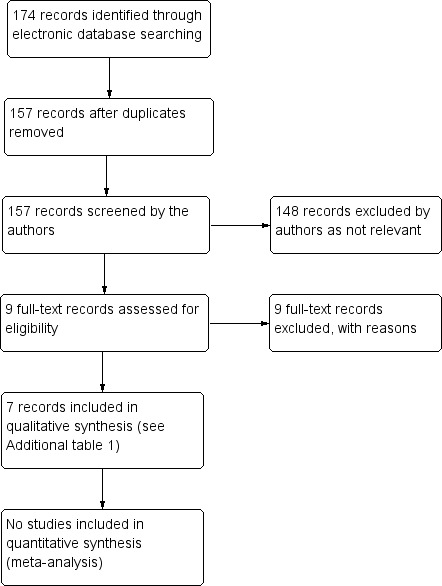
Results from searching for studies for inclusion in the review
Included studies
No studies were included.
Excluded studies
We reviewed nine full‐text articles: seven were retrospective studies, one was a case report and one an editorial. None met the inclusion criteria.
Risk of bias in included studies
We did not complete 'Risk of bias' assessment as no studies were included in the review.
Effects of interventions
We did not complete an assessment of the effects of the intervention as no studies were included in the review.
Discussion
We found no randomised controlled trials (RCTs) that investigated the adjuvant use of aspirin therapy for giant cell arteritis (GCA). Since no RCTs were found, we have described the other relevant studies identified in the searches in order to comment on the current evidence base for clinical practice (Table 1).
1. Summary of current medical evidence reporting on aspirin use in GCA.
| Article | Number of participants with GCA | Methods |
GCA Biopsy‐proven (%) |
Number on ASA at time of diagnosis | Number started on ASA after diagnosis | Comment on adjunctive therapy | Conclusion |
| Berger 2009 | 85 | Retrospective case series | 78% | 22 | ‐ | 22 participants took ASA treatment prior to GCA diagnosis | No benefit of established use of ASA on rate of ischaemic complications |
| Chuang 1982 | 15 | Retrospective case series of PMR and GCA | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lee 2006 | 143 | Retrospective case series | 73% | ASA not reported separately | ASA not reported separately | 60.1% received long‐term antiplatelet or anticoagulation | 16.2% versus 48% had ischaemic complication (P value < 0.0005) in favour of antiplatelet/coagulation |
| Liozon 2001 | 174 | Prospective observational case series investigating permanent visual loss | 84.5% | ‐ | ‐ | Low molecular weight heparin and intravenous heparin were used for visual loss | Thrombocytosis was strongly associated with risk of permanent visual loss |
| Narvaez 2008 | 121 | Retrospective | 73% | 30 | ‐ | 30.5% were on ASA and 7% on another antiplatelet agent prior to symptoms/signs of GCA | No observed benefit of antiplatelet therapy on the incidence of ischaemic complications or disease outcome |
| Nesher 2004a | 175 | Retrospective | 87% | 36 | 41 | 21% were already using low‐dose ASA at time of GCA diagnosis All given prednisolone at time of GCA diagnosis |
At diagnosis 8% with ASA had ischaemic complications compared to 29% who did not have ASA (P value = 0.01) At 3 months 3% with ASA had ischaemic complications compared to 13% who did not have ASA (P value = 0.02) |
| Souza 2013 | 45 | Retrospective | Not known | ASA not reported separately | ASA not reported separately | 32 (71.1%) were reported on aspirin | Aspirin was of statistical benefit in preventing relapse (P value = 0.023) |
ASA: aspirin GCA: giant cell arteritis PMR: polymyalgia rheumatica
Indirect support for the use of aspirin in GCA that is commonly cited includes a number of observations. First, aspirin has well‐documented antiplatelet and anti‐inflammatory effects. Second, thrombocytosis is a characteristic finding in GCA and has been associated with ischaemic complications such as risk of visual loss (Liozon 2001). Third, chimeric mouse models of GCA suggest that very high‐dose aspirin reduces interferon gamma levels to a greater extent than corticosteroids (Weyand 2002). Fourth, aspirin has an established role in the reduction of ischaemic complications in atherosclerotic disease. Finally, three retrospective studies are cited as supporting the use of aspirin as an adjunctive therapy in GCA where they collectively analyse 136 participants who presented with established aspirin use (Lee 2006; Nesher 2004a; Souza 2013). These studies, although not eligible for analysis within this review, are briefly discussed below.
Nesher 2004a found that around 20% presented with GCA whilst already using low‐dose aspirin exclusively for secondary prevention (i.e. all had previously had a cardiovascular event). There was no difference found between the established aspirin‐treated and non‐aspirin group in terms of their characteristics of presentation with GCA. What Nesher and colleagues did find was that ischaemic complications at presentation and subsequently at three months post‐presentation in the aspirin‐treated group were significantly reduced, but not absent. As commented on by Espinoza 2005, this study is limited as it was retrospective with no pre‐defined treatment protocol: participants could be started on aspirin at the physician's discretion. The incidence of ischaemic complications in this study was high at 33% of those studied, 25% prior to diagnosis and 8% post‐diagnosis (Hellmann 2004). Other confounding issues include the participant's underlying predisposition to cardiovascular risk and the directed use of cardiovascular‐modifying medications, such as statins and antihypertensive medications. Participants in Lee 2006 used both antiplatelet and anticoagulation therapy and found use of either significantly reduced the risk of an ischaemic event (P value > 0.0005). Souza 2013 retrospectively reviewed 45 patients with GCA, 32 of whom took aspirin. In analysis aspirin was statistically protective against disease relapse. However, what is not known by the authors is how many of their patients had been on long‐term aspirin prior to the diagnosis of GCA.
Others have not found low‐dose aspirin to be advantageous (Berger 2009; Gonzalez‐Gay 2004; Narvaez 2008; Salvarani 2009). Berger 2009 found 26% of their cohort were using aspirin at time of diagnosis of GCA. In their analysis they found no significant association between those who were on aspirin and those who were not in terms of ischaemic complications. Likewise, Narvaez 2008 did not observe a significant benefit in terms of incidence of ischaemic complications or disease outcome.
The use of low‐dose aspirin is common practice and is recommended (Dasgupta 2010), despite inadequate evidence to support this. The uncertainty of benefit must be weighed up with concerns over the potential morbidity associated with its use, particularly when used concurrently with corticosteroids. A range of haemorrhagic side effects are reported including gastrointestinal haemorrhage and occipital haemorrhage (Lee 2006).
This review highlights an evidence gap for the potential benefit and harm of concurrent use of aspirin in GCA. Major evidence is required to provide information on whether the theoretical benefit, as investigated by Weyand 2002, can be translated into a therapeutic benefit. This evidence would be required prior to consideration of recommending a RCT. It is acknowledged that this evidence would be challenging to accrue due to the low incidence of GCA and the relatively low rate of ischaemic complications in the context of prompt immunosuppression. Such treatment studies are likely to require increased national and international collaboration and the formation of networks directed towards increasing our understanding and improving our treatment in GCA.
Summary of main results
No studies met the inclusion criteria for this review. A summary of the current evidence is recorded in Table 1.
Overall completeness and applicability of evidence
Our search strategy (outlined earlier) is likely to have returned all relevant articles in this area. Our expert knowledge and handsearching did not return any additional references. We did not identify any relevant non‐English articles. The applicability of this review is limited by the lack of studies of sufficient quality to be included.
Quality of the evidence
No studies met the inclusion criteria for this review. The quality of the available evidence is poor in terms of both methodology and numbers observed.
Potential biases in the review process
We used standard Cochrane systematic review methodology to define the inclusion and exclusion criteria and conduct the searches for this review. Since no studies met the inclusion criteria, further comment is not possible.
Agreements and disagreements with other studies or reviews
We have not found any other reviews investigating aspirin use in GCA.
Authors' conclusions
Implications for practice.
There is insufficient evidence to determine the safety and efficacy of aspirin as an adjunctive treatment in giant cell arteritis (GCA). Indirect support for its usage may be provided by its known antiplatelet and anti‐inflammatory effects, the presence of thrombocytosis in GCA, the beneficial effect of high‐dose aspirin in laboratory studies, the established benefit of aspirin in atherosclerotic disease and from two retrospective studies that appear to show a beneficial effect. Clinicians must recognise, however, that despite its widespread use, none of these studies provide sufficient evidence to confirm benefit in people with GCA.
Clinicians who are considering the usage of aspirin as an adjunctive treatment in GCA must also recognise the established risks associated with aspirin, especially in the context of corticosteroid treatment. In this context there is a need to not only define whether there is a benefit to the use of aspirin in GCA, but also the size of any such benefit. Until then, in contrast to the situation with aspirin usage for the prevention or treatment of atherosclerotic disease, it will not be possible for the clinician or the patient to reliably estimate the relative benefit against harm in GCA.
Implications for research.
This review demonstrates the lack of well‐designed randomised controlled trials (RCTs) to support the use of low‐dose aspirin in GCA, and highlights the need for such large‐scale effectiveness trials to guide the management of this life‐threatening condition. Such studies need to be powered both to assess the direction (i.e. benefit or harm) and size of any effect to enable appropriate clinical decisions to be made. Additionally, the outcome measures should include the major life‐ or sight‐threatening complications of both GCA and of the treatment(s) and be conducted over a sufficient time‐scale to ensure that both early and late effects are captured.
Acknowledgements
The Cochrane Eyes and Vision Group (CEVG) created and executed the search strategies for the electronic databases. We thank Len Levin for his support in developing the protocol and Michael Marrone and Catey Bunce for their comments on the protocol. We thank Kate Cahill on her comments on this review. In addition we thank Anupa Shah, Managing Editor for CEVG for her assistance throughout the review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Giant Cell Arteritis] explode all trees #2 giant near/2 cell near/2 arteritis #3 (temporal or cranial) near/2 (arteritis) #4 GCA #5 #1 or #2 or #3 #6 MeSH descriptor: [Aspirin] explode all trees #7 acetylsalicylic near/2 acid #8 aspirin or ASA #9 #6 or #7 or #8 #10 #5 and #9
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomized).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. Giant Cell Arteritis/ 14. (giant adj2 cell adj2 arteritis).tw. 15. ((temporal or cranial) adj2 arteritis).tw. 16. GCA.tw. 17. or/13‐16 18. exp aspirin/ 19. (acetylsalicylic adj2 acid).tw. 20. (aspirin or ASA).tw. 21. or/18‐20 22. 17 and 21 23. 12 and 22
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. giant cell arteritis/ 34. (giant adj2 cell adj2 arteritis).tw. 35. ((temporal or cranial) adj2 arteritis).tw. 36. GCA.tw. 37. or/33‐36 38. acetylsalicylic acid/ 39. (acetylsalicylic adj2 acid).tw. 40. (aspirin or ASA).tw. 41. or/38‐40 42. 37 and 41 43. 32 and 42
Appendix 4. LILACS search strategy
giant cell arteritis and aspirin or ASA or acetylsalicylic acid
Appendix 5. metaRegister of Controlled Trials search strategy
giant cell arteritis
Appendix 6. ClinicalTrials.gov search strategy
(Giant Cell Arteritis) AND (Aspirin OR ASA OR Acetylsalicylic)
Appendix 7. ICTRP search strategy
Giant Cell Arteritis = Condition AND Aspirin OR ASA OR Acetylsalicylic = Intervention
Appendix 8. FDA search strategy
Giant Cell Arteritis AND Aspirin AND random OR randomly OR randomised OR randomized
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berger 2009 | Retrospective case series study of both clinical suspected giant cell arteritis and biopsy‐proven giant cell arteritis |
| Chuang 1982 | Retrospective case series with 15/96 patients diagnosed with giant cell arteritis |
| Dubey 2011 | Case report of 2 patients with biopsy‐proven giant cell arteritis, who were managed with intravenous heparin and intravenous methylprednisolone |
| Espinoza 2005 | Editorial on Nesher 2004a |
| Lee 2006 | Retrospective study, with 73% biopsy‐proven giant cell arteritis |
| Liozon 2001 | Prospective case series, with 84% biopsy‐proven giant cell arteritis |
| Narvaez 2008 | Retrospective study |
| Nesher 2004a | Retrospective study |
| Souza 2013 | Retrospective study |
Contributions of authors
Conceiving the review: Susan Mollan (SPM), Alastair Denniston (AKD), Michael Burdon (MAB) Designing the review: SPM, AKD Co‐ordinating the review: SPM Data collection for the review:
Designing electronic search strategies: Cochrane Eyes and Vision Group editorial base
Screening search results: SPM, Noor Sharrack (NS)
Organising retrieval of papers: NS
Screening retrieved papers against inclusion criteria: SPM, NS
Appraising quality of papers: SPM, NS
Extracting data from papers: SPM, NS
Writing to authors of papers for additional information: SPM
Providing additional data about papers: SPM
Interpretation of data: SPM, AKD Providing a clinical perspective: SPM, AKD, MAB Providing a policy perspective: SPM, AKD Writing the review: SPM Providing general advice on the review: MAB Performing previous work that was the foundation of the current study: SPM, AKD, MAB
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
Richard Wormald (Co‐ordinating Editor for CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
Declarations of interest
SPM none known. NS none known. MAB none known. AKD none known.
New
References
References to studies excluded from this review
Berger 2009 {published data only}
- Berger CT, Wolbers M, Meyer P, Daikeler T, Hess C. High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology 2009;48(3):258‐61. [DOI] [PubMed] [Google Scholar]
Chuang 1982 {published data only}
- Chuang TY, Hunder GG, Ilstrup DM, Kurland LT. Polymyalgia rheumatica: a 10 year epidemologic and clinical study. Annals of Internal Medicine 1982;97(5):672‐80. [DOI] [PubMed] [Google Scholar]
Dubey 2011 {published data only}
- Dubey R, Bhardwaj G, Sanli E, Kalapesi F, Francis IC. Temporal arteritis reversal of blindness using anticoagulation and steroids. Neuro‐Ophthalmology 2011;35(5‐6):264‐8. [Google Scholar]
Espinoza 2005 {published data only}
- Espinoza LR. Clinical trials in giant cell arteritis. Current Rheumatology Reports 2005;7(4):263‐4. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee MS, Smith SD, Galor A, Hoffman GS. Antiplatelet and anticoagulant therapy in patients with giant cell arteritis. Arthritis and Rheumatism 2006;54(10):3306‐9. [DOI] [PubMed] [Google Scholar]
Liozon 2001 {published data only}
- Liozon E, Herrmann F, Ly K, Robert PY, Loustaud V, Soria P, et al. Risk factors for visual loss in giant cell (temporal) arteritis: a prospective study of 174 patients. American Journal of Medicine 2001;111(3):211‐7. [DOI] [PubMed] [Google Scholar]
Narvaez 2008 {published data only}
- Narvaez J, Bernad B, Gomez‐Vaquero C, Garcia‐Gomez C, Roig‐Vilaseca D, Juanola X, et al. Impact of antiplatelet therapy in the development of severe ischaemic complications and in the outcome of patients with giant cell arteritis. Clinical Experimental Rheumatology 2008;26(3 (Suppl 49)):S57‐62. [PubMed] [Google Scholar]
Nesher 2004a {published data only}
- Nesher G, Berkun Y, Mates M, Baras M, Rubinow A, Sonnenblick M. Low‐dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis and Rheumatism 2004;50(4):1332‐7. [DOI] [PubMed] [Google Scholar]
Souza 2013 {published data only}
- Souza AW, Okamoto KY, Abrantes F, Schau B, Bacchiega AB, Shinjo SK. Giant cell arteritis: a multicenter observational study in Brazil. Clinics 2013;68(3):317‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Birkhead 1957
- Birkhead NC, Wagener HP, Shick RM. Treatment of temporal arteritis with adrenal corticosteroids: Results in 55 cases in which the lesion was proved at biopsy. Journal of the American Medical Association 1957;163:821. [DOI] [PubMed] [Google Scholar]
Borchers 2012
- Borchers AT, Gershwin ME. Giant cell arteritis: a review of classification, pathophysiology, geoepidemiology and treatment. Autoimmune Reviews 2012;11(6‐7):A544‐54. [DOI] [PubMed] [Google Scholar]
Carmona 2013
- Carmona FD, González‐Gay MA, Martín J. Genetic component of giant cell arteritis. Rheumatology 2014;53(1):6‐18. [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan CC, Paine M, O'Day J. Predictors of recurrent ischemic optic neuropathy in giant cell arteritis. Journal of Neuro‐Ophthalmology 2005;25(1):14‐7. [DOI] [PubMed] [Google Scholar]
Crow 2009
- Crow RW, Katz BJ, Warner JE, Alder SC, Zhang K, Schulman S, et al. Giant cell arteritis and mortality. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2009;64(3):365‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasgupta 2010
- Dasgupta B, Borg FA, Hassan N, Alexander L, Barraclough K, Bourke B, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010;49(8):1594‐7. [DOI] [PubMed] [Google Scholar]
De Silva 1986
- Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double‐blind study. Annals of the Rheumatic Diseases 1986;45(2):136‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Evans 1995
- Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population‐based study. Annals of Internal Medicine 1995;122(7):502‐7. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Gay 2004
- Gonzalez‐Gay MA, Piñeiro A, Gomez‐Gigirey A, Garcia‐Porrua C, Pego‐Reigosa R, Dierssen‐Sotos T, et al. Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine 2004;83(6):342‐7. [DOI] [PubMed] [Google Scholar]
Hayreh 1998
- Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. American Journal of Ophthalmology 1998;125(4):521‐6. [DOI] [PubMed] [Google Scholar]
Hayreh 2003
- Hayreh SS, Zimmerman B. Management of giant cell arteritis. Our 27‐year clinical study: new light on old controversies. Ophthalmologica 2003;217(4):239‐59. [DOI] [PubMed] [Google Scholar]
Hellmann 2004
- Hellmann DB. Low dose aspirin in the treatment of giant cell arteritis. Arthritis and Rheumatism 2004;50(4):1026‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 2009
- Hill CL, Cole A, Lester S, Whittle SL. Steroid sparing drug treatments for giant cell arteritis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005323.pub2] [DOI] [Google Scholar]
Hoffman 2002
- Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, et al. A multicenter, randomized, double blind placebo controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis and Rheumatism 2002;46(5):1309‐18. [DOI] [PubMed] [Google Scholar]
Hoffman 2007
- Hoffman GS, Cid MC, Rendt‐Zagar KE, Merkel PA, Weyand CM, Stone JH, et al. Infliximab for maintenance of glucocorticosteroid‐induced remission of giant cell arteritis: a randomized trial. Annals of Internal Medicine 2007;146(9):621‐30. [DOI] [PubMed] [Google Scholar]
Hussain 2011
- Hussain M, Javeed A, Ashraf M, Zhao Y, Mukhtar MM, Rehman MU. Aspirin and immune system. International Immunopharmacology 2012;12(1):10‐20. [DOI] [PubMed] [Google Scholar]
Kim 2003
- Kim N, Trobe JD, Flint A, Keoleian G. Late ipsilateral recurrence of ischemic optic neuropathy in giant cell arteritis. Journal of Neuro‐Ophthalmology 2003;23(2):122‐6. [DOI] [PubMed] [Google Scholar]
Lawrence 1998
- Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis and Rheumatism 1998;41(5):778‐99. [DOI] [PubMed] [Google Scholar]
Ly 2010
- Ly KH, Régent A, Tamby MC, Mouthon L. Pathogenesis of giant cell arteritis: More than just an inflammatory condition?. Autoimmune Reviews 2010;9(10):635‐45. [DOI] [PubMed] [Google Scholar]
Machado 1988
- Machado EB, Michet CJ, Ballard DJ, Hunder GG, Beard CM, Chu CP, et al. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950‐85. Arthritis and Rheumatism 1988;31(6):745‐9. [DOI] [PubMed] [Google Scholar]
Mackie 2011
- Mackie SL, Dasgupta B, Hordon L, Gough A, Green M, Hollywood J, et al. Ischaemic manifestations in giant cell arteritis are associated with area level socio‐economic deprivation, but not cardiovascular risk factors. Rheumatology 2011;50(11):2014‐22. [DOI] [PubMed] [Google Scholar]
Mahr 2006
- Mahr A, Saba M, Kambouchner M, Polivka M, Baudrimont M, Brochériou I, et al. Temporal artery biopsy for diagnosing giant cell arteritis: the longer, the better?. Annals of the Rheumatic Diseases 2006;65(6):826‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahr 2007
- Mahr AD, Jover JA, Spiera RF, Hernández‐García C, Fernández‐Gutiérrez B, Lavalley MP, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta‐analysis. Arthritis and Rheumatism 2007;56(8):2789‐97. [DOI] [PubMed] [Google Scholar]
Mazlumzadeh 2006
- Mazlumzadeh M, Hunder GG, Easley KA, Calamia KT, Matteson EL, Griffing WL, et al. Treatment of giant cell arteritis using induction therapy with high‐dose glucocorticoids: a double‐blind, placebo‐controlled, randomized prospective clinical trial. Arthritis and Rheumatism 2006;54(10):3310‐8. [DOI] [PubMed] [Google Scholar]
Nesher 2004b
- Nesher G, Berkun Y, Mates M, Baras M, Nesher R, Rubinow A, et al. Risk factors for cranial ischemic complications in giant cell arteritis. Medicine 2004;83(2):114‐22. [DOI] [PubMed] [Google Scholar]
Niederkohr 2005
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis: a decision‐analytic approach. Ophthalmology 2005;112(5):744‐56. [DOI] [PubMed] [Google Scholar]
Niederkohr 2007
- Niederkohr RD, Levin LA. A Bayesian analysis of the true sensitivity of a temporal artery biopsy. Investigative Ophthalmology and Visual Science 2007;48(2):675‐80. [DOI] [PubMed] [Google Scholar]
Nordborg 1989
- Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ 1989;299(6698):549‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petursdottir 1999
- Petursdottir V, Johansson H, Nordborg E, Nordborg C. The epidemiology of biopsy‐positive giant cell arteritis: special reference to cyclic fluctuations. Rheumatology 1999;38(12):1208‐12. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robson 2013
Rüegg 2003
- Rüegg S, Engelter S, Jeanneret C, Hetzel A, Probst A, Steck AJ, et al. Bilateral vertebral artery occlusion resulting from giant cell arteritis: report of 3 cases and review of the literature. Medicine 2003;82(1):1‐12. [DOI] [PubMed] [Google Scholar]
Salvarani 2005
- Salvarani C, Cimino L, Macchioni P, Consonni D, Cantini F, Bajocchi G, et al. Risk factors for visual loss in an Italian population‐based cohort of patients with giant cell arteritis. Arthritis and Rheumatism 2005;53(2):293‐7. [DOI] [PubMed] [Google Scholar]
Salvarani 2008
- Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant‐cell arteritis. Lancet 2008;372(9634):234‐45. [DOI] [PubMed] [Google Scholar]
Salvarani 2009
- Salvarani C, Della Bella C, Cimino L, Macchioni P, Formisano D, Bajocchi G, et al. Risk factors for severe cranial ischaemic events in an Italian population‐based cohort of patients with giant cell arteritis. Rheumatology 2009;48(3):250‐3. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Serrano 2013
- Serrano A, Márquez A, Mackie SL, Carmona FD, Solans R, Miranda‐Filloy JA, et al. Identification of the PTPN22 functional variant R620W as susceptibility genetic factor for giant cell arteritis. Annals of the Rheumatic Diseases 2013;71(11):1882‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smetana 2002
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis?. JAMA 2002;287(1):92‐101. [DOI] [PubMed] [Google Scholar]
Unizony 2013
- Unizony SH, Dasgupta B, Fisheleva E, Rowell L, Schett G, Spiera R, et al. Design of the tocilizumab in giant cell arteritis trial. International Journal of Rheumatology 2013 Apr 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Weisman 2002
- Weisman SM, Graham DY. Evaluation of the benefits and risks of low‐dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Archives of Internal Medicine 2002;162(19):2197‐202. [DOI] [PubMed] [Google Scholar]
Weyand 2002
- Weyand CM, Kaiser M, Yang H, Younge B, Goronzy JJ. Therapeutic effects of acetylsalicylic acid in giant cell arteritis. Arthritis and Rheumatism 2002;46(2):457‐66. [DOI] [PubMed] [Google Scholar]
Weyand 2004
- Weyand CM, Ma‐Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmune Reviews 2004;3(1):46‐53. [DOI] [PubMed] [Google Scholar]
Weyand 2011
- Weyand CM, Younge BR, Goronzy JJ. IFN‐γ and IL‐17: the two faces of T‐cell pathology in giant cell arteritis. Current Opinion in Rheumatology 2011;23(1):43‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mollan 2013
- Mollan SP, Marrone M, Burdon MA, Levin LA, Denniston AK. Aspirin as adjunctive treatment for giant cell arteritis. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010453] [DOI] [PMC free article] [PubMed] [Google Scholar]


