Abstract
The root-associated soil microbiome contributes immensely to support plant health and performance against abiotic and biotic stressors. Understanding the processes that shape microbial assembly in root-associated soils is of interest in microbial ecology and plant health research. In this study, 37 plant species were grown in the same soil mixture for 10 months, whereupon the root-associated soil microbiome was assessed using amplicon sequencing. From this, the contribution of direct and indirect plant effects on microbial assembly was assessed. Plant species and plant-induced changes in soil physicochemistry were the most significant factors that accounted for bacterial and fungal community variation. Considering that all plants were grown in the same starting soil mixture, our results suggest that plants, in part, shape the assembly of their root-associated soil microbiome via their effects on soil physicochemistry. With the increase in phylogenetic ranking from plant species to class, we observed declines in the degree of community variation attributed to phylogenetic origin. That is, plant-microbe associations were unique to each plant species, but the phylogenetic associations between plant species were not important. We observed a large degree of residual variation (> 65%) not accounted for by any plant-related factors, which may be attributed to random community assembly.
Keywords: microbial assembly, microbial selection, plant effects, plant phylogeny, root-associated soil microbiome
Laboratory gut bacterial communities are not representative for natural field conditions.
Introduction
The functional activities of the root-associated soil microbiome are of fundamental importance for plant health. The root-associated soil microbiome influences plant nutrient acquisition, pathogen defense, induced systemic resistance, growth, drought tolerance, and other traits (Berendsen et al. 2012, Philippot et al. 2013, Pieterse et al. 2014). Given the importance of the microbiome to plant fitness and health, soil fertility, and ecosystem productivity, defining the key processes that shape microbial assembly has been an ongoing pursuit within microbial ecology (Marschner et al. 2001, Lundberg et al. 2012, Pérez-Jaramillo et al. 2016, Fitzpatrick et al. 2018). Whilst many studies have performed observational research to describe patterns of microbial assembly and community ecology (Prosser 2020), fewer studies have performed direct manipulations in controlled experiments to examine the factors shaping root-associated soil microbial assembly during a plant’s early phases of growth and establishment.
Several studies have proposed that plant species (PS) themselves are the primary determinants of their associated microbiome (Becklin et al. 2012, Mendes et al. 2013, Bouffaud et al. 2014, Chaparro et al. 2014). Theoretically, as the phylogenetic relatedness of PS influences their degree of shared developmental and functional traits, it may also influence the phylogenetic similarity of the microorganisms that they recruit. Thus, with increasing phylogenetic similarity among PS, one may observe an increased relatedness of their microbiome. Findings supporting this hypothesis have been observed in several studies (Bouffaud et al. 2014, Lambais et al. 2014, Lei et al. 2019, Hartman et al. 2023). In contrast, other studies have observed that the abiotic conditions of the soil environment (i.e. soil type, pH, nutrient availability, and C:N ratio) are of greater influence on microbial community assembly in the rhizosphere and root-associated soil environment (Girvan et al. 2003, Ulrich and Becker 2006, Lauber et al. 2009, Xiao et al. 2017, Yeoh et al. 2017, Veach et al. 2019, Ren et al. 2020).
This ecological conundrum draws parallels to the nature-versus-nurture debate that has shaped research around human development for decades. Several studies have proposed an assembly model more akin to nature-via-nurture, whereby both the PS and soil shape the microbiome, and the relative strength of these different drivers will vary depending on the specific ecological context (Garbeva et al. 2008, Berg and Smalla 2009, Tkacz et al. 2015, Müller et al. 2016, Lee and Hawkes 2020). In the nature-via-nurture model, soil provides the primary source of microbial inoculum available to plants and sets the boundaries from which plants may select their microbiome. The dominant influence of soil type and edaphic properties on determining the broad patterns of microbial biogeography was recognized by Fierer and Jackson (2006) and Lauber et al. (2009). However, throughout their development and life span, plants and their root systems exert species-specific influences on the rhizosphere and root-associated soil environment, which drives environmental filtering of their microbiome (Berg and Smalla 2009, Chaparro et al. 2014, Reinhold-Hurek et al. 2015, Hu et al. 2018). Additionally, the symbiotic associations of plant hosts (e.g. N2-fixing rhizobia, arbuscular mycorrhizal fungi) have been identified to shape the assembly of the root microbiome (Hartman et al. 2023).
There have been two primary processes proposed that shape microbiomes: deterministic and stochastic (Goss-Souza et al. 2017). Niche-based, deterministic models propose that the biotic and abiotic conditions of the local environment drive microbial selection (Carroll et al. 2011, Goss-Souza et al. 2017). Deterministic models can be further split into primary and secondary processes. Primary deterministic processes constitute a more direct mechanism, whereby the release of plant-specific rhizo-deposits selects or favours microbial taxa from the wider soil microbial community (Hu et al. 2018, Sasse et al. 2018, Zhalnina et al. 2018). Secondary deterministic processes function indirectly whereby plant roots modify the general rhizosphere and soil conditions (pH, available P, nitrogen, etc.), and these changes, in turn, encourage the growth of microorganisms best adapted to that modified habitat space (Hinsinger 2001, Liang et al. 2002, Bell et al. 2015, van Veelen et al. 2020, Hernández-Cáceres et al. 2022). In contrast to deterministic models, stochastic models propose an element of randomness to community assembly (Dini-Andreote et al. 2015, Goss-Souza et al. 2017). These deterministic and stochastic processes do not occur independently of each other, and the challenge is determining the relative contribution of these under different experimental and ecological contexts.
We performed a plant experiment whereby a broad phylogenetic range of 37 different PS were grown in an identical blended soil medium. Following 10 months of growth, the root-associated soil microbiome was characterized using the 16S rRNA gene and ITS region sequencing. The differences in the structural variance of the root-associated soil microbiome between PS were related to their phylogenetic and functional traits, as well as to any plant-induced changes in soil physicochemistry (SC) that occurred throughout the experiment. By performing this experiment, our research aimed to partition the influences of primary and secondary deterministic processes (i.e. direct and indirect plant effects) and stochastic processes on microbial assembly in the root-associated soil environment. Furthermore, we hypothesize that the phylogenetic relatedness of the PS will be positively correlated to the phylogenetic similarity of their root-associated soil microbiome.
Materials and methods
Plant and soil sample collection
Plants from 37 different species were grown in a blended soil media or obtained from a commercial nursery (Southern Woods Plant Nursery, New Zealand). The selected PS covered a broad range of phylogenetic groups and included representatives from three plant classes, 12 orders, 14 families, and 31 genera. The PS covered a range of different life spans (annual, perennial, or long-lived), functional groups (e.g. grass, shrub, or tree), and provenances (exotic or native to Aotearoa New Zealand). They also included species with different mycorrhizal associations (arbuscular, AMF, ectomycorrhizal, EMF, and no association) and N2 fixation (presence or absence). Plant metadata was primarily obtained from PS profiles on the New Zealand Plant Conservation Network (https://www.nzpcn.org.nz/) and from literature searches where additional information was needed. The full list of PS used in this study and their associated metadata are provided in Supplementary Table S1.
The seeds or cuttings of each PS were planted in individual 10-L pots containing a blend of field-collected live soils mixed with a pasteurized soil: sand carrier for bulk. Between 12 and 20 replicate pots were established for each PS. The collection of the live soils was conducted across 12 sub-alpine, grass, and shrub-dominated sites that included the ranges of the plants being experimentally evaluated. This sampling design was performed to allow for the microbiomes associated with these species to be available for plant ‘recruitment’. However, it is important to state that we did not examine the microbial background of these ‘live’ soils before setting up the experiment. Details regarding the collection, handling, treatment, and mixing of these soils were first provided by Wakelin et al. (2021). The plants were randomized within a glasshouse and grown with regular watering and supplemental lighting when required. No fertilizers or other chemicals were added to the pots, and weeds were removed when apparent.
After 10 months of plant growth, root-associated soil samples were collected from each plant pot. Samples were collected from between four and seven replicate pots of each plant depending on plant availability [i.e. plants that had grown to full health and were not required for other research (Wakelin et al. 2021)]. All root-associated soil samples were collected aseptically by pressing an open 50-mL conical centrifuge tube into the soil adjacent to the stem(s) of the plant in each pot directly into the root zone. Three samples from around the circumference of each pot were collected and pooled to provide a single sample for each replicate of each PS. Pooled soil samples were sieved to 2 mm and stored at either 4°C until physicochemical analysis or -80°C until DNA extraction.
Measurements of soil edaphic properties
The edaphic properties of all root-associated soil samples were characterized at Hill Laboratories (Christchurch, New Zealand), where soil pH, Olsen phosphorus (mg/L), sulphate sulphur (mg/kg), total carbon (TC; %), organic matter (%), total nitrogen (TN; %), C:N ratio, potentially available N (kg/ha), anaerobically mineralizable N (AMN; %), AMN:TN ratio, and volume weight (g/mL) were determined using the protocols described by Wakelin et al. (2013).
Plant DNA extraction and matK gene sequencing
The DNA of each PS was extracted using the DNeasy Plant Mini Kit (QIAGEN), utilizing cryogenic tissue grinding of plant leaves with a sterilized mortar and pestle in liquid nitrogen. For phylogenetic inference, the Maturase K gene (matK) was amplified using the primers MatK472F (5′-CCRTCATCTGGAAATCTTGGTT-3′) and MatK1248R (5′-GCTRTRATAATGAGAAAGATT TCTGC-3′) (Fatima et al. 2019). PCR conditions consisted of an initial denaturation step of 94°C for 5 min followed by 35 cycles of 94°C for 30 sec, 56°C for 30 sec, and 72°C for 42 sec, followed by a final extension at 72°C for 10 min. The PCR reaction mixture consisted of 1 × PCR buffer, 0.5 mmol L−1 dNTPs, 0.25 μmol L−1 of each primer, 1 U Taq polymerase, and 5–50 ng of template DNA. The PCR products were purified using the QIAquick PCR Purification Kit, and the purified DNA was sequenced using Sanger sequencing at Macrogen (Seoul, Korea). The quality of the sequencing data was checked and edited using Sequencer software version 5.4.6 (Genecodes Corp, Ann Arbor, MI, USA). MEGA X (Kumar et al. 2018) was then used for sequence alignment and phylogenetic analysis. Briefly, matK gene‐based sequences were aligned using MUSCLE, and overhanging nucleotides were removed and then re-aligned. Distance matrices and phylogenetic trees were constructed using the maximum likelihood method and the Tamura–Nei model (Tamura and Nei 1993).
Soil DNA extraction and 16S rRNA gene/ITS region sequencing
Soil DNA was extracted from 0.25 g of soil using a DNeasy PowerSoil Kit (QIAGEN) according to the manufacturer’s protocol and quantified using a Nanodrop spectrophotometer. Subsequent Illumina amplicon sequencing followed the Earth Microbiome Project’s (EMP) protocol (Caporaso et al. 2012). In short, the bacterial 16S rRNA gene was amplified using the primers 515F (5′- GTGYCAGCMGCCGCGGTAA -3′) and 806R (5′- GGACTACNVGGGTWTCTAAT -3′) targeting the V4–V5 regions as described previously (Apprill et al. 2015, Parada et al. 2016). The fungal ITS region was amplified using the primers ITS1f (5′- CTTGGTCATTTAGAGGAAGTAA -3′) and ITS2 (5′- GCTGCGTTCTTCATCGATGC -3′) as described previously (Bokulich and Mills 2013, Hoggard et al. 2018). After PCR amplification, samples were purified using a Magnetic Bead PCR Cleanup Kit (GeneaidTM) and pooled in equimolar concentrations. The purified PCR products were used to prepare DNA libraries following the Illumina TruSeq DNA library preparation protocol using the Illumina MiSeq Reagent Kit v2. Illumina sequencing was performed at the Australian Genome Research Facility (Melbourne, Australia) using 2 × 150 bp pair-end chemistry on a MiSeq platform following the manufacturer’s guidelines.
Statistical analysis
Following sequencing, paired-end fastQ files were processed into amplicon sequence variants (ASVs) using the DADA2 version 1.18 workflow (Callahan et al. 2016). Briefly, the forward and reverse reads were quality-filtered, trimmed, and denoised before being merged into ASVs. Chimeric ASVs were removed, and taxonomies were assigned to each ASV using the Ribosomal Database Project (RDP) Classifier (Wang et al. 2007) and the UNITE (Abarenkov et al. 2021) databases. Following DADA2 processing, ASV count tables were filtered to remove unidentified and unwanted phyla (i.e. Cyanobacteria/Chloroplasts) and singletons. The ASV count tables were rarefied to adjust for differences in library size between samples. Before rarefaction, samples with low read counts were removed to avoid excessive data loss. Rarefaction curves displaying the number of ASVs in each sample have been provided in Supplementary Figs S1 and S2. The number of replicates per PS that were included in the rarefied 16S (henceforth reported as ‘bacterial’) and ITS (henceforth reported as ‘fungal’) ASV datasets is displayed in Supplementary Table S1. In total, all the PS had at least three replicates in the rarefied fungal ASV dataset. In the rarefied bacterial ASV dataset, 35 out of the 37 PS had at least three replicates; however, only two replicates remained for the PS Chionochloa conspicua and Trifolium repens following rarefaction.
The rarefied bacterial and fungal ASV datasets were analysed separately using the multivariate statistical analyses outlined below. Maximum likelihood phylogenetic trees were built using FastTree2 (Price et al. 2010). To provide estimates of alpha diversity, Faith’s phylogenetic diversity (PD) and species richness (SR) were calculated for each sample in Picante R (Kembel et al. 2010). The PD index assesses the PD of a community and is defined as the sum of the total phylogenetic branch length separating taxa in a community (Faith 1992, Kembel et al. 2010). In contrast, the SR index calculates the total number of taxa in a community based on their identity alone—no phylogenetic information is factored into the calculation. The differences in the PD and SR index between plant host-related factors were tested for significance using Kruskal–Wallis tests and pairwise Wilcoxon tests with Bonferroni correction.
To estimate the phylogenetic distances in microbial community composition between samples, weighted UniFrac distances were calculated on rarefied bacterial and fungal ASV count tables (Lozupone et al. 2011). Differences in bacterial and fungal community composition between the plant-related factors were tested for significance using permutational multiple analysis of variance (PERMANOVA) on distance metrics using the adonis2 (by = ‘terms’) function in vegan R (Oksanen et al. 2019) and pairwiseAdonis (Martinez Arbizu 2020). The differences in community composition were visualized using non-metric multidimensional scaling (NMDS) ordination plots. To estimate the within-group variance amongst samples, the average distance of individual samples to the group centroid (beta dispersion) was calculated using the betadisper function in phyloseq R (McMurdie and Holmes 2013). Permutation tests were used to determine significant differences in the within-group variance between plant-related factors.
The weighted UniFrac distances were correlated to differences in soil physicochemical properties using Mantel tests. In addition, weighted UniFrac distances were correlated to matrices of matK sequence similarity using Mantel tests. MatK similarity matrices were constructed to represent the phylogenetic relatedness between the different PS under investigation. Observations of the phylogenetic tree generated from the matK phylogeny showed sensible grouping of PS to their taxonomic positioning. Hierarchical clustering analysis was performed on the weighted UniFrac distance matrices and matK distance matrices using the complete linkage method in Stats R. Following this, dendrograms were constructed to visually compare differences in the clustering patterns of PS based on the weighted UniFrac distances of their fungal and bacterial communities versus their matK distances in ape R (Paradis and Schliep 2019).
Variance partitioning (VP) analysis was performed in vegan R to partition the variance observed in bacterial and fungal community composition (as represented by weighted UniFrac distances) to the plant-related factors (Oksanen 2019). Four explanatory matrices were constructed to represent the different influencing factors. These were: PS; plant life history (PLH) (i.e. provenance + life span + functional group); plant rhizosphere traits (PRT) (i.e. mycorrhizal association + N2 fixation); and SC. All unexplained (residual) variation from VP analysis was tentatively assigned to represent the influence of stochastic processes. Following VP analysis, distance-based redundancy analysis (db-RDA) was performed to test the significance of each explanatory matrix whilst conditioning for the other three matrices. In addition, forward stepwise selection was performed to identify the soil physicochemical properties that best accounted for the community variance that was partitioned to the influence of SC. Pairwise differences in the soil physicochemical properties selected by the forward selection model between the 37 different PS were identified using pairwise t-tests with Holm correction.
The rarefied bacterial and fungal ASV tables were used as input for differential abundance analysis. First, the R package pime was used to select bacterial and fungal ASVs that best defined the microbiome of each PS (Luiz Fernando 2020). Prevalence intervals with an out-of-bag (OOB) error rate of 0% were selected as cut-offs. For fungal ASVs, this was a prevalence of 75%, which retained 217 ASVs and 1 168 389 sequences. For bacterial ASVs, this was a prevalence of 80%, which retained 771 ASVs and 433 795 sequences. PIME-filtered ASV count tables were used as input for differential abundance analysis using metagenomeSeq R (Paulson et al. 2013), where the log change estimate of each ASV between different PS was calculated using the fitLogNormal function. Significant differences in the log change estimates of ASVs between PS were determined using permutation tests (n = 999) with correction for multiple comparisons using the Holm–Bonferroni method (holm). Heatmaps were produced using pheatmap R (Kolde and Kolde 2018) to display (a) the bacterial and fungal ASVs with significant log change estimates across PS and (b) the correlation shared between different PS (Pearson’s) based on the log change estimates of their bacterial and fungal ASVs.
Results
Microbial species richness and phylogenetic diversity
There were no significant differences in the SR of bacterial communities across any of the plant-related factors (Table 1). However, the Faith’s diversity of bacterial communities was significantly higher in native versus exotic plants and in non-N2 fixing versus N2 fixing plants.
Table 1.
The results of Kruskal–Wallis tests that were performed to identify significant differences in the SR and Faith’s diversity of root-associated bacterial and fungal ASVs across different plant-related factors.
| Bacterial ASVs | Fungal ASVs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | SR | Faith’s diversity | SR | Faith’s diversity | ||||||||
| H value | P value | DF | H value | P value | DF | H value | P value | DF | H value | P value | DF | |
| PS | 44.16 | 0.17 | 36 | 49.94 | 0.06 | 36 | 54.02 | 0.03* | 36 | 54.46 | 0.02* | 36 |
| Plant genus | 35.26 | 0.23 | 30 | 41.12 | 0.08 | 30 | 47.28 | 0.02* | 30 | 44.50 | 0.04* | 30 |
| Plant family | 14.22 | 0.36 | 13 | 16.80 | 0.21 | 13 | 18.25 | 0.15 | 13 | 19.58 | 0.11 | 13 |
| Plant order | 13.59 | 0.26 | 11 | 15.03 | 0.18 | 11 | 17.67 | 0.09 | 11 | 19.17 | 0.06 | 11 |
| Plant class | 2.43 | 0.30 | 2 | 1.91 | 0.39 | 2 | 6.45 | 0.04* | 2 | 4.64 | 0.10 | 2 |
| Provenance1 | 1.50 | 0.22 | 1 | 5.89 | 0.02* | 1 | 0.90 | 0.34 | 1 | 0.42 | 0.52 | 1 |
| N2 fixing | 2.13 | 0.14 | 1 | 4.96 | 0.03* | 1 | 0.43 | 0.51 | 1 | 0.79 | 0.37 | 1 |
| Life span2 | 1.34 | 0.51 | 2 | 2.18 | 0.34 | 2 | 6.28 | 0.04* | 2 | 7.11 | 0.03* | 2 |
| Functional group3 | 2.25 | 0.52 | 3 | 2.42 | 0.49 | 3 | 6.68 | 0.08 | 3 | 8.60 | 0.04* | 3 |
| Mycorrhizal association4 | 0.41 | 0.81 | 2 | 2.44 | 0.29 | 2 | 4.37 | 0.11 | 2 | 6.27 | 0.04* | 2 |
* P value < 0.05
exotic or native to Aotearoa New Zealand
annual, perennial, or long-lived
tree, forb, grass, or shrub
Arbuscular mycorrhizal fungi (AMF), ectomycorrhizal fungi (EMF), or no mycorrhizal association DF = degrees of freedom.
For fungal communities, both Faith’s diversity and SR were significantly higher in annual versus long-lived plants (Table 1). The PD of fungal communities was significantly lower in trees versus shrubs and grasses and significantly higher in non-mycorrhizal versus ectomycorrhizal plants. The mean (± SD) values for Faith's diversity and SR of fungal and bacterial across the different plant metadata factors can be seen through Supplementary Tables S2–S11.
Microbial beta-diversity and community composition
Bacterial and fungal microbial community composition was significantly different between all plant-related factors (Table 2). PS and genus were the factors that reported the highest R2 values, thus accounting for most of the explained variation in bacterial and fungal community composition (Table 2; see also Supplementary Figs S3 and S4 in Supplementary Data). Although significant, the R2 values for many of the plant-related factors representing functional plant traits (i.e. provenance, functional group, primary mycorrhizal association, life span, and N2 fixation) were all low (R2 < 0.07). Bacterial and fungal communities both exhibited a heterogeneous dispersion and a high degree of within-group variability. The degree of beta-dispersion (‘F value’) observed in bacterial communities was significantly different across the following factors: PS, plant genus, plant family, plant order, and mycorrhizal association (Table 2). For fungal communities, significant beta-dispersion values were observed by plant family, plant order, plant class, provenance, and mycorrhizal association (Table 2).
Table 2.
The results of PERMANOVA tests (R2 values) that were performed to identify significant differences in the composition of root-associated microbial communities across different plant-related factors.
| Bacterial ASVs | Fungal ASVs | |||
|---|---|---|---|---|
| Factor | R 2 | F value | R 2 | F value |
| PS | 0.342*** | 1.883** | 0.301*** | 1.165 |
| Plant genus | 0.295*** | 2.263*** | 0.259*** | 1.388 |
| Plant family | 0.150*** | 3.182*** | 0.120*** | 3.858*** |
| Plant order | 0.138*** | 3.401** | 0.107*** | 5.020*** |
| Plant class | 0.032*** | 2.531 | 0.020** | 3.892* |
| Provenance | 0.029*** | 0.688 | 0.023*** | 10.460** |
| N2 fixing | 0.012** | 0.961 | 0.018*** | 0.636 |
| Life span | 0.047*** | 2.654 | 0.022*** | 1.461 |
| Functional group | 0.069*** | 1.057 | 0.041*** | 0.764 |
| Mycorrhizal association | 0.048*** | 7.906** | 0.030*** | 3.299* |
* P value < 0.05; ** P value < 0.01; *** P value < 0.001
PERMANOVA tests were performed on weighted UniFrac distance matrices, which were calculated using bacterial and fungal ASV tables. The results of permutation-based tests of beta-dispersion are displayed (F values), were performed to identify significant differences in the within-group variance of bacterial and fungal communities for each plant-related factor.
MatK gene sequence similarity
The distances in matK gene sequence similarity between the different PS did not significantly correlate to the corresponding weighted UniFrac distances for their bacterial (Mantel r = 0.134, P value = 0.075) or fungal (Mantel r = 0.040, P value = 0.306) communities. This is illustrated in Supplementary Figs S5 and S6, as the hierarchical clustering patterns of the different PS based on their matK gene sequences versus their bacterial and fungal community composition had little correspondence.
Variance partitioning (VP) analysis
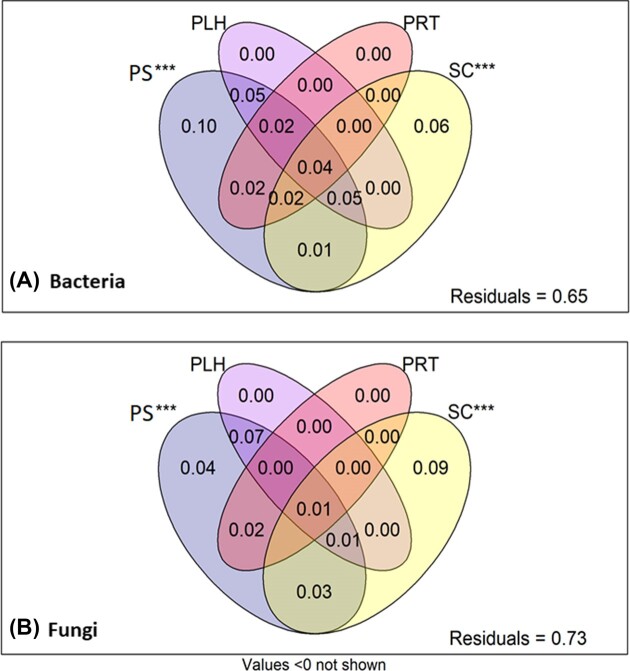
VP analysis identified that, cumulatively, PS, PLH, PRT, and SC explained 34.59% of bacterial community variance and 27.27% of fungal community variance (Fig. 1). Thus, both bacterial and fungal communities exhibited a high degree of residual, unexplained variation (65.41% and 72.73%, respectively). When individual explanatory matrices were tested for significance using partial db-RDA, both plant identity (Bacteria: F value = 1.40, P value < 0.001, Fungi: F value = 1.32, P value < 0.001) and SC (Bacteria: F value = 1.39, P value < 0.001, Fungi: F value = 1.41, P value < 0.001) significantly accounted for community variance.
Figure 1.
The proportion of explained variance attributed to each explanatory matrix, as identified using VP analysis. VP analysis was performed on weighted UniFrac distances of (A) bacterial ASVs and (B) fungal ASVs. Explanatory matrices used as input were PS, PLH, PRT, and SC. The significance of each explanatory matrix in accounting for community variation is displayed, whereby *** denotes P value < 0.001.
When the other explanatory matrices were conditioned out of the model, plant identity alone accounted for 9.52% of bacterial community variance and 3.65% of fungal community variance. In contrast, SC accounted for 5.67% of bacterial community variance and 9.40% of fungal community variance. PLH (Bacteria: F value = 0.00, P value > 0.05, Fungi: F value = 0.00, P value > 0.05) and PRT (Bacteria: F value = 0.00, P value > 0.05, Fungi: F value = 0.00, P value > 0.05) did not significantly account for any bacterial or fungal community variation.
The composition of the root-associated soil microbiome may be indirectly influenced by plant-induced modification of the physicochemical environment. When looking at soil physicochemical properties that best accounted for bacterial community variation, forward selection models identified Olsen P (F value = 10.76, P value < 0.05), sulphate sulphur (F value = 2.54, P value < 0.05), and pH (F value = 4.54, P value < 0.05) to be significant. For fungal communities, forward selection models identified Olsen P (F value = 9.95, P value < 0.05), AMN:TN (F value = 3.69, P value < 0.01), and volume weight (F value = 2.85, P value < 0.05) to be significant. The values for these soil properties were variable between the 37 different PS (Fig. 2). Pairwise t-tests identified that Olsen P was significantly higher (P adjusted < 0.05) in Acaena caesiiglauca (vs. Achillea millefolium, Dactylis glomerata, and Poa colensoi), Alnus glutinosa (vs. Ach. millefolium, D. glomerata, Holcus lanatus, Ozothamnus leptophyllus, and P. colensoi), and Pinus radiata (vs. Ach. millefolium, D. glomerata, and Po. colensoi). Volume weight was significantly higher in Ho. lanatus (vs. Hebe odora, O. leptophyllus, Olearia virgata, and Sophora microphylla) and Muehlenbeckia complexa (vs. He. odora and S. microphylla). Soil pH was significantly higher in D. glomerata and Ho. lanatus (vs. O. leptophyllus, Pi. contorta, Pi. radiata, Brachyglottis greyi, Coprosma robusta, and Ulex europaeus), Ach. millefolium (vs. Pi. radiata), and Po. colensoi and P. cita (vs. Pi. radiata, O. leptophyllus, and B. greyi). Although forward selection models identified AMN:TN and sulphate sulphur to significantly influence fungal and bacterial community composition, no significant pairwise differences were determined between the 37 PS for these properties. The mean ± SD values for all soil physicochemical properties associated with each PS are presented in Supplementary Table S12.
Figure 2.
The mean ± SE values of the soil properties that were identified by forward selection models to significantly account for the variation in root-associated soil microbial communities. The soil properties Olsen P (mg/L), sulphate sulphur (mg/kg), and pH significantly accounted for bacterial community variation, whilst Olsen P (mg/L), AMN:TN ratio, and volume weight (g/mL) significantly accounted for fungal community variation.
Taxonomic differentiation across plant species
Out of the 771 bacterial ASVs that were retained following PIME filtering and used as input for differential abundance analysis, only 10.12% (78 ASVs) were identified to be differentially abundant amongst PS (P adjusted < 0.05). Furthermore, out of the 217 fungal ASVs retained following PIME filtering, only 16.59% (36 ASVs) were differentially abundant amongst PS (P adjusted < 0.05). Figures 3 and 4 display the bacterial and fungal ASVs that had significantly different log change estimates across the PS under investigation. These results highlight that there were no large patterns of taxonomic differentiation amongst PS, that is, PS did not have markedly distinct taxonomic compositions. One exception was Agrostis capillaris (common bent or brown top grass), whose bacterial and fungal taxa were more evidently differentiated compared to the other PS. All the PS shared a significant (P adjusted < 0.05) positive correlation based on the log change estimates of their bacterial (Pearson’s r correlation; 0.70 ± 0.08 SD) and fungal ASVs (Pearson’s r correlation; 0.69 ± 0.08 SD). These results indicate a low divergence of PS based on their root-associated soil microbiome (Supplementary Figs S7 and S8).
Figure 3.
The log change estimates of the 78 bacterial ASVs that were identified to have significant differential abundance values (P adjusted < 0.05) across the PS under investigation. The taxonomic identity of each bacterial ASV is presented, with each bacterial ASV represented by the most refined taxonomic rank that could be accurately assigned.
Figure 4.
The log change estimates of the 36 fungal ASVs that were identified to have significant differential abundance values (P adjusted < 0.05) across the PS under investigation. The taxonomic identity of each fungal ASV is presented, with each fungal ASV represented by the most refined taxonomic rank that could be accurately assigned.
Discussion
The root-associated soil microbiome provides fundamental roles in supporting plant health, productivity, and resilience against abiotic and biotic stressors (Mendes et al. 2011, Berendsen et al. 2012, Penton et al. 2014). Thus, pinpointing how different components of the plant-root-soil interface drive microbial selection and establishment is key for us to manage plant and soil health into the future. However, identifying the primary processes that drive microbial assembly is complex and is suggested to be by the interacting influences of plant genotype, developmental stage, root exudates, root morphology, PLH, soil type, and previous soil history (Chaparro et al. 2014, Zhao et al. 2019, Zhou et al. 2020, Cordovez et al. 2021). By controlling for the starting soil mixture and surrounding environmental conditions, our research aimed to identify how the different phylogenetic, functional, and ecological traits of PS were related to the assembly of their root-associated soil microbiome.
The phylogenetic relatedness of plant hosts shared no relationship to the similarity in their root-associated soil microbiome
Our research aimed to test whether the phylogenetic relatedness of PS was correlated with the phylogenetic similarity of their root-associated soil microbiome—a hypothesis that has been supported by previous research (Bouffaud et al. 2014, Lambais et al. 2014, Yeoh et al. 2017, Lei et al. 2019, Kaplan et al. 2020, Hartman et al. 2023). Our results did not support this hypothesis, as the phylogenetic similarity in root-associated soil microbiomes did not correlate with the phylogenetic similarity between different PS. With the increase in phylogenetic ranking from PS to class level, we observed a consistent decline in the degree of microbial community variation that could be accounted for by plant phylogenetic origin. That is, higher phylogenetic rankings such as plant class and order only explained a small amount of compositional variation compared to PS-level identity. This suggests that, whilst PS may be used as a predictor of the root-associated soil microbiome, higher taxonomic rankings of PS cannot. Similar findings were observed by Fitzpatrick et al. (2018), who identified that although PS identity was a significant factor shaping rhizosphere assembly, the emergent structure of the rhizosphere microbiome shared no relationship with the phylogenetic relatedness between plant hosts.
Plant species and plant-induced changes in soil physicochemistry were the strongest predictors of microbial assembly
Although patterns in microbial assembly did not relate to the phylogenetic relationships among PS, species identity and differences in SC were the two most significant factors that accounted for bacterial and fungal community variation—a finding also observed by Burns et al. (2015). Whilst both factions were significant, PS identity accounted for a greater proportion of bacterial community variance than SC. Several studies have reported PS identity to be a significant factor in shaping microbial assembly and community structure (Garbeva et al. 2008, Berg and Smalla 2009, Becklin et al. 2012, Burns et al. 2015). These plant-species-dependent effects on microbial assembly have been attributed to the release of carbon-rich root exudates, which selectively enrich and recruit specific root-associated soil microorganisms (Bais et al. 2006), with the quality and composition of root exudates varying according to PS and plant developmental stage (Badri and Vivanco 2009, Zhalnina et al. 2018).
For fungal communities, SC was identified to account for a higher amount of community variance than PS identity. In our experiment, all PS were planted in the same starting soil mixture. As such, these effects are not associated with differences in soil type or edaphic properties per se but are changes that the plants themselves have directly expressed on the rhizosphere and soil environment. Furthermore, plant-driven changes in the composition of root-associated microorganisms throughout the early stages of microbial assembly may also have indirectly driven the shifts observed in SC. Plants can directly modify the conditions of their surrounding soil physicochemical environment via nutrient uptake/loss or by the chemical signatures of their leaf litter, roots, and root exudates. Plants can also shape their soil physicochemical environment indirectly by driving changes in the activity and composition of their root-associated microorganisms (Rengel and Marschner 2005, Waring et al. 2015, Henneron et al. 2020). Root-associated microorganisms have key roles in the transformation and mobilization of inorganic and organic substrates into more plant-accessible soil nutrients, meaning that they can have a transformative impact on soil nutrient cycling (Finzi et al. 2015, Dlamini et al. 2022; Dotaniya and Meena 2015). Plant-induced changes in SC provide an example of how secondary deterministic processes can indirectly shape microbial assembly. As plant roots modify the conditions of the rhizosphere and root-associated soil environment, this encourages the growth of microorganisms that can occupy the modified habitat space (Hinsinger 2001, Liang et al. 2002, Bell et al. 2015, van Veelen et al. 2020, Hernández-Cáceres et al. 2022).
Plant-available P, such as that measured by Olsen P (bicarbonate extractable), is a key measure of soil fertility and ecosystem productivity (Vitousek et al. 2010). In our research, Olsen P had a particularly strong relationship with changes in root-associated soil fungal and bacterial communities. In particular, the root-associated soils from the PS Pi. radiata, Al. glutinosa, and Ac. caesiiglauca had high Olsen P values compared to the other PS. These observations may demonstrate the process of plants mobilizing soil nutrients essential for their individual growth and fitness (Will 1978, Chen et al. 2002, Tallec et al. 2009, Varin et al. 2009) and how these are linked to changes in soil microbiology. When root-induced changes in soil chemistry influence microbial assembly, this ultimately impacts plant health and performance, and thereby success in the ecosystem. These form plant-soil feedback mechanisms that amplify over successive life cycles (van der Putten et al. 2013, Bennett and Klironomos 2019) and are profoundly connected with ecosystem-level processes.
The functional traits of plant species did not influence microbial community assembly
In our study, plant functional traits such as life span, functional group, provenance, N2 fixation, and mycorrhizal association were not identified as strong drivers in microbial community assembly. It is important to consider that we examined root-associated soil microbes, but not microbes that colonize and develop symbiotic relationships with plant roots such as endophytes or mycorrhizal fungi. Had we examined the assembly patterns of plant symbiotic microbes and not free-living soil microbes, we may have observed the functional traits of host plants to have had a more pronounced impact on patterns of microbial assembly. Our findings are complementary to Hartman et al. (2023), who identified that the symbiotic associations of plant hosts significantly impact the root microbiome. Unlike Hartman et al. (2023) and Bodenhausen et al. (2019), who sampled the root-associated microbiome, our study examined soil adjacent to plant roots. Thus, discrepancies between the findings of our research and Hartman et al. (2023) are due to the clearly different sampling methodologies, as we sampled soils at a greater physical distance from the root. Additionally, our research investigated root-associated soil microbial assembly following (a) a single life cycle of the plant and (b) at a single time point during the plant’s developmental stage. Thus, the absence of clear divergences in microbial assembly between plants with contrasting functional traits may be a consequence of our experiment’s relatively short duration or other factors. For example, although we studied plants with different life cycle strategies (i.e. annual vs. perennial), we did not study them over repeated life cycles, where the outcomes of their contrasting life histories may have modified their soil environment to a degree that influenced microbial assembly. Several studies have reported the soil microbiome to shift according to plant development, influenced by changes in plant root morphology and exudate release with each developmental stage (Micallef et al. 2009, Chaparro et al. 2014). Furthermore, the divergence in microbial assembly between PS may amplify over successive life cycles (Cordovez et al. 2021). This increasing divergence is driven by plant-soil feedback mechanisms, whereby successive modifications in soil biotic and abiotic conditions by plants exert greater selection pressures on their root-associated soil microbiota (Hu et al. 2018).
Root-associated soil microbiomes exhibited a large degree of unexplained variation
Niche-based theories of microbial community assembly assert that deterministic processes govern community structure, such as adaptive species traits, biotic interactions, and environmental filtering (Dini-Andreote et al. 2015, Zhou and Ning 2017). As discussed, aside from plant identity and plant-induced changes in SC, the functional plant traits measured in our study accounted for very little of the variation observed in root-associated soil microbial communities. Our results identified that a large amount of the compositional variation in root-associated soil communities remained unexplained, with over 73% of fungal community variation and 65% of bacterial community variation unaccounted. Given the breadth of variables we assessed, much of this variation may represent elements of stochastic processes driving random community assemblage. More recently, there has been a growing body of literature recognizing the degree to which stochastic processes may govern the resulting structure of microbial communities (Caruso et al. 2011, Zhang et al. 2016, Zhou and Ning 2017, Chen et al. 2019, Hou et al. 2021, Huang et al. 2022).
The PS under investigation in this study were at relatively early stages of succession and growth (plants were grown for 10 months), which may explain the large amount of unexplained compositional variation we observed. Stochastic processes are reported to dominate microbial assembly during the early stages of community establishment, as the roots of plant seedlings release an abundant supply of exudates, which reduces competitive biotic interactions (Dini-Andreote et al. 2015). However, throughout community development, microbiomes transition from random community assembly to more highly structured, niche-differentiated assemblages because of functional adaptations to the environmental selection pressures (Aguilar and Sommaruga 2020, Hu et al. 2020). As plants develop, they alter the bioavailability of resources according to their needs; thus, deterministic processes increasingly dominate microbial community assembly as the surrounding environment is increasingly modified by plant growth (Dini-Andreote et al. 2015). The modification of soil physicochemical properties by PS was observed for several of the PS in our study, with Pi. radiata, Ac. caesiiglauca, and Al. glutinosa driving changes in Olsen P, for example. It is possible that if the microbial communities were measured over longer periods, community assembly would be more evidently niche-differentiated as each plant exerted unique selection pressures within the root-associated soil environment.
Conclusion
Our research findings identified that during the early stages of plant growth and establishment, PS identity and plant-induced changes in SC were the most significant factors that shaped root-associated soil microbial assembly. The functional traits of the PS under investigation, such as their life span, provenance, growth form, and mycorrhizal associations, did not significantly account for any of the structural variation observed in bacterial or fungal communities between plants. Although PS identity was determined to be a significant factor driving microbial assembly, the phylogenetic relationships shared between the 37 PS under investigation shared no relationship to the similarity of their root-associated soil microbiomes. Thus, our findings reject the hypothesis that plant phylogenetic relatedness can be used to predict the emergent structure of the root-associated soil microbiome.
Supplementary Material
Acknowledgements
Thank you to Xiaoben Jiang, Charlotte Armstrong, and Sarah Addison for their assistance in the delivery of the project data. Thank you to the two anonymous reviewers for volunteering their time and expertise to review this manuscript—your valuable comments and suggestions have helped to improve the quality of this manuscript and are sincerely appreciated.
Contributor Information
Alexa-Kate Byers, Bioprotection Aotearoa, Lincoln University, PO Box 85084, Lincoln 7647, New Zealand.
Leo M Condron, Bioprotection Aotearoa, Lincoln University, PO Box 85084, Lincoln 7647, New Zealand.
Maureen O'Callaghan, AgResearch Ltd, 1365 Springs Road, Lincoln 7674, New Zealand.
Lauren Waller, Biosecurity New Zealand, Ministry for Primary Industries, 34-38 Bowen Street, PO Box 2526, Wellington 6140, New Zealand.
Ian A Dickie, Bioprotection Aotearoa, School of Biological Sciences, University of Canterbury, PO Box 4800, Christchurch 8140, New Zealand.
Steve A Wakelin, Ecology and Environment, Scion Research Ltd, 10 Kyle Street, Riccarton, Christchurch 8011, Canterbury, New Zealand.
Author contributions
Alexa-Kate Byers (Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing), Leo M. Condron (Funding acquisition, Project administration, Supervision, Writing – review & editing), Maureen O'Callaghan (Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing), Lauren Waller (Investigation, Methodology, Writing – review & editing), Ian A. Dickie (Funding acquisition, Project administration, Writing – review & editing), and Steve A. Wakelin (Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing)
Conflict of interest
None declared.
Funding
This work was supported by Programme C04X2002 ‘The Tree Microbiome Project: at the root of climate proofing forests’, supported by the New Zealand Ministry of Business, Innovation, and Employment (MBIE), and the Forest Growers Levy Trust. New Zealand Tertiary Education Commission (TEC) support for Bioprotection Aotearoa Centre for Research Excellence.
Data Availability
The R scripting code used for the data analysis presented in this research can be found at https://github.com/akbyers/rhizosphere_microbiome_assemblage
References
- Abarenkov K, Zirk A, Piirmann Tet al. UNITE general FASTA release for Fungi. Unite Community2021. Available: https://plutof.ut.ee/#/doi/10.15156/BIO/786368. (21 Februrary 2022, date last accessed).
- Aguilar P, Sommaruga R. The balance between deterministic and stochastic processes in structuring lake bacterioplankton community over time. Mol Ecol. 2020;29:3117–30. 10.1111/mec.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apprill A, McNally S, Parsons Ret al. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Microb Ecol. 2015;75:129–37. 10.3354/ame01753. [DOI] [Google Scholar]
- Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environ. 2009;32:666–81. 10.1111/j.1365-3040.2009.01926.x. [DOI] [PubMed] [Google Scholar]
- Bais HP, Weir TL, Perry LGet al. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006;57:233–66. 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Becklin KM, Hertweck KL, Jumpponen A. Host identity impacts rhizosphere fungal communities associated with three alpine plant species. Microb Ecol. 2012;63:682–93. 10.1007/s00248-011-9968-7. [DOI] [PubMed] [Google Scholar]
- Bell CW, Asao S, Calderón FJet al. Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol Biochem. 2015;85:170–82. 10.1016/j.soilbio.2015.03.006. [DOI] [Google Scholar]
- Bennett JA, Klironomos J. Mechanisms of plant–soil feedback: interactions among biotic and abiotic drivers. New Phytol. 2019;222:91–6. 10.1111/nph.15603. [DOI] [PubMed] [Google Scholar]
- Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–86. 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009;68:1–13. 10.1111/j.1574-6941.2009.00654.x. [DOI] [PubMed] [Google Scholar]
- Bodenhausen N, Somerville V, Desiro Aet al. Petunia-and Arabidopsis-specific root microbiota responses to phosphate supplementation. Phytobiomes J. 2019;3:112–24. 10.1094/PBIOMES-12-18-0057-R. [DOI] [Google Scholar]
- Bokulich NA, Mills DA. Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol. 2013;79:2519–26. 10.1128/AEM.03870-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffaud ML, Poirier MA, Muller Det al. Root microbiome relates to plant host evolution in maize and other Poaceae. Environ Microbiol. 2014;16:2804–14. 10.1111/1462-2920.12442. [DOI] [PubMed] [Google Scholar]
- Burns JH, Anacker BL, Strauss SYet al. Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB Plants. 2015;7:plv030. 10.1093/aobpla/plv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJet al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WAet al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4. 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IT, Cardinale BJ, Nisbet RM. Niche and fitness differences relate the maintenance of diversity to ecosystem function. Ecology. 2011;92:1157–65. 10.1890/10-0302.1. [DOI] [PubMed] [Google Scholar]
- Caruso T, Chan Y, Lacap DCet al. Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J. 2011;5:1406–13. 10.1038/ismej.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro JM, Badri DV, Vivanco JM. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014;8:790–803. 10.1038/ismej.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Condron LM, Davis MRet al. Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don). Soil Biol Biochem. 2002;34:487–99. 10.1016/S0038-0717(01)00207-3. [DOI] [Google Scholar]
- Chen W, Ren K, Isabwe Aet al. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome. 2019;7:1–16. 10.1186/s40168-019-0749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordovez V, Rotoni C, Dini-Andreote Fet al. Successive plant growth amplifies genotype-specific assembly of the tomato rhizosphere microbiome. Sci Total Environ. 2021;772:144825. 10.1016/j.scitotenv.2020.144825. [DOI] [PubMed] [Google Scholar]
- Dini-Andreote F, Stegen JC, Elsas JDet al. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc Natl Acad Sci. 2015;112:E1326–32. 10.1073/pnas.1414261112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlamini SP, Akanmu AO, Babalola OO. Rhizospheric microorganisms: the gateway to a sustainable plant health. Front Sustain Food Syst. 2022;6:925802. 10.3389/fsufs.2022.925802. [DOI] [Google Scholar]
- Dotaniya ML, Meena VD. Rhizosphere effect on nutrient availability in soil and its uptake by plants: a review. Proc Natl Acad Sci India Sect B Biol Sci. 2015, 85:1–12. 10.1007/s40011-013-0297-0. [DOI] [Google Scholar]
- Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1–10. 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- Fatima T, Srivastava A, Somashekar Pet al. Development of DNA-based species identification and barcoding of three important timbers. Bull Natl Res Cent. 2019;43:1–17. 10.1186/s42269-019-0116-8. [DOI] [Google Scholar]
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci. 2006;103:626–31. 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi AC, Abramoff RZ, Spiller KSet al. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Global Chang Biol. 2015;21:2082–94. 10.1111/gcb.12816. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CR, Copeland J, Wang PWet al. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc Nat Acad Sci USA. 2018;115:E1157–65. 10.1073/pnas.1717617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbeva P, van Elsas JD, van Veen JA. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil. 2008;302:19–32. 10.1007/s11104-007-9432-0. [DOI] [Google Scholar]
- Girvan MS, Bullimore J, Pretty JNet al. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl Environ Microbiol. 2003;69:1800–9. 10.1128/AEM.69.3.1800-1809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss-Souza D, Mendes LW, Borges CDet al. Soil microbial community dynamics and assembly under long-term land use change. FEMS Microbiol Ecol. 2017;93:fix109. 10.1093/femsec/fix109. [DOI] [PubMed] [Google Scholar]
- Hartman K, Schmid MW, Bodenhausen Net al. A symbiotic footprint in the plant root microbiome. Environ Microbiome. 2023;18:65. 10.1186/s40793-023-00521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneron L, Kardol P, Wardle DAet al. Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol. 2020;228:1269–82. https://doi.org/101111/nph.16760. [DOI] [PubMed] [Google Scholar]
- Hernández-Cáceres D, Stokes A, Angeles-Alvarez Get al. Vegetation creates microenvironments that influence soil microbial activity and functional diversity along an elevation gradient. Soil Biol Biochem. 2022;165:108485. 10.1016/j.soilbio.2021.108485. [DOI] [Google Scholar]
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil. 2001;237:173–95. 10.1023/A:1013351617532. [DOI] [Google Scholar]
- Hoggard M, Vesty A, Wong Get al. Characterizing the Human mycobiota: a comparison of small subunit rRNA, ITS1, ITS2, and large subunit rRNA genomic targets. Front Microbiol. 2018;9:2208. 10.3389/fmicb.2018.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Zhou R, Zeng Set al. Stochastic processes shape the bacterial community assembly in shrimp cultural pond sediments. Appl Microbiol Biotechnol. 2021;105:5013–22. 10.1007/s00253-021-11378-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Wei Z, Kowalchuk GAet al. Rhizosphere microbiome functional diversity and pathogen invasion resistance build up during plant development. Environ Microbiol. 2020;22:5005–18. 10.1111/1462-2920.15097. [DOI] [PubMed] [Google Scholar]
- Hu L, Robert CAM, Cadot Set al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun. 2018;9:2738. 10.1038/s41467-018-05122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bai J, Wang Jet al. Different stochastic processes regulate bacterial and fungal community assembly in estuarine wetland soils. Soil Biol Biochem. 2022;167:108586. 10.1016/j.soilbio.2022.10858. [DOI] [Google Scholar]
- Kaplan I, Bokulich NA, Caporaso JGet al. Phylogenetic farming: can evolutionary history predict crop rotation via the soil microbiome?. Evol Appl. 2020;13:1984–99. 10.1111/eva.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MRet al. Picante: r tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–4. 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Kolde R, Kolde MR. Package ‘pheatmap’. R package2018. Available: https://CRAN.R-project.org/package=pheatmap. (21 February 2022, date last accessed).
- Kumar S, Stecher G, Li Met al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambais MR, Lucheta AR, Crowley DE. Bacterial community assemblages associated with the phyllosphere, dermosphere, and rhizosphere of tree species of the Atlantic Forest are host taxon dependent. Microb Ecol. 2014;68:567–74. 10.1007/s00248-014-0433-2. [DOI] [PubMed] [Google Scholar]
- Lauber CL, Hamady M, Knight Ret al. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol. 2009;75:5111–20. 10.1128/AEM.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Hawkes CV. Plant and soil drivers of whole-plant microbiomes: variation in switchgrass fungi from coastal to mountain sites. Phytobiomes J. 2020;5:69–79. 10.1094/PBIOMES-07-20-0056-FI. [DOI] [Google Scholar]
- Lei S, Xu X, Cheng Zet al. Analysis of the community composition and bacterial diversity of the rhizosphere microbiome across different plant taxa. Microbiologyopen. 2019;8:e00762. 10.1002/mbo3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BC, Wang XL, Ma BL. Maize root-induced change in soil organic carbon pools. Soil Sci Soc Am J. 2002;66:845–7. 10.2136/sssaj2002.8450. [DOI] [Google Scholar]
- Lozupone C, Lladser ME, Knights Det al. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–72. 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz Fernando WR. Pime: A package for discovery of novel differences among microbial communities. Mol Ecol Resour. 2020;20:415–28. 10.1111/1755-0998.13116. [DOI] [PubMed] [Google Scholar]
- Lundberg DS, Lebeis SL, Paredes SHet al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner P, Yang C, Lieberei Ret al. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem. 2001;33:1437–45. 10.1016/S0038-0717(01)00052-9. [DOI] [Google Scholar]
- Martinez Arbizu P. 2020. Pairwise Adonis: pairwise multilevel comparison using adonis. R package. Available: https://github.com/pmartinezarbizu/pairwiseAdonis. (21 February 2022, date last accessed).
- McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–63. 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- Mendes R, Kruijt M, De Bruijn Iet al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–100. 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Micallef SA, Channer S, Shiaris MPet al. Plant age and genotype impact the progression of bacterial community succession in the Arabidopsis rhizosphere. Plant Signal Behav. 2009;4:777–80. 10.4161/psb.4.8.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller DB, Vogel C, Bai Yet al. The plant microbiota: systems-level insights and perspectives. Annu Rev Genet. 2016;50:211–34. 10.1146/annurev-genet-120215-034952. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Friendly Met al. Vegan: community ecology package (version 2.5-6). The Comprehensive R Archive Network2019. Available: https://CRAN.R-project.org/package=vegan. (21 February 2022, date last accessed).
- Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18:1403–14. 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–8. 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Paulson JN, Olson ND, Braccia DJet al. metagenomeSeq: statistical analysis for sparse high-throughput sequencing. Bioconductor package2013. Available: http://www.cbcb.umd.edu/software/metagenomeSeq. (21 February 2022, date last accessed).
- Penton CR, Gupta VVSR, Tiedje JMet al. Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS One. 2014;9:e93893. 10.1371/journal.pone.0093893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Jaramillo JE, Mendes R, Raaijmakers JM. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol. 2016;90:635–44. 10.1007/s11103-015-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot L, Raaijmakers JM, Lemanceau Pet al. Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol. 2013;11:789–99. 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Zamioudis C, Berendsen RLet al. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52:347–75. 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JI. Putting science back into microbial ecology: a question of approach. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190240. 10.1098/rstb.2019.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Bünger W, Burbano CSet al. Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol. 2015;53:403–24. 10.1146/annurev-phyto-082712-102342. [DOI] [PubMed] [Google Scholar]
- Ren Y, Xun W, Yan Het al. Functional compensation dominates the assembly of plant rhizospheric bacterial community. Soil Biol Biochem. 2020;150:107968. 10.1016/j.soilbio.2020.107968. [DOI] [Google Scholar]
- Rengel Z, Marschner P. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol. 2005;168:305–12. 10.1111/j.1469-8137.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Sasse J, Martinoia E, Northen T. Feed your friends: do plant exudates shape the root microbiome?. Trends Plant Sci. 2018;23:25–41. 10.1016/j.tplants.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Tallec T, Diquélou S, Avice JCet al. Availability of N and S affect nutrient acquisition efficiencies differently by Trifolium repens and Lolium perenne when grown in monoculture or in mixture. Environ Exp Bot. 2009;66:309–16. 10.1016/j.envexpbot.2009.02.002. [DOI] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–26. 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tkacz A, Cheema J, Chandra Get al. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 2015;9:2349–59. 10.1038/ismej.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich A, Becker R. Soil parent material is a key determinant of the bacterial community structure in arable soils. FEMS Microbiol Ecol. 2006;56:430–43. 10.1111/j.1574-6941.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- van der Putten WH, Bardgett RD, Bever JDet al. Plant–soil feedbacks: the past, the present and future challenges. J Ecol. 2013;101:265–76. 10.1111/1365-2745.12054. [DOI] [Google Scholar]
- van Veelen A, Koebernick N, Scotson CSet al. Root-induced soil deformation influences Fe, S and P: rhizosphere chemistry investigated using synchrotron XRF and XANES. New Phytol. 2020;225:1476–90. 10.1111/nph.16242. [DOI] [PubMed] [Google Scholar]
- Varin S, Leveel B, Lemauviel-Lavenant Set al. Does the white clover response to sulphur availability correspond to phenotypic or ontogenetic plasticity?. Acta Oecologica. 2009;35:452–7. 10.1016/j.actao.2009.01.002. [DOI] [Google Scholar]
- Veach AM, Morris R, Yip DZet al. Rhizosphere microbiomes diverge among Populus trichocarpa plant-host genotypes and chemotypes, but it depends on soil origin. Microbiome. 2019;7:76. 10.1186/s40168-019-0668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Porder S, Houlton BZet al. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl. 2010;20:5–15. 10.1890/08-0127.1. [DOI] [PubMed] [Google Scholar]
- Wakelin SA, Matson A, Wigley Ket al. High maintenance of rhizosphere soil C and N equilibrium regardless of plant species or species traits. Front Soil Sci. 2021;1:762510. 10.3389/fsoil.2021.762510. [DOI] [Google Scholar]
- Wakelin SA, van Koten C, O'Callaghan Met al. Physicochemical properties of 50 New Zealand pasture soils: a starting point for assessing and managing soil microbial resources. NZ J Agric Res. 2013;56:248–60. 10.1080/00288233.2013.822003. [DOI] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JMet al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring BG, Álvarez-Cansino L, Barry KEet al. Pervasive and strong effects of plants on soil chemistry: a meta-analysis of individual plant ‘Zinke’ effects. Proc Biol Sci. 2015;282:201521001. 10.1098/rspb.2015.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will G. Nutrient deficiencies in Pinus radiata in New Zealand. N Z J For Sci. 1978;8:4–14. Available: https://www.scionresearch.com/__data/assets/pdf_file/0003/37182/NZJFS811978WILL4_14.pdf. [Google Scholar]
- Xiao X, Chen W, Zong Let al. Two cultivated legume plants reveal the enrichment process of the microbiome in the rhizocompartments. Mol Ecol. 2017;26:1641–165. 10.1111/mec.14027. [DOI] [PubMed] [Google Scholar]
- Yeoh YK, Dennis PG, Paungfoo-Lonhienne Cet al. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat Commun. 2017;8:215. 10.1038/s41467-017-00262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhalnina K, Louie KB, Hao Zet al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol. 2018;3:470–80. 10.1038/s41564-018-0129-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Johnston ER, Liu Wet al. Environmental changes affect the assembly of soil bacterial community primarily by mediating stochastic processes. Global Change Biol. 2016;22:198–207. 10.1111/gcb.13080. [DOI] [PubMed] [Google Scholar]
- Zhao M, Yuan J, Shen Zet al. Predominance of soil vs root effect in rhizosphere microbiota reassembly. FEMS Microbiol Ecol. 2019;95:fiz139. 10.1093/femsec/fiz139. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ning D. Stochastic Community Assembly: does it matter in microbial ecology?. Microbiol Mol Biol Rev. 2017;81:e00002–17. 10.1128/MMBR.00002-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Coventry DR, Gupta VVSRet al. The preceding root system drives the composition and function of the rhizosphere microbiome. Genome Biol. 2020;21:89. 10.1186/s13059-020-01999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R scripting code used for the data analysis presented in this research can be found at https://github.com/akbyers/rhizosphere_microbiome_assemblage