Introduction
Transpulmonary pressure (PL) monitoring is becoming increasingly popular for estimating mechanical stress applied to injured lungs or tailoring ventilator settings during ARDS.1–5 The computation of PL relies on pleural pressure, for which the esophageal pressure (Pes) is an acceptable regional approximation.2,6
Clinicians typically measure Pes using a balloon catheter filled with air. The conventional methodology for balloon volume calibration involves filling it with a standardized volume and validating its position using the Baydur modified test, also known as the occlusion test (OT).1,3 However, this methodology has recently been called into question at the bedside. Indeed, Mojoli and colleagues7,8 proposed an original method based on the assessment of pressure-volume (PV) curve of the esophageal balloon catheter, which yielded promising results. Whereas this calibration procedure is attractive, it remains cumbersome and time consuming at the bedside, hindering widespread adoption for PL monitoring.2,7
In this short report, we propose a simplified method based on Mojoli's hypothesis. We developed an experimental protocol to dynamically build and record the PV curve of the esophageal balloon. Our method involves filling the balloon at a slow and continuous rate using an automatic process that does not require any intervention during recording.
Methods
The study was approved by the ethics committee of Rennes University Hospital and the French national authority. It is part of a larger observational study (NCT05697666) investigating the impact of neuromuscular relaxants on respiratory mechanics. Subjects or their family provided written informed consent after receiving a detailed protocol.
Subjects included had moderate to severe ARDS according to the Berlin definition and were eligible for muscular paralysis. Subjects were monitored using the NutriVent (Sidam, Mirandola, Italy) Pes catheter device (to evaluate the partitioning of respiratory mechanics) which was positioned according to recommended guidelines.1–3 Ventilation was conducted using protective volume controlled–continuous mandatory ventilation mode, with a tidal volume approximately 6 mL/kg of predictive body weight, and the PEEP level was at the discretion of the clinician.
An air-filled syringe was connected to the Pes monitoring circuit using a Y-branch and controlled using a syringe driver. Baseline pressure was zeroed before air injection. Pes and balloon volume were continuously recorded up to 8 mL inflation rate at 100 mL/h (Fig. 1A–B) using a manometric sensor coupled with respiratory variables (airway pressure and flow) via a FluxMed device (MBMed, Buenos Aires, Argentina). The balloon was then emptied and zeroed. A step-by-step OT sequence was then performed by filling the esophageal balloon with fixed volumes from 0.5–8 mL, and an OT was conducted for each static volume level according to Mojoli's description (Fig. 1B and 1E).7 OT ratio was defined as the ratio of increment of Pes (ΔPes) to the increment in airway pressure (ΔPaw) during expiratory (exp) occlusion and compression of the chest, expressed as ΔPes/ΔPaw ratio (with usual validated values ranging from 0.8 to 1.2). The value associated with the ratio closest to 1 was the most suitable volume balloon for assessing Pes according to Mojoli's method1,2,7 (Fig. 1E–F).
Fig. 1.
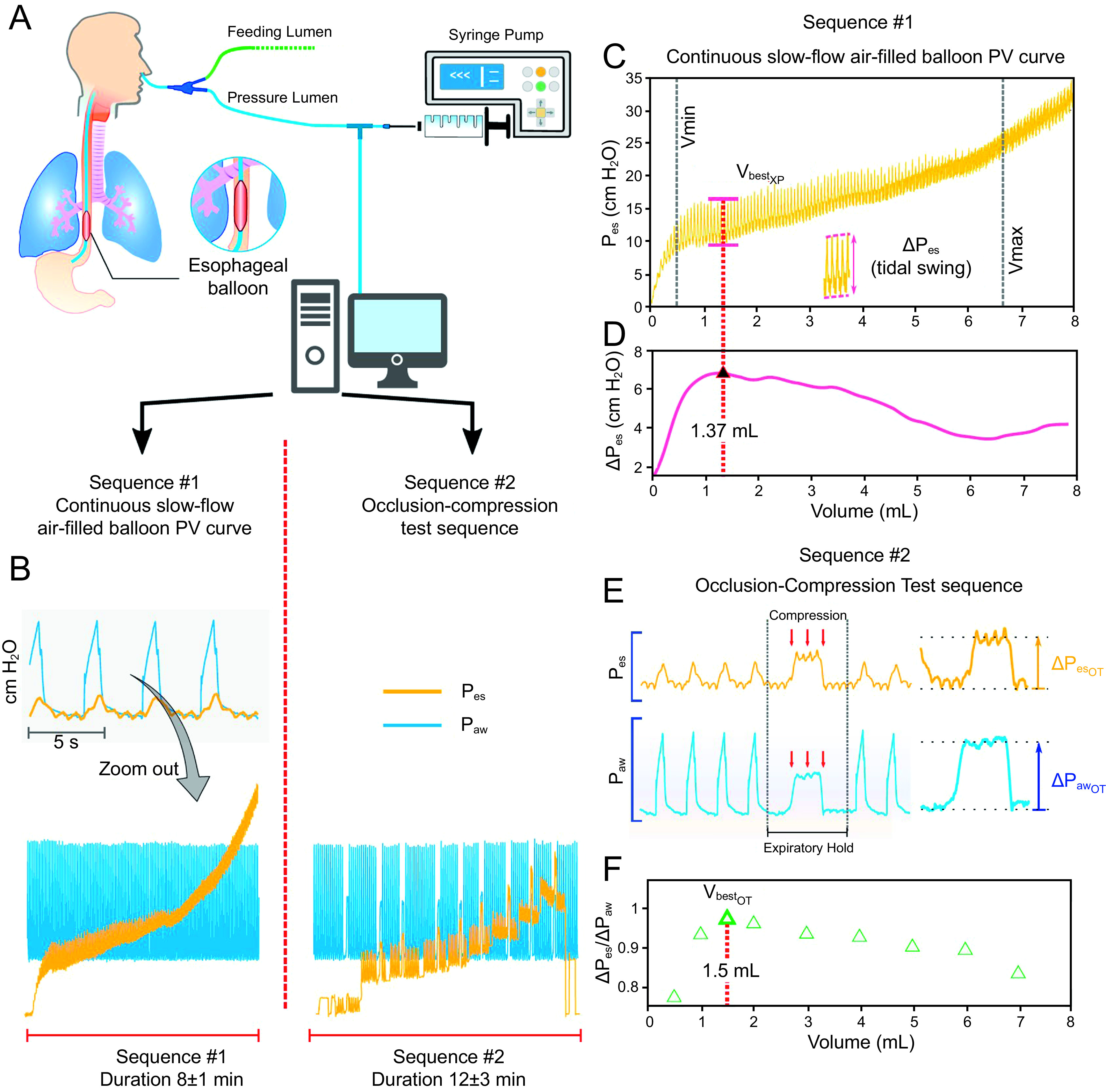
Synopsis of experimental protocol. Fig 1A: Set-up of experimental protocol for high-resolution dynamic esophageal balloon pressure-volume (PV) curve. Esophageal balloon was filled with air with a piloted syringe (filling balloon at rate of 100 mL/h, ie, 1.67 mL/min). Fig 1B: Air-filled esophageal balloon PV curve built with a continuous slow-flow inflation sequence (and subsequent occlusion test [OT] maneuver): recording and graphical/computerized analysis. Fig 1C–D: Continuous slow-flow PV curve graphics focus on VbestXP: recording and graphical/computerized analysis. C: Volume balloon with maximal Pes tidal variation. D: Analytics of dynamic PV curve leading to an estimate of VbestXP = 1.37 mL. Fig 1E–F: Detailed description focused on OT sequence. E: Computation of OT (as ΔPes/ΔPaw ratio) at each volume step (see text for detailed description of OT sequence). F: Maximal ratio defining VbestOT (top green triangle): here VbestOT = 1.5 mL, which closely match with VbestXP (1.37 mL) estimated by our experimental method (panel D). PV = pressure-volume; Pes = esophageal pressure; esp = expiratory; Paw = airway pressure; OT = occlusion test.
The esophageal balloon signals were processed and cleaned using software programs (Graphysio and MATLAB [MathWorks, Natick, Massachusetts]), and esophageal balloon PV curves were calculated (Fig. 1B). The experimental variables (XP variables) including VbestXP (mL), Eew (cm H2O/mL), and Pes insp/expXP (cm H2O), were compared to OT variables [VbestOT (mL), Pes insp/expOT (cmH2O)] derived from static PV steps using Mojoli's method as a standard. VbestXP represents maximal tidal variation of Pes; VbestOT is the balloon volume closer to 1 for OT ratio; Eew is esophageal wall elastance, and Pes insp/exp are the values of insp/exp Pes at Vbest with OT method and at volume = 4 mL (V4).
Statistical comparison between continuous variables (VbestXP and VbestXP) and Pes (PesOT and PesV4) were performed using the non-parametric Mann-Whitney test with a significance level of .05. Correlations between volumes (VbestXP and VbestOT) and Pes analyzed using Pearson correlation coefficient and linear regression statistics (R2).
Results
Complete acquisition and recording of esophageal balloon PV curve were performed in 6 subjects, resulting in a total of 10 PV curves. Demographic and clinical characteristics were as follows: age 44 ± 11 y; moderate to severe ARDS with a PaO2/FIO2 116 ± 35; plateau pressure 26 ± 4 cm H2O; PEEP level 12 ± 3 cm H2O; and etiology of ARDS including pneumonia (n = 2), pancreatitis (n = 3), and aspiration (n = 1). A typical pattern of dynamic low-flow inflation esophageal balloon PV curve is illustrated in Figure 1B and 1C. Of 6 subjects, 4 were included in the comparison of continuous esophageal balloon PV curve to complete OT. One subject was excluded due to high OT ratio (> 1.2 for every volume tested) and another due to lack of consent. One subject was included 5 times during ARDS. Eight recordings were analyzed.
Parameters including VbestXP, Eew, VbestOT, and Pes (insp/exp) were analyzed. VbestXP and Eew were identified in all 8 recordings. VbestOT and VbestXP were comparable (1.6 ± 0.6 mL and 1.9 ± 0.8 mL, respectively, P = .21) (Fig. 2A) with a high correlation (R2 = 0.84). Pesexp at VbestXP and VbestOT were closely related (R2 = 0.78; Fig. 2B). VbestXP and VbestOT varied between subjects and were different from the standardized fixed volume of 4 mL (V4) (P < .01). The OT ratio at V4 was 0.9 ± 0.1 (range 0.8–1.2). Furthermore, Pes (exp/insp) computed at VbestOT and V4 were different, with Pes at V4 being > Pes at VbestOT, with Pes,expOT at 10.4 ± 3 and Pes,expV4 at 15.5 ± 3.9 cm H2O (P = .02) and Pes,inspOT at 16.3 ± 3.9 and Pes,inspV4 at 19 ± 3.9 cm H2O (P = .01). Figure 2C illustrated the discrepancy between Pes,expOT and Pes,expV4, (R2 = 0.34). Intra-individual variability in volume calibration was observed in subject 4, with VbestXP ranging from 1.5–2.3 mL over 5 days of ARDS evolution.
Fig. 2.
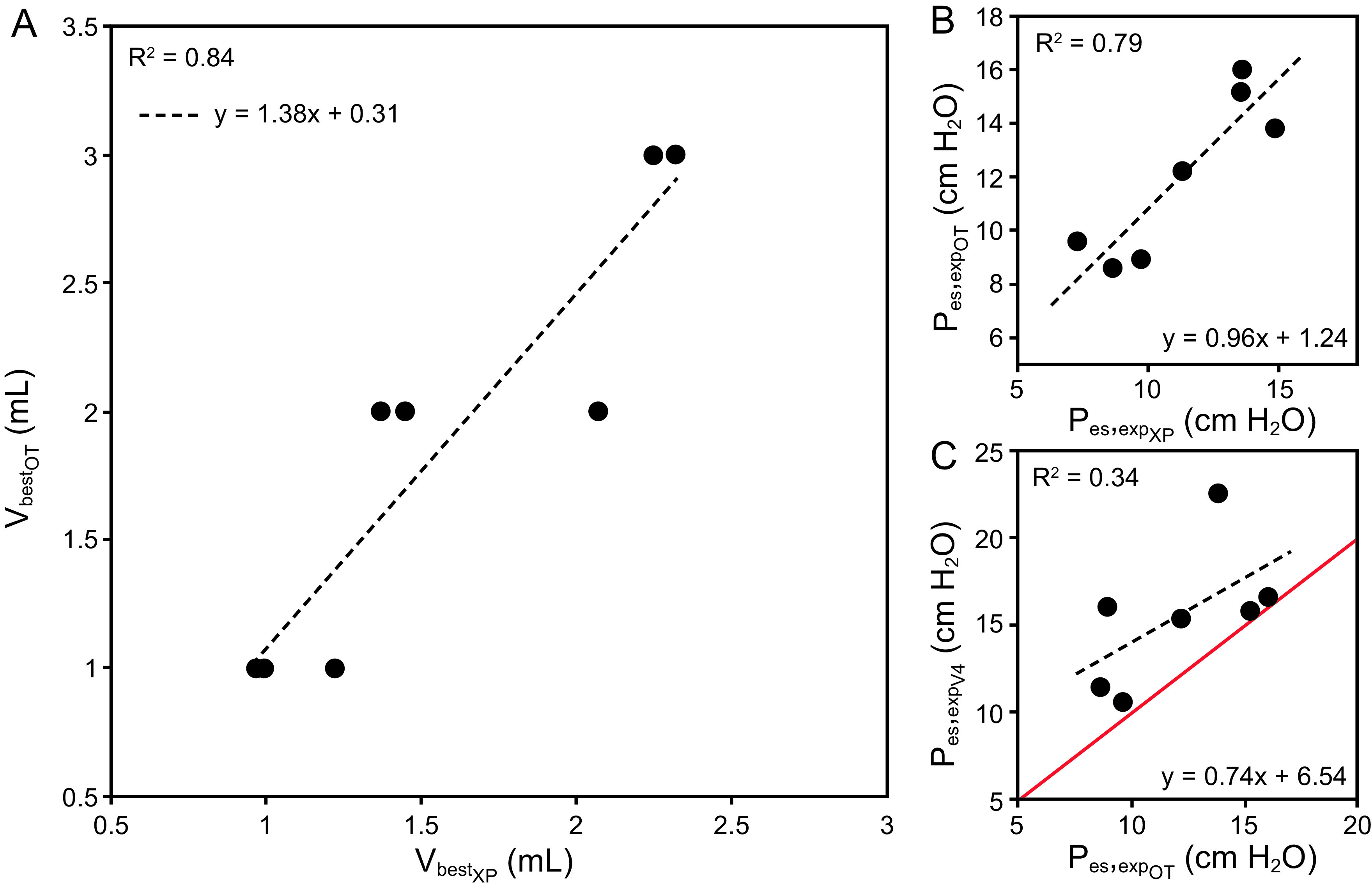
Reliability of experimental data versus reference. A: Scatterplot of the VbestOT versus VbestXP relationship (dots) superimposed with its associated linear fit (dashed line). B: Scatterplot of the Pes,expOT versus Pes,expXP relationship (dots) superimposed with its associated linear fit (dashed line). C: Scatterplot of the Pes,expOT versus Pes,expV4 relationship (dots) superimposed with its associated linear fit (dashed line). Linear coefficients, intercepts, and R2 statistics are displayed for each panel. V = volume; OT = occlusion test; XP = experimental slow-flow method variables; Pes = esophageal pressure; exp = expiratory; V4 = 4 mL volume.
We found that it took 8 ± 1 min (4.8 min for the syringe filling process alone) to set up the experimental part and record data for the PV method. Furthermore, the average time to perform OT at each step (n = 10) and compute the OT ratio closest to 1 was 12 ± 3 min (for trained practitioners). The time required to perform the PV procedure was significantly shorter than that for the OT (P = .01).
Discussion
In this report, we propose a new process to evaluate the optimal esophageal balloon among 4 subjects compared to the standard methodology of Mojoli et al. VbestXP and VbestOT were similar in our cohort (Fig. 2A), and dynamic esophageal balloon PV curve reproduced graphically static esophageal balloon PV curve framework described by Mojoli et al, with a high-resolution added value7 (Fig. 1B–C). We found that our process was less time consuming than the complete Mojoli et al procedure, which required setting various volumes and performing Baydur tests at each level.
Our approach advocates for individual volume assessment instead of standardized volume (4 mL), which may lead to an overestimation of Pes (Fig. 2C).7,8 It should be noted that our population showed lower calibration volumes compared to those found in literature,7–9 as well as more variable esophageal wall elastance (range 0.6–4 cm H2O/mL). OT ratio at V4 was in acceptable range in our cohort (0.8–1.2), but our data of Pes values captured at different volumes (Vbest and V4) were significantly different.1–3,7 Beyond inter-individual variability, we can also speculate on the possibility of an intra-patient variability as suggested by the several measurements performed in the same subject during the course of ARDS (optimal volumes ranging from 1.4–2.3 mL).
Our study had a descriptive and exploratory nature, aimed at assessing the feasibility of the PV protocol. Due to the small sample size and the fact that it was conducted in a single-center, our experimental protocol needs to be validated in a larger cohort. We can note that previous studies did not perform direct pleural manometry when using static or dynamic balloon volume PV curve to guide volume calibration. Therefore, although the use of Vbest for calibration seems physiologically plausible, its improved reliability for Pes monitoring remains hypothetical.10–12 Additionally, the computation and analysis of esophageal balloon PV curve were performed off line, and informative results for volume titration were not readily available. To address this issue, future research could focus on developing dedicated software tools and considering Baydur modified OT at VbestXP as a confirmatory rule. Our study suggests that a dynamic esophageal balloon inflation procedure may help monitor PL with greater accuracy and simplicity in clinical practice. Personalized and repeated measurements are important due to observed inter- and intra-individual variability of optimal balloon volume. Our preliminary results are exploratory but can contribute to refining Pes monitoring, leading to potential automation.
Acknowledgments
We thank PLUG Working Group Executive Committee, and Dr Luciano Gattinoni for his wise advice on our hypothesis and draft. We also thank all medical and nursing teams of our ICU department.
Footnotes
The authors have disclosed no conflicts of interest.
A version of this paper was presented at the PLUG Working Goup meeting, held virtually April 20, 2022.
REFERENCES
- 1. Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai R, et al. ; PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine). Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness, and perspectives. Intensive Care Med 2016;42(9):1360–1373. [DOI] [PubMed] [Google Scholar]
- 2. Gattinoni L, Giosa L, Bonifazi M, Pasticci I, Busana M, Macri M, et al. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev Respir Med 2019;13(8):737–746. [DOI] [PubMed] [Google Scholar]
- 3. Pham T, Telias I, Beitler JR. Esophageal manometry. Respir Care 2020;65(6):772–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, et al. ECMO criteria for influenza A (H1N1)–associated ARDS: role of the transpulmonary pressure. Intensive Care Med 2012;38(3):395–403. [DOI] [PubMed] [Google Scholar]
- 5. Sarge T, Baedorf-Kassis E, Banner-Goodspeed V, Novack V, Loring SH, Gong MN, et al. ; EPVent-2 Study Group. Effect of esophageal pressure–guided positive end-expiratory pressure on survival from acute respiratory distress syndrome: a risk-based and mechanistic re-analysis of the EPVent-2 trial. Am J Respir Crit Care Med 2021;204(10):1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshida T, Amato MBP, Luca Grieco D, Chen L, Lima CAS, Roldan R, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med 2018;197(8):1018–1026. [DOI] [PubMed] [Google Scholar]
- 7. Mojoli F, Iotti GA, Torriglia F, Pozzi M, Alberto Volta C, Bianzina S, et al. In vivo calibration of esophageal pressure in the mechanically ventilated patient makes measurements reliable. Crit Care 2016;20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cammarota G, Lauro G, Santangelo E, Sguazzotti I, Perucca R, Verdina F, et al. Mechanical ventilation guided by uncalibrated esophageal pressure may be potentially harmful. Anesthesiology 2020;133(1):145–153. [DOI] [PubMed] [Google Scholar]
- 9. Coudroy R, Vimpere D, Aissaoui N, Younan R, Bailleul C, Couteau-Chardon A, et al. Prevalence of complete airway closure according to body mass index in acute respiratory distress syndrome. Anesthesiology 2020;133(4):867–878. [DOI] [PubMed] [Google Scholar]
- 10. Tilmont A, Coiffard B, Yoshida T, Daviet F, Baumstarck K, Brioude G, et al. Esophageal pressure as a surrogate of pleural pressure in mechanically ventilated patients. ERJ Open Res 2021;7(1):00646-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ranieri MV, Tonetti T, Nava S. Transpulmonary pressure to guide mechanical ventilation: art or science? Am J Respir Crit Care Med 2021;204(10):1120–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung YC. Bioviscoelastic solids. In: biomechanics. New York: Springer; 1993: 242–320. [Google Scholar]


