Abstract
Presenting research at scientific meetings is an important part of the dissemination of research findings. Abstracts are an abbreviated form of a research study presented at a meeting of a professional society. Common elements include background, methods, results, and conclusions. Each section should be carefully written to maximize the chances of acceptance. This paper will cover how to write an abstract for a presentation at a scientific meeting and common mistakes that authors make when writing abstracts.
Keywords: research, respiratory care, abstract, national meeting, research methodology
Introduction
Research is critical to the application of evidence-based medicine.1,2 Dissemination of individual study findings allows the results to be used to inform clinical practice.3 Studies are frequently presented in abstract form at local, national, or international meetings of professional societies.4 An abstract is an abbreviated format that contains the major findings of each study. Presenting an abstract at a meeting is often a career highlight and an important milestone for clinician-scientists or burgeoning investigators. For many projects, submitting an abstract is an important step toward a full manuscript and can provide clarity for the authors on the major findings of their study before writing the paper.5 Unlike a full manuscript, editors and meeting organizers usually do not permit revision and resubmission based on reviewers' comments. Thus, it is important to submit a polished abstract. This paper will review the purpose, elements, and process of writing an abstract for presentation at a scientific meeting.
Purpose of the Abstract
An abstract is a shortened version of a research study that communicates the key findings. Scientific manuscripts also contain an abstract, but they are not identical to those presented at a national meeting.5 Meeting abstracts are usually more lenient in formatting and may allow tables, figures, or references.5 The goal is to report the major study findings, and the abstract should include all major outcomes. Formatting varies among journals, meetings, and societies, but the major elements are similar across most settings. Individual sections may vary depending on the type of study presented, for example, quality improvement projects include different elements than a clinical study. Common elements include background, methods, results, and conclusion. These elements are used by Respiratory Care as part of the annual Open Forum abstracts at the American Association for Respiratory Care Congress.
Each section has a different purpose. The background section includes what is known about the topic. It usually includes the research question and/or hypothesis being tested.6 Descriptive studies may not have a specific hypothesis but rather have a purpose statement.7 The methods section is how the study was done and should include enough detail for reviewers to evaluate the methodology. For submission as a stand-alone abstract, a statement of institutional review board (IRB) approval is required for studies that include human subjects and approval from the Institutional Animal Care and Use Committee for Animal Studies either within the text or as an acknowledgment during submission. Failure to secure IRB approval will result in the rejection of the abstract. The results section includes the findings of the study and must include data. The conclusion is a summary of the study findings, and no new data should be included in this section.
Before Writing the Abstract
Having a clear process for research and quality improvement projects within your department will increase your chances of success.8,9 A written proposal should be prepared for each study, and authorship, including the order of authors, should be determined in advance.9 This process has been shown to increase the quality of respiratory care–related research.8 How to execute individual study types is beyond the scope of this paper, and, for the rest of this paper, we will assume that the study was well designed and conducted, and that the results are worthy of dissemination. A well-written study proposal with a clear plan for executing the study gives authors a strong foundation for sharing their results in abstract form.
Before starting to write your abstract, there are several important considerations that need to be made. Other papers published as part of this series provide a solid background on stating why this research is important, getting started, and framing the research question.2,6,9 The first step is to determine the target meeting for submission. For respiratory therapists, the target conference is usually the Respiratory Care Open Forum at the annual American Association for Respiratory Care Congress. Respiratory therapists may also present at other meetings, such as the Society for Critical Care Medicine, the American Thoracic Society, the American College of Chest Physicians, or others. Abstracts may also be presented at local or regional meetings.
The second step before writing the abstract is to review the instructions for authors of the intended meeting or journal. This critical step is often overlooked but is important to maximize your chances of acceptance.10 The instructions include the submission deadline, proper sections, and details on the use of tables and figures, and provide the maximum number of characters or word count. Respiratory Care allows 300 words in an abstract as part of a full manuscript. For an abstract submitted to the Open Forum, Respiratory Care allows 2,500 characters, including spaces and 2 tables or figures total. Other meetings have different standards, and it can be challenging to include all the relevant information about a study within the constraints of an abstract. Most societies do not allow duplicate publication; for example, Respiratory Care does not allow abstracts previously published or presented at national or international meetings but will accept those presented at local or regional meetings.
Writing the Abstract
Choosing the title may not always be the first step when writing an abstract, but it is an important one. A good title draws potential readers to your study, describes the study, establishes the content and tone, and contains key words so it can be found by those searching the topic online.5,11 The title should not include results, be too long, be clever, mislead the reader, include abbreviations, be a question, or use trademarks.4 The conclusion of the study should not be part of the title. Examples of titles with strengths and weaknesses are summarized in Table 1.12–14 When writing the abstract, it is reasonable to include 3–5 alternative titles and allow the team to choose. There may be a word limit for the title, which depends on where the abstract is submitted.
Table 1.
Example Titles from the Author's Papers
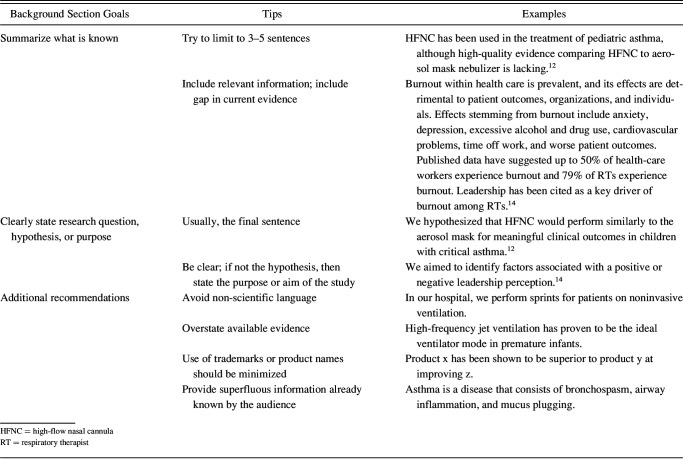
The background section includes what is known about the topic, the research question, the hypothesis being tested, or the purpose of the study. This section should include 1–2 sentences on what is known, including a statement of what is unknown to help the reader understand how the study contributes to knowledge on the topic. The level of detail will depend on the audience reading the abstract; for example, you do not need to describe commonly known respiratory care terms such as asthma, COPD, high-flow nasal cannula, or ARDS if submitting to a respiratory or critical care conference. The goal is for this section to be a maximum of 3–5 sentences in length but is dependent on how much background information readers will need to understand the rest of the abstract. Additional tips and examples for the background section are included in Table 2.
Table 2.
Background Section Tips
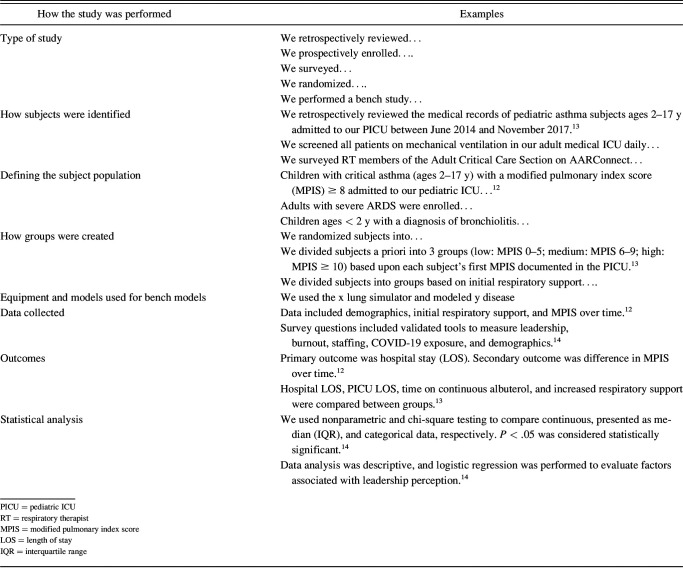
The methods section includes details on how the study was done, subject identification, interventions performed, equipment used for bench models, primary outcome, secondary outcomes, and statistical analysis performed. The population being studied will need to be defined (ie, asthma patients ages 2 to 17 years or adults with ARDS after trauma). Interventions should be described briefly if the study was a randomized controlled trial or a prospective observational study. For studies with complex statistical analysis, it can be challenging to include all the important information due to character limitations, but some information on statistical analysis should be included. Details and examples of parts of the methods section are included in Table 3.
Table 3.
Methods Section
The results section is where the key findings from the study are presented. If the background and methods sections are succinctly written, then there should be adequate space for the results. The number of subjects enrolled should be reported and, in most cases, at least some demographic data. Any differences that may affect the results should be clearly noted within the text. After demographics, the primary outcome should be reported, followed by any secondary outcomes. Any multivariable analysis and subgroup analysis should be included after the primary and secondary outcomes. Examples of results sections are included in Table 4. It is critical to report actual data because reviewers only have access to what is in the abstract, in contrast to a manuscript in which the complete study results are available.
Table 4.
Results Section
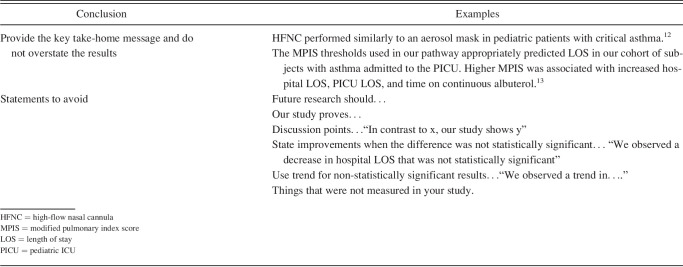
The final section is the conclusion. It should include 1–3 sentences that summarize the main study findings. It is important not to overstate the findings or include data not already reported in the previous sections. Authors are understandably enthusiastic about their study results, but it is important to avoid concluding that clinical practice should change based on a bench study, small observational study, or survey. Avoid ending with statements of future research that needs to be done because this applies to nearly all studies, including large multi-center randomized controlled trials. One exception is if the authors have a specific type of study that would help guide others in following up the study, such as confirming the results in a randomized controlled trial or a larger multi-center study, or translating a bench study into clinical practice. Examples of conclusion sections are included in Table 5.
Table 5.
Conclusion Section
Editing and Feedback
Usually, the first author writes the first draft of the abstract and sends it to the rest of the team for editing. In some cases, the senior author or mentor will provide edits before sending it to the whole group. Importantly, all the authors should be aware of the work and approve its submission. It is important to finish the first draft with adequate time, usually at least 2 weeks before the deadline, to allow all team members enough time to approve or suggest edits. Rushing at the last minute can result in mistakes that would otherwise be caught in a more deliberate process. The minimum time frame for editing should be discussed during the planning stage of the project to minimize the risk of missing the deadline.
Most abstracts require at least some revisions after feedback from the team. It is unusual for an abstract to require no edits, even for experienced and accomplished researchers or teams. For novice researchers, an abstract may go through many revisions before submission. I have had abstracts go through > 10 revisions before submission to ensure they were well written and the results presented clearly. Having a written plan at the beginning and following your process should improve the quality of the initial draft. Much of the background and methods sections can be adapted from the original written study proposal.
Receiving feedback can be challenging for new authors because they often have a lot of time and effort invested in the project. Some projects take years of work before completion, and it can be emotionally charged when the project is criticized. This process is particularly difficult for new investigators and may cause an intense emotional response. It is important to remember that the feedback is intended to improve the work, and the author should try to avoid taking edits and criticism personally. When team members are not constructive in their feedback, it should be addressed professionally by the project leader, principal investigator, or another member of the team. It may be necessary to take 1–2 d to allow time to process negative emotions before making the suggested edits.
Use of Tables and Figures
Tables and figures are allowed by certain meetings as part of an abstract submission. Respiratory Care allows 2 tables or figures total. Tables should be simple and include the major outcomes of the study. Including too many data points will make the table hard to read and distract from the main findings. Figures should also be simple and add to the body of the abstract. A run or control chart from a quality-improvement project is an excellent example of a figure. Pictures or drawings of experimental setups can also be helpful for reviewers and readers. Tables and figures supplement the body of the abstract and do not replace a section of the abstract. For example, in the results section, it is not appropriate to simply write “See Table.” Tables and figures should be called out in the results and not repeat data already reported within the text.
Peer Review of Abstracts
Abstracts are peer reviewed before acceptance. Abstracts submitted to the Respiratory Care Open Forum are reviewed by at least 3 persons in addition to the managing editor and editor in chief. Peer reviewers are selected based on their expertise and are given 3 options: accept, reject, or accept with conditions. Accept with conditions usually indicates that the authors did not note that they had received IRB approval. This issue will result in the authors being contacted by the managing editor for clarification. In contrast to manuscripts, authors are not given the chance to revise abstracts submitted to the Open Forum and reviewers will only have the abstract itself to judge the study, which is why it is important to include all the relevant details. In contrast to a full manuscript, the threshold for acceptance is generally lower for abstracts.
Reasons for Abstract Rejection
Although the acceptance rate for abstracts is higher than for full manuscripts, some abstracts are rejected. Not following instructions is a common reason for rejection, especially if the submitted sections are different than the format used by the conference. The main reasons for rejection put forth by Hess et al4 include the following: an unclear hypothesis or study question, ethics concerns or failure to obtain IRB approval, flawed methodology, no data reported, no statistical analysis of the data, conclusions not supported by the data, and perception of commercial influence. Not getting IRB approval for studies that enroll human subjects automatically results in rejection. Note that the IRB, not the investigator, decides if the study does not require IRB oversight.
Other reasons for rejection could be if it is too similar to the author's previous work or other submitted work. Authors may break a large project into multiple abstracts, but each abstract should be able to stand on its own. Occasionally, peer reviewers will discover that an abstract was presented at another conference or had been previously published, both of which will result in rejection by Respiratory Care. Another potential reason for rejection is unreported conflicts of interest. An abstract will not be rejected if the authors have conflicts but will be rejected if there are unreported conflicts as the ethics lapse occurs when the relationship is not reported. This could relate to industry authors not reporting that they work for the company or an author does not include that the study was funded by a company. Respiratory Care also does not accept abstracts written solely by investigators whose primary job is with industry. Industry authors can be co-authors; however, they need to clearly report their conflicts of interest.
Summary
Getting an abstract accepted at a national meeting is an important milestone for researchers. Having a strong process in place and following the instructions are keys to success. Each section of the abstract requires care to execute clearly. Most abstracts will go through significant edits before submission.
Footnotes
Mr Miller presented a version of this paper at AARC Congress 2022 held November 9–12, 2022 in New Orleans, Louisiana.
Mr Miller is Section Editor for Respiratory Care, received honorarium from Saxe Communications, S2N Health, and Fisher & Paykel.
REFERENCES
- 1. Hess DR. Evidence-based respiratory care. Respir Care 2021;66(7):1105–1119. [DOI] [PubMed] [Google Scholar]
- 2. Hess DR. Research and publication in respiratory care. Respir Care 2023;68(8):1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson PM, Petticrew M, Calnan MW, Nazareth I. Disseminating research findings: what should researchers do? A systematic scoping review of conceptual frameworks. Implement Sci 2010;5(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hess DR, Branson RD, Moore S, Masferrer R. Reflections on the Respiratory Care Open Forum. Respir Care 2018;63(10):1311–1313. [DOI] [PubMed] [Google Scholar]
- 5. Pierson DJ. How to write an abstract that will be accepted for presentation at a national meeting. Respir Care 2004;49(10):1206–1212. [PubMed] [Google Scholar]
- 6. Willis LD. Formulating the research question and framing the hypothesis. Respir Care 2023;68(8):1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller AG, Roberts KJ, Smith BJ, Burr KL, Hinkson CR, Hoerr CA, et al. Prevalence of burnout among respiratory therapists amidst the COVID-19 pandemic. Respir Care 2021;66(11):1639–1648. [DOI] [PubMed] [Google Scholar]
- 8. Miller AG, Wilson MD, Davies JD, Gentile MA, Thalman JJ, MacIntyre NR. Impact of a formal research committee on respiratory therapists' publications. Respir Care 2021;66(8):1229–1233. [DOI] [PubMed] [Google Scholar]
- 9. Miller AG. Getting started in research: the role of mentorship, forming the team, and developing a process. Respir Care 2023;68(8):1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore S. Submitting a manuscript to a scientific journal. Respir Care 2023;68(9):1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tullu MS. Writing the title and abstract for a research paper: being concise, precise, and meticulous is the key. Saudi J Anaesth 2019;13(Suppl 1):S12–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gates RM, Haynes KE, Rehder KJ, Zimmerman KO, Rotta AT, Miller AG. High-flow nasal cannula in pediatric critical asthma. Respir Care 2021;66(8):1240–1246. [DOI] [PubMed] [Google Scholar]
- 13. Miller AG, Haynes KE, Gates RM, Zimmerman KO, Bartlett KW, McLean HS, Rehder KJ. Initial modified pulmonary index score predicts hospital length of stay for asthma subjects admitted to the pediatric intensive care unit. Respir Care 2020;65(9):1227–1232. [DOI] [PubMed] [Google Scholar]
- 14. Burr KL, Hinkson CR, Smith BJ, Roberts KJ, Strickland SL, Hoerr CA, et al. Factors associated with a positive view of respiratory care leadership. Respir Care 2022;67(10):1236–1245. [DOI] [PubMed] [Google Scholar]