Abstract
Aneuploidy—the karyotype state in which the number of chromosomes deviates from a multiple of the haploid chromosome set—is common in cancer, where it is thought to facilitate tumor initiation and progression. However, it is poorly tolerated in healthy cells: during development and tissue homeostasis, aneuploid cells are efficiently cleared from the population. It is still largely unknown how cancer cells become, and adapt to being, aneuploid. P53, the gatekeeper of the genome, has been proposed to guard against aneuploidy. Aneuploidy in cancer genomes strongly correlates with mutations in TP53, and p53 is thought to prevent the propagation of aneuploid cells. Whether p53 also participates in preventing the mistakes in cell division that lead to aneuploidy is still under debate. In this review, we summarize the current understanding of the role of p53 in protecting cells from aneuploidy, and we explore the consequences of functional p53 loss for the propagation of aneuploidy in cancer.
Keywords: Aneuploidy, p53, Cancer, Chromosomal instability
Introduction
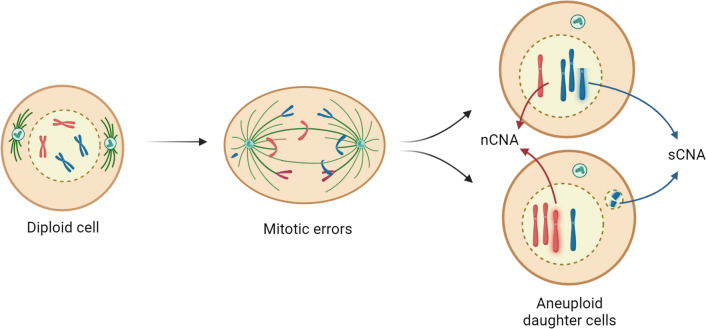
Changes to the genome are rarely tolerated by healthy cells. They possess elaborate mechanisms to prevent accumulation of, for example, single nucleotide substitutions or copy number alterations (CNAs). CNAs can appear in various forms, most notably focal and arm-level CNAs (hereafter referred to as structural CNAs—sCNAs) and whole chromosome CNAs (hereafter referred to as numerical CNAs—nCNAs). sCNAs involve copy number changes to parts of chromosomes, while nCNAs involve gains or losses of entire chromosomes (Fig. 1). CNAs result mainly from errors in chromosome segregation during mitosis or meiosis, creating daughter cells with aneuploid karyotypes (Gordon et al. 2012; Sheltzer and Amon 2011). Aneuploidy—a karyotype state in which the genome deviates from a multiple of the haploid set of chromosomes—is highly prevalent in cancer (sCNA and/or nCNA are present in ~ 90% of tumor samples), where ~ 66% of all tumor samples from The Cancer Genome Atlas (TCGA) dataset carry at least one nCNA (Knouse et al. 2017; Taylor et al. 2018). Aneuploidy in cancer cells is a genomic manifestation of the increased rate at which cells mis-segregate chromosomes, a phenotype known as chromosomal instability (CIN). CIN is prevalent in cancer but its frequencies can vary greatly, even within the same tumor type (Lengauer et al. 1997; Bolhaqueiro et al. 2019). CIN not only leads to aneuploidy but also drives karyotype heterogeneity in tumor cell populations (Shoshani et al. 2021; Sansregret and Swanton 2017; Watkins et al. 2020; Bolhaqueiro et al. 2019). Depending on the degree of CIN, this can result in opposing effects on tumor initiation and progression: while CIN can accelerate tumor evolution by creating favorable karyotypes that increase cancer plasticity, fueling metastasis and therapy resistance (Gerstung et al. 2020; Sansregret and Swanton 2017; Watkins et al. 2020; Ben-David and Amon 2020; Chunduri and Storchová 2019), it can also suppress tumor initiation or progression in several mouse models when induced to high levels (Sheltzer et al. 2017; Godek et al. 2016; Rowald et al. 2016; Silk et al. 2013; Hoevenaar et al. 2020; Zasadil et al. 2016). Selection pressure for CNAs to gain or lose driver oncogenes or tumor suppressor genes, respectively, is one way in which the tumor-promoting effects are achieved (Davoli et al. 2013; Sack et al. 2018; Trakala et al. 2021; Shih et al. 2023). For example, in a lymphoma mouse model, CIN was followed by clonal selection of recurrent CNAs that confer a proliferative advantage (Shoshani et al. 2021; Trakala et al. 2021), including gains of any chromosome (natural or engineered) that carried the c-Myc gene (Trakala et al. 2021). As a result of selection for oncogenic drivers, whole chromosome CNA patterns are context-dependent, being conserved within tumors of a tissue type but divergent between tumors of different tissues (Sack et al. 2018; Hoadley et al. 2018; Ben-David and Amon 2020; Davoli et al. 2013).
Fig. 1.
Aneuploidy: numerical vs. structural CNAs. Aneuploidy can arise from erroneous cell divisions. We define aneuploidy as a karyotype state in which the number of chromosomes deviates from a multiple of the haploid chromosome set. CNAs present in aneuploid cells can come in different flavors: numerical CNAs (nCNAs), which are whole-chromosome gains or losses, and structural CNAs (sCNAs), which are sub-chromosomal gains and losses ranging from arm-level alterations to smaller fractions of the genome (focal alterations)
Intriguingly, while aneuploidy is common in cancer, it is detrimental to cellular fitness (Sheltzer and Amon 2011; Ben-David and Amon 2020) and rare in healthy cells: less than 5% of cells across healthy tissues are aneuploid (Knouse et al. 2014; Chunduri and Storchová 2019). While much is known about how cancer cells circumvent the detrimental effects of mutations, much less is known about how they deal with aneuploidy. A prime candidate to guard against propagation of aneuploid cells is p53, a transcription factor with a central role in ensuring genome stability by responding to various stresses (Levine 2020). Inactivation of the TP53 gene at early stages of tumorigenesis, irrespective of tumor type, emphasizes its broad role as a tumor suppressor (Gerstung et al. 2020). p53 is referred to as the guardian of the genome, and in line with this, strong co-occurrence of aneuploidy and TP53 gene inactivation in cancer suggests that p53 plays a role in guarding against aneuploidy (Taylor et al. 2018; Ciriello et al. 2013). However, the interplay between aneuploidy and p53 in cancer is still poorly understood. In this review, we summarize the current understanding of the role of p53 activation in response to aneuploidy in healthy cells and explore the consequences of functional p53 loss for propagation of aneuploidy in cancer.
TP53 gene mutations and aneuploidy in cancer
Several large-scale cancer genome analyses showed that of all common oncogenic mutations, aneuploidy most strongly correlates with mutations in the TP53 gene (Taylor et al. 2018; Donehower et al. 2019; Hoadley et al. 2014; Ciriello et al. 2013; Davoli et al. 2017; Zack et al. 2013). These analyses were performed on TCGA datasets, using a definition of aneuploidy that included not only numerical CNAs but also arm-level and/or focal CNAs. For most cancer types, occurrence of CNAs correlates with overall mutation burden, with TP53 being the most significantly associated mutant gene (Taylor et al. 2018), and higher prevalence of TP53 mutations correlates with higher frequencies of CNAs (Hoadley et al. 2014). When comparing, within cancer types, samples with mutations in TP53 to those without, CNAs are enriched in the cancers carrying TP53 mutations. This is irrespective of gains or losses (Donehower et al. 2019). Of note, since the definition of aneuploidy used in the analyses included sCNAs, it is unclear whether TP53 mutations correlate with nCNAs. This is potentially relevant since events leading to sCNAs are expected to include DNA breaks and activate a p53 response, while those leading to nCNAs are not necessarily expected to do so. It would thus be of interest to re-assess TP53 mutation status in relation strictly to nCNAs. Interestingly, evidence of whole-genome doubling (WGD)—the duplication of at least 50% of the current set of chromosomes—is seen in 30–40% of all cancers, and it is correlated with prior loss of p53 function in more than half of the cases (Gerstung et al. 2020; Bielski et al. 2018). For example, in TCGA datasets, a subgroup of colorectal cancer tumors was characterized by early loss of p53 and WGD (Kim et al. 2021). WGD is considered an unstable state that is often followed by accumulation of sCNAs and nCNAs (Gerstung et al. 2020). Overall, both TP53 mutations and aneuploidy are prevalent in tumors and show intriguing correlations, which may signal causal links between the two.
The role of p53 in maintaining genome stability
To better understand the correlation between TP53 gene mutations and aneuploidy in cancer, we first look at p53’s role as the guardian of the genome. During interphase, p53 can respond to a plethora of cellular stresses, including DNA damage, oxidative stress, replication stress, and hypoxia (Gambino et al. 2013; Bieging et al. 2014). This response ensures that only cells with an intact genome progress into mitosis, which is widely regarded as a main reason for the tumor suppressor function of p53 (Liebl and Hofmann 2021; Boutelle and Attardi 2021; Janic et al. 2018). The various intricacies of the regulation and function of p53 have been extensively reviewed elsewhere (Kastenhuber and Lowe 2017; Kruiswijk et al. 2015); for the context of this review, we will briefly outline what is known about p53 activation and consequent outcomes in response to an insult.
In healthy cells, p53 is maintained at low levels by interaction with MDM2, which targets p53 for ubiquitin-dependent degradation (Fig. 2). Therefore, activation of p53, for example, in response to DNA damage, is achieved by disrupting this interaction, leading to p53 protein stabilization and regulation of target genes (Chène 2003; Levine 2020) (Fig. 2). DNA breaks activate the DNA damage response mediated by the ATM/CHK2 pathway, culminating in phosphorylation of p53 and MDM2 (Abuetabh et al. 2022; Banin et al. 1998; Chène 2003; Maya et al. 2001). Phosphorylation of p53 and MDM2 lowers the affinity for each other, resulting in p53 stabilization (Banin et al. 1998; Maya et al. 2001) (Fig. 2). The activation of the p53 pathway upon an insult can lead to distinct cell fates: cell cycle arrest, apoptosis, or senescence (Hafner et al. 2019). What determines a specific outcome upon p53 activation in response to, e.g., DNA damage depends on several variables. First, different tissues can express different isoforms of p53, expressed through alternative splicing. Although still poorly understood, different isoforms present distinct functions that in turn affect the outcome of p53 responses (Joruiz and Bourdon 2016). Second, post-translational modifications of the p53 protein, particularly phosphorylation, can significantly affect the degree of protein stability (via MDM2), conformation, localization, or affinity for binding partners (Liu et al. 2019). These modifications can also determine p53 transcriptional activity and consequently the cell fate: for example, while acetylation of p53 at residue K382 increases its activity after sustained DNA damage, a transient source of DNA damage leads to inhibition of p53 activity through methylation at the same residue (Loewer et al. 2010). Likewise, glucose deprivation can lead to G1 cell cycle arrest via p53: MDH1—an enzyme involved in glycolysis and mitochondrial respiration—stabilizes p53 by impinging on p53-MDM2 interaction and triggers post-translational modifications (acetylation and phosphorylation) that determine p53 activation (Lee et al. 2009; Kruiswijk et al. 2015; Wang et al. 2023). Third, p53 temporal dynamics can strongly determine cell fate (Jiménez et al. 2022; Purvis et al. 2012). Pulsatile p53 dynamics govern activation of p53 depending on the level of stress induced: while high levels of DNA damage lead to sustained p53 response and apoptosis, low levels of DNA damage result in a pulsed activation and milder effects, such as cell cycle arrest (Mönke et al. 2017; Purvis et al. 2012). Additionally, p53-dependent cell fate decisions hinge on pulsing activity of upstream and downstream players (Jiménez et al. 2022; Batchelor et al. 2008; Hanson et al. 2019; Paek et al. 2016; Stewart-Ornstein and Lahav 2017).
Fig. 2.
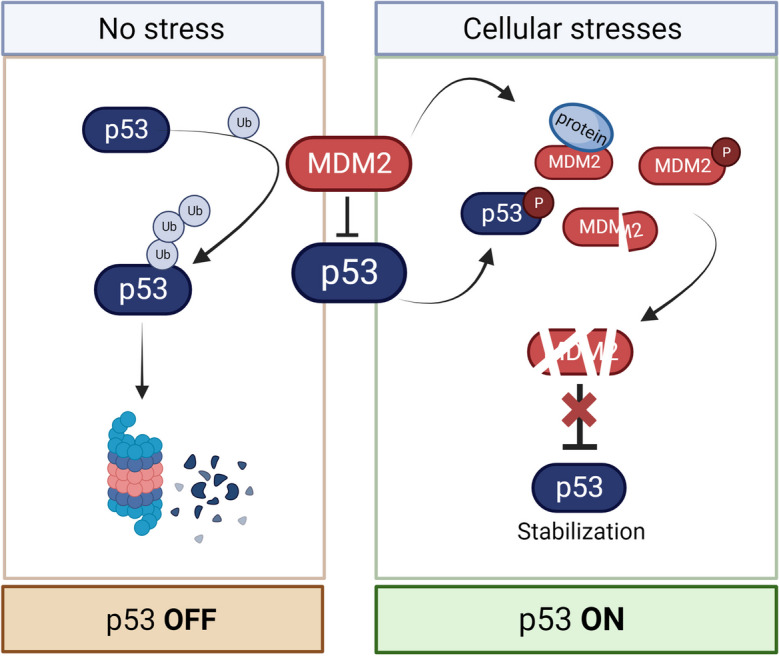
Regulation of p53. In physiological conditions, p53 interacts with its negative regulator MDM2, an E3 ubiquitin-protein ligase, which targets p53 for proteasomal degradation. Upon stress, upstream factors impinge on the p53-MDM2 interaction by inhibiting MDM2 function (e.g., target for degradation, phosphorylation of interaction site, cleavage, inhibition by interaction with other proteins (e.g., LATS1/2 or ribosome biogenesis subunits)). P53 can also be directly targeted, for example, by phosphorylation, to reduce affinity for MDM2. Disruption of MDM2-p53 interaction stabilizes p53 and leads to p53 activation in response to specific insults. Ub, ubiquitin.
To summarize, the cellular stress response pathways commonly impinge on the regulation of the interaction between p53 and MDM2 (Fig. 2), and, although highly complex and not fully understood, their effect on p53 activation and cell fates depend on the level of stress and the context of the affected cell (Vousden and Prives 2009; Mönke et al. 2017).
TP53 mutations: a cause of aneuploidy?
The role of p53 in maintaining genome stability and its frequent inactivation in aneuploid cancers raises the possibility that loss of p53 can cause aneuploidy (Table 1; Fig. 3). In support of this, TP53 knockout human intestinal organoids display CIN (Drost et al. 2015). This may be an indirect effect of the role of p53 in protecting cells from entering mitosis with damaged DNA (Fig. 3) (Bunz et al. 1998). Similarly, deletion of TP53 in an acute myeloid leukemia (AML) cell line leads to CIN and aneuploidy (Cazzola et al. 2019). Recently, human gastric organoids carrying TP53 gene mutations and followed for genome alterations over a 2-year period showed progressive and ordered accumulation of CNAs that are recurrently observed in gastric tumors, establishing a strong causal connection between TP53 mutations and recurrent CNAs in cancer (Karlsson et al. 2023).
Table 1.
P53 loss as a potential cause for aneuploidy
| Model of study | Mode of p53 inactivation | CIN/aneuploidy | Mode of measuring CIN/aneuploidy | Other factors | Citations |
|---|---|---|---|---|---|
| Primary culture of mammary mouse model | p53 + / + ; p53 + / − ; p53 − (LOH); p53 − / − | No | Metaphase spreads | Combination with transgenic Wnt leads to aneuploidy | Donehower et al. (2019) |
| HCT116 (mostly); RKO; DLD1 | p53 shRNA and p73 shRNA | No | IF to detect lagging chromosomes | Combination with loss of p73 caused CIN | Schmidt et al. (2021) |
| ACC-LC-176 near-diploid lung cancer and HCT116 (non-CIN control) | Human papilloma virus 16-E6-directed inactivation of p53 | No | FISH | - | Haruki et al. (2001) |
| HCT116 | p53 + / + ; p53 − / − | No | FISH | Combination with loss of pRB promotes CIN and aneuploidy | Manning et al. (2014) |
| HCT116 and primary fibroblasts | p53 KO by targeted homologous recombination | No (small tendency towards tetraploid) | M-FISH; FISH with centromeric probes | - | Bunz et al. (2002) |
| MEFs and lymphomas (in vivo) | p53 − / − or p53 mutated at 515C (lack induction of apoptosis) | p53 − / − : Yes; p53515C/515: No | Flow cytometric (DNA content); metaphase spreads | Complete loss of p53 leads to centrosome amplification and sCNAs (mostly lymphomas) | Liu et al. (2004) |
| Human intestinal WT organoids | CRISPR-Cas9 mediated p53 KO | CIN: Yes; Aneuploidy: No | CIN: live cell imaging; aneuploidy: metaphase spreads | Combination with loss of APC showed increase in aneuploidy | Drost et al. (2015) |
| 184hTERT diploid breast epithelial cell line | CRISPR-Cas9 mediated p53 KO | Yes (increased heterogeneity and fitness of aneuploid populations) | Whole genome single cell sequencing | - | Salehi et al. (2021) |
| MEFs | Germline p53 − / − | Yes (cytokinesis failure and chromosomal abnormalities) | Live cell imaging; metaphase spreads; flow cytometry; FISH | Combination with loss of Rassf1a leads to stronger increase in ploidy (Loss of p53 alone leads mostly to lymphomas) | Tommasi et al. (2011) |
| Primary MEFs and spleen cells mouse intestinal tissue (in vivo) | Germline p53 − / − | Yes (lagging chromosomes) | Metaphase spreads in MEFs; IF for abnormal mitosis in intestinal tissue sections | Combination with Cenpe + / − | Funk et al. (2021) |
| Single cell suspensions of spleen, bone marrow, and thymus; spleen and skin fibroblasts (in vivo) | Germline p53 − / − | Yes (plus abnormal centrosome amplification) | Metaphase spreads | Combination with c-Myc overexpression | Fukasawa et al. (1997) |
| Pancreatic ductal adenocarcinoma (PDAC) mouse model | Germline p53 + / − (with LOH) | Yes (mostly losses and WGD) | Single-cell genome sequencing; FISH | p53 loss is combined with KrasG12D mutation | Baslan et al. (2022) |
| Lymphomas (in vivo) | Conditional p53 knockout in T-cells (p53 + / − ; p53 − / −) | Yes | aCGH, interphase FISH | Combination of p53 loss with Mps1 inhibition enhanced aneuploidy and tumor development | Foijer et al. (2014) |
| Lymphomas (in vivo) | Germline p53 − / − | Yes (nCNAs) | Metaphase spreads | Loss of p53 alone was enough to induce thymic lymphomas with nCNAs to similar levels of p53 loss combined with reduction of Bub1 protein levels | Baker et al. (2009) |
| Mouse mammary epithelial cells (MMECs); Xenografts models of tetraploid cells (in vivo) | Germline p53 − / − | Yes (diploid and tetraploid p53 − / − MMECs develop nCNA and sCNAs) | Flow cytometry (DNA content); metaphase spreads | WGD was induced by blocking cytokinesis (DCB treatment); diploid p53 − / − cells become spontaneously tetraploid in culture | Fujiwara et al. (2005) |
| Biopsies of patients with BE; BAR-T and BAR-T10 BE patient-derived cell lines | p53 mutations (dysplastic samples); p53 siRNA | Yes (plus abnormal centrosome number) | Centrosome/centriole number and DNA content analysis by IF | - | Lopes et al. (2018) |
Summary of literature that examined the development of aneuploidy upon p53 loss. The table includes the strategies used to induce p53 inactivation, whether aneuploidy was detected when only p53 was lost and methods used to measure aneuploidy. Where relevant, co-occurrence of other factors that contributed to aneuploidy development is noted. LOH, loss of heterozygosity; IF, immunofluorescence; FISH, fluorescence in situ hybridization; M-FISH, multiplex-FISH; CIN, chromosomal instability; MEFs, mouse embryonic fibroblasts; WGD, whole genome doubling; DCB, dihydrocytochalasin-B; aCGH, array comparative genomic hybridization; shRNA, short hairpin RNA; KO, knockout; BE, Barrett’s esophagus; WT, wild type
Fig. 3.
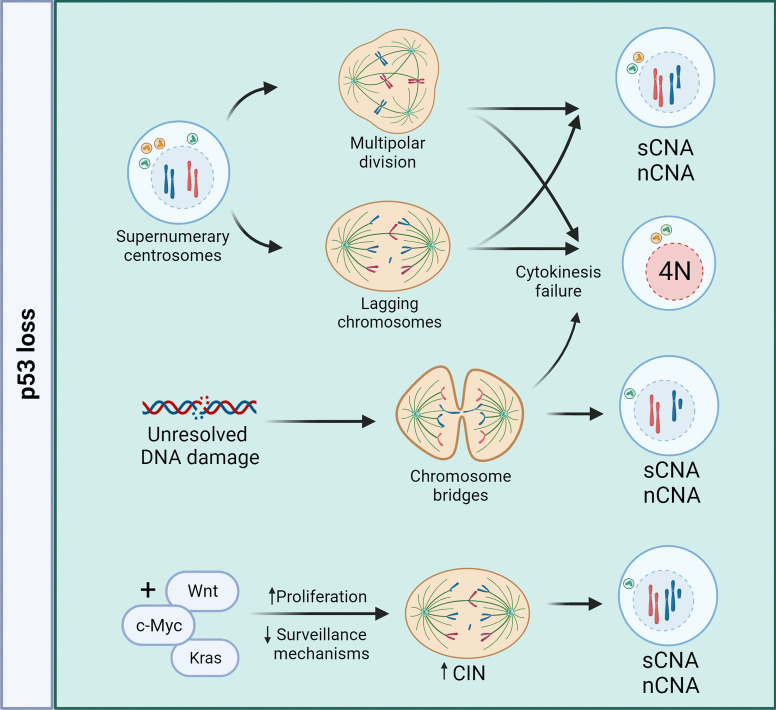
Consequences of p53 loss. Cells that lose p53 function can become aneuploid by various means, including supernumerary centrosomes, DNA damage, and altered proliferation and cellular surveillance mechanisms. Supernumerary centrosomes can result in multipolar spindles and/or lagging chromosomes, causing CNAs and WGD. Unresolved DNA damage can contribute to replication stress and mitotic errors (e.g., chromosome anaphase bridges) likely resulting in structural CNAs (sCNAs) or WGD. The combination of p53 loss with alterations in driver genes affects proliferation and surveillance mechanisms, resulting in aneuploid progeny with sCNA and/or numerical CNAs (nCNA)
How might loss of p53 cause aneuploidy? Mouse embryonic fibroblasts lacking p53 have amplified centrosomes, as a result of misregulated CDK2/cyclin-E compromising the centrosome duplication cycle (Fukasawa et al. 1996; Liu et al. 2004; Tarapore et al. 2001). Interestingly, likely due to a more stringent regulation of cyclin-E, loss of p53 in human non-transformed cells does not lead to centrosome amplification (Kawamura et al. 2004). Yet there is an intriguing correlation between p53 loss and supernumerary centrosomes in human cancers (Lopes et al. 2018; Fukasawa 2005; Chan 2011). Supernumerary centrosomes can lead to CIN by creating multipolar spindles that predispose cells to lagging anaphase chromosomes, resulting in aneuploid progeny (Silkworth et al. 2009; Ganem et al. 2009) (Table 1; Fig. 3). In agreement with this, cells in human TP53 knockout hepatocyte organoids display multipolar spindles (Artegiani et al. 2020). Loss of p53 can also predispose cells to WGD by allowing the survival of cells that underwent cytokinesis failure (Baslan et al. 2022; Fujiwara et al. 2005; Tommasi et al. 2011). An additional mechanism may involve cytokinesis failure as a result of DNA damage-induced anaphase bridges at the cleavage furrow (Andreassen et al. 2001a, b; Bunz et al. 1998; Bakhoum et al. 2017; Janssen et al. 2011) (Table 1; Fig. 3). WGD can further promote CIN: in most cancers with evidence of early WGD, subsequent near-triploidy and extensive CNAs are common (Gerstung et al. 2020; Knouse et al. 2017; Storchova and Pellman 2004; Storchova and Kuffer 2008; Baslan et al. 2022; Dewhurst et al. 2014; Zeng et al. 2023).
Contrasting these observations are studies reporting that TP53 mutations are not sufficient to cause aneuploidy: monolayer cancer or non-transformed cell lines often remain diploid after p53 deletion/inactivation (Simoes-Sousa et al. 2018; Bunz et al. 2002; Haruki et al. 2001). Loss of p53 in HCT116 cells induces CIN only when combined with the loss of p73 (Schmidt et al. 2021) or loss of pRB (Manning et al. 2014), and the CIN seen upon TP53 knockout in human intestinal organoids was not accompanied by a significant increase in aneuploidy (Drost et al. 2015) (Table 1).
What could explain the seemingly contradictory findings about the appearance of aneuploid cells upon p53 inactivation? TP53 knockout in an AML cell line resulted in appearance of aneuploid cells containing both sCNAs and nCNAs (Cazzola et al. 2019). Conversely, cells with functional p53 can accumulate some nCNAs but rarely sCNAs. In line with this, inducing CIN in p53-proficient HCT116 or RPE1 cells resulted in cycling aneuploid cells with mostly whole-chromosome gains (e.g., trisomies), while most whole-chromosome losses and sCNAs could be detected only when p53 was inactivated (Soto et al. 2017; Chunduri et al. 2021). So, like the analysis of cancer genome datasets, the discrepancy may lie in the definition of aneuploidy: even though nCNAs survive better in p53-inactivated cells, it is possible that loss of p53 leads predominantly to sCNAs rather than nCNAs.
In vivo p53-deficient germline mouse models display CIN and aneuploidy in tissues such as lymphoid (thymus, bone marrow, and spleen) (Fukasawa et al. 1997; Funk et al. 2021) and intestine (Funk et al. 2021) (Table 1). Likewise, inducible loss of p53 in mouse esophagus progenitor cells leads to accumulation of giant poly-aneuploid like cells—aneuploid cells that have undergone WGD (Murai et al. 2022). Nevertheless, oncogenic transformation in vivo upon p53 loss happens predominantly in blood cells (lymphomas) and/or connective tissue (sarcomas), and these cancers are aneuploid (Funk et al. 2021; Liu et al. 2004; Tommasi et al. 2011; Chi et al. 2009; Baker et al. 2009; Foijer et al. 2014) (Table 1). Induction of CIN by deleting the spindle assembly checkpoint (SAC) kinases Mps1 or Bub1 in mice heterozygous for functional p53 (p53 + / −) accelerated loss of heterozygosity (p53 − / −) and the development of aneuploid lymphomas (Foijer et al. 2014; Baker et al. 2009) (Table 1). Other in vivo p53 mutant cancer models also show aneuploidy, but loss of p53 was often combined with additional oncogenic events. Examples include transgenic Wnt overexpression (mammary cancer) (Donehower et al. 1995), Kras mutation (pancreatic cancer) (Baslan et al. 2022), or spontaneous c-Myc overexpression (lymphoma) (Fukasawa et al. 1997) (Table 1, Fig. 3). The combination of multiple factors makes it challenging to dissect the contribution of loss of p53 alone to the development of aneuploidy in these tissues.
To summarize, depending on the model and conditions (species, culture or tissue type, additional oncogenic alterations), p53 loss by itself can cause aneuploidy. Whether this is a result of increased CIN or of a higher probability of survival of aneuploid cells in vivo is unclear. Resolving this requires single cell analysis of CIN and aneuploidy in vitro and in vivo upon acute inactivation of p53.
A healthy cell’s response to aneuploidy
TP53 mutations correlate with aneuploidy in cancer, but as outlined earlier, it is unclear whether p53 loss can directly cause chromosome segregation errors. Moreover, loss of functional p53 does not invariably lead to aneuploidy. Instead, loss of p53 might be a permissive characteristic for propagation of aneuploidy. In order to explore this possibility we will first outline how healthy cells respond to aneuploidy.
Aneuploidy-induced stresses
Aneuploidy is rare in healthy cells (Knouse et al. 2014; Chunduri and Storchová 2019). Cells are protected from becoming aneuploid by various cell cycle checkpoints (e.g., replication or chromosome segregation checkpoints) (Chunduri and Storchová 2019; Gaillard et al. 2015; McAinsh and Kops 2023), and if somehow cells escape such checkpoints and become aneuploid, they appear to be eliminated from the population. Immune surveillance can drive the elimination of senescent aneuploid cells, mostly mediated by natural killer cells (Santaguida et al. 2017; Wang et al. 2021). Whether immune cells can recognize specifically aneuploid cells, and if so how, is largely unknown. Aneuploidy induces severe stresses that cause a substantial fitness decline (Sheltzer et al. 2017; Wang et al. 2021; Santaguida et al. 2017; Zhu et al. 2018; Gordon et al. 2012; Sheltzer and Amon 2011; Torres et al. 2007; Williams et al. 2008). As extensively reviewed elsewhere (Chunduri and Storchová 2019; Gordon et al. 2012; Zhu et al. 2018), aneuploidy can cause DNA damage (Janssen et al. 2011; Sheltzer et al. 2011; Passerini et al. 2016), replication stress (Ohashi et al. 2015; Passerini et al. 2016; Garribba et al. 2023), proteotoxic stress (Ohashi et al. 2015; Santaguida and Amon 2015b; Oromendia et al. 2012; Torres et al. 2007), hypo-osmotic stress (Tsai et al. 2019), metabolic stress (Stingele et al. 2012; Foijer et al. 2014), lysosomal stress (Santaguida and Amon 2015a; Santaguida et al. 2015), and an inflammatory response (Santaguida et al. 2017; Wang et al. 2021). These often lead to cell cycle arrest and/or cell death. The mechanisms by which aneuploidy causes these stresses are not completely resolved, but have been elucidated in some cases. Aneuploid cells can accumulate DNA damage as a result of DNA breaks on lagging chromosomes or anaphase bridges, or from mis-segregated chromosomes that end up in micronuclei, which are prone to rupture and exposure of the entrapped chromosome to cytoplasmic exonucleases (Umbreit et al. 2020; Zhang et al. 2015; Janssen et al. 2011; Hatch et al. 2013). Through imbalances in gene content from nCNAs, aneuploidy can affect transcription and translation (Chunduri and Storchová 2019), leading to, for example, a shortage of replication factors (such as MCM2–7) that in turn induce replication stress and cause DNA damage in under-replicated regions (Passerini et al. 2016; Garribba et al. 2023). Although dosage compensation mechanisms exist in aneuploid cells to negate the protein imbalances that result from chromosome gains (Stingele et al. 2012), they suffer from proteotoxic stress: imbalances in protein complex stoichiometries put a strain on protein folding and degradation machineries, leading to aggregation of misfolded proteins in the cytoplasm (Chunduri and Storchová 2019). Interestingly, aneuploidy-induced stresses are not the same for all types of CNAs, given that, for example, monosomies do not induce proteotoxic stress (Chunduri et al. 2021). Therefore, further efforts are needed to elucidate the mechanisms by which CNAs trigger the aforementioned stress responses.
p53 pathway response to aneuploidy
The overlap in stresses that arise from aneuploidy and those that activate p53 raises the question whether p53 is activated in response to aneuploidy and hence may impact the fate of aneuploid cells. Indeed, several studies have shown that p53 can be activated in response to CIN and/or aneuploidy (Table 2). This can occur regardless of the strategy used to induce CIN. For example, disrupting the SAC, alone or in combination with disrupting chromosome congression (by inhibiting MPS1), the master kinase of the SAC, − / + the mitotic kinesin CENP-E) in RPE-1 or HCT116 cells leads to sCNAs and nCNAs followed by p53 activation and consequent cell cycle arrest (Santaguida et al. 2017; Soto et al. 2017; Narkar et al. 2021; Janssen et al. 2011; Simoes-Sousa et al. 2018; Li et al. 2010) (Table 2). Similarly, induction of WGD in RPE-1 cells also causes cell cycle arrest mediated by p53 (Crockford et al. 2017; Ganem et al. 2014; Gemble et al. 2022), and mitotic arrest-and-release strategies induce p53 activation after mis-segregation events and result in cell cycle arrest (Thompson and Compton 2010; Dalton et al. 2010). P53 activation has also been observed in cell lines derived from mouse models of CIN (Li et al. 2010; Silk et al. 2013). However, aneuploidy does not invariably lead to p53 activation in monolayer cultures. For example, certain nCNAs or low levels of aneuploidy can propagate in p53-proficient RPE-1 cells (Soto et al. 2017; Santaguida et al. 2017), and even high aneuploidy levels can lead to cell cycle arrest in a p53-independent manner (Santaguida et al. 2017).
Table 2.
p53 responses to aneuploidy
| Cell type/mouse genotype | Mode of CIN/aneuploidy induction | CIN/aneuploidy phenotype | DNA damage | p53 activation | Mode of measuring p53 activation | Citations |
|---|---|---|---|---|---|---|
| EEB (transformed acute myeloid leukemia cell line) |
Spontaneous; MPS1ia + CENP-Eib |
p53 WT cells: nCNAs | Yes | No (only tested in cycling aneuploid cells with nCNAs) | Western blot | Cazzola et al. (2019) |
| 2D: RPE-1, HCT116; Nalm6 (suspension cell line) | MPS1ia; nocodazole | MPS1i: nCNAs; nocodazole: multipolar divisions | No | Yes | Western blot; IF | Narkar et al. (2021) |
| 3D: hMO and mCO | No | Western blot | ||||
| RPE-1 cells | MPS1ia,c + CENP-Eib | sCNAs and nCNAs; micronucleus formation | NA | Yes (in ~ 16% of cells) | IF | Soto et al. (2017) |
| RPE-1 cells | MPS1ia,d | Arrested cells: genomic imbalances involving more than 20% of their genomes (sCNA in 47% of cells and also nCNAs) | Yes | Yes (50% of arrested cells) | Western blot; IF | Santaguida et al. (2017) |
| Cycling cells: genomic imbalances involving less than 5% of their genomes (sCNA in 18% of cells and also nCNAs) | Inconclusive | No | ||||
| HCT116 | MPS1ie; MPS1ie + CENP-Eib to minimize DNA damage | Mostly nCNAs; 4N | No | Yes | Western blot; time-lapse imaging of GFP-p53 fusion biosensor | Simoes-Sousa et al. (2018) |
| RPE-1 cells | Cytokinesis failure: siRNA mediated KD of anillin; Chemical inhibition: Aurora Bif; mitotic arrest with colcemid | WGD | No (only in micronuclei) | Yes | Real-time qPCR and Western blot | Potapova et al. (2016) |
| RPE-1 cells | MPS1id | All cells became aneuploid (mostly nCNAs and a small fraction of sCNAs) | Yes | Yes | RNA sequencing | (Garribba et al. 2023) |
| RPE-1 cells | DCB; siRNA-mediated depletion of ECT2; Aurora Big | WGD | No | Yes | IF and Western blot | Ganem et al. (2014) |
| HCT116 and IMR90 | Mitotic arrest (Nocodazole) | Mitotic slippage and increase in ploidy | Yes | Yes | IF | Dalton et al. (2010) |
| HCT116 | Monastrol washout strategy; MAPK siRNA | Monastrol washout strategy—33% whole chromosomes; MAPK siRNA—33% lagging chromosomes | No | Yes | IF and Western blot | Thompson and Compton (2010) |
| HCT116 | MPS1id and siRNA screening | Chromosome gains | - | Yes | Western blot | Lopez-Garcia et al. (2017) |
| MEFS derived from Cdc20+/AAA mice; HCT116 | MEFs: Cdc20 with deficient binding for Mad2 (impaired SAC); HCT116: siRNA depletion of MAD2, BUBR1, and CENP-E | FISH for p53 high cells: aneuploidy 5% for chromosome 4 and 10% for chromosome 10 | No | Yes (mediated by ROS) | IF and Western blot | Li et al. (2010) |
| RPE-1; U2OS; MCF7; SW480 and BJs | Monastrol washout strategy; MPS1ic | Mostly lagging chromosomes | Yes | Yes | Western blot | Janssen et al. (2011) |
| RPE-1, BJ and, HCT116 | Cyclin B1, actin or cyclin A2 depletion | WGD | Yes | Yes | Western blot | Gemble et al. (2022) |
| RPE-1 cells overexpressing cyclin D1; spontaneous 4N HCT116 cells | DCB | WGD | No (absence of p53 phosphorylated in Ser15) | Yes | Western blot | Crockford et al. (2017) |
Summary of literature investigating p53 activation upon induction of aneuploidy along with the methods used to induce aneuploidy and to measure p53 activation. Detection of DNA damage is also included. mCO, mouse colon organoids; hMO, human mammary organoids; MEFs, mouse embryonic fibroblasts; ROS, reactive oxygen species; DCB, dihydrocytochalasin-B; IF, immunofluorescence; siRNA, small interfering RNA; WT, wild type
aMPS1 inhibitor NMS-P715
bCENP-E inhibitor GSK923295
cMPS1 inhibitor Cpd-5
dMPS1 inhibitor Reversine
eMPS1 inhibitor AZ3146
fAurora B inhibitor ZM447439
gAurora B inhibitor hesperadin
Additionally, a recent study using 3D cultures further challenged the hypothesis that p53 is activated in response to aneuploidy (Table 2). While p53 activation and cell cycle arrest upon chromosome mis-segregation was seen in monolayer cell cultures, no p53 activation was seen in either mouse colon or human mammary 3D organoid cultures (Narkar et al. 2021). In this study, chromosome segregation errors in the organoids were not accompanied by DNA damage, which may in part explain the lack of a p53 response and is in line with the requirement for p53 inactivation in the accumulation of sCNAs, as described earlier. Another possibility is that, since tissue architecture was proposed to be essential for chromosome segregation fidelity (Knouse et al. 2018), this may also somehow impact the p53 response. However, growing HCT116 cells in 3D did not alter the p53 response (Narkar et al. 2021), although it is debatable to what extent such cancer cells grown in 3D recapitulate tissue architecture. A point of consideration is the components of the medium used for organoid culture that might affect cellular responses: in human organoid lines specifically, p38 inhibitors are often used which might inhibit aneuploidy-induced stress response mediated by p38–p53 activation (Simoes-Sousa et al. 2018). Nonetheless, the 3D culture study emphasizes the importance of examining whether p53 is activated in response to aneuploidy in vivo. To our knowledge, while p53 activation has been detected in fibroblasts derived from mouse cancer models of CIN/aneuploidy, it has not been examined directly in vivo or in tissues of such models. Interestingly, p53 activation and elimination of aneuploid cells in mouse brains upon induction of CIN were reported in the context of mouse embryonic development (Shi et al. 2019).
Taken together, the inconsistent observations of a p53 response to aneuploidy in monolayer cultures and the lack of p53 response observed in 3D organoid cultures expose a gap in our understanding of the mechanisms by which cells respond to and surpass aneuploidy-induced stresses and the role of p53 in this.
The mechanisms of p53 activation by aneuploidy
The observation that aneuploidy, at least in monolayer cultures, often results in p53 activation raises the question of the underlying mechanism. We envision two possibilities: either p53 senses aneuploidy per se or it senses the stresses that result from aneuploidy.
Direct sensing of aneuploidy by p53
The first possibility is supported by a study reporting that cells sense lagging or misaligned chromosomes by marking them with phosphorylation of histone 3 (H3.3) on serine at position 31 (Ser31), which in turn activates p53 by an unknown mechanism (Hinchcliffe et al. 2016). Ser31 of H3.3 can be phosphorylated by the DNA damage response kinase CHK-1 in cancer cells that rely on the alternative lengthening of telomeres (ALT) pathway (Chang et al. 2015), although in the aforementioned study p53 activation from H3.3. Ser31 phosphorylation was apparently independent of DNA damage (Hinchcliffe et al. 2016). Alternatively, H3.3 Ser31 can be phosphorylated by the mitotic kinase Aurora B (Li et al. 2017), which is active at the cell’s midzone during anaphase and telophase (Fuller et al. 2008) and as such might mark a lagging chromosome. Paradoxically, however, one study showed that Aurora B can phosphorylate p53 at centromeres, resulting in accelerated p53 degradation (Gully et al. 2012). It thus remains unclear how H3.3-Ser31 is phosphorylated on mis-segregated chromosomes or how that impacts p53, and no subsequent studies have addressed this. P53 has also been reported to respond directly to mitotic defects that are connected to prolonged mitosis: a pool of p53 (phosphorylated at Ser15) located at centrosomes is released in the cytoplasm upon centrosome fragmentation during mitosis and recruits 53BP1, that in turn activates a mitotic surveillance pathway composed of 53BP1 and USP28 (Contadini et al. 2019). Similarly, a study proposed the existence of a “stopwatch” composed of USP28, 53BP1, and p53 that limits the proliferation of daughter cells that arise after a prolonged mitosis (Meitinger et al. 2016). A new pre-print study suggests that this “stopwatch” is a result of gradual MDM2 degradation that eventually leads to p53 activation in the following G1 (Fulcher et al. 2023). p53 has also been proposed to participate in a “tetraploidy checkpoint” in G1, able to detect and limit the proliferation of tetraploid cells. This concept resulted from the observation that newly formed tetraploid cells (generated by chemical induction of cytokinesis failure) would arrest in G1 in a p53-dependent manner (Andreassen et al. 2001a, b). However, the existence of such a checkpoint was challenged by other studies showing that G1 cell cycle arrest was not an obligatory outcome in tetraploid cells (Uetake and Sluder 2004; Wong and Stearns 2005). Taken together, the evidence for p53 as a direct sensor of the aneuploid state is currently thin.
Indirect sensing of aneuploidy by p53
In the second possibility, p53 senses the stresses that result from aneuploidy (Fig. 4). The observation that low levels of aneuploidy do not activate p53 but higher aneuploidy levels do lead to p53 activation, cell cycle arrest, and senescence supports this notion (Li et al. 2010; Santaguida et al. 2017). Although there is currently little evidence for it, cells with low CIN levels in vivo may escape p53 surveillance mechanisms, while those with high CIN levels may elicit a p53-mediated apoptosis response, with differential outcomes on tumorigenesis (Li et al. 2010). If indeed p53 is activated in response to aneuploidy-induced stresses, there is likely a threshold for p53 activation upon a mis-segregation event (Santaguida et al. 2017; Soto et al. 2017), much like the thresholds proposed to dictate p53 response dynamics and cell fates upon DNA damage (Mönke et al. 2017; Loewer et al. 2010; Paek et al. 2016). A “just right” level of aneuploidy might then be sufficiently high to promote tumorigenesis while being sufficiently low to allow escape from p53-mediated cell cycle arrest or apoptosis.
Fig. 4.
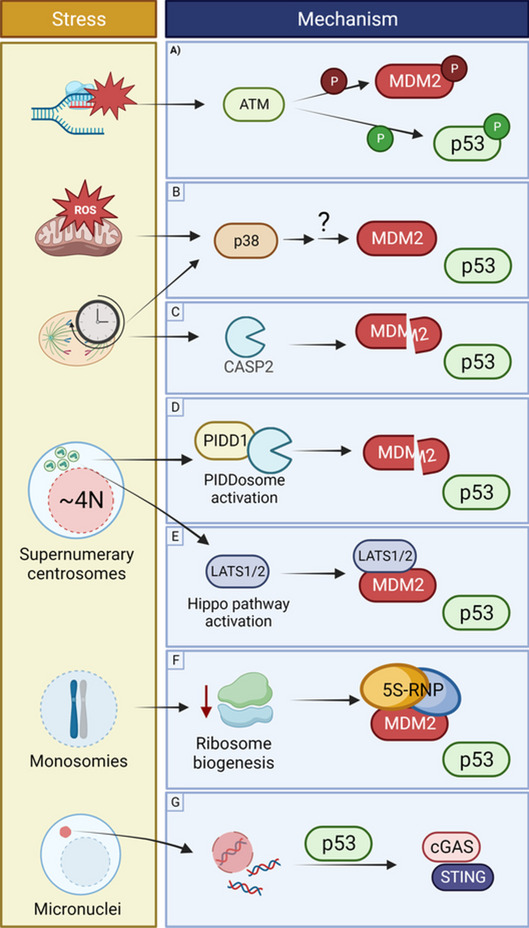
P53 activation in response to aneuploidy-induced stresses. Aneuploidy-induced stress responses often impinge on p53 activation by disrupting p53-MDM2 interaction. A Replication stress and DNA damage activate ATM, which can directly phosphorylate MDM2 or p53. B Metabolic stress and prolonged mitosis can trigger a p38 stress response, which indirectly targets MDM2 for degradation. C Prolonged mitosis can also activate CASP2, which can cleave MDM2. (D) Supernumerary centrosomes, often co-occurring with WGD, can trigger the PIDDosome (mediated by CASP2) or (E) the Hippo pathway: LATS1/2 binds MDM2 and reduces its affinity for p53. (F) Monosomies cause deficient ribosome biogenesis, which creates imbalances in ribosomal proteins, some of which then interact with MDM2 and reduce its affinity for p53. Although poorly understood, (G) the presence of cytoplasmatic DNA triggers p53 activation which in turn mediates the activation of the cytoplasmatic DNA sensing machinery (cGAS/STING) for immune activation
As said, there is substantial overlap between stresses known to activate p53 and those elicited by aneuploidy. Are all of them part of the mechanism by which aneuploidy triggers p53 activation, or do some contribute more than others? This question is largely unanswered. The clearest link between aneuploidy-induced stress and p53 activation is again DNA damage, as described earlier (Fig. 4). CIN and WGD commonly cause DNA damage with ensuing p53 activation (Janssen et al. 2011; Santaguida et al. 2017; Soto et al. 2017; Dalton et al. 2010; Krzywicka-Racka and Sluder 2011; Kuffer et al. 2013; Ganem et al. 2014; Gemble et al. 2022). The fact that DNA damage underlies sCNAs (Janssen et al. 2011; Li et al. 2010; Santaguida et al. 2017) and that sCNAs are eliminated in p53-proficient cells (Soto et al. 2017; Dalton et al. 2010; Cazzola et al. 2019) suggests that aneuploidy-associated DNA damage, at least in part, explains the p53 response to aneuploidy. In fact, loss of p53 or DNA damage repair genes (e.g., MLH1 and MSH2) lead to accumulation of sCNAs and single nucleotide alterations, suggesting that p53 acts mostly through the DNA damage response to prevent propagation of sCNAs (Janic et al. 2018). Although likely, for most of the other aneuploidy-related stresses like metabolic stress, replication stress, hypo-osmotic stress, proteotoxic stress, and autophagy stress, there is currently no evidence that they are a crucial intermediate between aneuploidy and p53. Examining this requires assessment of p53 activity (dynamics) following aneuploidy after specific elimination of one type of stress. This would also help understanding how p53 limits the survival of cells that acquire nCNAs, since nCNAs, in contrast to sCNAs, are less likely to accumulate DNA breaks. Of note, for one type of nCNA the mode of p53 activation seems resolved: monosomies are incompatible with functional p53. This is likely due to defects in ribosome biogenesis (Chunduri et al. 2021). Because ribosomal genes are spread across the genome, monosomies cause imbalances in ribosomal protein complexes, some of which can interact with MDM2 and activate the p53 response (Lindström et al. 2022) (Fig. 4). Some DNA damage-independent mechanisms have been proposed to link CNAs to p53 activation (Fig. 4). p38 works side by side with p53 to limit progression of cells in response to stress stimuli. Upon mis-segregation events, p38 causes apoptosis, at least in part through p53 stabilization and suppression of HIF-1α (a master regulator of the hypoxia response) (Simoes-Sousa et al. 2018). P38 has been shown to indirectly target MDM2 (unknown mechanism) leading to p53 stabilization (Zhang et al. 2021) (Fig. 4). Similarly, a postmitotic stress response by p38–p53 can trigger p53-dependent cell cycle arrest in G1 after prolonged mitosis or mitotic slippage without proper cytokinesis (Uetake and Sluder 2018; Vogel et al. 2004). In Drosophila, the p38–p53 axis participates in the response to metabolic stress induced by ROS formation as a consequence of CIN (Clemente-Ruiz et al. 2016). Recently, p53 was linked to the activation of the cytosolic DNA-sensing cGAS/STING pathway (Ghosh et al. 2023). Micronuclei formed after a mis-segregation event are prone to rupture, leading to accumulation of cytosolic DNA that can trigger cGAS/STING activation (Kwon et al. 2020) (Fig. 4). In the presence of cytosolic DNA, p53 induces the degradation of the exonuclease TREX1 (a DNA degrading enzyme), leading to the accumulation of cytosolic DNA and consequent detection by the sGAS/STING pathway (Ghosh et al. 2023). P53 can also limit proliferation of cells with supernumerary centrosomes after WGD (Ganem et al. 2009; Darp et al. 2022) through activation of the Hippo pathway (Ganem et al. 2014) or the PIDDosome (a caspase-2 activator) (Ganem et al. 2009; Fava et al. 2017) (Fig. 4). Activation of CASP2 (caspase-2) in response to CNAs, mitotic delay or DNA damage during mitosis, causes MDM2 cleavage, p53 stabilization, and thus aneuploid cell clearance through mitotic cell death (Fig. 4) (Dawar et al. 2017; Lopez-Garcia et al. 2017; Castedo et al. 2004; Lim et al. 2021). In colorectal cancer cell lines, repression of CASP2 activity by loss of BCL9L (a component of Wnt signaling pathway) allowed survival of CIN cells (Lopez-Garcia et al. 2017). In summary, several mechanisms can lead to p53 activation upon a mis-segregation event in vitro. However, more studies are necessary to clarify both the mechanisms of activation of p53 and the outcomes thereof in response to aneuploidy in 3D and in vivo.
Conclusions and future outlook
P53 participates in a complex network of cellular responses to diverse stresses. As we discuss in this review, many of these stresses overlap with the ones induced by aneuploidy. It is clear that p53 activation is a recurrent outcome of aneuploidy but not an obligatory one as, for example, nCNAs can propagate in p53 proficient cells. Nevertheless, loss of p53 creates a more permissive context for proliferation of aneuploid cells when compared to p53 proficient counterparts (Salehi et al. 2021; Adell et al. 2023; Fujiwara et al. 2005). Therefore, there is still much to learn about how exactly aneuploidy triggers p53 and how p53 limits survival of aneuploid cells (Fig. 5). Some outstanding questions remain: first, does p53 get activated in response to aneuploidy in vivo? The fact that loss of p53 in vivo promotes survival of aneuploid cells suggests a role for p53, but direct evidence for p53 activation in response to aneuploidy in vivo is scarce. Given the absence of p53 response in mouse- and human-derived 3D organoid cultures, it will be important to examine direct p53 activation in aneuploid cells in 3D organoid cultures and validate it in in vivo models of CIN/aneuploidy. Second, it is currently unknown whether, and if so how, different types of mitotic errors (e.g., lagging chromosomes, chromatin bridges, supernumerary centrosomes, micronuclei) trigger p53 activation. A major distinction seems to be between errors that lead to sCNAs vs. nCNAs: whereas nCNAs are still tolerated to some extent in cells with functional p53, sCNAs often arise in p53-deficient cells. It will therefore be very informative to re-assess the correlation between TP53 mutations and nCNAs. Third, it is still largely elusive whether p53 can work as a direct sensor for aneuploidy. Can p53, for example, be directly activated by a mis-segregating chromosome? Or is it perhaps more indirectly activated when a threshold is exceeded of the fraction of the genome that is altered (e.g., when more than 20% of the genome is gained or lost)? It could be of added value to use directed whole chromosome mis-segregation strategies such as KaryoCreate (Bosco et al. 2023) to explore whether p53 responds to a threshold of mis-segregation events (e.g., gene content, fraction of genome altered), in the absence of DNA damage. Lastly, it is also unresolved how different types of aneuploidy-induced stresses result in p53 activation. A direct link has been established between DNA damage responses and occurrence of sCNAs, but no other stresses were individually tested as mandatory intermediates for p53 activation in aneuploid cells. Understanding what mechanisms limit proliferation of aneuploid cells can open up therapeutic opportunities to target aneuploid tumor cells.
Fig. 5.
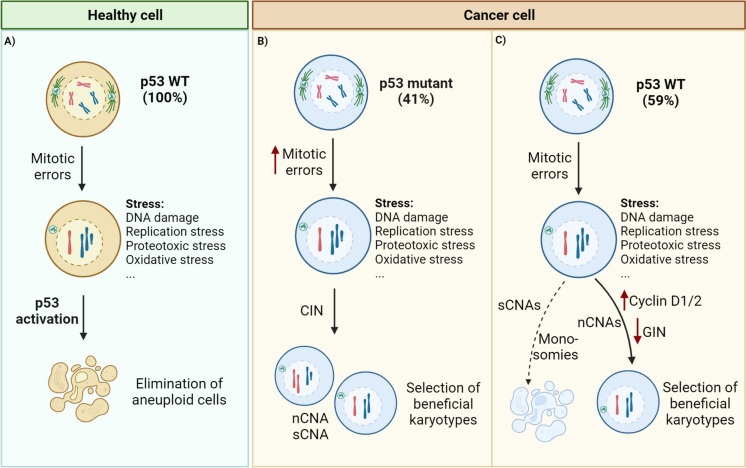
P53 as a gatekeeper for aneuploidy. In healthy cells (A), aneuploidy-induced stresses activate a p53 response, which prevents the propagation of aneuploid cells and causes their cell cycle arrest or death. In cancer cells, however, aneuploidy as well as p53 mutations are highly prevalent. In p53 mutant cancer cells (B) (~ 41% with TP53 gene mutations) (Hoadley et al. 2014), aneuploid cells can proliferate with low/moderate levels of aneuploidy. Propagation of replication stress, for example, can induce DNA damage and consequently further promote CIN. This leads to increased heterogeneity, which enables selection of optimal karyotypes. Approximately 59% of tumors are WT for p53 (C). In these tumors, aneuploidy can still be propagated by either developing mechanisms to surpass p53 activation (e.g., cyclin D1/2 upregulation in WGD) or by upregulating mechanisms to prevent accumulation of genomic instability (GIN) (e.g., upregulate DNA damage response pathways). As a result, such cells are more likely to accumulate numerical CNAs (nCNAs), mostly gains, while cells with structural CNAs (sCNAs) and/or monosomies are probably eliminated or overtaken by cells with more beneficial karyotypes
Most consequences of aneuploidy seem to impinge on the p53 pathway. However, p53 alterations are present at a pan-cancer frequency of approximately 41% while aneuploidy is present in ~ 90% of solid tumors, as obtained from TCGA database (Hoadley et al. 2014; Gerstung et al. 2020). This suggests that p53-proficient cells can propagate aneuploidy by either overcoming p53 activation or through p53-independent mechanisms (Fig. 5). For example, tetraploid human tumors (47% of which are wild-type for p53) (Crockford et al. 2017) can upregulate cyclin D1/2 which in turn sequesters p21, a downstream effector of p53, resulting in continued proliferation (Potapova et al. 2016; Crockford et al. 2017). Likewise, loss of BRG1, part of a chromatin remodeling complex, also seems to overcome p53 activation in aneuploid cells by upregulating cyclin D1 (Schiavoni et al. 2022). In another example, upregulation of HIF-1α can inhibit post-mitotic apoptosis and potentiate tolerance to aneuploidy in a p53-independent manner (Simoes-Sousa et al. 2018). Even if p53 itself is intact, alterations to components of the p53 network can also allow cells to overcome p53 pathway activation. For example, CDKN2A (encoding for p21) is a known cancer driver, frequently altered at early stages of tumor development (Donehower et al. 2019; Gerstung et al. 2020). Additionally, selection of sCNAs or nCNAs that affect genes involved in the p53 network can be another way by which aneuploid cells surpass p53 activation. In line with this, trisomy of chromosome 1q, recurrently present in cancer, leads to overexpression of MDM4 that in turn inhibits p53 function (Girish et al. 2023). Interestingly, gain of chromosome 1q was shown to be mutually exclusive with mutations in TP53. Likewise, deletion of CDKN2A and amplification of MDM2 are mutually exclusive with TP53 alterations in glioblastoma multiforme cancers (Donehower et al. 2019). Finally, since aneuploid cells can trigger further genomic instability (Sheltzer et al. 2011; Passerini et al. 2016), another way to adapt to aneuploidy might be by preventing accumulation of stresses (Clemente-Ruiz et al. 2016; Clarke et al. 2022). For example, aneuploid cells can improve DNA replication by activating DDK-mediated firing and mitotic DNA synthesis (MIDAS), in order to cope with replication stress and maintain mitotic fidelity (Garribba et al. 2023). It will be of great interest to examine in detail the general response to aneuploidy in p53 proficient cells and the pathways that such cells use to overcome aneuploidy’s detrimental effects. Given the prevalence of aneuploidy in cancer, such insights will likely reveal new targetable vulnerabilities of aneuploid cells.
Acknowledgements
We thank all members of the Kops group for the critical reading of the manuscript and the reviewers for constructive comments. All figures were created with Biorender.com.
Abbreviations
- 53BP1
P53-binding protein 1
- aCGH
Array comparative genomic hybridization
- ALT
Alternative lengthening of telomeres
- AML
Acute myeloid leukemia
- ATM
Ataxia-telangiectasia mutated
- BCL9L
B-cell CLL/lymphoma 9 ligand
- BE
Barrett’s esophagus
- BRG1
Brahma-related gene-1
- BUB1
Budding uninhibited by benzimidazoles 1
- CDK2
Cyclin-dependent kinase 2
- CENP-E
Centromere protein E
- cGAS/STING
Cyclic GMP–AMP synthase/stimulator of interferon genes
- CHK1/2
Checkpoint kinase 1/2
- CIN
Chromosomal instability
- CNA
Copy number alterations
- DCB
Dihydrocytochalasin-B
- DDK
DBF4-dependent kinase
- FISH
Fluorescence in situ hybridization
- GIN
Genomic instability
- HIF-1α
Hypoxia inducible factor 1 subunit alpha
- hMO
Human mammary organoids
- IF
Immunofluorescence
- KO
Knockout
- LATS1/2
Large tumor suppressor 1/2
- LOH
Loss of heterozygosity
- mCO
Mouse colon organoids
- MDH1
Nucleocytoplasmic malate dehydrogenase-1
- MDM2
Murine double minute 2
- MDM4
Murine double minute 4
- MEFs
Mouse embryonic fibroblasts
- M-FISH
Multiplex fluorescence in situ hybridization
- MIDAS
Mitotic DNA synthesis
- nCNA
Numerical copy number alterations
- P53
Tumor protein P53
- ROS
Reactive oxygen species
- SAC
Spindle assembly checkpoint
- sCNA
Structural copy number alterations
- shRNA
Short hairpin RNA
- siRNA
Small interfering RNA
- TCGA
The Cancer Genome Atlas
- TP53
Tumor protein 53 gene
- TREX1
Three prime repair exonuclease 1
- USP28
Ubiquitin specific peptidase 28
- WGD
Whole-genome doubling
- WT
Wild-type
Author contribution
Conceptualization and initial draft preparation, J.F.M.; review, editing, discussions, and suggestions, J.F.M. and G.J.P.L.K.; supervision and funding acquisition, G.J.P.L.K. All authors reviewed the manuscript.
Funding
This study was funded by the Dutch Cancer Society (KWF Kankerbestrijding) (no. KWF 12728).
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abuetabh Y, Wu HH, Chai C, Al Yousef H, Persad S, Sergi CM, Leng R. DNA damage response revisited: the p53 family and its regulators provide endless cancer therapy opportunities. Exp Mol Med. 2022;54:1658–1669. doi: 10.1038/s12276-022-00863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell MAY, Klockner TC, Höfler R, Wallner L, Schmid J, Markovic A, Martyniak A, Campbell CS. Adaptation to spindle assembly checkpoint inhibition through the selection of specific aneuploidies. Genes Dev. 2023;37(5–6):171–190. doi: 10.1101/gad.350182.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen PR, Lacroix FB, Lohez OD, Margolis RL. Neither p21WAF1 nor 14-3-3sigma prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001;61:7660–7668. [PubMed] [Google Scholar]
- Andreassen PR, Lohez OD, Lacroix FB, Margolis RL. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell. 2001;12:1315–1328. doi: 10.1091/mbc.12.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B, Hendriks D, Beumer J, Kok R, Zheng X, Joore I, Chuva de Sousa Lopes SC, van Zon J, Tans S, Clevers H. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR-Cas9 precision genome editing. Nat Cell Biol. 2020;22:321–31. doi: 10.1038/s41556-020-0472-5. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Jin F, Jeganathan KB, van Deursen JM. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–486. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Kabeche L, Compton DA, Powell SN, Bastians H. Mitotic DNA damage response: at the crossroads of structural and numerical cancer chromosome instabilities. Trends Cancer. 2017;3:225–234. doi: 10.1016/j.trecan.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- Baslan T, th Morris JP, Zhao Z, Reyes J, Ho YJ, Tsanov KM, Bermeo J, Tian S, Zhang S, Askan G, Yavas A, Lecomte N, Erakky A, Varghese AM, Zhang A, Kendall J, Ghiban E, Chorbadjiev L, Wu J, Dimitrova N, Chadalavada K, Nanjangud GJ, Bandlamudi C, Gong Y, Donoghue MTA, Socci ND, Krasnitz A, Notta F, Leach SD, Iacobuzio-Donahue CA, Lowe SW. Ordered and deterministic cancer genome evolution after p53 loss. Nature. 2022;608:795–802. doi: 10.1038/s41586-022-05082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U, Amon A. Context is everything: aneuploidy in cancer. Nat Rev Genet. 2020;21:44–62. doi: 10.1038/s41576-019-0171-x. [DOI] [PubMed] [Google Scholar]
- Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielski CM, Zehir A, Penson AV, Donoghue MTA, Chatila W, Armenia J, Chang MT, Schram AM, Jonsson P, Bandlamudi C, Razavi P, Iyer G, Robson ME, Stadler ZK, Schultz N, Baselga J, Solit DB, Hyman DM, Berger MF, Taylor BS. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet. 2018;50:1189–1195. doi: 10.1038/s41588-018-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhaqueiro ACF, Ponsioen B, Bakker B, Klaasen SJ, Kucukkose E, van Jaarsveld RH, Vivié J, Verlaan-Klink I, Hami N, Spierings DCJ, Sasaki N, Dutta D, Boj SF, Vries RGJ, Lansdorp PM, van de Wetering M, van Oudenaarden A, Clevers H, Kranenburg O, Foijer F, Snippert HJG, Kops G. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat Genet. 2019;51:824–834. doi: 10.1038/s41588-019-0399-6. [DOI] [PubMed] [Google Scholar]
- Bosco N, Goldberg A, Zhao X, Mays JC, Cheng P, Johnson AF, Bianchi JJ, Toscani C, Di Tommaso E, Katsnelson L, Annuar D, Mei S, Faitelson RE, Pesselev IY, Mohamed KS, Mermerian A, Camacho-Hernandez EM, Gionco CA, Manikas J, Tseng YS, Sun Z, Fani S, Keegan S, Lippman SM, Fenyö D, Giunta S, Santaguida S, Davoli T. KaryoCreate: A CRISPR-based technology to study chromosome-specific aneuploidy by targeting human centromeres. Cell. 2023;186:1985–2001.e19. doi: 10.1016/j.cell.2023.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31:298–310. doi: 10.1016/j.tcb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Bunz F, Fauth C, Speicher MR, Dutriaux A, Sedivy JM, Kinzler KW, Vogelstein B, Lengauer C. Targeted inactivation of p53 in human cells does not result in aneuploidy. Cancer Res. 2002;62:1129–1133. [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Valent A, Raslova H, Yakushijin K, Horne D, Feunteun J, Lenoir G, Medema R, Vainchenker W, Kroemer G. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene. 2004;23:4362–4370. doi: 10.1038/sj.onc.1207572. [DOI] [PubMed] [Google Scholar]
- Cazzola A, Schlegel C, Jansen I, Bochtler T, Jauch A, Krämer A. TP53 deficiency permits chromosome abnormalities and karyotype heterogeneity in acute myeloid leukemia. Leukemia. 2019;33:2619–2627. doi: 10.1038/s41375-019-0550-5. [DOI] [PubMed] [Google Scholar]
- Chan JY. A clinical overview of centrosome amplification in human cancers. Int J Biol Sci. 2011;7:1122–1144. doi: 10.7150/ijbs.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FT, Chan FL, McGhie JDR, Udugama M, Mayne L, Collas P, Mann JR, Wong LH. CHK1-driven histone H3.3 serine 31 phosphorylation is important for chromatin maintenance and cell survival in human ALT cancer cells. Nucleic Acids Res. 2015;43:2603–14. doi: 10.1093/nar/gkv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chène P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102–109. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- Chi YH, Ward JM, Cheng LI, Yasunaga J, Jeang KT. Spindle assembly checkpoint and p53 deficiencies cooperate for tumorigenesis in mice. Int J Cancer. 2009;124:1483–1489. doi: 10.1002/ijc.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunduri NK, Storchová Z. The diverse consequences of aneuploidy. Nat Cell Biol. 2019;21:54–62. doi: 10.1038/s41556-018-0243-8. [DOI] [PubMed] [Google Scholar]
- Chunduri NK, Menges P, Zhang X, Wieland A, Gotsmann VL, Mardin BR, Buccitelli C, Korbel JO, Willmund F, Kschischo M, Raeschle M, Storchova Z. Systems approaches identify the consequences of monosomy in somatic human cells. Nat Commun. 2021;12:5576. doi: 10.1038/s41467-021-25288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45:1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MN, Marsoner T, Adell MAY, Ravichandran MC, Campbell CS (2022) Adaptation to high rates of chromosomal instability and aneuploidy through multiple pathways in budding yeast’. Embo J 42(8):e111500 [DOI] [PMC free article] [PubMed]
- Clemente-Ruiz M, Murillo-Maldonado JM, Benhra N, Barrio L, Pérez L, Quiroga G, Nebreda AR, Milán M. Gene dosage imbalance contributes to chromosomal instability-induced tumorigenesis. Dev Cell. 2016;36:290–302. doi: 10.1016/j.devcel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Contadini C, Monteonofrio L, Virdia I, Prodosmo A, Valente D, Chessa L, Musio A, Fava LL, Rinaldo C, Di Rocco G, Soddu S. p53 mitotic centrosome localization preserves centrosome integrity and works as sensor for the mitotic surveillance pathway. Cell Death Dis. 2019;10:850. doi: 10.1038/s41419-019-2076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford A, Zalmas LP, Grönroos E, Dewhurst SM, McGranahan N, Cuomo ME, Encheva V, Snijders AP, Begum J, Purewal S, Cerveira J, Patel H, Renshaw MJ, Swanton C. Cyclin D mediates tolerance of genome-doubling in cancers with functional p53. Ann Oncol. 2017;28:149–156. doi: 10.1093/annonc/mdw612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton WB, Yu B, Yang VW. p53 suppresses structural chromosome instability after mitotic arrest in human cells. Oncogene. 2010;29:1929–1940. doi: 10.1038/onc.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darp R, Vittoria MA, Ganem NJ, Ceol CJ. Oncogenic BRAF induces whole-genome doubling through suppression of cytokinesis. Nat Commun. 2022;13:4109. doi: 10.1038/s41467-022-31899-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, Xu AW, Mengwasser KE, Sack LM, Yoon JC, Park PJ, Elledge SJ. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell. 2013;155:948–962. doi: 10.1016/j.cell.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355(6322):eaaf8399. doi: 10.1126/science.aaf8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawar S, Lim Y, Puccini J, White M, Thomas P, Bouchier-Hayes L, Green DR, Dorstyn L, Kumar S. Caspase-2-mediated cell death is required for deleting aneuploid cells. Oncogene. 2017;36:2704–2714. doi: 10.1038/onc.2016.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst SM, McGranahan N, Burrell RA, Rowan AJ, Gronroos E, Endesfelder D, Joshi T, Mouradov D, Gibbs P, Ward RL, Hawkins NJ, Szallasi Z, Sieber OM, Swanton C. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discov. 2014;4:175–185. doi: 10.1158/2159-8290.CD-13-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Godley LA, Aldaz CM, Pyle R, Shi YP, Pinkel D, Gray J, Bradley A, Medina D, Varmus HE. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 1995;9:882–895. doi: 10.1101/gad.9.7.882. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, Li X, Babur O, Hsu TK, Lichtarge O, Weinstein JN, Akbani R, Wheeler DA. Integrated analysis of TP53 gene and pathway alterations in The Cancer Genome Atlas. Cell Rep. 2019;28:3010. doi: 10.1016/j.celrep.2019.08.061. [DOI] [PubMed] [Google Scholar]
- Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, Sachs N, Overmeer RM, Offerhaus GJ, Begthel H, Korving J, van de Wetering M, Schwank G, Logtenberg M, Cuppen E, Snippert HJ, Medema JP, Kops GJ, Clevers H. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- Fava LL, Schuler F, Sladky V, Haschka MD, Soratroi C, Eiterer L, Demetz E, Weiss G, Geley S, Nigg EA, Villunger A. The PIDDosome activates p53 in response to supernumerary centrosomes. Genes Dev. 2017;31:34–45. doi: 10.1101/gad.289728.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foijer F, Xie SZ, Simon JE, Bakker PL, Conte N, Davis SH, Kregel E, Jonkers J, Bradley A, Sorger PK. Chromosome instability induced by Mps1 and p53 mutation generates aggressive lymphomas exhibiting aneuploidy-induced stress. Proc Natl Acad Sci U S A. 2014;111:13427–13432. doi: 10.1073/pnas.1400892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Fukasawa K, Wiener F, Vande Woude GF, Mai S. Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene. 1997;15:1295–1302. doi: 10.1038/sj.onc.1201482. [DOI] [PubMed] [Google Scholar]
- Fulcher LJ, Sobajima T, Gibbs-Seymour I, Barr FA (2023) MDM2 acts as a timer reporting the length of mitosis: 2023.05.26.542398
- Fuller BG, Lampson MA, Foley EA, Rosasco-Nitcher S, Le KV, Tobelmann P, Brautigan DL, Stukenberg PT, Kapoor TM. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk LC, Wan J, Ryan SD, Kaur C, Sullivan R, Roopra A, Weaver BA. p53 is not required for high CIN to induce tumor suppression. Mol Cancer Res. 2021;19:112–123. doi: 10.1158/1541-7786.MCR-20-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard H, García-Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15:276–289. doi: 10.1038/nrc3916. [DOI] [PubMed] [Google Scholar]
- Gambino V, De Michele G, Venezia O, Migliaccio P, Dall’Olio V, Bernard L, Minardi SP, Dellafazia MA, Bartoli D, Servillo G, Alcalay M, Luzi L, Giorgio M, Scrable H, Pelicci PG, Migliaccio E. ‘Oxidative stress activates a specific p53 transcriptional response that regulates cellular senescence and aging. Aging Cell. 2013;12:435–45. doi: 10.1111/acel.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Cornils H, Chiu SY, O’Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garribba L, De Feudis G, Martis V, Galli M, Dumont M, Eliezer Y, Wardenaar R, Ippolito MR, Iyer DR, Tijhuis AE, Spierings DCJ, Schubert M, Taglietti S, Soriani C, Gemble S, Basto R, Rhind N, Foijer F, Ben-David U, Fachinetti D, Doksani Y, Santaguida S. Short-term molecular consequences of chromosome mis-segregation for genome stability. Nat Commun. 2023;14:1353. doi: 10.1038/s41467-023-37095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemble S, Wardenaar R, Keuper K, Srivastava N, Nano M, Macé AS, Tijhuis AE, Bernhard SV, Spierings DCJ, Simon A, Goundiam O, Hochegger H, Piel M, Foijer F, Storchová Z, Basto R. Genetic instability from a single S phase after whole-genome duplication. Nature. 2022;604:146–151. doi: 10.1038/s41586-022-04578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, Mitchell TJ, Rubanova Y, Anur P, Yu K, Tarabichi M, Deshwar A, Wintersinger J, Kleinheinz K, Vázquez-García I, Haase K, Jerman L, Sengupta S, Macintyre G, Malikic S, Donmez N, Livitz DG, Cmero M, Demeulemeester J, Schumacher S, Fan Y, Yao X, Lee J, Schlesner M, Boutros PC, Bowtell DD, Zhu H, Getz G, Imielinski M, Beroukhim R, Sahinalp SC, Ji Y, Peifer M, Markowetz F, Mustonen V, Yuan K, Wang W, Morris QD, Spellman PT, Wedge DC, Van Loo P. The evolutionary history of 2,658 cancers. Nature. 2020;578:122–128. doi: 10.1038/s41586-019-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Saha S, Li J, Montrose DC, Martinez LA. p53 engages the cGAS/STING cytosolic DNA sensing pathway for tumor suppression. Mol Cell. 2023;83:266–80.e6. doi: 10.1016/j.molcel.2022.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girish V, Lakhani AA, Thompson SL, Scaduto CM, Brown LM, Hagenson RA, Sausville EL, Mendelson BE, Kandikuppa PK, Lukow DA, Yuan ML, Stevens EC, Lee SN, Schukken KM, Akalu SM, Vasudevan A, Zou C, Salovska B, Li W, Smith JC, Taylor AM, Martienssen RA, Liu Y, Sun R, Sheltzer JM. Oncogene-like addiction to aneuploidy in human cancers. Science. 2023;381(6660):eadg4521. doi: 10.1126/science.adg4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godek KM, Venere M, Wu Q, Mills KD, Hickey WF, Rich JN, Compton DA. Chromosomal instability affects the tumorigenicity of glioblastoma tumor-initiating cells. Cancer Discov. 2016;6:532–545. doi: 10.1158/2159-8290.CD-15-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13:189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- Gully CP, Velazquez-Torres G, Shin JH, Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams HP, Choi HH, Guma S, Phan L, Chou PC, Su CH, Zhang F, Chen JS, Yang TY, Yeung SC, Lee MH. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci U S A. 2012;109:E1513–E1522. doi: 10.1073/pnas.1110287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20:199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- Hanson RL, Porter JR, Batchelor E. Protein stability of p53 targets determines their temporal expression dynamics in response to p53 pulsing. J Cell Biol. 2019;218:1282–1297. doi: 10.1083/jcb.201803063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki N, Harano T, Masuda A, Kiyono T, Takahashi T, Tatematsu Y, Shimizu S, Mitsudomi T, Konishi H, Osada H, Fujii Y, Takahashi T. Persistent increase in chromosome instability in lung cancer: possible indirect involvement of p53 inactivation. Am J Pathol. 2001;159:1345–1352. doi: 10.1016/S0002-9440(10)62521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47–60. doi: 10.1016/j.cell.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe EH, Day CA, Karanjeet KB, Fadness S, Langfald A, Vaughan KT, Dong Z. Chromosome missegregation during anaphase triggers p53 cell cycle arrest through histone H3.3 Ser31 phosphorylation. Nat Cell Biol. 2016;18:668. doi: 10.1038/ncb3348. [DOI] [PubMed] [Google Scholar]
- Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MDM, Niu B, McLellan MD, Uzunangelov V, Zhang J, Kandoth C, Akbani R, Shen H, Omberg L, Chu A, Margolin AA, Van’t Veer LJ, Lopez-Bigas N, Laird PW, Raphael BJ, Ding L, Robertson AG, Byers LA, Mills GB, Weinstein JN, Van Waes C, Chen Z, Collisson EA, Benz CC, Perou CM, Stuart JM. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929–44. doi: 10.1016/j.cell.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, Akbani R, Bowlby R, Wong CK, Wiznerowicz M, Sanchez-Vega F, Robertson AG, Schneider BG, Lawrence MS, Noushmehr H, Malta TM, Stuart JM, Benz CC, Laird PW. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173:291–304.e6. doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoevenaar WHM, Janssen A, Quirindongo AI, Ma H, Klaasen SJ, Teixeira A, van Gerwen B, Lansu N, Morsink FHM, Offerhaus GJA, Medema RH, Kops G, Jelluma N. Degree and site of chromosomal instability define its oncogenic potential. Nat Commun. 2020;11:1501. doi: 10.1038/s41467-020-15279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janic A, Valente LJ, Wakefield MJ, Di Stefano L, Milla L, Wilcox S, Yang H, Tai L, Vandenberg CJ, Kueh AJ, Mizutani S, Brennan MS, Schenk RL, Lindqvist LM, Papenfuss AT, O’Connor L, Strasser A, Herold MJ. DNA repair processes are critical mediators of p53-dependent tumor suppression. Nat Med. 2018;24:947–953. doi: 10.1038/s41591-018-0043-5. [DOI] [PubMed] [Google Scholar]
- Janssen A, van der Burg M, Szuhai K, Kops GJ, Medema RH. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science. 2011;333:1895–1898. doi: 10.1126/science.1210214. [DOI] [PubMed] [Google Scholar]
- Jiménez A, Lu D, Kalocsay M, Berberich MJ, Balbi P, Jambhekar A, Lahav G. Time-series transcriptomics and proteomics reveal alternative modes to decode p53 oscillations. Mol Syst Biol. 2022;18:e10588. doi: 10.15252/msb.202110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joruiz SM, Bourdon JC (2016) p53 isoforms: key regulators of the cell fate decision. Cold Spring Harb Perspect Med 6 [DOI] [PMC free article] [PubMed]
- Karlsson K, Przybilla MJ, Kotler E, Khan A, Xu H, Karagyozova K, Sockell A, Wong WH, Liu K, Mah A, Lo YH, Lu B, Houlahan KE, Ma Z, Suarez CJ, Barnes CP, Kuo CJ, Curtis C. Deterministic evolution and stringent selection during preneoplasia. Nature. 2023;618:383–393. doi: 10.1038/s41586-023-06102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062–1078. doi: 10.1016/j.cell.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Izumi H, Ma Z, Ikeda R, Moriyama M, Tanaka T, Nojima T, Levin LS, Fujikawa-Yamamoto K, Suzuki K, Fukasawa K. Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression. Cancer Res. 2004;64:4800–4809. doi: 10.1158/0008-5472.CAN-03-3908. [DOI] [PubMed] [Google Scholar]
- Kim JE, Choi J, Sung CO, Hong YS, Kim SY, Lee H, Kim TW, Kim JI. High prevalence of TP53 loss and whole-genome doubling in early-onset colorectal cancer. Exp Mol Med. 2021;53:446–456. doi: 10.1038/s12276-021-00583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse KA, Wu J, Whittaker CA, Amon A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci U S A. 2014;111:13409–13414. doi: 10.1073/pnas.1415287111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse KA, Lopez KE, Bachofner M, Amon A. Chromosome segregation fidelity in epithelia requires tissue architecture. Cell. 2018;175:200–11.e13. doi: 10.1016/j.cell.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouse KA, Davoli T, Elledge SJ, Amon A (2017) Aneuploidy in cancer: seq-ing answers to old questions 1:335–54
- Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- Krzywicka-Racka A, Sluder G. Repeated cleavage failure does not establish centrosome amplification in untransformed human cells. J Cell Biol. 2011;194:199–207. doi: 10.1083/jcb.201101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffer C, Kuznetsova AY, Storchová Z. Abnormal mitosis triggers p53-dependent cell cycle arrest in human tetraploid cells. Chromosoma. 2013;122:305–318. doi: 10.1007/s00412-013-0414-0. [DOI] [PubMed] [Google Scholar]
- Kwon M, Leibowitz ML, Lee JH. Small but mighty: the causes and consequences of micronucleus rupture. Exp Mol Med. 2020;52:1777–1786. doi: 10.1038/s12276-020-00529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kim JH, Cho EJ, Youn HD. A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differ. 2009;16:738–748. doi: 10.1038/cdd.2009.5. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53: 800 million years of evolution and 40 years of discovery. Nat Rev Cancer. 2020;20:471–480. doi: 10.1038/s41568-020-0262-1. [DOI] [PubMed] [Google Scholar]
- Li M, Fang X, Baker DJ, Guo L, Gao X, Wei Z, Han S, van Deursen JM, Zhang P. The ATM-p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:14188–14193. doi: 10.1073/pnas.1005960107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Dong Q, Zhu B. Aurora kinase B phosphorylates histone H3.3 at serine 31 during mitosis in mammalian cells. J Mol Biol. 2017;429:2042–2045. doi: 10.1016/j.jmb.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Liebl MC, Hofmann TG (2021) The role of p53 signaling in colorectal cancer. Cancers (Basel) 13 [DOI] [PMC free article] [PubMed]
- Lim Y, Dorstyn L, Kumar S. The p53-caspase-2 axis in the cell cycle and DNA damage response. Exp Mol Med. 2021;53:517–527. doi: 10.1038/s12276-021-00590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström MS, Bartek J, Maya-Mendoza A. p53 at the crossroad of DNA replication and ribosome biogenesis stress pathways. Cell Death Differ. 2022;29:972–982. doi: 10.1038/s41418-022-00999-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tavana O, Gu W. p53 modifications: exquisite decorations of the powerful guardian. J Mol Cell Biol. 2019;11:564–577. doi: 10.1093/jmcb/mjz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewer A, Batchelor E, Gaglia G, Lahav G. Basal dynamics of p53 reveal transcriptionally attenuated pulses in cycling cells. Cell. 2010;142:89–100. doi: 10.1016/j.cell.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CAM, Mesquita M, Cunha AI, Cardoso J, Carapeta S, Laranjeira C, Pinto AE, Pereira-Leal JB, Dias-Pereira A, Bettencourt-Dias M, Chaves P. Centrosome amplification arises before neoplasia and increases upon p53 loss in tumorigenesis. J Cell Biol. 2018;217:2353–2363. doi: 10.1083/jcb.201711191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Sansregret L, Domingo E, McGranahan N, Hobor S, Birkbak NJ, Horswell S, Gronroos E, Favero F, Rowan AJ, Matthews N, Begum S, Phillimore B, Burrell R, Oukrif D, Spencer-Dene B, Kovac M, Stamp G, Stewart A, Danielsen H, Novelli M, Tomlinson I, Swanton C. BCL9L dysfunction impairs caspase-2 expression permitting aneuploidy tolerance in colorectal cancer. Cancer Cell. 2017;31:79–93. doi: 10.1016/j.ccell.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AL, Benes C, Dyson NJ. Whole chromosome instability resulting from the synergistic effects of pRB and p53 inactivation. Oncogene. 2014;33:2487–2494. doi: 10.1038/onc.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, Moas M, Buschmann T, Ronai Z, Shiloh Y, Kastan MB, Katzir E, Oren M. ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001;15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh AD, Kops GJPL. Principles and dynamics of spindle assembly checkpoint signalling. Nat Rev Mol Cell Biol. 2023;24(8):543–559. doi: 10.1038/s41580-023-00593-z. [DOI] [PubMed] [Google Scholar]
- Meitinger F, Anzola JV, Kaulich M, Richardson A, Stender JD, Benner C, Glass CK, Dowdy SF, Desai A, Shiau AK, Oegema K. 53BP1 and USP28 mediate p53 activation and G1 arrest after centrosome loss or extended mitotic duration. J Cell Biol. 2016;214:155–166. doi: 10.1083/jcb.201604081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mönke G, Cristiano E, Finzel A, Friedrich D, Herzel H, Falcke M, Loewer A. Excitability in the p53 network mediates robust signaling with tunable activation thresholds in single cells. Sci Rep. 2017;7:46571. doi: 10.1038/srep46571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai K, Dentro S, Ong SH, Sood R, Fernandez-Antoran D, Herms A, Kostiou V, Abnizova I, Hall BA, Gerstung M, Jones PH. p53 mutation in normal esophagus promotes multiple stages of carcinogenesis but is constrained by clonal competition. Nat Commun. 2022;13:6206. doi: 10.1038/s41467-022-33945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar A, Johnson BA, Bharne P, Zhu J, Padmanaban V, Biswas D, Fraser A, Iglesias PA, Ewald AJ, Li R. On the role of p53 in the cellular response to aneuploidy. Cell Rep. 2021;34:108892. doi: 10.1016/j.celrep.2021.108892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi A, Ohori M, Iwai K, Nakayama Y, Nambu T, Morishita D, Kawamoto T, Miyamoto M, Hirayama T, Okaniwa M, Banno H, Ishikawa T, Kandori H, Iwata K. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat Commun. 2015;6:7668. doi: 10.1038/ncomms8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26:2696–2708. doi: 10.1101/gad.207407.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek AL, Liu JC, Loewer A, Forrester WC, Lahav G. Cell-to-cell variation in p53 dynamics leads to fractional killing. Cell. 2016;165:631–642. doi: 10.1016/j.cell.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini V, Ozeri-Galai E, de Pagter MS, Donnelly N, Schmalbrock S, Kloosterman WP, Kerem B, Storchová Z. The presence of extra chromosomes leads to genomic instability. Nat Commun. 2016;7:10754. doi: 10.1038/ncomms10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova TA, Seidel CW, Box AC, Rancati G, Li R. Transcriptome analysis of tetraploid cells identifies cyclin D2 as a facilitator of adaptation to genome doubling in the presence of p53. Mol Biol Cell. 2016;27:3065–3084. doi: 10.1091/mbc.e16-05-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowald K, Mantovan M, Passos J, Buccitelli C, Mardin BR, Korbel JO, Jechlinger M, Sotillo R. Negative selection and chromosome instability induced by mad2 overexpression delay breast cancer but facilitate oncogene-independent outgrowth. Cell Rep. 2016;15:2679–2691. doi: 10.1016/j.celrep.2016.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack LM, Davoli T, Li MZ, Li Y, Xu Q, Naxerova K, Wooten EC, Bernardi RJ, Martin TD, Chen T, Leng Y, Liang AC, Scorsone KA, Westbrook TF, Wong KK, Elledge SJ. Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns. Cell. 2018;173:499–514.e23. doi: 10.1016/j.cell.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S, Kabeer F, Ceglia N, Andronescu M, Williams MJ, Campbell KR, Masud T, Wang B, Biele J, Brimhall J, Gee D, Lee H, Ting J, Zhang AW, Tran H, O’Flanagan C, Dorri F, Rusk N, de Algara TR, Lee SR, Cheng BYC, Eirew P, Kono T, Pham J, Grewal D, Lai D, Moore R, Mungall AJ, Marra MA, McPherson A, Bouchard-Côté A, Aparicio S, Shah SP. Clonal fitness inferred from time-series modelling of single-cell cancer genomes. Nature. 2021;595:585–590. doi: 10.1038/s41586-021-03648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L, Swanton C (2017) The role of aneuploidy in cancer evolution. Cold Spring Harb Perspect Med 7(1):a028373 [DOI] [PMC free article] [PubMed]
- Santaguida S, Amon A. Aneuploidy triggers a TFEB-mediated lysosomal stress response. Autophagy. 2015;11:2383–2384. doi: 10.1080/15548627.2015.1110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Amon A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat Rev Mol Cell Biol. 2015;16:473–485. doi: 10.1038/nrm4025. [DOI] [PubMed] [Google Scholar]
- Santaguida S, Vasile E, White E, Amon A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 2015;29:2010–2021. doi: 10.1101/gad.269118.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Richardson A, Iyer DR, M’Saad O, Zasadil L, Knouse KA, Wong YL, Rhind N, Desai A, Amon A. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev Cell. 2017;41:638–51.e5. doi: 10.1016/j.devcel.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni F, Zuazua-Villar P, Roumeliotis TI, Benstead-Hume G, Pardo M, Pearl FMG, Choudhary JS, Downs JA. Aneuploidy tolerance caused by BRG1 loss allows chromosome gains and recovery of fitness. Nat Commun. 2022;13:1731. doi: 10.1038/s41467-022-29420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AK, Pudelko K, Boekenkamp JE, Berger K, Kschischo M, Bastians H. The p53/p73 - p21(CIP1) tumor suppressor axis guards against chromosomal instability by restraining CDK1 in human cancer cells. Oncogene. 2021;40:436–451. doi: 10.1038/s41388-020-01524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Amon A. The aneuploidy paradox: costs and benefits of an incorrect karyotype. Trends Genet. 2011;27:446–453. doi: 10.1016/j.tig.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Blank HM, Pfau SJ, Tange Y, George BM, Humpton TJ, Brito IL, Hiraoka Y, Niwa O, Amon A. Aneuploidy drives genomic instability in yeast. Science. 2011;333:1026–1030. doi: 10.1126/science.1206412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheltzer JM, Ko JH, Replogle JM, Habibe Burgos NC, Chung ES, Meehl CM, Sayles NM, Passerini V, Storchova Z, Amon A. Single-chromosome gains commonly function as tumor suppressors. Cancer Cell. 2017;31:240–255. doi: 10.1016/j.ccell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Qalieh A, Lam MM, Keil JM, Kwan KY. Robust elimination of genome-damaged cells safeguards against brain somatic aneuploidy following Knl1 deletion. Nat Commun. 2019;10:2588. doi: 10.1038/s41467-019-10411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J, Sarmashghi S, Zhakula-Kostadinova N, Zhang S, Georgis Y, Hoyt SH, Cuoco MS, Gao GF, Spurr LF, Berger AC, Ha G, Rendo V, Shen H, Meyerson M, Cherniack AD, Taylor AM, Beroukhim R. Cancer aneuploidies are shaped primarily by effects on tumour fitness. Nature. 2023;619:793–800. doi: 10.1038/s41586-023-06266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani O, Bakker B, de Haan L, Tijhuis AE, Wang Y, Kim DH, Maldonado M, Demarest MA, Artates J, Zhengyu O, Mark A, Wardenaar R, Sasik R, Spierings DCJ, Vitre B, Fisch K, Foijer F, Cleveland DW. Transient genomic instability drives tumorigenesis through accelerated clonal evolution. Genes Dev. 2021;35:1093–1108. doi: 10.1101/gad.348319.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk AD, Zasadil LM, Holland AJ, Vitre B, Cleveland DW, Weaver BA. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc Natl Acad Sci USA. 2013;110:E4134–E4141. doi: 10.1073/pnas.1317042110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Sousa S, Littler S, Thompson SL, Minshall P, Whalley H, Bakker B, Belkot K, Moralli D, Bronder D, Tighe A, Spierings DCJ, Bah N, Graham J, Nelson L, Green CM, Foijer F, Townsend PA, Taylor SS. The p38alpha stress kinase suppresses aneuploidy tolerance by inhibiting Hif-1alpha. Cell Rep. 2018;25(749–60):e6. doi: 10.1016/j.celrep.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M, Raaijmakers JA, Bakker B, Spierings DCJ, Lansdorp PM, Foijer F, Medema RH. p53 prohibits propagation of chromosome segregation errors that produce structural aneuploidies. Cell Rep. 2017;19:2423–2431. doi: 10.1016/j.celrep.2017.05.055. [DOI] [PubMed] [Google Scholar]
- Stewart-Ornstein J, Lahav G (2017) p53 dynamics in response to DNA damage vary across cell lines and are shaped by efficiency of DNA repair and activity of the kinase ATM. Sci Signal [DOI] [PMC free article] [PubMed]
- Stingele S, Stoehr G, Peplowska K, Cox J, Mann M, Storchova Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol. 2012;8:608. doi: 10.1038/msb.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121:3859–3866. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- Tarapore P, Horn HF, Tokuyama Y, Fukasawa K. Direct regulation of the centrosome duplication cycle by the p53–p21Waf1/Cip1 pathway. Oncogene. 2001;20:3173–3184. doi: 10.1038/sj.onc.1204424. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, Schumacher SE, Wang C, Hu H, Liu J, Lazar AJ, Cherniack AD, Beroukhim R, Meyerson M. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell. 2018;33:676–89.e3. doi: 10.1016/j.ccell.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Sarah L, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188(3):369–81. doi: 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi S, Besaratinia A, Wilczynski SP, Pfeifer GP. Loss of Rassf1a enhances p53-mediated tumor predisposition and accelerates progression to aneuploidy. Oncogene. 2011;30:690–700. doi: 10.1038/onc.2010.440. [DOI] [PubMed] [Google Scholar]
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- Trakala M, Aggarwal M, Sniffen C, Zasadil L, Carroll A, Ma D, Su XA, Wangsa D, Meyer A, Sieben CJ, Zhong J, Hsu PH, Paradis G, Ried T, Holland A, Van Deursen J, Amon A. Clonal selection of stable aneuploidies in progenitor cells drives high-prevalence tumorigenesis. Genes Dev. 2021;35:1079–1092. doi: 10.1101/gad.348341.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Nelliat AR, Choudhury MI, Kucharavy A, Bradford WD, Cook ME, Kim J, Mair DB, Sun SX, Schatz MC, Li R. Hypo-osmotic-like stress underlies general cellular defects of aneuploidy. Nature. 2019;570:117–121. doi: 10.1038/s41586-019-1187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. ‘Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a "tetraploidy checkpoint"‘. J Cell Biol. 2004;165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Activation of the apoptotic pathway during prolonged prometaphase blocks daughter cell proliferation. Mol Biol Cell. 2018;29:2632–2643. doi: 10.1091/mbc.E18-01-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit NT, Zhang TJ, Lynch, Blaine LJ, Cheng AM, Tourdot R, Sun L, Almubarak HF, Judge K, Mitchell TJ, Spektor A, Pellman D. Mechanisms generating cancer genome complexity from a single cell division error. Science . 2020;368(6488):eaba0712. doi: 10.1126/science.aba0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C, Kienitz A, Hofmann I, Müller R, Bastians H. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene. 2004;23:6845–6853. doi: 10.1038/sj.onc.1207860. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wang RW, Viganò S, Ben-David U, Amon A, Santaguida S. Aneuploid senescent cells activate NF-κB to promote their immune clearance by NK cells. EMBO Rep. 2021;22:e52032. doi: 10.15252/embr.202052032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo M, Wei H, Chen Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 2023;8:92. doi: 10.1038/s41392-023-01347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TBK, Lim EL, Petkovic M, Elizalde S, Birkbak NJ, Wilson GA, Moore DA, Grönroos E, Rowan A, Dewhurst SM, Demeulemeester J, Dentro SC, Horswell S, Au L, Haase K, Escudero M, Rosenthal R, Bakir MA, Xu H, Litchfield K, Lu WT, Mourikis TP, Dietzen M, Spain L, Cresswell GD, Biswas D, Lamy P, Nordentoft I, Harbst K, Castro-Giner F, Yates LR, Caramia F, Jaulin F, Vicier C, Tomlinson IPM, Brastianos PK, Cho RJ, Bastian BC, Dyrskjøt L, Jönsson GB, Savas P, Loi S, Campbell PJ, Andre F, Luscombe NM, Steeghs N, Tjan-Heijnen VCG, Szallasi Z, Turajlic S, Jamal-Hanjani M, Van Loo P, Bakhoum SF, Schwarz RF, McGranahan N, Swanton C. Pervasive chromosomal instability and karyotype order in tumour evolution. Nature. 2020;587:126–132. doi: 10.1038/s41586-020-2698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Stearns T. Mammalian cells lack checkpoints for tetraploidy, aberrant centrosome number, and cytokinesis failure. BMC Cell Biol. 2005;6:6. doi: 10.1186/1471-2121-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhsng CZ, Wala J, Mermel CH, Sougnez C, Gabriel SB, Hernandez B, Shen H, Laird PW, Getz G, Meyerson M, Beroukhim R. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasadil LM, Britigan EM, Ryan SD, Kaur C, Guckenberger DJ, Beebe DJ, Moser AR, Weaver BA. High rates of chromosome missegregation suppress tumor progression but do not inhibit tumor initiation. Mol Biol Cell. 2016;27:1981–1989. doi: 10.1091/mbc.E15-10-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Hills SA, Ozono E, Diffley JFX. Cyclin E-induced replicative stress drives p53-dependent whole-genome duplication. Cell. 2023;186:528–42.e14. doi: 10.1016/j.cell.2022.12.036. [DOI] [PubMed] [Google Scholar]
- Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Mo Z, Duan G, Tang R, Zhang F, Lu M. (125)I Seed promotes apoptosis in non-small lung cancer cells via the p38 MAPK-MDM2-p53 signaling pathway. Front Oncol. 2021;11:582511. doi: 10.3389/fonc.2021.582511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Tsai HJ, Gordon MR, Li R. Cellular stress associated with aneuploidy. Dev Cell. 2018;44:420–431. doi: 10.1016/j.devcel.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.