Abstract
There is little research about the stress, quality of life (QOL) and gut microbiota in newly diagnosed breast cancer patients. In this study addressing the dearth of research on stress, quality of life (QOL), and gut microbiota in newly diagnosed breast cancer patients, 82 individuals were prospectively observed. Utilizing the Functional Assessment of Chronic Illness Therapy (FACT)-Breast questionnaire to assess health-related quality of life (HRQOL) and the Distress Thermometer (DT) to gauge distress levels, the findings revealed a mean FACT-B score of 104.5, underscoring HRQOL's varied impact. Significantly, 53.7% reported moderate to severe distress, with a mean DT score of 4.43. Further exploration uncovered compelling links between distress levels, FACT-B domains, and microbial composition. Notably, Alcaligenaceae and Sutterella were more abundant in individuals with higher DT scores at the family and genus levels (p = 0.017), while Streptococcaceae at the family level and Streptococcus at the genus level were prevalent in those with lower DT scores (p = 0.028 and p = 0.023, respectively). This study illuminates the intricate interplay of stress, QOL, and gut microbiota in newly diagnosed breast cancer patients, offering valuable insights for potential interventions of biomarker or probiotics aimed at alleviating stress and enhancing QOL in this patient cohort.
Subject terms: Cancer, Microbiology, Psychology, Health care, Medical research, Oncology
Introduction
Female breast cancer has surpassed lung cancer as the most common diagnosed cancer, with an estimated 2.3 million new cases (11.7%), and with 68.5 thousand deaths1. Due to the early screening and improvement in the diagnosis and treatment, breast cancer patients’ survival outcomes including disease-free survival and overall survival improved progressively2. This improvement has also led to the need for more intensive management in terms of psychological issues such as quality of life (QOL), stress, anxiety and depression in breast cancer patients. The anxiety and depression negatively affect the quality of life and survival rates in breast cancer patients3.
The human microbiota is the collection of microbes that inhabit various parts of the body, primarily the gut, skin, vagina, mouth, and among others. Each body site has a distinct microbiota and there is significant inter-individual variability in microbiomes, which can contribute to diseases such as metabolic disorders, inflammatory diseases, allergies, and cancer4–6. The gut microbiota appears to influence breast cancer risk, response to treatment, and recurrence by affecting human health through metabolic, neural, and endocrine signaling, and immune activity7. The gut microbiota dysbiosis (imbalance) may lead to the development of breast cancer through the crosstalk among microbiota and both endogenous hormones and estrogen-like compounds might synergize to provide protection from disease but also to increase the risk of developing hormone-related diseases8–11. Besides, diversity and specific microbiota were linked to chemotherapy response as well as prognosis in breast cancer patients12. Microbiota diversity was also predictive of side effects such as neurological symptoms, weight gain, and constipation. Emerging evidence indicates that gut microbiota affects the response to anticancer therapies by modulating the host immune system and gut microbiota involvement in trastuzumab efficacy represents the foundation for new therapeutic strategies aimed at manipulating commensal bacteria to improve response in trastuzumab-resistant breast cancer patients13. Fu et al. found that depletion of intratumor bacteria significantly reduced lung metastasis without affecting primary tumor growth, offering new methods for improved breast cancer management14. Furthermore, modulating microbiota by nutritional treatment with probiotics and prebiotics is as emerging and promising strategies for prevention and treatment of breast cancer in the future15.
Many of the breast cancer patients experience fatigue, depression, and/or anxiety months to years after their breast cancer diagnosis with these symptoms being associated with greater disability and a poorer quality of life16. The gut microbiome plays a role between stress response, inflammation, and depression, and anxiety through the microbiome-gut-brain axis, which plays a key role in the regulation of brain function and behavior17–19. Recent study revealed gut bacteria composition may play a role in depression through the production of neurotransmitters, such as serotonin and glutamate20. A meta-analysis of 34 controlled clinical trials found that probiotics showed small but significant benefits for depression and anxiety, while prebiotics did not differ from placebo in their effects on depression or anxiety21. Additional randomized clinical trials with psychiatric samples are necessary fully to evaluate their therapeutic potential. There is little research about the quality of life22, stress, and gut microbiota in newly diagnosed breast cancer patients, so we designed this prospective study hoping to find potential probiotics for decreasing stress and improving quality of life in breast cancer patients.
Methods
Patient population
The study was designed as a prospective observational research project and was approved by the Institutional Review Board of MacKay Memorial Hospital (MMH), Taipei, Taiwan (19MMHIS061e). The breast cancer patients with stage I-IV who were diagnosed by core biopsy and age greater than 20 years old were included. All patients were treated in MMH and provided written informed consent. Recurrent breast cancer patients or those patients with a history of mental illness were excluded. The patients were recruited as convenience samples. The quality of life was evaluated by FACT-Breast questionnaire23 (supplement Table 1). The distress scale was evaluated by Distress Thermometer (Chinese version)24,25. All the fecal sample collection and FACT-Breast questionnaire and Distress Thermometer evaluation were performed on the first admission for breast cancer treatment. Every patient also completed a lifestyle habits survey, which included questions about alcohol consumption, use of gastroenterology medications and antibiotics, bowel habits, presence of blood in stools, and history of gastrointestinal conditions such as gastroenteritis, irritable bowel syndrome, chronic diarrhea of constipation and colon polyps.
Quality of life and stress evaluation methods
Functional assessment of chronic illness therapy (FACT)-breast questionnaire
The Functional Assessment of Chronic Illness Therapy (FACT)-Breast is a health-related quality of life (HRQOL) questionnaire specifically designed to assess the impact of breast cancer on an individual's daily functioning23. The FACT-Breast questionnaire consists of a set of questions that measure different aspects of HRQOL such as physical, emotional, and social functioning, as well as overall well-being. The FACT-B consists of two parts, including the FACT-General (FACT-G) with 27 questions and the Breast Cancer Supplement (BCS) with 10 questions. It uses a 5-point scoring system, where 0 represents no at all and 4 represents a lot. The FACT-G includes four sub-scales: Physical Well-Being (PWB) with 7 questions, Social/Family Well-Being (SWB) with 7 questions, Emotional Well-Being (EWB) with 6 questions, and Functional Well-Being (FWB) with 7 questions. The BCS domain includes additional specific items about breast cancer: physical, psychological and aesthetical disorders due to cancer and therapies. The score of the FACT-B is the total of all life quality scores, with a higher score indicating higher satisfaction with life quality. The FACT-Breast questionnaire is widely used in research and clinical practice and has been shown to have good reliability and validity and the FACT-B in the Chinese version were confirmed26.
Distress thermometer
The Distress Thermometer24,25 is a single-item, 11-point visual analogue scale, with respondents indicating how distressed they have felt over the past week (from “No Distress” to “Extreme Distress”). The most recent version of the NCCN practice guidelines for the management of distress recommends that a DT score of 5 or higher indicates moderate-to-severe distress. It is a simple, self-report, pencil-and-paper measure, using a thermometer format line to rate the level of distress and is an accurate, valid screening tool for depression, anxiety27.
Fecal samples collection and DNA extraction of microbiota
Each fecal samples were collected before treatment when breast cancer confirmed by core biopsy and store in − 20 °C refrigerator before use. DNA extraction from fecal samples using QIAamp Fast DNA stool mini kit (QIAGEN GmbH, Hilden Germany) followed by user’s manual. Briefly, 0.2 g sample in 1 mL InhibitEX buffer with glass beads, homogenized by precellys homogenizer (Bertin Instruments, Montigny-le-Bretonneux France) 4500 beat per min, 2 min. Heat the suspension for 10 min at 70 °C centrifuge sample for 1 min to pellet stool particles. 25 uL proteinase K in a new 2 mL centrifuge tube add 600 uL supernatant from stool pellet. Then add 600 AL buffer and mix-well. Incubate at 70 °C for 10 min. Add 600 uL 100% ethanol and mix-well. Filtrate sample by QIAamp spin column 13,000 rpm, 1 min. Wash filter by AW1, AW2 buffer. Elute DNA sample by 100 uL ATE buffer. DNA amount and quality was measured by nanodrop 2000 (Thermo Scientific, MA USA).
16S rRNA library construction and sequencing
Variable regions of 16S rRNA are frequently used in phylogenetic classifications such as genus or species in diverse microbial populations. 2.5 μl (50 ng) of DNA was used to set up the first PCR with 0.2 μM V3 + V4 forward and reverse primers (Forward:TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG, Reverse: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) and 12.5 μl 2X Kapa HiFi HotStart ReadyMix (KapaBiosystems) in 25 μl reactions. The PCR cycling conditions were 3 min at 95 °C, 25 cycles of 30 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C, followed by 5 min at 72 °C. The amplified DNA was purified with Agencourt AMPure XP Reagent beads (Beckman Coulter Inc., CA, USA). The second PCR was set up to add indexes to the amplified DNA by adding 5 μl of purified DNA to 25 μl 2X Kapa HiFi HotStart ReadyMix (KapaBiosystems, MA, USA), 5 μl Nextera XT Index 1 and 2 primers (Illumina, CA, USA) in 50 μl reactions. The reaction was set at 3 min at 95 °C, 8 cycles of 30 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C, followed by 5 min at 72 °C on an Applied Biosystems 2720 thermocycler (Thermo Fisher Scientific, CA, USA), followed by another Agencourt AMPure XP Reagent beads purification (Beckman Coulter Inc., CA, USA).
We used qPCR (KAPA SYBR FAST qPCR Master Mix) to quantify each library using Roche LightCycler 480 system and pooled then equally to 4nM for illumina MiSeq NGS system (illumina, CA, USA). More than 80,000 reads with paired-end sequencing (2*300bp) were generated, and the metagenomics workflow classified organisms from the amplicon using a database of 16S rRNA data (https://www.basespace.illumina.com). The classification was based on the NCBI database (https://www.ncbi.nlm.nih.gov). The output of the workflow was a classification of reads at several taxonomic levels: kingdom, phylum, class, order, family, genus, and species. Then the sequences were analyzed using the QIIME2 software package version 2017.10 (https://qiime2.org/). Potential chimeric sequences were removed using DADA228, followed by trimming 30 and 90 bases of the 3′ region of the forward and the reverse reads, respectively. Taxonomical classification was performed using Naive Bayes classifier trained on the Greengenes13.8 with a 99% threshold of OTU full-length sequences.
Statistical analysis
All data are presented as the means ± SD. Student's t‐test was used for comparison between two groups. One‐way ANOVA or two‐way ANOVA was performed for comparisons between multiple groups. Statistical analyses were performed using SPSS 26.0 software. A p‐value < 0.05 was considered statistically significant.
We presented bacterial compositions at the Family, Genus and Species levels and calculated the alpha, beta-diversity indices by MicrobiomeAnalyst (https://www.microbiomeanalyst.ca)29,30. Alpha-diversity is measured within a single sample using Shannon index with the QIIME software package version 2017.10 (https://qiime2.org/)31. For genera with a median relative abundance exceeding 1%, we conducted multiple regression analysis with adjustment for confounders to examine the association between FCR and the bacterial composition. We excluded from the analysis bacterial taxa that were not detected in 5% or more of the final participants. Furthermore, we calculated the skewness and kurtosis of the bacterial compositions and transformed the distribution of any bacterial compositions that did not assume a normal distribution using Box-Cox transformation. A p-value and T-test are calculated for each genus and species to assess statistical significance. Beta-diversity is measured between different samples using the Bray–Curtis index, and PCoA is used to visualize the results. PERMANOVA is used to test the significance of differences between samples, and F-value, r-squared, and p-value are reported. The taxonomy labels using QIIME. The stacked plot shows the percentage abundance (PA) of different genera and species in each sample, and the Top 20 genera and species are presented in separate graphs to highlight the most abundant taxa. Linear discriminant analysis (LDA) effect size (LEfSe) is a statistical method used to identify features that are differentially abundant between groups of samples. It calculates a p-value and Log LDA score for each feature and reports the results in a graphical format. The original sample pool is divided into Family, Genus, and Species categories, and features with a p-value < 0.05 and Log LDA score > 1.0 are considered significant.
Results
Patients characteristics
From May 2019 to May 2022, total 82 female breast cancer patients proved by core biopsy were included in this study. All 82 patients had fecal sample collection and FACT-Breast questionnaire and Distress Thermometer evaluation on the first admission for breast cancer treatment prospectively. The age ranged from 30 to 75 years old (average 45.7 years old). As in supplement Table 2, most of the patients had early-stage disease, including 2 (2.4%) with stage 0, 19 (23.2%) with stage I, 48 (58.5%) with stage II, 9 (11.0%) with stage III, and 4 (4.9%) with stage IV. All patients except stage IV cases (total 78 cases) received breast operation. Chemotherapy was applied in 71 out of 82 patients (86.6%) and radiotherapy in 34 out of 82 patients (41.5%). Total 49 patients (59.8% of 82 patients) received hormone therapy in this series.
QOL of newly diagnosed breast cancer patients evaluated by FACT-B
Descriptive statistics for FACT-B different domain scores at diagnosis of breast cancer patients are shown in Table 1. The mean score of the FACT-B was 104.5 (SD, 19.76).
Table 1.
FACT-B scores at diagnosis of breast cancer patients (n = 82).
| Minimal | Maximal | Mean | SD | Score range | |
|---|---|---|---|---|---|
| Physical well being (PWB) | 13 | 28 | 23.8 | 3.28 | 0–28 |
| Social/Family well being (SWB) | 0 | 28 | 19.5 | 6.09 | 0–28 |
| Emotional well being (EWB) | 6 | 108 | 18.6 | 10.72 | 0–24 |
| Functional well-being (FWB) | 2 | 28 | 18.5 | 5.89 | 0–28 |
| Breast cancer subscale (BCS) | 11 | 35 | 24.1 | 5.24 | 0–40 |
| TOI | 40 | 87 | 66.4 | 11.44 | 0–96 |
| FACT-G | 46 | 177 | 80.4 | 17.30 | 0–108 |
| FACT-B | 68 | 201 | 104.5 | 19.76 | 0–148 |
TOI FACT-B trial outcome index, (PWB score) + (FWB score) + (BCS score) = FACT-B TOI; FACT-G Total score = (PWB score) + (SWB score) + (EWB score) + (FWB score); FACT-B total score = (PWB score) + (SWB score) + (EWB score) + (FWB score) + (BCS score) (Please refer to supplement Table 1), SD standard deviation.
Patients endorsing variable on distress thermometer
An initial DT was completed by all 82 patients. The mean score was 4.43 (range 0–10), with 53.7% (44/82) of the patients reporting moderate to severe distress (score 5 or above). Table 2 presents the problems indicated at presentation. Practical concerns (72% of patients) and emotional concerns (62.2% of patients) are the most sources of distress that can be identified by patients using the Distress Thermometer (DT). The most prevalent problem indicated at presentation was the treatment decisions in 51.2% (42/82) patients. In the emotional category, the nervousness and worry presented in more than 30% of the patients.
Table 2.
Percentage of at diagnosis of breast cancer patients endorsing variable on Distress Thermometer problem list (n = 82).
| Problem list | No | Yes (%) | |
|---|---|---|---|
| Practical concerns | Practical concerns | 59 | 72.0 |
| Child care | 12 | 14.6 | |
| Housing | 3 | 3.7 | |
| Insurance/Finances | 7 | 8.5 | |
| Transportation | 3 | 3.7 | |
| Work/School | 13 | 15.9 | |
| Treatment decisions | 42 | 51.2 | |
| Family concerns | Family concerns | 17 | 20.7 |
| Dealing with children | 6 | 7.3 | |
| Dealing with spouse or partner | 2 | 2.4 | |
| Ability to have children | 0 | 0.0 | |
| Family health issues | 8 | 9.8 | |
| Spiritual or religious concerns | Spiritual or religious concerns | 8 | 9.8 |
| Physical concerns | Physical concerns | 40 | 48.8 |
| Changes in appearance | 7 | 8.5 | |
| Taking care of myself | 2 | 2.4 | |
| Constipation/Diarrhea | 18 | 22.0 | |
| Memory or concentration | 3 | 3.7 | |
| Nose dry/congested | 4 | 4.9 | |
| Pain | 14 | 17.1 | |
| Sexual health | 3 | 3.7 | |
| Skin dry/itching | 3 | 3.7 | |
| Sleep | 15 | 18.3 | |
| Tingling in hands/feet | 4 | 4.9 | |
| Fatigue | 6 | 7.3 | |
| Emotional concerns | Emotional concerns | 51 | 62.2 |
| Depression | 12 | 14.6 | |
| Fear | 11 | 13.4 | |
| Nervousness | 34 | 41.5 | |
| Sadness | 15 | 18.3 | |
| Worry | 31 | 37.8 | |
| Loss of interest or enjoyment | 4 | 4.9 |
Comparison of DT scores with FACT-B, stage, treatment modality and lifestyle variants
To determine the risk factors of DT score, we compared the DT score (DT score of 5 or higher indicates moderate-to-severe distress) with FACT-B subscale scores, stage, treatment modality and lifestyle variants separately. There is no difference of DT score in FACT-B subscale scores (Table 3), stage, treatment modality (supplement Table 2) and lifestyle variants (supplement Table 3) separately in our newly diagnosed breast cancer patients. But age and education status had the significantly different. Patients with age less than 50 years old and education above bachelor’s degree had higher DT score in our series.
Table 3.
Comparison the DT and FACT-B subclass assessment dimensions.
| Dimension | DT score | No | Mean | S.D | t |
|---|---|---|---|---|---|
| PBW | 0–4 | 38 | 24.08 | 3.283 | 0.606 |
| 5–10 | 44 | 23.64 | 3.307 | ||
| SWB | 0–4 | 38 | 20.37 | 5.782 | 1.221 |
| 5–10 | 44 | 18.73 | 6.307 | ||
| EWB | 0–4 | 38 | 18.21 | 3.757 | − 0.273 |
| 5–10 | 44 | 18.86 | 14.292 | ||
| FWB | 0–4 | 38 | 19.21 | 5.818 | 0.998 |
| 5–10 | 44 | 17.91 | 5.953 | ||
| BCS | 0–4 | 38 | 24.66 | 5.148 | 0.919 |
| 5–10 | 44 | 23.59 | 5.324 | ||
| FACT-B | 0–4 | 38 | 106.53 | 16.945 | 0.867 |
| 5–10 | 44 | 102.73 | 21.949 |
DT distress thermometer, PBW physical well being, SWB social/family well being, EWB emotional well being, FWB functional well being, BCS breast cancer subscale, FACT-B functional assessment of chronic illness therapy –breast.
Index of alpha-, beta-diversity of different study groups in genus, species level
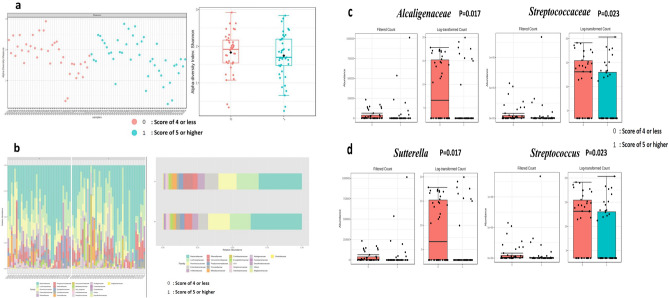
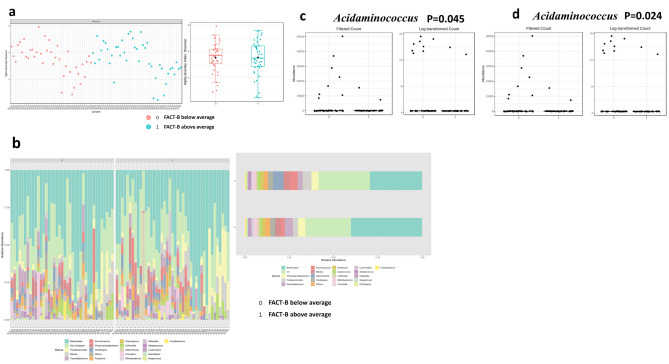
The index of alpha- and beta-diversity of different study groups in genus and species levels are presented in supplement Table 4 based on their DT scores, FACT subclass scores, depression and worry mentioned in DT problems, respectively. All the alpha-, beta-diversity parameters do not reach statistical significance. This may indicate that the differences between our samples of microbiota are small in this series. The alpha-diversity assessed by richness (Chao1, left box) and diversity (Shannon, right box) in the family level of DT score of 4 or less (0, pink dot color) and score of 5 or higher (1, blue dot) are shown in Fig. 1a. Barplots of the relative abundance of the 20 most abundant taxa identified to family level, found in DT score of 4 or less (0, lower row) and score of 5 or higher (1, upper row) are shown in Fig. 1b. Alpha-diversity by Shannon index and inter-quantile distribution indicate the richness and evenness divided with FACT-B criteria (Fig. 2a). Top 20 bacteria composition in genus level for each sample divided by FACT-B (Fig. 2b, left). Each label represents the percentage abundance of top 20 taxonomy. The most abundant genus of bacteria found in merged FACT-B group were Bacteroides (Fig. 2b, right).
Table 4.
Significant top 5 relatively abundant bacterial taxa of DT groups in Family and Genus level.
| DT 0–4 | DT 5–10 | LDA score | FDR | p values | |
|---|---|---|---|---|---|
| Family | |||||
| Alcaligenaceae | 37,302 | 50,003 | 3.8 | 0.28134 | 0.017* |
| Streptococcaceae | 74,404 | 54,195 | − 4 | 0.28134 | 0.023* |
| Erysipelotrichaceae | 306,340 | 141,720 | − 4.92 | 0.9568 | 0.164 |
| Burkholderiaceae | 8520.6 | 11,606 | 3.19 | 0.9568 | 0.176 |
| V5 | 62,940 | 67,250 | 3.33 | 0.9568 | 0.197 |
| Genus | |||||
| Sutterella | 37,302 | 50,003 | 3.8 | 0.39387 | 0.017* |
| Streptococcus | 74,404 | 54,195 | − 4 | 0.39387 | 0.023* |
| Dorea | 41,974 | 23,079 | − 3.98 | 0.61968 | 0.054 |
| Holdemania | 6583.6 | 3062.6 | − 3.25 | 0.61968 | 0.071 |
| Lachnospira | 66,054 | 31,091 | − 4.24 | 0.70652 | 0.102 |
DT distress thermometer, LDA latent Dirichlet allocation, FDR false discovery rate.
*p < 0.05.
Figure 1.
Identified potential bacteria biomarker in DT of breast cancer (Potential bacterial biomarkers in Family and Genus level with LEfSe analysis). Alpha-diversity by Shannon index and inter-quantile distribution indicate the richness and evenness divided with DT score (1a). Top 20 bacteria composition in genus level of each sample by percentage abundance ((1b), left). The most abundant genus of bacteria found in merge sample group were Bacteroides ((1b), right). In family level, Alcaligenaceae is significant in DT score less than 4 (p = 0.017, (1c) left), while Streptococcaceae is associated in DT score over 5 (p = 0.023, (1c) right). Further in genus level, A. Sutterella is a specific biomarker in DT score under 4 (p = 0.017, (1d) left). On the other hand, S. Streptococcus is a specific biomarker in DT score oner 5 (P = 0.023, (1d) right). Detailed LDA score list in Table 4. Using Linear discriminant analysis (LDA) Effect Size (LEfSe) under condition with p < 0.05, Log LDA score > 1.0. We identified some potential biomarker associated with DT score. However, we cannot identify more significant species in LEfSe due to bacteria diversity. (0: represent DT score less than 4; 1: represent DT score over than 5).
Figure 2.
Identified potential bacteria biomarker in FACT-B of breast cancer. Alpha-diversity by Shannon index and inter-quantile distribution indicate the richness and evenness divided with FACT-B criteria (2a). Top 20 bacteria composition in genus level for each sample divided by FACT-B ((2b), left). Each label represents the percentage abundance of top 20 taxonomy. The most abundant genus of bacteria found in merged FACT-B group were Bacteroides ((2b), right). Acidaminococcus is a specific bacteria biomarker in FACT-G below average (p = 0.045, (2c)) and FACT-B below average (p = 0.024, (2d)). Detailed LDA score list in Table 5.
Significant top 5 relatively abundant bacterial taxa in different study groups
The top 5 bacterial taxa that are relatively abundant in different study groups were determined using LEfSe criteria, with a p value < 0.05 and log LDA score > 1. Table 5 and Supplement Tables 5 and 6 show the significant top 5 bacterial taxa in each group. Specifically, Alcaligenaceae (p = 0.017) at the family level and Sutterella (p = 0.017) at the genus level were found to be significantly more abundant in individuals with higher scores on the DT scale (Fig. 1c), while Streptococcaceae (p = 0.028) at the family level and Streptococcus (p = 0.023) at the genus level were significantly more abundant in individuals with lower scores on the DT scale (Fig. 1d). Moreover, Christensenellaceae (p = 0.008) and Ruminococcaceae (p = 0.025) at the family level, and Faecalibacterium (p = 0.014), Coprococcus (p = 0.046) at the genus level, and Obeum (p = 0.001), Prausnitzii (p = 0.014), and Plebeius (p = 0.0018) at the species level were significantly more abundant in breast cancer patients who reported having depression in the DT questionnaire. The relative abundance of Eubacterium (p = 0.019) at the family level and dolichum (p = 0.019) at the genus level were significantly higher in individuals who did not report having depression in the DT questionnaire.
Table 5.
Significant top 5 relatively abundant bacterial taxa of FACT-G and FACT-B groups in Family and Genus level.
| Family | FACT-G below average | FACT-G above average | LDA score | FDR | p values |
|---|---|---|---|---|---|
| Clostridiaceae | 46,152 | 11,877 | − 4.23 | 0.8018 | 0.048* |
| Prevotellaceae | 143,960 | 219,760 | 4.58 | 0.8018 | 0.091 |
| Veillonellaceae | 335,740 | 314,110 | − 4.03 | 0.8018 | 0.107 |
| Gemellaceae | 298.09 | 967.95 | 2.53 | 0.8018 | 0.158 |
| Mogibacteriaceae | 19,960 | 15,280 | − 3.37 | 0.8018 | 0.160 |
| Genus | |||||
| Acidaminococcus | 4409.5 | 566.21 | − 3.28 | 0.95114 | 0.045* |
| Clostridium | 47,328 | 12,309 | − 4.24 | 0.95114 | 0.051 |
| Granulicatella | 413.74 | 1585.2 | 2.77 | 0.97095 | 0.099 |
| Prevotella | 103,280 | 121,340 | 3.96 | 0.97095 | 0.136 |
| Holdemania | 5361.9 | 4014.9 | − 2.83 | 0.97095 | 0.152 |
| FACT-B group | |||||
|---|---|---|---|---|---|
| Family | FACT-B below average | FACT-B above average | LDA score | FDR | p values |
| Alcaligenaceae | 21,127 | 64,473 | 4.34 | 0.80561 | 0.099 |
| Mogibacteriaceae | 20,608 | 15,019 | − 3.45 | 0.80561 | 0.136 |
| Prevotellaceae | 155,030 | 204,420 | 4.39 | 0.80561 | 0.178 |
| Odoribacteraceae | 46,711 | 91,040 | 4.35 | 0.80561 | 0.203 |
| Ruminococcaceae | 1,309,000 | 1,045,200 | − 5.12 | 0.80561 | 0.240 |
| Genus | |||||
| Acidaminococcus | 4748.7 | 526.7 | − 3.32 | 0.83702 | 0.024* |
| Anaerostipes | 45,465 | 16,553 | − 4.16 | 0.83702 | 0.142 |
| Granulicatella | 445.56 | 1474.6 | 2.71 | 0.83702 | 0.172 |
| Ruminococcus | 274,200 | 357,390 | 4.62 | 0.83702 | 0.218 |
| Parabacteroides | 416,770 | 756,920 | 5.23 | 0.83702 | 0.222 |
TOI FACT-B Trial Outcome Index, (PWB score) + (FWB score) + (BCS score) = FACT-B TOI; FACT-G Total score = (PWB score) + (SWB score) + (EWB score) + (FWB score); FACT-B total score = (PWB score) + (SWB score) + (EWB score) + (FWB score) + (BCS score) (Please refer to supplement Table 1), SD standard deviation, LDA latent Dirichlet allocation, FDR false discovery rate.
*p < .05.
In the PWB above average group, there was a significantly higher relative abundance of Alcaligenaceae (p = 0.022) at the family level and Sutterella (p = 0.022) at the genus level. In the SWB group, the relative abundance of Adlercreutzia (p = 0.005) was significantly higher in individuals with below-average scores. In the EWB group, the relative abundance of Carnobacteriaceae (p = 0.044) at the family level and Granulicatella (p = 0.044) at the genus level were significantly higher in individuals with above-average scores. Conversely, in the EWB below average group, there was a significantly higher relative abundance of Distasonis (p= 0.032) and V2 (p = 0.037) at the species level. The relative abundance of Prevotellaceae (p = 0.045) at the family level and Prevotella (p = 0.045) at the genus level, and Copri (p = 0.045) at the species level were significantly higher in individuals with above-average scores of FWB group patients. Conversely, in the BCS below average group, there was a significantly higher relative abundance of Lachnospiraceae (p = 0.033) and Pasteurellaceae (p = 0.037) at the family level, and Acidaminococcus (p = 0.018) and Haemophilus (p = 0.037) at the genus level, and Parainfluenzae (p = 0.037) and Catus (p = 0.041) at the species level. The FACT-G below average group had a significantly higher relative abundance of Clostridiaceae (p = 0.048) at the family level, and Acidaminococcus (p = 0.045, Fig. 2c) at the genus level. The FACT-B below average group had a significantly higher relative abundance of Acidaminococcus (p = 0.024, Fig. 2d) at the genus level. Detailed LDA score list in Table 5.
Discussion
To our knowledge, this is the first study to investigate the relationship between QOL, distress and the gut microbiome in newly diagnosed breast cancer patients. We sought to determine the relationship of distress and FACT-B different domain and fecal microbial composition among newly diagnosed breast cancer patients. Several associations between distress, FACT-B different domain and microbial taxa were observed among this sample of breast cancer patients.
From Table 2, treatment decisions, nervousness and worry are the most popular sources of distress that can be identified by patients using the Distress Thermometer (DT) in our series. Patients who rate their level of distress as 5 or higher on the DT and identify emotional concerns as a source of distress may benefit from further assessment or intervention to address these concerns. This may include referral to a mental health professional, such as a psychologist or psychiatrist, who can provide counseling or other forms of psychotherapy to help the patient manage their emotional distress. Other interventions that may be helpful for emotional concerns identified on the DT include support groups, relaxation techniques, and stress-reduction strategies, such as mindfulness meditation or yoga. Healthcare providers may also provide education and information about coping strategies and resources that are available to help patients manage emotional distress related to cancer and its treatment. Moreover, treatment decisions can be a significant source of anxiety and worry for many patients with breast cancer. Patients may feel overwhelmed by the complexity of treatment options, uncertain about the potential outcomes and side effects of different treatments. In clinical practice, healthcare providers should take steps to support patients in making informed decisions that are aligned with their goals and values. This may include providing clear and accurate information about treatment options, discussing the risks and benefits of different treatments, and engaging in shared decision-making with patients and their families32,33.
In this study, we observed significant differences in the abundance of certain bacterial families and genera in relation to the role of gut microbiota in distress and LOQ of newly diagnosed breast cancer patients. Specifically, Alcaligenaceae in the family level and Sutterella in the genus level were found to be significantly more abundant in individuals with higher scores on the DT scale, while individuals with below-average scores on the Functional Assessment of Cancer Therapy—General (FACT-G) scale had a significantly higher relative abundance of Clostridiaceae. Alcaligenaceae, a bacterial family within the gut microbiome, is implicated in a range of health and disease contexts. It shows associations with conditions such as inflammatory bowel diseases34, chronic kidney disease35, cholelithiasis36, thyroid cancer37, colorectal cancer38, esophageal squamous cell carcinoma39, and breast cancer40, suggesting potential roles in disease development or progression. The families Pseudomonadaceae, Sphingomonadaceae, Alcaligenaceae, Ruminococcaceae, and Clostridia were reported to be decreased in adjacent breast tissue compared with breast cancer tissue41. Additionally, the presence of Alcaligenaceae, a proinflammatory bacterial family, was found to be higher in depressed patients without anxiety compared to those with anxiety symptoms and the showed the proportion of Alcaligenaceae and Sutterella in the anxiety-negative depressed group was significantly higher than in the anxiety-positive group in first-episode depression of Chinese patients42. This suggests that the composition of the gut microbiota, including Alcaligenaceae, may influence the manifestation and severity of depression. However, more research is required to establish causality and understand the underlying mechanisms. Sutterella, belonging to Betaproteobacteria, are Gram-negative, non-spore-forming rods that grow in a microaerophilic atmosphere or under anaerobic conditions. Emerging research highlights the intricate relationship between the Sutterella and various aspects of health and disease, including irritable bowel disease, Crohn’s disease, autism spectrum disorder, Down syndrome and multiple sclerosis43, cancer therapy outcomes44, and sleep duration45. In the context of cancer therapy, particularly CAR-T cell therapy for hematologic malignancies, the presence of Sutterella in the gut microbiota has been associated with treatment responses and survival, emphasizing its potential role as a therapeutic target44. In contrast, studies related to autism spectrum disorder (ASD) have revealed differences in gut microbiota composition, including the presence of Sutterella, in children with ASD, suggesting a link between the microbiome and neurodevelopmental disorders. Conversely, lower abundance of Sutterella was been observed in people with depression46. Sutterella has demonstrated varying abundance levels in different psychiatric conditions, including lower levels in individuals with depression and higher levels in some children with autism47. This suggests its potential role in the intricate microbial-brain-gut axis. Additionally, Sutterella may possess immunomodulatory properties and contribute to Th-17 differentiation43. In a pilot study, lower relative abundance of Sutterella was consistently observed in adults with shorter sleep durations, suggesting a connection between this bacterium and sleep patterns45. Clostridiaceae plays a multifaceted role in health and disease. In menopause, it contributes to alterations in the gut microbiome, potentially affecting cardiometabolic health, with certain members like Clostridium lactatifermentans associated with protective effects against cardiovascular risk factors48. Additionally, Clostridiaceae, particularly Clostridium species, has been implicated in the gut microbiome of children with ASD, emphasizing its link to neurodevelopmental conditions49. The bidirectional relationship between stress, the hypothalamus-pituitary-adrenocortical axis, and the gut microbiome involves Clostridium species, potentially influencing overall well-being and distress50. Furthermore, emerging research suggests that Clostridiaceae bacteria may impact breast cancer outcomes by interacting with the immune system, highlighting their relevance in the context of cancer treatment and quality of life for survivors51.These findings suggest that the microbiome may play an important role in the development of distress and impacts of LOQ of newly diagnosed breast cancer patients.
The Streptococcaceae was significantly (p = 0.028) more abundant in individuals with lower scores on the DT scale in this study. Accompanied by inflammation, Streptococcus mutans (S. mutans), an oral bacterium, invades endothelial cells (ECs) and disrupts their integrity, thereby promoting tumor cell extravasation and ultimately facilitating metastasis of breast cancer cells to the lungs52. The role of neurotransmitter imbalance, particularly insufficient levels of monoamine neurotransmitters like serotonin, dopamine, and norepinephrine, in contributing to emotional distress and depression. Serotonin, a key neurotransmitter in the brain-gut axis, is mainly synthesized in the gut by certain bacteria53. Various bacteria such as Streptococcus spp., Enterococcus spp., Escherichia spp., Lactobacillus plantarum, Klebsiella pneumoniae, and Morganella morganii were reported to have the ability to produce serotonin54. The high abundance of Streptococcaceae was observed in people with depression and the linkage between gut microbiota pattern and depression may be through the brain-gut microbiome axis46. One animal study revealed that a combination of living Bifidobacterium, Lactobacillus and Streptococcus may be used for treatment of anxiety55. But high abundance of Streptococcaceae was observed in people with depression46. It needs further study to define the role of Streptococcaceae in the distress and QOL in newly diagnosed breast cancer patients and evaluate its potential interventions of biomarker.
The primary limitation of the Distress Thermometer (DT) in this study lies in its potential inadequacy for assessing the complex relationship between psychological distress and different problem list variables, such as pain, in newly diagnosed breast cancer patients. For example, while the study aims to screen for distress in this population using the DT scale, it faces challenges in capturing the nuances of pain experiences and their psychological impact. The DT's single-item nature remains subjective and may not sufficiently differentiate between different sources and origins of pain, making it less suitable for assessing pain-related distress comprehensively. Moreover, the study's diverse breast cancer patient population and the potential variations in distress of newly diagnosed cancer patients highlight the need for a more tailored and multidimensional assessment approach. Therefore, while the DT is recommended routine screening for distress in all cancer patients since1999 by the National Comprehensive Cancer Network (NCCN)56. The DT was developed as a simple tool to effectively screen for symptoms of distress and offers a user-friendly screening tool57, its limitations in addressing the multifaceted nature of different problems, such as pain, and distress in newly diagnosed breast cancer patients should be recognized, and supplementary assessments or tools may be necessary for a more in-depth understanding of this complex relationship. We would like to clarify that our study primarily aimed to explore the microbiome's potential links with depressive tendencies as measured by the DT scale, rather than to establish a direct causative relationship.
In conclusion, this prospective study defines the relationships among QOL, stress and gut microbiota in newly diagnosed breast cancer patients and provides many useful information to find potential interventions of biomarker or probiotics for decreasing stress and for improving quality of life in breast cancer patients.
Supplementary Information
Acknowledgements
The English edition was aided by ChatGPT partially.
Author contributions
C.-C.L., H.-W.Y. and P.-S.Y. drafted the manuscript and contributed to the conception of the study; F.L., W.-C.K. and Y.-C.C. helped perform the analysis with constructive discussions; H.-W.Y. and C.-J.L. contributed significantly to analysis; P.-S.Y. gave financial help and approved the final version. All authors reviewed the manuscript.
Funding
This study was supported by grants from the National Science and Technology Council, Taiwan (MOST 110-2321-B-195-001) and MacKay Memorial Hospital (MMH-109-86 and MMH-112-11), Taipei, Taiwan. Role of the Funder/Sponsor: The sponsor acted as the corresponding author and approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-45123-1.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 3.Setyowibowo H, et al. Psychoeducation for breast cancer: A systematic review and meta-analysis. Breast. 2022;62:36–51. doi: 10.1016/j.breast.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature486, 207–214 (2012). 10.1038/nature11234 [DOI] [PMC free article] [PubMed]
- 5.Selber-Hnatiw S, et al. Human gut microbiota: Toward an ecology of disease. Front. Microbiol. 2017;8:1265. doi: 10.3389/fmicb.2017.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rea D, et al. Microbiota effects on cancer: From risks to therapies. Oncotarget. 2018;9:17915–17927. doi: 10.18632/oncotarget.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampsell K, Hao D, Reimer RA. The gut microbiota: A potential gateway to improved health outcomes in breast cancer treatment and survivorship. Int. J. Mol. Sci. 2020;21:9239. doi: 10.3390/ijms21239239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong H, Bording-Jorgensen M, Dijk S, Wine E. The complex interplay between chronic inflammation, the microbiome, and cancer: Understanding disease progression and what we can do to prevent it. Cancers (Basel) 2018;10:83. doi: 10.3390/cancers10030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goedert JJ, et al. Postmenopausal breast cancer and oestrogen associations with the IgA-coated and IgA-noncoated faecal microbiota. Br. J. Cancer. 2018;118:471–479. doi: 10.1038/bjc.2017.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shvets YV, Lykhova OO, Chekhun VF. Human microbiota and breast cancer. Exp. Oncol. 2022;44:95–106. doi: 10.32471/exp-oncology.2312-8852.vol-44-no-2.17855. [DOI] [PubMed] [Google Scholar]
- 11.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl. Cancer Inst. 2016 doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csendes D, et al. Gastrointestinal microbiota and breast cancer chemotherapy interactions: A systematic review. Cureus. 2022;14:e31648. doi: 10.7759/cureus.31648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Modica M, et al. Gut microbiota condition the therapeutic efficacy of Trastuzumab in HER2-positive breast cancer. Cancer Res. 2021;81:2195–2206. doi: 10.1158/0008-5472.CAN-20-1659. [DOI] [PubMed] [Google Scholar]
- 14.Fu A, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–1372 e1326. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Ganguly S, Tollefsbol TO. Modulating microbiota as a new strategy for breast cancer prevention and treatment. Microorganisms. 2022;10:1727. doi: 10.3390/microorganisms10091727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers LQ, et al. Effects of a multicomponent physical activity behavior change intervention on fatigue, anxiety, and depressive symptomatology in breast cancer survivors: Randomized trial. Psychooncology. 2017;26:1901–1906. doi: 10.1002/pon.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petra AI, et al. Gut-Microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther. 2015;37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Wouw M, Boehme M, Dinan TG, Cryan JF. Monocyte mobilisation, microbiota & mental illness. Brain Behav. Immun. 2019;81:74–91. doi: 10.1016/j.bbi.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Bear T, et al. The microbiome-gut-brain axis and resilience to developing anxiety or depression under stress. Microorganisms. 2021;9:723. doi: 10.3390/microorganisms9040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radjabzadeh D, et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022;13:7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith KS, et al. Health-related quality of life is associated with fecal microbial composition in breast cancer survivors. Support Care Cancer. 2022;31:10. doi: 10.1007/s00520-022-07496-3. [DOI] [PubMed] [Google Scholar]
- 23.Brady MJ, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J. Clin. Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 24.Roth AJ, et al. Rapid screening for psychologic distress in men with prostate carcinoma: A pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Tang LL, Zhang YN, Pang Y, Zhang HW, Song LL. Validation and reliability of distress thermometer in Chinese cancer patients. Chin. J. Cancer Res. 2011;23:54–58. doi: 10.1007/s11670-011-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan C, et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res. Treat. 2007;106:413–418. doi: 10.1007/s10549-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 27.Civilotti C, Acquadro Maran D, Santagata F, Varetto A, Stanizzo MR. The use of the distress thermometer and the hospital anxiety and depression scale for screening of anxiety and depression in Italian women newly diagnosed with breast cancer. Support Care Cancer. 2020;28:4997–5004. doi: 10.1007/s00520-020-05343-x. [DOI] [PubMed] [Google Scholar]
- 28.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong J, Liu P, Zhou G, Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020;15:799–821. doi: 10.1038/s41596-019-0264-1. [DOI] [PubMed] [Google Scholar]
- 30.Dhariwal A, et al. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolyen E, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cincidda C, Pizzoli SFM, Ongaro G, Oliveri S, Pravettoni G. Caregiving and shared decision making in breast and prostate cancer patients: A systematic review. Curr. Oncol. 2023;30:803–823. doi: 10.3390/curroncol30010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeRosa AP, Demetres MR, McComas RR. Shared decision-making among women diagnosed with breast cancer: A phenomenological study and exploration into health literacy education. J. Consum. Health Intern. 2022;26:259–273. doi: 10.1080/15398285.2022.2093086. [DOI] [Google Scholar]
- 34.Humbel F, et al. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 2020;18:2019–2029 e2011. doi: 10.1016/j.cgh.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 35.Luo M, et al. Causal effects of gut microbiota on the risk of chronic kidney disease: A Mendelian randomization study. Front. Cell Infect. Microbiol. 2023;13:1142140. doi: 10.3389/fcimb.2023.1142140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng R, et al. Patients with primary and secondary bile duct stones harbor distinct biliary microbial composition and metabolic potential. Front. Cell Infect. Microbiol. 2022;12:881489. doi: 10.3389/fcimb.2022.881489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishaq HM, et al. Gut-thyroid axis: How gut microbial dysbiosis associated with euthyroid thyroid cancer. J. Cancer. 2022;13:2014–2028. doi: 10.7150/jca.66816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha R, et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS One. 2016;11:e0152126. doi: 10.1371/journal.pone.0152126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao W, et al. Association between alcohol consumption and oesophageal microbiota in oesophageal squamous cell carcinoma. BMC Microbiol. 2021;21:73. doi: 10.1186/s12866-021-02137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, et al. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget. 2017;8:88122–88138. doi: 10.18632/oncotarget.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieleman S, et al. Exploring the potential of breast microbiota as biomarker for breast cancer and therapeutic response. Am. J. Pathol. 2021;191:968–982. doi: 10.1016/j.ajpath.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Zheng S, et al. A correlation study of intestinal microflora and first-episode depression in Chinese patients and healthy volunteers. Brain Behav. 2021;11:e02036. doi: 10.1002/brb3.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiippala K, Kainulainen V, Kalliomaki M, Arkkila P, Satokari R. Mucosal prevalence and interactions with the epithelium indicate commensalism of Sutterella spp. Front. Microbiol. 2016;7:1706. doi: 10.3389/fmicb.2016.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat. Commun. 2022;13:5313. doi: 10.1038/s41467-022-32960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agrawal R, et al. Habitual sleep duration and the colonic mucosa-associated gut microbiota in humans-a pilot study. Clocks Sleep. 2021;3:387–397. doi: 10.3390/clockssleep3030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry. 2020;11:541. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, et al. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters BA, et al. Menopause is associated with an altered gut microbiome and estrobolome, with implications for adverse cardiometabolic risk in the hispanic community health study/study of latinos. mSystems. 2022;7:e0027322. doi: 10.1128/msystems.00273-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding HT, Taur Y, Walkup JT. Gut microbiota and autism: Key concepts and findings. J. Autism Dev. Disord. 2017;47:480–489. doi: 10.1007/s10803-016-2960-9. [DOI] [PubMed] [Google Scholar]
- 50.Bailey MT, et al. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson I, et al. Assessing the performance of a novel stool-based microbiome test that predicts response to first line immune checkpoint inhibitors in multiple cancer types. Cancers (Basel) 2023;15:3268. doi: 10.3390/cancers15133268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L, et al. The oral bacterium Streptococcus mutans promotes tumor metastasis by inducing vascular inflammation. Cancer Sci. 2022;113:3980–3994. doi: 10.1111/cas.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barandouzi ZA, et al. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022;12:1648. doi: 10.1038/s41598-022-05756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie J, et al. The combination of living Bifidobacterium, Lactobacillus, and Streptococcus improves social ranking and relieves anxiety-like behaviors in competitive mice in a social dominance tube test. Brain Behav. 2022;12:e2453. doi: 10.1002/brb3.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ownby KK. Use of the distress thermometer in clinical practice. J. Adv. Pract. Oncol. 2019;10:175–179. [PMC free article] [PubMed] [Google Scholar]
- 57.Ekman H, Pettersson A, Jakobsson L, Garmy P. A cross-sectional study of distress: A cancer response. Nurs. Open. 2020;7:850–856. doi: 10.1002/nop2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.