Abstract
Tripartite motif 17 (TRIM17) belongs to a subfamily of the RING-type E3 ubiquitin ligases, and regulates several cellular processes and pathological conditions including cancer. However, its potential function in gastric cancer (GC) remains obscure. Here, we have found TRIM17 mRNA and protein levels are both upregulated in human GC compared with normal specimens, and TRIM17 upregulation indicates poor survival for GC patients. Functionally, TRIM17 was found to act as an oncogene by promoting the proliferation and survival of GC cell lines AGS and HGC-27. Mechanistically, TRIM17 acts to interact with BAX and promote its ubiquitination and proteasomal degradation, leading to a deficiency in BAX-dependent apoptosis in GC cells in the absence and presence of apoptosis stimuli. Moreover, TRIM17 and BAX expression levels are inversely correlated in human GC specimens. Our data thus suggest TRIM17 contributes to gastric cancer survival through regulating BAX protein stability and antagonizing apoptosis, which provides a promising therapeutic target for GC treatment and a biomarker for prognosis.
Subject terms: Tumour biomarkers, Oncogenes
Introduction
Gastric cancer (GC) represents one of the top five lethal causes of cancer worldwide, with its incidence and mortality maintaining at a high level in China [1]. Although the diagnosis and treatment of GC have improved, a 5-year event-free survival rate for GC patients remains low [2, 3]. Thus, it is of great clinical importance to identify novel molecular mechanisms underlying gastric carcinogenesis.
Tripartite motif (TRIM) 17 is a member of TRIM family proteins that are a class of the RING-type E3 ubiquitin ligases known to regulate various cellular processes [4–7]. Data have suggested that TRIM17 plays an important role in regulating apoptosis and autophagy, as well as in regulating pathologic conditions such as Parkinson’s disease and cancer, with E3 ubiquitin ligase-dependent and -independent manner [7]. Thus far, studies have demonstrated an either tumor-suppressing or oncogenic role of TRIM17 in different cancerous contexts, as TRIM17 inhibits the proliferation of MCF-7 via inducing the degradation of a kinetochore protein ZWINT whereas promotes the melanoma survival and chemoresistance by blocking TRIM28 from mediating the degradation of BCL2A1 [8, 9]. Despite the findings, the potential function of TRIM17 in other cancer types remains largely unknown.
Apoptosis defect is one of the hallmarks of cancer that promotes carcinogenesis and renders cancer cells resistant to chemotherapy [10]. The multi-domain proapoptotic BCL2 family member BAX is a pivotal regulator to control mitochondrial apoptotic signaling pathways and serves as a tumor suppressor [11, 12]. Decreased levels or loss of function of BAX have been observed in several cancer types, conferring cancer survival and/or chemoresistance [13–16]. Several E3 ligases including Parkin, IBRDC2 and KPC1 are found to mediate the ubiquitination and proteasomal degradation of BAX under physiological and pathological contexts [17–21]. Although the enhanced BAX degradation has been observed in many cancer types and indicates poor prognosis [14, 16, 22, 23], potential E3 ubiquitin ligase accounting for its degradation in cancer remains obscure.
Here, by analyzing the Cancer Genome Atlas (TCGA) dataset and GC tissue microarray, we find that TRIM17 mRNA and protein levels are significantly upregulated in GC patients repative to normal controls, and high TRIM17 expression predicts poor prognosis. Our investigation further reveals that TRIM17 promotes GC cell proliferation and survival. Importantly, we establish TRIM17 as an unidentified E3 ubiquitin ligase that promotes the ubiquitination and degradation of BAX, consequently antagonizing apoptosis in the absence or presence of anticancer treatment in GC cells.
Results
TRIM17 expression is upregulated in clinical GC specimens and correlates with poor prognosis in patients
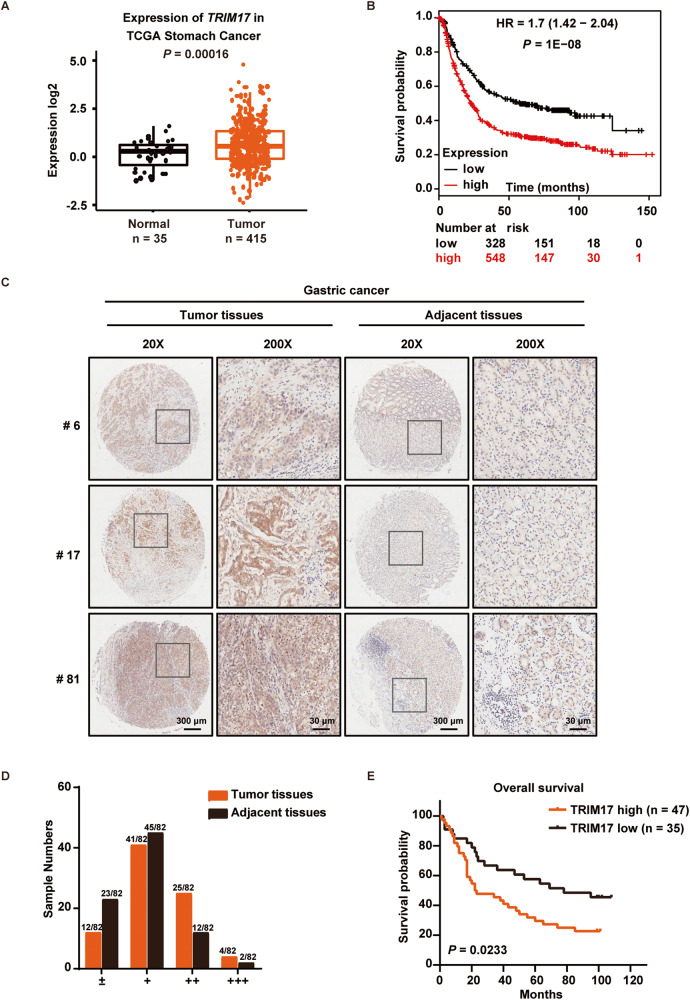
We first analyzed the bioinformatics data from the TCGA dataset with patients having a variety of cancer types in digestive system (Supplementary Fig. S1A, B) and found a higher abundance of TRIM17 mRNA is seen in the tumor tissues from GC patients than in normal tissues, with high TRIM17 expression being associated with poor survival in GC patients (Fig. 1A, B). To further investigate the expression and prognostic value of TRIM17 protein, we performed IHC staining of a human GC tissue microarray. As shown in Fig. 1C, much stronger staining for TRIM17 was observed in the tumors (left) versus that in adjacent tissues (right). All samples were classified into four groups based on the IHC scores, with the medium (++) to strongest staining (+++) for TRIM17 in approximately 35.37% tumor tissues compared with 17.07% in adjacent counterparts (Fig. 1D). High TRIM17 protein expression was found to be strongly associated with poorer 5-year overall survival of the GC patients (P = 0.0233) (Fig. 1E). These data indicate a potential oncogenic role of TRIM17 in GC.
Fig. 1. TRIM17 is upregulated in GC patients with poor clinical prognosis.
A Expression of TRIM17 mRNA level in gastric cancer (GC) tissues (n = 415) compared with normal tissues (n = 35) from the Cancer Genome Atlas (TCGA) dataset. B Kaplan-Meier curve depicting overall survival according to TRIM17 expression level from www.kmplot.com (Affymetrix id = 220279_at). C Representative samples of immunohistochemical (IHC) staining of TRIM17 expression in human GC tissue microarray. Enlarged image in square is shown in right panel in each group. D Classification of TRIM17 protein IHC score in the GC tissue microarray. Sample numbers are listed compared with total of 82 samples. E Kaplan-Meier survival analysis of the GC patients from the tissue microarray classified by TRIM17 protein expression. High and low expression of TRIM17 for each specimen was defined relative to the expression level of paired adjacent tissue.
TRIM17 deficiency inhibits the proliferation and survival as well as induces apoptosis in GC cells
To investigate the biological function of TRIM17, effects of TRIM17 knockdown or overexpression on cell proliferation or survival were assessed. As TRIM17 protein expression is generally higher in several human GC lines than that in GES-1, a human gastric epithelial cell line, AGS and HGC-27 cell lines were used for further investigation (Supplementary Fig. S2A). The colony formation and growth curve assay indicated that TRIM17 knockdown by siRNAs or shRNA dramatically inhibited the proliferation of AGS or HGC-27 cells compared with that of the control (Fig. 2A–C; Supplementary Fig. S2B). Conversely, ectopic TRIM17 expression promoted cell survival (Supplementary Fig. S2C-E). Further FACS analysis indicated that significantly increased apoptosis was found in the GC cells upon TRIM17 knockdown by siRNAs for 72 h (Fig. 2D), whereas cell cycle distribution remained unchanged (Supplementary Fig. S2F). Consistently, cleaved PARP and Caspase 3 levels manifesting apoptotic responses were induced by TRIM17 depletion for 72 h in AGS and HGC-27 cells (Supplementary Fig. S2G), and negligible changes were observed regarding the expression levels of cell cycle regulators such as p21, p27 and cyclin D1 (Supplementary Fig. S2H). We further assessed the effects of TRIM17 in GC cells xenograft mouse model. Depletion of TRIM17 in AGS cells was found to significantly suppress the tumor progression as judged by the decreased tumor weight and volume (Fig. 2E–G), whereas ectopic expression of TRIM17 in HGC27 cells significantly promoted the tumor growth (Supplementary Fig. S2I-K). Consistent growth inhibitory effects were found by the proportion of Ki-67 positive staining cells demonstrating proliferation index in tumor tissue from the TRIM17-depleted group compared to the control one (Fig. 2H, I). We also noticed a markedly increase of apoptosis evidenced by the positive cleaved Caspase 3 staining via IHC in TRIM17-depleted tumor tissue (Fig. 2H, I). Collectively, these results suggest that TRIM17 promotes GC proliferation and survival whereas antagonizes apoptosis.
Fig. 2. TRIM17 deficiency suppresses the proliferation and induces apoptosis in GC cells.
A Colony formation assay upon TRIM17 knockdown. Cells were transfected with si-Control or si-TRIM17 every three days to maintain the knockdown efficiency. The percentage of colonies number is quantified in the lower panel (Mean ± SD; n = 3). B, C TRIM17 knockdown suppressed cell proliferation in AGS (B) and HGC-27 (C) cells. AGS cells transiently transfected with si-Control or si-TRIM17, or HGC-27 cells infected with sh-Control or sh-TRIM17 lentivirus, were seeded onto 12-well plates. The siRNAs in (B) were transfected every 3 days to maintain the knockdown efficiency. Growth curve was measured at 24 h intervals up to 7 days (mean ± SD; n = 3). D GC cells were transfected with si-Control or si-TRIM17s for 72 h, followed by flow cytometry analysis with Annexin V/PI staining. Representative data were shown on the left and apoptotic rates were quantifided in right panel (Mean ± SD; n = 3). E–G AGS cells stably expressing pRS-sh-Control or pRS-sh-TRIM17 were inoculated into NOD/SCID mice. The tumor growth was recorded as described in Materials and Methods. Average tumor weight on the 90th day after inoculation and tumor volume during the indicated time were calculated in (F) and (G), respectively. H, I Immunohistochemical analysis of TRIM17, Ki-67, Cleaved Caspase 3 and BAX in tumor tissues from the NOD/SCID mice. Representative images were shown in (H) and quantification of the integrated optical density (IOD) was shown in (I). Scale bars :100 μm. For all data throughout this figure, *P < 0.05; **P < 0.01; ***P < 0.001.
TRIM17 binds to BAX
Next, we set out to investigate the underlying mechanism by which TRIM17 promotes GC survival. As TRIM17 is an E3 ubiquitin ligase in the TRIMs family [7], we aim to identify its potential substrates. We expressed Flag-tagged TRIM17 in HEK293T cells and subjected the anti-Flag immunoprecipitates to mass spectrometry analysis. Among the proteins identified in the TRIM17 complex (Supplementary Table S1), we found several peptides corresponding to BAX, which hasn’t been previously known as a TRIM17’s interactor. BAX exerts an essential tumor-suppressing function in the regulation of apoptosis, highlighting its priority as a potential target. Meanwhile, BAX peptides have not been listed in the contaminant repository for affinity purification-mass spectrometry data (https://www.crapome.org), indicating the specificity of the interaction. Supportively, gene set enrichment analysis (GSEA) from two enrichment plots in TCGA dataset for GC demonstrated a significantly inverted correlation between TRIM17 and apoptosis signaling pathways (Fig. 3A, Supplementary Fig. S3A). In light of these findings, we hypothesized that BAX could be a potential target for TRIM17 to regulate apoptosis.
Fig. 3. TRIM17 interacts with and regulates BAX stability.
A Gene Set Enrichment Analysis (GSEA) demonstrating the correlation between TRIM17 and apoptosis signaling pathway in the enrichment plot. NES, normalized enrichment score. FDR, false discovery rate. B GC cells were subjected to IP analyses with BAX antibody or normal rabbit IgG. C, D HEK293T cells co-expressed TRIM17-Flag or a control vector and HA-BAX for 72 h were subjected to IP and IB analyses. E GST pull-down assay indicating the direct interaction of purified GST-TRIM17 protein with His-BAX protein. F, G Cells were transfected with pCMV6-TRIM17-Flag or a control vector (F) or transfected with pRS-sh-TRIM17 or pRS-sh-Control (G) for 72 h prior to IB analyses. H Increasing amounts of pCMV6-TRIM17-Flag (1 μg, 3 μg) were transfected into HGC-27 cells for 72 h followed by IB analyses. I HGC-27 cells transfected with pCMV6-TRIM17-Flag or a control vector and AGS cells transfected with pRS-sh-TRIM17 or pRS-sh-Control for 72 h were treated with CHX (50 μg/ml) for the indicated time interval prior to IB analyses. The BAX protein half-lives were quantified and presented. J HGC-27 cells were transfected with pCMV6-TRIM17-Flag or a control vector for 72 h and treated with MG132 (20 μM) for another 6 h prior to IB analyses.
To verify the interaction between TRIM17 and BAX, we performed co-IP analysis with anti-BAX antibody and endogenous binding between TRIM17 and BAX proteins in AGS and HGC-27 cells was observed (Fig. 3B). Also expression of Flag-tagged TRIM17 in AGS cells was able to co-immunoprecipitate endogenous BAX (Supplementary Fig. S3B). Supportively, co-IP analysis indicated that Flag-tagged TRIM17 and HA-tagged BAX could form a complex in HEK293T cells (Fig. 3C, D). Moreover, in an in vitro GST pull-down assay using the recombinant GST-TRIM17 protein and His-BAX protein, TRIM17 bound to BAX directly (Fig. 3E). These data confirm BAX is a bona fide binding partner of TRIM17.
TRIM17 promotes the ubiquitination and degradation of BAX via proteasome
We then asked whether TRIM17 affects BAX protein level. Expectedly, TRIM17 overexpression downregulated, whereas TRIM17 knockdown upregulated, BAX static protein levels in AGS and HGC-27 cells (Fig. 3F, G). In addition, TRIM17 has no observable effect on BAX mRNA expression in the GC cells as revealed by RT-qPCR analysis (Supplementary Fig. S3C, D), indicating the regulation occurs at a post-transcriptional level. Since two BCL2 family members MCL1 and BCL2A1 have been reported to be a direct or an indirect substrate for TRIM17 [9, 24], we assessed whether TRIM17 may affect the protein levels of other BCL2 family members. As seen in Fig. 3H, increasing amounts of TRIM17 expression only suppressed BAX protein levels without affecting that of other BCL2 family members including BAK, MCL1, BCL2, and BCL2L1, suggesting its specific regulation towards BAX. Consistently, a markedly increase of BAX expression by IHC staining in TRIM17-depleted tumor tissue in vivo was observed (Fig. 2H, I).
Next, we determined whether TRIM17 might regulate the stability of BAX protein by examining the half-lives of BAX protein in cycloheximide (CHX)-based chase experiment. Upon TRIM17 overexpression, the half-life of BAX in HGC-27 cells was significantly reduced compared to that of the control groups, whereas TRIM17 knockdown in AGS cells caused a marked increase in the steady-state levels of BAX protein (Fig. 3I). Interestingly, we noticed endogenous BAX half-lives varied from less than 10 h to longer than 24 h in a panel of GC cell lines (Supplementary Fig. S3F), complicating the regulation of background BAX levels under different cell lines. Moving forward, we found TRIM17-induced endogenous or exogenous BAX degradation was blocked by the proteasome inhibitor MG132 in HGC-27 and HEK293T cells (Fig. 3J; Supplementary Fig. S3E), suggesting that TRIM17 controls endogenous BAX protein stability via proteasome.
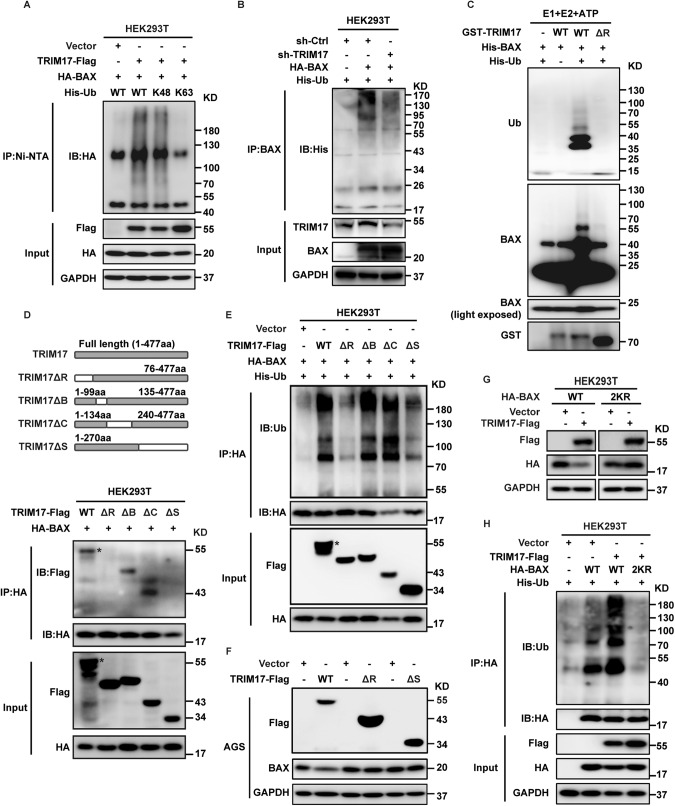
We then investigated the function of TRIM17 on BAX protein ubiquitination in vivo and in vitro. We co-expressed BAX, TRIM17 along with wild-type or mutant His-Ub by transient transfection in HEK293T cells. The polyubiquitinated BAX was purified by Ni-NTA beads under denaturing conditions and detected by IB with anti-HA antibody. As shown in Fig. 4A, ectopic expression of TRIM17 strongly stimulated the polyubiquitination of BAX. In contrast, TRIM17 depletion in HEK293T reduced the ubiquitination of BAX pulled down by anti-BAX antibody (Fig. 4B). We also found ectopic expression of TRIM17 facilitated the Lys 48(K48)-linked, but not Lys 63(K63)-linked, polyubiquitin chains on BAX protein, supporting the proteasome-mediated substrate degradation (Fig. 4A; Supplementary Fig. S4A) [25, 26]. Furthermore, purified GST-TRIM17 protein robustly promoted, whereas deletion of the RING domain in TRIM17 (TRIM17ΔR) failed to stimulate, the in vitro polyubiquitination of BAX (Fig. 4C).
Fig. 4. TRIM17 ubiquitinates BAX in vivo and in vitro, with its E3 liagase activity dependent on the RING or SPRY domain.
A HEK293T cells were co-expressed TRIM17-Flag or a control vector with HA-BAX and wild type (WT) or mutant His-tagged ubiquitin (His-Ub) for 72 h. The polyubiquitylated proteins were purified by Ni-NTA beads and detected with anti-HA antibody. His-Ub K48 and K63 represent all lysines were mutated except for the K48 or K63, respectively. B HEK293T cells co-expressed sh-TRIM17 or sh-Control with HA-BAX and His-Ub for 72 h followed by in vivo ubiqutination analyses. C TRIM17 instead of TRIM17ΔR promoted the polyubiquitination of BAX in vitro. D Interaction of BAX with a series of deletion mutants of TRIM17. HEK293T cells co-expressing BAX and wild-type (WT) or deletion mutants of TRIM17 (with schematic diagram shown on the upper panel) were cultured for 72 h, prior to IP and IB analyses. Asterisk indicates taget band. E In vivo ubiquitination assay of BAX in HEK293T cells after co-transfection with the indicated constructs. F AGS cells were transfected with indicated plasmids or a control vector for 72 h prior to IB analyses. G HEK293T cells were co-expressed with TRIM17-Flag or a control vector along with the indicated HA-BAX for 72 h prior to IB analyses. H HEK293T cells were co-expressed with the indicated plasmids for 72 h prior to ubiquitination analyses.
To map the BAX-interacting regions in TRIM17, a series of deletion mutants were constructed. As shown in Fig. 4D, deletion of RING domain (TRIM17ΔR) or SPRY domain (TRIM17ΔS) instead of B-box domain (TRIM17ΔB) or Coiled coil domain (TRIM17ΔC) markedly failed to interact with BAX protein, suggesting both domains are responsible for the substrate binding. Consistently, either overexpression of TRIM17ΔR or ΔS mutant markedly abolished the ubiquitination state of BAX compared with the wild type (WT) TRIM17 and two other deletion mutants (Fig. 4E). Furthermore, ectopic expression of TRIM17ΔR or ΔS failed to mediate the degradation of BAX in GC cells (Fig. 4F; Supplementary Fig. S4B). These results suggested the RING and SPRY domain on TRIM17 protein are critical for its binding and ubiquitin ligase activity towards BAX. Functional analysis by colony formation assay demonstrated a loss of proliferative effect for TRIM17ΔR compared with WT TRIM17 after re-expressing either plasmid in GC cells depleted of TRIM17 (Supplementary Fig. S4D), confirming a pivotal role of the RING domain in mediating the pro-survival function of TRIM17.
As K21 and K123 have been reported to be vital for the ubiquitination and stability of BAX [27], we assessed whether either K21R or K123R mutation affects the TRIM17-induced BAX degradation in HEK293T cells, and found either mutant failed to rescue the decreased BAX level (Supplementary Fig. S4C). Another previously reported K128R substitution also failed to rescue TRIM17’s effect (Supplementary Fig. S4C) [19]. However, mutations with both K21R and K123R (2KR) did protect BAX from TRIM17-mediated degradation, with a similar effect as that of all the nine lysine mutations (K-R) (Fig. 4G; Supplementary Fig. S4C). Supportively, the 2KR substitution substantially reduced TRIM17-mediated BAX polyubiquitination (Fig. 4H), indicating both K21 and K123 sites are critical for mediating the ubiquitin-binding and degradation of the substrate. Collectively, our data clearly supported that TRIM17 targets BAX for proteasome-mediated degradation by conjugating polyubiquitin chains onto the substrate.
BAX knockdown abrogates TRIM17 depletion-induced tumor-suppressing function in vitro
We next checked whether alteration of BAX expression may affect the biological function of TRIM17. As demonstrated by colony formation and growth curve assay in Fig. 5A, B; Supplementary Fig. S5A, simultaneous BAX knockdown greatly abrogated the growth inhibitory effects caused by TRIM17 depletion in the GC cells. Moreover, the enhanced apoptotic rate by TRIM17 knockdown was markedly brought down upon BAX depletion as well (Fig. 5C, D). Similar tendency was observed for Caspase 3 activity measurement (Fig. 5E). These data confirm that TRIM17 safeguards cancer survival by suppressing a BAX-dependent apoptosis.
Fig. 5. BAX knockdown abrogates the TRIM17 depletion-induced tumor suppressing function in vitro.
A Cells stably expressing pRS-sh-Control, pRS-sh-TRIM17 and/or pRS-sh-BAX plasmids were seeded onto 6-well plates and cultured for three weeks. The colonies were counted and analyzed (Mean ± SD; n = 3). B Proliferation of the cells as in (A) was measured at 24 h intervals up to 7 days (Mean ± SD; n = 3). C Representative data of apoptosis data in GC cells. Cells were transfected with pRS-sh-Control, pRS-sh-TRIM17, pRS-sh-BAX plasmid alone or in combination for 72 h prior to Annexin V/PI staining. D Quantitative analysis of apoptosis in (C) (Mean ± SD; n = 3). E Caspase 3 activity upon TRIM17 and/or BAX knockdown in GC cells. Cells as in (C, D) were detected by Caspase 3 assay kit (Mean ± SD; n = 3). The pRS-sh-Control group was set as a control. For all data in this figure, *P < 0.05; **P < 0.01; ***P < 0.001.
TRIM17 negatively regulates apoptotic response induced by anticancer treatments, with its depletion promoting chemosensitization
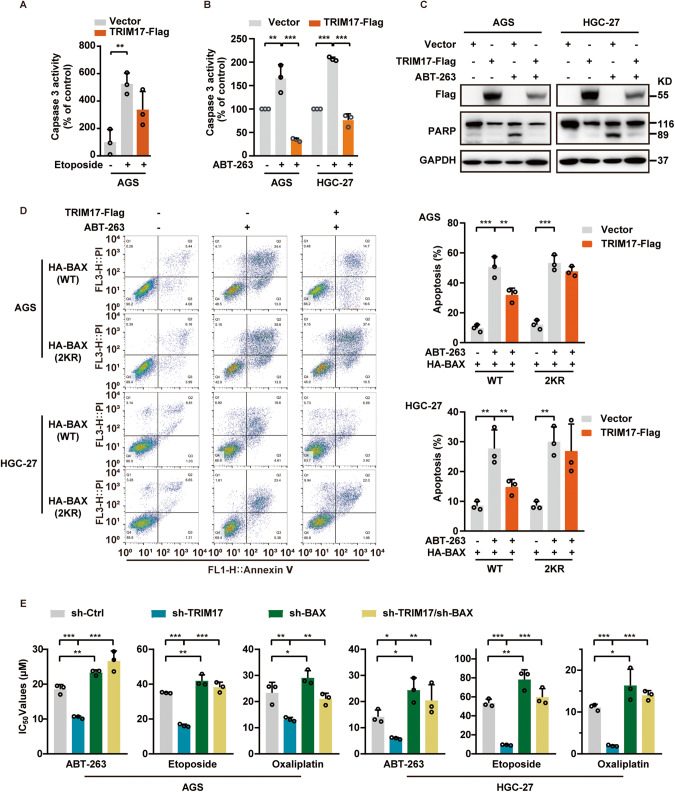
We next analyzed whether TRIM17 may affect apoptotic responses induced by anticancer drugs. TRIM17 overexpression reduced whereas knockdown enhanced the Etoposide-induced Caspases 3 activity in AGS cells (Fig. 6A; Supplementary Fig. S5B). We also treated the GC cells with a BCL2 family inhibitor ABT-263 (Navitoclax) [28] for 24 h and observed a significant reduction of drug-induced Caspases 3 activity and PARP cleavage by TRIM17 overexpression (Fig. 6B, C). The data confirm TRIM17 negatively regulates apoptotic responses induced by anticancer drugs.
Fig. 6. TRIM17 negatively regulates apoptotic responses induced by anticancer drugs and its depletion promotes chemosensitization.
A, B Cells were transfected with pCMV6-TRIM17-Flag or a control vector for 48 h and then treated with 100 μM Etoposide (A) or 2 μM ABT-263 (B) for 24 h, followed by Caspase 3 assay (Mean ± SD; n = 3). The control vector group without drug treatment was set as a control. C Cells were transfected with pCMV6-TRIM17-Flag or a control vector for 48 h and treated with 2 μM ABT-263 for 24 h prior to IB analyses. D AGS and HGC27 cells were co-transfected with pCMV6-TRIM17-Flag or a control vector along with HA-BAX (either WT or 2KR) expressing plasmids for 24 h, and then treated with ABT-263 for 48 h prior to apoptosis analyses (Mean ± SD; n = 3). E GC cells were transfected with pRS-sh-Control or pRS-sh-TRIM17 along with pRS-sh-BAX plasmids for 6 h, and then seeded onto 96-well plates and treated with the anticancer drugs for 72 h. IC50 values were plotted (Mean ± SD; n = 3). For all data in this figure, *P < 0.05; **P < 0.01; ***P < 0.001.
To further address the possible role of BAX in TRIM17-regulated apoptosis upon drug treatment, we transfected AGS and HGC27 cells with pcDNA4.0-HA-BAX or pcDNA3.0-HA-BAX-2KR in the absence and presence of TRIM17 overexpression, and compared the apoptotic rate after ABT-263 treatment for 48 h. Apoptosis was found to be prominent in both groups without TRIM17 upregulation; however, the apoptotic rate was greatly reduced by TRIM17 overexpression in cells expressing WT BAX instead of 2KR BAX (Fig. 6D; Supplementary Fig. S5C). This proves that the anti-apoptotic effect of TRIM17 depends on its degradation of WT BAX instead of the mutant one.
As decreased BAX expression levels normally confer chemoresistance in cancer therapy [29, 30], we further tested whether TRIM17 may affect the sensitivity of GC cells to anticancer therapy, and found TRIM17 depletion sensitized the GC cells to cytotoxicity induced by ABT-263, Etoposide or Oxaliplatin treatment for 72 h, as manifested by the markedly decreased IC50s of the drugs (Fig. 6E). Expectedly, BAX co-silencing abrogates the chemosensitizing effects caused by TRIM17 downregulation (Fig. 6E). Altogether, these findings clearly indicate that TRIM17 negatively regulates apoptosis in response to chemotherapy and its depletion contributes to chemosensitization in a BAX-dependent manner.
Expression levels of TRIM17 and BAX are inversely correlated in human GC specimens and cell lines
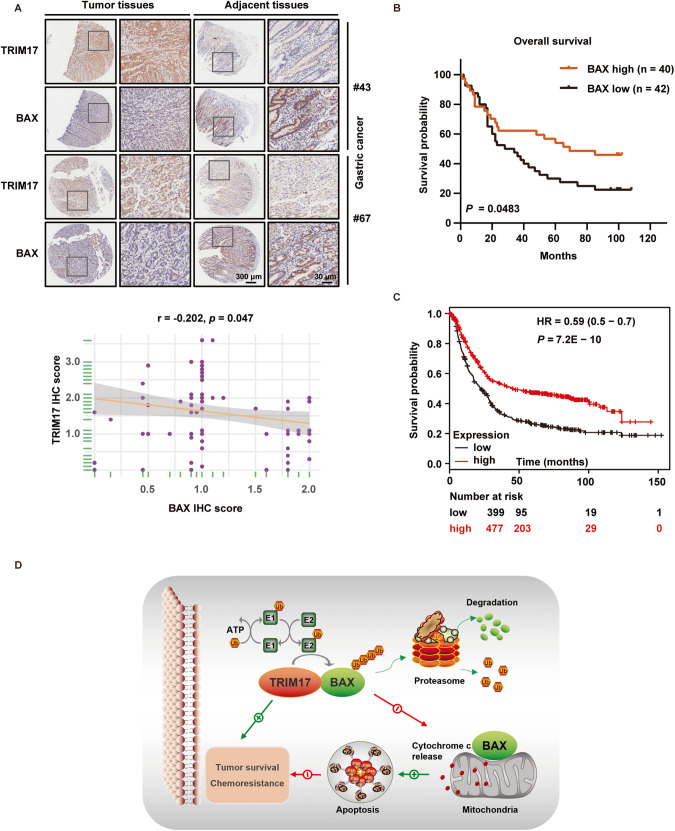
We lastly analyzed BAX protein expression in the human GC tissue microarray by IHC staining, and found a lower abundance of BAX in tumor tissues than that in adjacent tissues (Supplementary Fig. S6A). By comparing the expression levels of TRIM17 and BAX in the same set of GC tissues, a significant inverse correlation between both proteins was found (P = 0.047) (Fig. 7A). Supportively, IB analysis of multiple GC cell lines demonstrated a significantly inverted correlation between TRIM17 and BAX protein levels (Supplementary Fig. S6B). Also low BAX protein expression was significantly associated with poor 5-year survival in the GC patients (P = 0.0483) (Fig. 7B). Interestingly, low BAX mRNA expression was found to be associated with poor survival in GC patients as well based on TCGA dataset analysis (Fig. 7C). The clinical data support a negative regulation of TRIM17 towards BAX static levels in GC specimens.
Fig. 7. Expression levels of TRIM17 and BAX are inversely correlated in human GC specimens and cell lines.
A Comparison of the IHC staining of TRIM17 and BAX in human GC tissue microarray. Representative staining results of two GC samples are shown on the upper panel, with enlarged mages demonstrated in the right part in each group. Correlation of the proteins’ expression is shown on the lower panel by Spearman correlation coefficient analysis of the IHC scores. B Kaplan-Meier survival analysis of the overall survival for GC patients from the tissue microarra. High and low expression of BAX was defined for each specimen relative to the expression level of paired adjacent tissue. C Kaplan-Meier survival curve according to BAX expression in GC dataset retrieved at https://www.kmplot.com (Affymetrix id = 208478_s_at). D Working model depicting that TRIM17 functions as an E3 ubiquitin ligase that promotes the proteasomal degradation of BAX, thereby contributing to tumor survival and chemoresistance in GC.
Discussion
Previous studies have established a context-dependent tumor-suppressing or oncogenic function of TRIM17 in breast cancer and melanoma by regulating the expression of target proteins in E3 ubiquitin ligase-dependent or -independent manner [7–9]. In the present work, we find that TRIM17 expression is highly expressed in GC specimens relative to the normal tissues that denotes a poor prognosis, indicating a potential association of TRIM17 with GC progression. Our overexpression and silencing data have proved that TRIM17 exerts a pro-survival role in GC. Importantly, we establish TRIM17 as an unidentified E3 ubiquitin ligase to target BAX for proteasomal degradation, based on the following findings: 1) TRIM17 directly interacts with BAX; 2) TRIM17 negatively and specifically regulates BAX protein half-life without affecting its transcriptional level as well as the protein levels of BAK and several other BCL2 family members; 3) TRIM17 promotes BAX polyubiquitination and degradation in vivo and in vitro; 4) K48-polyubiquitinated BAX, instead of K63 modification, is regulated by TRIM17. Accordingly, TRIM17 safeguards GC survival by suppressing a BAX-dependent apoptosis under either static or drug-induced conditions, as seen in the working model (Fig. 7D).
Predominantly localized in the cytosol under static conditions while translocated into mitochondria once activated by apoptotic stresses, BAX can be regulated at both transcriptional and post-transcriptional levels [17–20, 31]. An E3 ligase Parkin has been proved to target cytosolic BAX for proteasomal degradation [17]. Moreover, Parkin has been suggested to ubiquitylate BAX at two lysines K21 and K64 to inhibit the protein’s translocation to the mitochondria, or at K128 to promote BAX’s degradation on the mitochondria [18, 19]. Here, we found the K21 and K123 of BAX protein are critical for TRIM17-mediated BAX ubiquitination and degradation in GC cells. Consistently the K21R and K123R mutations on BAX are found to be resistant to Parkin-dependent degradation in HCT116 cells [27]. As Parkin and IBRDC2 are known to promote the degradation of BAX on mitochondria to avoid excessive mitochondrial toxicity caused by the activated BAX [19, 20], whether TRIM17 is able to reduce the endogenous or activated BAX on the mitochondria remains to be investigated. Notably, while we failed to observe the K63-linked polyubiquitination of BAX by TRIM17, the possibility that BAX might serve as an autophagic target for TRIM17 cannot be excluded, since the latter protein is known to positively or negatively regulate autophagy, depending on selective substrate targets [32, 33]. Altogether, our work has added TRIM17 to the established E3 ligases targeting BAX for degradation. It is intriguing to understand whether and how these E3 ligases may delicately coordinate to regulate the homeostasis and functionality of the substrate under tumoral or non-tumoral contexts.
Our work demonstrates that, by controlling the homeostatic level of BAX, TRIM17 promotes the survival and antagonizes apoptosis in GC based on the following findings: 1) TRIM17 reduced the endogenous BAX levels and negatively regulated the apoptotic responses as well as apoptosis in the absence or presence of anticancer drugs; 2) TRIM17’s pro-survival function was blocked by the TRIM17ΔR mutant construct that failed to bind BAX to mediate its degradation; 3) the reduced cell survival and enhanced apoptosis upon TRIM17 depletion was abrogated by BAX co-downregulation. Given that BAX instability normally confers chemoresistance [29, 30], it is straightforward to observe that TRIM17 knockdown sensitized GC cells to anticancer treatment, which was abrogated by BAX co-silencing as well (Fig. 6E). Also the chemosensitization effect upon TRIM17 deficiency is supported by a recent study that revealed TRIM17 knockdown increased the sensitivity of human non-small cell lung cancer cells to cisplatin treatment [34]. In addition, given the central role of BCL-XL and/or BCL2 in maintaining cell survival and mediating chemoresistance in non-solid malignances and solid tumors including GC, selective BCL2 family inhibitors in combination with TRIM17 targeted strategy remains to be a promising direction for GC treatment [35, 36].
Notably, the anti-apoptotic effect of TRIM17 in cancer has been supported by S Desagher et al.’s work, demonstrating that TRIM17 prevents TRIM28 from ubiquitinating and degrading BCL2A1 in melanoma cells, thereby stabilizing BCL2A1 [9]. Interestingly, the team has also established a proapoptotic effect of TRIM17 in neurons [37], which is either mediated by MCL1 as its direct substrate or regulated at a transcriptional level by NFATc3 [24, 38]. However, despite BAX, we failed to find TRIM17 exerted any effect towards the protein level of MCL1 as well as other BCL2 family members in HGC27 cells. This suggests the regulatory role of TRIM17 during apoptosis is complicated and subjected to different cell contexts-dependent substrates. Thus, by identifying BAX as a novel substrate for TRIM17, our work sheds more light on the current understanding regarding its function in regulating apoptosis and cancer.
Importantly, analysis of human GC samples and cell lines supports a significant negative correlation between TRIM17 and BAX protein levels; GC patients with high TRIM17 or low BAX expression exhibit poor clinical prognosis. In light of these findings, the suppressed BAX level in GC patients could possibly reflect enhanced degradation of the protein by TRIM17. Collectively, our findings establish the unrevealed function of TRIM17 to promote GC survival and progression by controlling BAX stability and antagonizing apoptosis. TRIM17 may thus serve as a potential therapeutic target for GC treatment and a biomarker for prognosis.
Materials and methods
Cell lines
The GC cell lines AGS, HGC-27, MKN45, NCI-N87 and HEK293T cell line were purchased from the Institute of Basic Medicine, Chinese Academy of Medical Sciences. SUN1 and SUN16 cell lines were purchased from Korean Cell Line Bank (KCLB) and MKN1, MKN7 and MKN74 cell lines were from Japanese Collection of Research Bioresources Cell Bank (JCRB). Human gastric mucosal epithelial cell line GES-1 was purchased from Enogene Biotechnology Company. Cells were maintained in DMEM, RPMI 1640 or MEM medium with 10% fetal bovine serum (FBS) and incubated at 37°C in 5% CO2 incubator. GC cell lines were authenticated by STR profiling every half-year regularly.
Antibodies and reagents
The anti-BAX (#2774), anti-BAX (2D2) (#89477), anti-BAK (#12105), anti-MCL1 (#5453), anti-BCL2L1 (#2764), anti-BCL2 (#3498), anti-p21 (#2947), anti-p27 (#3686), anti-Cyclin D1 (#55506), anti-GST (#5475), anti-Ubiquitin (Ub) (#3936), anti-PARP (#9532), anti-Cleaved Caspase-3 (Asp175) (#9664), anti-HA (#3724), anti-Flag (#14793), anti-His (#12698), Rabbit Anti-Mouse IgG (Light-Chain Specific) (#58802), Mouse Anti-Rabbit IgG (Light-Chain Specific) (#93702) and Mouse Anti-rabbit IgG (Conformation Specific) (#5127) antibodies were obtained from Cell Signaling Technology (CST). Anti-β-Tubulin Monoclonal antibody (#K200059M) was purchased from Solarbio Life Sciences (China). Normal rabbit IgG (#sc-2027) and normal mouse IgG (#sc-2025) were obtained from Santa Cruz Biotechnology. The anti-TRIM17 (#13663-1-AP), anti-Ki-67 (#27309-1-AP), anti-GAPDH (#60004-1-Ig) and anti-β-actin (#66009-1-Ig) antibodies were obtained from Proteintech. The anti-Flag (#B26102), anti-HA (#B26202) and Protein A/G (#B23202) magnetic beads was obtained from Bimake (China). Horseradish peroxidase conjugated goat anti-rabbit IgG (#ZB-2301) and goat anti-mouse IgG (#ZB-5305) were obtained from ZSGB-BIO (China). MG132 (#S2619), Cycloheximide (CHX, #S7418), Etoposide (#S1225), ABT-263 (#S100) and Oxaliplatin (#S1224) were obtained from Selleck Chemicals.
Plasmids and siRNAs
The pCMV6-TRIM17-Flag (#RC213994), pCMV6-BAX-GFP (#RG204369), pRS-sh-TRIM17 (#TR308653, target sequence: 5’-GGAAGTGTCCTTCTACAGTGTAAGCGATG-3’) and pRS-sh-BAX (#TR306440, target sequence: 5’-ATGCGTCCACCAAGAAGCTGAGCGAGTGT-3’) plasmids were obtained from OriGene Technologies. pcDNA4.0-HA-BAX, pGEX-4T-1-GST-TRIM17, pGEX-4T-1-GST-TRIM17ΔR and pCMV6-TRIM17-Flag deletion mutants and pLKO.1-shTRIM17 plasmids (target sequence: 5’-GACCAAGTACTTATCCACCTT-3’) were constructed in our lab. pcDNA3.0-HA-BAX point mutant plasmids were constructed by Sangon Biotech (China). Wild type and the mutated except Lys-48 (K48) or Lys-63 (K63) His-tagged Ubiquitin expressing plasmids (His-Ub, His-Ub K48 and His-Ub K63) were gifted by Prof. Hu Ronggui in the Center for Excellence in Molecular Cell Science, Chinese Academy of Science. The His-Ub K48R (#P45617) and His-Ub K63R (#45929) plasmids were obtained from MiaoLing Biology (China). The siRNAs duplexes were synthesized by RiboBio (China). The sequences of siRNAs for TRIM17 or BAX were as follows: si-TRIM17#1: 5’-CGGACAGATTGAAGTGCTA-3’, si-TRIM17#2: 5’-GCATTGTGCTGGAGTTTGA-3’, si-TRIM17#3: 5’-GAACCTCTACCTGGTGGAA-3’; si-BAX: 5’-GTGCCGGAACTGATCAGAA-3’.
Knockdown and overexpression of target genes
siRNAs and plasmids were transfected with lipofectamine 2000 (Thermo, #11668019) and DNA transfection reagent (Neofect, China, #TF20121201), respectively. Cells were transfected with siRNAs or pRS-shRNA plasmids for the indicated time to transiently deplete TRIM17. Cells stably expressing sh-TRIM17 were established via infection or transfection. During infection process, the pLKO.1-shTRIM17 or pLKO.1 scramble control vector was co-transfected with lentivirus package plasmids (psPAX2 and PMD2.0 G) into HEK293T cells and the supernatant containing viruses were collected at 72 h after transfection. During transfection process, pRS-sh-TRIM17 and/or pRS-sh-BAX plasmid was transfected into cells and selected with 5 μg/mL puromycin (Sigma-Aldrich, #P8833). All stably knocked down cells were maintained with 1 μg/mL puromycin. Cells were transfected with pCMV6-TRIM17-Flag plasmid for TRIM17 overexpression. Stably overexpressed cells were selected with 1 mg/ml Geneticin (Selleck Chemicals, #S3028) and maintained at 500 μg/mL Geneticin.
Colony formation and growth proliferation
For colony formation assay, cells were seeded onto 6-well plates (3 × 103 cells/well). After three weeks, the colonies were stained with 0.1% (w/v) crystal violet (#G1064, Solarbio) and counted. For growth proliferation assay, cells were seeded onto 12-well plates (0.5-1 × 104 cells/well) and counted at 24 h intervals up to 7 days.
Cell cycle and apoptosis analysis
For cell cycle analysis, GC cells were seeded onto 6-well plates (5 × 105 cells/well) and transfected with pRS-sh-TRIM17 or pRS-sh-Control plasmid for 72 h. The cell cycle distribution was performed as described before [39]. For apoptosis analysis, cells were seeded onto 6-well plates (5 × 105 cells/well) and transfected with indicated siRNAs or pRS-shRNA plasmids for 72 h, followed by detection with Annexin V/PI staining kit (Beijing 4 A Biotech Co., Ltd, China, #FXP022) by flow cytometry. Caspase 3 activity was measured with a Caspase 3 activity assay kit (CST, #5723) according to manufacturer’s instruction. The fluorescence intensity was detected using a fluorescence reader (2104 Multilabel Reader, PerkinElemer EnVision).
IC50 measurement for anticancer drugs
AGS and HGC-27 cells were planted into 6-well plates (5 × 105 cells/well) overnight and transfected with the pRS-shRNA plasmids for 6 h. The cells were re-seeded into 96-well plate (8 × 103 cells/well) and treated with different concentration of anticancer drugs for 72 h, followed by MTT assay and IC50 measurement.
Xenograft tumor model
5-week-old female NOD/SCID mice and 6- to 8-week-old female BALB/c nude mice were purchased from SPF Biotechnology Co. Ltd in China. For knockdown experiment, the NOD/SCID mice were divided equally into two groups (n = 6 per group) and subcutaneously injected with AGS cells stably expressing pRS-sh-TRIM17 or pRS-sh-Control (8 × 106 cells/per mice). The body weight of the mice, length and width of tumors were measured twice a week. All mice were sacrificed at the 90th day post inoculation, and the tumors were collected, photographed and weighted. For overexpression experiment, the BALB/c nude mice were randomly divided into two groups (n = 5 per group) and subcutaneously implanted into the flank with xenografted HGC27 tumor mass (cut into equal pieces, 2 mm × 2 mm) stably expressing pCMV6-TRIM17-Flag or a control vector. After 7 days, the tumors were examined every 2 days. Mice were sacrificed at the 45th day after implantation, and the tumors were collected, photographed and weighted. During these procedures, measurement and analyses were performed in a double-blinded manner.
Immunoblotting and immunoprecipitation
Immunoblotting (IB) was performed as described before [40]. For protein half-life analysis, cells were treated with 50 μg/ml CHX for the indicated time. The band intensities were quantified by ImageJ software. For immunoprecipitation (IP) analysis,
cells were treated with MG132 (20 μM) for 6 h prior to harvest and lysed with Triton X-100 buffer (150 mM NaCl, 50 mM Tris, and 1% Triton X-100, pH 7.5) for 30 mins at 4 °C. After incubation with 20 μl anti-Flag/anti-HA magnetic beads or 20 μl Protein A/G magnetic beads containing 4 μl indicated antibodies overnight at 4 °C, the immunocomplexes were washed five times with lysis buffer and analyzed by IB.
RT-qPCR analysis
Total mRNA extraction and the RT-qPCR analysis were performed as described before [40]. Sequences of primer pairs were as follows. TRIM17: GACATGGAGTACCTTCGGGA (forward), GCAGTCTCCTCTTCTTCCGT (reverse); BAX: CCCGAGAGGTCTTTTTCCGAG (forward), CCAGCCCATGATGGTTCTGAT (reverse); GAPDH: CATGAGAAGTATGACAACAGCCT (forward), AGTCCTTCCACGATACCAAAGT (reverse).
Immunohistochemistry (IHC) analysis
IHC in human GC tissue microarray (purchased from Shanghai Outdo Biotech Co., Ltd., China, #HstmA180Su15) containing 82 pairs of clinical GC tissues and adjacent tissues was stained with TRIM17 or BAX at 1:25 dilution. The IHC score was calculated by multiplying the staining intensity by the percentage of positive staining. For TRIM17 assessed, the staining intensity was evaluated with scores ranging from 0 to 4 as follows: 0 ≤ IHC score < 1 (±); 1 ≤ IHC score < 2 (+); 2 ≤ IHC score < 3 (++); and 3 ≤ IHC score < 4 (+++). According to the staining results and survival data, the Kaplan-Meier survival curve was plotted using GraphPad Prism 8. The xenografted tumor tissues for IHC staining were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 3-μm thickness. The slides were stained with anti-TRIM17 (1:1500), anti-Ki-67 (1:4000), anti-Cleaved Caspase 3 (1:1000), or anti-BAX (1:200) antibody. All stained slides were digitized with a slice scanning system (Panoramic MIDI, 3DHISTECH). Cells with brown intracellular granules (cytoplasm or nucleus) were considered as positively stained. Five high-power fields (HPF) at × 250 magnification were captured from each section for semi-quantitative analysis. The integrated optical densities (IOD) of positive expression were measured by Image-Pro Plus (IPP) software.
Protein expression and purification
Transetta (DE3) chemically competent cells (TransGen Biotech Co., Ltd, China, #CD801-02) transformed with pGEX-4T-1-GST-TRIM17 or pGEX-4T-1-GST-TRIM17-ΔR were plated on LB agar plates (containing 100 μg/ml ampicillin) and incubated at 37 °C overnight. The grown cell colony was inoculated into 5 ml liquid LB medium (containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol). After incubated at 37 °C for 24 h, the cell suspensions were transferred into 1 L liquid LB medium for incubation until OD600 reached 0.5. The protein expression was induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 22 °C overnight. Then the cells were collected by centrifugation and resuspended in 50 ml PBS. The resuspension was lysed by the Continuous Flow Cell Disruptor (Constant Systems Limited, UK) and centrifuged at 12000 rpm for 30 mins, followed by loading on 5 ml GSTrapTM 4B (GE Healthcare, #28-4017-47) column and incubation at 4 °C for 2 h. The bound proteins were then eluted by elution buffer (20 mM reduced glutathione, 10 mM Tris-HCl, PH 8.0), followed by dialysis at 4 °C overnight and storage at -80 °C in 50% glycerol.
GST pull-down assay
An equal amount of GST or GST-TRIM17 was mixed with His-BAX (OriGene Technologies, #TP720866), and incubated with glutathione-Sepharose 4B beads (GE Healthcare, #17-0756-01) at 4 °C overnight. After washing, the bound proteins were detected by IB with antibodies against GST and BAX.
Ubiquitination assay
For in vivo ubiquitination assays, the wild-type and mutant constructs were co-transfected into HEK293T cells for 72 h and treatment with MG132 (20 μM) for 6 h. For the HA-beads capture assay, the cells were harvested and lysed with RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.1% SDS, 5 mM EDTA, and 0.5% deoxycholic acid sodium salt, pH 7.4) containing protease inhibitors (Roche, #11697498001). After sonicating, 20% of the lysates were added with 5 × loading buffer, boiled at 100 °C for 10 mins and served as input samples. The remaining lysates were incubated with 5 μl anti-BAX antibody (#89477) or 30 μl anti-HA magnetic beads at 4 °C overnight. After washing, the beads-protein complex was boiled for 10 mins at 100 °C prior to IB analysis.
For the Ni-nitrilotriacetic acid (NTA) capture assay, 20% of the cells were harvested and lysed with RIPA lysis buffer. After sonicating and centrifuging, the lysates were added with 5 × loading buffer and boiled for 10 mins at 100 °C and stored at -80 °C as input samples. The remaining cells were lysed in 1 ml highly denaturing buffer A1 (6 M guanidium-HCl, 10 mM Tris-HCl, 100 mM Na2HPO4/NaH2PO4, 5 mM imidazole, pH 8.0). After sonicating and incubating on the ice for 30 mins, the lysis solution was added with 50 μl Ni-NTA His-tag Purification Agarose (MCE, #HY-K0210) and incubated at 4 °C for 16 h. The beads-protein complex was washed with buffer A2 (6 M guanidium-HCl, 10 mM Tris-HCl, 100 mM Na2HPO4/NaH2PO4, 10 mM β-mercaptoethanol, pH 8.0), buffer B (10 M Urea, 10 mM Tris-HCl, 100 mM Na2HPO4/NaH2PO4, 10 mM β-mercaptoethanol, pH 8.0), buffer C1 (8 M Urea, 10 mM Tris-HCl, 100 mM Na2HPO4/NaH2PO4, 10 mM β-mercaptoethanol, 0.2% Triton X-100, pH 6.3), and buffer C2 (8 M Urea, 10 mM Tris-HCl, 100 mM Na2HPO4/NaH2PO4, 10 mM β-mercaptoethanol, 0.1% Triton X-100, pH 6.3), respectively. After washing, the ubiquitinated proteins were eluted with 50 μl 1 × elution buffer (20 mM pH 6.8 Tris-HCl, 10% Glycerine, 0.8% SDS, 0.1% Bromophenol blue, 720 mM β-mercaptoethanol, 300 mM imidazole) and boiled at 100 °C for 5 mins. The complex was centrifuge at 12000 rpm for 2 mins and supernatants were collected as IP samples.
For in vitro ubiquitination assay, the recombinant proteins of His-BAX and GST, GST-TRIM17 or GST-TRIM17ΔR were added into a reaction system comprised of 20 × E1 ubiquitin-activating enzyme, 10 × E2 ubiquitin-conjugation enzyme (UbcH6), ATP and 20 × biotinylated ubiquitin according to the manufacturer’s instructions in the Ubiquitination Kit from Enzo Life Science (#BML-UW9920-0001). After incubation at 37 °C for 8 h, the reaction system was added with 2 × non-reducing gel loading buffer and boiled at 100 °C for 10 mins prior to IB analysis.
Mass spectrometry analysis
After IP and fractionation on SDS-PAGE, proteins were stained with Coomassie stain and target strips were cut, followed by mass spectrometry analysis using high-resolution orbitrap LC/MS instrument in Human Phenome Institute, Fudan University.
Statistical analysis
Data were expressed as Mean ± SD from at least three independent experiments. All the statistical analyses were performed using independent-samples t-test between two groups and one-way ANOVA in multiple groups via SPSS 23.0 for data comparison. For IB analysis, representative data from at least three independent experiments was shown. The Kaplan-Meier method was used to draw overall survival curves, and the difference in survival was analyzed using the Log-rank test. Differences were identified as significant at three levels: *P < 0.05, **P < 0.01, ***P < 0.001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This project is sponsored by the National Natural Science Foundation of China (Nos. 82002966; 82073241), the CAMS Innovation Fund for Medical Sciences (Nos. 2021-I2M-1-030; 2022-I2M-2-002; 2022-I2M-1-016) and the National Key Research and Development Program (2022YFC2804700).
Author contributions
ZW, JS, and HY designed the experiments; JS and HY performed most of the animal, functional, and biochemical experiments; HY performed bioinformatical analyses and tissue IHC staining; XQ, YC, LZ, and JL(in) performed some animal and biochemical experiments; JL(ang), QY and ZW assisted in the analysis of some experiments and interpreted the data; ZW, JS and HY drafted the manuscript; ZW conceived and supervised the project. All authors have reviewed and approved the submission.
Data availability
The TGCA data in Fig. 1A and supplementary Fig. S1A-B were downloaded from http://xena.ucsc.edu/public/. The Kaplan-Meier survival analysis in Figs. 1B and 7C was downloaded from https://www.kmplot.com. The GSEA data in Fig. 3A and supplementary Fig. S3A were retrieved from http://www.gsea-msigdb.org/gsea/downloads.jsp. All of the original immunoblots were provided as Supplementary material. Other raw data that support the findings of this study can be made available upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics
All animal experiments were approved by the Ethics Committee of the Institute of Medicinal Biotechnology, Peking Union Medical College and Chinese Academy of Medical Sciences in China, and conducted in accordance with the regulations and operational procedures of experimental animal management.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiajia Shen, Hang Yang.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01221-1.
References
- 1.World health organization, international agency for research on cancer 2023. GLOBOCAN 2020. Accessed January 2, 2023. gco.iarc.fr.
- 2.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264–79. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selim JH, Shaheen S, Sheu WC, Hsueh CT. Targeted and novel therapy in advanced gastric cancer. Exp Hematol Oncol. 2019;8:25. doi: 10.1186/s40164-019-0149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42:297–311. doi: 10.1016/j.tibs.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 6.Venuto S, Merla G. E3 ubiquitin ligase TRIM proteins, cell cycle and mitosis. Cells. 2019;8:510. doi: 10.3390/cells8050510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu SM, Kozoriz A, Desagher S, Lassot I. To ubiquitinate or not to ubiquitinate: TRIM17 in cell life and death. Cells. 2021;10:1235. doi: 10.3390/cells10051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo H, Ikeda K, Urano T, Horie-Inoue K, Inoue S. Terf/TRIM17 Stimulates degradation of kinetochore protein ZWINT and regulates cell proliferation. J Biochem. 2012;151:139–44. doi: 10.1093/jb/mvr128. [DOI] [PubMed] [Google Scholar]
- 9.Lionnard L, Duc P, Brennan MS, Kueh AJ, Pal M, Guardia F, et al. TRIM17 and TRIM28 antagonistically regulate the ubiquitination and anti-apoptotic activity of BCL2A1. Cell Death Differ. 2019;26:902–17. doi: 10.1038/s41418-018-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 13.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–9. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 14.Nunes CT, Miners KL, Dolton G, Pepper C, Fegan C, Mason MD, et al. A novel tumor antigen derived from enhanced degradation of Bax protein in human cancers. Cancer Res. 2011;71:5435–44. doi: 10.1158/0008-5472.CAN-11-0393. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of Bax protein for cancer therapy. Med Res Rev. 2016;36:313–41. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA. 2000;97:3850–5. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson BN, Berger AK, Cortese GP, Lavoie MJ. The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc Natl Acad Sci USA. 2012;109:6283–8. doi: 10.1073/pnas.1113248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charan RA, Johnson BN, Zaganelli S, Nardozzi JD, LaVoie MJ. Inhibition of apoptotic Bax translocation to the mitochondria is a central function of parkin. Cell Death Dis. 2014;5:e1313. doi: 10.1038/cddis.2014.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cakir Z, Funk K, Lauterwasser J, Todt F, Zerbes RM, Oelgeklaus A, et al. Parkin promotes proteasomal degradation of misregulated BAX. J Cell Sci. 2017;130:2903–13. doi: 10.1242/jcs.200162. [DOI] [PubMed] [Google Scholar]
- 20.Benard G, Neutzner A, Peng G, Wang C, Livak F, Youle RJ, et al. IBRDC2, an IBR-type E3 ubiquitin ligase, is a regulatory factor for Bax and apoptosis activation. EMBO J. 2010;29:1458–71. doi: 10.1038/emboj.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan Y, Wang YY, Liu X, Luo B, Zhang L, Zheng F, et al. KPC1 alleviates hypoxia/reoxygenation-induced apoptosis in rat cardiomyocyte cells though BAX degradation. J Cell Physiol. 2019;234:22921–34. doi: 10.1002/jcp.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrawal SG, Liu FT, Wiseman C, Shirali S, Liu H, Lillington D, et al. Increased proteasomal degradation of Bax is a common feature of poor prognosis chronic lymphocytic leukemia. Blood. 2008;111:2790–6. doi: 10.1182/blood-2007-10-110460. [DOI] [PubMed] [Google Scholar]
- 23.Liu FT, Agrawal SG, Gribben JG, Ye H, Du MQ, Newland AC, et al. Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood. 2008;111:2797–805. doi: 10.1182/blood-2007-08-110445. [DOI] [PubMed] [Google Scholar]
- 24.Magiera MM, Mora S, Mojsa B, Robbins I, Lassot I, Desagher S. Trim17-mediated ubiquitination and degradation of Mcl-1 initiate apoptosis in neurons. Cell Death Differ. 2013;20:281–92. doi: 10.1038/cdd.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grice GL, Nathan JA. The recognition of ubiquitinated proteins by the proteasome. Cell Mol Life Sci. 2016;73:3497–506. doi: 10.1007/s00018-016-2255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D, Wu R, Zheng J, Li P, Yu L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018;28:405–15. doi: 10.1038/s41422-018-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng R, Zhu J, Deng S, Shi H, Xu S, Wu H, et al. Targeting BAX ubiquitin-binding sites reveals that BAX activation is essential for its ubiquitin-dependent degradation. J Cell Biochem. 2020;121:2802–10. doi: 10.1002/jcb.29505. [DOI] [PubMed] [Google Scholar]
- 28.Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: navitoclax as a pro-apoptotic and anti-fibrotic agent. Front Pharm. 2020;11:564108. doi: 10.3389/fphar.2020.564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manoochehri M, Karbasi A, Bandehpour M, Kazemi B. Down-regulation of BAX gene during carcinogenesis and acquisition of resistance to 5-FU in colorectal cancer. Pathol Oncol Res. 2014;20:301–7. doi: 10.1007/s12253-013-9695-0. [DOI] [PubMed] [Google Scholar]
- 30.Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15:8617–22. doi: 10.7314/APJCP.2014.15.20.8617. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Xing D, Liu L, Gao B. Regulation of Bax activation and apoptotic response to UV irradiation by p53 transcription-dependent and -independent pathways. Cancer Lett. 2008;271:231–9. doi: 10.1016/j.canlet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Mandell MA, Jain A, Arko MJ, Chauha S, Kimura T, Dinkins C, et al. TRIM proteins regulate authphagy and can target autophagic substrates by direct recognition. Dev Cell. 2014;30:394–409. doi: 10.1016/j.devcel.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandell MA, Jain A, Kumar S, Castleman MJ, Anwar T, Eskelinen EL, et al. TRIM17 contributes to autophagy of midbodies while activety sparing other targets from degradation. J Cell Sci. 2016;129:3562–73. doi: 10.1242/jcs.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong T, Zhang J, Liu X, Li H TRIM17-mediated ubiquitination and degradation of RBM38 promotes cisplatin resistance in non-small cell lung cancer. Cell Oncol (Dordr). 2023;10.1007/s13402-023-00825-6. [DOI] [PubMed]
- 35.Wei Y, Zhang L, Wang C, Li Z, Luo M, Xie G, et al. Anti-apoptotic protein BCL-XL as a therapeutic vulnerability in gastric cancer. Anim Model Exp Med. 2023;6:245–54. doi: 10.1002/ame2.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nor Hisam NS, Ugusman A, Rajab NF, Ahmad MF, Fenech M, Liew SL, et al. Combination therapy of navitoclax with chemotherapeutic agents in solid tumors and blood cancer: a review of current evidence. Pharmaceutics. 2021;13:1353. doi: 10.3390/pharmaceutics13091353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassot I, Robbins I, Kristiansen M, Rahmeh R, Jaudon F, Magiera MM, et al. Trim17, a novel E3 ubiquitin-ligase, initiates neuronal apoptosis. Cell Death Differ. 2010;17:1928–41. doi: 10.1038/cdd.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mojsa B, Mora S, Bossowski JP, Lassot I, Desagher S. Control of neuronal apoptosis by reciprocal regulation of NFATc3 and Trim17. Cell Death Differ. 2015;22:274–86. doi: 10.1038/cdd.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sha MQ, Zhao XL, Li L, Li LH, Li Y, Dong TG, et al. EZH2 mediates lidamycin-induced cellular senescence through regulating p21 expression in human colon cancer cells. Cell Death Dis. 2016;7:e2486. doi: 10.1038/cddis.2016.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen J, Li P, Shao X, Yang Y, Liu XJ, Feng M, et al. The E3 ligase RING1 targets p53 for degradation and promotes cancer cell proliferation and survival. Cancer Res. 2018;78:359–71. doi: 10.1158/0008-5472.CAN-17-1805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The TGCA data in Fig. 1A and supplementary Fig. S1A-B were downloaded from http://xena.ucsc.edu/public/. The Kaplan-Meier survival analysis in Figs. 1B and 7C was downloaded from https://www.kmplot.com. The GSEA data in Fig. 3A and supplementary Fig. S3A were retrieved from http://www.gsea-msigdb.org/gsea/downloads.jsp. All of the original immunoblots were provided as Supplementary material. Other raw data that support the findings of this study can be made available upon reasonable request.