Abstract
In the past decades the incidence of colorectal cancer (CRC) in people under the age of 50 has increased, which is referred to as early-onset CRC or young-onset CRC (YO-CRC). YO-CRC is expected to account for 11% of colon cancers and 23% of rectal cancers by 2030. This trend is observed in different parts of the world and in both men and women. In 20% of YO-CRC cases, a hereditary cancer syndrome is found as the underlying cause; however, in the majority of cases no genetic predisposition is present. Beginning in the 1950s, major changes in lifestyle such as antibiotic use, low physical activity and obesity have affected the gut microbiome and may be an important factor in YO-CRC development. Owing to a lack of screening, patients with YO-CRC are often diagnosed with advanced-stage disease. Long-term treatment-related complications should be taken into account in these younger patients, making the more traditional sequential approaches of drug therapy not always the most appropriate option. To better understand the underlying mechanism and define relationships between environmental factors and YO-CRC development, long-term prospective studies are needed with lifestyle data collected from childhood.
ToC blurb
Young-onset colorectal cancer occurs in individuals younger than 50 years of age and is increasing in incidence worldwide. This Primer provides an overview of the epidemiology, diagnosis, screening, prevention, pathophysiology and management of this cancer and it’s impact on patient quality of life.
Introduction
Colorectal cancer (CRC) is the third most common cancer and cause of cancer-related death in both men and women worldwide [1]. In the past 10 years, the incidence of colorectal cancer has remained stable or declined in high-income countries, while it has increased in low- and middle-income countries [2, 3]. The decline may partially be explained by the implementation of effective CRC screening programmes in high-income countries [4], which generally target average-risk populations 50–75 years of age.[5] While CRC incidence stabilized in high-income countries in individuals ≥50 years of age, it increased rapidly in individuals <50 years of age, which has been defined as young-onset CRC (YO-CRC) [6, 7]. This increase in YO-CRC has occurred in both women and men globally [7–9]. The threshold of 50 years of age for the definition of young-onset could be reconsidered. For example, in a study from Europe the largest increase in YO-CRC incidence rate was observed within the age group 20–39 years. [7] It was also shown that considerable differences were present in clinicopathological features in CRCs diagnosed in the 20–39 year group compared with the 40–49-year group. [10] Based on these differences in incidence between the different age groups of YO-CRC, and strengthend by differences in clinicopathological findings, this age-threshold may change over time. In the USA, population-based screening is currently initiated at the age of 45 years. In time, this programme will probably also affect the trends in incidence of YO- CRC, especially for the 45–50 years.
As the majority of YO-CRC studies utilize an age threshold of 50 years and this age is currently the inflection point for the age-dependent change in CRC incidence and also the age at which most countries initiate CRC screening for the average-risk population, we have used the threshold of <50 years of age to define YO-CRC in this Primer.
The reasons for the increase in YO-CRC are not well-understood and seem to reflect a birth-cohort effect [G] [7, 11, 12]. Most patients with YO-CRC do not have a genetic predisposition, therefore it is thought that lifestyle risk factors such as obesity, antibiotic use, low physical activity and diet may be important in YO-CRC development. These risk factors can also be linked to the major changes that took place in lifestyle and social trends due to the economic prosperity from the 1950s.
Individuals with hereditary colon cancer syndromes such as Lynch syndrome [G] (also known as hereditary non-polyposis colorectal cancer (HNPCC)) or familial adenomatous polyposis [G] (FAP) have a higher risk of CRC development and these tumours occur most often at an earlier age. Although the age of onset (<50 years) may be similar for sporadic YO-CRC and hereditary syndrome-associated YO-CRC, there are differences in time of diagnosis, prevention and management. Detection of YO-CRC at an early stage remains challenging, especially for sporadic YO-CRC, because individuals below the age of 50 years without a family history of CRC or hereditary syndrome are not routinely screened for CRC.
In this Primer, we discuss the epidemiology, risk factors as well as diagnosis and management of sporadic and hereditary YO-CRC. We also highlight the effect of this disease on patient quality of life and consider outstanding research and clinical questions.
Epidemiology
Global incidence trends
The increasing incidence of CRC in young adults was first reported in 2003 in the USA. This observation was based on data from the nine Surveillance, Epidemiology, and End Results (SEER) registries for the period 1973–1999. [13] In this time frame, colon cancer incidence in older adults remained stable and rectal cancer incidence decreased, whereas colon and rectal cancer incidence in younger adults increased by 17% and 75%, respectively. Despite the low absolute numbers of CRC cases in the young population, the rise in CRC incidence is significant, and younger patients present with more advanced staged tumours in the colon (25.8% versus 35.3%, P <0.001) and the rectum (38.4% vs. 41.7%, p = 0.005). [13]
In 2009, the American Cancer Society (ACS) followed up on this observation with an analysis of 13 SEER cancer registries to examine CRC trends in the period 1992–2005 among 20–49-year-old participants.[14] Using joinpoint analysis [G] to detect changes in incidence patterns over time by 10-year age groups, the results suggested that overall incidence of CRC per 100,000 young adults increased by 1.5% per year in men and by 1.6% in women in the period 1992–2005. This sharp increase was most notable in non-Hispanic white individuals. In this group, the largest annual percentage increase in CRC incidence was observed in the 20–29-year age group [14]: 5.2% in men and 5.6% in women. [14]
In 2017, CRC incidence for five-year age groups were examined by age-period-cohort modelling [G] of SEER cancer registry data for the period 1974–2013 [15] Age-specific relative risk of CRC declined for birth cohorts from 1890 until 1950, but increased for cohorts born in the period 1950–1990. Compared with adults born around 1950, those born around 1990 have 2-fold higher risk of colon cancer (IRR=2.40, 95% CI 1.11–5.19) [15] and 4-fold higher risk of rectal cancer (IRR=4.32, 95% CI 2.19–8.51). [15] Globally, studies in high-income countries illustrate that CRC incidence is increasing in adults under the age of 50 in nearly all countries, except for Japan, where despite having the highest overall CRC incidence in the world, CRC incidence decreased in individuals under 50 years of age since the early 1990s. [16] A population-based annual incidence analysis from the Cancer Incidence in Five Continents (CI5plus) database concluded that, in the 42 countries examined, CRC incidence in individuals 20–49 years of age was highest in South Korea (12.9 cases per 100,000 population; 95% CI 12.6–13.3), Australia (11.2 cases per 100,000 population; 95% CI 10.9–11.5) and the USA (10.0 cases per 100,000 population, 95% CI 9.8–10.3). [8]
YO-CRC incidence is projected to continue rising. By 2030, ~11% of all colon cancers and ~23% of all rectal cancers are predicted to occur in individuals under 50 years of age. [5, 17] According to an analysis of SEER registry data, colon cancer incidence in Americans 20–34 years of age is expected to increase by 90% by 2030 and by 124.2% for rectosigmoid and rectal cancers. [18] A 2020 report suggests that half of all new CRC diagnoses are in individuals 66 years of age or younger.[19] Whereas the incidence of CRC in individuals over 65 years of age continues to decline (by 3.3% annually), the incidence of tumours in the proximal and distal colon and the rectum in younger individuals in the USA continues to rise (by approximately 2% annually). [19, 20]
Hypotheses of sporadic YO-CRC incidence trends
The rise in YO-CRC may be attributed to an overall increase in CRC incidence within all ages. The fact that the increase is not seen in the older population may be explained by the fact that most of the older population are screened and benefit from the preventative effects of colonoscopy.[21] In addition, in developed countries CRC-related healthcare are often performed in high-resource settings with easy access to care which also may attribute to a decrease in CRC incidence in the older ages. In the past decades, economic migration has increased. A recent review has pointed to the impact of disparites to YO-CRC.[22] There are notable environmental risk factors and exposures to consider for such disparities. Obesity and type-two diabetes are especially prevalent among African Americas and Hispanics, especially with respect to childhood obesity. These populations may also have poorer quality diets and increased rates of television viewing and decreased physical activity.
Age-period cohort analysis in the USA suggests an increased risk of YO-CRC in sequential birth cohorts starting in 1950 with a continuing rise in incidence in subsequent birth cohorts. [15] The rise started with people born in 1946–1964 and is currently the highest for those born in 1965–1980. [5, 15] In the short period of time in which the incidence changes manifested, lifestyle and environmental factors would have seen more dramatic changes than would have been possible by population genetics. [5] These effects suggest exposures occurring early in life, as well as other different exposures over time. Alternative hypothesis clarifying the opposing incidence trends of increasing YO-CRC and decreasing overall CRC may include three hypotheses. First, within the (genetic) diathesis of CRC, recent environmental changes might have led to earlier disease expression. The early cases substitute for cases that, previously, would have been observed years or decades later in the same person. Current guidelines recommend colonoscopy screening at an earlier age for individuals with a heriditary colorectal cancer syndrome, familial CRC or a long history of inflammatory bowel disease. Second, the two incidence trends represent separate events, each with their own etiopathogenesis, and overlap in affected age groups partially obscures the real trend dynamics. Third, both preceding models account for some portion of the cases. As understanding of the interplay between genetic and environmental triggers of pathogenesis increases, the importance of the three hypotheses will be clarified.
Risk factors
Sex and race.
There are notable differences in the risk of YO-CRC between males and females. SEER-based studies covering the period 2004–2015 reported an increased mortality risk for YO-CRC among males compared with females (odds ratio (OR) 1.09; 95% CI 1.08–1.11). [23] Studies of US-based electronic health records reached similar conclusions, with males more likely than females to develop YO-CRC (OR 1.44; 95% CI 1.11–1.87). [24] More research is needed to determine the aetiology of these sex-specific differences.
The incidence of young-onset CRC also differs by race, although these differences are not necessarily biological. Increases in YO-CRC incidence have been driven primarily by the rise in incidence of rectal cancer, mostly among white individuals. A 2019 analysis noted that in 2010–2014, rectal cancer incidence and relative survival were similar in black and white populations, suggesting that differences in the aetiology of colon and rectal cancer might provide important clues to the rising incidence of CRC among younger adults. [25] However, relative survival remains significantly worse among black than white individuals, even among black patients who present with early-stage CRC. [26] As noted earlier, the incidence of YO-CRC is rising exponentially among the Hispanic population — the fasting growing demographic in the USA — with an annual rise of 2.4% versus 2.0% for the white population. [22] This trend is not unique to the USA and is also observed in Asia, with the incidence of YO-CRC increasing among men and women in Taiwan and Korea. Annual increases in YO-CRC incidence are noted in Japan as well, although these increases (both in males and females) do not seem to be statistically significant. In Hong Kong, colon cancer incidence among females is rising whereas it is stable among males. [27]
Lifestyle and diet.
Data from the US Food and Agricultural Office suggest that the consumption of sugar, meat and alcohol increased markedly after World War II.[28, 29]. Interestingly, the most rapid increases in YO-CRC diagnoses were observed in countries with the highest overall CRC incidence, such as South Korea. The reasons for the increased rates of CRC in South Korea have not yet been conclusively elucidated but may be related to the change in diet that occurred during the economic boom following the Korean War, which ended in 1953. Analyses from the Korean National Nutrition Survey illustrate that in response to food shortages in the late 1960s, many wheat-derived processed foods were introduced in the 1970s. The fast-food movement in South Korea, which was especially popular among the youth, began shortly after the introduction of these processed foods in the late 1970s. [8] Consumption of ultra-processed, high-fat foods – such as fast-food – are associated with a 10-fold increased risk of developing CRC, and high-fat diets increase the risk of YO-CRC by almost 2-fold (OR = 1.98, CI = 1.13–3.49). [30, 31]
Obesity and diabetes.
While more age-period cohort studies are needed to ascertain long-term generational exposures with respect to YO-CRC, the incidence of many risk factors such as obesity and type 2 diabetes mellitus are rising at the same time. Increasing evidence points to obesity as a risk factor for YO-CRC.[32, 33] Moreover, sedentary jobs have increased by 83% since 1950, and according to US Bureau of Labor Statistics, deskbound service-related professions currently comprise 43% of all US-based jobs.[34] [16, 35] According to the Framingham Heart Study, the prevalence of obesity has been on the rise since the 1950s, with the incidence of overweight increasing 2-fold and obesity more than 3-fold over the span of five decades.[36] A Nurses Health Study prospectively examined the association of weight gain and obesity in early adulthood with the risk of YO-CRC. Women who were overweight early in life had a higher risk of YO-CRC compared to those with leaner BMIs (RR 1.63; 95% CI 1.01–2.61).[37] An analysis of the Nurses’ Health Study II showed that obesity and weight gain since early adulthood were associated with increased YO-CRC risk. [37] In a German case-control study, elevated BMI at 20 or 30 years of age or 10 years prior to YO-CRC diagnosis were risk factors for YO-CRC development, even in persons without a family history of CRC. [38] A meta-analysis of six studies showed similar trends of YO-CRC incidence with obesity and weight gain. [33] Obesity also results in prenatal programming to YO-CRC. A prospective cohort study in California examined the associations of maternal obesity, pregnancy weight gain, and birth weight to subsequent CRC diagnosis in adult offspring. [39] Maternal overweight (hazard ratio (HR) 2.12; 95%CI 1.18, 3.82) and obesity (HR 2.51; 95% CI 1.05–6.02) were associated with increased risk of CRC in offspring. More age-period cohort studies are needed to elucidate the magnitude of these risk factors over a long period of time.
A 2022 review [21] postulated that the increase of YO-CRC incidence mirrors the global trends in the prevalence of diabetes, which has increased from 30 million cases of diabetes in 1964 to 171 million cases 40 years later. A Swedish cohort study observed that individuals with a type-two diabetes mellitus diagnosis before 50 years of age had a 3.5-fold (95% CI 2.3–5.1) increased risk of YO-CRC.[40]
Genetic risk factors.
An inherited component is thought to be present in up to 30% of all CRC cases, mainly owing to a first -degree relative with CRC, whereas a germline mutation is present in around 5% of all CRC cases. [41] Currently, no true population-based studies exist that have assessed the prevalence of germline variants in YO-CRC. However, some studies suggest that a pathogenic germline variant associated with a higher colorectal cancer risk is present in up to 20% of patients with YO-CRC. [42, 43] Data are limited regarding whether the incidence of these inherited variants is increasing in the general population. [42] Two multigene panel testing studies examined the incidence of germline mutations in large cohorts of patients with YO-CRC (450 patients in the Ohio study and 403 in the clinic-based Michigan study). [43, 44] The studies found that 16% (Ohio) and 25% (Michigan) of the YO-CRC cases have germline mutations associated with polyposis syndromes, including 8.4% and 13.9% with Lynch syndrome, 1.1% and 2.5% with FAP, 0.9% and 0.5% with MUTYH-associated polyposis (MAP), respectively.
Mechanisms/pathophysiology
Oncogenesis pathways
CRC pathogenesis generally involves genetic alterations that disrupt DNA repair mechanisms, resulting in aberrant crypt formation in the colon, followed by adenomatous or serrated polyps formation and accumulation of additional mutations in signalling pathway components. Further mutations can result in adenomatous/serrated polyps forming colorectal tumours. The chromosomal instability (CIN) pathway is involved in oncogenesis of 85 % of CRCs. This pathway leads to mutations in the gene encoding adenomatous polyposis coli (APC), which is involved in WNT/β-catenin signalling, resulting in the formation of aberrant crypt foci in the colon. Aberrant crypt foci formation is one of the earliest changes in the colon and is followed by adenomatous polyp formation. If KRAS and/or TP53 mutations occur within the adenomatous polyp, the transition to CRC will occur. A TP53 mutation is present in approximately half of all CRC cases. The prevalence of TP53 mutations is reported to be higher in YO-CRC, whereas that of mutations in APC and KRAS is significantly lower than in later-onset CRC. [45, 46]
CRC oncogenesis can also occur via alternate pathways involving the formation of mutations in BRAF and NRAS. BRAF mutations are considered drivers of the serrated pathway [G] [and are present in 7% of CRCs. [47] NRAS suppresses apoptosis [48] and pathogenic variants are less prevalent in this gene than in BRAF in CRC. For example, in a small study of 69 patients with YO-CRC, 4% had a NRAS mutation. [49] The exact prevalence of pathogenic variants in KRAS, NRAS and BRAF in YO-CRC and how this relates to their prevalence in later-onset CRC is difficult to determine, as the data are conflicting and not all studies were able to exclude patients with Lynch syndrome, who may have biased the results [10].
Microsatelite instability [50] is found in more than 90% of patients with Lynch syndrome, while most sporadic YO-CRCs are microsatellite stable (MSS) and lack DNA repair mechanism abnormalities. [21] However, in comparison to late-onset CRC, YO-CRC tumours show a higher prevalence of the MSI-high phenotype and lower prevalence of BRAF mutations and the CpG island methylator phenotype-high (CIMP-high). [51] CpG island methylation is another oncogenic pathway in CRC, as this post-translational modification causes silencing of genes. CIMP overlaps with CIN or MSI pathways, and is associated with the sessile serrated pathway. CIMP is absent in Lynch syndrome-related CRC regardless of age of diagnosis. In contrast to late-onset CRC, CIMPlow is more often present in YO-CRC.[52].
While the underlying genetic defects differ significantly between Lynch syndrome and FAP, the pathogenetic mechanisms in these two disorders represent canonical pathways of CRC carcinogenesis. The main signalling pathways underlying the adenoma to carcinoma sequence are the DNA repair, WNT, PTEN/PI3K, TGF/BMP and RAS/MAPK pathways. (see also figure 3).
Figure 3. Pathogenetic mechanism of sporadic YO-CRC.
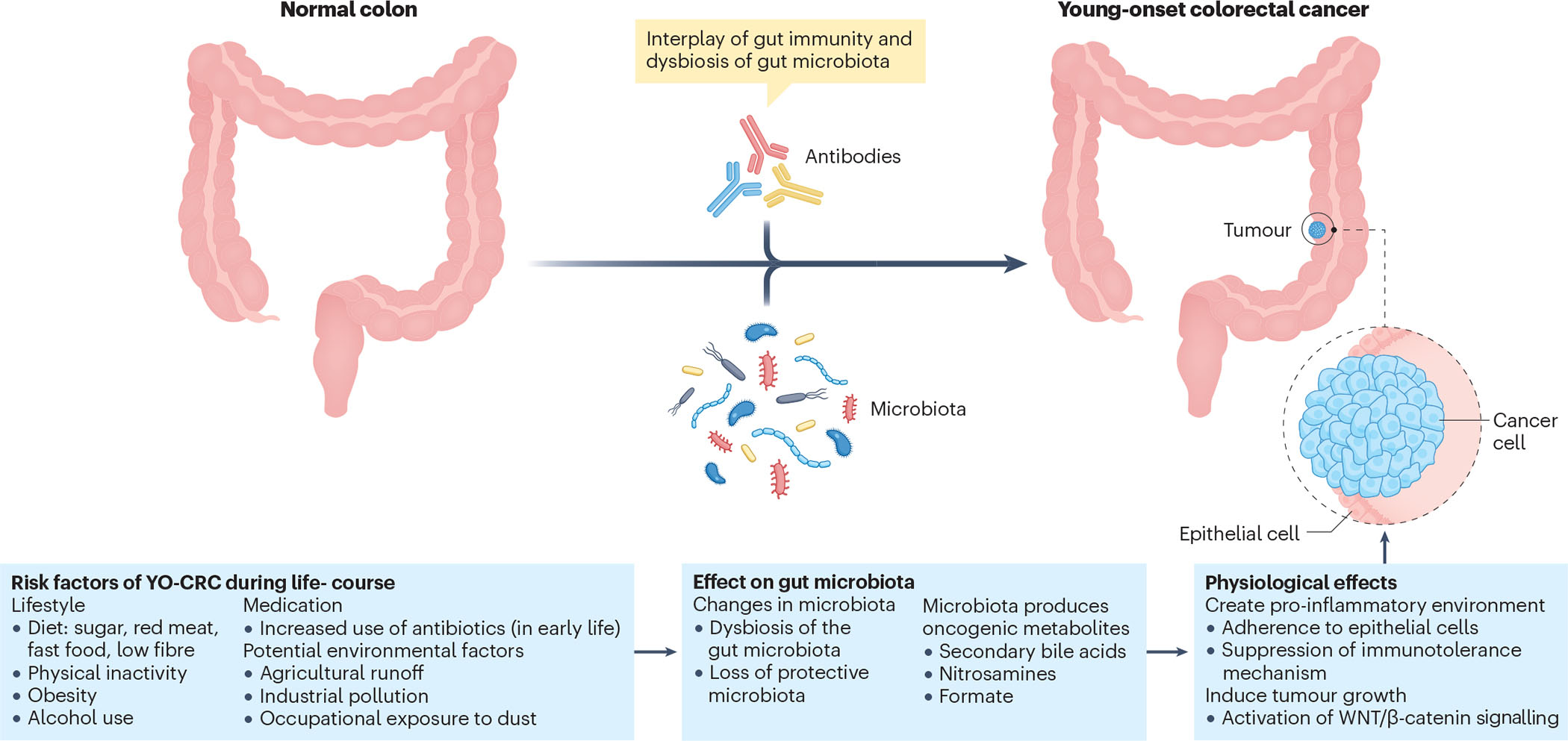
Examples of risk factors, during the life-course that may be involved in the development of young-onset colorectal cancer (YO-CRC), including environmental exposures, medication use and diet. All these factors influence the gut microbiota, creating a susceptible environment for CRC by inducing inflammation, suppressing immunity and promoting tumour growth.
Sporadic YO-CRC
In contrast to inherited YO-CRC, where a germline pathogenic variant in a cancer susceptibility gene is identified, the pathogenesis of sporadic YO-CRC is unknown. [53]. Half of all CRC cases and deaths in all ages in the USA are attributable to modifiable risk factors such as smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body fat.[54] Another risk factor for YO-CRC is chronic inflammation of the colon, as has been shown in patients with a long history of inflammatory bowel disease.[24] The most plausible explanation for the trend of increasing sporadic YO-CRC prevalence is probably an interplay of lifestyle changes and environmental risk factors (Box 1) initiating oncogenetic pathways related to the development of CRC.
Box 1. Lifestyle and environmental risk factors associated with CRC.
Positive association
Red and/or processed meat consumption
Poor dietary patterns
Obesity
Antibiotic use
Changes in gut microbiota
Diabetes mellitus
Alcohol consumption
Inflammatory bowel disease
Negative association
Micronutrient consumption
Physical activity
Aspirin use
Other possible risk factors
Low birth weight
Childhood obesity
Cesarean delivery
Breastfeeding
Infectious agents
In the USA, geographical differences in environmental exposures could be associated with risk of YO-CRC and provide clues to causation. These exposures could include agricultural runoff, industrial pollution and occupational exposure to dusts. [5] The mechanisms underlying the most common risk factors for the development of YO-CRC are described below.
Lifestyle and diet.
Processed foods and red meat contain carcinogenic compounds that are mainly generated during processing and/or cooking. The most studied class of carcinogenic compound is N-nitroso compounds, polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (HAAs). N-nitroso compounds and PAHs may become apparent in cured or smoked red meat, while HAAs are produced when meat is heated at high temperatures. [55] Aside from these compounds, other chemicals could be responsible for the carcinogenicity of red and processed meat, including environmental pollutants (such as heavy metals) and organic contaminants that may be present in greater or smaller quantities in raw and unprocessed meat or be modified by coocking.[56]. The underlying mechanism of these compounds and increased CRC risk is caused by a direct carcinogenic effect on the colonic epithelium. [57] and may be summarized in three mechanism [58]: increased levels of N-nitroso compounds leads to DNA adducts that result in abnormal replication and development of cancerous cells; haem- and food metabolites induce proliferation of the colonic epithelium; and an inflammatory response that may trigger several malignant processes. [56]
Alcohol use is another lifestyle risk factor that is associated with increased risk of CRC development, although the exact mechanism is largely unknown. Proposed mechanisms include dysbiosis of the gut microbiota that results in inflammation and exposure of the colonic epithelium to carcinogenic alcohol metabolites causing DNA damage of the exposed colonic epithelium. [59]
Obesity.
Obesity could be an independent YO-CRC risk factor or a surrogate for other factors such as red meat consumption or low consumption of high-fibre foods. The mechanisms for this effect are unknown but might include insulin resistance, systemic inflammation, and/or an altered microbiota. [21] Hyperglycaemia and hyperinsulinaemia and abnormalities in the insulin-like growth factor I axis have a role in carcinogenesis. Intestinal cell proliferation can be induced by both hyperinsulinaemia and hyperglycaemia, by stimulation of MAPK pathways through the insulin-like growth factor receptor and the WNT/β-catenin signalling pathway.[60] Subclinical inflammation is associated with carcinogenesis, and obesity is a state of chronic inflammation.[61] Endotoxinaemia owing to leakage of bacterial products from the intestines into the blood and hypoxia ensued by adipose tissue may have important roles in the chronic inflammatory state in individuals with obesity [62]. The hypoxia also triggers alterations in adipokines, which in combination with an increased endoplasmic reticulum stress response and mitochondrial dysfunction, might be associated with CRC development in individuals with obesity. [63] Fusobacteria, one of the gut microbiota are elevated in the saliva and intestines of individuals with obesity.[64] Fusobacterium causes inflammation by inducing production of several proinflammatory factors such as IL-1, IL-6, IL-8, TNF, COX2 and matrix metalloproteinase 3, which are associated with CRC development [7]. Promotion of β-catenin, TLR4 and p21-activated kinase 1 signalling are other mechanisms whereby Fusobacteria might be linked to CRC development.
Antibiotics.
As mentioned previously, a strong birth-cohort phenomenon has been demonstrated, with CRC falling in cohorts born earlier in the twentieth century and rising in those born later (YO-CRC); the inflection point is 1952. [15] This timing is consistent with the widespread introduction of antibiotic use in children, beginning in the late 1940s and increasing thereafter. If antibiotic use was critical for YO-CRC oncogenesis, then a likely mechanism would be via their effects on the colonic microbiota. Two hypotheses have been proposed. First, antibiotic use selects for one or more taxa in early life, which over years or decades promote oncogenic events. This model resembles the hypothesis regarding the carcinogenic role of Helicobacter pylori in the stomach, where its persistence drives or accelerates the normal senescent sequence from dysplasia toward malignancy.[66] Alternatively, early-life antibiotic exposure leads to loss of protective microbiota members that curtail a pathogenetic pathway, analogous to the relationship between the loss of H. pylori, especially cagA+ strains, and the development of oesophageal adenocarcinoma. [66, 67] The absence of these H. pylori strains has been associated with this cancer and its precursor lesions, reflux oesophagitis, and Barrett’s oesophagus. [66] How can loss of an organism fuel an oncogenic process? Persistent H. pylori gradually reduces the ability of the stomach to produce acid; in its absence, full acid production continues, damaging the adjacent oesophagus. H. pylori is also involved in regulation of gastric hormones, which participate in oncogenesis in the distal oesophagus. [68] The opposing trends in gastric cancer falling in the elderly, new gastric cancers rising in the young, and rising incidence of oesophageal adenocarcinoma may represent microbe-induced phenomena paralleling oncogenesis in the colon. [69]
A second hypothesis is that antibiotics have reshaped colonic microbiota composition toward increased oncogenesis. Support comes from the Nurses study that identified 16,642 individuals who had screening colonoscopy performed after the age of 60 years and who had extensive medical records beginning decades earlier. [70] Of these individuals, 1,195 had colonic adenomas, which are well-recognized CRC pre-malignant lesions. Exposure to antibiotics between the ages of 40 and 59 years was significantly associated with adenomas after the age of 60, with a strong dose–response relationship; antibiotic exposure between the age of 20 and 39 years showed parallel relationships. Thus, pre-malignant lesions were associated with antibiotic exposure years or decades earlier. A meta-analysis of six studies provides further support for the role of antibiotic exposure in colonic neoplasia in general, with a stronger association for colonic than for rectal cancers and associations with certain antibiotics (penicillins, cephalosporins) but not others, often relating to wide confidence intervals. [71–74]
Microbiota.
The microbiota produces metabolites that may be oncogenic with long periods of exposure, including secondary bile acids, nitrosamines and formate. [75, 76] The microbiota also has strong bidirectional interactions with host, involving both innate and adaptive immune responses. [77, 78] Diet strongly influences the microbiota, so that dietary links to CRC may be operating via an altered microbiome. [79] Fusobacterium nucleatum, Bacteroides fragilis and Escherichia coli are the most common gut bacteria that are related to late-onset CRC.[80], and below we discuss their involvement in YO-CRC.
F. nucleatum is a gram-negative anaerobe that can induce inflammation in the colon and favours tumour growth. [64] From their niche in the oral mucosa, F. nucleatum bacteria may reach the colon haematogenously and/or by transit through the gastrointestinal lumen. [81] The presence of F. nucleatum has been associated with CRC lesions in some but not all studies; the reasons for these discrepancies include heterogeneity of bacterial strains studied, differences in populations and tissues sampled, and study power. [82] F. nucleatum may create a pro-inflammatory environment in the colon, via adherence to epithelial cells and/or by suppressing immunotolerance mechanisms, and it activates WNT/β-catenin signalling and thereby accelerates cellular proliferation. [83–86] F. nucleatum is now well-recognized as being present in both pre-malignant lesions and tumours, but whether it is an early initiating factor or a later tumour-promoter has not been clearly elucidated. [87] Aside from the higher prevalence of F. nucleatum in the gut microbiota of individuals with obesity than of those without obesity, there is currently no evidence that F. nucleatum is directly related to YO-CRC. Although the gut microbiota contains commensal E. coli strains, some of which have pathogenic potential.[88] One of these strains is polyketide synthase-positive (pks+) E. coli, which promotes carcinogenesis and facilitates CRC progression by producing colibactin (the pks gene product). Colibactin is a genotoxin that induces double-strand DNA breaks, cell cycle arrest, senescence and chromosomal abnormalities.[89]. In one study, pks+ E. coli colonization of the gut microbiota was substantially lower in patients with YO- CRC than those with late-onset CRC (20% versus 52%, respectively). [90] However, this result does not rule out the possibility that pks+ E. coli may have been present earlier in life and eliminated, while the effect on CRC development manifests later in life.[91]
In summary, antibiotic exposure, an altered microbiota and obesity have all been linked to increased YO-CRC prevalence. A unifying hypothesis is that antibiotic exposure, essentially a birth cohort phenomenon since the early 1950s, perturbed the gut microbiome in early life, which predisposes individuals to both obesity and YO-CRC. The more proximate mechanisms are more obscure but operate over years and decades.
Hereditary YO-CRC
Familial CRC.
While ‘familial’ CRC has not been formally defined in the setting of YO-CRC, this group has been used to include patients who do not harbour an identifiable germline pathogenic variant by clinical multiplex testing but report a family history of CRC in at least one first- or second-degree relative. Around 25% of patients with YO-CRC have a first degree relative with CRC. [43] [24] A small proportion of patients with familial CRC harbour rare, low-frequency germline variants that confer elevated CRC risk, albeit substantially lower than that for germline variants associated with a hereditary syndrome. For example, the United Kingdom National Colorectal Cancer Group analysed high-coverage exome sequencing data for 1,006 familial YO-CRC cases and found highly penetrant [G] rare mutations in 16% of these cases.[92] While several genes, such as POT1, POLE2 and MRE11, were considered new candidate CRC predisposition genes, the molecular mechanism of oncogenesis remains unknown in the majority of patients with familial YO-CRC. Current clinical genetic risk assessment relies on family history, tumour phenotype and age of diagnosis to identify individuals for whom germline DNA sequencing is warranted. Single-nucleotide polymorphism (SNP) data from large genome-wide association studies (GWAS) has been analysed to generate a polygenic risk score (PRS) that can be used to predict cancer risk. For example, a GWAS analysed data for 12,197 individuals <50 years of age and 95,865 individuals >50 years of age and identified 95 SNPs that were used to generate a PRS, which more accurately predicted risk of YO-CRC than of late-onset CRC. A follow-up of this analysis identified individuals who would most benefit from anticipatory screening at 45 years of age. [242, 243]
Inherited CRC syndromes.
The incidence of a germline pathogenic variant in a cancer susceptibility gene is substantially higher in YO-CRC (50% of people with YO-CRC and a hereditary cancer syndrome having no family history of CRC in a first-degree relative) than in CRC overall (20% of cases). [43, 93] For these reasons, genetic testing with a multigene panel has been advocated in patients with YO-CRC. [94] Inherited CRC syndromes have classically been broadly categorized into those that cause polyposis [G] and those that do not. FAP is the most common polyposis syndrome and Lynch syndrome is the most common non-polyposis syndrome. These highly penetrant CRC predisposition syndromes are associated with a substantial lifetime risk of CRC, ranging from 15–52% for different Lynch syndrome pathogenic variants to almost 100% in classic FAP. [95] Other hereditary CRC predisposition syndromes include MUTYH-associated polyposis, polymerase proofreading-associated polyposis (PPAP), Peutz–Jeghers syndrome (PJS), juvenile polyposis syndrome (JPS), PTEN hamartoma tumour syndrome (PHTS; also known as Cowden syndrome), mixed polyposis, and serrated polyposis. [96, 97] These less penetrant CRC predisposition syndromes are associated with lower lifetime risk of CRC than for the more penetrant syndromes, albeit still substantially higher than for sporadic CRC (4.5%). Both highly- and less penetrant syndromes are associated with a higher risk of being diagnosed with CRC at much younger ages than the average age of onset for sporadic CRC. [96]
In patients with YO-CRC, the most prevalent germline pathogenic variants are in the Lynch syndrome-associated genes MLH1, MSH2, EPCAM, MSH6 and PMS2, which occur in 2–5% of patients. [98] Although less common than Lynch syndrome-associated pathogenic variants, other pathogenic variants occur in moderate- and low-penetrance genes, such as heterozygous MUTYH and APC p.I1307K, and high-penetrance genes, including APC, SMAD4, BMPR1A, biallelic MUTYH, biallelic NTHL1, STK11, TP53, GREM1, POLE, and POLD1.[96] New evidence suggests that pathogenic variants in ATM and germline sequence variation in other base excision repair pathway genes such as Neil1 may also increase CRC risk. [99]Although some of these genes have been associated with increased CRC risk and have specific screening recommendations, the clinical significance of many of the other variants in CRC has not been determined. [92, 100–105]
Lynch syndrome is the most common inherited CRC and accounts for approximately 2–16% of YO-CRC cases. [43, 101, 103–105] Lynch syndrome is an autosomal dominant condition characterized by highly penetrant germline pathogenetic variants, and an increased risk of extracolonic cancers, including gynaecological (endometrial and ovarian), gastrointestinal (gastric, small intestinal and pancreatic), and genitourinary (transitional cell tumours of ureters, bladder and prostate) cancers, sebaceous adenomas and skin carcinomas. Germline alterations include pathogenic variants in the mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2 or deletions in EPCAM. In case of EPCAM, germline deletions of the last few exons of the EPCAM gene (EPCAM 3’end deletions) are involved and result in Lynch syndrome by epigenetic silencing of MSH2.[106] Pathogenic variants in Lynch sydnrome genes lead to a loss of functional mismatch repair proteins, which results in MSI [50]. [107] Mismatch repair genes are important for repairing incorrect pairing of nucleotide bases during DNA replication. When mismatches are not corrected, the resulting copy of the gene does not function correctly, leading to an increased cancer risk. Lynch syndrome-associated CRC tends to develop at younger ages (before 50 years of age) and progress more rapidly compared with sporadic CRC. [108, 109]
Familial adenomatous polyposis (FAP) is an autosomal dominant condition that accounts for approximately 1% of all CRC cases and is associated with pathogenic variants in the adenomatous polyposis coli (APC) gene. In approximately 30% of FAP cases, the index case is the first individual diagnosed in the family and represents a de novo mutation. [110] In these cases, the offspring of the index case carry a 50% risk of inheriting the pathogenic variants and the associated high risk of young-onset adenomas and CRC. The classic form of FAP is associated with hundreds to thousands of polyps throughout the colon, whereas 20–100 polyps are present in attenuated FAP, a form of FAP with later onset than classic FAP. AFAP is associated with mutations in three parts of the APC gene (in the first 5 exons, exon 9 and the last 3 exons). Early identification and surveillance is important, as adenomas may start to form at ~12 years of age in classic FAP, with ~50% of patients with classic FAP develop adenomas by age 15 and 95% by age 35.[111]
In most hereditary CRC syndromes, polyps undergo malignant transformation, but the exact route to CRC seems to differ among the various conditions. Discovery of the key pathogenic variants in these syndromes has been instrumental to our understanding of the underlying molecular mechanisms of CRC oncogenesis.
Diagnosis, screening and prevention
Diagnosis
Most CRCs in young patients are identified due to the onset of signs and symptoms rather than incidentally or during screening, probably because most guidelines recommend colonoscopic screening for CRC from the age of 50 years; or 45 years in the American Cancer Society guidelines (Grade B recommendation). [95, 96, 112–114]
The lack of screening in younger patients, more aggressive tumour features, and patients and clinicians ignoring or misinterpreting symptoms [98] lead to a higher percentage (85%) of patients with YO-CRC presenting symptomatically at diagnosis and, therefore, with later stage cancers than for CRC overall (50%), of which many cases are detected by screening. [98] Furthermore, because clinical strategies that rely on family history and tumour phenotypes for germline testing often miss carriers of pathogenic variants, it is suggested that germline testing with multigene cancer panels should be considered for all YO-CRC cases. [43]
The most common presenting symptoms in YO- CRC are abdominal pain (46%) and rectal bleeding (47%), while weight loss is the least common symptom (8%). [115–120] Other common symptoms at diagnosis include abdominal distension, change in bowel habits, and fatigue. [116, 121–123]
If a patient presents with CRC-related symptoms, a colonoscopy with biopsy of the malignant tumour is indicated, except when an endoscopically resectable tumour is expected during colonoscopy, in which case the biopsy should be omitted because it might hinder radical removal of the malignant tumour.
Same as in late-onset CRC, YO-CRC are staged according to the American Joint Committee on Cancer (AJCC) TNM system. The TNM system categorizes patients based on the extent of the tumor into the wall of the colon or rectum (T), nearby lymph nodes involvement(N) and if distant metastasis are present (M)
Screening
The rising incidence of YO-CRC can be addressed by preventive measures (see later discussion) as well as early diagnosis. Primary prevention measures prevent the onset of the disease and will decrease CRC incidence, secondary prevention measures usually focus on early detection of the disease (that is, screening) and will decrease CRC-related mortality. In the case of CRC, secondary prevention measures will both decrease CRC mortatlity by detecting CRC at an early stage, and decrease CRC incidence by detection and removal of polyps preventing the onset of the disease.
General screening considerations.
There is a wealth of evidence that screening reduces the incidence and mortality of colorectal cancer. [124] This evidence pertains to both non-imaging screening (that is, stool-based), predominantly performed by immunochemical testing for occult blood in the faeces, as well as imaging-based screening, predominantly performed by endoscopy. CRC screening has been implemented in many countries, with most organized population screening programmes focusing on individuals 50–75 years of age. [125, 126] Some programmes, such as the Dutch and the English national CRC screening programmes, even use a higher starting age (55 and 60 years, respectively), the latter due to limited colonoscopy capacity. Since 2021, however, the UK bowel screening program is expanding to make it available to everyone from the age of 50 onwards, which will be gradually implemented over 4 years. Organized programmes that offer screening before the age of 50 years were first implemented in Italy and Japan. [125] In the United States, the US Multi-Society Task Force on Colorectal Cancer in 2017 recommended to start screening at the age of 50, but consider to start at age 45 in individuals of African-American descent. [127] This guideline was updated in 2022 to recommend starting screening at age 45 in all individuals with average risk of CRC. [128] This update followed similar earlier recommendations by the American Cancer Society and the US Preventive Services Task Force. [129, 130] Other guidelines, such as the Canadian and the Asia-Pacific guidelines, maintain 50 years as the recommended age for starting widespread CRC screening. [131, 132]
Lowering the starting age of population screening for sporadic CRC to 45 years seems to be cost-effective. [133, 134] In a Markov model analysis [G], screening 1,000 persons between 45 and 50 years of age is cost-effective, but a greater benefit, and at lower cost, was observed when higher participation rates in unscreened older individuals (55–75 years of age) and higher-risk people was achieved. [133] A study using three microsimulation models found that of 49 screening strategies that were efficient according to all three models, 41 (83%) had a starting age of 45 years. [135] These strategies encompassed screening with stool tests, endoscopy and CT-colonography. A modelling study focused on the Australian FIT screening programme concluded that lowering the starting age from 50 years to 45 years could be cost-effective but would increase the demand for colonoscopy by 3–14%. [136] The authors concluded that their results support the existing Australian practice to start population screening at age 50.
Screening for patients with Lynch syndrome.
All CRC or advanced adenomas should be assessed for evidence of MMR deficiency (dMMR) as an initial screening test for possible underlying Lynch syndrome. A dMMR CRC is identified by immunochemical staining for MMR protein and/or PCR-based MSI testing. In patients with dMMR CRC, somatic causes of dMMR, such as BRAFV600E mutation or MLH1 promoter hypermethylation, should be sought, but in the absence of such evidence, germline testing should be performed to confirm a Lynch syndrome diagnosis.[137–139] Universal MSI testing for Lynch syndrome in all cases of CRC is recommended, to increase the diagnosis of Lynch syndrome and reduce morbidity and mortality owing to Lynch syndrome-associated cancers. [95, 112, 140–142] However, in clinical practice MSI testing rates remain low, even in high-risk individuals.[143] Insufficient MSI testing will lead to unidentified patients with Lynch syndrome (and their relatives) or those with dMMR CRC. The underdiagnosis of dMMR CRC will affect CRC management, as MSI-high and dMMR CRCs are more likely to respond to immune-checkpoint inhibitors. [[144] As universal germline genetic testing for Lynch syndrome is not currently accepted practice and is not cost-effective, tools such as the risk assessment algorithms PREMM5 and MMRpro can aid in the identification of individuals with Lynch syndrome based on personal and family history before a cancer diagnosis. [139, 140]
Cascade testing.
The discovery of a pathogenic variant in an affected proband provides an opportunity for at-risk relatives to pursue genetic testing, known as cascade testing, which can lead to personalized cancer risk management and risk reduction. Owing to privacy concerns, affected patients must directly communicate their genetic test results to at-risk relatives. While effective communication is necessary, the likelihood of cascade testing occuring is influenced by many other factors, including family dynamics, health knowledge, and the motivation and health status of the index patient. Cascade testing enables identification of shared mutations and medical management options for the newly identified pathogenic variant carrier, including risk reduction procedures and screening, which can be life-saving, [145] and results in reduced cancer mortality and healthcare costs. One analysis estimated that targeted cascade testing of at-risk relatives of all US carriers with pathogenic variants in 18 cancer susceptibility genes could be identified within 9.9 years of initial pathogenic variant discovery in the index patient if there was an uptake of 70% among first-, second- and third- degree relatives, compared with 59.5 years if the absence of cascade testing. However, other studies found that only 15–20% of at-risk relatives become aware of critical information that might motivate them to pursue genetic testing. In addition, only ≤30% of informed first- and second-degree relatives of tested pathogenic-variant- positive index patients contact genetic services to arrange their own genetic counselling and consider genetic testing. [146–148] Individuals who do pursue their own genetic testing are more likely to be female and more likely first- and second- degree relatives [149–151], with uptake drastically decreasing beyond the first-degree relative of the index patient. [152] Barriers to cascade testing include those at the provider, patient and system level, [153] such as lack of physician knowledge and/or lack of genetic counseling support, little communication between family members, poor understanding of the genetic condition or lack of awareness of the benefit to relatives, medical mistrust, and limited financial reimbursement or limited insurance coverage for testing. [154–162] If we are to realize the full potential of personalized genetic medicine, then improved strategies to offer genetic services to at-risk relatives are needed.
Surveillance
YO-CRC, a family history of CRC, and multiple polyps and/or primary tumours are all features associated with hereditary CRC syndromes. In addition, patients may present with a polyposis phenotype, or right sided polyps and/or serrated adenomas; therefore, colonoscopy is the optimal tool to screen these patients. Several expert groups have formulated detailed recommendations for surveillance of CRC and extracolonic cancers for the different inherited CRC syndromes. [95, 112] The age to start CRC screening differs between the hereditary syndromes.
Surveillance in patients with Lynch syndrome.
For patients with confirmed Lynch syndrome, surveillance colonoscopies should be performed every 1–2 years. Emerging data demonstrate that the age of CRC onset and overall CRC risk can differ depending on the specific MMR gene that is mutated. [140, 163] In patients with pathogenic variants of MSH6 or PMS2, CRC onset was delayed by 10 years compared with that in carriers of pathogenic variants in MLH1 or MSH2.[163–166] Based on the literature, the 2019 European and 2020 British guidelines suggest starting CRC screening at 25 years of age for individuals with pathogenic variants in MLH1 or MSH2, and at 35 years for those with pathogenic variants in MSH6 or PMS2.[167, 168] CRC screening by colonoscopy and removal of polyps seemed to be effective for CRC prevention in patients wth Lynch syndrome, with more than a 50% reduction in CRC risk and prevention of CRC-related mortality and a decrease in overall mortality by ~65% in families with Lynch syndrome. [169–171] Identification of new carriers by testing biopsy samples of advanced adenomas and CRC tumours from population-based CRC screening programmes for MMR dd not seem to be effective. [172] Patients with Lynch syndrome should also consider regular screening for other Lynch syndrome-associated cancers, based on personal and family history.
Surveillance in patients with classic FAP.
The cumulative risk of CRC development in patients with classic FAP can approach 100%. The standard of care for classic FAP includes dilient endoscopic screening combined with prophylactic surgery when the polyp burden is no longer endoscopically manageable. Diagnosis of CRC before the age of 20 years is unusual (~1.3%) [173], so colonoscopic screening is generally recommended every 1 to 2 years beginning at age 10–12 years. Most colonoscopies in children are performed under general anaesthesia, and to better assess polyp distribution throughout the colon a colonoscopy is recommended in all patients with classic FAP. [41, 140, 167] After colectomy, annual surveillance by flexible sigmoidoscopy of remaining rectal mucosa or ileal pouch is recommended every 6–12 months. Colon screening and colectomy decrease both CRC incidence and mortality in patients with classic FAP, and survival is improved in relatives who undergo screening. [171, 173–175]
Surveillance in AFAP and MAP.
In AFAP, the development of adenoma and CRC is delayed by 10-–20 years compared with classic FAP, with an average age at CRC diagnosis of 58 years of age (range 29–81 years). [176] For patients with AFAP or MAP, [CRC screening follows the same principle as for classic FAP: as a more proximal colonic polyp distribution can be present, colonoscopy every 1–2 years from the age of 18 years to the mid-20s is the CRC screening approach. Prophylactic surgery is indicated when the polyp burden becomes no longer endoscopically manageable.
Awareness and early diagnosis.
The high prevalence (10–35%) of pathogenic variants in patients with YO-CRC [6, 43, 44, 102] underlines the need for early identification of these carriers by means of adequate medical history taking, low threshold mutation assessment (by, for example, immunohistochemistry of tumour samples), and genetic analysis of family members of index cases. [177] Carriers of germline pathogenic variants should be offered close surveillance and, when required, targeted intervention. This approach can reduce cancer incidence as well as cancer stage at diagnosis. Furthermore, clinicians should be aware of the rising incidence of YO-CRC, which should alert them to include CRC in the differential diagnosis and take adequate diagnostic steps when seeing younger patients with symptoms compatible with CRC.
Prevention
A third to a half of all CRC cases, including YO-CRC, are attributable to modifiable risk factors, such as obesity, smoking, lack of physical exercise, and an unhealthy diet, which can be addressed through primary prevention measures. [178, 179] Emerging evidence shows that adherence to a Mediterranean diet and its related micronutrients [180], long-term aspirin use, [181] and physical exercise [182] are inversely associated with CRC risk.
Life-style measures in sporadic YO-CRC.
The highest CRC incidence is found in countries in which the diet includes predominantly red and processed meat and low amounts of fibre. This correlation implies that alimentary factors might have a role in both CRC development and prevention.[183] [184]. A meta-analysis found that CRC risk increases by 14 % for every 100g increase in daily red meat intake [185]. In addition, the processing of red meat by grilling or frying at high temperatures results in the formation of numerous carcinogenic proteins [186] These results indicate that dietary intake of fish, poultry, or legumes instead of red meat and avoiding high temperature methods to cook red meat would be beneficial in decreasing CRC risk. [183] A high-fibre diet consists of grains, fruits and vegetables, it dilutes colonic contents, and it increases bacterial fermentation by decreasing the colon transit time.[187] In a study including over 500,000 individuals 25–70 years of age and recruited from 10 European countries, total dietary fibre consumption was inversely associated with CRC risk[188]. However, the majority of data on lifestyle measures and CRC risk are based on CRC in older adults. In a prospective study from the Nurses’ Health Study II, dietary and lifestyle patterns during adulthood were studied in women (25–42 years of age at baseline), and CRC risk before the age of 50 years (YO-CRC) and after the age of 50 years were examined.[189] Both dietary and lifestyle patterns with high insulinaemic potential were associated with higher CRC risk in younger women. Insulin response is related to overall health, and diet modulates insulin response. The findings from this study suggest that a healthy diet and lifestyle with low insulinemic potential might be effective measures to reduce CRC risk in younger women. Hyperglycaemia and/or hyperinsulinaemia also have an important role in CRC development in individuals wth obesity and patients with diabetes mellitus. High levels of glycated haemoglobin (HbA1c) which reflect the levels of hyperglycaemia over time, are associated with increased CRC risk. [190] Adequate regulation of glucose levels might be important to reduce CRC risk. A controlled dietary intake and daily physical activity [179] are simultaneously demanding and require long-term commitment and adherence, both from the individual as well as from communities and public health organizations. The persistent uptake of these interventions to reduce cancer incidence is further impaired by the fact that they reduce relative risks and population incidence yet their effects cannot be extrapolated to an individual level. Increasing physical activity, changing diet, stopping smoking, reducing alcohol comsumption, and losing weight might all improve general well-being but cannot individually be translated into a number of cancers prevented. The same is true for the other risk factors, such as the gut microbiota and antibiotic use. Intervention studies evaluating changes in the gut microbiome and their preventive effect on CRC development would be of interest but are difficult to conduct. Apart from addressing the effects on CRC risk, broad preventive measures such as promoting healthy diets and physical activity and restricting prescription of antibiotics, are also relevant efforts to reduce the ever-increasing demand on healthcare in various parts of the world, which is already pushing towards its limitations in workforce, infrastructure, and finance.
In conclusion, various measures are needed to reverse the increasing incidence of YO-CRC. These include broad lifestyle measures as well as focussing on reducing health inequality, identifying carriers of pathogenic variants, increasing awareness and improving early diagnosis, and implementing population screening. These measures augment each other and together could have a considerable effect on YO-CRC incidence.
Management
Sporadic YO-CRC
The management of sporadic YO-CRC often requires a balanced consideration of multimodality therapy that includes surgical resection of tumours, systemic therapy, and judicious use of radiotherapy, aiming at optimizing disease cure while minimizing potential long-term complications of medical and surgical treatments. A patients’ medical fitness, clinical staging of the disease and molecular typing of the tumours are crucial factors in the formulation of a treatment plan. Traditional sequential approaches with different lines of drug therapy may not necessarily offer the best outcome and a personalized approach based on patient and tumour characteristics is preferred. International management guidelines for the treatment of YO-CRC have been drawn up by a group of 69 experts using a consensus process.[191] This guideline advocates germline genetic testing of multiple genes (including APC, BMPR1A, EPCAM, MLH1, MSH2, MSH6, MUTYH, POLD1, POLE, PMS2, PTEM, SMAD4, STK11 and TP53) in all patients with newly diagnosed YO-CRC, preferably before any modality of therapy is started. However, there is no evidence that endoscopic, surgical and systemic therapy should differ between YO-CRC and late-onset CRC, except in the case of individuals with a pathogenic germline variant. [191]
Endoscopic and surgical treatment.
Patients with YO-CRC tend to present with advanced-stage disease. Nevertheless, surgical treatment of YO-CRC follows the same stage-appropriate oncological principles as those for CRC in older patients, while often favouring minimally-invasive approaches. Five-year survival, recurrence, mortality, and complication rate differ for the different treatment option (Table 1), including endoscopic resection, laparoscopic colectomy, open colectomy and total meso-rectal excison.
Table 1.
Surgical treatment options in colorectal cancer
| Approach | 5-year survival | Recurrence | Mortality | Complications |
|---|---|---|---|---|
| Endoscopic resection | 90–100% | 13.6–18.7% (5-year) | 1.6–3.8% (5-year) | 0–9% |
| Laparoscopic resection | 94.2% | 16% (3-year) | <1% (30-day) | 19% |
| Open colectomy | 89.17% | 18% (3-year) | 1% (30-day) | 19% |
| Total mesorectal excision | 91.4% | 7.3% (5-year) | 0.8% (30-day) | 15–20% |
For selected early-stage lesions with only mucosal invasion (Tis-T1), several advanced endoscopic resection techniques have gained popularity, with data demonstrating technical safety, earlier procedural recovery and short-term outcomes comparable t standard surgical resection. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have become the standard practice in the treatment of early-stage colorectal cancer,as first recommended by the Japanese Gastroenterological Endoscopy Society in 2015 [192] and subsequently by the guidelines of the European Society of Gastrointestinal Endoscopy [193], the American Gastroenterological Association[194] and those of other nations. During these procedures, submucosal fluid injection creates a cushion below the mucosal layer that prevents perforation during resection. A protruding polypoid lesion without submucosal invasion can usually be treated with EMR. Depressed lesions, those >15mm in diameter and those that fail to lift after submucosal fluid injection are likely to have submucosal or deeper invasion, and therefore requir early complete surgical excision or ESD. In the last 8 years, endoscopic full-thickness resection (EFTR) has been used for deeply invading lesions in the colon and rectum. [195] As these are specialist techniques, they should only be performed in centres with ample experience of local resection. No head-to-head comparisons of endoscopic resection and surgery have been reported and long-term results of endoscopic treatment approaches are not yet available. A large series from 96 centres in the USA that included 1,234 patients undergoing EFTR reported a technical success rate of >90%, with a mean procedure time of <1h. [195]
The goal of curative-intent surgical resection for CRC is to resect the involved bowel segment and an appropriate margin a well as regional lymphadenectomy. [196, 197] Key oncological surgery approaches to minimize local recurrence and optimizing survival outcomes are complete meso-colic excision (CME), involving dissection of the embryological plane, and central vascular ligation, ensuring complete regional lymphadenectomy. [198] It has been argued that the whole colonic tumour and mesocolon belong to one distinct lymphovascular entity that is created separately in the same embryological origin. Therefore, in addition to removing the tumour and bowel segment, CME includes resection of the mesentry enveloping the tumour, lymph nodes draining the tumour, and vasculature of the segment. [199] During surgical exploration, the entire peritoneal cavity, including the pelvis and diaphragm, should be examined thoroughly. Extensive peritoneal metastasis undetected during preoperative clinical staging studies is rare but should prompt consideration of aborting CRC resection in favour of expeditious initiation of systemic therapy, particularly if the primary tumour is asymptomatic. [200]
Laparoscopic colectomy had showed favourable short- term outcomes (intraoperative complications, 30-day mortality and post-surgical complications) and equivalent long- term oncologic outcomes as open resection. [201]. A randomized controlled study in the USA compared laparoscopic colectomy to open colectomy and found that the rates of intraoperative complications, 30-day mortality and readmission were comparable for the two approaches but hospital stay was shorter (5 days versus 6 days) and use of parenteral narcotics was also shorter (3 days versus 4 days) with laparoscopic colectomy. [201] After a median long-term follow-up of 7-years, 5-year disease-free survival (68.4% versus 69.2%, P=0.94; respectively), overall survival (open versus laparoscopic: and 74.6 vs 76.4%, P=0.93; respectively), and overall recurrence rate (21.8 versus 19.4%, P=0.25; respectively) were similar. [50] Laparoscopic colectomy should be performed only by a surgeon skilled in laparoscopic surgery and experienced in CRC surgery. Patients who have bowel distention owing to obstruction; advanced malignancy not suitable for R0 resection; or an inability to tolerate pneumoperitoneum should not be offered laparoscopic colectomy.
Systemic therapy.
Systemic therapy using fluoropyrimidine-based chemotherapy agents (such as 5-fluorouracil, capecitabine, uracil–tegafur, TS-1 and TAS-102) as adjuvant therapy improves survival in stage III and selected cases of stage II disease compared with no adjuvant therapy.[202] Adding oxaliplatin to a fluoropyrimidine agent further enhances treatment efficacy, so the combination has become the standard adjuvant regimen. In certain low-risk (T1–3N1) CRCs (which comprises 60% of stage III tumours), a 3-month treatment regimen instead of the standard 6-month treatment regimen may reduce drug-related neuropathy without jeopardizing clinical outcome. [203] A pooled analysis of six randomized controlled phase III trials confirmed the non-inferiority of 3-month FOLFLOX (fluorouracil, leucovorin and oxalipatin) compared with 6-month therapy. [204]
Recurrent or metastatic CRC
For the treatment of metastatic YO-CRC, the approach is similar to late-onset CRC; that is, tumour characteristics and patient factors decide the choice of therapy. Systemic therapy using chemotherapy (fluoropyrimidine, oxaliplatin and irinotecan) as backbone combined with targeted agents (anti-VEGF and anti-EGFR antibodies) or immunotherapy (PD1 or CTLA4 antibodies) are recommended.[199, 205] CRCs with KRAS or NRAS mutations do not respond to anti-EGFR treatment; CRCs with BRAF mutations have poorer outcomes with anti-EGFR treatment than wild-type BRAF CRCs. In metastatic CRC with wild-type RAS and RAF, first-line treatment with anti-EGFR antibodies (cetuximab or pantimumab) or an anti-VEGF antibody (becavizumab) has comparable results.[206] The majority of BRAF mutations result in the V600E substitution. Compared with standard chemotherapy, patients with metastatic BRAFV600E CRC show improved progression-free and overall survival when treated with encoragenib plus cetuximab with or without binimetinib. [207] In a randomized study comparing addition of cetuximab or bevacizumab to FOLFOX or FOLFIRI (leucovorin, fluorouracil and irinotecan) for treatment of metastatic wild-type KRAS CRC, there was no difference in overall survival. [206]
In addition to tumour mutations, the anatomical location of CRC is an important consideration in the choice of therapy. A study analysing the pooled results from six randomized trials (CRYSTAL, FIRE-3, CALGB, PRIME, PEAK and 20050181) of metastatic wild-type RAS CRC treated with chemotherapy plus EGFR antibody or bevacizumab found that patients with left-sided CRC have more favorable responses to anti-EGFR antibodies and longer survival than those with right-sided CRC. [208] The biological basis for this difference has not been fully elucidated but is probably related to genomic, embryological and microbial factors. The proximal colon, from the caecum to the transverse colon, is derived from the embryonic midgut, which may have a different genomic mutations and microbiome distribution than the distal transverse colon to the rectum, which is derived from the embryonic hindgut. A trial comparing panitumumab and bevacizumab for metastatic, left-sided, wild-type-RAS CRC showed that anti-EGFR treatment achieved better overall survival than anti-VEGF treatment. [209]
Metastatic MSI-high or dMMR CRCs are more likely to respond to inhibitors targeting the immune-checkpoint proteins PD1 and CTLA4.[210] Since most YO-CRC cases of this type are associated with Lynch syndrome, management is as for YO-CRC in hereditary syndromes (described below ).
Rectal cancer
Because the complex anatomy, rich lymphatic network and limited space in the pelvis affect accessibility, surgery for rectal cancer is complex and requires expertise. Sphincter-preserving operative options include stapled low anterior resection or hand-sewn coloanal anastomosis, whereas non-sphincter operations include abdominal–perineal resection and/or any form of pelvic exenterative resection. Although the operative mortality and morbidity rates are very low today, the functional consequences of proctectomy or permanent ostomy remain significant and often require lifelong adaptation.
The optimal surgical approach for rectal dissection involves sharp dissection along embryological planes in the form of either tumour-specific or total mesorectal excision (TME), taking care to avoid blunt disruption of tissue planes, which may result in incomplete excision of tumour and/or nodal tissue. In tumours that involve structures beyond the TME plane, appropriate planes of dissection that provide an adequate radial margin should be followed. [211, 212] Patients with young-onset rectal cancer often present with locally advanced disease showing high risk features including threatened circumferential resection margin, extensive mesorectal and/or lateral pelvic adenopathy, extramural vascular invasion, and/or T4 disease; consequently, optimal curative-intent resection can adversely affect functional outcomes including bowel, bladder and sexual function, and should be proactively addressed. Minimally invasive platforms, either laparoscopic or robotic, have been well utilized in rectal cancer. Level I evidence has demonstrated non-inferior long-term oncological outcomes when comparing laparoscopic to open resections, whereas observational studies have reported similar outcomes for robotics versus open resections. [213] Finally, although transanal local excision or microsurgery enables more full-thickness local excision of rectal tumours without compromising anorectal function, they are only suitable for very early stage rectal lesions, which unfortunately are rare among patients with young-onsetrectal cancer.
The neoadjuvant and adjuvant therapies for locally advanced rectal cancer have evolved considerably over the past decade and show worldwide variation. Traditionally, in patients with clinical stage II and III rectal cancer, neoadjuvant long-course chemoradiation had been the most commonly used approach. The German Rectal Cancer Study Group had established the advantages of pre-operative versus post-operative chemoradiotherapy for locally advanced rectal cancer in reducing the risk of local recurrence of rectal cancer. [214] Pre-operative radiation also has a more favorable adverse event profile and helps in tumour debulking and in facilitating R0 surgical resection. Fluoropyrimidine treatment has typically been used to sensitize the tumours to radiotherapy.[215] Neoadjuvant short-course radiotherapy followed by either immediate or delayed surgery have also been utilized in some countries.[216] In the past 20 years, a total neoadjuvant apporach using oxaliplatin-based chemotherapy combined with long-course chemoradiation has been advocated for patients with high-risk locally advanced disease. [217] Evidence suggests that induction chemoradiation followed by consolidation systemic chemotherapy is not only safe and feasible but can result in a high rate of complete clinical response, providing an opportunity for the surgeon and the patient to discuss a watch and wait approach that allows preservation of the rectum.[218, 219] This approach is often attractive to patients with young-onset rectal cancer who face trade-offs of altered bowel function and/or permanent ostomy, but patient selection, close monitoring, and prompt salvage surgery remain key components of care. [220]
YO-CRC in hereditary syndromes
Germline cancer risk assessment should be considered a key component of patient-centric management of YO-CRC. Management of hereditary YO-CRC requires individualized consideration and should aim to optimize the outcome of the current YO-CRC, but also to address the potential and personal risks of other syndromic malignancies. In addition to management of the YO-CRC, clinical cancer genetics care, involving inclusion of a clinical geneticist as part of the multidisciplinary team, should be provided. [221] Management of the various hereditary syndromes is complex; the individualized planning and management for Lynch syndrome is discussed here in detail as an example. [221–223]
dMMR is the molecular hallmark of these CRC tumours in Lynch syndrome, and the management of dMMR YO-CRCs has been revolutionized by the advent of immunotherapy. For patients with Lynch syndrome who have stage IV YO-CRC, the current first-line therapy is PD1 blockade. [224] In the KEYNOTE 117 trial, immunotherapy demonstrated significantly longer progression-free survival (median, 16.5 vs. 8.2 months; hazard ratio, 0.60; 95% confidence interval [CI], 0.45 to 0.80; P = 0.0002)and fewer adverse events than chemotherapy. [225] Favorable response to upfront immunotherapy in the metastatic setting can potentially translate to improved conversion to and candidacy for potential curative-intent resection of metastatic disease. For patients with Lynch syndrome who present with locally advanced (stage II or III) YO-CRC, ongoing clinical trials are elucidating the affect of immunotherapy in the neoadjuvant and adjuvant settings. [226, 227] Previous data showed that, in the setting of stage II dMMR colon cancer, an adjuvant single-agent 5-FU-based regimen conferred little benefit, whereas in the setting of stage II or III dMMR rectal cancer, a neoadjuvant regimen of 5-FU-based radiosensitization and pelvic radiotherapy was associated with a complete pathological response rate of 27%. [228, 229] Neoadjuvant immunotherapy has been associated with excellent clinical and pathological response rates, highlighting a potential for non-operative management of dMMR localized solid tumours. Indeed, a landmark proof-of concept trial demonstrated that single-agent PD1 blockade achieved a clinical response rate of 100% in 12 patients with locally advanced dMMR rectal cancer, allowing organ preservation. [230] For patients Lynch syndrome who present with early-stage YO-CRC, surgical resection remains the mainstay of therapy.
In all cases where there is a role for curative-intent surgical management of Lynch syndrome-related YO-CRC, surgery should be planned and conducted with two considerations in mind. The first is to gain maximal local control and disease-free survival for the current CRC. Thus, oncological surgical principles of tumour handling with integrity, adequate regional lymphadenectomy, and en-bloc resection of involved adjacent viscera are respected. The second is to consider opportunities for preventing potential future metachronous CRC and for affecting syndrome-related cancers other than the index CRC. Extended resections reduce metachronous CRC risk but confer no proven survival benefit and are associated with worse long-term bowel function. [231–233] The risk of extra-CRC syndromic cancers can depend on the specific mutation, but no genotype–phenotype link has been firmly established. This risk is typically most pertinent for women with Lynch syndrome, whose pelvic organs (particularly the uterus and ovaries) are at risk. In these patients, endometrial surveillance should be considered and the question of prophylactic surgery when operating for YO-CRC is often raised and should involve an individualized, shared decision-making discussion. [234]
Quality of life
Quality of life can be profoundly impacted by cancer, and patients with YO-CRC may be uniquely affected (Box 2, Box 3). [235] Compared with their older counterparts, patients wth YO-CRC are often diagnosed with later-stage disease, arising from the distal colon and rectum. [236] Multimodality therapy including extensive pelvic surgery, pelvic radiation and systemic chemotherapy is, therefore, the standard management experience for many patients with YO-CRC. They may also face a higher risk for overtreatment, showing a tendency to receive more extensive and aggressive treatments. [237, 238] In addition, the cancer often disrupts the formative years of their adulthood, when values and expectations are being formulated while skills to cope with or decipher adverse life events may not yet have matured. [239–241] Thus, patients with YO-CRC likely experience unique medical, psychosocial, relationship and economic concerns, and their management must include care for the multiple dimensions of the whole patient.
Box 2 |. Experience of a patient with YO-CRC and a hereditary cancer syndrome.
“I am a two-time colorectal cancer survivor. I was first diagnosed at the early age of twenty years old. Not knowing how drastically cancer would change my life was indescribable. I truly don’t believe that I fully understood what cancer was, and what road it would take me down. As much as I tried to block that part of life out, the aftereffects of radiation, chemotherapy, and surgery always brought me back to reality: multiple trips to the bathroom, missing family events, get-togethers, feeling sick, exhausted, and drained, was my life. The radiation damaged my ovaries seventeen years ago, and the feeling of loss and failure as a woman was forever. After nineteen years, the fear of my cancer returning had a permanent residence in the back of my mind. One day after my colonoscopy, my fear had become my reality. I then learned that the only way for me to survive and live life without going to the restroom on myself was to have a permanent colostomy. For weeks, I couldn’t bring myself to believe how I would exist in my new way of life. I will always feel that with surgery and a permanent ostomy, a part of me died that day, while another part of me was born. When I awoke, I was literally a new person with a new way of living. Learning to fly again was not easy, both mentally and physically. I battled so many thoughts that were telling me this was a big mistake and there is no way I could do this. I contemplated back before the ostomy, on how I obsessed about every little thing about my body I didn’t like. Even though it took me time to accept, I told myself that no matter what, I would love myself for who I am, because what it took for me to get here, and the things that I’ve lost would never outweigh the things that I’ve gained. This is my new second chance at life, and I dare to love every single bit of it. I now know that it’s ok to dislike things that aren’t perfect, but I must love the things that are imperfect, because those are the things that make us who we are. Most of all I want to thank my new way of life, because without it, I wouldn’t get to see all the wonderful things it has brought me.”
Box 3. Experience of a patient with non-hereditary YO-CRC.
“I am a forty-five-year-old woman who has always been healthy, nor do I have a history of cancer in my family. When I started experiencing minor digestive issues, having cancer was the furthest thing from my mind. Receiving a cancer diagnosis is overwhelming, daunting, and filled with many different emotions; you worry about yourself, you worry about your family, and you start to contemplate your own mortality. It’s a truly unique experience. However, after the initial shock, I made the conscious choice to not fixate on my disease. I couldn’t change my diagnosis, but I could decide how I would react to it. At this critical time in my life, I knew that one of the most important decisions I would ever make was who I would trust to direct my care. I was pleasantly surprised that when I didn’t focus on cancer, and allowed my physicians to do their work, that cancer didn’t define me. This optimistic outlook allowed me to work, travel, spend time with my family, exercise, pursue my hobbies and interests and find a new kind of joy. I believe that this perspective really made a difference in my body’s ability to recover and heal. Cancer inevitably changes you, enlarging your empathy and awareness and giving you a new perspective on the fragility of life. While we do not choose cancer, we do choose how it shapes us going forward.”
Patients with YO-CRC with hereditary cancer syndromes have to contend with not only their current cancer diagnosis but also their underlying genetic condition. The underlying genetic condition can confer risks of multiple cancers in different organs, along with uncertainty regarding timing of onset, disease prognosis, and life expectations. [242] Key issues for patients include chronic fear of cancer diagnosis or recurrence, chronic dread and uncertainty, and limited resources to help them cope with the existential challenge posed by a cancer syndrome. Specifically, grief and anticipatory loss have emerged as essential distinguishing features in patients with hereditary versus sporadic cancer diagnoses. Thus, long-term engagement with bereavement training has been suggested.[242] Oncofertility considerations may be particularly complex in patients with Lynch syndrome, because they not only face decreased fecundity because of cancer treatments but also must consider genetic risks of endometrial cancer. [243] Nonetheless, long-term quality of life adaptation likely occurs, as a study comparing survivors of Lynch syndrome-related CRC versu. sporadic CRC found no statistical difference in self-reported QOL measured by the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) instrument (mean score of 84.8 versus 85.8; respectively). [244]
Multimodality therapy disproportionately affects patients with YO-CRC. Surgical treatment of the distal colon and rectum can lead to permanent bowel dysfunction, characterized as low anterior resection syndrome [G], and/or can result in sphincter loss and permanent ostomy. [245] Additional concerns include urinary and sexual dysfunction, perianal or peristomal disorders, and stricture formation. These adverse effects restrict diet, clothing, work, sports, travel, and social relationships. Indeed, social and emotional functional domains of QOL are most disproportionately affected. [246] In a survey of 830 long-term CRC survivors enriched for those surviving YO-CRC, profound differences in functional outcomes were identified, including persistently worse anxiety, body image, embarrassment about bowel movements, and social functioning, even after a median interval of 10 years after diagnosis. [247] Finally, oncofertility and financial toxicity are two critical areas requiring devoted attention in patients with YO-CRC. The multitude of options for sperm and embryo and oocyte preservation should be discussed prior to cancer treatments are formulated. [248]. A survey of CRC survivors in the USA found that young adults faced more out-of-pocket costs and medical debt during and after receiving cancer care than older adults. [249] In addition, increased depletion of coping resources, such as skipping medication and reduced spending on food, and higher psychological burden occurred more frequently in patients who were diagnosed before 40 years of age than in those diagnosed later in life. [249] The psychosocial consequences of financial toxicity can also affect quality of life. Taken together, the management of YO-CRC should encompass the whole patient. A proactive approach to understand and address these multi-dimensional needs would likely help improve the quality of their cancer survivorship.
Outlook
The rise in YO-CRC incidence was first described approximately 20 years ago in the USA. [13] Since then, a similar trend has been detected in many other parts of the world. [7–9] The cause of this increase in YO-CRC incidence is still not completely clear. In 20–25 % of YO-CRC cases, a hereditary cancer syndrome or family history of CRC is present, but whether hereditary cancer syndromes are also increasing in incidence in the general population is not known.
Studies on YO-CRC usually pool data from all patients with CRC under the age of 40 or 50 years and do not make a distinction between the different age groups or are unable to exclude patients with Lynch syndrome. It will therefore be important that future studies take these age groups into account and exclude hereditary cancer syndromes, especially considering most patients with YO-CRC do not have a genetic predisposition. This observation suggests that early and subsequent environmental exposures or lifestyle factors have an important role in YO-CRC development, of which the four most notable are obesity, antibiotic use, low physical activity, and dietary patterns, all of which affect the gut microbiota. Furthermore, the interplay between gut dysbiosis and gut immunity might also have a key role in CRC pathogenesis and YO-CRC development. However, which changes in the intestinal microbiota contribute to the development of CRC and YO-CRC and which prevent or protect against their developmentis are currently unknown. Given the preventive and therapeutic potential of interventions targeting the gut microbiota, further research on antibiotic use and its effect on the gut microbiota is needed. Collection of fecal samples at different time points in life might reveal the influence of environmental factors on the gut microbiota and provide insights into the underlying mechanism of CRC development.
Of note, data on environmental factors and YO-CRC risk are mostly derived from cross-sectional cohort studies, which can only detect associations but not infer causality. To better understand the underlying mechanisms and define the relationships between environmental factors, changes in the gut microbiota and YO-CRC development and life-long risk, long-term prospective studies collecting detailed lifestyle, antibiotic use and dietary data from childhood are needed. [6] Although it will take some time before outcomes of these long-term prospective studies become available, progress towards the implementation of these studies should not be impeded. This strategy will probably also elucidate other relationships between risk factors, the gut microbiota and any other disease occurrence.
Several large prospective cohort studies collecting data from children and pregnant women were initiated around the year 2000. [250–253] These studies were set up to provide population-relevant evidence about child development. The Child Health and Development Studies initiated in 1959 examined prenatal determinants of pregnancy outcomes, child health and development. These data can be a starting point to examine early-life or even pre- and post-natal risk factors for CRC.
Assessing whether CRC risk is reduced after elimination of risk factors, such as smoking, consumption of alcohol, highly saturated fats, high-sugar beverages, and red meat, and low physical activity, is of interest, particularly from a preventive perspective, but is also challenging to address. Interventional prospective studies might provide some insights but confounders owing to differences between individuals in CRC risk should be taken into account. The additional effect of environmental factors on CRC risk in patients with a hereditary syndrome is also not fully elucidated. For this group of patients, several large prospective cohort studies are underway [254–256] and the datasets from these studies could be harmonized and/or integrated to increase patient numbers and the validity of any findings. The studies of polygenic risk scores have been limited to white individuals of European ancestry and the clinical utility of these scores has not yet been determined. The use of polygenic risk scores should therefore be limited to research settings until further research is conducted. A better understanding of the pathogenetic mechanisms underlying YO-CRC will enable tailored screening and targeted interventions and might also provide new insights leading to the development of new therapies, which could eventually result in improved clinical outcomes for this group of young patients.
Figure 1. Global incidence of YO-CRC.
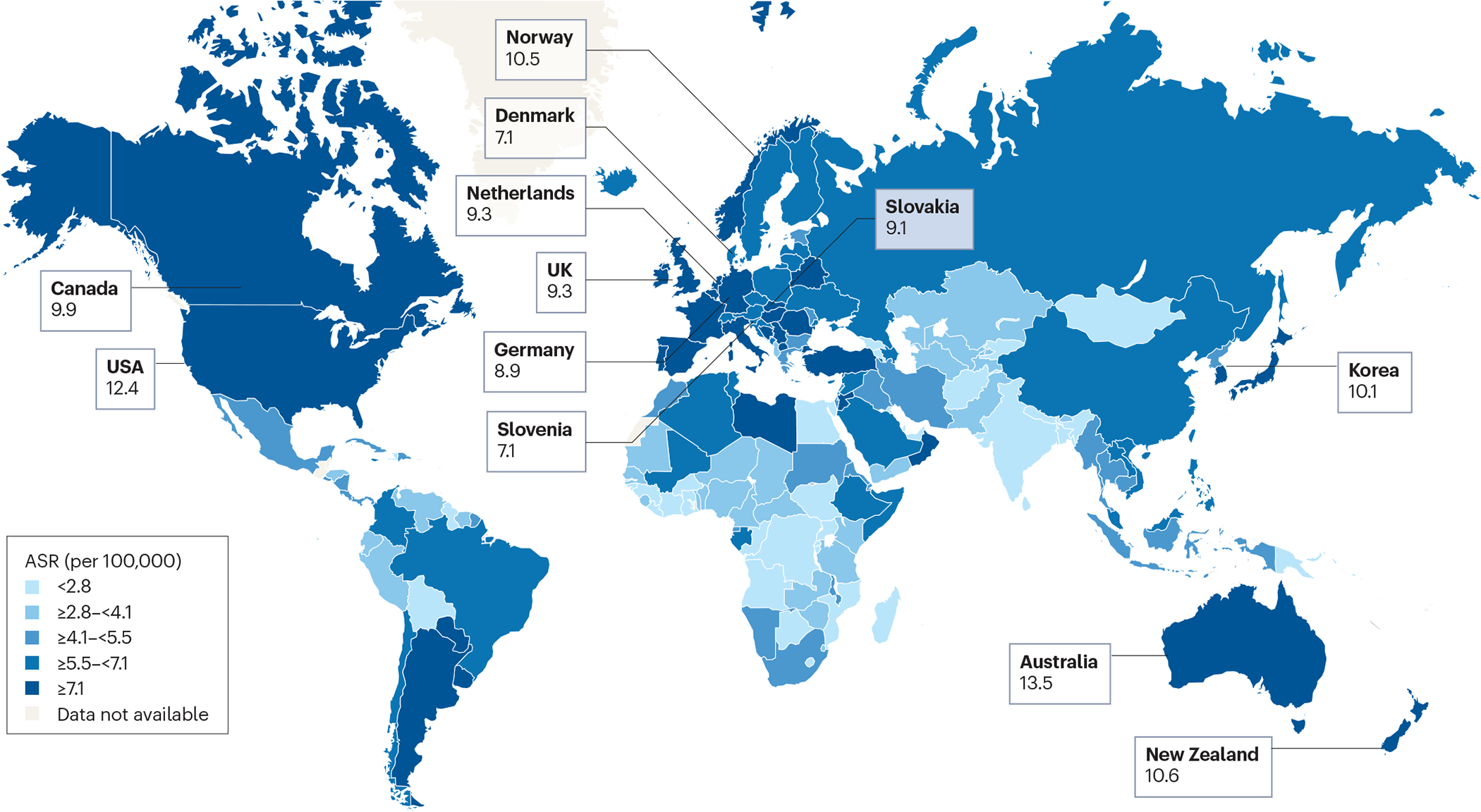
Age-standardized incidence of young-onset colorectal cancer (YO-CRC; age 20–49 years) in both sexes worldwide for the year 2020. Countries/regions with the greatest percentage increase for years 2008–2012 are depicted in red.[8]
Figure 2. Pathogenetic mechanisms of hereditary YO-CRC.
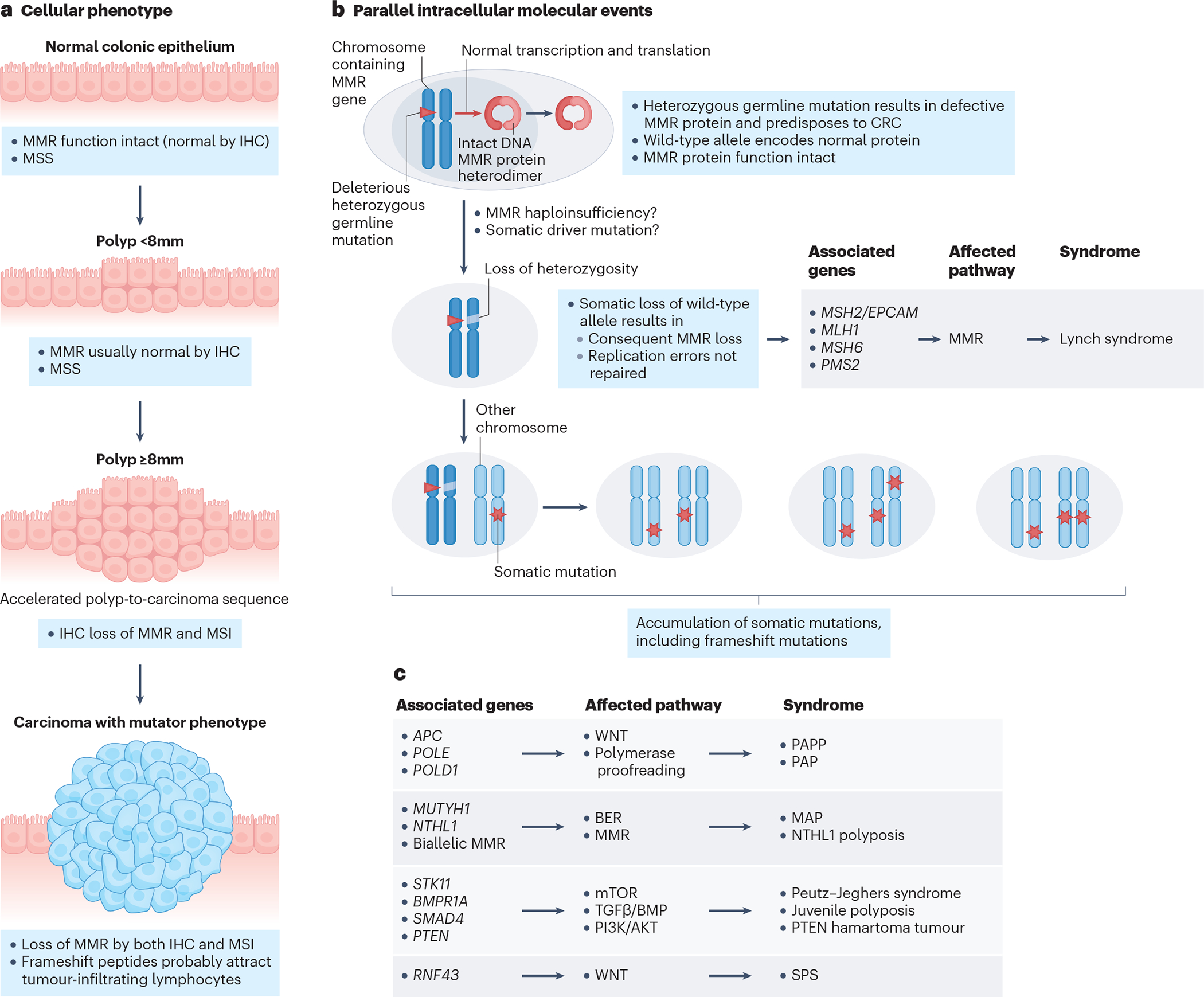
The schematic depicts the cellular phenotype and intracellular events in hereditary colorectal cancer (CRC) development. Hereditary CRC syndromes are caused by pathogenic variants in many different genes, which affect different molecular pathways in each hereditary syndrome. APC, adenomatous polyposis coli; FAP, familial adenomatous polyposis; IHC, immunohistochemistry; MAP, MUTYH-associated polyposis; MMR, mismatch repair; MSI, microsatellite instability; MSS,: microsatellite stability PPAP, polymerase proofreading-associated polyposis; SPS, serrated polyposis syndrome.
Figure 4. Endoscopic imaging and histology of a YO-CRC case.
a, b| Endoscopic imaging of a tumour in the sigmoid colon of a 45- year old woman diagnosed with sporadic young-onset colon cancer (YO-CRC). c, d| Histology of a surgically resected tumour specimen from the same patient.
Figure 5. Proposed algorithm for the management of YO-CRC.
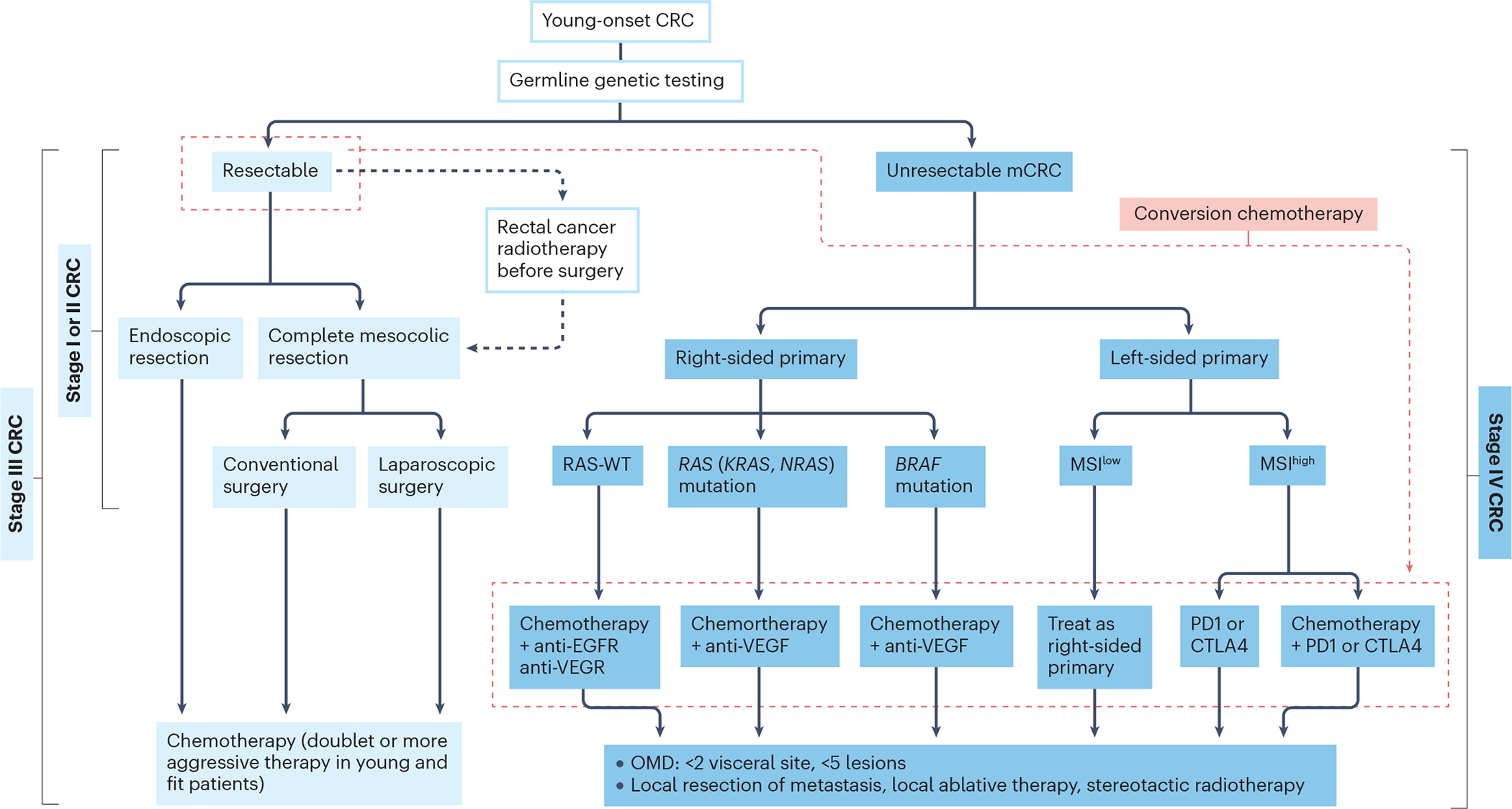
Treatment follows the same principles as for later-onset colorectal cancer (CRC) and the approach depends on whether the tumour is resectable and the stage and location of the primary tumour. Left-sided CRC arises from the descending colon, and sigmoid colon or distal third of the transverse colon, whereas right-sided CRC arises from the ascending colon or proximal two thirds of the transverse colon. Stages are defined by the TNM staging X, Y and Z. Surgical removal of tumours is followed by consolidation chemotherapy. Non-resectable tumours are treated with induction chemotherapy combined with immunotherapy.
Acknowledgements
The authors thank A. Hahn for her contribution to the article.
Glossary terms
- Birth-cohort effect
The effect that having been born in a certain time, period, or having experienced the same life experiences has on the development or perceptions of a particular group.
- Joinpoint analysis
A regression analysis that fits a series of joined straight lines on a log scale with respect to trends in the cancer incidence and mortality rates, adjusted for age. Line segments are joined at points called joinpoints.
- Age-period-cohort modelling
An analysis that examines three distinct time occurrences: age, period, and cohort effects. The analysis seeks to estimate the independent effect of these three phenomena with respect to the outcome of interest for the study.
- Markov model analysis
An analysis to forecast the value of a variable, which assumes that future states depend only on the current state and not on events that have been occurred before it or by prior activity.
- Polyposis
The formation of a substantially greater number of colorectal polyps than in the general population.
- Familial adenomatous polyposis
(FAP). A hereditary disorder characterized by the presence of hundreds to thousands of (precancerous) colorectal adenomatous polyps caused by mutations of the suppressor gene APC (adenomatous polyposis coli)
- Lynch syndrome
-
A hereditary disorder with an increased risk of many types of cancer, particularly colorectal cancers caused by mutations in the DNA mismatch repair genes (MLHL, MSH2, MSH6, PMS2, and EPCAM).
The so-called serrated pathway describes the progression of sessile serrated adenomas and traditional serrated adenomas to colorectal cancer.
- Penetrance
The proportion of people with a particular genetic variant (or mutation) who exhibit signs and symptoms of a genetic disorder.
- Conversion chemotherapy
Chemotherapy administered to patients with borderline or unresectable liver metastases originating from colorectal cancer, with the aim of achieving resectability.
- Low anterior resection syndrome
A constellation of symptoms that may develop after a low anterior resection and includes faecal incontinence, urgency or feelings of incomplete emptying.
Footnotes
Competing interests
The authors declare no competing interests.
Nature Reviews Disease Primers thanks [Referee#1 name], [Referee#2 name] and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2018. CA Cancer J Clin, 2018. 68(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, et al. , Global patterns and trends in colorectal cancer incidence and mortality. Gut, 2017. 66(4): p. 683–691. [DOI] [PubMed] [Google Scholar]
- 3.Malvezzi M, et al. , European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol, 2018. 29(4): p. 1016–1022. [DOI] [PubMed] [Google Scholar]
- 4.Doubeni CA, et al. , Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut, 2018. 67(2): p. 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoffel EM and Murphy CC, Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology, 2020. 158(2): p. 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akimoto N, et al. , Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol, 2021. 18(4): p. 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vuik FE, et al. , Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut, 2019. 68(10): p. 1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegel RL, et al. , Global patterns and trends in colorectal cancer incidence in young adults. Gut, 2019. 68(12): p. 2179–2185. [DOI] [PubMed] [Google Scholar]
- 9.Lui RN, et al. , Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev, 2019. 28(8): p. 1275–1282. [DOI] [PubMed] [Google Scholar]
- 10.Vuik FER, et al. , Clinicopathological characteristics of early onset colorectal cancer. Aliment Pharmacol Ther, 2021. 54(11–12): p. 1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner DR, et al. , Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med, 2017. 105: p. 345–349. [DOI] [PubMed] [Google Scholar]
- 12.Chung RY, et al. , A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol, 2019. 59: p. 29–36. [DOI] [PubMed] [Google Scholar]
- 13.O’Connell JB, et al. , Rates of colon and rectal cancers are increasing in young adults. Am Surg, 2003. 69(10): p. 866–72. [PubMed] [Google Scholar]
- 14.Siegel RL, Jemal A, and Ward EM, Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev, 2009. 18(6): p. 1695–8. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, et al. , Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst, 2017. 109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potter JD, Rising rates of colorectal cancer in younger adults. BMJ, 2019. 365: p. l4280. [DOI] [PubMed] [Google Scholar]
- 17.Collaborative R, et al. , Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg, 2021. 156(9): p. 865–874. [DOI] [PubMed] [Google Scholar]
- 18.Bailey CE, et al. , Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg, 2015. 150(1): p. 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RL, et al. , Colorectal cancer statistics, 2020. CA Cancer J Clin, 2020. 70(3): p. 145–164. [DOI] [PubMed] [Google Scholar]
- 20.Araghi M, et al. , Global trends in colorectal cancer mortality: projections to the year 2035. Int J Cancer, 2019. 144(12): p. 2992–3000. [DOI] [PubMed] [Google Scholar]
- 21.Venugopal A and Carethers JM, Epidemiology and biology of early onset colorectal cancer. EXCLI J, 2022. 21: p. 162–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller C, et al. , Disparities in Early-Onset Colorectal Cancer. Cells, 2021. 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClelland PH, Liu T, and Ozuner G, Early-Onset Colorectal Cancer in Patients under 50 Years of Age: Demographics, Disease Characteristics, and Survival. Clin Colorectal Cancer, 2022. 21(2): p. e135–e144. [DOI] [PubMed] [Google Scholar]
- 24.Gausman V, et al. , Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol, 2020. 18(12): p. 2752–2759 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy CC, et al. , Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology, 2019. 156(4): p. 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holowatyj AN, et al. , Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol, 2016. 34(18): p. 2148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung JJY, et al. , Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am J Gastroenterol, 2019. 114(2): p. 322–329. [DOI] [PubMed] [Google Scholar]
- 28.Holmes AJ, Anderson K, Convergence in National Alcohol Consumption Patterns: New Global Indicators*. Journal of Wine Economics,, 2017. 12( 2): p. 117–148 [Google Scholar]
- 29.Nishida CKS, Shetty P, The Joint WHO/FAO Expert Consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Helath Nutrition, 2007. 7(1a): p. 245–250. [DOI] [PubMed] [Google Scholar]
- 30.Romaguera D, et al. , Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr, 2021. 40(4): p. 1537–1545. [DOI] [PubMed] [Google Scholar]
- 31.Carroll KL, et al. , Diet as a Risk Factor for Early-Onset Colorectal Adenoma and Carcinoma: A Systematic Review. Front Nutr, 2022. 9: p. 896330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon JW, et al. , Effects of age, time period, and birth cohort on the prevalence of diabetes and obesity in Korean men. Diabetes Care, 2008. 31(2): p. 255–60. [DOI] [PubMed] [Google Scholar]
- 33.Li H, et al. , Association of Body Mass Index With Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am J Gastroenterol, 2021. 116(11): p. 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gremaud AL, et al. , Gamifying Accelerometer Use Increases Physical Activity Levels of Sedentary Office Workers. J Am Heart Assoc, 2018. 7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Church TS, et al. , Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One, 2011. 6(5): p. e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parikh NI, et al. , Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med, 2007. 120(3): p. 242–50. [DOI] [PubMed] [Google Scholar]
- 37.Liu PH, et al. , Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol, 2019. 5(1): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, et al. , Associations of Body Mass Index at Different Ages With Early-Onset Colorectal Cancer. Gastroenterology, 2022. 162(4): p. 1088–1097 e3. [DOI] [PubMed] [Google Scholar]
- 39.Murphy CC, et al. , Maternal obesity, pregnancy weight gain, and birth weight and risk of colorectal cancer. Gut, 2022. 71(7): p. 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali Khan U, et al. , Personal History of Diabetes as Important as Family History of Colorectal Cancer for Risk of Colorectal Cancer: A Nationwide Cohort Study. Am J Gastroenterol, 2020. 115(7): p. 1103–1109. [DOI] [PubMed] [Google Scholar]
- 41.Jasperson KW, et al. , Hereditary and familial colon cancer. Gastroenterology, 2010. 138(6): p. 2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SG, et al. , The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol, 2022. 7(3): p. 262–274. [DOI] [PubMed] [Google Scholar]
- 43.Stoffel EM, et al. , Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology, 2018. 154(4): p. 897–905 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearlman R, et al. , Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol, 2017. 3(4): p. 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JE, et al. , High prevalence of TP53 loss and whole-genome doubling in early-onset colorectal cancer. Exp Mol Med, 2021. 53(3): p. 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauri G, et al. , Early-onset colorectal cancer in young individuals. Mol Oncol, 2019. 13(2): p. 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanz-Garcia E, et al. , BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol, 2017. 28(11): p. 2648–2657. [DOI] [PubMed] [Google Scholar]
- 48.Haigis KM, et al. , Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet, 2008. 40(5): p. 600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perea J, et al. , Frequency and impact of KRAS mutation in early onset colorectal cancer. Hum Pathol, 2017. 61: p. 221–222. [DOI] [PubMed] [Google Scholar]
- 50.Colon Cancer Laparoscopic or Open Resection Study, G., et al. , Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol, 2009. 10(1): p. 44–52. [DOI] [PubMed] [Google Scholar]
- 51.Ugai T, et al. , Molecular Characteristics of Early-Onset Colorectal Cancer According to Detailed Anatomical Locations: Comparison With Later-Onset Cases. Am J Gastroenterol, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perea J, et al. , Age at onset should be a major criterion for subclassification of colorectal cancer. J Mol Diagn, 2014. 16(1): p. 116–26. [DOI] [PubMed] [Google Scholar]
- 53.Stigliano V, et al. , Early-onset colorectal cancer: a sporadic or inherited disease? World J Gastroenterol, 2014. 20(35): p. 12420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islami F, et al. , Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin, 2018. 68(1): p. 31–54. [DOI] [PubMed] [Google Scholar]
- 55.Bouvard V, et al. , Carcinogenicity of consumption of red and processed meat. Lancet Oncol, 2015. 16(16): p. 1599–600. [DOI] [PubMed] [Google Scholar]
- 56.Domingo JL and Nadal M, Carcinogenicity of consumption of red meat and processed meat: A review of scientific news since the IARC decision. Food Chem Toxicol, 2017. 105: p. 256–261. [DOI] [PubMed] [Google Scholar]
- 57.Fahrer J and Kaina B, Impact of DNA repair on the dose-response of colorectal cancer formation induced by dietary carcinogens. Food Chem Toxicol, 2017. 106(Pt B): p. 583–594. [DOI] [PubMed] [Google Scholar]
- 58.Hammerling U, et al. , Consumption of Red/Processed Meat and Colorectal Carcinoma: Possible Mechanisms Underlying the Significant Association. Crit Rev Food Sci Nutr, 2016. 56(4): p. 614–34. [DOI] [PubMed] [Google Scholar]
- 59.Johnson CH, et al. , Molecular Mechanisms of Alcohol-Induced Colorectal Carcinogenesis. Cancers (Basel), 2021. 13(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grega T, et al. , Pathophysiological Characteristics Linking Type 2 Diabetes Mellitus and Colorectal Neoplasia. Physiol Res, 2021. 70(4): p. 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deng T, et al. , Obesity, Inflammation, and Cancer. Annu Rev Pathol, 2016. 11: p. 421–49. [DOI] [PubMed] [Google Scholar]
- 62.Tarasiuk A, Mosinska P, and Fichna J, The mechanisms linking obesity to colon cancer: An overview. Obes Res Clin Pract, 2018. 12(3): p. 251–259. [DOI] [PubMed] [Google Scholar]
- 63.Avgerinos KI, et al. , Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism, 2019. 92: p. 121–135. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, et al. , Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology, 2017. 152(4): p. 851–866.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, et al. , Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget, 2017. 8(19): p. 31802–31814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peek RM Jr. and Blaser MJ, Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer, 2002. 2(1): p. 28–37. [DOI] [PubMed] [Google Scholar]
- 67.Islami F and Kamangar F, Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila), 2008. 1(5): p. 329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francois F, et al. , The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol, 2011. 11: p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson WF, et al. , The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst, 2018. 110(6): p. 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao Y, et al. , Long-term use of antibiotics and risk of colorectal adenoma. Gut, 2018. 67(4): p. 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aneke-Nash C, et al. , Antibiotic use and colorectal neoplasia: a systematic review and meta-analysis. BMJ Open Gastroenterol, 2021. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, et al. , Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case-control study. Gut, 2019. 68(11): p. 1971–1978. [DOI] [PubMed] [Google Scholar]
- 73.Kilkkinen A, et al. , Antibiotic use predicts an increased risk of cancer. Int J Cancer, 2008. 123(9): p. 2152–5. [DOI] [PubMed] [Google Scholar]
- 74.Boursi B, et al. , Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf, 2015. 24(5): p. 534–42. [DOI] [PubMed] [Google Scholar]
- 75.Louis P, Hold GL, and Flint HJ, The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol, 2014. 12(10): p. 661–72. [DOI] [PubMed] [Google Scholar]
- 76.Ternes D, et al. , The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat Metab, 2022. 4(4): p. 458–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu HJ and Wu E, The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 2012. 3(1): p. 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng D, Liwinski T, and Elinav E, Interaction between microbiota and immunity in health and disease. Cell Res, 2020. 30(6): p. 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.David LA, et al. , Diet rapidly and reproducibly alters the human gut microbiome. Nature, 2014. 505(7484): p. 559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad Kendong SM, et al. , Gut Dysbiosis and Intestinal Barrier Dysfunction: Potential Explanation for Early-Onset Colorectal Cancer. Front Cell Infect Microbiol, 2021. 11: p. 744606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abed J, et al. , Colon Cancer-Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front Cell Infect Microbiol, 2020. 10: p. 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sears CL, The who, where and how of fusobacteria and colon cancer. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen T, et al. , TOX expression decreases with progression of colorectal cancers and is associated with CD4 T-cell density and Fusobacterium nucleatum infection. Hum Pathol, 2018. 79: p. 93–101. [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Li Q, and Fu X, Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl Oncol, 2019. 12(6): p. 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubinstein MR, et al. , Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe, 2013. 14(2): p. 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubinstein MR, et al. , Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep, 2019. 20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flanagan L, et al. , Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis, 2014. 33(8): p. 1381–90. [DOI] [PubMed] [Google Scholar]
- 88.Kaper JB, Nataro JP, and Mobley HL, Pathogenic Escherichia coli. Nat Rev Microbiol, 2004. 2(2): p. 123–40. [DOI] [PubMed] [Google Scholar]
- 89.Homburg S, et al. , Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol Lett, 2007. 275(2): p. 255–62. [DOI] [PubMed] [Google Scholar]
- 90.Oliero M, et al. , Prevalence of pks + bacteria and enterotoxigenic Bacteroides fragilis in patients with colorectal cancer. Gut Pathog, 2022. 14(1): p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee-Six H, et al. , The landscape of somatic mutation in normal colorectal epithelial cells. Nature, 2019. 574(7779): p. 532–537. [DOI] [PubMed] [Google Scholar]
- 92.Chubb D, et al. , Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nat Commun, 2016. 7: p. 11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.You YN, et al. , Germline Cancer Risk Profiles of Young-Onset Colorectal Cancer Patients: Findings from a Prospective Universal Germline Testing and Tele-Genetics Program. Dis Colon Rectum, 2022. [DOI] [PubMed] [Google Scholar]
- 94.Boardman LA, et al. , AGA Clinical Practice Update on Young Adult-Onset Colorectal Cancer Diagnosis and Management: Expert Review. Clin Gastroenterol Hepatol, 2020. 18(11): p. 2415–2424. [DOI] [PubMed] [Google Scholar]
- 95.Provenzale D, et al. , NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J Natl Compr Canc Netw, 2018. 16(8): p. 939–949. [DOI] [PubMed] [Google Scholar]
- 96.Valle L, et al. , Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol, 2019. 247(5): p. 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma H, et al. , Pathology and genetics of hereditary colorectal cancer. Pathology, 2018. 50(1): p. 49–59. [DOI] [PubMed] [Google Scholar]
- 98.Willauer AN, et al. , Clinical and molecular characterization of early-onset colorectal cancer. Cancer, 2019. 125(12): p. 2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Broderick P, et al. , Evaluation of NTHL1, NEIL1, NEIL2, MPG, TDG, UNG and SMUG1 genes in familial colorectal cancer predisposition. BMC Cancer, 2006. 6: p. 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yurgelun MB, et al. , Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J Clin Oncol, 2017. 35(10): p. 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LaDuca H, et al. , A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med, 2020. 22(2): p. 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mork ME, et al. , High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol, 2015. 33(31): p. 3544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhunussova G, et al. , Mutation Spectrum of Cancer-Associated Genes in Patients With Early Onset of Colorectal Cancer. Front Oncol, 2019. 9: p. 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.You YN, et al. , Detection of Pathogenic Germline Variants Among Patients With Advanced Colorectal Cancer Undergoing Tumor Genomic Profiling for Precision Medicine. Dis Colon Rectum, 2019. 62(4): p. 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mork ME, et al. , Outcomes of disease-specific next-generation sequencing gene panel testing in adolescents and young adults with colorectal cancer. Cancer Genet, 2019. 235–236: p. 77–83. [DOI] [PubMed] [Google Scholar]
- 106.Tutlewska K, Lubinski J, and Kurzawski G, Germline deletions in the EPCAM gene as a cause of Lynch syndrome - literature review. Hered Cancer Clin Pract, 2013. 11(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ligtenberg MJ, et al. , Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet, 2009. 41(1): p. 112–7. [DOI] [PubMed] [Google Scholar]
- 108.Edelstein DL, et al. , Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol, 2011. 9(4): p. 340–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vasen HF, Review article: The Lynch syndrome (hereditary nonpolyposis colorectal cancer). Aliment Pharmacol Ther, 2007. 26 Suppl 2: p. 113–26. [DOI] [PubMed] [Google Scholar]
- 110.Valle L, Genetic predisposition to colorectal cancer: where we stand and future perspectives. World J Gastroenterol, 2014. 20(29): p. 9828–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aelvoet, et al. , Management of familial adenomatous polyposis and MUTYH-associated polyposis; new insights. Best Pract Res Clin Gastroenterol, 2022. 58–59: p. 101793. [DOI] [PubMed] [Google Scholar]
- 112.Peterse EFP, et al. , The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer, 2018. 124(14): p. 2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giardiello FM, et al. , Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol, 2014. 109(8): p. 1159–79. [DOI] [PubMed] [Google Scholar]
- 114.Knudsen AB, et al. , in Colorectal Cancer Screening: An Updated Decision Analysis for the U.S. Preventive Services Task Force. 2021: Rockville (MD). [PubMed]
- 115.Ko CW, et al. , AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology, 2020. 159(3): p. 1085–1094. [DOI] [PubMed] [Google Scholar]
- 116.Vajravelu RK, et al. , Understanding Characteristics of Who Undergoes Testing Is Crucial for the Development of Diagnostic Strategies to Identify Individuals at Risk for Early-age Onset Colorectal Cancer. Gastroenterology, 2021. 160(4): p. 993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Low EE, et al. , Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology, 2020. 159(2): p. 492–501 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Syed AR, et al. , Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol, 2019. 11(11): p. 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frostberg E and Rahr HB, Clinical characteristics and a rising incidence of early-onset colorectal cancer in a nationwide cohort of 521 patients aged 18–40 years. Cancer Epidemiol, 2020. 66: p. 101704. [DOI] [PubMed] [Google Scholar]
- 120.Krigel A, et al. , Symptoms and demographic factors associated with early-onset colorectal neoplasia among individuals undergoing diagnostic colonoscopy. Eur J Gastroenterol Hepatol, 2020. 32(7): p. 821–826. [DOI] [PubMed] [Google Scholar]
- 121.Dozois EJ, et al. , Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore), 2008. 87(5): p. 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Glover M, et al. , Epidemiology of Colorectal Cancer in Average Risk Adults 20–39 Years of Age: A Population-Based National Study. Dig Dis Sci, 2019. 64(12): p. 3602–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Di Leo M, et al. , Risk factors and clinical characteristics of early-onset colorectal cancer vs. late-onset colorectal cancer: a case-case study. Eur J Gastroenterol Hepatol, 2021. 33(9): p. 1153–1160. [DOI] [PubMed] [Google Scholar]
- 124.Kuipers EJ, et al. , Colorectal cancer. Nat Rev Dis Primers, 2015. 1: p. 15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schreuders EH, et al. , Colorectal cancer screening: a global overview of existing programmes. Gut, 2015. 64(10): p. 1637–49. [DOI] [PubMed] [Google Scholar]
- 126.Colorectal Cancer Screening. IARC Handbooks of Cancer Prevention. Vol. 17. 2019. [Google Scholar]
- 127.Rex DK, et al. , Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol, 2017. 112(7): p. 1016–1030. [DOI] [PubMed] [Google Scholar]
- 128.Patel SG, et al. , Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology, 2022. 162(1): p. 285–299. [DOI] [PubMed] [Google Scholar]
- 129.Wolf AMD, et al. , Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin, 2018. 68(4): p. 250–281. [DOI] [PubMed] [Google Scholar]
- 130.Davidson KW, et al. , Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama, 2021. 325(19): p. 1965–1977. [DOI] [PubMed] [Google Scholar]
- 131.Kalyta A, et al. , Canadian Colorectal Cancer Screening Guidelines: Do They Need an Update Given Changing Incidence and Global Practice Patterns? Curr Oncol, 2021. 28(3): p. 1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sung JJY CH, Liebermand D, Kuipers EJ, Rutter M, Macrae F, et al. , Third Asia Pacific consensus recommendation on colorectal cancer screening and surveillance. submitted. [DOI] [PubMed]
- 133.Ladabaum U, et al. , Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology, 2019. 157(1): p. 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ladabaum U, et al. , Strategies for Colorectal Cancer Screening. Gastroenterology, 2020. 158(2): p. 418–432. [DOI] [PubMed] [Google Scholar]
- 135.Knudsen AB, et al. , Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. Jama, 2021. 325(19): p. 1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lew JB, et al. , Benefits, Harms, and Cost-Effectiveness of Potential Age Extensions to the National Bowel Cancer Screening Program in Australia. Cancer Epidemiol Biomarkers Prev, 2018. 27(12): p. 1450–1461. [DOI] [PubMed] [Google Scholar]
- 137.Hampel H, et al. , Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol, 2008. 26(35): p. 5783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Snowsill T, et al. , A model-based assessment of the cost-utility of strategies to identify Lynch syndrome in early-onset colorectal cancer patients. BMC Cancer, 2015. 15: p. 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Snowsill T, et al. , Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess, 2017. 21(51): p. 1–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Syngal S, et al. , ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol, 2015. 110(2): p. 223–62; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hunter JE, et al. , Universal screening for Lynch syndrome among patients with colorectal cancer: patient perspectives on screening and sharing results with at-risk relatives. Fam Cancer, 2017. 16(3): p. 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clarke EV, et al. , Implementation of a Systematic Tumor Screening Program for Lynch Syndrome in an Integrated Health Care Setting. Fam Cancer, 2019. 18(3): p. 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Papke DJ, et al. , Underutilization of Guideline-Recommended Mismatch Repair/Microsatellite Instability Biomarker Testing in Advanced Colorectal Cancer. Cancer Epidemiol Biomarkers Prev, 2022. 31(9): p. 1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mei WJ, et al. , Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: A narrative review. Front Immunol, 2022. 13: p. 1019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Evaluation of Genomic Applications in, P. and G. Prevention Working, Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med, 2009. 11(1): p. 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beard VK, et al. , Genetic testing in families with hereditary colorectal cancer in British Columbia and Yukon: a retrospective cross-sectional analysis. CMAJ Open, 2020. 8(4): p. E637–E642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bednar EM, et al. , Assessing relatives’ readiness for hereditary cancer cascade genetic testing. Genet Med, 2020. 22(4): p. 719–726. [DOI] [PubMed] [Google Scholar]
- 148.Ponz de Leon M, et al. , Genetic testing among high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Br J Cancer, 2004. 90(4): p. 882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koehly LM, et al. , A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev, 2003. 12(4): p. 304–13. [PubMed] [Google Scholar]
- 150.McGivern B, et al. , Family communication about positive BRCA1 and BRCA2 genetic test results. Genet Med, 2004. 6(6): p. 503–9. [DOI] [PubMed] [Google Scholar]
- 151.Sanz J, et al. , Uptake of predictive testing among relatives of BRCA1 and BRCA2 families: a multicenter study in northeastern Spain. Fam Cancer, 2010. 9(3): p. 297–304. [DOI] [PubMed] [Google Scholar]
- 152.Stoffel EM, et al. , Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol, 2008. 6(3): p. 333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whitaker KD, et al. , Cascade Genetic Testing for Hereditary Cancer Risk: An Underutilized Tool for Cancer Prevention. JCO Precis Oncol, 2021. 5: p. 1387–1396. [DOI] [PubMed] [Google Scholar]
- 154.Forrest K, et al. , To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet, 2003. 64(4): p. 317–26. [DOI] [PubMed] [Google Scholar]
- 155.Stoffel EM, Cascade Genetic Testing in Families with Lynch Syndrome 2016, MiGRC. [Google Scholar]
- 156.George R, Kovak K, and Cox SL, Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J Genet Couns, 2015. 24(3): p. 388–99. [DOI] [PubMed] [Google Scholar]
- 157.Menko FH, et al. , The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer, 2019. 18(1): p. 127–135. [DOI] [PubMed] [Google Scholar]
- 158.Hann KEJ, et al. , Awareness, knowledge, perceptions, and attitudes towards genetic testing for cancer risk among ethnic minority groups: a systematic review. BMC Public Health, 2017. 17(1): p. 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srinivasan S, et al. , Stakeholder Perspectives on Overcoming Barriers to Cascade Testing in Lynch Syndrome: A Qualitative Study. Cancer Prev Res (Phila), 2020. 13(12): p. 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Whitworth P, et al. , Impact of Payer Constraints on Access to Genetic Testing. J Oncol Pract, 2017. 13(1): p. e47–e56. [DOI] [PubMed] [Google Scholar]
- 161.Radford C, et al. , Factors which impact the delivery of genetic risk assessment services focused on inherited cancer genomics: expanding the role and reach of certified genetics professionals. J Genet Couns, 2014. 23(4): p. 522–30. [DOI] [PubMed] [Google Scholar]
- 162.Gustafson SL, Pfeiffer G, and Eng C, A large health system’s approach to utilization of the genetic counselor CPT(R) 96040 code. Genet Med, 2011. 13(12): p. 1011–4. [DOI] [PubMed] [Google Scholar]
- 163.Moller P, et al. , Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut, 2018. 67(7): p. 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Plaschke J, et al. , Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol, 2004. 22(22): p. 4486–94. [DOI] [PubMed] [Google Scholar]
- 165.Dominguez-Valentin M, et al. , Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med, 2020. 22(1): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hendriks YM, et al. , Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology, 2004. 127(1): p. 17–25. [DOI] [PubMed] [Google Scholar]
- 167.Monahan KJ, et al. , Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut, 2020. 69(3): p. 411–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Leerdam ME, et al. , Endoscopic management of Lynch syndrome and of familial risk of colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy, 2019. 51(11): p. 1082–1093. [DOI] [PubMed] [Google Scholar]
- 169.Jarvinen HJ, et al. , Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology, 2000. 118(5): p. 829–34. [DOI] [PubMed] [Google Scholar]
- 170.Renkonen-Sinisalo L, et al. , Surveillance improves survival of colorectal cancer in patients with hereditary nonpolyposis colorectal cancer. Cancer Detect Prev, 2000. 24(2): p. 137–42. [PubMed] [Google Scholar]
- 171.Barrow P, et al. , Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg, 2013. 100(13): p. 1719–31. [DOI] [PubMed] [Google Scholar]
- 172.Goverde A, et al. , Routine Molecular Analysis for Lynch Syndrome Among Adenomas or Colorectal Cancer Within a National Screening Program. Gastroenterology, 2018. 155(5): p. 1410–1415. [DOI] [PubMed] [Google Scholar]
- 173.Vasen HF, et al. , Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut, 2008. 57(5): p. 704–13. [DOI] [PubMed] [Google Scholar]
- 174.Bjork JA, et al. , Risk factors for rectal cancer morbidity and mortality in patients with familial adenomatous polyposis after colectomy and ileorectal anastomosis. Dis Colon Rectum, 2000. 43(12): p. 1719–25. [DOI] [PubMed] [Google Scholar]
- 175.Heiskanen I, Luostarinen T, and Jarvinen HJ, Impact of screening examinations on survival in familial adenomatous polyposis. Scand J Gastroenterol, 2000. 35(12): p. 1284–7. [DOI] [PubMed] [Google Scholar]
- 176.Burt RW, et al. , Genetic testing and phenotype in a large kindred with attenuated familial adenomatous polyposis. Gastroenterology, 2004. 127(2): p. 444–51. [DOI] [PubMed] [Google Scholar]
- 177.Leenen CH, et al. , Cost-effectiveness of routine screening for Lynch syndrome in colorectal cancer patients up to 70 years of age. Genet Med, 2016. 18(10): p. 966–73. [DOI] [PubMed] [Google Scholar]
- 178.LoConte NK, et al. , Lifestyle Modifications and Policy Implications for Primary and Secondary Cancer Prevention: Diet, Exercise, Sun Safety, and Alcohol Reduction. Am Soc Clin Oncol Educ Book, 2018. 38: p. 88–100. [DOI] [PubMed] [Google Scholar]
- 179.ed.)., U.S.D.o.H.a.H.S.P.a.g.n., Physical Activity Guidelines 2nd edition. 2019-September.
- 180.Farinetti A, et al. , Mediterranean diet and colorectal cancer: A systematic review. Nutrition, 2017. 43–44: p. 83–88. [DOI] [PubMed] [Google Scholar]
- 181.Cao Y, et al. , Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol, 2016. 2(6): p. 762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Oruc Z and Kaplan MA, Effect of exercise on colorectal cancer prevention and treatment. World J Gastrointest Oncol, 2019. 11(5): p. 348–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Durko L and Malecka-Panas E, Lifestyle Modifications and Colorectal Cancer. Curr Colorectal Cancer Rep, 2014. 10: p. 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Derry MM, et al. , Identifying molecular targets of lifestyle modifications in colon cancer prevention. Front Oncol, 2013. 3: p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chan DS, et al. , Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One, 2011. 6(6): p. e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sugimura T, et al. , Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci, 2004. 95(4): p. 290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aune D, et al. , Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ, 2011. 343: p. d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bingham SA, et al. , Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet, 2003. 361(9368): p. 1496–501. [DOI] [PubMed] [Google Scholar]
- 189.Yue Y, et al. , Prospective evaluation of dietary and lifestyle pattern indices with risk of colorectal cancer in a cohort of younger women. Ann Oncol, 2021. 32(6): p. 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Beer JC and Liebenberg L, Does cancer risk increase with HbA1c, independent of diabetes? Br J Cancer, 2014. 110(9): p. 2361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cavestro GM, et al. , Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines. Clin Gastroenterol Hepatol, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tanaka S, et al. , JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc, 2015. 27(4): p. 417–434. [DOI] [PubMed] [Google Scholar]
- 193.Pimentel-Nunes P, et al. , Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy, 2015. 47(9): p. 829–54. [DOI] [PubMed] [Google Scholar]
- 194.Draganov PV, et al. , AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol, 2019. 17(1): p. 16–25 e1. [DOI] [PubMed] [Google Scholar]
- 195.Kuellmer A, et al. , Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc, 2019. 89(6): p. 1180–1189 e1. [DOI] [PubMed] [Google Scholar]
- 196.Costas-Chavarri A, et al. , Treatment of Patients With Early-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. J Glob Oncol, 2019. 5: p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Labianca R, et al. , Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2013. 24 Suppl 6: p. vi64–72. [DOI] [PubMed] [Google Scholar]
- 198.Heald RJ, The ’Holy Plane’ of rectal surgery. J R Soc Med, 1988. 81(9): p. 503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Emmanuel A and Haji A, Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? A review of the literature. Int J Colorectal Dis, 2016. 31(4): p. 797–804. [DOI] [PubMed] [Google Scholar]
- 200.Kanemitsu Y, et al. , Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients With Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J Clin Oncol, 2021. 39(10): p. 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Clinical Outcomes of Surgical Therapy Study, G., et al. , A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med, 2004. 350(20): p. 2050–9. [DOI] [PubMed] [Google Scholar]
- 202.Carrato A, Adjuvant treatment of colorectal cancer. Gastrointest Cancer Res, 2008. 2(4 Suppl): p. S42–6. [PMC free article] [PubMed] [Google Scholar]
- 203.Lieu C, et al. , Duration of Oxaliplatin-Containing Adjuvant Therapy for Stage III Colon Cancer: ASCO Clinical Practice Guideline. J Clin Oncol, 2019. 37(16): p. 1436–1447. [DOI] [PubMed] [Google Scholar]
- 204.Grothey A, et al. , Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med, 2018. 378(13): p. 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Van Cutsem E, et al. , ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol, 2016. 27(8): p. 1386–422. [DOI] [PubMed] [Google Scholar]
- 206.Venook AP, et al. , Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA, 2017. 317(23): p. 2392–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tabernero J, et al. , Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J Clin Oncol, 2021. 39(4): p. 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Arnold D, et al. , Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol, 2017. 28(8): p. 1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yoshino T, et al. , Rationale for and Design of the PARADIGM Study: Randomized Phase III Study of mFOLFOX6 Plus Bevacizumab or Panitumumab in Chemotherapy-naive Patients With RAS (KRAS/NRAS) Wild-type, Metastatic Colorectal Cancer. Clin Colorectal Cancer, 2017. 16(2): p. 158–163. [DOI] [PubMed] [Google Scholar]
- 210.Overman MJ, et al. , Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol, 2018. 36(8): p. 773–779. [DOI] [PubMed] [Google Scholar]
- 211.Peacock O, et al. , Complications After Extended Radical Resections for Locally Advanced and Recurrent Pelvic Malignancies: A 25-Year Experience. Ann Surg Oncol, 2020. 27(2): p. 409–414. [DOI] [PubMed] [Google Scholar]
- 212.You YN, et al. , The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum, 2020. 63(9): p. 1191–1222. [DOI] [PubMed] [Google Scholar]
- 213.Giordano L, et al. , Robotic-Assisted and Laparoscopic Sigmoid Resection. JSLS, 2020. 24(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sauer R, et al. , Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med, 2004. 351(17): p. 1731–40. [DOI] [PubMed] [Google Scholar]
- 215.Gerard JP, et al. , Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol, 2006. 24(28): p. 4620–5. [DOI] [PubMed] [Google Scholar]
- 216.McCarthy K, et al. , Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev, 2012. 12: p. CD008368. [DOI] [PubMed] [Google Scholar]
- 217.Garcia-Aguilar J, et al. , Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol, 2015. 16(8): p. 957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Garcia-Aguilar J, et al. , Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol, 2022. 40(23): p. 2546–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yuval JB and Garcia-Aguilar J, Watch-and-wait Management for Rectal Cancer After Clinical Complete Response to Neoadjuvant Therapy. Adv Surg, 2021. 55: p. 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Weiser MR, et al. , A Dynamic Clinical Calculator for Estimating Conditional Recurrence-Free Survival After Total Neoadjuvant Therapy for Rectal Cancer and Either Surgery or Watch-and-Wait Management. JAMA Netw Open, 2022. 5(9): p. e2233859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stoffel EM, et al. , Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol, 2015. 33(2): p. 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Herzig D, et al. , The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes. Dis Colon Rectum, 2017. 60(9): p. 881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Herzig DO, et al. , Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis Colon Rectum, 2017. 60(2): p. 137–143. [DOI] [PubMed] [Google Scholar]
- 224.Grothey A, Pembrolizumab in MSI-H-dMMR Advanced Colorectal Cancer - A New Standard of Care. N Engl J Med, 2020. 383(23): p. 2283–2285. [DOI] [PubMed] [Google Scholar]
- 225.Andre T, et al. , Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med, 2020. 383(23): p. 2207–2218. [DOI] [PubMed] [Google Scholar]
- 226.Sinicrope F, Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III colon cancer and deficient mismatch repair (ATOMIC, Alliance A021502). Journal of Clinical Oncology, 2019. 37: p. e15169–e. [Google Scholar]
- 227.Andre T, Immune Checkpoint Blockade Therapy in Patients with Colorectal Cancer Harboring Microsatellite Instability/Mismatch Repair Deficiency in 2022. American Society of Clinical Oncology Educational Book, 2022: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 228.Sargent DJ, et al. , Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol, 2010. 28(20): p. 3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.de Rosa N, et al. , DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J Clin Oncol, 2016. 34(25): p. 3039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cercek A, et al. , PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med, 2022. 386(25): p. 2363–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Heneghan HM, Martin ST, and Winter DC, Segmental vs extended colectomy in the management of hereditary nonpolyposis colorectal cancer: a systematic review and meta-analysis. Colorectal Dis, 2015. 17(5): p. 382–9. [DOI] [PubMed] [Google Scholar]
- 232.Anele CC, et al. , Risk of metachronous colorectal cancer following colectomy in Lynch syndrome: a systematic review and meta-analysis. Colorectal Dis, 2017. 19(6): p. 528–536. [DOI] [PubMed] [Google Scholar]
- 233.You YN, et al. , Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum, 2008. 51(7): p. 1036–43. [DOI] [PubMed] [Google Scholar]
- 234.Etchegary H, et al. , Decisions about prophylactic gynecologic surgery: a qualitative study of the experience of female Lynch syndrome mutation carriers. Hereditary Cancer in Clinical Practice, 2015. 13(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Velikova G, Stark D, and Selby P, Quality of life instruments in oncology. Eur J Cancer, 1999. 35(11): p. 1571–80. [DOI] [PubMed] [Google Scholar]
- 236.Ahnen DJ, et al. , The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc, 2014. 89(2): p. 216–24. [DOI] [PubMed] [Google Scholar]
- 237.Kneuertz PJ, et al. , Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg, 2015. 150(5): p. 402–9. [DOI] [PubMed] [Google Scholar]
- 238.You YN, et al. , Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Ann Surg Oncol, 2011. 18(9): p. 2469–76. [DOI] [PubMed] [Google Scholar]
- 239.Soliman H and Agresta SV, Current issues in adolescent and young adult cancer survivorship. Cancer Control, 2008. 15(1): p. 55–62. [DOI] [PubMed] [Google Scholar]
- 240.Perl G, et al. , Young patients and gastrointestinal (GI) tract malignancies - are we addressing the unmet needs? BMC Cancer, 2016. 16: p. 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Avis NE, et al. , Quality of life among younger women with breast cancer. J Clin Oncol, 2005. 23(15): p. 3322–30. [DOI] [PubMed] [Google Scholar]
- 242.Werner-Lin A, et al. , Waiting and "weighted down": the challenge of anticipatory loss for individuals and families with Li-Fraumeni Syndrome. Fam Cancer, 2020. 19(3): p. 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Stupart D, et al. , Fertility after young-onset colorectal cancer: a study of subjects with Lynch syndrome. Colorectal Dis, 2015. 17(9): p. 787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Burton-Chase AM, et al. , Health-related quality of life in colorectal cancer survivors: are there differences between sporadic and hereditary patients? J Patient Rep Outcomes, 2017. 2(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sun R, et al. , The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer, 2021. 29(12): p. 7249–7258. [DOI] [PubMed] [Google Scholar]
- 246.Al Rashid F, et al. , The impact of bowel dysfunction on health-related quality of life after rectal cancer surgery: a systematic review. Tech Coloproctol, 2022. [DOI] [PubMed] [Google Scholar]
- 247.Bailey CE, et al. , Functional deficits and symptoms of long-term survivors of colorectal cancer treated by multimodality therapy differ by age at diagnosis. J Gastrointest Surg, 2015. 19(1): p. 180–8; discussio 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rashedi AS, et al. , Survey of Third-Party Parenting Options Associated With Fertility Preservation Available to Patients With Cancer Around the Globe. JCO Glob Oncol, 2020. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Corrigan KL, et al. , Financial toxicity impact on younger versus older adults with cancer in the setting of care delivery. Cancer, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Morton SM, et al. , Cohort profile: growing up in New Zealand. Int J Epidemiol, 2013. 42(1): p. 65–75. [DOI] [PubMed] [Google Scholar]
- 251.Connelly R and Platt L, Cohort profile: UK Millennium Cohort Study (MCS). Int J Epidemiol, 2014. 43(6): p. 1719–25. [DOI] [PubMed] [Google Scholar]
- 252.Kooijman MN, et al. , The Generation R Study: design and cohort update 2017. Eur J Epidemiol, 2016. 31(12): p. 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Cirillo PM and Cohn BA, Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation, 2015. 132(13): p. 1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fuchs CS, et al. , A prospective study of family history and the risk of colorectal cancer. N Engl J Med, 1994. 331(25): p. 1669–74. [DOI] [PubMed] [Google Scholar]
- 255.Clavel-Chapelon F, et al. , E3N, a French cohort study on cancer risk factors. E3N Group. Etude Epidemiologique aupres de femmes de l’Education Nationale. Eur J Cancer Prev, 1997. 6(5): p. 473–8. [DOI] [PubMed] [Google Scholar]
- 256.Moller P, et al. , Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut, 2017. 66(3): p. 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]


