Abstract
OBJECTIVE:
In the SVR trial (Single Ventricle Reconstruction), newborns with hypoplastic left heart syndrome were randomly assigned to receive a modified Blalock-Taussig-Thomas shunt (mBTTS) or a right ventricle-to-pulmonary artery shunt (RVPAS) at Norwood operation. Transplant-free survival was superior in the RVPAS group at 1 year, but no longer differed by treatment group at 6 years; both treatment groups had accumulated important morbidities. In the third follow-up of this cohort (SVRIII [Long-Term Outcomes of Children With Hypoplastic Left Heart Syndrome and the Impact of Norwood Shunt Type]), we measured longitudinal outcomes and their risk factors through 12 years of age.
METHODS:
Annual medical history was collected through record review and telephone interviews. Cardiac magnetic resonance imaging (CMR), echocardiogram, and cycle ergometry cardiopulmonary exercise tests were performed at 10 through 14 years of age among participants with Fontan physiology. Differences in transplant-free survival and complication rates (eg, arrhythmias or protein-losing enteropathy) were identified through 12 years of age. The primary study outcome was right ventricular ejection fraction (RVEF) by CMR, and primary analyses were according to shunt type received. Multivariable linear and Cox regression models were created for RVEF by CMR and post-Fontan transplant-free survival.
RESULTS:
Among 549 participants enrolled in SVR, 237 of 313 (76%; 60.7% male) transplant-free survivors (mBTTS, 105 of 147; RVPAS, 129 of 161; both, 3 of 5) participated in SVRIII. RVEF by CMR was similar in the shunt groups (RVPAS, 51±9.6 [n=90], and mBTTS, 52±7.4 [n=75]; P=0.43). The RVPAS and mBTTS groups did not differ in transplant-free survival by 12 years of age (163 of 277 [59%] versus 144 of 267 [54%], respectively; P=0.11), percentage predicted peak Vo2 for age and sex (74±18% [n=91] versus 72±18% [n=84]; P=0.71), or percentage predicted work rate for size and sex (65±20% versus 64±19%; P=0.65). The RVPAS versus mBTTS group had a higher cumulative incidence of protein-losing enteropathy (5% versus 2%; P=0.04) and of catheter interventions (14 versus 10 per 100 patient-years; P=0.01), but had similar rates of other complications.
CONCLUSIONS:
By 12 years after the Norwood operation, shunt type has minimal association with RVEF, peak Vo2, complication rates, and transplant-free survival. RVEF is preserved among the subgroup of survivors who underwent CMR assessment. Low transplant-free survival, poor exercise performance, and accruing morbidities highlight the need for innovative strategies to improve long-term outcomes in patients with hypoplastic left heart syndrome.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT0245531.
Keywords: hypoplastic left heart syndrome, longitudinal outcomes, single ventricle reconstruction
Clinical Perspective.
What Is New?
For children enrolled in the SVR trial (Single Ventricle Reconstruction) who underwent the Norwood procedure with right ventricle-to-pulmonary artery shunt or modified Blalock-Taussig-Thomas shunt, shunt type had no effect on right ventricular ejection fraction, post-Fontan transplant-free survival, or exercise performance 12 years after the procedure.
Transplant-free survival at 12 years was 59% in the right ventricle-to-pulmonary artery shunt group and 54% in the modified Blalock-Taussig-Thomas shunt group, a difference that was not statistically significant.
Patients whose Norwood operation was performed with a right ventricle-to-pulmonary artery shunt had higher cumulative incidence rates of protein-losing enteropathy and interventional catheterization, but other morbidities were similar in the shunt groups.
What Are the Clinical Implications?
Despite surgical, transcatheter, and pharmacological innovations that have transformed care of previously fatal congenital heart defects, long-term morbidity and mortality remain high for children with hypoplastic left heart syndrome undergoing the Norwood operation.
There is a critical need for investment in innovative strategies to improve outcomes for individuals with hypoplastic left heart syndrome.
Hypoplastic left heart syndrome (HLHS) and other related single-ventricle anomalies are among the highest-risk congenital cardiac defects, with 1-year mortality rates approaching 100% in the absence of either infant cardiac transplantation or staged surgical palliation. Staged palliation begins with the Norwood operation, first described in 1980,1 in the neonatal period, followed by an initial cavopulmonary connection of the superior vena cava to the branch pulmonary arteries (stage 2), typically performed between 4 and 6 months of age, and then a total cavopulmonary connection, including the inferior vena cava, to the pulmonary arteries (Fontan procedure [stage 3]), usually performed between 18 and 36 months of age. Typically performed in the first week of life, the Norwood operation converts the proximal pulmonary artery into a new aorta to ensure adequate blood flow to the body. Perfusion of the pulmonary arteries requires creation of a systemic to pulmonary artery connection. For >2 decades, this connection was made using a modified Blalock-Taussig-Thomas shunt (mBTTS) from the right innominate artery to the right pulmonary artery. In 2004, the right ventricle-to-pulmonary artery shunt (RVPAS) was promoted as an alternative to mBTTS, with the intent to reduce diastolic runoff into the pulmonary arteries from the systemic circulation and important end organs and to improve coronary perfusion pressure.2
In the original SVR trial (Single Ventricle Reconstruction), neonates with HLHS or other related single right ventricular anomalies from 15 centers across North America were randomly assigned to receive either mBTTS or RVPAS at the time of the Norwood operation.3 In this first-ever multicenter clinical trial comparing surgical strategies in congenital heart disease, transplant-free survival at 12 months was better for those with RVPAS.4 However, by 6 years of age, children assigned to RVPAS compared with mBTTS no longer differed significantly in transplant-free survival (64% versus 59%, respectively) and had similar hazards of death or transplant.5 In addition, through 6 years of age, children who received RVPAS required more catheter-based interventions than those who received mBTTS.5 It remained unclear whether differences in cardiac function, morbidity, or survival would emerge between the shunt groups as the cohort aged.
SVR III (Long-Term Outcomes of Children With Hypoplastic Left Heart Syndrome and the Impact of Norwood Shunt Type) was designed to determine whether shunt type was associated with right ventricular function, transplant-free survival, exercise performance, or morbidities by the preteen years. Specific aims also included identification of risk factors associated with lower right ventricular ejection fraction (RVEF), the primary outcome, or with worse transplant-free survival after the Fontan procedure through early adolescence.
METHODS
In SVR, newborns with HLHS were randomly assigned to receive either mBTTS or RVPAS during the Norwood operation. The primary aim was to determine whether RVPAS improved transplant-free survival in the first year of life. SVR trial survivors were eligible to participate in this multicenter, prospective, longitudinal cohort study. Details of the methods were previously reported.6 In brief, participation included one-time cardiac magnetic resonance imaging (CMR), echocardiogram, and ramped cardiopulmonary exercise testing with cycle ergometry performed between June 2015 and September 2020 for each participant at a target age of 10 to 12 years. However, some participants did not complete study measures until 14 years of age due to scheduling impediments related to COVID-19–associated limitations on research participation and complexities of school schedules. Research CMR was performed without anesthesia or contrast. However, some participants underwent CMR during the study window for clinical purposes with exposure to sedation or anesthesia; in these instances, the clinical CMR was used for study purposes.6 Interim medical history was collected annually through interviews and medical record review to learn about medical course, including complications such as development of arrhythmias, protein-losing enteropathy (PLE), plastic bronchitis, neurological events, and the need for additional interventions (surgical or catheter-based). This study was approved by the institutional review board (or its equivalent) of each participating center; informed consent from parents or legal guardians and assent as appropriate were obtained for each participant. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical Analysis
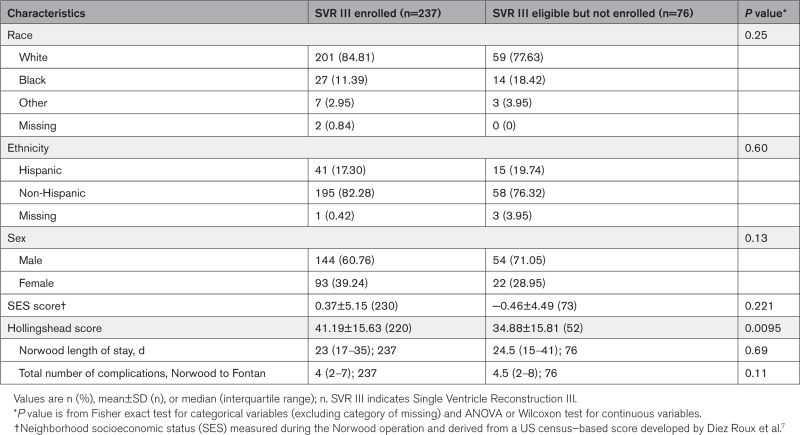
The primary outcome of the study was RVEF measured by CMR. Analyses were performed as shunts were received, excluding 5 participants in whom both shunts were in place at the conclusion of the index operation, and secondarily as intention to treat. We used the Fisher exact test and t tests to compare sociodemographic and medical factors between eligible patients who were enrolled versus those who were not enrolled in SVR III.
Regression modeling was performed to assess the effects of patient factors and medical history on the primary outcome of RVEF by CMR. Predictors significant at level 0.20 in univariable modeling were identified for potential inclusion in multivariable modeling with backward elimination. The effect of outliers was considered by eliminating them from univariate modeling and assessing for statistical significance. Predictors with frequencies <10 were not deemed eligible for further modeling. When different forms of predictors were considered (eg, continuous variables versus categorical quartiles), association with outcome was considered to determine which variable form to include. The possibility of collinearity among potential predictors was explored, and some variables with a variance inflation factor (VIF) >10 were excluded from the considered list of candidate variables. To preserve sample size for multivariable modeling, a category of “missing” was created for categorical variables. Four continuous covariates with >15% missing data were initially excluded from multivariable modeling, but later added, one by one, in sensitivity analyses to final models and found to be nonsignificant. All other continuous covariates had <1% missing.
Time to death or transplant was estimated with the Kaplan-Meier method (log-rank test), using all available data across the entire SVR study period (time 0 at randomization) and separately for the period after Fontan completion (time 0 at Fontan procedure). Competing-risks methodology was used to estimate the cumulative incidence rate of death or transplant by shunt type. Cox proportional hazards modeling was performed to assess the association of shunt type on transplant-free survival; due to violation of the proportional hazards assumption, a time-dependent treatment indicator was used, with 3 time intervals: before stage 2 surgery; from stage 2 surgery until Fontan surgery; and after Fontan surgery. Cox proportional hazards modeling was also performed to assess the association of covariates with post-Fontan survival. Predictors significant at level 0.20 in univariable modeling were identified for potential inclusion in multivariable modeling with backward elimination, with the same model considerations listed previously for the primary outcome. The C statistic using the Harrell C index was calculated for each survival model.
Exercise testing results were compared between shunt groups by t tests and Wilcoxon rank sum tests. Incidence rates of clinical events (eg, PLE, atrial arrhythmias, or ventricular arrhythmias) at 12 years were compared between shunt groups. The incidence rate and proportion comparisons were based on Poisson regression and Fisher exact test, respectively. We compared time to events between shunt groups up to 12 years after randomization using a Wald test of the pointwise Kaplan-Meier event rate estimates.
SAS EG v7.15 statistical software was used for all analyses.
RESULTS
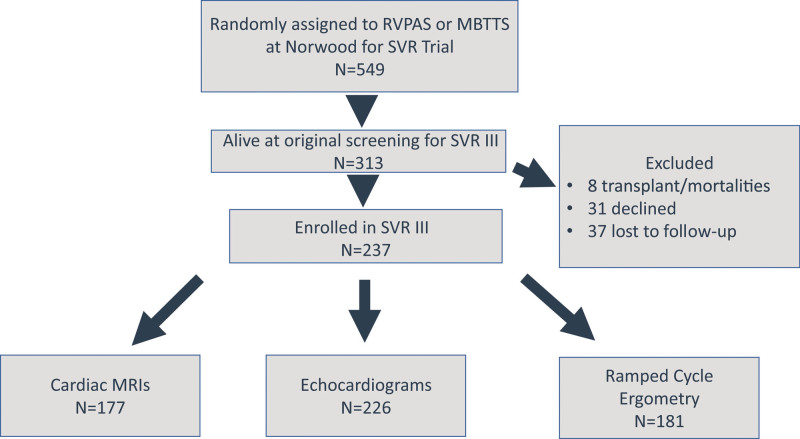
Among 549 participants randomly assigned to RVPAS or mBTTS who underwent a Norwood operation as neonates in the SVR trial, 313 (57.0%) were alive and transplant-free at the time of screening for the SVR III study. Of these eligible participants, 237 (75.7%), (109/153 [71%] with a mBTTS, 128/160 [80%] with a RVPAS, and 3/5 [60%] with both shunt types) enrolled in the SVR III study. Eight (2.6%) were excluded due to death or transplant following screening but prior to enrollment, 31 (9.9%) declined participation, and 37 (11.8%) were lost to follow-up (Figure 1). Race or ethnicity did not differ between participants and eligible nonparticipants. Participants had higher socioeconomic status by Hollingshead score at 14 months of age, but no difference in US census-based neighborhood socioeconomic status7 (Table 1). Among participants, there was a greater percentage of Hispanic ethnicity among the RVPAS shunt recipients compared with those who received an mBTTS (22.5% versus 11.4%; P=0.037; Table S1).
Figure 1.
SVR overall study participation. A total of 549 individuals were enrolled and randomly assigned to a right ventricle-to-pulmonary artery shunt (RVPAS) or a modified Blalock-Taussig-Thomas shunt (mBTTS), and 76% of transplant-free survivors (237 of 313) were enrolled in SVR III (Single Ventricle Reconstruction III). Cardiac magnetic resonance imaging (CMR) was completed for 177 participants, echocardiograms for 226 participants, and ramped cycle ergometry exercise testing for 181 participants. SVR indicates Single Ventricle Reconstruction.
Table 1.
Comparison of SVR III Participants With Patients Who Were Eligible But Not Enrolled
Right Ventricular Function
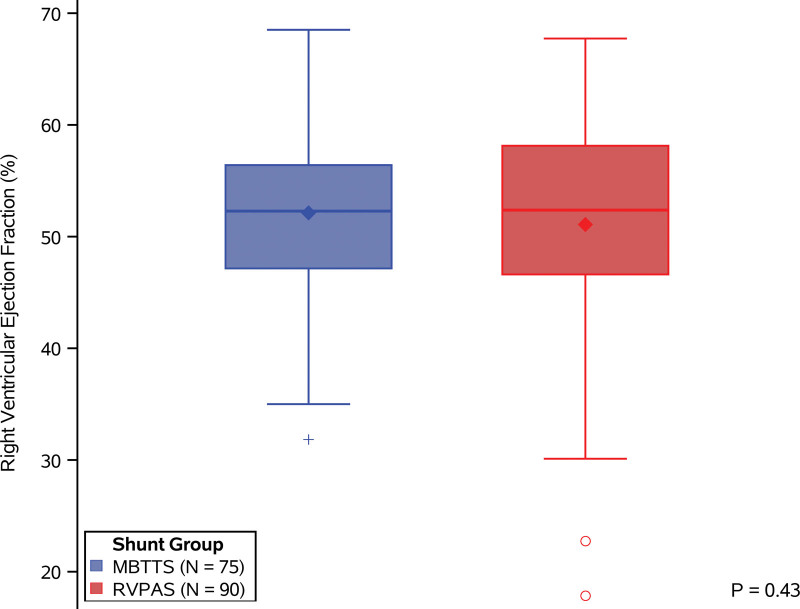
Right ventricular function was measured with CMR as well as with 2-dimensional transthoracic echocardiography. Participants who underwent CMR were 10.9 years of age (interquartile range, 10.4–11.5) at the time of CMR, and, compared with those who did not have CMR, they had a significantly shorter hospital length of stay after the Norwood procedure (22 [interquartile range, 16–33] versus 28 days [interquartile range, 20–39]; P=0.02) and were more likely to be male (66% versus 47%; P=0.01; Table S2). Among the 177 participants who underwent CMR, RVEF was measurable in 168 (95%); the mean±SD for the overall cohort was 51.5±8.6% (RVPAS 51±9.6 [n=90] and mBTTS 52±7.4 [n=75]; P=0.43), without difference between shunt groups (Figure 2). Three of the 168 had both an RVPAS and mBTTS in place at the completion of the Norwood operation, and thus these participants were excluded from the comparison of RVEF by shunt type. Echocardiograms were performed in 226 participants, of whom only 78 (35%) had measurable RVEF by this modality; overall mean±SD of echocardiographic RVEF was 49.9±6.2%, again without differences between the RVPAS (49.5±6.9) and mBTTS (50.2±5.7) groups.
Figure 2.
Comparison by received shunt type of right ventricle ejection fraction as measured by cardiac magnetic resonance imaging. There is no significant difference in right ventricular ejection fraction between those with a modified Blalock-Taussig-Thomas shunt (mBTTS; blue) and those with a right ventricle-to-pulmonary artery shunt (RVPAS; red). For each shunt type, the median is marked by a bold center line and the mean is marked by a diamond. Interquartile range is demarcated by the rectangle, and whiskers extend to the 5th and 95th percentiles. Outliers are marked by blue+ for the mBTTS group and red circles for the RVPAS group. Comparison by Student t test (P=0.43).
An analysis was performed to determine whether CMR RVEF was associated with demographic and pre-Fontan medical factors. Factors associated with RVEF on univariable modeling are summarized in Table S3, but in multivariable modeling, only exposure to anesthesia during the CMR (β coefficient, change in RVEF=−8.6; P=0.003) and lower fractional area change measured at the time of the pre–stage 2 echocardiogram (β coefficient, change in RVEF=0.28 per unit fractional area change; P=0.001) were independent risk factors associated with lower CMR RVEF (R2=0.12).
Survival
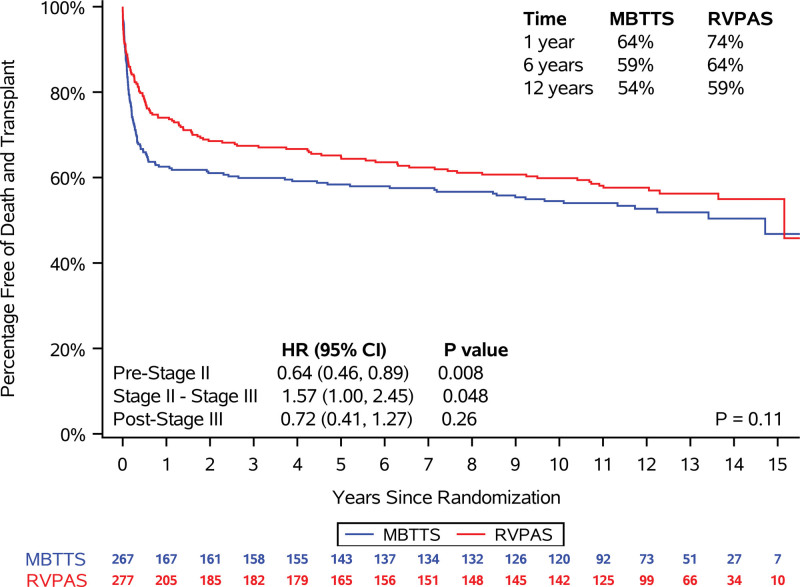
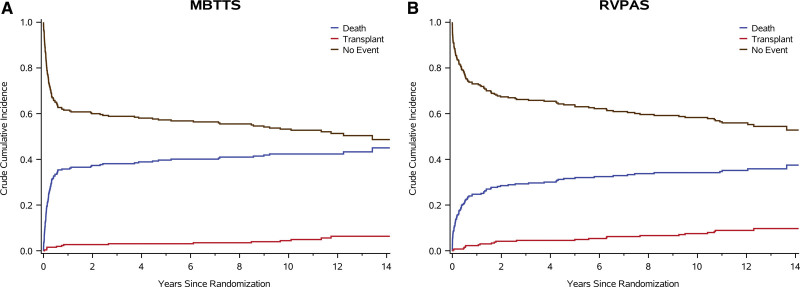
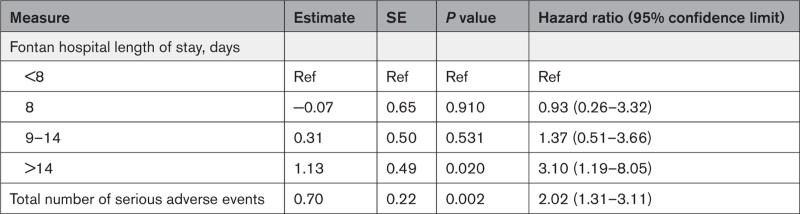
In the combined shunt groups, from the Norwood procedure to 12 years of age, 201 patients (36.9%) died, and 36 (6.6%) underwent an orthotopic heart transplant. Transplant-free survival at 12 years was 59% for RVPAS versus 54% for mBTTS (P=0.11; Figure 3). In addition, no difference was identified in transplant-free survival conditional on Fontan survival (P=0.93). Compared with the mBTTS group, the RVPAS group had a lower hazard of transplant-free survival before stage 2 (hazard ratio, 0.64; P=0.008), a higher hazard between stages 2 and 3/Fontan (hazard ratio, 1.57; P=0.048), and no difference in hazard after the Fontan (hazard ratio, 0.72; P=0.26; Figure 3). Competing risk plots demonstrate ongoing risk for transplant and death that is similar in RVPAS and mBTTS recipients (Figure 4). Factors associated with increased risk of death or heart transplant on bivariate modeling are summarized in Table S4. In analyses conditional on survival to hospitalization for the Fontan procedure, multivariable modeling demonstrated that independent factors associated with worse post-Fontan transplant-free survival included a post-Fontan hospital length of stay >14 days and a greater total number of serious adverse events during the first year of life (Table 2).
Figure 3.
Comparison of received shunt type in freedom from the composite end point of death or cardiac transplantation. Comparison by log-rank test shows no difference with all data considered (P=0.11). Percentage of survival at 1, 6, and 12 years of age by shunt type is shown in the top right quadrant. Hazard ratios (HRs) of right ventricle-to-pulmonary artery shunt (RVPAS) vs modified Blalock-Taussig-Thomas shunt (mBTTS) and CIs during 3 time periods (before stage 2 operation, between stage 2 and Fontan [stage 3], and after Fontan [stage 3]) are compared in the lower left quadrant. Because this analysis was by shunt type received, the 5 participants who had both an RVPAS and mBTTS at the conclusion of the Norwood operation were excluded from this portion of the analysis.
Figure 4.
Competing risks of death, transplant, and no event (transplant-free survival). A, Competing risk for those who received a modified Blalock-Taussig-Thomas shunt (mBTTS). B, Competing risk for those who received a right ventricle-to-pulmonary artery shunt (RVPAS).
Table 2.
Multivariable Cox Regression Model for Death or Cardiac Transplant After the Fontan Procedure (n=326; C statistic=0.69)
Exercise Performance
Ramped cardiopulmonary cycle ergometry was performed in 181 (76%) of the SVR III participants. Exercise performance was below expected, with peak Vo2 at 73±18% predicted for age and sex. There was no difference by shunt type in peak Vo2 (RVPAS 29.0±7.0 mL·kg·min versus mBTTS 29.7±7.7 mL·kg·min; P=0.26), percentage predicted peak Vo2 (74±18% [n=91] versus 72±18% [n=84]; P=0.71), in-peak work rate (RVPAS 65.3±23.4 W versus mBTTS 68.8±19.8 W; P=0.61), or percentage predicted peak work rate (RVPAS 64.8±20.0 versus mBTTS 63.5±18.7; P=0.65). Among those with mBTTS, 38% had a peak Vo2 >80% predicted, compared with 30.4% of those with RVPAS (P=0.33).
Cardiac Events and Complications
Analyses of cardiac events and complications included data for all SVR participants from trial enrollment through 12 years of age. Overall, the rate of interventional catheterizations was 12.2 per 100 patient-years. Those with RVPAS had a higher rate than those with mBTTS (13.7 versus 10.6 per 100 patient-years, respectively; P=0.01). The frequency of unplanned cardiac operations was similar between the RVPAS and mBTTS groups (41.2 versus 41.8 per 100 patient-years, respectively; P=0.78; Table S5).
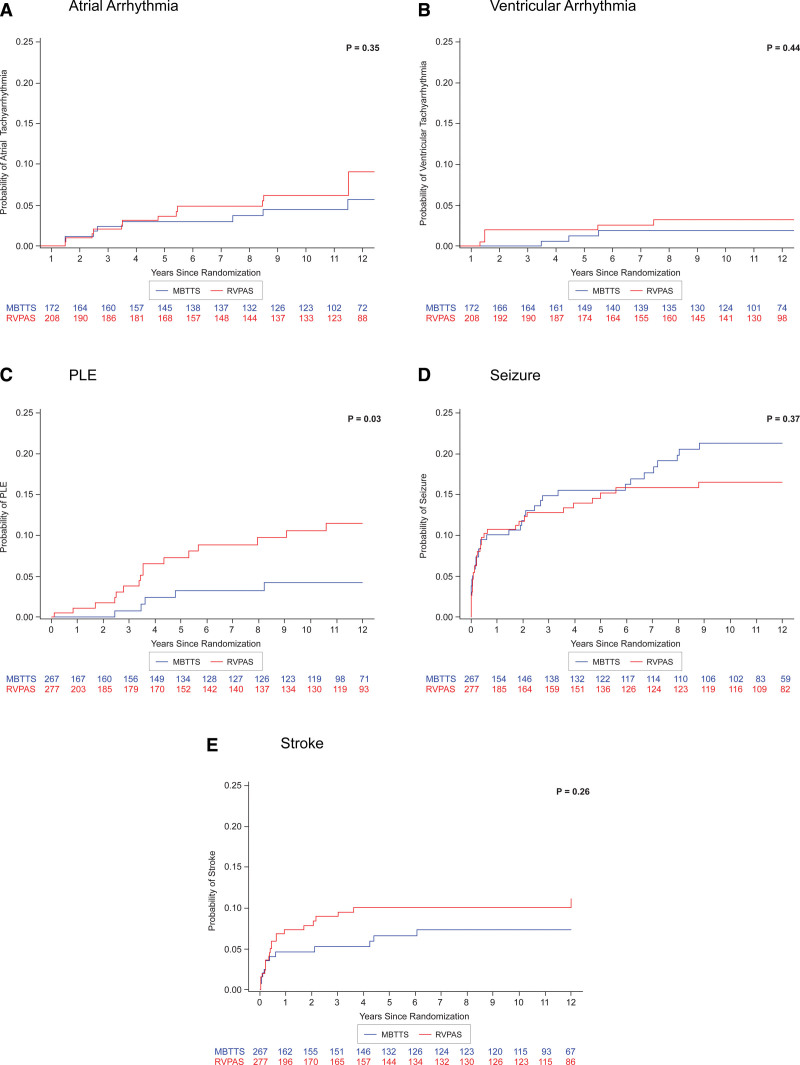
From the Norwood operation to 12 years of age, among all who participated in the SVR trial, 14 (2.6%) required pacemaker placement, without a significant difference between those with RVPAS (n=9 [3.3%]) or mBTTS (n=5 [1.8%]; P=0.42). Atrial tachyarrhythmias were reported in 5.7% of participants (22 of 380) who completed annual follow-up starting at 2 years of age. Ventricular tachyarrhythmias occurred in 2.3% (9 of 380). There was no difference in cumulative incidence of atrial or ventricular tachyarrhythmias by shunt group (Figure 5A and 5B).
Figure 5.
Comparison of probability of events over time. A, Atrial arrhythmia. B, Ventricular arrhythmia. C, Protein-losing enteropathy (PLE). D, Seizure. E, Stroke. P value for each comparison by log-rank test. Probability of atrial arrhythmias and of ventricular arrhythmias starts at 1 year, as seen by the x axis in A and B. For PLE, seizure, and stroke, data collection and x axis starts at the time of the Norwood operation. mBTTS indicates modified Blalock-Taussig-Thomas shunt; and RVPAS, right ventricle-to-pulmonary artery shunt.
By 12 years of age, among all SVR trial participants, PLE and plastic bronchitis were reported in 21 (3.8%) and 4 (0.7%), respectively. SVR participants who had RVPAS had a greater cumulative risk of PLE than those who had mBTTS (5% versus 2%; P=0.03; Figure 5C). There was no difference by shunt type in the reported rate of plastic bronchitis, although the small number of events limited power to detect a difference.
Cerebrovascular events were reported in 6.9% of the overall cohort, and seizures were reported in 13.8%, without a difference between shunt groups in cumulative incidence for either of these neurological events (Figure 5D and 5E).
DISCUSSION
The SVR trial was the first multicenter randomized controlled trial of a surgical intervention for children with congenital heart disease, and its participants constitute the largest prospective cohort of children with HLHS and other related single-ventricle abnormalities from the Norwood operation to early adolescence.3–5,8 Previous analyses in the SVR cohort showed a higher hazard of death or transplant before the stage 2 procedure among patients in the mBTTS group.5,8–10 However, longitudinal follow-up of this landmark cohort to the preteen years demonstrated no significant differences between the RVPAS and mBTTS nontransplanted survivors in cardiac function as measured by RVEF by either CMR (primary outcome) or echocardiography, post-Fontan transplant-free survival up to 12 years of age, or exercise performance, which was lower than predicted for age and height in both groups, consistent with previous findings in patients undergoing the Fontan procedure.11–13 By 12 years of age, the only difference between the shunt types was a higher cumulative incidence of PLE and of interventional cardiac catheterization procedures in the RVPAS group. The rate of ventricular arrhythmias was very low, similar to that found in other large cohorts of patients undergoing the Fontan procedure,14 with no difference seen between the 2 shunt groups, consistent with findings at 6 years of age in this same cohort.15
Because the insertion and implantation of the RVPAS requires an incision in the right ventricle, we had originally hypothesized that patients treated with RVPAS would be more susceptible to right ventricular dysfunction and ventricular arrhythmias. In general, RVEF by CMR was within the normal range at 10 to 14 years of age among transplant-free survivors regardless of shunt type. These findings are consistent with the findings of Sobh et al,16 who found that RVEF by CMR was 54% among 6- to 11-year-old children with HLHS and Fontan circulation, and with previous findings from the SVR cohort showing no difference in RVEF between shunt groups as measured by echocardiography at 6 years of age.17 Whereas systolic function is relatively preserved, further evaluation of the CMR and echocardiography data will be important to understand the effect of age and of shunt type on diastolic function and other indicators of cardiac dysfunction. The preteen period may be too early to detect systolic dysfunction by CMR. Ghelani et al18 recently described longitudinal changes in ventricular function and showed that on consecutive CMR, ejection fraction decreased over time among patients undergoing the Fontan procedure who were followed into early adulthood. Moreover, despite many risk factors considered and comprehensive prospective collection of data, even extensive modeling provided little insight into risk factors for lower RVEF. The inability to predict >12% of the variance in RVEF from routinely measured sociodemographic and clinical variables highlights the complexity of this patient cohort and the contributors to progressive right ventricular dysfunction. Genetic factors and epigenetic variants may play a role and are an important area for future investigation.
Perhaps the most striking and sobering finding in this longitudinal study was the ongoing risk for both death and heart transplantation in the overall cohort of SVR survivors; by 12 years of age, nearly half of the cohort was either deceased or had undergone an orthotopic heart transplant. A greater number of adverse events after the Norwood operation and a post-Fontan longer length of stay >14 days were independent factors associated with death or a heart transplant.
PLE is one of the most serious complications of the Fontan physiology and is characterized by loss of enteric protein. It is possible that the higher rate of PLE among the RVPAS group is related to a greater distortion of the pulmonary arteries, reflected in part by the significantly higher rate of interventional catheterizations in the RVPAS group noted at 6 years of age,5 and here, in the preteen years. Patients who receive RVPAS, compared with mBTTS, have lower pulmonary blood flow before stage 2 and an increased risk of pulmonary artery stenoses,19 and it is possible that less early growth of the pulmonary arterial tree plays a role. The significantly greater cumulative incidence of PLE among those who received RVPAS could reflect higher diastolic pressures in the systemic right ventricle and resultant increased systemic venous pressures. A final possibility is that the statistically significant difference was simply attributable to chance (type 1 error). Further studies are needed to investigate these hypotheses.
These findings should be considered in light of study limitations. No difference was identified in RVEF between shunt groups in the SVR III study cohort, but we cannot exclude survival bias such that if right ventricular dysfunction contributed to transplant-free death to a greater extent in one shunt group than in the other, this could contribute to the comparison of RVEF between shunt groups. However, this hypothesis was not supported by echocardiographic data from earlier phases of the trial.5 Having more than mild right ventricular dysfunction by CMR was uncommon in SVR III participants; however, those few who could not undergo CMR may have been at higher risk of lower RVEF because of pacemakers or other medical complexity. However, such a selection bias would not have affected comparisons of RVEF between shunt types. Enrolled participants were more likely to have had a higher Hollingshead score at 14 months of age and a tendency to be identified as an underrepresented minority, limiting generalizability of overall outcomes. Future studies will require improved equity in recruitment and retention strategies. Finally, detailed genotype information was not incorporated into modeling for survival or for cardiac function; in the future, genetic markers may facilitate precision medical care.
Through extensive follow-up and evaluation of the SVR cohort, we found that shunt type has little effect on cardiac function, post-Fontan transplant-free survival, or exercise performance through early adolescence. Those who received RVPAS had higher cumulative incidence rates of PLE and interventional catheterization, but other morbidities are similar in the shunt groups. Of foremost concern, the risk of overall long-term morbidity and mortality remain high for children with HLHS undergoing the the Norwood operation as neonates. There is a critical need for further investment in innovative strategies to reduce the risks for morbidity and mortality and to improve outcomes for these children.
ARTICLE INFORMATION
Acknowledgments
The authors thank Gail Pearson, Victoria Pemberton, Kristen Burns, Mario Stylianou, and D’Andrea Freemon (National Heart, Lung, and Blood Institute); Lynn Mahony (protocol chair, University of Texas Southwestern Medical Center); Felicia Trachtenberg (principal investigator), Julie Miller (principal investigator), Melissa Lamberti, Russell Gongwer, Allison Crosby-Thompson, Melissa Allen, Ayesha Amarnath, Bahar Coley, Lozan Eyob, Beverly Slayton, Tanya Olesker (no longer with institution), Chenwei Hu, Danielle Hollenbeck-Pringle (no longer with institution), Paul Stark (no longer with institution), Barbara Winrich (no longer with institution), Shreya Divatia (no longer with institution), Andrew Morrison (no longer with institution), and Chitra Kinhikar (no longer with institution; HealthCore [New England Research Institutes]; data coordinating center); Jane W. Newburger (principal investigator), David Bellinger, Ashwin Prakash, Jamie Levine, Jonathan Rhodes, Carolyn Dunbar-Masterson, and Lisa Jean Buckley (core clinical site investigators; Boston Children’s Hospital); Amee Shah (principal investigator), Margaret Challenger, Annette Zygmunt, Robert Garofano, Kathleen Gilmartin (no longer with institution), Katrina Golub, Rosalind Korsin, and Chanel Rojas (Children’s Hospital of New York); J. William Gaynor (principal investigator), Chitra Ravishankar (principal investigator), Katherine Lupton, Tonia Morrison, Meryl Cohen, Stephen Paridon, Thomas Flynn, Kevin Whitehead, Donna Sylvester (no longer with institution), Somaly Srey, and Angela Winters (Children’s Hospital of Philadelphia); James Cnota (principal investigator), Teresa Barnard (deceased), Michelle Hamstra (no longer with institution), and Kathleen Rathge (Cincinnati Children’s Medical Center); Kevin Hill (principal investigator), Jennifer Li, Michael Campbell, Michael Carboni, Jennifer Martin, Melanie Simms, Kathryn Gustafson, Kimberly Carroll (no longer with institution), and Mingfen Xu (Duke University Medical Center); Andrew Atz (principal investigator), Shahryar Chowdhury, Kimberly McHugh, Mary Kral, Ryan Butts (no longer with institution), Geoffrey Forbus, Arni Nutting, Carolyn Taylor, Shameeka Bowman (no longer with institution), Kaylan Chundru (no longer with institution), Layla Al Sarraf, Mary Freeman, and Patricia Infinger (no longer with institution; Medical University of South Carolina); Thomas Miller (co-principal investigator [no longer with institution]), L. LuAnn Minich (co-principal investigator), Philip Burch (no longer with institution), Richard Williams, Sean Cunningham, Linda Lambert, Bergen Lindauer (no longer with institution), and Marian Shearrow (Primary Children’s Hospital and the University of Utah); Steven Schwartz (principal investigator), Brian McCrindle, Martha Rolland, Patricia Walter, Patricia Arseneau, Wei Hui, Susan Iori, Cameron Slorach, Andreea Dragulescu, Renee Sananes, and Mike Seed (Hospital for Sick Children, Toronto); Caren S. Goldberg (principal investigator), Mark Russell, Katherine Afton, Chelsea Stewart, Kimberly Heinrich, Jimmy Lu, and Adam Dorfman (University of Michigan); Michele Frommelt (principal investigator), Kathy Mussatto (no longer with institution), Peter Frommelt, Nancy Ghanayem (no longer with institution), Michelle Loman, Michelle Otto, Jessica Stelter, Jennifer Dixon, Andrea Moker, Michael Danduran, Stacy Leibham, and Megan Schoessling (Children’s Hospital of Wisconsin); Jon Detterich (principal investigator), Alan Lewis, Sharon O’Neill, Hesham Mahmoud (no longer with institution), and Taqwa Ramadan (no longer with institution; auxiliary site: Children’s Hospital Los Angeles); Marguerite Crawford (principal investigator), Jeffrey P. Jacobs (no longer with institution), Jade Hanson, Lexie Dallas, and Dawn Morelli (no longer with institution; auxiliary site: John Hopkins All Children’s Heart Institute); William Mahle (principal investigator), Dawn Ilardi, Ritu Sachdeva, Timothy Slesnick, William Border, Courtney Fyock (no longer with institution), Susie Gentry (no longer with institution), Rae Ashley (no longer with institution), Amanda Graham (no longer with institution), and Leslie Smitley (no longer with institution; auxiliary site: Emory University); Christian Pizarro, Jeanne Baffa, Michael McCulloch, Brad Robinson, Erica Sood, and Carol Prospero (auxiliary site: Nemours Cardiac Center); Peter Frommelt, Jessica Stelter, and Meagan Schoessling (echocardiography core laboratory: Children’s Hospital of Wisconsin); Stephen Paridon (exercise core laboratory: Children’s Hospital of Philadelphia); Michael Taylor, Joshua Germann, and Peace Madueme (MRI core laboratory: Cincinnati Children’s Medical Center); Michael Artman (former chair), Timothy Feltes, Jogarao Gobburu, Sally Hunsberger, and Paul Matherne (protocol review committee); and Dianne Atkins, Preetha Balakrishnan, Craig Broberg, David J. Driscoll, David J. Gordon (former executive secretary), Frank Evans (data and safety monitoring board executive secretary), Sally A. Hunsberger, Liza-Marie Johnson, Mark Galantowicz, Thomas J. Knight, John Kugler (former chair), Paul Lipkin, J. Philip Saul (data and safety monitoring board chair), and Holly Taylor (data and safety monitoring board).
Sources of Funding
Funding for this study was provided by the National Heart, Lung, and Blood Institute through the Pediatric Heart Network, supporting the clinical sites and the data coordinating center, including grants HL135680, HL135685, HL135683, HL135689, HL135646, HL135665, HL135678, HL135682, HL135666, and HL135691. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
Disclosures
None.
Supplemental Material
Tables S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CMR
- cardiac magnetic resonance imaging
- HLHS
- hypoplastic left heart syndrome
- mBTTS
- modified Blalock-Taussig-Thomas shunt
- PLE
- protein-losing enteropathy
- RVEF
- right ventricular ejection fraction
- RVPAS
- right ventricle-to-pulmonary artery shunt
- SVR
- Single Ventricle Reconstruction
- VIF
- variance inflation factor
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.065192.
For Sources of Funding and Disclosures, see page 1338.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Felicia Trachtenberg, Email: Felicia.Trachtenberg@carelon.com.
J. William Gaynor, Email: gaynor@chop.edu.
William T. Mahle, Email: mahlew@kidsheart.com.
Chitra Ravishankar, Email: ravishankar@chop.edu.
Steven M. Schwartz, Email: steven.schwartz@sickkids.ca.
James F. Cnota, Email: james.cnota@cchmc.org.
Richard G. Ohye, Email: ohye@umich.edu.
Michael Taylor, Email: michael.taylor1@austin.utexas.edu.
Stephen Paridon, Email: paridon@email.chop.edu.
Peter C. Frommelt, Email: pfrommelt@chw.org.
Katherine Afton, Email: kafton@med.umich.edu.
Andrew M. Atz, Email: atzam@musc.edu.
Kristin M. Burns, Email: kristin.burns@nih.gov.
Jon A. Detterich, Email: jdetterich@chla.usc.edu.
Kevin D. Hill, Email: kevin.hill@duke.edu.
Antonio G. Cabrera, Email: antonio.cabrera@hsc.utah.edu.
Alan B. Lewis, Email: alewis@chla.usc.edu.
Christian Pizarro, Email: cpizarro@nemours.org.
Amee Shah, Email: ams79@cumc.columbia.edu.
Binu Sharma, Email: gtmbini@gmail.com.
Jane W. Newburger, Email: jane.newburger@cardio.chboston.org.
REFERENCES
- 1.Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45:87–91. doi: 10.1016/0002-9149(80)90224-6 [DOI] [PubMed] [Google Scholar]
- 2.Sano S, Ishino K, Kawada M, Honjo O. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:22–31. doi: 10.1053/j.pcsu.2004.02.023 [DOI] [PubMed] [Google Scholar]
- 3.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, Tabbutt S, Wernovsky G, et al. ; Pediatric Heart Network Investigators. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, et al. ; Pediatric Heart Network Investigators. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newburger JW, Sleeper LA, Gaynor JW, Hollenbeck-Pringle D, Frommelt PC, Li JS, Mahle WT, Williams IA, Atz AM, Burns KM, et al. ; Pediatric Heart Network Investigators. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. doi: 10.1161/CIRCULATIONAHA.117.029375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg CS, Gaynor JW, Mahle WT, Ravishankar C, Frommelt P, Ilardi D, Bellinger D, Paridon S, Taylor M, Hill KD, et al. ; Pediatric Heart Network Investigators. The Pediatric Heart Network’s study on long-term outcomes of children with HLHS and the impact of Norwood shunt type in the Single Ventricle Reconstruction trial cohort (SVRIII): design and adaptations. Am Heart J. 2022;254:216–227. doi: 10.1016/j.ahj.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 7.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, et al. ; Pediatric Heart Network Investigators. Transplantation-free survival and interventions at 3 years in the Single Ventricle Reconstruction trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, et al. ; Pediatric Heart Network Investigators. Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, et al. ; Pediatric Heart Network Investigators. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. doi: 10.1016/j.jtcvs.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrindle BW, Williams RV, Mitchell PD, Hsu DT, Paridon SM, Atz AM, Li JS, Newburger JW; Pediatric Heart Network Investigators. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation. 2006;113:1123–1129. doi: 10.1161/CIRCULATIONAHA.105.576660 [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DJ, Avitable CM, McBride MG, Paridon SM. Exercise capacity in the Fontan circulation. Cardiol Young. 2013;23:824–830. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg DJ, Zak V, McCrindle BW, Ni H, Gongwer R, Rhodes J, Garofano RP, Kaltman JR, Lambert LM, Mahony L, et al. ; Pediatric Heart Network Investigators. Exercise capacity and predictors of performance after Fontan: results from the Pediatric Heart Network Fontan 3 Study. Pediatr Cardiol. 2021;42:158–168. doi: 10.1007/s00246-020-02465-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, et al. ; Pediatric Heart Network Investigators. Arrhythmias in a contemporary Fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol. 2010;56:890–896. doi: 10.1016/j.jacc.2010.03.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cain N, Saul JP, Gongwer R, Trachtenberg F, Czosek RJ, Kim JJ, Kaltman JR, LaPage MJ, Janson CM, Singh AK, et al. Relation of Norwood shunt type and frequency of arrhythmias at 6 years (from the Single Ventricle Reconstruction trial). Am J Cardiol. 2022;169:107–112. doi: 10.1016/j.amjcard.2021.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobh M, Freitag-Wolf S, Schewe J, Kanngiesser LM, Uebing AS, Gabbert DD, Voges I. Serial right ventricular assessment in patients with hypoplastic left heart syndrome: a multiparametric cardiovascular magnetic resonance study. Eur J Cardiothorac Surg. 2021;61:36–42. doi: 10.1093/ejcts/ezab232 [DOI] [PubMed] [Google Scholar]
- 17.Frommelt PC, Hu C, Trachtenberg F, Baffa JM, Boruta RJ, Chowdhury S, Cnota JF, Dragulescu A, Levine JC, Lu J, et al. Impact of initial shunt type on echocardiographic indices in children after single right ventricle palliations. Circ Cardiovasc Imaging. 2019;12:e007865. doi: 10.1161/CIRCIMAGING.118.007865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghelani SJ, Lu M, Sleeper LA, Prakash A, Castellanos DA, Clair NS, Powell AJ, Rathod RH. Longitudinal changes in ventricular size and function are associated with death and transplantation late after the Fontan operation. J Cardiovasc Magn Reson. 2022;24:56. doi: 10.1186/s12968-022-00884-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiyagari R, Rhodes JF, Shreader P, Radtke WA, Bandisode VM, Bergersen L, Gillespie MJ, Gray RG, Guey LT, Hill KD, et al. Impact of pre-stage II hemodynamics and pulmonary artery anatomy on 12-month outcomes in the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2014;148:1467–1474. doi: 10.1016/j.jtcvs.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]