Abstract
Herein, we present 2 patients with lower limb ischemia caused by complicated popliteal aneurysms with thrombosis and distal embolization, compromising blood flow to the foot. In both cases, covered stents were first implanted guided by intravascular ultrasound and computed tomography angiography, respectively. After “trapping” the thrombi, mechanical thrombectomy or further stent implantations were performed, “fixing” the remaining lesions and preventing embolization. (Level of Difficulty: Intermediate.)
Key Words: computed tomography, intravascular ultrasound (IVUS), peripheral circulation, peripheral vascular disease, thrombus
Central Illustration
Popliteal arterial aneurysm (PAA) is a localized, irreversible dilation of the popliteal artery wall, exceeding the normal vessel diameter by more than 50%. PAA is a rare pathology, although it is the most common after abdominal aortic aneurysm.1,2 Herein, we present 2 cases of a nonsurgical candidates, with multiple comorbidities, who presented with critical limb-threatening ischemia and acute limb ischemia (ALI), respectively, caused by thrombosed PAAs.
Learning Objectives
-
•
Peripheral arterial embolism has a multitude of potential etiologies.
-
•
Atrial fibrillation, abdominal aneurysm, and popliteal aneurysms are 3 common causes and sources of arterial embolism that need to be considered.
-
•
A “trap and fix” strategy may reduce the rate of potential thromboembolic complications during endovascular repair of complicated popliteal aneurysms, obviating the need for catheter-directed thrombolysis in such complex cases.
-
•
Intravascular ultrasound and/or preprocedural computed tomography angiography is of great importance to guide endovascular management successfully and safely.
Case Presentation 1
The first 85-year-old patient was referred to our department after failed intervention in an external hospital. Clinically, a wound with gangrene of his right great toe was present, Rutherford category (RC) 5 (Figure 1A).
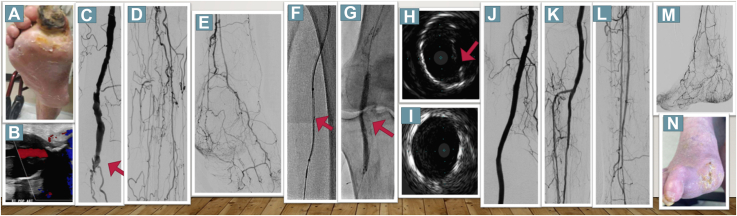
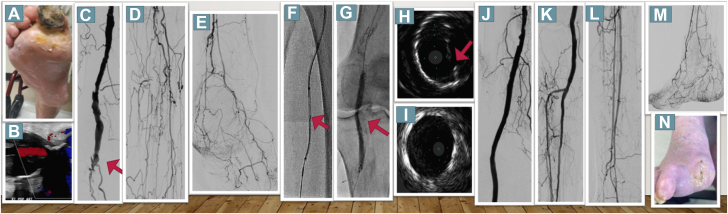
Figure 1.
Duplex Sonography, Angiography and IVUS Findings in the First Patient
(A) Patient 1 presenting with a gangrene. (B) Duplex sonography showed a thrombosed popliteal arterial aneurysm. (C) Angiography showed occlusion of the P2 segment (red arrow). (D, E) Long occlusions of all tibioperoneal vessels were observed. (F) A bidirectional approach was used for recanalization, and 2 Viabahn endoprostheses were implanted. (G, H) Fluoroscopy and intravascular ultrasound depicted significant recoil. (I) After deployment of a Supera stent, intravascular ultrasound images exhibited improved luminal gain. (J to M) The final angiographic result with a 2-vessel run-off. (N) The wound healed completely after 60 days.
He had a history of smoking, chronic obstructive lung disease, obstructive sleep apnea, and surgically resected stage IIA signet ring gastric adenocarcinoma. Duplex sonography revealed a thrombosed right PAA (Figure 1B) with faint but audible signals in all 3 pedal arteries. Because of inadequate wound healing, a second revascularization attempt was performed. Digital subtraction angiography showed an occluded PAA at the P2 segment of the popliteal artery (red arrow in Figure 1C). Additionally, long occlusions of all tibioperoneal vessels were present (Figures 1D and 1E). Because of lesion complexity, retrograde puncture of the right peroneal vessel was performed, followed by insertion of a low-profile 2.9-F sheath. After subintimal passage of the antegrade wire, a 0.035-inch support catheter was inserted at the level of the proximal peroneal artery. Then, a 0.018-inch hydrophilic guidewire wire was passed through the distal cap from retrograde into the proximal support catheter (Figure 1F). After externalization, an Emboshield Nav6 (Abbott Vascular) embolic protection device was deployed in the peroneal artery. Subsequently, intravascular ultrasound (IVUS) was performed to evaluate the thrombosed PAA, and the diameters of the proximal and distal landing zones were measured (6.5 and 5.5 mm, respectively). Because of partly thrombotic and calcified components on IVUS, predilatation was performed with a small-diameter balloon to allow delivery of a 6.0 × 15-mm and a 7.0 × 100-mm Viabahn (Gore), thus oversizing by 10% to 15% based on IVUS measures. Fluoroscopy and IVUS depicted significant recoil caused by calcium compression (Figures 1G and 1H), and the Viabahn was relined using a 5.5 × 150-mm Supera (Abbott Vascular) stent, which resulted in substantially improved luminal gain by IVUS (Figure 1I). Afterward, 2 4.0 × 38-mm and 3.0 × 28-mm Onyx zotarolimus-eluting stents were implanted in the tibioperoneal trunk and the anterior tibial artery to improve perfusion to the foot (Figures 1J to 1M), which is essential to prevent covered stent occlusion in the long term. Six days after revascularization, minor amputation of his right great toe was performed, and the wound completely healed after 60 days (Figure 1N).
Case Presentation 2
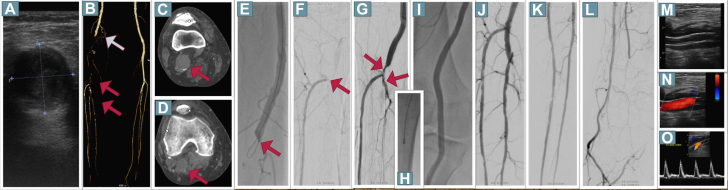
A 74-year-old man presented with acute intense pain of his right lower limb during the last 7 days. In addition, mild paresthesia was present without motoric deficits (RCIIA) or myonecrosis. His foot was pale and pulseless. He had coronary artery disease and heart failure. Duplex sonography revealed thrombotic occlusion of the distal superficial femoral (SFA) and popliteal arteries. In addition, a thrombosed PAA with a diameter of 2.8 × 2.5 cm was diagnosed (Figure 2A). Faint but audible Doppler signals were detected in the foot arteries. Computed tomography angiography confirmed the occlusion of the distal SFA (blue arow) with refilling at the level of the proximal crural arteries (Figure 2B). In addition, the presence of the PAA with a maximal diameter of 2.8 × 3.0 cm was confirmed (Figures 2C and 2D). Because of the older age and high comorbidities, endovascular repair was offered. After antegrade puncture, angiography confirmed the occlusion of the distal SFA (Figures 2E and 2F). Crossing was easily performed from antegrade using a 0.018-inch hydrophilic guidewire. Based on preprocedural computed tomography angiography images, two 6.0 × 150-mm and 7.0 × 150-mm Viabahn endoprostheses were placed in the popliteal artery and the distal SFA to exclude the underlying thrombus. It should be noted that in contrast to balloon-expandable stents, Viabahn endoprostheses are appropriate for treatment of mobile segments. Herewith, antegrade flow was re-established in the popliteal artery, and remaining thrombus formations were detected distally (Figure 2G), which were effectively removed by 6-F Rotarex (Becton Dickinson) thrombectomy (Figure 2H), resulting in brisk flow to the foot (Figures 2I to 2L). The patient was put on dual platelet inhibition with aspirin and clopidogrel. Duplex sonography on day 3 revealed exclusion of the PAA by the Viabahn protheses with good triphasic flow in the popliteal artery (Figures 2M to 2O). Duplex sonography showed patency of the stent grafts and of all tibioperoneal arteries at 3 months, with reopening also of the posterior tibial artery during antiplatelet treatment. The patient remained uneventful during the following 9 months.
Figure 2.
Duplex Sonography, Angiography and CT Findings in the Second Patient.
(A) Thrombosed popliteal arterial aneurysm with a diameter of 2.8 × 2.5 cm. (B) Computed tomography angiography confirmed the occlusion of the distal superficial femoral artery (blue arrow) with refilling at the level of the proximal crural arteries (red arrows). (C, D) Popliteal arterial aneurysm was confirmed (red arrows). (E, F) Occlusion of the distal superficial femoral artery with contrast refilling in the proximal anterior tibial artery. Two overlapping Viabahn endoprostheses were placed. Because of (G) remaining thrombus formations (red arrows), (H) thrombectomy was performed, resulting in (I to L) brisk flow. (M to O) Duplex sonography revealed exclusion of the popliteal arterial aneurysm with triphasic flow in the popliteal artery.
Discussion
Advanced age, male, and history of smoking represent classical epidemiologic risk factors associated with PAAs.2,3 Both patients exhibited symptoms of limb ischemia at admission. In addition, faint but audible Doppler signals were detected. Lower extremity ischemia is the most common symptom occurring because of thrombosis or embolization from PAAs, with variable clinical symptoms ranging from claudication and chronic limb-threatening ischemia to ALI.4 With ALI, different stages are differentiated, including RCIIA with minimal sensory and without motor loss and often with inaudible arterial signals but audible venous signals. RCIIB is associated with evident sensory and motor loss as well as usually inaudible arterial signals but also audible venous signals.
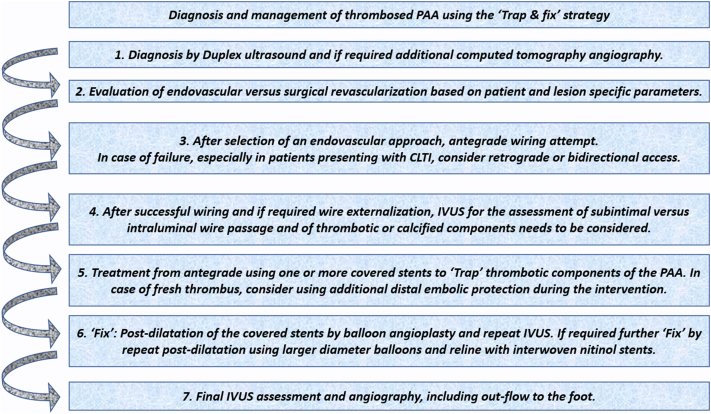
Indications for repair depend on the PAA diameter (more than 2 cm) and clinical symptoms.5,6 Treatment of thrombosed PAAs encompasses both open repair and endovascular revascularization, with the type of treatment depending on patient-specific factors.5 Our patients both exhibited extensive comorbidities, not being optimal surgical candidates. During an endovascular approach, embolization of thrombotic burden is a feared complication, which may result in trash foot syndrome, requiring major amputation. In both cases reported herein, covered stents were implanted first to trap the thrombus. This “trap and fix” strategy (all steps are provided in Figure 3) in both cases effectively prevented distal embolization. In acute cases with “fresh thrombus,” however, even with this technique, embolic protection and catheter-directed thrombolysis may represent additional useful tools to further reduce the risk of embolization or improve distal perfusion to the foot. Thus, in our second case, the use of embolic protection would have possibly prevented distal embolization, which was subsequently treated by thrombectomy. Furthermore, the role of pre- and periprocedural imaging needs to be highlighted, which in both cases helped to select the distal landing zone of the covered stents. In the case of a covered stent implantation distal to the crural trifurcation, the flow of one or more tibioperoneal arteries would have been compromised. Furthermore, Viabahn endoprostheses are not available with <5 mm, which limits their use in crural vessels. Intravascular or preprocedural imaging can help to define the landing zones for implantation of covered stents, keeping the procedure safe. In addition, the role of bidirectional access is demonstrated in our first case.7,8 After the endovascular treatment, outcomes in terms of symptom improvement and wound healing were excellent in both cases.
Figure 3.
Steps of the “Fix and Trap” Technique
CLTI = chronic limb-threatening ischemia; IVUS = intravascular ultrasound; PAA = popliteal arterial aneurysm.
The suggested “trap and fix” strategy (Figure 3) represents a novel way to avoid distal embolization during the endovascular treatment of thrombosed PAA.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Cecenarro R.R., Allende J.N., Barreras Molinelli L., Antueno F.J., Gramatica L. Popliteal artery aneurysms: literature review and presentation of case. Article in Spanish. Rev Fac Cien Med Univ Nac Cordoba. 2018;75:41–45. doi: 10.31053/1853.0605.v75.n1.16097. [DOI] [PubMed] [Google Scholar]
- 2.Kassem M.M., Gonzalez L. StatPearls Publishing; 2023. Popliteal artery aneurysm. StatPearls. [PubMed] [Google Scholar]
- 3.Mikhaylov I.P., Lavrenov V.N., Isaev G.A., Kokov L.S., Trofimova E.Y. Ruptures of popliteal artery aneurysms. Article in Russian. Khirurgiia (Mosk) 2018;(4):57–62. doi: 10.17116/hirurgia2018457-62. [DOI] [PubMed] [Google Scholar]
- 4.Shortell C.K., DeWeese J.A., Ouriel K., Green R.M. Popliteal artery aneurysms: a 25-year surgical experience. J Vasc Surg. 1991;14:771–776. doi: 10.1067/mva.1991.33214. discussion 776-779. [DOI] [PubMed] [Google Scholar]
- 5.Kainth A., Smeds M.R. StatPearls Publishing; 2023. Popliteal aneurysm repair. StatPearls. [PubMed] [Google Scholar]
- 6.Tsilimparis N., Dayama A., Ricotta J.J., 2nd Open and endovascular repair of popliteal artery aneurysms: tabular review of the literature. Ann Vasc Surg. 2013;27:259–265. doi: 10.1016/j.avsg.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Korosoglou G., Schmidt A., Lichtenberg M., et al. Crossing algorithm for infrainguinal chronic total occlusions: an interdisciplinary expert opinion statement. J Am Coll Cardiol Intv. 2023;16:317–331. doi: 10.1016/j.jcin.2022.11.036. [DOI] [PubMed] [Google Scholar]
- 8.Korosoglou G., Schmidt A., Stavroulakis K., et al. Retrograde access for the recanalization of lower-limb occlusive lesions: a German EXPERIENCE REPORT in 1,516 consecutive patients. J Am Coll Cardiol Intv. 2022;15:348–351. doi: 10.1016/j.jcin.2021.09.034. [DOI] [PubMed] [Google Scholar]