Abstract
Introduction:
Glioblastoma (GBM) is the most common invasive brain tumor composed of diverse cell types with poor prognosis. The highly complex tumor microenvironment (TME) and its interaction with tumor cells play important roles in the development, progression, and durability of GBM. Angiogenic and immune factors are two major components of TME of GBM; their interplay is a major determinant of tumor vascularization, immune profile, as well as immune unresponsiveness of GBM. Given the ineffectiveness of current standard therapies (surgery, radiotherapy, and concomitant chemotherapy) in managing patients with GBM, it is necessary to develop new ways of treating these lethal brain tumors. Targeting TME, altering tumor ecosystem may be a viable therapeutic strategy with beneficial effects for patients in their fight against GBM.
Materials and Methods:
Given the potential therapeutic effects of cannabidiol (CBD) in a wide spectrum of diseases, including malignancies, we tested, for the first time, whether inhalant CBD can inhibit GBM tumor growth using a well-established orthotopic murine model. Optical imaging, histology, immunohistochemistry, and flow cytometry were employed to describe the outcomes such as tumor progression, cancer cell signaling pathways, and the TME.
Results:
Our findings showed that inhalation of CBD was able to not only limit the tumor growth but also to alter the dynamics of TME by repressing P-selectin, apelin, and interleukin (IL)-8, as well as blocking a key immune checkpoint—indoleamine 2,3-dioxygenase (IDO). In addition, CBD enhanced the cluster of differentiation (CD) 103 expression, indicating improved antigen presentation, promoted CD8 immune responses, and reduced innate Lymphoid Cells within the tumor.
Conclusion:
Overall, our novel findings support the possible therapeutic role of inhaled CBD as an effective, relatively safe, and easy to administer treatment adjunct for GBM with significant impacts on the cellular and molecular signaling of TME, warranting further research.
Keywords: glioblastoma, CBD, tumor microenvironment, immune checkpoint, P-selectin, IDO
Introduction
Glioblastoma (GBM), the most common malignant brain tumor, is highly invasive locally, recurs often, and has poor prognosis.1–3 Despite advances in cancer therapies, GBM remains incurable, with a median survival of only 15 months.1–5 Current standards of care for GBM, including surgery, radiotherapy, and chemotherapy, produce only limited responses.4 Therefore, an urgent need exists for the development of novel, more effective alternative therapeutic modalities in the treatment of GBM.
Tumor microenvironment (TME), a complex network of many cell types, blood vessels, lymphatics, and immune signaling, and extracellular matrices, plays a significant and integral role in the progression of cancer.5–8 The interplay between angiogenic and immunogenic compartments within TME is of fundamental importance to tumor survival and thus poor patient outcomes.7,9 Therefore, targeting angiogenesis and immunologic components may alter the ecosystem of TME with beneficial outcomes for patients with GBM.
Recent studies have suggested a central role for apelin, an inotropic peptide with proangiogenic features during the progression of GBM.10–13 While there was a minimal level of apelin expression in normal brain tissues, however, apelin expression was significantly elevated in GBM. Inhibition of apelin has resulted in the decrease in growth rate of GBM tumor volume.12,13
Several studies have reported a very distinctive immune profile within TME of GBM, characterized by heightened immune checkpoint signaling, accumulated suppressive myeloid cells, and a decrease in effector lymphoid cells. There is a particularly low frequency of cytotoxic T cells.7,9,14 The reciprocal communication between immune compartment and nonimmune components of TME (e.g., tumor cells, endothelial cells, and vascular system) in GBM not only determines the status of the immune profile of TME but also affects the vascularization, angiogenesis, and ultimately the longevity and viability of the tumor itself.7,9,15,16
Importantly, cellular immunity, mediated by effector T cells, is the major arm of immune system against tumor antigens and cancer progression.17,18 Inhibiting T cell activation, particularly auto-reactive cluster of differentiation (CD) 8+ cytotoxic T cells, is a central immunomodulatory strategy by which immune checkpoints exert their suppressive role against antitumor immunity within GBM.5,19–21
As an immune checkpoint, indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme with inhibitory effects on T cells, has emerged as a very attractive potential target in the immunotherapy of several types of cancer, including GBM.4,22–25 Due to its unique potential immunomodulatory function, IDO is considered a nonconventional immune checkpoint with overarching effects on chronic inflammation, antigen presentation, and immune tolerance for tumor ecosystem.
Along with IDO, recent studies have demonstrated that P-selectin may serve as an immune checkpoint within TME, promoting tumor growth in GBM.26,27 P-selectin is a transmembrane protein acting as a cell adhesion molecule on the surfaces of activated endothelial cells and platelets, providing the foundation for interplay between tumor cells and cellular components of the blood.28
Furthermore, the phylogenetically ancient, but newly discovered members of TME are innate lymphoid cells (ILCs).29–31 These special lymphoid cells mirror T-helper cells, but possess neither T cell receptors nor lymphoid surface markers, except CD45.32–34 The role of ILCs in tumor development and cancer progression is controversial and yet to be elucidated.30,31,35 However, increasing evidence suggests a central role for ILCs (including natural killer, NK, cells) in GBM.36 Given the complexity and heterogeneity of GBM, an alternative treatment to alter the TME by inhibiting immune checkpoints may be a potential therapeutic modality with significant beneficial effects for patients with GBM.
Cannabidiol (CBD) is a relatively safe, nonpsychoactive phytocannabinoid produced by Cannabis plants. Recent work by our laboratory and others suggest a beneficial effect of CBD alone or in combination with other cannabinoids in the treatment of malignancies.37–41 Increasing evidence suggests therapeutic and potential antitumor effects of cannabinoids in the treatment of GBM.42–56 Multiple pre-clinical studies, including in vitro as well as in vivo animal models, have demonstrated very encouraging results by inhibiting the growth of GBM.42–52,57,58 Several investigations on human subjects and clinical trials have supported the notion that cannabinoids may have the potential as therapeutic modalities in GBM.44–49,53,56
In this study, we tested, for the first time, the potential effect of inhaled CBD in the progression of GBM in a murine model, and whether such treatment could alter the TME of GBM. Our findings demonstrated the potential of CBD inhalation in the inhibition of tumor growth with alterations of TME in GBM.
Materials and Methods
Animals
Wild-type (total of 11 mice from 3 independent cohorts), 9- to 11-week-old male C57BL/6 mice (obtained from Jackson Laboratories, Bar Harbor, ME) were used to generate the orthotopic GBM model. The animals were housed in the laboratory animal facilities of the Augusta University with free access to food and water. All the experiments were performed according to the National Institutes of Health (NIH) guidelines and regulations. The Institutional Animal Care and Use Committee (IACUC) of Augusta University (protocol No. 2011–0062) approved all the experimental procedures.
Tumor cells and orthotopic animal model of GBM
To generate the orthotopic GBM model in mice, syngeneic GL261 cells were used as described previously.59 In brief, luciferase positive GL261 cells were grown in standard growth media (RPMI-1640 plus 10% fetal bovine serum) and collected in serum-free media on the day of implantation. Mice were anesthetized using 3% isoflurane and maintained with 1.5–2% isoflurane throughout all surgical procedures. After preparation and drilling a hole at 2.25 mm to the right and 1 mm posterior to the bregma, taking care not to penetrate the dura, a 10 μL Hamilton syringe with a 26G-needle containing tumor cells (30,000) in a volume of 3 μL was lowered to a depth of 4 mm and then raised to a depth of 3 mm.
During and after the injection, a careful note was made for any reflux from the injection site. The needle was withdrawn 1 mm at a time in a stepwise manner 2–3 min after completing the injection. The surgical hole was sealed with bone wax. Finally, the skull was swabbed with Betadine before suturing the skin. Post-operative analgesia was provided with a single injection of buprenorphine (1 mg/kg sc).
Tumor growth was determined by optical imaging (bioluminescence imaging after injecting luciferin) on day 8 post-implantation. In vivo, optical images were obtained to keep track of primary tumor and metastasis development by injecting 100 μL of luciferin (dose 150 mg/kg) intraperitoneally followed by the acquisition of bioluminescence signal by spectral AmiX optical imaging system (Spectral Instruments Imaging, Inc., Tucson, AZ). The photon intensity per mm per second was determined by Aura imaging software by Spectral Instruments Imaging, LLC (version 4.0.0).
Treatment protocol
The animals were further subdivided to receive either inhalant CBD or placebo (10 mg/day), delivered through an inhaler (ApelinDx; TM Global Bioscience USA) as described. The dose was calculated based on effectiveness and tolerability of CBD to achieve antitumor effect.60,61 Inhalant CBD or placebo was applied to the animals every day for a period of 8 days, first dose on day 9 and last dose on day 16 post-surgery. At day 17 post-implantation, another set of imaging was performed before all animals were sacrificed and tumor tissues were harvested for histology and immunohistochemical analysis, as well as all flow cytometry-based assays.
Metered dose tincture inhaler: composition and pharmacokinetics
TM Global Bioscience USA provided the metered dose tincture inhaler, ApelinDx. As depicted in Figure 1, ApelinDx was modified by adding an extra nozzle piece to adjust to the mouse model and to further control the intake of CBD. ApelinDx contained 985 mg of broad-spectrum CBD (winterized crude hemp extract) plus 15 mg of cosolvent, surfactant, and propellant, total volume of 1000 mg (5 mg dose per spray, with 200 mL/min flow rate). For the placebo, the 985 mg of broad-spectrum CBD was replaced with 985 mg of hemp seed oil.
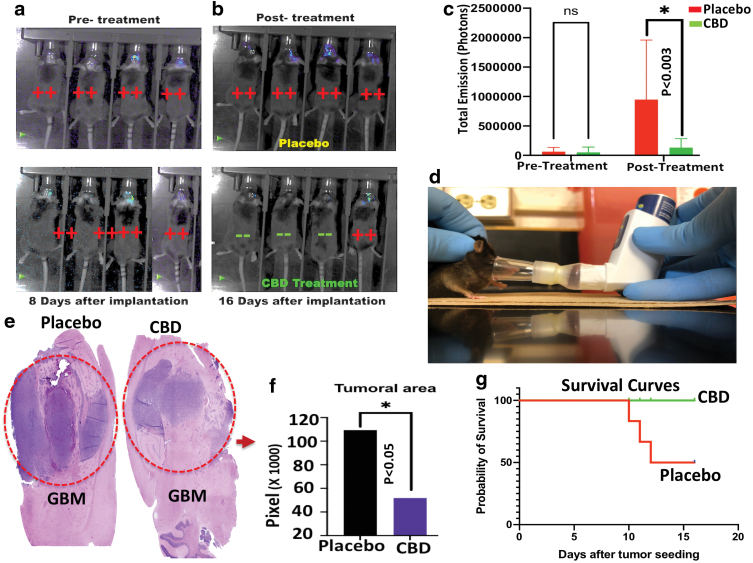
FIG. 1.
Inhalant CBD inhibits tumor growth and improved survival in GBM. (a) Optical imaging (bioluminescence imaging after injecting luciferin) showed the establishment and growth of GBM tumor on day 8 post-implantation. (b) Tumor growth was inhibited after eight consecutive daily treatments of inhalant CBD compared to the placebo-treated group as optical bioluminescence images were quantified (*p>0.05) (c). (d) Mice were treated with CBD/placebo using inhaler ApelinDx. (e) H&E staining suggested significant decrease in tumor size after CBD treatment compared to placebo. The difference in tumor size between CBD- and placebo-treated groups (p<0.05) was quantified using ImageJ Java-based image processing program (*p>0.05) (f). (g) Survival was evaluated (n=6/group), showed that, while all animals in CBD-treated group were alive at 16 days after orthotopic implantation of the tumor, however, 50% of animals from placebo group (3/6) died by day 13 post-implantation, suggesting beneficial effects of CBD treatment. GBM, glioblastoma; H&E, hematoxylin and eosin. Color images are available online.
The plasma concentration of inhalant CBD is reached to the peak rapidly within 3–10 min and maximum concentration is higher relative to oral ingestion.60 Several studies have shown an average systemic bioavailability of 31% for CBD.62 Furthermore, it is reported that inhalant CBD reduces concentrations of an inactive circulating metabolite and enhanced CBD bioavailability compared with oral administration.62–64 These values improve therapeutic ratio by preventing erratic absorption and bypasses first-pass hepatic metabolism, reducing local and systemic side effects.65
Histology and immunohistochemistry
Freshly harvested GBM tumor tissues were fixed with 10% neutral buffered formalin (HT50-1-128; Sigma), processed, and then embedded with conventional dehydrated paraffin. All subsequent procedures were performed at room temperature. Fixed paraffin-embedded tumoral tissues were cut in 4 μm sections and stained with hematoxylin and eosin (H&E) based on standard protocol of H&E staining, observed and analyzed by a bright field light Zeiss microscope. To analyze the tumor size, we cut all tumors in half from the location with longest diameter to send for histology sectioning. We then measured and quantified the area of tumor invasion by using NIH ImageJ software (version 1.53g).
Further immunohistochemical assessment was carried out as described previously.66 In short, all slides were rehydrated and endogenous peroxidase activity was blocked using hydrogen peroxide diluted 1:10 with distilled water for 10 min. Sections were treated with proteinase K for 10 min and washed twice in phosphate buffered saline (PBS).
Next, slides were incubated with antibodies against apelin (Cat. No. BS-2425R-A750; Bioss), interleukin (IL)-8 (Cat. No. Orb360891; Biorbyt), P-selectin (Cat. No. 148309; Biolegend), IDO (Cat. No. SC-53978 AF594; Santa Cruz Biotechnology), CD103 (Cat. No. 121415; Biolegend), and CD8 (Cat. No. 553032; BD Biosciences) for 2 h at room temperature. Biotinylated immunoglobulins (Cat. No. HK340-9K; Biogenex) were added to all slides for 20 min. After two washes with PBS, all slides were incubated with peroxidase-conjugated streptavidin (Cat. No. HK330-9K; Biogenex) for 20 min followed by two washes in PBS.
All slides were then treated with chromogen (Cat. No. K3461; Dako) until clearly detectable color appeared. Excess chromagen was decanted and all slides were washed by distilled water. All preparations were counterstained with hematoxylin (Cat. No. 812; ANATECH Ltd.) for 3 min and mounted in an aqueous mountant (Cat. No. 13800; LERNER Laboratories) before the analysis using bright field Zeiss (AXIO Imager M2) light microscope.
Analytical flow cytometry
For flow cytometry analysis, tumor tissues were placed in a tissue culture dish with 1 mL PBS +2% fetal calf serum, 2 mg/mL of collagenase type II, and 1 mg/mL of DNase type I for 30 min at 37°C. Samples were then sieved through a cell strainer (BD Biosciences), followed by centrifugation (252 g, 5 min, 4°C) to prepare single-cell suspensions. Cells then were subjected to flow cytometry analysis using a NovoCyte Quanteun and analyzed by FlowJo analytical software.
Briefly, cells were gated as Lin−CD45+ (mouse, catalog 103114, clone 30-F11) lymphocytes and a lineage cocktail of antibodies (all antibodies from BioLegend, unless otherwise noted), including FITC-conjugated anti-CD3 (mouse, catalog 100204, clone 17A2), anti-CD4 (mouse, catalog 100406, clone GK1.5), anti-CD14 (mouse, catalog 123308, clone Sa14-2), anti-CD16 (mouse, catalog 101305, clone 93), anti-CD19 (mouse, catalog 152404, clone 1D3/CD19), anti-CD8 (mouse, catalog 140404, clone 53-5.8), anti-CD15 (human/mouse, catalog 125611, clone MC-480), anti-CD20 (mouse, catalog 152108, clone SA271G2), was used for negative selection.
Subsequently, ILC1s were identified as mouse (Lin−CD127+IL-12Rβ2+ [mouse/human, catalog FAB1959P-100, clone 305719; R&D Systems]) cells, ILC2s were identified as mouse (Lin−CD127+GATA3+) cells, and ILC3s were identified as mouse (Lin−CD127+RORγt+; mouse/human, catalog 17-6988-82, clone AFKJS-9; Thermo Fisher Scientific) cells (all antibodies from BioLegend). Isotype-matched controls were analyzed to set the appropriate gates for each sample. For each marker, samples were analyzed in duplicate.
To minimize false-positive events, the number of double-positive events detected with the isotype controls was subtracted from the number of double-positive cells stained with corresponding antibodies (not isotype control). Cells expressing a specific marker were reported as a percentage of the number of gated events. A population was considered positive for a specific marker if the population exceeded a 2% isotypic control threshold.
Statistics
For statistical analysis, Brown-Forsythe and Welch analysis of variance (ANOVA) was used to establish significance (p<0.05) among groups. For tissue quantification statistical analysis, we compared the area of expression in both placebo and CBD-treated groups by using two-way ANOVA followed by post-hoc Sidak test for multiple comparison (p<0.05). Survival between groups was compared using Kaplan-Meier analysis and the Log-rank Mantel-Cox test.
Results
Inhalant CBD inhibited tumor growth and improved survival rate in GBM
Tumor establishment was shown by optical imaging at pre-treatment stage (Fig. 1a) before dividing mice into two groups of placebo or CBD-treated groups. As displayed in Figure 1b, and quantified in Figure 1c, photon intensities of optical imaging demonstrated that inhalant CBD (Fig. 1d) was able to inhibit tumor growth in GBM compared to the placebo group. In addition, histological assessment using H&E staining revealed a significant inhibition of tumor growth (p<0.05) in CBD-treated mice versus placebo control group (Fig. 1e, f).
Survival was evaluated (Fig. 1g), for total of eight animals (two independent of four animals per cohort), and showed that, while all animals in CBD-treated group were alive at 16 days after orthotopic implantation of the tumor, three animals (38%) from placebo group died by day 13 post-implantation, suggesting beneficial effects of CBD treatment.
Inhalant CBD suppressed angiogenic factors within GBM tumor
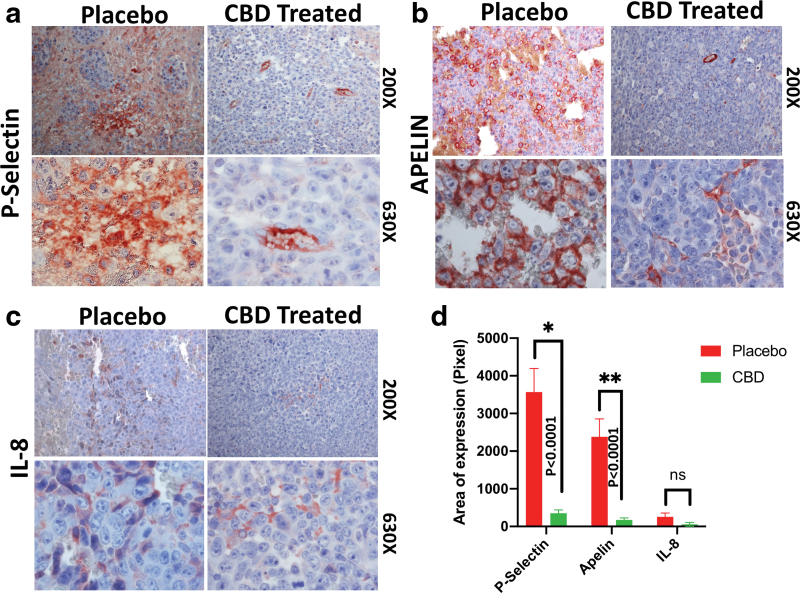
Immunohistochemistry staining showed that inhalant CBD was able to repress the expression of P-selectin (Fig. 2a) and apelin (Fig. 2b), as well as IL-8 (Fig. 2c), in CBD-treated group compared to the placebo group as quantified (Fig. 2d). Downregulation of these proteins can potentially alter the TME, affecting the tumor angiogenesis negatively.
FIG. 2.
Immunohistochemical staining of paraffin-embedded GBM tumor tissues showed inhalant CBD decreased expression of angiogenic factors: (a) P-selectin, (b) apelin, and (c) IL-8 significantly compared to the placebo treated group as quantified (*p>0.05; **p>0.01) (d). All images have been obtained using bright field Zeiss (AXIO Imager M2) light microscope, magnifications of 200×and 630×. IL, interleukin; TME, tumor microenvironment. Color images are available online.
Inhalant CBD blocked immune checkpoint signaling, altering the immune profile in GBM
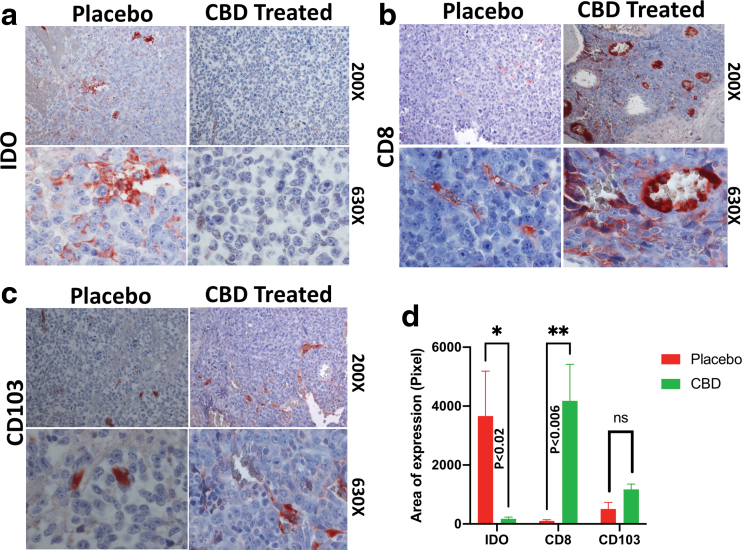
Further immunohistochemistry analysis showed that inhalant CBD blocked the IDO expression (Fig. 3a), while it enhanced CD8 and CD103 expression compared to placebo-treated group (Fig. 3b, c). IDO reduction might have promoted antitumor immunity by increasing frequency of CD8+ cells and improving antigen presentation through heightened CD103 expression as quantified (Fig. 3d). Besides IDO, CBD was able to repress the expression of P-selectin, a nonclassic immune checkpoint with immunosuppressive role, enhancing antitumor immunity.
FIG. 3.
Inhalant CBD modulates immune checkpoints within TME in GBM, altering the intratumoral immune profile. Immunohistochemical staining of paraffin-embedded GBM tumor tissues showed inhalant CBD blocked the immune checkpoint, IDO (a), while enhancing CD8 (b) and CD103 (c) expression compared to the placebo-treated group as quantified (*p > 0.05; **p > 0.01) (d). All images have been obtained using bright field Zeiss (AXIO Imager M2) light microscope, magnifications of 200× and 630×. CD, cluster of differentiation; IDO, indoleamine 2,3-dioxygenase. Color images are available online.
Inhalant CBD regulated ILCs, affecting local proliferation and activation of ILCs within TME
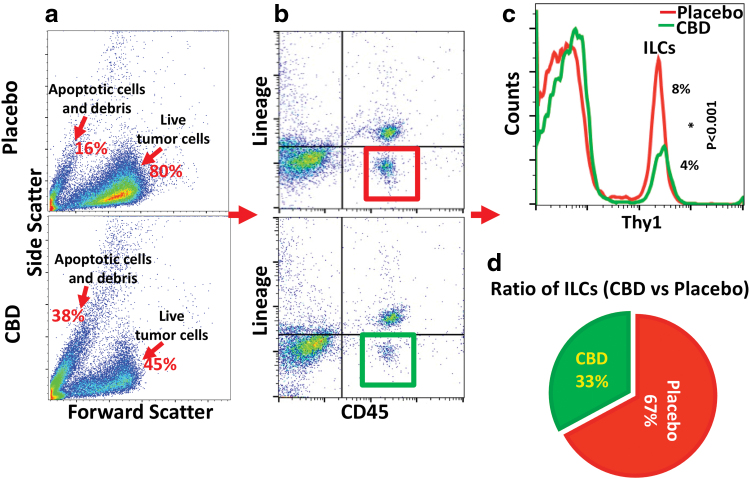
Flow cytometry analysis showed that ILCs were downregulated significantly (p<0.001) in group treated with inhalant CBD compared to the placebo group (Fig. 4). Since each cytokine produced by each class of ILCs can affect TME in a specific way, the reduction in ILCs would affect the interaction between ILCs and TME, altering the intratumor vascularization and immune profile.
FIG. 4.
Inhalant CBD decreased ILC frequencies in GBM, regulating local proliferation and activation of ILCs within TME. Flow cytometry analysis showed that inhalant CBD was able to reduce frequencies of ILCs within TME of GBM significantly (p<0.001), potentially improving antitumor immunity. ILCs were characterized by live gating of total tumor cells (a) based on FSC/SSC, and further gating (b) as CD45+ lineage negative (CD3e, CD11b, CD24, CD5, CD11c, CD19, NK1.1, Gr-1, TER119, and gd TCR), followed by additional gating (*p > 0.05) (c) as Lin-CD45+Thy1+. A pie chart (d) displays the representation of the ratio of ILC counts of CBD-treated (green 33%) vs placebo group (red 67%). FSC, forward scatter; ILC, innate lymphoid cell; NK, natural killer; SSC, side scatter. Color images are available online.
Discussion
Our findings are significant and novel at several levels. This is the first study in which CBD is applied in an experimental model for the treatment of GBM. CBD is a relatively safe and naturally occurred compound.67–70 Therefore, such inhibitory effect of CBD on tumor growth offers a novel therapeutic modality with high relevance and significant clinical values in the treatment of GBM, the most fatal primary brain tumor. More importantly, the use of inhaler to deliver the CBD in noninvasive, precisely metered doses is highly translational to human trials.
GBM is a hypervascular type of tumor, highly depending on angiogenesis and vascularization.59,71 Therefore, targeting angiogenic factors has emerged as an attractive and potentially effective therapeutic modality in the treatment of GBM. In addition to cellular heterogeneity and matrix complexity of TME in GBM, the strategic location of GBM at central nervous system within the blood-brain barrier (BBB) zone has added to the intricacy and existing challenges of therapy for GBM. Results of our current studies suggested that inhalant CBD could penetrate into the BBB, blocking angiogenic factors and altering the balance between stimulating versus inhibitory forces during the tumor angiogenesis within TME, resulting in the tumor growth inhibition.
Our findings revealed, for the first time, that inhalant CBD could block apelin, P-selectin, and IL-8; all are able to influence angiogenesis and vascularization in GBM. Apelin, a neuroangiogenic peptide, promotes cancer metastasis through contribution to the tumor angiogenesis, promoting cancer stem cells and drug resistance.72 Furthermore, the role of IL-8 and its receptors CXCR1/2 in the progression of several cancers, including GBM, has been demonstrated.73
Accordingly, as a potent angiogenic factor, IL-8 plays a crucial role in the progression as well as invasion of GBM, altering TME in GBM, affecting angiogenesis process in an autocrine/endocrine manner. Regulation of NF-κB, NO (nitric oxide) signaling, as well as the inhibition of cross talk between IL-8 and the intratumor IL-6/VEGF are examples of potential mechanisms by which targeting IL-8 may limit the tumor growth in GBM.74–77
While several previous studies had shown the suppressive effect of CBD on IL-8,78 however, our findings, for the first time, showed the downregulation of IL-8 in GBM by inhalant CBD, supporting the notion that CBD may be used as an immunotherapeutic agent in the treatment of GBM.
The other novel finding of our studies here was the blockade of P-selectin expression in GBM after CBD treatment. Several reports have already indicated the role of P-selectin in the progression of GBM.26,27 P-selectin is a vascular adhesion molecule contributing to cancer development by facilitating the cancer-endothelial cell interactions, enhancement of myeloid cell recruitment, and promoting cross talk between cancer cells and platelets.28,79 By blocking P-Selectin, inhalant CBD not only interrupted the basic P-selectin functions but also re-structured the GBM TME.
In addition, besides the traditional role of P-selectin as a cell adhesion molecule and conciliator of cellular recruitment, several recent studies have reported that P-selectin may function as an immune checkpoint through its receptor, PSGL-1, by regulating T cells and curtailing the immunoinflammatory responses.80 Interestingly, our findings revealed that in addition to P-selectin, CBD was able to reduce the IDO expression within TME. IDO functions as an immune checkpoint by depletion of tryptophan, an essential amino acid, regulating T effector cells and promoting Tregs induction.
Given the previous reports indicating the significance of IDO inhibition in limiting GBM development,4,80–82 the potential of CBD in downregulation of IDO may be an effective immunotherapeutic strategy in the treatment of GBM, requires further investigations.
Importantly, the suppression of immune checkpoints in CBD-treated animals was associated with higher CD103 and CD8 expression. As a member of integrin family, integrin αE (ITGAE), also known as CD103, plays crucial roles in a variety of biological processes, including limiting tumor growth.83,84 Several studies have demonstrated the association between heightened intratumoral level of CD103 and improved outcomes for cancer patients.84,85 Consistent with our findings, CD103-expressing dendritic cells have been shown to possess higher quality of antigen presentation, resulting in more effective recruitment of CD8+ T cells and greater antitumor immunity, with better prognosis as well as more favorable clinical consequences.84,85
The interactions between cannabinoids and their receptors are essential for cannabinoid functions, including their impact on the tumor and malignancies.86–88 While CBD has the minimal direct and low affinity with CB receptors (CB1 and CB2), it can modulate the binding of CB receptors with other cannabinoids, including THC. CBD, however, can bind and activate a wide range of non-CB receptors with potential antitumor functions.
Transient receptor potential (TRP) channels are responsible for calcium signaling and cell homeostasis, are shown to play a crucial role in antitumor function of cannabinoids, particularly regarding CBD beneficial impact on brain tumors.87,89
TRP superfamily constitutes of over 30 members; among them, 2 receptors of TRPV1 and TRPV2 (vanilloid family) and their high affinity binding with CBD appear to play a central role in antitumor features of CBD.88,89 In fact, our recent findings showed that the nonpsychotropic cannabinoid, cannabichromene (CBC), can modulate transient receptor potential ankyrin-type1 (TRPA1) in an inflammatory process, may have implications for treatment of malignancies such as GBM.90 Peroxisome proliferator- activated receptor gamma (PPAR-γ) is also a non-CB type II nuclear receptor that has been shown to interact with CBD.
Several studies have reported that regulation of PPAR-γ has resulted in increased median survival for GBM.86,87,91 Interestingly, G-protein-coupled receptor 55 (GPR55) is suggested to play a role in the progression and growth of GBM. CBD is shown to have an antagonistic effects on GPR55, proposing the cross-talk between CBD and GPR55 as a potential novel therapeutic target in the treatment of GBM.86,91
In addition, our studies here showed that CBD reduced the frequencies of ILCs within TME of GBM. Due to their fast-reacting feature to the microenvironmental stimulators, ILCs are considered central modulatory cells during inflammatory responses.92,93 While increasing evidence indicates crucial roles for ILCs in cancer, however, the exact roles of ILCs in cancer are controversial and not fully understood.35,93–95
Several studies have reported that TME of certain cancers is enriched with ILCs compared to the scant numbers of ILCs in normal tissues and circulation.93 While our findings here showed high frequencies of ILCs within TME in GBM, CBD treatment was able to reduce ILCs significantly in GBM compared to the placebo group. Furthermore, ILCs are heterogenic and plastic innate cells with high capabilities in cross talking with all components of TME.93–95 Therefore, CBD-induced regulation of ILCs in GBM tumors has the potential to be considered an effective immunotherapeutic strategy in the treatment of GBM.
In conclusion, our findings suggest that inhalation of CBD can inhibit the tumor growth of GBM by re-shaping and establishing an antitumor dynamic within TME of GBM. Given the urgency to explore new therapeutic strategies for more effective treatment of GBM, leveraging the modulatory and protective capacities of CBD seems a potent option to help patients with GBM. Our data definitively warrant further research in this area.
Acknowledgment
Authors are thankful to ThriftMaster Holding Group (THG) for providing the inhalant CBD for this study.
Abbreviations Used
- ANOVA
analysis of variance
- BBB
brain–blood barrier
- CBD
cannabidiol
- CD
cluster of differentiation
- FSC
forward scatter
- GBM
glioblastoma
- GPR55
G-protein-coupled receptor 55
- H&E
hematoxylin and eosin
- IDO
indoleamine 2,3-dioxygenase
- IL
interleukin
- ILC
innate lymphoid cell
- NIH
National Institutes of Health
- NK
natural killer
- PBS
phosphate buffered saline
- PPAR-γ
peroxisome proliferator- activated receptor gamma
- SSC
side scatter
- THC
tetrahydrocannabinol
- THG
Thriftmaster Holding Group
- TME
tumor microenvironment
- TRP
transient receptor potential
Authors' Contributions
All authors contributed to the study, commented on the article, and approved the final version.
Author Disclosure Statement
All authors declare no conflict of interest. THG is the provider of CBD inhalers and has a licensing contract with Augusta University. THG had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Funding Information
This work was partially supported by Institutional Seed Money from Dental College of Georgia at Augusta University as well as by awards from the NIH (NS110378 to K.M.D./B.B., and NS114560 to K.V.).
Cite this article as: Khodadadi H, Salles ÉL, Alptekin A, Mehrabian D, Rutkowski M, Arbab AS, Yeudall WA, Yu JC, Morgan JC, Hess DC, Vaibhav K, Dhandapani KM, Baban B (2023) Inhalant cannabidiol inhibits glioblastoma progression through regulation of tumor microenvironment, Cannabis and Cannabinoid Research 8:5, 824–834, DOI: 10.1089/can.2021.0098.
References
- 1. Kanderi T, Gupta V. Glioblastoma multiforme. StatPearls Publishing: Treasure Island, FL, 2021. [PubMed] [Google Scholar]
- 2. D'Alessio A, Proietti G, Sica G, et al. . Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers (Basel). 2019;11:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim JH, Bae Kim Y, Han JH, et al. . Pathologic diagnosis of recurrent glioblastoma: morphologic, immunohistochemical, and molecular analysis of 20 paired cases. Am J Surg Pathol. 2012;36:620–628. [DOI] [PubMed] [Google Scholar]
- 4. Ladomersky E, Zhai L, Lenzen A, et al. . IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res. 2018;24:2559–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen PY, Wu CY, Fang JH, et al. . Functional change of effector tumor-infiltrating CCR5+CD38+HLA−DR+CD8+ T cells in glioma microenvironment. Front Immunol. 2019;10:2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Araya RE, Goldszmid RS. Characterization of the tumor immune infiltrate by multiparametric flow cytometry and unbiased high-dimensional data analysis. Methods Enzymol. 2020;632:309–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roma-Rodrigues C, Mendes R, Baptista PV, et al. . Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffer D, Annovazzi L, Casalone C, et al. . Glioblastoma: microenvironment and niche concept. Cancers (Basel). 2018;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gargini R, Segura-Collar B, Sánchez-Gómez P. Cellular plasticity and tumor microenvironment in gliomas: the struggle to hit a moving target. Cancers (Basel). 2020;12:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishimaru Y, Shibagaki F, Yamamuro A, et al. . An apelin receptor antagonist prevents pathological retinal angiogenesis with ischemic retinopathy in mice. Sci Rep. 2017;7:15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helker CS, Eberlein J, Wilhelm K, et al. . Apelin signaling drives vascular endothelial cells toward a pro-angiogenic state. Elife. 2020;9:e55589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mastrella G, Hou M, Li M, et al. . Targeting APLN/APLNR improves antiangiogenic efficiency and blunts proinvasive side effects of VEGFA/VEGFR2 blockade in glioblastoma. Cancer Res. 2019;79:2298–2313. [DOI] [PubMed] [Google Scholar]
- 13. Harford-Wright E, Andre-Gregoire G, Jacobs KA, et al. . Pharmacological targeting of apelin impairs glioblastoma growth. Brain. 2017;140:2939–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pombo Antunes AR, Scheyltjens I, Duerinck J, et al. . Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife. 2020;9:e52176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31:326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hambardzumyan D, Bergers G. Glioblastoma: defining tumor niches. Trends Cancer. 2015;1:252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen D, Zhang X. Cellular immunity augmentation in mainstream oncologic therapy. Cancer Biol Med. 2017;14:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leclerc M, Voilin E, Gros G, et al. . Regulation of antitumour CD8 T-cell immunity and checkpoint blockade immunotherapy by Neuropilin-1. Nat Commun. 2019;10:3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Principe N, Kidman J, Goh S, et al. . Tumor infiltrating effector memory antigen-specific CD8+ T cells predict response to immune checkpoint therapy. Front Immunol. 2020;11:584423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang I, Tihan T, Han SJ, et al. . CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17:1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown ZJ, Yu SJ, Heinrich B, et al. . Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol Immunother. 2018;67:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhai L, Ladomersky E, Lenzen A, et al. . IDO1 in cancer: a Gemini of immune checkpoints. Cell Mol Immunol. 2018;15:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romani M, Pistillo MP, Carosio R, et al. . Immune checkpoints and innovative therapies in glioblastoma. Front Oncol. 2018;8:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhai L, Ladomersky E, Lauing KL, et al. . Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clin Cancer Res. 2017;23:6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeini E, Ofek P, Pozzi S, et al. . P-selectin axis plays a key role in microglia immunophenotype and glioblastoma progression. Nat Commun. 2021;12:1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nolo R, Herbrich S, Rao A, et al. . Targeting P-selectin blocks neuroblastoma growth. Oncotarget. 2017;8:86657–86670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borsig L. Selectins in cancer immunity, Glycobiology. 2018;28:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells—how did we miss them? Nat Rev Immunol. 2013;13:75–87. [DOI] [PubMed] [Google Scholar]
- 30. Hagerling C, Casbon AJ, Werb Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015;25:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tugues S, Ducimetiere L, Friebel E, et al. . Innate lymphoid cells as regulators of the tumor microenvironment. Semin Immunol. 2019;41:101270. [DOI] [PubMed] [Google Scholar]
- 32. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. [DOI] [PubMed] [Google Scholar]
- 33. Bennstein SB, Uhrberg M. Biology and therapeutic potential of human innate lymphoid cells. FEBS J. 2021. [Epub ahead of print]; DOI: 10.1111/febs.15866. [DOI] [PubMed] [Google Scholar]
- 34. Baban B, Braun M, Khodadadi H, et al. . AMPK induces regulatory innate lymphoid cells after traumatic brain injury. JCI Insight. 2021;6:e126766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghaedi M, Ohashi PS. ILC transdifferentiation: roles in cancer progression. Cell Res. 2020;30:562–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedgwick AJ, Ghazanfari N, Constantinescu P, et al. . The role of NK cells and innate lymphoid cells in brain cancer. Front Immunol. 2020;11:1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dariš B, Tancer Verboten M, Knez Ž, et al. Cannabinoids in cancer treatment: therapeutic potential and legislation. Bosn J Basic Med Sci. 2019;19:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285:6–12. [DOI] [PubMed] [Google Scholar]
- 39. Dell DD, Stein DP. Exploring the use of medical marijuana for supportive care of oncology patients. J Adv Pract Oncol. 2021;12:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Griffiths C, Aikins J, Warshal D, et al. . Can cannabidiol affect the efficacy of chemotherapy and epigenetic treatments in cancer? Biomolecules. 2021;11:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simmerman E, Qin X, Yu JC, et al. . Cannabinoids as a potential new and novel treatment for melanoma: a pilot study in a murine model. J Surg Res. 2019;235:210–215. [DOI] [PubMed] [Google Scholar]
- 42. López-Valero I, Torres S, Salazar-Roa M, et al. . Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem Pharmacol. 2018;157:275–284. [DOI] [PubMed] [Google Scholar]
- 43. López-Valero I, Saiz-Ladera C, Torres S, et al. . Targeting glioma initiating cells with a combined therapy of cannabinoids and temozolomide. Biochem Pharmacol. 2018;157:266–274. [DOI] [PubMed] [Google Scholar]
- 44. Likar R, Koestenberger M, Stultschnig M, et al. . Concomitant treatment of malignant brain tumours with CBD—a case series and review of the literature. Anticancer Res. 2019;39:5797–5801. [DOI] [PubMed] [Google Scholar]
- 45. Solinas M, Massi P, Cinquina V, et al. . Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multi target effect. PLoS One. 2013;8:e76918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott KA, Shah S, Dalgleish AG, et al. . Enhancing the activity of cannabidiol and other cannabinoids in vitro through modifications to drug combinations and treatment schedules. Anticancer Res. 2013;33:4373–4380. [PubMed] [Google Scholar]
- 47. Huang T, Xu T, Wang Y, et al. . Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy. 2021;17:3592–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torres S, Lorente M, Rodríguez-Fornés F, et al. . A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol Cancer Ther. 2011;10:90–103. [DOI] [PubMed] [Google Scholar]
- 49. Dall'Stella PB, Docema MFL, Maldaun MVC, et al. . Case report: clinical outcome and image response of two patients with secondary high-grade glioma treated with chemoradiation, PCV, and cannabidiol. Front Oncol. 2019;8:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13:2955–2967. [DOI] [PubMed] [Google Scholar]
- 51. Hernán Pérez de la Ossa D, Lorente M, Gil-Alegre ME, et al. . Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS One. 2013;8:e54795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deng L, Ng L, Ozawa T, et al. . Quantitative analyses of synergistic responses between cannabidiol and DNA-damaging agents on the proliferation and viability of glioblastoma and neural progenitor cells in culture. J Pharmacol Exp Ther. 2017;360:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doherty GJ, de Paula BHR. Cannabinoids in glioblastoma multiforme-hype or hope? Br J Cancer. 2021;124:1341–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang K, Wang Q, Li Q, et al. . Cannabinoid WIN 55,212–2 inhibits human glioma cell growth by triggering ROS-mediated signal pathways. Biomed Res Int. 2021;2021:6612592. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55. Volmar MNM, Cheng J, Alenezi H, et al. . Cannabidiol converts NFκB into a tumor suppressor in glioblastoma with defined antioxidative properties. Neuro Oncol. 2021;23:1898–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dumitru CA, Sandalcioglu IE, Karsak M. Cannabinoids in glioblastoma therapy: new applications for old drugs. Front Mol Neurosci. 2018;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Singer E, Judkins J, Salomonis N, et al. . Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015;6:e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soroceanu L, Murase R, Limbad C, et al. . Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic target. Cancer Res. 2013;73:1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ali S, Borin TF, Piranlioglu R, et al. . Changes in the tumor microenvironment and outcome for TME-targeting therapy in glioblastoma: a pilot study. PLoS One. 2021;16:e0246646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nelson KM, Bisson J, Singh G, et al. . The essential medicinal chemistry of cannabidiol (CBD). J Med Chem. 2020;63:12137–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Phillips JE. Inhaled efficacious dose translation from rodent to human: a retrospective analysis of clinical standards for respiratory diseases. Pharmacol Ther. 2017;178:141–147. [DOI] [PubMed] [Google Scholar]
- 62. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84:2477–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lichtman AH, Poklis JL, Poklis A, et al. . The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend. 2001;63:107–116. [DOI] [PubMed] [Google Scholar]
- 64. Devinsky O, Kraft K, Rusch L, et al. . Improved Bioavailability with Dry Powder Cannabidiol Inhalation: a phase 1 clinical study. J Pharm Sci. 2021;110:3946–3952. [DOI] [PubMed] [Google Scholar]
- 65. Meyer P, Langos M, Brenneisen R. Human pharmacokinetics and adverse effects of pulmonary and intravenous THC-CBD formulations. Med Cannabis Cannabinoids. 2018;1:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khodadadi H, Salles ÉL, Jarrahi A, et al. . Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 2020;5:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guzmán M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745–755. [DOI] [PubMed] [Google Scholar]
- 68. Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shohami E, Horowitz M. Cannabinoids in health and disease. J Basic Clin Physiol Pharmacol. 2016;27:175–179. [DOI] [PubMed] [Google Scholar]
- 70. Chesney E, Oliver D, Green A, et al. . Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahir BK, Engelhard HH, Lakka SS. Tumor development and angiogenesis in adult brain tumor: glioblastoma. Mol Neurobiol. 2020;57:2461–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masoumi J, Jafarzadeh A, Khorramdelazad H, et al. . Role of Apelin/APJ axis in cancer development and progression. Adv Med Sci. 2020;65:202–213. [DOI] [PubMed] [Google Scholar]
- 73. Sharma I, Singh A, Siraj F, et al. . IL-8/CXCR1/2 signalling promotes tumor cell proliferation, invasion and vascular mimicry in glioblastoma. J Biomed Sci. 2018;25:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NF-κB induced invasion by gliomas. J Neurooncol. 2011;101:227–235. [DOI] [PubMed] [Google Scholar]
- 75. Guequén A, Zamorano P, Córdova F, et al. . Interleukin-8 secreted by glioblastoma cells induces microvascular hyperpermeability through NO signaling involving S-nitrosylation of VE-cadherin and p120 in endothelial cells. Front Physiol. 2019;10:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Luo X, Xu S, Zhong Y, et al. . High gene expression levels of VEGFA and CXCL8 in the peritumoral brain zone are associated with the recurrence of glioblastoma: a bioinformatics analysis. Oncol Lett. 2019;18:6171–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pasi F, Facoetti A, Nano R. IL-8 and IL-6 bystander signalling in human glioblastoma cells exposed to gamma radiation. Anticancer Res. 2010;30:2769–2772. [PubMed] [Google Scholar]
- 78. Anil SM, Shalev N, Vinayaka AC, et al. . Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci Rep. 2021;11:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Natoni A, Macauley MS, O'Dwyer ME. Targeting selectins and their ligands in cancer. Front Oncol. 2016;6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tinoco R, Otero DC, Takahashi AA, et al. . PSGL-1: a new player in the immune checkpoint landscape. Trends Immunol. 2017;38:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhai L, Ladomersky E, Lauing KL, et al. . Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clin Cancer Res. 2017;23:6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhai L, Lauing KL, Chang AL, et al. . The role of IDO in brain tumor immunotherapy. J Neurooncol. 2015;123:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kilshaw PJ, Higgins JM. Alpha E: no more rejection? J Exp Med. 2002;196:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Banchereau R, Chitre AS, Scherl A, et al. . Intratumoral CD103+ CD8+ T cells predict response to PD-L1 blockade. J Immunother Cancer. 2021;9:e002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Harjunpää H, Llort Asens M, Guenther C, et al. . Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol. 2019;10:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Laezza C, Pagano C, Navarra G, et al. . The endocannabinoid system: a target for cancer treatment. Int J Mol Sci. 2020;21:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Oesch S, Gertsch J. Cannabinoid receptor ligands as potential anticancer agents—high hopes for new therapies? J Pharm Pharmacol. 2009;61:839–853. [DOI] [PubMed] [Google Scholar]
- 88. Velasco G, Carracedo A, Blázquez C, et al. . Targeting cannabinoid receptors in brain tumors. In: Köfalvi A (ed.). Cannabinoids and the brain. Springer: Boston, MA, 2008, pp. 361–374. [Google Scholar]
- 89. Chinigò G, Castel H, Chever O, et al. . TRP channels in brain tumors. Front Cell Dev Biol. 2021;9:617801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khodadadi H, Salles ÉL, Shin E, et al. . A potential role for cannabichromene in modulating TRP channels during acute respiratory distress syndrome. J Cannabis Res. 2021;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Byrne KF, Pal A, Curtin JF, et al. . G-protein-coupled receptors as therapeutic targets for glioblastoma. Drug Discov Today. 2021;26:2858–2870. [DOI] [PubMed] [Google Scholar]
- 92. Kotas ME, Locksley RM. Why innate lymphoid cells? Immunity. 2018;48:1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ducimetière L, Vermeer M, Tugues S. The interplay between innate lymphoid cells and the tumor microenvironment. Front Immunol. 2019;10:2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bruchard M, Ghiringhelli F. Deciphering the roles of innate lymphoid cells in cancer. Front Immunol. 2019;10:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. An Z, Flores-Borja F, Irshad S, et al. . Pleiotropic role and bidirectional immunomodulation of innate lymphoid cells in cancer. Front Immunol. 2020;10:3111. [DOI] [PMC free article] [PubMed] [Google Scholar]