Abstract
Background:
Activation of signaling effectors by G-protein coupled receptors (GPCRs) depends on different molecular mechanisms triggered by conserved amino acid residues. Although studies have focused on the G-protein signaling state, the mechanism for β-arrestin signaling by CB1 is not yet well defined. Studies have indicated that transmembrane helix 7 (TMH7) and the highly conserved NPXXY motif can be subject to different conformational changes in response to biased ligands and could therefore participate in a molecular mechanism to trigger β-arrestin recruitment.
Objective:
To investigate the effect of mutations in the NPXXY motif on different signaling pathways activated by the CB1 receptor.
Materials and Methods:
Point mutations of the NPXXY motif and associated residues were generated in the CB1 receptor using site-directed mutagenesis and transfection into HEK-293 cells. Signaling by wild-type and mutant receptors was analyzed by quantifying inhibition of cAMP, and by β-arrestin recruitment assays.
Results:
We found that N7.49 and Y7.53 are essential for β-arrestin recruitment by CB1. N7.49A and Y7.53F impair β-arrestin signaling, with no effect on G-protein signaling. We found a regulatory role for residue I2.43; I2.43 interacts with Y7.53, affecting its positioning. Reducing steric bulk at I2.43 (I2.43A) enhances β-arrestin1 recruitment, while introducing a polar residue (I2.43T) reduces β-arrestin recruitment.
Conclusions:
These findings point to a novel mechanism for β-arrestin recruitment, implicating amino acids in the NPXXY motif as critical for the putative β-arrestin biased conformational state of Class A GPCRs.
Keywords: CB1, beta-arrestin, biased signaling, GPCR, NPXXY
Introduction
The CB1 cannabinoid receptor is a Class A G-protein coupled receptor (GPCR) that was first identified as the receptor that mediates the biological effects of (-)-trans-Δ9-THC, the main psychoactive component in Cannabis sativa.1 Later, the endogenous ligands 2-arachidonoylglycerol (2-AG) and anandamide were identified.2–4 Upon agonist binding, GPCRs can activate heterotrimeric G-proteins and recruit β-arrestins. Certain ligands preferentially stimulate coupling to and activation of either G-proteins or β-arrestins, which led to characterization of the concept of biased signaling.5,6 The use of biased ligands has been proposed as a pharmacological strategy to dissociate therapeutic effects from adverse effects in various GPCR-targeted therapies.7
CB1 mainly couples to Gαi/o proteins and recruits β-arrestin1 (Arrestin2) and β-arrestin2 (Arrestin3).8–10 High expression of CB1 in the central nervous system, as well as its modulatory effects on neurotransmitters such as dopamine, serotonin, GABA, glutamate, and endogenous opioids, renders CB1 a promising target for the treatment of several different neurological disorders.11,12 Yet, endeavors to advance CB1 agonists into the clinic have long been hindered by their associated psychoactive side effects. CB1 is capable of biased signaling,9,10,13,14 but known orthosteric agonists trigger both pathways in a relatively balanced way. Understanding the conformational changes involved in G-protein versus β-arrestin signaling through CB1 will be an important tool to design novel biased ligands with the potential to refine cannabinoid therapeutics.
Structural elucidation of many Class A GPCRs, including CB1, in their antagonist-bound (inactive) and in their agonist-bound (G-protein coupling active) states provides a static picture of the conformational differences between these two states, suggesting a common mechanism of activation.15–17 Class A GPCRs are activated when an agonist enters the binding pocket and triggers the aromatic “toggle switch” (F3.36/W6.48 in CB1), forcing W6.48 in the CWXP motif to change conformation, resulting in an outward movement of transmembrane helix 6 (TMH6) at the intracellular (IC) domain of the receptor.18–21 The Ballesteros–Weinstein numbering is used for amino acid residues throughout the rest of the text.22 The outward movement of TMH6 creates an opening at the IC end of the GPCR into which the end of the α-5 helix of Gαi/o can insert.17,23 This initiates G-protein signal transduction.
Considerable progress has been made in our understanding of how various conformations of GPCR-bound arrestins regulate receptor signaling and desensitization.24 GPCR kinases (GRKs) phosphorylate Ser and Thr residues on the C-terminus of the GPCR, which can then interact with the highly positively charged N-domain of the β-arrestin. For the CB1 receptor, GRK2 and GRK3 were shown to phosphorylate S425 and S42925; while GRK5-6 phosphorylate residues at the end of the C-terminus T460, S462, S464, T465 T467, and S468.13 In addition to these sites, the CB1 C-terminus has six negatively charged residues (E/D) that could interact with β-arrestin. Interactions with phosphorylated residues on ICL3 have also been shown for other GPCRs, but not yet for CB1.26,27
After β-arrestin binding, the GPCR/β-arrestin complex can form a partial engagement, when only the phosphorylated C-terminal tail of the GPCR interacts directly with the N-domain of the β-arrestin, or a core engagement, which also involves insertion of the finger loop of the β-arrestin into the opening at the transmembrane core of the GPCR.24 Both forms of interaction can promote receptor internalization and signaling, but the core engagement was shown to mediate G-protein desensitization.28,29
The molecular mechanism that leads to GRK phosphorylation and GPCR recruitment of β-arrestin is under investigation. Biophysical studies of Class A GPCRs, demonstrate that β-arrestin-biased ligands affect the conformation of transmembrane helix 7 (TMH7) and helix 8 (Hx8), rather than TMH6.6,30–33 The NPXXY motif, located on TMH7 on the IC side of the receptor, is highly conserved across class A GPCRs and has been shown to participate in a hydrogen bond network responsible for stabilizing the active state for G-protein signaling.23,34,35 On the contrary, studies have revealed that the NPXXY motif is also involved in β-arrestin function, as point mutations affected receptor phosphorylation and internalization, ERK1/2 phosphorylation, and β-arrestin recruitment with little to no effect on G-protein signaling.36–38
In addition, the Y7.53 side chain was shown to adopt different rotameric states during G-protein and β-arrestin-biased signaling.39,40 However, whether NPXXY motif residues are involved in β-arrestin recruitment by the CB1 receptor has not been investigated. In this study, we report that N7.49 and Y7.53 are essential for β-arrestin recruitment and signaling by CB1.
Materials and Methods
Mutagenesis and cell culture
The I2.43T, I2.43A, S7.57E, and Y7.53F mutations were generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA). Synthetic mutagenic oligonucleotides used were 27–43 base pairs long. DNA sequencing was then performed to confirm the presence of the desired mutation. Established cell lines such as HEK293 and AD293 do not require IRB approval, because they do not meet the definition of human subjects under IRB purview.
Human embryonic kidney (HEK) cells (HEK293; American Type Culture Collection, Rockville, MD) and AD293 cells (Agilent) were grown in Dulbecco's modified Eagle's medium (DMEM; Corning, Corning, NY) supplemented with 10% fetal bovine serum (FBS; characterized; GE Healthcare Hyclone Laboratories, Logan, UT) at 37°C and 5% CO2. HEK293 cells stably expressing wild-type (WT) and mutated CB1 receptors were generated as previously described.41,42 N-terminally GFP-tagged rat CB1 receptor in pcDNA3 (GFP-rCB1) was transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and clonal cells were selected in a growth medium containing G418 (Geneticin, 800 μg/mL; Enzo Life Sciences, Farmingdale, NY).
Two weeks after transfection, clones were isolated and CB1 expression was verified by fluorescence microscopy. Similarly, HEK293 cell lines stably expressing β-arrestin1 or β-arrestin2 constructs were generated by transfection with Lipofectamine 2000 and selection of clonal cells with 200 μg/mL of hygromycin B (Enzo Life Sciences). Expression of β-arrestin constructs in isolated clonal cells was assessed by β-arrestin recruitment chemiluminescent assay, described below. Selected cell lines were maintained in a growth medium with 800 μg/mL of G418 or 100–200 μg/mL of hygromycin B and were not used after passage 25.
Detection of cAMP
Cells stably expressing GFP-rCB1 were cultured until 70–80% confluent, and then serum starved overnight in the absence or presence of 100 ng/mL of pertussis toxin (PTx; MilliporeSigma, Burlington, MA). Then, cells were dissociated in Trypsin (0.05%)-EDTA (0.1%; Quality Biological, Gaithersburg, MD), centrifuged at 200 g for 5 min, and resuspended in stimulation buffer: Hank's Balanced Salt Solution (HBSS; Corning) containing 0.1% bovine serum albumin (BSA) and 0.5 mM IBMX (MilliporeSigma). The cell suspension was transferred to a white 384-well plate at a density of 500 cells/well and treated with 5 μM of forskolin (Cayman Chemical, Ann Arbor, MI) along with 2-AG for 30 min. After drug treatment, cAMP levels were detected using the LANCE Ultra cAMP detection kit (PerkinElmer, Waltham, MA) according to the manufacturer's instructions.
Emissions at 665 and 615 nm were measured in an EnVision 2104 multilabel plate reader (PerkinElmer). Emission at 665 nm was used to estimate cAMP concentration in samples based on a cAMP standard curve. The cAMP concentration in each sample was normalized to levels in samples treated only with forskolin.
β-arrestin recruitment chemiluminescent assay
Assessment of β-arrestin recruitment was performed using a β-galactosidase complementation assay, as previously described.43,44
Briefly, HEK293 cells were transfected with plasmids encoding either β-arrestin1 or β-arrestin2 fused to a fragment of the β-galactosidase enzyme (Acceptor) and WT or mutated human CB1 receptor fused to a complementary fragment of the β-galactosidase enzyme (Donor) on the C-terminus. Receptor–arrestin interaction results in an irreversible β-galactosidase complementation, and enzyme activity can be measured. HEK293 cells stably expressing β-arrestin1 or β-arrestin2 were transiently transfected with the WT or mutated CB1 receptor using Lipofectamine 2000 for 24 h. Cells were serum starved overnight and treated with 2-AG or CP55,940 for 90 min. After drug treatment, cells were lysed with the Tropix® Lysis Solution (Applied Biosystems, Foster City, CA) and β-galactosidase enzyme activity was detected after a 1-h incubation with the Galacto-Star™ One-Step β-Galactosidase Reporter Gene Assay System (Applied Biosystems) detection reagents.
Luminescence was measured in an EnVision 2104 multilabel plate reader (PerkinElmer), and relative luminescence units were normalized to vehicle-treated samples. No increase in basal β-arrestin recruitment was detected with any of the mutations, as luminescence from vehicle-treated samples was compared by Welch's t test. For β-arrestin1 assays (N=3), p-values were ∼0.10 for I2.43A; 0.23 for I2.43T; 0.41 for S7.57E; 0.29 for N7.49A; and 0.91 for Y7.53F. For β-arrestin2 assays (N=3–4), p-values were ∼0.21 for I2.43A; 0.44 for S7.57E; 0.30 for N7.49A; and 0.08 for Y7.53F. Reduced basal β-arrestin2 recruitment was found for I2.43T, as discussed below.
Bioluminescence resonance energy transfer
CB1-RLuc8 (Donor) was generated by inserting rat CB1, synthesized by Genewiz (South Plainfield, NJ), into pcDNA3-EGFR-Rluc8, prepared previously from complementary DNA (cDNA), kindly provided by Walter G. Thomas (The University of Queensland, Queensland, Australia). Rat β-arrestin1-mEYFP or β-arrestin2-mEYFP (Acceptor) plasmids were originally from Dr. Robert Lefkowitz's laboratory (Duke University, Durham, NC). Protein–protein interaction between the donor and acceptor results in bioluminescence resonance energy transfer (BRET), promoting fluorescence emission from the acceptor. This interaction is reversible and allows for kinetic measurement of β-arrestin recruitment. AD293 cells were seeded in a 6-well plate at a density of 400,000 cells per well. After 24 h, cells were transfected using X-tremeGENE™ 9 (MilliporeSigma) and 160 ng of WT or I2.43A CB1-RLuc8, along with 800 ng of β-arrestin1-mEYFP or β-arrestin2-mEYFP, at a 3:1 transfection reagent to DNA ratio.
Twenty-eight hours after transfection, cells were trypsinized and resuspended in DMEM supplemented with 5% FBS, transferred to a poly-d-lysine-coated white 96-well plate at a density of 100,000 cells per well, and incubated overnight. Cells were then serum-starved in FluoroBrite™ DMEM (Thermo Fisher Scientific, Waltham, MA) for 6 h, before being incubated at 37°C with 5 μM of coelenterazine-h (Biotium, Fremont, CA) in HBSS with 0.1% glucose. Light emissions at 480 and 535 nm were measured in an M1000 multimode plate reader (Tecan) for ∼7 min to determine baseline. Then, cells were treated with vehicle, CP55,940 (10 μM), or 2-AG (30 μM), while light emissions were measured for each well in intervals of ∼1 min.
The BRET signal was determined by the ratio of fluorescence emissions at 535 nm divided by luminescence emissions at 480 nm. Ligand-induced BRET (ΔBRET) was calculated by subtracting the BRET ratios from vehicle-treated samples at individual time points. An area under the curve analysis from stimulation time 0–31 min was used to quantify CB1-β-arrestin interactions over the course of treatment.
Radioligand binding
Membrane preparations were harvested from HEK293 cells expressing WT and mutated human CB1 receptors and assayed as previously described.45,46 Briefly, radioligand saturation binding assay was initiated by adding 50 μg of membrane lysate to glass tubes containing [3H]SR141716A, and binding buffer A (50 mM Tris-Base, 1 mM EDTA, 3 mM MgCl2, and 5 mg/mL BSA, pH 7.4) to a final volume of 500 μL. Nonspecific binding was determined in the presence of excess unlabeled SR141716A (10 μM). Reactants were allowed to reach equilibrium for ∼1 h. Subsequently, free and bound radioligands were separated by vacuum filtration through Whatman GF-C filters, and the radioactivity retained on the filters was quantified in a liquid scintillation counter. The Kd (equilibrium dissociation constant) and Bmax (maximal binding) values were determined by analyzing saturation binding data by nonlinear regression and fitted to a one-site binding model.
Statistical analysis
Data are reported as mean±standard error of the mean. In dose–response curves, data were fit to a three-parameter nonlinear regression curve. Potency and efficacy were compared between WT and mutant receptors using unpaired Welch's t test. When parameters could not be calculated with a 95% confidence interval, the difference between WT and mutated receptors at the same dose of agonist was calculated by two-way analysis of variance (ANOVA) followed by Šídák's multiple comparisons test. Furthermore, the area under the curve after agonist treatment was calculated for BRET assays and used to compare β-arrestin recruitment between genotypes using two-way ANOVA with Tukey's multiple comparisons test. Data analysis was performed on GraphPad Prism 9 software (GraphPad, San Diego, CA), and p<0.05 was considered statistically significant.
Results
N7.49 is critical for β-arrestin recruitment by CB1
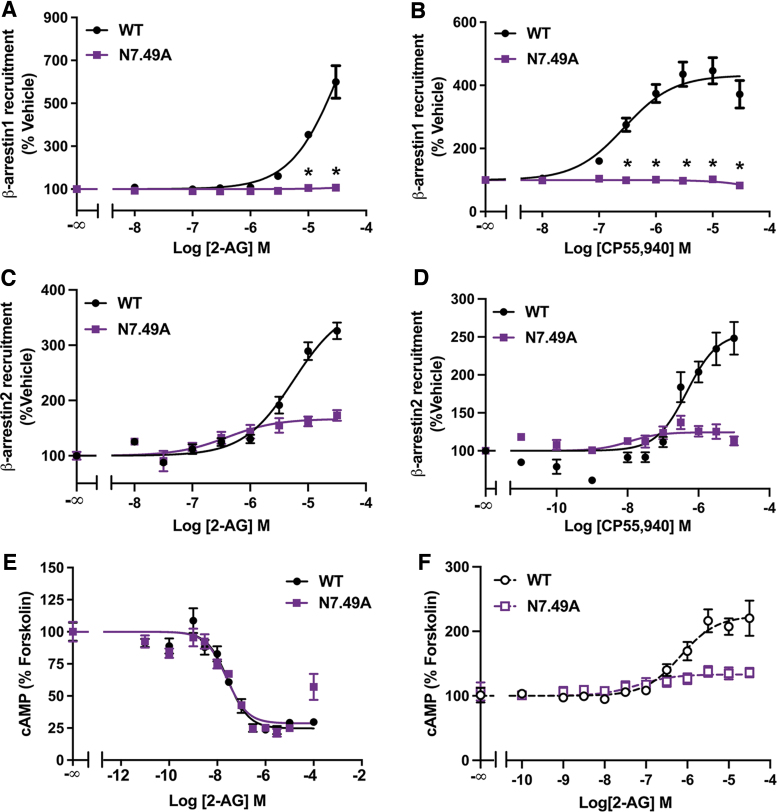
In the current study, we investigated if NPXXY motif residues, located on TMH7, are important for β-arrestin recruitment by the CB1 receptor. The effect of point mutations on CB1 signaling was determined by measuring downstream signaling in HEK293 cells expressing the WT or mutated CB1 receptor. The endogenous agonist 2-AG was the main ligand used for characterization of CB1 mutations, since it is a full agonist that was previously described to show little bias for activation of signaling pathways downstream of CB1.9,14 Remarkably, we found that mutating N7.49 to an Ala (N7.49A) substantially altered the CB1 response to 2-AG and the exogenous agonist CP55,940 in a β-arrestin recruitment assay. N7.49A abolished β-arrestin1 recruitment (Fig. 1A, B) and partially impaired β-arrestin2 recruitment (Fig. 1C, D) in response to either agonist.
FIG. 1.
N7.49A mutation impairs β-arrestin recruitment. (A) Impaired 2-AG stimulated recruitment of β-arrestin1 by N7.49A analyzed by the β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Effect at each dose was compared by two-way ANOVA (dose, genotype, and interaction: p<0.0001) followed by Šidák's multiple comparisons test: *p<0.0001. (B) CP55,940 stimulated recruitment of β-arrestin1 by N7.49A assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Effect at each dose compared by two-way ANOVA (p<0.0001) followed by Šidák's multiple comparisons test: *p<0.0001. (C) Reduced β-arrestin2 recruitment in N7.49A. Data from four biological replicates performed in three technical replicates (N=12) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (14.3-fold shift, p=0.0075) and Emax (55% reduction, p=0.0005). (D) CP55,940 stimulated β-arrestin2 recruitment by S7.57E. Data from four biological replicates performed in three technical replicates (N=12) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (42.6-fold shift, p=0.025) and Emax (51% reduction, p=0.0011). (E) No change in the inhibition of cAMP accumulation by 2-AG in forskolin-treated cells expressing the WT or N7.49A CB1 receptor. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: IC50 (p=0.65) and Emax (p=0.34). (F) N7.49A reduced 2-AG-mediated stimulation of cAMP accumulation in cells pretreated with PTx. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (11.1-fold shift, p=0.049) and Emax (73% reduction, p=0.0025). All data shown as mean±SEM. 2-AG, 2-arachidonoylglycerol; ANOVA, analysis of variance; PTx, pertussis toxin; SEM, standard error of the mean; WT, wild type.
To measure CB1-mediated G-protein signaling, we assessed the level of cAMP accumulation in response to 2-AG in cells expressing CB1. Interestingly, when compared with WT CB1, the N7.49A receptor showed no difference in the potency or efficacy of 2-AG for inhibition of forskolin-stimulated cAMP (Fig. 1E). This indicates that Gαi/o signaling is unaffected by this mutation. Low-efficiency CB1-Gαs coupling has been previously described and is evident under conditions where Gαi/o signaling is inhibited,47–49 such as in cells exposed to PTx, which inactivates Gαi/o proteins.50 Pretreatment with PTx abolished cAMP inhibition, showing that Gαi/o signaling was successfully eliminated (Fig. 1F).
In fact, we found that 2-AG increased cAMP accumulation in PTx-exposed cells above the level stimulated by forskolin, which is attributed to CB1-stimulated Gαs signaling.48 Lower potency for stimulation of cAMP relative to inhibition of cAMP is likely due to the lower efficacy of Gαs coupling compared with Gαi/o.48,51 The stimulation of cAMP production in PTx-exposed cells was lessened by N7.49A, indicating reduced Gαs signaling with this mutation (Fig. 1F). Collectively, these results show that N7.49 is a key residue for β-arrestin1 recruitment by CB1 with little impact on G-protein signaling.
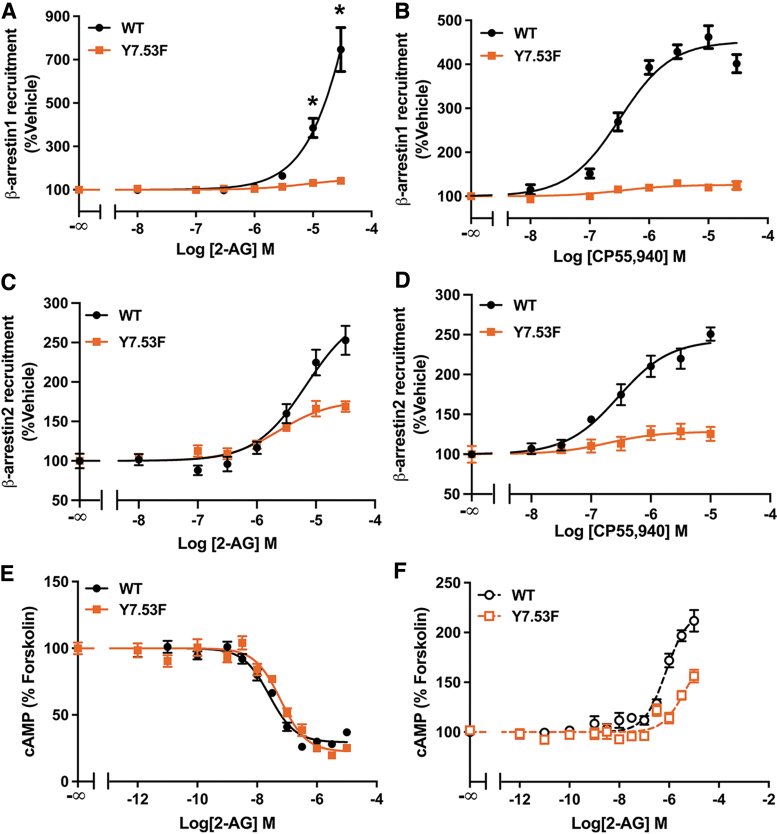
Y7.53 is important for CB1 β-arrestin recruitment
To investigate whether Y7.53 is important for β-arrestin recruitment by CB1, we mutated Y7.53 to Phe (Y7.53F). This mutation eliminated 2-AG- and CP55,940-mediated β-arrestin1 recruitment (Fig. 2A, B) and significantly decreased β-arrestin2 recruitment (Fig. 2C, D). In the cAMP inhibition response, Y7.53F caused a slight decrease in 2-AG potency, but also an increase in efficacy (Fig. 2E), suggesting that this mutation has little to no effect on Gαi/o signaling. In cells pretreated with PTx, stimulation of cAMP accumulation was partially decreased by Y7.53F (Fig. 2F), indicating that Gαs signaling is inhibited. Altogether, these findings demonstrate that the hydroxyl group on Y7.53 is crucial for β-arrestin recruitment by CB1, while G-protein signaling remains largely unchanged.
FIG. 2.
Y7.53F mutation impaired β-arrestin recruitment. (A) 2-AG-mediated β-arrestin1 recruitment is eliminated in Y7.53F. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Each dose was compared between genotypes by two-way ANOVA followed by Šidák's multiple comparisons test: *p<0.0001. (B) CP55,940 stimulated recruitment of β-arrestin1 in Y7.53F assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.96) and Emax (72% decrease, p=0.0006). (C) Impaired 2-AG-mediated β-arrestin2 recruitment in Y7.53F. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.081) and Emax (40% reduction, p=0.024). (D) CP55,940 stimulated recruitment of β-arrestin2 in Y7.53F. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.72) and Emax (47% reduction, p=0.004). (E) Inhibition of cAMP accumulation by 2-AG in forskolin-treated cells expressing WT or Y7.53F CB1 receptor. Data from four biological replicates performed in three technical replicates (N=12) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: IC50 (2.4-fold increase, p=0.013) and Emax (10% increased inhibition of cAMP, p=0.038). (F) Y7.53F reduced 2-AG-mediated stimulation of cAMP accumulation in cells pretreated with PTx. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (5.2-fold increase, p=0.007) and Emax (35% reduction, p=0.015). All data shown as mean±SEM.
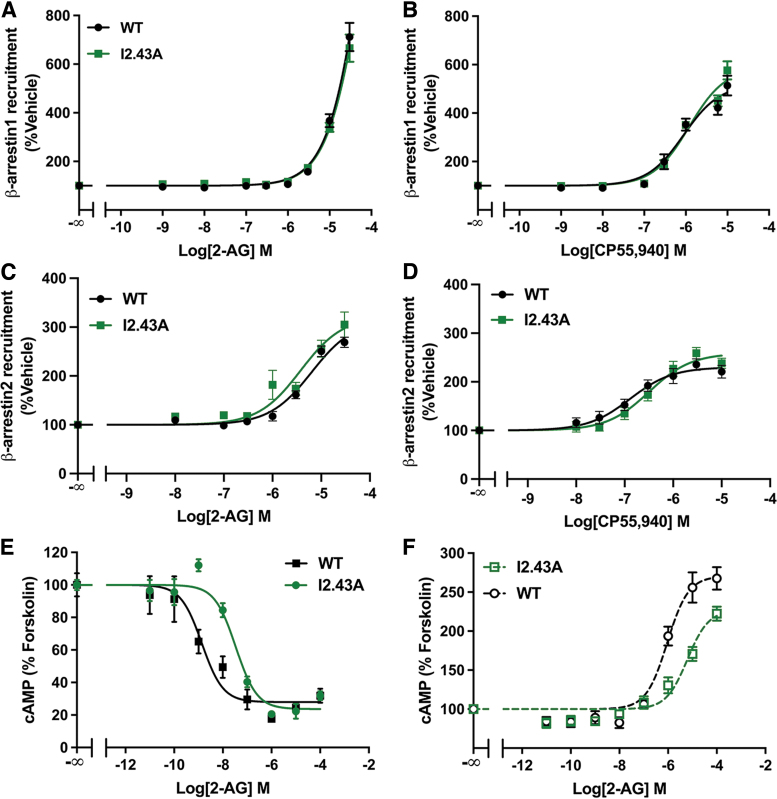
I2.43 regulates CB1 β-arrestin1 recruitment
The I2.43 residue is part of the hydrophobic layer that maintains the inactive state52 and can affect the NPXXY motif via vVan der Waals interactions with Y7.53.15 To investigate the role of this residue in CB1 signaling, we mutated I2.43 to an Ala (I2.43A) or a Thr (I2.43T). In the I2.43A mutation, we found no significant difference in 2-AG- or CP55,940-stimulated recruitment of β-arrestin1 (Fig. 3A, B) or recruitment of β-arrestin2 (Fig. 3C, D). In the dose–response curve for cAMP inhibition, we noted a rightward shift, showing a decrease in the potency of 2-AG for Gαi/o signaling in I2.43A (Fig. 3E). Similarly, I2.43A also partially decreased 2-AG-mediated stimulation of cAMP in PTx-exposed cells, showing inhibition of low-efficiency CB1-Gαs activation (Fig. 3F).
FIG. 3.
I2.43A mutation inhibited G-protein signaling. (A) 2-AG stimulated recruitment of β-arrestin1 assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Effects of 2-AG doses were compared by two-way ANOVA (treatment: p<0.0001; genotype: p=0.99; interaction: p=0.85). (B) CP55,940 stimulated β-arrestin1 recruitment assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression analysis. Parameters were compared by Welch's t test: EC50 (p=0.30) and Emax (p=0.056). (C) 2-AG stimulated recruitment of β-arrestin2. Data from four biological replicates performed in three technical replicates (N=12) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.82) and Emax (p=0.25). (D) No effect of I2.43A on CP55,940-stimulated β-arrestin2 recruitment. Data from four biological replicates performed in three technical replicates (N=12) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.060) or Emax (p=0.058). (E) Reduced potency but not efficacy (IC50: 24.7-fold increase, p=0.0041; Emax: p=0.40) of 2-AG for the inhibition of cAMP accumulation in forskolin-treated cells expressing WT or I2.43A CB1 receptor. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. (F) I2.43A reduced 2-AG-mediated stimulation of cAMP accumulation in cells pretreated with PTx. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (6.7-fold increase, p=0.0003) and Emax (16% reduction, p=0.0038). All data are shown as mean±SEM.
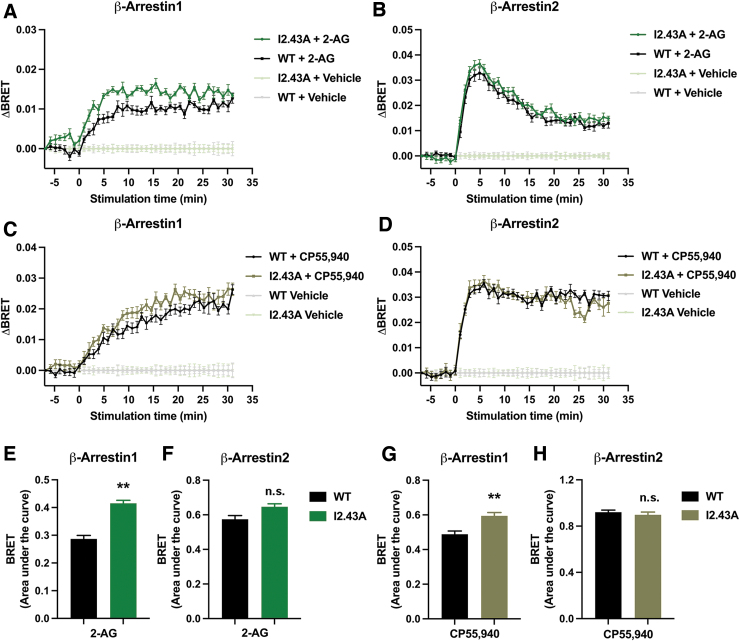
Although no difference between WT and I2.43A was observed in β-arrestin recruitment, one limitation of this assay is that it measures the cumulative response over the course of a 90-min treatment. This eliminates our ability to detect changes in the kinetics of β-arrestin recruitment. Therefore, we applied BRET to measure β-arrestin recruitment in real time. We found that I2.43A significantly enhanced β-arrestin1 ligand-induced BRET (ΔBRET) in response to 2-AG (Fig. 4A, E) and CP55,940 (Fig. 4C, G). Conversely, we found no changes in β-arrestin2 ΔBRET stimulated by WT or I2.43A after treatment with 2-AG (Fig. 4B, F) or CP55,940 (Fig. 4D, H). These results suggest that introducing a less bulky residue at position 2.43 selectively enhanced the kinetics of β-arrestin1 recruitment.
FIG. 4.
I2.43A mutation enhanced β-arrestin1 recruitment. (A) Ligand-induced BRET (ΔBRET) in cells expressing β-arrestin1 and WT or I2.43A CB1 receptor treated with 30 μM 2-AG. (B) ΔBRET in cells expressing β-arrestin2 and WT or I2.43A CB1 receptor treated with 30 μM 2-AG. (C) ΔBRET in cells expressing β-arrestin1 and WT or I2.43A CB1 receptor treated with 10 μM CP55,940. (D) ΔBRET in cells expressing β-arrestin2 and WT or I2.43A CB1 receptor treated with 10 μM CP55,940. (E) Area under the curve analysis for 2-AG-stimulated β-arrestin1 ΔBRET. Difference between genotypes determined by unpaired Welch's t test: **p=0.0016. (F) Area under the curve analysis for 2-AG-stimulated β-arrestin2 ΔBRET. Difference between genotypes determined by unpaired Welch's t test: p=0.059. (G) Area under the curve analysis for CP55,940-stimulated β-arrestin1 ΔBRET. Difference between genotypes determined by unpaired Welch's t test: **p=0.0012. (H) Area under the curve analysis for CP55,940-stimulated β-arrestin2 ΔBRET. Difference between genotypes determined by unpaired Welch's t test: p=0.45. All data are shown as mean±SEM from three biological replicates performed in three technical replicates (N=9). BRET, bioluminescence resonance energy transfer.
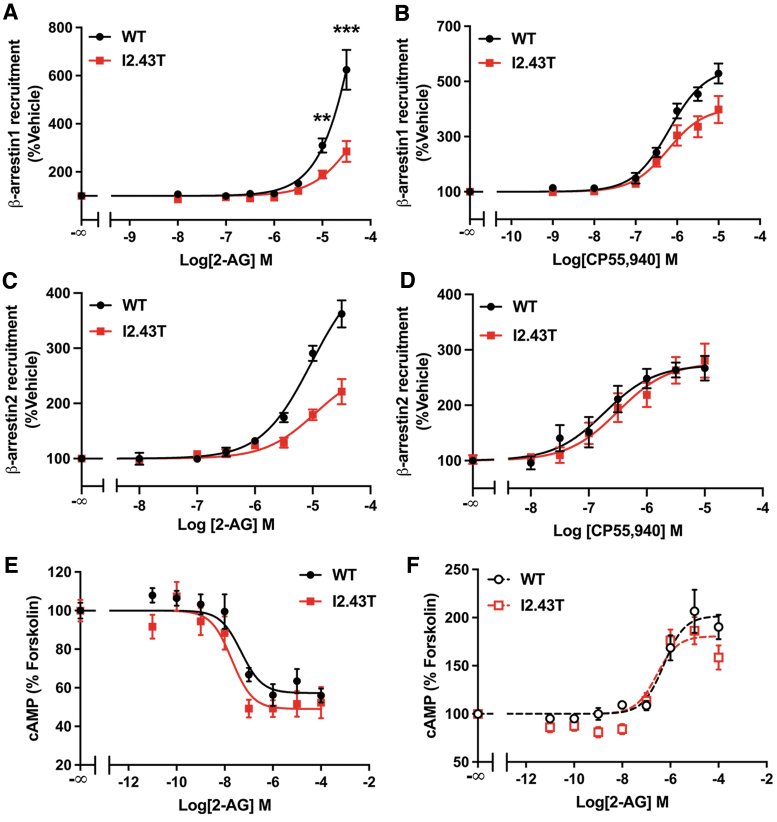
The I2.43T mutation introduced a residue of similar size to the Ile, which also has a hydroxyl. In this case, we found that I2.43T diminished 2-AG- and CP55,940-mediated recruitment of β-arrestin1 (Fig. 5A) and β-arrestin2 (Fig. 5C). I2.43T also decreased CP55,940-stimulated β-arrestin1 recruitment (Fig. 5B), but not β-arrestin2 recruitment (Fig. 5D). However, basal β-arrestin2 recruitment was reduced in I2.43T, as luminescence from vehicle-treated samples was ∼61% lower in I2.43T compared with WT (Welch's t test: p=0.017; N=3). This likely resulted in the overestimated rates of ligand-induced β-arrestin2 recruitment from I2.43T. Therefore, these data indicate that β-arrestin recruitment is reduced by the I2.43T mutation. In the cAMP inhibition response, 2-AG potency and efficacy were unchanged in I2.43T (Fig. 5E), suggesting that this mutation does not affect Gαi/o signaling.
FIG. 5.
I2.43T mutation reduced β-arrestin recruitment. (A) I2.43T decreased 2-AG-stimulated β-arrestin1 recruitment. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Response at each dose compared by two-way ANOVA (treatment, genotype, and interaction: p<0.0001) followed by Šídák's multiple comparisons test: **p=0.0069; ***p<0.0001. (B) CP55,940-stimulated recruitment of β-arrestin1 assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression analysis. Parameters compared by Welch's t test: EC50 (p=0.88) and Emax (32% decrease, p=0.013). (C) I2.43T decreased 2-AG-stimulated β-arrestin2 recruitment. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.75) and Emax (53% reduction, p=0.0089). (D) CP55,940-stimulated recruitment of β-arrestin2. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression analysis. Parameters compared by Welch's t test: EC50 (p=0.34) and Emax (p=0.86). (E) Inhibition of cAMP accumulation by 2-AG in forskolin-treated cells expressing WT or I2.43T CB1 receptor. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: IC50 (p=0.30), Emax (p=0.17). (F) I2.43T did not change 2-AG-mediated stimulation of cAMP accumulation in cells pretreated with PTx. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.37) and Emax (p=0.12). All data shown as mean±SEM.
In addition, no difference was found between WT and I2.43T in the stimulation of cAMP production in cells pretreated with PTx (Fig. 5F), indicating that Gαs signaling is unchanged. Introducing a large polar residue at the 2.43 position hindered CB1-mediated β-arrestin recruitment.
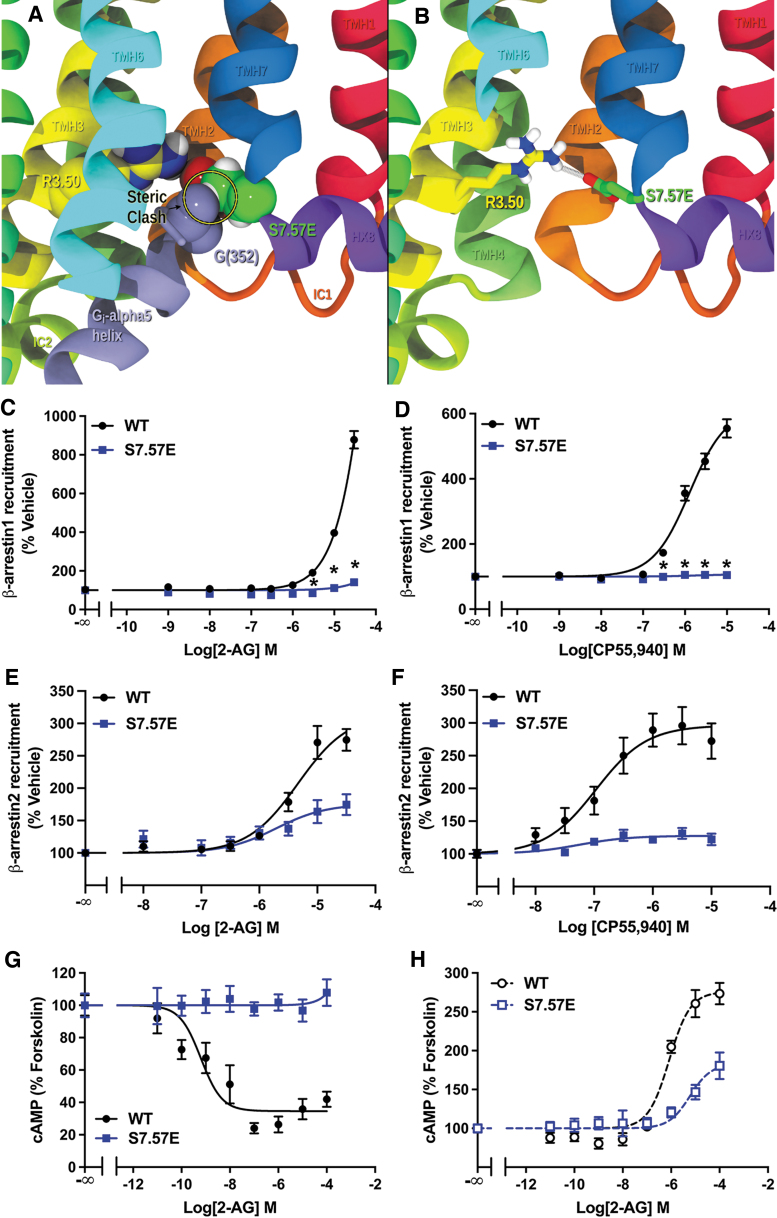
S7.57E mutation hampers CB1 signaling
Finally, we evaluated the impact of a residue, S7.57, that likely does not participate in the NPXXY effects. Instead, mutating S7.57 to Glu (S7.57E) may cause a clash with the α-5 helix on the C terminus of the G-protein as it tries to insert (Fig. 6A). E7.57 may also be able to form a salt-bridge with R3.50, tethering TMH7 near TMH3 and preventing a conformational change in the TMH7/8 elbow region (Fig. 6B). Therefore, it is likely to have negative effects on both G-protein and β-arrestin pathways. We found that S7.57E eliminated β-arrestin1 recruitment (Fig. 6C) and significantly reduced β-arrestin2 recruitment (Fig. 6D) in response to both 2-AG and CP55,940, suggesting an impairment in the ability to recruit β-arrestins.
FIG. 6.
S7.57E mutation impairs CB1 signaling. (A) S7.57E creates a steric clash with the G-protein in the CB1 G-protein bound state. PDB-ID: 6KPG is shown here with S7.57 mutated to a Glu. Here S7.57E carbons are in green CPK display and Gαi α-5 residue G(352) in the β-hairpin turn is shown is lavender. (B) S7.57E is also likely to form a salt-bridge with R3.50, preventing any conformational change of the TMH7/Hx8 region. Here S7.57E carbons are shown in green tube display and R3.50 carbons are in yellow (PDB-ID: 6KPG). TMH6 is partially removed for clarity. (C) Reduced 2-AG-stimulated recruitment of β-arrestin1 by S7.57E measured by the β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Effect at each dose compared by two-way ANOVA (treatment, genotype, and interaction: p<0.0001) followed by Šidák's multiple comparisons test: *p<0.001. (D) CP55,940-stimulated recruitment of β-arrestin1 by S7.57E assayed by a β-galactosidase enzyme complementation assay. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Effect of each dose was compared by two-way ANOVA (p<0.0001) followed by Šidák's multiple comparisons test: *p<0.001. (E) Reduced 2-AG-stimulated β-arrestin2 recruitment by S7.57E. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.30) and Emax (44% reduction, p=0.006). (F) CP55,940-stimulated β-arrestin2 recruitment by S7.57E. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.49) and Emax (57% reduction, p=0.0051). (G) Inhibition of cAMP accumulation by 2-AG in forskolin-treated cells expressing WT or S7.57E CB1 receptor. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. No effect of S7.57E. (H) S7.57E reduced 2-AG-mediated stimulation of cAMP accumulation in cells pretreated with PTx. Data from three biological replicates performed in three technical replicates (N=9) and fit to a three-parameter nonlinear regression curve. Parameters compared by Welch's t test: EC50 (p=0.070) and Emax (53% reduction, p=0.034). All data shown as mean±SEM. Hx8, helix 8; PDB, Protein Data Bank; TMH, transmembrane helix.
Furthermore, no inhibition of cAMP accumulation was found (Fig. 6E), indicating that Gαi/o signaling is impaired. Stimulation of cAMP production in PTx-exposed cells is also significantly reduced, suggesting an impairment in Gαs signaling. These data show that the S7.57E mutation had a negative impact on all the signaling effectors.
To evaluate if receptor expression levels could impact signaling effector coupling, we analyzed the level of receptor expression in cell lines stably expressing WT and mutated CB1, which were used in the assessment of cAMP accumulation. The total level of receptor expression was measured by radioligand binding (Table 1). No cell line showed a significant difference in Kd compared with WT, indicating no change in ligand affinity. The Bmax values for I2.43A and Y7.53F were not different from WT. However, I2.43T showed a slightly lower Bmax compared with WT. Conversely, we found a significantly increased Bmax for S7.57E and N7.49A compared with WT. The level of receptor expression is similar between S7.57E and N7.49A, however, the latter shows normal Gαi/o signaling. This indicates that the lack of Gαi/o signaling in S7.57E is not related to receptor expression levels.
Table 1.
Radioligand Binding in HEK293 Cells Stably Expressing Wild-Type or Mutated CB1 Receptor
| CB1 receptor | Kd (nM) | Bmax (pmol/mg) |
|---|---|---|
| WT | 3.48 ± 0.24 | 29.16 ± 0.62 |
| I2.43A | 2.67 ± 0.28 | 27.32 ± 0.83 |
| I2.43T | 3.46 ± 0.75 | 21.28 ± 1.42a |
| Y7.53F | 4.22 ± 0.42 | 33.44 ± 1.06 |
| S7.57E | 4.88 ± 0.38 | 53.47 ± 1.41b |
| N7.49A | 4.76 ± 0.40 | 51.31 ± 1.51b |
All data shown as mean ± SEM.
Data from three biological replicates performed in three technical replicates (N = 9) and analyzed by Brown–Forsythe and Welch's ANOVA with Dunnett's post-test: ap = 0.049; bp < 0.01.
ANOVA, analysis of variance; HEK, human embryonic kidney; SEM, standard error of the mean; WT, wild type.
A mechanism for NPXXY-regulated β-arrestin recruitment
To investigate a possible molecular mechanism for the effects on β-arrestin recruitment observed with NPXXY motif mutations, we analyzed published X-ray crystal and cryo-electron microscopy (EM) structures of the CB1 receptor. By comparing the inactive state (inverse agonist bound) to both active G-protein signaling state (orthosteric agonist and Gαi bound) and ORG27569 bound receptor, we propose that the NPXXY motif participates in a hydrogen bond network that favors β-arrestin recruitment. ORG27569 (ORG) is an allosteric modulator of CB1 that inhibits G-protein signaling,53 but enhances orthosteric ligand binding54 and promotes recruitment of β-arrestin1.55 A crystal structure of CB1 in complex with ORG has been recently resolved in the presence of CP55,940 in the orthosteric binding site.56
This structure (Protein Data Bank [PDB]-ID: 6KQI) provides insight into the negative modulator function of ORG on CP55,940-mediated G-protein signaling, via inhibition of the active rotameric state of the key “toggle switch” residues (F3.36 and W6.48) and allosteric agonist site residue F4.46.57–60 As a result, binding of ORG stabilizes TMH6 in an inactive state conformation. A closer examination of the IC end of TMH7 suggests that ORG also affects NPXXY motif residues. Table 2 compares the distances between relevant NPXXY residues and D2.50 in inverse agonist- or agonist-bound CB1 structures, as well as in the ORG/CP55,940-bound CB1 crystal structure (PDB-ID: 6KQI). These data reveal that ORG induces formation of a direct hydrogen bond between N7.49 and D2.50, and a lengthening of the distance between D2.50 and Y7.53, such that a bridging water hydrogen bond could tie those residues together.
Table 2.
Distance Between Select Residues in CB1 Crystal and Cryo-EM Structures
| Residue-atom | PDB-ID | Bound ligand | Distance (Å) |
|---|---|---|---|
| D2.50-OD1 and N7.49-ND2 | |||
| 5U09 | Taranabant | 3.62 | |
| 5XRA | AM11542 | 4.52 | |
| 6KQI | ORG/CP55,940 | 4.50 | |
| 6KPG | AM841/Gi | 5.04 | |
| D2.50-OD2 and N7.49-ND2 | |||
| 5U09 | Taranabant | 3.61 | |
| 5XRA | AM11542 | 3.62 | |
| 6KQI | ORG/CP55,940 | 2.77a | |
| 6KPG | AM841/Gi | 3.91 | |
| D2.50-OD2 and Y7.53-OH | |||
| 5U09 | Taranabant | 3.63 | |
| 5XRA | AM11542 | 9.16 | |
| 6KQI | ORG/CP55,940 | 5.06b | |
| 6KPG | AM841/Gi | 9.45 | |
Data from the Worldwide PDB database (X-ray crystallography and cryo-EM structures). Taranabant: inverse agonist. AM11542, AM841, and CP55,940: orthosteric agonists. ORG: ORG27569, allosteric β-arrestin-biased ligand.
Indicates a distance permissive to direct hydrogen bonding.
Indicates a distance permissive to hydrogen bonding via a water molecule.
EM, electron microscopy; PDB, Protein Data Bank.
A similar arrangement of NPXXY motif residues is also observed in a molecular dynamics (MD) simulation of CB1 bound to ORG alone.61 To be clear, the binding site occupied by ORG in the MD simulation has not been confirmed biologically. Stornaiuolo and coworkers identified a binding exosite for ORG located in the TMH1/2/4 IC lipid face,62 which was later also found by Shao and coworkers when they cocrystallized ORG and CP55,940 bound to CB1.56 CB1 has been shown to have more than one exosite that can modulate signaling pathways,58,63 and whether ORG can also bind to the site identified in the MD simulation, particularly in the absence of an orthosteric ligand, is yet to be confirmed.
However, in the ORG/CB1 MD simulation, ORG binding caused a similar positioning of N7.49 and D2.50, suggesting the formation of a hydrogen bond, as was observed in the ORG/CP55,940-bound CB1 crystal structure, further supporting the role of the NPXXY motif in CB1-mediated β-arrestin recruitment. Moreover, a direct hydrogen bond between N7.49 and D2.50 is only found in ORG-bound CB1, since inverse agonist- and orthosteric agonist-bound CB1 structures published to date do not find these two residues directly interacting (Table 2). N7.49 and D2.50 may only interact directly when a β-arrestin/CB1 signal is induced.
Data from N7.49A mutation in the current study corroborate this hypothesis. In addition, a distance between Y7.53 and D2.50 that is permissive to the formation of a water-bridged hydrogen bond is only observed in ORG-bound CB1. Data from Y7.53F mutation in the current article support an important role for this interaction in CB1-mediated β-arrestin recruitment, since Y7.53F eliminates a key hydroxyl. The selective inhibitory effect on β-arrestin recruitment could be explained by the inability of the F7.53 to form a hydrogen bond with D2.50.
Discussion
Class A GPCRs share a set of highly conserved residues and/or motifs (one per helix). These are TMH1: N1.50, TMH2: D2.50, TMH3: D/ERY, TMH4: W4.50, TMH5: P5.50, TMH6: CWXP, and TMH7: NPXXY. These highly conserved residues have important functions in the molecular mechanism of receptor activation. For instance, the CWXP motif in TMH6 is involved in CB1 G-protein signaling, where W6.48 forms a “toggle switch” with F3.36.60 In addition, for G-protein signaling, an IC “ionic lock,” formed by the highly conserved R3.50 and D/E6.30, also needs to be broken. Use of such highly conserved residues assures that while the EC side of the receptors are quite different in sequence (to recognize different ligand classes), most use the same toggle switch/ionic lock mechanism to change the conformation of TMH6 to activate and accept the end of the α-5 helix of the G-protein.
Because β-arrestin activation has already been shown to stimulate a movement of TMH7/Hx8, we reasoned that the NPXXY motif, with its Pro that would give the helix the ability to bend/flex, may be part of an analogous gating mechanism for β-arrestin.
In this study, we found that N7.49, one of the residues in the NPXXY motif, is crucial for CB1-mediated β-arrestin recruitment. The N7.49A mutation has also been shown to impair β-arrestin recruitment and function in other GPCRs. In the β2-adrenergic receptor (β2-AR), N7.49A abolished receptor phosphorylation and internalization as well as G-protein signaling.64 In the DOR, N7.49A greatly reduced or eliminated β-arrestin recruitment, but only slightly reduced G-protein signaling.65 Interestingly, in the DOR, N7.49A also caused an efficacy switch, turning an antagonist into a β-arrestin-biased agonist.65 Furthermore, in the N-formyl peptide receptor (FPR1), N7.49A impaired receptor phosphorylation, receptor internalization, and desensitization, and did not affect the G-protein-mediated response, suggesting that β-arrestin recruitment is impaired but not G-protein signaling.36
Our results for this mutation in the CB1 receptor agree with findings for the FPR1 and the DOR. However, our data partially differed from findings in the β2-AR, since in CB1, Gαi/o signaling was not eliminated by N7.49A.
The Y7.53 residue, also in the NPXXY motif, has been proposed to be important for GPCR-mediated β-arrestin recruitment and function. In the β2-AR, Y7.53 is also an important residue for β-arrestin activation, as mutating Y7.53 to Ala or Phe impaired receptor internalization.64,66,67 However, in these studies, Y7.53F also partially reduced G-protein signaling.64,66,67 More recently, Ragnarsson et al. showed that Y7.53F impaired β-arrestin2 recruitment to the β2-AR and the α1B-adrenergic receptor (α1B-AR) and inhibited G-protein signaling.38 Similarly, in the angiotensin receptor 1 (AT1R), Y7.53F decreased the rate of receptor internalization and impaired β-arrestin2 recruitment, but also partially reduced G-protein signaling.68,69
In contrast, in the FPR1 and the vasopressin V2 receptor (V2R), Y7.53F impaired receptor internalization without affecting G-protein signaling.70,71 Furthermore, in the DOR, Y7.53F impaired not only receptor internalization but also ERK1/2 signaling, with no change in G-protein activation.72 The results presented in the current study are in agreement with findings for FPR1, V2R, and DOR, and in partial agreement with findings for the β2-AR, α1B-AR, and AT1R, since in the CB1 receptor, Y7.53F greatly impaired β-arrestin recruitment, while G-protein signaling is largely unchanged.
Although our findings on β-arrestin recruitment are consistent with studies on N7.49A and Y7.53F mutations in other GPCRs, our results on G-protein signaling differ from some, but not all, findings in the literature. This could be due to a stronger effect of these mutations on Gs and Gq/11 signaling, to which the β2-AR and the AT1R preferentially couple, than on Gi/o signaling, to which CB1, FPR1, and DOR preferentially couple. The fact that, in CB1, N7.49A and Y7.53F disrupted stimulation of cAMP supports a greater impact of these mutations on Gs rather than Gi/o activation. On the contrary, Gs signaling was not impacted by Y7.53F in V2R.71 Therefore, further studies are required to investigate why this mutation affects G-protein signaling in some class A GPCRs but not others.
Altogether, these studies and the data presented here support an important function for N7.49 and Y7.53 in β-arrestin activation that could apply to several Class A GPCRs.
GPCR signaling has been shown to involve D2.50 interaction with positions 3.39 and 7.49.73 The CB1 R (PDB-ID: 5U09) and R* (PDB-ID: 5XRA) crystal structures show that the S3.39 side chain oxygen is 4.68 Å from the nearest D2.50 carboxyl oxygen in the R state and moves to 3.01 Å in the R* state, creating a hydrogen bond in the G-protein signaling state.15,74 Conversely, N7.49 decreases interaction with D2.50 as the residues move apart in the R* state compared with the R state. Distances increase for the D2.50 carboxyl oxygen, from 3.62 to 4.52 Å and 3.61 to 3.62 Å, to the nitrogen of the N7.49 side chain. Our N7.49A mutation did not impact G-protein signaling due to this natural lessening of interaction in the WT.
In contrast, N7.49A severely impaired recruitment of both β-arrestin1 and β-arrestin2. In the CB1-ORG MD simulation61 and the ORG/CP55,940-bound CB1 structure (PDB-ID: 6KQI), N7.49 forms a hydrogen bond with D2.50. This interaction may act as a hinge hydrogen bond for TMH7 to bend at P7.50, leading to conformational changes at the TMH7/Hx8 elbow. Without the interaction at N7.49A, the conformational change of the IC end of TMH7 is impaired and as a result β-arrestin recruitment is also impaired. Y7.53 may also form a water-bridged hydrogen bond with D2.50 as suggested by the distance between the two residues in the ORG/CP55,940-bound CB1 structure (PDB-ID: 6KQI). Eliminating a hydroxyl with the Y7.53F mutation makes this interaction impossible and disrupts β-arrestin recruitment, indicating that this hydrogen bond is also important for β-arrestin recruitment by CB1.
While the NPXXY motif is highly conserved in Class A GPCRs, residue 2.43 is functionally conserved, in that a relatively large hydrophobic residue is typically located there, such as Ile or Met. Reducing steric bulk from the residue at 2.43 had an interesting impact on CB1 signaling. First, I2.43A hindered G-protein signaling. To explain this effect, we examined the cryo-EM structure of Gαi-bound CB1 R.*17 Indeed, in G-protein signaling, I2.43 packs against A7.54 and forms a wall in this structure, while it is likely that in I2.43A, the R* state is destabilized. Second, I2.43A enhanced the kinetics of β-arrestin1 recruitment. Since I2.43 interacts with Y7.53, this effect could be due to a lessening of steric hindrance on the positions of Y7.53 and TMH7. Therefore, I2.43A would facilitate the β-arrestin1 recruitment mechanism triggered by the NPXXY motif.
On the contrary, the I2.43T mutation added a β-branching polar residue in position 2.43, which could hinder the correct positioning of Y7.53 that triggers β-arrestin recruitment. Accordingly, despite similar G-protein signaling to WT, I2.43T reduced β-arrestin recruitment. These findings demonstrate that I2.43 negatively regulates β-arrestin activation through control of the NPXXY motif in CB1.
Interestingly, S7.57E resulted in impairment of both G-protein signaling and β-arrestin recruitment. The effect on G-protein signaling likely results from the bulk of the E7.57, which will clash with the Gα protein, underscoring the importance of insertion of the α-5 helix into the transmembrane core17,23 for G-protein activation. The β-arrestin pathway is likely impacted through formation of a salt-bridge between R3.50 and E7.57. This interaction would impair the ability of TMH7 to bend/flex by tethering it to TMH3, supporting a role for movement of the IC end of TMH7 in β-arrestin recruitment.
In conclusion, our results demonstrate that N7.49 and Y7.53, two residues in the NPXXY motif, are essential in inducing β-arrestin recruitment via CB1. Furthermore, I2.43 is distinctly positioned to hinder this function through its steric bulk and interactions with Y7.53. Our findings suggest, for the first time, features of a CB1 conformational state that promotes β-arrestin recruitment, where highly conserved residues on TMH7 serve as key participants in a molecular mechanism that may control conformational changes at the IC end of TMH7. All mutations had a greater impact on the recruitment of β-arrestin1 than the recruitment of β-arrestin2, indicating that this mechanism induces a CB1 conformation that favors β-arrestin1. This may also be applicable to other class A GPCRs where amino acids in the NPXXY motif interact to regulate β-arrestin activation.
Data Availability
This study includes no data deposited in external repositories. Materials can be obtained through an Material Transfer Agreement.
Acknowledgments
We thank Viren Patwa for technical assistance and Dr. Lee-Yuan Liu-Chen for intellectual contributions. The work performed by Luciana Magalhaes Leo was in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Temple University (DOI: 10.34944/dspace/6826).
Abbreviations Used
- α1B-AR
α1B-adrenergic receptor
- β2-AR
β2-adrenergic receptor
- Δ9-THC
delta-9-tetrahydrocannabinol
- 2-AG
2-arachidonoylglycerol
- ANOVA
analysis of variance
- AT1R
angiotensin receptor 1
- BRET
bioluminescence resonance energy transfer
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- FPR1
N-formyl peptide receptor
- GPCR
G-protein-coupled receptor
- GRK
GPCR kinase
- HBSS
Hank's Balanced Salt Solution
- HEK
human embryonic kidney
- Hx8
helix 8
- IC
intracellular
- MD
molecular dynamics
- PDB
Protein Data Bank
- PTx
pertussis toxin
- SEM
standard error of the mean
- TMH
transmembrane helix
- V2R
vasopressin v2 receptor
- WT
wild type
Authors' Contributions
L.M.L. designed and performed the experiments and data analysis for mutagenesis experiments. R.A.-Z. proposed CB1 mutations. D.P.H. analyzed CB1 receptor structures. A.P.S. performed In-Cell Western and β-arrestin recruitment assays for Y7.53F, N7.49A, and S7.57E mutations. P.Z. performed and analyzed radioligand binding assays. D.G.T. provided resources used in BRET assays. E.M. and S.S. provided DNA constructs used in β-arrestin recruitment assays. M.E.A. and P.H.R. supervised the experiments. L.M.L., R.A.-Z., D.P.H., M.E.A., and P.H.R. wrote the article. All authors reviewed the article.
Author Disclosure Statement
The authors declare they have no conflicts of interest.
Funding Information
This work was supported by the National Institutes of Health (R01 DA003934, R01 DA045698, T32 DA007237, and P30 DA013429).
Cite this article as: Leo LM, Al-Zoubi R, Hurst DP, Stephan AP, Zhao P, Tilley DG, Miess E, Schulz S, Abood ME, Reggio PH (2023) The NPXXY motif regulates β-arrestin recruitment by the CB1 cannabinoid receptor, Cannabis and Cannabinoid Research 8:5, 731–748, DOI: 10.1089/can.2021.0223.
References
- 1. Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. [DOI] [PubMed] [Google Scholar]
- 2. Devane W, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. [DOI] [PubMed] [Google Scholar]
- 3. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. [DOI] [PubMed] [Google Scholar]
- 4. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. [DOI] [PubMed] [Google Scholar]
- 5. Shenoy SK, Drake MT, Nelson CD, et al. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. [DOI] [PubMed] [Google Scholar]
- 6. Liu JJ, Horst R, Katritch V, et al. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tan L, Yan W, McCorvy JD, et al. Biased ligands of G protein-coupled receptors (GPCRs): structure-functional selectivity relationships (SFSRs) and therapeutic potential. J Med Chem. 2018;61:9841–9878. [DOI] [PubMed] [Google Scholar]
- 8. Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. [DOI] [PubMed] [Google Scholar]
- 9. Laprairie RB, Bagher AM, Kelly MEM, et al. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem. 2014;289:24845–24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ibsen MS, Finlay DB, Patel M, et al. Cannabinoid CB1 and CB2 receptor-mediated arrestin translocation: species, subtype, and agonist-dependence. Front Pharmacol. 2019;10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020;16:9–29. [DOI] [PubMed] [Google Scholar]
- 12. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665–1703. [DOI] [PubMed] [Google Scholar]
- 13. Delgado-Peraza F, Ahn KH, Nogueras-Ortiz C, et al. Mechanisms of biased β-arrestin-mediated signaling downstream from the cannabinoid 1 receptor. Mol Pharmacol. 2016;89:618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khajehali E, Malone DT, Glass M, et al. Biased agonism and biased allosteric modulation at the CB1 cannabinoid receptor. Mol Pharmacol. 2015;88:368–379. [DOI] [PubMed] [Google Scholar]
- 15. Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Hua T, Vemuri K, Pu M, et al. Crystal structure of the human cannabinoid receptor CB1. Cell. 2016;167:750..e14–762.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishna Kumar K, Shalev-Benami M, Robertson MJ, et al. Structure of a signaling cannabinoid receptor 1-G protein complex. Cell. 2019;176:448..e12–458.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carpenter B, Tate CG. Active state structures of G protein-coupled receptors highlight the similarities and differences in the G protein and arrestin coupling interfaces. Curr Opin Struct Biol. 2017;45:124–132. [DOI] [PubMed] [Google Scholar]
- 19. Gurevich VV, Gurevich EV. Molecular mechanisms of GPCR signaling: a structural perspective. Int J Mol Sci. 2017;18:2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weis WI, Kobilka BK. The molecular basis of G protein–coupled receptor activation. Annu Rev Biochem. 2018;87:897–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ji S, Yang W, Yu W. Understanding the role of the CB1 toggle switch in interaction networks using molecular dynamics simulation. Sci Rep. 2021;11:22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ballesteros JA, Weinstein H. [19] Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Receptor molecular biology, Sealfon SC, ed. New York, NY: Academic Press, 1995, pp. 366–428. [Google Scholar]
- 23. Hua T, Li X, Wu L, et al. Activation and signaling mechanism revealed by cannabinoid receptor-G(i) complex structures. Cell. 2020;180:655..e18–665.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranjan R, Dwivedi H, Baidya M, et al. Novel structural insights into GPCR–β-arrestin interaction and signaling. Trends Cell Biol. 2017;27:851–862. [DOI] [PubMed] [Google Scholar]
- 25. Jin W, Brown S, Roche JP, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang W, Masureel M, Qu Q, et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020;579:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staus DP, Hu H, Robertson MJ, et al. Structure of the M2 muscarinic receptor–β-arrestin complex in a lipid nanodisc. Nature. 2020;579:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cahill TJ, 3rd, Thomsen AR, Tarrasch JT, et al. Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A. 2017;114:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumari P, Srivastava A, Ghosh E, et al. Core engagement with beta-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Mol Biol Cell. 2017;28:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fay JF, Farrens DL. A key agonist-induced conformational change in the cannabinoid receptor CB1 is blocked by the allosteric ligand Org 27569. J Biol Chem. 2012;287:33873–33882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fay JF, Farrens DL. Structural dynamics and energetics underlying allosteric inactivation of the cannabinoid receptor CB1. Proc Natl Acad Sci U S A. 2015;112:8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rahmeh R, Damian M, Cottet M, et al. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc Natl Acad Sci U S A. 2012;109:6733–6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wingler LM, Elgeti M, Hilger D, et al. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell. 2019;176:468..e11–478.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erlandson SC, McMahon C, Kruse AC. Structural basis for G protein-coupled receptor signaling. Annu Rev Biophys. 2018;47:1–18. [DOI] [PubMed] [Google Scholar]
- 35. Huang W, Manglik A, Venkatakrishnan AJ, et al. Structural insights into μ-opioid receptor activation. Nature. 2015;524:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gripentrog JM, Jesaitis AJ, Miettinen HM. A single amino acid substitution (N297A) in the conserved NPXXY sequence of the human N-formyl peptide receptor results in inhibition of desensitization and endocytosis, and a dose-dependent shift in p42/44 mitogen-activated protein kinase activation and chemotaxis. Biochem J. 2000;352:399–407. [PMC free article] [PubMed] [Google Scholar]
- 37. Kalatskaya I, Schüssler S, Blaukat A, et al. Mutation of tyrosine in the conserved NPXXY sequence leads to constitutive phosphorylation and internalization, but not signaling, of the human B2 bradykinin receptor. J Biol Chem. 2004;279:31268–31276. [DOI] [PubMed] [Google Scholar]
- 38. Ragnarsson L, Andersson Å, Thomas WG, et al. Mutations in the NPxxY motif stabilize pharmacologically distinct conformational states of the α 1B- and β 2-adrenoceptors. Sci Signal. 2019;12:eaas9485. [DOI] [PubMed] [Google Scholar]
- 39. Wacker D, Wang C, Katritch V, et al. Structural features for functional selectivity at serotonin receptors. Science. 2013;340:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suomivuori C-M, Latorraca NR, Wingler LM, et al. Molecular mechanism of biased signaling in a prototypical G protein–coupled receptor. Science. 2020;367:881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gamage TF, Anderson JC, Abood ME. CB1 allosteric modulator Org27569 is an antagonist/inverse agonist of ERK1/2 signaling. Cannabis Cannabinoid Res. 2016;1:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tao Q, Abood ME. Mutation of a highly conserved aspartate residue in the second transmembrane domain of the cannabinoid receptors, CB1 and CB2, disrupts G-protein coupling. J Pharmacol Exp Ther. 1998;285:651–658. [PubMed] [Google Scholar]
- 43. Miess E, Gondin AB, Yousuf A, et al. Multisite phosphorylation is required for sustained interaction with GRKs and arrestins during rapid μ-opioid receptor desensitization. Sci Signal. 2018;11:eaas9609. [DOI] [PubMed] [Google Scholar]
- 44. Garai S, Leo LM, Szczesniak A-M, et al. Discovery of a biased allosteric modulator for cannabinoid 1 receptor: preclinical anti-glaucoma efficacy. J Med Chem. 2021;64:8104–8126. [DOI] [PubMed] [Google Scholar]
- 45. Kapur A, Hurst DP, Fleischer D, et al. Mutation studies of Ser7.39 and Ser2.60 in the human CB1 cannabinoid receptor: evidence for a serine-induced bend in CB1 transmembrane helix 7. Mol Pharmacol. 2007;71:1512–1524. [DOI] [PubMed] [Google Scholar]
- 46. Marcu J, Shore DM, Kapur A, et al. Novel insights into CB1 cannabinoid receptor signaling: a key interaction identified between the extracellular-3 loop and transmembrane helix 2. J Pharmacol Exp Ther. 2013;345:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abadji V, Lucas-Lenard JM, Chin CN, et al. Involvement of the carboxyl terminus of the third intracellular loop of the cannabinoid CB1 receptor in constitutive activation of G(s). J Neurochem. 1999;72:2032–2038. [DOI] [PubMed] [Google Scholar]
- 48. Eldeeb K, Leone-Kabler S, Howlett AC. CB1 cannabinoid receptor-mediated increases in cyclic AMP accumulation are correlated with reduced Gi/o function. J Basic Clin Physiol Pharmacol. 2016;27:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mangmool S, Kurose H. Gi/o protein-dependent and -independent actions of pertussis toxin (PTx). Toxins. 2011;3:884–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eldeeb K, Leone-Kabler S, Howlett AC. Mouse neuroblastoma CB1 cannabinoid receptor-stimulated [35S]GTPɣS binding: total and antibody-targeted Gα protein-specific scintillation proximity assays. In Methods in enzymology, Reggio PH, ed. New York, NY: Academic Press, 2017, pp. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yuan S, Filipek S, Palczewski K, et al. Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway. Nat Commun. 2014;5:4733. [DOI] [PubMed] [Google Scholar]
- 53. Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem. 2012;287:12070–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Price MR, Baillie GL, Thomas A, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. [DOI] [PubMed] [Google Scholar]
- 55. Ahn KH, Mahmoud MM, Shim JY, et al. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J Biol Chem. 2013;288:9790–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shao Z, Yan W, Chapman K, et al. Structure of an allosteric modulator bound to the CB1 cannabinoid receptor. Nat Chem Biol. 2019;15:1199–1205. [DOI] [PubMed] [Google Scholar]
- 57. Schneider M, Kasanetz F, Lynch DL, et al. Enhanced functional activity of the cannabinoid type-1 receptor mediates adolescent behavior. J Neurosci. 2015;35:13975–13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hurst DP, Garai S, Kulkarni PM, et al. Identification of CB1 receptor allosteric sites using force-biased MMC simulated annealing and validation by structure-activity relationship studies. ACS Med Chem Lett. 2019;10:1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh R, Hurst DP, Barnett-Norris J, et al. Activation of the cannabinoid CB1 receptor may involve a W6 48/F3 36 rotamer toggle switch. J Pept Res. 2002;60:357–370. [DOI] [PubMed] [Google Scholar]
- 60. McAllister SD, Hurst DP, Barnett-Norris J, et al. Structural mimicry in class A G protein-coupled receptor rotamer toggle switches: the importance of the F3.36(201)/W6.48(357) interaction in cannabinoid CB1 receptor activation. J Biol Chem. 2004;279:48024–48037. [DOI] [PubMed] [Google Scholar]
- 61. Lynch DL, Hurst DP, Shore DM, et al. Molecular dynamics methodologies for probing cannabinoid ligand/receptor interaction. Methods Enzymol. 2017;593:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stornaiuolo M, Bruno A, Botta L, et al. Endogenous vs exogenous allosteric modulators in GPCRs: a dispute for shuttling CB1 among different membrane microenvironments. Sci Rep. 2015;5:15453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vallée M, Vitiello S, Bellocchio L, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barak LS, Menard L, Ferguson SSG, et al. The conserved seven-transmembrane sequence NP(X)2,3Y of the G-protein-coupled receptor superfamily regulates multiple properties of the beta2-adrenergic receptor. Biochemistry. 1995;34:15407–15414. [DOI] [PubMed] [Google Scholar]
- 65. Fenalti G, Giguere PM, Katritch V, et al. Molecular control of δ-opioid receptor signalling. Nature. 2014;506:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barak LS, Tiberi M, Freedman NJ, et al. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 67. Gabilondo AM, Krasel C, Lohse MJ. Mutations of Tyr326 in the β2-adrenoceptor disrupt multiple receptor functions. Eur J Pharmacol. 1996;307:243–250. [DOI] [PubMed] [Google Scholar]
- 68. Laporte SA, Servant G, Richard DE, et al. The tyrosine within the NPXnY motif of the human angiotensin II type 1 receptor is involved in mediating signal transduction but is not essential for internalization. Mol Pharmacol. 1996;49:89–95. [PubMed] [Google Scholar]
- 69. Bonde MM, Hansen JT, Sanni SJ, et al. Biased signaling of the angiotensin II type 1 receptor can be mediated through distinct mechanisms. PLoS One. 2010;5:e14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. He R, Browning DD, Ye RD. Differential roles of the NPXXY motif in formyl peptide receptor signaling. J Immunol. 2001;166:4099–4105. [DOI] [PubMed] [Google Scholar]
- 71. Bouley R, Sun T-X, Chenard M, et al. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol. 2003;285:C750–C762. [DOI] [PubMed] [Google Scholar]
- 72. Kramer HK, Andria ML, Kushner SA, et al. Mutation of tyrosine 318 (Y318F) in the delta-opioid receptor attenuates tyrosine phosphorylation, agonist-dependent receptor internalization, and mitogen-activated protein kinase activation. Brain Res Mol Brain Res. 2000;79:55–66. [DOI] [PubMed] [Google Scholar]
- 73. Katritch V, Fenalti G, Abola EE, et al. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014;39:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shao Z, Yin J, Chapman K, et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study includes no data deposited in external repositories. Materials can be obtained through an Material Transfer Agreement.