Abstract
BACKGROUND:
We report the case of a patient with aplastic anemia and pancytopenia on immune-suppressive therapy who developed invasive pulmonary infection with mucormycosis and was treated with immune adjuvant therapy.
CASE SUMMARY:
Given the patient’s profound lymphopenia and progressive invasive mucor despite dual antifungal drug therapy, interleukin (IL)-7, a cytokine that induces lymphocyte activation and proliferation, was instituted and resulted in normalization of absolute lymphocyte counts and was temporally associated with clearance of fungal pathogens and resolution of clinical symptoms.
CONCLUSION:
Patients with life-threatening fungal infections are frequently immune suppressed and immune adjuvant therapies should be considered in patients who are not responding to antifungal drugs and source control. Well-designed, double-blind, placebo-controlled trials are needed to advance the field. Although a number of immune adjuvants may be beneficial in fungal sepsis, IL-7 is a particularly attractive immune adjuvant because of its broad immunologic effects on key immunologic pathways that mediate enhanced antifungal immune system activity.
Keywords: fungal, immunity, infection, lymphocyte, sepsis
KEY POINTS
Question: New immune adjuvant therapies that boost patients’ immune systems are being increasingly used in patients with serious infections who are failing conventional therapy. Do patients with refractory invasive fungal infections who are immunosuppressed benefit from the addition of immune adjuvant drug therapies?
Findings: Interleukin (IL)-7, a cytokine that induces lymphocyte activation and proliferation, was used in a patient with aplastic anemia and lymphopenia who developed mucormycosis. There was a temporal association of IL-7 therapy with restoration of the patient’s lymphocyte counts to normal and with clearing of the fungal infection.
Meaning: This case report adds to the growing number of cases in which immune adjuvant therapies were associated with an apparent improvement in the clinical course of patients with life-threatening invasive fungal infections.
CASE REPORT
Invasive fungal infections are an increasing cause of morbidity and mortality (1, 2). Candidemia is now the third to fourth most common nosocomial bloodstream infection causing more than 28,000 infections per year with an attributable mortality ranging from 25% to 57% (3). Fungal infections are also of particular concern because of the resistance that these pathogens are developing to current antimicrobial therapies. Mucormycosis is an opportunistic fungal infection of the mucormycetes family (previously zygomycetes). Some of the most frequently identified Mucorales include Rhizopus species, Mucor species, and Rhizomucor species. These infections typically occur in immunocompromised individuals and have a high mortality rate that is at least partially attributed to the rapid growth rate and ability of the mold to invade blood vessels. During the COVID-19 pandemic, invasive mucormycosis was particularly problematic in patients with diabetes and individuals treated with high doses of corticosteroids (4).
Drugs that augment host immunity are increasingly being used in patients with life-threatening fungal infections that have been refractory to standard therapies (5–10). In some cases, the initiation of therapies that boost the immune system (immune adjuvant therapies) has restored indices of immune function and resulted in clearance of the infections (9, 10). These immune adjuvant therapies include granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), checkpoint inhibitors (antiprogrammed cell death 1 [anti-PD-1]), and interleukin (IL)-7 (7–10). IL-7 is a lymphocyte growth factor that is essential for the formation and survival of CD4 and CD8 T cells (11). IL-7 not only causes lymphocyte proliferation but also causes lymphocyte activation leading to increased IFN-γ, a cytokine that plays a key role in fungal infections (8). Herein, we describe the use of IL-7 in a severely lymphopenic patient with aplastic anemia and invasive pulmonary mucormycosis.
PATIENT CASE
A 53-year-old man with a medical history of squamous cell carcinoma of the left oral cavity and COVID-19 infection requiring mechanical ventilation was admitted for evaluation and therapy of pancytopenia. Workup was consistent with aplastic anemia vs. hypoplastic myelodysplastic syndrome, and therapy with antithymocyte globulin, cyclosporine, and eltrombopag (a platelet growth factor) was initiated. The patient did well with this therapy for approximately 2 months until developing fever, dry cough, and tachypnea requiring hospital admission. Extensive workup for the etiology of his pulmonary infection was negative for COVID-19, influenza, respiratory syncytial virus, cytomegalovirus, and other viral and bacterial pathogens. Aspergillus galactomannan antigen was initially elevated but repeat testing 5 days later was negative. Invasive pulmonary mucormycosis was diagnosed based upon an induced sputum culture positive for Mucor species and a chest CT demonstrating necrotizing regions radiographically consistent with invasive fungal infection. The patient’s mucormycosis was treated with liposomal amphotericin B and posaconazole. Therapy with granulocyte infusions, Granulocyte-Colony Stimulating Factor (G-CSF), and GM-CSF was also initiated because of low absolute neutrophil and monocyte counts. Despite these therapies, the patient’s clinical course worsened with acute hypercapnic, hypoxemic respiratory distress, fevers, and progressive changes on chest radiographs. He was transferred to the ICU and initiated on noninvasive positive pressure ventilation.
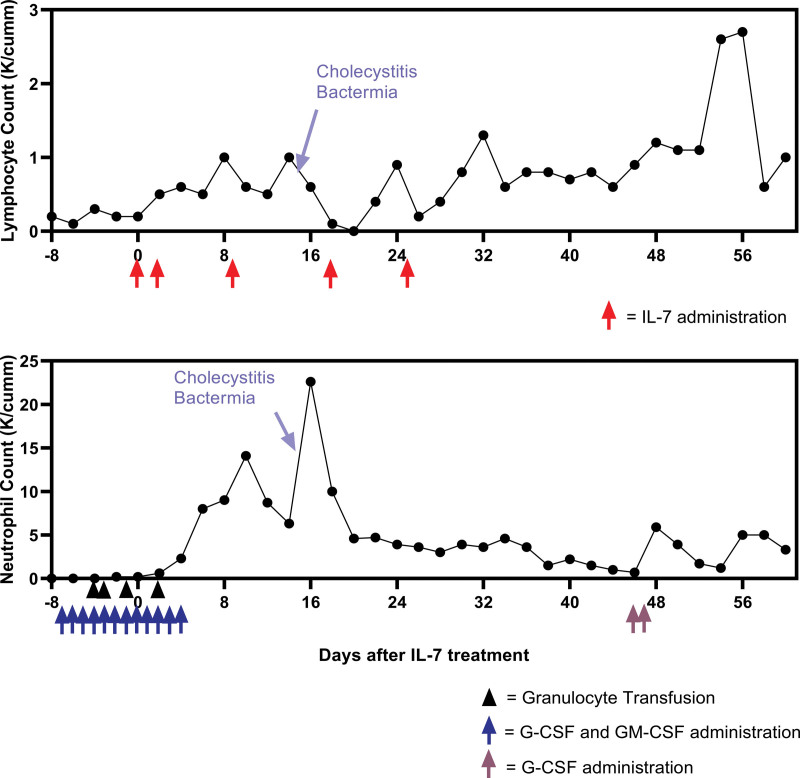
Because of the patient’s worsening clinical course and persistently low lymphocyte counts, IL-7 was instituted on a compassionate basis after obtaining informed consent from the patient for treatment with IL-7 and publication of case details and images. A test dose of IL-7 (3 µg/kg ideal body weight, intramuscularly) (kindly provided by Dr. Michel Morre, RevImmune) was administered and was well-tolerated (Fig. 1). Forty-eight hours later, the dosage was increased to 10 µg/kg and continued weekly for three additional doses. Following the initiation of IL-7 therapy, there was a temporal association with an increase in the patient’s absolute lymphocyte count. Over the subsequent 48 hours, he continued on intermittent noninvasive positive pressure ventilation support alternating with supplemental oxygen of 10–13 L/min to maintain Spo2 greater than 92%. Approximately 48 hours following his first IL-7 injection, his oxygen requirement objectively began to decrease to 7–9 L/min to maintain Spo2 greater than 92%, and the patient was able to spend longer periods off noninvasive positive pressure ventilation support. By 4 days following IL-7, his oxygen requirement had objectively decreased to 4–6 L/min to maintain Spo2 greater than 92%. Within 12 days from the first IL-7 injection, his respiratory status had improved to the point he was able to transfer out of the ICU to the floor and his course continued to improve.
Figure 1.
Top, Absolute lymphocyte counts over time: This timeframe of the absolute lymphocyte counts before, during, and after IL-7 administration shows a transient increase followed by an expected decrease in response to acute acalculous cholecystitis with bacteremia, after which the lymphocyte count continued to trend upward until returning to a near normal level approximately 60 d following injection. The peak value between days 48 and 56 likely reflects the impact of IL-7 given the absence of other associated factors, including administration of G-CSF, GM-CSF, and granulocyte infusions during that period. Bottom, Absolute granulocyte counts over time: This timeframe of the absolute granulocyte counts before, during, and after IL-7 therapy. Note the large increase in the absolute granulocyte count with the onset of cholecystitis and bacteremia. G-CSF = granulocyte-colony stimulating factor, GM-CSF = granulocyte macrophage colony-stimulating factor, IL = interleukin.
The patient’s hospital course was further complicated by the development of acalculous cholecystitis and Enterococcus faecium bacteremia which was treated with biliary duct sphincterotomy, biliary stent placement, and antibiotics. As is typically observed during the onset of sepsis, the patient’s absolute lymphocyte count decreased transiently during his acute septic episode and bacteremia (Fig. 1) (12). The patient subsequently recovered and was discharged to a nursing home approximately ten weeks after admission.
DISCUSSION
This is the first reported case of the therapeutic use of IL-7 in a patient with severe lymphopenia because of aplastic anemia and a life-threatening refractory infection that was nonresponsive to standard medical therapies. Patients’ ability to successfully eradicate invading pathogens depends on a coordinated response between the different cellular components of the innate and adaptive immune systems. Patients with aplastic anemia have defects in the production of granulocytes, monocytes, and lymphocytes and, therefore, have severely impaired host immunity. The patient’s decreased granulocytes and monocytes were successfully treated with cytokine growth factors, including G-CSF, GM-CSF, and granulocyte infusions. Although these therapies were effective in restoring absolute granulocyte and monocyte counts, the patient’s absolute lymphocyte counts remained critically low and his infection continued to progress. Lymphocytes play a key role in containing and eliminating pathogens and decreased absolute lymphocytes have been shown to closely correlate with mortality in patients with Candidemia (13, 14). Consequently, therapy with IL-7, a lymphocyte growth factor, was instituted to increase the absolute lymphocyte counts and to improve lymphocyte functional capabilities. IL-7 leads to a significant increase in the patient’s absolute lymphocyte counts and subsequent successful elimination of the invasive fungal infection.
There is a growing recognition that therapies that boost the patients’ immune system can be beneficial in infectious diseases (5, 6, 8) (Table 1). Patients with invasive fungal infections or patients who have multidrug-resistant bacterial infections are good candidates for immune adjuvant therapies because they are highly likely to be immune suppressed and infections with these pathogens carry a high mortality despite treatment with antibiotic drug therapies. In this regard, many patients with disseminated fungal infections die despite antimicrobial drug therapies that may have good in vitro inhibitory and/or killing activity against the particular fungal pathogen. These facts underscore the need for new therapeutic approaches that harness the host’s intrinsic immune system as an additional means to manage these challenging infections.
TABLE 1.
Immune Adjuvant Therapies for Refractory Fungal Infections
| Drug | Indications | Contra-indications | Dose | Side Effects | Comments | References |
|---|---|---|---|---|---|---|
| G-CSF | Neutrophils <500/μL | Hypersensitivity | 5 µg/kg daily | Injection site reactions, fever, cough, SOB, rash, headache, fatigue, arthralgia | Onset is variable depending upon condition; may combine with GM-CSF in severe cases | (6–8) |
| GM-CSF | Neutrophils <500/μL and decreased monocytes | Hypersensitivity | 250 μg/m2 daily |
Injection site reactions, fever, headache, chills, malaise, asthenia, arthralgia, myalgia, rash | Onset is variable depending upon condition; may combine with G-CSF in severe cases | (7, 8) |
| IFN-γ | Failing conventional therapy | Hypersensitivity | 100 μg three times weekly for 5 doses | Injection site reactions, fever, headache, chills, fatigue, rash, diarrhea, vomiting |
ongoing trial of IFN-γ in invasive candidiasis nct04979052-; Case reports of combining IFN-γ and anti-PD-1 Consider administering with acetaminophen and/or at bedtime to minimize side effects |
(8, 9, 15, 16) |
| IL-7 | Failing conventional therapy, lymphopenia |
Autoimmunity, lymphoid malignancy organ transplant |
10 μg/kga Q 3–4 d up to 8 doses |
Injection site reactions; fever, chills, rash, | Currently available for compassionate use only—see website revimmune.com to request drug; currently in multiple clinical trials as immune adjuvant in oncology; IL-7 reported to accelerate T cell recovery after allogenic stem cell transplant (17) | (10) |
| Anti-PD-1 | Failing conventional therapy | Autoimmunity organ transplant |
Nivolumab 250 mg |
Numerous potentially serious autoimmune complicationsb | Case reports combining anti-PD-1 with IFN-γ; may use lymphocyte PD-1 expression determined via flow cytometry to guide therapy; half-life of Nivolumab is approximately 2 wk | (9, 15) |
Anti-PD-1 = antiprogrammed cell death 1, IFN-γ = interferon gamma, G-CSF = granulocyte-colony stimulating factor, GM-CSF = granulocyte macrophage colony-stimulatingfactor, IL = interleukin, SOB = shortness of breath.
Test dose of 3 μg/kg IL-7 ideal body weight can be administered 24 hr prior to the therapeutic dose of 10 μg/kg ideal body weight if concern for adverse effects. Rare complication of posterior reversible encephalopathy syndrome reported in patients.
Serious autoimmune complications with anti-PD-1 therapy usually occur after multiple doses of anti-PD-1. Thus, the risk of autoimmune reactions is less likely if only a single dose of anti-PD-1 is administered.
Comments regarding therapy of patients with life-threatening fungal infections:
1) Reduce immunosuppressive medications: consider reducing corticosteroids and other immunosuppressive agents if possible.
2) Source control: remove invasive lines and hardware if possible and rule out undrained foci of infection.
Several immune adjuvants have been reported to be effective in patients with life-threatening invasive fungal infections. There are two reported cases of concomitant therapy with anti-PD-1 and IFN-γ in patients with refractory mucormycosis despite maximal therapy, including surgical debridement and systemic antifungal therapy (9, 15). Initiation of immune adjuvant therapy with anti-PD-1 (Nivolumab) and IFN-γ was associated with rapid clinical improvement, enhanced immune phenotypic markers, and eradication of mucormycosis. Currently, there is an ongoing clinical trial of IFN-γ in patients with invasive candidiasis (NCT05235711). Recombinant human GM-CSF has also been employed in adult and pediatric patients with refractory fungal infections with a reported response rate of approximately 80% (7).
Another immuno-adjuvant that is likely to be highly efficacious in life-threatening fungal infections is IL-7. Two independent groups have reported that IL-7 ameliorates fungal sepsis-induced immune suppression and improves survival in well-controlled animal models of fungal sepsis (18, 19). IL-7 has been called the “maestro of the immune system” because of its effects on an array of immune cells that play critical roles in fungal pathogen elimination (20). IL-7 activates CD4 and CD8 T cells to produce IFN-γ that stimulates monocytes/macrophages to phagocytose and kill fungal pathogens. Neutrophils also play an essential role in eliminating fungal organisms. IL-7 induces the formation and stimulation of Th17 lymphocytes to produce IL-17, a cytokine that causes neutrophil mobilization and enhances neutrophil migration to sites of fungal infection (21). IL-7 also activates a recently described subset of T cells termed “mucosal-associated invariant T cells” that line the gastrointestinal and respiratory tracts and serve to prevent invasion by fungal pathogens (22).
A recent remarkable case report detailing the efficacy of IL-7 in an immunocompetent patient who was dying of intractable fungal infection provides compelling support for the use of IL-7 immunotherapy in patients who are not responding to conventional therapy (10). In that case, a previously healthy 21-year-old trauma victim patient sustained catastrophic degloving injuries of his buttocks and perineum following a motorcycle accident. Approximately 1 week after injury, the patient developed invasive muscle and soft-tissue infection with Trichosporon asahii and Saksenaea species. Blood and tissue cultures remained positive for approximately 1 month despite aggressive surgical debridement and triple antifungal drug therapy. Because of the patient’s continuing deterioration and persistent low absolute lymphocyte counts, IL-7 was started on a compassionate-use basis. Blood and tissue cultures became negative within 5 days of the initiation of treatment with IL-7, and there was a concomitant rapid improvement in the patient’s clinical course. Immunohistochemical staining of biopsies of the infected wound margin showed a marked increase in the number of CD3+ lymphocytes after beginning IL-7 immune therapy consistent with an effect of IL-7 to expand the lymphocyte compartment and increase trafficking of lymphocytes to areas of inflammation and/or infection.
Limitations
Although there was a temporal association of the patient’s clinical course improving with the initiation of IL-7, the patient was also treated with a number of other immune-modulating therapeutic agents, including GM-CSF, G-CSF, and granulocyte infusions. It is likely that all of these therapies played an important role in the patient’s ability to survive his infection. It is also possible that the patient’s adaptive immune system recovered from some of the immune-suppressive therapies used to treat his underlying disease.
In conclusion, patients with life-threatening fungal infections are frequently immune suppressed, and immune adjuvant therapies should be considered in patients who are not responding to antifungal drugs and source control. A number of immune adjuvants have been reported to be effective in case reports, but a well-designed, double-blind, placebo-controlled trial is needed to advance the field. Although a number of immune adjuvants may be beneficial in fungal sepsis, IL-7 is a particularly attractive immune adjuvant because of its effects on key immunologic pathways which mediate enhanced antifungal immune system activity and because it can be particularly impactful in patients with lymphopenia.
ACKNOWLEDGMENTS
We thank Dr. Michel Morre, RevImmune, for kindly providing recombinant human IL-7 (CYT-107) for compassionate use in this patient. Dr. DiPersio thanks NIH award 5R35CA210084-06, and Dr. Hotchkiss thanks NIH grant R35GM126928.
Footnotes
RevImmune kindly provided CYT-107, recombinant humanized IL-7, for compassionate use in this patient.
Dr. Hotchkiss has been an investigator on several trials of IL-7 in sepsis. The remaining authors have not disclosed any potential conflicts of interest.
Drs. Crees, Kim, and DiPersio were involved in caring the patient and helped write and review the article. Drs. Crees, Patel, Dram, Bern, Eberly, Augustin, and Hotchkiss all assisted in writing and reviewing the article.
REFERENCES
- 1.Watkins RR, Gowen R, Lionakis MS, et al. : Update on the pathogenesis, virulence, and treatment of candida auris. Pathog Immun 2022; 7:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lionakis MS, Drummond RA, Hohl TM: Immune responses to human fungal pathogens and therapeutic prospects. Nat Rev Immunol 2023; 23:433–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsay S, Williams S, Mu Y, et al.: National Burden of Candidemia, United States, 2017. Open Forum Infect Dis 2018; 5:S142–S143 [Google Scholar]
- 4.Kumar R, Misra A, Kumar D, et al. : A Systematic review of mucormycosis cases in COVID-19: Is it an unholy trilogy of COVID-19, diabetes mellitus, and corticosteroids? J Family Med Primary Care 2022; 11:2573–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionakis MS: Exploiting antifungal immunity in the clinical context. Semin Immunol 2023; 67:101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiani G, McCormick TS, Leal LO, et al. : Recombinant human granulocyte macrophage-colony stimulating factor expressed in yeast (sargramostim): A potential ally to combat serious infections. Clin Immunol 2020; 210:108292. [DOI] [PubMed] [Google Scholar]
- 7.Chen TK, Batra JS, Michalik DE, et al. : Recombinant human granulocyte-macrophage colony-stimulating factor (rhu GM-CSF) as adjuvant therapy for invasive fungal diseases. Open Forum Infect Dis 2022; 9:ofac535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullberg BJ, van de Veerdonk F, Netea MG: Immunotherapy: A potential adjunctive treatment for fungal infection. Curr Opin Infect Dis 2014; 27:511–516 [DOI] [PubMed] [Google Scholar]
- 9.Grimaldi D, Pradier O, Hotchkiss RS, et al. : Nivolumab plus interferon-gamma in the treatment of intractable mucormycosis. Lancet Infect Dis 2017; 17:18. [DOI] [PubMed] [Google Scholar]
- 10.Turnbull IR, Mazer MB, Hoofnagle MH, et al. : IL-7 immunotherapy in a nonimmunocompromised patient with intractable fungal wound sepsis. Open Forum Infect Dis 2021; 8:ofab256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barata JT, Durum SK, Seddon B: Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol 2019; 20:1584–1593 [DOI] [PubMed] [Google Scholar]
- 12.Francois B, Jeannet R, Daix T, et al. : Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight 2018; 3:e98960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega-Loubon C, Cano-Hernandez B, Poves-Alvarez R, et al. : The overlooked immune state in candidemia: A risk factor for mortality. J Clin Med 2019; 8:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Opal SM: Activating immunity to fight a foe—A new path. N Engl J Med 2020; 382:1270–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukaszewicz AC, Venet F, Boibieux A, et al. : Nivolumab and interferon-gamma rescue therapy to control mixed mould and bacterial superinfection after necrotizing fasciitis and septic shock. Med Mycol Case Rep 2022; 37:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tawfik DM, Dereux C, Tremblay J-A, et al. : Interferon gamma as an immune modulating adjunct therapy for massive mucormycosis after severe burn—a case report. Front Immunol 2022; 13:883638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perales M-A, Goldberg JD, Yuan J, et al. : Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogenic stem cell transplantation. Blood 2012; 120:4882–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unsinger J, Burnham CA, McDonough J, et al. : Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis 2012; 206:606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lelu K, Dubois C, Evlachev A, et al. : Viral delivery of IL-7 is a potent immunotherapy stimulating innate and adaptive immunity and confers survival in sepsis models. J Immunol 2022; 209:99–117 [DOI] [PubMed] [Google Scholar]
- 20.Sprent J, Surh CD: Interleukin 7, maestro of the immune system. Semin Immunol 2012; 24:149–150 [DOI] [PubMed] [Google Scholar]
- 21.Kasten KR, Prakash PS, Unsinger J, et al. : Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun 2010; 78:4714–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sortino O, Richards E, Dias J, et al. : IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 2018; 32:825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]