Abstract
Background:
To systematically evaluate the effects of vitamin D supplementation in patients with nonalcoholic fatty liver disease (NAFLD).
Methods:
National Library of Medicine, Cochrane Library, Elsevier, China National Knowledge Infrastructure, Web of Science, WANFANG databases, and Google Scholar were retrieved to collect relevant randomized controlled trials, which are published from the earliest records the time the database was created to April 2023. Meta-analysis was conducted by using Review Manager 5.4 software after evaluating in terms of inclusion and exclusion criteria. The outcome indicators include 25-hydroxyvitamin D [25(OH)D] levels, insulin resistance index (homeostasis model assessment of insulin resistance), fasting blood glucose, and fasting insulin levels (FINS).
Results:
Eight randomized controlled trials with a total of 657 patients are included. Vitamin D supplementation increased 25(OH)D levels significantly (mean difference [MD] = 2.01, 95% confidence intervals [CI]: 0.94 to 3.08, P < .05) and vitamin D supplementation had a significant effect on insulin resistance index (MD = −0.54, 95% CI: −1.28 to 0.20, P = .16), fasting glucose (MD = −0.59, 95% CI: −1.50 to 0.32, P = .20), and FINS levels (MD = −0.30, 95% CI: −0.77 to 0.17, P = .21) had no significant effect.
Conclusion:
Vitamin D supplementation improves 25(OH)D levels in patients with nonalcoholic fatty liver disease, but there is no effect on homeostasis model assessment of insulin resistance, fasting blood glucose, or FINS.
Keywords: 25(OH)D, meta-analysis, nonalcoholic fatty liver, randomized controlled trial, vitamin D
1. Introduction
Vitamin D is fat-soluble. It has effects in type 2 diabetes mellitus, metabolic syndrome, cardiovascular disease, cancer, and autoimmune diseases.[1] Nonalcoholic fatty liver disease (NAFLD) is a metabolic disease of liver.[2] The incidence of NAFLD increases in the whole world.[3] Lifestyle interventions, dietary restrictions and physical exercise are recommended; however, the effects are usually limited and short-lived.[4] It shows that insulin resistance (IR), oxidative stress, and impaired glucolipid metabolism play a key role in the development of NAFLD; patient with NAFLD has a high risk of developing type 2 diabetes mellitus or metabolic syndrome.[5] Insulin resistance is the biggest contributor to this condition.[6] It shows that a reduced vitamin D level is strongly associated with the pathogenesis of IR, so vitamin D supplementation may have a significant improvement in glycemic control.[7] In pancreatic β-cells, 25-hydroxyvitamin D [25(OH)D] binds to the vitamin D receptor, which stimulates the insulin secretion.[8] Vitamin D in patients with NAFLD has controversial findings. This study intends to conduct a systematic review and meta-analysis to clarify the role of vitamin D in patients with NAFLD.
2. Materials and Methods
2.1. Search strategy
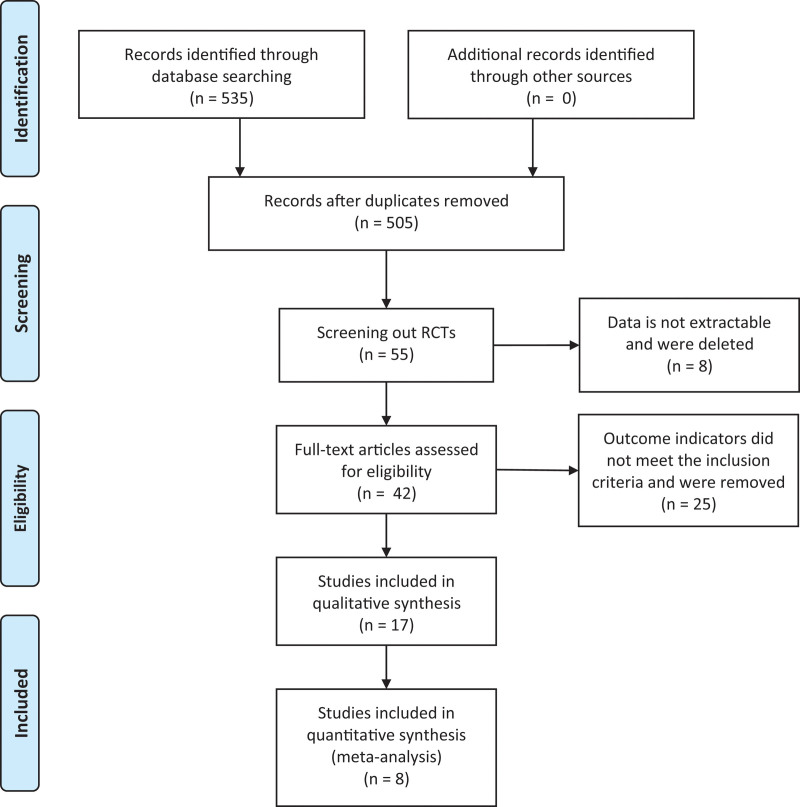
National Library of Medicine, Cochrane Library, China National Knowledge Infrastructure, Web of Science, WANFANG databases, and Google Scholar were retrieved to collect relevant randomized controlled trials (RCTs), which are published from the earliest records to April 2023. The search terms are “vitamin D,” “nonalcoholic fatty liver disease,” “vitamin D3,” “cholecalciferol,” “ergocalciferol,” “NAFLD” in combination. The literature screening process is shown in Figure 1.
Figure 1.
Literature screening process.
2.2. Inclusion criteria
(1) NAFLD or nonalcoholic steatohepatitis aged > 18 years; (2) placebo or lifestyle intervention in the control group, and oral or injectable vitamin D or D3 in the vitamin D group; (3) outcome indicators include the levels of 25(OH)D, homeostasis model assessment of insulin resistance (HOMA-IR), fasting blood glucose (FBG), and fasting insulin (FINS); (4) study design: RCTs.
2.3. Exclusion criteria
(1) Inaccessible abstracts or full text; (2) studies for which data could not be accurately extracted; (3) duplicate published studies.
2.4. Literature quality assessment
The evaluator first independently completes an initial screening of the included literature by reading the title and abstract. After reading the full text, literatures met the inclusion criteria were included. The following evaluation criteria for RCTs in Review Manager 5.4 were used: (1) generation of random sequences; (2) allocation concealment; (3) blinding of subjects and intervention providers; (4) blinding of outcome assessments; (5) completeness of outcome data; (6) selective outcome reporting; and (7) other biases. The 7 items above were evaluated using “low risk,” “high risk,” and “uncertain risk.”
2.5. Data extraction
Data extracted from the included literatures included the following: (1) general information such as title, first author, year of publication, sample size and trial quality score; (2) comparability of data and interventions across patient data groups; and (3) outcome indicators: levels of 25(OH)D, HOMA-IR, FBG and FINS.
2.6. Statistical processing
Data were analyzed using Review Manager 5.4 software at a test level of α = 0.05. Continuous variables and units were analyzed by mean difference (MD) and its 95% confidence intervals (CI). Clinical heterogeneity of included studies was first analyzed, followed by statistical heterogeneity using the I2 test.[9] When P > .1, I2 < 50%, homogeneity among several similar studies could be considered and a fixed-effects model was chosen for meta-analysis. Heterogeneity was considered when P < .1, I2 > 50%; and a random-effect model was used. P < .05 indicates a statistically significant difference and I2 ≥ 50% suggests high heterogeneity.[10]
3. Results
3.1. Study characteristics
Figure 2 is the flow chart. The initial review obtained 535 papers, and finally 8 RCTs are included.[11–18] There are 657 patients totally. 329 patients are in the control group and 328 patients are in the experimental group. The basic characteristics of includes studies are shown in Table 1.
Figure 2.
Flow chart.
Table 1.
Basic information of inclusion in the study.
| Author | Number of patients | Vitamin D supplementation dose | Periodicity | Cross referencing measures | Study outcome indicators |
|---|---|---|---|---|---|
| Hamid L (2016) | T:40 C:40 |
25 μg/d | 12 week | Placebo | ①②③④ |
| Hamid Y (2021) | T:64 C:64 |
0.25 μg/d | 17 week | Placebo | ①②③ |
| Mahdi (2014) | T:30 C:30 |
5000 U/w | 10 week | Placebo | ④ |
| Mazhar (2019) | T:51 C:51 |
5000 U/w | 12 week | Placebo | ①④ |
| Dai Jiale (2022) | T:50 C:50 |
400 U/d | 12 week | Lifestyle interventions | ①②③④ |
| Han Yuanping (2022) | T:14 C:15 |
Single intramuscular injection of vitamin Vitamin D3 600,000 U |
1 month | No intervention | ③ |
| Li Jinqiang (2022) | T:42 C:42 |
0.25 μg/d | 12 week | Lifestyle interventions | ④ |
| Lu Yongwen (2019) | T:37 C:37 |
0.5 μg/d | 12 week | Lifestyle interventions | ①②③④ |
① Insulin resistance index. ② Fasting blood glucose. ③ Insulin level. ④ 25(OH)D.
C = control group, T = test group.
3.2. Quality assessment
All 8 studies are low risk according to the randomized allocation. Seven studies are low risk and one study is unclear in the allocation concealment. Three studies are low risk and 5 studies are unclear in the double-blind method. Two studies are low risk and 6 studies are unclear in the evaluation of blindness. Four studies are low risk and 4 studies are unclear in the data integrity. Five studies are low risk and 3 studies are unclear in the selective report. Six studies are low risk and 2 studies are unclear in the others. Since there is no high risk in these 7 criteria, the quality of all included studies is good. The quality assessment table is shown in Table 2.
Table 2.
Quality assessment table.
| Included studies | Random allocation | Allocation concealment | Double blind method | Evaluation of blindness | Data integrity | Selective report | Others |
|---|---|---|---|---|---|---|---|
| Hamid L (2016) | Low risk | Low risk | Unclear | Unclear | Low risk | Unclear | Low risk |
| Hamid Y (2021) | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk |
| Mahdi (2014) | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk |
| Mazhar (2019) | Low risk | Low risk | Low risk | Unclear | Unclear | Low risk | Unclear |
| Dai Jiale (2022) | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk | Unclear |
| Han Yuanping (2022) | Low risk | Low risk | Unclear | Unclear | Low risk | Unclear | Low risk |
| Li Jinqiang (2022) | Low risk | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
| Lu Yongwen (2019) | Low risk | Low risk | Unclear | Unclear | Unclear | Low risk | Low risk |
3.3. Meta-analysis results
3.3.1. 25(OH)D.
Six studies report changes in 25(OH)D in patients. The results are shown in Figure 3. There is a heterogeneity as I2 = 96% and a random-effect model was used. Under a leave-one-out approach, the heterogeneity is possibly caused by the research of Mahdi (Fig. 4). Vitamin D supplementation significantly increased serum 25(OH)D levels (MD = 2.01, 95% CI: 0.94 to 3.08, P < .05).
Figure 3.
Changes of 25(OH)D between 2 groups. 25(OH)D = 25-hydroxyvitamin D.
Figure 4.
Effect sizes of 25(OH)D under leave-one-out approach. 25(OH)D = 25-hydroxyvitamin D.
3.3.2. HOMA-IR.
Five studies are included. The results are shown in Figure 5. There is a heterogeneity as I2 = 92% and a random-effect model was used. Under a leave-one-out approach, the heterogeneity is possibly caused by the research of Hamind L and Hamind Y (Fig. 6). There is no statistically significant difference in the effect of vitamin D supplementation on HOMA-IR (MD = -0.54, 95% CI: -1.28 to 0.20, P = .16).
Figure 5.
Changes of HOMA-IR between 2 groups. HOMA-IR = homeostasis model assessment of insulin resistance.
Figure 6.
Effect sizes of HOMA-IR under leave-one-out approach. HOMA-IR = homeostasis model assessment of insulin resistance.
3.3.3. FBG.
Four studies are included. The results are shown in Figure 7. There is a heterogeneity as I2 = 94% and a random-effect model was used. Under a leave-one-out approach, the heterogeneity is possibly caused by the research of Hamind L and Hamind Y (Fig. 8). There is no statistically significant difference in the effect of vitamin D supplementation on FBG (MD = −0.59, 95% CI: −1.50 to 0.32, P = .20).
Figure 7.
Changes of FBG between 2 groups. FBG = fasting blood glucose.
Figure 8.
Effect sizes of FBG under leave-one-out approach. FBG = fasting blood glucose.
3.3.4. FINS.
Five studies are included. The results are shown in Figure 9. There is a heterogeneity as I2 = 81% and a random-effect model was used. Under a leave-one-out approach, the heterogeneity is possibly caused by the research of Hamind L and Han (Fig. 10). There is no statistically significant difference in the effect of vitamin D supplementation on FINS (MD = −0.30, 95% CI: −0.77 to 0.17, P = .21).
Figure 9.
Changes of FINS between 2 groups. FINS = fasting insulin levels.
Figure 10.
Effect sizes of FINS under leave-one-out approach. FINS = fasting insulin levels.
3.3.5. Publication bias.
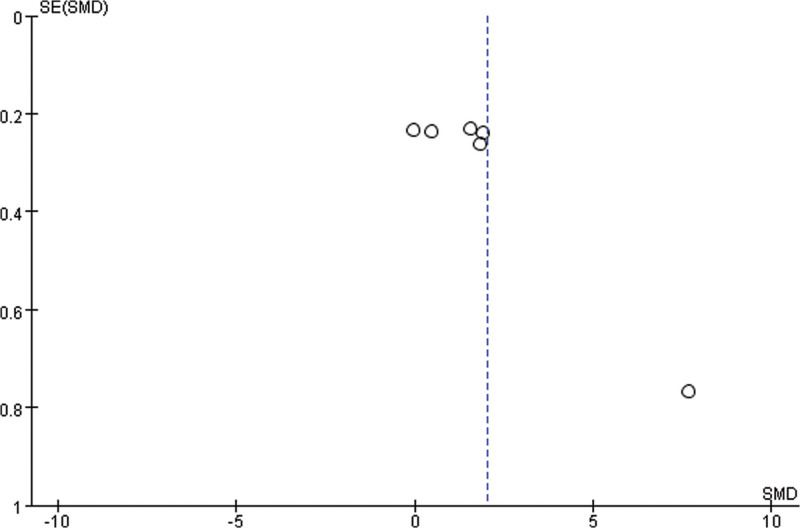
As shown in Figure 11, based on the 25(OH)D level, funnel plot is applied to evaluate the publication biases of 6 studies. It shows that the publication bias is small.
Figure 11.
Funnel plot.
4. Discussion
In this meta-analysis, vitamin D supplementation increased 25(OH)D levels significantly. However, there is no significant effect on HOMA-IR, FBG, or FINS. Also, heterogeneity exists in all indicators. In the included studies, patients in both groups have only placebo and lifestyle interventions without taking statins or any other medicines. The reason for no treatment of statins in related RCTs is probably that statins have no influence on plasma vitamin D levels.[19]
Since 25(OH)D has a half-life of approximately 3 weeks, heterogeneity within the HOMA-IR group disappears and FINS reduces when the duration of administration is prolonged (>16 weeks).[4] It suggests that vitamin D supplementation in improving IR is based on a low baseline level of vitamin D, so vitamin D sufficiency may lead to a relative insensitivity to vitamin D supplementation.[20] In addition, a recent RCT suggests that vitamin D supplementation may not improve glycemic index or anthropometry in patients with NAFLD.[21] It found that 25(OH) D levels are lower in the NAFLD group than in the control group, thus suggesting a negative correlation between 25(OH) D levels and NAFLD.[1] A meta-analysis shows that lower serum 25(OH)D concentrations (mainly in patients with vitamin D deficiency) are the only significant predictor of NAFLD.[22] These are generally consistent with the findings in this meta-analysis.
This meta-analysis focuses on assessing indexes related to glucose metabolism but not liver enzymes or lipids. Active vitamin D is excluded from this study, since supplementation of active vitamin D cannot increase circulating levels of 25(OH)D. In extrarenal tissues, free 25(OH)D metabolized by 1-alpha-hydroxylase to 1,25(OH)2D, then it binds to the vitamin D receptor to regulate gene transcription and perform physiological functions.[23]
This study has some limitations: (1) the number of populations included in the study is relatively small; (2) there is heterogeneity, which may be related to duration of dosing, route of administration and the differences within baseline levels of 25(OH)D.
5. Conclusion
Vitamin D supplementation improves 25(OH)D levels in patients with NAFLD, but there is no effect on HOMA-IR, FBG, or FINS.
Acknowledgments
The authors thank Dr Bin Wang for assistance with data extraction.
Author contributions
Conceptualization: Xuemeng Chen, Ye Zhao, Yan Zhao.
Funding acquisition: Ye Zhao, Liheng Dai.
Investigation: Xuemeng Chen.
Methodology: Liheng Dai.
Project administration: Ran Zhang.
Resources: Ye Zhao, Yan Zhao.
Supervision: Liheng Dai.
Validation: Yan Zhao, Liheng Dai.
Writing – original draft: Xuemeng Chen, Ye Zhao.
Writing – review & editing: Ran Zhang, Yan Zhao, Liheng Dai.
Abbreviations:
- 25(OH)D
- 25-hydroxy vitamin D
- CI
- confidence intervals
- FBG
- fasting blood glucose
- FINS
- fasting insulin
- HOMA-IR
- homeostasis model assessment of insulin resistance
- IR
- insulin resistance
- MD
- mean difference
- NAFLD
- nonalcoholic fatty liver disease
- RCT
- randomized controlled trial
This project was supported by the Krirk University Science Foundation for Young Scholars: 2022 (107).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Chen X, Zhao Y, Zhang R, Zhao Y, Dai L. The effect of vitamin D supplementation on some metabolic parameters in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of 8 RCTs. Medicine 2023;102:42(e35717).
References
- [1].Li L. Correlation analysis of serum 25(OH)D and non-alcoholic fatty liver in physical examiners. Inner Mongolia Med J. 2021;53:776–9. [Google Scholar]
- [2].Lian CY, Zhai ZZ, Li ZF, et al. High fat diet-triggered non-alcoholic fatty liver disease: a review of proposed mechanisms. Chem Biol Interact. 2020;330:109199. [DOI] [PubMed] [Google Scholar]
- [3].Carlsson B, Lindén D, Brolén G, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2020;51:1305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guo L, Cao J, Chen Y. A meta-analysis of the effect of vitamin D supplementation on glucose metabolism-related indicators in patients with nonalcoholic fatty liver disease. J Clin Pharmacother. 2022;20:59–65. [Google Scholar]
- [5].Yu TP, Zhao TC, Chen MY, et al. Relationship between nonalcoholic fatty liver and metabolic syndrome in adult patients with occult autoimmune diabetes mellitus. Chin Med J (Engl). 2018;98:2398–402. [DOI] [PubMed] [Google Scholar]
- [6].Armandi A, Schattenberg JM. Beyond the paradigm of weight loss in non-alcoholic fatty liver disease: from pathophysiology to novel dietary approaches. Nutrients. 2021;13:1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li X, Liu Y, Zheng Y, et al. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raza S, Tewari A, Rajak S, et al. Vitamins and non-alcoholic fatty liver disease: a molecular insight. Liver Res. 2021;5:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cordero CP, Dans AL. Key concepts in clinical epidemiology: detecting and dealing with heterogeneity in meta-analyses. J Clin Epidemiol. 2021;130:149–51. [DOI] [PubMed] [Google Scholar]
- [10].Khan MS, Li L, Yasmin F, et al. Assessment of heterogeneity in heart failure-related meta-analyses. Circ Heart Fail. 2020;13:e007070. [DOI] [PubMed] [Google Scholar]
- [11].Dai J, Xu H, Wang J, et al. Effects of vitamin D supplementation on insulin resistance and inflammatory factor levels in patients with nonalcoholic fatty liver disease. Chin Pharm. 2022;25:458–61. [Google Scholar]
- [12].Lu YW. Effect of vitamin D supplementation on insulin resistance in nonalcoholic fatty liver. Chin Foreign Med Res. 2019;17:39–41. [Google Scholar]
- [13].Han Y, Xu S, Jiang X. Effect of vitamin D supplementation on intestinal flora in patients with nonalcoholic fatty liver disease. J South China Univ Technol Nat Sci Ed. 2022;50:62–73. [Google Scholar]
- [14].Li J, Wang H, Xie Q, et al. Effects of vitamin D supplementation therapy on biochemical indices, IL-18, IL-37 and liver fibrosis and steatosis in patients with non-alcoholic steatohepatitis. J Hunan Normal Univ Med Ed. 2022;19:117–20. [Google Scholar]
- [15].Yaghooti H, Ghanavati F, Seyedian SS, et al. The efficacy of calcitriol treatment in non-alcoholic fatty liver patients with different genotypes of vitamin D receptor FokI polymorphism. BMC Pharmacol Toxicol. 2021;22:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hussain M, Iqbal J, Malik SA, et al. Effect of vitamin D supplementation on various parameters in non-alcoholic fatty liver disease patients. Pak J Pharm Sci. 2019;32(3 Special):1343–8. [PubMed] [Google Scholar]
- [17].Lorvand Amiri H, Agah S, Mousavi SN, et al. Regression of non-alcoholic fatty liver by vitamin D supplement: a double-blind randomized controlled clinical trial. Arch Iran Med. 2016;19:631–8. [PubMed] [Google Scholar]
- [18].Foroughi M, Maghsoudi Z, Ghiasvand R, et al. Effect of vitamin D supplementation on C-reactive protein in patients with nonalcoholic fatty liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- [19].Sahebkar A, Reiner Z, Simental-Mendia LE, et al. Impact of statin therapy on plasma vitamin D levels: a systematic review and meta-analysis. Curr Pharm Des. 2017;23:861–9. [DOI] [PubMed] [Google Scholar]
- [20].Zou J, Wang Y, Wang Y, et al. Research progress of vitamin D to improve insulin resistance. China Pharm. 2023;32:127–32. [Google Scholar]
- [21].Hariri M, Zohdi S. Effect of vitamin D on nonalcoholic fatty liver disease: a systematic review of randomized controlled clinical trials. Int J Prev Med. 2019;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gad AI, Elmedames MR, Abdelhai AR, et al. Efficacy of vitamin D supplementation on adult patients with non-alcoholic fatty liver disease: a single-center experience. Gastroenterol Hepatol Bed Bench. 2021;14:44–52. [PMC free article] [PubMed] [Google Scholar]
- [23].Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and ex-traskeletal actions of vitamin D: current evidence and out-standing questions. Endocr Rev. 2019;40:1109–51. [DOI] [PMC free article] [PubMed] [Google Scholar]