Significance
Major changes in the history of life are correlated with exceptional environmental change but independent tests of cause and effect have been elusive. We addressed this for Caribbean faunal turnover and extinction following isolation from the Pacific by Central American uplift which reduced coastal upwelling and planktonic productivity. These environmental changes drove predicted changes in abundance of taxa with different feeding modes and life histories based upon biological characteristics of affected taxa. But extinctions lagged by up to 2 Myr due to spatial heterogeneity of species distributions as expected from metapopulation theory. Quantitative abundance data rather than simple compilations of taxonomic occurrences are essential for basic understanding of the causes of macroevolutionary change.
Keywords: macroevolution, Isthmus of Panama, Caribbean Sea, paleoecology, extinction
Abstract
Isolation of the Caribbean Sea from the tropical Eastern Pacific by uplift of the Isthmus of Panama in the late Pliocene was associated with major, taxonomically variable, shifts in Caribbean biotic composition, and extinction, but inferred causes of these biological changes have remained elusive. We addressed this through falsifiable hypotheses about how independently determined historical changes in oceanographic conditions may have been responsible. The most striking environmental change was a sharp decline in upwelling intensity as measured from decreases in intra-annual fluctuations in temperature and consequently in planktonic productivity. We then hypothesized three general categories of biological response based upon observed differences in natural history between the oceans today. These include changes in feeding ecology, life histories, and habitats. As expected, suspension feeders and predators became rarer as upwelling declined. However, predicted increases in benthic productivity by reef corals, and benthic algae were drawn out over more than 1 Myr as seagrass and coral reef habitats proliferated; a shift that was itself driven by declining upwelling. Similar time lags occurred for predicted shifts in reproductive life history characteristics of bivalves, gastropods, and bryozoans. Examination of the spatial variability of biotic change helps to understand the time lags. Many older species characteristic of times before environmental conditions had changed tended to hang on in progressively smaller proportions of locations until they became extinct as expected from metapopulation theory and the concept of extinction debt. Faunal turnover may not occur until a million or more years after the environmental changes ultimately responsible.
Major pulses of radiation and extinction in the history of life are associated with exceptional environmental change (1, 2). But excepting extreme events such as asteroid impact or the exceptionally well-dated Permo-Triassic extinction (3), it is commonly difficult to pinpoint close stratigraphic correspondence between environmental change and the inferred biological response. An even greater challenge is to go beyond simple temporal correlations linking environmental and evolutionary change to establish independent evidence of cause and effect based on falsifiable hypotheses of how changes in environmental conditions would be expected to differentially affect organisms in terms of their specific energy requirements, feeding strategies, or life histories. This has been particularly challenging because paleontologists until recently recorded only the presence or absence of taxa through space and time rather than changes in their abundance in relation to independently measured environmental conditions from the same samples (4–6).
Isolation of the tropical Western Atlantic from the Eastern Pacific by gradual uplift of the Central American Isthmus provides a model system for understanding mechanisms of biological responses to environmental change. Late Pliocene closure of the last Central American seaways (7) caused profound changes in Caribbean oceanography and was accompanied by widespread shifts in quantitative community composition from dominance by mollusks to increases in corals and coralline algae and rise of coral reefs (8, 9). Rates of speciation and extinction of major Caribbean taxa including reef corals, mollusks, cheilostome bryozoans, fishes, and sharks also increased, in some cases involving total turnover of species (10–17). However, timing and magnitude of taxonomic replacement varied among groups and peaks in rates of taxonomic turnover commonly lagged 1 to 2 Myr after changes in Caribbean environments had stabilized (8), raising questions as to what other factors may have been responsible.
In this paper, we move beyond previous analyses of correlation to test specific hypotheses about changes in abundance and life history patterns of different ecological guilds in response to decreased planktonic productivity. We begin by synthesizing ecological differences between Caribbean and Eastern Pacific coastal ecosystems today as a frame of reference for understanding past changes in Caribbean ecosystems. We then update changes in Caribbean environmental conditions and patterns of extinction over the past 10 Myr to set the stage for testing hypotheses of expected shifts in trophic ecology and life histories among multiple higher taxa as well as changes in biogenic habitat structure associated with protracted development of coral reefs. We conclude with an analysis of spatial heterogeneity in temporal shifts in proportions of extant species of scallops and bryozoans in relation to their modes of reproduction that may help to explain the 1-to-2-Myr lag in species extinctions after oceanographic conditions had largely stabilized.
Data for this paper include a combination of previously published and original information and analyses as documented in the supplementary online data set and references (Dataset S1). Data are typically based on extensive replicate sampling of Recent and fossil Caribbean locations, principally in Panama, Costa Rica, the Dominican Republic, and Venezuela. Recent data come from dredge and grab samples from ships, whereas fossil data come from geological bulk samples from outcrops on land. To avoid bias due to patchy distributions, numerous replicate samples from the same modern location and geological samples from the same location, age, and geological stratum were grouped into ecological units which we call faunules for analysis. A faunule is a limited lithological unit in space and time, typically ~10 to 100 m in exposure length and ~1 to 10 m in stratigraphic thickness (18) with a mean chronostratigraphic resolution of ∓0.4 Myr (Dataset S1). We employed proportional abundances in our analyses to account for disparities in sampling intensity and sedimentation rates across different faunules. Absolute abundance measurements yielded comparable results, but with higher levels of noise.
Recent Environmental and Ecological Conditions across the Central American Isthmus
The Isthmus of Panama is a mere 60 km wide at its narrowest point (Fig. 1) but the Eastern Pacific and Caribbean exhibit extreme differences in physical and chemical oceanography, biological production, life history characteristics, geographic extent of ecosystems, and species richness of major taxa (Fig. 2).
Fig. 1.

Geography and oceanography of Tropical America and the Isthmus of Panama highlighting contrasting environmental conditions in the TEP and Caribbean. Ocean color reflects mean February SST from 1985 to 2009 (19), revealing the strong coastal upwelling zones activated along the Pacific coast of Central America where topography is low enough for wind-jets to form (20). In contrast, upwelling is absent in the Caribbean except in patches along the Northern coast of South America (21). Size of points indicate clusters of major sampling regions for fossil (yellow diamonds) and modern (blue circles) samples as part of the Panama Paleontology Project (See SI Appendix for data).
Fig. 2.
Major abiotic and biotic differences across the Isthmus of Panama. Seasonal upwelling drives highly variable temperatures in the TEP, whereas the Caribbean is thermally stable. Salinity is lower on average in the TEP than the Caribbean because of moisture transport from the Atlantic to the Pacific (A). Higher dissolved nutrients on the Pacific coast (B) results in trophic amplification with chlorophyll concentrations, phytoplankton and zooplankton abundances, fouling biomass from caged panels, reef fish biomass, and numbers of seabird nests increasingly higher in the Pacific compared to the Caribbean side of the Isthmus of Panama (C). The early life stages (eggs, larvae, or ovicells) of fish, scallops, arcid bivalve shells, turritelline gastropods, cheilostome bryozoans and echinoids) are all larger in the Caribbean than the TEP (D). On a regional scale, the area occupied by coral reefs and seagrasses are more that 300 and 20,000-times greater in the Caribbean, respectively. In contrast, the extent of mangrove is 2.5-times greater in the TEP (E). Regional species richness is higher in the Caribbean for stony corals, echinoids, mollusks, and fish (F). Points with error bars are means and error bars 95% CIs. Points without error bars are totals. See SI Appendix and Dataset S1 for data and sources.
The most striking physical oceanographic difference is extreme temperature variability in excess of 15 °C in the Eastern Pacific due to seasonal pulses of upwelling and interannual variability in relation to El Niño–Southern Oscillation events. In comparison, inter and intra-annual temperature variation rarely exceeds 4 °C in the Caribbean (Fig. 2A). The Eastern Pacific is also fresher (Fig. 2A) and more turbid than the characteristically salty, clear waters of the Caribbean.
Pacific upwelling increases nutrient concentrations (Fig. 2B) that support several fold greater concentrations of phytoplankton and even greater differences in abundance of zooplankton than occur in the Caribbean (Fig. 2C) where upwelling is limited to two small areas in eastern and western Venezuela and the Guajira Peninsula in easternmost Colombia (Fig. 1). These differences in planktonic productivity in turn cascade up the food web to greater rates of recruitment and biomass accumulation of encrusting organisms on the seafloor (Fig. 2C). Abundances of fish and nesting seabirds are 1 to 2 orders of magnitude greater along the Eastern Pacific coast than the Caribbean (Fig. 2C).
Patterns of reproduction are also affected. Eggs of Caribbean fishes, echinoderms, mollusks, and bryozoans are typically larger than their corresponding taxa in the Eastern Pacific (Fig. 2D), a difference that promotes more rapid larval settlement and metamorphosis that offers advantages in the comparatively nutrient-poor and unproductive Caribbean waters (22–25). Moreover, numerous Caribbean gastropods and reef corals lack planktonic development entirely and develop directly into miniature adults (26, 27).
Coral reefs and seagrass meadows dominate Caribbean coastlines, whereas Eastern Pacific reefs are small, scattered, and highly ephemeral and seagrasses are almost entirely absent (Fig. 2E). Mangroves are abundant on both coasts although more extensive in the Eastern Pacific. Total species richness of corals, mollusks, echinoderms, and fishes is also greater in the Caribbean than Eastern Pacific (Fig. 2F). But there are interesting ecological exceptions. Infaunal bivalves and high intertidal mollusks are more diverse in the Eastern Pacific, whereas epifaunal bivalves are more diverse in the Caribbean in relation to much greater development of Caribbean coral reefs (28).
Caribbean Environments and Diversity through Time
Caribbean environments and biotas were transformed over the past 6 Myr with the greatest changes including strong pulses of extinction coinciding with the final closing of Central American seaways in the late Pliocene (Fig. 3). Although most of our data are from the Central American Isthmus, the trends extended throughout the Caribbean. In particular, data on mean annual ranges in temperature (MART) include abundant previously unpublished data from the northern Dominican Republic (Fig. 3D) (SI Appendix) and coral data were compiled from the entire region (9).
Fig. 3.

Environmental change and extinction over the last 11 Myr. Pleistocene glaciation caused global eustatic sea levels (A) to fall and increasingly fluctuate in amplitude, and SST (B) to decline, substantially in the TEP but minimally in the Caribbean. Formation of the Isthmus of Panama in the Pliocene resulted in a decline in the effect of upwelling in both northern and southern Caribbean coastal waters, reflected in a reduced range of δ18O within multiserially sampled mollusks (C) and a decline in the MART estimated by intracolony variations in bryozoan zooid sizes (29) (D). In response to declining upwelling, productivity in Caribbean coastal waters collapsed between 6 and 2.8 Ma (7) and carbonate content in shelf sediments increased substantially (E). Per capita extinction rates in Caribbean bivalve genera (F), bryozoan species (G), gastropod genera (H), and coral species (I) peaked between 4 and 1 Ma (8, 30, 31). Error bars represent 95% CIs and fitted lines are Generalized Additive Models (32). Note that points in the Middle panel are jittered on the y axis. See Materials and Methods, SI Appendix and Supplementary Dataset for further information on published and previously unpublished samples and data.
Global sea level began to decline about 3 Ma (33) contributing to final isolation of the Caribbean from the Eastern Pacific (Fig. 3A). Sea surface temperatures (SST) began to decline around 6 Ma in the tropical Eastern Pacific (TEP) while remaining relatively constant in the Caribbean until roughly 3 Ma when they began to decline more slowly (Fig. 3B). However, seasonal fluctuations in Caribbean coastal environments as observed by declining intra-shell variation of mollusks in δ18O (34) (a measure of both temperature and salinity) has steadily declined (Fig. 3C). This is corroborated by an approximately 50% decline in mean annual range in temperature based upon fluctuations in size of cheilostome bryozoan zooids that decreased by half between 6 and 3 Myr and subsequently stabilized (Fig. 3D) (ref. 8 and our data, SI Appendix).
The only explanation for such an extreme drop in tropical seasonality is a sharp decline in intensity of upwelling that, by analogy to the recent, must have resulted in a corresponding drop in Caribbean planktonic productivity. This decline is well-documented basin-wide in independent sedimentological, faunal, and isotopic records from both coastal sections and open ocean sediment cores (34–39). In contrast, calcium carbonate in coastal sediments consisting primarily of calcareous algal and coral debris has progressively increased over the past 6 Myr (8) (Fig. 3E), again reflecting declining oceanic productivity and mirroring open ocean core data (33, 40).
Rates of Caribbean taxonomic extinction greatly increased between 4 and 1 Ma (8, 30, 31). Extinction of bivalves and bryozoans that are almost exclusively suspension feeders was spread evenly throughout the 3 Myr (Fig. 3 F and G). In contrast, extinction of reef corals and gastropods, which exhibit a wide variety of feeding modes, was concentrated between 3 and 1 Ma with turnovers of approximately 50 to 70% (Fig. 3 H and I).
Changes in Trophic Structure, Life Histories, and Benthic Habitats in Response to Collapse of Upwelling
Based on known ecological and life history characteristics of recent Caribbean biotas we hypothesized three broadly defined shifts in ecology and community composition in response to the collapse of Caribbean upwelling and inferred reduction in planktonic productivity. These include 1) an overall shift in trophic structure from suspension feeding upon plankton to other forms of nutrition coupled with a general collapse in large apex predators with high energetic demands, 2) decrease in larval life spans and clonal reproduction that depend on high productivity coupled with an increase in direct modes of development, and 3) increase in the extent of biogenic benthic habitats dominated by coral reefs and seagrasses.
Decline in Suspension Feeders and Predators and Increases in Alternate Modes of Feeding.
Most bivalves are suspension feeders on phytoplankton (31), and their abundance relative to other modes of nutrition sharply and significantly declined after upwelling collapsed (Fig. 4A and SI Appendix, Fig. S2). This mirrors the previously documented and striking decrease in maximum body size and growth rates of suspension feeding Caribbean oysters from the Miocene to Pliocene to Pleistocene (41).
Fig. 4.
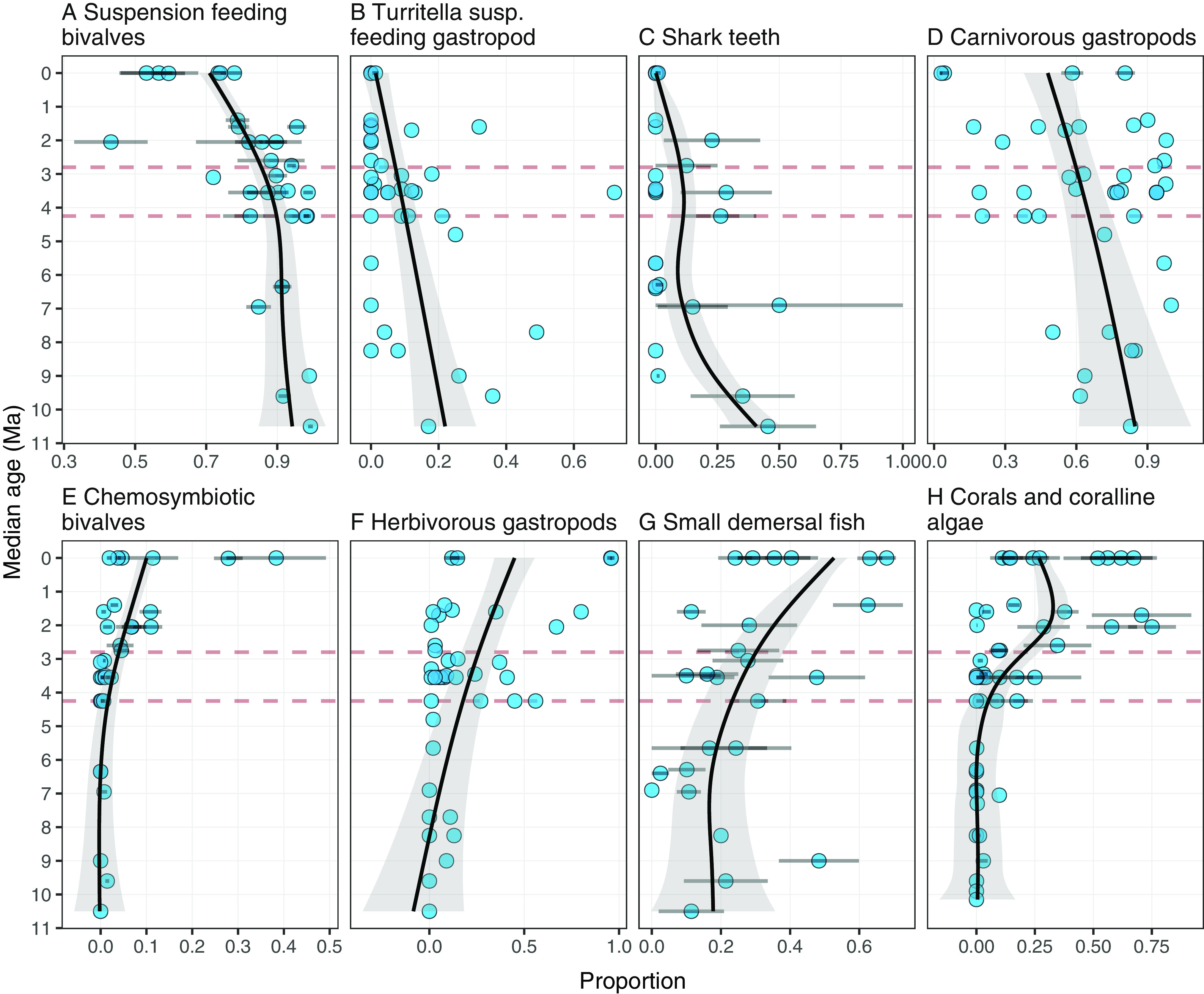
Relative abundances of different ecological groups (A–H) reveal how coastal ecosystems changed in the southwestern Caribbean over the last 11 Myr. Points represent means of proportions in geological and modern faunules and error bars 95% confidence limits. Proportions are calculated relative to their major guilds (Materials and Methods). Fitted lines are Generalized Additive Models (32). Dashed red horizontal lines represent bins used in SI Appendix, Fig. S2 for statistical analyses. See Materials and Methods, SI Appendix and Supplementary Dataset for further information on published and previously unpublished samples and data.
There was also a significant decline in abundance of suspension feeding gastropods (Fig. 4B and SI Appendix, Fig. S2). Most snails inhabiting the seafloor are predators or grazers except for turritellids that are suspension feeders (31). Typically large species of turritellids were the most abundant snails in many Miocene and Early Pliocene deposits throughout the Caribbean where they often exceeded abundance of all other mollusks combined (18, 42). But just as for suspension feeding bivalves, the proportion of turritellids decreased significantly toward the recent (Fig. 4B and SI Appendix, Fig. S2). Moreover, surviving turritellids are small compared to earlier taxa (43, 44).
The Late Miocene and Early Pliocene were also characterized by an extraordinary abundance and diversity of large-bodied and energetically demanding vertebrates including sharks and marine mammals that vanished in the Late Pliocene after the collapse in upwelling (15, 45). However, it is difficult to quantify these changes because of a lack of abundance data for osteological material. Instead, we documented the changing abundances of shark’s teeth in faunules over time. As expected, the relative abundance of shark’s teeth to other icthyoliths (principally teleost otoliths) declined significantly through time (Fig. 4C and SI Appendix, Fig. S2). The proportional abundance of predatory gastropods relative to all other gastropods also declined (31) (Fig. 4D), although not significantly (SI Appendix, Fig. S2).
In contrast to the above declines, proportional abundance of bivalves, gastropods, and fishes with different modes of feeding increased. Chemosymbiotic bivalves, that are nourished by sulfur bacteria living within their gills, became significantly more abundant after 2.8 Myr and proliferated in some, but not all, habitats in the late Pleistocene (Fig. 4E and SI Appendix, Fig. S2). Herbivorous gastropods that feed primarily on various algae and detritus also proportionally increased (Fig. 4F and SI Appendix, Fig. S2). Feeding modes of teleost fishes also shifted based upon analyses of the abundance of otoliths identified to family or genera (46) (SI Appendix). Small demersal fishes that are primarily micropredators, grazers, or deposit feeders that interact closely with the benthos (47) increased significantly relative to all other fishes in our samples (Fig. 4G and SI Appendix, Fig. S2). The most abundant of these fishes are Gobiidae and Apogonidae that were represented by >4,000 otoliths (SI Appendix).
Reef corals are nourished primarily by photosynthetic dinoflagellates within their tissues and associated calcareous algae are entirely photosynthetic. Weight percentage of reef corals and calcareous algae in bulk geological samples from the Central American isthmus and recent dredging along the isthmian coast (8) increased significantly approximately 4.25 Ma as shallow seaways between the oceans were closing (SI Appendix, Fig. S2), and then increased significantly again, another twofold-to-threefold, near the beginning of the Pleistocene ~2.8 Ma (Fig. 4H and SI Appendix, Fig. S2).
Shifts in Reproductive Mode.
Most invertebrates and fishes in strongly upwelling and highly variable environments such as the TEP produce small eggs that metamorphose into planktonic larvae that feed in the plankton for weeks to months before metamorphosis. In contrast, most species in nutrient-poor and stable environments such as the Caribbean today produce larger eggs that either metamorphose into larvae that drift for shorter intervals before metamorphosis or forgo the larval stage entirely in favor of direct maternal development of brooded young that crawl away or settle within hours to a few days of release (22–26, 48) (Fig. 2D). Asexual (clonal) development is also more common in highly productive environments (49, 50). Thus, as Caribbean upwelling and planktonic productivity declined, we expect to see a shift from predominantly Pacific to Caribbean modes of reproduction. Preliminary results to that effect have been demonstrated for Caribbean strombinid and turritelline gastropods which exhibit increase in protoconch size following oceanographic isolation from the Eastern Pacific (51–53). We tested this in greater detail for three groups with an exceptional fossil record including scallops, “strombinid” gastropods, and cupuladriid cheilostome bryozoans.
Scallops.
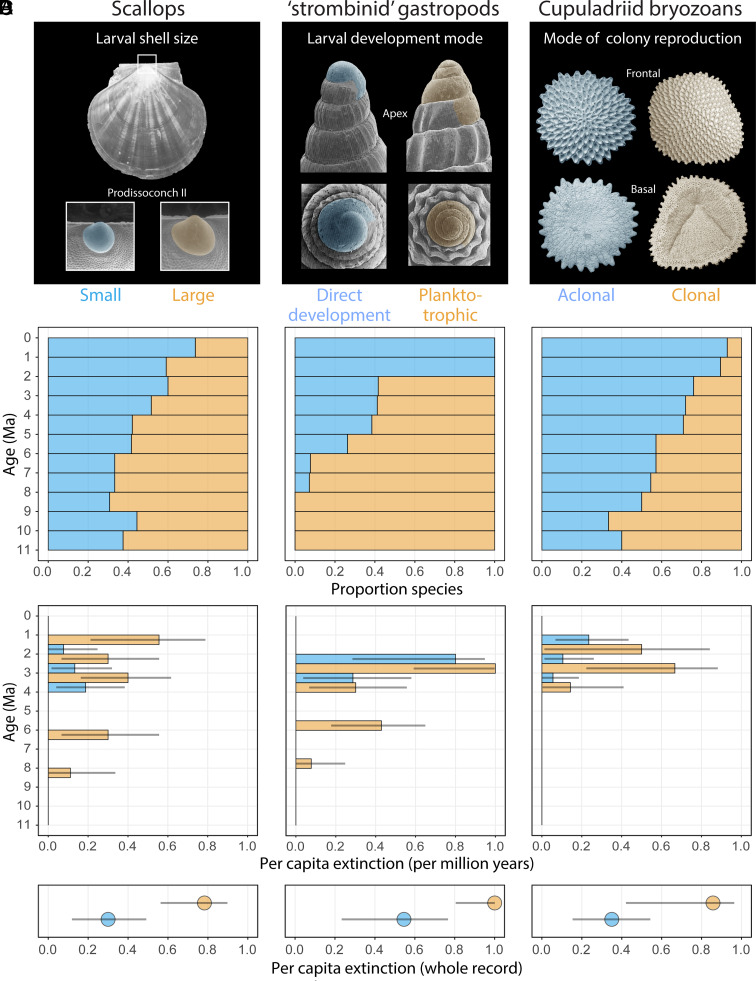
Scallops have an exceptional fossil record because their shells are composed entirely of calcite (54), which is more stable during diagenesis in comparison to most bivalves that have shells that are wholly or partially aragonitic. Caribbean scallops experienced a total turnover of species within the past 4 Myr (11). Scallops are also commercially important so their reproduction has been studied in detail in the laboratory. Egg size is closely reflected in the dimensions of the prodissoconch PI pre-larval shell (SI Appendix, Fig. S3 A and B), whereas larval swimming duration is highly positively correlated with the size of the prodissoconch PII larval shell (SI Appendix, Fig. S3 A and C). The ratio of PII size to PI size closely corresponds to differences in life histories in the two oceans today (SI Appendix, Fig. S3D). We therefore gathered PI and PII size data from fossil scallops to determine reproductive trends over the past 10 Myr (Materials and Methods and SI Appendix). Results are based primarily on PII shells because PI shells are more rarely preserved as fossils and hence patterns are harder to interpret. Scallop species whose mean PII shell size was larger than the mean of all species’ PII sizes through the entire record were assigned as “Large PII’’ species and those less than the mean were assigned “Small PII” species (Fig. 5A). As expected, the proportion of Caribbean species with smaller than average PII size increased toward the recent (Fig. 5B). This trend is due to strikingly higher extinction rates for species with larger than average PII size (Fig. 5D) that peaked in the Late Pliocene and Early Pleistocene (Fig. 5C).
Fig. 5.
Diversity, extinction, and the modes of reproduction in scallops, strombinid gastropods, and cupuladriid bryozoans in the Caribbean over the last 11 Myr. (A) Prodissoconch II size in scallops is positively related to duration of larval swimming time in the plankton and nutrient availability. Protoconchs of strombinids with less than 1.5 whorls indicate direct development, which is favored when planktonic food is low, while those with more than 3 whorls are planktotrophic, favored when planktonic food is high. Cupuladriid bryozoans can propagate clonally via fragmentation or aclonally through sexual reproduction, both of which are morphologically preserved in fossil colonies (12, 55), and clonality is favored when planktonic food availability is higher (12, 49, 50). (B) Proportional richness of species with different reproductive modes in scallop, strombinid gastropods, and cupuladriid bryozoans per million years. Per capita species-level extinction of each reproductive “mode” per million years (C) and the whole record (D). Error bars represent the inverse of the cumulative beta probability density function. Fisher’s Exact test reveals extinction levels in reproductive modes are significantly different within all taxonomic groups (P < 0.01, P < 0.01, and P < 0.05 respectively). Error bars represent binomial CIs (56). Occurrence data for all species used in this analysis and their reproductive modes is available in Supplementary Dataset.
Strombinid gastropods.
The diameter of the apical whorl on strombinid shells is correlated with the mode of development in living species (27, 51, 53, 57). Species with 1.5 whorls or less exhibit direct development of crawl-away juveniles, whereas those with three or more whorls have planktotrophic larvae (Fig. 5A). Species with intermediate numbers of whorls exhibit variable amounts of time in the plankton. We compiled published data on the occurrences of species and their mode of development derived from the number of apical whorls across the Caribbean (27, 58, 59) (SI Appendix). As expected, the proportion of species with direct development increased, reaching 100% in the past 2 Myr (Fig. 5B). This trend reflects higher extinction rates of planktotrophic species that peaked 3 to 2 Ma (Fig. 5 C and D).
Cupuladriid bryozoans.
Cupuladriids are abundant saucer-shaped cheilostomes that reproduce by both sexual (aclonal) larvae and asexual (clonal) fragmentation of adult colonies. Species that primarily adopt sexual reproduction tend to have small, robust colonies that limit colony fragmentation, while species that undergo prevalent clonal reproduction have thinner skeletons that promote fragmentation, and may adopt traits that further enhance clonality such as auto-fragmentation (55). The specific mode of reproduction of colonies is preserved in the colony’s skeleton such that sexually produced colonies retain the original zooids that form when the larva metamorphoses, while colonies that formed via clonal reproduction retain the junctures of fragmentation and clonal separation (49, 55) (Fig. 5A). Thus, the proportion of colonies formed by clonal vs. aclonal modes can be quantified in modern and fossil assemblages. Clonal reproduction is more common in productive waters (12, 49, 50), and, as expected, the proportion of Caribbean species that reproduce clonally more frequently than average declined throughout the last 10 Myr and especially the last 5 Myr (Fig. 5B), reflecting the selective loss of species that clone more than average (Fig. 5 C and D) (12).
Increase in Extent of Biogenic Habitats and Associated Taxa.
The most important shallow water biogenic habitats in the Caribbean today are mangroves, seagrasses, and coral reefs (Fig. 2) (60, 61). Corals are abundantly preserved in the fossil record (Fig. 4H). Mangrove plant remains are only sporadically preserved, but there is an excellent recent and fossil record of mangrove pollen (62). However, seagrass remains including pollen are so rare that their presence can only be inferred by occurrence of taxa commonly restricted to seagrass environments (63). For example, many chemosymbiotic lucinid bivalves are especially characteristic of sulfur-rich rhizome mats of shallow-water turtlegrass beds today (64–66).
Mangroves flourish in embayments along most tropical shores (67–69) including both the Caribbean and Pacific coasts of Central America (Fig. 2E), and this has been true along Caribbean shores for at least the last 19 Myr based on their extensive palynological record (70). In contrast, coral reefs and seagrasses are most extensive today in clear, nutrient-poor waters where planktonic productivity is low (71–74). We therefore hypothesized that Caribbean reefs and seagrasses, and their associated biota, should have increased with decreased upwelling and this is overwhelmingly the case.
We classified all of our fossil faunules as either unvegetated sediment or biogenic based upon occurrence of corals, coralline algae, and proxies for seagrasses. Recent samples were similarly classified including the presence of seagrasses and corals in dredge/grab samples. Biogenic habitats first appeared in any abundance ~5 Ma and predominated the record after approximately 2 Ma (Fig. 6A). The extent of coral reefs also greatly increased but not until the end of the Pliocene and early Pleistocene with the development of the first great Caribbean reef tracts and barrier reefs since the mass extinction of Caribbean corals at the end of the Oligocene (9) (Fig. 6B). These increases in corals and reef development account for most of the increase in weight percent carbonate at the same time (Fig. 3E).
Fig. 6.

Long-term increase in proportion of biogenic habitats in the Caribbean. Biogenic faunules increased in the SWC over the last 5 Myr (A), coral reefs proliferated 2 to 0 Ma (B) (9), epifaunal bivalves increased in relative abundance (C), and the proportion of muricid to naticid gastropods rose sharply (D). Points represent means of proportions in geological and modern faunules and error bars 95% confidence limits. Fitted lines are Generalized Additive Models (32).
Shallow-water seagrasses also increased based on indirect evidence. This includes the rise in chemosymbiotic bivalves (Fig. 4E) and the striking absence of large lucinid bivalves such as Codakia and Phacoides from our collections until the Late Pleistocene (18, 75).
The proportion of epifaunal bivalves that are characteristic of hard substrates and seagrass beds relative to infaunal bivalves more characteristic of soft sediments also increased significantly after 4.25 Ma and again after 2.8 Ma (Fig. 6C and SI Appendix, Fig. S2). Even among scallops, all of which are epifaunal, the proportional abundance of species that live byssally attached to hard substrates increased relative to species that live unattached on soft sediments such as Argopecten that dominated Caribbean scallop faunas for more than 6 Myr before becoming uncommon in the Pleistocene (11). Similar changes occurred for predatory muricid and naticid gastropods that drill through bivalve shells to eat their flesh. Muricids predominate on hard substrates, whereas naticids favor soft sediments (76), and, as expected, our analyses show that the proportion of muricids increased significantly relative to naticids in parallel with increased coral reef development after 2.8 Ma (Fig. 6D and SI Appendix, Fig. S2).
Time Lags in Extinction Following Collapse in Upwelling and Planktonic Productivity
Most of the decline in Caribbean seasonality and upwelling occurred before about 3 Ma as the path between the seas became severely constricted and then closed (Fig. 3), but many of the most pronounced biological changes including major pulses of extinction did not occur until after 3 Ma and especially in the Early Pleistocene (Figs. 3–6). These include, most strikingly, the proportional increase in abundance of corals and coralline algae (Fig. 4H) and the index of coral reef development and associated taxa (Fig. 6 B–D), as well as intense pulses of coral species and gastropod generic extinction between 2 and 1 Ma Fig. 3 H and I).
These time lags between physical environmental change and inferred biological responses raise questions as to what other physical factors might also have played a role? SST continued their gradual and slight decline and seasonality and carbonate deposition had largely stabilized after 3 Ma (Fig. 3 C–E). Increased Pleistocene fluctuations in sea level and especially during the last 1 Myr have been linked to the rise of acroporid corals whose exceptionally rapid growth rates and propensity for reproduction by fragmentation made them the most important reef builders on modern reefs (77). This proliferation of acroporids helps to explain the remarkable explosion of Caribbean reef development over the past 1.5 Myr (Fig. 6B), but no convincing mechanism linking sea level and the other biotic changes can be constructed, particularly given the observed selective shifts in diets and life histories (Figs. 4 and 5) that would be unaffected by sea level.
Instead, increasing heterogeneity of benthic habitats appears to have been an important factor, but to understand this, we need to distinguish between the initial consequences of the decline in Caribbean upwelling and primary productivity associated with the closure of the isthmian seaways and its subsequent prolonged effects. Clearer, nutrient-poor waters favored development of biogenic habitats that gradually and profoundly changed the character of Caribbean coastal environments (76). Importantly, the rise of seagrass meadows and coral reefs increased regional habitat heterogeneity evidenced by the transition from exclusively soft sediment faunules prior to 5 Ma and the subsequent increasing frequency of biogenic faunules thereafter (Fig. 6A).
Changes in proportions of different habitats are important to the metapopulation dynamics of species. All species exist as spatially disjunct metapopulations with high abundance in some locations and low abundance or absence in others (78). Species persistence depends on the number and size of locations occupied, the rate of colonization of new locations, and the rate of extinction in locations already occupied. Changes in these parameters in turn depend on the life history characteristics of the species. For example, decrease in the number of favorable locations may favor species A with greater colonizing ability even if it is an inferior competitor with another species B that colonizes new habitats more slowly. Thus, over time, any location may be occupied by A alone, by B alone, by both A and B, or neither species may be present. But when the number of suitable locations falls below some critical threshold, the poorer colonizer species B will eventually go extinct even if it occurs abundantly at some locations when the habitat change occurs (79). Ecologists call this extinction debt (80).
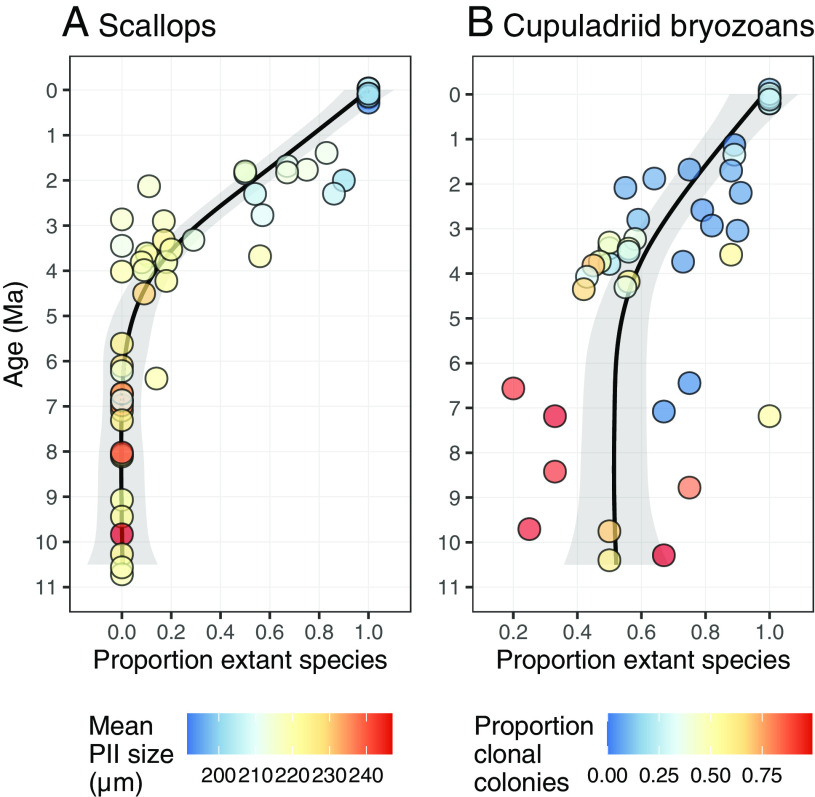
We can explore these ideas in relation to turnover of species of scallops and cupuladriid bryozoans for which we have abundant quantitative occurrence data throughout the past 11 Myr (Fig. 7 and SI Appendix, Fig. S5). We have already seen that scallop species with larger than average prodissoconchs (longer swimming times in the plankton) suffered higher extinction rates than species with shorter swimming times in the plankton (Fig. 5A). Average PII size in different sampled locations was consistently larger than 220 μ until about 4 Ma (Fig. 7A) when many of the species with larger than average PII size became extinct (Fig. 5C). But afterward, until about 1.5 Ma, some locations were dominated by species with relatively large PII larval shells (pale orange to yellow faunules in Fig. 7A) while other faunules of the same approximate age were dominated by species with smaller PII larval shells (pale yellow to pale blue faunules in Fig. 7A). Proportions of extant species in different faunules during these nearly 3 Myr ranged from zero to 90%. All of the species with larger than average planktonic larvae were doomed by 4 to 3 Ma when planktonic productivity had collapsed, but many of them hung on for another 2 Myr until the proportion of faunules in which they occurred dropped below some critical level.
Fig. 7.
Proportion per faunule of extant species of scallops (A) and cupuladriid bryozoans (B). The color of each faunule represents the mean size of the Prodissoconch II by abundance of shells in scallops, and the proportion of colonies that exhibit clonal reproduction in cupuladriids. See Materials and Methods for further information.
Cupuladriid bryozoans display a similar pattern over time in terms of the extent of clonal fragmentation exhibited by different species (Fig. 7B). But the underlying story is more complex because some species exhibited great plasticity in reproductive mode in response to declining planktonic productivity. There are three patterns. Some species primarily reproduced by clonal fragmentation up until they became extinct between 4 and 2 Ma. Other species that have survived switched from predominantly clonal to predominantly sexual reproduction as planktonic productivity declined, and still others that originated during the final closure of the seaway exhibited predominantly sexual reproduction from the start (12). On a per-faunule basis, clonal reproduction ranged from 50 to 100% until 5 Ma. Between 5 and 3 Ma, contemporaneous faunules ranged widely from about 0 to 75% clonally produced colonies. After 3 Ma no faunule had more than 30% of clonally produced colonies—a pattern that persists in the modern Caribbean (SI Appendix, Fig. S4) (49). During this time of shifting dominance in the mode of reproduction, the proportion of extant species within faunules ranged widely from 40 to 100% before the ultimate extinctions occurred (Fig. 7B). This reflects the prolonged survival of predominantly clonal species that eventually became extinct but hung on in ever-dwindling numbers and geographic distribution for as long as 2 Myr (12).
There was therefore a period characterized by highly variable modes of reproduction in both scallops and cupuladriids. This prolonged 2 to 3 Myr shift began when Caribbean upwelling effectively ceased and ended with the ultimate extinctions.
Evolution and Environment Revisited
Major shifts in the composition of Caribbean nearshore communities over the past 11 Myr closely correspond with biological predictions based upon the decline in upwelling and associated planktonic primary productivity caused by isolation of the Caribbean from the Eastern Pacific (8). But different taxa responded very differently to environmental change. For example, per capita extinctions of suspension feeding bryozoans and bivalves and predatory sharks began to increase 4 Ma in keeping with oceanographic change (Fig. 3 F and G). But increased extinction among reef corals and gastropods occurred 1.5 to 2 Myr after oceanographic seawater conditions had stabilized (Fig. 3 H and I).
Analysis of changes in proportional abundance helps to resolve this paradox. All of the trends in Fig. 4 continued well after the interval of oceanographic change between 4.25 and 2.8 Ma, and the proportional abundance in six of the eight ecological guilds changed significantly after 2.8 Ma (SI Appendix, Fig. S2). The same was true for epifaunal bivalves and the ratio of muricid to naticid gastropods (Fig. 6 C and D and SI Appendix, Fig. S2). The case of reef corals is especially striking because species diversity progressively increased throughout the Late Pliocene before the mass extinction that eliminated two thirds of all Caribbean coral species at the beginning of the Pleistocene, just as coral abundance was greatly increasing (Fig. 4H).
The key point is that faunal turnover was not instantaneous. This is especially striking for the rise of Acropora palmata and Acropora cervicornis that originated nearly 6 Ma (81) but did not become dominant reef builders until the Lower Pleistocene about 2 Ma, the same time as the mass extinction of roughly two thirds of all Caribbean reef coral species (9, 17, 82). Moreover, acroporid dominance continued to increase until the recent collapse of both Acropora species due to overfishing, pollution, disease, and climate change (83, 84).
An even greater time lag occurred in the appearance of the molluscan assemblages characteristic of shallow water turtlegrass environments that are dominated today by a unique assemblage of mostly lucinid chemosymbiotic bivalves including the genera Codakia, Lucina, and Parvilucina, as well as Diplodonta, among others (64, 65). All of these except Parvilucina are rare or absent in the Caribbean fossil record until the Upper Pleistocene 125 Ka (Fig. 4E) (10, 85, 86).
Both of these ecological revolutions were precipitated by the oceanographic changes associated with the rise of the Panamanian isthmus but occurred long afterward because increases in extent of biogenic habitats and the rise of their associated biotas continued long after physical environmental conditions had stabilized.
Materials and Methods
Environmental and Ecological Conditions across the Isthmus Today.
Caribbean and TEP surface temperature and salinity data used in Fig. 2A were from refs. 87–89. Caribbean salinity (n = 59,054), Caribbean temperature (n = 41,905), TEP salinity (n = 114,090), TEP temperature (n = 114,090) (Dataset S1). Dissolved nutrients, chlorophyll a concentrations, and phytoplankton and zooplankton abundances were derived from weekly sampling of waters in Caribbean and Pacific coasts of the Isthmus of Panama from October 1993 to December 1996 reported in ref. 90. Fouling biomass was the average wet weight that accumulated in 3 mo on numerous caged panels lowered into shallow water on coastal habitats on either side of the Isthmus of Panama from ref. 91. Fish biomass was taken from standardized Reef Life Surveys (92) and includes both ray-finned fish and elasmobranchs. Total seabird nests along the entire Caribbean and Pacific coasts of Panama by ref. 93. Published data on the sizes of early life stages of extant species in oceans across the Isthmus of Panama include fish species egg sizes (23), scallop species prodissoconch I sizes (11), arcid bivalve species egg sizes (25), protoconch sizes in turritelline gastropod species (52), ovicell sizes (a proxy for egg size) in cheilostome Bryozoa species (94), and echinoid species egg volumes (22) in the wider Caribbean and TEP. Greater Caribbean coral reef area from Burke and Maidens (95) and Miloslavich et al. (61). TEP (southern Mexico to Ecuador) coral reef area from ref. 96. Caribbean seagrass area from Jackson (97) and Miloslavich et al. (61). Estimated TEP (southern Mexico to Ecuador) seagrass area from ref. 96. TEP Mangrove area from World Database on Protected Areas (98) was calculated as the sum of the three regions: Southern Mesoamerican Pacific Mangroves, South American Pacific Mangroves and Northern Mesoamerican Pacific Mangroves (7,874, 13,551 and 8,212 km2 respectively). Greater Caribbean Mangrove area was from Wilkie et al. (99). Species numbers in the Greater Caribbean and TEP were taken from refs. 61 and 100–106. See Dataset S1.
Late Neogene to Modern Tropical American Ocean and Coastal Environmental Changes and Caribbean Extinctions.
Global eustatic sea levels (Fig. 3A) were taken from ref. 107. Corrected SST (Fig. 3B) in the Caribbean and TEP were derived from Alkenone, faunal analyses, Mg/Ca ratios, TEX86, and UK’37 proxies from refs. 108–113. Compiled data and further information are presented in Dataset S1.
The mean range of δ18O in serially sampled mollusks in Caribbean faunules (Fig. 3C) was compiled from 173 shells reported in refs. 34, 42, and 114–117. MART (Fig. 3D) was estimated from zooid size variation in 396 bryozoan colonies (29, 118–120). MARTs from the southwestern Caribbean are from ref. 8 while northern Caribbean (Honduras, Jamaica, Dominican Republic, and Puerto Rico), and southern Caribbean (Venezuela) are all previously unpublished data. Mean carbonate content in nearshore shelf fossil bulk and modern grab sediments (Fig. 3D) was measured following methods in ref. 8. Dataset S1 contains compiled δ18O range data, all MART data, percent CaCO3 in sediment data, and information on ages of faunules.
Per capita extinction rates in Caribbean coral species, gastropod genera, bivalve genera, and bryozoan species (Fig. 3 F–I) were taken from refs. 8, 12, 30, and 121.
Late Neogene to Modern Caribbean Coastal Ecological Changes.
Change and variation in the relative abundances of different ecological groups in the southwestern Caribbean over the last 11 Myr were compiled using bulk samples collected from geological faunules (see previous description) (18) and dredge and grab samples from modern faunules (essentially an ecosystem of shared depth, oceanic conditions and sediment characteristics, typically on the order of 100 to 1,000 m in size). Faunule ages are listed in Dataset S1. Both modern and fossil samples were washed to 2 mm and all skeletal remains picked and identified to family, genera or species, assigned to ecological groups to calculate mean proportions of different ecological groups through time. Abundances of suspension feeding bivalves (Fig. 4A), chemosymbiotic bivalves (Fig. 4E), and epifaunal bivalves (Fig. 6C) were calculated as valve counts proportional to all bivalves by faunule (75). Turritella (Fig. 4B), carnivorous gastropods (Fig. 4D), and herbivorous gastropods (Fig. 4F) calculated as shell counts proportional to all other mollusk genera (18) and previously unpublished data. Muricids to naticid data (Fig. 6D) calculated as the proportional counts of gastropod shells in the families Muricidae to those in Naticidae (18) and previously unpublished data. Amounts of coral and coralline algae were weights relative to all other skeletal remains of heterotrophic guilds (including mollusks, sea urchins, bryozoans, crustaceans, fish, etc) (8). Numbers of small demersal fish were calculated as the number of fish otoliths in that functional group proportional to all other icthyoliths (otoliths and shark teeth) and shark teeth were calculated as numbers of teeth proportional to all other icthyoliths (i.e. otoliths) (46). Molluscan life habits and feeding modes were derived from refs. 31 and 122. Otoliths assigned functional groups from ref. 123. Faunules and formations were assigned as either “biogenic” (typically formed by reef or seagrass accumulations) or “soft sediment” (typically constructed by siliciclastic sediments) (Fig. 6A). Caribbean reef growth (Fig. 6B) was calculated as the proportion of 126 faunules across the Greater Caribbean that had extensive coral reef development. Data in Fig. 3 are from ref. 9.
To test for statistical differences through time, samples were binned into the following three time bins based on the median ages of faunules; (a) >4.25 Ma (defined by ref. 8 as the start of declining upwelling in the southwestern Caribbean), (b) 4.25 to 2.8 Ma, and (c) <2.8 Ma defined as the time of final isolation of the Caribbean from the TEP (7). Differences in proportions across all three time bins were compared using χ2 Kruskal Wallace from the package “ggstatsplot” (124) in R and results reported in SI Appendix, Fig. S2, which also shows which time bins were significantly different for each other. Ecological data used in Figs. 4 and 6 and SI Appendix, Fig. S2 are available in Dataset S1. Code used to produce χ2 Kruskal Wallace tests is in SI Appendix.
Reproductive Life Histories, Extinction, and Per-Faunule Changes.
Published and previously unpublished records of scallop (11, 125, 126), strombinid (27, 58, 59), and cupuladriid species (127–129) occurrences were used to calculate species richness, first occurrences and last occurrences and estimate extinction levels in the Caribbean from 11 to 0 Ma (Fig. 5). For each species in each group, metrics on mode of reproduction were gathered. These include the PI and PII larval shell size in scallops, mode of reproduction in strombinids, and the proportion of clonal to aclonal colonies in cupuladriids. To explore selectivity in extinction, we assigned each species a dominant mode of reproduction (large/small PII in scallops, direct/planktotrophic in strombinids, and clonal/aclonal in cupuladriids). We could then calculate per capita extinction levels within different modes of reproduction for each group per 1 Myr and for the entire record (11 to 0 Ma) using first and last occurrences of each species. Binomial CIs (56) were calculated at alpha = 0.05 (Fig. 5). First and last occurrences of all species in each group and their dominant mode of reproduction are listed in Dataset S1. See SI Appendix for more information on data and analyses.
For scallops and cupuladriid bryozoans, we compiled the numbers of individuals and their PII sizes and proportion of clonal colonies by abundance on a per-faunule basis. We could then plot changes in modes of reproduction through time and in the context of the proportion of extant species per faunule (Fig. 7).
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
Anthony G. Coates was instrumental in documenting the geological and sedimentological context of the fossils used in this paper. His friendship and generous support of countless students and researchers involved in the Panama Paleontology Project is greatly missed. Ira Rubinoff provided critical encouragement, free ship time, field vehicles, and technician support from the Smithsonian Tropical Research Institute throughout the early phases of our research. We especially thank Travis Smith for his extensive support in the field and sharing unpublished data from his PhD thesis on the scallops of Tropical America. Felix Rodriguez supported fieldwork and supervision of all of the processing of biological and paleontological samples in Panama. Seth Finnegan kindly allowed us to use unpublished mollusk data. Dozens of people have given critical support to the Panama Paleontology and Project and related studies. We especially acknowledge the support of Alan Cheetham, Amalia Herrera, Brigida de Gracia, Ethan Grossman, Helena Fortunato, Jill Leonard-Pingel, JoAnn Sanner, Kenneth Johnson, Laurel Collins, and Orangel Aguilera. We also thank Andrew Fernandez, Betzy Rovero, Carmen Schlöder, Catalina Pimiento, crew of the R/V Urraca, Dick Norris, Divya Saxena, Dolores Piperno, Ebgert Leigh, Eldredge Bermingham, Erin Dillon, Evelyn York, Gabriel Jacome, Graciela Quijano, Harilaos Lessios, Javier Jara, Jessica Lueders-Dumont, Jon Cybulski, Jorge Morales, Kimberli García, Lauren Graniero, Lisa Levin, Marcos Alvarez, Mark Torchin, Maybelline Ureña, Milton Solano, Nagayasu Nakanishi, Nancy Budd, Nancy Knowlton, Nicholas Pyenson, Owen McMillan, Phil Hastings, Plinio Gondola, Richard Cooke, Suzanne Williams, Urania Gonzalez and Yadixa del Valle. Finally, we wish to express our gratitude to the three reviewers: Douglas H. Erwin, Geerat J. Vermeij, and Rachel A. Wood, for their valuable comments and prompt handling of our manuscript. This study was supported by the Sistema Nacional de Investigadores (SENACYT), NSF (BSR90-06523, DEB93-00905, DEB96-96123, DEB97-05289, EAR03-45471, and EAR13-25683).
Author contributions
J.B.C.J. and A.O. designed research; performed research; analyzed data; and wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: D.H.E., Smithsonian National Museum of Natural History; G.J.V., University of California Davis; and R.A.W., University of Edinburgh.
Contributor Information
Jeremy B. C. Jackson, Email: jjackson@amnh.org.
Aaron O’Dea, Email: odeaa@si.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Zachos J., Pagani M., Sloan L., Thomas E., Billups K., Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Knoll A. H., Life on a Young Planet (Princeton University Press, 2015). [Google Scholar]
- 3.Shen S.-Z., et al. , A sudden end-Permian mass extinction in South China. GSA Bulletin 131, 205–223 (2019). [Google Scholar]
- 4.McKinney F. K., Lidgard S., Sepkoski J. J. Jr., Taylor P. D., Decoupled temporal patterns of evolution and ecology in two post-Paleozoic clades. Science 281, 807–809 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Wagner P. J., Kosnik M. A., Lidgard S., Abundance distributions imply elevated complexity of post-Paleozoic marine ecosystems. Science 314, 1289–1292 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Jackson J. B. C., Erwin D. H., What can we learn about ecology and evolution from the fossil record? Trends Ecol. Evol. 21, 322–328 (2006). [DOI] [PubMed] [Google Scholar]
- 7.O’Dea A., et al. , Formation of the Isthmus of Panama. Sci. Adv. 2, e1600883 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Dea A., et al. , Environmental change preceded Caribbean extinction by 2 million years. Proc. Natl. Acad. Sci. U.S.A. 104, 5501–5506 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson K. G., Jackson J. B. C., Budd A. F., Caribbean reef development was independent of coral diversity over 28 million years. Science 319, 1521–1523 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Jackson J. B., Jung P., Coates A. G., Collins L. S., Diversity and extinction of Tropical American mollusks and emergence of the Isthmus of Panama. Science 260, 1624–1626 (1993). [DOI] [PubMed] [Google Scholar]
- 11.Smith J. T., Travis Smith J., Jackson J. B. C., Ecology of extreme faunal turnover of tropical American scallops. Paleobiology 35, 77–93 (2009). [Google Scholar]
- 12.O’Dea A., Jackson J., Environmental change drove macroevolution in cupuladriid bryozoans. Proc. Biol. Sci. 276, 3629–3634 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrillo-Briceño J. D., Carrillo J. D., Aguilera O. A., Sanchez-Villagra M. R., Shark and ray diversity in the Tropical America (Neotropics)-an examination of environmental and historical factors affecting diversity. PeerJ 6, e5313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domning D. P., Sirenians, seagrasses, and Cenozoic ecological change in the Caribbean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 166, 27–50 (2001). [Google Scholar]
- 15.Pimiento C., et al. , The Pliocene marine megafauna extinction and its impact on functional diversity. Nat. Ecol. Evol. 1, 1100–1106 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Jackson J. B. C., Cheetham A. H., Phylogeny reconstruction and the tempo of speciation in cheilostome Bryozoa. Paleobiology 20, 407–423 (1994). [Google Scholar]
- 17.Budd A. F., Johnson K. G., Origination preceding extinction during late Cenozoic turnover of Caribbean reefs. Paleobiology 25, 188–200 (1999). [Google Scholar]
- 18.Jackson J. B. C., Todd J., Fortunato H., Jung P., “Diversity and assemblages of Neogene Caribbean mollusca of lower Central America” in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, Collins L. S., Coates A. G., Eds. (Bulletins of American Paleontology, 1999), pp. 193–230. [Google Scholar]
- 19.Saha K., et al. , AVHRR Pathfinder version 5.3 level 3 collated (L3C) global 4km sea surface temperature for 1981-present (2018) 10.7289/V52J68XX. [DOI]
- 20.O’Dea A., Hoyos N., Rodríguez F., Degracia B., De Gracia C., History of upwelling in the Tropical Eastern Pacific and the paleogeography of the Isthmus of Panama. Palaeogeogr. Palaeoclimatol. Palaeoecol. 348–349, 59–66 (2012). [Google Scholar]
- 21.Rueda-Roa D. T., Muller-Karger F. E., The southern Caribbean upwelling system: Sea surface temperature, wind forcing and chlorophyll concentration patterns. Deep Sea Res. Part I: Oceanogr. Res. Pap. 78, 102–114 (2013). [Google Scholar]
- 22.Lessios H. A., Adaptation and phylogeny as determinants of egg size in echinoderms from the two sides of the Isthmus of Panama. Am. Nat. 135, 1–13 (1990). [Google Scholar]
- 23.Robertson D. R., Collin R., Inter- and Intra-specific variation in egg size among reef fishes across the Isthmus of Panama. Front. Ecol. Evol. 2, 84 (2015). [Google Scholar]
- 24.Levitan D. R., Optimal egg size in marine invertebrates: Theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. Am. Nat. 156, 175–192 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Moran A. L., Egg size evolution in tropical American arcid bivalves: The comparative method and the fossil record. Evolution 58, 2718–2733 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Richmond R. H., Hunter C. L., Reproduction and recruitment of corals: Comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 60, 185–203 (1990). [Google Scholar]
- 27.Fortunato H., Reproductive strategies in gastropods across the Panama seaway. Invertebr. Reprod. Dev. 46, 139–148 (2004). [Google Scholar]
- 28.Leigh E. G., O’Dea A., Vermeij G. J., Historical biogeography of the Isthmus of Panama. Biol. Rev. Camb. Philos. Soc. 89, 148–172 (2014). [DOI] [PubMed] [Google Scholar]
- 29.O’Dea A., Okamura B., Intracolony variation in zooid size in cheilostome bryozoans as a new technique for investigating palaeoseasonality. Palaeogeogr. Palaeoclimatol. Palaeoecol. 162, 319–332 (2000). [Google Scholar]
- 30.Jackson J. B. C., Johnson K. G., Life in the last few million years. Paleobiology 26, 221–235 (2000). [Google Scholar]
- 31.Todd J. A., et al. , The ecology of extinction: Molluscan feeding and faunal turnover in the Caribbean Neogene. Proc. R. Soc. London. Series B: Biol. Sci. 269, 571–577 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickham H., “Programming with ggplot2” in ggplot2: Elegant Graphics for Data Analysis, Wickham H., Ed. (Springer International Publishing, 2016), pp. 241–253. [Google Scholar]
- 33.Bartoli G., et al. , Final closure of Panama and the onset of northern hemisphere glaciation. Earth Planet. Sci. Lett. 237, 33–44 (2005). [Google Scholar]
- 34.Grossman E. L., et al. , Freshwater input, upwelling, and the evolution of Caribbean coastal ecosystems during formation of the Isthmus of Panama. Geology 47, 857–861 (2019). [Google Scholar]
- 35.Keller G., Zenker C. E., Stone S. M., Late Neogene history of the Pacific-Caribbean gateway. J. South Am. Earth Sci. 2, 73–108 (1989). [Google Scholar]
- 36.Ortega-Ariza D., Franseen E. K., Boudagher-Fadel M. K., Effects of sea level and upwelling on development of a Miocene shallow-water tropical carbonate ramp system, Ponce, Puerto Rico. J. Sediment. Res. 91, 1227–1256 (2021). [Google Scholar]
- 37.Huguet C., Jaeschke A., Rethemeyer J., Paleoclimatic and palaeoceanographic changes coupled to the Panama Isthmus closing (13–4 Ma) using organic proxies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 601, 111139 (2022). [Google Scholar]
- 38.Steph S., et al. , Early Pliocene increase in thermohaline overturning: A precondition for the development of the modern equatorial Pacific cold tongue. Paleoceanography 25, PA2202 (2010). [Google Scholar]
- 39.Karatsolis B.-T., Lougheed B. C., De Vleeschouwer D., Henderiks J., Abrupt conclusion of the late Miocene-early Pliocene biogenic bloom at 4.6-4.4 Ma. Nat. Commun. 13, 353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haug G. H., Tiedemann R., Effect of the formation of the Isthmus of Panama on Atlantic Ocean thermohaline circulation. Nature 393, 673–676 (1998). [Google Scholar]
- 41.Kirby M. X., Jackson J. B. C., Extinction of a fast-growing oyster and changing ocean circulation in Pliocene tropical America. Geology 32, 1025–1028 (2004). [Google Scholar]
- 42.Anderson B. M., Hendy A., Johnson E. H., Allmon W. D., Paleoecology and paleoenvironmental implications of turritelline gastropod-dominated assemblages from the Gatun Formation (Upper Miocene) of Panama. Palaeogeogr. Palaeoclimatol. Palaeoecol. 470, 132–146 (2017). [Google Scholar]
- 43.Pietsch C., Gigliotti M., Anderson B. M., Allmon W. D., Patterns and processes in the history of body size in turritelline gastropods, Jurassic to recent. Paleobiology, 1–21 (2023). [Google Scholar]
- 44.Andersson B. M., Allmon W., High calcification rates and inferred metabolic trade-offs in the largest turritellid gastropod, Turritella abrupta (Neogene). Palaeogeogr. Palaeoclimatol. Palaeoecol. 544, 109623 (2020). [Google Scholar]
- 45.Aguilera O., de Aguilera D. R., An exceptional coastal upwelling fish assemblage in the Caribbean Neogene. J. Paleontol. 75, 732–742 (2001). [Google Scholar]
- 46.Aguilera O., Rodrigues de Aguilera D., “Bathymetric distribution of Miocene to Pleistocene Caribbean teleostean fishes from the coast of Panama and Costa Rica” in A Paleobiotic Survey of Caribbean Faunas from the Neogene of the Isthmus of Panama, Collins L. S., Coates A. G., Eds. (Bulletins of American Paleontology, 1999), pp. 251–269. [Google Scholar]
- 47.Mora C., Ecology of Fishes on Coral Reefs (Cambridge University Press, 2015). [Google Scholar]
- 48.Jackson J. B. C., Modes of dispersal of clonal benthic invertebrates: Consequences for species’ distributions and genetic structure of local populations. Bull. Mar. Sci. 39, 588–606 (1986). [Google Scholar]
- 49.O’Dea A., Herrera-Cubilla A., Fortunato H., Jackson J. B. C., Life history variation in cupuladriid bryozoans from either side of the Isthmus of Panama. Mar. Ecol. Prog. Ser. 280, 145–161 (2004). [Google Scholar]
- 50.O’Dea A., Asexual propagation in the marine bryozoan Cupuladria exfragminis. J. Exp. Mar. Bio. Ecol. 335, 312–322 (2006). [Google Scholar]
- 51.Jackson J. B. C., Jung P., Fortunato H., “Paciphilia revisited: Transisthmian evolution of the Strombina group (Gastropoda: Columbellidae)” in Evolution and Environment in Tropical America, Jackson J. B. C., Jung P., Fortunato H., Budd A. F., Eds. (University of Chicago Press, Chicago, 1996), pp. 234–270. [Google Scholar]
- 52.Sang S., Friend D. S., Allmon W. D., Anderson B. M., Protoconch enlargement in Western Atlantic turritelline gastropod species following the closure of the Central American Seaway. Ecol. Evol. 9, 5309–5323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fortunato H., “Reproduction and larval development of the Strombina-group (Buccinoidea: Columbellidae) and related gastropods: Testing the use of the larval shell for inference of development in fossil species” in Systematics, Phylogeny and Biology of Neogastropoda, Oliverio M., Chemello R., Eds. (Bolletino Malacologico, 2002), suppl. 4, pp. 111–126. [Google Scholar]
- 54.Waller T. R., “The functional significance of some shell microstructures in the Pectinacea (Mollusca: Bivalvia)” in International Geological Congress, 24th Session, Montreal, Canada, Gill J. E., Goodwin A. M., Wynne-Edwards H. R., Eds. (International Geological Congress, 1972), vol. 7, pp. 48–56. [Google Scholar]
- 55.O’Dea A., Jackson J. B. C., Taylor P. D., Rodriguez F., Modes of reproduction in recent and fossil cupuladriid bryozoans. Palaeontology 51, 847–864 (2008). [Google Scholar]
- 56.Wilson E. B., Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22, 209–212 (1927). [Google Scholar]
- 57.Jablonski D., Lutz R. A., Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol. Rev. Camb. Philos. Soc. 58, 21–89 (1983). [Google Scholar]
- 58.Fortunato H., Phylogenetic relationships of the columbellid taxa Cotonopsis and Cosmioconcha (Neogastropoda: Buccinoidea: Columbellidae). Am. Mal. Bull. 23, 33–42 (2007). [Google Scholar]
- 59.Houbrick R. S., A new Strombina species (Gastropoda: Prosobranchia) from the tropical western Atlantic. Proc. Biol. Soc. Wash. 96, 349–354 (1983). [Google Scholar]
- 60.Harborne A. R., et al. , The functional value of Caribbean coral reef, seagrass and mangrove habitats to ecosystem processes. Adv. Mar. Biol. 50, 57–189 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Miloslavich P., et al. , Marine biodiversity in the Caribbean: Regional estimates and distribution patterns. PLoS One 5, e11916 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellison J. C., Long-term retrospection on mangrove development using sediment cores and pollen analysis: A review. Aquat. Bot. 89, 93–104 (2008). [Google Scholar]
- 63.Reich S., Di Martino E., Todd J. A., Wesselingh F. P., Renema W., Indirect paleo-seagrass indicators (IPSIs): A review. Earth Sci. Rev. 143, 161–186 (2015). [Google Scholar]
- 64.Jackson J. B. C., The ecology of the molluscs of Thalassia communities, Jamaica, West Indies. II. Molluscan population variability along an environmental stress gradient. Mar. Biol. 14, 304–337 (1972). [Google Scholar]
- 65.Jackson J. B. C., Ecology of mollusks of Thalassia communities, Jamaica, West-Indies. 1. distribution, environmental physiology, and ecology of common shallow-water species. Bull. Mar. Sci. 23, 313–350 (1972). [Google Scholar]
- 66.Taylor J. D., Coral reef and associated invertebrate communities (mainly molluscan) around Mahé, Seychelles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 254, 129–206 (1968). [Google Scholar]
- 67.Rützler K., Feller I. C., Caribbean mangrove swamps. Sci. Am. 274, 94–99 (1996).8643952 [Google Scholar]
- 68.Krauss K. W., et al. , Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 89, 105–127 (2008). [Google Scholar]
- 69.Feller I. C., et al. , Biocomplexity in mangrove ecosystems. Ann. Rev. Mar. Sci. 2, 395–417 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Jaramillo C. A., et al. , “Palynological record of the last 20 million years in Panama” in Paleobotany and Biogeography: A Festschrift for Alan Graham in His 80th Year, Stevens W. D., Montiel O. M., Raven P., Eds. (Missouri Botanical Garden Press, St. Louis, 2014), pp. 134–253. [Google Scholar]
- 71.McGlathery K. J., Macroalgal blooms contribute to the decline of seagrass in nutrient-enriched coastal waters. J. Phycol. 37, 453–456 (2001). [Google Scholar]
- 72.Birkeland C., Others, nutrient availability as a major determinant of differences among coastal hard-substratum communities in different regions of the tropics. UNESCO Rep. Mar. Sci. 46, 45–97 (1987). [Google Scholar]
- 73.Muscatine L., Porter J. W., Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27, 454–460 (1977). [Google Scholar]
- 74.Stoddart D. R., Ecology and morphology of recent coral reefs. Biol. Rev. Camb. Philos. Soc. 44, 433–498 (1969). [Google Scholar]
- 75.Leonard-Pingel J. S., Jackson J. B. C., O’Dea A., Changes in bivalve functional and assemblage ecology in response to environmental change in the Caribbean Neogene. Paleobiology 38, 509–524 (2012). [Google Scholar]
- 76.Leonard-Pingel J. S., Jackson J. B. C., Drilling predation increased in response to changing environments in the Caribbean Neogene. Paleobiology 42, 394–409 (2016). [Google Scholar]
- 77.Renema W., et al. , Are coral reefs victims of their own past success? Sci. Adv. 2, e1500850 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanski I., Metapopulation Ecology (OUP Oxford, 1999). [Google Scholar]
- 79.Nee S., May R. M., Dynamics of metapopulations: Habitat destruction and competitive coexistence. J. Anim. Ecol. 61, 37–40 (1992). [Google Scholar]
- 80.Tilman D., May R. M., Lehman C. L., Nowak M. A., Habitat destruction and the extinction debt. Nature 371, 65–66 (1994). [Google Scholar]
- 81.Budd A. F., Klaus J. S., Johnson K. G., Cenozoic diversification and extinction patterns in caribbean reef corals: A review. Paleontol. Soc. Pap. 17, 79–93 (2011). [Google Scholar]
- 82.Klaus J. S., McNeill D. F., Budd A. F., Coates A. G., Neogene reef coral assemblages of the Bocas del Toro region, Panama: The rise of Acropora palmata. Coral Reefs 31, 191–203 (2012). [Google Scholar]
- 83.Pandolfi J. M., Jackson J. B. C., Ecological persistence interrupted in Caribbean coral reefs. Ecol. Lett. 9, 818–826 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Cramer K. L., et al. , Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6, eaax9395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rehder H. A., The pleistocene mollusks of grand Cayman Island, with notes on the geology of the Island. J. Paleontol. 36, 583–585 (1962). [Google Scholar]
- 86.Noble R. S., Curran H. A., Wilson M. A., Paleoenvironmental and paleoecologic analyses of a Pleistocene mollusc-rich lagoonal facies, San Salvador Island, Bahamas. Geol. Soc. Am. Spec. Pap. 300, 91–104 (1995). [Google Scholar]
- 87.Paton S., Robertson R., SST_San Blas (2019). 10.25573/data.10068065.v1 (12 February 2023). [DOI]
- 88.Paton S., Bay of Panama water quality monitoring project, site 1 of 7 (2021). 10.25573/data.10042703.v2 (12 February 2023). [DOI]
- 89.Paton S., Bocas and Galeta water quality 2002-05 (2022). 10.25573/data.19111301.v2 (12 February 2023). [DOI]
- 90.D’Croz L., Robertson D. R., “Coastal oceanographic conditions affecting coral reefs on both sides of the Isthmus of Panama” in Proceedings of the 8th International Coral Reef Symposium, Lessios H. A., Macintyre I. G., Eds. (Smithsonian Tropical Research Institute, Balboa, Panama, 1997), vol. 2, pp. 2053–2058. [Google Scholar]
- 91.Torchin M. E., et al. , Asymmetry of marine invasions across tropical oceans. Ecology 102, e03434 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Edgar G. J., Stuart-Smith R. D., Systematic global assessment of reef fish communities by the reef life survey program. Sci. Data 1, 140007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kushlan J. A., Angehr G. R., Hines K., Seabirds and other colonial waterbirds of the Caribbean coast of Panama. J. Caribbean Ornithol. 30, 145–153 (2017). [Google Scholar]
- 94.Jackson J. B. C., Herrera Cubilla A., “Adaptation and constraint as determinants of zooid and ovicell size among encrusting ascophoran cheilostome Bryozoa from opposite sides of the Isthmus of Panama” in Proceedings of the 11th International Bryozoology Association Conference. Smithsonian Tropical Research Institute, Panamá, Herrera-Cubilla A., Jackson J. B. C., Eds. (Smithsonian Tropical Research Institute, Balboa, Panama, 2000), pp. 249–258. [Google Scholar]
- 95.Burke L. M., Maidens J., Reefs at Risk in the Caribbean (World Resources Institute (WRI), Washington, DC, 2004). [Google Scholar]
- 96.Lyons M., Larsen K., Skone M., CoralMapping/AllenCoralAtlas: DOI for paper at ~ v1.3 (2022), 10.5281/zenodo.6622015. [DOI]
- 97.Jackson J. B. C., Reefs since Columbus. Coral Reefs 16, S23–S32 (1997). [Google Scholar]
- 98.World Database on Protected Areas. Choice Reviews Online 47, 47–2935 (2010). [Google Scholar]
- 99.Wilkie M. L., Fortuna S.; Food and Agriculture Organization of the United Nations. Forestry Department, Forest Resources Assessment Programme, Status and Trends in Mangrove Area Extent Worldwide (Forestry Department, Food and Agriculture Organization of the United Nations, 2003). [Google Scholar]
- 100.Alvarado J. J., Echinoderm diversity in the Caribbean Sea. Mar. Biodivers. 41, 261–285 (2010). [Google Scholar]
- 101.Alvarado J. J., Solís-Marín F. A., Ahearn C. G., Echinoderm (Echinodermata) diversity in the Pacific coast of Central America. Mar. Biodivers. 40, 45–56 (2009). [Google Scholar]
- 102.Robertson D. R., Van Tassell J., Shorefishes of the greater Caribbean: Online information system (Version 2.0, Smithsonian Tropical Research Institute, Balboa, Panama, 2019). Accessed 4 April 2022. [Google Scholar]
- 103.Robertson D. R., Allen G. R., Shorefishes of the tropical Eastern Pacific: Online information system (Version 2.0, Smithsonian Tropical Research Institute, Balboa, Panama, 2015). Accessed 4 April 2022. [Google Scholar]
- 104.Allen G. R., Robertson D. R., Fishes of the Tropical Eastern Pacific (University of Hawaii Press, 1994). [Google Scholar]
- 105.Cortés J., et al. , “Marine biodiversity of eastern tropical pacific coral reefs” in Coral Reefs of the Eastern Tropical Pacific, Enochs I. C., Glynn P. W., Manzello D. P., Eds. (Springer, Netherlands, 2017), pp. 203–250. [Google Scholar]
- 106.Glynn P. W., et al. , “Eastern pacific coral reef provinces, coral community structure and composition: An overview” in Coral Reefs of the Eastern Tropical Pacific, Enochs I. C., Glynn P. W., Manzello D. P., Eds. (Springer, Netherlands, 2017), pp. 107–176. [Google Scholar]
- 107.Miller K. G., et al. , The Phanerozoic record of global sea-level change. Science 310, 1293–1298 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Seki O., et al. , Alkenone and boron-based Pliocene pCO2 records. Earth Planet. Sci. Lett. 292, 201–211 (2010). [Google Scholar]
- 109.Martinez J. I., Ignacio Martinez J., Mora G., Barrows T. T., Paleoceanographic conditions in the western Caribbean Sea for the last 560 kyr as inferred from planktonic foraminifera. Mar. Micropaleontol. 64, 177–188 (2007). [Google Scholar]
- 110.Schmidt M. W., Vautravers M. J., Spero H. J., Western Caribbean sea surface temperatures during the late quaternary. Geochem. Geophys. Geosyst. 7, Q02P10 (2006), 10.1029/2005GC000957. [DOI] [Google Scholar]
- 111.Sosdian S. M., et al. , Constraining the evolution of Neogene ocean carbonate chemistry using the boron isotope pH proxy. Earth Planet. Sci. Lett. 498, 362–376 (2018). [Google Scholar]
- 112.O’Brien C. L., et al. , High sea surface temperatures in tropical warm pools during the Pliocene. Nat. Geosci. 7, 606–611 (2014). [Google Scholar]
- 113.Zhang Y. G., Pagani M., Liu Z., A 12-million-year temperature history of the tropical Pacific Ocean. Science 344, 84–87 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Bemis B. E., Geary D. H., The usefulness of bivalve stable isotope profiles as environmental indicators: Data from the eastern Pacific Ocean and the southern Caribbean Sea. Palaios 11, 328–339 (1996). [Google Scholar]
- 115.Key M. M. Jr., Hollenbeck P. M., O’Dea A., Patterson W. P., Stable isotope profiling in modern marine bryozoan colonies across the Isthmus of Panama. Bull. Mar. Sci. 89, 837–856 (2013). [Google Scholar]
- 116.Tao K., Robbins J. A., Grossman E. L., O’Dea A., Quantifying upwelling and freshening in Nearshore tropical American environments using stable isotopes in modern gastropods. Bull. Mar. Sci. 89, 815–835 (2013). [Google Scholar]
- 117.Teranes J. L., et al. , “The oxygen isotopic record of seasonality in Neogene bivalves from the Central American Isthmus” in Evolution and Environment in Tropical America, Jackson J. B. C., Budd A. F., Coates A. G., Eds. (University of Chicago Press, Chicago, IL, 1996), pp. 105–129. [Google Scholar]
- 118.O’Dea A., Seasonality and zooid size variation in Panamanian encrusting bryozoans. J. Mar. Biol. Assoc. U. K. 83, 1107–1108 (2003). [Google Scholar]
- 119.Okamura B., Bishop J. D. D., Zooid size in cheilostome bryozoans as an indicator of relative palaeotemperature. Palaeogeogr. Palaeoclimatol. Palaeoecol. 66, 145–152 (1988). [Google Scholar]
- 120.O’Dea A., Jackson J. B. C., Bryozoan growth mirrors contrasting seasonal regimes across the Isthmus of Panama. Palaeogeogr. Palaeoclimatol. Palaeoecol. 185, 77–94 (2002). [Google Scholar]
- 121.Cheetham A. H., Sanner J., Jackson J. B. C., Metrarabdotos and related genera (Bryozoa: Cheilostomata) in the late Paleogene and Neogene of tropical America. J. Paleontol. 81, 1–91 (2007). [Google Scholar]
- 122.Todd J., Jackson J., Johnson K., Fortunato H., “Plio-Pleistocene faunal turnover in the Caribbean: Molluscan food webs implicate plummeting nutrient supply” in GSA Annual Meeting Abstracts with Programs (Geological Society of America, 2001), vol. 33, p. 1811. [Google Scholar]
- 123.Froese R., Pauly D., Fishbase as a tool for comparing the life history patterns of flatfish. Neth. J. Sea Res. 32, 235–239 (1994). [Google Scholar]
- 124.Patil I., Visualizations with statistical details: The “ggstatsplot” approach. J. Open Source Softw. 6, 3167 (2021). [Google Scholar]
- 125.Smith J. T., Jackson J. B. C., Fortunato H., Diversity and abundance of tropical American scallops (Bivalvia: Pectinidae) from opposite sides of the central American Isthmus. Veliger 48, 26–45 (2006). [Google Scholar]
- 126.Smith J. T., “Ecology and environments of an extreme faunal turnover in topical American scallops,” PhD thesis, University of California, San Diego: (2006). [Google Scholar]
- 127.Herrera-Cubilla A., Dick M. H., Sanner J., Jackson J. B. C., Neogene Cupuladriidae of tropical America. II: Taxonomy of recent Discoporella from opposite sides of the Isthmus of Panama. J. Paleontol. 82, 279–298 (2008). [Google Scholar]
- 128.Herrera-Cubilla A., Jackson J. B. C., Phylogeny of genus Cupuladria (Bryozoa, Cheilostomata) in the Neogene of tropical America. J. Paleontol. 88, 851–894 (2014). [Google Scholar]
- 129.Herrera-Cubilla A., Dick M. H., Sanner J., Jackson J. B. C., Neogene Cupuladriidae of Tropical America. I: Taxonomy of recent Cupuladria from opposite sides of the Isthmus of Panama. J. Paleontol. 80, 245–263 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All study data are included in the article and/or supporting information.