Significance
The regulation of sleep has been a central question in neurobiology of the past decades. Lack of sleep seems to not only affect brain function but has effects on other organs, suggestive of functional interplay between brain and the periphery during sleep. In this study, we probe the involvement of the blood–brain barrier (BBB), which controls molecular exchanges between the blood and the brain, in Drosophila sleep. We find that BBB permeability is fine-tuned to an animal’s sleep status: Sleep deprivation (SD) causes defined increases in BBB permeability occurring irrespective of the mode of SD, while lowering an animal’s sleep pressure homeostatically restores BBB integrity. Our data suggest that cellular and molecular BBB status is a biomarker for sleep.
Keywords: sleep, blood-brain-barrier, GPCR
Abstract
Sleep is vital for most animals, yet its mechanism and function remain unclear. We found that permeability of the BBB (blood–brain barrier)—the organ required for the maintenance of homeostatic levels of nutrients, ions, and other molecules in the brain—is modulated by sleep deprivation (SD) and can cell-autonomously effect sleep changes. We observed increased BBB permeability in known sleep mutants as well as in acutely sleep-deprived animals. In addition to molecular tracers, SD–induced BBB changes also increased the penetration of drugs used in the treatment of brain pathologies. After chronic/genetic or acute SD, rebound sleep or administration of the sleeping aid gaboxadol normalized BBB permeability, showing that SD effects on the BBB are reversible. Along with BBB permeability, RNA levels of the BBB master regulator moody are modulated by sleep. Conversely, altering BBB permeability alone through glia-specific modulation of moody, gαo, loco, lachesin, or neuroglian—each a well-studied regulator of BBB function—was sufficient to induce robust sleep phenotypes. These studies demonstrate a tight link between BBB permeability and sleep and indicate a unique role for the BBB in the regulation of sleep.
Sleep is conserved across many animal phyla (1–3), but its physiological function and the facilitation of its rapid onset are not yet fully understood. Sleep is considered vital because prolonged sleep deprivation (SD) is lethal across different animal species and in most paradigms (4–7). Studies examining the function and regulation of sleep yielded several genetic and neuronal correlates that, if perturbed, affect sleep duration and other sleep parameters. As different neurons, brain regions, peripheral tissues, genes, and cell types have been implicated in sleep, it remains unclear how the observed phenomena are linked and, together, create sleep.
The fruit fly Drosophila melanogaster displays hallmarks of sleep that are similar to those of mammals (8, 9), and this insect has been used as a genetically tractable model organism to unravel the molecular and physiological bases of sleep (10, 11). Studies of sleep regulation in the fly have taken either a genetic or a neurobiological approach (11). The latter has uncovered different brain regions and neuronal subtypes that are involved in sleep, including subpopulations of clock neurons, the mushroom body, and dorsal fan-shaped bodies (12), which all promote or inhibit sleep via neurotransmitter systems that are largely evolutionarily conserved and modulated by the time of day, oxidative status, temperature, light information, feeding, and age (13–16). Still, how the different brain regions and neuronal signaling pathways interact has yet to be elucidated. Forward genetic screens in Drosophila yielded mutants that sleep less, but the affected genes do not follow an obvious molecular pattern and encode products that fall into different protein classes, including ion transport, neurotransmitter systems, cell cycle regulation, proteasomal degradation, circadian clock, and various intracellular signaling pathways (11).
Recent advances regarding the function of sleep have uncovered that sleep is required for many physiological processes in mammals as well as in insects, including glymphatic clearance, suppression of motor activity, reduction of oxidative stress, memory consolidation, and synaptic homeostasis (4, 17–24). However, the relative importance and potential coordination of these processes are not well understood. Sleep appears to not only be regulated by and target the brain, as studies link peripheral tissues such as the gut and muscle as fundamental participants in the process of sleep. How the brain integrates signals from the body and communicates with the periphery is unclear.
Research in the last 5 to 10 y uncovered dynamics of tissues, organs, and physiological processes at the interface of brain and body. Studies in mammals and flies revealed changes of cerebrospinal fluid, blood flow in the brain, extracellular ion composition, and xenobiotic blood–brain barrier (BBB) transport during sleep/wake cycles or in a time-of-day dependent fashion. These dynamics seem to involve neuronal signaling, astrocytic water transport, and circadian rhythms in the BBB (17, 25–29). As the organ required for brain homeostasis and preserving the metabolic and ionic microenvironment required for neuronal function (30), the BBB could be serving a prime role in regulating molecular exchange affecting sleep, which is the subject of this study.
Transport mechanisms across the BBB include paracellular diffusion as well as receptor, carrier, adsorptive-mediated, and lipophilic transcytosis (31, 32). We focus on the most basic mechanism, the paracellular, passive diffusion pathway formed by tight junction belts between endocytes or, in invertebrates, septate junction belts formed by subperineurial glia (33) limiting transport between blood and brain to water-soluble molecules <500 Dalton.
To better understand the interaction of BBB dynamics and sleep, we employed acute or chronic/genetic forms of SD and measured BBB permeability using a previously described tracer dye assay (34–36). We found that the BBB dynamically reflects sleep need in a genotype-dependent manner. We then sought to investigate how the BBB regulates sleep on a cellular level and found that modulating components of a known BBB function pathway directly affects sleep, demonstrating that the BBB is an integral part of sleep regulation.
Results
Acute SD, Regardless of Method, Reversibly Increases BBB Permeability.
Studies in mice and rats have shown that acute and chronic, genetic SD alter aspects of BBB integrity including disrupting tight junctions and leading to the detachment of pericytes, a cell type located on the outside of capillaries, from the microvessel (37, 38). The mammalian and fly BBBs share functional and molecular similarities, but also crucial differences. Drosophila does not have a closed circulatory system; therefore, the BBB is located on the outside of the central and peripheral nervous systems. While some of the junctional proteins are conserved between invertebrates and vertebrates, the barrier is formed by specialized glia and not endocytes in the fly. The fly lacks the complexity of additional cell types including muscle, astrocytes, and pericytes forming the mammalian BBB (39). Sleep, too, encompasses shared and divergent aspects across invertebrate and mammalian model organisms. Similarities include the hallmarks of sleep—different sleep posture, decreased responsiveness to external stimuli, circadian and homeostatic regulation, and altered brain activity. Differences include the absence of a distinct sleep architecture in flies, which includes Rapid Eye Movement (REM) and non-REM sleep in mammals, and differences in the underlying genetic and neuronal correlates (40, 41).
To investigate the role of the BBB in sleep regulation and homeostasis using the fly model system, we first sought to understand whether SD alters BBB permeability in Drosophila, given the similarities and differences between mammalian and Drosophila sleep regulation and BBB morphology. To test the hypothesis that SD causes an increase in BBB permeability, we used three different methods of acute SD—activation of wake-promoting dopaminergic and octopaminergic neurons as well as mechanical stimulation—and subsequently monitored BBB permeability. To measure BBB permeability we employed tracer injection assays (Fig. 1A), which are routinely used to assess BBB permeability in invertebrate and mammalian model systems (34, 35, 42, 43). We used the tracer Dextran TexasRed 10 kDa, which does not significantly penetrate the BBB in wild-type flies [(34, 36), Fig. 1B], but has been shown to penetrate the BBB under conditions of BBB disruption (35, 36).
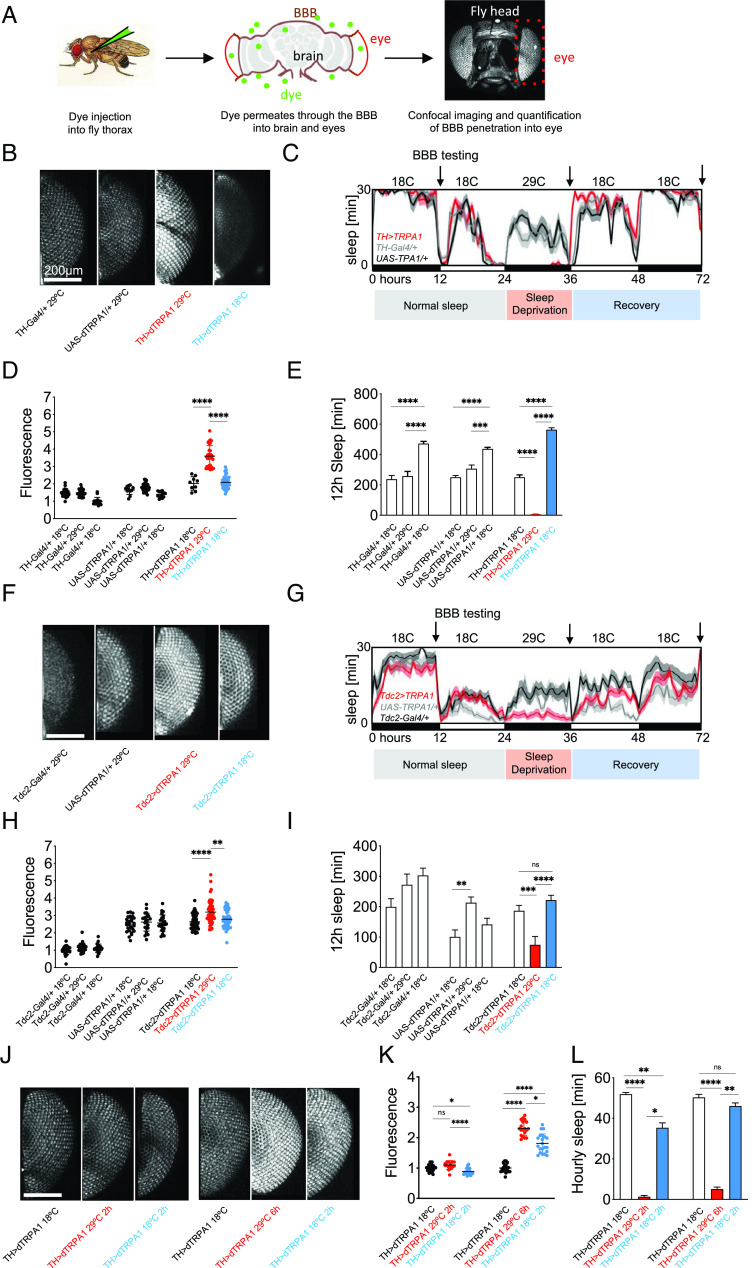
Fig. 1.
Neurogenic SD reversibly increases BBB permeability. (A) Workflow illustration of the BBB injection assay. Drosophila males are injected with 10kDa Dextran dye. Dye penetration into the brain and eyes depend on BBB permeability. Flies are decapitated, and heads are imaged using confocal microscopy. Fluorescence in each eye is quantified as a measure of dye penetration across the BBB. (B) Flies were sleep deprived for one night using thermogenetic activation of dopaminergic neurons (TH>dTRPA1) at 29 °C and injected with 10 kDa Dextran-TexasRed, red, or allowed to recover sleep for 24 h at 18 °C before injection and imaging, blue. Hemizygous controls are in black. Fluorescence intensity in the eye shows increased BBB permeability after SD but closure after sleep recovery. (C) Sleep plot for thermogenetic SD. TH>TRPA1 flies and heterozygous controls were sleep deprived for 12 h at 29 °C and injected the next morning or left to recover lost sleep at 18 °C for 24 h before injection the next day. Average sleep in 16 flies is shown, binned as sleep in 30 min. Arrows denote timepoints of BBB testing shown in (B). (D) Quantification of BBB permeability in (B). Each point represents normalized fluorescence in one eye, n = 30 to 40; red, sleep-deprived flies; blue, flies after recovery sleep; black, hemizygous controls. (E) Quantification of sleep data in (C). Shown is total sleep in the 12 h prior to injection for normal sleep, SD and recovery groups for the indicated genotypes. n = 16; red, sleep-deprived flies; blue, flies after recovery sleep; black, hemizygous controls. (F) Flies were sleep deprived for one night using thermogenetic activation of octopaminergic neurons (Tdc2>dTRPA1) at 29 °C and injected with 10 kDa Dextran-TexasRed, red, or additionally allowed to recover for 24 h at 18 °C before injection and imaging, blue. Hemizygous controls are in black. Fluorescence intensity in the eye shows increased BBB permeability after SD, but closure after recovery. n = 30 to 40; red, sleep-deprived flies; blue, flies after recovery sleep; black, hemizygous controls. (G) Sleep plot for octopaminergic SD. Tdc2>TRPA1 flies and heterozygous controls were sleep deprived for 12 h at 29 °C and injected the next morning or left to recover lost sleep at 18 °C for 24 h before injection the next day. Average sleep in 16 flies is shown, binned as sleep in 30 min. Arrows denote timepoints of BBB testing shown in (F). (H) Quantification of BBB permeability in (F). Each point represents normalized fluorescence in one eye, n = 30 to 40; red, sleep-deprived flies; blue, flies after recovery sleep; black, hemizygous controls. (I) Quantification of sleep data in (G). Shown is total sleep in 12 h prior to injection for normal sleep, SD and recovery groups for the indicated genotypes. n = 16; red, sleep-deprived flies; blue, flies after recovery sleep; black, hemizygous controls. (J) Flies were sleep deprived for 2 h or 6 h using thermogenetic activation of dopaminergic neurons (TH>dTRPA1) and injected with 10 kDa Dextran-TexasRed, red, or allowed to recover sleep for 2 h before injection and imaging, blue. Controls are in black. Fluorescence intensity in the eye shows increased BBB permeability after 6 h but not 2 h of SD and partial closure after 2 h sleep recovery. (K) Quantification of BBB permeability in (J). Each point represents normalized fluorescence in one eye, n = 30 to 40; red, sleep-deprived flies; blue, flies after recovery sleep; black, controls. (L) Hourly sleep for the indicated groups from (J)/(K) during the day before SD, during SD, and during the 2 h of recovery sleep for the indicated genotypes. n = 16; red, sleep-deprived flies; blue, flies after recovery sleep; black, controls. Statistical significance was calculated using one-way ANOVA with Dunn’s post hoc testing. Significance levels are P < 0.05: *, <0.01: **, <0.001: ***, <0.0001: ****. SD: Sleep deprivation.
Dopaminergic SD is highly effective and causes reversible increases in BBB permeability.
Thermogenetic dopaminergic hyperexcitation using TH-Gal4 to drive expression of the thermosensitive trpA1 channel in arousal-promoting dopamine neurons results in complete sleep loss [(44), Fig. 1 C and E]. After 12 h of dopaminergic SD, the flies showed a ~1.7-fold increase in BBB permeability compared with temperature-matched controls (Fig. 1 B and D). TRPA1 was inactivated by shifting the flies from 29 °C to 18 °C (Fig. 1 C and E), and the flies were allowed to recover sleep for 24 h before BBB permeability was measured. BBB integrity was restored to baseline permeability (Fig. 1 B and D). These results indicate that sleep loss–induced BBB opening is a dynamic and reversible physiological process.
SD–induced changes in BBB permeability are dose-dependent.
Considering the processes seemed so dynamic and quick, we experimented with different amounts of SD and sleep recovery time. We used dopaminergic SD to sleep-deprive animals for 2 h or 6 h (Fig. 1L) and either immediately probed their BBB permeability or let them recover sleep for another 2 h before BBB testing (Fig. 1 J and K). Two hours of dopaminergic SD did not cause a significant increase of BBB permeability; however, a ~2.3-fold increase was observed after 6 h of SD, suggesting that SD causes BBB permeability increases in a dose-dependent manner. The BBB partially closed again when flies were allowed to recover sleep for 2 h, compared with flies who were injected immediately after 6 h of SD. This shows that the BBB closes rapidly after SD-induced opening, but only after sleep is recovered.
Mechanical SD increases BBB permeability.
To confirm that BBB opening was an effect of SD, and not merely an effect of the method of SD, we also tested other methods of SD. We employed mechanical SD during the night using mild randomized shaking (45). No effect on BBB permeability was observed after one night of mechanical SD (Fig. 2 C and D), which might be due to the lower efficiency of mechanical SD when compared to the very strong and potentially somewhat artificial SD activating all dopaminergic neurons (4, 7, 44, 46). However, two and three consecutive nights of mechanical SD caused a BBB permeability increase of ~1.5-fold and ~1.8-fold, respectively (Fig. 2 C and D). Similar to dopaminergic SD (Fig. 1), a 24-h period when flies were allowed to recover sleep was sufficient to fully reverse the mechanical SD-induced BBB permeability increase (Fig. 2 C and D). This reversal remained stable after 3 and 6 d post SD (Fig. 2 C and D), which provides evidence that mechanical SD did not cause lasting effects on BBB permeability.
Fig. 2.
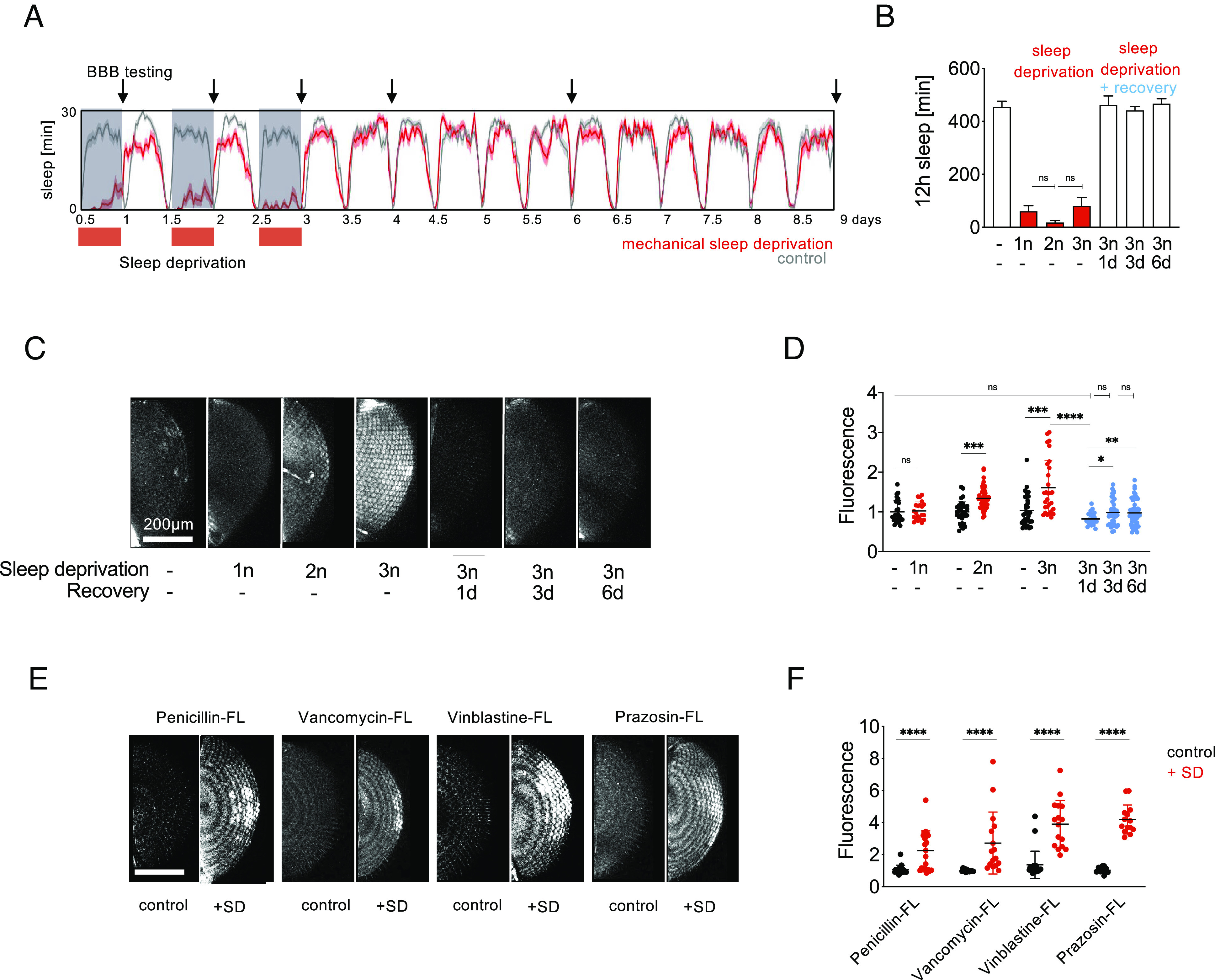
Mechanical SD reversibly increases BBB permeability and improves drug penetration into the brain. (A) Sleep plot for mechanical SD. Wild-type flies were sleep deprived using a custom-built machine by randomized shaking for 2x2 s every 5 m for 1, 2, or 3 subsequent nights (n) during their normal sleep time between ZT 12–24, plotted in red, and left to recover for up to 6 d. Average sleep in 16 flies is shown, binned as sleep in 30 min. Arrows denote timepoints of BBB testing shown in (C). (B) Quantification of sleep data in (A). Shown is total sleep in 12 h prior to injection for each group. n = 16. Statistical significance was calculated using one-way ANOVA with Dunn’s post hoc testing. Red, sleep-deprived flies. —| indicates comparison to 1n SD. (C) Flies were sleep deprived as shown in (A) and injected with 10 kDa Dextran-TexasRed to assess BBB permeability after 1, 2, or 3 nights of SD or let to recover sleep for 1, 3, or 6 d prior to BBB testing. (D) Quantification of BBB permeability in (C). n = 30 to 40. Red, sleep-deprived flies; blue, flies after recovery sleep; black, controls. Statistical significance was calculated using t tests for pairwise and one-way ANOVA with Dunn’s post hoc testing for multiple comparisons. —| indicates comparisons to non-sleep-deprived flies. (E) Three nights of SD increase BBB penetration of fluorescein-labeled penicillin, vancomycin, vinblastine, and prazosin. Flies were mechanically sleep deprived or left to sleep during Zeitgeber time 12 to 24 h for three consecutive nights and injected with fluorescently labeled bioactive drugs the next morning for BBB assessment. (F) Quantification of BBB permeability in (E). n = 30 to 40. Red, sleep-deprived flies; black, controls. For quantification of BBB permeability, fluorescence was measured for both eyes of each fly. Each dot represents normalized fluorescence in one eye. Statistical significance was calculated using t tests. Significance levels are P < 0.05: *, <0.01: **, <0.001: ***, <0.0001: **. SD: sleep deprivation.
Octopaminergic SD increases BBB permeability.
Activation of octopaminergic neurons has been shown to be arousal-promoting, similar to dopaminergic activation. However, in contrast to dopaminergic and mechanical SD methods, octopaminergic activation causes deficiencies in sleep homeostasis and the flies do not exhibit rebound sleep [Fig. 1 G and I; (47)]. We observed that after 12 h of octopaminergic SD, the BBB permeability increase was attenuated (~1.15-fold increase of BBB permeability, Fig. 1 F and H) when compared to dopaminergic SD (~1.7-fold Fig. 1D) but was still significant, despite the less efficient SD (Fig. 1 G and I), suggesting that BBB responses to SD are affected by, but not completely precluded by, a lack of rebound sleep.
Drug penetration into the brain is increased after SD.
The BBB poses a major obstacle to treatment of brain pathologies, because 100% of large molecules (>0.5 kDa), like the brain cancer drug vinblastine (molecular weight: 1 kDa), and 98% of small molecule drugs (<0.5 kDa), like the nonsteroidal antiinflammatory drug paracetamol, cannot penetrate it in therapeutic amounts (48). Current solutions to this problem include pharmacologically increasing the diffusion barrier permeability, implantation of ultrasonic devices to temporarily permeabilize the diffusion barrier, fusing molecules to receptor ligands to engage the transcellular route, or intracranial administration via injection (49–51).
Based on our finding that SD increases penetration of fluorescent dyes into the brain (Figs. 1 and 2 A–D), we tested whether drug penetration would be enhanced after SD. We tested brain penetration after thorax injection of four drugs typically excluded from entering the brain due to their large size over 0.5 kDa and engagement of xenobiotic transporters (52, 53). All four drugs are conjugated with the fluorescein derivate Bodipy-FL for BBB tracing, while retaining bioactivity (52, 54–56). All four drugs are required to cross the BBB in certain conditions: The antibiotics Penicillin and Vancomycin are used in treating meningitis (57); Vinblastine is used to treat brain cancer (58); and Prazosin is an antianxiety drug acting on the central nervous system (59). The four drugs showed ~2.2- to 4.2-fold increases in brain penetration in our injection assay after three nights of mechanical SD (Fig. 2 E and F). While the eye images appear more banded here than the TexasRed experiments, we believe that might be due to altered hydrophobicity, cellular uptake or binding of these compounds. The increase in BBB drug penetration after SD suggests that sleep status could be a consideration for drug delivery into the brain.
Genetic Sleep Mutants Display Increased BBB Permeability.
We next tested how mutations that chronically reduce daily sleep affect BBB permeability. We tested 8 genetic sleep mutants displaying reductions in daily sleep duration (Fig. 3C and SI Appendix, Fig. S2B): redeye (rye) or nicotinic acetylcholine aeceptor α4 (60), shaker5 (sh), which encodes the beta subunit of a potassium channel (61), hyperkinetic (hk), which encodes the alpha subunit of the same channel (62), fumin (fmn), a dopamine transporter (63), sleeplessP1(sss), a Ly-6 protein involved in protein trafficking (64), insomniac1 (inc), a putative BTB (for BR-C, ttk and bab) or POZ (for Pox virus and Zinc finger)-domain protein (65), and wide awakeD1 (wakeD1) and wide awakeD2 (wakeD2), two alleles of a Gamma-Aminobutyric Acid (GABA) receptor modulator (66). Each mutation, with the exception of rye, exhibited increases in BBB permeability when compared to control wild-type flies (Fig. 3 A and B and SI Appendix, Fig. S2A), indicating there might be a broad link between sleep loss and BBB permeability (Fig. 3D). To explore whether conversely restoring sleep in a sleep mutant could affect BBB permeability, we fed inc flies the hypnotic gaboxadol (67), which increased sleep, and measured BBB permeability the next day. Fifteen hours of gaboxadol administration increased sleep by ~50% compared to untreated flies (Fig. 3G and SI Appendix, Fig. S2D) and restored BBB permeability by ~20% (Fig. 3 E and F). While the effect on the BBB was smaller than on sleep, these results illustrate that short-term sleep restoration can, in principle, restore BBB permeability in chronically sleep-deprived genetic sleep mutants. Populations of flies of the same genotype display variability both in sleep as well as BBB permeability (e.g. SI Appendix, Fig. S2A). To test whether sleep duration tracks with BBB permeability on an individual level, we measured sleep and BBB while tracking individual inc flies. We observe that flies that sleep more tend to have a less permeable BBB, and vice versa (SI Appendix, Fig. S2C, R2 = 0.6), suggesting that flies’ BBB state reflects both genotype and individual experience. Taken together, these data further support the notion of a functional link between BBB and sleep states.
Fig. 3.
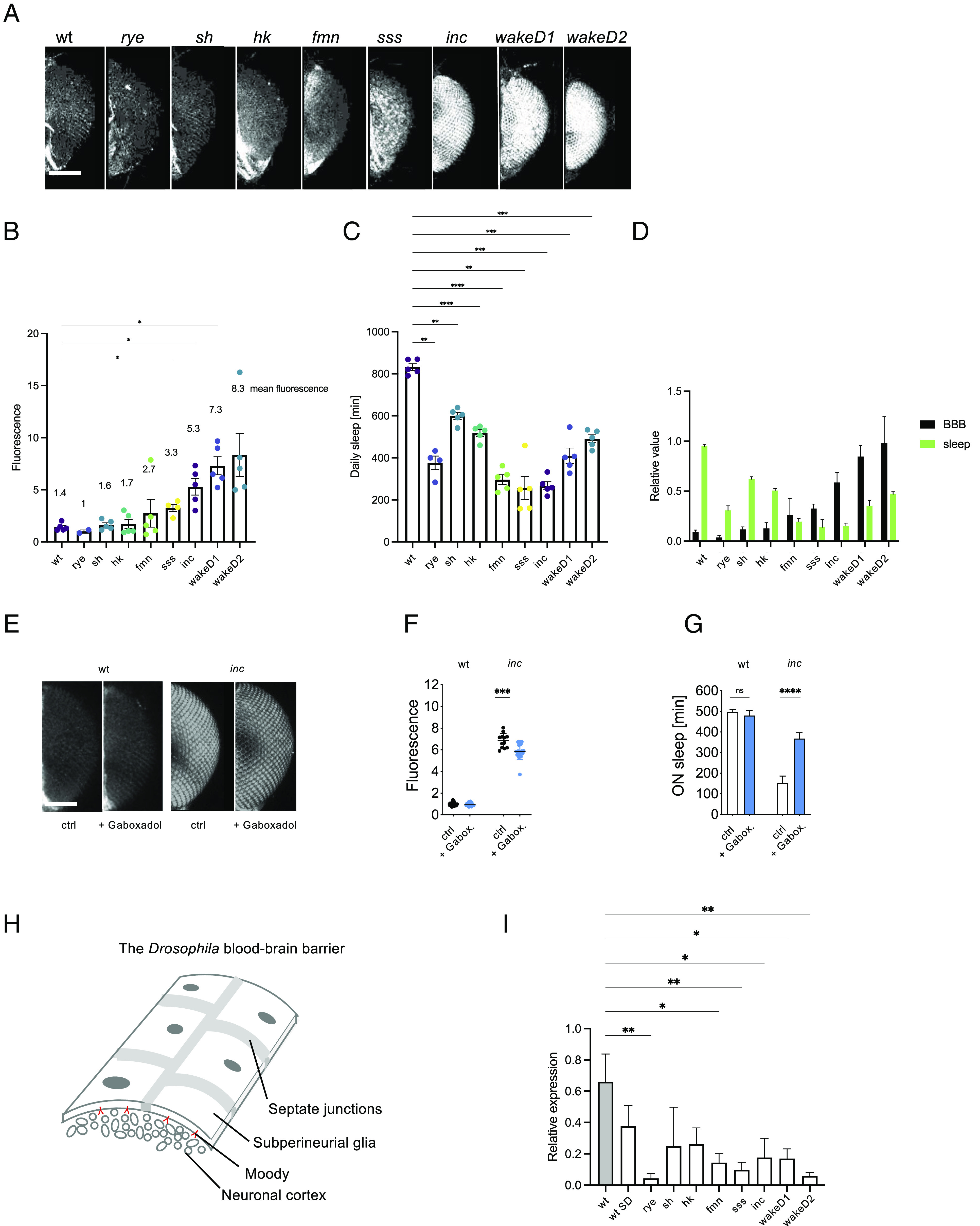
Sleep mutants display increased BBB permeability. (A) BBB permeability via tracer dye assay in sleep duration mutants. (B) Quantification of BBB permeability in (A). n = 30 to 40, shown are averages for 2 to 5 independent experiments. Significance was assessed using one-way ANOVA and post hoc Dunnett’s test. (C) Total daily sleep amounts for mutants in (A) and (B). n = 20 to 32, shown are averages for 4 to 5 independent experiments. Statistical significance was calculated using one-way ANOVA with Dunnett’s post hoc testing. (D) Bar chart displaying a largely inverse relationship between sleep and BBB permeability in sleep mutants. Shown are averages of 3 to 5 independent experiments. For comparability, data were normalized to the averages of daily sleep and fluorescence, respectively. To this end, the respectively lowest value was subtracted from the individual measurements and the result was divided by the difference between highest and lowest values, for sleep and BBB data, respectively. n = 2 to 5 independent experiments. Statistical significance was calculated using one-way ANOVA with Dunnett’s post hoc testing. (E) BBB permeability in inc and wild-type flies after administration of 0.1 mg/mL gaboxadol sleep aid overnight. (F) Quantification of BBB permeability in (E). n = 20 to 30. Significance was assessed using t–testing. (G) Sleep after gaboxadol administration. n = 16. Significance was assessed using t–testing. (H) Schematic representation of the Drosophila BBB. (I) Shown are qRT-PCR results of relative moody expression normalized to gapdh in wild-type, sleep deprived wild-type, and 8 sleep mutants. Shown is a bar graph of averages of 3 independent experiments. Significance was assessed using one-way ANOVA and post hoc Dunnett’s test. For quantification of BBB permeability, fluorescence was measured for both eyes of each fly. Each dot represents normalized fluorescence in one eye. Significance levels are P < 0.05: *, P < 0.01: **, <0.001: ***, <0.0001: ****. Bar: 200 μm.
SD Modulates Moody GPCR Expression.
We next sought to understand the molecular basis of BBB changes occurring during sleep homeostasis. Past studies established the orphan G protein-coupled receptor (GPCR) Moody as a major regulator of BBB development and function that acts cell-autonomously and exclusively in the BBB (35, 36, 68–73). Hypothesizing that moody might be involved in BBB regulation during sleep homeostasis, we mechanically sleep-deprived wild-type flies for two consecutive nights and then measured moody expression using qRT-PCR (quantitative real-time PCR) in sleep-deprived flies and normal sleeping controls (3 independent experiments, 30 to 60 fly heads each). moody levels appeared to be lower in wild-type flies after SD (Fig. 3I).
To further test whether moody levels dynamically change during sleep homeostasis, we measured moody expression using qRT-PCR in 8 sleep mutants. moody RNA levels were ~60 to 90% lower in all sleep mutants tested compared to isogenic wild-type control flies (Fig. 3I). The observation that lower moody expression correlates with chronic/genetic SD (as well as acute SD) suggests that a lowered moody expression may be causing BBB phenotypes that in turn negatively affect sleep.
The moody Signaling Cascade Affects Sleep in a Glia-Autonomous Manner.
In Drosophila and other insects, the formation and function of the BBB resides within the subperineurial glia (SPG), a cell type enclosing the central and peripheral nervous systems. These cells form a polarized epithelium with a septate junction belt along their lateral surface creating pericellular permeability barriers. SPGs are highly specialized cells requiring the action of a GPCR signaling cascade during embryonic and larval development to properly localize junctional components, vesicular traffic, and the cytoskeleton for proper formation of a contiguous seal (35, 68, 73). Specifically, the orphan GPCR Moody is localized on the basal, brain-facing surface of the SPG, and is required for proper SPG development and BBB function (Fig. 3H). Downstream effectors of moody include the G-protein subunit gao, the negative regulator of G-protein signaling loco and the protein kinase C catalytic subunit 1, pkaC1, which transmits the moody signal to the cytoskeleton, modulating spatiotemporal actomyosin contractility for correct cell shaping and polarized cell function to deliver septate junction components via vesicular trafficking to the lateral cell surface and create the BBB (35). The end result is a ladder-like septate junction belt consisting of large transmembrane protein complexes composed of over 20 septate junction proteins, forming regularly spaced homophilic interactions with their counterparts on the neighboring cell. These lateral cell surfaces fold onto each other, increasing the surface area and elongating the ladder-like septate junction belts to hundreds of “rungs” formed by the septate junctions (33).
Given that sleep homeostasis seems to involve BBB permeability changes, we wanted to test whether modifying the BBB itself would affect sleep. Null mutations of genes required for BBB development and function are frequently developmentally lethal, e.g., moody, loco, PKAC1, lachesin (35, 68, 74) preventing adult sleep experiments. We hypothesized that utilizing transgenic RNAi against genes of interest in the BBB might result in adult viability due to the incomplete removal of gene function (75). RNAi of gao, loco, pkac1, and the septate junction components lachesin and neuroglian (35, 74, 76) in the BBB using the subperineurial glia-specific moody-Gal4 driver (SI Appendix, Fig. S3 A–C) resulted in significant increases in BBB permeability (Fig. 4 A–C) and significant sleep deficits (Fig. 4 F and H). This shows that cell-autonomous BBB disruption directly affects sleep.
Fig. 4.
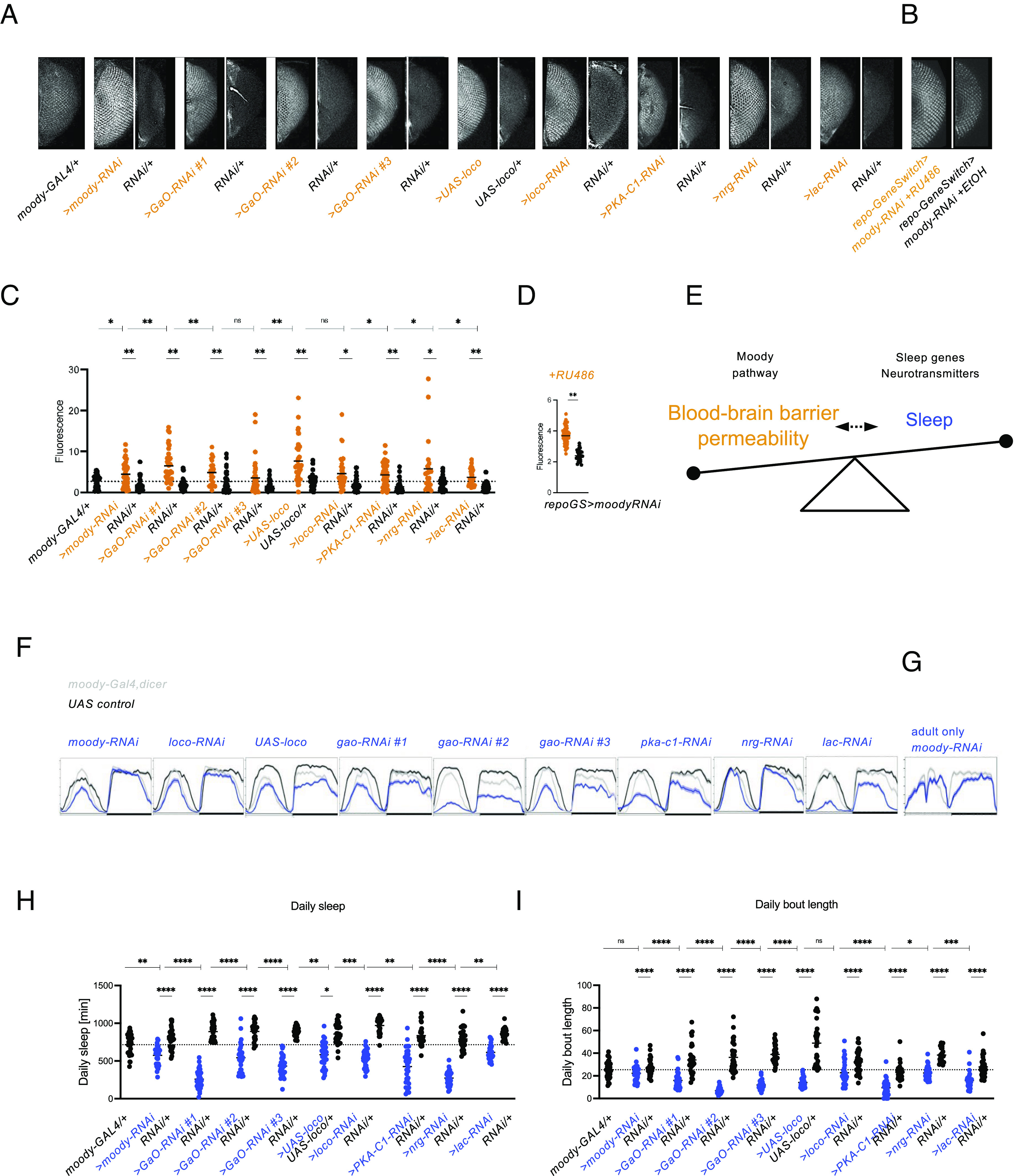
Glial GPCR signaling is required for BBB integrity and sleep. (A) Tracer injection assay for SPG-specific RNAi using moody-Gal4 driving moody, GaO, loco, PKA-C1, lachesin, and neuroglian, and driving overexpression of loco, shows increased BBB permeability. (B) Tracer injection assay for adult glia-specific knockdown of the GPCR Moody using repo-GeneSwitch shows increased BBB permeability. (C and D) Quantification of BBB data shown in (A and B). n = 40 - 45. Significance was assessed using one-way ANOVA and post hoc Tukey test (C) and t-testing (D). (E) Model of bidirectiontal relationship between the BBB and sleep. The BBB seems to sense sleep states and respond to SD by increasing permeability and to sleep increases with BBB closure. As opening the BBB itself causes SD, it seems that a particular level of opening, or even its dynamics, signal sleep states. Future work ideally measuring BBB changes and yet to be identified real time sleep biomarkers concomitantly will hopefully elucidate the interplay of BBB dynamics and sleep, but for now, we believe our data suggest that the BBB is at least part of sleep regulation. The moody pathway, neurotransmitters such as dopamine and octopamine as well as sleep genes are known to contribute to the function of the BBB and sleep, respectively, and we observe that modifying them changes the balance between BBB and sleep. (F) BBB-specific knockdown using moody-Gal4 of moody, GaO, loco, PKA-C1, lachesin, and neuroglian and overexpression of loco shows decreased sleep. n = 32. (G) Adult glia-specific knockdown of the GPCR Moody using repo-GeneSwitch leads to decreased sleep. n = 16. (H) Quantification of daily sleep for data shown in (F). Significance was assessed using one-way ANOVA and post hoc Tukey test. (I) Daily bout length quantification for data shown in (F). For quantification of BBB permeability, fluorescence was measured for both eyes of each fly. Each dot represents normalized fluorescence in one eye. For all sleep plots, average 24 h sleep over 4 d binned as sleep in 30 min is shown. Significance levels are P < 0.05: *, <0.01: **, <0.001: ***, <0.0001: ****. For C, H, I, horizontal dashed line indicates mean of moody-Gal4/+ control, and —| indicates comparison to moody-GAL4/+, SPG: subperineurial glia.
To test whether sleep effects were a remnant of BBB knockdown’s overall effect on animal health, we used the GeneSwitch system to only reduce moody levels in adult flies (77). Adult-only knockdown of moody also caused a BBB opening (Fig. 4 B and D) and sleep loss (Fig. 4G), suggesting a dynamic function for the moody pathway during adult sleep.
Overall sleep loss (Fig. 4 F–H) was accompanied by a reduction in the average sleep bout length for most genotypes (Fig. 4I), indicating that BBB disruption affects total sleep duration via sleep fragmentation. The other sleep and activity parameters tested—number of sleep bouts, sleep latency, and waking activity—showed less robust departures from control values (SI Appendix, Fig. S3 D–G).
These results show that the BBB can cell-autonomously control sleep duration and, together with the data from Figs. 1–3, suggest a feedback mechanism where sleep state is reflected in and altered through BBB permeability changes.
Discussion
Our data establish the BBB as a sleep regulatory center in Drosophila. We observe that the BBB dynamically and rapidly—both functionally and molecularly—responds to acute as well as chronic-genetic SD and that altering BBB function alone, through mutations of the moody signaling pathway, can affect sleep, suggesting that the BBB senses sleep need and responds to it by altering its permeability. The correct BBB permeability state seems to be required for normal sleep, as disrupting BBB function causes sleep loss.
In normal physiology, the BBB controls influx and efflux of molecules in and out of the brain, including ions, amino acids, neurotransmitters, vitamins, proteins, carbohydrates, lipids, and hormones (78). Transport is facilitated via various mechanisms which include active transporters, transcytosis, and passive diffusion. The function of the BBB is not static but is affected by circadian rhythms, neuronal activity, and the metabolism (27, 79, 80). Our data add sleep as an additional parameter modulating BBB function, as has been suggested by studies in mammals (37, 42, 81), and we found that the relationship between the BBB and sleep is reciprocal. Our results show that the BBB rapidly and dynamically responds to SD by increasing its permeability and that permeability increases conversely cause sleep loss (Fig. 4E). It is conceivable that BBB permeability changes allow the passage of one or multiple molecules that affect sleep into or out of the brain. Defective xenobiotic exclusion of steroid hormones from the brain via the BBB has been shown to affect sleep in Drosophila, demonstrating that molecules outside the CNS can affect sleep (82). Future studies may determine whether the BBB mediates a rapid global switch from a sleeping to an awake brain, for example, by controlling ionic diffusion and potassium concentrations, which could broadly raise neuronal activation thresholds and lead to the observed extracellular ionic changes in the sleeping mammalian brain (25).
Functional Significance of BBB Changes with Respect to Disease States.
The BBB creates a highly controlled microenvironment in the brain, perturbations of which affect neuronal function, as evidenced by neuropathologies at least in part caused by BBB disruption including stroke, Alzheimer’s, Multiple Sclerosis, epilepsy and brain tumor metastasis (83, 84). BBB disruption also occurs during normal aging, under chronic stress and during systemic inflammation and infection (85–87); however, whether the relationship between BBB disruption and these pathologies is causal or merely correlative is less clear. The magnitude of BBB changes in disease states can be an order of magnitude larger than the observed changes after sleep loss described in this study (88), illustrating the physiological relevance of our experiments. All of the pathologies listed above are also marked by sleep disruption, which in some instances increases the risk for developing the disease in the first place and the severity of which correlates with disease severity (89–94). The fly ortholog of a human autism gene has been reported to act in the BBB and elicit sleep fragmentation via developmental hyperserotonemia (95). However, a direct involvement of the BBB in sleep regulation had not been shown until this present study.
BBB and sleep disruptions have been, so far independently, associated with causing illness, and our study provides additional evidence for the notion that the two processes are linked. If BBB permeability and sleep regulation are fundamentally connected as part of the same system, it will be interesting to study how they relate to disease risk, development, and prognosis, and whether interventions that affect one also augment the other.
Acute SD and Drug Delivery.
Adequate drug delivery to the brain is a problem plaguing the treatment of many neuropathologies. This puts strong importance on finding possible regulatory sites for controlling the BBB as a means of finding better ways to deliver drugs to the brain. The BBB has distinct mechanisms to exclude molecules from the brain including xenobiotic transporters in the cell membrane that efflux most small hydrophobic molecules [<0.4 kDa (96)] taken up transcellularly through the membrane, and the paracellular barrier formed by junctions between the BBB forming cells. The latter creates a diffusion barrier for hydrophilic molecules in a size-dependent manner with a maximum passage size of about 0.5 kDa (97). Due to these mechanisms, the BBB poses a major obstacle to treatment of brain pathologies, because 100% of large molecules (>0.5 kDa), like the brain cancer drug vinblastine (molecular weight: 1 kDa), and 98% of small molecule drugs (<0.5 kDa), like the non-steroidal anti-inflammatory drug paracetamol, cannot penetrate it in therapeutic amounts (48). Current solutions to this problem include pharmacologically increasing the diffusion barrier permeability, implantation of ultrasonic devices to temporarily permeabilize the diffusion barrier, fusing molecules to receptor ligands to engage the transcellular route, or intracranial administration via injection (49–51). Provided our observed effects are conserved in humans, a patient’s sleep and nighttime administration of drugs could have a significant effect on drug penetration into the CNS and possibly treatment outcomes.
Molecular and Cellular Mechanism.
In Drosophila, the BBB’s subperineurial glia form a layer of polarized epithelial cells, with septate junctions between them creating a seal for water-soluble molecules over 500 Da. Our observation that SD rapidly induces increases in BBB permeability raises the question: What are the molecular and cellular mechanisms that underlie the remodeling of septate junctions on the scale of hours? The Moody GPCR pathway has been shown to play a key role in the development of the epithelial barrier, as well as its maintenance in adulthood (35, 36, 68, 73). Moody activates a signaling cascade which, via PKA activation, controls the developmental assembly of the septate junction belt in a highly coordinated spatiotemporal manner that involves rearrangements of the actin cytoskeleton and vesicular trafficking (35, 68, 73). We have shown that RNAi-based suppression of moody pathway genes causes increased BBB permeability. We also observed strong downregulation of moody in every genetic sleep mutant tested. These findings suggest that altered moody pathway function may be primarily responsible for the leaky barriers of these mutants.
During SD and recovery, it is conceivable that similar processes occur. Indeed, it has been shown that perturbation of endocytosis at the Drosophila BBB alters sleep (98), possibly interfering with Moody-activated remodeling during sleep/wake cycles. We have shown that acute SD rapidly depresses moody expression in wild-type Drosophila, which is accompanied by increased BBB permeability. Moody is expressed on the neuronal-facing apical cell surface of the subperineurial glia (68). While its ligand is not known, this topology could permit neuronal states related to sleep to be communicated to glia via this GPCR pathway. As many known sleep genes in Drosophila have been shown to be expressed in neurons and we observe BBB changes upon sleep-suppressing neuronal activation, neuron–glia signaling seems likely to be part of the process, and it will be interesting to elucidate the involved signaling molecules including Moody’s ligand.
BBB State and Sleep Need.
The two-step model of sleep regulation posits that circadian rhythm and sleep homeostasis control the timing of sleep (99). Sleep homeostasis is a process that controls sleep duration to meet an organism’s daily sleep need. Rebound sleep after SD is interpreted as proof of the existence of sleep homeostasis. The physiological and cellular nature of the sleep homeostat is—like the nature of sleep itself—the subject of current research. Sleep homeostasis has been shown to be affected by oxidative stress, neuronal, glial, and microglial activity, neuropeptide signaling, infection, the circadian clock, temperature, and starvation (4, 12, 13, 100, 101). Synaptic downscaling, glymphatic clearance of toxins, peripheral glucose homeostasis, reactive oxygen species clearance, and inhibition of motor function have been proposed as processes occurring during sleep homeostasis, constituting functions of sleep (4, 13, 17, 19, 23, 102–104). As at least some of these processes must involve yet to be identified body–brain signals (4, 19, 102, 103, 105), future studies will reveal how the BBB changes shown here affect known or novel functions of sleep.
Together our data indicate a system where an increase of sleep need is measured by the BBB, resulting in an adjustment of moody levels, an opening of the BBB, and the initiation of rebound sleep. This process is likely to involve neuronal to BBB signaling (dopaminergic SD also alters the BBB, and many of the sleep mutants are expressed in neurons) as well as yet-to-be-characterized molecular exchange across the BBB.
Materials and Methods
Fly Genetics.
Flies were raised on standard cornmeal/molasses food at 25 °C in a 12-h light/12-h dark (LD) cycle. Wild-type strains used as controls were isogenic w1118 (iso1CJ) strains (106, 107) for sleep. ln all experiments, Gal4 and UAS parental controls were tested as hemizygotes. The following fly stocks were used: redeye (rye, Bloomington #80692) or nicotinic Acetylcholine Receptor α4 (60), shaker5 (sh, Bloomington #111) (61), hyperkinetic2 (hk, Bloomington #55) (62), fumin (fmn, was a lab stock), (63), sleeplessP1(sss, gift from A. Sehgal), (64), insomniac1 (inc) (65), and wide awakeD1 (wakeD1) and wide awakeD2 (wakeD2, gifts from Mark Wu), (66), moody-Gal4 (36); UAS-loco (68); GaO-RNAi #3, and repo-GeneSwitch (98) were gifts from U. Gaul, TH-Gal4 was a lab stocks; Tdc2-Gal4 and UAS-dTRPA1 are from the Bloomington stock center (#9313 and #26264, respectively). nrgRNAi, GaO-RNAi#1, #2 and #3, lachesin-RNAi, pkaC1-RNAi, and moodyRNAi are VDRC #9248, #28201gd, #19124, #28010, #28940, #31277, #36821, respectively. moody-Gal4 was used with UAS-dicer (Bloomington #24651) to enhance RNAi efficiency. All genetic sleep mutants were outcrossed 5 generations to the wild-type strain. For repo-GeneSwitch induction, 5- to 8-d-old flies were placed on food containing 5mM RU486 in ethanol (Abcam; ab120356) for 3 d or only on food containing the corresponding volume of the solvent ethanol, followed by BBB and sleep analysis. Males were used for all experiments except tdc2>TRPA1 (47).
qRT-PCR.
Seven-day-old flies were frozen in liquid nitrogen at ZT4. Heads were homogenized with TRIzol (Invitrogen) and RNA was extracted with RNeasy Mini Kit (QIAGEN). cDNA was prepared using iScript Reverse Transcription Supermix (BIO-RAD). qRT-PCR was performed with Fast SYBR Green Master Mix (Applied Biosystems) and 7500 Fast Real-Time PCR System (Applied Biosystems). The following primers were used: moody forward: TCCTTCGTCGTCTGCTACTTG, moody reverse: ATTGTGCGGCTGTGGTTGTTG, gapdh forward: CCACTGCCGAGGAGGTCAACTAC, gapdh reverse: ATGCTCAGGGTGATTGCGTATGC
BBB Injection Assay.
Modified after Bainton et al. (36) and Li (35). CO2-anesthetized adult flies were injected using a MPPI-3 pressure injector (Applied Scientific Instrumentation) with 1-mm borosilicate needles (FHC-Co) containing fluorescent dyes under a dissecting microscope. An average volume of 100 ± 25 nL (range 70 to 130 nL) of dye was injected into the lateral thorax between wing socket and haltere. Moderately varying dye concentration does not affect brain penetration: up to fourfold changes in Texas Red 10 kDa dye concentration do not significantly affect brain penetration 30 min after injection. All dyes were diluted in injection buffer containing 0.1 mM sodium phosphate, pH 6.8, and 5 mM KCl. For all BBB permeability assessments, flies were injected with 2.5 mM Dextran TexasRed (MW 10,000, Thermo Fisher D1863). Fluorescent drugs were injected at the following concentrations: Bocillin FL Penicillin (Thermo Fisher, B13233): 10 mM, Bodipy FL Prazosin (Thermo Fisher, B7433): 15 mM, Vinblastine Bodipy FL (Thermo Fisher V12390): 3 mM, Vancomycin Bodipy FL (Thermo Fisher, V34850): 5 mM. All fluorescent drug derivatives retain bioactivity (23, 25–27). Flies were allowed to recover for precisely 30 min before decapitation and imaging on a Zeiss LSM710 confocal microscope. For each fly, both eyes were imaged as interindividual differences in BBB permeability are not larger than intraindividual differences (F-test, Fig. S1). Laser and acquisition settings remained unchanged for all samples in the same experiment. Stacks of 6 to 20 confocal slices of 16 µm were taken and maximum projection images generated using a custom Metamorph (Molecular Devices) script (gift from T. Tong, Bioimaging Resource Center, Rockefeller University). Average pixel intensity in the eye was measured using ImageJ software. For each experiment, data were normalized to mock injected flies. For individual assessment of sleep and BBB permeability, inc flies were loaded into Drosophila activity monitors (Trikinetics) and sleep was recorded for 4 d before injection and BBB assessment. All injections were conducted at ZT0.5-1.5 unless otherwise noted.
Live Imaging of Subperineurial Glia.
Cephalic complexes from inc;lachsesinGFP or lachesinGFP third instar larvae were dissected in Schneider’s S2 cell media (Thermo Fischer, #21720024), mounted in saline, and imaged live using a on a Zeiss LSM710 confocal microscope. Laser and acquisition settings remained unchanged for all samples in the same experiment. Image stacks consisting of 20 to 40 1-µm slices were acquired. For 3D visualization of SPG, we used Imaris software (Bitplane).
Sleep Analysis.
First, 5 to 7-d-old animals enclosing from light:dark cycle-entrained cultures were loaded into glass tubes and assayed for 5 to 7 d at 25 °C in light:dark cycles. Locomotor activity levels were monitored using the Drosophila Activity Monitoring System (DAM, Trikinetics). For sleep measurements, activity counts were collected in 1-min bins for at least 4 d in light:dark cycles and sleep was identified as at least 5 min of inactivity (8, 108) using a sliding window. Sleep parameters were determined using an R-script (109).
SD and Gaboxadol Administration.
Flies were mechanically stimulated overnight during their nighttime sleep hours (Zeitgeber time 12 to 24 h) using a custom-built machine (45). Mechanical stimuli were randomly applied for 2x2 s every 5 m. Gaboxadol administration was performed as previously described (67, 110). Briefly, flies were placed into glass tubes with food containing either water or 1 mg/mL of gaboxadol (or THIP) hypnotic. Tubes were loaded into DAM monitors and sleep was monitored for 15 h prior to BBB assessment via dye injection. Thermogenetic SD was induced by moving TH>TRPA1 or Tdc2>TRPA1 crosses from 18 °C to 29 °C for different lengths of time and back to 18 °C for recovery sleep.
Statistics.
Statistical analysis was performed using GraphPad Prism. For comparisons between two genotypes, Student’s t tests were used. For comparisons between three or more genotypes, One-way ANOVAs with Dunn’s post hoc or Dunnett’s post hoc tests were used as described in the figure legends associated with each experiment. For all bar graphs, error bars indicated calculated SEM.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank T. Tong (Rockefeller Bioimaging Resource Center, Rockefeller University) for help with image analysis; P. Kidd for help with Python and W. Wang for help with R programming; M. Wu, U. Gaul and the Bloomington, NIG-Fly, and Vienna Drosophila Resource Center (VDRC) Drosophila stock centers for fly stocks; L. Vosshall, H. Steller, D. Botstein, and D. Cabrera for comments on the manuscript and postdocs and faculty of The Rockefeller University for support and helpful discussions. This work was supported by NIH grants 5R37 NS053087 and 5R35 GM136237 to M.W.Y.
Author contributions
S.A., X.L., and M.W.Y. designed research; S.A., X.L., Y.S., S.L., A.T., J.O., Z.W., A.N., A.V., C.S., and B.S. performed research; S.A., X.L., Y.S., S.L., A.T., J.O., Z.W., A.N., A.V., C.S., B.S., and M.W.Y. analyzed data; S.A. wrote the paper; and S.A., C.S., B.S., and M.W.Y. revised the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: M.B.A., HHMI; and J.R., University College London.
Contributor Information
Sofia Axelrod, Email: saxelrod@rockefeller.edu.
Michael W. Young, Email: young@rockefeller.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix. Data have been deposited to Dryad (111).
Supporting Information
References
- 1.Cirelli C., The genetic and molecular regulation of sleep: From fruit flies to humans. Nat. Rev. Neurosci. 10, 549–560 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols A. L. A., Eichler T., Latham R., Zimmer M., A global brain state underlies C. elegans sleep behavior. Science 356, eaam6851 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Kanaya H. J., et al. , A sleep-like state in Hydra unravels conserved sleep mechanisms during the evolutionary development of the central nervous system. Sci. Adv. 6, eabb9415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccaro A., et al. , Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 181, 1307–1328.e15 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Rechtschaffen A., Bergmann B. M., Sleep deprivation in the rat: An update of the 1989 paper. Sleep 25, 18–24 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Driver R. J., Lamb A. L., Wyner A. J., Raizen D. M., DAF-16/FOXO regulates homeostasis of essential sleep-like behavior during larval transitions in C. elegans. Curr. Biol. 23, 501–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann Q., Beckwith E. J., Gilestro G. F., Most sleep does not serve a vital function: Evidence from Drosophila melanogaster. Sci Adv. 5, eaau9253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw P. J., Cirelli C., Greenspan R. J., Tononi G., Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Hendricks J. C., et al. , Rest in Drosophila is a sleep-like state. Neuron 25, 129–138 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Axelrod S., Saez L., Young M. W., “Chapter one–studying circadian rhythm and sleep using genetic screens in Drosophila” in Methods in Enzymology, Circadian Rhythms and Biological Clocks, Sehgal A., Ed. (Academic Press, 2015), pp. 3–27. [DOI] [PubMed] [Google Scholar]
- 11.Tomita J., Ban G., Kume K., Genes and neural circuits for sleep of the fruit fly. Neurosci. Res. 118, 82–91 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Shafer O. T., Keene A. C., The regulation of Drosophila sleep. Curr. Biol. 31, R38–R49 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Kempf A., Song S. M., Talbot C. B., Miesenböck G., A potassium channel β-subunit couples mitochondrial electron transport to sleep. Nature 568, 230–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzotta G. M., Damulewicz M., Cusumano P., Better sleep at night: How light influences sleep in Drosophila. Front. Physiol. 11, 997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckwith E. J., French A. S., Sleep in Drosophila and its context. Front. Physiol. 10, 1167 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh K., Evans J. M., Hendricks J. C., Sehgal A., A Drosophila model for age-associated changes in sleep:wake cycles. Proc. Natl. Acad. Sci. U.S.A. 103, 13843–13847 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie L., et al. , Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bushey D., Tononi G., Cirelli C., Sleep and synaptic homeostasis: Structural evidence in Drosophila. Science 332, 1576–1581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D., Dan Y., A motor theory of sleep-wake control: Arousal-action circuit. Annu. Rev. Neurosci. 42, 27–46 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Maret S., Faraguna U., Nelson A. B., Cirelli C., Tononi G., Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 14, 1418–1420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donlea J. M., Thimgan M. S., Suzuki Y., Gottschalk L., Shaw P. J., Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332, 1571–1576 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G., et al. , Sleep promotes branch-specific formation of dendritic spines after learning. Science 344, 1173–1178 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vivo L., et al. , Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diering G. H., et al. , Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding F., et al. , Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352, 550–555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fultz N. E., et al. , Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulido R. S., et al. , Neuronal activity regulates blood-brain barrier efflux transport through endothelial circadian genes. Neuron 108, 937–952.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S. L., Yue Z., Arnold D. M., Artiushin G., Sehgal A., A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130–139.e10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hablitz L. M., et al. , Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 11, 4411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbott N. J., Rönnbäck L., Hansson E., Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Terstappen G. C., Meyer A. H., Bell R. D., Zhang W., Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Abbott N. J., Patabendige A. A. K., Dolman D. E. M., Yusof S. R., Begley D. J., Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Limmer S., Weiler A., Volkenhoff A., Babatz F., Klämbt C., The Drosophila blood-brain barrier: Development and function of a glial endothelium. Front. Neurosci. 8, 365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinsonneault R. L., Mayer N., Mayer F., Tegegn N., Bainton R. J., “Novel models for studying the blood-brain and blood-eye barriers in Drosophila” in The Blood-Brain and Other Neural Barriers: Reviews and Protocols, Nag S., Ed. (Humana Press, 2011), pp. 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., et al. , The cAMP effector PKA mediates Moody GPCR signaling in Drosophila blood-brain barrier formation and maturation. Elife 10, e68275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bainton R. J., et al. , Moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 123, 145–156 (2005). [DOI] [PubMed] [Google Scholar]
- 37.He J., et al. , Sleep restriction impairs blood-brain barrier function. J. Neurosci. 34, 14697–14706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurtado-Alvarado G., Velázquez-Moctezuma J., Gómez-González B., Chronic sleep restriction disrupts interendothelial junctions in the hippocampus and increases blood-brain barrier permeability. J. Microsc. 268, 28–38 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Dunton A. D., Göpel T., Ho D. H., Burggren W., Form and function of the vertebrate and invertebrate blood-brain barriers. Int. J. Mol. Sci. 22, 12111 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki S., Liu C.-Y., Hayashi Y., Sleep in vertebrate and invertebrate animals, and insights into the function and evolution of sleep. Neurosci. Res. 118, 3–12 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Joiner W. J., Unraveling the evolutionary determinants of sleep. Curr. Biol. 26, R1073–R1087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina-Flores F., et al. , Sleep loss disrupts pericyte-brain endothelial cell interactions impairing blood-brain barrier function. Brain Behav. Immun. 89, 118–132 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Kaya M., Ahishali B., Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol. Biol. 763, 369–382 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Ueno T., et al. , Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 15, 1516–1523 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Rogulja D., Young M. W., Control of sleep by cyclin A and its regulator. Science 335, 1617–1621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Q., Liu S., Kodama L., Driscoll M. R., Wu M. N., Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr. Biol. 22, 2114–2123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seidner G., et al. , Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Curr. Biol. 25, 2928–2938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Waterbeemd H., Camenisch G., Folkers G., Chretien J. R., Raevsky O. A., Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Target. 6, 151–165 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Hersh D. S., et al. , Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 22, 1177–1193 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carpentier A., et al. , Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 8, 343re2 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Power E. A., et al. , Drug delivery across the blood-brain barrier for the treatment of pediatric brain tumors–An update. Adv. Drug Deliv. Rev. 185, 114303 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Dey S., Ramachandra M., Pastan I., Gottesman M. M., Ambudkar S. V., Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 94, 10594–10599 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutsar I., McCracken G. H. Jr., Friedland I. R., Antibiotic pharmacodynamics in cerebrospinal fluid. Clin. Infect. Dis. 27, 1117–1127, quiz 1128–9 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Daniel R. A., Errington J., Control of cell morphogenesis in bacteria. Cell 113, 767–776 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Atilano M. L., et al. , Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 107, 18991–18996 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kicheva A., et al. , Kinetics of morphogen gradient formation. Science 315, 521–525 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Smith D. S., Basistha M., Bacterial meningitis empiric therapy. Medscape (2022). https://emedicine.medscape.com/article/1953067-overview. Accessed 14 September 2023.
- 58.Lassaletta A., et al. , Phase II weekly vinblastine for chemotherapy-naïve children with progressive low-grade glioma: A Canadian pediatric brain tumor consortium study. J. Clin. Oncol. 34, 3537–3543 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Koola M. M., Varghese S. P., Fawcett J. A., High-dose prazosin for the treatment of post-traumatic stress disorder. Ther. Adv. Psychopharmacol. 4, 43–47 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi M., Yue Z., Kuryatov A., Lindstrom J. M., Sehgal A., Identification of Redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. Elife 3, e01473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cirelli C., et al. , Reduced sleep in Drosophila Shaker mutants. Nature 434, 1087–1092 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Bushey D., Huber R., Tononi G., Cirelli C., Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J. Neurosci. 27, 5384–5393 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kume K., Kume S., Park S. K., Hirsh J., Jackson F. R., Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25, 7377–7384 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koh K., et al. , Identification of SLEEPLESS, a sleep-promoting factor. Science 321, 372–376 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stavropoulos N., Young M. W., Insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron 72, 964–976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu S., et al. , WIDE AWAKE mediates the circadian timing of sleep onset. Neuron 82, 151–166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berry J. A., Cervantes-Sandoval I., Chakraborty M., Davis R. L., Sleep facilitates memory by blocking dopamine neuron-mediated forgetting. Cell 161, 1656–1667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwabe T., Bainton R. J., Fetter R. D., Heberlein U., Gaul U., GPCR signaling is required for blood-brain barrier formation in drosophila. Cell 123, 133–144 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Hatan M., Shinder V., Israeli D., Schnorrer F., Volk T., The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J. Cell Biol. 192, 307–319 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoxha V., et al. , Sex-specific signaling in the blood-brain barrier is required for male courtship in Drosophila. PLoS Genet. 9, e1003217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stork T., et al. , Organization and function of the blood–brain barrier in Drosophila. J. Neurosci. 28, 587–597 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Babatz F., Naffin E., Klämbt C., The Drosophila blood-brain barrier adapts to cell growth by unfolding of pre-existing septate junctions. Dev. Cell 47, 697–710.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Schwabe T., Li X., Gaul U., Dynamic analysis of the mesenchymal-epithelial transition of blood-brain barrier forming glia in Drosophila. Biol. Open 6, 232–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strigini M., et al. , The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol. Cell. Neurosci. 32, 91–101 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Dietzl G., et al. , A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Hindle S. J., Bainton R. J., Barrier mechanisms in the Drosophila blood-brain barrier. Front. Neurosci. 8, 414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osterwalder T., Yoon K. S., White B. H., Keshishian H., A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. U.S.A. 98, 12596–12601 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbott N. J., Friedman A., Overview and introduction: The blood-brain barrier in health and disease. Epilepsia 53, 1–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang S. L., et al. , A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat. Commun. 12, 617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Segarra M., Aburto M. R., Acker-Palmer A., Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 44, 393–405 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Sun J., et al. , Sleep deprivation induces cognitive impairment by increasing blood-brain barrier permeability via CD44. Front. Neurol. 11, 563916 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hindle S. J., et al. , Evolutionarily conserved roles for blood-brain barrier xenobiotic transporters in endogenous steroid partitioning and behavior. Cell Rep. 21, 1304–1316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Profaci C. P., Munji R. N., Pulido R. S., Daneman R., The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 217, e20190062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tiwary S., et al. , Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci. Rep. 8, 8267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hussain B., Fang C., Chang J., Blood-brain barrier breakdown: An emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 15, 688090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee S., et al. , Real-time in vivo two-photon imaging study reveals decreased cerebro-vascular volume and increased blood-brain barrier permeability in chronically stressed mice. Sci. Rep. 8, 13064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galea I., The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 18, 2489–2501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sweeney M. D., Sagare A. P., Zlokovic B. V., Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin D., Chen S., Liu J., Sleep disturbances in autoimmune neurologic diseases: Manifestation and pathophysiology. Front. Neurosci. 15, 687536 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garbarino S., Lanteri P., Bragazzi N. L., Magnavita N., Scoditti E., Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 4, 1304 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lloret M.-A., et al. , Is sleep disruption a cause or consequence of Alzheimer’s disease? Reviewing its possible role as a biomarker. Int. J. Mol. Sci. 21, 1168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winer J. R., et al. , Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J. Neurosci. 39, 6315–6324 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berisha A., Shutkind K., Borniger J. C., Sleep disruption and cancer: Chicken or the egg? Front. Neurosci. 16, 856235 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jensen L. D., Oliva D., Andersson B.-Å., Lewin F., A multidisciplinary perspective on the complex interactions between sleep, circadian, and metabolic disruption in cancer patients. Cancer Metastasis Rev. 40, 1055–1071 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coll-Tané M., et al. , The CHD8/CHD7/Kismet family links blood-brain barrier glia and serotonin to ASD-associated sleep defects. Sci. Adv. 7, eabe2626 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banks W. A., Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 9, S3–S5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Artiushin G., Zhang S. L., Tricoire H., Sehgal A., Endocytosis at the Drosophila blood-brain barrier as a function for sleep. Elife 7, 523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borberly A. A., Achermann P., Sleep homeostasis and models of sleep regulation. J. Biol. Rhythms 14, 559–570 (1999). [DOI] [PubMed] [Google Scholar]
- 100.Davla S., et al. , AANAT1 functions in astrocytes to regulate sleep homeostasis. Elife 9, e53994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toda H., Williams J. A., Gulledge M., Sehgal A., A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science 363, 509–515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tingley D., McClain K., Kaya E., Carpenter J., Buzsáki G., A metabolic function of the hippocampal sharp wave-ripple. Nature 597, 82–86 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melnattur K., Zhang B., Shaw P. J., Disrupting flight increases sleep and identifies a novel sleep-promoting pathway in Drosophila. Sci. Adv. 6, eaaz2166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Alphen B., Semenza E. R., Yap M., van Swinderen B., Allada R., A deep sleep stage in Drosophila with a functional role in waste clearance. Sci. Adv. 7, eabc2999 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Titos I., et al. , A gut-secreted peptide suppresses arousability from sleep. Cell 186, 1382–1397.e21 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin J. C., et al. , Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 79, 49–58 (1994). [DOI] [PubMed] [Google Scholar]
- 107.Yin J. C., Del Vecchio M., Zhou H., Tully T., CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell 81, 107–115 (1995). [DOI] [PubMed] [Google Scholar]
- 108.Huber R., et al. , Sleep homeostasis in Drosophila melanogaster. Sleep 27, 628–639 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Geissmann Q., Garcia Rodriguez L., Beckwith E. J., Gilestro G. F., Rethomics: An R framework to analyse high-throughput behavioural data. PLoS One 14, e0209331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dissel S., et al. , Sleep restores behavioral plasticity to Drosophila mutants. Curr. Biol. 25, 1270–1281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Axelrod S., Individual BBB, sleep and qRT-PCR data points for “The Drosophila blood-brain barrier regulates sleep via moody GPCR signaling” [Dataset]. Dryad. 10.5061/dryad.15dv41p39. Deposited 27 September 2023. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix. Data have been deposited to Dryad (111).