Significance
Bacterial genomes encode a wealth of natural products with potential applications in varied industries. However, obtaining the natural products predicted from bacterial genomes remains a barrier to translational efforts. Systematic characterization of talented bacteria using multiomics analyses and reverse genetics can reveal new natural products and their roles. A soil Burkholderia bacterium was previously used to produce antitumor spliceostatins for preclinical development. We obtained genome, metabolome, and epigenome datasets and harnessed the data to enable genetic engineering and to identify the natural product selethramide. Selethramide is a cyclic lipodepsipeptide that promotes bacterial motility due to its surfactant activity. The multiomic data and improved genetic engineering methods reported here are generalizable and will facilitate continued characterization of prolific strains.
Keywords: natural products, lipopeptide, bacterial host development
Abstract
Bacterial natural products have found many important industrial applications. Yet traditional discovery pipelines often prioritize individual natural product families despite the presence of multiple natural product biosynthetic gene clusters in each bacterial genome. Systematic characterization of talented strains is a means to expand the known natural product space. Here, we report genomics, epigenomics, and metabolomics studies of Burkholderia sp. FERM BP-3421, a soil isolate and known producer of antitumor spliceostatins. Its genome is composed of two chromosomes and two plasmids encoding at least 29 natural product families. Metabolomics studies showed that FERM BP-3421 also produces antifungal aminopyrrolnitrin and approved anticancer romidepsin. From the orphan metabolome features, we connected a lipopeptide of 1,928 Da to an 18-module nonribosomal peptide synthetase encoded as a single gene in chromosome 1. Isolation and structure elucidation led to the identification of selethramide which contains a repeating pattern of serine and leucine and is cyclized at the side chain oxygen of the one threonine residue at position 13. A (R)-3-hydroxybutyric acid moiety decorates the N-terminal serine. Initial attempts to obtain deletion mutants to probe the role of selethramide failed. After acquiring epigenome (methylome) data for FERM BP-3421, we employed a mimicry by methylation strategy that improved DNA transfer efficiency. Mutants defective in selethramide biosynthesis showed reduced surfactant activity and impaired swarming motility that could be chemically complemented with selethramide. This work unveils a lipopeptide that promotes surface motility, establishes improved DNA transfer efficiency, and sets the stage for continued natural product identification from a prolific strain.
Genetically encoded small molecules (natural products) have important applications in medicine and agriculture (1). Bioinformatics tools to predict biosynthetic gene clusters (BGCs) that encode new natural products have become very sophisticated, and genome mining is increasingly contributing to natural product discovery (2). A recent study showed that within bacteria, the Burkholderiales order is a major source, ranking top three in terms of predicted natural product biosynthetic diversity (3). Burkholderiales are largely generalists (4) with flagellar motility and metabolic versatility that allow them to adapt to diverse habitats. They can be found either free living in environments ranging from pristine soil to contaminated landfill or associated with eukaryotic hosts from fungi to humans (5). This complex ecology is underscored by diverse chemical scaffolds that can be translated into pharmaceutical and agricultural products (5, 6). Yet, while the known metabolites highlight the potential of Burkholderiales as a source of natural products, they represent only a very small fraction of the biosynthetic potential predicted from Burkholderiales genomes (3).
Obtaining the natural products predicted from bacterial genomes remains a bottleneck, in part because many BGCs are not expressed in quantities practical enough to allow detection and isolation (7) leading to repeat examination of the “low hanging fruit” of high-yielding and chromatographically tractable products (8). Yet, multiple natural products are commonly encoded in each bacterial strain with large genomes (8–10). It has been argued that synergy and contingency may explain why multiple BGCs are maintained as multiple natural products provide a selective advantage (9). However, traditional natural products discovery pipelines often reveal only one natural product class from a given bacterial strain, and the strain is then abandoned (8). Systematic strain characterization is a means to expand the known natural product (bio)chemical space (8).
The soil isolate Burkholderia sp. FERM BP-3421 was identified as a producer of the spliceostatin class of cytotoxic agents (Fig. 1A) (11–13). Spliceostatins are splicing modulators that were in preclinical development as antibody-drug conjugates, showing antitumor activity in animal models (11–14). High-cell density growth conditions have been developed to enable production of spliceostatins at gram per liter scale in an industrial setting (15, 16). While some Burkholderia species are pathogenic, FERM BP-3421 has the advantage of having a permissive growth temperature <35 °C that precludes pathogenicity to humans (11). In addition to producing autologous polyketide–nonribosomal peptide spliceostatins in high yields, FERM BP-3421 also supported heterologous production of the ribosomally synthesized and posttranslationally modified peptide (RiPP) capistruin from Burkholderia thailandensis at two orders of magnitude higher titers than with the B. thailandensis native producer (~100 mg/L vs. 0.7 mg/L) (17). Given the promise of this strain, we have been interested in further exploring its biosynthetic potential using multiomic approaches.
Fig. 1.
Natural products from Burkholderia sp. FERM BP-3421. (A) Known natural products previously reported (representative structures of the spliceostatin family) and detected in this study (romidepsin and aminopyrrolnitrin). Reported bioactivities are indicated. (B) Selethramide identified in this study.
Here, we report genomics, metabolomics, and epigenomics studies that revealed two known natural products and led to the identification of another product. In addition to spliceostatins, we show that FERM BP-3421 also produces the known antifungal agent aminopyrrolnitrin and the FDA-approved anticancer drug romidepsin in accordance with the presence of the known BGCs (Fig. 1A). From the orphan metabolite features, we identified a cyclic octadecalipodepsipeptide we named selethramide (Fig. 1B), which is encoded in a giant nonribosomal peptide synthetase. We then harnessed epigenome data to improve DNA transfer efficiency into FERM BP-3421, allowing the generation of selethramide synthetase knockout mutants and the identification of selethramide’s role in swarming motility. The multiomic dataset and new tools pave the way for further natural product discovery in the future.
Results
The Genome of Burkholderia sp. FERM BP-3421 Encodes At Least 29 BGCs.
The genome of FERM BP-3421 was sequenced using Illumina and Nanopore technologies. Hybrid assembly yielded a complete genome containing four circular replicons, i.e., two chromosomes and two plasmids (Fig. 2A and SI Appendix, Table S1) (18). The multireplicon nature is characteristic of Burkholderia bacteria (5). Based on the analysis obtained from the automated multilocus species tree web-based platform (19), FERM BP-3421 forms its own subclade in the phytopathogenic Burkholderia glumae clade of publicly available genomes (SI Appendix, Fig. S1), in agreement with what was previously reported based only on the 16S rRNA gene analysis (12). Moreover, the 16S rRNA gene of FERM BP-3421 shares 100% sequence identity with the soil isolate Burkholderia rinojensis A396, a producer of spliceostatins and romidepsin (Fig. 1A) (20, 21). Although the genome of B. rinojensis A396 is not publicly available, FERM BP-3421 likely belongs to the same species.
Fig. 2.

Genome and metabolome of Burkholderia sp. FERM BP-3421. (A) Genome map highlighting the type and distribution of BGCs. The genome contains four circular replicons, i.e., two chromosomes (Chr.) and two plasmids (p). Replicons are oriented with respect to replication genes dnaA or parA and parB as indicated. The sizes of the replicons are not to scale. The location of BGCs encoding natural products is indicated and color-coded by biosynthetic class as shown (lane 1 from the outside in). BGCs were numbered clockwise from the replication gene used to orient each replicon. Known BGCs (orb, ornibactin; prn, pyrrolnitrin; cay, caryoynencin; fr9, spliceostatins; dep, romidepsin) and selethramide (sel) reported here are indicated. BGCs for which we detected products are in bold. Predicted open reading frames (ORFs) on the leading (black) and lagging (gray) strands are shown on lanes 2 and 3, respectively. A normalized and skewed plot of guanosine + cytosine (G+C) content (yellow/purple) is depicted in lanes 4 and 5 respectively. PKS, polyketide synthase; NRPS, nonribosomal peptide synthetase; RiPP, ribosomally synthesized and posttranslationally modified peptides; Hserlactone, homoserine lactone. Genome maps were generated using DNAplotter (22). Genome accession codes CP117782 (chr 1), CP117781 (chr 2), CP117780 (p1), CP117779 (p2). (B) Molecular network analysis of metabolites produced by FERM BP-3421 in 2S4G medium. MS/MS spectra were window filtered by choosing only the top six fragment ions in the ±5.0-Da window throughout the spectrum. The precursor ion mass tolerance was set to 0.03 Da and a MS/MS fragment ion mass tolerance of 0.03 Da. A network was then created where edges were filtered to have a cosine score above 0.7 and more than 6 matched peaks.
To identify BGCs, we analyzed the genome of FERM BP-3421 using antiSMASH 6.0 (23). Because antiSMASH may miss BGCs encoding natural products with uncommon biosynthesis, we also manually searched for known Burkholderia gene clusters based on our prior review of Burkholderia natural products (5). Using BLAST analysis, we identified caryoynencin in addition to the BGCs identified by antiSMASH. In total, 29 BGCs were identified (Fig. 2A and SI Appendix, Table S2), which is well above the average of 15 reported for Burkholderia genomes (5, 24). As is known for other Burkholderia (5), BGCs are relatively more abundant in chromosome 2 and plasmids as opposed to chromosome 1, which instead contains a larger proportion of essential genes.
Based on sequence identity, five BGCs encode known natural products (Fig. 2A and SI Appendix, Fig. S2), i.e., ornibactin (BGC 1.6), pyrrolnitrin (2.6), caryoynencin (2.10), the spliceostatin family of natural products that includes FR901464 (3.3), and romidepsin (3.5). The remaining 24 BGCs are orphan.
Metabolomic Studies Identify Known Anticancer Romidepsin and Antifungal Aminopyrrolnitrin.
To date, only spliceostatins have been reported from FERM BP-3421 (12). A rich medium containing glycerol as the carbon source and soy peptone as the nitrogen source (2S4G) was previously developed that leads to high cell density and gram-per-liter spliceostatin titers (16). We analyzed the metabolome profile of the wild-type FERM BP-3421 strain using liquid chromatography and tandem mass spectrometry (LC-MS/MS) and molecular networking (25) after cultivation in the 2S4G medium (Fig. 2B). Using an authentic standard, we showed that FERM BP-3421 produces romidepsin (BGC 3.1) (Fig. 2B and SI Appendix, Fig. S3). Although pyrrolnitrin itself was not identified, we detected the precursor aminopyrrolnitrin (Fig. 2B and SI Appendix, Fig. S3). Pyrrolnitrin is biosynthesized in four steps from tryptophan (26). Conversion of the amino group to a nitro group is the final step catalyzed by the Rieske N-oxygenase PrnD that requires a flavin:NADH reductase partner PrnF (26). The lack of a prnF homolog in BGC 2.6 may explain the observed accumulation of aminopyrrolnitrin despite prnD itself being present in the BGC. Further, we did not detect the corresponding m/z features for ornibactin and caryoynencin under the tested cultivation conditions. Biosynthesis of siderophores such as ornibactin may require iron-depleted media, and detection of caryoynencin is challenging due to the highly reactive conjugated alkyne structure leading to a very short half-life (27, 28). Thus, the rich medium used and the long cultivation time (5 d) may have prevented expression and/or detection of ornibactins and caryoynencin.
Identification of Selethramide, a Cyclic Octadecalipodepsipeptide.
From the orphan metabolite features observed, m/z 965 [M+2H]2+ stood out because the fragmentation pattern suggested a large lipopeptide composed mainly of serines and leucines/isoleucines (Fig. 3). The target mass m/z 965 [M+2H]2+ was purified and the structure elucidated. In brief, a 2-L culture of FERM-BP-3421 was extracted, and selethramide was isolated using preparative mass-guided HPLC resulting in an isolated yield of 2.9 mg/L. The molecular formula of selethramide was established as C89H158N18O28 based on the HR-ESI-MS ([M+H]+ peak at 1928.1532). The only BGC that matched the product was the nonribosomal peptide synthetase (NRPS) BGC 1.2 (Figs. 2A and 3A). Based on the predicted biosynthesis of the nonribosomal peptide encoded in cluster 1.2, selethramide consisted of an acyl chain with 18 amino acid residues (six units of d-Ser, one unit of )-Ser, one unit of d-Thr/d-allo-Thr, eight units of )-Leu, and two units of D-Leu, Fig. 3A). The composition and order of the amino acids was confirmed by MS/MS fragmentation analysis of both the intact peptide and the linearized methanolysis product generated by treatment with 0.02M NaOMe in methanol (Fig. 3B and SI Appendix, Fig. S4). The absolute configurations of all amino acids were determined using Marfey’s analysis. The derivatized hydrolysate was annotated using an in-house derivatized amino acids standards library via ultra performance liquid chromatography-mass spectrometry (UPLC-MS) (SI Appendix, Figs. S5–S8 and Table S3). According to the Marfey’s analysis result, combined with predictions from the BGC analysis, the absolute configuration of the amino acids was assigned as D-Ser1-D-Ser2-D-Leu3-)-Leu4-D-Ser5-)-Ser6-D-Leu7-)-Leu8-D-Ser9-)-Leu10-)-Leu11-D-Ser12-D-allo-Thr13-)-Leu14-)-Leu15-D-Ser16-)-Leu17-)-Leu18.
Fig. 3.

Identification of selethramide. (A) Predicted biosynthesis of selethramide. Locus 1.2 contains one contiguous NRPS gene selB encoding 18 modules, in addition to a MbtH-like chaperone selA, that together are proposed to represent the sel BGC. Genes surrounding selAB are expected to be involved in primary metabolism, i.e., a glutathione-S-transferase, and a glycine betaine/l-proline transporter ProP upstream of selA, and a malonate-utilizing operon downstream of selB. The predicted domains of SelB are indicated. The stereoconfiguration of the amino acids was predicted based on the C-domain type present at each module. See also SI Appendix, Fig. S17 for a C-domain phylogenetic tree and sequence alignment. (B) MS/MS fragmentation pattern for selethramide. The amino acid sequences with major fragments consistent with the losses of acyl chain-Ser1 (b2 ion, calcd 1755.0873, expt 1755.0752), Ser2 (b3 ion, calcd 1668.0552, expt 1668.0560), Leu3 (b4 ion, calcd 1554.9712, expt 1544.9613), Leu4 (b5 ion, calcd 1441.8871, expt 1441.8886), Ser5 (b6 ion, calcd 1354.8551, expt 1354.8472), Ser6 (b7 ion, calcd 1267.8231, expt 1267.8250), Leu7 (b8 ion, calcd 1154.7390, expt 1154.7352), Leu8 (b9 ion, calcd 1041.6549, expt 1041.6518), Ser9 (b10 ion, calcd 954.6229, expt 954.6245), Leu10 (b11 ion, calcd 841.5388, expt 841.5395), Leu11 (b12 ion, calcd 728.4548, expt 728.4480), and Ser12 (b13 ion, calcd 641.4227, expt 641.4178) are indicated. For the full-length fragmentation pattern of the linear basic methanolysis product, see SI Appendix, Fig. S4. (C) Selected 2D NMR correlations.
The MS/MS fragmentation data suggested that part of the amino acid chain (from allo-Thr13 to Leu18) was cyclized through one of the free hydroxy groups. The cyclization position and acyl chain composition were determined by detailed interpretation of 1D and 2D NMR spectra (Fig. 3C and SI Appendix, Figs. S9–S15). For the acyl chain, combination of data from 1D and Heteronuclear Single Quantum Coherence NMR spectra clearly showed the presence of one methyl doublet ( H 1.24, C 23.2), one oxygenated methine ( H 4.20, C 66.0), and one diastereotopic methylene ( H 2.39, 2.47, C 45.9). 1H-1H COSY cross-peaks between H-2′ and H-3′, H-3′ and H-4′ along with HMBC cross-peaks of H-2′/C-1′ and H-2/C-1′ indicated the existence of a 3-hydroxybutyric acid moiety connected to Ser1 (Fig. 3C and SI Appendix, Fig. S15). Cyclization of the amino acids was determined from 1H-1H COSY cross-peaks between H-68 and H-69, and H-69 and H-70, and HMBC correlations from H-69 and H-98 to C-103, which together indicated the presence of a cyclized structure from Thr13 to the terminal amino acid, Leu18. This cyclization position was further confirmed by the structure of the basic hydrolysis product (SI Appendix, Fig. S4) which placed the methyl ester from ring opening on the terminal leucine residue (Leu18). Finally, an HMBC correlation between H-68/C-66 showed the connection between the cyclized amino acids and Ser12, defining the position of the amino acid sidechain (Fig. 3C and SI Appendix, Fig. S15).
The absolute configuration of the hydroxy group at C-3′ was determined by synthetic derivatization and comparison to synthetic standards. The free fatty acid was obtained by hydrolysis and derivatized to afford 3-hydroxy-N-((S)-1-phenylethyl)butanamide. The retention time of this 3-hydroxy-N-((S)-1-phenylethyl)butanamide was compared with derivatized commercial standards of (R)-3-hydroxybutyric acid and (S)-3-hydroxybutyric acid via UPLC-MS. The retention time of the derivatized selethramide component aligned with the (R)-3-hydroxy-N-((S)-1-phenylethyl)butanamide reference material from the commercial standard (SI Appendix, Fig. S16). Therefore, the hydroxy group was defined as 3′(R), and the full absolute configuration of selethramide was assigned as shown in Figs. 1B and 3A.
The Epigenome of FERM BP-3421, Prediction of Restriction-Modification Systems, and Mimicry by Methylation Strategy to Improve DNA Transfer Efficiency.
To unequivocally connect selethramide with the NRPS BGC 1.2 (chromosome 1) and to probe its role, we designed a gene deletion construct for homologous recombination using vector pMo130 (29) to generate plasmid pSBR002. However, multiple attempts to transfer pSBR002 into FERM BP-3421 and obtain single cross-over mutants failed. In contrast, we were able to transfer a construct targeting the spliceostatin gene cluster located in plasmid 1 as previously reported (15), indicating that either the product of NRPS 1.2 is essential—which seemed rather unlikely to us—or that there may be a decrease in genome editing efficiency when targeting the chromosome. To improve DNA transfer efficiency, we set out to obtain epigenome data for FERM BP-3421 and to predict Restriction-Modification (R-M) systems.
DNA methylation is the main type of epigenetic modification in bacteria. Three methylated bases can be found in bacterial DNA, i.e., N6-methyladenine (m6A—most common), N4-methylcytosine (m4C), and C5-methylcytosine (m5C). The first described role for DNA methylation in bacteria was in genome defense as part of R-M systems that prevent the uptake of foreign DNA. R-M systems include a restriction endonuclease (R) that recognizes a particular DNA sequence and cleaves the DNA at or around that sequence and a cognate DNA methyltransferase (M) that protects the host’s sequence via methylation. R-M systems are subdivided into four types depending on characteristics such as oligomerization state, sequence recognition, required cofactors, and partner requirement. Moreover, types I, II, and III Rs cleave nonmethylated DNA, whereas type IV Rs cleave only methylated DNA. Type I and III systems are present as R-M pairs, whereas type II and IV systems can be present as R-M pairs or as solitary R or M genes (30).
We obtained methylome data for the FERM BP-3421 wild-type strain by sequencing the genome with Single Molecule, Real-Time (SMRT) sequencing and deriving methylated positions from kinetic variation of nucleotide incorporation. Analysis of the methylome data with REBASE (31) revealed seven genes encoding proteins that are putatively associated with R-M systems, i.e., two R-M pairs of types II and III, two solitary M genes belonging to type II, and one solitary R gene belonging to type IV. The distribution of methylated motifs across the genome and the identified R-M systems are depicted in Fig. 4A. The unsuitability of SMRT sequencing for m5C identification may explain why no methylation motif was predicted for M.BurV.
Fig. 4.
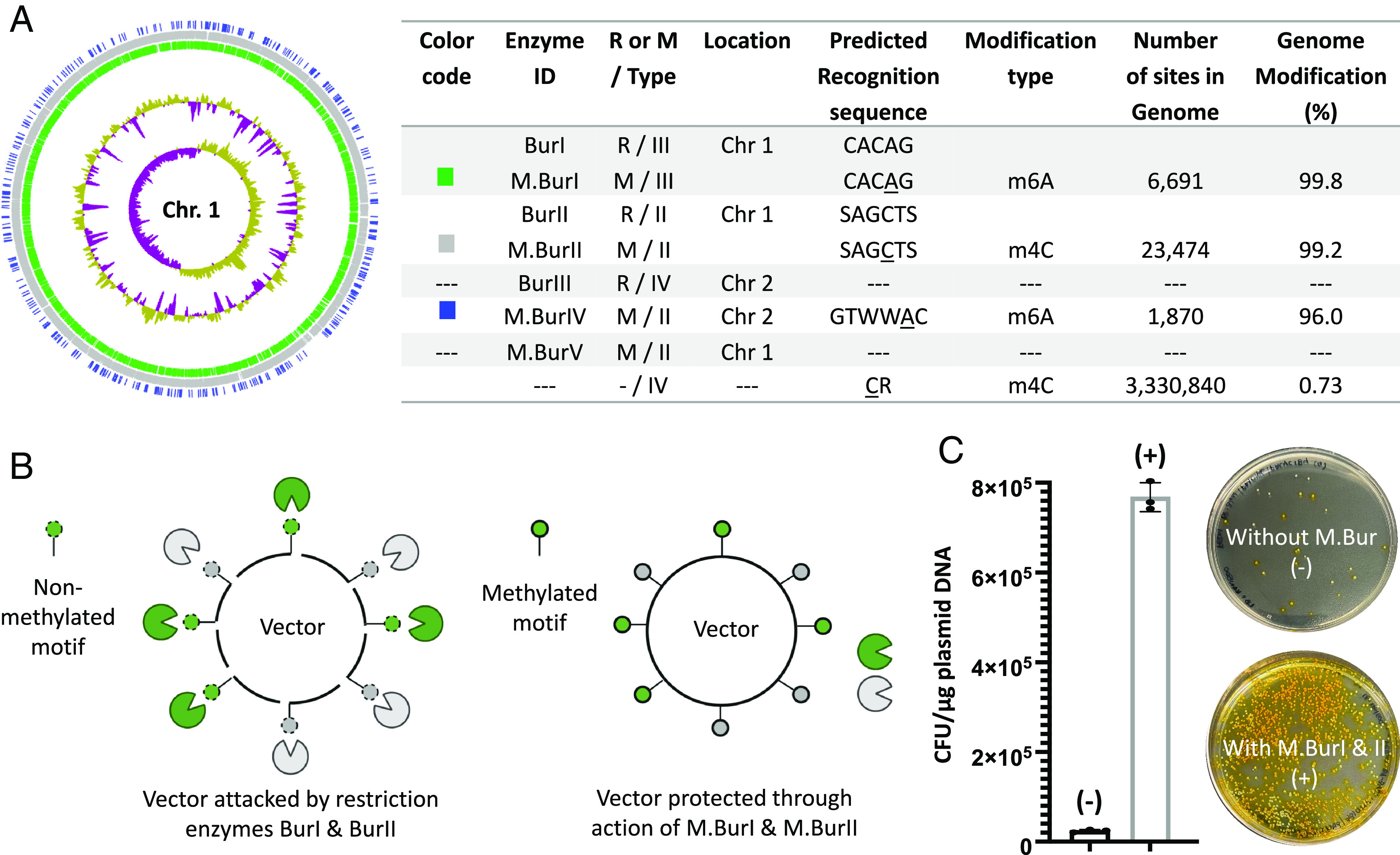
Harnessing epigenome data to improve DNA transfer efficiency. (A) Methylome and predicted R-M systems of Burkholderia sp. FERM BP-3421. Putative R-M systems predicted using REBASE are shown on the table to the right, where underlined bases represent m6A and m4C. S, G, or C; W, A, or T. To illustrate the density of each methylation site, the distribution of methylated motifs is shown on the left for chromosome 1, in lane 1 (M.BurIV, blue), lane 2 (M.BurII, gray), and lane 3 (M.BurI, green), from the outside in. The CR motif is not shown because it appears to be a sequencing artifact. The normalized and skewed plots of G+C content (yellow/purple) are depicted in lanes 4 and 5 respectively. (B) Schematic representation of the mimicry by methylation strategy used to improve DNA transfer efficiency. (C) Comparison of electroporation efficiency calculated as yellow colony forming units (CFU)/µg plasmid DNA. Burkholderia FERM BP-3421 was electroporated with plasmid pMo168 prepared from E. coli carrying either pACYC184_MBurI_MBurII (+) or the empty vector pACYC184 as negative control (−). The error bars indicate SDs (N = 3, P-value < 0.001 using a two-tailed t test). Representative plate results are shown (LB agar with kanamycin selection and catechol metabolism via the xylE reporter present in pMo168, i.e., yellow indicates pMo168 uptake).
To improve DNA transfer efficiency into FERM BP-3421, we employed a mimicry by methylation strategy (Fig. 4B and SI Appendix, Fig. S18) (32). The genes encoding DNA methytransferases M.BurI and M.BurII, associated with restriction enzyme genes, were cloned into pACYC184 (ATCC 37033) to yield pACYC184_MBurI_MBurII (SI Appendix, Figs. S19 and S20). We then used the replicative vector pMo168 containing the broad-host-range replicon from pBBR1 (29) to test whether prior methylation of plasmid DNA improves electroporation efficiency into FERM BP-3421. We observed a 32-fold improvement in electroporation efficiency when using pMo168 isolated from Escherichia coli containing pACYC184_MBurI_MBurII (7.7 × 105 CFU per µg vector DNA) compared to E. coli without M.Bur genes (2.4 × 104 CFU per µg vector DNA) (Fig. 4C and SI Appendix, Fig. S21).
Selethramide Is Required for Swarming Motility.
By using the mimicry by methylation strategy described above, we were able to obtain markerless deletion mutants of NRPS selB by homologous recombination using pSBR002 (SI Appendix, Fig. S22). Comparative metabolite analysis showed that ΔselB mutants are devoid of selethramide production, confirming the link between the sel BGC and selethramide (Fig. 5A). We next tested the hypothesis that selethramide may be involved in mediating surface motility as has been shown for other lipopeptides, which given their amphiphilic nature often act as surfactants (33). Indeed, in contrast to the wild-type, no swarming motility was observed for ΔselB mutants when swarming assays were performed using LB containing 0.5% Bacto agar. Chemical complementation assays were then performed in which phenotypic concentrations of selethramide were added to the media prior to pouring agar plates. Swarming was restored in mutants in the presence of selethramide indicating that selethramide is required for swarming motility under the experimental conditions tested (Fig. 5B and SI Appendix, Fig. S23).
Fig. 5.
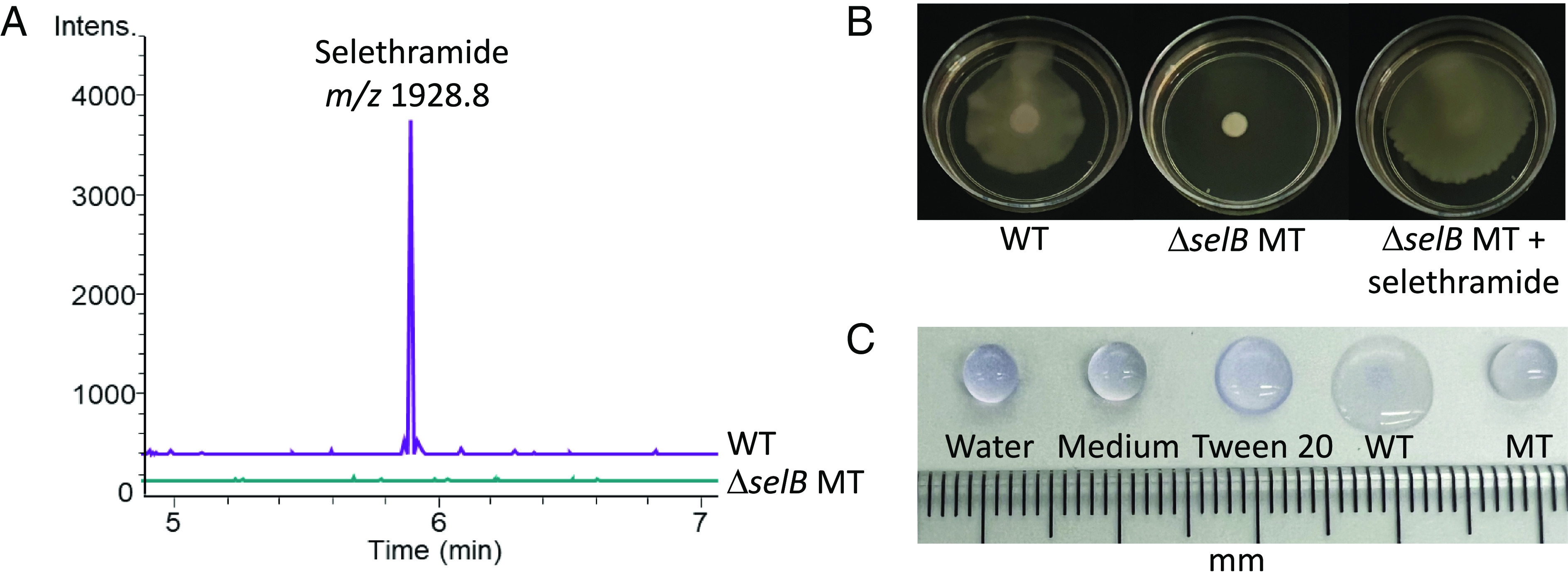
Selethramide is required for swarming motility. (A) Liquid chromatography-mass spectrometry analysis of culture extracts from the wild-type FERM BP-3421 (WT) and the selethramide deletion mutant (ΔselB MT). Extracts were obtained after cultivation in 2S4G medium for 5 d. Extracted ion chromatograms at 1928.84. (B) Swarming assay on LB containing Bacto agar at 0.5% w/v. Pictures taken after 72 h. For chemical complementation, phenotypic concentrations of selethramide were used (3 µg/mL added to plate prior to inoculation). (C) Drop collapse assay. To 20-µL droplets of water containing crystal violet at 0.0025% to facilitate visualization, 5 µL of water or 2S4G production medium was added as negative control (4 mm drop diameter observed), whereas the same volume of aqueous Tween 20 at 0.1% w/v served as positive control (5 mm drop diameter observed). WT (7 mm drop diameter observed) and MT (4 mm drop diameter observed) samples consisted of 5 µL of culture normalized to the same OD600.
Next, we compared the surfactant activity of ΔselB mutants and the wild type using established drop collapse assays (34) (Fig. 5C and SI Appendix, Fig. S24). Water and culture media were used as negative controls and the nonionic surfactant polysorbate 20 (Tween 20) as a positive control. The water droplet collapsed with the wild-type strain culture (7 mm), whereas the drop containing the ΔselB mutant culture showed no collapse, having the same diameter as the negative controls (4 mm). Results were identical between whole culture and supernatant (SI Appendix, Fig. S24). Results obtained with the drop collapse assay support selethramide acting as a surfactant.
Selethramide Shows Only Weak Antibacterial Activity.
Because lipopeptide surfactants often have antimicrobial activity (35), we tested the activity of selethramide against a panel of gram-negative and gram-positive bacteria. Selethramide showed minimum inhibitory concentration (MIC90) against Listeria ivanovii at 64 μM and >64 μM for all other strains tested (SI Appendix, Fig. S25).
Discussion
Advancements in genomics, bioinformatics, and metabolomics are revitalizing natural products as a source of molecules for industrial applications (36, 37). However, translating bioinformatics predictions into natural products remains a barrier. The systematic analysis of promising strains can help expand the known natural product space (8). The genome analysis reported here demonstrates that Burkholderia sp. FERM BP-3421 is prolific not only in terms of autologous spliceostatins and heterologous capistruin product yields (16, 17) but also in terms of the number of BGCs encoded in its genome (29 versus the 15 average reported for Burkholderiaceae) (5, 24). Moreover, various biosynthetic classes are represented (Fig. 2A), indicating its potential capability to produce various products. Metabolomics analyses identified known romidepsin (Istodax®) and aminopyrrolnitrin and led to the isolation of selethramide (Figs. 1 and 2B).
Selethramide has a repeating pattern of hydrophilic (7 serines, 1 allo-threonine) and hydrophobic (10 leucine) amino acids in addition to a N-terminal, short-chain fatty acid we identified as (R)-3-hydroxybutyric acid. The surfactant activity of selethramide is in line with other lipopeptides (33). Although no identical structures have been published, selethramide is reminiscent of the viscosin group of lipononadepsipeptides from Pseudomonas spp. (38–41) and of the lipodecadepsipeptides poaeamide (42) and orfamide H (43), all of which are rich in leucines and serines (although other amino acids may be present such as glutamate and glutamine), are shorter than selethramide, and contain a (R)-3-hydroxy fatty acid albeit with a longer carbon chain (C10-C15) than selethramide (C4) (SI Appendix, Fig. S26).
The selethramide synthase SelB appears to be the largest, contiguous NRPS reported thus far. It is encoded in a single gene of 59.9 kbp yielding a protein (2.2 MDa) that is nearly as large as the ribosome (2.7 MDa). The closest in size according to BLAST searches is the kolossin NRPS (1.8 MDa) from Photorhabdus laumondii subsp. laumondii TTO1 with 50% identity at the protein level (WP_011146892). Kolossin is likewise encoded in one NRPS gene (49.1 kb) but contains 15 modules (44). In addition to kolossin and related NRPS genes from Photorhabdus spp., we identified a similar sel locus in a draft genome of Burkholderia multivorans that includes selA and surrounding genes. However, the draft nature of that genome precluded a full comparison of the partial NRPS gene and selB (SI Appendix, Table S4).
Although our experimental data do not permit assignment of the sel BGC borders (Fig. 3A), it is reasonable to include the MbtH-like gene selA as part of the BGC because MbtH-like proteins have been shown to interact with adenylation domains to stimulate their function (45, 46). Genes surrounding selAB are likely involved in primary metabolism, i.e., glutathione-S-transferase upstream of selA and malonate-utilizing operon downstream of selB. Thus, SelA and SelB are expected to be sufficient for biosynthesis of the core peptide structure since only proteinogenic amino acids would be used and epimerization of l- to d-amino acids would be performed by dual epimerization/condensation domains. Thus, all the required enzymatic activities to select, activate, epimerize, and condense the 18 amino acids are contained in SelB, along with a starter condensation domain to cap serine-1 with a (R)-3-hydroxybutyryl moiety. Biosynthesis of (R)-3-hydroxybutyryl-CoA as the substrate for the starter condensation domain can be accomplished from acetoacetyl-CoA catalyzed by NADPH-dependent (R)-3-hydroxybutyryl-CoA dehydrogenases such as PhaB (47). A PhaB homolog is present in the genome of FERM BP-3421 (locus tag: Bsp3421_000898) as part of an operon likely involved in the synthesis of polyhydroxyalkanoates. Polyhydroxyalkanoates are polyesters of commercial significance as biodegradable plastics such as poly-(R)-3-hydroxybutyrate (48).
To generate selB deletion mutants and probe the role of selethramide, we first needed to improve the DNA transfer efficiency into the wild type. R-M systems have been explored to improve DNA transfer efficiency into diverse bacteria (49–51), but, to the best of our knowledge, not into Burkholderia. Isolates of Burkholderia have been shown to harbor clade-specific R-M systems, which may explain the restriction of gene exchange between clades (52). We first obtained methylome data which revealed three methylation motifs (Fig. 4A). The methylated motifs CACAG and GTWWAC have been previously identified in Burkholderia cenocepacia (53) and in Burkholderia pseudomallei (52). Motif SAGCTS has been previously identified in Robbsia andropogonis (Burkholderiaceae family) and in the γ-Proteobacterium Beggiatoa leptomitiformis (54).
Strategies to evade restriction of incoming DNA can be divided into host-based or vector-based. A host-based strategy is the deletion of restriction enzyme genes from the host’s genome, which can be accomplished only once genetic tractability has been established. The two described vector-based strategies are mimicry by methylation where the vector DNA is methylated to resemble the host DNA prior to transformation and a stealth-based approach where restriction enzyme recognition motifs are removed via synonymous DNA synthesis (55). We view the classical mimicry by methylation strategy as ideal because it does not require DNA synthesis, making it simpler and more affordable than the stealth-based approach. Moreover, because the current knowledge of context-dependent transcription and translation performance is incomplete (56, 57), we prefer to avoid sequence variation as they may affect expression performance (58).
From the four putative DNA methyltransferase genes identified, the two that are colocalized with restriction enzyme genes were employed in a mimicry by methylation strategy (Fig. 4B) that led to a 32-fold improvement in DNA transfer efficiency as measured with a self-replicative vector (Fig. 4C). The electroporation efficiency with the replicative vector (nearly 106 CFU/µg DNA) is still at least two orders of magnitude lower than commercial protocols developed for model E. coli bacteria (108 to 1010 CFU/µg DNA). Future method optimization in addition to the identification of other defense systems against DNA entry (59, 60) could be targeted to further improve DNA transfer efficiency into the Burkholderia strain.
Nevertheless, the improvement we attained was sufficient for our purposes, allowing the generation of a ΔselB mutant (Fig. 5A) and the identification of the role of selethramide in promoting surface motility by acting as a surfactant (Fig. 5 B and C) as reported for other lipopeptides (38–43). The question remains, however, of why the homologous recombination efficiency appears to drop when targeting the main chromosome for gene deletion as done here with selB in comparison to targeting plasmid 1 as we have previously done without major problems when studying spliceostatin biosynthesis (15). Using the model plant Arabidopsis thaliana, Monroe et al. (61) recently challenged the paradigm that mutations are random. Consequently, the ability to engineer different regions of a genome may vary. The authors showed that mutation rates are reduced in essential regions of the genome and that epigenomic and other features such as GC content explain most of the mutation bias (61). The difficulty we encountered when trying to knockout selB aligns with this recent paradigm shift. While still speculative, it makes sense that mechanisms evolved that protect Burkholderia species’ main chromosome against homologous recombination given that it is known to contain a larger proportion of essential genes (62). For example, the GC content of the chromosomes is higher than that of the plasmids (SI Appendix, Table S1). Moreover, different mutation rates have been observed for main chromosomes and smaller replicons within multipartite genomes (63).
In conclusion, the genome, metabolome, and epigenome analyses reported here enabled improvements in DNA transfer efficiency and the identification of selethramide (Fig. 1). Selethramide is required for swarming motility, and as such, it is important to the physiology of the organism, allowing it to reach environments beneficial for survival. The combined data and analyses presented here will facilitate future natural product discovery from a prolific Burkholderia strain. Moreover, the multiomics datasets and improved tools bring us closer to developing this strain into a synthetic biology chassis for natural product identification (64) which we are currently exploring.
Methods
Chemicals, Enzymes, and General Procedures.
Oligonucleotide primers were synthesized by Sigma-Aldrich. Restriction enzymes and T4 ligase were from New England Biolabs. DNA polymerase used in PCR reactions was either from New England Biolabs (Q5 polymerase) or Sigma-Aldrich (Dream Taq polymerase). dNTPs were from Thermo Fisher and DMSO from Sigma Aldrich. PCR amplifications were performed using a Bio-Rad T100 thermal cycler. Agarose gels were prepared with 1% (w/v) agarose in TAE buffer. Gel electrophoresis experiments were performed using a Bio-Rad Sub-Cell GT Agarose Gel Electrophoresis System at 110 V for 60 min. DNA staining after electrophoresis was performed with GelRed Nucleic Acid Gel Stain (Biotium) for 30 min prior to visualization. The accuracy of constructed plasmids was confirmed by Sanger sequencing or whole plasmid sequencing, performed by UIC’s DNA Facility services and Primordium Labs, respectively.
For selethramide isolation and characterization, optical rotations were measured on a Model 341 Polarimeter (PerkinElmer). Ultraviolet absorption spectra were recorded on a Cary 300 UV-Vis spectrophotometer (Agilent Technologies). HR-ESI-MS and MS/MS fragmentation spectra were recorded on a SYNAPT UPLC-ESI-qTOF mass spectrometer (Waters) using an ACQUITY I-Class UPLC® and HSS T3 column (Waters). Marfey’s analysis spectra were performed on a RDa (Waters) ESI-TOF mass spectrometer using an ACQUITY UPLC® and HSS T3 column (Waters). NMR spectra were measured on an AVANCE II 600-MHz spectrometer equipped with a 5-mm QNP cryoprobe (Bruker). MPLC (CombiFlash, Teledyne ISCO) was carried out on RediSep Rf solid load cartridge (5 g, Teledyne ISCO). HPLC separations were performed on an Agilent 1200 series HPLC equipped with a binary pump and a diode array detector using VyDAC C4 and C8 (Hichrom) and Kinetex XB-C18 columns (Phenomenex). Hydrolysis of selethramide was performed using a CEM Discover LabMate microwave reactor. Separation of culture broth and cells and resin was performed by JLA-8.1000 (Beckman Coulter).
Bacterial Strains and Cultivation Conditions.
Burkholderia sp. FERM BP-3421 was obtained from the International Patent Organism Depository at the National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan, and was routinely cultured in Luria-Bertani (LB) broth or LB agar at 30 °C, unless otherwise stated. All bacterial strains were preserved in 20% sterile glycerol in LB at –80 °C.
Genome Sequencing and Assembly.
Illumina and Oxford Nanopore sequencing and hybrid assembly of FERM BP-3421 was performed as described (18). The complete genome sequence is available from GenBank under accession codes CP117779, CP117780, CP117781, and CP117782. Raw reads are available under SAMN31673526.
Methylome Sequencing and Data Analysis.
Genomic DNA of FERM BP-3421 was prepared as described under SI Appendix, Methods, and 28 µg was submitted to the Institute for Genome Sciences at the University of Maryland, School of Medicine for PacBio SMRT sequencing. The quality of the DNA was assessed by a fragment analyzer, and the library was sequenced using a PacBio RS II System. SMRTbell sequencing templates of reads >10 kb were created with the PacBio preparation kit. SMRT sequencing resulted in 322,245 reads and a read N50 value of 4,998 bp. De novo assembly of the genome was achieved by using CANU with default parameters in SMRT Analysis Suite version 9.0 (PacBio). Base modification analysis was performed by mapping SMRT sequencing reads to the assembled genome using the SMRT Analysis Suite version 8.0. Motif Finder was used to identify methylated sequence motifs.
MS2 Data Acquisition and Molecular Networking Analysis.
Production cultures of FERM BP-3421 were prepared, extracted, and analyzed as described under SI Appendix, Methods using a Bruker Compact qTOF mass spectrometer. A molecular network was created using the online workflow (https://ccms-ucsd.github.io/GNPSDocumentation/) on the GNPS website (http://gnps.ucsd.edu) (25). The data were filtered by removing all MS/MS fragment ions within ±17 Da of the precursor m/z. MS/MS spectra were window filtered by choosing only the top 6 fragment ions in the ±50 Da window throughout the spectrum. The precursor ion mass tolerance was set to 0.03 Da and a MS/MS fragment ion tolerance of 0.03 Da. A network was then created where edges were filtered to have a cosine score above 0.7 and more than 6 matched peaks. Further, edges between two nodes were kept in the network if and only if each of the nodes appeared in each other's respective top 10 most similar nodes. Consensus spectra with less than two spectra accumulation were not considered in this analyses. Finally, the maximum size of a molecular family was set to 100, and the lowest-scoring edges were removed from molecular families until the molecular family size was below this threshold. The spectra in the network were then searched against GNPS' spectral libraries. The library spectra were filtered in the same manner as the input data. All matches kept between network spectra and library spectra were required to have a score above 0.7 and at least 6 matched peaks. The obtained data was deposited at MassIVE repository under accession code MSV000090637 (https://massive.ucsd.edu/ProteoSAFe/private-dataset.jsp?task=986136bcba6f4551a085c10f8687fc43).
Isolation and Structure Elucidation of Selethramide.
Two 1-L production cultures of FERM BP-3421 were prepared using 2S4G medium and Amberlite XAD-16 adsorbent resin as described under SI Appendix, Methods. After extraction of the cells and resin mixture with 1:1 dichloromethane and methanol the dried crude extract was fractionated using a CombiFlash system and three rounds of reversed-phase HPLC to give selethramide (5.81 mg). For Marfey’s analysis, selethramide was treated as described under SI Appendix, Methods. Derivatives of selethramide hydrolysate were annotated by the amino acid standards library (SI Appendix, Table S3 and Figs. S5–S8), and the amino acids in the structure were assigned using Waters UNIFI software. See SI Appendix, Methods for acyl chain derivatization and synthesis of selethramide methyl ester for MS/MS analysis of the linear product.
Chemical Characterization of Selethramide.
The observed specific rotation was [α]D20= −20.6 (c 0.20, MeOH). The observed HR-ESI-MS m/z was 1928.1532 [M+H]+ (calcd for C89H159N18O28, 1928.1566). See SI Appendix, Figs. S9–S14 for NMR spectra.
Mimicry by Methylation Studies.
Plasmid pACYC184_MBurI_MBurII was constructed starting from the low copy number plasmid pACYC184 (65) as described under SI Appendix, Methods (SI Appendix, Fig. S19). After whole plasmid sequencing, four sequence differences were identified on the vector backbone compared to the deposited sequence (GenBank accession code X06403) for pACYC184 (SI Appendix, Fig. S20), but M.BurI and M.BurII sequences matched the ones we had on file. The sequence of plasmid pACYC184_MBurI_MBurII was deposited in GenBank under accession code OP823197. To compare transformation efficiency, the pBBR1-based, broad-host-range, replicative plasmid pMo168 (29) was used. Plasmid DNA was isolated from E. coli TOP10 containing pACYC184_MBurI_MBurII and pMo168 or from E. coli TOP10 containing pMo168 and the empty pACYC184. Plasmids purified from each strain were digested and analyzed by gel electrophoresis to confirm the coexistence of pMo168 and the helper plasmid. Each plasmid preparation was analyzed with Qubit to obtain accurate DNA concentrations before the electroporation step. Triplicate electroporations were performed for each plasmid preparation. After incubation for 2 d at 30 °C, the plates were sprayed with a 0.45M catechol aqueous solution [based on the reporter gene xylE (29)] and yellow colonies were counted (SI Appendix, Fig. S21).
Generation of the Selethramide Knockout Mutant via Homologous Recombination.
Plasmid pSBR002 was constructed and transferred into Burkholderia sp. FERM BP-3421 via conjugation from E. coli S17/pACY184_MBurI_MBurII/pSBR002 as described under SI Appendix, Methods. Burkholderia sp. FERM BP-3421/pSBR002 single cross-over mutants were confirmed by PCR and screened for double cross-over events where 15% sucrose was used for vector backbone counterselection. PCR was used to distinguish between reversion to the parent strain or ΔselB deletion mutants. Three independent ΔselB deletion mutants were obtained, the genome of one of which was sequenced to confirm the deletion (SI Appendix, Fig. S22). For comparative metabolite analysis, see SI Appendix, Methods.
Swarming Motility, Drop Collapse Assays, and Selethramide Antibacterial Activity Assays.
Please refer to SI Appendix, Methods and Figs. S23–S25.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank the International Patent Organism Depository of the National Institute of Technology and Evaluation (Japan) for strain FERM BP-3421, M. Voskuil (Addgene) for vectors pMo130 and pMo168 and the American Type Culture Collection for vector pACYC184. Financial support for this work was provided by the National Institute of General Medical Sciences (GM129344 to R.G.L. and A.S.E.) and by the Office of the Director and the National Center for Complementary and Integrative Health (T32 AT007533 to S.B.R.), NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
R.G.L. and A.S.E. designed research; S.B.R., S.L., S.K., B.S.P., M.J.J.R., D.Y.L., and H.C. performed research; S.B.R., S.L., S.K., B.S.P., M.J.J.R., D.Y.L., H.C., R.G.L., and A.S.E. analyzed data; and A.S.E. wrote the paper with input from all authors.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Metabolomics data for FERM BP-3421 have been deposited in MassIVE (MSV000090637 at https://massive.ucsd.edu/ProteoSAFe/private-dataset.jsp?task=986136bcba6f4551a085c10f8687fc43) (66). The NMR data for selethramide have been deposited in the Natural Products Magnetic Resonance Database (www.np-mrd.org) and can be found at NP0331836 (67).
Supporting Information
References
- 1.Newman D. J., Cragg G. M., Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Chevrette M. G., et al. , The confluence of big data and evolutionary genome mining for the discovery of natural products. Nat. Prod. Rep. 38, 2024–2040 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Gavriilidou A., et al. , Compendium of specialized metabolite biosynthetic diversity encoded in bacterial genomes. Nat. Microbiol. 7, 1324 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Sriswasdi S., Yang C. C., Iwasaki W., Generalist species drive microbial dispersion and evolution. Nat. Commun. 8, 1162 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunakom S., Eustáquio A. S., Burkholderia as a source of natural products. J. Nat. Prod. 82, 2018–2037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach E., Passaglia L. M. P., Jiao J., Gross H., Burkholderia in the genomic era: From taxonomy to the discovery of new antimicrobial secondary metabolites. Crit. Rev. Microbiol. 48, 121–160 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Scherlach K., Hertweck C., Mining and unearthing hidden biosynthetic potential. Nat. Commun. 12, 3864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bader C. D., Panter F., Müller R., In depth natural product discovery–Myxobacterial strains that provided multiple secondary metabolites. Biotechnol. Adv. 39, 107480 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Challis G. L., Hopwood D. A., Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. U.S.A. 100, 14555–14561 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegemann J. D., Birkelbach J., Walesch S., Müller R., Current developments in antibiotic discovery: Global microbial diversity as a source for evolutionary optimized antibacterials. EMBO Rep. 24, e56184 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima H., et al. , New antitumor substances, FR901463, FR901464 and FR901465. I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 49, 1196–1203 (1996). [DOI] [PubMed] [Google Scholar]
- 12.He H., et al. , Cytotoxic spliceostatins from Burkholderia sp. and their semisynthetic analogues. J. Nat. Prod. 77, 1864–1870 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Liu X., et al. , Genomics-guided discovery of thailanstatins A, B, and C as pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43. J. Nat. Prod. 76, 685–693 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthenveetil S., et al. , Natural product splicing inhibitors: A new class of antibody-drug conjugate (ADC) payloads. Bioconjug. Chem. 27, 1880–1888 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Eustáquio A. S., Janso J. E., Ratnayake A. S., O’Donnell C. J., Koehn F. E., Spliceostatin hemiketal biosynthesis in Burkholderia spp. is catalyzed by an iron/α-ketoglutarate-dependent dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 111, E3376–E3385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eustáquio A. S., Chang L. P., Steele G. L., O’Donnell C. J., Koehn F. E., Biosynthetic engineering and fermentation media development leads to gram-scale production of spliceostatin natural products in Burkholderia sp. Metab. Eng. 33, 67–75 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Kunakom S., Eustáquio A. S., Heterologous production of lasso peptide capistruin in a Burkholderia host. ACS Synth. Biol. 9, 241–248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunakom S., Adaikpoh B. I., Tran T. A., Eustáquio A. S., Complete genome sequence of soil bacterium Burkholderia sp. strain FERM BP-3421, a producer of spliceostatins. Microbiol. Resour. Announc. 12, e0011123 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alanjary M., Steinke K., Ziemert N., AutoMLST: An automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 47, W276–W282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordova-Kreylos A. L., et al. , Isolation and characterization of Burkholderia rinojensis sp. nov., a non-Burkholderia cepacia complex soil bacterium with insecticidal and miticidal activities. Appl. Environ. Microbiol. 79, 7669–7678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens D. K., et al. , The contribution of romidepsin to the herbicidal activity of Burkholderia rinojensis biopesticide. J. Nat. Prod. 83, 843–851 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Carver T., Thomson N., Bleasby A., Berriman M., Parkhill J., DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 25, 119–120 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blin K., et al. , AntiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 49, W29–W35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X., Cheng Y. Q., Genome-guided discovery of diverse natural products from Burkholderia sp. J. Ind. Microbiol. Biotechnol. 41, 275–284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M., et al. , Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 34, 828–837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J. K., Zhao H., Identification and characterization of the flavin:NADH reductase (PrnF) involved in a novel two-component arylamine oxygenase. J. Bacteriol. 189, 8556–8563 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agnoli K., Lowe C. A., Farmer K. L., Husnain S. I., Thomas M. S., The ornibactin biosynthesis and transport genes of Burkholderia cenocepacia are regulated by an extracytoplasmic function sigma factor which is a part of the Fur regulon. J. Bacteriol. 188, 3631–3644 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross C., Scherlach K., Kloss F., Hertweck C., The molecular basis of conjugated polyyne biosynthesis in phytopathogenic bacteria. Angew. Chem. Int. Ed Engl. 53, 7794–7798 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Hamad M. A., Zajdowicz S. L., Holmes R. K., Voskuil M. I., An allelic exchange system for compliant genetic manipulation of the select agents Burkholderia pseudomallei and Burkholderia mallei. Gene 430, 123–131 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts R. J., et al. , A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 31, 1805–1812 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts R. J., Vincze T., Posfai J., Macelis D., REBASE–a database for DNA restriction and modification: Enzymes, genes and genomes. Nucleic Acids Res. 43, D298–D299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasui K., et al. , Improvement of bacterial transformation efficiency using plasmid artificial modification. Nucleic Acids Res. 37, e3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamley I. W., Lipopeptides: From self-assembly to bioactivity. Chem. Commun. (Camb) 51, 8574–8583 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Youssef N. H., et al. , Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods. 56, 339–347 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Schneider T., Müller A., Miess H., Gross H., Cyclic lipopeptides as antibacterial agents–potent antibiotic activity mediated by intriguing mode of actions. Int. J. Med. Microbiol. 304, 37–43 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Baltz R. H., Genome mining for drug discovery: Progress at the front end. J. Ind. Microbiol. Biotechnol. 48, kuab044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panter F., Bader C. D., Müller R., Synergizing the potential of bacterial genomics and metabolomics to find novel antibiotics. Chem. Sci. 12, 5994–6010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiramoto M., Okada K., Nagai S., The revised structure of viscosin, a peptide antibiotic. Tetrahedron Lett. 13, 1087–1090 (1970). [DOI] [PubMed] [Google Scholar]
- 39.Nielsen T. H., Christophersen C., Anthoni U., Sørensen J., Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J. Appl. Microbiol. 87, 80–90 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Sinnaeve D., et al. , The solution structure and self-association properties of the cyclic lipodepsipeptide pseudodesmin A support its pore-forming potential. Chemistry 15, 12653–12662 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Gerard J., et al. , Massetolides A-H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J. Nat. Prod. 60, 223–229 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Zachow C., et al. , The novel lipopeptide poaeamide of the endophyte Pseudomonas poae RE*1-1-14 is involved in pathogen suppression and root colonization. Mol. Plant Microbe Interact. 28, 800–810 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Ma Z., Zhang S., Liang J., Sun K., Hu J., Isolation and characterization of a new cyclic lipopeptide orfamide H from Pseudomonas protegens CHA0. J. Antibiot. 73, 179–183 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Bode H. B., et al. , Structure elucidation and activity of kolossin A, the d-/l-pentadecapeptide product of a giant nonribosomal peptide synthetase. Angew. Chem. Int. Ed. Engl. 54, 10352–10355 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Zhang W., Heemstra J. R., Walsh C. T., Imker H. J., activation of the pacidamycin PacL adenylation domain by MbtH-like proteins. Biochemistry 49, 9946–9947 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felnagle E. A., et al. , MbtH-like proteins as integral components of bacterial nonribosomal peptide synthetases. Biochemistry 49, 8815–8817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J., Chang J. H., Kim E. J., Kim K. J., Crystal structure of (R)-3-hydroxybutyryl-CoA dehydrogenase PhaB from Ralstonia eutropha. Biochem. Biophys. Res. Commun. 443, 783–788 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Reusch R. N., Physiological importance of poly-(R)-3-hydroxybutyrates. Chem. Biodivers. 9, 2343–2366 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Dong H., Zhang Y., Dai Z., Li Y., Engineering Clostridium strain to accept unmethylated DNA. PLoS One 5, e9038 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B., Yu J., Zhang W., Meldrum D. R., Premethylation of foreign DNA improves integrative transformation efficiency in Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 81, 8500–8506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huff F., Muth C., Naumer C., Meinhardt F., The restriction modification system of Bacillus licheniformis MS1 and generation of a readily transformable deletion mutant. Appl. Microbiol. Biotechnol. 101, 7933–7944 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Nandi T., et al. , Burkholderia pseudomallei sequencing identifies genomic clades with distinct recombination, accessory, and epigenetic profiles. Genome Res. 25, 608 (2015). [PMC free article] [PubMed] [Google Scholar]
- 53.Vandenbussche I., et al. , DNA methylation epigenetically regulates gene expression in Burkholderia cenocepacia and controls biofilm formation, cell aggregation, and motility. mSphere 5, e00455-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fomenkov A., et al. , Complete genome sequence of the freshwater colorless sulfur bacterium Beggiatoa leptomitoformis neotype strain D-402T. Genome Announc. 3, e01436-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston C. D., et al. , Systematic evasion of the restriction-modification barrier in bacteria. Proc. Natl. Acad. Sci. U.S.A. 116, 11454–11459 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kent R., Halliwell S., Young K., Swainston N., Dixon N., Rationalizing context-dependent performance of dynamic RNA regulatory devices. ACS Synth. Biol. 7, 1660–1668 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Biziaev N., et al. , Recognition of 3’ nucleotide context and stop codon readthrough are determined during mRNA translation elongation. J. Biol. Chem. 298, 102133 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang Y., et al. , How synonymous mutations alter enzyme structure and function over long timescales. Nat. Chem. 15, 308–318 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doron S., et al. , Systematic discovery of antiphage defense systems in the microbial pangenome. Science. 359, eaar4120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tesson F., et al. , Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monroe J. G., et al. , Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 602, 101–105 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.diCenzo G. C., Mengoni A., Perrin E., Chromids aid genome expansion and functional diversification in the family Burkholderiaceae. Mol. Biol. Evol. 36, 562–574 (2019). [DOI] [PubMed] [Google Scholar]
- 63.diCenzo G. C., Finan T. M., The divided bacterial genome: Structure, function, and evolution. Microbiol. Mol. Biol. Rev. 81, e00019-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Lorenzo V., Krasnogor N., Schmidt M., For the sake of the bioeconomy: Define what a synthetic biology chassis is! N. Biotechnol. 60, 44–51 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Chang A. C. Y., Cohen S. N., Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134, 1141–1156 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanowski S. B., et al. , Identification of the lipodepsipeptide selethramide encoded in a giant nonribosomal peptide synthetase from a Burkholderia bacterium. MassIVE Database. https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=986136bcba6f4551a085c10f8687fc43. Deposited 3 November 2022. [DOI] [PMC free article] [PubMed]
- 67.Romanowski S. B., et al. , Identification of the lipodepsipeptide selethramide encoded in a giant nonribosomal peptide synthetase from a Burkholderia bacterium. Natural Products Magnetic Resonance Database. https://np-mrd.org/natural_products/NP0331836. Deposited 20 September 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Metabolomics data for FERM BP-3421 have been deposited in MassIVE (MSV000090637 at https://massive.ucsd.edu/ProteoSAFe/private-dataset.jsp?task=986136bcba6f4551a085c10f8687fc43) (66). The NMR data for selethramide have been deposited in the Natural Products Magnetic Resonance Database (www.np-mrd.org) and can be found at NP0331836 (67).



