Significance
Evidence for functional recovery using in vivo neuronal conversion technology after brain injury is limited, and a direct contribution of induced neuronal cells to recovery remains unclear. Because microglia/macrophages converge internally at the site of injury where substantial neuronal death occurs, a lesion core convergent cell population composed of microglia/macrophages may be an ideal target for direct conversion and recovery of lost neurons following brain injury. Our results show that expression of the transcription factor NeuroD1 converts microglia/macrophages into striatal projection neurons in ischemic regions of transient middle cerebral artery occlusion (tMCAO) mice. This neuronal conversion directly contributes to functional recovery after tMCAO.
Keywords: direct reprogramming, microglia, NeuroD1, stroke
Abstract
Although generating new neurons in the ischemic injured brain would be an ideal approach to replenish the lost neurons for repairing the damage, the adult mammalian brain retains only limited neurogenic capability. Here, we show that direct conversion of microglia/macrophages into neurons in the brain has great potential as a therapeutic strategy for ischemic brain injury. After transient middle cerebral artery occlusion in adult mice, microglia/macrophages converge at the lesion core of the striatum, where neuronal loss is prominent. Targeted expression of a neurogenic transcription factor, NeuroD1, in microglia/macrophages in the injured striatum enables their conversion into induced neuronal cells that functionally integrate into the existing neuronal circuits. Furthermore, NeuroD1-mediated induced neuronal cell generation significantly improves neurological function in the mouse stroke model, and ablation of these cells abolishes the gained functional recovery. Our findings thus demonstrate that neuronal conversion contributes directly to functional recovery after stroke.
Stroke is often associated with severe disabilities, high recurrence rate, and other poor outcomes. Since neuronal loss is a major pathological hallmark of brain injury, regenerating new neurons to replenish lost neurons in the injured area is critical for functional recovery. An advanced approach toward achieving the replacement of damaged neurons is to induce in vivo fate conversion of non-neuronal cells residing within the injured brain into neurons. Several groups have achieved in vivo fate conversion within the mouse brain by instructing endogenous astrocytes or oligodendrocyte progenitor cells to generate induced neuronal (iN) cells (1–7). Moreover, a recent study suggests the conversion of astrocytes to iN cells in a focal ischemia model induced by the vasoconstrictive peptide endothelin-1, resulting in the improvement of some neurological dysfunctions (8). However, most thromboembolic infarcts in humans occur in the territory of the middle cerebral artery (MCA) (9), and transient MCA occlusion (tMCAO) induces massive loss of astrocytes as well as neurons in the injured area (10), which makes it difficult to target astrocytes for neuronal reprogramming in this context. Thus, it is important to assess the effect of iN cell generation by targeting non-neuronal cells residing within the injured area other than astrocytes using a tMCAO model. Furthermore, it has yet to be shown whether the iN cells participate directly in functional recovery following brain injury, which is an outstanding critical question to be solved before we can move forward to clinical application using this in situ neuronal conversion technology.
Microglia are CNS-resident macrophages and are derived from primitive macrophages (11), which arise from early erythro-myeloid progenitors in the yolk sac during the early embryonic stage (12–14). With the establishment of the blood circulation, these primitive macrophages migrate into the developing CNS, and a combination of ontogeny and CNS environment confers the microglial signature on the migrated cells (15–17). Although microglia maintain their CNS population by self-renewal in the normal brain, CNS injury prompts peripheral macrophages to infiltrate into the brain by passing through a disrupted blood–brain barrier. CNS-infiltrated macrophages initially retain peripheral identity, but they eventually acquire a highly similar gene expression pattern to that of brain-resident microglia and act similarly to microglia after injury (18, 19).
In response to ischemic stroke within the MCA, although astrocytes become reactive and form an astrocytic scar in the peri-injured region, microglia/macrophages converge more internally within the area of insult, i.e., in the lesion core, where neuronal loss is prominent (10, 20–24). Since microglia as well as macrophages regenerate rapidly (25), microglia/macrophages that converge at the lesion core should be an ideal target for direct conversion to replenish lost neurons after brain injury without exhaustion of their source in the brain. We have previously revealed that a single transcription factor, NeuroD1 (ND1), directly converts microglia into neurons both in vitro and in the mouse adult brain under normal conditions (26). Here, we report that ND1 can convert microglia/macrophages into iN cells in the injured striatum of tMCAO mice. ND1-converted iN cells expressed a marker for striatal projection neurons (SPNs) and were functionally integrated into the existing brain circuits in the ischemic area. In addition, iN cell-specific ablation abolished the restored neurological function in tMCAO mice, indicating that iN cells make a direct and major contribution to the attained functional recovery after ischemic injury.
Results
Microglia/Macrophages Accumulate at the Ischemic Core After tMCAO.
We first examined the condition of the lesion site induced by 30 min of right tMCAO, using immunohistochemistry. The brain damage is largely restricted to the striatum after 30 min of tMCAO in mice (10, 27, 28). Consistent with these reports, we observed considerable loss of DARPP32-positive SPNs in the striatum at 7 d after tMCAO (Fig. 1A) and also found that cells positive for Iba1 (a marker for both microglia and peripheral macrophages) converged in the lesion core (Fig. 1A). By contrast, GFAP-positive astrocytes resided in the peri-infarct area and surrounded Iba1-positive cells in the core region (Fig. 1A), in agreement with previous studies (10, 22, 23). Next, we checked the spatiotemporal distribution of microglia/macrophages in the injured striatum after tMCAO by chronological immunohistochemistry using an additional antibody for a microglial marker, Tmem119 (18, 29), which is also expressed in microglia-like cells derived from peripheral macrophages but not in peripheral macrophages themselves. Although only a small number of Iba1/Tmem119-double-positive cells were observed in the injured region at day 3 after tMCAO, these cells dramatically increased in number by day 7 and were sustained at day 10 (Fig. 1 B and C). We also detected cells displaying sustained peripheral macrophage-type marker expression (Iba1-positive and Tmem119-negative) in the injured region by day 10, and these cells were virtually absent on day 28 (Fig. 1 B and C).
Fig. 1.
Microglia/macrophages accumulate in the ischemic core after tMCAO. (A) Representative images of staining for GFAP (yellow), DARPP32 (red), and Iba1 (cyan) in the ischemic striatum at 7 d after tMCAO. White dashed enclosure shows the peri-infarct area. Nuclei were stained with Hoechst (Scale bars, 500 μm). CC, corpus callosum. (B) Spatiotemporal distribution of microglia/macrophages after tMCAO. Representative images of staining for Tmem119 (red) and Iba1 (cyan) in the ischemic core of the striatum at 3, 7, 10, and 28 d after tMCAO. Nuclei were stained with Hoechst (Scale bar, 50 μm). (C) Quantification of indicated marker-positive cells in the ischemic core in (B) (n = 4 per group).
ND1 Converts Microglia/Macrophages to iN Cells in the Ischemic Region.
Having observed accumulation of microglia/macrophages in the lesion core after tMCAO, we then assessed whether ND1 can induce neuronal conversion from these cells. To this end, we employed a lentivirus, used previously to induce microglia-to-neuron conversion in the intact brain (26), expressing ND1–P2A–EGFP under the promoter of CD68, a gene expressed in the homeostatic state of microglia/macrophages (30) and further upregulated after tMCAO (18). We injected CD68–EGFP virus as control or CD68–ND1–P2A–EGFP virus into the ischemic area at 1 wk after tMCAO, when microglia/macrophages greatly accumulate (Fig. 1 B and C). Two weeks post-injection (wpi) in tMCAO mice, microglia-type cells (Tmem119/Iba1-double-positive) rather than peripheral macrophage-type cells (Tmem119-negative and Iba1-positive) became predominant in the injured area (SI Appendix, Fig. S1 A and B), and over 80% of the control virus-infected cells were microglia-type cells (Fig. 2A). We also found that very few ND1-transduced cells were microglia-type cells at 2 wpi (SI Appendix, Fig. S1C and Fig. 2A), implying that they had already begun to be reprogrammed into iN cells. In addition, only a small percentage of control and ND1-expressing virus-infected cells were GFAP-positive astrocytes and APC-positive oligodendrocytes, and almost no difference in the ratio was observed between cells infected with control and ND1-expressing viruses (SI Appendix, Fig. S1 D and E, and Fig. 2A). In contrast, similar to the case of intact brain (26), we found that approximately 60% and 30% of ND1-transduced cells were positive for βIII-tubulin and Map2ab, respectively (Fig. 2 B–D, and SI Appendix, Fig. S1 F and G), suggesting that ND1-transduced cells turned into iN cells, partially if not completely, by 2 wpi in the injured striatum. These iN cells also expressed Foxp1 (SI Appendix, Fig. S1 H and I), which is highly expressed in SPNs (31). Moreover, when we depleted microglia-type cells (Tmem119/Iba1-double-positive) from the brain by feeding mice with PLX5622 (PLX)-formulated diet continuously from 2 wk prior to tMCAO and until the mice were killed (Fig. 2 E and F), the appearance of ND1-converted Map2ab-positive iN cells was virtually abolished in the ischemic area (Fig. 2 G and H). Peripheral macrophage-type cells (Tmem119-negative and Iba1-positive) remained present in PLX-treated tMCAO mouse striatum and their number was higher than in PLX-untreated tMCAO mouse striatum (SI Appendix, Fig. S1 C, J and K), in agreement with a previous study (32). Taking these observations together with the knowledge that PLX (a CSF1R inhibitor) specifically ablates microglia but not peripheral macrophages (32, 33) (Fig. 2F and SI Appendix, Fig. S1 C, J, and K), it is conceivable that the majority of original cells converted into iN cells by CD68–ND1–P2A–EGFP virus were microglia-type cells composed of resident microglia and microglia-like cells derived from peripheral macrophages in the ischemic brain. However, it remains unclear whether either or both cell populations were actually converted into iN cells.
Fig. 2.
ND1 programs neuronal conversion from microglia/macrophages in the ischemic striatum. (A) Quantification of the indicated marker-positive cells in the striatum at 2 wpi (n = 4 per group). *P < 0.05, two-tailed Mann–Whitney U test. (B and C) Representative images of staining for EGFP (green)-, βIII-tubulin (red)-, and Map2ab (cyan)-positive cells in the ischemic area of control (B) and ND1 (C) virus-treated mice at 2 wpi (Scale bars, 50 μm). (D) Quantification of the indicated marker-positive cells in (B) and (C) (n = 4 per group). *P < 0.05, two-tailed Mann–Whitney U test. (E) Schematic representation of microglial depletion from the brain. (F) Representative images of staining for Tmem119 (red) in the striatum contralateral to the lesion in tMCAO mice treated with control or PLX diets at 2 wpi (Scale bar, 50 μm). (G) Representative images of staining for EGFP (green) and Map2ab (red) in the ischemic area of the striatum in tMCAO mice treated with control or PLX diets at 2 wpi (Scale bar, 50 μm). (H) Quantification of the indicated marker-positive cells in (G) (n = 4 per group). *P < 0.05, two-tailed Mann–Whitney U test.
We also asked whether neuronal conversion from microglia-type cells reduces their number in the injured region. Although the number of microglia-type cells decreased in the core of the virus-infected region of ND1 virus-injected tMCAO mice at 2 wpi compared with control, there was no significant difference in the periphery of the virus-infected region between them (SI Appendix, Fig. S1L).
Neural Stem/Precursor Cells in the Subventricular Zone Are not the Origin of iN Cells.
In the ischemic brain, it has been reported that a limited population of new neurons derived from neural stem/precursor cells (NS/PCs) in the subventricular zone (SVZ) migrate toward the injured site (34). To exclude the possibility that a subpopulation of ND1-converted iN cells originate from such NS/PCs, we crossed Nestin–CreERT2 mice with Stop–EGFP mice and administered their offspring with tamoxifen orally before tMCAO surgery to permanently label Nestin-positive NS/PCs and their progeny (SI Appendix, Fig. S2A). EGFP-positive cells appeared in the SVZ after tMCAO, but no tdTomato-positive iN cells induced by CD68–ND1–P2A–tdT virus in the ischemic area were positive for EGFP (SI Appendix, Fig. S2 B–D), indicating that NS/PCs could not be the source of iN cells converted by ND1 expression in this experimental setting.
ND1-converted iN Cells Acquire Functional Properties of SPN-like Cells.
Since considerable numbers of DARPP32-positive SPNs are lost after tMCAO (Fig. 1A), their regeneration is paramount for adequate functional recovery. When we performed immunostaining with an antibody for DARPP32, the DARPP32-negative area decreased in the ND1-treated striatum after tMCAO compared with control. Moreover, no change occurred in the soma size of DARPP32-positive cells in the periphery of the ischemic core (Fig. 3 A and B). These data suggest that ND1 transduction could repopulate a subtype of cells expressing the SPN-specific marker in the injured striatum. Next, we asked whether ND1-converted iN cells are indeed functionally integrated into brain circuits as SPN-like cells. When assessed at 4 wk after CD68–ND1–P2A–EGFP lentivirus injection into the striatum of tMCAO mice, 70% of ND1-transduced cells had become positive for DARPP32 (Fig. 3 C and D). ND1-converted iN cells in the striatum also expressed Ctip2, which controls the maturation and survival of SPNs (31), whereas these cells did not express Cux1 (a marker for cortical neurons) or calretinin (a marker for interneurons) (SI Appendix, Fig. S3 A and B). We also performed patch-clamp recordings of iN cells in the striatum and observed spontaneous action potential firing following depolarization under the current-clamp condition, whereas control virus-infected microglia/macrophages lacked such firing (Fig. 3 E and F, and SI Appendix, Fig. S4 A and B). Furthermore, iN cells showed long latencies of spike generation in response to current pulse injections (SI Appendix, Fig. S4C), consistent with the behavior of typical SPNs (35, 36). The median resting membrane potential of iN cells (–87.9 ± 5.5 mV) was similar to what is typical for SPNs (37–40), whereas iN cells had higher mean input resistance (537 ± 71 MΩ) and slightly lower mean whole-cell capacitance (90.3 ± 11.9 pF) compared to typical SPNs (SI Appendix, Fig. S4 A and D). Moreover, the input resistance of iN cells varied widely, ranging from 180 to 950 MΩ. We also detected spontaneous synaptic events, both excitatory and inhibitory, in iN cells (Fig. 3G), in contrast with control virus-infected microglia/macrophages (SI Appendix, Fig. S4E). The frequency and amplitude with these iN cells were similar to those with iN cells in the intact mice (26), indicating that iN cells receive synaptic inputs from regions outside the striatum such as the cortex or thalamus, as well as from within the striatum. These data suggest that microglia/macrophages were converted into functional iN cells, although some iN cells may not have yet fully matured by 4 wk after viral injection, in agreement with a previous report (41).
Fig. 3.
ND1 converts microglia/macrophages into SPN-like cells in the ischemic striatum. (A) Representative images of staining for DARPP32 (red) and NeuN (cyan) in control and ND1 virus-treated ischemic hemispheres from bregma to 0.96 mm rostral at 8 wpi. White dashed enclosures show the DARPP32-negative area in the striatum (Scale bar, 1 mm). (B) Quantification of total DARPP32-negative area (Upper) in the striatum of the five slices in (A) (range from bregma to 0.96 mm rostral) (n = 8 mice per group), and soma area of DARPP32-positive cells in the periphery of the ischemic core (lower) within the same range (n = 100 cells per group). *P < 0.05, two-tailed Mann–Whitney U test. (C) Representative images of staining for EGFP (green), DARPP32 (red), and NeuN (cyan) in ND1 virus-injected striatum at 4 wpi (Scale bar, 10 μm). (D) Percentage of DARPP32-positive cells among EGFP-positive cells in control or ND1 virus-treated ischemic regions at 4 wpi (n = 4 per group). *P < 0.05, two-tailed Mann–Whitney U test. (E) Representative image of a recorded EGFP-positive iN cell labeled with biotin at 4 wk after ND1 transduction (Scale bar, 10 μm). (F) Representative traces of spontaneous firing activity of action potentials by the depolarizing current steps in an iN cell in the striatum under the current-clamp condition. The inset indicates the configuration of step-pulses elicited from the patch pipette (cumulative step stimulation from the resting potential with 10 pA for 500 ms duration). (G) Representative traces of spontaneous excitatory postsynaptic currents (sEPSCs) and spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from iN cells, and bar graphs showing the frequency (Left) and amplitude (Right) of sEPSCs of control (n = 11 cells from 3 animals) and iN (n = 10 cells from 3 animals) cells and sIPSCs of control (n = 11 cells from 3 animals) and iN (n = 8 cells from 3 animals) cells in the striatum under the voltage-clamp condition.
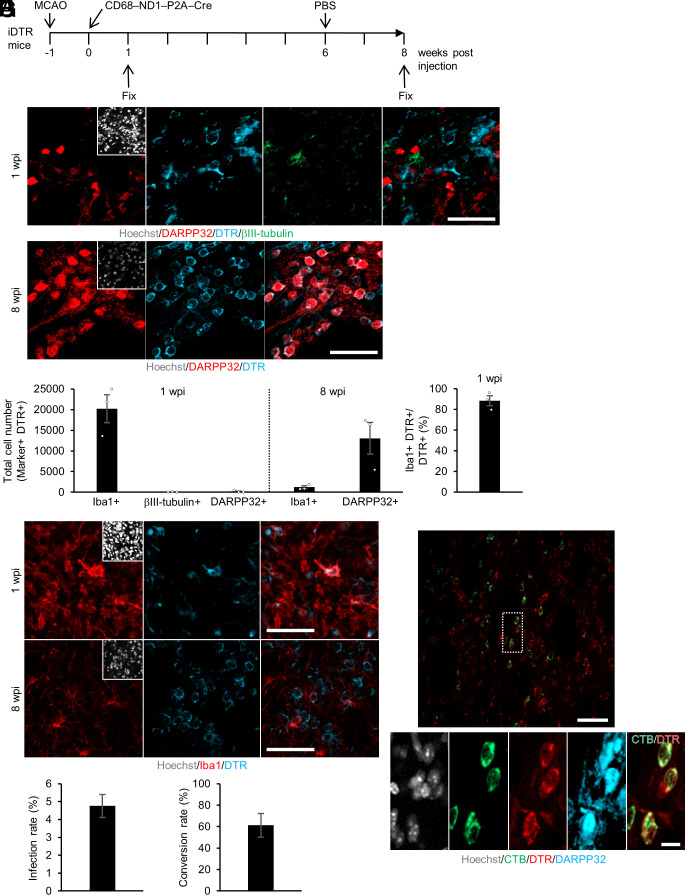
We then investigated how many microglia/macrophages were converted into iN cells in the injured area and maintained for a long period after tMCAO (Fig. 4A). We employed Cre-inducible diphtheria toxin receptor (iDTR) transgenic mice. Cre-transduced cells in iDTR mice permanently express DTR, even after neuronal conversion, which enables us to trace as well as ablate iN cells. We injected CD68–ND1–P2A–Cre lentivirus into the striatum of iDTR mice at 1 wk after tMCAO and traced at two different time points (1 wpi and 8 wpi). Although iN cells were detected at 2 wpi (Fig. 2 C and D), almost no DTR and βIII-tubulin double-positive iN cells were observed at 1 wpi (Fig. 4 B, Upper panels, and C), indicating that ND1-transduced cells had not yet converted into iN cells at this early time point. Furthermore, there were about 20,000 Iba1/DTR-double-positive cells in total (88% among total DTR-positive cells) in the lesion site at 1 wpi, whereas there were about 2,700 DTR-positive other cell types (12% among total DTR-positive cells) (Fig. 4C). Moreover, although only a small fraction of Iba1/DTR-double-positive microglia/macrophages expressed Ki67 (a marker for cell proliferation), no or almost no other DTR-positive cells including GFAP-positive astrocytes and APC-positive oligodendrocytes did so (SI Appendix, Fig. S5 A–E). Additionally, in contrast to microglia/macrophages, GFAP and Ki67 dual-positive astrocytes were almost absent in the lesion core at all investigated time points (day 3, 7, 10, and 28) after tMCAO (SI Appendix, Fig. S5 F–I), leading us to conclude that the majority of the iN cells were derived from the 20,000 Iba1/DTR-double-positive microglia/macrophages and not from the 2,700 Iba1-negative/ DTR-positive non-microglial/macrophage proliferating cells.
Fig. 4.
iN cells survive in the injured region and project their axons into the globus pallidus. (A) Schematic representation of infected microglia/macrophages at 1 wpi and iN cells at 8 wpi in tMCAO mice injected with CD68–ND1–P2A–Cre lentivirus into the striatum. (B) Representative images of staining for DARPP32 (red), DTR (cyan), and βIII-tubulin (green) in the ischemic area of ND1–P2A–Cre virus-injected striatum at 1 wpi or staining for DARPP32 (red) and DTR (cyan) in the ischemic area of ND1–P2A–Cre virus-injected striatum at 8 wpi (Scale bars, 50 μm). (C) Quantification of the indicated marker-positive cells in the virus-injected area of the striatum (n = 3 per group). (D) Representative images of staining for Iba1 (red) and DTR (cyan) in the ischemic area of ND1–P2A–Cre virus-injected striatum at 1 or 8 wpi (Scale bars, 50 μm). (E) Infection rate of microglia/macrophages following virus injection into the striatum (n = 3 per group). (F) Neuronal conversion rate of microglia/macrophages in the striatum (n = 3 per group). (G) Representative images of staining for CTB (green), DTR (red), and DARPP32 (cyan) in the striatum of iDTR tMCAO model mice administered PBS. The mice were injected with CTB into the globus pallidus at 10 wk after CD68–ND1–P2A–Cre lentivirus injection. The Lower image group comprises magnified views of the white dashed box in the Upper panel [Scale bars, 50 μm (Upper), 10 μm (Lower)].
Among microglia/macrophages residing in the area depleted of neurons, 5% were infected with ND1 lentivirus and expressed both DTR and Iba1 at 1 wpi (Fig. 4 D and E). The lineage-tracing experiment revealed that about 60% of ND1-transduced cells were consequently converted into DARPP32-positive iN cells and maintained in the injured striatum until at least 8 wpi (Fig. 4 B, Lower panels, and F). The expression, if any, of βIII-tubulin in mature SPNs is very low compared to that in immature neurons generated from NSCs in the SVZ (42). In agreement with this, almost all iN cells at 8 wpi exhibited no or very little expression of βIII-tubulin (SI Appendix, Fig. S6 A–D), even though iN cells at 2 wpi were clearly expressing βIII-tubulin (Fig. 2 C and D), indicating that iN cells had become more mature SPN-like cells by 8 wpi. The total number of iN cells in the striatum was about 13,000 at 8 wpi (Fig. 4C), a level at which functional recovery of tMCAO mice can be expected by analogy with a previous study of endogenous NS/PC-derived neurons (43). We next checked the anatomical connectivity of iN cells at 8 wpi. The striatum receives projections from dopaminergic neurons in the substantia nigra pars compacta (44). We used an antibody against the rate-limiting enzyme for dopamine synthesis, tyrosine hydroxylase (TH), which can detect the somas of dopaminergic neurons in the substantia nigra pars compacta and their projections in the striatum. We observed that almost all somas of DARPP32/DTR-double-positive iN cells in the injured striatum contacted TH-positive neuronal processes (SI Appendix, Fig. S6 E and F). In addition, some iN cells were labeled with cholera toxin B (CTB, a retrograde axonal tracer) that had been injected into the globus pallidus, a major target area of SPNs (Fig. 4G and SI Appendix, Fig. S6G), suggesting that they projected their axons into the globus pallidus in the same way as intrinsic SPNs do.
Together, these results indicate that ND1-converted iN cells were functionally and anatomically integrated into the neural circuitry in the injured brain.
iN Cells can be Converted from Microglia by ND1 Expression.
Two recent studies have reported that in the adult brain, neurons considered to be converted from glial cells, including astrocytes and microglia, are mislabeled pre-existing neurons (45, 46), although the conclusions are currently the subject of debate (47–49). Therefore, to check the presence of mislabeled pre-existing neurons in our experimental setting, we injected CD68–ND1–P2A–EGFP lentivirus into the ischemic area at 1 wk after tMCAO in adult mice and killed them at 2 and 8 wpi. In contrast to a previous report (45), although EGFP-labeled cells were still maintained in the ischemic core of ND1 virus-injected striatum at 2 wpi, we could hardly detect these cells at 8 wpi (SI Appendix, Fig. S7A), probably because CD68 promoter-driven EGFP expression had already stopped in iN cells. In addition, when we employed iDTR mice, which enabled us to permanently label Cre-transduced cells even after neuronal conversion, we observed DARPP32/DTR-double-positive iN cells that were maintained in the injured striatum until at least 8 wk after CD68–ND1–P2A–Cre lentivirus injection (Fig. 4 B and C). Furthermore, to confirm that ND1-induced iN cells in the tMCAO brain are neither mislabeled pre-existing neurons nor pre-existing neurons fused with ND1-transduced microglia/macrophages in the ischemic striatum, we injected AAV expressing EGFP under the control of the Synapsin promoter into the striata bilaterally (intact and injured sides) at 1 wk before tMCAO (SI Appendix, Fig. S7B) to label pre-existing neurons. One week after tMCAO, we injected ND1 lentivirus into the ischemic core and fixed the mice after 2 wk. In the intact striatum without ND1 virus injection, most EGFP-positive cells were positive for NeuN and DARPP32 (SI Appendix, Fig. S7C), indicating that this AAV system indeed allows us to track pre-existing neurons in the striatum. On the other hand, in the ischemic core injected with ND1 virus, EGFP-positive pre-existing neurons were much fewer than in the contralateral intact striatum, most likely due to neuronal death induced by the injury, and all the βIII-tubulin-positive iN cells were negative for EGFP (SI Appendix, Fig. S7 D and E). These results indicate that pre-existing neurons are not a major cell source for ND1-induced iN cells in the injured striatum.
We next examined whether ND1-converted iN cells in our study originated from microglia. To this end, we employed hexosaminidase subunit beta (Hexb)–CreERT2::Stop–YFP mice, which enable us to specifically label microglia rather than macrophages without ectopic leakage into other CNS cells, including astrocytes, oligodendrocytes, and neurons (50). We have previously revealed that ND1-transduced microglia can be successfully converted into neurons in vitro using this lineage-mapping strategy (51). We administered Hexb–CreERT2::Stop–YFP mice with tamoxifen intraperitoneally at postnatal days P7 and P9 (Fig. 5A). In the virus-uninjected contralateral striatum, almost all YFP-positive cells were Iba1-positive microglia (Fig. 5 B and C, and SI Appendix, Fig. S8), in agreement with the original paper describing Hexb–CreERT2 mice (50). In the ischemic core injected with ND1 virus at 2 wpi, YFP-positive cells exhibited neuron-like morphology, which was different from that of YFP-positive cells in the periphery of the ND1 virus-injected area and in the ischemic core injected with control virus (Fig. 5 D and E). These ND1-induced cells were positive for βIII-tubulin, Map2 ab, and Foxp1, and almost all βIII-tubulin-positive iN cells were positive for YFP (Fig. 5 F and G, and SI Appendix, Fig. S9 A and B), whereas no YFP/βIII-tubulin-double-positive cells were detected in the control virus-injected striatum (Fig. 5 E and F). These data suggest that the majority of ND1-induced iN cells originated from microglia. In addition, almost all YFP-positive cells were Iba1-positive microglia in the ischemic core after control virus injection (SI Appendix, Fig. S9 C and D). None of the YFP-positive cells were DARPP32-positive neurons in the ischemic core injected with the control virus at 2 wpi (SI Appendix, Fig. S9 E and F). These results support our conclusion that microglia but not endogenous neurons are the cell source for newly generated neurons in the ischemic core.
Fig. 5.
Microglia can be converted into iN cells. (A) Schematic representation of tamoxifen-triggered labeling of Hexb-expressing cells for fate mapping. Hexb–CreERT2::Stop–YFP mice were given tamoxifen intraperitoneally at P7 and P9, to permanently label Hexb-positive cells. (B) Representative images of staining for YFP (green) and Iba1 (magenta) in the contralateral striatum at 2 wpi (Scale bar, 50 μm). (C) Quantification of the indicated marker- and YFP-positive cells in the contralateral intact striatum (n = 3). (D and E) Representative images of staining for YFP (green) and βIII-tubulin (magenta) in the control or ND1 virus-injected ischemic area of the striatum at 2 wpi. The Right panels (E) are enlargements of the white dashed boxes in (D) (ischemic core and periphery) (Scale bars, 500 μm) (D), 50 μm (E). CC, corpus callosum. (F) Quantification of βIII-tubulin- and YFP-positive cells in the control or ND1 virus-injected ischemic area of the striatum (n = 3 per group). (G) Quantification of βIII-tubulin- and YFP-positive cells in ND1 virus-injected ischemic area of the striatum (n = 3 per group).
Neuronal Conversion Restores Functional Impairment Induced by tMCAO.
We next asked whether the neuronal conversion restores behavioral impairments caused by tMCAO (Fig. 6A). tMCAO mice exhibited asymmetric body movement in the elevated body swing test, corner test, cylinder test, and corner rotation, in agreement with previous reports (28, 43). We found that iN cell generation significantly improved these neurological dysfunctions from 3 wpi (Fig. 6B and Movies S1–S6). The cylinder test demonstrated spontaneous recovery of control virus-injected tMCAO mice. This test is mainly associated with the motor area in the cortex, where almost no neuronal loss is usually incurred by 30-min tMCAO (10, 27) (Fig. 3A). Considering that 90-min tMCAO in mice induces neuronal loss in the cortex in addition to the striatum, and persistently impairs the behavior associated with the cylinder test (52), 30-min tMCAO-induced neuronal loss in the striatum only transiently affects the cylinder test (28), most likely because the cortex is almost intact. Therefore, we did not observe any significant difference between control and ND1 virus-injected tMCAO mice over time. These data indicate that the generation of iN cells in the injured striatum by ND1 improves neurological dysfunctions in the tMCAO mouse model.
Fig. 6.
ND1-mediated microglia/macrophage-to-neuron conversion restores functional impairment after tMCAO. (A) Experimental timeline to investigate the functional recovery of tMCAO mice. (B) Time course of changes in scores in the elevated body swing test, cylinder test, corner test, and corner rotation test as indicated. Red lines, ND1 virus-treated tMCAO (tMCAO + ND1) group; blue lines, control virus-treated tMCAO (tMCAO + Control) group; gray lines, control virus-infected sham operation (Sham + Control) group (n = 8 per group). Dot plots display each value, with vertical lines representing mean ± SEM. ***P < 0.001, two-way repeated-measures ANOVA, post hoc Bonferroni’s multiple comparison test for tMCAO + ND1 vs. tMCAO + Control. #P < 0.05, ##P < 0.01, ###P < 0.001, two-way repeated-measures ANOVA, post hoc Bonferroni’s multiple comparison test for tMCAO + Control vs. Sham + Control. See also Movies S1–S6.
Ablation of iN Cells Abolishes Gained Functional Recovery.
To reveal whether iN cells contribute directly to the recovery, we ablated them using iDTR transgenic mice. We injected CD68–ND1–P2A–Cre lentivirus into the striatum of iDTR mice after tMCAO and confirmed the dramatic decrease of DTR-positive iN cells following DT administration (Fig. 7 A–C). When we ablated iN cells after functional rescue had been observed, the attained recovery was almost completely abolished in ND1-treated tMCAO mice (Fig. 7D). Moreover, when we eliminated ND1- and Cre-transduced cells at 1 wk before functional recovery was expected (Fig. 6B), these mice showed no sign of functional recovery (SI Appendix, Fig. S10), indicating that iN cell maturation in the injured striatum is prerequisite for the recovery. We also investigated whether DT-induced cell death itself impairs neurological function, and found that ablation of iN cells in intact mice at 2 wpi (SI Appendix, Fig. S11 A–C) did not affect neurological function (SI Appendix, Fig. S11D). Furthermore, if there existed pre-existing neurons mislabeled with CD68–ND1–P2A–Cre virus, their removal should result in impaired behavioral function. However, this was not the case (SI Appendix, Fig. S11D), suggesting that there were few, if any, mislabeled pre-existing neurons, in agreement with the immunostaining results (SI Appendix, Fig. S7D). Thus, these findings indicate that iN cells make a direct and major contribution to the functional recovery induced by ND1-mediated direct neuronal conversion of microglia/macrophages.
Fig. 7.
Ablation of iN cells abolishes gained functional recovery. (A) Schematic representation of the ablation of DTR-expressing cells after functional recovery was observed in tMCAO mice. (B) Representative images of staining for DARPP32 (red) and DTR (cyan) in the striatum of iDTR mice administered DT or PBS. Each rightmost panel is a magnified view of the white square in the panel to its left [Scale bars, 500 μm (Left), 50 μm (Right)]. CC, corpus callosum, LV, lateral ventricle. (C) Ablation rate of iN cells following DT administration in the virus-injected area of the striatum (n = 3 per group). (D) Time course of changes in scores in the indicated tests. iDTR tMCAO model mice were administered DT intraperitoneally at 6 wk after CD68–ND1–P2A–Cre (magenta line, ND1–Cre + DT at 6 wpi, n = 8) or CD68–ND1–P2A–EGFP (black line, ND1–EGFP + DT at 6 wpi, n = 8) lentiviral injection. Dot plots display each value, with vertical lines representing mean ± SEM. ***P < 0.001, two-way repeated-measures ANOVA and post hoc Bonferroni’s multiple comparison test.
Discussion
In this study, we have shown that ND1 enables microglia/macrophages to convert into SPN-like cells, which integrate into existing brain circuits to restore neurological function in the ischemic area of tMCAO mice. After tMCAO, microglia/macrophages have been shown to exhibit both the classical pro-inflammatory and the wound-healing reparative anti-inflammatory state. Anti-inflammatory microglia/macrophages increase during 7 d after tMCAO and subside around day 14, whereas pro-inflammatory microglia/macrophages increase and persist until at least day 14 (24, 53). We have previously shown that in addition to microglia in the normal condition, pro-inflammatory microglia induced by lipopolysaccharide stimulation can be converted into iN cells by ND1 transduction (26). In the present study, we targeted microglia/macrophages residing in the injured area at 1 wk after tMCAO by injecting CD68–ND1–P2A–EGFP virus and observed ND1-converted iN cells at 2 wpi (day 21 after tMCAO) (Fig. 2 C and D), although we found only very few iN cells at 1 wpi (day 14 after tMCAO) (Fig. 4 B and C). Overall, it seems likely that pro-inflammatory microglia/macrophages are mainly converted into iN cells after tMCAO.
Since these microglia/macrophages produce pro-inflammatory cytokines and thus induce further damage to CNS tissue, reducing these neurotoxic cells by neuronal conversion may benefit brain repair by increasing the survival of remaining neurons (54). If this is the case, functional recovery should be retained to some extent, even after the ablation of ND1-transduced cells. However, the recovery attained by neuronal conversion of microglia/macrophages at 3 wpi completely disappeared when we eliminated iN cells at 1 wk before functional recovery was expected (SI Appendix, Fig. S10B), excluding the above possibility.
Microglia/macrophages provide disease-modifying regulation of various types of cells including astrocytes, oligodendrocytes, and leukocytes (55). In this study, only 5% of microglia/macrophages that converged in the ischemic area were infected by ND1-expressing virus (Fig. 4E). In addition, at 2 wpi, neuronal conversion from microglia/macrophages affected the density of microglia/macrophages only in a very limited area of the injury (SI Appendix, Fig. S1L). Since this small number of lost microglia/macrophages can be replenished by repopulation from residual microglia (25) or the phenotypic differentiation of peripheral macrophages to microglia-like cells (18, 19), neuronal conversion from microglia/macrophages causes little exhaustion of the source in the brain and should thus have little adverse effect on the behavior of other cells after tMCAO.
We found that depletion of microglia-type cells (Tmem119/ Iba1-double-positive) by PLX5622 treatment virtually abolished the appearance of ND1-converted iN cells, indicating that the major original cell source converted into iN cells is microglia-type cells and not peripheral macrophage-type cells. However, microglia-like cells originally derived from peripheral macrophages in the lesion area could contribute as a cell source for ND1-mediated neuronal conversion. Although intraperitoneal administration of clodronate liposomes can ablate peripheral macrophages but not microglia (56), it remains to be examined whether this microglia-like population can indeed be converted to neurons.
ND1 is a proneural basic helix–loop–helix transcription factor that is essential for neuronal differentiation from NS/PCs in the CNS. We have previously found that microglia can be directly converted into neurons by expression of this single transcription factor in the intact mouse brain (26). ND1 penetrates closed chromatin regions associated with bivalent histone modifications (both the active histone mark H3K4me3 and the repressive mark H3K27me3 simultaneously) in microglia to induce the expression of neuronal genes. These regions are resolved to a monovalent H3K4me3 mark at later stages of reprogramming to establish neuronal identity. Cells such as non-reactive astrocytes that do not retain the bivalent signature in ND1-bound loci around neuronal genes, on the other hand, cannot be reprogrammed by ND1 (26). In the present study, we have shown that ND1 converts microglia/macrophages accumulated in the injured site to neurons; this is probably because these cells have bivalent histone modifications in ND1-bound loci. Further experiment will determine the epigenomic profile in vivo to examine whether ND1 acts as a pioneer factor in vivo as well as in vitro (26). As we and others have shown, the technology of in situ conversion of non-neuronal cells into neurons has opened a broad avenue for developing therapeutic strategies for neurological disorders. However, regional and characteristic differences regarding the response to conversion-inducing factors among the original cell source must be taken into account to obtain desired neuronal subtypes effectively. For example, astrocytes in the intact striatum (5) but not in the cortex (4) can be reprogrammed by Sox2. Furthermore, the efficiency of conversion into DARPP32-positive SPN-like cells from astrocytes by ND1 in the striatum is quite low (2%) (57), but can be improved by the combined expression of ND1 with Dlx2 (41). In contrast, expression of ND1 alone in microglia in the same brain region can efficiently induce conversion into DARPP32-positive SPN-like cells (75%) (26). In the present study, we found that striatal microglia/macrophages that have converged in the ischemic area can also convert into DARPP32-positive SPN-like cells with high efficiency (70%) (Fig. 3D). These observations suggest that the epigenetic profiles in the original cells and/or external cues from niches in each brain region can determine the response to conversion-inducing transcriptional factors and affect the efficiency of reprogramming to the desired neuronal subtypes. Therefore, although our in situ neuronal conversion technology based on ND1 transduction in microglia/macrophages to treat ischemic injury should be applicable to CNS injuries and diseases other than stroke as long as endogenous microglia/macrophages are available and have bivalent histone modifications in ND1-bound loci, regional variation in the response to conversion-inducing transcriptional factors may limit the efficacy of appropriate iN cell production. In such cases, selection of more suitable conversion-inducing transcription factors than ND1 for region-specific microglia would become essential, warranting further investigation to provide a strategy best matched to each neurological disorder.
Many challenges still remain for the clinical application of neuronal conversion. For example, it has not yet been determined whether ND1 alone can convert human microglia into neurons as well as mouse microglia: ND1 may require collaboration with other transcription factors to efficiently induce neuronal conversion of human microglia. In addition, although lentiviral vectors were used to infect mouse microglia in this study, viral toxicity, side effects of gene transfer, and route of administration must be examined and optimized using higher mammals such as nonhuman primates before actual clinical application.
Nevertheless, we believe our finding, that forced neurogenesis from microglia/macrophages (microglia-type cells) in the injured area through in vivo direct reprogramming reinstates neurological function, holds great promise as a therapeutic strategy to treat ischemic injury and other CNS diseases characterized by neuronal loss, such as traumatic brain injury and spinal cord injury.
Materials and Methods
Mice.
All efforts were made to minimize animal suffering and to reduce the number of animals used. Animals were housed under a 12/12-h light/dark cycle and fed ad libitum. Male 8-wk-old wild-type mice (C57BL/6, obtained from Japan SLC), male Nestin–CreERT2::Stop–EGFP (a generous gift from Dr. I. Imayoshi), male iDTR mice (purchased from the Jackson Laboratory), and male and female Hexb–CreERT2::Stop–YFP mice (50) were used for a mouse model of ischemic stroke. All experiments were carried out according to the animal experimentation guidelines of Kyushu University, Tokai University, or University of Tokyo, which comply with the NIH Guide for the Care and Use of Laboratory Animals.
Mouse Model of Ischemic Stroke.
tMCAO was performed as described previously (58, 59). Briefly, 8-wk-old mice were anesthetized with a mixture of 4 mg/kg midazolam, 0.3 mg/kg medetomidine, and 5 mg/kg butorphanol, and tMCAO was generated by the insertion of a silicon-coated monofilament (6-0 medium MCAO suture L34 No. 602334, Doccol Corporation) via the right proximal external carotid artery into the internal carotid artery. The distance from the suture tip to the right common carotid artery bifurcation was 9–10 mm. Thirty minutes after the right tMCAO, the inserted monofilament was withdrawn to allow reperfusion. After the operation, the effects of medetomidine were reversed with atipamezole. Mice that displayed more than 80% of biased swing behavior in the elevated body swing test and more than 80% of biased turning behavior in the corner test 1 wk after tMCAO were used as tMCAO mice in this study. For sham group animals, vessels were visualized and cleared of overlying connective tissue as would be done in normal surgical dissection, but no additional manipulations were made. The operator assigned numbers to mice at random and assessed behavioral testing by managing the numbers so as to be blind to the treatments during the testing.
Behavioral Testing.
Elevated body swing test.
By the base of the tail, the mouse was elevated to approximately 10 cm from the bottom of the cage and the direction of the first body swing, more than 10° bending of the upper body from the vertical axis to either side, was recorded (60). The procedure was repeated 20 times. The laterality index was computed as follows: [Swings (left)]/[Swings (left) + Swings (right)]. The experimenter was blinded to the treatment group throughout behavioral testing. Other investigators blinded to the treatments also checked the movies of behavioral testing.
Corner test.
A modified version of the corner test was used (28). The testing apparatus consisted of four connecting board walls joined at 30° and 150° angles. To start the trial, animals were placed halfway in and facing a corner. When the mouse entered the deep part of the corner, both sides of the vibrissae were stimulated together by the two boards. In response, the mouse reared forward and upward and then turned back to face the open end. Ten trials were performed for each mouse and the percentage of right turns was calculated. Only turns involving full rearing along either board were recorded. Occasional turns that did not involve the initial rearing motion were not counted. The laterality index was computed as follows: [Turns (right)]/[Turns (left) + Turns (right)]. The experimenter was blinded to the treatment group throughout behavioral testing. Other investigators blinded to the treatments also checked the movies of behavioral testing.
Corner rotation.
To quantify turning behavior independently from the strict rearing and turning paradigm used in the corner test, every complete 180° turn to the left or right with or without rearing during the first 5 min of the corner test was scored (28). Incomplete turns were ignored. The laterality index was computed as follows: [Turns (right)]/[Turns (left) + Turns (right)]. The experimenter was blinded to the treatment group throughout behavioral testing. Other investigators blinded to the treatments also checked the movies of behavioral testing.
Cylinder test.
The mouse was placed in a transparent cylinder 9 cm in diameter and 15 cm in height and videotaped during the test (52). A mirror was placed at an angle behind the cylinder to enable the rater to record forelimb movements when the mouse was turned away from the camera. After the mouse was put into the cylinder, forelimb use in the first contact against the wall after rearing and during lateral exploration was recorded by the following criteria. i) The first forelimb to contact the wall during a full rear was recorded as an independent wall placement for that limb. ii) Simultaneous use of both the left and right forelimb by contacting the wall of the cylinder during a full rear and for lateral movements along the wall was recorded as “both” movement. iii) After the first forelimb (for example, the right forelimb) contacted the wall and then the other forelimb was placed on the wall, but the right forelimb was not removed from the wall, a “right forelimb independent” movement and a both movement were recorded. However, if the other (left) forelimb made several contacting movements on the wall, a right forelimb independent movement and only one both movement were recorded. iv) When the mouse explored the wall laterally, alternating both forelimbs, it was recorded as a both movement. A total of 20 movements were recorded during the 10-min test. The laterality index was calculated as follows: [Contacts (right) – Contacts (left)]/[Contacts (left) + Contacts (right) + Contacts (both)]. The experimenter was blinded to the treatment group throughout behavioral testing. Other investigators blinded to the treatments also checked the movies of behavioral testing.
iN Cell Induction.
To induce iN cells, we used lentiviral vectors in which gene expression is controlled under the human CD68 promoter. The human CD68 promoter (30) was cloned into the lentiviral vector FG12 (Addgene) using XbaI and XhoI sites. ND1–P2A was then replaced with the UbiC promoter using XhoI and AgeI sites. EGFP in the CD68–ND1–P2A–EGFP was replaced with Cre or tdTomato using AgeI and BsrgI sites. Lentiviruses were produced by transfecting HEK293T cells with the lentivirus constructs pCMV–VSV–G–RSV–Rev and pCAG–HIVgp using polyethylenimine. Culture supernatants containing lentivirus were collected 48 h after transfection and centrifuged at 6,000g overnight at 4 °C. The virus was concentrated using Lenti-X Concentrator (Clontech), and suspended in PBS.
Stereotactic Brain Injections.
Striata of adult mice were stereotactically injected with 2 μL lentivirus with a titer of 0.5–2 × 109 particles/mL. Injection coordinates were as follows: anterior/posterior, +0.8 mm; medial/lateral, ±2.0 mm; and dorsal/ventral from skull, −3.0 mm.
To label pre-existing neurons, 2 μL of pAAV–Syn–EGFP with a titer of 1.9 × 1012 particles/mL was injected into the bilateral striata.
To label iN cells that projected into the globus pallidus, 0.3 μL of Alexa Fluor 488-conjugated CTB (Invitrogen, C34775) was injected into the globus pallidus of iDTR tMCAO model mice at 10 wk after CD68–ND1–P2A–Cre lentivirus injection. Injection coordinates were as follows: anterior/posterior, −0.5 mm; medial/lateral, ±1.9 mm; and dorsal/ventral from skull, −4.0 mm. Five days later, the animals were fixed.
Microglial Ablation.
For the ablation of brain microglia, mice were fed with PLX5622-formulated D12450J diet (12 mg PLX5622 per kg of diet, Research Diets) ad libitum during 5 wk.
Tamoxifen Administration.
Tamoxifen (Sigma-Aldrich, T5648) was dissolved in sesame oil. For tamoxifen-inducible Cre-mediated EGFP expression, 5 mg of tamoxifen/0.2 mL of oil was orally administered to 6-wk-old Nestin–CreERT2::Stop–EGFP mice for four consecutive days.
For tamoxifen-inducible Cre-mediated YFP expression, 0.4 mg of tamoxifen/20 μL of oil was intraperitoneally administered to Hexb–CreERT2::Stop–YFP (homozygous for Hexb–CreERT2) mice at P7 and P9.
Ablation of iN Cells.
Since mouse cells are much less sensitive to diphtheria toxin (DT) than human cells, we could ablate only Cre-transduced cells in the inducible DT receptor (iDTR) mouse by injecting DT. DT (FUJIFILM Wako Pure Chemical Corporation) was diluted in PBS (5 μg/mL). Two or six weeks after viral injection, 1 μg of toxin or 0.2 mL of PBS was given by intraperitoneal injection for three consecutive days. To detect DTR expression, we used an anti-human heparin-binding EGF-like growth factor (HB-EGF) antibody.
Immunohistochemistry.
Adult mouse brains were fixed in 4% paraformaldehyde, and 40-μm sections were cut with a cryostat (Leica). Cryosections were washed with PBS and blocked for 1 h at room temperature (RT) with blocking solution (5% FBS, 0.3% Triton X-100), and incubated overnight at 4 °C with primary antibodies diluted in blocking solution. The following primary antibodies were used in this study: rabbit anti-Tmem119 (1:200, ab209064; Abcam), goat anti-Iba1 (1:500, ab5076; Abcam), rabbit anti-Iba1 (1:500, 019-19741; FUJIFILM Wako Pure Chemical Corporation), rabbit anti-βIII-tubulin (1:500, PRB-435P; Covance), mouse anti-Map2ab (1:500, M1406; Sigma), rabbit anti-Foxp1 (1:500, ab16645; Abcam), mouse anti-NeuN (1:500, MAB377; Merck Millipore), rabbit anti-DARPP32 (1:500, ab40801; Abcam), chick anti-GFAP (1:500, AB5541; Merck Millipore), rabbit anti-GFAP (1:500, G9269; Sigma), mouse anti-APC (1:1000, OP80; Calbiochem), chick anti-GFP (1:500, GFP-1010; Aves Laboratories), rat anti-GFP (1:500, 04404-84; Nacalai Tesque), rat anti-RFP (1:500, RFP 5F8; ChromoTek), goat anti-HB-EGF (1:200, AF259; R&D Systems), mouse anti-HB-EGF (1:500, 013-27191; FUJIFILM Wako Pure Chemical Corporation), rabbit anti-Ki67 (1:500, ab15580; Abcam), chick anti-tyrosine hydroxylase (1:1000, TYH; Aves Laboratories), rabbit anti-calretinin (1:500, AB5054; Merck Millipore), rat anti-Ctip2 (1:500, ab18465; Abcam), and rabbit anti-Cux1 (1:100, ABE217; Merck Millipore). Nuclei were stained using Hoechst 33258 (Nacalai Tesque). Stained sections were visualized with a confocal microscope (LSM800, Zeiss).
Evaluation of Cell Number and Area.
Marker-positive cell number in the striatum was quantified using every sixth hemisphere section. The number of marker-positive cells was counted and multiplied by 6 to estimate the total number. The infarct area in the striatum was detected by DARPP32 staining and measured using ImageJ software (NIH) and ZEN lite (Zeiss). The infection rate of microglia/macrophages following virus injection in the striatum shows the proportion of Iba1- and DTR-positive cells at 1 wpi among Iba1-positive cells at 7 d after tMCAO. The total number of Iba1-positive cells at 7 d after tMCAO was estimated from cell density multiplied by total area where Iba1-positive cells in the striatum accumulated. The neuronal conversion rate shows the proportion of DARPP32- and DTR-positive cells at 8 wpi among Iba1- and DTR-positive cells at 1 wpi. The ablation rate of iN cells following DT administration shows the proportion of DARPP32- and DTR-positive cells at 8 wpi following DT administration in the virus-injected area of the striatum compared with PBS administration.
Electrophysiology.
Electrophysiological recording was performed at 4 wpi. For ex vivo electrophysiology, striatum slices were cut into 200-μm-thick sections with a VT1000 vibratome (Leica) using ice-cold cutting solution consisting of (in mM) 234 sucrose, 2.5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 25 NaHCO3, 11 glucose, and 0.5 myoinositol. Whole-cell patch-clamp recording was performed in voltage-clamp mode on EGFP-positive cells according to a similar method described previously (61). Signals were recorded using a patch-clamp amplifier (Axopatch200B; Molecular Devices). The external solution consisted of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgCl2, 1.6 CaCl2, 10 glucose, and 25 NaHCO3 saturated with 95% O2 and 5% CO2. Patch pipettes (8–10 MΩ) were filled with an internal solution of (in mM) 120 K-gluconate, 10 HEPES, 0.2 EGTA, 20 KCl, 2 MgCl2, 7 Na2-phosphocreatine, 4 Mg-ATP, and 0.3 Na2-GTP, pH adjusted to 7.3 with KOH. Spontaneous excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) were recorded at a holding potential of −70 mV and 0 mV, respectively, in the presence 10 μM bicuculline methiodide (a GABAA receptor antagonist). The evoked action potentials were recorded in current-clamp mode. Current steps (0–120 pA, increasing in increments of 10 pA) were applied for 500 ms. The spontaneous EPSCs, IPSCs, and evoked action potentials were analyzed by Mini Analysis Program (Synaptosoft) and Clampfit (Molecular Devices). For biocytin labeling, intracellular solution was supplemented with 0.3% biocytin (Vector Laboratories, SP-1120).
Quantification and Statistical Analysis.
No statistical methods were used to pre-determine sample sizes. Statistical analyses were done afterward without interim data analysis. No data points were excluded. We did not use any methods to determine whether the data met assumptions of the statistical approach. All data were collected and processed randomly. A two-tailed Mann–Whitney U test was used to calculate the p value for pairwise comparisons. For multiple comparisons, P values were calculated using two-way repeated-measures ANOVA and post hoc Bonferroni’s multiple comparison test. The values of n (sample size) are provided in the figures and figure legends. We considered P < 0.05 to be statistically significant. Data represent mean ± SEM.
Study Approval.
All experiments were carried out according to the animal experimentation guidelines of Kyushu University, Tokai University School of Medicine, or University of Tokyo, which comply with the NIH Guide for the Care and Use of Laboratory Animals.
Supplementary Material
Appendix 01 (PDF)
Elevated body swing test in tMCAO + Control mouse at 8 wpi, Related to Fig. 6B
Elevated body swing test in tMCAO + ND1 mouse at 8 wpi, Related to Fig. 6B
Elevated body swing test in sham-operated mouse, Related to Fig. 6B
Corner test and corner rotation in tMCAO + Control mouse at 8 wpi, Related to Fig. 6B
Corner test and corner rotation in tMCAO + ND1 mouse at 8 wpi, Related to Fig. 6B
Corner test and corner rotation in sham-operated mouse, Related to Fig. 6B
Acknowledgments
We thank H. Nakashima, S. Katada, and T. Imamura for discussions; Y. Nakagawa for excellent secretarial assistance; I. Smith for proofreading the manuscript; I. Imayoshi for sharing Nestin-CreERT2 mice; L. Cong, S. Yeung, P. Singh, and A. Rymar (Plexxikon) for providing PLX5622; I. Goto (EPS EKISHIN) for providing the PLX5622-formulated diet; and M. Tachibana, T. Shibahara, T. Ago, K. Tanaka, Y. Kawamura, and N. Kaneko for teaching us how to make and evaluate tMCAO model mice. We appreciate the technical assistance from the Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences, which is partially supported by the Mitsuaki Shiraishi Fund for Basic Medical Research. This work was supported by the Kaibara Morikazu Medical Science Promotion Foundation (to T.I.), a Grant-in-Aid for Young Scientists (B) JP18K14820 (to T. Matsuda), a Grant-in-Aid for Scientific Research (B) JP21H02808 (to T. Matsuda), a Grant-in-Aid for Exploratory Research JP23K18451 (to T. Matsuda), the Takeda Science Foundation (to T. Matsuda), the Qdai-jump Research Program (Wakaba Challenge) of Kyushu University (to T. Matsuda), a research grant from The Noguchi Institute (to T. Matsuda), AMED JP21bm0404057 (to T. Matsuda and K.N.), AMED-CREST JP20gm1310008 (to K.N.), the Suzuken Memorial Foundation (to K.N.), the Naito Foundation (to K.N.), a Grant-in-Aid for Scientific Research on Innovative Areas JP16H06527 (to K.N.), JP16K21734 (to K.N.), and a Grant-in-Aid for Challenging Research (Exploratory) JP19K22473 (to K.N.).
Author contributions
T.I., T. Matsuda, and K.N. designed research; T.I., T. Matsuda, Y.H., and K.M.-I. performed research; A.K., T. Masuda, and M.P. contributed new reagents/analytic tools; T.I., T. Matsuda, Y.H., K.M.-I., N.I., J.-i.K., and K.N. analyzed data; and T.I., T. Matsuda, and K.N. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Taito Matsuda, Email: matsuda.taito.344@m.kyushu-u.ac.jp.
Kinichi Nakashima, Email: nakashima.kinichi.718@m.kyushu-u.ac.jp.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Niu W., et al. , In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 15, 1164–1175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torper O., et al. , Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 7038–7043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Z., et al. , In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14, 188–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich C., et al. , Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 3, 1000–1014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu W., et al. , SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Rep. 4, 780–794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torper O., et al. , In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 12, 474–481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brulet R., et al. , NEUROD1 instructs neuronal conversion in non-reactive astrocytes. Stem Cell Rep. 8, 1506–1515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y. C., et al. , A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol. Ther. 28, 217–234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer C. J., Ischemic stroke: Experimental models and reality. Acta Neuropathol. 133, 245–261 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buscemi L., Price M., Bezzi P., Hirt L., Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model. Sci. Rep. 9, 507 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginhoux F., et al. , Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kierdorf K., et al. , Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Gomez Perdiguero E., et al. , Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeffel G., et al. , C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buttgereit A., et al. , Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397–1406 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Bennett F. C., et al. , A combination of ontogeny and CNS environment establishes microglial identity. Neuron 98, 1170–1183.e1178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shemer A., et al. , Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun. 9, 5206 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajan W. D., et al. , Dissecting functional phenotypes of microglia and macrophages in the rat brain after transient cerebral ischemia. Glia 67, 232–245 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Grassivaro F., et al. , Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J. Neurosci. 40, 784–795 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price C. J., et al. , Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke 37, 1749–1753 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Gulyas B., et al. , Evolution of microglial activation in ischaemic core and peri-infarct regions after stroke: A PET study with the TSPO molecular imaging biomarker [((11))C]vinpocetine. J. Neurol. Sci. 320, 110–117 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Annunziato L., Boscia F., Pignataro G., Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J. Cereb. Blood Flow Metab. 33, 969–982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fumagalli S., Perego C., Pischiutta F., Zanier E. R., De Simoni M. G., The ischemic environment drives microglia and macrophage function. Front. Neurol. 6, 81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wattananit S., et al. , Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci. 36, 4182–4195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y., et al. , Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21, 530–540 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Matsuda T., et al. , Pioneer factor NeuroD1 rearranges transcriptional and epigenetic profiles to execute microglia-neuron conversion. Neuron 101, 472–485.e477 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Denes A., et al. , Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. 27, 1941–1953 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Balkaya M., Krober J., Gertz K., Peruzzaro S., Endres M., Characterization of long-term functional outcome in a murine model of mild brain ischemia. J. Neurosci. Methods 213, 179–187 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Bennett M. L., et al. , New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U.S.A. 113, E1738–E1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal A. J., et al. , Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124, e33–e44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arlotta P., Molyneaux B. J., Jabaudon D., Yoshida Y., Macklis J. D., Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J. Neurosci. 28, 622–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin W. N., et al. , Depletion of microglia exacerbates postischemic inflammation and brain injury. J. Cereb. Blood Flow Metab. 37, 2224–2236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilla A. M., Diekmann H., Fischer D., Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J. Neurosci. 37, 6113–6124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O., Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Kita T., Kita H., Kitai S. T., Passive electrical membrane properties of rat neostriatal neurons in an in vitro slice preparation. Brain Res. 300, 129–139 (1984). [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi Y., Wilson C. J., Emson P. C., Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J. Neurophysiol. 62, 1052–1068 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Ade K. K., Janssen M. J., Ortinski P. I., Vicini S., Differential tonic GABA conductances in striatal medium spiny neurons. J. Neurosci. 28, 1185–1197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gertler T. S., Chan C. S., Surmeier D. J., Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. 28, 10814–10824 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibanez-Sandoval O., et al. , Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J. Neurosci. 30, 6999–7016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willett J. A., et al. , Electrophysiological properties of medium spiny neuron subtypes in the caudate-putamen of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. eNeuro 6, ENEURO.0016–0019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z., et al. , Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington’s disease. Nat. Commun. 11, 1105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menezes J. R., Luskin M. B., Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J. Neurosci. 14, 5399–5416 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaneko N., et al. , New neurons use Slit-Robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration. Sci. Adv. 4, eaav0618 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu W., Zang T., Wang L. L., Zou Y., Zhang C. L., Phenotypic reprogramming of striatal neurons into dopaminergic neuron-like cells in the adult mouse brain. Stem Cell Rep. 11, 1156–1170 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L. L., et al. , Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 184, 5465–5481.e5416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao Y., et al. , NeuroD1 induces microglial apoptosis and cannot induce microglia-to-neuron cross-lineage reprogramming. Neuron 109, 4094–4108.e4095 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Bocchi R., Masserdotti G., Gotz M., Direct neuronal reprogramming: Fast forward from new concepts toward therapeutic approaches. Neuron 110, 366–393 (2021), 10.1016/j.neuron.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Chen G., In vivo confusion over in vivo conversion. Mol. Ther. 29, 3097–3098 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuda T., Nakashima K., Clarifying the ability of NeuroD1 to convert mouse microglia into neurons. Neuron 109, 3912–3913 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Masuda T., et al. , Novel Hexb-based tools for studying microglia in the CNS. Nat. Immunol. 21, 802–815 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Irie T., et al. , Lineage tracing identifies in vitro microglia-to-neuron conversion by NeuroD1 expression. Genes Cells 28, 526–534 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., et al. , Chronic behavioral testing after focal ischemia in the mouse: Functional recovery and the effects of gender. Exp. Neurol. 187, 94–104 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Hu X., et al. , Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Gascon S., Masserdotti G., Russo G. L., Gotz M., Direct neuronal reprogramming: Achievements, hurdles, and new roads to success. Cell Stem Cell 21, 18–34 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Greenhalgh A. D., David S., Bennett F. C., Immune cell regulation of glia during CNS injury and disease. Nat. Rev. Neurosci. 21, 139–152 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A., et al. , Targeting different monocyte/macrophage subsets has no impact on outcome in experimental stroke. Stroke 48, 1061–1069 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Liu M. H., et al. , Differential neuronal reprogramming induced by NeuroD1 from astrocytes in grey matter versus white matter. Neural Regen. Res. 15, 342–351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara H., Huang P. L., Panahian N., Fishman M. C., Moskowitz M. A., Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J. Cereb. Blood Flow Metab. 16, 605–611 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Percie du Sert N., et al. , The IMPROVE guidelines (ischaemia models: Procedural refinements of in vivo experiments). J. Cereb. Blood Flow Metab. 37, 3488–3517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borlongan C. V., Sanberg P. R., Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J. Neurosci. 15, 5372–5378 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hayashi Y., et al. , BK channels in microglia are required for morphine-induced hyperalgesia. Nat. Commun. 7, 11697 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Elevated body swing test in tMCAO + Control mouse at 8 wpi, Related to Fig. 6B
Elevated body swing test in tMCAO + ND1 mouse at 8 wpi, Related to Fig. 6B
Elevated body swing test in sham-operated mouse, Related to Fig. 6B
Corner test and corner rotation in tMCAO + Control mouse at 8 wpi, Related to Fig. 6B
Corner test and corner rotation in tMCAO + ND1 mouse at 8 wpi, Related to Fig. 6B
Corner test and corner rotation in sham-operated mouse, Related to Fig. 6B
Data Availability Statement
All study data are included in the article and/or supporting information.