Significance
Functional MRI (fMRI) has proven valuable at mapping cortical activity in the brain. However, many fMRI studies may be underestimating the extent of activation due to low signal-to-noise-ratio or modeling assumptions. Further, fMRI signal in the white matter of the brain is often removed or treated as an artifact. In a complementary study to previous works which showed that nearly all cortex responds to a task, we show both white and gray matter show widespread blood oxygenation level–dependent signal changes. This suggests that many reports of fMRI studies may not only underestimate the true extent of brain activation but also exclude and/or neglect half of all brain tissue (white matter) and may miss crucial information from the MRI signal.
Keywords: fMRI, white matter, activation, hemodynamic response
Abstract
Recent studies have revealed the production of time-locked blood oxygenation level–dependent (BOLD) functional MRI (fMRI) signals throughout the entire brain in response to tasks, challenging the existence of sparse and localized brain functions and highlighting the pervasiveness of potential false negative fMRI findings. “Whole-brain” actually refers to gray matter, the only tissue traditionally studied with fMRI. However, several reports have demonstrated reliable detection of BOLD signals in white matter, which have previously been largely ignored. Using simple tasks and analyses, we demonstrate BOLD signal changes across the whole brain, in both white and gray matters, in similar manner to previous reports of whole brain studies. We investigated whether white matter displays time-locked BOLD signals across multiple structural pathways in response to a stimulus in a similar manner to the cortex. We find that both white and gray matter show time-locked activations across the whole brain, with a majority of both tissue types showing statistically significant signal changes for all task stimuli investigated. We observed a wide range of signal responses to tasks, with different regions showing different BOLD signal changes to the same task. Moreover, we find that each region may display different BOLD responses to different stimuli. Overall, we present compelling evidence that, just like all gray matter, essentially all white matter in the brain shows time-locked BOLD signal changes in response to multiple stimuli, challenging the idea of sparse functional localization and the prevailing wisdom of treating white matter BOLD signals as artifacts to be removed.
Functional MRI (fMRI) based on blood oxygenation level–dependent (BOLD) contrast is well established for mapping cortical activity in the brain. By characterizing BOLD signal changes in response to known events or stimuli, task-based fMRI has proven successful at identifying and localizing brain regions that are functionally involved in response to these tasks or stimulations. Despite the common notion that sparse, specific brain regions are associated with specific tasks, evidence has also emerged that whole-brain hemodynamic changes may be evoked by brain neuronal responses to stimuli. Specifically, by using extensive fMRI acquisitions, resulting in high signal-to-noise ratio data, Gonzalez-Castillo et al. (1) showed that BOLD signal changes correlate with task timings throughout a majority of the cortex, with different BOLD responses in different regions of the gray matter in the brain. They demonstrate that the sparse activations observed in traditional fMRI maps result from elevated noise, and overly strict assumptions of BOLD response functions, in agreement with (and validated by) other studies showing increased activation with signal averaging (2–6), or regional differences in BOLD responses (3, 6–10). Thus, there is strong evidence that “whole-brain, time-locked activation” can be revealed with higher signal to noise ratio acquisitions, and that common reports of sparse, highly localized brain responses miss the detection of lower levels of activity across large expanses of the cortex.
In this context, “whole-brain” refers to only the gray matter of the brain and studies such as that of Gonzalez-Castillo et al. (1) explicitly ignore possible BOLD effects in white matter. Indeed, over the past 30+ y, white matter BOLD signals have been rarely reported and in fact are often regressed out as nuisance covariates. However, multiple reports have demonstrated successful detection and analysis of BOLD signals in white matter (11, 12). For example, previous studies have reported white matter BOLD signal responses to tasks and at rest (13–16), relationships between white matter signals and the gray matter regions to which they connect (17–19), alterations of white matter BOLD signal in disease/disorder (20–25), and finally, robust, reproducible network properties of white matter BOLD signals (18, 26). Importantly, recent reports have confirmed that white matter responses to stimuli are similar to, but different from, those in gray matter (13, 14, 27, 28), generally indicating a slower response, smaller percent signal change, and variation across regions (29). Together, the explicit removal of white matter BOLD signal as a nuisance regressor (30), inaccuracies in modeling white matter BOLD response functions (28), and decreased detection sensitivity due to low SNR, has made white matter a blind spot in the fMRI literature (30).
In comparisons with the gray matter, it is unknown whether white matter displays a time-locked variation of BOLD signals across all structural pathways, and whether such changes in BOLD signals vary based on stimuli. Motivated by findings of whole-brain gray matter BOLD responses (1), in combination with recent works demonstrating reliable white matter BOLD signals, we hypothesize that there additionally exist widespread white matter BOLD signal changes, even in pathways that are not thought to be specifically associated with a given task. Such changes would underline the findings in the cortex that there are widespread vascular changes that may or may not correspond to neural activities. To evaluate this, we averaged multiple trials of sustained stimuli to generate image data with high signal-to-noise ratio and use an analysis that avoids assumptions about the precise shape of the signal response to assess signal changes in both gray matter regions and white matter pathways derived from diffusion tractography. We test whether significant signal changes are found throughout the white and gray matter and additionally assess differences in BOLD signal changes across regions and across different stimuli.
Methods
Image Acquisition.
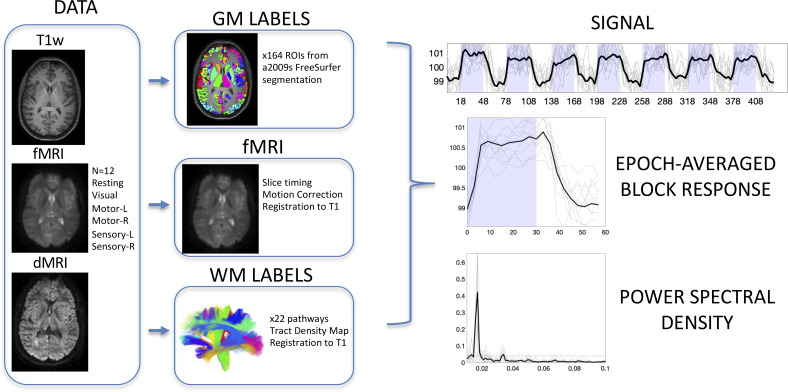
All imaging (Fig. 1, DATA) was performed on a 3-T Philips Achieva CRX scanner (Philips Healthcare) at Vanderbilt University Institute of Imaging Science, using a 32-channel head coil. All human datasets from Vanderbilt University were acquired after informed consent, and the full study protocol was approved by Vanderbilt Institutional Review Board (IRB #020623). To provide anatomical references, high-resolution T1-weighted images were also acquired using a multishot, 3D gradient echo sequence at voxel size of 1 × 1 × 1 mm3. Next, for white matter pathway delineation, diffusion MRI images were acquired using a pulsed gradient spin echo (PGSE) echo planar imaging (EPI) sequence at a voxel size of 2 × 2 × 2 mm3, with 32 diffusion-weighted images at a b-value of 1,000 s/mm2, and a single nondiffusion weighted image.
Fig. 1.
Image acquisition, preprocessing, region of interest delineation, and time-locked activation. Data include T1-weighted structural images, fMRI data including resting state and 5 stimuli, and diffusion MRI. Gray matter labels were extracted from structural images, while white matter labels were derived from diffusion MRI fiber tractography. Functional MRI data were corrected for slice-timing and motion, and all data were registered to T1 space for analysis. Analysis included extracting region-averaged signal, from which the epoch-averaged block response can be visualized. Time-locked activation was determined by the power spectral density at task frequency (0.0167 Hz) with statistically significant activation determined by bootstrap analysis at P < 0.01 with FDR correction.
Six sets of images sensitive to BOLD contrast were acquired from each subject using a single shot, T2*-weighted gradient echo, echo planar imaging sequence with repetition time of 3 s, echo time of 45 ms, SENSE factor of 2, matrix size of 80 × 80, field of view of 240 × 240 mm2, 43 axial slices of 3-mm thick with zero gap, and 145 sequential volumes. The BOLD images contained a resting state acquisition (Resting) and sets of images acquired with sensory stimulations to the right hand and the left hand (Sensory-R and Sensory-L), motor tasks by the subjects’ right hand and left hand (Motor-R and Motor-L), and visual stimulations (Visual). All six runs had the same time duration of 435 s. During the image acquisitions, subjects lay in a supine position with eyes closed in a resting state or fixed on a screen mounted at the end of the scanner bore, viewed through mirrors mounted on the head coil. Head motions during image acquisitions were reduced by placing restricting pads within the head coil.
All stimulations and tasks were prescribed in a block design format, with five additional volumes acquired without stimulations or tasks at the beginning of each run. Sensory stimulations started with 30 s of hand stimulations by repeatedly brushing the palm followed by 30 s of no stimulations. The subjects fixed on an arrow sign pointing to the side of hand stimulation or a cross sign in the middle of the screen when there were no stimulations. Motor tasks started with 30 s of finger movement, in which subjects successively tapped each finger of the hand indicated by the arrow, followed by 30 s of no finger movements. In visual stimulations, the subjects continuously viewed the projection screen, which displayed 30 s of a checkerboard flashing at 8 Hz followed by 30 s of a cross sign on black ground. The blocks of stimulation or task were repeated seven times for each of the functional acquisitions. The total acquisition time was 435 s, yielding 145 volumes for each task/stimulation.
Fifteen subjects were included in this study (7 male, 8 female; age range 23 to 37), although not all BOLD contrasts were acquired on all subjects. Each BOLD contrast was represented N = 12 times in our cohort. Data is deposited and made available at https://www.nitrc.org/projects/vuiis-bold/ (31).
Image Preprocessing.
BOLD images were preprocessed (Fig. 1, fMRI) using the statistical parametric mapping software package SPM12 (www.fil.ion.ucl.ac.uk/spm/software). The preprocessing performed corrections only for slice timing and head motions, with no subjects indicating large head motion (>2 mm in translation or >2° in rotation). Importantly, no additional spatial smoothing beyond that introduced by head motion correction and realignment was applied to any fMRI data so as to minimize partial volume effects. Signal was temporally normalized and converted to a percent signal change in each voxel. The diffusion data were preprocessed to correct for motion and eddy currents using the FSL software package (32).
Region of Interest Delineation.
Subject-specific labels for gray and white matter were created. FreeSurfer (33) was run on all T1-weighted images, and gray matter labels were extracted from the Destrieux (34) atlas parcellation, resulting in 164 gray matter labels (Fig. 1, GM LABELS) already in the space defined by the anatomical T1-weighted image.
Fiber tractography was performed on the diffusion MRI data using the TractSeg tool (35) that performed probabilistic bundle-specific tractography to generate streamlines representing the spatial trajectories of white matter pathways. Twenty-two major association, projection, and commissural pathways were selected for this study (Fig. 1, WM LABELS), and tract density images were generated that described the density of streamlines in each imaging voxel. Diffusion data were registered with the T1-weighted images using FSL’s epi_reg tool for EPI to structural registration, and white matter labels were transformed to the structural image space.
Finally, the mean FMRI image was registered to the T1-weighted image using FSL’s epi_reg tool. BOLD signal, for all contrasts, was transformed to the structural image space. At this point, white and gray matter labels and fMRI signal are all aligned within the same space.
Time-Locked Activation.
Our aim was to determine which regions of interest indicated time-locked activation with the stimulus/task across a population. To do this, we first extracted the averaged fMRI signal within each of the white and gray matter regions of interest (Fig. 1, SIGNAL). From this, the power spectral density was estimated via the periodogram method (periodogram function in MatLab) which estimates the distribution of signal power per unit frequency (Fig. 1, POWER SPECTRAL DENSITY). In this study, the fundamental frequency of the tasks occurred at 0.0167 Hz (1/60 s). To determine whether there was significant time-locked activation (i.e., statistically significant power at this frequency), we performed a bootstrap analysis of the resting state data to generate a null distribution of power at this frequency. Here, 10,000 bootstrap signals were generated from volumes of 39 × 39 × 39 mm3 (13 × 13 × 13 voxels) within 12 subjects, and the average power spectral density at 0.0167 Hz was calculated. From this, given the real data (i.e., the subject averaged power spectral density in a region of interest), we generated a P-value for statistically significant time-locked activation. Regions with P < 0.01 after false discovery rate correction are considered time-locked, or activated, by our tasks, and the epoch-averaged response function (Fig. 1, EPOCH-AVERAGED BLOCK RESPONSE) can be visualized. By detecting only the fundamental periodicity some sensitivity may be lost, but by ignoring higher harmonics, we avoided assumptions about the specific time course of signals within each epoch.
Results
Widespread Gray Matter Time-locked Activation.
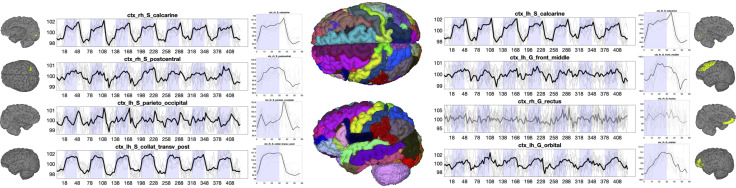
Fig. 2 shows the subject-averaged signal and epoch-averaged responses for several selected gray matter regions for the visual task. Many regions show both qualitatively observable and statistically significant, time-locked activation with the task. While some regions show the canonical sustained boxcar response, or a sustained response with small transitory response at the onset or offset of the task, other trends are also apparent, including delayed responses, or multiple peaks. The observation of widespread gray matter time-locked activation agrees with the previous study that motivated this work (1).
Fig. 2.
Time-locked activation in gray matter. The signal and epoch-averaged response to the visual task are shown for eight selected gray matter regions. Plots are shown in BOLD if statistically significant time-locked activation is present, and mean value is shown as a thick line, with data from all subjects (N = 12) as thin lines.
Widespread White Matter Time-locked Signal Changes.
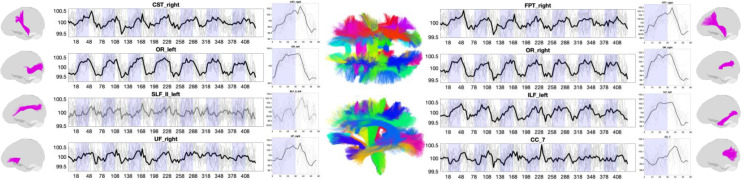
Beyond the traditional gray matter analysis for the visual stimulus task, we show white matter signals for several selected pathways in Fig. 3. White matter also shows widespread time-locked signal changes across projection, commissural, and association pathways. Here, several pathways show strong, canonical boxcar shapes, while others have a somewhat smaller signal change, or different shapes including, for example, negative signal changes during stimulation.
Fig. 3.
Time-locked BOLD signal changes in white matter. The signal and epoch-averaged responses to the visual task are shown for eight selected white matter regions. Plots are shown in BOLD if statistically significant time-locked signal changes are present, and mean value is shown as a thick line, with data from all subjects (N = 12) as thin lines.
Variation in Tasks.
Fig. 4 shows the signal for two selected gray matter and two selected white matter regions across all BOLD contrasts/tasks, including resting state. First, resting state does not show time-locked activation at 0.0167 Hz. However, all other tasks, for both gray matter and both white matter regions, show statistically significant signal changes. Notably, the signal response for all regions varies dramatically based on the tasks or hemisphere in which the task is performed.
Fig. 4.
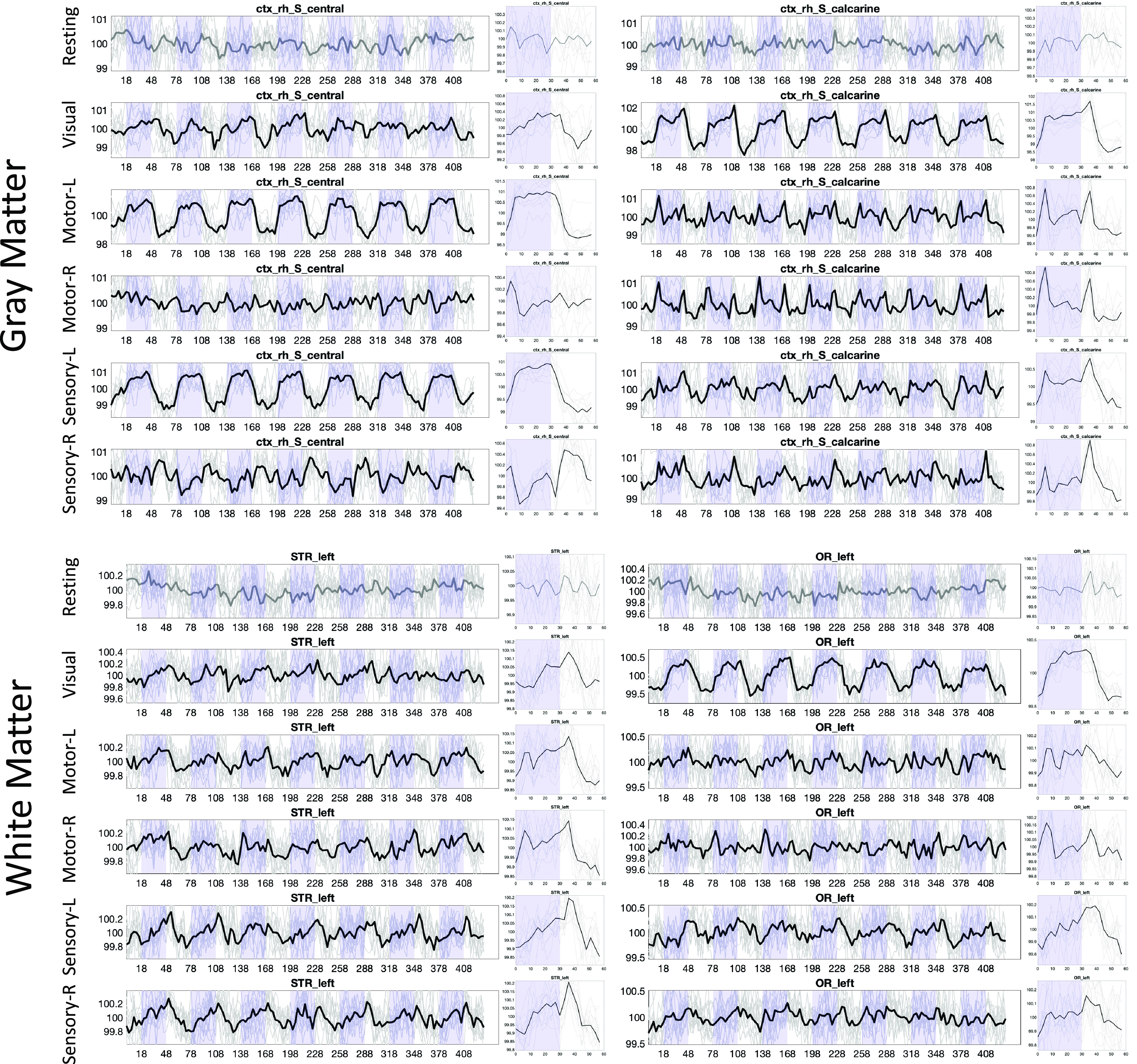
Time-locked activation varies across regions and across stimuli, in both white and gray matter. Example signal and epoch-averaged signal are shown for two gray matter and two white matter regions, across all tasks. Plots are shown in BOLD if statistically significant time-locked activation is present.
Whole-Brain Time-Locked Activation.
Fig. 5 visualizes regions based on the power spectral density (showing only statistically significant regions) for all tasks. Here, the whole-brain response, for all tasks, is clearly visible, importantly in both gray and white matters. The traditional regions of interest associated with each function indeed show the strongest activations, for example, the occipital lobe for visual tasks or pre/post-central sulci for motor tasks. Additionally, so does the white matter directly projecting to/from these regions, the optic radiations in visual tasks, the corticospinal tract for motor/sensory tasks. However, for all tissues, nearly all regions show significant signal changes, including those that play accessory roles in tasks or are not hypothesized to play any role at all.
Fig. 5.
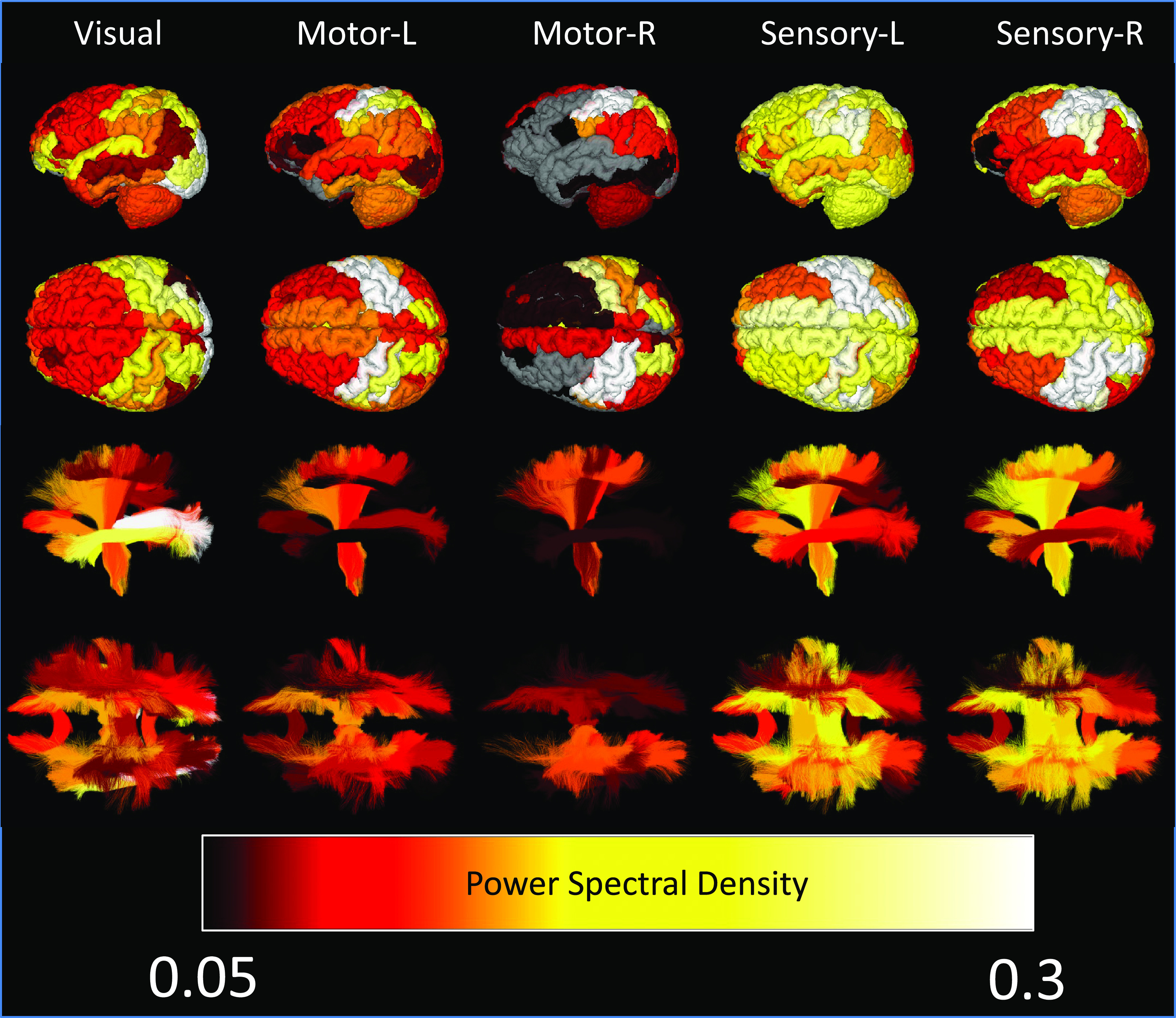
Whole brain time-locked signal changes in both white and gray matter. Gray matter regions (Top) and white matter pathways (Bottom) are shown color-coded based on subject-averaged power-spectral density for five tasks. Nonsignificant changes are shown in gray for gray matter, and not visualized in white matter.
Discussion
White Matter BOLD Signal.
Using simple tasks and analysis, we show here that task-locked BOLD signal changes may be evoked across the whole brain, in both white and gray matter. These findings complement and extend the previous reports of whole brain responses to tasks (1) and emphasize that white matter BOLD signals may be reliably detected. BOLD signal in white matter remains controversial (11, 12), as the blood flow and volume in white matter are much lower than those in the gray matter (36, 37), and the biological basis of BOLD signal mechanisms in white matter is unclear. There is evidence that the fMRI signal in gray matter corresponds to the metabolic demands required to produce changes in local post-synaptic potentials (38), but it is not clear that electrophysiological activity such as the propagation of action potentials also produces BOLD effects (12). However, white matter also contains a large number of glial cells which are engaged in the tissue response to neural activity, and whose various functions consume energy, so it is possible that BOLD effects in white matter is driven by different needs and cell types. It is also possible that BOLD effects in white matter reflect a vascular drainage effect from the cortex, though the widespread distribution of white matter BOLD makes this seem unlikely. Moreover, a similar uncertainty would also apply to cortical BOLD responses. Notably, reports of white matter fMRI signal changes are becoming more frequent, with studies commonly describing activations in the corpus callosum (39–42), internal capsule (43, 44), and optic radiations, among other large white matter pathways (13, 45). Here, we confirm detectable signal fluctuations in a large number of projection, commissural, and association pathways.
Gawryluk et al. (12) and Gore et al. (11) describe several explanations for why white matter BOLD signals may have not been detected in previous studies or are dismissed entirely as artifacts. First, white matter BOLD signals and signal changes are lower in magnitude than gray matter activation (12) as expected by reason of lower blood volume. We therefore performed multiple within-subject and across-subject averaging to improve SNR. Moreover, in conventional analyses, clustering of voxels to increase SNR usually is performed over cubic or spherical volumes that are unsuited to thin white matter tracts. Here, we average the signal over the entire length of each white matter pathway. Indeed, comparing region-averaged white and gray matter BOLD signal in Fig. 4 shows white matter BOLD fluctuations on the order of 1/3rd to 1/6th that of gray matter. To detect white matter “activation” at the same confidence level as gray matter signals would require significantly increased SNR (e.g., 9 to 36 repeats if done through averaging alone). This largely explains why white matter signals are rarely observed.
Second, fMRI analysis has been tailored towards gray matter data. For example, white matter signals may be treated as nuisance regressors (30, 46), or used as a “zero activity” reference, and a specific form is usually assumed for the hemodynamic response function in General Linear Models which may not be appropriate for white matter (47) (see below for more discussion). Here, we used a spectral analysis that made no assumptions about the detailed shape of the BOLD signal changes. In line with our results, recent work that estimates the BOLD response directly from the signal (28), permitting flexibility of hemodynamic profile and shapes, revealed hemodynamic activity in white matter that was not found when using the canonical gray matter hemodynamic response function. Similar approaches that relax the overly strict response models may be necessary to improve detection sensitivity in white matter.
BOLD signal changes in white matter could also be attributed to task-related motion (12). However, voxels near the boundaries of tissue would be most susceptible to this artifact, whereas we have weighted the white matter signal by density within the white matter, and there is no obvious reason why white matter would be any more susceptible to this than gray matter. Second, changes in white matter BOLD signal could be due to residual effects of oxygenation changes in gray matter vasculature through draining veins. This should be studied in detail in future studies, possibly using susceptibility weighted imaging to look at venous distributions within white matter. However, this concern is partially alleviated due to the fact that white matter tracts are drained through subependymal veins close to the lateral ventricles with little interaction between vasculature of gray and white matter tissue types in the healthy brain (48).
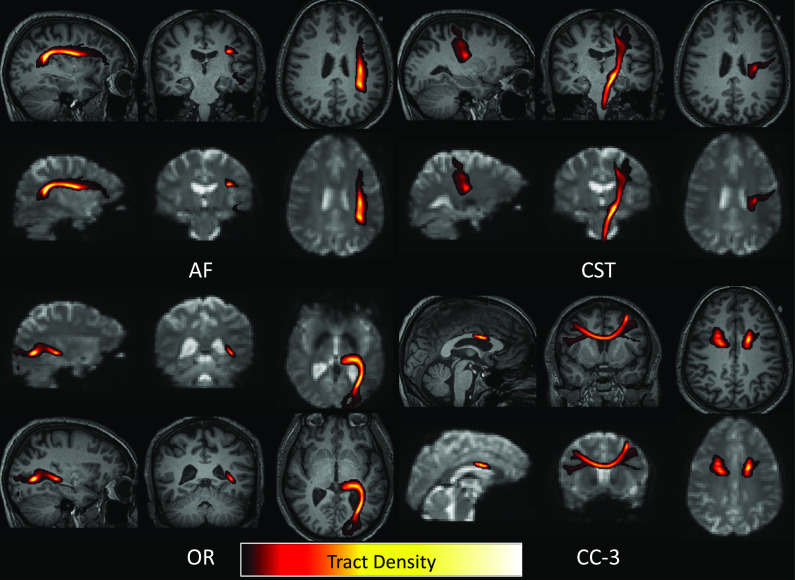
Finally, white matter activation could be attributed to partial volume effects from gray matter, especially if using larger voxels in image acquisitions, or after smoothing data. Here, to minimize partial volume effects, we did not smooth the data. Moreover, by weighting by tract density, the white matter signal was heavily weighted by the dense core of deep white matter bundles for the large pathways studied here (Fig. 6), reducing potential gray matter confounds. SI Appendix describes additional experiments performed to investigate potential partial volume effects and their effect on the time-locked BOLD signal changes. SI Appendix, Experiment #1 is an erosion experiment that shows that when pathway segmentations are iteratively eroded, the signal still shows time-locked activations even when fully eroded to the white matter skeleton. This suggests that the core of the bundle is driving these signals. SI Appendix, Experiment #2 evaluates heterogeneity of white matter and gray matter signals by evaluating the similarity of white matter signals and all neighboring gray matter regions. Here, the similarity between white matter and neighboring regions is always less than the relationship between gray matter signals and their neighboring regions. While this does not explicitly exclude partial volume effects, it is nonetheless convincing that white matter signal is at least as unique as differences in BOLD signal across different gray matter regions.
Fig. 6.
Weighting white matter BOLD signal by tract density reduces partial volume effects with gray matter regions. Four example pathways are shown as tract density maps overlaid on T1 images (at 1 mm isotropic resolution) and overlaid on BOLD contrast (at 3 mm isotropic resolution), where the highest density of streamlines is consistently in deep white matter regions.
We provide strong evidence that evoked white matter BOLD signals are near ubiquitous within the brain when a task or stimulus is presented and that they occur concurrently with widespread activity in gray matter as has been previously reported. Their origins remain to be more clearly established, but they share multiple features with the hemodynamic responses that are attributed to neural activity in gray matter. Signal propagation along axons reportedly requires only a small fraction of the total white matter energy budget (36, 37), but other processes within white matter, including those in other cell types, may provoke a BOLD response to functional activity in the brain.
Brain Wide Activation.
Our study was motivated in part by findings from ref. 1, which found nonrandom gray matter-wide modulations of BOLD responses to tasks. These results raise the issue that many fMRI studies may overlook involvement of many brain areas due to insufficient power to detect activations. Statistical parametric maps, which describe involvement of brain regions in a task, are usually derived by fitting empirical BOLD signal data to a model of the BOLD response function and evaluating quality of fit. As highlighted throughout the literature, inappropriate models of the hemodynamic response function may decrease statistical power to detect true effects, resulting in an underestimation of the amount of variance explained by the signal (and overestimation of noise) and substantial number of false negative results (47).
Even for a simple task such as a visual discrimination task (1), almost the entire brain shows BOLD signal changes, and the act of labeling brain areas as active versus inactive is likely overly ambiguous. Here, we extend this work, showing that in addition to visual stimulation, also sensory and motor tasks produce wide-spread signal changes outside of the traditional locations associated with these tasks. These widespread effects may reflect neural engagement of multiple areas or some form of vascular coupling between regions. Intuitively, if the entire cortex is experiencing changes, the white matter substrates linking these cortical regions must also be consequently affected (or effecting these changes). Regardless of the biological basis of the signal in WM, it is clear that some change in blood volume, oxygenation, or relaxation rate occurs in a large portion of the white matter pathways of the brain. These white matter pathways also do not have single-task functions but show BOLD signal changes in a multitude of tasks. However, here, there is significant spatial overlap of structural pathways within the deep white matter (49, 50) which may confound the identity of the origins of some white matter signals.
As our study acts as an extension and white matter analogue of ref. 1, it is important to directly compare results. Gonzalez-Castillo et al., employed massive averaging (100 runs) of a single subject to improve SNR, enabling a model-free analysis of voxel-wise activation, whereas we employed multiple trials (6 runs on 12 subjects) and averaging over large (but anatomically meaningful) spatial extents. Despite methodological differences, results in gray matter are in agreement, with task-locked signal changes observable across the entire cortex, with shape varying substantially across regions. Interestingly, their Fig. 4 apparently shows no white matter activation. However, their Supporting Information states “Clustering was attempted using only gray matter voxels that span both the cortical gray-matter ribbon and subcortical gray-matter structures,” again reflecting the blind spot for fMRI signal in the literature. Their follow-up study on both 3T and 7T systems did show BOLD signal changes throughout the white matter (51). Again, in agreement with our results, activation varied across regions—with responses described as positively sustained, negatively sustained, and transient—and across tasks. Notably, the higher resolution 7T data displayed widespread white matter activation, even in regions where partial volume with gray matter is not expected. However, white matter activation was dramatically reduced with reduced task demand (and thus reduced contrast and signal to noise ratios). This could still be an SNR effect due to the voxel-wise analysis in combination with decreased white matter SNR (particularly at 7T).
Linking Structure and Function.
One goal of brain mapping by MRI is to establish links between neuronal substrates and their structural connections in order to better understand their functional relevance. Structure and function in neuroimaging have traditionally been studied through independent measures. While functional engagement has traditionally been identified through BOLD signals and focused on gray matter, structural connections have been quantified through diffusion MRI and focused on white matter. We propose that both BOLD and diffusion contrasts can provide useful information on structural and functional characteristics of both tissue types. There are several excellent examples of linking behavior/phenotypes to not just gray matter function but also to localized white matter function and further investigating the biological and microstructural basis of findings using diffusion MRI—for example, to investigate neuroplasticity with motor learning (52, 53). Further studies may elucidate relationships between white and gray matter signals, including potential time delays and differences in stimuli. It may be possible, using high temporal resolution and high signal to noise ratio data, to identify which white matter voxels are associated with connections to specific cortical regions, providing validation for diffusion tractography, which also suffers from both false positive and false negative detections (54–57). Studies have shown that BOLD signals in white matter pathways are highly correlated to other voxels within the same pathway (58), offering a way to either parcellate white matter based on functional signals or perform fiber tractography directly on functional data (59).
Finally, we do not impose a constraint on the hemodynamic response function. While our analysis detected a time-locked frequency component at the task frequency, it is possible that higher-order harmonic analysis could reveal further regions that are not only time but also phase-locked with the task. We also rely on a priori white (and gray) matter regions for spatial averaging to increase SNR. While these are well-validated fiber pathways of the brain (60), there certainly remain partial volume issues within white matter as these pathways are known to share strong overlap (49). A more fine-grained parcellation of the white matter signal is possible using dimensionality reduction or clustering techniques (18, 61, 62). For example, a model-free exploratory analysis which sorts data based on the power spectrum and clusters based on similarity to cluster centroids has proven useful (and highly sensitive) at detecting white matter activation (63). Overall, not only are white matter signals heterogeneous in nature, but our results show that there are unique signal fluctuations across pathways and across tasks. Similar dimensionality reductions and clustering techniques may enable new structure-function parcellations of the white matter and open broad avenues of research in neuroscience.
Diffusion MRI has also been claimed to detect functional activations as changes in apparent diffusion coefficients (64). Task-correlated changes in diffusivity have been hypothesized to represent structural changes such as cell-swelling during neuronal activation (64), and have shown activation in similar regions to that of traditional BOLD contrast (65, 66). These types of diffusion experiments can be utilized in the white matter (67–69), and future investigations may enable teasing out microstructural contributions to the white matter signal described in the current study.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by the NSF Career Award #1452485, the NIH under award numbers R01EB017230, K01EB032898, and in part by ViSE/VICTR VR3029 and the National Center for Research Resources, Grant UL1 RR024975–01.
Author contributions
K.G.S., M.L., F.R., Y.G., Y.Z., L.X., Z.D., A.W.A., B.A.L., and J.C.G. designed research; K.G.S. performed research; K.G.S., M.L., and L.C. analyzed data; and K.G.S., Z.D., B.A.L., and J.C.G. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. P.B. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Anonymized Neuroimage formats data have been deposited in NITRC (https://www.nitrc.org/projects/vuiis-bold/) (31).
Supporting Information
References
- 1.Gonzalez-Castillo J., et al. , Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc. Natl. Acad. Sci. U.S.A. 109, 5487–5492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cremers H. R., Wager T. D., Yarkoni T., The relation between statistical power and inference in fMRI. PLoS One 12, e0184923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor A. J., Kim J. H., Ress D., Characterization of the hemodynamic response function across the majority of human cerebral cortex. Neuroimage 173, 322–331 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad Z. S., Ropella K. M., DeYoe E. A., Bandettini P. A., The spatial extent of the BOLD response. Neuroimage 19, 132–144 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Huettel S. A., McCarthy G., The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport 12, 2411–2416 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Calhoun V. D., Stevens M. C., Pearlson G. D., Kiehl K. A., fMRI analysis with the general linear model: Removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage 22, 252–257 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Handwerker D. A., Gonzalez-Castillo J., D’Esposito M., Bandettini P. A., The continuing challenge of understanding and modeling hemodynamic variation in fMRI. Neuroimage 62, 1017–1023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handwerker D. A., Ollinger J. M., D’Esposito M., Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21, 1639–1651 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Aguirre G. K., Zarahn E., D’Esposito M., The variability of human, BOLD hemodynamic responses. Neuroimage 8, 360–369 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Tyler C. W., Kontsevich L. L., Ferree T. C., Independent components in stimulus-related BOLD signals and estimation of the underlying neural responses. Brain Res. 1229, 72–89 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore J. C., et al. , Functional MRI and resting state connectivity in white matter–A mini-review. Magn. Reson. Imaging 63, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gawryluk J. R., Mazerolle E. L., D’Arcy R. C., Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions Front. Neurosci. 8, 239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M., Newton A. T., Anderson A. W., Ding Z., Gore J. C., Characterization of the hemodynamic response function in white matter tracts for event-related fMRI. Nat. Commun. 10, 1140 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser L. M., Stevens M. T., Beyea S. D., D’Arcy R. C. N., White versus gray matter: fMRI hemodynamic responses show similar characteristics, but differ in peak amplitude. BMC Neurosci. 13, 91 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behjat H., Aganj I., Abramian D., Eklund A., Westin C. F., Characterization of spatial dynamics of fMRI data in white matter using diffusion-informed white matter harmonics. Proc. IEEE Int. Symp. Biomed. Imaging 2021, 1586–1590 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabri M., Pierpaoli C., Barbaresi P., Polonara G., Functional topography of the corpus callosum investigated by DTI and fMRI. World J. Radiol. 6, 895–906 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y., et al. , Lower functional connectivity of white matter during rest and working memory tasks is associated with cognitive impairments in schizophrenia. Schizophr. Res. 233, 101–110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P., et al. , White matter functional connectivity in resting-state fMRI: Robustness, reliability, and relationships to gray matter. Cereb. Cortex 32, 1547–1559 (2022), 10.1093/cercor/bhab181. [DOI] [PubMed] [Google Scholar]
- 19.Celeghin A., et al. , Intact hemisphere and corpus callosum compensate for visuomotor functions after early visual cortex damage. Proc. Natl. Acad. Sci. U.S.A. 114, E10475–E10483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Q., et al. , Desynchronized white matter function and structure in drug-naive first-episode major depressive disorder patients. Front. Psychiatry 13, 1082052 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., et al. , Desynchronized functional activities between brain white and gray matter in major depression disorder. J. Magn. Reson. Imaging 53, 1375–1386 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Astafiev S. V., et al. , Abnormal white matter blood-oxygen-level-dependent signals in chronic mild traumatic brain injury. J. Neurotrauma. 32, 1254–1271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu X., et al. , Exploring white matter functional networks in children with attention-deficit/hyperactivity disorder. Brain. Commun. 2, fcaa113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Long J., Yang S., He B., Atypical functional covariance connectivity between gray and white matter in children with autism spectrum disorder. Autism Res. 14, 464–472 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Chen X., et al. , Extraction of dynamic functional connectivity from brain grey matter and white matter for MCI classification. Hum. Brain Mapp. 38, 5019–5034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., et al. , Functional engagement of white matter in resting-state brain networks. Neuroimage 220, 117096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtemanche M. J., Sparrey C. J., Song X., MacKay A., D’Arcy R. C. N., Detecting white matter activity using conventional 3 Tesla fMRI: An evaluation of standard field strength and hemodynamic response function. Neuroimage 169, 145–150 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chen G., et al. , BOLD response is more than just magnitude: Improving detection sensitivity through capturing hemodynamic profiles. bioRxiv [Preprint] (2023). 10.1101/2023.02.13.528362 (Accessed 1 February 2023). [DOI] [PMC free article] [PubMed]
- 29.Schilling K. G., et al. , Anomalous and heterogeneous characteristics of the BOLD hemodynamic response function in white matter. Cereb. Cortex. Commun. 3, tgac035 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grajauskas L. A., Frizzell T., Song X., D’Arcy R. C. N., White matter fMRI activation cannot be treated as a nuisance regressor: Overcoming a historical blind spot. Front. Neurosci. 13, 1024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schilling K. G., et al. , VUIIS - 3T - Functional Contrasts - Resting/MotorStim/SensoryStim/Visual. vuiis-bold. https://www.nitrc.org/projects/vuiis-bold/. Deposited 26 September 2023.
- 32.Jenkinson M., Beckmann C. F., Behrens T. E., Woolrich M. W., Smith S. M., FSL. Neuroimage 62, 782–790 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Fischl B., FreeSurfer. Neuroimage 62, 774–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Destrieux C., Fischl B., Dale A., Halgren E., Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasserthal J., Neher P., Maier-Hein K. H., TractSeg–Fast and accurate white matter tract segmentation. Neuroimage 183, 239–253 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Helenius J., et al. , Cerebral hemodynamics in a healthy population measured by dynamic susceptibility contrast MR imaging. Acta Radiol. 44, 538–546 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Rostrup E., et al. , Regional differences in the CBF and BOLD responses to hypercapnia: A combined PET and fMRI study. Neuroimage 11, 87–97 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Rauch A., Rainer G., Augath M., Oeltermann A., Logothetis N. K., Pharmacological MRI combined with electrophysiology in non-human primates: Effects of Lidocaine on primary visual cortex. Neuroimage 40, 590–600 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Tettamanti M., et al. , Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J. Neurophysiol. 88, 1051–1058 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Fabri M., Polonara G., Functional topography of human corpus callosum: An FMRI mapping study. Neural. Plast. 2013, 251308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gawryluk J. R., D’Arcy R. C., Mazerolle E. L., Brewer K. D., Beyea S. D., Functional mapping in the corpus callosum: A 4T fMRI study of white matter. Neuroimage 54, 10–15 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Newman A. J., Supalla T., Hauser P., Newport E. L., Bavelier D., Dissociating neural subsystems for grammar by contrasting word order and inflection. Proc. Natl. Acad. Sci. U.S.A. 107, 7539–7544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazerolle E. L., et al. , Sensitivity to white matter FMRI activation increases with field strength. PLoS One 8, e58130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gawryluk J. R., Mazerolle E. L., Brewer K. D., Beyea S. D., D’Arcy R. C., Investigation of fMRI activation in the internal capsule. BMC Neurosci. 12, 56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weis S., et al. , Functional neuroanatomy of sustained memory encoding performance in healthy aging and in Alzheimer’s disease. Int. J. Neurosci. 121, 384–392 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Van Dijk K. R. A., et al. , Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J. Neurophysiol. 103, 297–321 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stelzer J., Lohmann G., Mueller K., Buschmann T., Turner R., Deficient approaches to human neuroimaging. Front. Hum. Neurosci. 8, 462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz D. S., Yilmaz H., Gailloud P., Cerebral developmental venous anomalies: Current concepts. Ann. Neurol. 66, 271–283 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Schilling K. G., et al. , Prevalence of white matter pathways coming into a single white matter voxel orientation: The bottleneck issue in tractography. Hum. Brain Mapp. 43, 1196–1213 (2022), 10.1002/hbm.25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girard G., et al. , On the cortical connectivity in the macaque brain: A comparison of diffusion tractography and histological tracing data. Neuroimage 221, 117201 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Castillo J., et al. , Task dependence, tissue specificity, and spatial distribution of widespread activations in large single-subject functional MRI datasets at 7T. Cereb. Cortex 25, 4667 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frizzell T. O., et al. , White matter neuroplasticity: Motor learning activates the internal capsule and reduces hemodynamic response variability. Front. Hum. Neurosci. 14, 509258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frizzell T. O., et al. , Imaging functional neuroplasticity in human white matter tracts. Brain Struct. Funct. 227, 381–392 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schilling K. G., et al. , Challenges in diffusion MRI tractography–Lessons learned from international benchmark competitions. Magn. Reson. Imaging 57, 194–209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schilling K. G., et al. , Limits to anatomical accuracy of diffusion tractography using modern approaches. Neuroimage 185, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donahue C. J., et al. , Using diffusion tractography to predict cortical connection strength and distance: A quantitative comparison with tracers in the Monkey. J. Neurosci. 36, 6758–6770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knösche T. R., Anwander A., Liptrot M., Dyrby T. B., Validation of tractography: Comparison with manganese tracing. Hum. Brain Mapp. 36, 4116–4134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Z., et al. , Detection of synchronous brain activity in white matter tracts at rest and under functional loading. Proc. Natl. Acad. Sci. U.S.A. 115, 595–600 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Z., et al. , Visualizing functional pathways in the human brain using correlation tensors and magnetic resonance imaging. Magn. Reson. Imaging 34, 8–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catani M., de Schotten M. T., Atlas of Human Brain Connections (Oxford University Press, Oxford, UK, 2012). [Google Scholar]
- 61.Peer M., Nitzan M., Bick A. S., Levin N., Arzy S., Evidence for functional networks within the human brain’s white matter. J. Neurosci. 37, 6394–6407 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J., et al. , Exploring the functional connectome in white matter. Hum. Brain Mapp. 40, 4331–4344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Arcy R. C., Hamilton A., Jarmasz M., Sullivan S., Stroink G., Exploratory data analysis reveals visuovisual interhemispheric transfer in functional magnetic resonance imaging. Magn. Reson. Med. 55, 952–958 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Le Bihan D., Diffusion, confusion and functional MRI. Neuroimage 62, 1131–1136 (2012). [DOI] [PubMed] [Google Scholar]
- 65.De Luca A., Schlaffke L., Siero J. C. W., Froeling M., Leemans A., On the sensitivity of the diffusion MRI signal to brain activity in response to a motor cortex paradigm. Hum. Brain Mapp. 40, 5069–5082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nunes D., Ianus A., Shemesh N., Layer-specific connectivity revealed by diffusion-weighted functional MRI in the rat thalamocortical pathway. Neuroimage 184, 646–657 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicolas R., Gros-Dagnac H., Aubry F., Celsis P., Comparison of BOLD, diffusion-weighted fMRI and ADC-fMRI for stimulation of the primary visual system with a block paradigm. Magn. Reson. Imaging 39, 123–131 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Lin T. H., et al. , Diffusion fMRI detects white-matter dysfunction in mice with acute optic neuritis. Neurobiol. Dis. 67, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spees W. M., Lin T. H., Song S. K., White-matter diffusion fMRI of mouse optic nerve. Neuroimage 65, 209–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Anonymized Neuroimage formats data have been deposited in NITRC (https://www.nitrc.org/projects/vuiis-bold/) (31).