Abstract
Hypoglycemia is known as a sudden diminution in blood glucose level <50 mg/dL. Hypoglycemic encephalopathy is a metabolic encephalopathy that is usually observed in patients treated for diabetes or chronic alcoholism. Neurological manifestations may range from transient deficits to prolonged coma, depending on the duration and severity of hypoglycemia. Neuroradiological features of hypoglycemia are variable involving the cerebral white and gray matter regions. Acquired metabolic or toxic conditions can cause hypoglycemia-like damage to the cerebral white matter and basal ganglia. Widespread lesions in the brain parenchyma or basal ganglia have a poor prognosis. In this report, we present a patient with widespread brain damage secondary to profound and prolonged hypoglycemia.
Keywords: Hypoglycemic encephalopathy, Coma, Glucose, Basal ganglia, Brain injury
Introduction
Hypoglycemia-related neurologic and mental disorders known as hypoglycemic encephalopathy (HE) have been recognized since the 1920s. Hypoglycemia is defined as an abrupt diminution in blood glucose level <50 mg/dL [1]. Hypoglycemia is usually observed in chronic alcoholism, severe metabolic disorders, or in patients treated for diabetes. Its neurological signs might range from focal and reversible signs including convulsions or hemiplegia to more severe presentations such as a vegetative state or persistent coma [2].
Neuroradiological findings are limited because most neurological signs improve after glucose normalization. The main neuroradiological feature of HE is impairment of the white matter, hippocampus, and cerebral cortex. In this report, we describe a case of HE with extensive damage to the central nervous system.
Case presentation
A 67-year-old man who had previously been treated for diabetes mellitus with a long-acting insulin analog was admitted to the emergency department. Two days after he had been last seen, the patient was discovered comatose at home. On admission, the man was unconscious with no response to pain and his Glasgow Coma Scale score was 3 (eye opening: 1; verbal response: 1; motor response: 1). Endotracheal intubation was performed using mechanical ventilation. The vital parameters were normal, and the initial blood glucose level was 18 mg/dL. After normalization of blood glucose to 125 mg/dL by 30% dextrose, there was no clinical improvement.
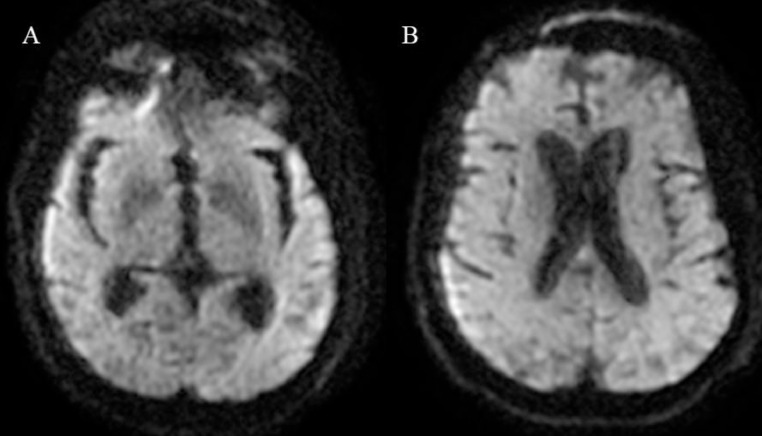
Cranial computed tomography revealed bilateral and confluent hypodensities of the periventricular and cerebral white matter (Fig. 1). Cerebral magnetic resonance imaging performed 24 hours after the admission showed on diffusion-weighted imaging (DWI) no cortical or subcortical compromise (Fig. 2). The fluid-attenuated inversion recovery (FLAIR) sequence revealed high signal intensity in the pons with bilateral hyperintensities involving the basal ganglia associated with widespread lesions of the corona radiata sparing the cerebral cortex (Fig. 3).
Fig. 1.
Cranial computed tomography showing symmetrical homogeneous hypodensities of the periventricular and subcortical white matter (arrows).
Fig. 2.
Cerebral magnetic resonance imaging on diffusion-weighted imaging shows no lesion of the basal ganglia (A), with no cortical or cerebral white matter abnormality (B).
Fig. 3.
Cranial magnetic resonance imaging on axial fluid-attenuated inversion recovery sequence showing pontine and temporal lesions (A) (arrows), with roughly symmetrical hyperintensities involving the caudoputamens and thalami (B) (arrows), with extensive lesions of the deep and subcortical white matter sparing the cerebral cortex (C) (arrow).
Laboratory testing (full blood count, renal and liver function tests, calcium, serum electrolyte, arterial blood gas analysis, ammonia level, thyroid hormone, thiamine test, vit B9 and B12 levels, toxicology screening, serology for hepatitis, HIV and syphilis) found no evidence of carbon monoxide poisoning, drug toxicity, infection, acidosis, or hepatic or renal dysfunction. A diagnosis of HE was made. Two weeks after admission, the initial neurological status of the patient did not improve and he died later due to septicemia.
Discussion
Given that glucose serves as the primary energy source for the nervous system, severe hypoglycemia may result in neuronal death. Clinical signs of hypoglycemia are intricate and are related to the severity, duration, and reactivity of serum glucose levels. Patients with HE exhibit specific neurologic symptoms such as aphasia, hemiplegia, hemianopsia, and convulsions with lethargy and coma as the hypoglycemia persists [3].
Brain energy deficit reduces the intracellular synthesis of proteins, leading to cell membrane ionic pump dysfunction, which causes the transfer of water from the extracellular to the intracellular sector. This results in cytotoxic edema involving the white matter, basal ganglia, hippocampus, and cerebral cortex. The brainstem is usually untouched because of the increment in local glucose transport systems [4].
Modifications in neurotransmitter regulation are also significant in the physiopathology of hypoglycemic neuronal injury. The glucose oxidation produces precursors for neurotransmitters including glutamate and acetylcholine. Glutamate and glutamine constitute another energy source leading to intracellular alkalosis by ammonia and accumulation of aspartate in the extracellular sector, which may cause selective neuronal necrosis. More, glutamate's excitotoxic activity has been linked to glial cell and myelin sheath edema [5].
The main neuroradiological features reported of hypoglycemia are specific lesions of white matter or impairment of both white and gray matter. White matter selective lesions have usually been reported on the corona radiata, corpus callosum, and internal capsule but rarely on the pontomesencephalic region [6]. Hypoglycemia-related lesions are presented as hyperintensities on T2/FLAIR and DWI sequences. These neuroradiological lesions may be reversible after normalization of serum glucose levels [7]. The neuroradiological findings in this study remain particular with the normality of the DWI and the extensive lesions on the FLAIR sequence, which could be related to the reversibility of the diffusion abnormalities after the normalization of blood glucose. This hypothesis needs to be confirmed.
Acquired metabolic or toxic conditions may damage the cerebral white matter and basal ganglia in approximatively manner to hypoglycemia. Hyponatremic Encephalopathy MRI findings include central pontine myelinolysis with trident-shaped lesion on T2/FLAIR in the central pons, or extra pontine myelinolysis with bilateral hyperintensities on DWI involving the cerebellum, cerebral cortex or basal ganglia. The radiological features of hypernatremic osmotic encephalopathy are central or extra pontine myelinolysis and arterial or venous thrombosis. Uremic encephalopathy is a metabolic condition linked to renal failure with specific MRI patterns including the posterior reversible encephalopathy syndrome or cytotoxic oedema of basal ganglia [8]. Hyperammonemic encephalopathy shows particular MRI characteristics with bilateral symmetrical hyperintensities on both DWI and FLAIR interesting the dorsomedial thalamus and the cingulate and insular cortices [9]. Thiamine deficiency usually occurs with symmetrical T2/FLAIR hyperintensity in the regions around the third ventricle [10].
Moreover, carbon monoxide intoxication is characterized by confluent high signal lesions on DWI and Flair sequences involving the centrum semiovale and sparing the brainstem [11]. Methanol toxicity and cocaine encephalopathy are among the possible diagnoses. A combination of clinical history, neurological symptoms, and laboratory investigations can aid in differentiating these disorders [12].
Studies indicate that clinically profound and persistent hypoglycemia is linked with poor prognosis [7,13]. There has been several research looking for an association between neuroradiological patterns and outcome. The research results found that only isolated injuries in the internal capsule are associated with full recovery, whereas widespread lesions in the cerebral white matter or the basal ganglia have an unfavorable short-term outcome [13], [14], [15].
Conclusion
HE is a critical condition characterized by low blood glucose levels leading to brain dysfunction and potential damage. Early detection of hypoglycemia, particularly in high-risk populations, is paramount to ensure appropriate glucose supplementation and prevent the progression of encephalopathy. Cerebral neuroimaging is an important parameter that can help differentiate HE from other conditions. Further research is needed to better understand the underlying mechanisms, optimize diagnostic approaches, and develop innovative therapeutic interventions to mitigate the impact of HE on affected individuals.
Patient consent
I qualify as the corresponding author to this manuscript and warrant that I have informed the patient of this scientific manuscript and confirm that I obtained his written and informed consent for the publication of this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Guarantor of Submission: The corresponding author is the guarantor of submission.
References
- 1.Guler A, Kumral E, Sirin TC, Sirin H, Kitis O. Magnetic resonance imaging characteristics of persistent vegetative state due to prolonged hypoglycemia. J Clin Diagn Res. 2015;9:TD1–TD2. doi: 10.7860/JCDR/2015/10478.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang KI, Hsieh KL, Chen CY. Hypoglycemic encephalopathy mimicking acute ischemic stroke in clinical presentation and magnetic resonance imaging: a case report. BMC Med Imaging. 2019;19:11. doi: 10.1186/s12880-019-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28:584–587. doi: 10.1161/01.str.28.3.584. [DOI] [PubMed] [Google Scholar]
- 4.Pelligrino D, Almquist LO, Siesjö BK. Effects of insulin-induced hypoglycemia on intracellular pH and impedance in the cerebral cortex of the rat. Brain Res. 1981;221:129–147. doi: 10.1016/0006-8993(81)91068-4. [DOI] [PubMed] [Google Scholar]
- 5.Kang EG, Jeon SJ, Choi SS, Song CJ, Yu IK. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2010;31:559–564. doi: 10.3174/ajnr.A1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JH, Kim YJ, Yoo WJ, Ihn YK, Kim JY, Song HH, et al. MR imaging of hypoglycemic encephalopathy: lesion distribution and prognosis prediction by diffusion-weighted imaging. Neuroradiology. 2009;51:641–649. doi: 10.1007/s00234-009-0544-5. [DOI] [PubMed] [Google Scholar]
- 7.Yanagawa Y, Isoi N, Tokumaru AM, Sakamoto T, Okada Y. Diffusion-weighted MRI predicts prognosis in severe hypoglycemic encephalopathy. J Clin Neurosci. 2006;13:696–699. doi: 10.1016/j.jocn.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Eesa M, Scott JN. Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol. 2009;193:879–886. doi: 10.2214/AJR.08.2257. [DOI] [PubMed] [Google Scholar]
- 9.U-King-Im JM, Yu E, Bartlett E, Soobrah R, Kucharczyk W. Acute hyperammonemic encephalopathy in adults: imaging findings. AJNR Am J Neuroradiol. 2011;32:413–418. doi: 10.3174/ajnr.A2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke's encephalopathy: a review. Biomed Res Int. 2014;2014 doi: 10.1155/2014/503596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR Am J Neuroradiol. 2014;35:625–631. doi: 10.3174/ajnr.A3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Eesa M, Scott JN. Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol. 2009;193:879–886. doi: 10.2214/AJR.08.2257. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T, Takahashi T, Sato A, Tanaka H, Igarashi S, Fujita N, et al. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101:159–163. doi: 10.1016/j.diabres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Johkura K, Nakae Y, Kudo Y, Yoshida TN, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. AJNR Am J Neuroradiol. 2012;33:904–909. doi: 10.3174/ajnr.A2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witsch J, Neugebauer H, Flechsenhar J, Jüttler E. Hypoglycemic encephalopathy: a case series and literature review on outcome determination. J Neurol. 2012;259:2172–2181. doi: 10.1007/s00415-012-6480-z. [DOI] [PubMed] [Google Scholar]