Summary
Background
Birth defects are a leading cause of neonatal, infant, and childhood mortality, but recent population-based survival estimates for a spectrum in the U.S. are lacking.
Methods
Using the statewide Texas Birth Defects Registry (1999–2017 births) and vital records linkage to ascertain deaths, we conducted Kaplan–Meier analyses to estimate survival probabilities at 1, 7, and 28 days, and 1, 5, and 10 years. We evaluated survival in the full cohort of infants with any major defect and for 30 specific conditions. One-year survival analyses were stratified by gestational age, birth year, and case classification.
Findings
Among 246,394 live-born infants with any major defect, the estimated survival probabilities were 98.9% at 1 day, 95.0% at 1 year, and 93.9% at 10 years. Ten-year survival varied by condition, ranging from 36.9% for holoprosencephaly to 99.3% for pyloric stenosis. One-year survival was associated with increasing gestational age (e.g., increasing from 46.9% at <28 weeks to 95.8% at ≥37 weeks for spina bifida). One-year survival increased in more recent birth years for several defect categories (e.g., increasing from 86.0% among 1999–2004 births to 93.1% among 2014–2017 births for unilateral renal agenesis/dysgenesis) and was higher among infants with an isolated defect versus those with multiple defects.
Interpretation
This study describes short- and long-term survival outcomes from one of the largest population-based birth defect registries in the world and highlights improved survival over time for several conditions. Our results may lend insight into future healthcare initiatives aimed at reducing mortality in this population.
Funding
This study was funded in part by a Centers for Disease Control and Prevention (CDC) birth defects surveillance cooperative agreement with the Texas Department of State Health Services and Health Resources and Services Administration (HRSA) Block Grant funds.
Keywords: Birth defects, Cohort study, Child mortality, Registries, Survival analysis
Research in context.
Evidence before this study
We searched PubMed and Ovid Medline for articles published between January 1, 1999 and March 31, 2023 on survival outcomes among infants with birth defects using search terms such as ((congenital anomal∗) OR (birth defect∗)) AND ((survival) OR (mortality) OR (Kaplan)). A meta-analysis published in 2020 and analyses from pooled European cohorts provided survival probability estimates for a number of specific defects. Results suggested that some conditions demonstrated improved survival over time and that the presence of additional defects was an important factor in survival. Recent population-based survival estimates for a spectrum of major birth defects in the U.S. are lacking.
Added value of this study
We conducted a cohort analysis looking at short- and long-term survival outcomes for 30 specific conditions and for all infants with a major birth defect. These analyses used data from the Texas Birth Defects Registry, one of the largest active-surveillance population-based birth defect registries in the world, and covered statewide births from 1999 to 2017. We additionally analyzed one-year survival probabilities across gestational age, birth year, and case classification subgroups. While survival varied widely by defect-type, one-year survival was consistently associated with gestational age at birth across all conditions studied. Survival improved in more recent years for some of the defects studied and, among nonsyndromic cases, infants with an isolated defect generally had a higher probability of one-year survival than those with additional defects. Our key findings are among the first assessments of their nature to describe defect-specific survival probabilities across subgroups with this level of detail.
Implications of all the available evidence
We confirmed that preterm birth and co-occurring birth defects are important contributors to mortality among infants with birth defects, and provided detailed defect-specific survival probabilities by gestational age group, birth year, and defect classification. Taken in context with prior research, these survival estimates may be useful to clinicians, parents, and care teams assessing short-term and long-term prognosis among infants with specific birth defects. Additionally, these findings may be used to prioritize future work on improving survival outcomes among infants with birth defects. Research focused on reducing preterm birth and improving outcomes when it does occur may be impactful ways to reduce mortality in this population.
Introduction
While the overall rate of infant mortality has declined over time, birth defects, which cumulatively affect an estimated 3–5% of pregnancies, remain the leading cause of infant mortality in the U.S.1,2 The deleterious sequelae of birth defects, including an increased risk of morbidity, mortality, and socioeconomic impacts, extend into early childhood and beyond.3,4 In fact, among children one to four years of age, birth defects are the second leading cause of mortality and the third leading cause of mortality among children five to nine years of age in the U.S.2,5 Survival estimates vary by defect type due to heterogeneity in the functional impact and organ systems affected,6 and although infant survival has improved over time for some specific defects (e.g., critical congenital heart defects7), comprehensive data on survival among U.S. births in recent years are lacking, yielding a fragmented understanding of current survival trajectories.
To identify opportunities for advancements in screening and treatment, population-based studies of survival among infants with a spectrum of birth defects are necessary, but in the U.S., the most recent of such analyses examined infants born prior to 2008.6,8 Given this ∼15-year data gap and improvements in neonatal care during this time (e.g., refinements in the use of antenatal corticosteroids and surfactants to prevent and treat respiratory distress syndrome,9 advances in the treatment of acute kidney injury,10 sutureless closure among infants with gastroschisis11), it is likely that the landscape of survival among infants with birth defects has changed during this period for at least some defects. In addition, other key factors that may be associated with differences in survival have not yet been examined for many defects, including co-occurrence with additional defects and preterm birth.
Population-based studies of long-term survival among children with rare birth defects are challenging to conduct because they require data from an extremely large catchment area (e.g., millions of total deliveries), as well as sufficient data linkages to track long-term survival. Therefore, few data sources are available to conduct such analyses, especially within single surveillance systems. To address these challenges and gaps in understanding birth defects mortality, we used one of the largest population-based registries in the world, the Texas Birth Defects Registry, to describe survival outcomes in the neonatal period, infancy, and childhood across a spectrum of major non-cardiac birth defects.
Methods
Study population
We used data from the Texas Birth Defects Registry (TBDR), which conducts active surveillance across the state to ascertain birth defects diagnosed in hospitals, birthing centers, and relevant clinics. The Registry is managed by the Birth Defects Epidemiology and Surveillance Branch of the Texas Department of State Health Services (DSHS), and it includes live births, stillbirths, and terminations with a monitored structural birth defect or chromosomal abnormality diagnosed within the first year of life.12 Given the statewide catchment area and the large size of the state, the available data is representative of birth defects among over seven million total live births since 1999. Mothers must be Texas residents at the time of delivery for inclusion in the TBDR. Diagnoses are recorded using Centers for Disease Control and Prevention-modified British Pediatric Association (BPA) codes for the reporting of birth defects.13 TBDR records are routinely linked to data from the Texas DSHS Center for Health Statistics (birth certificates, fetal death certificates, and death certificates) to establish maternal residence, provide additional sociodemographic data, and determine vital status.
This project was approved by the Institutional Review Board of the Texas DSHS and the UTHealth Committee for the Protection of Human Subjects. The staff of the TBDR has legislative authority to collect the TBDR data on all deliveries in Texas without individual consent (Texas Health and Safety Code, Chapter 87; Texas Administrative Code, Title 25, Part 1, Chapter 37, Subchapter P, Rules 37.301–37.306).
Case data
Data were obtained for live-born infants in the TBDR with a gestational age ≥20 weeks with at least one major birth defect delivered between January 1, 1999 and December 31, 2017. We excluded birth defects considered minor by the National Birth Defects Prevention Study14 or the TBDR.15 We determined vital status based on the presence of a linked death certificate, including out-of-state deaths reported back to Texas from states with inter-agency agreements,16 or death recorded in the abstracted medical record. Individuals without a linked death certificate or death recorded in the medical record were presumed to be alive at the time of censoring (at 10 years of age or December 31, 2018). We calculated the number of follow-up days/years by either (1) subtracting the date of birth from the date of death, or (2) for infants without death records, subtracting the date of birth from the date of censoring (10th birthday or December 31, 2018, the last date available for linked vital records at the time of analysis). Infants with a death recorded, but who were missing a valid date of death were excluded from analyses (n = 88). Data were also available for infant sex, maternal race/ethnicity, gestational age at birth (20–<28 weeks, 28–<32 weeks, 32–<37 weeks, ≥37 weeks), birth year (1999–2004, 2005–2009, 2010–2013, 2014–2017), and case classification group (presence of isolated, multiple, chromosomal, or syndromic defects17). Data for infant and maternal characteristics came from the linked birth certificate when available, and were otherwise drawn from abstracted medical records. Infants with only nonsyndromic defects that were all in the same system (e.g., only eye defects) or part of known sequences (e.g., clubfoot co-occurring with spina bifida) were classified as having isolated defects.18
Statistical analyses
We used SAS software (Version 9.4, SAS Institute Inc, Cary, NC, USA) and Stata/IC (Version 14.2, StataCorp LLC, College Station, TX, USA) to conduct analyses among infants with (1) any major birth defect in the TBDR, and, in defect-specific analyses, (2) 30 selected major non-cardiac birth defects, based on those reported annually by the TBDR13 (BPA codes detailed in Supplementary Table S1). For each analytic group (full cohort and 30 defect-specific groups), we calculated the median follow-up time and the interquartile range for each estimate. We then calculated the survival probability and 95% confidence interval (CI) at six time points (1 day, 7 days, 28 days, 1 year, 5 years, and 10 years) using Kaplan–Meier survival analysis, with 95% CIs from the pointwise confidence limits computed for the survivor function in PROC LIFETEST. Survival estimates started at the day of birth (origin time) for all analytic time points and included deaths observed during the time period of interest to calculate the survival probability that an individual survives from the time origin (birth) to the end of each specified time. For instance, the estimated 7-day survival probability was calculated based on the observed survival experience during days 0–6. All infants had at least one year of follow-up (via restriction to births through December 31, 2017), and, for 5- and 10-year mortality analyses, cases born respectively after December 31, 2013 and 2008 contributed follow-up time from birth until the time of censoring (death or December 31, 2018). To visualize the timing of when first-year deaths occurred during infancy for specific defects, we plotted on a bar chart the proportion of cases that died at age <1 day (on the day of birth), 1–6, 7–27, and 28–364 days old.
In the full cohort, we calculated Kaplan–Meier survival estimates at all time points by infant sex and maternal race/ethnicity (Hispanic, non-Hispanic Black, non-Hispanic other [includes American Indian or Alaska Native, Asian, Pacific Islander, and other specified groups], and non-Hispanic White). In the full cohort, we plotted Kaplan–Meier survival curves, stratified by gestational age, birth year, and case classification group (categories defined above), with log-rank tests to evaluate survival differences across strata. Finally, in the full cohort and in defect-specific groups, we calculated one-year Kaplan–Meier survival estimates within strata defined by gestational age, birth year, and case classification group, and these survival estimates were plotted on bar plots. We assessed linear trends in one-year survival across ordinal gestational age and birth year categories using two-sided Cochran–Armitage tests for trend, and we assessed differences in survival probability between case classification groups using chi-square tests.
In post-hoc secondary analyses, we assessed trends in survival by birth year among infants with congenital hip dislocation (BPA 754.300). In additional post-hoc analyses, we conducted Cox proportional hazard regression analyses, assessing model assumptions graphically and using Schoenfeld residual-based diagnostics, across categorized stratification variables to calculate the hazard ratio (HR) of death during the first seven days and repeated the models with an ordinal term for 4-level categories to assess the linear relationship across groups.
Role of the funding source
The study funder was not involved in study design, data collection or analysis, interpretation and writing of the report, or the decision to submit the manuscript for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
There were 246,394 live-born neonates with at least one major defect recorded in the TBDR (1999–2017). Over half of neonates were male (55.9%) and 48.4% of included neonates were born to Hispanic women, 35.5% to non-Hispanic White women, 11.7% to non-Hispanic Black women, and 4.4% to women of other race/ethnicity (Supplementary Table S2). Overall, 14,474 (5.9%) of cases had a death prior to ten years of age recorded, either noted only on a linked death certificate (38.0%), only in the abstracted medical record (5.0%), or both (57.0%). Individuals without a recorded death prior to age ten (n = 231,920; 94.1%) were censored at 10 years of age or at the end of follow-up (December 31, 2018). The median follow-up time among all individuals until death, age 10, or December 31, 2018 was 8.4 years (interquartile range 4.1–10.0 years) and ranged from 0.9 (holoprosencephaly) to 10.0 years (pyloric stenosis and congenital hip dislocation without hip dysplasia) among the specific defects analyzed (Supplementary Table S3).
Among all individuals with a major birth defect in the TBDR, the probability of survival decreased from 98.9% at 1 day to 93.9% at 10 years (Table 1). Survival probabilities among males decreased from 98.9% at 1 day to 94.3% at 10 years, while survival probabilities among females decreased from 98.8% at 1 day to 93.6% at 10 years (Supplementary Table S2). At ten years, survival probabilities were 93.5% among children born to Hispanic women, 92.7% among children born to non-Hispanic Black women, 94.8% among children born to non-Hispanic White women, and 94.9% among children born to women of other race/ethnicity (Supplementary Table S2).
Table 1.
Kaplan–Meier survival estimates by birth defect, Texas Birth Defects Registry, 1999–2017 births.
| Total N | 1 D (95% CI) | 7 D (95% CI) | 28 D (95% CI) | 1 Y (95% CI) | 5 Y (95% CI) | 10 Y (95% CI) | |
|---|---|---|---|---|---|---|---|
| All infantsa | 246,394 | 98.9 (98.8–98.9) | 97.8 (97.7–97.9) | 96.9 (96.8–96.9) | 95.0 (94.9–95.1) | 94.2 (94.2–94.3) | 93.9 (93.8–94.0) |
| Central nervous system | |||||||
| Spina bifida without anencephaly | 2453 | 97.1 (96.4–97.7) | 95.0 (94.0–95.8) | 94.0 (92.9–94.8) | 91.9 (90.8–92.9) | 90.5 (89.2–91.6) | 89.8 (88.5–91.0) |
| Encephalocele | 528 | 84.8 (81.5–87.6) | 76.3 (72.5–79.7) | 72.7 (68.7–76.3) | 68.8 (64.6–72.5) | 65.4 (61.2–69.3) | 63.7 (59.3–67.7) |
| Holoprosencephaly | 633 | 86.1 (83.2–88.6) | 72.7 (69.0–76.0) | 64.0 (60.1–67.6) | 49.1 (45.2–53.0) | 40.4 (36.5–44.2) | 36.9 (32.9–40.8) |
| Eye and ear | |||||||
| Anophthalmia | 162 | 77.8 (70.6–83.4) | 65.4 (57.6–72.2) | 59.9 (51.9–67.0) | 54.3 (46.3–61.6) | 47.8 (39.9–55.3) | 45.6 (37.5–53.4) |
| Microphthalmia | 1934 | 96.4 (95.5–97.2) | 87.7 (86.1–89.1) | 81.2 (79.4–82.9) | 69.7 (67.6–71.7) | 66.3 (64.2–68.4) | 65.4 (63.2–67.5) |
| Cataract | 1386 | 99.6 (99.0–99.8) | 98.3 (97.5–98.9) | 96.9 (95.8–97.7) | 93.9 (92.5–95.0) | 91.2 (89.5–92.6) | 90.5 (88.7–91.9) |
| Anotia or microtia | 2431 | 98.6 (98.0–99.0) | 96.3 (95.5–97.0) | 93.9 (92.9–94.8) | 90.8 (89.6–91.9) | 89.5 (88.2–90.7) | 89.1 (87.7–90.3) |
| Respiratory | |||||||
| Choanal atresia or stenosis | 910 | 99.3 (98.5–99.7) | 98.0 (96.9–98.7) | 95.5 (93.9–96.7) | 90.5 (88.5–92.3) | 87.9 (85.6–89.9) | 86.7 (84.2–88.8) |
| Agenesis or aplasia of the lung | 79 | 79.7 (69.1–87.1) | 72.2 (60.9–80.7) | 63.3 (51.7–72.8) | 50.6 (39.2–61.0) | 49.4 (38.0–59.8) | 47.2 (35.6–58.0) |
| Oral clefts | |||||||
| Cleft palate alone (without cleft lip) | 3873 | 97.7 (97.2–98.1) | 95.0 (94.2–95.6) | 92.7 (91.9–93.5) | 88.1 (87.0–89.1) | 86.7 (85.6–87.7) | 86.2 (85.0–87.2) |
| Cleft lip with or without cleft palate | 7224 | 97.3 (96.9–97.6) | 94.4 (93.8–94.9) | 92.5 (91.9–93.1) | 89.8 (89.1–90.5) | 88.8 (88.0–89.5) | 88.5 (87.8–89.3) |
| Gastrointestinal | |||||||
| Tracheoesophageal fistula/esophageal atresia | 1557 | 97.4 (96.4–98.1) | 90.9 (89.4–92.3) | 87.4 (85.7–89.0) | 82.3 (80.4–84.1) | 79.7 (77.6–81.6) | 79.0 (76.8–81.0) |
| Pyloric stenosis | 11,994 | NR | NR | 99.9 (99.9–100.0) | 99.6 (99.4–99.7) | 99.4 (99.2–99.5) | 99.3 (99.1–99.4) |
| Stenosis or atresia of the small intestine | 2389 | 99.6 (99.2–99.8) | 98.9 (98.4–99.3) | 97.7 (97.1–98.3) | 93.5 (92.4–94.4) | 91.4 (90.2–92.5) | 90.8 (89.5–91.9) |
| Stenosis or atresia of large intestine, rectum, or anal canal | 3620 | 94.3 (93.5–95.0) | 89.8 (88.8–90.7) | 87.9 (86.8–88.9) | 84.4 (83.2–85.6) | 83.0 (81.7–84.2) | 82.7 (81.4–83.9) |
| Hirschsprung disease | 1029 | NR | NR | 99.0 (98.2–99.5) | 96.3 (95.0–97.3) | 95.0 (93.5–96.2) | 94.6 (93.0–95.8) |
| Biliary atresia | 522 | NR | NR | 99.0 (97.7–99.6) | 86.0 (82.7–88.7) | 83.0 (79.5–86.0) | 82.2 (78.5–85.2) |
| Genitourinary | |||||||
| Hypospadias | 12,050 | 99.7 (99.5–99.7) | 98.9 (98.7–99.1) | 98.2 (98.0–98.5) | 97.0 (96.7–97.3) | 96.6 (96.3–96.9) | 96.5 (96.1–96.8) |
| Epispadias | 775 | NR | 99.1 (98.1–99.6) | 98.7 (97.6–99.3) | 98.6 (97.5–99.2) | 98.3 (97.1–99.0) | 98.3 (97.1–99.0) |
| Renal agenesis or dysgenesis (unilateral or not otherwise specified) | 3084 | 97.3 (96.6–97.8) | 94.5 (93.6–95.2) | 92.8 (91.8–93.6) | 89.5 (88.4–90.5) | 88.3 (87.2–89.4) | 87.9 (86.7–89.0) |
| Bladder exstrophy | 126 | 93.7 (87.7–96.8) | 92.1 (85.8–95.6) | 90.5 (83.8–94.5) | 86.5 (79.2–91.4) | 85.7 (78.3–90.7) | 85.7 (78.3–90.7) |
| Musculoskeletal | |||||||
| Congenital hip dislocation without hip dysplasia | 1941 | 98.8 (98.2–99.2) | 97.2 (96.3–97.8) | 96.1 (95.1–96.9) | 93.7 (92.5–94.7) | 92.8 (91.6–93.9) | 92.5 (91.2–93.6) |
| Talipes equinovarus/clubfoot | 11,148 | 96.8 (96.4–97.1) | 94.5 (94.0–94.9) | 93.2 (92.7–93.6) | 90.4 (89.9–91.0) | 89.5 (88.9–90.1) | 89.2 (88.6–89.8) |
| Reduction defects of the upper limbs | 2736 | 96.2 (95.4–96.9) | 93.0 (92.0–93.9) | 91.0 (89.9–92.1) | 87.8 (86.5–88.9) | 86.3 (84.9–87.5) | 85.7 (84.3–87.0) |
| Reduction defects of the lower limbs | 1239 | 92.8 (91.2–94.1) | 90.4 (88.6–91.9) | 88.7 (86.8–90.3) | 86.0 (83.9–87.8) | 84.7 (82.6–86.6) | 84.6 (82.5–86.5) |
| Craniosynostosis | 3878 | 99.6 (99.3–99.7) | 99.2 (98.8–99.4) | 98.8 (98.4–99.1) | 98.0 (97.5–98.4) | 97.6 (97.0–98.0) | 97.2 (96.6–97.7) |
| Achondroplasia | 247 | NR | NR | 97.6 (94.7–98.9) | 93.1 (89.2–95.7) | 93.1 (89.2–95.7) | 92.3 (88.0–95.1) |
| Diaphragmatic hernia | 1945 | 92.1 (90.8–93.2) | 83.0 (81.2–84.6) | 75.1 (73.1–76.9) | 68.2 (66.1–70.2) | 66.8 (64.6–68.8) | 66.5 (64.4–68.6) |
| Omphalocele | 1173 | 88.2 (86.3–89.9) | 80.4 (78.0–82.6) | 76.0 (73.4–78.3) | 68.7 (66.0–71.3) | 67.6 (64.8–70.2) | 67.6 (64.8–70.2) |
| Gastroschisis | 3462 | 98.8 (98.4–99.1) | 97.5 (97.0–98.0) | 96.9 (96.2–97.4) | 94.6 (93.8–95.3) | 93.8 (93.0–94.6) | 93.6 (92.8–94.4) |
D, day; Y, year; CI, confidence interval; NR, not reported (n < 5).
All infants with a major birth defect; cases with more than one birth defect were only counted once in the “All infants” group and contributed to each applicable defect-specific estimate.
Over 20% of neonates with anophthalmia or lung agenesis/aplasia and approximately 14–15% of infants with encephalocele or holoprosencephaly died on the first day of life (Table 1). One-year survival probabilities varied substantially by defect type, from approximately 50% among infants with holoprosencephaly or lung agenesis/aplasia to ≥98% among infants with pyloric stenosis, epispadias, or craniosynostosis (Table 1). Ten-year survival ranged from 36.9% for holoprosencephaly to 99.3% for pyloric stenosis and survival probabilities among neonates, infants, and children with spina bifida were 94.0% at 28 days, 91.9% at one year, and 89.8% at ten years (Table 1).
For nearly all defects, the majority of deaths occurred during the neonatal period (<28 days; Fig. 1). Of note, among neonates with lung agenesis/aplasia, bladder exstrophy, encephalocele, anophthalmia, and reduction defects of the lower limbs, 40–50% of first-year mortality specifically occurred on the first day of life (Fig. 1). By contrast, over 50% of deaths occurred during the postneonatal period (28–364 days) among individuals with choanal atresia/stenosis and stenosis/atresia of the small intestine (Fig. 1).
Fig. 1.
Mortality proportion in the first year of life by age at the time of death, Texas Birth Defects Registry, 1999–2017 births.
Stratification by gestational age, birth year, and case classification group
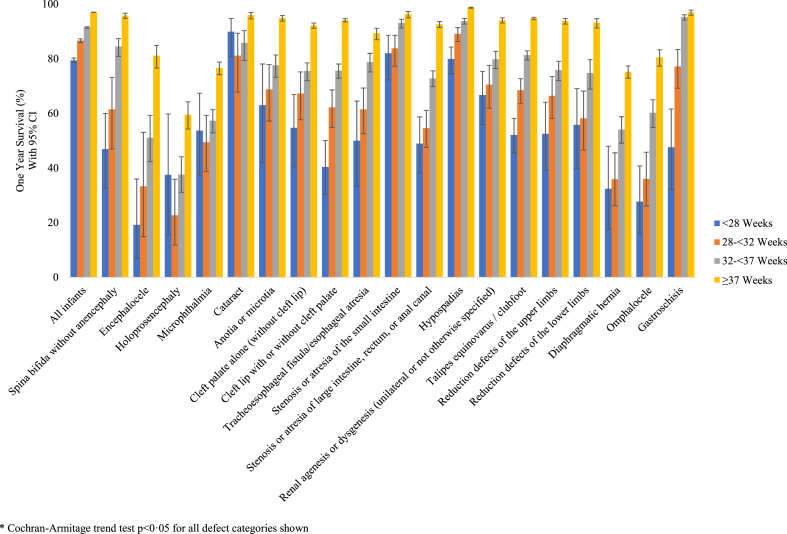
In analyses stratified by gestational age, one-year survival estimates increased with increasing gestational age in the full cohort and for nearly all defects analyzed (all tests for trend p < 0.0001; Fig. 2 and Supplementary Table S4). In the full cohort, one-year survival probabilities increased across gestational age categories, from 79.4% (95% CI: 78.6–80.3) among infants delivered at <28 weeks to 97.1% (95% CI: 97.0–97.1) among infants delivered at ≥37 weeks (Fig. 2 and Supplementary Table S4; log-rank p < 0.0001). In defect-specific results, infants delivered at <28 weeks had the lowest survival probability for all but three defects (holoprosencephaly, microphthalmia, and cataract), among which confidence intervals overlapped widely with other gestational age categories due to small numbers (Fig. 3). Infants delivered at ≥37 weeks had the highest survival probability across all defect groups studied (Fig. 3 and Supplementary Table S4). These relationships were similarly reflected in HR estimates, with the highest hazards of death in the first seven days among infants born at <28 weeks versus ≥37 weeks for all defect groups analyzed (Supplementary Table S5).
Fig. 2.
One-year Kaplan–Meier survival curves by (a) gestational age, (b) birth year, and (c) classification, Texas Birth Defects Registry, 1999–2017 births.
Fig. 3.
One-year Kaplan–Meier survival estimates by gestational age at birth, Texas Birth Defects Registry 1999–2017 births.
For the full cohort and for most defects, the highest one-year survival estimates were observed among infants born in more recent years (2010–2013 or 2014–2017; Fig. 4 and Supplementary Table S6). Among all infants, one-year survival estimates increased across birth year groups, from 94.2% (95% CI: 94.0–94.4) among infants delivered 1999–2004 to 95.7% (95% CI: 95.6–95.9) among infants delivered 2014–2017 (Fig. 2 and Supplementary Table S6; log-rank p < 0.0001). Among 26 defects with adequate sample size to assess trends across birth year cohorts (≥5 infants in each comparison group), one-year survival was associated with increasing birth year group for seven defects (Supplementary Table S6): (1) anotia/microtia (p = 0.03), (2) stenosis/atresia of the small intestine (p = 0.01), (3) stenosis/atresia of large intestine, rectum, or anal canal (p = 0.01), (4) renal agenesis/dysgenesis (unilateral or not otherwise specified [NOS]) (p < 0.0001), (5) craniosynostosis (p = 0.01), (6) diaphragmatic hernia (p = 0.02), and (7) gastroschisis (p = 0.02). This improvement was particularly striking for unilateral/NOS renal agenesis/dysgenesis, increasing from 86.0% for 1999–2004 births to 93.1% for 2014–2017 births. Conversely, one defect, congenital hip dislocation without hip dysplasia (BPA 754.300 without 755.665–755.667), had a significantly decreasing (instead of increasing) test for trend in survival across birth years. Because this finding may be due in part to changes in the TBDR coding definition for this defect over time (i.e., exclusion of infants with co-occurring hip dysplasia from the congenital hip dislocation group only in recent years), we conducted post-hoc secondary analyses among infants with congenital hip dislocation (BPA 754.300), in which we found no significant trend in survival across years (data not shown). Similar HR trends of decreasing hazards of death in the first seven days across increasing birth year group were observed for anotia/microtia, renal agenesis/dysgenesis, and gastroschisis (Supplementary Table S7).
Fig. 4.
One-year Kaplan–Meier survival estimates by birth year, Texas Birth Defects Registry, 1999–2017 births.
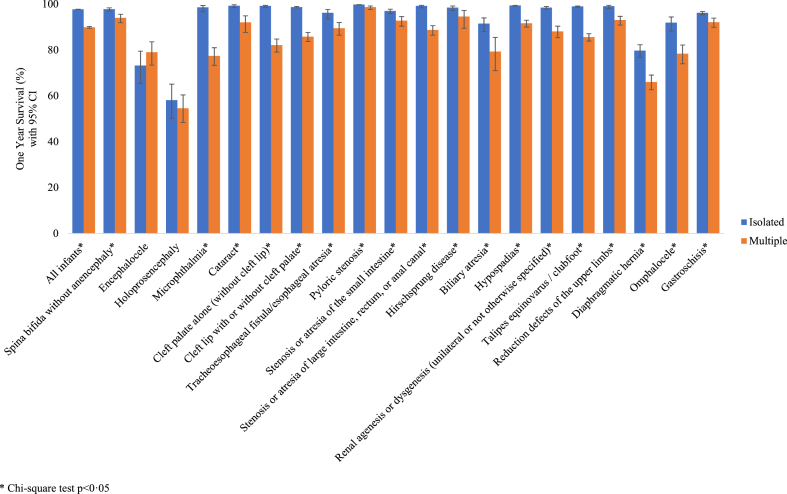
For all defect categories except encephalocele, infants with an isolated defect had higher survival probabilities compared to infants with multiple defects, which was also reflected in HRs for death by seven days of age (Fig. 5 and Supplementary Tables S8 and S9). In the full cohort, infants with an isolated defect had a 97.7% (95% CI: 97.7–97.8) probability of surviving to one year, while infants with multiple defects had an 89.9% (95% CI: 89.5–90.3) probability of survival (Supplementary Table S8). Infants with a syndrome diagnosis had the lowest first-year survival estimate (81.2%; Fig. 2, log-rank p < 0.0001). Among infants with isolated defects, one-year survival ranged from 58.1% for infants with holoprosencephaly to 99.8% for infants with pyloric stenosis. Of note, one-year survival estimates among isolated cases were >99% for seven defects (cataract; cleft palate alone; pyloric stenosis; stenosis/atresia of large intestine, rectum, or anal canal; hypospadias; talipes equinovarus/clubfoot; reduction defects of the upper limbs).
Fig. 5.
One-year Kaplan–Meier survival estimates in nonsyndromic infants by case classification group, Texas Birth Defects Registry, 1999–2017 births.
Discussion
Survival probabilities varied widely by defect type, and we observed trends of higher survival probabilities with increasing gestational age at birth, both within the overall cohort and within all specific defect types analyzed. Consistent with previous studies, differences in survival by maternal race/ethnicity widened during the postneonatal period and into childhood8 and female infants had slightly lower survival probabilities.6 Survival results by birth year groups showed an overall improvement in survival across years in the full cohort, and these trends were observed in seven defect-specific trend analyses, with substantially improved survival among infants with unilateral/NOS renal agenesis/dysgenesis in particular.
Our key findings of higher overall and defect-specific survival by increasing gestational age, increasing birth year cohort, and less severe case classification group (e.g., cases with isolated defects) are somewhat difficult to directly compare to prior literature. One study assessed survival by birth year group in New York,6 but was limited to 2006 or earlier birth years and grouped defects by organ system. One Australian study described survival by isolated versus multiple case classification,19 but also lumped defects by system rather than presenting data for specific defects. In these studies that grouped analyses by organ system, results were consistent with our findings; infants born in earlier birth years had higher hazards of death across all defect groups analyzed6 and infants with isolated defects had higher survival estimates than those with multiple defects across all defect groups.19 An early Texas study (1995–1997 births) also looked at one-year defect-specific survival by classification for 13 of the same defects; survival probabilities in that study were generally similar to or slightly lower among isolated cases and generally lower among infants with additional defects compared to our results.20 Some of the overall improvements in survival we observed in more recent years may be related to overall improvements in neonatal care over the years studied. Infant mortality in the U.S. declined by 21% between 2005 and 2020 and mortality among infants born at <32 weeks declined by 9% between 2015 and 2020.21 Management and repair of birth defects has likely changed over the years studied (e.g., shift to sutureless umbilical closure among infants with gastroschisis, which reduces ventilation and anaesthesia exposure11), as well in improvements in screening and diagnosis (e.g., pulse oximetry screening in newborns to identify critical congenital heart defects22). Future work should focus on better understanding factors associated with improved survival and identifying opportunities to continue to improve the diagnosis, management, and treatment of birth defects.
Our findings of higher survival among term versus preterm infants in the full cohort were consistent with previous studies, which have reported increased mortality among preterm infants at up to five years.19,23 Infants with birth defects are at increased risk of preterm delivery compared to infants without birth defects, with differences in the risk of premature delivery by defect type.24 Prematurity is an established risk factor for mortality in the general population,21 and the overall proportion of mortality estimated to be attributable to preterm birth among all infants with birth defects is high (51.7%).25 Thus, our findings further support the importance of gestational age at delivery to survival outcomes among infants with birth defects and support the need for further research to reduce preterm birth and mortality risk among infants with birth defects.
We also observed differences by defect type in the timing of when deaths occurred during the first year, which may shed light on both the likely etiologies at play as well as the most impactful time points to prioritize for future interventions designed to decrease neonatal and infant mortality among infants with different types of birth defects. For example, our results suggest that successful interventions targeting mortality prevention soon after delivery may be of particular importance among neonates with specific defects (e.g., lung agenesis/aplasia, anophthalmia), whereas postneonatal interventions may be beneficial among infants with defects such as choanal atresia/stenosis and stenosis/atresia of the small intestine. To improve postneonatal survival, it may be especially important to improve understanding of the extent to which social determinants of health (e.g., those related to access to care) may play a role.
Although it is difficult to directly compare our overall, unstratified findings to previous studies due to differences in birth years across studies (e.g., 1983–2006,6 1999–2007,8 2004–200919), some general parallels between our findings and previous studies can be drawn. Looking at long-term survival estimates (>1 year) for the full cohort, our results were similar to older cohort studies from the U.S. (1992–1998; 1996–2003) and Canada (1979–1986),23,26, 27, 28 and slightly higher than those reported in Scotland (1980–1997)29 and New York (1983–2006).6 First-year survival estimates were similar, with overlapping confidence intervals, for nearly all defects analyzed, compared to an earlier study that pooled data across 12 birth defect surveillance programs in the U.S., including Texas (1999–2007 births)8 and results for 1999–2004 births in this study were similar to survival probabilities from an earlier Texas study (1995–1997 births).20 Finally, compared to recent European studies using pooled EUROCAT data, our survival estimates generally were slightly lower, although EUROCAT analyses for common defects were restricted to 2005–2014 births.30,31 Of note, the estimated one-year survival probabilities for infants with spina bifida were slightly higher in our study (91.9%) compared to an earlier study that included birth years prior to folic acid fortification (88.5% for 1983–2006 births6), which may support the notion of a connection between fortification and improved survival.32,33 Improvements in survival among infants with spina bifida are thought to be due to both increases in periconceptional folic acid intake, which is associated with decreased spina bifida severity,34 and improvements in treatment.35 Survival among infants with spina bifida did not significantly change across the years studied and continued efforts to ensure adequate folic acid intake prior to conception may both reduce the prevalence of spina bifida and improve survival outcomes.34
Additional similar studies have not been conducted in the U.S. in recent years (2008 births or later), and the U.S. may have important unique characteristics compared to other countries (e.g., healthcare system landscape, folic acid fortification, lack of national birth defects surveillance or national vital records). Minor differences in overall, unstratified survival estimates across studies may be driven, in part, by these and other differences in the underlying populations (maternal factors associated with infant survival), access to care and healthcare utilization (antenatal diagnosis and access to termination), and access to and quality of postnatal care (access to appropriate intensive care and skilled surgical intervention). Thus, our findings may be most generalizable to U.S. populations, for which ∼1 in 10 births occur in Texas.21
Strengths of this study include the use of a large, diverse population-based registry that uses active surveillance to ascertain birth defects. The TBDR conducts routine linkage with state vital records and out-of-state deaths are additionally obtained through cooperative agreements. The registry has conducted statewide surveillance and vital records linkage since 1999, thereby providing adequate follow-up data to assess multiple age-specific survival probabilities. Our study had several limitations. First, we relied on the underlying assumption of the Kaplan–Meier survival method that the survival experience of censored cases would be the same as those cases contributing longer follow-up time. Since survival estimates for certain defects have improved over time, the calculated survival estimates may be slightly lower than the true expected survival outcomes for an infant born today. Future changes in pregnancy outcomes (e.g., prenatal detection and termination rates) or the landscape of defect severity may also impact survival probabilities. Additionally, analyses conducted in the full TBDR cohort included infants with defects we did not study in select defect-specific analyses. This may, in part, explain why we observed an overall trend in improving survival outcomes over successive birth year groups in the full cohort, while only some of the specific defects we analyzed in this report had significant improvement in survival over the years studied. Specifically, heart defects were not analyzed for this study because they are to be the subject of a companion survival paper under development. Finally, as a descriptive study of statewide registry data, our sample size was limited to all birth defect cases ascertained during the study period. Some estimates were not calculated due to small case numbers or few deaths during certain time windows (e.g., biliary atresia 1-day and 7-day survival probabilities). Nevertheless, we included a larger number of cases than recent EUROCAT studies30,31 and similar and/or larger numbers than previous U.S. studies.6,8
Conclusions
The detailed survival information provided in this study may be useful to clinicians, parents, and care teams assessing short-term and long-term prognosis among infants with specific birth defects. To our knowledge, these estimates represent the most up-to-date survival data available from a cohort in the US, and our results particularly highlight the important contribution of co-occurring birth defects and gestational age at birth to mortality risk among infants with birth defects, as well as improvement in survival over time both overall and for select defects. Given the elevated risk of preterm birth among infants with birth defects and the contribution of preterm birth to mortality in this population, reducing preterm birth and improving outcomes when it does occur may be potentially impactful areas to focus efforts to reduce mortality in this population.
Contributors
RHB contributed to study conceptualization, methodology, data curation, and formal analyses. AJA contributed to study conceptualization, methodology, funding acquisition, project administration, and validation of analyses. RHB and AJA wrote the first draft of the manuscript. JMN contributed to methodology, funding acquisition, and supervision. MAC and CJS contributed to study conceptualization, methodology, funding acquisition, resources, and data collection. JMN, MAC, and CJS reviewed and edited the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The data used in this study are not publicly available due to the confidentiality of the Texas Birth Defects Registry and Texas Vital Statistics data. They may be accessed by submitting a request to the Texas Department of State Health Services and receiving approval to obtain these data.
Declaration of interests
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was funded in part by a Centers for Disease Control and Prevention (CDC) birth defects surveillance cooperative agreement with the Texas Department of State Health Services (NU50DD000102, HHS 00096260001) and Health Resources and Services Administration (HRSA) Block Grant funds. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA or CDC.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100617.
Appendix ASupplementary data
References
- 1.Almli L.M., Ely D.M., Ailes E.C., et al. Infant mortality attributable to birth defects - United States, 2003-2017. MMWR Morb Mortal Wkly Rep. 2020;69:25–29. doi: 10.15585/mmwr.mm6902a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1–77. [PubMed] [Google Scholar]
- 3.Arth A.C., Tinker S.C., Simeone R.M., Ailes E.C., Cragan J.D., Grosse S.D. Inpatient hospitalization costs associated with birth defects among persons of all ages - United States, 2013. MMWR Morb Mortal Wkly Rep. 2017;66:41–46. doi: 10.15585/mmwr.mm6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razzaghi H., Oster M., Reefhuis J. Long-term outcomes in children with congenital heart disease: National Health Interview Survey. J Pediatr. 2015;166:119–124. doi: 10.1016/j.jpeds.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J., Murphy S.L., Kochanek K.D., Arias E. Deaths: final data for 2019. Natl Vital Stat Rep. 2021;70:1–87. [PubMed] [Google Scholar]
- 6.Wang Y., Hu J., Druschel C.M., Kirby R.S. Twenty-five-year survival of children with birth defects in New York State: a population-based study. Birth Defects Res A Clin Mol Teratol. 2011;91:995–1003. doi: 10.1002/bdra.22858. [DOI] [PubMed] [Google Scholar]
- 7.Oster M.E., Lee K.A., Honein M.A., Riehle-Colarusso T., Shin M., Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–e1508. doi: 10.1542/peds.2012-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Liu G., Canfield M.A., et al. Racial/ethnic differences in survival of United States children with birth defects: a population-based study. J Pediatr. 2015;166:819–826.e1–2. doi: 10.1016/j.jpeds.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polin R.A., Carlo W.A., Committee on Fetus and Newborn, American Academy of Pediatrics Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–163. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 10.Starr M.C., Charlton J.R., Guillet R., et al. Advances in neonatal acute kidney injury. Pediatrics. 2021;148 doi: 10.1542/peds.2021-051220. [DOI] [PubMed] [Google Scholar]
- 11.Diyaolu M., Wood L.S., Bruzoni M. Sutureless closure for the management of gastroschisis. Transl Gastroenterol Hepatol. 2021;6:31. doi: 10.21037/tgh-20-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller E. Evaluation of the Texas Birth Defects Registry: an active surveillance system. Birth Defects Res A Clin Mol Teratol. 2006;76:787–792. doi: 10.1002/bdra.20331. [DOI] [PubMed] [Google Scholar]
- 13.Texas Department of State Health Services. Birth Defects Epidemiology and Survillance Branch Annual report of birth defects among Texas deliveries. https://www.dshs.texas.gov/texas-birth-defects-epidemiology-surveillance/birth-defects-data-publications
- 14.Rasmussen S.A., Olney R.S., Holmes L.B., et al. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 15.Langlois P.H., Marengo L., Lupo P.J., et al. Evaluating the proportion of isolated cases among a spectrum of birth defects in a population-based registry. Birth Defects Res. 2023;115:21–25. doi: 10.1002/bdr2.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Association for Public Health Statistics and Information Systems (NAPHSIS) State and territorial exchange of vital events (STEVE) 2023. https://www.naphsis.org/steve
- 17.Benjamin R.H., Mitchell L.E., Scheuerle A.E., et al. Identifying syndromes in studies of structural birth defects: guidance on classification and evaluation of potential impact. Am J Med Genet A. 2023;191:190–204. doi: 10.1002/ajmg.a.63014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garne E., Dolk H., Loane M., et al. Paper 5: Surveillance of multiple congenital anomalies: implementation of a computer algorithm in European registers for classification of cases. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S44–S50. doi: 10.1002/bdra.20777. [DOI] [PubMed] [Google Scholar]
- 19.Schneuer F.J., Bell J.C., Shand A.W., Walker K., Badawi N., Nassar N. Five-year survival of infants with major congenital anomalies: a registry based study. Acta Paediatr. 2019;108:2008–2018. doi: 10.1111/apa.14833. [DOI] [PubMed] [Google Scholar]
- 20.Nembhard W.N., Waller D.K., Sever L.E., Canfield M.A. Patterns of first-year survival among infants with selected congenital anomalies in Texas, 1995-1997. Teratology. 2001;64:267–275. doi: 10.1002/tera.1073. [DOI] [PubMed] [Google Scholar]
- 21.Ely D.M., Driscoll A.K. Infant mortality in the United States, 2020: data from the period linked birth/infant death file. Natl Vital Stat Rep. 2022;71:1–18. [PubMed] [Google Scholar]
- 22.Mahle W.T., Newburger J.W., Matherne G.P., et al. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the AHA and AAP. Pediatrics. 2009;124:823–836. doi: 10.1542/peds.2009-1397. [DOI] [PubMed] [Google Scholar]
- 23.Nembhard W.N., Salemi J.L., Ethen M.K., Fixler D.E., Canfield M.A. Mortality among infants with birth defects: joint effects of size at birth, gestational age, and maternal race/ethnicity. Birth Defects Res A Clin Mol Teratol. 2010;88:728–736. doi: 10.1002/bdra.20696. [DOI] [PubMed] [Google Scholar]
- 24.Miquel-Verges F., Mosley B.S., Block A.S., Hobbs C.A. A spectrum project: preterm birth and small-for-gestational age among infants with birth defects. J Perinatol. 2015;35:198–203. doi: 10.1038/jp.2014.180. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin R.H., Canfield M.A., Marengo L.K., Agopian A.J. Contribution of preterm birth to mortality among neonates with birth defects. J Pediatr. 2022;253:270–277.e1. doi: 10.1016/j.jpeds.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Agha M.M., Williams J.I., Marrett L., To T., Dodds L. Determinants of survival in children with congenital abnormalities: a long-term population-based cohort study. Birth Defects Res A Clin Mol Teratol. 2006;76:46–54. doi: 10.1002/bdra.20218. [DOI] [PubMed] [Google Scholar]
- 27.Berger K.H., Zhu B.P., Copeland G. Mortality throughout early childhood for Michigan children born with congenital anomalies, 1992-1998. Birth Defects Res A Clin Mol Teratol. 2003;67:656–661. doi: 10.1002/bdra.10118. [DOI] [PubMed] [Google Scholar]
- 28.Glinianaia S.V., Morris J.K., Best K.E., et al. Long-term survival of children born with congenital anomalies: a systematic review and meta-analysis of population-based studies. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dastgiri S., Gilmour W.H., Stone D.H. Survival of children born with congenital anomalies. Arch Dis Child. 2003;88:391–394. doi: 10.1136/adc.88.5.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coi A., Santoro M., Pierini A., et al. Survival of children with rare structural congenital anomalies: a multi-registry cohort study. Orphanet J Rare Dis. 2022;17:142. doi: 10.1186/s13023-022-02292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glinianaia S.V., Rankin J., Pierini A., et al. Ten-year survival of children with congenital anomalies: a European cohort study. Pediatrics. 2022;149 doi: 10.1542/peds.2021-053793. [DOI] [PubMed] [Google Scholar]
- 32.Bol K.A., Collins J.S., Kirby R.S., National Birth Defects Prevention N. Survival of infants with neural tube defects in the presence of folic acid fortification. Pediatrics. 2006;117:803–813. doi: 10.1542/peds.2005-1364. [DOI] [PubMed] [Google Scholar]
- 33.Shin M., Kucik J.E., Siffel C., et al. Improved survival among children with spina bifida in the United States. J Pediatr. 2012;161:1132–1137. doi: 10.1016/j.jpeds.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mai C.T., Evans J., Alverson C.J., et al. Changes in spina bifida lesion level after folic acid fortification in the US. J Pediatr. 2022;249:59–66.e1. doi: 10.1016/j.jpeds.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho P., Quigley M.A., Tatwavedi D., Britto C., Kurinczuk J.J. Neonatal and infant mortality associated with spina bifida: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.