Summary
JAK inhibitors impact multiple cytokine pathways simultaneously, enabling high efficacy in treating complex diseases such as cancers and immune-mediated disorders. However, their broad reach also poses safety concerns, which have fuelled a demand for increasingly selective JAK inhibitors.
Deucravacitinib, a first-in-class allosteric TYK2 inhibitor, represents a remarkable advancement in the field. Rather than competing at kinase domain catalytic sites as classical JAK1-3 inhibitors, deucravacitinib targets the regulatory pseudokinase domain of TYK2. It strikingly mirrors the functional effect of an evolutionary conserved naturally occurring TYK2 variant, P1104A, known to protect against multiple autoimmune diseases yet provide sufficient TYK2-mediated cytokine signalling required to prevent immune deficiency.
The unprecedentedly high functional selectivity and efficacy-safety profile of deucravacitinib, initially demonstrated in psoriasis, combined with genetic support, and promising outcomes in early SLE clinical trials make this inhibitor ripe for exploration in other autoimmune diseases for which better, safe, and efficacious treatments are urgently needed.
Keywords: JAK inhibitors, TYK2, Autoimmune disease, Deucravacitinib
Introduction
JAK-STAT proteins and pathways
Janus tyrosine kinases (JAKs) are non-receptor tyrosine kinases and encompass four mammalian members: JAK1, JAK2, JAK3, and TYK2 that act together with signal transducer and activator of transcription (STAT) factors in mediating intracellular signal transduction (Fig. 1). The JAK-STAT pathway orchestrates cellular responses to growth factors and cytokines. Both JAK deficiencies and sustained activation of the JAK-STAT pathway are linked with numerous autoimmune diseases and malignancies, which has established this intracellular pathway as an important therapeutic target.
Fig. 1.
The JAK-STAT proteins and pathway. (a) Homology and functional domain structure of JAKs. (b) The conserved domain structure of the seven STAT family members, with the SH2 domain determining the specificity for a particular receptor (c) Canonical JAK-STAT signal transduction. The binding of a ligand to its cognate receptor at the cell surface induces receptor oligomerization (i) and activation of two or more non-covalently associated JAKs through trans- and autophosphorylation (ii). The activated JAKs phosphorylate tyrosine residues in the receptors' intracellular domains creating docking sites for recruited STATs (iii). Once docked, phosphorylation by JAKs leads to a conformational change that allows STAT dimerization (iv). Activated STATs translocate to the nucleus, where they exert their activating or suppressing effect on gene expression (v). Abbreviations: FERM, four-point-one, ezrin, radixin, moesin; SH2, Src-homology 2; TAD, tyrosine activation domain.
The JAKs are composed of seven homology domains (JH) organized into four functional domains. The tandem architecture of the structurally similar JH1 and JH2 domains is a hallmark of JAKs and has given rise to the name after the Roman God, Janus with two faces.
Structures of single-domain components, such as JH1 and JH2, or JH1-JH2 and FERM-SH2 compound domains, and recently that of the full-length JAK1 complexed with a segment of the IFNgR1 receptor1 have been extensively described and provide valuable insights into JAK functions as well as being key to the development of drugs targeting the JAK-STAT pathways. Studies of natural gain of function mutations have linked genetic, structural, and functional features of JAK variants whilst structure-based investigations of cytokine-receptor interactions have contributed to the understanding of how extracellular assemblies activate the intracellular JAKs.
The closely associated FERM-SH2 domains define receptor specificity and their interactions with either of the cytokine receptor motifs, Boxes 1 and 2, are required for stability in vitro2 and maintenance of kinase integrity.3 The JH1 kinase domain is catalytically active and possesses a conserved kinase fold with two lobes, between which the ATP binding site is situated in the cleft, and characteristic features enabling ATP binding and catalysis (Fig. 2, left). The TYK2 JH2 pseudokinase also has a canonical kinase fold and can bind ATP but is catalytically inactive due to amino acid substitutions of key catalytic residues, such as T659E, P760F in the D-F-G motif, and G733R and N734D in the H-R-D motif (Fig. 2, right). Structures of JH1-JH2 complexes have revealed interactions between the N-lobes of the JH1 and JH2 domains, by which the JH2 domain mediates autoinhibition of the JH1 kinase domains, stabilizing its inactive state until receptor dimerization promotes the transition to an active conformation.4,5 This is consistent with many activating mutations associated with human myeloproliferative neoplasms mapping to this JH1-JH2 interface4,5 as well as the increased basal kinase activity upon deletion of JH2 in JAK2 and JAK3.6
Fig. 2.
Crystal structures of the TYK2 JH1 kinase (left, PDB ID: 4GVJ), and TYK2 JH2 pseudokinase domains (right, PDB ID: 6NZP). The ATP binding site of JH1 is situated in the cleft between the N and C lobes connected by two regulatory spines. The N-terminal lobe comprises a five-stranded beta-sheet and an alpha-C helix crucial for kinase activation. A glycine-rich loop (purple) serves to bind ATP through hydrogen bond formation between the alpha-C helix and the ATP. The C-terminal lobe is mainly helical and contains an H-R-D motif of the catalytic loop (yellow), in which the aspartic acid acts as the catalytic base during phosphoryl transfer, and a D-F-G motif (green) in which the aspartic acid binds the Mg2+ that coordinates ATP. The phosphorylation of two adjacent tyrosine residues (turquoise) is a key step in JAK activation. The location of the natural variant P1104A is shown in pink. The TYK2 JH2 pseudokinase domain (right) has a canonical kinase fold and can bind ATP but lacks key catalytic residues (blue), harbouring instead T658 that cannot form a salt bridge between the αC-helix and β-strand3, P760 in the pseudokinase D-P-G motif and G733 and N734 that form the pseudokinase H-G-N motif. The JH2 structure is shown with deucravacitinib (red).
Multitude of JAK-STAT functions
In vivo evidence for JAKs in cytokine signalling was first demonstrated through loss of function mutations in JAK3 or its associated common γ cytokine receptor chain, which rendered lymphocytes unable to respond to their ligands and thereby impaired T and NK cell development and B cell function; subsequently termed severe combined immunodeficiency (SCID).7,8 Since then, numerous mutations, including germline and somatic, loss of function and gain of functions mutations, and naturally occurring variants in both JAK and STATs genes have been linked to various phenotypes depending on the gene and mutation, including immunodeficiencies, susceptibility to intracellular bacterial, viral, or fungal infections, as well as autoimmune diseases and multiple malignancies.9 For example, most patients with myeloproliferative neoplasms have the somatic V617F mutation in the JH2 pseudokinase domain of JAK2, which abolishes the normal inhibitory effect on the JH1 kinase domain and thereby causes constitutive kinase activity.10 Genome-wide association studies for autoimmune diseases have likewise identified numerous variants in both JAK and STAT genes, among which a single TYK2 SNP, rs34536443, confers protection against multiple autoimmune diseases.11 Thus, here we explore further the functional role of TYK2 and why this JAK could be a preferential candidate for drug development.
Complexities of JAK-STAT pathway
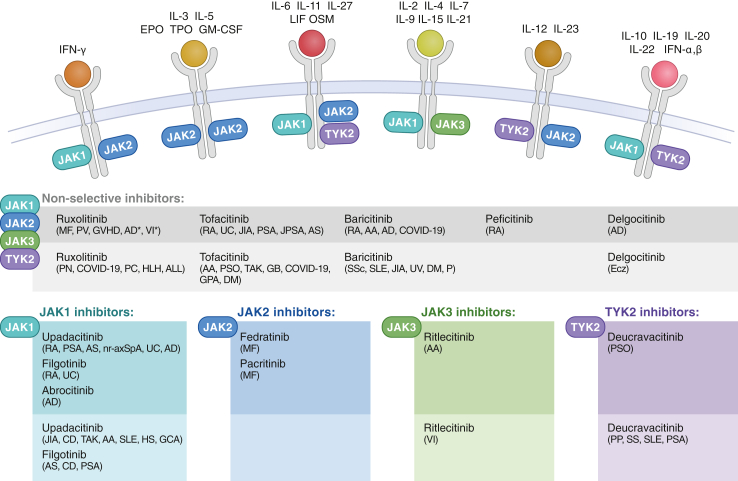
The biological impact of therapeutic JAK inhibition is highly complex due to the unbalanced ratio of JAKs and cytokines, with only four JAKs combining to transduce signalling from 57 cytokines, as well as their capacity to crosstalk with other signalling pathways. Their differential impact is also dependent on the JAKs themselves. For instance, JAK2 acts as a homodimer during hematopoietic growth factor signalling, but can also form heterodimers with TYK2, JAK1, or JAK1/JAK2. In turn, JAK1 and TYK2 both pair with multiple JAKs and are each involved in several pathways (Fig. 3). STATs also exhibit partial signalling redundancy and have additional functions beyond the JAK-STAT pathways.12 Moreover, JAKs undertake non-kinase functions such as scaffolding and stabilizing cytokine receptor complexes, thereby facilitating their expression and ligand binding.13,14 Furthermore, in addition to the intramolecular regulation by the pseudokinase domains, the JAK-STAT pathway is intermolecularly regulated by factors such as suppressors of cytokine signalling (SOCSs), protein tyrosine phosphatases (PTPs), and protein inhibitors of activated STAT (PIAs).
Fig. 3.
Cytokines signal via combinations of JAKs. Currently, 12 JAK inhibitors are approved for the treatment of a variety of diseases (dark-shaded boxes) and investigated in phase 3 clinical trials (light-shaded boxes) for additional conditions. Abbreviations: MF, myelofibrosis; PV, polycythaemia vera; GVHD, graft versus host disease; AD, atopic dermatitis; VI, vitiligo; RA, rheumatoid arthritis; UC, ulcerative colitis; JIA, juvenile idiopathic arthritis; PSA, psoriatic arthritis; JPSA, juvenile psoriatic arthritis; AS, ankylosing spondylitis; PN, prurigo nodularis; PC, pancreatic cancer; HLH, haemophagocytic lymphohistiocytosis; ALL, acute lymphoblastic leukemia; AA, alopecia areata; PSO, psoriasis; TAK, Takayasu arteritis; GB, glioblastoma; GPA, granulomatosis with polyangiitis; DM, dermatomyositis; SSc, systemic sclerosis; SLE, systemic lupus erythematosus; UV, uveitis; P, pneumonia; Ecz, eczema; nr-axSpA, non-radiographic axial spondylitis; CD, Crohn’s disease; HS, hidradenitis suppurativa; GCA, giant cell arteritis; PP, palmoplantar postulosis; Sjogren’s syndrome.
TYK2
TYK2 is constitutively expressed in immune cells and transduces signals downstream of type I IFN, gp130, interleukin (IL)-10R2, IL-13Rα, and IL-12Rβ1 cytokine receptor families. Accordingly, TYK2 orchestrates an extensive array of cellular responses to a variety of cytokines such as IFNa, IFNb, The IL-10/20 families, the IL-12 family, IL-4/IL-13, and the IL-6gp130 upon binding to their cognate receptors (Fig. 3).
TYK2 pairs with JAK2 and engages mainly STAT4 to mediate signal transduction of IL-12. This signal cooperates with TCR signals to express T-bet promoting differentiation of naïve CD4+ T cells into Th1 cells.15 The TYK2/JAK2 heterodimer, acting through STAT1, STAT3, and STAT4, is likewise required for IL-23 signalling, which regulates the differentiation and survival of Th17 cells.16 TYK2 also combines with JAK1 and engages STAT1 and STAT2 to mediate signalling of the potent antiviral type 1 IFNs17 that enhance various proinflammatory immune processes such as dendritic cell, macrophage, regulatory T cell differentiation and function, as well as B cell activation and antibody production.
Clinical phenotypes of genetic variants predict therapeutic approach
TYK2 deficiency can be both beneficial and harmful, consistent with TYK2’s critical role in providing immunity to microbial infections and being detrimental in autoinflammatory and autoimmune diseases. In mice, TYK2 deficiency increases susceptibility to viral and bacterial infections16,18 and impairs tumour surveillance.19 Conversely, TYK2 deficiency confers protection against collagen antibody-induced arthritis, colitis, and EAE in animal models.20
In humans, TYK2 deficiency has been described in patients with homozygous or heterogeneous compound loss of function mutations.21, 22, 23, 24, 25, 26 While complete TYK2 deficiency is rare and generally increases susceptibility to intracellular infections, a partial modulation of TYK2 function, typically caused by naturally occurring variants, is more common with consequences varying in both nature and extent. Such hypomorphic variants play a notable role in modulating immune responses and susceptibility to immune-mediated diseases by selectively reducing, without abrogating, TYK2-mediated cytokine signalling. Specifically, genome-wide association studies in European populations have linked at least seven TYK2 SNPs with a range of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, inflammatory bowel diseases, psoriasis, psoriatic arthritis, type 1 diabetes, systemic sclerosis, ankylosing spondylitis, and primary biliary cirrhosis.11,27 Among these, the missense SNP rs12720356 (I684S) located in the regulatory JH2 domain confers risk of some autoimmune diseases and protection against others, potentially indicating a cell type- or target tissue TYK2-dependency that is disease-specific.11,28, 29, 30, 31, 32
Conversely, the variant, rs34536443 (P1104A), located in the JH1 kinase domain, displays a uniformly protective effect across at least ten autoimmune diseases,11 prompting the authors of this study to propose the SNP as a priority variant for guiding the development of cross-disease therapeutics. Crucially, their genotyping of 116,732 individuals from the UK Biobank resource revealed no heightened risk for serious infections or cancer. A Mendelian randomization study based on summary genetic association data from GWAS meta-analyses has reported a slightly elevated risk of lung cancer and non-Hodgkin lymphoma, which was not supported by functional, tissue-specific gene expression or colocalization analyses.33 Another Mendelian randomization study using the P1104A variant as a proxy for TYK2 inhibition supported TYK2 inhibition as a therapeutic target for autoimmune diseases. This study reported nominal associations with prostate and breast cancer risk that were not confirmed by tissue-specific gene expression and colocalization analyses.34 Thus, based on these studies and considering that the P1104A variant in Mendelian studies reflects a lifelong exposure, while pharmaceutically TYK2 inhibition would typically be commenced during the second or third decade of life, it remains unknown and requires randomized clinical trials with a long-term follow-up period to determine if TYK2 inhibition contributes to increased cancer risk.
Mechanistically, the P1104A variant reduces the enzymatic activity, while not affecting the expression and scaffolding capacity of TYK2.11,30,31 This is in agreement with comparative molecular dynamics simulations on the kinase domains from WT and the P1104A variant suggesting that the low enzymatic activity is due to the stabilization of the inactive conformation.35 Homozygous carriers of the P1104A variant are enriched in British tuberculosis (TB) cases, suggesting that this variant may underlie the disease in approximately 1% of European patients,36 suggested to be due to reduced IL-23-dependent28,37 and possibly IL-12-dependent11 IFNɣ production. Intriguingly, the P1104 variant, originating from the common ancestors of West Eurasians, has shown large fluctuations in frequency over the past 10,000 years with a sharply decreasing allele frequency during the last 2000 years, concomitant with TB endemicity, from ∼9% to ∼4% in modern Europe, where a decline in TB prevalence likely prevents further negative selection.38 Healthy heterozygous carriers of the P1104 variant have fewer circulating Tfh cells and decreased IFN-signalling in naïve but not effector T cells.39 Other mutational investigations have demonstrated intact IL-6 and IL-10, and IFN-a/b signalling in TYK2 P1104A cells although catalytically impaired.11,31,32,39
However, not all hypomorphic mutations in TYK2 are advantageous; the heterozygous compound mutations rs770927552 and rs201917359 (R231W in the FERM domain), cause reduced TYK2 protein levels and impaired IL-23 signalling without affecting type I IFN, IL-6, IL-10, and IL-12 signalling, which have been reported in a sibling case with primary immunodeficiency with T cell lymphopenia as well as EBV-associated B cell lymphoma.40
The ability to predict whether an effect of hypermorphic (i.e., increased activity) TYK2 variants, which increase the response to cytokines through signalling efficacy and stabilization of their receptors, can be translated into protection or susceptibility in autoimmune disease, is not yet clear. For example, the common non-synonymous TYK2 splice variant, rs2304256 (V362F) confers protection against autoimmune diseases such as systemic lupus erythematosus, psoriasis, and type 1 diabetes in Caucasians27 but is associated with worse COVID-19 outcomes.41 Additionally, multi-omics studies are increasingly linking TYK2 mutations and overexpression to many types of cancer including leukemia, suggesting that TYK2 could also be an oncogene.42
Collectively, these selected examples show that naturally occurring variants in various domains of TYK2 can cause either increased or decreased kinase activity and exemplify how an evolutionarily conserved homozygous variant, P1104A, may define a signalling optimum between immunodeficiency and autoimmunity.11,36 Likewise, TYK2 balances functions in tumour immunosurveillance and inflammatory cytokine signalling in cancer and metastasis development.43 These examples also highlight that each genetic disease association may represent a unique mechanism, and therefore accentuate the need for fine-mapping each causal SNP, validating their effect on TYK2 expression and function, pinpointing the affected pathways, and identifying the cell types and cell states that drive the disease association. Given the ubiquitous expression and the redundant role of TYK2 in cytokine signalling, this compulsory strategy represents significant undertakings.
JAK1-3 and TYK2 as targets for immunotherapy
Small molecule JAK inhibitors: is selectivity possible?
The idea of exploiting the therapeutic potential of JAK modulation was fostered by the discovery of JAK3 deficiency in patients with severe combined immune deficiency,7,8 while the later recognition of the frequent gain of function JAK2 V617F mutation in myeloproliferative disorders10 provided a further rationale for developing therapeutics to suppress exaggerated JAK-STAT signalling. Extensive preclinical and clinical research efforts eventually led to the development of the first JAK inhibitors, ruxolitinib and tofacitinib, which both inhibit multiple kinases although with different potencies.9
Since then, a continuous strive for selectivity has characterized the development of JAK inhibitors with the overall aim of increasing safety by minimizing off-target effects without losing efficacy. This aim is far from trivial. First, the heterodimeric pairings and the involvement of a given JAK in several pathways complicate the selection of the best target. Cytokines are pleiotropic and redundant, and even a highly JAK1 selective inhibitor will, due to the heteromeric JAK pairing, affect cytokine signalling that also depends on JAK2, JAK3, and TYK2. Further, mouse studies using both non-selective and next-generation selective JAK1-3 inhibitors demonstrate overlapping immunogenomic effects at the network level rather than blocking specific pathways.44 Second, the approach for all currently licensed JAK1-3 inhibitors has been to target the JH1 domain encompassing the ATP catalytic site, which is highly conserved across many kinases. Third, one such ATP competitive inhibitor will exist in equilibrium with the substrate and ATP implying that an increase in intracellular inhibitor dose, which is hard to control as it depends on patient-specific parameters such as age, sex, and co-medications, will likely affect the ATP binding to the other JAKs thereby causing loss of selectivity in vivo. Fourth, in vivo selectivity cannot directly be inferred from in vitro studies; in vitro and ex vivo data from enzymatic and biochemical assays vary widely across studies as they depend on the substrates cell lines used, the cytokine and/or STAT being measured, and experimental dosages.45, 46, 47 Ultimately, JAK selectivity is relative, and the translation into clinical efficacy and safety is highly complicated. Collectively, these complexities challenge the development and use of selective inhibitors yet represent a major therapeutical advantage for clinical efficacy in the treatment of complex diseases, in which multiple cytokines are typically involved, and for which the targeting of a single cytokine is likely insufficient.
Multiple mechanisms of JAK inhibition
To date, all approved small molecule JAK1-3 inhibitors are reversible ATP-competitive inhibitors, whose mode of action depends on their competition with ATP in JAKs (Fig. 4). They can bind to the ATP binding site in the activated (conventional type I inhibitors) or inactive JH1 configuration (type II inhibitors). Allosteric JAK inhibitors are also emerging and include the recently approved deucravacitinib that selectively inhibits TYK2, as described further below and the JAK1-specific inhibitor VVD-118313 under development that targets the pseudokinase domain (JH2) cysteine C817 in JAK1.48 Irreversible JAK3 inhibitors, whose mode of action depends on the covalent interaction with a unique cysteine residue at position 909 in the ATP binding pocket of JAK3 are also in development. One such compound, Z583, an irreversible JAK3 highly selective inhibitor forms a covalent bond with Cys909, thereby irreversibly inhibiting JAK3-mediated signalling.49
Fig. 4.
The TYK2 allosteric inhibitor deucravacitinib mimics the functional effect of the natural variant P1104A. (a) Ligand-receptor binding induces TYK2 activation. Currently licensed JAK1-3 type I inhibitors (green) target JH1 and work via ATP competition. (b) The natural variant P1104A stabilizes the inactive JH1 conformation. (c) The TYK2 allosteric inhibitor (shown by its molecular structure) binds to JH2, locking this regulatory domain into an inhibitory interaction with JH1, thereby preventing TYK2 activation and downstream signalling. Abbreviation: WT, wild-type allele.
Is co-targeting multiple kinases useful?
While immense efforts are dedicated to increasing the selectivity of JAK inhibitors to minimize adverse effects, efficacy in terms of broader responses is also being pursued by co-targeting multiple kinases. For instance, ritlecitinib (PF06651600), an irreversible dual inhibitor blocking both JAK3 and five TEC family kinases, was recently approved for the treatment of alopecia areata and is investigated in clinical trials for vitiligo (NCT03715829), and rheumatoid arthritis (NCT02969044). Another example is gusacitinib, a dual pan-JAK, and spleen tyrosine kinase (SYK) inhibitor, which was granted FDA Fast Track designation in 2021 for the treatment of moderate to severe chronic hand eczema. Gusacitinib also improves systemic inflammation in patients with atopic dermatitis (NCT03139981).
Tissue-specific kinase inhibitors might improve therapy
The small molecular size (350–450 g/mol) of JAK inhibitors generally favours the penetration of tissue, such as the epidermis. Tissue-targeted administration, which can increase the local effect while potentially minimizing adverse effects caused by systemic JAK inhibition, has been explored in topicals; delgocitinib is approved in Japan for atopic dermatitis, and ruxolitinib/Opzelura is approved by FDA for atopic dermatitis and vitiligo. Other investigational applications include ruxolitinib in inhalers for asthma (Australian New Zealand Clinical Trials Registry, ACTRN12617001227381, ACTRN12619000227190), and ophthalmic solutions containing the dual JAK/SYK inhibitor (R348) for ocular surface disease in graft versus host disease (NCT02040623). Moreover, the gut-selective inhibitor for inflammatory bowel diseases, TD-1473, which displays low plasma and high colonic tissue concentration, is currently being evaluated for safety and efficacy in clinical trials NCT03758443 and NCT03635112 for ulcerative colitis and Crohn’s disease, respectively.
It is currently not clear to what extent individual JAK inhibitors can cross the blood-brain barrier.50, 51, 52 Although a large body of evidence suggests a pathogenic role of the JAK-STAT pathway in multiple sclerosis,53 and multiple JAK inhibitors have been tested in EAE models, these efforts have not yet been translated into clinical trials. Nevertheless, the genetic stepping stones on which deucravacitinib was developed could accelerate the development of therapies for multiple sclerosis; rs34536443 (P1104A) represents the largest genetic protection outside of the HLA for developing multiple sclerosis (OR = 0.3511), and the protection unfolds without an increased risk for serious infections. Mice harbouring the orthologue multiple sclerosis-protective P1124A variant are likewise protected against MOG-induced EAE.11
How efficacious and safe are approved JAK1-3 inhibitors? Comparison with TNF inhibitors
Consistent with the pleiotropic effects of JAKs on pro-inflammatory cascades, their inhibitors show efficacy and high repurposing potential across a multitude of immune-inflammatory and haematological conditions, cancers, and inborn errors of immunity. However, as many of these effects are also critical for host defence, infections remain a safety concern for the use of JAK inhibitors. Generally, JAK inhibitors seem well tolerated in clinical trials. Infection risks, comparable to that observed with biological therapeutics such as TNF inhibitors except for varicella zoster virus reactivation being more common with JAK inhibitors, are dose-dependent and compounded by other risk factors such as medication, underlying disease, and age.54,55 Other concerns raised by clinical trials include dyslipidemia and liver toxicities, thromboembolic events, gastrointestinal perforation, major adverse cardiovascular events, and malignancies.45 Most safety data inherently stem from first-generation non-selective inhibitors and direct comparisons with more selective inhibitors are urgently needed. Finally, without an understanding of the mechanisms underlying reported adverse effects, it remains unclear whether further JAK specificity will improve their safety profiles.
The approval of the TNF-targeting monoclonal antibody, infliximab, in 1998 marked a milestone as the first monoclonal antibody approved for the treatment of Crohn’s disease and in 1999 rheumatoid arthritis. The TNF-Receptor Ig fusion protein, etanercept was approved in 1998 for rheumatoid arthritis, before infliximab despite starting trials later. Thenceforth, five different anti-TNF therapeutics have been licensed, transforming the management of autoimmune diseases, such as rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis, psoriasis arthritis, and psoriasis. Although head-to-head comparisons for individual JAK inhibitors and TNF inhibitors are still very scarce, TNF inhibitors and JAK inhibitors are on average considered to have similar efficacies in treating rheumatoid arthritis.56 Compared to TNF inhibitors, small molecule JAK inhibitors may have further benefits such as oral delivery, lack of immunogenicity and shorter half-life. TNF inhibitors are considered well-tolerated, both alone and in combination with conventional non-biologic therapeutics such as methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine,57 but a subset of patients do not respond significantly, or gradually lose responsiveness to TNF inhibitors. Current evidence suggests that JAK inhibitors may be a suitable choice in such cases.58,59 In this regard, it is worth noting that TNF signalling is not mediated by JAK1-3 nor TYK2, underscoring the intricacies of treating complex disorders.
Clinical trials do not readily translate to routine clinical practice60 and long-term and real-world studies are still limited. One such post-approval surveillance study (ORAL) comparing safety profiles for tofacitinib versus TNF-alpha inhibitor for the treatment of patients with rheumatoid arthritis over 50 years of age with at least one additional cardiovascular risk factor, revealed an increased risk of major adverse cardiovascular events and cancers and confirmed the risk for infections61 for tofacitinib. These data led to changed recommendations and an updated black box warning on all approved JAK1-3 inhibitors indicated for the treatment of arthritis and other inflammatory conditions irrespective of selectivity, as similar modes of action are assumed.62 This and other studies63,64 emphasize the importance of real-world surveillance studies and highlight the need for risk stratification when tailoring therapy. Similar studies are also needed for individual JAK inhibitors and indications. Given the growing collection of JAK inhibitors, future head-to-head clinical trials of these should be included to inform the clinical choice of individual JAK inhibitors.
What are the unresolved clinical issues?
Although JAK inhibitors are considered at least as effective and safe as biologics, many questions of clinical importance remain unanswered; For instance, can they be safely used in combination with other drugs including biologics,65,66 and when in the disease course JAK inhibitors are optimally introduced? E.g., to efficiently modulate established disease, or as a frontline therapeutic to prevent or delay disease progression? As most autoimmune diseases are characterized by evolving immune phenotypes across the disease course, a dynamic dosing strategy might even be beneficial. The optimal sequence of medications and/or safety of co-administration with other medications should be determined for each disease. In rheumatoid arthritis and other inflammatory diseases, JAK inhibitors are currently reserved for patients, who have an inadequate or intolerance to TNF-alpha inhibitors. Several studies support such switching in rheumatoid arthritis56 and vice versa.67 Recently, a study of real-world patients with rheumatoid arthritis showed that switching from one JAK inhibitor to another JAK inhibitor resulted in higher drug retention than when switching to a TNF-alpha inhibitor.68 Notably, the novel allosteric JAK inhibitors may provide an advantage in facilitating drug retention, as their targets lie outside the JH1 catalytic site, are less conserved, and therefore will be easier to combine to overcome drug failure.
In conclusion, selecting optimal disease-modifying therapy for a patient goes far beyond pairing JAK inhibitors with diseases, but requires careful consideration of the patient's history including co-medications and co-morbidities, cautious attention to risk factors, and vigilant surveillance for adverse effects, such as serious infections and malignancies. Due to JAK inhibitors' short half-lives, some adverse effects may resolve quickly, while others may be prevented, such as through vaccination. Moreover, the identification of reliable biomarkers of JAK-STAT dysregulation would be useful, both to help clinicians match a patient with a particular JAK inhibitor and to monitor immune regulation along with the assessment of disease activity.
TYK2 inhibitors
Why generate small molecule TYK2 inhibitors?
The rationale for specifically targeting TYK2 is based on multiple achievements, such as the development of approved JAK1-3 inhibitors with favourable benefit-safety profiles, structural, biochemical, and functional knowledge of TYK2, and the increasingly more detailed understanding of the role of type I IFNs, IL-6, IL-10, IL12, and IL-23 signalling in many inflammatory and autoimmune diseases. Furthermore, the therapeutic targeting of these pathways has already shown clinical relevance as evidenced by the beneficial effects of antibody therapies such as ustekinumab (anti-IL-12/23p40), guselkumab (anti-IL-23p19), and tocilizumab (anti-IL-6R) in the treatment of psoriasis and psoriasis arthritis, and rheumatoid arthritis, respectively.69 Finally, but not least, the approach of TYK2 inhibition for the treatment of autoimmune diseases finds strong support in genetic and functional data.11
As for other JAK inhibitors, the first approach was to target the kinase domain of TYK2. One such molecule, ropsacitinib has been reported to show efficacy and tolerability in a randomized placebo-controlled phase 2 trial of psoriasis (NCT03895372), whereas the dual JAK1/TYK2 inhibitor, brepocitinib is currently tested for dermatomyositis in phase 3 and for a variety of other, mostly autoimmune conditions in phase 2 clinical trials (Table 1).
Table 1.
TYK2 inhibitors under development that have advanced to phase 2 and 3 clinical trials.
| Inhibitor type | Name | Selectivity | Kinase inhibition IC50 (uM) |
Phase 3–4 clinical trials | Phase 2 clinical trials |
|---|---|---|---|---|---|
| Allosteric (JH2) | Deucravacitinib BMS-986165 Approved for treatment of psoriasis FDA 2022 |
TYK2 JH2 | JAK1: >10,000 JAK2: >10,000 JAK3: >10,000 TYK2 (JH1): >10,000 TYK2 (JH2): 0.2 nM |
Psoriasis (NCT04772079, NCT05702995, NCT05478499, NCT004036435, NCT03611751, NCT03624127, NCT03924427, NCT04167462, NCT05478499, NCT05701995) Palmoplantar postulosis (NCT05710185) Systemic lupus erythematosus (NCT05617677, NCT05620407) Psoriatic arthritis (NCT04908202, NCT04908189) |
Alopecia areata (NCT05556265, NCT03252587) Lupus erythematosus discoid/subacute cutaneous (NCT04857034) Lupus nephritis (NCT03943147) Ulcerative colitis (NCT03934216, NCT04613518, NCT04877990) Crohn's disease (NCT04877990, NCT03599622) |
| NDI-034858 TAK-279 | TYK2 JH2 | Psoriatic arthritis (NCT05153148) Psoriasis (NCT04999839) |
|||
| VTX958 | TYK2 JH2 | Psoriasis (NCT05655299) Crohn's disease (NCT05688852) Psoriatic arthritis (NCT05715125) |
|||
| ESK-001 | TYK2 JH2 | Psoriasis (NCT05739435, NCT05600036) | |||
| BMS-986202 | TYK2 JH2 | TYK2 JH2: 0.19 nM | None | None | |
| ATP competitive (JH1) | Ropsacitinib PF-06826647 | TYK2 (>JAK2 > JAK1) | JAK1: 383 nM JAK2: 74 nM JAK3: >10,000 nM TYK2: 17 nM |
None | Psoriasis (NCT03895372) Ulcerative colitis (NCT04209556 withdrawn) Hidradenitis suppurativa (NCT04092452) |
| SAR-20347 | TYK2(>JAK1 > JAK2 > JAK3) | JAK1: 23 nM JAK2: 26 nM JAK3: 41 nM TYK2: 0.6 nM |
None | None | |
| NDI-031407 | TYK2(>JAK3 > JAK2 > JAK1) | JAK1: 46 nM JAK2: 31 nM JAK3: 4.2 nM TYK2: 0.21 nM |
None | None | |
| GLPG3667 | TYK2 | None | Dermatomyositis (NCT05695950) | ||
| ATP competitive (JH1) dual | Brepocitinib PF-06700841 | JAK1, TYK2 (>JAK2) | JAK1: 17 nM JAK2: 77 nM JAK3: 6494 nM TYK2: 23 nM |
Dermatomyositis (NCT05437263) | Cicatricial alopecia (NCT05076006, NCT05076006) Atopic dermatitis (NCT03903822) Systemic lupus erythematosus (NCT03845517) Psoriatic arthritis (NCT03963401) Psoriasis (NCT02969018, NCT03850483) Crohn's disease (NCT03395184) Ulcerative colitis (NCT02958865) Alopecia areata (NCT02974868) Hidradenitis suppurativa (NCT04092452) Vitiligo (NCT03715829) Uveitis (NCT05523765) |
| TLL018 | JAK1/TYK2 | JAK1: 4 nM JAK2: >1000 nM JAK3: >1000 nM TYK2: 5 nM |
None | Ulcerative colitis (NCT05121402) withdrawn Rheumatoid arthritis (NCT05133297) |
|
| ATP competitive (JH1) tissue restricted | OST-122 | JAK3/TYK2/ARK5 gut-selective | None | Ulcerative colitis (NCT04353791) |
Only trials of the highest phase number are included.
Development of an effective allosteric TYK2 inhibitor
The strategy of allosteric inhibition, based on distinguishing the catalytically inactive pseudokinase domains in the JAK family, has recently emerged with the first allosteric inhibitor, deucravacitinib, recently approved for psoriasis, and others such as ESK-001, NDI-034858 (TAK-279), and BMS-986202 in development. Deucravacitinib further displays promising efficacy-safety profiles in early clinical trials across a range of autoimmune conditions, for which it may have the potential to transform the therapeutic landscape.
Seminal complementing work moved the field quickly forward70, 71, 72, 73, 74, 75, 76; Similar to the other JAKs, the TYK2 JH2 pseudokinase domain controls the catalytic activity of the JH1 kinase domain,4 and when pharmacologically stabilized abrupt signal transduction of TYK2-dependent cytokine signalling in T cells.71 Crystallization of TYK2 JH2 in complex with adenosine 5′-0-(thiotriphosphate) (ATP-gS) demonstrated that the JH2 domain, albeit catalytically inactive, can bind the nucleotide and stabilize the protein without inducing major conformational changes, and hence, is accessible to ATP-competitive compounds.70 Subsequently, Liu et al.74 identified metabolically stable TYK2 JH2 selective Imidazo(1,2-b) pyridazine derivates, which inhibited IL-12/IL/18-induced IFN-g production in a rat pharmacodynamic model. A second series of inhibitors with the incorporation of a deuterated methyl amide improved selectivity over the other JAKs.75 Optimizations led to the identification of the first-in-class oral TYK2 allosteric JAK inhibitor, BMS-986165 (deucravacitinib) engineered to target the JH2 domain without cross-active blocking of JAK1, 2, 3. In vitro studies have confirmed a ≥100-fold selectivity for TYK2 versus JAK1 and JAK3 and ≥2000-fold versus JAK2 in cells.73,76 Deucravacitinib blocks IL-23 signalling in human T cells and IL-17 production in murine T cells in vitro, and when administered prophylactically shows efficacy in animal models of IBD and lupus73 and psoriasis.76
The first placebo-controlled double-blind phase 2 trial in psoriasis77 showed a significantly higher percentage of participants achieving a 75–90% reduction in (PASI) skin scores when treated with 3–12 mg deucravacitinib daily compared to placebo. Mild adverse events were more frequent in the active-treatment groups and one case of melanoma was reported with a dose of 3 mg daily but no cases of herpes zoster infection, opportunistic infections, tuberculosis, or cardiovascular events. Neither were neutropenia, elevations in liver enzyme and creatinine levels, nor dyslipidemia, well-known adverse effects of JAK inhibitors, observed.77
Deucravacitinib received FDA approval for the treatment of psoriasis in 2022 based on two large phase 3, double-blinded, 52 weeks trials (POETYK PSO-1, NCT03624127; POETYK PSO-2, NCT03611751). Both trials confirmed efficacy with significantly higher (PASI75) response rates after 16 weeks with deucravacitinib compared to placebo and apremilast and favourable tolerability and safety.78 At the end of the 52 weeks of POETYK trials, 1221 patients were switched to an open-label deucravacitinib extension trial for up to 240 weeks, during which period the safety profile remained constant except for an increase in serious infections attributable to the COVID-19 pandemic.79
In psoriatic arthritis, a randomized, double-blind, placebo-controlled phase 2 study (NCT03881059) demonstrated efficacy, and phase 3 trials (NCT04908202 and NCT04908189) are currently recruiting to evaluate the efficacy and safety of deucravacitinib compared with placebo. In systemic lupus erythematosus, deucravacitinib treatment of whole blood from patients suppresses IFN-dependent gene expression,73 and 45–58% of patients treated with 3–12 mg daily deucravacitinib compared to 34% of placebo-treated patients reached the endpoint SLE responder index (SRI-4) in the phase 2 PAISLEY trial. Rates of adverse events were similar across groups, except for infections and cutaneous events frequently observed among patients treated with deucravacitinib. No major adverse cardiovascular or thrombotic events, deaths, or opportunistic or tuberculosis infections were reported.80 Phase 3 randomized, double-blind placebo-controlled studies are currently recruiting participants to evaluate efficacy and safety in active systemic lupus erythematosus (NCT05617677 and NCT05620407).
Notwithstanding the high hopes for deucravacitinib, the first phase 2 double-blind, placebo-controlled clinical trial for ulcerative colitis (LATTICE-UC, NCT03934216) did not meet its primary endpoint of clinical remission, but safety and laboratory parameters were consistent with those observed in trials for psoriasis and psoriatic arthritis. Additional trials further explore the therapeutical potential in ulcerative colitis (NCT04613518, NCT04877990) and Crohn's disease (LATTICE-CD, NCT03599622).
Conclusions and outstanding questions
Inhibitors of JAK1-3 have rapidly gained traction to treat a wide range of diseases from cancers to autoimmune diseases, and most recently they have been approved to treat cytokine release syndrome in severe COVID-19. Hypothetically, JAK inhibitors could be useful for any condition, in which JAK-dependent cytokine signalling plays a causal role in the pathophysiology such as cytokine release syndrome, obesity-related metabolic disorders and diabetic kidney disease,81 giant cell arteritis,82 morphea and systemic sclerosis,83 dermatomyositis,84 itch and pruritus85 and for overcoming IFN-driven resistance to immune checkpoint blockade in melanoma.86 Whether JAK inhibitors offer therapeutic promise for the treatment of neuroinflammatory diseases is not yet conclusive.87, 88, 89, 90, 91, 92 Notably, emerging research in Alzheimer's disease93, 94, 95 aiming to repurpose approved JAK inhibitors has led to a currently recruiting phase 1/2 clinical trial (NCT05189106) with baricitinib.95
While JAK1 and JAK2 are associated with broad cytokine profiles, comparatively few cytokines depend on TYK2 for intracellular signalling, which provides TYK2 inhibitors with a basic level of selectivity, while also restricting their use to conditions, in which cytokines such as type 1 IFN, IL-12, and IL-23 play a pathogenic role. Until recently, TYK2 inhibitors seemed early in their development but deucravacitinib, a first-in-class- allosteric inhibitor has recently taken centre stage due to its unprecedented high functional selectivity and promising efficacy-safety profile for the treatment of psoriasis. Key features distinguish deucravacitinib from all approved JAK1-3 inhibitors. First, rather than targeting the JH1 catalytic site, deucravacitinib binds to the JH2 pseudokinase domain and thereby stabilizes the inhibitory interaction between the regulatory and catalytic domains. Notably, a direct in vitro comparison of novel JH2-targeting TYK2 inhibitors with JH1-targeting TYK2 inhibitors ropsacitinib and brepocitinib confirmed both higher selectivity and potency of the JH2 ligands to block TYK2-mediated signalling.96
Second, deucravacitinib mimics the effect of an evolutionary conserved naturally occurring TYK2 variant P1104A evidenced by GWASs to protect against ten autoimmune diseases. Further desiccation of their effects on cytokine signalling across cell types and diseases is required to fully understand potential differences. It is roughly estimated that one out of ten drug candidates entering clinical trials will eventually obtain approval,97 while genetically supported drug targets increase this likelihood of success.98 Much genomic-driven drug discovery focuses on repurposing, as exemplified by the retrospectively identified correlations between GWAS-identified rheumatoid arthritis susceptibility genes and targets of tofacitinib99 while deucravacitinib illustrates the power of using genetic data to drive drug development. Intriguingly, P1104A has undergone strong Mycobacterium tuberculosis-mediated negative selection during the last 2000 years and is in linkage disequilibrium with rs74956615 that confer risk for COVID-19 critical illness and hospitalization, hence functionally balancing disparate risks for infectious and autoimmune diseases through time and space.11,36,100
In conclusion, while anticipated to represent a paradigm shift in the treatment of complex disorders, where the simultaneous targeting of multiple molecules may be advantageous, potential safety risks currently restrict the full clinical benefits of licensed JAK1-3 inhibitors. The development of more selective drugs aims to address these safety concerns. As these inhibitors all target the catalytic site of the JH1-domain, selectivity depends on dosing, which therefore could be key to improving the efficacy-safety profiles. Current evidence suggests that the alternative targeting of the pseudokinase JH2 domain may circumvent the safety issue of traditional JH1-targeting JAK inhibitors. Deucravacitinib, one such first-in-class drug, has proven effective and safe in the treatment of psoriasis by selectively inhibiting TYK2-mediated cytokine signalling without affecting other JAK-mediated cytokine signalling. These findings hold great promise for the treatment of numerous other immunological and inflammatory diseases. Furthermore, while TYK2 hitherto has been primarily recognized for its role in tumour surveillance, emerging data suggest an oncogenic potential,42,101 which could make TYK2 inhibitors also attractive in treating cancers.
Search strategy and selection criteria
Data for this review were identified by searches of Pubmed and references from relevant articles using the search terms: “Janus kinases”, “JAK inhibitors”, and “TYK2”. Data were also collected from the ClinicalTrial.Gov website.
Contributors
All authors contributed to the writing, reviewed, and approved the final manuscript.
Declaration of interests
L.F. and K.E.A. have an unrestricted grant from Bristol Myers Squibb. L.T.J. and M.F. declare no competing interests.
Acknowledgements
The work was supported by Wellcome (no. 100308/Z/12/Z), Medical Research Council UK (no. MC_UU_12010/3), the Oak Foundation (no. OCAY-15-520), and NIHR Oxford BRC. Funders were not involved in the writing of this review.
References
- 1.Glassman C.R., Tsutsumi N., Saxton R.A., Lupardus P.J., Jude K.M., Garcia K.C. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science. 2022;376(6589):163–169. doi: 10.1126/science.abn8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallweber H.J., Tam C., Franke Y., Starovasnik M.A., Lupardus P.J. Structural basis of recognition of interferon-alpha receptor by tyrosine kinase 2. Nat Struct Mol Biol. 2014;21(5):443–448. doi: 10.1038/nsmb.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y.J., Chen M., Cusack N.A., et al. Unexpected effects of FERM domain mutations on catalytic activity of Jak3: structural implication for Janus kinases. Mol Cell. 2001;8(5):959–969. doi: 10.1016/s1097-2765(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 4.Lupardus P.J., Ultsch M., Wallweber H., Bir Kohli P., Johnson A.R., Eigenbrot C. Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition. Proc Natl Acad Sci U S A. 2014;111(22):8025–8030. doi: 10.1073/pnas.1401180111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan Y., Gnanasambandan K., Ungureanu D., et al. Molecular basis for pseudokinase-dependent autoinhibition of JAK2 tyrosine kinase. Nat Struct Mol Biol. 2014;21(7):579–584. doi: 10.1038/nsmb.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saharinen P., Silvennoinen O. The pseudokinase domain is required for suppression of basal activity of Jak2 and Jak3 tyrosine kinases and for cytokine-inducible activation of signal transduction. J Biol Chem. 2002;277(49):47954–47963. doi: 10.1074/jbc.M205156200. [DOI] [PubMed] [Google Scholar]
- 7.Russell S.M., Tayebi N., Nakajima H., et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270(5237):797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 8.Macchi P., Villa A., Giliani S., et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377(6544):65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 9.O'Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kralovics R., Passamonti F., Buser A.S., et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 11.Dendrou C.A., Cortes A., Shipman L., et al. Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8(363) doi: 10.1126/scitranslmed.aag1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awasthi N., Liongue C., Ward A.C. STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. J Hematol Oncol. 2021;14(1):198. doi: 10.1186/s13045-021-01214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragimbeau J., Dondi E., Alcover A., Eid P., Uze G., Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003;22(3):537–547. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone C.J., Fuchs S.Y. Eliminative signaling by Janus kinases: role in the downregulation of associated receptors. J Cell Biochem. 2014;115(1):8–16. doi: 10.1002/jcb.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait Wojno E.D., Hunter C.A., Stumhofer J.S. The immunobiology of the interleukin-12 family: room for discovery. Immunity. 2019;50(4):851–870. doi: 10.1016/j.immuni.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 17.Stark G.R., Darnell J.E., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura R., Shibata K., Yamada H., Shimoda K., Nakayama K., Yoshikai Y. Tyk2-signaling plays an important role in host defense against Escherichia coli through IL-23-induced IL-17 production by gammadelta T cells. J Immunol. 2008;181(3):2071–2075. doi: 10.4049/jimmunol.181.3.2071. [DOI] [PubMed] [Google Scholar]
- 19.Stoiber D., Kovacic B., Schuster C., et al. TYK2 is a key regulator of the surveillance of B lymphoid tumors. J Clin Invest. 2004;114(11):1650–1658. doi: 10.1172/JCI22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizaki M., Muromoto R., Akimoto T., et al. Tyk2 deficiency protects joints against destruction in anti-type II collagen antibody-induced arthritis in mice. Int Immunol. 2011;23(9):575–582. doi: 10.1093/intimm/dxr057. [DOI] [PubMed] [Google Scholar]
- 21.Kreins A.Y., Ciancanelli M.J., Okada S., et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212(10):1641–1662. doi: 10.1084/jem.20140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minegishi Y., Saito M., Morio T., et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25(5):745–755. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs S., Kaiser-Labusch P., Bank J., et al. Tyrosine kinase 2 is not limiting human antiviral type III interferon responses. Eur J Immunol. 2016;46(11):2639–2649. doi: 10.1002/eji.201646519. [DOI] [PubMed] [Google Scholar]
- 24.Sarrafzadeh S.A., Mahloojirad M., Casanova J.L., et al. A new patient with inherited TYK2 deficiency. J Clin Immunol. 2020;40(1):232–235. doi: 10.1007/s10875-019-00713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv G., Sun G., Wu P., et al. Novel mutations of TYK2 leading to divergent clinical phenotypes. Pediatr Allergy Immunol. 2022;33(1) doi: 10.1111/pai.13671. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q., Matuozzo D., Le Pen J., et al. Recessive inborn errors of type I IFN immunity in children with COVID-19 pneumonia. J Exp Med. 2022;219(8) doi: 10.1084/jem.20220131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellenz F.M., Dieter C., Lemos N.E., Bauer A.C., Souza B.M., Crispim D. Association of TYK2 polymorphisms with autoimmune diseases: a comprehensive and updated systematic review with meta-analysis. Genet Mol Biol. 2021;44(2) doi: 10.1590/1678-4685-GMB-2020-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boisson-Dupuis S., Ramirez-Alejo N., Li Z., et al. Tuberculosis and impaired IL-23-dependent IFN-gamma immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol. 2018;3(30) doi: 10.1126/sciimmunol.aau8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enerback C., Sandin C., Lambert S., et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8(1):7043. doi: 10.1038/s41598-018-25282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasson M.H., Xiang Z., Walgren R., et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood. 2008;111(9):4797–4808. doi: 10.1182/blood-2007-09-113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Couturier N., Bucciarelli F., Nurtdinov R.N., et al. Tyrosine kinase 2 variant influences T lymphocyte polarization and multiple sclerosis susceptibility. Brain. 2011;134(Pt 3):693–703. doi: 10.1093/brain/awr010. [DOI] [PubMed] [Google Scholar]
- 32.Li Z., Gakovic M., Ragimbeau J., et al. Two rare disease-associated Tyk2 variants are catalytically impaired but signaling competent. J Immunol. 2013;190(5):2335–2344. doi: 10.4049/jimmunol.1203118. [DOI] [PubMed] [Google Scholar]
- 33.Yarmolinsky J., Amos C.I., Hung R.J., et al. Association of germline TYK2 variation with lung cancer and non-Hodgkin lymphoma risk. Int J Cancer. 2022;151(12):2155–2160. doi: 10.1002/ijc.34180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan S., Wang L., Zhang H., et al. Mendelian randomization and clinical trial evidence supports TYK2 inhibition as a therapeutic target for autoimmune diseases. EBioMedicine. 2023;89 doi: 10.1016/j.ebiom.2023.104488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesgidou N., Eliopoulos E., Goulielmos G.N., Vlassi M. Insights on the alteration of functionality of a tyrosine kinase 2 variant: a molecular dynamics study. Bioinformatics. 2018;34(17):i781–i786. doi: 10.1093/bioinformatics/bty556. [DOI] [PubMed] [Google Scholar]
- 36.Kerner G., Ramirez-Alejo N., Seeleuthner Y., et al. Homozygosity for TYK2 P1104A underlies tuberculosis in about 1% of patients in a cohort of European ancestry. Proc Natl Acad Sci U S A. 2019;116(21):10430–10434. doi: 10.1073/pnas.1903561116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogishi M., Arias A.A., Yang R., et al. Impaired IL-23-dependent induction of IFN-γ underlies mycobacterial disease in patients with inherited TYK2 deficiency. J Exp Med. 2022;219(10) doi: 10.1084/jem.20220094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerner G., Laval G., Patin E., et al. Human ancient DNA analyses reveal the high burden of tuberculosis in Europeans over the last 2,000 years. Am J Hum Genet. 2021;108(3):517–524. doi: 10.1016/j.ajhg.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorman J.A., Hundhausen C., Kinsman M., et al. The TYK2-P1104A autoimmune protective variant limits coordinate signals required to generate specialized T cell subsets. Front Immunol. 2019;10:44. doi: 10.3389/fimmu.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nemoto M., Hattori H., Maeda N., et al. Compound heterozygous TYK2 mutations underlie primary immunodeficiency with T-cell lymphopenia. Sci Rep. 2018;8(1):6956. doi: 10.1038/s41598-018-25260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dieter C., de Almeida Brondani L., Lemos N.E., et al. Polymorphisms in ACE1, TMPRSS2, IFIH1, IFNAR2, and TYK2 genes are associated with worse clinical outcomes in COVID-19. Genes. 2022;14(1):29. doi: 10.3390/genes14010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leitner N.R., Witalisz-Siepracka A., Strobl B., Muller M. Tyrosine kinase 2 - surveillant of tumours and bona fide oncogene. Cytokine. 2017;89:209–218. doi: 10.1016/j.cyto.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Karjalainen A., Shoebridge S., Krunic M., et al. TYK2 in tumor immunosurveillance. Cancers. 2020;12(1):150. doi: 10.3390/cancers12010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moodley D., Yoshida H., Mostafavi S., et al. Network pharmacology of JAK inhibitors. Proc Natl Acad Sci U S A. 2016;113(35):9852–9857. doi: 10.1073/pnas.1610253113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harigai M., Honda S. Selectivity of Janus kinase inhibitors in rheumatoid arthritis and other immune-mediated inflammatory diseases: is expectation the root of all headache? Drugs. 2020;80(12):1183–1201. doi: 10.1007/s40265-020-01349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy E.H. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology. 2019;58(6):1122. doi: 10.1093/rheumatology/kez002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traves P.G., Murray B., Campigotto F., Galien R., Meng A., Di Paolo J.A. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80(7):865–875. doi: 10.1136/annrheumdis-2020-219012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavanagh M.E., Horning B.D., Khattri R., et al. Selective inhibitors of JAK1 targeting an isoform-restricted allosteric cysteine. Nat Chem Biol. 2022;18(12):1388–1398. doi: 10.1038/s41589-022-01098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C., Yin Y., Shi G., et al. A highly selective JAK3 inhibitor is developed for treating rheumatoid arthritis by suppressing gammac cytokine-related JAK-STAT signal. Sci Adv. 2022;8(33) doi: 10.1126/sciadv.abo4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker D., Hadjicharalambous C., Gnanapavan S., Giovannoni G. Can rheumatologists stop causing demyelinating disease? Mult Scler Relat Disord. 2021;53 doi: 10.1016/j.msard.2021.103057. [DOI] [PubMed] [Google Scholar]
- 51.Fukuyama T., Tschernig T., Qi Y., Volmer D.A., Baumer W. Aggression behaviour induced by oral administration of the Janus-kinase inhibitor tofacitinib, but not oclacitinib, under stressful conditions. Eur J Pharmacol. 2015;764:278–282. doi: 10.1016/j.ejphar.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 52.Richardson P.J., Ottaviani S., Prelle A., Stebbing J., Casalini G., Corbellino M. CNS penetration of potential anti-COVID-19 drugs. J Neurol. 2020;267(7):1880–1882. doi: 10.1007/s00415-020-09866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y., Gibson S.A., Benveniste E.N., Qin H. Opportunities for translation from the bench: therapeutic intervention of the JAK/STAT pathway in neuroinflammatory diseases. Crit Rev Immunol. 2015;35(6):505–527. doi: 10.1615/CritRevImmunol.2016015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adas M.A., Alveyn E., Cook E., Dey M., Galloway J.B., Bechman K. The infection risks of JAK inhibition. Expert Rev Clin Immunol. 2022;18(3):253–261. doi: 10.1080/1744666X.2022.2014323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoisnard L., Lebrun-Vignes B., Maury S., et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci Rep. 2022;12(1):7140. doi: 10.1038/s41598-022-10777-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smolen J.S., Landewe R.B.M., Bijlsma J.W.J., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 57.Monaco C., Nanchahal J., Taylor P., Feldmann M. Anti-TNF therapy: past, present and future. Int Immunol. 2015;27(1):55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burmester G.R., Blanco R., Charles-Schoeman C., et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 59.Genovese M.C., Fleischmann R., Combe B., et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 60.Heneghan C., Goldacre B., Mahtani K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18(1):122. doi: 10.1186/s13063-017-1870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ytterberg S.R., Bhatt D.L., Connell C.A. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. reply. N Engl J Med. 2022;386(18):1768. doi: 10.1056/NEJMc2202778. [DOI] [PubMed] [Google Scholar]
- 62.U.S. Food and Drug Administration . 2021. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions.https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death Available from: [Google Scholar]
- 63.Salinas C.A., Louder A., Polinski J., et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with baricitinib compared to TNFi: a multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther. 2023;10(1):201–223. doi: 10.1007/s40744-022-00505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charles-Schoeman C., Buch M.H., Dougados M., et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL Surveillance. Ann Rheum Dis. 2023;82(1):119–129. doi: 10.1136/ard-2022-222259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alayo Q.A., Khatiwada A., Patel A., et al. Effectiveness and safety of combining tofacitinib with a biologic in patients with refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2021;27(10):1698–1702. doi: 10.1093/ibd/izab112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L., Yan Y.D., Shi F.H., Lin H.W., Gu Z.C., Li J. Comparative efficacy and safety of JAK inhibitors as monotherapy and in combination with methotrexate in patients with active rheumatoid arthritis: a systematic review and meta-analysis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.977265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fleischmann R.M., Blanco R., Hall S., et al. Switching between Janus kinase inhibitor upadacitinib and adalimumab following insufficient response: efficacy and safety in patients with rheumatoid arthritis. Ann Rheum Dis. 2021;80(4):432–439. doi: 10.1136/annrheumdis-2020-218412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amstad A., Papagiannoulis E., Scherer A., et al. Comparison of drug retention of TNF inhibitors, other biologics and JAK inhibitors in RA patients who discontinued JAK inhibitor therapy. Rheumatology. 2022;62(1):89–97. doi: 10.1093/rheumatology/keac285. [DOI] [PubMed] [Google Scholar]
- 69.Fugger L., Jensen L.T., Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63–80. doi: 10.1016/j.cell.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Min X., Ungureanu D., Maxwell S., et al. Structural and functional characterization of the JH2 pseudokinase domain of JAK family tyrosine kinase 2 (TYK2) J Biol Chem. 2015;290(45):27261–27270. doi: 10.1074/jbc.M115.672048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tokarski J.S., Zupa-Fernandez A., Tredup J.A., et al. Tyrosine kinase 2-mediated signal transduction in T lymphocytes is blocked by pharmacological stabilization of its pseudokinase domain. J Biol Chem. 2015;290(17):11061–11074. doi: 10.1074/jbc.M114.619502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moslin R., Gardner D., Santella J., et al. Identification of imidazo[1,2-b]pyridazine TYK2 pseudokinase ligands as potent and selective allosteric inhibitors of TYK2 signalling. Medchemcomm. 2017;8(4):700–712. doi: 10.1039/c6md00560h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burke J.R., Cheng L., Gillooly K.M., et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502) doi: 10.1126/scitranslmed.aaw1736. [DOI] [PubMed] [Google Scholar]
- 74.Liu C., Lin J., Moslin R., et al. Identification of Imidazo[1,2-b]pyridazine derivatives as potent, selective, and orally active Tyk2 JH2 inhibitors. ACS Med Chem Lett. 2019;10(3):383–388. doi: 10.1021/acsmedchemlett.9b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moslin R., Zhang Y., Wrobleski S.T., et al. Identification of N-methyl nicotinamide and N-methyl pyridazine-3-carboxamide pseudokinase domain ligands as highly selective allosteric inhibitors of tyrosine kinase 2 (TYK2) J Med Chem. 2019;62(20):8953–8972. doi: 10.1021/acs.jmedchem.9b00443. [DOI] [PubMed] [Google Scholar]
- 76.Wrobleski S.T., Moslin R., Lin S., et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973–8995. doi: 10.1021/acs.jmedchem.9b00444. [DOI] [PubMed] [Google Scholar]
- 77.Papp K., Gordon K., Thaçi D., et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321. doi: 10.1056/NEJMoa1806382. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong A.W., Gooderham M., Warren R.B., et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39. doi: 10.1016/j.jaad.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Warren R., Sofen H., Imafuku S., et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program. Ann Rheum Dis. 2022;81(Suppl 1):841. [Google Scholar]
- 80.Morand E., Pike M., Merrill J.T., et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2023;75(2):242–252. doi: 10.1002/art.42391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brosius F.C., Tuttle K.R., Kretzler M. JAK inhibition in the treatment of diabetic kidney disease. Diabetologia. 2016;59(8):1624–1627. doi: 10.1007/s00125-016-4021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watanabe R., Hashimoto M. Perspectives of JAK Inhibitors for large vessel vasculitis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.881705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McGaugh S., Kallis P., De Benedetto A., Thomas R.M. Janus kinase inhibitors for treatment of morphea and systemic sclerosis: a literature review. Dermatol Ther. 2022;35(6) doi: 10.1111/dth.15437. [DOI] [PubMed] [Google Scholar]
- 84.Paik J.J., Lubin G., Gromatzky A., Mudd P.N., Jr., Ponda M.P., Christopher-Stine L. Use of Janus kinase inhibitors in dermatomyositis: a systematic literature review. Clin Exp Rheumatol. 2023;41(2):348–358. doi: 10.55563/clinexprheumatol/hxin6o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han Y., Woo Y.R., Cho S.H., Lee J.D., Kim H.S. Itch and Janus kinase inhibitors. Acta Derm Venereol. 2023;103 doi: 10.2340/actadv.v103.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen H., Huang F., Zhang X., et al. Selective suppression of melanoma lacking IFN-gamma pathway by JAK inhibition depends on T cells and host TNF signaling. Nat Commun. 2022;13(1):5013. doi: 10.1038/s41467-022-32754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosseini A., Gharibi T., Mohammadzadeh A., et al. Ruxolitinib attenuates experimental autoimmune encephalomyelitis (EAE) development as animal models of multiple sclerosis (MS) Life Sci. 2021;276 doi: 10.1016/j.lfs.2021.119395. [DOI] [PubMed] [Google Scholar]
- 88.Dang C., Lu Y., Chen X., Li Q. Baricitinib ameliorates experimental autoimmune encephalomyelitis by modulating the Janus kinase/signal transducer and activator of transcription signaling pathway. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.650708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshida H., Kimura A., Fukaya T., et al. Low dose CP-690,550 (tofacitinib), a pan-JAK inhibitor, accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. Biochem Biophys Res Commun. 2012;418(2):234–240. doi: 10.1016/j.bbrc.2011.12.156. [DOI] [PubMed] [Google Scholar]
- 90.Jang Y., Lee W.J., Lee H.S., Chu K., Lee S.K., Lee S.T. Tofacitinib treatment for refractory autoimmune encephalitis. Epilepsia. 2021;62(4):e53–e59. doi: 10.1111/epi.16848. [DOI] [PubMed] [Google Scholar]
- 91.Massoud F., Ismail I.I., Al-Hashel J.Y., Abboud H. CNS demyelination during tofacitinib therapy: first report. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102568. [DOI] [PubMed] [Google Scholar]
- 92.Ercoban R., Islamoglu Z.G.K. New developing multiple sclerosis in a patient using tofacitinib due to alopesia areata. Dermatol Ther. 2022;35(6) doi: 10.1111/dth.15477. [DOI] [PubMed] [Google Scholar]
- 93.Chen X., Holtzman D.M. Emerging roles of innate and adaptive immunity in Alzheimer's disease. Immunity. 2022;55(12):2236–2254. doi: 10.1016/j.immuni.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sui S., Lv H. Cognitive improving actions of tofacitinib in a mouse model of Alzheimer disease involving TNF-alpha, IL-6, PI3K-Akt and GSK-3beta signalling pathway. Int J Neurosci. 2022:1–9. doi: 10.1080/00207454.2022.2151712. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez S., Hug C., Todorov P., et al. Machine learning identifies candidates for drug repurposing in Alzheimer's disease. Nat Commun. 2021;12(1):1033. doi: 10.1038/s41467-021-21330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y., Li X., Shen R., et al. Novel small molecule tyrosine kinase 2 pseudokinase ligands block cytokine-induced TYK2-mediated signaling pathways. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.884399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dowden H., Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18(7):495–496. doi: 10.1038/d41573-019-00074-z. [DOI] [PubMed] [Google Scholar]
- 98.King E.A., Davis J.W., Degner J.F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019;15(12) doi: 10.1371/journal.pgen.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okada Y., Wu D., Trynka G., et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woss K., Simonovic N., Strobl B., Macho-Maschler S., Muller M. TYK2: an upstream kinase of STATs in cancer. Cancers. 2019;11(11):1728. doi: 10.3390/cancers11111728. [DOI] [PMC free article] [PubMed] [Google Scholar]