Key Points
-
•
With 5 years of follow-up, nivolumab had lasting benefits in patients with R/R cHL after auto-HCT.
-
•
It appears feasible to discontinue nivolumab in patients with persistent CR and reinitiate treatment upon disease progression.
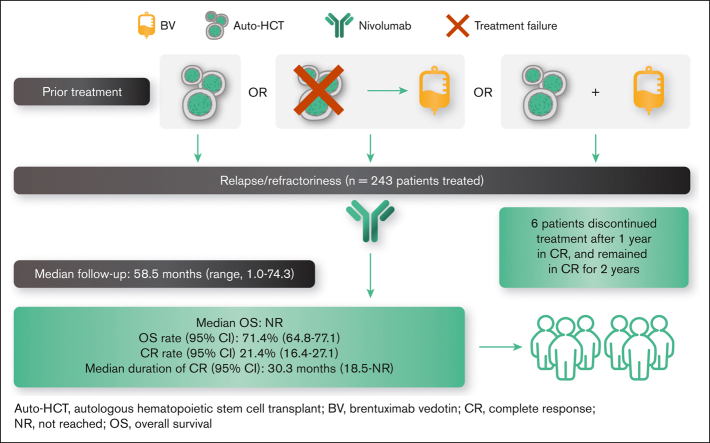
Visual Abstract
Abstract
Patients with relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL) for whom autologous hematopoietic cell transplantation (auto-HCT) had failed experienced frequent and durable responses to nivolumab in the phase 2 CheckMate 205 trial. We present updated results (median follow-up, ∼5 years). Patients with R/R cHL who were brentuximab vedotin (BV)–naive (cohort A), received BV after auto-HCT (cohort B), or received BV before and/or after auto-HCT (cohort C) were administered with nivolumab 3 mg/kg IV every 2 weeks until progression or unacceptable toxicity. Patients in cohort C with complete remission (CR) for 1 year could discontinue nivolumab and resume upon relapse. Among 243 patients (cohort A, n = 63; B, n = 80; and C, n = 100), the objective response rate (ORR) was 71.2% (95% confidence interval [CI], 65.1-76.8); the CR rate was 21.4% (95% CI, 16.4-27.1). Median duration of response, CR, and partial remission were 18.2 (95% CI, 14.7-26.1), 30.3, and 13.5 months, respectively. Median progression-free survival was 15.1 months (95% CI, 11.3-18.5). Median overall survival (OS) was not reached; OS at 5 years was 71.4% (95% CI, 64.8-77.1). In cohort C, all 3 patients who discontinued in CR and were subsequently re-treated achieved objective response. No new or unexpected safety signals were identified. This 5-year follow-up of CheckMate 205 demonstrated favorable OS and confirmed efficacy and safety of nivolumab in R/R cHL after auto-HCT failure. Results suggest patients may discontinue treatment after persistent CR and reinitiate upon progression. This trial was registered at www.clinicaltrials.gov as #NCT02181713.
Introduction
Patients with relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL) after autologous hematopoietic cell transplantation (auto-HCT) have a poor prognosis, with a historical median overall survival (OS) of ∼2 years.1, 2, 3, 4, 5 Brentuximab vedotin (BV) may provide benefits in this setting, but only 10% maintain complete remission (CR) after 5 years of follow-up,6,7 and most patients relapse or progress after BV therapy, with few available treatment options.
Genetically driven overexpression of programmed death–1 (PD-1) ligands 1 (PD-L1) and 2 (PD-L2) are characteristic of cHL and downregulate T-cell immune responses by binding to PD-1.8, 9, 10 PD-1 immune checkpoint inhibitors have shown strong activity in patients with R/R cHL11, 12, 13 and are a recommended treatment option in this setting per the clinical practice guidelines of the National Comprehensive Cancer Network (NCCN), the European Society for Medical Oncology, and the Chinese Society of Clinical Oncology.14, 15, 16 One such inhibitor is nivolumab, a fully human immunoglobulin G4 anti–PD-1 monoclonal antibody that augments antitumor immune responses by blocking signaling through the PD-1 pathway, releasing T-cell inhibition.11,17 However, the long-term survival benefit of anti–PD-1 therapy and optimal treatment duration are unknown.
The pivotal, single-arm, phase 2 CheckMate 205 trial (NCT02181738) demonstrated frequent and durable responses with nivolumab in patients with R/R cHL for whom auto-HCT had failed.18, 19, 20 A favorable objective response rate (ORR) was demonstrated after a median follow-up of 18.0 months19 and 33.0 months.20 Here, we present updated efficacy and safety results from CheckMate 205 with an extended median follow-up of 58.5 months, including exploratory analyses of progression-free survival (PFS) and OS as well as CR maintenance after nivolumab discontinuation.
Methods
Trial details have been described previously and are summarized later in the article.18,19
Study design and patients
In the multicenter, multicohort, single-arm, phase 2 CheckMate 205 trial, patients aged ≥18 years with cHL for whom auto-HCT had failed were enrolled at 38 sites across Europe and North America. Patients were enrolled in cohort A if they had received no prior treatment with BV, cohort B if they received BV as salvage therapy after failure of auto-HCT, or cohort C if they received BV before and/or after auto-HCT. Exclusion criteria included autoimmune disease, radiation therapy within 3 weeks or chest radiation within 24 weeks of the first dose of nivolumab, auto-HCT within 90 days of the first dose of nivolumab, and allogeneic HCT (allo-HCT) or checkpoint blockade at any time before nivolumab treatment. This study was conducted in accordance with the Declaration of Helsinki. Before enrollment, all patients provided written informed consent. Before trial initiation at each site, approval from the appropriate institutional review board and independent ethics committee was obtained.
Treatment
Patients received nivolumab 3 mg/kg IV every 2 weeks until disease progression or unacceptable toxicity, or patients could elect to discontinue nivolumab and proceed to receive allo-HCT. Per a protocol amendment in July 2014, patients could continue treatment after investigator-determined disease progression. Patients in cohort C who achieved CR for ≥1 year were to discontinue treatment, with the option to reinitiate therapy if they relapsed within 2 years.
Determination of tumor response was made by an independent review committee (IRC) using the 2007 International Working Group criteria for malignant lymphoma. Tumor assessments were performed at screening, week 9 (± 7 days) after treatment initiation, weeks 17, 25, 37, and 49, then every 16 weeks (± 14 days) up to week 97, and every 26 weeks (± 21 days) thereafter until disease progression was documented or the patient initiated a preparative regimen for auto- or allo-HCT, whichever occurred earlier.
Adverse events (AEs), serious AEs, and treatment-related AEs (TRAEs) were reported throughout the treatment period and between screening and 100 days after the last dose. Immune-mediated AEs, including specific events such as diarrhea/colitis, hepatitis, pneumonitis, nephritis and renal dysfunction, rash, and endocrine-related events, were reported within 100 days of the last dose. Late-emergent TRAEs, defined as TRAEs (including immune-mediated AEs) with an onset date >100 days after the last dose of study therapy, were also reported. AEs were coded using the Medical Dictionary for Regulatory Activities version 23.1. AEs and laboratory values were tabulated using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
End points
The primary end point was IRC-assessed ORR. Secondary end points included the IRC-assessed duration of response (DOR), CR and partial remission (PR) rates, and CR and PR duration. Exploratory end points included IRC-assessed PFS according to the cohort and by best overall response (BOR), OS according to the cohort and by BOR, and safety.
Statistical analysis
The planned sample size was 60 patients each for cohorts A and B and 100 patients for cohort C. Assuming an ORR of 40%, this would provide ∼93% power to reject the null hypothesis that the true ORR was ≤20%, considering a 2-sided α of 5%. The sample size for cohort C was planned to provide an 87% probability of observing at least 1 occurrence of any AE that would have occurred with 2% incidence.
All patients who received at least 1 dose of nivolumab were included in the primary efficacy and safety analyses. Primary efficacy analyses were performed independently for each cohort; safety was analyzed in the combined population. ORRs were summarized using binomial response rates, and 2-sided 95% exact confidence intervals (CIs) were calculated using the Clopper-Pearson method.
Results
Baseline patient characteristics and disposition
Between August 2014 and August 2015, a total of 276 patients were enrolled, and 243 were treated across cohorts A (n = 63), B (n = 80), and C (n = 100). At database lock (22 January 2021), the median follow-up was 58.5 months for all patients treated: 61.9 months for cohort A, 58.5 months for cohort B, and 53.5 months for cohort C. As reported previously,19 baseline characteristics were generally similar across cohorts (Table 1), with a median age of 34 years (range, 18-72) in the treated population.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | Cohort A (BV naive) (n = 63) |
Cohort B (BV after auto-HCT) (n = 80) |
Cohort C (BV before and/or after auto-HCT) (n = 100) |
Overall (N = 243) |
|---|---|---|---|---|
| Age, median (range), y | 33 (18-65) | 37 (18-72) | 32 (19-69) | 34 (18-72) |
| Female, n (%) | 29 (46.0) | 29 (36.3) | 44 (44.0) | 102 (42.0) |
| Stage at study entry, n (%) | ||||
| I | 1 (1.6) | 1 (1.3) | 2 (2.0) | 4 (1.6) |
| II | 19 (30.2) | 11 (13.8) | 20 (20.0) | 50 (20.6) |
| III | 18 (28.6) | 14 (17.5) | 17 (17.0) | 49 (20.2) |
| IV | 24 (38.1) | 54 (67.5) | 61 (61.0) | 139 (57.2) |
| Not reported | 1 (1.6) | 0 | 0 | 1 (0.4) |
| B symptoms at study entry, n (%) | 10 (15.9) | 18 (22.5) | 25 (25.0) | 53 (21.8) |
| Bulky disease at study entry, n (%) | 10 (15.9) | 17 (21.3) | 22 (22.0) | 49 (20.2) |
| Extralymphatic involvement, n (%) | 24 (38.1) | 36 (45.0) | 45 (45.0) | 105 (43.2) |
| Median prior lines of therapy (IQR) | 2 (2-3) | 4 (4-7) | 4 (3-5) | 4 (3-5) |
| Time from diagnosis to first dose of nivolumab, median (IQR), y | 3.1 (2.0-7.5) | 6.2 (3.3-8.3) | 3.5 (2.3-6.4) | 4.5 (2.4-7.6) |
IQR, interquartile range.
At database lock, 7.8% of patients remained on treatment (supplemental Figure 1). The most common reason for treatment discontinuation was disease progression. Median duration of treatment was 14.3 months (95% CI, 12.7-16.3) and was similar across cohorts.
Objective response and CR
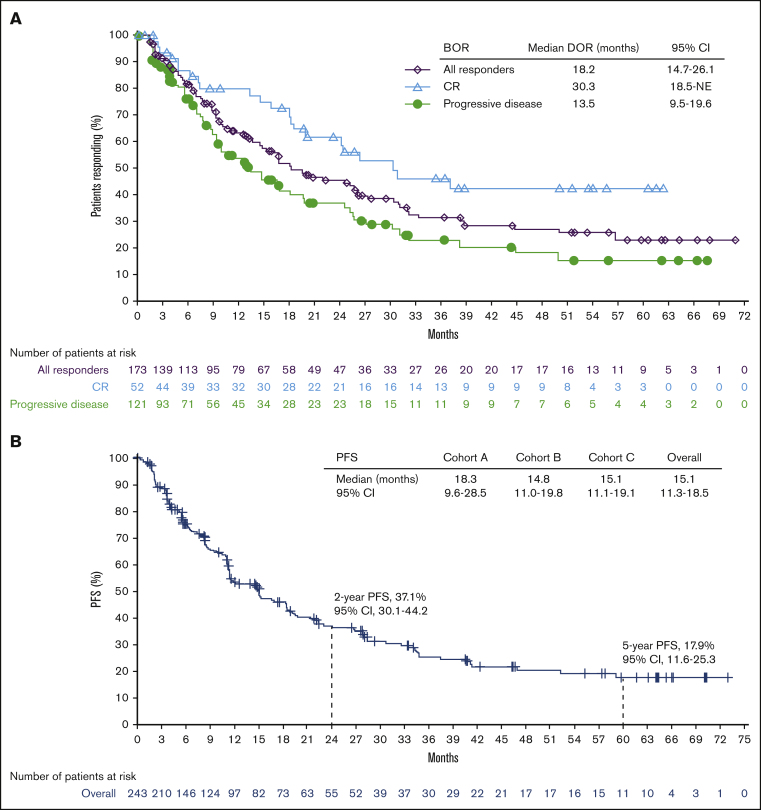
At a median follow-up of 58.5 months (range, 1.0-74.3), the IRC-assessed ORR was 71.2% (95% CI, 65.1-76.8), with a CR rate of 21.4% (95% CI, 16.4-27.1; Table 2). CR rate was the highest in patients who were naive to BV (cohort A, 31.7%) compared with those who had prior BV exposure (cohort B, 13.8%; cohort C, 21.0%). The overall population had a median time to response of 2.1 months (range, 0.8-17.9) and a median time to CR of 4.0 months (range, 1.7-34.4), both similar in all cohorts. The median DOR was 18.2 months (95% CI, 14.7-26.1; Figure 1A), and the median durations of CR and PR were 30.3 months and 13.5 months, respectively.
Table 2.
BOR
| Response | Cohort A (BV naive) (n = 63) |
Cohort B (BV after auto-HCT) (n = 80) |
Cohort C (BV before and/or after auto-HCT) (n = 100) |
Overall (N = 243) |
|---|---|---|---|---|
| ORR, % (95% CI) | 65.1 (52.0-76.7) | 71.3 (60.0-80.8) | 75.0 (65.3-83.1) | 71.2 (65.1-76.8) |
| BOR, n (%) | ||||
| CR | 20 (31.7) | 11 (13.8) | 21 (21.0) | 52 (21.4) |
| PR | 21 (33.3) | 46 (57.5) | 54 (54.0) | 121 (49.8) |
| SD | 14 (22.2) | 14 (17.5) | 12 (12.0) | 40 (16.5) |
| Progressive disease | 8 (12.7) | 7 (8.8) | 11 (11.0) | 26 (10.7) |
| Median time to response, mo (range) | 2.0 (1.5-4.6) | 2.2 (1.6-11.1) | 2.1 (0.8-17.9) | 2.1 (0.8-17.9) |
| IQR | 1.9–2.3 | 1.9–3.0 | 1.9–3.8 | 1.9–3.6 |
| Median time to CR, mo (range) | 3.9 (1.7-34.4) | 4.4 (1.9-22.8) | 4.2 (1.8-17.9) | 4.0 (1.7-34.4) |
| IQR | 3.7–5.2 | 3.7–19.1 | 3.7–6.5 | 3.7–8.3 |
| Median DOR, mo (95% CI) | 26.2 (15.2–NE) | 16.6 (9.3-25.7) | 18.2 (11.6-30.9) | 18.2 (14.7-26.1) |
NE, not evaluable; SD, stable disease.
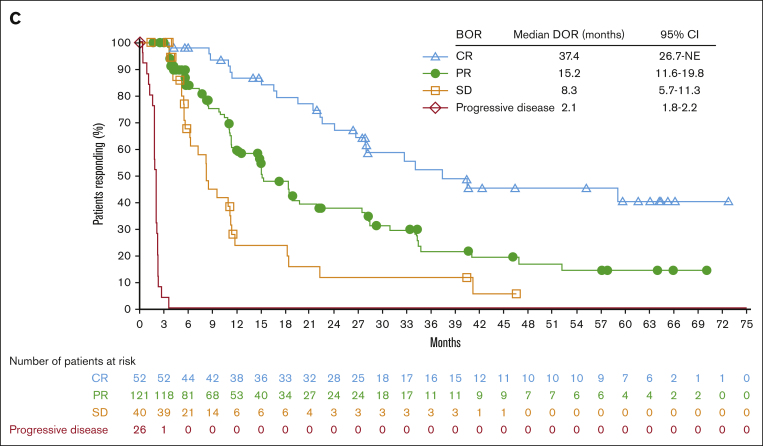
Figure 1.
Efficacy endpoints: duration of response and progression-free survival. DOR according to the BOR (A), PFS according to the cohort (B), and PFS according to the BOR (C). NE, not estimable; SD, stable disease.
PFS
Median PFS was 15.1 months (95% CI, 11.3-18.5) and was the longest in patients who were naive to BV (18.3 months in cohort A, 14.8 months in cohort B, and 15.1 months in cohort C; Figure 1B). The PFS rate was 37.1% (95% CI, 30.1-44.2) at 2 years and 17.9% (95% CI, 11.6-25.3) at 5 years. When compared based on the BOR, the median PFS was highest in patients who achieved CR, at 37.4 months (95% CI, from 26.7 to not estimable; Figure 1C). For those with a BOR of PR, stable disease, and progressive disease, median PFS was 15.2 months (95% CI, 11.6-19.8), 8.3 months (95% CI, 5.7-11.3), and 2.1 months (95% CI, 1.8-2.2), respectively.
OS
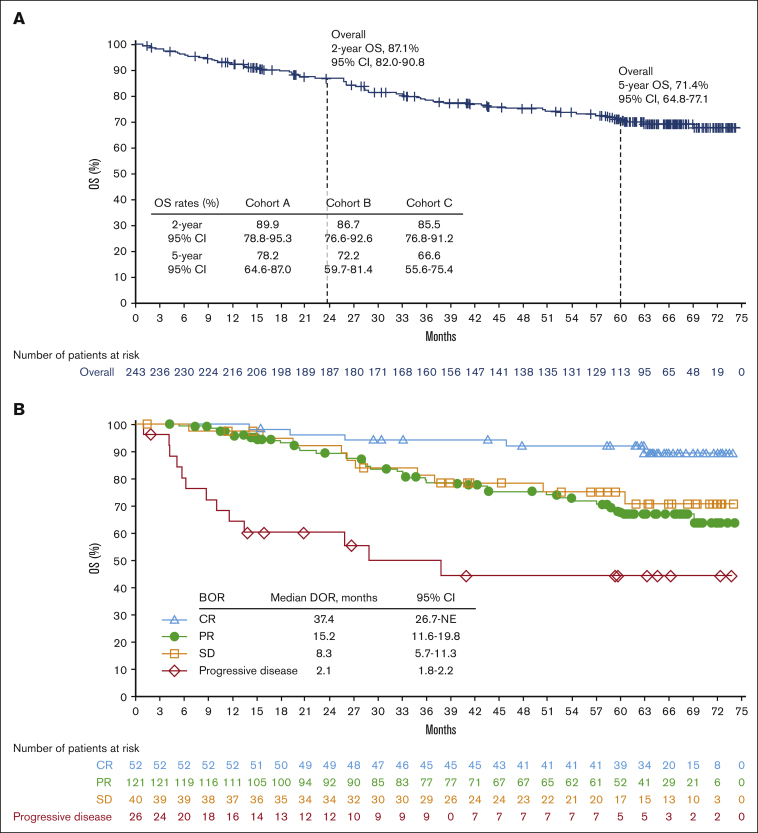
A total of 65 patients died, including 36 from disease progression. The median OS was not reached in any cohort (Figure 2A). The OS rate was 87.1% (95% CI, 82.0-90.8) at 2 years and 71.4% (95% CI, 64.8-77.1) at 5 years. The median OS was 28.8 months (95% CI, from 10.0 to not reached) in patients whose BOR was progressive disease and not reached in patients whose BOR was CR, PR, or stable disease (Figure 2B).
Figure 2.
Efficacy endpoints: Overall survival and best overall responses. OS according to the cohort (A) and BOR (B). Of the 65 deaths, causes included disease progression (n = 36), graft-versus-host disease (n = 5), sepsis and/or septic shock (n = 3), pneumonia (n = 3), cardiac arrest (n = 2), multiple organ failure (n = 2), lung cancer (n = 1), Epstein-Barr virus-positive T-cell lymphoma with multiple organ failure (n = 1), adverse reaction to allo-HCT (n = 1), allo-HCT complicated by graft-versus-host disease (n = 1), heart failure (n = 1), post-transplant complications (n = 1), respiratory infection (n = 1), hemorrhagic cystitis (n = 1), acute hypoxemic respiratory failure secondary to pneumocystic pneumonia (n = 1), and unknown causes (n = 5).
Patients who received subsequent allo-HCT
A total of 58 (23.9%) patients received subsequent HCT (allo-HCT, n = 57; auto-HCT, n = 1) more commonly in cohort C (n = 30) than in cohorts A (n = 13) or B (n = 14). Patients received allo-HCT at a median of 63.0 days (range, 16-716) after their last nivolumab dose. Among the patients who received allo-HCT, 29 (51%) were in CR 2 years after transplant (cohort A, n = 7; cohort B, n = 9; and cohort C, n = 13; supplemental Table 1).
Patients in cohort C who discontinued nivolumab after CR
Another exploratory end point was to evaluate the risk and benefit of discontinuing nivolumab after persistent CR. Among the 21 patients in cohort C who achieved CR, 12 had persistent CR for at least 1 year (supplemental Table 2), discontinued treatment, and entered the observational follow-up (maximum of 2 years) and survival follow-up phases. The median follow-up period for these patients since discontinuation for persistent CR was 48.3 months (range, 36.1-54.5). Six patients (50%) completed the follow-up; 6 patients did not complete the follow-up because of progressive disease (n = 4), withdrawal of consent (n = 1), and suspicion of progression that did not meet the criteria for progressive disease (n = 1). Of the 4 patients with progressive disease, 3 were re-treated with nivolumab per protocol (the remaining patient received commercial nivolumab off-study), of whom 1 achieved and maintained CR but discontinued because of treatment-related grade 3 pneumonitis after 2.0 months, 1 achieved PR but discontinued because of disease progression (DOR, 11.1 months), and 1 achieved CR and was still receiving treatment after 35.4 months at database lock.
Safety
In the treated population, TRAEs occurred in 198 patients (81.5%) and 67 patients (27.6%) had a grade 3 or 4 TRAE (Table 3). The most common TRAEs (≥10% of patients) were fatigue (25.1%), diarrhea (16.0%), infusion-related reaction (14.0%), rash (11.9%), nausea (11.1%), and pruritus (10.3%). Thirty-two patients (13.2%) had a serious TRAE, most commonly pneumonitis (2.1%) and infusion-related reaction (2.1%); 7.8% had a grade 3 or 4 serious TRAE. A total of 22 patients (9.1%) discontinued treatment because of a TRAE, and 13 (5.3%) discontinued because of a grade 3 or 4 TRAE. Pneumonitis was the most common TRAE leading to discontinuation (2.9%). The most common immune-mediated AEs were hypothyroidism (14.4%) and rash (11.9%). Late-onset TRAEs (>100 days after the last dose of study therapy) occurred in 1.6% of patients and included decreased lymphocyte count, increased blood glucose, hyperbilirubinemia, and autoimmune nephritis. There were no deaths related to the study treatment.
Table 3.
TRAEs
| TRAEs (N = 243) | Any grade | Grade 3 or 4 |
|---|---|---|
| Patients with TRAEs, n (%) | 198 (81.5) | 67 (27.6) |
| TRAEs occurring in ≥10% of patients, n (%) | ||
| Fatigue | 61 (25.1) | 2 (0.8) |
| Diarrhea | 39 (16.0) | 2 (0.8) |
| Infusion-related reaction | 34 (14.0) | 1 (0.4) |
| Rash | 29 (11.9) | 2 (0.8) |
| Nausea | 27 (11.1) | 0 |
| Pruritus | 25 (10.3) | 0 |
| Immune-mediated AEs within 100 d of last dose | ||
| Hypothyroidism/thyroiditis | 35 (14.4) | 0 |
| Rash | 29 (11.9) | 4 (1.6) |
| Hepatitis | 15 (6.2) | 12 (4.9) |
| Pneumonitis | 15 (6.2) | 2 (0.8) |
| Hypersensitivity/infusion reactions | 13 (5.3) | 2 (0.8) |
| Diarrhea/colitis | 6 (2.5) | 5 (2.1) |
| Hyperthyroidism | 6 (2.5) | 0 |
| Nephritis and renal dysfunction | 3 (1.2) | 1 (0.4) |
| Diabetes mellitus | 2 (0.8) | 1 (0.4) |
| Adrenal insufficiency | 1 (0.4) | 0 |
Discussion
These long-term results with 5-years of follow-up for CheckMate 205 are consistent with previous findings of median follow-ups of 18 months19 and 33 months20 and confirm the durable benefit and safety of nivolumab in patients with cHL who progressed or relapsed after auto-HCT. After a median follow-up of 5 years, the observed safety profile of nivolumab was consistent with previous reports, with no newly identified late effects or unexpected safety signals.19,20 Additionally, in this extended follow-up study, the observed DOR (18.2 months) and duration of CR (30.3 months) indicated that responses remained durable across all 3 cohorts, particularly for patients who achieved CR. Although the median OS was not reached, the 5-year OS was highest for patients who achieved CR. The AE profile was similar to that in previous reports, with no new or unexpected safety findings.19,20
The CR rate was numerically higher than it was at the median follow-up of 33 months20; it was highest in patients who were naive to BV (cohort A) and lowest in patients who received BV after failure of auto-HCT (cohort B). The high CR rate observed in this study in patients who were naive to BV is consistent with previous data showing that first-line nivolumab monotherapy in patients with early-stage cHL resulted in a CR rate of 51%.21 It should be noted, however, that patients in cohort A had only received a median of 2 prior lines of therapy, whereas patients in cohorts B and C had received a median of 4 prior lines of therapy. The OS was favorable in all cohorts and all patients who did not experience progressive disease as a BOR to nivolumab. Recent data support the superiority of PD-1 inhibitors over BV in this setting,12 and this study supports the earlier use of PD-1 inhibitors in R/R cHL.
The optimal duration of PD-1 inhibitor therapy in R/R cHL remains unknown. Discontinuation of nivolumab in patients who achieved CR for at least 1 year was explored, and although patient numbers were limited, all patients responded to re-treatment, suggesting that this approach may be feasible. A larger sample size is necessary, however, to understand the frequency, depth, and durability of responses in this setting.
A limitation of this study was the absence of quantitative scoring of positron emission tomography scans because of the use of 2007 International Working Group criteria, because it was designed before the availability of the 2014 Lugano criteria.22
The results presented herein confirm data from previous studies reporting the efficacy of PD-1 pathway inhibition in R/R cHL for which auto-HCT had failed. In the phase 3 KEYNOTE-204 trial in patients with R/R cHL ineligible for auto-HCT or for whom auto-HCT had failed, pembrolizumab, the only other PD-1 inhibitor approved in the United States for the treatment of R/R cHL,23 significantly improved PFS compared with BV, with up to a 2-year follow-up.12 Additionally, the phase 2 KEYNOTE-087 and phase 1 KEYNOTE-013 trials demonstrated comparably favorable ORR and CR rates in patients with R/R cHL at median follow-ups of 2 years and 4 years, respectively.13,24 To our knowledge, these analyses represent the longest phase 2 or 3 study follow-up of anti–PD-1 blockade in patients with cHL.
Recent studies have shown excellent results with the combination of PD-1 blockade and chemotherapy before auto-HCT in R/R cHL.25, 26, 27 There is also increasing evidence that PD-1 blockade combined with chemotherapy may be beneficial as first-line therapy.28,29 The increased use of PD-1 blockade in either setting could affect its efficacy and role after a failure of auto-HCT. For patients who achieve PR with nivolumab, subsequent chemotherapy or allo-HCT may be beneficial, because evidence suggests that both are effective after PD-1 blockade.30, 31, 32, 33
In conclusion, this 5-year follow-up of CheckMate 205 confirmed durable responses, particularly a long duration of CR and favorable OS with nivolumab in patients with R/R cHL for whom auto-HCT had failed, irrespective of prior BV treatment. These results also suggest that it may be feasible for patients to discontinue treatment after 1 year of CR and reinitiate treatment upon disease progression. Recent and ongoing trials including NCT03004833, NCT03016871, and NCT01100502 are examining the benefits of nivolumab in earlier lines of therapy.
Conflict-of-interest disclosure: S.M.A. reports research support (institutional) from ADC Therapeutics, Bristol Myers Squibb, Regeneron, Seagen, Takeda, and Trillium. P.J.B. reports grants (institutional) from BeiGene, Bristol Myers Squibb, Merck Sharp and Dohme, and Takeda; consulting fees from Takeda; honoraria from BeiGene, Merck Sharp and Dohme, and Takeda; and travel/meeting support from Celgene. G.v.K. reports consulting fees from Merck. H.J.L. reports grants from Bristol Myers Squibb, Merck, Oncternal, Seagen, and Takeda; consulting fees from Century Therapeutics; and honoraria from Aptitude Health and the Korean Society of Cardio-oncology. A.S. reports consulting fees from Arquile, Incyte, and Sanofi; honoraria from AbbVie, Amgen, Arquile, AstraZeneca, Bayer, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Gilead, Merck Sharp and Dohme, Novartis, Pfizer, Roche, Sandoz, Servier, and Takeda; and advisory board participation for Bayer, Bristol Myers Squibb, Eisai, Gilead, Merck Sharp and Dohme, Pfizer, and Servier. P.L.Z. reports honoraria from AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Kyowa Kirin, Merck, Novartis, Roche, Takeda, and Sanofi. G.P.C. reports research support from Amgen, AstraZeneca, Bristol Myers Squibb, and Pfizer; and honoraria from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Gilead, Incyte, Kyowa Kirin, Novartis, Pfizer, Roche, SecuraBio, and Takeda. J.B.C. reports research support (institutional) from Bristol Myers Squibb/Celgene. J.K. reports research support (institutional) from AstraZeneca, Bristol Myers Squibb, and Merck; reports consulting fees from AbbVie, Antengene, Bristol Myers Squibb, Gilead, Karyopharm, Medison Ventures, Merck, Roche, and Seagen; reports honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, Incyte, Janssen, Karyopharm, Merck, Novartis, Pfizer, Roche, and Seagen; and has chaired an advisory board for Lymphoma Canada. K.J.S. reports research support from Bristol Myers Squibb; consulting fees from Bristol Myers Squibb; research support (institutional) from Roche; honoraria from AbbVie, BeiGene, Bristol Myers Squibb, Janssen, Kyowa Kirin, Novartis, and Seagen; and advisory board participation for Regeneron. M.T. reports consulting fees and honoraria from AbbVie, AstraZeneca, Bristol Myers Squibb, Gilead, Janssen, MorphoSys, Novartis, Roche, and Takeda; and travel/meeting support from AstraZeneca, Gilead, Janssen, Roche, and Takeda. M.P. reports consulting fees from AstraZeneca, Bristol Myers Squibb, Merck Sharp and Dohme, and Roche; honoraria from Takeda and Thermo Fisher Scientific; and travel/meeting support from AstraZeneca, Merck Sharp and Dohme, and Roche. U.J. reports honoraria from Bristol Myers Squibb. W.W. is an employee of Syndena and reports research support from Amgen, AstraZeneca, Bristol Myers Squibb/Celgene, Bundesland Tirol Programm, European Commission, Janssen, Novartis, Roche, Sanofi, and Takeda; advisory board participation for Amgen, AbbVie, Bristol Myers Squibb/Celgene, EUSA Pharma, Fujimoto, Gilead, GlaxoSmithKline, Incyte, Janssen, Kite, Merck, Myelom- und Lymphomselbsthilfe Österreich, Novartis, Pfizer, Roche, Sandoz, Sanofi, and Takeda; and steering/safety committee participation for Amgen, Bristol Myers Squibb/Celgene, DSM, European Health Data & Evidence Network, Harmony, Honeur, and MorphoSys. J.M.-G. is an employee and holds stock in Bristol Myers Squibb. M.A.S. reports research support from AbbVie, AstraZeneca, Bayer, Bristol Myers Squibb, and Merck; and advisory board participation for AstraZeneca and Immunitas Therapeutics. A.E. reports consulting fees from AstraZeneca, Merck Sharp and Dohme, Takeda, and Tessa Pharma; grants from Bristol Myers Squibb; and honoraria from AstraZeneca, Hexal AG, Innovent, Janssen, Takeda, and TS Oncology. P.A. reports research funding from Kite; research funding (institutional) from Adaptive, Affimed, Bristol Myers Squibb, Genentech/Roche, IGM, Merck, Otsuka, Sigma Tau, and Tensha; honoraria from Bristol Myers Squibb and Merck; and consulting fees from Adaptive, ADC Therapeutics, Affimed, AstraZeneca, Bristol Myers Squibb, C4, Celgene, Daiichi Sankyo, Enterome, Epizyme, Genentech, GenMab, Infinity, Merck, Miltenyi, MorphoSys, Pfizer, Regeneron, Tessa, and Xencor. The remaining authors declare no competing financial interests.
The current affiliation for G.v.K. is Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, MA.
Acknowledgments
The authors thank the patients and families for making this trial possible and the clinical study teams who participated. This study was supported by Bristol Myers Squibb. Professional medical writing support for this manuscript was provided by Richard Sora, of Caudex, funded by Bristol Myers Squibb.
Authorship
Contribution: S.M.A., P.J.B., G.v.K., H.J.L., A.S., P.L.Z., G.P.C., J.B.C., J.P.d.B., J.K., K.J.S., M.T., M.P., U.J., W.W., M.A.S., A.E., and P.A. conceptualized and designed the study and collected and assembled data; P.J.B. was responsible for the provision of study materials or for patients; and all authors analyzed, interpreted, and approved the data, wrote the manuscript, provided the final approval for the manuscript, and are accountable for all aspects of the work.
Footnotes
Data are available on request through the Bristol Myers Squibb policy webpage at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program. 2008;2008(1):326–333. doi: 10.1182/asheducation-2008.1.326. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Tresckow B, Müller H, Eichenauer DA, et al. Outcome and risk factors of patients with Hodgkin Lymphoma who relapse or progress after autologous stem cell transplant. Leuk Lymphoma. 2014;55(8):1922–1924. doi: 10.3109/10428194.2013.854888. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18(6):1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 5.Moskowitz AJ, Hamlin PA, Jr., Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460. doi: 10.1200/JCO.2012.45.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roemer MG, Advani RH, Redd RA, et al. Classical Hodgkin lymphoma with reduced β2M/MHC class I expression is associated with inferior outcome independent of 9p24.1 status. Cancer Immunol Res. 2016;4(11):910–916. doi: 10.1158/2326-6066.CIR-16-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 11.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuruvilla J, Ramchandren R, Santoro A, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512–524. doi: 10.1016/S1470-2045(21)00005-X. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. 2019;134(14):1144–1153. doi: 10.1182/blood.2019000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(suppl 4):iv19–iv29. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Ma J, Union for China Lymphoma Investigators of Chinese Society of Clinical Oncology Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version) Chin J Cancer Res. 2021;33(3):289–301. doi: 10.21147/j.issn.1000-9604.2021.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas Version 2.2023 © National Comprehensive Cancer Network, Inc. 2023. All rights reserved.
- 17.Opdivo (nivolumab). Prescribing information. Bristol Myers Squibb. http://packageinserts.bms.com/pi/pi_opdivo.pdf
- 18.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armand P, Engert A, Younes A, et al. 2018. Nivolumab for relapsed or refractory classical Hodgkin lymphoma after autologous hematopoietic cell transplantation: extended follow-up of the phase 2 single-arm CheckMate 205 study. Paper presented at: 60th American Society of Hematology (ASH) Annual Meeting and Exposition; 1-4 December. San Diego, CA. [Google Scholar]
- 21.Bröckelmann PJ, Goergen H, Keller U, et al. Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: the randomized phase 2 German Hodgkin Study Group NIVAHL trial. JAMA Oncol. 2020;6(6):872–880. doi: 10.1001/jamaoncol.2020.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keytruda (pembrolizumab). Prescribing information. Merck. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- 24.Armand P, Kuruvilla J, Michot JM, et al. KEYNOTE-013 4-year follow-up of pembrolizumab in classical Hodgkin lymphoma after brentuximab vedotin failure. Blood Adv. 2020;4(12):2617–2622. doi: 10.1182/bloodadvances.2019001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero S, Balaguer-Rosello A, Montoro J, et al. Addition of chemotherapy to nivolumab after PD-1 inhibitor failure as bridge to allogeneic stem cell transplantation in classical Hodgkin's lymphoma: report on three cases and literature review. Ther Adv Hematol. 2021;12 doi: 10.1177/20406207211038181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mei MG, Lee HJ, Palmer JM, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. 2022;139(25):3605–3616. doi: 10.1182/blood.2022015423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moskowitz AJ, Shah G, Schoder H, et al. Phase II trial of pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin as second-line therapy for relapsed or refractory classical Hodgkin lymphoma. J Clin Oncol. 2021;39(28):3109–3117. doi: 10.1200/JCO.21.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bröckelmann PJ, Bühnen I, Meissner J, et al. Nivolumab and doxorubicin, vinblastine, and dacarbazine in early-stage unfavorable Hodgkin lymphoma: final analysis of the randomized German Hodgkin Study Group phase II NIVAHL trial. J Clin Oncol. 2023;41(6):1193–1199. doi: 10.1200/JCO.22.02355. [DOI] [PubMed] [Google Scholar]
- 29.Allen PB, Savas H, Evens AM, et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced-stage classical Hodgkin lymphoma. Blood. 2021;137(10):1318–1326. doi: 10.1182/blood.2020007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabretta E, Guidetti A, Ricci F, et al. Chemotherapy after PD-1 inhibitors in relapsed/refractory Hodgkin lymphoma: outcomes and clonal evolution dynamics. Br J Haematol. 2022;198(1):82–92. doi: 10.1111/bjh.18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casadei B, Argnani L, Morigi A, et al. Effectiveness of chemotherapy after anti-PD-1 blockade failure for relapsed and refractory Hodgkin lymphoma. Cancer Med. 2020;9(21):7830–7836. doi: 10.1002/cam4.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito A, Kim SW, Matsuoka KI, et al. Safety and efficacy of anti-programmed cell death-1 monoclonal antibodies before and after allogeneic hematopoietic cell transplantation for relapsed or refractory Hodgkin lymphoma: a multicenter retrospective study. Int J Hematol. 2020;112(5):674–689. doi: 10.1007/s12185-020-02960-4. [DOI] [PubMed] [Google Scholar]
- 33.Merryman RW, Castagna L, Giordano L, et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia. 2021;35(9):2672–2683. doi: 10.1038/s41375-021-01193-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.