Summary
Neurosurgical robots have developed for decades and can effectively assist surgeons to carry out a variety of surgical operations, such as biopsy, stereo-electroencephalography (SEEG), deep brain stimulation (DBS), and so forth. In recent years, neurosurgical robots in China have developed rapidly. This article will focus on several key skills in neurosurgical robots, such as medical imaging systems, automatic manipulator, lesion localization techniques, multimodal image fusion technology, registration method, and vascular imaging technology; introduce the clinical application of neurosurgical robots in China, and look forward to the potential improvement points in the future based on our experience and research in the field.
Subject areas: Neurosurgery, Surgical equipment, Robotics
Graphical abstract

Neurosurgery; Surgical equipment; Robotics
Introduction
Brain tumors, medically intractable epilepsy, movement disorders, and other neurological conditions have a worldwide impact on healthcare and often require neurosurgical treatment. The brain has several characteristics: (i) it is held in the skull allowing fixation of devices to hold it in position during the procedure; (ii) the anatomical topology is stable, and functional areas are well known; (iii) the brain is the organ where the highest precision is required during surgery to avoid collateral damage to the surrounding anatomy.1
Due to the characteristics of the brain, stereotaxy and stereotactic frames are created for neurosurgery. The stereotactic technique relates to a Cartesian coordinate system and employs mathematical concepts to identify points in space that result from the intersection of 3 orthogonal planes: the anteroposterior, lateral, and vertical.2 The fusion of mathematical, anatomical, and neurological fundamentals enables neurosurgeons to identify and access stereotactic targets without any direct visualization.3 After the first apparatus for human stereotaxy was reported in 19474 stereotaxy rapidly became a subject of interest, and over 40 different stereotactic frames were designed and reported in the 1950s. Gabriel and Nashold listed several approaches which were divided into 5 categories: (i) translational systems; (ii) burr-hole mounted systems; (iii) arc-centered; (iv) interlocking arcs; and most recently; (v) frameless. Despite the conceptual differences between stereotactic frames, they all share the common goal of establishing a rigid relationship between the patient’s head/brain and the operation space where screws, drills, probes, and other devices are handled. The frame is often considered to be bulky and complex. The fixation screws employed for rigid skull fixation bring pain to the patient. The large size of the frame limits the space for examination and surgery. Therefore, neural stereotactic technology has been developing toward a frameless direction since then.2
The development of neurosurgical robots is a long-term process with the continuous progress of imaging technology and mechanical control technology. Many scholars have introduced the development history of neurosurgical robots in chronological order, so next, we will introduce the robots that have brought significant changes to neurosurgery in the order of technology development (Table 1).
Table 1.
Summary of the robots that have brought significant changes to neurosurgery
| Robot model | Year of application | Manufacturer | Application | DoF | Features |
|---|---|---|---|---|---|
| PUMA | 1985 | UNIMATION (US) | CT-guided biopsies | 6 | Earliest use in neurosurgery, suitable for various procedures |
| Neuromate | 1998 | Renishaw PLC (UK) | Stereotactic neurosurgery | 5 | Suitable for a wide range of neurosurgical procedures, frameless surgery |
| NeuroArm | 2008 | IMRIS & U of Calgary (Canada) | Microsurgery and stereotactic neurosurgery | 7 | Force sensing, haptic feedback, MRI compatibility |
| ROSA Brain/Spine | 2011 | Medtech S.A. (France) | Stereotactic neurosurgery | 6 | Precise execution of brain or spinal stereotactic surgery |
| ROSA One | 2014 | Medtech S.A. (France) | Spinal and brain Stereotactic neurosurgery | 6 | Fusion of ROSA Brain and Spine, capable of both brain and spinal surgery |
Programmable Universal Manipulator for Assembly
The neurosurgical robot initially originated from the industrial Programmable Universal Manipulator for Assembly (PUMA) robot. The prototype of PUMA was developed by UNIMATION, INC., which consists of six non-parallel joints and allows the robotic arm to move independently in three vertical directions. On April 11, 1985, Kwoh et al. completed their first clinical surgery using PUMA under CT guidance. Although PUMA could only perform a simple stereotactic biopsy then, its application was still groundbreaking in history.5
NeuroMate
In 1987, the NeuroMate surgical robot, developed by Integrated Surgical Systems (Sacramento, CA), was introduced. This robot featured five joints, also known as 5° of freedom (DOF), providing multi-axis motion capabilities. With its increased flexibility and maneuverability, NeuroMate facilitated coordinated movements, allowing surgeons to navigate challenging angles and perform various surgical procedures. The NeuroMate system was complemented by the Voxim control system (IVS Technology GmbH/Chemnitz, Germany), which integrated volumetric image data from CT or MRI scans. This enabled the system to assist surgeons and the robot in navigation and localization during procedures, supporting a range of neurosurgical stereotactic techniques, including biopsy, deep brain stimulation, and brain tumor resection. In 1999, NeuroMate achieved frameless neurosurgery, allowing preoperative registration by projecting CT and MRI images onto the patient’s anatomy. With precision comparable to traditional frame-based surgeries, NeuroMate became a significant milestone in frameless surgery and set a standard for subsequent advancements in the field.6
NeuroArm
In 2001, the NeuroArm surgical robot, developed by the University of Calgary, Canada, appeared, bringing significant innovation in robotic arms and control systems. The NeuroArm robotic arm is equipped with a three-dimensional force sensor in 7-DOF, which can measure the force applied to the tissue in real-time and transmit the data to the control lever of the remote console.7 Through the arrester in the control lever, the surgeon can simulate tactile feedback, greatly enhancing the safety and operability of the surgery. In addition, as the NeuroArm robot is made of special materials and has nuclear magnetic compatibility, it can complete surgical operations in a magnetic resonance environment; NeuroArm’s manipulation platform supports remote operation, so it can transmit MRI images obtained during surgery and 3D visual information obtained from other image data processing to surgeons through a monitor, achieving the visualization of the surgical operation process. These technologies allow doctors to operate the surgical robot outside the operating room, providing a technical reference for developing ultra-remote neurosurgical robot therapy.8
Modern robots
Over the past two decades, neurosurgical robots have undergone several iterations, and currently, one of the most widely used systems internationally is the ROSA One robot. It integrates and advances the capabilities of the ROSA Brain and ROSA spine robots, supporting both cranial and spinal surgical procedures. ROSA One inherits the inherent characteristics and advantages of its predecessors, offering high precision, repeatability, and stable motion. Its rapid response to force variations, remote operation capability, long-term position holding, and endurance enable it to handle a wide range of neurosurgical procedures.
China initially had limited involvement in the early development of neurosurgical robots, but in the past decade, it has made remarkable progress. China has taken a leading position in certain local technologies, such as 3D structured light technology. The application of neurosurgical robots in China has also increased significantly, with participation in tens of thousands of surgeries of various types. They are increasingly becoming the ideal assistants for surgeons in the field (Figure 1).
Figure 1.
Some milestones of neurosurg navigation robotsery robots
The development of neurosurgical robots
The improvements of neurosurgical robots, which follows the growing trends of general surgical robotics, is driven by improvements of medical imaging systems, automatic manipulator, lesion localization techniques, multimodal image fusion technology, registration method, and vascular imaging technology.
Medical imaging systems
Medical imaging systems, including Magnetic Resonance Imaging (MRI), Computed Tomography (CT), and Positron Emission Tomography (PET) and so on, provide detailed information about the brain. These imaging technologies have laid the foundation for the development of neurosurgical robots.
Magnetic resonance imaging
MRI provides information on the soft tissue structure of the brain. It has a high spatial resolution and no radiation damage to the human body, and the advantage of rich information makes it an important position in clinical diagnosis.
Functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) are special forms of magnetic resonance imaging (MRI). fMRI can be used to determine the spatial relationships between blood vessels and eloquent brain areas based on the structural MRI or CT image, and this can be applied in the neurosurgery field for counseling.9 fMRI is a time series of brain activity based on blood oxygen. As stimuli are introduced or the subjects perform paradigm operations, fMRI will record the entire brain’s blood oxygen data every few seconds. Due to the need for greater oxygenation by stimulated neurons, it increases the flow of brain blood to the activated areas of the brain, which is the physiological basis for the formation of fMRI signals. The advantage of fMRI lies in its non-invasive ability to record local brain activity and specific brain functions compared to electrical stimulation mapping.10 However, DTI is an MR imaging method based on the directional movement of water molecules along neural tissues. The diffusion of water along the fiber axis of the white matter bundle is less restricted than through the white matter bundle. Capturing this information will point in the direction of specific fibers, and post-processing of the image allows for studying specific fibers while isolating other fibers in the vicinity. In this way, capturing white matter bundles passing through the identified regions of interest is even possible.11 Besides, DTI tractography can provide important information to reduce the visual field defects by visualizing Meyer’s loop.12 This information is crucial to the epilepsy surgery team during pre-operative counseling.
X-ray and computed tomography
CT equipment evolved from X-ray technology. It providing clear images of bone tissue due to its high-density absorption rate, but show limited cartilage information due to the low permeability of X-rays in soft tissue. This advancement in CT technology has also facilitated the development of nuclear medicine imaging techniques.
SPECT (Single-Photon Emission Computed Tomography) and PET (Positron Emission Tomography) are two CT technologies in nuclear medicine. SPECT is a functional image that displays the metabolism of human tissues and organs and the blood flow of arteries and veins. It provides good and malignant information on tumors and is widely used in the diagnosis of various tumor diseases, but the resolution of SPECT is low, and the positioning ability is poor.13 PET image reveals the true blood flow information and can accurately identify the location of the patient’s lesion. Its principle is using positrons to generate photons colliding with the tissue’s electrons. The purpose of PET is to detect the number of photons, showing a color image of brain function information, suitable for tumor detection; its sensitivity is high, but it is difficult to obtain accurate brain structure position information; soft tissue and bone boundary resolution are lacking, so the spatial resolution is very low and the spatial distortion is highly probable. PET-CT is a complete imaging system that integrates PET and CT on a single instrument, allowing for comprehensive disease assessment by combining anatomical and functional metabolic images. PET/CT technology is also valuable in observing intracranial brain metabolism and localizing specific epileptic lesions. In neurosurgery assistant robot applications, thin slice CT scanning is often combined with MRI-3D images for preoperative positioning and postoperative result reconstruction.14
Since the first combination of imaging technology with stereotactic stents, great progress has been made in the field of neurosurgery. Many classic surgical procedures have been created, such as frame-based DBS surgery and deep electrode implantation surgery, which are still performed in some hospitals today. Nowadays, MRI, CT, and PET can all be imported into the programs and operation interfaces of neurosurgical robots to assist in surgical planning and execution.
Automatic manipulator (robotic arm) and sensor
The stereotactic frame has been used in neurosurgery for many years. The design paradigm for the first and many current neurosurgical robots are based on employing the stereotactic frames. But there are still many disadvantages: the stereotactic frame needs to be fixed on patient’s head; its weight brings a great burden to the head; some patients are unwilling to accept; infants are not suitable for use due to their incomplete skull development; the complex structure of the stereotactic frame blocks the line of sight and hinders the operation space; the installation time is long. In the surgical space, surgical robots did not emerge until the mid-1980s, when the confluence of higher precision and non-hydraulic automatic manipulator technology and favorable social factors permitted the development of surgical robots to occur.15
The robotic arm is a crucial component of the neurosurgical robotic system, serving not only as the structure that executes the instructions given by the doctor but also playing an essential role in collecting sensory information such as touch and vision and providing feedback to the doctor. Higher degrees of freedom in the robotic arm result in a greater precision of operational space and movement. Therefore, angle sensors are necessary for the active movement of the robotic arm, as they enable the control system to adjust and manipulate each joint based on their relative angles. To ensure safer task completion, some robotic arms are equipped with force sensors. Similar to how applying force on natural tissue generates specific resistance, the presence of force sensors allows the robotic arm to detect changes in resistance. When an increase in tactile resistance is detected, the robotic arm immediately performs actions such as emergency stops and obstacle avoidance; thereby preventing potential damage to neural and vascular tissues caused by improper planning or manual manipulation. In terms of vision, it is common for a camera to be installed at the front end of the robotic arm, allowing the surgeon to visualize information within the surgical field that would not be visible in traditional surgery and assess their own input.16,17
Regarding products, most robotic arms were already mature industrial robots before being used in medical surgical instruments. Companies that produce these industrial robots have an independent product system that provides robotic arm technology for various industries both domestically and internationally. Only after a series of secondary processing can it be considered a qualified robot product. Due to the high demand for accuracy in neurosurgery, only a few enterprises provide qualified neurosurgical robotic arm equipment in China. They mainly include manufacturers of robotic arms such as KUKA robotic arms, DENSO arms, and Universal Robot (Denmark), each with some different parameters. The specific details of these robotic arms are shown in the Table 2 later in discussion.18,19,20
Table 2.
Products and parameters of some robotic arm manufacturers
| Brand | KUKA | DENSO robotics | Universal robot |
|---|---|---|---|
| Model | LBR iiwa | VS-060 | 5 |
| Load weight | 7kg | 4kg | 5kg |
| Repeatability | ±0.1 mm | ±0.02 mm | ±0.1 mm |
| Free degree | 7 | 6 | 6 |
Lesion localization techniques
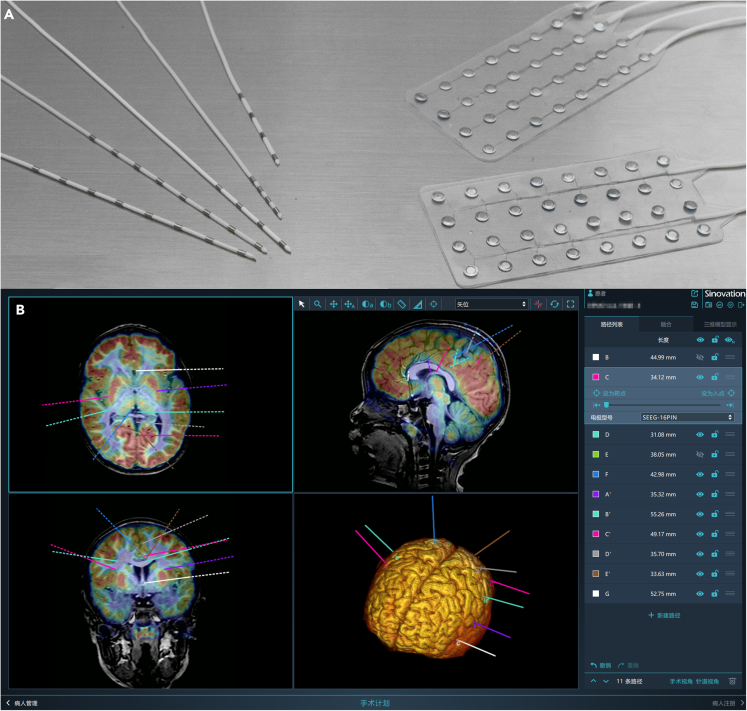
Surgical removal of epileptogenic brain tissue is indicated for treating many medically refractory focal seizure disorders. The degree of accuracy in identifying epileptogenic foci is the most important factor for getting good results. Doctors use electrical contacts to sense the intracranial electrical activity. Standard scalp contacts have been used for many years, but accurate localization is usually very difficult with recordings obtained from such contacts. Therefore, some epilepsy centers have adopted intracranial contacts to better define regions of cortical epileptogenicity since the 1980s. Intracranial sensing techniques have used two different kinds of members for engagement with brain tissue. Such tissue-engagement members include flexible flat surface members and depth probes.
Flexible flat surface members are multi contact electrodes that can directly record the electrical activity of the cerebral cortex and can be implanted into the brain for a short period of time. They are mainly used for cortical electroencephalography and can minimize the influence of structures such as the scalp, skull, and dura mater on the recording of brain electrical activity. But it cannot effectively monitor deep brain tissue, and Depth probes have effectively overcome this drawback. Depth probes, which are often referred to as "depth electrodes," penetrate deep into the brain tissue in direct contact with such tissue. Doctors can accurately find the location of epileptic foci with the help of intracranial electro-encephalography (IEEG) using depth electrodes. It is still the "gold standard" for determining the location of epileptic foci up to date (Figure 2).
Figure 2.
Display of Depth probes and Flexible flat surface members
(A) Depth probes (left), Flexible flat surface members (right); (B) Several depth electrodes and designed pathways showed in the model.
We compare the traditional Scalp contacts electrode with the Flexible flat surface members and Depth probes electrode in Table 3.21
Table 3.
Comparison of differences between scalp contacts, flexible flat surface members, and depth probes
| Electrode type | Scalp contacts | Flexible flat surface members | Depth probes |
|---|---|---|---|
| Position | extracranial | Intracranial | Intracranial |
| Accuracy | inaccurate | accurate | accurate |
| Electrical signal acquisition | indirect | direct | direct |
| Signal interference | Strong | medium Strong | Weak |
| installation time | Short | Long | Long |
| Applicable situation | EEG | ECoG | SEEG |
Multimodal image fusion technology
The multimodal image fusion means combining images from multiple imaging modalities to obtain a fused image with additional clinical applicability. Imaging techniques have provided clinicians with the information about the human body’s structural and functional characteristics. Different imaging methods reveal different characteristics of the same body part.
The purpose of the fusion is to obtain better contrast, fusion quality, and perceived experience. The result of the fusion should meet the following conditions: (i) the fused image should retain the information of source images completely; (ii) the fused image should not produce any synthetic information, such as artifacts; and (iii) bad states should be avoided, such as misregistration and noise.22 There are various combinations in medical image fusion, such as MRI and PET, MRI and CT, MRI and SPECT, CT and PET, CT and SPECT, SPECT and PET, and MRI-T1 and MRI-T2. Different ways of integration keep their own characteristics. For example, MRI/PET fusion is beneficial for detecting liver metastasis, Alzheimer’s disease, and brain tumor diagnosis; MRI/SPECT fusion is helpful for the localization of lesions and vertebral bone metastasis in patients with tinnitus; CT/PET fusion image energy improves the diagnosis of lung cancer; SPECT/PET for abdominal research; and ultrasound/MRI for vascular blood flow diagnosis.23
We will provide some examples to further illustrate the role of image fusion technology in doctors and robotic surgeries. The fusion operation of images is often related to the surgical requirements in clinical and is also an important preoperative step for certain surgeries. Common surgical planning software includes ELEKTA, Framelink, Brainlab, and so forth. They mainly rely on CT and MRI fusion to ensure accurate localization. The post-processing of epilepsy images often uses SPM (Statistical Parameter Mapping) software, which is a MATLAB-based software that integrates PET/CT and MRI, allowing doctors to see the patient’s epileptic focus and metabolic status visually. The specific instructions are as follows.
Target during deep brain stimulation surgery
Firstly, patients should undergo a preoperative MRI examination. In our center, it requires at least one 3DT1 sequence of 192 images and one T2 sequence of 60 images. On the day of surgery, install a stereotactic framework for the patient and move him to the CT room for a 0.625mm or 1mm thin layer scan. Next, import the MRI and CT images into the ELEKTA surgical planning system. The system can recognize the headrest indicator points on CT images and perform matching, calibration, and homing on T1 and T2 images respectively. After determining the position of the AC-PC line on the system, information such as the distance to the target point (such as STN, GPi), the input angle path, and the specific scale displayed on the headrest can be obtained, guiding surgeons or robots to begin surgery (Figure 3).
Figure 3.
MRI-CT intraoperative fusion process and effect presentation
(A) Brief flowchart of MRI-CT fusion during DBS surgery; (B) An example of MRI and CT image fusion from different perspectives. MRI is in red, and CT is in green.
Post-processing of epilepsy images
For patients with epilepsy to be operated on, PET/CT is a routine preoperative examination, and its image fusion with MRI is also one of the most commonly used fusions. Pure PET images are relatively blurry and can only reflect the metabolic status of some brain regions, with many limitations. When fused with the T1, T2, and FLAIR sequences of MRI, it can provide a more intuitive view of the adjacent relationship between metabolic reduction lesions and surrounding tissues and important functional areas, thereby helping doctors determine the type of surgery, develop surgical pathways and plans, and ultimately guide patients in their subsequent treatment. After the patient completes MRI and PET scans, we will import the structural image data and PET into the SPM software. SPM will automatically match and calibrate the PET and MRI’s images and coordinate positions. Since the T1, T2, and FLAIR sequences in MRI have the same coordinate positions, matching and calibration only need to be performed once. Doctors can use Mricron software to overlay and display the fusion results of PET and different sequences of MRI images in order to determine the patient’s future surgical plan (Figure 4).
Figure 4.
Epilepsy Image Post Processing fusion process and effect presentation
(A) Brief Flow Chart of Epilepsy Image Post-processing Fusion; (B) An example of MRI and PET image fusion from different perspectives. MRI is in green and blue, while PET is in white and black.
Registration method
Registration allows a surgeon to compare the real world with the model shown in the display device, and it is the core step of robotic neurosurgery. Up to now, three generations of registration methods have been used in this field, as shown in Figure 5. The first generation, registration with markers, since 2005; the second generation, laser point cloud registration, since 2009; and the third generation, 3D structured light registration, since 2019. We will show the details with examples in a subsequent post.
Figure 5.
Comparison of the three-generation registration methods
Vascular imaging technology
Cerebral hemorrhage is one of the major challenges of minimally invasive brain surgery. Obtaining an accurate model of blood vessels is crucial for designing safe surgical channels in neurosurgery.
At present, cerebral vascular imaging mainly relies on phase-contrast magnetic resonance angiography (PC-MRA) for implementation. PC-MRA is sensitive to blood flow velocity, so it can detect arteries and veins. Besides, the reconstructed structural images of PC-MRA correspond strictly to the vascular images, and no additional registration is required. Therefore, it is possible to visualize the cerebral cortex and blood vessels on the basis of one MRI scan, to shorten the scan time and help patients keep prohibited during the scan.24 The scanned PC-MRA image cannot be directly imported into the robot for use, and a series of data processing is required. Firstly, 'Skull striping' needs to be performed, and this step is to visualize the blood vessels in the cortex and eliminate blood vessels unrelated to surgery. Then, the image data is combined with a filter, and the threshold is adjusted to improve the signal-to-noise ratio to reduce noise in the vascular image.25 In this way, the program can accurately distinguish arterial and venous vessels according to different blood flow rates. And the data can be imported into the neurosurgical robots, and the doctor can present the reconstructed cerebral vessels on the console automatically or manually, and further verify them during the operation.
For example, Figure 6A shows a model of blood vessels and brain, Figure 6B shows the corresponding actual tissue. It can be seen that the imaging results of the blood vessels are consistent with the vascular orientation shown in the intraoperative photos.
Figure 6.
A comparison of a blood vessel model and the picture of actual tissue
(A) It shows a model of blood vessels and brain; (B) It shows the corresponding actual tissue. The probe is pointing at the blood vessel, and the green box area in Figure 6A corresponds with Figure 6B.
Control system
Despite technological differences, all robot systems follow a very similar structured workflow, where a control system orchestrates the planning of various components. Just as the human body needs the brain to coordinate its limbs, the control system of a robot is as important as the robot itself. A good control method can significantly affect the quality of user-device interaction and subsequent surgical results, whether direct or remote. Computer software is an essential component of robot control systems. The software provides a connection between medical imaging images, sensors, and databases with actual surgical procedures, allowing surgeons to accurately and predictably plan and execute surgeries using real-time and preoperative information from patients. The robot control system also includes computer control of motion. Through various programming of the control system, the robotic arm can move materials, parts, tools, or other specialized equipment to perform various tasks. The connections and rigid links of each joint on the robotic arm, as well as their motion modes, can be calculated mathematically and connected to the "end effector" through the last joint. Then, the control system can calculate the links' position to control each joint’s movement.
Different robots have varying requirements for their control systems. Robots such as ROSA, Sinovation, and Remebot produced by Chinese companies are categorized as stereotactic-navigation robots. The robotic arm component of these systems does not directly perform manipulations involving contact with tissues, such as cutting or hemostasis. This approach offers increased safety: the robot provides positioning information, surgical plans, and implant channels, while the high-risk surgical procedures are carried out by the surgeon. These robots are primarily used in minimally invasive treatments and minimally invasive-based extended procedures.
On the other hand, some robot control systems, such as the da Vinci Surgical System or NeuroArm, require intuitive and clear control consoles for the surgeon to operate. For example, due to inherent biological limitations, surgeons often experience slight hand tremors when holding or manipulating the robotic arm control interface, which can be fatal in neurosurgery. The control systems of such master-slave robots often incorporate tremor filtering capabilities, which remove low-frequency tremors from the surgeon’s hand, preventing the robotic arm from mimicking the same tremors. Additionally, these operating systems include motion scaling functionality, allowing surgeons to make gross movements with their hands while the robot scales down the corresponding movements proportionally. Currently, motion scaling typically ranges from 1/1 to 1/20.26
While there are pros and cons to each type of robot, both require surgeons to undergo a certain amount of learning to operate their control panels. Regardless of the age or level of surgical training of the practitioners, proper training and ongoing practice contribute to the safe and effective performance of surgeries by the surgeons.
Neurosurgical robot in China
Six neurosurgical robots from four companies (MEDTECH S.A., Sinovation, Remebot and Hozmedical), which have been approved in China, are shown in Figure 7.
Figure 7.
The neurosurgical robots have received NMPA approval in China
SR1 series and Q300 come from Sinovation; CAS comes from Hozmedical; RM comes from Remebot. Abbreviation: NMPA: National Medical Products Administration of China.
We summarized and compared the neurosurgical robots approved in China in Table 4 and then made a representative introduction. From a parameter perspective, the robotic arm of Hozmedical’s robots lacks 1° of flexibility, resulting in slight limitations on the operating range and content. Other manufacturers have no significant differences in robot models, only differences in registration methods and other auxiliary functions. Next, we will compare ROSA robots and introduce some features and novelty of Chinese neurosurgical robots.
Table 4.
Main parameters of neurosurgery robots approved in China
| Company | MEDTECH S.A. | Sinovation | Remebot | Hozmedical | |||
|---|---|---|---|---|---|---|---|
| Product name | ROSA one | SR1 | SR1-3D | RM-100 | RM-200 | CAS-R-2 | |
| Suitable crowd | adult and children | adult and children | adult and children | adult | adult and children | adult | |
| Mechanical arm | free degree(DoF) | 6 | 6 | 6 | 6 | 6 | 5 |
| Movement control mode | Automotive manipulator | Automotive manipulator | Automotive manipulator | Automotive manipulator | Automotive manipulator | Passive actuating arm | |
| System accuracy | 0.75mm | 0.5mm | 0.5mm | 0.5mm | 0.5mm | – | |
| Registration method | Fiducial markers | YES | YES | YES | YES | YES | YES |
| Laser surface matching | YES | YES | YES | NO | NO | NO | |
| Optical 3D scanner surface matching | NO | NO | YES | NO | NO | NO | |
Rosa, Zimmer Biomet
The ROSA systems include ROSA Brain, ROSA Spine and ROSA One. Thus far, the ROSA systems have been used in more than 1200 neurosurgical procedures and have received US FDA, EU CE, and Health Canada approval. The ROSA robot can be manually guided by surgical personnel and can also perform autonomously. The ROSA Spine was studied during visually guided pedicle screw insertions, and performance was equivalent to standard practices.
ROSA received the China National Medical Products Administration (NMPA, formerly the China Food and Drug Administration, or CFDA) approval in 2014, and ROSA one received NMPA approval in 2019. The ROSA mainly comprises an electromechanical part, a 6-DOF serial manipulator and a control software for neurosurgery planning, registration and guidance. It also has a laser automatic registration function without marker points. The robot arm of ROSA is installed on the mobile trolley connected to the head bracket. It has a wide range of operation, 6° of freedom and a force sensing device.27 The ROSA one equipped with an image guidance device has advanced navigation function and tactile function of stereotactic. Surgeons can supervise the robot to perform autonomously in a high-precision mode, and can also manually control and move surgical instruments during surgery.28
SR1 and SR1-3D, Sinovation
Sinovation is one of the suppliers of neurosurgical robots in China, so we take SR1 and SR1-3D as representative examples of domestic neurosurgical robots in China to introduce their structure and usage.29
SR1
SR1 consists of a robotic arm and a computer workstation. The robotic arm is used to correlate registered marker points in space to the patient’s image in the computer terminal. For registration, one option is to utilize a probe mounded at the end of the robotic arm to sample the marker points; another registration option is to be assisted with a laser rangefinder at the end of the robotic arm, which carries out point cloud registration.
The robotic arm has six motor-driven joints and each joint can move in certain range, which allows for the robotic arm to move to different positions in space. A force sensor is mounted at the end of the robotic arm senses 6-DOF external forces. The robotic arm can work in different modes, including full automatic motion mode and cooperation mode (Figure 8).
Figure 8.
A photo of SR1 during the operation
The workflow of the SR1 robotic system includes the following steps: (i) image data acquisition, from MRI scanning and/or CT scanning; (ii) surgical trajectory planning, the obtained medical images are used for multimodal fusion and 3D reconstruction, the 3D model can show blood vessels, skin, lesions, and so forth, and physicians can make surgical plans based on these images; (iii) intraoperative frameless registration, select the image marker points in the 3D model on software, and the robotic arm drives the probe to obtain at least 3 corresponding markers’ spatial coordinates, register and verify the registration results; or, use the laser rangefinder to get point cloud, extract surface point cloud from 3D model, register and verify registration results; (iv) intraoperative navigation, according to the surgical plan, the robotic arm moves to the pre-planned position, with the adapter installed at the end of the robotic arm, the surgeon drills holes on the skull following the pathway of the adapter, and then guides the surgical instrument to reach the lesion.
SR1-3D
Sinovation launched SR1-3D in 2020. Its main upgrade from SR1 is that it can use 3D structured light for registration.
The 3D structured light adopted by SR1-3D can scan the patient’s head in an extensive range, at multiple angles and in multiple poses to obtain a complete 3D point cloud of the head and face, making the registration technology without markers no longer limited by the patient’s position. This marker-free method no longer needs to acquire pre-op CT or manually pick up markers with a robotic arm during surgery. It can achieve fast and automatic scanning registration with accuracy verification, saving preoperative preparation time. In addition, the self-positioning accuracy of the 3D structured light is less than 0.1mm, which can achieve a registration positioning accuracy of less than 0.5mm. Compared with the first and second generations of registration technologies, the 3D-structured light significantly improves registration accuracy, dramatically shortens the registration time and improves surgical accuracy (Figure 9).
Figure 9.
Comparison of workflow between conventional stereotactic neurosurgery using frames and robot-assisted stereotactic neurosurgery
Registration method is the most important technical feature in distinguishing different neurosurgical robots. We introduce and compare the three-generation registration methods used by different neurosurgical robots as follows.
First generation, registration with markers, since 2005
All neurosurgical robots can support this registration method. The difference is that ROSA one, SR1, SR1-3D and CAS-R-2 use the probe mounted at the end of the robotic arm to pick the markers. The registration accuracy of this method depends on the positioning accuracy of the robotic arm itself. The repeated positioning error of the robotic arm used by SR1 does not exceed 0.02mm, ensuring a high level of registration accuracy. The markers can be skin markers or bone markers. The skin marker is non-invasive and economical, but it is easy to cause errors due to the deformation and movement of the scalp. The accuracy of bone marker registration is higher, but it will cause additional damage, and the patient’s 1) compliance is relatively poor. RM-100 and RM-200 use visible light cameras to identify the corner points connected to the end of the probe, and the positioning error is less than 0.2mm. This method does not require high precision for the repetitive positioning of the robotic arm itself. It is based on the optical tracking coordinate system, and has low costs. However, the ambient light in the operation room may affect the recognition of visible light cameras.
Second generation, laser point cloud registration, since 2009
Lasers were used to scan the target surface to obtain the point cloud for registration. Specifically, a robotic arm can be used to drive a laser locator or a hand-held laser transmitter to project multiple laser points to the location to be registered. Hundreds to thousands of laser points can be collected in more than 10 min, and the camera can take photos to form the point cloud information. Then, it can be registered with the medical image points cloud. This method does not need to install bone nails or paste markers of transdermal patches, omits the steps of installing markers and preoperative positioning images, saves operation time, and has high patient acceptance.30 Specifically, ROSA one and SR1 both adopt the scheme that the robotic arm drives the laser locator.
Third generation, 3D structured light registration, since 2019
3D structured light registration is the third-generation registration method. The laser point cloud registration has high resolution and measurement accuracy, but the measurement range is small, and the measurement time is long. However, 3D structured light can obtain millions of point cloud data in seconds, compared to the long measurement time and small range of laser registration. This method can effectively solve the problem of step change of target surface slope while ensuring high spatial resolution and has a large field of view.31
Liu et al. conducted clinical trials on 3D structured light registration technology using Sinovation’s SR1-3D at Beijing Tiantan Hospital. The results showed that the 3D structured light registration method had the advantages of short registration time, small positioning error and excellent safety performance.32
New technologies
In this section, we will briefly introduce several neurosurgical robots technologies and related auxiliary technologies that are still in the research stage, development or experimental testing.
Yang et al. developed a neurosurgery surgical electrode placement robot system, a surgical assistant robot guided by XMR (X-ray/MR) image, and has recently applied for a patent.33 The driving force of the robot can be hydraulic pressure and/or aerodynamic force, which will not affect the accuracy of MRI, and can further provide accurate positioning information for the robot through XMR real-time image guidance technology. This system enables the robot to break through technical bottlenecks such as multi-functional multi-material electrodes, magnetic resonance compatible mute drive, real-time magnetic resonance imaging, and robot system integration in the magnetic resonance environment, so that the neurosurgical robot can carry out autonomous precise positioning in order to achieve safer and more accurate robot-assisted DBS electrode placement.
Another example is the artificial intelligence vision technology developed by Tsinghua University in China, which relies on the existing robot assembly visual artificial recognition technology and structured light recognition technology. It projects horizontal and vertical stripes onto the patient’s head, and uses two stereo cameras to obtain the patient’s 3D point cloud information, matching and locating it with the patient’s previous MRI images. Then, the lesion, blood vessels, their anatomical positions, and adjacent relationships can be reconstructed in 3D and projected onto the patient’s head with considerable accuracy, assisting the doctor in preoperative preparation and preliminary positioning. At present, this technology has been attempted for epilepsy lesion resection surgery, greatly facilitating doctors' preoperative preparation and path selection. Due to its fast and convenient projection and reconstruction, it may also be applied in areas such as emergency intracerebral hemorrhage drilling and drainage in the future.34
With the emergence of magnetic resonance compatible devices and products, the application of magnetic resonance imaging technology has been further expanded. For example, in the past, due to the particular limitations of deep electrode implantation in the brain, such as in a magnetic field environment, the electrode would release heat and damage brain tissue. Therefore, many examinations, even after surgery, could not afford. The emergence of magnetic resonance compatible electrodes means that patients after DBS can complete morphological and functional magnetic resonance imaging scans while the machine turned on, providing many favorable conditions for patients to judge postoperative recovery effect, disease improvement degree, and prognosis.35
On the other hand, magnetic resonance compatible robots are also constantly developing. As important components of surgical robots, robotic arms and operating computers naturally rely on the participation of metal parts. However, this also leads to the inability of traditional surgical robots to work in magnetic resonance operating rooms. The development of MRI-compatible surgical robots focuses on solving this challenge. Researchers have replaced the original metal components with materials such as polypropylene, nylon, and paraformaldehyde. Some robots also use gas as their power system, allowing surgical robots to perform high-precision operations in high magnetic flux situations, further ensuring the safety and accuracy of surgery. Unfortunately, currently magnetic resonance compatible surgical robots have not been widely used and are only occasionally used in some surgeries requiring biopsy.36,37
Finally, it is the progress of MRI itself. For a long time, 3.0T magnetic flux magnetic resonance robots have met the needs of most neurosurgery for preoperative diagnosis and surgical target positioning. With the advent of 7.0T high magnetic flux MRI equipment, the blood vessel routing in important functional areas or the internal structure and blood supply arteries of intracranial tumors can be more accurately depicted. So the accuracy of preoperative positioning and imaging diagnostic ability will increase and further facilitate the development of neurosurgical robots.38
Clinical application
After a series of development and upgrading, the Chinese-made robots can be used in neurosurgery operations, such as intracerebral lesion biopsy, ventriculoperitoneal shunt, Ommaya capsule implantation, intracerebral SEEG electrode implantation, and DBS electrode implantation. They have been used in more than 200 hospitals in China, with more than 50000 operations, and have accumulated a considerable amount of usage data.
In this section, we collected surgery data from some hospitals in China, covering different age groups and disease types, summarized and analyzed the literature of two neurosurgical robots (Sinovation and Remebot) listed in China (Tables 5 and 6).
Table 5.
Comparison between robot and traditional framework in DBS, SEEG and biopsy
| Type of surgery | Sources | Surgical assistant instrument used | Target error (mm) | p-Value | Operation time (min) | p-Value |
|---|---|---|---|---|---|---|
| DBS | Mei et al.39 | Sinovation SR1 | 1.52 ± 0.53 (0.20–2.39) | 0.1301 | – | – |
| Frame | 1.77 ± 0.67 (0.59–2.98) | – | ||||
| SEEG | Zhang et al.40 | Sinovation SR1 | 2.27 (0.79,3.05) | 0.991 | 7.60 (6.9,8.35) | <0.001 |
| Frame | 2.02 (1.03,3.04) | 13.55 (11.50,16.00) | ||||
| Qiao et al.41 | Sinovation SR1 | 1.4 (0.4–9.3) | >0.05 | 8.0 (5.9–12.8) | <0.01 | |
| Frame | 1.4 (0.0–4.6) | 14.7 (10.3–18.5) | ||||
| Biopsy | Hu et al.42 | Sinovation SR1 | 1.10 ± 0.30 (0.69–2.03) | <0.05 | 29.36 ± 13.64min | <0.05 |
| Frame | 1.63 ± 0.41 (0.74–2.65) | 50.57 ± 41.08 min | ||||
| Wu et al.43 | Remebot | – | – | 80.1 ± 12.3 | <0.01 | |
| Frame | – | 116.5 ± 14.1 |
Table 6.
Comparison between robot and traditional framework in ventriculoperitoneal shunting and Ommaya reservoir implantation
| Neurosurgery | Sources | Surgical assistant instrument used | Operation time (min) | P-Value | Bone hole diameter | P-Value | Intraoperative blood loss (mL) | P-Value |
|---|---|---|---|---|---|---|---|---|
| Ventriculoperitoneal shunting | (Liu et al.44) | Remebot | 29.75 ± 6.38 | <0.001 | 4.0 ± 0.3 | – | 10.0 ± 3.98 | <0.001 |
| Frame | 48.63 ± 6.60 | 11.0 ± 0.2 | 22.25 ± 4.52 | |||||
| Ommaya reservoir implantation | (Liu et al.45) | Remebot | 41.17 ± 11.09 | <0.01 | 4.1 ± 0.5 | <0.05 | 11.1 ± 3.08 | <0.01 |
| Frame | 65 ± 14.32 | 11.3 ± 0.3 | 19.9 ± 3.98 |
Deep brain stimulation
Deep brain stimulation, also known as brain pacemaker implantation, is a neurosurgery technique that aims at treating Parkinson’s disease, primary tremor, dystonia and other functional diseases. They require accurate implantation of electrodes that contact deep brain nuclei and change the excitability of corresponding nuclei, improving Parkinson’s disease and dystonia symptoms. DBS can control seizures, improve and relieve pain, dementia, mental illness and other clinical symptoms, and is a minimally invasive reversible adjustable surgical technique.46
Mei et al. compared the surgery assisted by SR1 of Sinovation with the traditional frame surgery. They found that the accuracy and precision of wire implantation in the frameless robot-assisted DBS surgery with awake state is equivalent to that in the traditional frame-based technology, and the vector error and coordinate error of the target point implanted with the electrode assisted by the frameless robot have no statistical significance with the traditional frame implantation (p = 0.13).39 Compared with foreign robots, SR1 has reached the world’s advanced level in error value with its 1.52 ± 0.53mm vector error. Previously, it was reported that some foreign research centers regarded 3 mm as the standard for implanting wires, while most studies believed that the accuracy below 2 mm was the ideal choice for wire placement.47 At the same time, according to the report of De Benedictis et al., it is found that the wire placement accuracy (vector error) using ROSA in DBS is 1.60 mm.48 Varma et al. evaluated the accuracy of 49 patients using frameless Neuromate robot-assisted DBS surgery, and reported that the average accuracy was 1.7 mm.49
Although Mei et al. did not specify the specific intraoperative time of traditional frame surgery and frameless robot-assisted surgery, robot-assisted surgery does not need to set up a heavy frame or manually set up coordinates. Its accurate and convenient positioning greatly simplifies the surgical process, so the overall manual operation time is shorter than frame surgery. At the same time, patients who have experienced robot-assisted DBS surgery also said it would be more comfortable to perform surgery without a headframe. The research fully shows that the domestic neurosurgical robot is feasible on DBS, and its accuracy has reached the standard of traditional framework or even above, which is a future development direction.
Stereoelectroencephalography
SEEG is one of the most common surgical methods completed by neurosurgical robots, which is widely used in the focus diagnosis and surgical planning of various types of epilepsy. Multiple electrodes (usually 8–10) need to be implanted during SEEG. The implantation positions cover all parts of the brain, and the implantation accuracy requirement is high, so SEEG electrodes have become the most complicated surgical method for the comprehensive performance of neurosurgical robots. The clinical application of neurosurgical robots also perfectly solves the continuous positioning problem of multi-electrode paths required in the surgical process.50
Zhang et al. of Hebei Provincial People’s Hospital used SR1 of Sinovation to compare SEEG with traditional frame surgery.40 It was concluded that the SEEG neurosurgery assisted by SR1 robot has a high accuracy. The average error of the target point is 2.27 (0.79, 3.05) mm, while the traditional framework is 2.02 (1.03, 3.04) mm, p = 0.991, which indicates that it meets the same accuracy required by the traditional framework. In addition, the average operation time was also shortened from 13.55 (11.50, 16.00) min for traditional frame surgery to 7.60 (6.9, 8.35) min for robot-assisted surgery, with a statistically significant difference (p < 0.001), indicating that the domestic SR1 neurosurgical robot can effectively shorten the time for SEEG surgery.
In 2018, Qiao et al. from Xuanwu Hospital also conducted SEEG research comparing domestic surgical robots with traditional frame surgery.41 In the robot group, the target error is 1.4 (0.4–9.3) mm, while that in the frame group is 1.4 (0.0–4.6) mm. There is no statistical difference between the two groups (p > 0.05), indicating that SR1 of Sinovation has matched the traditional frame surgery in target accuracy. At the same time, the implantation time of a single electrode in the robot group was also shortened from 14.7 (10.3–18.5) min in the traditional frame group to 8.0 (5.9–12.8) min, with the statistical difference (p < 0.01), which also shows that SR1 robot can not only achieve high accuracy in SEEG, but also effectively shortens the surgical process. In terms of postoperative complications, there was no difference between the robot group and the traditional frame group, and no bleeding or infection occurred after surgery.
Brain tissue biopsy
Stereotactic tissue biopsy is one of the earliest and most widely used projects in brain surgery, and it is also an essential aspect of evaluating neurosurgical robots. Stereotactic biopsy can safely obtain tissues for intracranial lesions, especially in deep intracranial or important functional areas, to achieve a pathological diagnosis and provide a basis for the determination of patients' next treatment plan.51 Many doctors considered direct stereotactic brain biopsy to have high morbidity and mortality decades ago, which easily led to high sampling error and misdiagnosis. With the application of neurosurgical robots, the operation of stereotactic biopsy has been further simplified and the operation time has been greatly shortened, and its safety and effectiveness have also been further improved.52
In 2020, Wu et al.43 compared the clinical data of traditional frame-based and robot-assisted frameless patients in the age, gender, symptoms, neuroimaging characteristics, prognosis, postoperative complications, operation time and hospital stay. Among them, there is no significant difference between the frame-based group and the Remebot robot group regarding patient age, gender, symptoms and signs, symptom duration, focus location, focus size, postoperative complication rate and overall hospital stay. In terms of the overall operation time, the total duration of the operation in the frame-based group was longer than that in the Remebot robot group (116.5 ± 14.1 vs. 80.1 ± 12.3 min, p < 0.001); In terms of Diagnostic yield, the robot group was 32/35, while the frame group was 29/31, and there was no statistical difference (p = 0.75). In fact, previous studies have shown that the diagnostic rates of traditional frame-based and frameless biopsy are similar: for example, Woodworth et al. found no difference between the two methods for frame-based and frameless stereotactic brain biopsy.53 The joint diagnostic rate is 90%, and there is no difference in the incidence of complications. Yi et al. reported a retrospective analysis of 288 consecutive brain biopsies.51 No significant difference was found in the diagnostic rate. The diagnostic rate of frame-based biopsy was 96.9%, and that of frameless biopsy was 91.8%. Therefore, the robot-assisted frameless biopsy with Remebot is as safe and effective as the traditional frame-based stereotactic biopsy (no statistical difference). Moreover, when the Remebot robot system is used, the overall operation time of patients is shorter, which can reduce the discomfort of patients, speed up the operation process and improve the operation efficiency.
Meanwhile, Hu et al. compared 47 patients with traditional frame brain biopsy and 104 patients with Sinovation SR1 robot-assisted brain biopsy, and made statistical analysis on the efficacy, accuracy and complications of the biopsy.42 The results showed that there was no significant difference in the diagnostic rate between the frame-based group and the Sinovation SR1 robot-assisted group (95.74% vs. 98.08%, p > 0.05). The mean operation time of the Sinovation SR1 robot-assisted group was significantly shorter than that of the frame-based group (29.36 ± 13.64 vs. 50.57 ± 41.08 min, p < 0.01). The entry point error and target point error of the frame-based group were also significantly higher than those of the robot-assisted group (p < 0.001), and there was no significant difference in postoperative complications between these two groups. This also shows that Sinovation SR1 robot can greatly reduce the operation time and improve the efficiency while ensuring the accuracy of brain biopsy (even significantly higher than the traditional frame group), which is likely to be a powerful alternative to frame brain biopsy in the future.
Ventriculoperitoneal shunting
Cranial hypertension is a common symptom of the nervous system. Failure to deal with the increased intracranial pressure in time may lead to severe consequences such as brain hernia, which may endanger patients' lives. In the case of poor drug treatment, ventriculoperictonic shunting is one of the solutions commonly encountered in patients with brain edema. Liu et al. summarized the situation of 60 patients who received ventriculoperitoneal shunt in Beijing Tiantan Hospital (including 20 patients assisted by robots and 40 patients treated by traditional methods) and conducted a series of statistical analyses.44 The operation time of the robot-assisted surgery group (from the completion of disinfection and towel laying to the fixation of drainage tube) was 29.75 ± 6.38 min, while the average operation time of traditional ventriculoperitoneal shunt group was 48.63 ± 6.60 min. The intraoperative blood loss in the robot-assisted group was 10.0 ± 3.98 mL, and the one-time puncture success rate was 100%, while the intraoperative blood loss in the traditional operation group was 22.25 ± 4.52 mL, and the one-time puncture success rate was 77.5%. The diameter of the bone hole in the robot-assisted surgery group was 4.0 ± 0.3 mm, while the average diameter of the bone hole in the traditional surgery group was 11.0 ± 0.2 mm, which was statistically significant (p < 0.01). This shows that under the simplified operation of surgical procedures such as automatically locating the path and target after registration, the average operation time of the robot-assisted group is significantly shorter than that of the traditional operation group, and the intraoperative blood loss is effectively reduced. Although there is no significant difference in hospital stay between the two groups, the reduction of wound size and the success rate of nearly 100% in the robotic surgery group can better satisfy patients, which further demonstrates the feasibility and great prospect of the application of neurosurgical robot in ventricular shunt surgery.
Ommaya reservoir implantation
One of the main functions of the Ommaya capsule is to enable drugs to directly bypass the blood brain barrier for intraventricular administration, or puncture and drain cerebrospinal fluid to improve and alleviate the symptoms of increased intracranial pressure. In addition, it can repeatedly extract cerebrospinal fluid for examination and analysis to understand the efficacy and determine the drug concentration in the cerebral ventricle. In recent years, as neurosurgical robot-assisted stereotactic technology played an increasingly important role in multiple operations, its application has also been extended to Ommaya capsule implantation.
Liu et al. conducted statistical analysis on 100 patients receiving Ommaya reserve implantation (50 were implanted with a Remebot robot, and the rest 50 were implanted with traditional surgical methods) and compared the surgical related data between these two groups.45 On the premise that there is no significant difference in the age and sex of patients, the average operation time of the robot-assisted surgery group was 41.17 ± 11.09 min, while that of the conventional Ommaya reserve implantation group was 65 ± 14.32 min. The diameter of bone pore in the robot-assisted surgery group was 4.1 ± 0.5mm, while that in the conventional Ommaya reserve implantation group was 11.3 ± 0.3mm. The intraoperative blood loss was 11.1 ± 3.08mL in the robot-assisted surgery group and 19.9 ± 3.98mL in the conventional Ommaya reserve implantation group. The above three groups of data were statistically significant (p < 0.01, <0.05, <0.01, respectively). The average hospitalization time of the two groups was 3.9 ± 1.2 days and 4.1 ± 0.5 days, respectively, with no statistical significance (p > 0.05). This shows that the Remebot robot can save more operation time and effectively reduce intraoperative bleeding compared with the traditional implantation method in Ommaya reserve implantation, and can better reduce the size of bone pores and accelerate patients' recovery time with the help of mechanical stability structure.
Compact robots
Many of the neurosurgical robots previously available on the market have achieved significant success in clinical applications, but there is still room for improvement. For instance, the current mainstream neurosurgical robots occupy a considerable amount of surgical space, which inconveniences surgeons during operations and increases the difficulty of integration with other imaging devices. The purchase price and maintenance costs are relatively high, limiting their usage to predominantly high-level hospitals. The clinical application of neurosurgical robots faces challenges in being widely promoted in lower-tier hospitals, and many patients are unable to benefit from technological advancements due to economic constraints. There have been several emerging small-scale neurosurgical robots both domestically and internationally. These robots are predominantly focused on compact robotics, each with their own distinct characteristics and advantages, as well as varying capabilities (Table 7).
Table 7.
Compact robot comparison
| Robot model | Year of application | Manufacturer | Reg. approval | Features |
|---|---|---|---|---|
| Pathfinder | 2004 | Prosurgics Ltd (UK) | Discontinued | One of the pioneers compact frameless surgical robot used primarily in neurosurgery. |
| Renaissance | 2004 | Mazor Robotics (Israel) | CE, US FDA, Korean FDA, NMPA | Capable of performing spinal surgeries and can also be mounted on the skull for neurosurgery. |
| Stealth Autoguide | 2019 | Medtronic (US) | US FDA | Offers greater versatility and can be used in conjunction with various collaborative devices. |
| Q300 | 2022 | Sinovation (China) | NMPA | Semi-open front-end structure provides a larger operational space, allowing for deeper and more accurate punctures. |
FDA, Food and Drug Administration; NMPA, National Medical Products Administration of China.
Pathfinder
Pathfinder, developed by Prosurgics Ltd. in High Wycombe, United Kingdom, was a neurosurgical robot created in response to the increasing demand for miniaturized robots in the surgical environment. It featured a 6-degree-of-freedom robotic arm and was mounted on a mobile cart, allowing for smooth movement within the operating room. During surgeries, Pathfinder could be securely attached to a Mayfield head frame, positioned opposite to the surgical side or parallel to the patient, maintaining flexibility without interfering with or obstructing the workspace of the surgeon. Additionally, Pathfinder was a frameless surgical robot that utilized embedded vision systems to continuously track and register its position, using black titanium spheres (a kind of marker) attached to the patient’'s scalp or skull. However, the most frequently reported issues with the Pathfinder system were skin movement between preoperative and intraoperative scans and registration failures caused by marker identification errors due to abnormal lighting conditions. Unfortunately, due to funding limitations and certification issues, the project was discontinued in early 2009.54,55
Renaissance
The Renaissance robotic system, originally developed by Mazor Robotics Ltd. (acquired by Medtronic in 2018). It was initially utilized for spinal pedicle screw placement and later extended to include minimally invasive neurosurgical procedures.
It consists of the MARS robot and its controller, a custom robot base, targeting rails, registration fixtures, a 3D laser scanner, and a PC. This system falls under the category of image-guided navigation and is primarily used for tool guidance and assisting in skull perforations. Renaissance is also a frameless, markerless system. The MARS robot it is equipped with can be securely fixed to the patient’s skull using the custom base or mounted on a Mayfield head frame, allowing for preoperative registration matching using CT/MRI and point cloud laser scanning. Moreover, the surface registration error of the Renaissance system is close to 1mm, while the target registration error is approximately 1.7mm. With a cost below $100,000, Renaissance offers a cost-effective solution while maintaining high convenience and precision, as compared to other robots at the time that ranged in cost from $300,000 to $500,000, with larger sizes and higher expenses.56,57
Stealth Autoguide
The Stealth Autoguide system is an innovative neurosurgical positioning and navigation system produced by Medtronic. This system provide real-time visual spatial positioning and orientation for surgeons during neurosurgical procedures, enables surgeons to perform precise placement or guidance of standardized neurosurgical instruments. The technology behind Stealth Autoguide is primarily derived from iSYS Corporation’s compact surgical robot, Micromate. Additionally, during surgery, Stealth Autoguide can be seamlessly integrated with Medtronic’s existing surgical navigation platform, the Stealth Station S8, enabling more precise registration, route calibration, and visualization operations. The Stealth Autoguide system features a compact design, occupying minimal space in the operating room, and can be utilized for stereotactic biopsies, stereotactic electroencephalography, and other procedures.58,59
Q300
Q300 is a Chinese-made mini neurosurgical robot that has just been approved by NMPA in early 2022. Q300 is composed of a workstation, a bracket and an infrared camera (Polaris), positioning components (probe, reference frame, connector, reflector, and surgical instrument adapter), a laser probe, a foot switch, and an automatic positioning device. The infrared camera tracks the reflective spheres on patients, surgical tools and automatic positioning devices through infrared light positioning technology. It obtains the position relationships of each reflective sphere in space. Using the marker point registration technology, can the actual spatial coordination of the patient be registered to the patient’s preoperative image coordination. Thus, the preoperative image and surgical plan are unified with the actual spatial position of the patient. Then, the surgical plan, the patient’s spatial position, and the spatial position of the automatic positioning device are all unified under the patient coordinate system. Thus the spatial position of the patient and the automatic positioning device is acquired, and their relative positional relationship is determined. Then, the automatic positioning device will automatically reach the position and direction of the surgical plan and guide the operator to conduct further puncture and other operations. Furthermore, the Q300 features a unique semi-open holder structure at its front end, allowing for the easy retraction of surgical instruments during puncture procedures. This design not only provides a more open surgical space but also offers a larger operating area. Additionally, the shorter length of the holder enables the surgical instruments to reach deeper lesions for effective treatment (Figure 10).
Figure 10.
Illustration of Q300 in clinical application
It can be seen that the newly developed Q300 robot from China falls into the category of compact neurosurgical robots, similar to Pathfinder and Renaissance, as they all belong to the stereotactic-navigation robots. To streamline the process, they all support frameless surgical registration technology while maintaining a high level of accuracy. Represented by the Q300, compact robots have smaller mechanical components, allowing doctors to have more operating space. They offer a more affordable overall price, making them suitable for hospitals with limited budgets to upgrade their equipment, providing a new choice for various hospitals to perform procedures such as tissue biopsy, hematoma aspiration, and other common surgeries.
Discussion
In 1979, the Robot Institute of America, an industrial trade organization, defined a robot as a "reprogrammable multifunctional manipulator" intended to move materials, parts, tools, or specialized equipment through various programmed motions to perform a range of tasks. In the field of neurosurgery, different types of robots have different roles. The Chinese-produced robots, similar to ROSA, belong to the category of stereotactic-navigation robots, primarily responsible for spatial localization, surgical planning, providing pathways for electrode implantation, and less involved in specific surgical procedures. In this mode, doctors play a subjective and active role, taking the lead in the surgery. At the same time, neurosurgical robots serve as auxiliary devices that greatly expand the surgical capabilities of doctors. With their high precision, repeatability, and stable motion capabilities, robots can assist doctors in performing difficult or otherwise impossible surgical operations. For example, in blind punctures, doctors in traditional surgery face challenges in adjusting the puncture angle based on preoperative images, whereas with the assistance of robots, suitable puncture angles can be automatically designed based on imaging data, reducing the probability of risks. It can be said that surgeons and surgical robots complement each other.
Neurosurgical robots are also becoming increasingly autonomous, with some robots capable of performing autonomous operations in certain minimally invasive surgeries. The previously mentioned ROSA, neuromate, and Renaissance have also been designed with autonomous interaction capabilities. With the growing use of intelligent algorithms, they can assist robots in performing tasks, detecting sensitive structures and boundaries, and issuing warnings to surgeons in case of danger. They can also assist in adjusting parameters such as cutting speed and applied pressure, rather than solely relying on the input from surgeons. It is believed that in the future, as the scope of autonomous tasks for surgical robots expands and they become more deeply involved in surgeries, they will be able to alleviate the pressure on surgeons to a greater extent.60
Safety
Safety is an ongoing concern when it comes to the clinical application of robots. Despite established workflows for stereotactic surgery over the past decades, it remains challenging, especially due to the high demands for precision and minimal invasiveness. Inaccurate instrument positioning can result in trajectory deviations and localization errors, significantly increasing the risk of bleeding, infections, and other complications. Common causes of positioning errors may include: 1) mechanical errors in the frame or registration scans, 2) inadequate sampling of registration landmarks, 3) inherent errors in image fusion, and so on.
Therefore, preparation from the perspectives of the surgeon, patient, and robot is essential to minimize intraoperative and postoperative complications. For example, performing high-precision vascular reconstruction preoperatively and completing thorough preoperative planning and discussions are necessary. Surgeons and robotic operators must undergo appropriate training, carefully register and configure equipment parameters before the surgery, and improve their surgical proficiency. Additionally, during the surgical process, the opening of the dura mater can lead to brain displacement and alterations in crucial target information due to factors such as gravity, intracranial pressure changes, and tissue resection. In procedures like DBS and SEEG, skull perforation can cause target displacement due to vibrations. Therefore, performing secondary localization/registration to correct target information during surgery and sealing dural defects with fibrin glue immediately after cannula insertion to minimize cerebrospinal fluid loss are effective methods for reducing errors and complications.61
Cost
The issue of cost and expenses has always been a significant challenge in the development and application of neurosurgical robots. The high price of surgical robots is one of the key barriers to their widespread adoption. However, there are several favorable factors that could help mitigate this hindrance:
-
(1)
Hospital budgets: The funds allocated for purchasing surgical robots often need to be calculated separately from more essential operational budgets.
-
(2)
Cost-effectiveness: The use of robots can save operating room time, resulting in significant economic returns by reducing overall surgical expenses.
-
(3)
Increasing demand: With China’s large population and the growing aging population, the prevalence of neurosurgical-related diseases is increasing. The increased demand can help lower the per-use cost of robots.
-
(4)
Accessibility and training: As neurosurgical robots are introduced to lower-tier hospitals and remote medical facilities, and with the dissemination of remote healthcare and personnel training, an increasing number of grassroots neurosurgeons can learn how to use robots effectively. This enables more patients to receive treatment and expands the overall market.
These factors collectively contribute to addressing the cost and expense challenges associated with neurosurgical robots and pave the way for their wider application and development.
Development environment
A few years ago, neurosurgical robots were mainly used in Europe and Americas. For decades, Chinese doctors had no way to deal with some intracranial diseases, or could only use stereotactic technology based on external stereotactic frames. Because neurosurgery requires accuracy, the cost and price of robots are high, and there are relatively few hospitals in China that introduce and apply neurosurgical robots. With the research and development of domestic robots, several groups of new domestic robots, such as Sinovation, Remebot and Hozmedical, have shown creativity and progress in various surgical fields. Regarding surgical accuracy, operation modes, registration methods and degrees of automation, the gap with foreign advanced robots has been rapidly narrowed and even surpassed in some aspects. At the same time, with the localization production of major components, the cost and funding requirements of domestically independently developed robots are also shrinking, which makes it possible for hospitals in remote and underdeveloped areas to apply surgical robots to relieve patients' pain. The future development of neurosurgery still needs the comprehensive support of all parties (including communities of industry, academia, insurance, and so forth)
Prospection
We are looking forward to upgrading and improving the neurosurgical robot in the following aspects.
Artificial intelligence
Artificial intelligence has been widely used in various fields, and the combination of artificial intelligence and neurosurgical robot system will be the development trend. In the further development of neurosurgery, the application of artificial intelligence is an indispensable element. At present, some institutions have tried to use artificial intelligence technology to rapidly analyze a large number of clinical data generated in the modern medical environment, to achieve appropriate diagnoses, correct clinical decisions and predictions of the future situation of patients, which are currently impossible. Suppose the verified artificial intelligence technology is used in the operating room. In that case, it can independently distinguish important neural structures and boundaries, provide suggestions to surgeons, or autonomously adjust the speed when removing lesions and then adjust the best pressure when using instruments. These are helpful for the precision and safety in operations using the surgical robot system. Finally, the application of AI can also help analyze and select the best surgical approach and method, which can further relieve the pressure on the surgeon, better serve the patients, and accelerate the process of surgical preparation.
Remote operation
The current neurosurgical robot is not yet fully automatic, and doctors are often required to operate and intervene in the operation process, which reflects the importance of neurosurgeons’ skill and experience. Neurosurgeons with rich clinical experience and excellent skills are still scarce resources. The severe imbalance of medical resources restricts the improvement of medical level in economically underdeveloped areas. With the development of Internet technology and the construction of 5G and other equipment and supporting facilities, the current network latency is decreased tremendously; Neurosurgeons can remotely use robots to perform surgery for patients who are physically far away with the help of the high data transmission bandwidth. In addition, automated robots can complete a series of preoperative preparations by themselves. Remote surgeons only need to complete some important steps, and the surgical process can be further accelerated. In the future, remote surgery technology will continue to develop, and complete surgeries with higher operational accuracy and less delay.
Virtual training system
At present, young doctors need to improve their surgical skills in the operation room for a long time. Their learning curve is long, while the cost of surgical training is high, and patients will also bear some inconvenience. Providing fast, low-cost training to young doctors has excellent value. With the rapid development of virtual reality and other technologies, inexperienced doctors who have never used neurosurgical robots can quickly acquire knowledge and skills in robot-assisted neurosurgery through virtual training systems.
Limitations of study
We have made every effort to collect information about the robots, but there might still be some missing details. We appreciate your understanding.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2022YFC2405100).
Author contributions
FGM conceptualized the scope and structure of the article, figures, and tables. SYZ and CLH wrote the first draft of the article. YG prepared the illustrations. JGZ and FGM supervised the article preparation and secured the funding. YL critically revised the article. HGL made great contributions in revision. All authors performed the literature screening and editing.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Huanguang Liu, Email: hg_liu@163.com.
Jianguo Zhang, Email: jianguozhang@ccmu.edu.cn.
Yang Li, Email: liyang@buaa.edu.cn.
Fangang Meng, Email: mengfg@ccmu.edu.cn.
References
- 1.Zamorano L., Li Q., Jain S., Kaur G. Robotics in neurosurgery: state of the art and future technological challenges. Int. J. Med. Robot. 2004;1:7–22. doi: 10.1002/rcs.2. [DOI] [PubMed] [Google Scholar]
- 2.Gildenberg P. McGraw-Hill Companies; 1998. The History of Stereotactic and Functional Neurosurgery. [Google Scholar]
- 3.Rhodes M.L., Glenn W.V., Azzawi Y.M., Slater R. Stereotactic neurosurgery using 3-D image data from computed tomography. J. Med. Syst. 1982;6:105–119. doi: 10.1007/BF00994124. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel E.A., Wycis H.T., Marks M., Lee A.J. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 5.Kwoh Y.S., Hou J., Jonckheere E.A., Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans. Biomed. Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 6.Beasley R.A. Medical robots: current systems and research directions. Journal of Robotics. 2012;2012 401613.401601-401613.401614. [Google Scholar]
- 7.Guo Z., Leong M.C.W., Su H., Kwok K.W., Chan D.T.M., Poon W.S. Techniques for stereotactic neurosurgery: beyond the frame, toward the intraoperative magnetic resonance imaging-guided and robot-assisted approaches. World Neurosurg. 2018;116:77–87. doi: 10.1016/j.wneu.2018.04.155. [DOI] [PubMed] [Google Scholar]
- 8.Mattei T.A., Rodriguez A.H., Sambhara D., Mendel E. Current state-of-the-art and future perspectives of robotic technology in neurosurgery. Neurosurg. Rev. 2014;37:357–366. doi: 10.1007/s10143-014-0540-z. discussion 366. [DOI] [PubMed] [Google Scholar]
- 9.Dimou S., Battisti R.A., Hermens D.F., Lagopoulos J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg. Rev. 2013;36:205–214. doi: 10.1007/s10143-012-0436-8. discussion 214. [DOI] [PubMed] [Google Scholar]
- 10.Fortugno A.P., Bakke J.R., Babajani-Feremi A., Newman J., Patel T.S. Functional Magnetic Resonance Imaging and Applications in Dermatology. JID Innov. 2021;1 doi: 10.1016/j.xjidi.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bari A.A., Thum J., Babayan D., Lozano A.M. Current and Expected Advances in Deep Brain Stimulation for Movement Disorders. Prog. Neurol. Surg. 2018;33:222–229. doi: 10.1159/000481106. [DOI] [PubMed] [Google Scholar]
- 12.Lilja Y., Nilsson D.T. Strengths and limitations of tractography methods to identify the optic radiation for epilepsy surgery. Quant. Imaging Med. Surg. 2015;5:288–299. doi: 10.3978/j.issn.2223-4292.2015.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhász C., John F. Utility of MRI, PET, and ictal SPECT in presurgical evaluation of non-lesional pediatric epilepsy. Seizure. 2020;77:15–28. doi: 10.1016/j.seizure.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sałyga A., Guzikowska-Ruszkowska I., Czepczyński R., Ruchała M. PET/MR - a rapidly growing technique of imaging in oncology and neurology. Nucl. Med. Rev. Cent. East. Eur. 2016;19:37–41. doi: 10.5603/NMR.2016.0007. [DOI] [PubMed] [Google Scholar]
- 15.Edwards M. Robots in industry: an overview. Appl. Ergon. 1984;15:45–53. doi: 10.1016/s0003-6870(84)90121-2. [DOI] [PubMed] [Google Scholar]
- 16.Bogue R. Robots in healthcare. Ind. Robot. 2011;38:218–223. [Google Scholar]
- 17.Ueda H., Suzuki R., Nakazawa A., Kurose Y., Marinho M.M., Shono N., Nakatomi H., Saito N., Watanabe E., Morita A. Paper presented at: CIRP Conference on BioManufacturing. 2017. Toward autonomous collision avoidance for robotic neurosurgery in deep and narrow spaces in the brain. [Google Scholar]
- 18.Almusawi A.R.J., Dülger L.C., Kapucu S. A New Artificial Neural Network Approach in Solving Inverse Kinematics of Robotic Arm (Denso VP6242) Comput. Intell. Neurosci. 2016;2016 doi: 10.1155/2016/5720163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enebuse I., Ibrahim B.K.S.M.K., Foo M., Matharu R.S., Ahmed H. Accuracy evaluation of hand-eye calibration techniques for vision-guided robots. PLoS One. 2022;17 doi: 10.1371/journal.pone.0273261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminski J. KUKA robot arms. 2023. https://www.mroelectric.com/blog/kuka-robot-arm
- 21.Fahimi Hnazaee M., Wittevrongel B., Khachatryan E., Libert A., Carrette E., Dauwe I., Meurs A., Boon P., Van Roost D., Van Hulle M.M. Localization of deep brain activity with scalp and subdural EEG. Neuroimage. 2020;223 doi: 10.1016/j.neuroimage.2020.117344. [DOI] [PubMed] [Google Scholar]
- 22.Meher B., Agrawal S., Panda R., Abraham A. A survey on region based image fusion methods. Inf. Fusion. 2019;48:119–132. [Google Scholar]
- 23.Huang B., Yang F., Yin M., Mo X., Zhong C. A Review of Multimodal Medical Image Fusion Techniques. Comput. Math. Methods Med. 2020;2020 doi: 10.1155/2020/8279342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du X., Ding H., Zhou W., Zhang G., Wang G. Cerebrovascular segmentation and planning of depth electrode insertion for epilepsy surgery. Int. J. Comput. Assist. Radiol. Surg. 2013;8:905–916. doi: 10.1007/s11548-013-0843-5. [DOI] [PubMed] [Google Scholar]
- 25.Frangi A.F., Niessen W.J., Vincken K.L., Viergever M.A. Lecture Notes in Computer Science; 2000. Multiscale Vessel Enhancement Filtering; p. 1496. [Google Scholar]
- 26.Edström E., Burström G., Persson O., Charalampidis A., Nachabe R., Gerdhem P., Elmi-Terander A. Does Augmented Reality Navigation Increase Pedicle Screw Density Compared to Free-Hand Technique in Deformity Surgery? Single Surgeon Case Series of 44 Patients. Spine. 2020;45 doi: 10.1097/BRS.0000000000003518. E1085–e1090. [DOI] [PubMed] [Google Scholar]
- 27.Chenin L., Peltier J., Lefranc M. Minimally invasive transforaminal lumbar interbody fusion with the ROSA(TM) Spine robot and intraoperative flat-panel CT guidance. Acta Neurochir. 2016;158:1125–1128. doi: 10.1007/s00701-016-2799-z. [DOI] [PubMed] [Google Scholar]
- 28.Elsabeh R., Singh S., Shasho J., Saltzman Y., Abrahams J.M. Cranial neurosurgical robotics. Br. J. Neurosurg. 2021;35:532–540. doi: 10.1080/02688697.2021.1950622. [DOI] [PubMed] [Google Scholar]
- 29.Li J.Y., Ren Z.W., Guo S., Yu K.J., hu Y.S., LI Y.J. Research on the positioning accuracy of deep brain electrode implantation assisted by the Tirobot Crane stereotactic surgical robot. Journal of Clinical Neurosurgery. 2021;18:485–488. [Google Scholar]
- 30.Jia P.F., Wu J.S. Application of the contour laser registration method in the neuronavigation. Chinese Journal of Minimally Invasive Neurosurgery. 2005;11:501–502. [Google Scholar]
- 31.Wu Z.N. Research and Application of Binocular Vision 3D Measurement Technology Based on Structured Light. University of Electronic Science and Technology of China; 2021. [DOI] [Google Scholar]
- 32.Liu H.G., Fan S.Y., Liu Y.H., Zhang H., Hu W.H., Wu D.L., Liu D.F., Zhang K., Zhang J.G., Yang A.C. Preliminary application of 3D-intelligent structured light registration in robot-assisted neurosurgical operations. Chin J Neurosurg. 2021;37:880–884. [Google Scholar]
- 33.Gao A.Z., Liu J.L., Huang S.P., Yang G.Z. XMR Image Guided Deep Brain Electrode Implantation Robot (China National Intellectual Property Administration). https://www.cnipa.gov.cn/col/col1510/
- 34.Hospital T.U.Y. Research on intelligent visual technology to assist in epilepsy surgery. 2023. http://www.yuquanhosp.com/Html/News/Articles/462.html
- 35.Shen L., Jiang C., Hubbard C.S., Ren J., He C., Wang D., Dahmani L., Guo Y., Liu Y., Xu S., et al. Subthalamic Nucleus Deep Brain Stimulation Modulates 2 Distinct Neurocircuits. Ann. Neurol. 2020;88:1178–1193. doi: 10.1002/ana.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Z.W., Jiang S., Qi H.J., Yan B. Machinability of structural materials of MR-compatible robots. Acta Armamentarii. 2013;34:1007–1012. [Google Scholar]
- 37.Yang B., Roys S., Tan U.X., Philip M., Richard H., Gullapalli R., Desai J.P. Design, Development, and Evaluation of a Master-Slave Surgical System for Breast Biopsy under Continuous MRI. Int. J. Rob. Res. 2014;33:616–630. doi: 10.1177/0278364913500365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng K., Duan Q., Hu J., Li C., Ma X., Bian X., Duan C., Xiong Y., Lin J., Lu H., et al. Evaluation of postcontrast images of intracranial tumors at 7T and 3T MRI: An intra-individual comparison study. CNS Neurosci. Ther. 2023;29:559–565. doi: 10.1111/cns.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei S., Yu K., Ren Z., Hu Y., Guo S., Li Y., Li J. Techniques of Frameless Robot-Assisted Deep Brain Stimulation and Accuracy Compared with the Frame-Based Technique. Brain Sci. 2022;12 doi: 10.3390/brainsci12070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D., Cui X., Zheng J., Zhang S., Wang M., Lu W., Sang L., Li W. Neurosurgical robot-assistant stereoelectroencephalography system: Operability and accuracy. Brain Behav. 2021;11:e2347. doi: 10.1002/brb3.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao L., Yu T., Ni D.Y., Wang X.Y., Xu C.P., Zhang G.J., LI Y.J. Application of robot-assisted stereoencephalography electrode implantation in epilepsy surgery. Chin. J. Neurosurg. 2019;10:1049–1053. [Google Scholar]
- 42.Hu Y., Cai P., Zhang H., Adilijiang A., Peng J., Li Y., Che S., Lan F., Liu C. A Comparation Between Frame-Based and Robot-Assisted in Stereotactic Biopsy. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.928070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S., Wang J., Gao P., Liu W., Hu F., Jiang W., Lei T., Shu K. A comparison of the efficacy, safety, and duration of frame-based and Remebot robot-assisted frameless stereotactic biopsy. Br. J. Neurosurg. 2021;35:319–323. doi: 10.1080/02688697.2020.1812519. [DOI] [PubMed] [Google Scholar]
- 44.Liu D.F., Liu H.G., Zhang K., Meng F.G., Yang A.C., Zhang J.G. The Clinical Application of Robot-Assisted Ventriculoperitoneal Shunting in the Treatment of Hydrocephalus. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.685142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H.G., Liu D.F., Zhang K., Meng F.G., Yang A.C., Zhang J.G. Clinical Application of a Neurosurgical Robot in Intracranial Ommaya Reservoir Implantation. Front. Neurorobot. 2021;15 doi: 10.3389/fnbot.2021.638633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., Matthews K., McIntyre C.C., Schlaepfer T.E., Schulder M., et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClelland S., 3rd, Ford B., Senatus P.B., Winfield L.M., Du Y.E., Pullman S.L., Yu Q., Frucht S.J., McKhann G.M., Goodman R.R., Goodman R.R. Subthalamic stimulation for Parkinson disease: determination of electrode location necessary for clinical efficacy. Neurosurg. Focus. 2005;19:E12. [PubMed] [Google Scholar]
- 48.von Langsdorff D., Paquis P., Fontaine D. In vivo measurement of the frame-based application accuracy of the Neuromate neurosurgical robot. J. Neurosurg. 2015;122:191–194. doi: 10.3171/2014.9.JNS14256. [DOI] [PubMed] [Google Scholar]
- 49.Varma T.R.K., Eldridge P.R., Forster A., Fox S., Fletcher N., Steiger M., Littlechild P., Byrne P., Sinnott A., Tyler K., Flintham S. Use of the NeuroMate stereotactic robot in a frameless mode for movement disorder surgery. Stereotact. Funct. Neurosurg. 2003;80:132–135. doi: 10.1159/000075173. [DOI] [PubMed] [Google Scholar]
- 50.Taha J.M., Favre J., Baumann T.K., Burchiel K.J. Tremor control after pallidotomy in patients with Parkinson's disease: correlation with microrecording findings. J. Neurosurg. 1997;2:e2. doi: 10.3171/foc.1997.2.3.5. [DOI] [PubMed] [Google Scholar]
- 51.Lu Y., Yeung C., Radmanesh A., Wiemann R., Black P.M., Golby A.J. Comparative effectiveness of frame-based, frameless, and intraoperative magnetic resonance imaging-guided brain biopsy techniques. World Neurosurg. 2015;83:261–268. doi: 10.1016/j.wneu.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall W.A. The safety and efficacy of stereotactic biopsy for intracranial lesions. Cancer. 1998;82:1749–1755. doi: 10.1002/(sici)1097-0142(19980501)82:9<1756::aid-cncr23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Woodworth G.F., McGirt M.J., Samdani A., Garonzik I., Olivi A., Weingart J.D. Frameless image-guided stereotactic brain biopsy procedure: diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J. Neurosurg. 2006;104:233–237. doi: 10.3171/jns.2006.104.2.233. [DOI] [PubMed] [Google Scholar]
- 54.Eljamel M.S. Validation of the PathFinder neurosurgical robot using a phantom. Int. J. Med. Robot. 2007;3:372–377. doi: 10.1002/rcs.153. [DOI] [PubMed] [Google Scholar]
- 55.Faria C., Erlhagen W., Rito M., De Momi E., Ferrigno G., Bicho E. Review of Robotic Technology for Stereotactic Neurosurgery. IEEE Rev. Biomed. Eng. 2015;8:125–137. doi: 10.1109/RBME.2015.2428305. [DOI] [PubMed] [Google Scholar]
- 56.Devito D.P., Kaplan L., Dietl R., Pfeiffer M., Horne D., Silberstein B., Hardenbrook M., Kiriyanthan G., Barzilay Y., Bruskin A., et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine. 2010;35:2109–2115. doi: 10.1097/BRS.0b013e3181d323ab. [DOI] [PubMed] [Google Scholar]
- 57.Shamir R., Freiman M., Joskowicz L., Shoham M., Zehavi E., Shoshan Y. Robot-assisted image-guided targeting for minimally invasive neurosurgery: planning, registration, and in-vitro experiment. Med. Image Comput. Comput. Assist. Interv. 2005;8:131–138. doi: 10.1007/11566489_17. [DOI] [PubMed] [Google Scholar]
- 58.Brandman D., Hong M., Clarke D.B. Preclinical Evaluation of the Stealth Autoguide Robotic Guidance Device for Stereotactic Cranial Surgery: A Human Cadaveric Study. Stereotact. Funct. Neurosurg. 2021;99:343–350. doi: 10.1159/000512508. [DOI] [PubMed] [Google Scholar]
- 59.Medtronic STEALTH AUTOGUIDETM cranial robotic guidance platform. 2019. https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/cranial-robotics/stealth-autoguide.html
- 60.Wang P., Becker A.A., Jones I.A., Glover A.T., Benford S.D., Greenhalgh C.M., Vloeberghs M. A virtual reality surgery simulation of cutting and retraction in neurosurgery with force-feedback. Comput. Methods Programs Biomed. 2006;84:11–18. doi: 10.1016/j.cmpb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Beerthuizen P.G., Kruidhof W. System and software safety analysis for the ERA control computer. Reliab. Eng. Syst. Saf. 2001;71:285–297. [Google Scholar]