Abstract
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder with a worldwide prevalence of 6–10 % of women of reproductive age. PCOS is a risk factor for cardiometabolic disorders such as type 2 diabetes, myocardial infarction, and stroke in addition to exhibiting signs of hyperandrogenism and anovulation. However, there is no known cure for PCOS, and medications have only ever been used symptomatically, with a variety of adverse effects. Drugs made from natural plant products may help treat PCOS because several plant extracts have been widely recognized to lessen the symptoms of PCOS. In light of this, 72 current studies on natural products with the potential to control PCOS were examined. By controlling the PI3K/AKT signaling pathway and decreasing NF-κB and cytokines such as tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6), certain plant-derived chemicals might reduce inflammation. Other substances altered the HPO axis, which normalized hormones. Additionally, other plant components increased glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) levels to reduce radiation-induced oxidative stress. The other substances prevented autophagy by impairing beclin 1, autophagy-related 5 (ATG5), and microtubule-associated protein 1A/1B-light chain 3 - II (LC3- II). The main focus of this comprehensive review is the possibility of plant extracts as natural bio-resources of PCOS treatment by regulating inflammation, hormones, reactive oxygen species (ROS), or autophagy.
Keywords: Polycystic ovary syndrome, Natural products, Traditional Chinese medicine, Traditional Korean medicine, PI3K, NF-κB
1. Introduction
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder that has a convoluted mechanism on the reproductive system arising from the interaction of genetic and environmental reasons [1]. World widely, approximately 4–20 % of women of reproductive age are affected by PCOS [2]. The classical clinical presentation of PCOS includes features of hyperandrogenism, chronic anovulation/oligomenorrhea, and ultrasonographic morphology of the ovary [3]. Furthermore, PCOS has been considered to be a risk factor for cardiometabolic diseases such as type 2 diabetes, myocardial infarction, and stroke, and PCOS may increase the risk of ovarian cancer in younger women [4,5]. Several studies have shown that women with PCOS have intrinsic insulin resistance regardless of obesity, which is the risk factor for type 2 diabetes [6]. Asian women, especially Korean women who don't manifest PCOS features such as hirsutism and obesity also showed higher type 2 diabetes prevalence compared to non-PCOS women [7]. The treatment of PCOS is performed as symptomatic treatment according to the desired clinical effects by using clomifene and metformin for fertility and oral contraceptives for restoring menstruation and reducing the risk of endometrial hyperplasia [8]. However, clomifene and metformin have limitations like poor efficacy, relatively high multiple-pregnancy rate (3%–8%), and side effects such as mood changes, hot flushes, and gastrointestinal symptoms [9,10]. Oral contraceptive use has been associated with an excess risk of benign liver tumors and a modest risk of liver cancer and cervical cancer. Also, oral contraceptives used before the first full-term pregnancy or using them longer than 5 years can induce the development of breast cancer [11].
Many studies have suggested natural products including Paeoniflorin, Catechins, Licorice, and Inositol as an effective alternative treatment for PCOS [[12], [13], [14], [15], [16], [17], [18], [19]]. Traditionally PCOS was treated by natural products such as Angelica gigas, Cnidium officinale, Rehmannia glutinosa, Leonurus japonicus, etc. for female fertility and menstruation [[20], [21], [22]]. Additionally, many studies have suggested various natural products as effective alternative treatments for PCOS patients. Recently, several systematic reviews on herbs for PCOS were published to organize effective treatment [[23], [24], [25]].
PCOS is assumed to be related to insulin resistance and has defects in insulin receptor signaling pathways such as PI3K-PKB/Akt and ERK/MAPK, which regulate gene expression [26]. Our study shed light on how natural products regulate these pathways. Potent anti-PCOS natural compounds were classified and reviewed by experimental methods. We classified experiment method types into human studies, in vitro, and in vivo, and in particular, in vivo experiments were subdivided according to the experimental mouse species. In this study, we aimed to review comprehensively the effects and biomechanisms of natural products that can be used in PCOS treatment, and the topics in this review have not yet been covered extensively in the literature.
2. Search strategy and study selection criteria
Research papers regarding polycystic ovary syndrome and natural products were collected from 2006 to 2021 using the PubMed and PubMed Central databases (www.ncbi.gov/pubmed), the Scopus databases (https://www.scopus.com), and the Web of Science databases (http://www.webofknowledge.com). “Polycystic ovary syndrome”, “natural products”, “traditional Chinese medicine” and “traditional Korean medicine” were combined to search for relevant articles.
The inclusion criteria were as follows: (1) articles that reported the efficacy of natural products of polycystic ovary syndrome, (2) research where in vitro, in vivo, or human studies were performed, (3) articles written in English, (4) articles published between 2006 and 2021. The exclusion criteria were (1) review articles, (2) case reports, and (3) articles written in languages other than English.
3. The promising evidence of natural products in modern drug discovery and their role in PCOS treatment
The results in the tables are categorized using experimental techniques such as in vitro, in vivo, and human studies.
3.1. In vitro studies reveal the potential of natural products in PCOS treatment
Two studies about the effects of natural products against PCOS using in vitro experiments have been reported (Table 1).
Table 1.
In vitro studies of natural products in PCOS treatment.
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Terpene | Paeoniflorin extract | Paeonia lactiflora Pall | Primary theca cells isolated from ovaries of non-pregnant female mice | 1, 10,100, 1000 μg/mL; 24 h | Reversion of dexamethasone-induced testosterone over-secretion | ↓CYP17A1, CYP11A1 | [15] |
| Decoction | Gui Zhu Yi Kun formula (Tu Si-Zi, Bai zhu, Dang Gui, Sha Shen, Che Qian-Zi, Qian Cao, Si Gua-Luo) | Semen cuscutae, Rhizoma atractylodis Macrocephalae, Angelica sinensis, Adenophora tetraphylla, Plantago asiatica, Rubia cordifolia, Luffa cyclindrica | Granulosa cells of PCOS rats | 5, 10 20 g/kg/day; 4 days | Reduction of PCOS risk | ↑ mTOR, AMPKα, p-mTOR ↓Beclin-1, LC3, p53, sestrin2 |
[27] |
CYP17A1, cytochrome p450 family 17 subfamily a member 1; CYP11A1, cytochrome p450 family 11 subfamily a member 1; mTOR, mechanistic target of rapamycin; AMPKα, AMP-activated protein kinase; p-mTOR, phospho-mechanistic target of rapamycin; LC3, light chain 3; ↑, up-regulation; ↓, down-regulation.
Ong et al. reported that Paeoniflorin extract could be used to treat ovarian hyperandrogenism related to polycystic ovary syndrome [15]. In the study, dexamethasone enhanced testosterone secretion, while Paeoniflorin extract reversed this over-production in a dose-dependent manner, in addition to normalizing progesterone production in dexamethasone-treated cells. All doses of paeoniflorin extract inhibited CYP17A1, and only 100 μg/mL of paeoniflorin extract downregulated CYP11A1, which are enzymes of the ovarian steroidogenic pathway.
Xing et al. discovered that the effects of the Gui Zhu Yi Kun formula in mediating the p53/AMPK pathway inhibited GC (granulosa cells) autophagy, which suggested a possible novel mechanism underlying the treatment of PCOS with the Gui Zhu Yi Kun formula (GZYKF) [27]. GZYKF significantly decreased the protein expression levels of Beclin-1, but only high dose GZYKF inhibited the mRNA expression of LC3. Furthermore, increasing the dose of GZYKF had a positive association with mTOR, p-mTOR, AMPKα, and sestrin activation, whereas increasing inhibition of p53 was observed when the GZYKF dosage was increased.
Paeoniflorin extract (PFE) and Gui Zhu Yi Kun formula, both from China, were used on primary theca cells and granulosa cells (GC) separately. The limitation of the paeoniflorin extract study was that the effect of the dexamethasone model with and without PFE was not observed in a co-culture of theca cells and granulosa cells. Additionally, this study only focused on the CYP11A1 and CYP17A1 protein expression and did not include the effects of PFE on T and P in theca cells under basal conditions [15]. On the other hand, the study using GZYKF was confirmed by mechanisms such as mTOR, AMPKα, p-mTOR, Beclin-1, LC3, p53, and sestrin2 on granulosa cells. Furthermore, GZYKF mediated the p53/AMPK signaling pathway and thereby inhibited GC autophagy, which might be the underlying mechanism of the therapeutic effects of GZYKF on PCOS [27]. However, the limitation of the study was that it was observed only in granulosa cells.
3.2. In vivo or preclinical trial studies provides further evidence of the benefits of natural products in the treatment of PCOS
In vivo or preclinical trial is an experiment that is done on living organisms such as laboratory animals like mice, rabbits, or rats.
3.2.1. In vivo mice studies
Until now, several studies have been performed to test the effectiveness of PCOS of natural products using female naval medical research institute (NMRI) mice or C57BL/6 mice. PCOS was induced by treatment of dihydrotestosterone (DHT), human chorionic gonadotrophin (hCG) and insulin combination, estradiol valerate, dimethyl sulfoxide, dehydroepiandrosterone (DHEA) or letrozole [16,17,[28], [29], [30], [31], [32], [33]].
3.2.1.1. C57BL/6 mice
Five studies were conducted in vivo experiments using C57BL/6 mice (Table 2).
Table 2.
In vivo studies in C57BL/6 mice.
| Classification | Compound/Extract | Source | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Phytosterol | -sitosterol | Rehmannia glutinosa DC., Dioscorea opposite Thunb., Cervi Cornu Colla, Angelica sinensis Diels, Dipsacus asper Wall., Ligustrum lucidum Ait., Astragalus membranaceus Moench. | 25 mg/kg/day; 14 days | Regulation of the pathological process of PCOS | ↑LIF, HOXA10, Integrin 3 ↓COX-2 |
[28] |
| Flavanol | Catechins | Oolong tea or Camellia sinensis | 25, 50, 100 mg/kg; | Alleviation of ovarian dysfunction and insulin resistance | ↑p-IRS1, p-PI3K ↓NF-κB, P65, IL-1α, IL-6, TNF-α, MMP2, MMP9, p-STAT3 |
[16] |
| Carotenoid | Crocetin | Crocus sativus Linné | 40 mg/kg/day; 4 weeks | Attenuation of PCOS | ↑POA-GnRH, AVPV-kiss1 ↓ARC-kiss1 |
[29] |
| Terpene | Tanshinone IIA | Salvia miltiorrhiza | 100 mg/kg/day; 4 weeks | Treatment of PCOS | ↑FSHR, Aromatase, cAMP | [30] |
| Decoction | FuFang ZhenZhu TiaoZhi | Ligustrum lucidum W.T.Aiton, Atractylodes macrocephala Koidz., Coptis chinensis Franch., Citrus medica L., Panax notoginseng F.H.Chen, Salvia miltiorrhiza Bunge, Eucommia ulmoides Oliv., Cirsium japonicum (Thunb.) | 2.892 g/kg/day; 5 weeks | Preventive benefits of PCOS | ↑Adiponectin, PPAR- | [31] |
PCOS, polycystic ovary syndrome; LIF, leukemia inhibitory factor; HOXA10, homeobox A10; COX-2, cyclooxygenase-2; p-IRS1, phosphorylated insulin receptor substrate 1; p-PI3K, phosphorylated phosphoinositide 3-kinase; NF-κB, nuclear factor kappa-B; IL-1, interleukin-1; IL-6, interleukin-6; TNF-a, tumor necrosis factor-a; MMP, matrix metallopeptidase; STAT3, signal transducer and activator of transcription 3; POA-GnRH, Gonadotropin-releasing hormone in the preoptic area at proestrus; AVPV-kiss1, kisspeptin in the anteroventral periventricular nucleus at proestrus; ARC-kiss1, kisspeptin in the arcuate nucleus at diestrus; FSHR, follicle-stimulating hormone receptor; cAMP, cyclic adenosine monophosphate; PPAR-, peroxisome proliferator-activated receptor-; ↑, up-regulation; ↓, down-regulation.
-sitosterol, the main component of BSYXF (Bu Shen Yang Xue formula), improved ovarian status, the balance of hormone levels, and endometrium receptivity. In addition, BSYXF altered the structure of gut microbiota, resulting in a protective effect on PCOS [28].
Hong et al. revealed that catechins from oolong tea improved the morphological conditions of PCOS mice by regulating STAT3 signaling [16]. Catechins inhibited NF-κB mediated inflammation by decreasing the expression of inflammatory markers and endometrial damage by reducing MMP2 and MMP9 expression.
Hu et al. reported that crocetin regulated GnRH by increasing kisspeptin neurons in anteroventral periventricular and reducing kisspeptin expression in the arcuate nuclei [29]. Thus, the changes in serum levels of hormones could be seen. Treated mice also showed structural and functional changes in ovaries.
Tanshinone IIA (TSIIA) restored the balance between estradiol and testosterone in PCOS mice by up-regulating the expression of follicle-stimulating hormone receptor (FSHR), aromatase, and FSH-induced cAMP levels [30].
When mice were treated with FTZ (FuFang ZhenZhu TiaoZhi formula), there was a smaller decrease in the number of corpora lutea and antral follicles and an increase in cystic follicles and circulating level and transcriptional abundance of adiponectin in adipose tissue. Also, PCOS-related insulin resistance was decreased [31].
A total of five studies have been conducted to test the therapeutic effect of natural products, using PCOS-induced C57BL/6 mice. All studies confirmed improved morphological changes using Hematoxylin and Eosin (H&E) staining. To identify the mechanism of how the compound acts on PCOS, immunohistochemical staining and Western blot analysis were used [16,[28], [29], [30], [31]]. Additionally, three studies used reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) [[29], [30], [31]]. Restored hormonal balance was seen in every study [16,[28], [29], [30], [31]]. Among them, one substance (crocetin) was involved in GnRH and estrogen feedback mechanism, and the other one (TSIIA) acted on the hormonal receptor to influence the transcription of aromatase [29,30]. Also, one substance treated PCOS-induced abnormal glucose and lipid metabolism by regulating related hormones [31]. In addition, one study showed the influence of gut microbiota on PCOS using 16S rDNA sequencing, suggesting a fresh approach to taking PCOS [28].
3.2.1.2. NMRI mice
Until now, three studies have been implemented to prove the potential effects of natural products against PCOS-induced NMRI mice (Table 3).
Table 3.
In vivo in NMRI mice.
| Classification | Compound/Extract | Source | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Plant | Hydro-alcoholic Licorice extract | Glycyrrhiza glabra | 100, 150 mg/kg/day; 3 weeks | Protective effects on PCOS | ↓ovarian cyst | [17] |
| Decoction | Lutein and nettle extract | Urtica dioica(nettle) | (1) 200, 400 mg/kg (2) 125, 250 mg/kg (3) Combination 200 & 125 mg/kg; 30 days |
Improvement of reproductive function | ↑TAC ↓MDA |
[32] |
| Decoction | Combination of Paeonia spp. and Glycyrrhiza glabra |
Paeonia spp., Glycyrrhiza glabra |
50, 100 mg/kg/day; 20 days | Synergistic effects on polycystic ovary | Improvement of follicles in the ovaries | [33] |
TAC, total antioxidant capacity; MDA, malondialdehyde; ↑, up-regulation; ↓, down-regulation.
Shamsi et al. found that licorice extract enhanced ovarian status, leading to help oocyst maturation [17]. Then, it also decreased testosterone and estrogen. There was an increase in fertilization rate and also in blastocyst stage embryos.
Bandariyan et al. reported that lutein and nettle extract, both alone and in combination, showed antioxidant activities in PCOS mice [32]. Also, serum estrogen levels increased in the treatment group, and reproduction parameters were improved.
When PCOS mice were treated with Paeonia spp. and Glycyrrhiza glabra, improvements in morphological changes were seen [33]. Also, the average level of fasting insulin and testosterone decreased significantly.
Two substances showed antioxidant activity on PCOS-induced oxidative stress [17,32]. Improved reproductive hormonal status and morphological changes were also seen in all studies. Only one study used H&E staining [17]. The fertility rate was verified using in vitro maturation or superovulation and in vitro fertilization to test the reproductive status [17,32]. However, none of the studies revealed the mechanism of action of the compounds, whit the exception of one of them, confirming only the results of the activity of the compounds These studies classified the dose of the compounds into high and low. In particular, the lutein and nettle study, which designed seven groups consisting of control, PCOS, PCOS treated with nettle of high or low dose, PCOS treated with lutein of high or low dose, and PCOS treated with the combination, was well-organized.
3.2.2. In vivo in rats model
To test the plant-derived compounds that are thought to be effective in PCOS, the below studies utilized in vivo experiments using two kinds of rats: Sprague Dawley (SD) rats and Wister rats.
3.2.2.1. Sprague Dawley rats
Fourteen in vivo studies using SD rats subjected to PCOS were reported (Table 4).
Table 4.
In vivo studies in Sprague Dawley rats.
| Classification | Compound/Extract | Source | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Alkaloid | Berberine | Coptis chinensis Franch, Phellodendron chinensis var. glabriusculum. | 95, 190 mg/kg/day; 4 weeks | Attenuation of PCOS | PI3K/AKT pathway | [34] |
| Stilbene | Resveratrol | Berberis integerrima | 20 mg/kg/day; 42 days | Treatment of PCOS | ↑SOD, TAC ↓MDA, TNF- |
[35] |
| Plant | Allium fistulosum root extract | Allium fistulosum | 500 mg/kg/body weight; 2 weeks | Improvement of ovarian function progression | ↑Kitl, Bmp, Pgr, ESR1, Cyp19a1 | [36] |
| Plant | Berberis integerrima hydroalcoholic extract | Berberis integerrima | 3 gr/kg/day; 42 days | Treatment of PCOS | ↑SOD, TAC ↓MDA, TNF- |
[35] |
| Plant | Flaxseed hydroalcoholic extract | Linum usitatissimum | 200 mg/kg; 30 days | Amelioration of PCOS sign | ↑Progesterone, preantral follicles, antral follicles, and corpus luteum, the thickness of the granulosa layer; ↓testosterone, the thickness of the theca layer, and tunica albuginea |
[37] |
| Decoction | Combination of Berberis integerrima hydroalcoholic extract and Resveratrol | Berberis integerrima, Resveratrol | 20 mg/kg+3 gr/kg/day; 42 days | Treatment of PCOS | ↑SOD, TAC ↓MDA, TNF- |
[35] |
| Decoction | Bao Gui capsule | Herba Epimedium, Rhizome polygonati, Fructus Psoraleae, Carapex et Plastrum Testudinis, Radix Rehmanniae, Rhizoma Anemarrhenae, Radix Angelicae Sinensis, Semen Persicae, Rhizoma Acori Tatarinowii, Radix Polygoni Cuspidati, Herba Verbenaeofficinalis, Radix Ophiopogonis | 0.28,0.57 g/kg/day; 21 days | Treatment of PCOS | ↑P450arom, GLUT4 ↓P450c17 , IL-6, TNF- NF-, IKK , SOCS3 |
[38] |
| Decoction | Cangfu Daotan Decoction | Atractylodis Rhizoma, Cyperi Rhizoma, Fructu Ponciri, Fabanxia, Pericarpium Citri Nobilis, Poria, Arisaematis Rhizoma, Glycyrrhizae Radix, Zingiberis Rhizoma. | 1.42,5.68 g/kg/day; 14 days | Treatment of PCOS | ↑OATP2B1, OATP3A1 ↓IL-1 , IL-6, TNF- |
[39] |
| Decoction | Cangfu Daotan Decoction | Atractylodis Rhizoma, Cyperi Rhizoma, Pinellia ternata, Pericarpium Citri Reticulatae, Poria Cocos, Arisaematis Rhizoma, Astragalus membranaceus, Aurantii Fructus, Codonopsis Radix, Epimrdii Herba, Crataegus pinnatifida Bge, Radix Salviae, licorice, white mustard, Endothelium Corneum Gigeriae Galli, Gleditsiae Spina | 15, 30 g/kg/day; 4 weeks | Regulation of PCOS | ↑bcl-2, p-Akt/Akt, p-PI3K/PI3K ↓TNF-α, IL-1β, IL-6, Bax, Bad, IGF-1 |
[40] |
| Decoction | Guizhi fuling wan | Cinnamomum cassia J. Presl, Poria cocos Wolf, Paeonia veitchii Lynch, Prunus persica Batsch, Paeonia suffruticosa Andr. | 0.31, 0.62, 1.24 g/kg/day; 30 days | Attenuation of ovulation disorder | ↑P62, p-P13K, p-AKT, p-mTOR ↓LC3-II, Beclin1, Atg5, c-PARP, c-caspase-3, c-caspase-9 |
[41] |
| Decoction | Heqi san |
Curculigo orchioides Gaertn, Schisandra chinensis Baill, Cynanchum podophyllum C. K. Schneid, Citrus medica L. var. sarcodactylis Swingle, Crataegus pinnatifida Bunge, Rhus chinensis Mill, Clinopodium megalanthum (Diels) C. Y. Wu & Hsuan ex H. W. Li, Cuscuta chinensis Lam, Poncirus trifoliata (L.) Raf, Hordeum vulgare L, Polygala tenuifolia Willd, Epimedium davidii Franch |
8.1 g/kg/day; 30 days | Improvement of PCOS | ↑GLUT4, PTEN, p-ERK, p-AKT, p-GSK3β, IRS-1 | [42] |
| Decoction | Liuwei Dihuang pills | Rehmanniaglutinosa DC. rhizome, Cornus officinalis Siebold & Zucc. fructus, Dioscorea opposita Thunb. Rhizome, Alisma plantagoaquatica Subsp. orientale Juz. tuber, Paeonia suffruticosa Andr. root bark, Poria cocos Wolf. sclerotium | 1.2, 3.6 g/kg/day; 21 days | Amelioration of PCOS | ↑P-IRS-1, FSHR, Cyp19a1 ↓P-PI3Kp85α, P-Akt, P-FoxO1a |
[43] |
| Decoction | Mahuang tang |
Ephedra Herba, Cinnamomi Ranulus, Glycyrrhizae Radix et Hizoama, Armeniacae Semen, Allii Radix, Zingiberis Rhizoma Crudus, |
500 mg/kg/day; 2 weeks | Protection of PCOS | ↑Cyp19a1, Hsd17b1 ↓Cyp11a1 |
[44] |
| Decoction | Spearmint and flaxseed extract | 40 mg/kg spearmint + 200 mg/kg flaxseed extract; 30 days | Treatment of PCOS | [45] | ||
| Decoction | Xiao-Yao-san | Radix bupleuri, Angelica gigas Nakai., Radix paeoniae Alba., Atractylodes macrocephala Koids., Poria cocos Wolf, Zingiber officinale Roscoe., Mentha haplocalyx Briq., Glycyrrhizae glabra L. | 0.505, 1.01 g/kg/day; 4 weeks | Amelioration of PCOS | ↑AKT and S6K1 ↓DbH, c-fos, b2R |
[46] |
| Decoction | Yingyanghuo, Xianmao | Epimedium brevicornum Maxim., Curculiginis Rhizoma | 7.5 g/kg; 7 days | Alleviation of PCOS | ↑AKT1 | [47] |
PCOS, polycystic ovary syndrome; SOD, superoxide dismutase; TAC, total antioxidant capacity; MDA, malondialdehyde; TNF-α, Tumor necrosis factor-α; Kitl, KIT ligand; Bmp, bone morphogenetic protein; Pgr, progesterone receptor; ESR1, estrogen receptor 1; CYP19a1, cytochrome P450, family 19, subfamily a, polypeptide 1; P450arom, cytochrome P450 aromatase; GLUT4, glucose transporter 4; P450c17α, cytochrome P450c17α; IL-6, interleukin-6; NF-κB, nuclear factor- κB; IKKβ, nuclear factor- κB kinase subunit β; SOCS3, suppressor of cytokine signaling 3; OATP2B1, organic anion transporting polypeptides 2B1; OATP3A1, organic anion transporting polypeptides 3A1; IL-1 , interleukin-1 ; IL-6, interleukin-6; Bcl-2, B-cell lymphoma 2; AKT, Protein kinase B; Bax, bcl-2-associated X protein; IGF-1, Insulin-like growth factor 1; p-P13K, phosphorylated-P13K; p-AKT, phosphorylated-AKT; p-mTOR, phosphorylated-mTOR; c-PARP, cleaved-PARP; c-caspase-3, cleaved-caspase-3; c-caspase-9, cleaved-caspase-9; GLUT4, Glucose transporter type 4; PTEN, Phosphatase and tensin homolog; IRS-1,Insulin receptor substrate 1; FSHR, Follicle Stimulating Hormone Receptor; FoxO1a, Forkhead box protein O1; Hsd17b1, Hydroxysteroid 17-Beta Dehydrogenase 1; Cyp11a1, Cytochrome P450 Family 11 Subfamily A Member 1; S6K1, Ribosomal protein S6 kinase beta-1; DbH, Dopamine beta-hydroxylase; b2R, Beta-2 adrenergic receptor; ↑, up-regulation; ↓, down-regulation.
After administration of Berberine to SD rats, fasting blood glucose, and homeostatic model assessment for Insulin Resistance (HOMA-IR), fasting insulin values decreased dose-dependently. Also, serum hormone levels and apoptosis of the ovary were restored [34].
Ashkar et al. reported that resveratrol and Berberis integerrima (B. integerrima) extract improved insulin resistance by decreasing HOMA-IR [35]. They had an antioxidant effect.
Allium fistulosum regulated the steroid biosynthesis pathway through up-regulating aromatase [36]. It also reacted with various hormone receptors and caused folliculogenesis by increasing the expression of KIT ligand and bone morphogenetic protein.
The hydroalcoholic extract of flaxseed was effective in serum hormone levels and polycystic ovarian morphology [37]. Bao Gui capsule (BGC) attenuated hyperandrogenism via increasing Cyp19a1 and down-regulating P450c17 [38]. It also ameliorated insulin resistance and detracted some inflammatory markers and IKK /NF- signaling.
Yi et al. revealed that Cangfu Daotan Decoction (CFD) could attenuate PCOS by increasing OATP2B1 and OATP3A1 expression in PCOS rats, leading to regulating hormone levels [39]. CFDTT also improved lipid metabolism, ovarian status, and inflammatory responses.
The treatment of Cangfu Daotan decoction (CFD) has been used clinically for gynecological diseases especially PCOS. Decoction (CFD) on SD rats, when intragastrically administrated, reduced body weight and the weight and diameter of the ovary. Besides, CFD caused decreasing in the serum levels of LH, Testosterone, E2, TNF-α, IL-1β, IL-6, and CRP. CFD also restored Bcl-2 abnormal expression and suppressed Bax and Bad expression. The expression of p-Akt/Akt, p-PI3K/PI3K was increased, which means that CFD can improve insulin resistance and inflammation through the PI3K/Akt pathway [40].
Guizhi fuling wan (GFW) affected reproductive hormone levels, ovarian status, and insulin resistance, as seen by follicular development, and the decrease in the serum levels of T, LH, and FINS in treated animals, as well as lower LH/FSH ratios and HOMA-IR values. These results were attributed to the activation of the PI3K/AKT/mTOR signaling pathway that promoted granulosa cell autophagy [41].
Cannula administration of Heqi san in an SD rats’ model of PCOS improved serum hormone level, insulin resistance, and morphological lesion of the ovary [42]. Progesterone level, insulin sensitivity index, and organ coefficient increased and LH level, testosterone level, estradiol level, HOMA-IR, and ovary volume decreased compared to the PCOS group. Moreover, the Heqi san group improved the response to insulin stimulation by up-regulating p-ERK, p-AKT, and p-GSK3β and recovered the decrease in IRS-1 and PTEN expression levels, which are all key factors in the PI3K/APT pathway. Hengxia et al. found some miRNAs including rno-miR-144-3p, rno-miR-30c-2-3p, rno-miR-486, rno-miR-3586-3p and rno-miR-146b-5p, which seems to be critical in PCOS.
Liwei dihawung pills (LWDH) were orally administrated to rats showing improved ovarian polycystic pathogenesis and development of follicles which seemed to be the effect of LWDH by acting on the PI3K/Akt signaling pathway [43]. LWDH regulated this signaling pathway through up-regulating p-IRS-1, down-regulating PI3Kp85α, Akt, and FoxO1, and up-regulating mRNA levels of FSHR and Cyp19a1. LWDH also regulated serum hormone levels, by up-regulating progesterone levels and down-regulating LH and testosterone levels.
Mahuang-Tang restored PCOS hormone levels by increasing estrogen levels and reducing LH levels and LH/FSH ratio [44]. Also, gonadotropin receptors; follicle-stimulating hormone receptor (Fshr), and luteinizing hormone receptor (Lhr) were decreased steroid receptors; progesterone receptor (Pgr), and estrogen receptor1(Esr1) were increased one of the steroidogenic enzymes, Cyp11a1 is reduced and some enzymes like Cyp19a1, Hsd17b1 are increased compared to PCOS included group.
Mina et al. found that the combination of spearmint extract and flaxseed extract is effective for improving the morphometric features of PCOS such as body weights, duration of estrous cycles, and the number of follicles [45]. Also, the combination showed changes in the endocrine profile by restoring serum levels of progesterone, estradiol, testosterone, and Dehydroepiandrosterone (DHEA) compared to PCOS induced group.
Injection of Xiao-Yao-San increased estradiol and progesterone level and suppressed cystic follicles, apoptosis, autophagy of granulosa cells, dopamine beta-hydroxylase (DbH), c-fos level in locus coeruleus, noradrenaline level and the expression of beta 2 adrenergic receptor (b2R) and induces Akt and S6K1 pathways [46].
Chang et al. reported that the combination of Yinyanghuo and Xianmao in rats improved PCOS conditions by regulating the key protein AKT1 [47]. The combination of Yinyanghuo and Xianmao influences the AGE-RAGE pathway, FoxO1 pathway, and estrogen signaling pathway. The most targeted proteins in these signaling pathways were AKT1, IL6, INSR, ESR, and GSK3B. Also, rats showed morphological changes such as restoration of normal tissue with oocytes and an increase in the number of granule cell layers.
In almost all 14 studies conducted to test the effect of natural compounds against polycystic ovary syndrome using SD rats, serum hormone levels were analyzed. Also, the ovarian micromorphological characteristics to identify the effect of the compounds were observed by staining the ovary. Every compound showed improved conditions. Three studies only conducted one of two methods [34,44,47]. Two studies that are blank in the table's mechanism column only researched ovarian morphological features or serum hormone levels [34,37,45]. Therefore, these studies may need additional research about the mechanism of plant-derived compounds to PCOS. Except for the previous studies that were limited to effects, other studies additionally conducted immunostaining, western blotting, real-time PCR analysis, etc, to investigate the mechanism of the experimented compound. Common features of PCOS were ameliorated by plant-derived compounds through various mechanisms. Discovered mechanisms were related to PI3K/AKT signaling [[41], [42], [43],46,47], the Bcl-2/Bax pathway [40], antioxidant enzyme activity [35], and aromatase regulation [36,44]. Unlike other studies, Chang et al. used network construction built by the Cytoscape program to identify key genes [47]. For future studies, this method can be an effective way to evaluate the mechanisms by making it easier to find the key genes the compound influences.
3.2.2.2. Wistar rats studies
Twenty studies have been conducted in vivo experiments with Wistar rats to test the efficacy of the compound on PCOS and Three studies have been conducted in vivo experiments with unspecified rats (Table 5).
Table 5.
In vivo studies on Wistar rats.
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Flavone | Baiclin | Scutellaria baicalensis | 1) NCI–H295R 2)Wistar rats |
1) 0.1, 1, 12.5, 25, 50, 100, 200 mmol/L; 24, 48, 72 h 2)20 mg/kg/day; 4 weeks |
Improvement of hyperandrogenism in PCOS | ↓HSD3B2, GATA1 | [48] |
| Alkaloid | Berberine | Coptis sp. and Berberis sp | Wistar rats | 100, 200 mg/kg/day; | Improvement of ovulation and endometrial receptivity in PCOS | ↑Cyp19a1 ↓ 3, LPAR3 |
[49] |
| Tannin | Hydrolyzable tannin | Wistar rats | 1.5, 2%/kg/body weight; 42 days | Improvement of PCOS | ↑IgM | [50] | |
| Isoflavone | Nanocurcumin | Curcuma longa | Wistar rats | 100, 200 mg/kg; 15 days | Reduction of PCOS risk | ↑miR-223-3p, GSH, CAT, β-cell ↓LC3II, p62, IL-6, NF-κB |
[51] |
| Flavonol | Quercetin | Plants polyphenolic flavonoid | Wistar rats | 100 mg/kg BW/day; 15 days | Alleviation of polycystic ovary | ↑SOD, CAT, GST, ATPase, Ca2+ ATPase, H+ ATPase, Bcl2, E-Cadherin ↓MDA, VEGF, TNF-, mRNA AR gene, Bax |
[52] |
| Terpene | Thymoquinone | Nigella sativa L. | Wister rats | 0.75 mg/100 mL olive oil/rat, or 15 mg/kg body weight; 16 h, 40 h | Improvement of PCOS | ↑Cyp19a1, Sqstm1, P62/SQSTM1 ↓AR, Beclin1, Atg5, Map-LC3- I, Map-LC3- II, P65 |
[53] |
| Vitamin | Vitamin C | Tropical fruits | Wistar rats | 150 mg/kg BW/day; 15 days | Protection against polycystic ovary | ↑CAT, ATPase enzymes activity, Bcl-2, E-cadherin ↓MDA, TNF-, VEGF, AR, Bax |
[54] |
| Plant | Bee pollen | Anthophila | Wistar rats | 50,100,200 mg/kg/day; 21 days | Therapeutic effect on PCOS | ↑Bax, Caspase-3, Sirt-1 ↓Bcl-2 |
[55] |
| Plant | Caesalpenia bonducella ethanol extract | Guilandina bonduc | Wistar rats | 200, 400 mg/kg; 28 days | Improvement of PCOS | ↑SOD CAT, GST, ↓GPx, GR |
[56] |
| Plant | Chamomile | Matricaria chamomilla L. | Wistar rats | 75 mg/kg/day; 30 days | Improvement of PCOS | ↑GPx, CAT ↓caspase-3, MDA, |
[57] |
| Plant | Fennel seed extract | Foeniculum vulgare | Wistar rats | 150 mg/kg/day; 15 days | Regulation of the hormonal and biochemical changes of PCOS | ↑FSH, HDL ↓Insulin, LH, FBS, testosterone, total triglycerides, total cholesterol |
[58] |
| Plant | F. religiosa 10 % fresh leaf extract | Ficus religiosa | Wistar rats | 1.0 mL/kg/day; 30 days (oral, spray) | Alleviation of PCOS | ↑GSH, GPx, SOD, CAT, Cyp19a1, PPAR- ↓3 -HSD, 17 -HSD, HmG-CoA reductase, TC |
[59] |
| Plant | F. religiosa aqueous dry leaf extract | Ficus religiosa | Wistar rats | 100, 300 mg/kg/day; 30 days | Alleviation of PCOS | ↑GSH, GPx, SOD, CAT, Cyp19a1, PPAR- ↓3 -HSD, 17 -HSD, HmG-CoA reductase |
[59] |
| Plant | Ginger extract | Zingiber officinale Roscoe | Wistar rats | 175,350 mg/kg/day; 88 days | Improvement of PCOS | ↑LH, estrogen; ↓progesterone, FSH |
[60] |
| Plant | Gymnema sylvestre ethanol extract | Gymnema sylvestre | Wistar rats | 100 mg/kg/day; 28 days | Inhibition in insulin resistance and androgen production | ↑Luteinizing hormone, testosterone, estrogen; ↓follicle-stimulating hormone, progesterone |
[61] |
| Plant | Hedge woundwort |
Stachys sylvatica L. |
Wistar rats | 100, 250, 500 mg/kg; 10 days |
Improved obesity. Reduced cystic follicles. |
Improvement of folliculogenesis and regulation of gonadotropins synthesis | [62] |
| Plant | Majoram extract | Origanum majorana | Wistar rats | 20 mg/kg/day; 24 days | Beneficial ameliorative effects against PCOS | ↑SOD, GPx ↓IL-6 |
[63] |
| Plant | Pergularia daemia ethanol extract | Pergularia daemia | Wistar rats | 300 mg/kg/day; 28 days | Improvement of ovulation and menstrual irregularity | ↓follicle-stimulating hormone, progesterone | [61] |
| Plant | Chitosan-engaged tripolyphosphate ions and concurrent loading of the biomolecules of the ethanolic extract of fennel seed | Foeniculum vulgare | Wistar rats | 50 mg/kg/day; 15 days | Regulation of the hormonal and biochemical changes of PCOS | ↑FSH, HDL ↓Insulin, LH, FBS, |
[58] |
| Decoction | Combination of Gymnema sylvestre, Pergularia daemia ethanol extract | Gymnema sylvestre, Pergularia daemia | Wistar rats | 400 mg/kg/day; 28 days | Synergistic effects against PCOS | ↑Luteinizing hormone, testosterone, estrogen | [61] |
| Plant | Actaea racemosa and Vitamin C | Cimicifuga racemosa | Wistar rats | AR extract 7.14 mg/kg, vitamin C 500 mg/kg; 28days | Alleviation of PCOS without hepatic adverse effect | ↑GSH, Ki-67, Cyp19a1 ↓MDA |
[64] |
| Decoction | Chia seed, Walnut | Salvia hispenica, Prunus dulcis | Wister rats | 40 g, 40 g, 20 g & 20 g/day; 15 days | Regulation of the hormone level | ↓body and ovarian weights | [65] |
| Decoction | Fennel essential oil, flaxseed oil | Foeniculum vulgare, Linum usitatissimum | Wistar rats | (1)100 mg/kg (2) 429 mg/kg (3) combination 100 mg/kg & 429 mg/kg; 50 days |
Improvement of the follicle conditions of PCOS | ↓cystic follicles | [66] |
| Decoction | Hochu-ekki-to | Wistar rats | 500 mg/kg/day; 7 days | Improvement of ovarian reproductive and immune functions. | ↑ER-, ER-, IL-2, IL-4, IFN-, ↓AR, NGFR, GR, MAP2K-2, Hsp 90 |
[67] | |
| Decoction | Kañcanāra Guggulu | Bauhinia variegate L., Terminalia chebula Retz., Terminalia bellerica, Emblica officinalis, Zingiber officinale, Pipernigrum, Piper longum Linn., Crataeva nurvala, Elettaria cardamomum, Cinnamomum zeylanicum, Commiphora mukul | Wistar rats | 100 mg/kg/day; 28 days | Regularization of the estrous cycle | ↓body weight, lipid profile, Blood glucose | [68] |
| Decoction | Niśāmalakī | Curcuma longa, Emblica officinalis | Wistar rats | 0.9 g/kg/day; 28 days | Correction of changes induced by PCOS | ↓body weight, lipid profile, Blood glucose | [68] |
| Decoction | Sahoyao-gancao decoction | Radix Paeoniae Alba., Glycyrrhizae uralensis | rats | 12.5, 25,50 g/kg; 14 days | Treatment of PCOS | ↑IκB. ↓TNF-α, IL-1β, IL-6, and IL-18, P– NF-κB, p- NF-κB p65 |
[69] |
| Decoction | Unkeito | Paeonia lactiflora, Cinnamomum cassia | rats | Improvement of the follicle conditions of PCOS | ↑gonadotropins | [70] |
PCOS, polycystic ovary syndrome; Bax, bcl-2-associated X protein; CYP19a1, cytochrome P450, family 19, subfamily a, polypeptide 1; LPAR3, lysophosphatidic acid receptor 3; GPx, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; miR, microRNAs; GSH, glutathione; LC3II, light chain 3 II; p62, Sequestosome 1; IL-6, interleukin 6; NF-κB, nuclear factor-kappa B; SOD, superoxide dismutase; GST, glutathione-S-transferase; MDA, malondialdehyde; VEGF, vascular endothelial growth factor; TNF-α, Tumor osis factor-α; mRNA AR gene, mRNA androgen receptor gene; Cyp19a1, Cytochrome P450 Family 19 Subfamily A Member 1; HSD3B2, Hydroxy-Delta-5-Steroid Dehydrogenase, 3 Beta- And Steroid Delta-Isomerase 2; GATA1, GATA Binding Protein 1; GSH, reduced glutathione; GPx, glutathione peroxidase; PPAR-, peroxisome proliferator-activated receptor-; 3 -HSD, 3 -Hydroxysteroid dehydrogenase; 17 -HSD, 17 - Hydroxysteroid dehydrogenase; GR, glucocorticoid receptor; Sqstm1, Sequestosome 1; AR, androgen receptor; Beclin1, Atg5, Autophagy Related 5; Map-LC3- I, Microtubule-associated proteins 1A/1B light chain 3B; ER-, estrogen receptor-; ER-, estrogen receptor ; IL-2, interleukin-2; IL-4, interleukin 4; IFN-, interferon-; NGFR, nerve growth factor receptor; MAP2K-2, mitogen-activated protein kinase kinase 2; Hsp 90, heat shock protein 90; IκB, IkappaB kinase; IL-1β, interleukin-1β; IL-18, Interleukin-18; ↑, up-regulation; ↓, down-regulation.
Baiclin from Scutellaria baicalensis, in a dose- and time-dependent manner, down-regulated testosterone concentration and inhibited the expression of HSD3b2, which is the key enzyme of androgen biosynthesis, in NCI–H295R [48]. Meanwhile, after baiclin treatment in Wistar rats, the serum androgen level was restored. A decrease in the expression of Hydroxy-Delta-5-Steroidal Dehydrogenase, 3 Beta and Steroid Delta-Isomerase 2 (HSD3B2) and GATA Binding Protein 1 (GATA1) which is the transcription factor that activates HSD3B2 was also identified.
Wang et al. revealed that berberine showed improved insulin resistance and endometrial receptivity [49]. Also, an increase of luteinizing hormone receptor, Cyp19a1, and enhanced morphological changes could be seen particularly in high doses, leading to normal ovulation.
1.5, 2 % of hydrolyzable tannin (HT) had been attributed to preventing metabolic disorders induced by PCOS [50]. Antioxidant activities and anti-inflammatory activity were shown. HT also influenced reproductive profiles and lipid profiles but had a negative impact on nutrient digestibility.
Abuelezz et al. suggested that nanocurcumin could be an inflammation and autophagy modulator for the possible better management of PCOS complications [51]. Compared to the PCOS group, the nanocurcumin modulated inflammatory – autophagy by upregulating microRNAs-223-3p (miR-223-3p), decreasing levels of light chain 3 II (LC3II), p62, interleukin 6 (IL-6), nuclear factor-kappa B (NF-κB), increasing in ß cell mass, restoring GSH and CAT enzyme levels. Moreover, nanocurcumin helped restore cellular homeostasis and combated the drastic impact of persistent high reactive oxygen species (ROS) levels.
One study revealed that Quercetin significantly increased antioxidant and anti-apoptosis activities by increasing the expression of BCl2 and E-Cadherin and decreasing Bax [52].
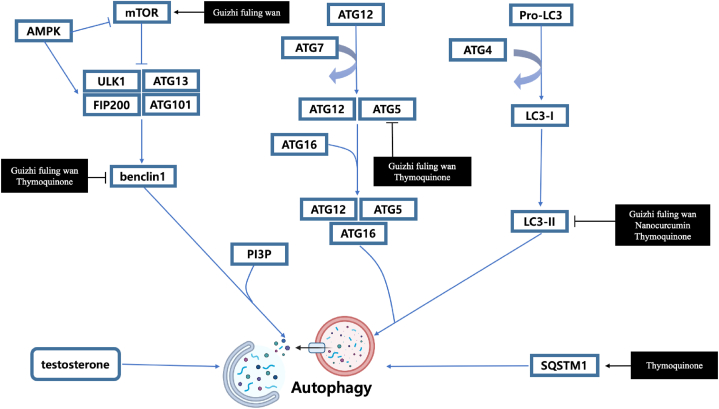
Wister rats were injected with Ru486 along with thymoquinone through intraperitoneal [53]. The Thymoquinone group showed reduced ovary size, LH: FSH ratio, and total testosterone level compared to the PCOS group. Thymoquinone down-regulated the androgen receptors, autophagic biomarker Beclin1, Map-LC3- I, and Map-LC3- II, and active NF-κBsubunit P65 levels and up-regulated PCO biomarker aromatase, cyp19A1, autophagy substrate p62/SQSTM1.
Olaniyan et al. reported that Vitamin C showed antioxidant activities in the polycystic ovary [54]. It also had anti-apoptotic effects in PCOS rats. Plus, improved immune functions and changes in hormone levels were also observed.
Naseri et al. found that higher bee pollen (BP) doses induced antioxidant activities and apoptosis of ovarian cysts [55]. BP also increased the expression of the Sirt-1 gene significantly, leading to improved insulin resistance. Moreover, BP and Metformin combination was more effective in apoptosis and antioxidant capacity.
Administrating Caesalpenia bonducella (CB) dose-dependently increased enzymatic antioxidants and non-enzymatic antioxidants [56]. PCOS rats showed decreased superoxide dismutase (SOD), catalase (CAT), and, glutathione-S-transferase (GST) and increased glutathione peroxidase (GPx) and glucocorticoid receptor (GR), which were restored by the treatment of CB. CB restored the disproportion of SOD, CAT, GST, GR, and GPx. Moreover, the treatment of CB showed changed morphological features such as a reduction in cysts, regular luteinization of follicles, and proliferation of healthy follicles with a clear thecal layer.
Alahmadi et al. revealed that chamomile improved the function and histological structure of the thyroid gland in PCOS rats [57]. Adult virgin female Wistar rats were orally administered M. chamomilla flower extract. Treatment of PCOS rats with chamomile extract increased the level of triiodothyronine (T3), thyroxine (T4), glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT) and decreased level of thyroid stimulating hormone (TSH), caspase-3, malondialdehyde (MDA).
Bayrami et al. suggested that Fennel seed extract (FSX) regulated reproductive hormone levels and lipid profiles [58]. However, FSX anchored on Chit-TPP, which helps efficient delivery to targeted molecules, was more effective in PCOS rats than using FSX alone concerning lipid metabolism.
Suriyakalaa et al. demonstrated that leaf extract of Ficus religiosa alleviated PCOS by correcting menstrual irregularities and serum hormone/lipid profiles, stimulating aromatase and peroxisome proliferator-activated receptor-γ (PPAR-, and up-regulating 3β-Hydroxysteroid (3 -HSD) and 17β- Hydroxysteroid dehydrogenase (17 -HSD) [59]. Additionally, this study found that 3-acetoxy-3-hydroxy-propionic acid, the component of Ficus religiosa, is the key component that caused Ficus religiosa to ameliorate PCOS.
Atashpour et al. found that ginger extracts at higher doses restored serum hormone levels to normal status without side effects compared to clomiphene citrate [60].
Pachiappan et al. reported that Gymnema sylvestre and Pergularia daemia affected insulin sensitivity, androgen production, anovulation, and menstrual irregularities [61]. However, a combination of G. sylvestre and P. daemia had a better synergistic effect.
Stachys sylvatica had been attributed to diuretic, digestive, emmenagogue, antispasmodic, anti-inflammatory, sedative, and tonic properties in folk medicine and phytotherapy [62]. Alizadeh et al. treated thirty adult female Wistar rats with an average weight of 180 g with S. sylvatica extract. The S. sylvatica extract improved obesity in the PCOS group. In addition, it was observed that the number of cystic follicles decreased significantly. In the group of animals that received 500 mg/kg of the extract, there was an increase in the level of FSH and a decrease in the concentration of estrogen.
Rababa'h et al. elucidated that majoram had anti-inflammatory and antioxidant effects [63]. They also showed improvement in insulin resistance and hormone levels. Furthermore, when using majoram with metformin, there was a synergistic effect on PCOS rats.
Azouz et al. reported that Actaea racemosa extracts and vitamin C regulated ovarian and uterine weights, lowered luteinizing hormone (LH) and testosterone (T) hormone levels, and changed lipid profiles such as total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol and very low-density lipoprotein (VLDL) cholesterol [64]. Oxidative stress biomarkers (MDA and GSH) were restored in the AR group. Moreover, vitamin C showed a prevention effect of mild hepatic oxidative stress in the combination group. Ki-67; a cell proliferation marker, increased significantly in the granuloma cell layer and aromatase Cyp19a1 increased significantly, especially in the combination group.
Wistar albino rats were administered either walnuts or chia seeds individually or in combination [65]. In all cases, the rats had reduced body weight and ovarian weight and changes in hormone levels that correspond with increased levels of progesterone and estradiol and decreased testosterone levels.
Ghasemi et al. found that fennel essential oil and flaxseed oil have anti-diabetic effects and anti-oxidative effects that can improve PCOS conditions [66]. Both fennel oil and flaxseed oil respectively decreased body mass index and restored hormone levels, serum glucose, insulin, homeostasis model assessment-estimated insulin resistance (HOMA-IR), homeostasis model assessment of β-cell function (HOMA-b) and quantitative insulin-sensitivity check index (QUICKI) indices. However, the combination of fennel essential oil and flaxseed oil adversely affected the fasting blood glucose and insulin resistance condition. The histomorphology study showed that oils reduced ovarian weights and increased the number of follicles.
Park et al. reported that Hochu-ekki-to treatment regulated steroid hormones receptors like estrogen receptor (ER), AR, and GR by reducing Hsp90 expression, leading to normalizing serum hormone levels [67]. It could also improve immune responses by reducing various inflammatory markers.
Dawane et al. showed that Niśāmalakī (NA) revised insulin resistance [68]. Kañcanāra Guggulu (KG) had less effect on insulin resistance, however, enhanced morphological changes were seen in all treatment groups. When using NA with metformin, it was also effective in insulin resistance.
Shaoyao-Gancao Decoction (SGD), which is consisted of Radix Paeoniae Alba and Glycyrrhizae uralensis is orally administered to rats [69]. As a result, body weight and ovarian weight were dose-dependently reduced, estrous cycles were improved, the number of cystic dilating follicles was decreased, and the layers of granular cells were increased. SGD reduced the serum levels of testosterone and LH and increased the serum level of E2 and FSH and in mRNA levels, SGD lowered inflammatory factors such as TNF-α, IL-1β, IL-6, and IL-18 and phosphorylation of NF-κB p65 and increased the expression of IkappaB kinase (IκB).
Unkeito-administrated rats showed increased numbers of small antral follicles and preovulatory follicles [70]. But there was no significant difference in serum hormone levels and the numbers of preantral follicles and corpora lutea between the control group and unkeito treated group.
A total of 20 studies have been performed to test the efficacy of the compound in polycystic ovary syndrome conditions using in vivo Wistar rat model experiments. A total of 3 studies didn't specify the rat model used in the experiment. Serum sex hormone level is restored by the treatment of plant-derived compounds in many studies [48,49,53,54,[57], [58], [59], [60], [61],[63], [64], [65],67,69]. Histomorphology features of PCOS-induced rats' ovaries were improved by the compounds in eight studies [49,56,57,61,65,66,68,70]. In one study that analyzed two compounds, compounds respectively showed improved PCOS conditions but in combination they adversely affected PCOS [66]. Therefore, this study may need future studies to evaluate the interactions between the two compounds on PCOS. Two studies experimented with the effect of the compound alone and the combination of the compound and the treatment drug: metformin [55,63]. In both studies, the combination of the compound and metformin showed synergetic effects on PCOS. Common features of PCOS were ameliorated by the compounds through various mechanisms. Some substances improved PCOS through antioxidant effects [50,52,56,59,63], some improved through anti-apoptosis [51,52,54], and others through restoration of the immune system [52,54,63]. Revealed mechanisms were related to the Bcl-2/Bax pathway [54,55] and NF-κB signaling [53,69]. Most of the studies were performed only in vivo, but there was one study that was performed both in vivo and in vitro [48]. Six studies were insufficient about the mechanism of the compound. Therefore, these studies may need additional research about the mechanism of the compounds to PCOS [58,60,61,65,66,68].
3.3. Human studies provide clinical evidence of natural products in the treatment of PCOS
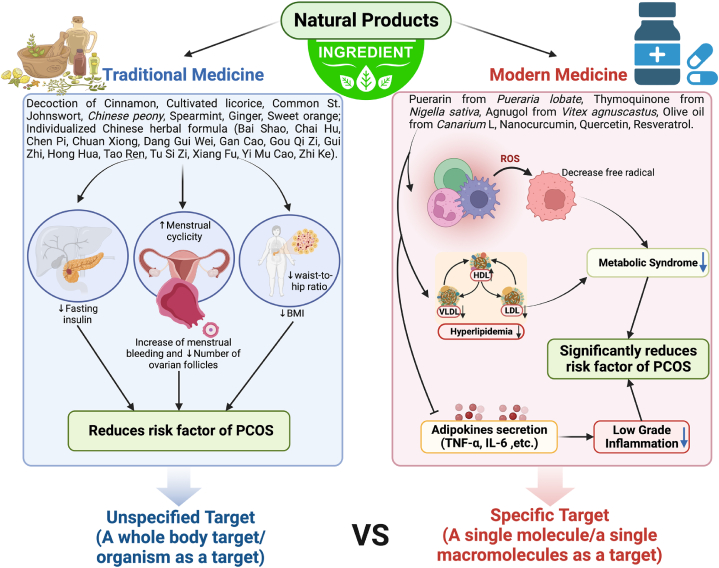
Polycystic ovary syndrome (PCOS) is a common, complex reproductive endocrinopathy characterized by menstrual irregularities, hyperandrogenism, and polycystic ovaries [71]. PCOS is a leading cause of infertility with an incidence of 6–26 % among women of childbearing age [72]. The symptoms of PCOS can be suppressed by engaging in lifestyle, but the intervention of natural products can further enhance its effect. Thus, the development of more effective natural products for PCOS is imperative. Twenty-five human studies regarding PCOS treatment with natural products were reported (Table 6) and compared between traditional medicine and modern drug discovery as shown in Fig. 1. The underlying difference between traditional medicine and modern drug discovery from natural products in PCOS treatment is the form of therapy given, in traditional medicine the target is more unspecified (a whole body target/organism as a target of natural product) and there can be more than one combination of natural products (Fig. 1). Whereas in modern drug discovery it is a specific target (a single molecule/a single macromolecule as a target), usually more about compounds observed in each natural product or plant used (Fig. 1).
Table 6.
Clinical studies in human trials verify the efficacy of natural products in the treatment of PCOS.
| Classification | Compound/Extract | Source | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Isoflavone | Puerarin | Pueraria lobate (Wild) Ohwi | 51 patients with PCOS, aged 18-39 | 150 mg/day; 3 months | Reduction of PCOS risk | ↑SHBG, SOD ↓TC, |
[73] |
| Terpene | Thymoquinone | Nigella sativa | 207 overweight and obese PCOS patients, aged 18-35 | 500 mg; 6 months. | Reduction of PCOS risk | ↑SOD; ↓Body weight, MDA |
[74] |
| Plant | Agnugol, Castleberry dry extract |
Vitex agnus-castus | 120 women with PCOS and oligomenorrhea, married, not pregnant, aged 20-45 | 3.2–4.8 mg/day; 3 months |
Reduction of PCOS risk | ↓menstrual cycle interval | [75] |
| Plant | Canola oil | Brassica napus L. | 72 patients with PCOS aged 18–45 years | 25 g; 10 weeks |
Reduce fatty liver grade. | ↓TG, TC/HDL, LDL/HDL, TG/HDL | [76] |
| Plant | Chamomile | Anthemis L. | 80 women of childbearing age with PCOS. | 370 mg; 3 months |
Decrease in testosterone level | ↓Testosterone | [77] |
| Plant | Cinnamon | Cinnamomum verum J. Presl | 66 women were diagnosed with PCOS | 1, 1.5 g/day; 12 weeks |
Reduce fasting insulin and insulin resistance | ↓HOMA-IR, LDL, HDL | [78] |
| Plant | Common flax | Linum usitatissimum L. | 48 women aged 18–44 years old with PCOS | 30 g/day; 12 weeks |
Increase of menstrual regularity, QUICKI. Decrease of fasting blood glucose, and body weight. |
↑QUICKI, HDL, adiponectin ↓TG, IL-6, TNF-α, insulin, HOMA-IR, hs-CRP, leptin |
[79] |
| Plant | Common flax | Linum usitatissimum L. | 60 women aged 18–40 years old with PCOS | 1000 mg; 12 weeks |
Decrease of Insulin level | ↓Insulin, serum triglycerides, VLDL-cholesterol, hs-CRP | [80] |
| Plant | Common sage | Salvia officinalis L. | 60 patients diagnosed with PCOS | 330 mg/day; 8 weeks | Inhibition of insulin resistance in patients with PCOS | ↑QUICKIE; ↓Body Mass Index, Insulin levels (P < 0.001), and HOMA-IR |
[81] |
| Plant | Fennel | Foeniculum vulgare Mill | 61 women with oligomenorrhea complaints and with PCOS, aged 18-35 | 5 g/day; 6 months | Reduction of oligomenorrhea and PCOS risk | ↓The mean pain severity | [82] |
| Plant | Lilac chaste tree | Vitex agnus-castus L. | A 21-year-old female with a history of PCOS | 400 mg; 10 months |
Increase of estrogen and progesterone activity. | ↓Endometrial hyperplasia | [83] |
| Plant | Marjoram | Origanum majorana | 25 patients diagnosed with PCOS, aged 16-35 | 1.3–1.5 g; 1 month. | Reduction of PCOS risk | ↓DHEA-S | [84] |
| Plant | Olive oil | Canarium L. | 72 patients with PCOS aged 18–45 years | 25 g; 10 weeks |
Reduce fatty liver grade. | ↓HOMA-IR | [76] |
| Plant | Sickle fruit fenugreek (Furocyst) | Trigonella foenum-graecum L. | 50 premenopausal women (18–45 years, BMI<42) with PCOS | 500 mg; 3 months |
Reduced ovary volume and cyst size. Return of regular menstrual cycle. |
↑ LH, FSH | [85] |
| Plant | Walnut | Juglans regia | 60 women with PCOS | 150 gm/day; 90 days | Reduction of PCOS risk | ↓TC, LDL, TG | [86] |
| Decoction | Cinnamon, Cultivated licorice, Common St. Johnswort, Chinese peony |
Cinnamomum verum J. Presl, Glycyrrhiza glabra L. Hypericum perforatum L. Paeonia lactiflora Pall. |
122 women aged 18–44 years with PCOS with a confirmed medical diagnosis | 3 months | Inhibition of oligomenorrhea, amenorrhoea. Improved insulin and luteinizing hormone, blood pressure, quality of life, depression, anxiety and stress, and pregnancy rates. |
↓fasting insulin | [71] |
| Decoction | Cinnamon, Spearmint, Ginger, Sweet orange | Cinnamomum aromaticum Nees, Mentha spicata L., Zingiber officinale Roscoe, Citrus × sinensis (L.) Osbeck | 60 women with PCOS aged 18–35 years old with primary and secondary infertility | 700 mg; 3 months |
Increase in rate of pregnancy, antioxidant, and anti-inflammatory properties | ↑CAT, GPx, SOD ↓Insulin, MDA, FBS |
[72] |
| Decoction | Celery, Anise burnet saxifrage |
Apium graveolens L. Pimpinella anisum L. |
72 women aged between 18 and 40 years from PCOS with a chief complaint of oligomenorrhea | 4.5 g; 15 days. |
Increase of menstrual cycle's regularity. Improvement of oligomenorrhea |
↓BMI, Testosterone | [87] |
| Decoction | Dihuang, Huanglian, Baishao, Huangqin, Ejiao, Fuling |
Radix Rehmanniae, Rhizoma Coptidis, Radix Paeoniae Alba, Radix Scutellariae Baicalensis, Colla Corii Asini, Poria |
95 women aged 18–33 years old with PCOS | 4 capsules; 6 months |
Decrease in BMI, WHR, and blood glucose levels. Improvement of insulin resistance. |
↑HDL-C, IS ↓TG, LDL-C, insulin, HOMA-IR |
[88] |
| Decoction | Femitex-SP4 | Saraca indica L., Vitex agnus castus L., Embelica officinalis L., Symplocos racemosa Roxb. | 150 patients, aged 18-44 | 500 mg; 1 year | Reduction of PCOS risk | ↓Testosterone | [89] |
| Decoction | Individualized Chinese herbal formula (Bai Shao, Chai Hu, Chen Pi, Chuan Xiong, Dang Gui Wei, Gan Cao, Gou Qi Zi, Gui Zhi, Hong Hua, Tao Ren, Tu Si Zi, Xiang Fu, Yi Mu Cao, Zhi Ke) | Paeonia japonica, Bupleurum falcatum L., Citri unshius Pericarpium, Cnidium officinale Makino, Angelicae sinensis Radix, Glycyrrhiza uralensis Fisch, Lycium chinense Mill., Cinnamomum cassia Presl, Carthamus tinctorius L., Prunus persica (L.) Batsch, Cuscuta japonica Choisy, Cyperus rotundus L., Leonurus sibiricus L., Citrus aurantium L. | 40 women presented with oligomenorrhea or amenorrhoea with a diagnosis of PCOS, aged 18–44 | 16 g/day; 6 months | Reduction of PCOS risk | ↓waist-to-hip ratio | [90] |
| Decoction | Lilac chaste tree, Sweet fennel, Queen Anne's lace |
Vitex agnus-castus L. Foeniculum vulgare Mill. Daucus carota L. |
150 women aged between 18 and 43 years | 500 mg; 3 months |
Induction of menstrual. Increase of menstrual bleeding. |
↑Menstrual cyclicity | [91] |
| Decoction | Persian herbal remedy | Foeniculum vulgare, Urtica dioica, Daucus carota, Trifolium pratense, Curcuman longa | 80 overweight women with PCOS | 5 g/day; 12 weeks | Reduction of PCOS risk | ↓TC, LDL-C, TG, AST, ALT, HDL-C, | [92] |
| Decoction | Shouwu Jiangqi Decoction | Fallopia multiflora, Radix puerariae, Batryticated silkworm, Astragalus propinquus Schischkin, Dioscorea oppositifolia L., Euonymus alatus, Cyperus rotundus L., Cuscutae semen. | 81 participants diagnosed with PCOS, aged 18-35 | 3 months | The expectation of efficacy of SWJQD on the treatment of PCOS with kidney deficiency, phlegm, and blood stasis | ↑Insulin sensitivity | [93] |
| Decoction | Yijin-tang |
Pinellia ternata Breitenbach, Citrus unshiu Markovich, Poria cocos Wolf, Glycyrrhiza uralensis Fisch, Zingiber officinale Roscoe |
15 PCOS women with oligomenorrhea | 6 g; 12 weeks |
Decrease in the menstrual cycle and follicle number. Improvement of oligomenorrhea. |
↓Number of ovarian follicles | [94] |
SHBG, sex hormone binding globulin; SOD, superoxide dismutase; TC, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, quantitative insulin-sensitivity check index; IL-6, interleukin 6; TNF-α, tumor necrosis factor-α; hs-CRP, high-sensitivity C-reactive protein; VLDL-cholesterol, very-low-density lipoprotein-cholesterol; DHEA-S, dehydroepiandrosterone sulfate; CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; FBS, Fast blood sugar; HDL-C, low-density lipoprotein cholesterol; LDL-C, low-density lipoprotein-cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ISI, insulin-sensitive index; ↑, up-regulation; ↓, down-regulation.
Fig. 1.
Comparison between traditional and modern drugs on PCOS treatment.
Li et al. suggested that puerarin could be considered for the management of metabolic disorders and hyperandrogenism in PCOS patients [73]. In the study, 70 patients were assigned into two groups, the obese group, and the non-obese group, and the obese group was divided into an obese treatment group and an obese control group. Patients in the obese treatment group and non-obese group orally received 150 mg/d of puerarin tablets for three months. As a result, improved blood levels of SHBG and SOD, and lower systolic blood pressure, TC, and testosterone blood levels were observed in patients who received puerarin regardless of their obese status and obese patients with puerarin respectively (Fig. 1).
Ammar et al. discovered that thymoquinone, black cumin oil supplementation constituted a beneficial added value to metformin in ameliorating PCOS-related disorders [74]. Two hundred seven overweight and obese PCOS patients were divided into two groups, group A received metformin 500 mg and group B received a combination of metformin 500 mg and thymoquinone in the form of black cumin oil 500 mg capsules three times daily for 6 months. Group B showed a significant decrease in the number of patients suffering from oligomenorrhea, weight reduction, and body fat redistribution, regaining oxidative balance with a significant increase in SOD activity and a decrease in MDA concentrations.
Shayan et al. reported that agnugol could be presented as an herbal alternative to treat oligomenorrhea in patients with polycystic ovary syndrome [75]. 120 women with PCOS and oligomenorrhea were randomly divided into two groups, Group A, patients taking Agnugol, and Group B, patients taking Metformin. After the treatment with Agnugol and Metformin, menstrual intervals decreased and the length of menstruation and the number of pads increased in both groups. But more side effects were reported in the group using metformin.
Previous studies showed the positive effects of olive and canola oils in dyslipidemia modulation [76]. The participants included 90 patients with PCOS aged 18–45 years. The Canola oil effect resulted in a significant reduction in serum levels of TG and TC/HDL, LDL/HDL, and TG/HDL ratios. Canola and olive oils could significantly reduce the fatty liver grade. Moreover, the Homeostasis model evaluation for insulin resistance index (HOMA-IR) in both canola and olive groups was significantly decreased.
According to traditional herbal medicine, chamomile has been considered one of the herbal remedies for patients with PCOS [77]. Heidary et al. demonstrated this for 80 women of childbearing age with PCOS. The intervention group received 370 mg oral capsules of chamomile three times a day for 3 months. The control group did receive starch capsules (three times a day). Decreased level of testosterone was observed in the intervention group (in women with PCOS) who received chamomile capsules.
Cinnamon is an herbal remedy used traditionally by patients with PCOS to improve their menstrual cycle [78]. Out of 80 women that were diagnosed with PCOS, 66 were enrolled in this randomized double-blind placebo-controlled clinical trial. Fasting insulin and insulin resistance were reduced after 12 weeks in the cinnamon group. There was also a significant lower in low-density lipoprotein (LDL), high-density lipoprotein (HDL), and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
Haidari et al. were conducted on women aged 18–44 years old with PCOS [79]. Flaxseed powder was effective in controlling weight since it decreases body weight, waist circumference, and insulin concentration. flaxseed powder increased Quantitative Insulin-Sensitivity Check Index (QUICKI), High-Density Lipoprotein (HDL), and adiponectin. It also causes a decrease in Triglycerides (TG), Interleukin 6 (IL-6), TNF-α, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), high-sensitivity C-Reactive Protein (hs-CRP), and leptin. Flaxseed in patients with PCOS could improve some biochemical and anthropometric markers, through amelioration of dyslipidemia, obesity, IR, and inflammation.
Mirmasoumi et al. conducted research for women aged 18–40 years on the treatment of PCOS in women [80]. After the 12-week intervention, compared to the placebo, flaxseed oil omega-3 supplementation significantly decreased insulin values, homeostasis model of assessment-estimated insulin resistance, mF-G scores, and increased quantitative insulin sensitivity check index. Also, supplementation with flaxseed oil omega-3 resulted in significant decreases in serum triglycerides, VLDL-cholesterol, and high-sensitivity C-reactive protein (hs-CRP) compared to the placebo.
Amini et al. reported that salvia officinalis extract prevented insulin resistance in euglycemic patients with PCOS [81]. Sixty PCOS patients were orally administered 330 mg S. officinalis extract daily for eight weeks. Results showed a significant decrease in BMI, insulin levels, HOMA-IR, and FBS value, and an increase in QUICKI in S. officinalis groups compared with placebo groups.
Mokaberinejad et al. found that fennel seed infusion plus dry cupping used a safe and effective therapeutic intervention in the management of oligomenorrhea in patients with polycystic ovary syndrome [82]. In the study, 61 patients were randomly divided into two groups, Group 1 received fennel tea and dry cupping, and Group 2 received metformin. The fennel seed infusion plus dry cupping reduced the days between two menstrual cycles compared to metformin. Moreover, the decrease in average pain was more significant in the fennel-dry cupping group. Body mass index was also reduced in both groups.
Vitex agnus-castus was an herb that was commonly known for its ability to occlude prolactin release from the pituitary gland [83]. Alois et al. reported that on a 21-year-old female with a history of PCOS. As a result of taking Vitex agnus-castus, total and free testosterone decreased. In addition, a persistently elevated LH to FSH ratio of just under 3:1. After 6 months, menstrual cycles improved and dysmenorrhea decreased further.
Husein et al. reported that marjoram tea had beneficial effects on the hormonal profile of PCOS women [84]. Twenty-five patients were assigned to receive marjoram tea or a placebo tea twice daily for 1 month. Compared to the placebo group, marjoram tea reduced DHEA-S and fasting insulin levels. The results also showed an improvement in HOMA-IR and GIR, although this was not significant.
Swaroop et al. included in this study, 50 premenopausal women (18–45 years, BMI <42) diagnosed with PCOS [85]. Swaroop et al. reported significant increases in luteinizing hormone (LH) and follicular stimulating hormone (FSH) levels were observed compared to the baseline values. In addition, significant reductions in ovary volumes were observed following treatment, demonstrating reduced cyst size. Also, it increased the menstrual cycle's regularity.
Rashid et al. discovered that treatment with high-dose walnuts had a benefit effect on oxidative stress [86]. In the study, 60 women with polycystic ovarian syndrome were divided into two groups, the metformin-treated group, and walnut treated group. Compared with the metformin-treated group, for 3 months, total serum cholesterol, and LDL cholesterol were significantly reduced in the walnut-treated group. In addition, serum triglyceride levels were reduced after one month of feeding with walnut.
Arentz et al. researched women aged 18–44 years on the treatment of PCOS in women [71]. When herbal mixture (Cinnamomum verum, Glycyrrhiza glabra, Hypericum perforatum, Paeonia lactiflora) the proportion with normal menstrual cycles was large. Follicular phase oestradiol was increased in the test group, and LH was lowered. There was a significant improvement in systolic and diastolic blood pressure for women taking the herbal mixture compared with controls. In addition, anxiety, depression, and stress decreased.
A randomized clinical trial was carried out on 60 infertile participants with polycystic ovary syndrome (PCOS) willing to be pregnant [72]. Herbal mixture (Cinnamomum aromaticum, Mentha spicata, Zingiber officinale, Citrus × sinensis) increased Catalase (CAT), Glutathione peroxidase (GPx), and Superoxide dismutase (SOD) in patients with PCOS. It also causes a decrease in insulin, Malondialdehyde (MDA), and Fast blood sugar (FBS). Consumption of an herbal mixture as a supplement alongside clomiphene citrate (CC) can improve the serum antioxidant levels, glycemic status, and pregnancy rate in PCOS patients. In addition, the effect of menstrual regulation can be seen.
The affirmative effects of Apium graveolens and Pimpinella anisum hydro alcoholic extracts on hormonal regulation, polycystic ovarian tissue, and fertility have been shown in a few animal studies [87]. The experimental Model included women aged between 18 and 40 years suffering from PCOS with a chief complaint of oligomenorrhea. In total, 72 patients participated in this clinical trial. CAC (Apium graveolens, Pimpinella anisum) fully increased the menstrual cycle's regularity and improved oligomenorrhea. In addition, CAC reduced the total serum testosterone and LH/FSH ratio in women with polycystic ovary syndrome.
One of the Chinese patented medicine Heyan Kuntai capsule (HYKT) consists of prepared Radix Rehmanniae (Dihuang), Rhizoma Coptidis (Huanglian), Radix Paeoniae Alba (Baishao), Radix Scutellariae Baicalensis (Huangqin), Colla Corii Asini (Ejiao), and Poria (Fuling) [88]. HYKT showed the BMI and WHR of all the patients were decreased. The fasting and postprandial 2 h blood glucose levels significantly declined when treated with HYKT. Likewise, serum sex hormones including luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone were lowered after being treated with HYKT. Besides, blood lipids outcomes such as total cholesterol, triglyceride, and low-density lipoprotein cholesterol (LDL-C), as well as insulin and HOMA-IR were decreased with significance in the HYKT group. Whereas high-density lipoprotein cholesterol (HDL-C) and insulin-sensitive index (ISI) increased.
Ishaq et al. discovered that Femitex-SP4 which included Saraca indica L., Vitex agnus castus L., Embelica officinalis L., and Symplocos racemosa Roxb was proven to be clinically effective in most of the patients, particularly PCOS patients with menstrual irregularities [89]. 150 patients aged between 18 and 44 received 500 mg of powdered herbs in capsule form twice daily. After treatment, there was a noticeable change in the menstrual cycle, ovarian size, morphology, and a notable reduction in free testosterone levels.
Wong et al. suggested that both individualized and standardized CHM could improve outcomes of patients with polycystic ovary syndrome [90]. 40 women who present with oligomenorrhea or amenorrhoea with a diagnosis of polycystic ovary syndrome participated in the study. Compared to standardized CHM, individualized CHM did not show significant improvement in the mean menstrual rate, mFG scores, and PCOS total scores at 6 months among women with polycystic ovary syndrome. However, individualized CHM showed a significant reduction in WHR when compared to standardized CHM.
Aslagh capsule (Vitex agnus-castus, Foeniculum vulgare, Daucus carota) is used for the management of oligomenorrhea and amenorrhoea in ITM clinics [91]. 150 patients aged between 18 and 43 years with oligomenorrhea due to PCOS were included in the study. Aslagh capsule was affecting in contributing to menstruation, being hepatoprotective and enhancing blood circulation in the uterus and ovaries. Also, menstrual cyclicity has been greatly improved. Aslagh capsule showed beneficial effects similar to metformin.
Rouhani et al. revealed that Persian herbal remedies and electroacupuncture could be used as a better treatment of metabolic complications and overweight problems in patients with polycystic ovary syndrome [92]. Eighty overweight women with PCOS were randomly divided into four groups, the first group received metformin, the second group received metformin and Persian herbal remedy, the third group received metformin and electroacupuncture, and the fourth group received both therapies. After 12 weeks of intervention, in the herbal medicine and (herbal medicine + electroacupuncture) groups, BMI, BF, WHR, fasting insulin, HOMA-IR, HOMA-β, total cholesterol, LDL, TG, AST, ALT, HDL decreased, and increased in QUICKI was observed.
Xu et al. found that the study will investigate whether the combination of Shouwu Jiangqi Decoction and acupuncture can alleviate the clinical symptoms and improve insulin resistance in patients with PCOS [93]. 81 participants with PCOS will be divided into 3 groups: group A, SWJQD and acupuncture, group B, SWJQD and sham acupuncture, and group C, metformin. The outcomes include the HOMA-IR index, the Oral Glucose Tolerance Test, sex hormone levels, body mass index, ovulation rate, clinical pregnancy rate, and complete sequencing data. Adverse events will be also recorded.
Yijin-tang (YJT) is a traditional medicine prescribed for the prevention and treatment of diverse diseases, which is composed of Pinellia ternata Breitenbach, Citrus unshiu Markovich, Poria cocos Wolf, Glycyrrhiza uralensis Fisch, and Zingiber officinale Roscoe [94]. 15 patients with oligomenorrhea due to PCOS were included in the study. The menstrual cycle length significantly decreased at week 12 compared to baseline. In addition, the number of ovarian follicles was reduced after the 12-week treatment.
A total of 25 human studies have been conducted to confirm the effect of natural products on polycystic ovary syndrome. Most studies were performed on women with PCOS, aged approximately 18–45, and were confirmed by mechanisms such as TC, LDL, TG, and AST [73,75,76,[78], [79], [80],84,86,88,89,92]. Five studies inhibit TC and six substances that inhibit T [73,76,77,83,[86], [87], [88], [89],92]. Studies using 12 substances improved the menstrual cycle's regularity and oligomenorrhea [71,74,75,79,82,85,87,[89], [90], [91],93,94]. In addition, 8 studies controlled the effect of hormones, and 3 studies increased the rate of pregnancy [71,72,77,81,83,84,89,92,93]. These studies also lowered insulin levels and fatty liver grade [71,76,[78], [79], [80], [81],84,88,92,93]. In particular, cinnamon was used in 3 studies, and common flax was used in duplicated 2 studies [71,72,[78], [79], [80]]. Flax was used alone, in contrast to cinnamon which was mixed with other natural products. Both experiments with flax showed lowered insulin levels in common. The drug doses in the two experiments differed by 30 times. Experiments conducted by mixing cinnamon and other natural extracts decreased insulin and increased pregnancy rates. It also showed antioxidant and anti-inflammatory properties. However, when cinnamon was used alone, only the insulin-reducing effect was shown. 6 studies were not confirmed by mechanisms [74,75,81,82,90,93].
4. Discussion
Polycystic ovary syndrome (PCOS) is one of the most common endocrine/metabolic disorders in women worldwide [73]. Although there have been significant advances in medicine and technology, PCOS therapeutic interventions like oral contraceptives, androgen-suppressing medications, ovulation stimulation, and metformin have serious side effects and complications. These include the risk of vascular thromboembolism, menorrhagia, polyvinyl chloride, and digestive problems like nausea and dizziness [82]. Natural products with high safety and selectivity, as well as few reports of adverse reactions, are being introduced to medical research and tests to see if they can treat PCOS because current efforts to control this condition are ineffective, particularly in adolescents and young women.
The effectiveness of natural remedies in treating PCOS has already been studied in several studies. For instance, Zhou et al. investigated the information on the effectiveness of Chinese herbal medicine (CHM) on the rates of live birth pregnancies and adverse events in subfertile women with polycystic ovarian syndrome (PCOS) [23]. However, compared to the present study, there is insufficient evidence to support the use of CHM for women with PCOS and subfertility. There is no information on live births, and there is no consistent evidence that CHM affects fertility. Furthermore, there isn't enough evidence on side effects to say whether CHM is safe. Linjing et al. reviewed the strength and validity of the existing evidence regarding the use of Chinese medicine for the treatment of PCOS in another study [95]. However, unlike the current study, this one only included evaluations in Chinese, which may have resulted in biased and incorrect conclusions. The current study is significant because it assesses the safety and pharmacological action of natural compounds in PCOS using a larger sample size, a longer study duration, and a more systematic methodology.
In this present review, natural products have been suggested as an alternative to treat PCOS, and their hepatoprotective effects have been extensively investigated in terms of pharmacological properties including anti-inflammatory, normalization of hormones, antioxidant, and inhibition of autophagy.
4.1. Inflammation and PCOS
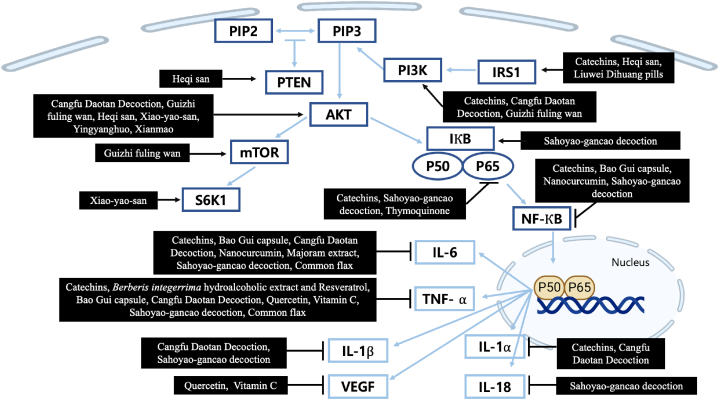
Inflammation is one of the potential risk factors of PCOS [96]. In the first stage of the inflammatory cascade, PAMPs and DAMPs detect infection or damage. Toll-like receptors (TLRs) can detect a variety of damage signals, activating common signaling pathways and NF–B. After signal transduction, IkB releases NF–B, which moves to the nucleus. Gene transcription and translation produce the third stage of the inflammatory cascade, the inducible expression of pro-inflammatory cytokines like IL-1β, IL-6, TNF, and others. The final stage is inflammation resolution, which minimizes host collateral damage [97]. According to recent studies mentioned earlier in our review several compounds regulated these inflammation factors. The mechanisms of compounds were elucidated in Fig. 2. Catechins, Sahoyao-gancao decoction, Bao Gui capsule, Cangfu Daotan decoction and nanocurcumin inhibited NF-ΚB mediated inflammation by decreasing the expression of phosphorylated NF-kappa-B p65 (p–NF–κB p65), IL-1α, IL-6, and TNF- α [16,38,39,51,69]. Moreover, studies found that Resveratrol, Berberis (B.) integerrima extract, a combination of berberis integerrrima hydroalcoholic extract and resveratrol, quercetin, and vitamin C improved anti-inflammatory activities by decreasing TNF- α [35,52,54]. Furthermore, common flax could down-regulate inflammatory pathways in the body and reduce the levels of inflammatory agents such as TNF- α, C-reactive protein (CRP), and IL-6 [79]. Rababa'h also reported that majoram extract reduced the level of IL-6 [63].
Fig. 2.
Schematic diagram of inflammation mechanisms of the natural compounds.
4.2. Hormones and PCOS
PCOS causes hormone imbalances. High androgen levels cause PCOS. Increasing luteinizing hormones (LH) and decreasing follicle-stimulating hormone (FSH) due to increased gonadotropin-releasing hormone (GnRH) also contribute to PCOS. Insulin resistance is a major cause of PCOS, which is linked to low sex hormone-binding globulin levels (SHBG) [98]. PCOS genes code for steroidogenic enzymes. PCOS women's ovarian theca cells overexpress enzymes involved in androgen biosynthesis, increasing 17-hydroxyprogesterone, testosterone, and androstenedione production. In PCO granulosa cells, decreased expression of 3-hydroxysteroid dehydrogenase (3β-HSD) after hCG and decreased aromatase activity increase androgens [99]. According to recent studies mentioned earlier in our review, several natural products regulated these hormone factors, especially in the Hypothalamic-Pituitary-Ovary axis and steroidogenesis pathway. The mechanisms of compounds were elucidated in Fig. 3. Lilac chaste tree and sickle fruit fenugreek etc. normalized the hormone level by increasing both LH and FSH levels [39,50,83,85]. Tanshinone IIA also increased the level of FSH [30]. Furthermore, a decoction of cinnamon, cultivated licorice, common St. Johnswort, and Chinese peony, etc. reduced PCOS risk by decreasing LH levels [31,36,[39], [40], [41], [42],44,49,59,64,71,87,88]. In addition, Fennel seed extract, Ginger extract, etc. decreased LH levels and increased FSH levels [28,29,43,58,60,61,69]. On top of that, Crocetin reduced the level of GnRH [29]. Studies also found that there are several natural products which improved the level of insulin in the serum of PCOS patients, such as Common flax, decoction of Cinnamon etc. [71,72,79,80,88]. Moreover, puerarin elevated the level of SHBG [73]. Marjoram tea also significantly reduced the level of DHEA-s [84]. In the steroidogenic pathway, Mahuang-Tang and Paeoniflorin extract inhibited the enzyme CYP11A [15,44]. Paeoniflorin extract and Bao Gui capsule also inhibited the enzyme CYP17A1 [15,38]. Numerous studies in our review reported that some compounds increased the level of progesterone such as the Hydroalcoholic extract of flaxseed, Guizhi fuling wan, etc. [37,41,42,46,50,54,[59], [60], [61]]. Furthermore, Allium fistulosum root extract, Bao Gui capsules, etc. increased the level of aromatase which is CYP19A1 [30,36,38,44,53,59,64]. Some studies also found that 10 % fresh leaf extract of F. religiosa, and F. religiosa aqueous dry leaf extract increased the reduced enzyme 3βHSD the enzyme 17βHSD [59]. Others related with estradiol, estrogen, androgen, and testosterone are shown in Fig. 3.
Fig. 3.
The modulation and regulation of PCOS-hormone by the natural product derivatives compounds.
4.3. ROS and PCOS
The imbalance between ROS and antioxidants causes the oxidation of lipids, protein, and DNA in spermatozoa, Leydig, and Sertoli cells in the testes, which can produce Malondialdehyde (MDA) [100]. GSH-Px, SOD, and CAT control antioxidant mechanisms in cells. Glutathione peroxidase is the most important antioxidant because H2O2 affects GSH [101]. These antioxidant enzymes also increase the expression of γ-glutamylcysteine synthetase (γGCS), the limiting enzyme in glutathione (GSH) synthesis [102,103]. Numerous studies in our review reported antioxidant efficacy and their mechanisms were organized in Fig. 4. In our study, Resveratrol, Chamomile, Quercetin, Vitamin C, the combination of Actaea racemosa and Vitamin C, and Puerarin downregulated MDA [35,52,54,57,64,73]. Resveratrol, Quercetin, Caesalpenia bonducella ethanol extract, F. religiosa leaf extract, Majoram extract, the combination of Cinnamon, Spearmint, Ginger and Sweet orange, and Puerarin upregulated SOD [35,52,56,59,63,72,73]. Chamomile, F. religiosa leaf extract, Majoram extract, and the Combination of Cinnamon, Spearmint, Ginger, and Sweet orange upregulated Gpx [57,59,63,72]. Chamomile, Nanocurcumin, Quercetin, Vitamin C, Caesalpenia bonducella ethanol extract, F. religiosa leaf extract, and the combination of Cinnamon, Spearmint, Ginger and Sweet orange upregulated CAT [51,52,54,56,57,59,72]. Nanocurcumin, F. religiosa leaf extract, and the combination of Actaea racemosa and Vitamin C upregulated GSH [51,52,59,64].
Fig. 4.
Schematic diagram of ROS inhibitory activities by natural product derivatives compounds.
4.4. Autophagy and PCOS
Autophagy is involved in regulating the growth of follicles at different developmental stages [104]. However, in PCOS patients due to functional ovarian hyperandrogenism, the autophagy system doesn't work normally [105]. In PCOS GCs, testosterone induces beclin-1 (BECN1), anti-thymocyte globulin (ATG), light chain 3 (LC3), and autophagy. In PCOS women, GCs have more ATG7, ATG5, Beclin1, and LC3-II:LC3-I mRNA and less SQSTM1. Testosterone propionate increases Beclin1 and LC3 in GCs, boosting autophagy [104]. In our study, some natural compounds regulated these autophagy pathways, and their mechanisms were illustrated in Fig. 5. ATG5 and Beclin1 were downregulated by Guizhi fuling wan and Thymoquinone [41,106]. LC3-II is down-regulated by Guizhi fuling wan and Nanocurcumin (Fig. 5) [41,51]. Recent studies of Guilandina bonduc L. reveal its potential as a herbal remedy that strengthens the immune system and helps regulate the menstrual cycle in PCOS [107].
Fig. 5.
Schematic diagram of autophagy modulatory mechanism by natural product derivatives compounds.
We used PubMed, Web of Science, and Scopus as documents, and our literature search was limited to five years. Furthermore, the results may have been influenced by the heterogeneity of the experiments. A protocol clinical experiment was also included in the review, which could not guarantee the efficacy of products against PCOS [93].
Additionally, several studies have linked fennel and berberine to a reduction in PCOS. These natural ingredients should be vigorously investigated as a potential clinical trial candidates because they have the potential to be future alternative therapeutics for PCOS. When taken as a whole, this assessment can act as a starting point for information on the creation of new PCOS medications and should be regarded as a suggestion for further investigation rather than a final judgment.
5. Conclusions
This review also highlighted and discussed the therapeutic benefits of several natural products on polycystic ovary syndrome comprehensively. Natural products were categorized using experimental techniques such as in vitro, in vivo, and others. Interestingly, 72 latest studies on natural products with the potential to control PCOS were examined. By controlling the PI3K/AKT signaling pathway and decreasing NF-κB and cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6, certain plant-derived chemicals might reduce inflammation. Other bioactive substances altered the HPO axis, which normalized hormones. More interestingly, other plant bioactive components increased glutathione, superoxide dismutase, catalase, and glutathione peroxidase levels to reduce radiation-induced oxidative stress and it helps to improve the condition of PCOS. The other bioactive substances prevented autophagy by impairing beclin 1, autophagy-related 5, and microtubule-associated protein 1A/1B-light chain 3 - II. It's exciting that natural remedies may one day be used to treat PCOS via modern drug discoveries. More clinical research on the use of such natural compounds is needed.
Funding
This research was supported by Graduate School Innovation office, Kyung Hee University, a grant from Kyung Hee University in 2023 (KHU-20230914), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), and the innovation network support Program through the INNOPOLIS funded by Ministry of Science and ICT (2022-IT-RD-0205-01-101).
Data availability statement
There is no data related to this review article. The data were only sourced from the literature listed in this article (Data included in article/supp. material/referenced in article).
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Woobin Jung: Conceptualization, Investigation, Visualization, Writing – original draft. Hyojoo Choi: Conceptualization, Investigation, Visualization, Writing – original draft. Jimin Kim: Conceptualization, Investigation, Visualization, Writing – original draft. Jeongwoo Kim: Conceptualization, Investigation, Visualization, Writing – original draft. Woojin Kim: Writing – review & editing. Fahrul Nurkolis: Visualization, Writing – review & editing. Bonglee Kim: Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Fahrul Nurkolis, Email: fahrul.nurkolis.mail@gmail.com.
Bonglee Kim, Email: bongleekim@khu.ac.kr.
References
- 1.Shaikh N., Dadachanji R., Mukherjee, S.J.I.j.o.m.g. 2014. Genetic Markers of Polycystic Ovary Syndrome: Emphasis on Insulin Resistance. 2014. [Google Scholar]
- 2.Deswal R., Narwal V., Dang A., Pundir C.S. The prevalence of polycystic ovary syndrome: a brief systematic review. J. Hum. Reprod. Sci. 2020;13:261–271. doi: 10.4103/jhrs.JHRS_95_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar N., Agarwal H. Early clinical, biochemical and radiological features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm. Metab. Res. 2022 Sep;54(9):620–624. doi: 10.1055/a-1880-1264. [DOI] [PubMed] [Google Scholar]
- 4.Harvey R.E., Coffman K.E., Miller, V.M.J.W.s.H. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. 2015;11:239–257. doi: 10.2217/whe.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z., Wang Y.H., Wang L.L., Hu D.T., Teng Y., Zhang T.Y., Yan Z.Y., Wang F., Zou Y.F. Polycystic ovary syndrome and the risk of endometrial, ovarian and breast cancer: an updated meta-analysis. Scot. Med. J. 2022;67:109–120. doi: 10.1177/00369330221107099. [DOI] [PubMed] [Google Scholar]
- 6.Ryu K.J., Kim M.S., Kim H.K., Kim Y.J., Yi K.W., Shin J.H., Hur J.Y., Kim T., Park H. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: the National Health Insurance Service-National Sample Cohort Study. Fertil. Steril. 2021;115:1569–1575. doi: 10.1016/j.fertnstert.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Ryu K.-J., Kim M.S., Kim H.K., Kim Y.J., Yi K.W., Shin J.H., Hur J.Y., Kim T., Park H.J.F. Sterility. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: the National Health Insurance Service-National Sample Cohort Study. 2021;115:1569–1575. doi: 10.1016/j.fertnstert.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Bednarska S., Siejka A.J.A.i.C., Medicine E. The pathogenesis and treatment of polycystic ovary syndrome: What's N. 2017;26 doi: 10.17219/acem/59380. [DOI] [PubMed] [Google Scholar]
- 9.Legro R., Barnhart H., Schlaff, W.J.N.E.J.M. Cooperative Multicenter Reproductive Medicine Network Clomiphene, metformin, or both for infertility in the PCOS. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 10.Legro R.S., Brzyski R.G., Diamond M.P., Coutifaris C., Schlaff W.D., Casson P., Christman G.M., Huang H., Yan Q., Alvero, R.J.N.E.J.M. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanadys W., Barańska A., Malm M., Błaszczuk A., Polz-Dacewicz M., Janiszewska M., Jędrych M. Use of oral contraceptives as a potential risk factor for breast cancer: a systematic review and meta-analysis of case-control studies up to 2010. Int J Environ Res Public Health. 2021:18. doi: 10.3390/ijerph18094638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genazzani, A.D.J.R.b.o. Inositol as putative integrative treatment for PCOS. 2016;33:770–780. doi: 10.1016/j.rbmo.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Unfer V., Carlomagno G., Dante G., Facchinetti F.J.G.E. Effects of myo-inositol in women with PCOS: a systematic review of randomized controlled trials. 2012;28:509–515. doi: 10.3109/09513590.2011.650660. [DOI] [PubMed] [Google Scholar]
- 14.Unfer V., Nestler J.E., Kamenov Z.A., Prapas N., Facchinetti F. Effects of inositol(s) in women with PCOS: a systematic review of randomized controlled trials. Int J Endocrinol. 2016;2016 doi: 10.1155/2016/1849162. Epub 2016 Oct 23. PMID: 27843451; PMCID: PMC5097808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong M., Cheng J., Jin X., Lao W., Johnson M., Tan Y., Qu X. Paeoniflorin extract reverses dexamethasone-induced testosterone over-secretion through downregulation of cytochrome P450 17A1 expression in primary murine theca cells. J. Ethnopharmacol. 2019;229:97–103. doi: 10.1016/j.jep.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Hong G., Wu H., Ma S.T., Su Z. Catechins from oolong tea improve uterine defects by inhibiting STAT3 signaling in polycystic ovary syndrome mice. Chin. Med. 2020;15 doi: 10.1186/s13020-020-00405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamsi M., Nejati V., Najafi G., Pour S.K. Protective effects of licorice extract on ovarian morphology, oocyte maturation, and embryo development in pcos-induced mice: an experimental study. International Journal of Reproductive BioMedicine. 2020;18:865–876. doi: 10.18502/ijrm.v13i10.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manouchehri A., Abbaszadeh S., Ahmadi M., Nejad F.K., Bahmani M., Dastyar N. Polycystic ovaries and herbal remedies: a systematic review. JBRA Assist Reprod. 2022 doi: 10.5935/1518-0557.20220024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azin F., Khazali H. Phytotherapy of polycystic ovary syndrome: a review. Int J Reprod Biomed. 2022;20:13–20. doi: 10.18502/ijrm.v20i1.10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon S., Kim, H.J.S.D.P.C. Donguibogam. 2006:297–2189. [Google Scholar]
- 21.Ried K. Chinese herbal medicine for female infertility: an updated meta-analysis. Complement Ther Med. 2015;23:116–128. doi: 10.1016/j.ctim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Akbaribazm M., Goodarzi N., Rahimi M. Female infertility and herbal medicine: an overview of the new findings. Food Sci. Nutr. 2021;9:5869–5882. doi: 10.1002/fsn3.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou K., Zhang J., Xu L., Lim C.E.D. Chinese herbal medicine for subfertile women with polycystic ovarian syndrome. Cochrane Database Syst. Rev. 2021:2021. doi: 10.1002/14651858.CD007535.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen W., Jin B., Pan Y., Han Y., You T., Zhang Z., Qu Y., Liu S., Zhang Y. The effects of traditional Chinese medicine-associated complementary and alternative medicine on women with polycystic ovary syndrome. Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/6619597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y., Guo X., Ma S., Ma H., Li H., Wang Y., Qin Z., Wu X., Han Y., Han Y. The treatment with complementary and alternative traditional Chinese medicine for menstrual disorders with polycystic ovary syndrome. Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/6678398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamanti-Kandarakis E., Dunaif A.J.E.r. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y., Liu Y.X., Liu X., Wang S.L., Li P., Lin X.H., Sui C.L., Xu C., Qi B., Tong Q. Effects of Gui Zhu Yi Kun formula on the P53/AMPK pathway of autophagy in granulosa cells of rats with polycystic ovary syndrome. Exp. Ther. Med. 2017;13:3567–3573. doi: 10.3892/etm.2017.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y.Y., Cao Y., Huang W.L., Liu Y.X., Lu Y., Zhao J.J. Beta-sitosterol ameliorates endometrium receptivity in PCOS-like mice: the mediation of gut microbiota. Front. Nutr. 2021:8. doi: 10.3389/fnut.2021.667130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Q., Jin J., Zhou H., Yu D., Qian W., Zhong Y., Zhang J., Tang C., Liu P., Zhou Y., et al. Crocetin attenuates DHT-induced polycystic ovary syndrome in mice via revising kisspeptin neurons. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;107:1363–1369. doi: 10.1016/j.biopha.2018.08.135. [DOI] [PubMed] [Google Scholar]
- 30.Jin J., Hu Q.Y., Xu W.W., Zhu W.J., Liu B., Liu J., Wang W., Zhou H.F.J.E., medicine t. Tanshinone IIA attenuates estradiol-induced polycystic ovarian syndrome in mice by ameliorating FSHR expression in the ovary. 2019;17:3501–3508. doi: 10.3892/etm.2019.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y., Tang J.L., Guo Q.Y., Xu Y.D., Yan K.X., Wu L.H., Xie K., Zhu A.M., Rong X.L., Ye D.W., et al. Traditional Chinese Medicine formula FTZ protects against polycystic ovary syndrome through modulating adiponectin-mediated fat-ovary crosstalk in mice. J. Ethnopharmacol. 2021:268. doi: 10.1016/j.jep.2020.113587. [DOI] [PubMed] [Google Scholar]
- 32.Bandariyan E., Mogheiseh A., Ahmadi A. The effect of lutein and Urtica dioica extract on in vitro production of embryo and oxidative status in polycystic ovary syndrome in a model of mice. BMC Complementary Medicine and Therapies. 2021:21. doi: 10.1186/s12906-021-03229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadinpour A., Seyedi Z.S., Arabdolatabadi A., Razavi Y., Ajdary M. The synergistic effect of Paeonia spp and Glycyrrhiza glabra on polycystic ovary induced in mice. Pak. J. Pharm. Sci. 2020;33:1665–1670. doi: 10.36721/PJPS.2020.33.4.REG.1665-1670.1. [DOI] [PubMed] [Google Scholar]
- 34.Yu J., Ding C., Hua Z., Jiang X., Wang C. Protective effects of berberine in a rat model of polycystic ovary syndrome mediated via the PI3K/AKT pathway. J. Obstet. Gynaecol. Res. 2021 doi: 10.1111/jog.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashkar F., Eftekhari M.H., Tanideh N., Koohpeyma F., Mokhtari M., Irajie C., Iraji A. Effect of hydroalcoholic extract of berberis integerrima and resveratrol on ovarian morphology and biochemical parameters in letrozole-induced polycystic ovary syndrome rat model: an experimental study. International Journal of Reproductive BioMedicine. 2020;18:637–650. doi: 10.18502/ijrm.v13i8.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y.H., Yang H., Lee S.R., Kwon S.W., Hong E.J., Lee H.W. Welsh onion root (Allium fistulosum) restores ovarian functions from letrozole induced-polycystic ovary syndrome. Nutrients. 2018;10 doi: 10.3390/nu10101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jelodar G., Masoomi S., Rahmanifar F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iranian Journal of Basic Medical Sciences. 2018;21:645–650. doi: 10.22038/ijbms.2018.25778.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian Y.L., Zhao F.G., Wang W.J. Use of Bao Gui capsule in treatment of a polycystic ovary syndrome rat model. Mol. Med. Rep. 2020;21:1461–1470. doi: 10.3892/mmr.2020.10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi W.M., Li X., Chen K., Zhu M., Cai X.H., Pan A.Z. Effects of Cangfu Daotan Decoction on obese polycystic ovary syndrome and its mechanism. Steroids. 2021;165 doi: 10.1016/j.steroids.2020.108740. [DOI] [PubMed] [Google Scholar]
- 40.Wang C.Y., Ding C.F., Hua Z.J., Chen C.Y., Yu J. Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome via IGF-1-PI3K/Akt-Bax/Bcl-2 pathway. Mediat. Inflamm. 2020:2020. doi: 10.1155/2020/8865647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M., Zhu H., Zhu Y., Hu X. Guizhi Fuling Wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 2021:270. doi: 10.1016/j.jep.2021.113821. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H., Zhou D., Chen Y., Liu D., Chu S., Zhang S. Beneficial effects of Heqi san on rat model of polycystic ovary syndrome through the PI3K/AKT pathway. Daru. 2017;25 doi: 10.1186/s40199-017-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu Z., Dong J., Xue C., Li X., Liu K., Liu B., Cheng J., Huang F. Liuwei Dihuang Pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/Akt signaling pathway. J. Ethnopharmacol. 2020:250. doi: 10.1016/j.jep.2019.111965. [DOI] [PubMed] [Google Scholar]
- 44.Yang H., Lee Y.H., Lee S.R., Kaya P., Hong E.J., Lee H.W. Traditional medicine (Mahuang-Tang) improves ovarian dysfunction and the regulation of steroidogenic genes in letrozole-induced PCOS rats. J. Ethnopharmacol. 2020:248. doi: 10.1016/j.jep.2019.112300. [DOI] [PubMed] [Google Scholar]
- 45.Mehraban M., Jelodar G., Rahmanifar F. A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J. Ovarian Res. 2020;13 doi: 10.1186/s13048-020-00633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun H.Y., Li Q., Liu Y.Y., Wei X.H., Pan C.S., Fan J.Y., Han J.Y. Xiao-Yao-San, a Chinese medicine formula, ameliorates chronic unpredictable mild stress induced polycystic ovary in rat. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C., Liu L.H., Li N., Xiu A.H., Zhang Z.X., Ai H. Efficacy of an Yinyanghuo (Herba Epimedii Brevicornus)-Xianmao (Rhizoma Curculiginis) drug pair in a rat model of polycystic ovary syndrome. J. Tradit. Chin. Med. 2021;41:588–599. doi: 10.19852/j.cnki.jtcm.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Yu J., Liu Y., Zhang D., Zhai D., Song L., Cai Z., Yu C. Baicalin inhibits recruitment of GATA1 to the HSD3B2 promoter and reverses hyperandrogenism of PCOS. J. Endocrinol. 2019;240:497–507. doi: 10.1530/JOE-18-0678. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z., Nie K., Su H., Tang Y., Wang H., Xu X., Dong H. Berberine improves ovulation and endometrial receptivity in polycystic ovary syndrome. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153654. [DOI] [PubMed] [Google Scholar]
- 50.Manzoor F., Nisa M.U., Hussain H.A., Ahmad N., Umbreen H. Effect of different levels of hydrolysable tannin intake on the reproductive hormones and serum biochemical indices in healthy female rats. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-77672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abuelezz N.Z., Shabana M.E., Rashed L., Morcos G.N.B. Nanocurcumin modulates mir-223-3p and nf-κb levels in the pancreas of rat model of polycystic ovary syndrome to attenuate autophagy flare, insulin resistance and improve ß cell mass. J. Exp. Pharmacol. 2021;13:873–888. doi: 10.2147/JEP.S323962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olaniyan O.T., Bamidele O., Adetunji C.O., Priscilla B., Femi A., Ayobami D., Okotie G., Oluwaseun I., Olugbenga E., Mali, P.C.J.J.o.b. 2020. Quercetin Modulates Granulosa Cell mRNA Androgen Receptor Gene Expression in Dehydroepiandrosterone-Induced Polycystic Ovary in Wistar Rats via Metabolic and Hormonal Pathways; p. 31. et-al. [DOI] [PubMed] [Google Scholar]
- 53.Saha P., Kumar S., Datta K., Tyagi R.K. Upsurge in autophagy, associated with mifepristone-treated polycystic ovarian condition, is reversed upon thymoquinone treatment. J. Steroid Biochem. Mol. Biol. 2021:208. doi: 10.1016/j.jsbmb.2021.105823. [DOI] [PubMed] [Google Scholar]
- 54.Olaniyan O.T., Femi A., Iliya G., Ayobami D., Godam E., Olugbenga E., Bamidele O., Mali P.C.J.P. Vitamin C suppresses ovarian pathophysiology in experimental polycystic ovarian syndrome. 2019;26:331–341. doi: 10.1016/j.pathophys.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Naseri L., Khazaei M.R., Khazaei M. Potential therapeutic effect of bee pollen and metformin combination on testosterone and estradiol levels, apoptotic markers and total antioxidant capacity in A rat model of polycystic ovary syndrome. International Journal of Fertility & Sterility. 2021;15:101–107. doi: 10.22074/ijfs.2020.134604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meera B., Muralidharan P., Hari R. Antioxidant potential of Caesalpenia bonducella seeds in the management of polycystic ovary syndrome (PCOS) using mifepristone induced rats model. J. Herbs, Spices, Med. Plants. 2020:1–12. doi: 10.1080/10496475.2020.1795041. [DOI] [Google Scholar]
- 57.Alahmadi A.A., Alzahrani A.A., Ali S.S., Alahmadi B.A., Arab R.A., El-Shitany N.A.E.A. Both matricaria chamomilla and metformin extract improved the function and histological structure of thyroid gland in polycystic ovary syndrome rats through antioxidant mechanism. Biomolecules. 2020;10 doi: 10.3390/biom10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayrami A., Shirdel A., Rahim Pouran S., Mahmoudi F., Habibi-Yangjeh A., Singh R., Abdul Raman A.A. Co-regulative effects of chitosan-fennel seed extract system on the hormonal and biochemical factors involved in the polycystic ovarian syndrome. Mater. Sci. Eng., C. 2020;117 doi: 10.1016/j.msec.2020.111351. [DOI] [PubMed] [Google Scholar]
- 59.Suriyakalaa U., Ramachandran R., Doulathunnisa J.A., Aseervatham S.B., Sankarganesh D., Kamalakkannan S., Kadalmani B., Angayarkanni J., Akbarsha M.A., Achiraman S. Upregulation of Cyp19a1 and PPAR-γ in ovarian steroidogenic pathway by Ficus religiosa: a potential cure for polycystic ovary syndrome. J. Ethnopharmacol. 2021:267. doi: 10.1016/j.jep.2020.113540. [DOI] [PubMed] [Google Scholar]
- 60.Atashpour S., Jahromi H.K., Jahromi Z.K., Maleknasab M. Comparison of the effects of Ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. International Journal of Reproductive Biomedicine. 2017;15:561–568. [PMC free article] [PubMed] [Google Scholar]
- 61.Pachiappan S., Ramalingam K., Balasubramanian A. Combined effects of Gymnema sylvestre and Pergularia daemia on letrozole-induced polycystic ovarian syndrome in rats. Asian Pacific Journal of Reproduction. 2021;10:68–74. doi: 10.4103/2305-0500.311610. [DOI] [Google Scholar]
- 62.Alizadeh F., Ramezani M., Piravar Z. Effects of Stachys sylvatica hydroalcoholic extract on the ovary and hypophysis-gonadal axis in a rat with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2020;25 doi: 10.1186/s43043-020-0015-9. [DOI] [Google Scholar]
- 63.Rababa'h A.M., Matani B.R., Ababneh M.A. The ameliorative effects of marjoram in dehydroepiandrosterone induced polycystic ovary syndrome in rats. Life Sci. 2020:261. doi: 10.1016/j.lfs.2020.118353. [DOI] [PubMed] [Google Scholar]
- 64.Azouz A.A., Ali S.E., Abd-Elsalam R.M., Emam S.R., Galal M.K., Elmosalamy S.H., Alsherbiny M.A., Hassan B.B., Li C.G., El Badawy S.A. Modulation of steroidogenesis by Actaea racemosa and vitamin C combination, in letrozole induced polycystic ovarian syndrome rat model: promising activity without the risk of hepatic adverse effect. Chin. Med. 2021:16. doi: 10.1186/s13020-021-00444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramachandran S., Nikitha J., Gopi C., Amala M., Dhanaraju M.D. Effect of Prunus dulcis and Salvia hispenica in the management of polycystic ovary syndrome in Wistar rats. Journal of Taibah University Medical Sciences. 2020;15:122–128. doi: 10.1016/j.jtumed.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghasemi M., Riasi A., Kowsar R., Mahdavi A.H., Asgary Dastjerdi S., Talebi A., Moshtaghian S.J. Effect of fennel essential oil and flaxseed oil on blood parameters, insulin resistance, and histological structure of ovaries in rats suffered polycystic ovary syndrome. Comp. Clin. Pathol. 2021;30:445–452. doi: 10.1007/s00580-021-03236-x. [DOI] [Google Scholar]
- 67.Park E., Choi C.W., Kim S.J., Kim Y.I., Sin S., Chu J.P., Heo J.Y. Hochu-ekki-to treatment improves reproductive and immune modulation in the stress-induced rat model of polycystic ovarian syndrome. Molecules. 2017;22 doi: 10.3390/molecules22060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dawane J.S., Pandit V., Khade K.S., Suryawanshi S.P., Wele A., Pawar M., Bhalerao S. Study the efficacy of herbal formulation Niśāmalakī in animal model of polycystic ovarian disease syndrome. Ancient Sci. Life. 2017;37(2):86–93. doi: 10.4103/asl.ASL_51_18. [DOI] [Google Scholar]
- 69.Shao Y.Y., Chang Z.P., Cheng Y., Wang X.C., Zhang J.P., Feng X.J., Guo Y.T., Liu J.J., Hou R.G. Shaoyao-gancao decoction alleviated hyperandrogenism in a letrozole-induced rat model of polycystic ovary syndrome by inhibition of NF-κB activation. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshita S., Osuka S., Sonehara R., Miyake N., Murakami M., Wei W., Hayashi S., Muraoka A., Ganieva U., Nakanishi N., Kasahara Y. REPRODUCTIVE SCIENCES(Vol. 27, No. SUPPL 1, Pp. 191A-192A). TIERGARTENSTRASSE 17. SPRINGER HEIDELBERG; D-69121 HEIDELBERG, GERMANY: 2020, March. Effect of unkeito, a traditional herbal medicine, on a rat model of polycystic ovary syndrome. [Google Scholar]
- 71.Arentz S., Smith C.A., Abbott J., Fahey P., Cheema B.S., Bensoussan A. Combined lifestyle and herbal medicine in overweight women with polycystic ovary syndrome (PCOS): a randomized controlled trial. Phytother Res. 2017;31:1330–1340. doi: 10.1002/ptr.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ainehchi N., Khaki A., Farshbaf-Khalili A., Hammadeh M., Ouladsahebmadarek E. The effectiveness of herbal mixture supplements with and without clomiphene citrate in comparison to clomiphene citrate on serum antioxidants and glycemic biomarkers in women with polycystic ovary syndrome willing to Be pregnant: a randomized clinical trial. Biomolecules. 2019;9 doi: 10.3390/biom9060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W., Hu H., Zou G., Ma Z., Liu J., Li F. Therapeutic effects of puerarin on polycystic ovary syndrome: a randomized trial in Chinese women. Medicine. 2021;100 doi: 10.1097/MD.0000000000026049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ammar I.M.M., Salem M.A.A. Amelioration of polycystic ovary syndrome-related disorders by supplementation of thymoquinone and metformin. Middle East Fertil. Soc. J. 2021;26 doi: 10.1186/s43043-021-00076-1. [DOI] [Google Scholar]
- 75.Shayan A., Masoumi S.Z., Shobeiri F., Tohidi S., Khalili A. Comparing the effects of agnugol and metformin on oligomenorrhea in patients with polycystic ovary syndrome: a randomized clinical trial. J. Clin. Diagn. Res. : J. Clin. Diagn. Res. 2016;10:Qc13–qc16. doi: 10.7860/jcdr/2016/22584.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yahay M., Heidari Z., Allameh Z., Amani R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: a randomized controlled trial. Lipids Health Dis. 2021;20:7. doi: 10.1186/s12944-021-01433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heidary M., Yazdanpanahi Z., Dabbaghmanesh M.H., Parsanezhad M.E., Emamghoreishi M., Akbarzadeh M. Effect of chamomile capsule on lipid- and hormonal-related parameters among women of reproductive age with polycystic ovary syndrome. J. Res. Med. Sci. 2018;23 doi: 10.4103/jrms.JRMS_90_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hajimonfarednejad M., Nimrouzi M., Heydari M., Zarshenas M.M., Raee M.J., Jahromi B.N. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: a randomized double-blind placebo controlled clinical trial. Phytother Res. : PTR. 2018;32:276–283. doi: 10.1002/ptr.5970. [DOI] [PubMed] [Google Scholar]
- 79.Haidari F., Banaei-Jahromi N., Zakerkish M., Ahmadi K. The effects of flaxseed supplementation on metabolic status in women with polycystic ovary syndrome: a randomized open-labeled controlled clinical trial. Nutr. J. 2020;19 doi: 10.1186/s12937-020-0524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirmasoumi G., Fazilati M., Foroozanfard F., Vahedpoor Z., Mahmoodi S., Taghizadeh M., Esfeh N.K., Mohseni M., Karbassizadeh H., Asemi Z. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes. 2018;126:222–228. doi: 10.1055/s-0043-119751. [DOI] [PubMed] [Google Scholar]
- 81.Amini L., Mojab F., Jahanfar S., Sepidarkish M., Raoofi Z., Maleki-Hajiagha A. Efficacy of Salvia officinalis extract on the prevention of insulin resistance in euglycemic patients with polycystic ovary syndrome: a double-blinded placebo-controlled clinical trial. Compl. Ther. Med. 2020;48 doi: 10.1016/j.ctim.2019.102245. [DOI] [PubMed] [Google Scholar]
- 82.Mokaberinejad R., Rampisheh Z., Aliasl J., Akhtari E. The comparison of fennel infusion plus dry cupping versus metformin in management of oligomenorrhoea in patients with polycystic ovary syndrome: a randomised clinical trial. J. Obstet. Gynaecol. 2019;39:652–658. doi: 10.1080/01443615.2018.1541232. [DOI] [PubMed] [Google Scholar]
- 83.Alois M., Estores I.M. Hormonal regulation in pcos using acupuncture and herbal supplements: a case report and review of the literature. Integr. Med. 2019;18:36–39. [PMC free article] [PubMed] [Google Scholar]
- 84.Haj-Husein I., Tukan S., Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J. Hum. Nutr. Diet. 2016;29:105–111. doi: 10.1111/jhn.12290. [DOI] [PubMed] [Google Scholar]
- 85.Swaroop A., Jaipuriar A.S., Gupta S.K., Bagchi M., Kumar P., Preuss H.G., Bagchi D. Efficacy of a novel fenugreek seed extract (trigonella foenum-graecum, furocyst) in polycystic ovary syndrome (PCOS) Int. J. Med. Sci. 2015;12:825–831. doi: 10.7150/ijms.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rashid A.I., Mohammed I.K., Kadhem R.A., Al-Bayati N., Al Mukhtar E.J. The metabolic effect of walnut in polycystic ovarian syndrome. International Journal of Drug Delivery Technology. 2019;9:593–596. doi: 10.25258/ijddt.v9i4.13. [DOI] [Google Scholar]
- 87.Jazani A.M., Nazemiyeh H., Tansaz M., Bazargani H.S., Fazljou S.M.B., Azgomi R.N.D., Hamdi K. Celery plus anise versus metformin for treatment of oligomenorrhea in polycystic ovary syndrome: a triple-blind, randomized clinical trial. Iran. Red Crescent Med. J. 2018;20 doi: 10.5812/ircmj.67181. [DOI] [Google Scholar]
- 88.Liang R., Liu Z., Li P., Fan P., Xu L., Sun X., Peng J., Peng X., Zhang M. Kuntai capsules improve glucolipid metabolism in patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Medicine (Baltim.) 2019;98 doi: 10.1097/md.0000000000016788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishaq S., Rizwani G.H., Shareef H., Fatima S., Anser H., Sarfraz S. A herbal treatment approach for the management of clinical, hormonal and ultrasound parameters in reproductive age group women with polycystic ovarian syndrome: a randomized clinical trial. Pak. J. Pharm. Sci. 2021;34:1097–1102. doi: 10.36721/PJPS.2021.34.3.SUP.1097-1102.1. [DOI] [PubMed] [Google Scholar]
- 90.Wong C.H.L., Cheung W.K.W., Chung V.C.H. Is individualised Chinese herbal formula effective in treating oligomenorrhoea and amenorrhoea among female with polycystic ovary syndrome as compared to standardised Chinese herbal formula? Advances in Integrative Medicine. 2017;4:40–41. doi: 10.1016/j.aimed.2017.04.002. [DOI] [Google Scholar]
- 91.Bahman M., Hajimehdipoor H., Bioos S., Hashem-Dabaghian F., Afrakhteh M., Tansaz M. Effect of aslagh capsule, a traditional compound herbal product on oligomenorrhea in patients with polycystic ovary syndrome: a three-arm, open-label, randomized, controlled trial. Galen Medical Journal. 2019;8 doi: 10.31661/gmj.v8i0.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rouhani M., Motavasselian M., Taghipoor A., Layegh P., Asili J., Hamedi S.S., Avval S.B. Efficacy of a Persian herbal remedy and electroacupuncture on metabolic profiles and anthropometric parameters in women with polycystic ovary syndrome: a randomized controlled trial. Galen Medical Journal. 2019;8 doi: 10.31661/gmj.v8i0.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu W., Tang M., Wang J., Wang L. Clinical effects of Shou-Wu Jiang-Qi Decoction combined acupuncture on the treatment of Polycystic Ovarian Syndrome with kidney deficiency, phlegm and blood stasisness: study protocol clinical trial (SPIRIT Compliant) Medicine (United States) 2020:99. doi: 10.1097/MD.0000000000019045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park K.S. Efficacy and safety of herbal medicine Yijin-tang on polycystic ovary syndrome: a single-arm pilot study. J. Herb. Med. 2021;28 doi: 10.1016/j.hermed.2021.100454. [DOI] [Google Scholar]
- 95.Wang L., Liang R., Tang Q., Zhu L. An overview of systematic reviews of using Chinese medicine to treat polycystic ovary syndrome. Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/9935536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham Gnanadass S., Divakar Prabhu Y., Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch. Gynecol. Obstet. 2021;303:631–643. doi: 10.1007/s00404-020-05951-2. [DOI] [PubMed] [Google Scholar]
- 97.Noah T Ashley R.J.N. Zachary M weil Inflammation: mechanisms, costs and natural variation. Annual review of ecology evolution and systematics November. 2012;43:385–406. [Google Scholar]
- 98.Zhu J.L., Chen Z., Feng W.J., Long S.L., Mo Z.C. Sex hormone-binding globulin and polycystic ovary syndrome. Clin. Chim. Acta. 2019;499:142–148. doi: 10.1016/j.cca.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Ashraf S., Nabi M., Rasool S.U., Rashid F., Amin S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egyptian Journal of Medical Human Genetics. 2019;20 doi: 10.1186/s43042-019-0031-4. [DOI] [Google Scholar]
- 100.Sudjarwo S.A., Anwar C., Wardani G., Eraiko, K.J.A.P.J.o.R. Antioxidant and anti-caspase 3 effect of chitosan-Pinus merkusii extract nanoparticle against lead acetate-induced testicular toxicity in rat. 2019;8:13. [Google Scholar]
- 101.Cokluk E., Sekeroglu M.R., Aslan M., Balahoroglu R., Bilgili S.G., Huyut Z.J.R.R. Determining oxidant and antioxidant status in patients with genital warts. 2015;20:210–214. doi: 10.1179/1351000215Y.0000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reiter R.J., Tan D.-x., Osuna C., Gitto, E.J.J.o.b.s. Actions of melatonin in the reduction of oxidative stress. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez C., Mayo J.C., Sainz R.M., Antolín I., Herrera F., Martín V., Reiter, R.J.J.J.o.p.r. Regulation of antioxidant enzymes: a significant role for melatonin. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 104.Kumariya S., Ubba V., Jha R.K., Gayen J.R.J.A. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. 2021;17:2706–2733. doi: 10.1080/15548627.2021.1938914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao K., Bian C., Zhao X.J.E., medicine t. Association of polycystic ovary syndrome with metabolic syndrome and gestational diabetes: Aggravated complication of pregnancy. 2017;14:1271–1276. doi: 10.3892/etm.2017.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saha P., Kumar S., Datta K., Tyagi, R.K.J.T.J.o.S.B. Biology M. 2021. Upsurge in Autophagy, Associated with Mifepristone-Treated Polycystic Ovarian Condition, Is Reversed upon Thymoquinone Treatment. [DOI] [PubMed] [Google Scholar]
- 107.Ayyadurai T., Moola A.K., Mohan P.K., et al. Ameliorative effects of Guilandina bonduc L. aqueous seed extract on letrozole induced polycystic ovary syndrome in female wistar albino rats. ADV TRADIT MED (ADTM) 2022;22:885–903. doi: 10.1007/s13596-022-00652-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no data related to this review article. The data were only sourced from the literature listed in this article (Data included in article/supp. material/referenced in article).