Abstract
A cytofluorometric assay that allowed assessment of damage to phagocytosed Aspergillus fumigatus conidia at the single-cell level was developed. After ingestion by monocyte-derived macrophages (MDMs), conidia were reisolated by treatment of the cells with streptolysin O, a pore-forming toxin with lytic properties on mammalian cells but not on fungi. The counts obtained by staining of damaged conidia with propidium iodide and quantification by cytofluorometry correlated with colony counts. By the use of this method, we demonstrate that MDMs differentiated in vitro by low-dose granulocyte-macrophage colony-stimulating factor and gamma interferon have only a limited capacity to damage Aspergillus conidia in vitro. The killing rate 12 h after phagocytosis was found to be only 10 to 15%. However, intracellular loading of the phagocytes with amphotericin B (AmB) dose dependently enhanced the anticonidial activity. Preincubation of macrophages with only 1 μg of AmB per ml resulted in an uptake of 18 fg of AmB/cell, leading to killing rates of 50 to 60%. The experimental protocol provides a new tool for the rapid quantification of anticonidial activity against A. fumigatus in vitro. Intracellular accumulation of AmB may represent an important factor underlying the efficacy of this antifungal drug in the prophylaxis and treatment of Aspergillus infections.
Aspergillus fumigatus is becoming increasingly important as a causative agent of life-threatening infections in immunocompromised hosts (for reviews, see references 4, 5, 12, 21, and 34). During the last three decades, the number of invasive Aspergillus infections has risen dramatically and the incidence has been reported to vary between 5 and 19% in transplantation patients (15, 19). A. fumigatus is the most prominent pathogen in the Aspergillus family, accounting for more than 90% of human Aspergillus infections.
Amphotericin B (AmB) has remained the drug of choice for treatment. However, the lethality of manifest infections remains high and the toxicity of full-scale treatment is considerable (7, 8, 11). This has prompted exploration of possibilities for the prophylactic use of AmB, with the result that clinical regimens for low-dose intravenous application are now available (28). Furthermore, the possibility of aerosol application of AmB by inhalation has been investigated (2, 23).
Uptake of conidia by the respiratory system is the initial event in Aspergillus infections, with survival of conidia in phagocytes and the onset of germination being requisites for establishing disease. The reported ingestion and conidial killing activities of alveolar macrophages are widely regarded as central to the first line of defense (32). The mechanisms of conidial elimination by macrophages are not fully understood (for reviews, see references 30 and 31), and this may partly be due to a lack of methods for measuring conidial damage and killing. To quantify the killing, the ingested conidia are usually liberated by lysis of phagocytes, and viability is assessed either by microscopic observation of conidial outgrowth (20, 32) or by performing colony count studies (24, 38).
Previous studies have shown that in addition to its action against extracellular fungi, AmB can accumulate intracellularly and enhance the phagocytic killing of Candida albicans in vitro (18). Because AmB is the drug of choice for the treatment of Aspergillus infection, we investigated whether macrophages would also be able to accumulate AmB and whether this would have an effect on damage to A. fumigatus conidia.
In the course of this work, we developed a novel method based on detergent-free reisolation of conidia from phagocytes, followed by flow cytometric analysis with propidium iodide (PI), which is used to stain dead conidia. This allowed quantification of conidial killing at the level of a single conidium. We were unable to confirm previous reports that had suggested efficient conidial elimination by macrophages (24, 38). The killing capacity of monocyte-derived macrophages differentiated in culture with low-dose granulocyte-macrophage colony-stimulating factor (GM-CSF) and gamma interferon is surprisingly low but can be markedly enhanced by preincubation and loading of the cells with nontoxic concentrations of AmB.
MATERIALS AND METHODS
Preparation of conidial suspensions.
Conidia were prepared from strain ATTC 46645. After subculture on Sabouraud agar (Becton Dickinson, Heidelberg, Germany), conidial suspensions were prepared as described by Roilides et al. (25). In brief, the plates were washed with a physiological saline solution (0.9% [wt/vol] NaCl), and the conidial suspension was filtered twice through a sterile 40-μm-pore-size nylon mesh (Falcon, Heidelberg, Germany). Penicillin (100 U/ml) and streptomycin (100 μg/ml) (antibiotic mix; Gibco, Karlsruhe, Germany) were added, and the suspensions were stored at 4°C.
Preparation of MDMs.
Human monocytes were isolated from buffy coats as described previously (16, 18) and were seeded at a concentration of 1.5 × 106/ml/well in minimal essential medium (MEM) containing 10% normal human serum (NHS) in 24-well plates (Nunc, Wiesbaden, Germany). Monocyte-derived macrophages (MDMs) were obtained by culture for 5 to 7 days at 37°C in 5% CO2 in the presence of 2.5 ng of GM-CSF (Essex Pharma, Munich, Germany) per ml and 0.5 ng of gamma interferon (Gammaferon 50; Bioferon GmbH, Laupheim, Germany) per ml.
Drugs and reagents.
AmB was purchased from Squibbs Pharma, Vienna, Austria, and was kept as a 5-mg/ml stock in distilled water at −20°C. Working solutions were prepared in water. Recombinant human gamma interferon (Gammaferon 50; Bioferon GmbH) was diluted 1:100 in phosphate-buffered saline (PBS)–0.1% human serum albumin (Behring, Marburg, Germany) to yield a stock solution of 500 μg/ml which was stored in portions (50 μl) at −70°C. Human recombinant GM-CSF (Leukomax; Essex Pharma, Munich, Germany) was kept as 20-μl portions of 25 mg/ml at −20°C. Streptolysin O (SLO) was prepared as described previously (39), dissolved at a concentration of 2 mg/ml in PBS–0.1% bovine serum albumin, and kept in 10-μl portions at −70°C. An RNase (R-6513) stock solution (1 mg/ml), a DNase stock solution (2,000 kU/ml), and a proteinase K stock solution (20 mg/ml) (all purchased from Sigma, Munich, Germany) were prepared in normal saline and were kept at −20°C. Deoxycholate (Roth, Karlsruhe, Germany) was prepared as a 5% (wt/vol) stock solution in distilled water. Stock solutions of sodium dodecyl sulfate (5% [wt/vol]), Triton X-100 (10% [vol/vol]), and Nonidet P-40 (5% [vol/vol]), all purchased from Sigma, were prepared in distilled water and were kept at room temperature.
Effects of SLO on metabolic activity of MDMs and conidia.
The potential damaging effects of SLO on either conidia or MDMs were tested by a colorimetric test (13, 14). Conidia (106/well) were incubated with SLO (20 μg/ml) in PBS–0.1% BSA for 30 min. Thereafter, supernatants were carefully aspirated and were replaced by 50 μl of RPMI 1640 (Biochrom, Berlin, Germany) containing 0.5 mg of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Serva, Heidelberg, Germany) per ml and 100 μM menadione (Sigma). Incubations were continued for another 3 h to allow the formation of formazan by viable conidia (13). Formazan crystals were dissolved by the addition of 150 μl of acidic isopropanol (95 ml of isopropanol, 5 ml of 1 N HCl) per well and vigorous shaking for 10 min. Aliquots of 150 μl were transferred to enzyme-linked immunosorbent assay reader plates (Greiner, Nürtingen, Germany), and the amount of formazan was measured immediately in a microplate reader (EAR400; Salzburger Labortechnik, Crailsheim, Germany) at 550 nm. Wells without conidia served as background controls. To determine the effects of SLO on MDMs, metabolic activity in the cells was assessed in parallel by phase-contrast microscopy. MDMs were incubated with SLO, supernatants were aspirated and replaced by 50 μl of RPMI 1640 containing MTT-menadione, and the assay was then continued as described above for the conidia. The percentage of formazan formed was calculated by the following equation: relative formazan formation = (ODs − ODb)/(ODC − ODb) × 100, where ODs is the optical density of the sample, ODb is the optical density of the background, and ODc is the optical density of the control sample (without toxin).
Measurement of cell-associated AmB.
AmB was quantified by scanning spectrophotometry as originally described by Shihabi et al. (33) with modifications for measurement of cell-associated AmB as described by Martin et al. (18). In brief, MDMs (3 × 106/well) were cultured in six-well culture plates (Delta plate; Nunc, Wiesbaden, Germany). After overnight incubation with AmB at 0.5 and 1.0 μg/ml in the culture medium, the supernatants were discarded and the cells were carefully washed three times with PBS. Then, 400 μl of acetonitrile was added to the wells. After vigorous mixing, the cells were scraped off, the suspension was transferred to an Eppendorf tube, and the tube was centrifuged at 10,000 × g for 2 min. The supernatants were transferred to quartz microcuvettes (Zeiss, Frankfurt on Main, Germany), and scanning photometry was performed at wavelengths of 350 to 450 nm with a Ultrospec II photometer (Pharmacia LKB, Freiburg, Germany) connected to a data processing unit. Calculations were performed by linear regression analysis on the basis of standard curves for AmB.
AmB pretreatment of MDMs.
Prior to incubation with conidia, the cells were incubated for 16 h in 1 ml of MEM–10% NHS containing various concentrations of AmB ranging from 0.1 to 1.0 μg/ml. Subsequently, the cells were washed three times with 1 ml of PBS (pH 7.3), and finally, 1 ml of MEM–10% NHS was added.
Incubation of MDMs with conidia and reisolation of conidia.
To each well containing MDMs, 1.5 × 106 conidia were added and the plates were centrifuged at 200 × g for 5 min. After incubation for 12 h, the medium was removed and stored at −20°C for later assessment of residual AmB activity. A total of 150 μl of 0.1% BSA containing SLO at a concentration of 20 μg/ml was added to the wells, and the plates were incubated at 37°C for 30 min. The content of each well was drawn three times through an Eppendorf pipette (200 μl), and the suspension was then transferred to an Eppendorf tube and kept at 37°C. After rinsing with 100 μl of PBS, each well was checked for complete removal of cells by microscopic inspection. The tubes were transferred to an Eppendorf thermomixer (37°C), and the following components were added: MgCl2 and CaCl2, each to final concentration of 0.5 mM; 10 μl of RNase (1 mg/ml); and 10 μl of DNase (2,000 kU/ml). The incubation was then continued for 10 min. Subsequently, 3 μl of proteinase K (20 mg/ml) was added for another 10 min. Finally, the tubes were transferred to a water bath sonifier (Bandelin Electronic, Berlin, Germany) for 10 min and then kept on ice. An aliquot of 100 μl was used for determination of the numbers of CFU, and the remaining suspension was stored at 4°C overnight before being subjected to cytofluorometric analysis. Cells incubated with conidia for 45 min to allow phagocytosis only were used as controls for the overall viability of the conidia. Wells containing MDMs only were treated as described above and served as background controls in cytofluorometric measurements.
Quantification of conidial killing by colony counting.
For each sample, aliquots corresponding to approximately 100 and 1,000 CFU were plated in duplicate on Sabouraud agar plates containing 0.03% deoxycholate, and the plates were incubated at 37°C for 36 h. Colonies were counted under a stereomicroscope and a kill index (KI) was calculated as 1 − (CFUs/CFUc), where CFUs is the colony count of the respective sample, and CFUc corresponds to the colony counts of the phagocytosis control.
PI staining and cytofluorometry.
PI was added to the reisolated conidial samples at a final concentration of 50 μg/ml. After incubation for 20 min at room temperature the sample volume was adjusted to 500 μl with PBS. Each sample was vigorously vortexed before cytofluorometric measurement in a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany). Acquisition was done with LysisII Software (Becton Dickinson), and the parameters were E-01 for forward scatter (FSC), 305 for side scatter (SSC), and 507 for the fluorescence 3 (FL-3) detector. A live gate was set for each experiment. A relative FL-3 intensity above 101 was considered to reflect positive PI staining, and a marker (M1) was set accordingly. The average measurement time for 2,500 events within the gate corresponding FSC/SSC characteristics was 15 s. Analysis was performed by using either LysisII software or WINMDI, version 2.3, a public-domain cytofluorometer analysis software package by J. Trotter (http://facs.scripps.edu).
Statistical analysis.
Statistical analysis was performed with Prism software (GraphPad Inc., San Diego, Calif.). Determination of significance was done by a two-tailed Mann-Whitney test.
RESULTS
Effect of detergents on PI staining of conidia.
Existing methods for reisolation of phagocytosed conidia include the use of detergents (24, 27, 37, 38). Hence, we first attempted to use deoxycholate as the lysis reagent. Resting conidia did not stain with PI. Unexpectedly, however, if the conidia were first incubated for several hours in medium to permit transition to the metabolically active stage, exposure to deoxycholate caused them to stain positively with PI. This is shown in Fig. 1. The same results were obtained when sodium dodecyl sulfate, Triton X-100, or Nonidet P-40 (all at a 0.5% final concentration) was used. This finding suggested that as the conidia became metabolically active, they became sensitive to the actions of the detergents. As a consequence, to exclude damaging effects on conidia during reisolation from macrophages, detergent-free cell lysis procedures had to be developed.
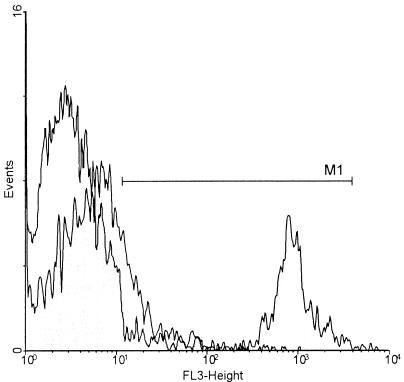
FIG. 1.
Deoxycholate promotes PI uptake in metabolically active conidia. Conidia were incubated in MEM for 4 h at 37°C and were then treated with deoxycholate (0.5%, 10 min, 23°C). When stained with PI, the number of PI-positive conidia increased by 30% (░⃞) compared to the number of control conidia before culture (□). The PI fluorescence intensity (FL-3) is plotted on the x axis against the number of cells (events) on the y axis. The marker (M1) defines the range of PI-positive conidia.
Reisolation of phagocytosed conidia from MDMs with the use of SLO.
We turned to the use of an agent that would selectively destroy mammalian cells while leaving fungi intact. SLO was chosen for this purpose. In a first set of experiments monocytes or conidia were incubated with SLO, and metabolic activity was assessed by the MTT assay. Incubation of MDMs with SLO at concentrations of 1 to 20 μg/ml for 10 min led to a dose-dependent reduction of MTT conversion. This was paralleled by disintegration of the cells, as observed by phase-contrast microscopy. In contrast, no effect on the metabolic activity of conidia was detected (Fig. 2). By combining SLO treatment with enzymatic digestion, the cells could be lysed extensively, so that phagocytosed conidia were liberated, and these became detectable as a single population by cytofluorometry. This is shown in a dot plot (Fig. 3A and B). The position within the plot was identical to that of the conidia analyzed prior to phagocytosis (Fig. 3A). The background, i.e., cell debris particles eliciting signals within the live gate defined for conidia, was determined for each experiment and ranged from 2 to 5% of the total counts, which was considered negligible. No uptake of PI was seen by conidia that had been incubated in medium for 4 h (Fig. 3C).
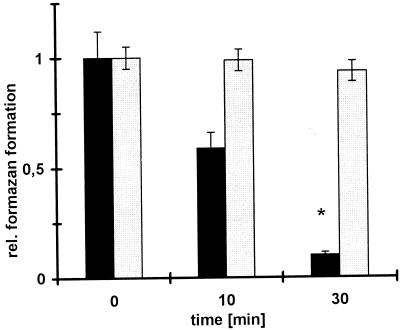
FIG. 2.
SLO affects metabolic activity in MDMs but not in conidia. MDMs and conidia were incubated with SLO (20 μg/ml) in PBS–0.1% BSA for the indicated times (x axis). Subsequently, the metabolic activity was assessed by formazan formation (optical density at 550 nm; y axis) by a menadione-augmented MTT test. The metabolic activity in MDMs (■) decreased by 90% but was unaffected in conidia (░⃞). A relative formazan formation value of 1 corresponds to 100%; values are given as means ± standard errors of the means from three independent experiments. ∗, P < 0.05.
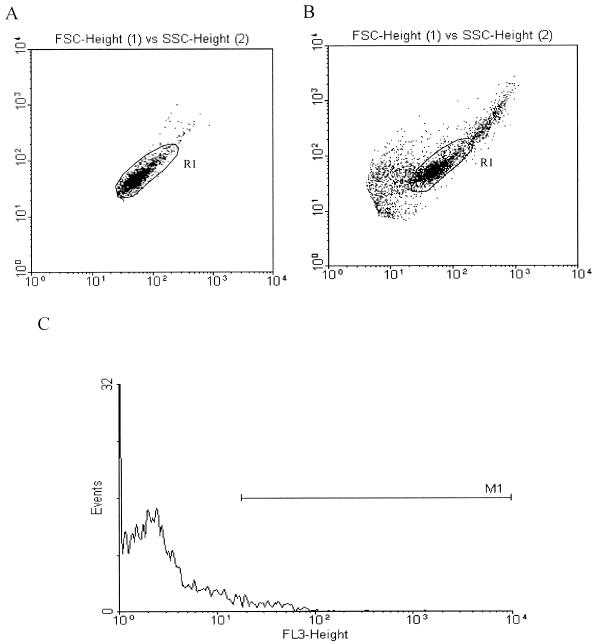
FIG. 3.
Conidia can be liberated from phagocytes through lysis with SLO: flow cytometric analysis. (A) A region (R1) was defined according to the size (FSC) and granularity (SSC) characteristics of the conidia. (B) After phagocytosis and an intracellular stay for 12 h, the conidia were isolated from MDMs by a combination of SLO lysis and mixing with enzymes. Conidia were detected as a distinct population within R1 as defined in panel A, and 2,500 events/sample were gated. (C) After phagocytosis and reisolation as described for panel B; PI staining and fluorescence-activated cell sorter analysis were performed. Histogram analysis of the population within region R1 (B) showed less than 8% PI-positive conidia, as defined by a marker (M1). The numbers of conidia (events; y axis) are plotted against PI fluorescence intensity (FL-3; x axis).
Use of detergents to reisolate phagocytosed conidia creates killing artifacts.
The pilot PI uptake experiments had suggested that detergents might damage metabolically active conidia, thus creating artifacts during reisolation procedures. This was confirmed by determination of the viability by obtaining colony counts for conidia reisolated from MDMs either by deoxycholate or by SLO treatment. When the detergent was used, a killing rate of approximately 90% (KI = 0.9) within 12 h after phagocytosis was observed. In contrast, much lower rates of killing (only 16% after 12 h and 27% after 24 h) were determined when the detergent-free procedure with SLO was used (Fig. 4). Thus, deoxycholate at concentrations required for solubilization damages A. fumigatus conidia unspecifically and cannot be used in these assays.
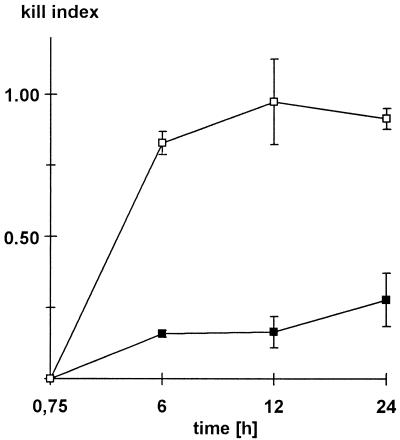
FIG. 4.
Deoxycholate susceptibility of conidia reisolated from MDMs. MDMs and conidia were incubated for the indicated times (x axis). Then, the conidia were reisolated from cells by either deoxycholate (□) or SLO (■) lysis and colony counts were determined. Killing rates are expressed as relative KI, with a value of 1 corresponding to 100% killing. Killing rates rose to approximately 90% within 12 h when deoxycholate was used. After SLO lysis significantly lower killing rates of 16% (12 h) and 27% (24 h) were detected (P = 0.02). Values are means ± standard errors of the means of three independent experiments.
Accumulation of AmB in MDMs.
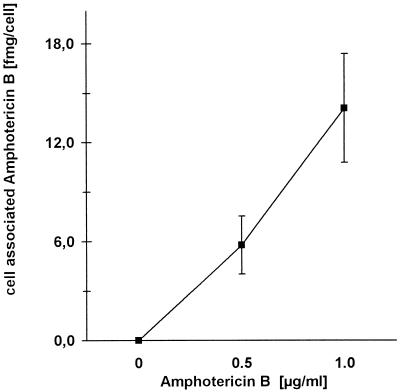
The accumulation of AmB in MDMs was determined after pretreating the cells with 0.5 or 1.0 μg of AmB per ml for 14 h. The cell-associated AmB was quantified by wavelength scan photometry. As shown in Fig. 5, a dose-dependent uptake by MDMs was observed. Pretreatment of the cells with the antimycotic at a final concentration of 1 μg/ml led to an accumulation of approximately 14 fg of AmB per cell (Fig. 5).
FIG. 5.
Association of AmB with MDMs. MDMs were incubated for 14 h with AmB at the given concentrations (x axis). After washing with PBS, AmB was extracted from the cells with acetonitrile and the concentration of cell-associated AmB was determined by photometry. Results are given as the amount of AmB per cell (mean ± standard deviation; n = 3).
Effect of cell-associated AmB on phagocytic killing of conidia as determined by flow cytometry.
MDMs were first incubated with 1 μg of AmB per ml to allow intracellular AmB accumulation as described above. Subsequently, the conidia were added. After 12 h, the conidia were reisolated from the cells by the SLO lysis procedure. As controls, conidia were reisolated from the cells just after complete phagocytosis, i.e., after 45 min of incubation with MDMs. The reisolated conidia were stored overnight at 4°C and were then stained with PI. Overnight storage proved to be essential in order for PI uptake to be observed. The proportion of PI-positive conidia in controls was in the range of 6 to 9% (Fig. 6). For controls incubated for 45 min, the proportion of PI-positive conidia was 8.03% ± 0.01% (mean ± standard error of the mean; n = 4 independent experiments). Prolongation of the intracellular stay to 12 h increased the percentage of PI-positive conidia to 17.96% ± 0.06% (mean ± standard error of the mean of four independent experiments (Fig. 6).
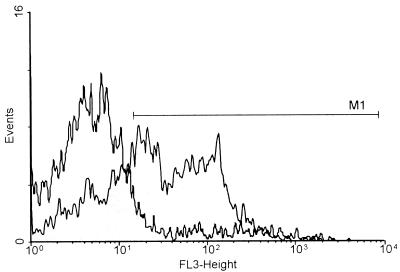
FIG. 6.
Histogram analysis of PI fluorescence in conidia reisolated from AmB-treated MDMs. After a 12-h incubation, the conidia were reisolated from untreated (□) and AmB (1 μg/ml)-pretreated (░⃞) MDMs. Histogram analysis of the PI-stained conidia was performed. An approximately fourfold increase in the numbers of PI-positive conidia was found in samples from AmB-treated MDMs compared with the numbers found in samples from untreated MDMs. The number of conidia (events; y axis) is plotted against the PI fluorescence intensity (FL-3; x axis, logarithmic scale). The marker (M1) defines the range for PI-positive staining.
As shown in the experiment whose results are presented in Fig. 6, the percentage of conidia staining positive for PI increased from 16% in untreated cells to 59% in MDMs that had been pretreated with 1 μg of AmB per ml. Similar results were obtained in all experiments; a marked increase in the numbers of PI-positive conidia after phagocytosis by AmB-loaded cells was observed, with a maximum of 65% (mean ± standard error of the mean of four independent experiments, 57.28% ± 0.04% [P = 0.02]). This indicated that pretreatment of MDMs with AmB led to a marked increase in anticonidial activity.
Determination of anticonidial activity by colony counting.
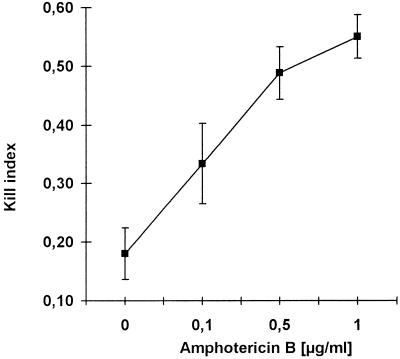
Colony counting, the “gold standard” for assessing conidial killing was performed with samples from the reisolated conidia. Aliquots were plated out directly at the end of the reisolation procedure. Each count was determined in duplicate. With untreated MDMs, a KI of 0.18 (median killing, 18%) was found (Fig. 7). In contrast, MDMs that had been preincubated with AmB for 14 h had much higher levels of anticonidial activity (Fig. 7). Augmentation of conidial killing was dependent on the drug concentration, and a maximum kill of 55% ± 7% was seen for cells that had been pretreated with 1 μg of AmB per ml. These results were similar to those obtained by the cytofluorometric assay (Fig. 6).
FIG. 7.
Effect of AmB pretreatment on conidial killing determined by colony counting. MDMs were preincubated with AmB at the indicated concentrations and washed; the conidia were then added. Twelve hours after phagocytic uptake, reisolated conidia were plated on Sabouraud agar and colonies were enumerated after 2 to 3 days. By comparison with the controls, which were incubated for only 45 min, a KI (y axis) was calculated for each sample. A dose-dependent increase in conidial killing was observed in AmB-pretreated MDMs (mean ± standard deviation; n = 5).
Correlation between cytofluorometric measurements and colony counting.
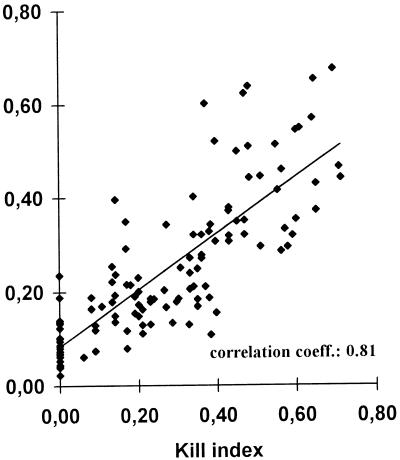
Killing rates obtained in parallel from cytofluorometric measurement and colony count determinations were correlated. Data derived from 107 kill determinations formed the basis for statistical evaluation by linear regression analysis, shown as a regression curve (Fig. 8). A correlation coefficient of 0.81 was calculated (Fig. 8). The results of the statistical evaluation indicated a clear correlation between the conidial killing assessed either by cytofluorometry or by determination of the numbers of CFU.
FIG. 8.
Comparison of conidial killing assessment by colony counting and PI cytofluorometry. The KIs, which were quantified by colony counting (x axis) and from the percentage of PI-positive conidia (y axis), were determined for the same samples (n = 107). Linear regression analysis was performed, and the correlation coefficient was determined to be 0.81 (P < 0.005).
DISCUSSION
Invasive pulmonary aspergillosis is a typical opportunistic infection in patients with sustained immunosuppression (4, 19, 34). AmB, the drug of choice, has considerable dose-related side effects, and despite adequate treatment, the rate of lethality remains high (5, 8, 11). Thus, the use of low-dose intravenous AmB has evolved as one strategy of prophylaxis (28). Furthermore, the local prophylactic administration of AmB at the port of entry, i.e., as an aerosol application to the lung, has also been reported (2, 23).
After the administration of therapeutic doses of AmB, the AmB accumulates in lung tissue, and the uptake of AmB by alveolar macrophages has been reported (1, 6). Previous studies by Martin and Bhakdi (16) demonstrated that AmB also accumulates in monocytes in vitro and that this enhances the ability of the cells to kill C. albicans in vitro. Here, we investigated whether macrophages would also be able to accumulate AmB in vitro and whether cell-associated AmB would have an effect on activity against the conidia of A. fumigatus, the main causative agent of invasive pulmonary aspergillosis.
Pulmonary alveolar macrophages are considered the first line of defense against Aspergillus conidia and are able to kill conidia in vitro (30–32). It has also been shown by Schaffner and colleagues (29, 32) that blood monocyte-derived macrophages from either rabbits or humans display comparable anticonidial activities (29, 32). Hence, human monocyte-derived macrophages have been used in in vitro studies with Aspergillus (20, 26, 29, 32, 38) and were also used as effector cells in our study. To obtain a constant degree of differentiation, low doses of gamma interferon and GM-CSF were present during culture.
Methods used to assess conidial killing are limited and involve either microscopic assessment of conidial germination (20, 32) or colony counting (24, 38). Microscopy is difficult to quantify, and determination of the numbers of CFU is tedious and time-consuming. Therefore, we developed a novel method based on cytofluorometry, which determines the level of killing at the single conidium level. Membrane permeability for PI was selected as the indicator for conidial damage. PI uptake can be detected by argon laser cytofluorometry and has been used for killing assessments with mammalian cells as well as fungal organisms, e.g., Candida (17, 35). Reisolation from phagocytes is a basic prerequisite for the assessment of conidial killing, and detergents have been used for this purpose (24, 38). However, we found that after transition of conidia from the dormant to the metabolically active state, PI uptake occurred upon treatment with deoxycholate, suggesting a damaging effect on the conidia.
Therefore, it became essential to develop a detergent-free reisolation procedure. To this end, the selective action of SLO on mammalian cells was exploited (3, 39). Exposure to SLO resulted in permeabilization and fragmentation of the phagocytes, paralleled by a sharp decline in their metabolic activity. In fungi, cholesterol is replaced by ergosterol (10); hence, the binding structure for SLO is absent. As expected, conidial metabolic activity and cell wall integrity were not affected by SLO.
The conidial killing rate 12 h after phagocytosis by untreated macrophages was in the range of 15 to 18% when the SLO reisolation method was used. This is in accordance with the killing rates derived from experiments in which hypotonic lysis with distilled water was used for reisolation (20, 29, 32). In contrast, colony counts for conidia reisolated by deoxycholate treatment indicated a much higher rate of killing (approximately 90%). Similar high killing rates (60%) after 2 to 3 h of phagocytosis have been found in other studies that used detergent lysis (24, 27, 37, 38). Our present data now indicate that the use of detergents can give rise to incorrect results. Hypotonic lysis with water is better, but it generates high levels of background cell debris so that cytofluorometry cannot be used to detect the conidia (data not shown). The SLO-based lysis procedure produced dispersed conidial suspensions, rendering these accessible to identification as a distinct population by flow cytofluorometry.
Preincubation of MDMs with AmB resulted in a dose-dependent cellular accumulation of the drug. The intracellular concentration, estimated on the basis of a cell volume of 500 fl and an amount of 14 fg/cell, was 7 μg/ml, which is in the therapeutic range (8, 11; unpublished observations). Similar results have been reported for human monocytes (18). The cellular accumulation of the antimycotic resulted in a marked increase in the percentage of PI-positive conidia. Notably, uptake of PI by the conidia was observed only after overnight incubation of the cells at 4°C. This finding suggested that breakdown of the membrane permeability barrier lagged behind the primary damaging event incurred by the phagocytes.
Conidial colony counts from AmB-pretreated MDMs were reduced correspondingly. Since a direct effect of AmB in the media was excluded by testing them for antifungal activity in a menadione-augmented MTT test (9, 13, 14) (data not shown), the observed enhancement of anticonidial activity must have been due to cell-associated AmB. This is in line with previous studies of Martin et al. (18) showing that the intracellular accumulation of AmB enhances the ability of the cells to kill C. albicans. To date, the effect of AmB on the antifungal properties of phagocytes has been studied exclusively with Candida. In their early studies, Perfect et al. (22) used much higher concentrations of AmB, and an indirect antifungal effect mediated by activation of the phagocytes was discussed. Martin et al. (18), however, have clearly shown that low-dose AmB pretreatment (the concentrations used in the present study) resulted in a marked increase in the candidacidal activity of human monocytes without activating the cells. The possibility of a direct antifungal effect of cell-associated AmB has also been implicated from results from studies by van Etten et al. (36), in which low-dose AmB (0.4 μg/ml) enhanced Candida killing by murine macrophages.
During cell culture, low-dose GM-CSF as well as gamma interferon were present, and both cytokines reportedly augmented the activity of monocytes against Aspergillus hyphae (26). In contrast, the anticonidial activity of MDMs is not influenced by gamma interferon (29; unpublished observations). To our knowledge, no studies are available on the effect of GM-CSF on conidial killing by MDMs. We share, however, an unpublished observation with Schaffner (31), who mentions that no significant change in the killing capability of MDMs can be induced by GM-CSF. Furthermore, the basal killing rates that we observed in MDMs are similar to the killing rates reported from studies in which no cytokines were present during in vitro differentiation. Thus, it can be assumed that the culture of freshly isolated monocytes with low-dose GM-CSF and gamma interferon does not significantly alter the basic anticonidial activity of MDMs. Whether or not cytokines may contribute to the increase in conidial killing after AmB accumulation in the phagocytes needs further investigation.
Conclusion.
Reisolation of conidia after ingestion by phagocytes and assessment of PI uptake by cytofluorometry were established as novel methods for the quantification of conidial killing by phagocytes. This new tool obviates the exposure of conidia to detergents, which may have an intrinsic damaging effect on phagocytosed conidia.
The present study is the first to investigate the effect of cell-associated AmB on the conidia of A. fumigatus. The results indicate that the cellular accumulation of AmB enhances the ability of human macrophages to kill the fungus. Cell-associated AmB may be a factor contributing to the prophylactic and therapeutic efficacies of this antifungal drug against Aspergillus infections.
ACKNOWLEDGMENTS
We thank M. Hussmann for helpful discussion.
This work was supported by the Ministerium für Umwelt und Forsten des Landes Rheinland-Pfalz.
REFERENCES
- 1.Benson J M, Nahata M C. Clinical use of systemic antifungal agents. Clin Pharm. 1988;7:424–438. [PubMed] [Google Scholar]
- 2.Beyer J, Barzen G, Risse G, Weyer C, Miksits K, Dullenkopf K, Huhn D, Siegert W. Aerosol amphotericin B for prevention of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1993;37:1367–1369. doi: 10.1128/aac.37.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S, Weller U, Walev I, Martin E, Jonas D, Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol Berlin. 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- 4.Bouchara J P, Tronchin G, Larcher G, Chabasse D. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 1995;3:327–330. doi: 10.1016/s0966-842x(00)88965-9. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W. Invasive aspergillosis in immunocompromised patients. Curr Opin Infect Dis. 1994;7:456–462. [Google Scholar]
- 6.Edmonds L C, Davidson L, Bertino J S. Effect of variation in infusion time and macrophage blockade on organ uptake of amphotericin B-deoxycholate. J Antimicrob Chemother. 1991;28:919–924. doi: 10.1093/jac/28.6.919. [DOI] [PubMed] [Google Scholar]
- 7.Ellis M E, al-Hokail A A, Clink H M, Padmos M A, Ernst P, Spence D G, Tharpe W N, Hillier V F. Double-blind randomized study of the effect of infusion rates on toxicity of amphotericin B. Antimicrob Agents Chemother. 1992;36:172–179. doi: 10.1128/aac.36.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser I S, Denning D W. Empiric amphotericin B therapy: the need for a reappraisal. Blood Rev. 1993;7:208–214. doi: 10.1016/0268-960x(93)90007-q. [DOI] [PubMed] [Google Scholar]
- 9.Garn H, Krause H, Enzmann V, Drossler K. An improved MTT assay using the electron-coupling agent menadione. J Immunol Methods. 1994;168:253–256. doi: 10.1016/0022-1759(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D H. Fungal physiology. New York, N.Y: Wiley-Liss; 1994. pp. 23–62. [Google Scholar]
- 11.Hoeprich, P. D. 1992. Clinical use of amphotericin B and derivatives: lore, mystique, and fact. Clin. Infect. Dis. 14(Suppl. 1):S114–S119. [DOI] [PubMed]
- 12.Holden D W, Tang C M, Smith J M. Molecular genetics of Aspergilluspathogenicity. Antonie Leeuwenhoek. 1994;65:251–255. doi: 10.1007/BF00871953. [DOI] [PubMed] [Google Scholar]
- 13.Jahn B, Martin E, Stüben A, Bhakdi S. Susceptibility testing of Candida albicans and Aspergillusspecies by a simple microtiter menadione-augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay. J Clin Microbiol. 1995;33:661–667. doi: 10.1128/jcm.33.3.661-667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn B, Stüben A, Bhakdi S. Colorimetric susceptibility testing for Aspergillus fumigatus: comparison of menadione augmented 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide and Alamar Blue tests. J Clin Microbiol. 1996;34:2039–2041. doi: 10.1128/jcm.34.8.2039-2041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer M R, Marshall S E, Starnes V A, Gamberg P, Amitai Z, Theodore J. Infectious complications in heart-lung transplantation. Analysis of 200 episodes. Arch Intern Med. 1993;153:2010–2016. [PubMed] [Google Scholar]
- 16.Martin E, Bhakdi S. Quantitative analysis of opsonophagocytosis and of killing of Candida albicansby human peripheral blood leukocytes by using flow cytometry. J Clin Microbiol. 1991;29:2013–2023. doi: 10.1128/jcm.29.9.2013-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin E, Maier F, Bhakdi S. Antagonistic effects of fluconazole and 5-fluorocytosine on candidacidal action of amphotericin B in human serum. Antimicrob Agents Chemother. 1994;38:1331–1338. doi: 10.1128/aac.38.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin E, Stüben A, Gorz A, Weller U, Bhakdi S. Novel aspect of amphotericin B action: accumulation in human monocytes potentiates killing of phagocytosed Candida albicans. Antimicrob Agents Chemother. 1994;38:13–22. doi: 10.1128/aac.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McWhinney P H, Kibbler C C, Hamon M D, Smith O P, Gandhi L, Berger L A, Walesby R K, Hoffbrand A V, Prentice H G. Progress in the diagnosis and management of aspergillosis in bone marrow transplantation: 13 years’ experience. Clin Infect Dis. 1993;17:397–404. doi: 10.1093/clinids/17.3.397. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Osusky I, Schoedon G, Blauer F, Schneemann M, Schaffner A. Comparison of the antimicrobial activity of deactivated human macrophages challenged with Aspergillus fumigatus and Listeria monocytogenes. J Infect Dis. 1996;174:651–654. doi: 10.1093/infdis/174.3.651. [DOI] [PubMed] [Google Scholar]
- 21.Musial C E, Cockerill F R, Roberts G D. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988;1:349–364. doi: 10.1128/cmr.1.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect J R, Granger D L, Durack D T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987;156:316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- 23.Purcell I F, Corris P A. Use of nebulised liposomal amphotericin B in the treatment of Aspergillus fumigatusempyema. Thorax. 1995;50:1321–1323. doi: 10.1136/thx.50.12.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson M D, Kerr K M, Seaton A. Killing of Aspergillus fumigatusspores by human lung macrophages: a paradoxical effect of heat-labile serum components. J Med Vet Mycol. 1989;27:295–302. doi: 10.1080/02681218980000401. [DOI] [PubMed] [Google Scholar]
- 25.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatushyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:4870–4877. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roilides E, Holmes A, Blake C, Venzon D, Pizzo P A, Walsh T J. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-gamma. J Infect Dis. 1994;170:894–899. doi: 10.1093/infdis/170.4.894. [DOI] [PubMed] [Google Scholar]
- 27.Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo P A, Walsh T J. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–329. [PubMed] [Google Scholar]
- 28.Rousey S R, Russler S, Gottlieb M, Ash R C. Low-dose amphotericin B prophylaxis against invasive Aspergillusinfections in allogeneic marrow transplantation. Am J Med. 1991;91:484–492. doi: 10.1016/0002-9343(91)90184-y. [DOI] [PubMed] [Google Scholar]
- 29.Schaffner A. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J Clin Invest. 1985;76:1755–1764. doi: 10.1172/JCI112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaffner A. Host defense in aspergillosis. In: Benett J E, Hay R J, Peterson P K, editors. New strategies in fungal disease. Edinburgh, United Kingdom: Churchill Livingstone; 1992. pp. 98–112. [Google Scholar]
- 31.Schaffner A. Macrophage-Aspergillusinteractions. Immunol Ser. 1994;60:545–552. [PubMed] [Google Scholar]
- 32.Schaffner A, Douglas H, Braude A I, Davis C E. Killing of Aspergillusspores depends on the anatomical source of the macrophage. Infect Immun. 1983;42:1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shihabi Z K, Wasilauskas B L, Peacock J E. Serum amphotericin-B assay by scanning spectrophotometry. Ther Drug Monit. 1988;10:486–489. doi: 10.1097/00007691-198804000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Stephan J L, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint Basile G, Durandy A, Griscelli C, Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993;123:564–572. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 35.Tanke H J, van der Linden P W, Langerak J. Alternative fluorochromes to ethidium bromide for automated read out of cytotoxicity tests. J Immunol Methods. 1982;52:91–96. doi: 10.1016/0022-1759(82)90353-2. [DOI] [PubMed] [Google Scholar]
- 36.van Etten E W, van de Rhee N E, van Kampen K M, Bakker-Woudenberg I A. Effects of amphotericin B and fluconazole on the extracellular and intracellular growth of Candida albicans. Antimicrob Agents Chemother. 1991;35:2275–2281. doi: 10.1128/aac.35.11.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washburn R G, Gallin J I, Bennett J E. Oxidative killing of Aspergillus fumigatusproceeds by parallel myeloperoxidase-dependent and -independent pathways. Infect Immun. 1987;55:2088–2092. doi: 10.1128/iai.55.9.2088-2092.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Washburn R G, Hammer C H, Bennett J E. Inhibition of complement by culture supernatants of Aspergillus fumigatus. J Infect Dis. 1986;154:944–951. doi: 10.1093/infdis/154.6.944. [DOI] [PubMed] [Google Scholar]
- 39.Weller U, Muller L, Messner M, Palmer M, Valeva A, Tranum-Jensen J, Agrawal P, Biermann C, Dobereiner A, Kehoe M A, Bhakdi S. Expression of active streptolysin O in Escherichia colias a maltose-binding-protein-streptolysin-O fusion protein. The N-terminal 70 amino acids are not required for hemolytic activity. Eur J Biochem. 1996;236:34–39. doi: 10.1111/j.1432-1033.1996.00034.x. [DOI] [PubMed] [Google Scholar]