Summary
In this comprehensive review, we will dissect the impact of research on proteoglycans focusing on recent developments involved in their synthesis, degradation, and interactions, while critically assessing their usefulness in various biological processes. The emerging roles of proteoglycans in global infections, specifically the SARS-CoV-2 pandemic, and their rising functions in regenerative medicine and biomaterial science have significantly affected our current view of proteoglycans and related compounds. The roles of proteoglycans in cancer biology and their potential use as a next-generation protein-based adjuvant therapy to combat cancer is also emerging as a constructive and potentially beneficial therapeutic strategy. We will discuss the role of proteoglycans in selected and emerging areas of proteoglycan science, such as neurodegenerative diseases, autophagy, angiogenesis, cancer, infections and their impact on mammalian diseases.
Subject areas: Cellular physiology, Neuroscience, Cancer
Graphical abstract
Cellular physiology; Neuroscience; Cancer
Introduction
General structural and functional considerations
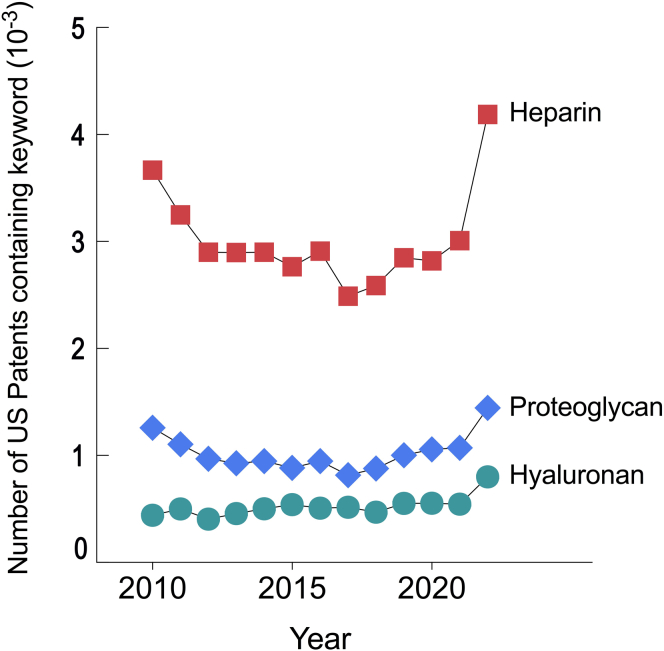
The world of proteoglycan science has markedly expanded in recent decades and has now reached an international stature. A wider knowledge of proteoglycan biology is a desirable goal that poses worthy and timely objectives of this comprehensive review article. A recent search of the total number of patents between 2010 and 2022 filed and logged in the US Patent Public Search database (USPTO) has revealed many patents involving proteoglycans and related molecules (Figure 1). Data were accumulated from 2010 to 2022 using keywords such as heparin, proteoglycan, or hyaluronan within the published document with the following search engine: https://ppubs.uspto.gov/pubwebapp/. It is not surprising that heparin, one of the most important anti-coagulant factors, is the most frequently used with over 4,000 patents in this dataset.
Figure 1.
Graphic showing increased number of US patents containing key proteoglycan words in their title
Compilation of the total number of patents per year filed and logged in the US Patent Public Search database (USPTO) that contain the keywords: “heparin,” “proteoglycan,” or “hyaluronan” within the published document. Data were accumulated from 2010 to 2022 using the following search engine: https://ppubs.uspto.gov/pubwebapp/.
Proteoglycans function in a variety of molecular networks1,2,3,4 and act on distinct signaling pathways mainly due to their complex and composite nature. Indeed, proteoglycans are unique and specialized protein/polysaccharide hybrid molecules that harbor protein cores of diverse dimensions and glycosaminoglycan (GAG) side chains of varied chemical composition, size, and structure.5,6
It is noteworthy that the family of full-time proteoglycans comprises a very small percentage of the human genome. Currently there are about 20,000 protein-encoding genes in the main human databases, GENCODE/Ensembl, RefSeq, and UniProtKB. However, the proteoglycan family contains only about 50 individual protein cores, or ∼0.25%. In addition, many protein cores are highly conserved across species.7 These features suggest that proteoglycans are functionally essential to organismal structure and function, and consequently are involved in the pathophysiology of many diseases.
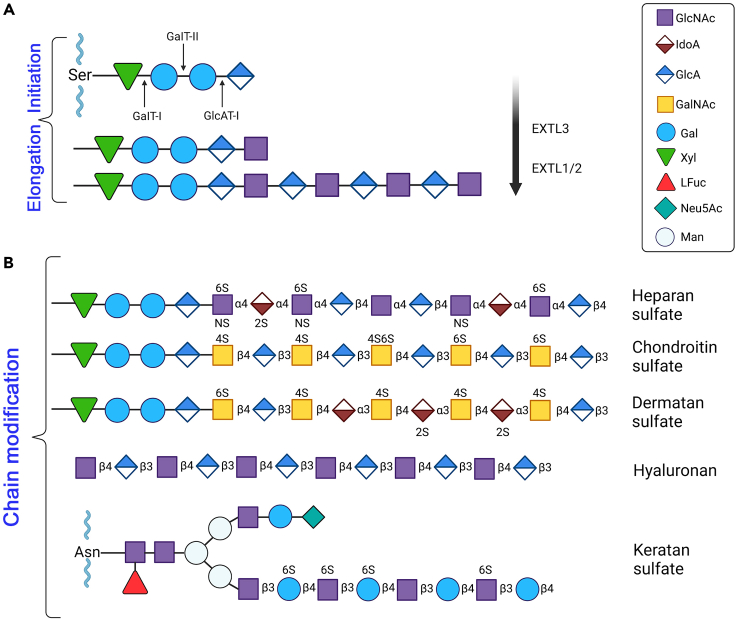
Proteoglycans can be comprehensively classified according to a taxonomy that integrates various characteristics including their cellular and subcellular localization, homology at the genetic and protein levels, and the presence of specific protein modules in their respective protein cores.5 Proteoglycans are often named and categorized after their GAG chains—long, linear carbohydrate chains that are constituted of alternating, repeating disaccharides.8 More specifically, these GAG chains cf. structural variation among proteoglycans as the quality, degree of sulfation, and number of attached chains can vary drastically,9,10 providing these complex macromolecules with copious biological activities. An example of the initial steps of GAG biosynthesis, in this case heparan sulfate (HS) synthesis, involving chain initiation and elongation is shown in Figure 2A. The polymerization of typical GAG chains follows a general pattern of an amino sugar: N-acetylglucosamine, N-substituted glucosamine, or N-acetylgalactosamine followed by an uronic acid: glucoronic acid or iduronic acid or alternatively a galactose.8 To better understand the variation within GAG chains, it is important to delve into the biosynthesis of these chains—more specifically HS and chondroitin sulfate (CS) as there is recent evidence for site-specific glycosylation of proteoglycans depending on the amino acid sequence of the specific attachment site.11,12 The process begins in the endoplasmic reticulum-Golgi apparatus interface with the addition of xylose to a serine residue in the protein core (Figure 2A). This is catalyzed by xylosoltransferase 1,2. Next, two galactoses are added along with one glucuronic acid which forms the characteristic Xyl-Gal-Gal-GlcA tetrasaccharide linkage region. The difference between CS and HS synthesis starts here, where there is either an addition of β1-4GalNAc for CS synthesis or α1-4GlcNAc for HS synthesis.13 Subsequently, the nascent GAGs chains are elongated and, for HS synthesis, this process involves the use of EXTL3 to transfer a GlcNAc to the established tetrasaccharide linkage (Figure 2A). EXT1 and EXT2 form an HS polymerase complex and extend the chain by continually adding repeating GlcA and GlcNAc residues. Importantly, post-polymerization, the chain undergoes a series of modifications sometimes referred to as maturation (Figure 2B). These modifying enzymes include N-deacetylase/N-sulfotransferases (NDSTs), O-sulfotransferases (2OST, 6OSTs, and 3OSTs), and D-glucuronyl C5-epimerase (GLCE). NDSTs act on GlcNAc residues by replacing acetyl groups with sulfate groups. GLCE converts GlcA to IdoA residues primarily on GlcA residues near the N-sulfated glucosamine units. The aptly named sulfotransferases transfer sulfate groups to the respective positions of GlcA/IdoA and glucosamine residues. Because the NDSTs produce N-sulfated glucosamine which are preferred substrates for the aforementioned enzymes that perform epimerization and O-sulfation,14 some regions become highly modified while others remain poorly modified; structural heterogeneity is achieved through this process.15
Figure 2.
General proteoglycan biosynthetic steps involved in GAG chain initiation, elongation and modifications
(A) Biosynthesis of the backbone structure of heparan sulfate. The protein core provides a Ser residue that forms a linkage with a Xyl-Gal-Gal-GlcA tetrasaccharide. For HS biosynthesis, a GlcNAc residue is added to the tetrasaccharide linkage via exostosin like glycosyltransferase 3 (EXTL3). Subsequently, EXTL1 and EXTL2 add alternating GlcA and GlcNAc to the chain.
(B) The progressive GAG chain modification leads to diverse structures of sulfated and unsulfated GAGS. The representative structures of heparan sulfate, chondroitin sulfate, dermatan sulfate, hyaluronan, and keratan sulfate (which are linked to an Asp residue instead of a Ser) are shown respectively. We note that we are not discussing the detailed biosynthesis and structure of hyaluronan since this subject has been covered extensively in recent literature.298,299,311 For additional information and details, please refer to the text. The schematic was adapted from Esko and Selleck8 and McMillan et al.15
Different GAG chains are uniquely modified and contain different disaccharide units—small changes can determine categorization as epimerization of GlcA to IdoA in CS converts it to dermatan sulfate (DS). Very broadly, HS is constituted of GlcA/IdoA and GlcNAc, CS consists of GlcA and GalNAc, and DS is epimerized so naturally it is composed of IdoA and GalNAc (Figure 2B). HA lacks sulfate groups and does not attach to a core protein and is made up of GlcA and GlcNAc. Keratan sulfate (KS) has a different tetrasaccharide linkage region and specific amino acid residue. KS contains galactose and GlcNAc.
We believe that the strategic location of various proteoglycans has a biological meaning. For example, most of the heparan sulfate proteoglycans (HSPGs) are adjacent to most cells, either bound to the cell surface through a hydrophobic transmembrane domain or via a glycosyl-phosphatidyl-inositol (GPI) anchor.10,16 In contrast, most of the chondroitin sulfate proteoglycans (CSPGs) are localized to the extracellular matrix spatially distant from the cellular compartment. In addition, both HSPGs and CSPGs bind to several morphogens and growth factors and present them in a bioactive form to their cognate receptors, during both developmental and pathological processes. Indeed, GAG modifications control general patterns of gene expression, cell specification and cell growth via morphogen regulation.17 A wide-ranging deduction from these emerging proteoglycan functions is that HSPGs may have a more prominent role in cellular regulation because of their proximity to the cell surface, whereas CSPGs may have additional functions as structural and mechanical constituents.
The complex GAG-protein interactions facilitate critical physiological and pathological processes, such as development, cancer invasion, angiogenesis, neuronal plasticity, and viral entry. However, mapping GAG-protein networks is a challenging task for biologists as these interactions need specific sulfation patterns often including ROS derived effects.18 Moreover, these global interactions involve several transmembrane receptors and extracellular matrix-associated proteins.19 Recent progress in establishing natural and synthetic GAG libraries has allowed us to decipher in detail specific GAG sequences interacting with proteins.20 These GAGs are generally spotted on microarrays and have led to the discovery of novel binding partners while concurrently determining their size and chemical composition that promotes protein binding.21 The recently updated GAG interactome contains about four times more GAG-binding proteins2 than the originally established GAG interactome,22 with a marked increase in the number of DS and HS/heparin-binding proteins.2 Moreover, advances in structural data related to GAG biosynthesis,23 novel chemical and biochemical tools for studying GAG sequencing,24 and chemical glycobiology including the generation of semi-synthetic proteoglycans25 have allowed the dissection of the structural heterogeneity and have ultimately provided powerful insights into proteoglycan functions. Surely, the exploration of the GAG interaction networks could be used for therapeutic purposes especially in the design of specific inhibitors targeting specific GAG/protein interactions.
Another important general feature of proteoglycans is that they often undergo an irreversible post-translational modification via proteolytic processing of their protein cores.26,27 While uncontrolled proteolysis is a noticeable feature of many diseases and pathological processes including cancer, angiogenesis and inflammation, proteolysis might also liberate protein core fragments with biological activity including endostatin and endorepellin.28,29
Cell surface GPI-anchored proteoglycans: The glypican family
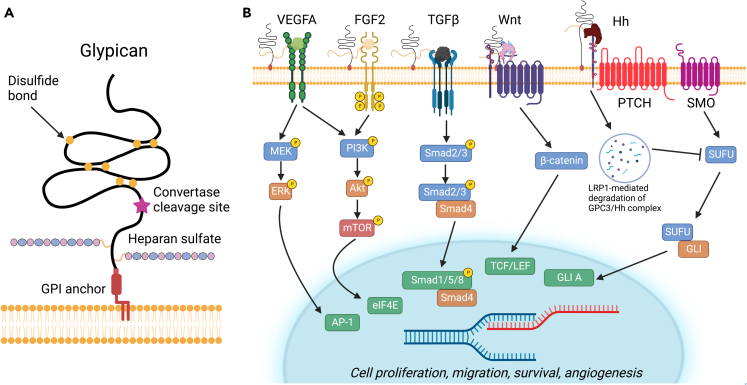
Glypicans are HS proteoglycans linked to the outer surface of the plasma membrane via a lipid moiety composed of GPI anchor30,31 (Figure 3A). There are six family members in mammals (GPC1 to GPC6) with no appreciable significant homology to other characterized structure, with exception of a short segment between residues 200 and 300 that is homologous to the Cys-rich domain of Frizzled proteins.32 Notably, the glypican protein core is highly compact with 14 highly conserved Cys residues forming disulfide bonds in all six members of the family.32 Another general structural feature of glypicans is that they almost exclusively carry HS chains, in contrast to Syndecans (see next section), and that the chains are near the C-terminus, near the plasma membrane (Figure 3A). This strategic localization suggests that these HS chains could mediate the interaction of glypicans with other cell-surface molecules, including growth factor receptors and co-receptors. Glypicans often undergo endoproteolytic cleavage of the proximal protein core by furin-like convertases (Figure 3A). The cleavage site is located at the C-terminal end of the Cys-rich domain generating two subunits that remain attached by disulfide bonds. Notably, the proteolytic processing of glypicans is required for modulation of cell survival, Wnt signaling, and gastrulation,33 and has a remarkable impact on both the binding properties and GAG-chain sulfation suppression of Hedgehog (Hh) signaling.34
Figure 3.
Graphical overview of the structure of glypican and downstream pathway signaling effects
(A) Structure of glypican and constituent parts. Glypicans are heparan sulfate proteoglycans attached to the cell surface via a GPI lipid anchor at the C-terminus. Glypican is composed of a ∼450 kDa N-terminal protein domain containing fourteen conserved Cys residues that form seven disulfide bonds as well as a stalk region containing heparan sulfate glycosaminoglycan chains.
(B) Glypicans serve a variety of functions and affect multiple signaling cascades including the Wnt and Hedgehog pathways. In the Wnt pathway, GPC3 acts as a co-receptor and attracts Wnt to the cell surface by forming a complex with Wnt and FZD, amplifying downstream β-catenin signaling. The HS chains are not necessary for activation but rather help with stabilization of the formation of the complex. In the Hedgehog signaling pathway, GPC3 competes with PTCH for Hh binding. Mediated by LRP1, the GPC3/Hh complex undergoes endocytosis and lysosomal degradation which results in an inhibition of Hedgehog signaling. Glypicans also act as co-receptors for various angiogenic growth factors, including VEGFA, FGF2, and this interaction is thought to stabilize assembly the multi-receptor complexes. This promotes downstream signaling leading to an increase in cell proliferation and migration. GPC1 also binds TGFβ as a co-receptor and promotes SMAD signaling. For additional details, please refer to the text. Figure adapted from Pam and Ho.44
Two seminal papers published in 1999 showed that the Drosophila Division abnormally delayed gene (Dally) encoded for a Gpc that regulates the morphogen Wingless signaling by cooperating with Frizzled 2 pathway.35,36 Notably, recent evidence has shown that the palmitoylated Wingless is shielded by the core domain of a Dally-like glypicans (Dlp) thereby providing a new paradigm for Gpc signaling.37 Specifically, in the presence of palmitoylated peptides, Dlps change conformation to create a hydrophobic space, thereby protecting the lipid of Wnt proteins from the aqueous environment and could function as a reservoir from which Wnt proteins can be handed over to their cognate receptors.37,38
Numerous genetic studies have clearly shown an important role for glypicans in developmental morphogenesis and patterning events.39,40 In vivo evidence indicates that the main function of membrane-attached glypicans is to act as growth factor co-receptors including Wnt signaling (see previous text), Hedgehogs (Hh), vascular and fibroblast growth factors (VEGFA and FGF2), and bone morphogenetic proteins (BMPs). Glypicans have been implicated in the pathogenesis of several types of cancer, notably hepatocellular carcinomas, and as potential biomarkers and potential targets for personalized therapy.41,42 The multiple signaling pathways where glypicans have been involved are schematically summarized in Figure 3B. As illustrated in the schematic, glypicans mostly act as co-receptors, that is, a cell surface receptor that binds a signaling molecule in addition to a primary receptor thereby facilitating ligand recognition and initiating biological processes regulating cell function.
Glypicans may have a stimulatory or inhibitory activity on signaling depending on the cellular context.43,44 For example, in the case of Wnt, the stimulatory mechanism is based on the ability of glypicans to facilitate and/or stabilize the interaction of Wnts with their signaling receptors, the Frizzled proteins. Signaling by the Wnt proteins is finely regulated to ensure proper development and tissue homeostasis. This has been in part conceptualized by the idea of shedding of glypican/Wnt complexes by Notum, thought to be a phospholipase.45 However, recent evidence in Drosophila has shown that Notum requires glypicans to suppress Wnt signaling but does not cleave their GPI anchor; it acts, instead, as a carboxylesterase that removes an essential palmitoylated moiety from Wnt proteins.46
A recent study has shown that targeting GPC1 expression by CRISPR/Cas9, RNAi or anti-GPC1 antibodies attenuates proliferation in a variety of cancer cells including bladder carcinomas, gliomas, and hepatocellular carcinomas.47 Moreover, systematic Ingenuity Pathway Analysis suggests that suppression of proliferation of cancer cells results in ECM-mediated inhibition of specific pro-cancer signaling pathways involving TGFβ and p38 MAPK.47 Indeed, it is well known that TGFβ affects ECM biology including various collagens and other proteoglycans.48,49,50,51 Collectively, the aforementioned observations data suggest that GPC effects on specific cancer types could be utilized as potential diagnostic and prognostic factors.
As aforementioned, glypicans are involved in the pathogenesis of many solid tumors. What is especially striking is the involvement of GPC3 in the pathophysiology of hepatocellular carcinoma, where the proteoglycan stimulates canonical Wnt signaling52 and can be a useful biomarker both in serum and tumor proper.53 In support of these original observations, a recent study has shown that GPC3 is highly enriched in the small extracellular vesicles from patients with hepatocellular carcinoma.54 Moreover, the furin site of GPC3 is exposed and readily available suggesting that furin-dependent GPC3 cleaved domains could be a promising biomarker for early detection of this lethal cancer and could also serve as a predictor for disease prognosis and progression.54
Another unexpected finding is the recent discovery that GPC1 is the main rate-limiting factor driving the unconventional secretion of FGF2.55 It has been known for some time that FGF2 is secreted by a pathway of unconventional protein secretion based on direct self-translocation across the plasma membrane. Surprisingly, GPC1, but no other HS proteoglycans such as SDC or perlecan, is required for unconventional secretion of FGF2 into the extracellular space. Collectively, these novel findings provide not only a molecular mechanism underlying this process but also reveal an intimate relationship between GPC1 and FGF2 that could play a role in tumor progression and metastasis.56
Intercalated proteoglycans: The syndecan family
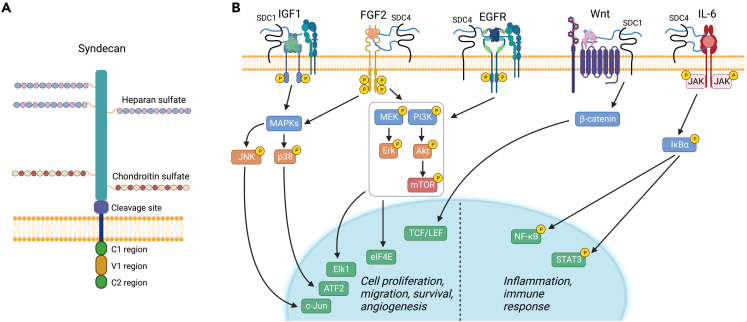
The SDC family comprises four homologous type I transmembrane proteoglycans members. SDC1 (Figure 4A) and SDC3 are the largest members with protein cores of ∼33 and ∼41 kDa, respectively, whereas SDC2 harbors smaller with protein cores of ∼23 and 22 kDa, respectively.57 The general structure of syndecans includes an ectodomain, a single hydrophobic transmembrane domain, and a short C-terminal cytoplasmic domain with two constant and one intervening variable region (Figure 4A). An interesting feature of syndecans is their GAG attachment sites and type as they harbor both HS and CS in the ectodomain, in contrast to glypicans which preferentially harbor HS chains. Specifically, SDC1 and SDC3 bear CS chains near the plasma membrane whereas the HS chains are in a more distal region (Figure 4A). Recently it has been shown that the HS chains of SDC1 and SDC2 induce changes in conformational dynamics depending on the number of the HS chains and their location on the protein core.58 The distal localization of the HS chains suggests a specific function of syndecans in modulating receptor activity vis-à-vis glypicans or other HSPGs such as perlecan, which is also located at the plasma membrane59 via the α2β1 integrin which specifically binds its C-terminal domain V/endorepellin.60,61,62
Figure 4.
Overview of the structure of syndecan 1–4 (SDC) and downstream signaling pathways
(A) Graphical depiction of a typical syndecan. Syndecans are type I, single-pass transmembrane proteoglycans. All syndecans contain HS chains covalently linked to protein core ectodomain; however, SDC1 and 3 also contain CS chains. The relative sizes of SDC 1–4 are 33, 23, 41, and 22 kDa respectively. The cytoplasmic domain contains two conserved regions, C1 and C2, separated by a variable (V1) region. The crystal structures for syndecans remain obscure because the ectodomains contain a significant amount of disorder.
(B) Syndecans serve a variety of functions but primarily act as co-receptors affecting multiple signaling pathways. SDC1 activates the IGF1 signaling pathway by stimulating docking of both IGF1-R and αvβ3/αvβ5 integrins and subsequently triggers angiogenesis. SDC4 mediates the assembly of EGFR and α6β4/α3β1 integrins which leads to downstream pro-migration signaling crucial for tumorigenesis. SDC4 binds FGF through heparan sulfate chains and is an essential co-receptor for FGF signaling. Interestingly, SDC4 can activate this signaling pathway in both an FGFR-dependent and FGFR-independent manner. Wnt signaling is modulated by SDC1 via its HS chains which bind Wnts and R-spondins to stimulate β-catenin signaling for cell growth. Syndecans play a role in recruiting pro-inflammatory molecules and modulating the progression of inflammation. This is done primarily through GAG chain binding to cytokines but there is evidence for a SDC1/IL-6 axis with the presence of a regulatory feedback loop that is yet to be elucidated. For additional details, please refer to the text. The graphic was generated with BioRender.com.
Another general feature of syndecans is that the protease-mediated shedding of their protein cores at a site located proximal to the plasma membrane (Figure 4A) can relocate the ectodomain to more distal sites,26 and the shed form of at least one transmembrane proteoglycan (SDC1) can be internalized and redirected to the nucleus.63,64,65 Proteolytic processing can extend the inherent molecular diversity of proteoglycans via the generation of bioactive fragments with new functional properties. Shed SDC1 binds to hepatocyte growth factor (HGF) and stimulates tumor growth while promoting angiogenesis via VEGF.66,67 The N-terminal SDC2 domain selectively enhances 6-O HS chains sulfation and promotes VEGFA-dependent neovascularization,68 while SDC2 selectively regulates VEGF-induced vascular permeability.69 Moreover, there is evidence that chemotherapy stimulates SDC1 shedding, and this would have a negative effect on treatment by promoting tumor relapse.70
Syndecans are key regulators of cell signaling71 by playing a major role in acting as co-receptors.72 Syndecans have been involved in regulating at least five distinct signaling pathways (Figure 4B). Four of them at times utilize overlapping signaling components such as MAPK/MEK and PI3 kinase, but they ultimately and collectively promote cell proliferation, survival, migration, and angiogenesis. SCD1 mediates HGF binding and promotes MET signaling in multiple myeloma.73 In addition, SCD1 promotes an aberrant Wnt/β-catenin signaling pathway by presenting Wnts and R-spondins to their receptors,74 suggesting that targeting this pathway in the tumor microenvironment could be a potential therapeutic target for multiple myeloma.75 Additionally, SDC4 has been recently implicated in regulating the IL-6/STAT3 signaling pathway in inflammatory breast carcinomas.76
Through utilization of the diverse and complex signaling pathways outlined previously, syndecans and its HS chains affect the homeostasis of various organs and the pathogenesis of several neoplastic diseases including those of the liver,77 colon,78 breast,79 plasma cells,74,75,80 and in bacterial infection.81,82 Syndecans are directly involved in inflammation (see the following text) and are often upregulated in inflammatory diseases concurrent with enhancement of cytokine and chemokine levels. This dual activation boosts leukocyte adhesion to endothelial cells which is fortified by integrin-controlled signaling and other cell adhesion molecules, leading to the arrest of leukocyte rolling. The adhesion of leukocytes to endothelial cells results in transmigration through the endothelial cell barrier and active inflammatory infiltrate.83 SDC2 and SDC4 engage the EGFR and RON tyrosine kinase receptors and promote carcinoma cell cycle progression.84 SDC1 activates the IGF1/IGFR1 signaling pathway by promoting docking of both IGF1-R and αvβ3/αvβ5 integrins thereby triggering angiogenesis.85 Notably, SDC4 mediates the assembly of EGFR and α6β4/α3β1 integrins which leads to a pro-migratory effect crucial for tumorigenesis. SDC4 binds FGF through its HS side chains and is a vital co-receptor for FGF signaling. Interestingly, SDC4 can activate this signaling pathway in both an FGFR-dependent and FGFR-independent manner. Recently, SDC4 has been recently implicated in the progression of gastric cancer and has been proposed to represent a potential therapeutic target for precision medicine.86
Another unique ability of SDC1 is its ability to act as a potential LDL receptor, by directly mediating ligand catabolism through a pathway distinct from classical coated pits, thereby acting as a receptor for atherogenic lipoproteins and other ligands in vivo.87 SCD1 can also mediate apoE-VLDL uptake in human fibroblasts with little or no contribution from LRP and the endocytic path taken by SCD1 is clathrin-independent and relies upon lipid raft function. Genetic studies utilizing Sdc1−/− mice have shown that these mutant animals accumulate plasma triglycerides and exhibit protracted half-life of injected human VLDL and intestinally derived chylomicrons, suggesting that SCD1 is the primary proteoglycan mediating hepatic triglyceride clearance.88 Indeed, shedding of SCD1 from human hepatocytes can alter VLDL clearance, thereby contributing to hypertriglyceridemia in septic patients.89 Finally, there is evidence that SCD4 may also participate in the control of lipid metabolism as exercise evokes the secretion of hepatokines that promote SDC4 expression which in turn reduces fatty acid uptake and hepatic steatosis.90
Proteoglycans in axonal guidance and neurodegenerative diseases
It is well established that the presentation of secreted axonal guidance factors plays a key function in shaping central nervous system (CNS) connectivity. One of the most intriguing roles of HSPGs is their direct involvement in regulating guidance factor activity. Through a genetic in vivo analysis it was shown that Sdc is critical for the fidelity of Slit repellent signaling at the midline of Drosophila CNS.91 Moreover, the Drosophila glypican Dally-like protein (Dlp) is required for proper axon guidance and visual-system function.92 Mosaic studies revealed that Dlp is necessary in both the retina and the brain for different aspects of visual system assembly and Sdc mutants also exhibited similar axonal guidance and visual-system defects.92 Notably, rescue experiments using Dlp+ transgenes were able to rescue some of the Sdc phenotypes, but the opposite was not effective.92 Collectively, these findings suggest that both the HS chains and the protein cores play a role in this CNS function. In support of these findings is the observation that viable hypomorphic mutations in the two C. elegans exostosin glycosyltransferase genes, Rib1 and Rib2, evoke abnormal cell and axonal migration.93 Moreover the C. elegans Ephrin4 functions non-cell autonomously with HSPGs to promote axonal growth and branching while Sdc and Gpc have cell-autonomous yet mutually antagonistic roles in ectopic neurite formation.94 Another interesting finding is that synaptogenesis is modulated by HSPGs in C. elegans male neural network as loss of 3-O sulfation results disrupts the formation of synapses in a component of the mating circuits.95 Overall, these studies point to a fundamental role for HSPGs not only in axonal guidance but also in the formation of synaptic connections.
During the past century, the rise in life expectancy has led to a significant increase in the incidence of age-related neurodegenerative disorders such as Alzheimer’s disease (AD) and several amyloid related disorders.96 Both senile plaques and vascular amyloid deposits harbor amyloid-β peptides15 and these aggregates contain PGs, mostly HSPGs.97 The central role played by HSPGs in AD has been demonstrated for a long time.98 There is also ample evidence to support a key role for HSPGs in Tauopathies: HSPGs directly bind to co-deposit with Tau and modulate Tau secretion, internalization, and aggregation.99 In support of these findings are several observations including: (1) Overexpression of heparanase (see the following) lowers amyloid burden in double transgenic mice overexpressing heparanase and β-amyloid precursor protein,100 (2) heparanase expression is upregulated in the brain of AD patients,101 and (3) heparanase overexpression impedes perivascular clearance of amyloid-β from murine brain.102 Moreover, HSPGs are directly involved in propagation of proteopathic peptides103 and in the prion-like spread of Tau pathology,104 and can serve as mediators between monomeric Tau and its subsequent ERK1/2 activation.105 Meanwhile, the expression of HSPGs is dysregulated in the disease, and this dysregulation may exacerbate the progression of Tauopathies.99 Furthermore, neuronal HSPGs modulate amyloid-β clearance and aggregation in AD patients.106 Specifically, GPC4 drives Tau hyper-phosphorylation suggesting that this HSPG can be a gateway for Tau spreading.107 Both the cell surface HSPGs syndecans and glypicans contribute to cellular uptake of α-synuclein and Tau,108,109,110 and specific GAG chain length and sulfation appear to modulate this process in a differential way.111 In an interesting C. elegans model of Parkinson’s disease, where worms were fed α-synuclein preformed fibrils causing neurodegeneration, knockdown of Sdc1 or enzymes involved in HS biosynthesis protected against α-synuclein aggregation, motor dysfunction and neurodegeneration.112 Overall, these data indicate that HSPGs are directly involved in mediating prion-like α-synuclein neurotoxicity and could be a potential therapeutic target.
Several lines of additional evidence implicate HSPGs and specific sulfation of the HS chains in neurodegenerative disease. For example, GAGs from the hippocampus of AD patients exhibit abnormal ability to bind growth factor activities and to bind Tau proteins.113 Internalization of Tau proteins is regulated by 6-O sulfation of HSPGs and this posttranslational modification can regulate Tau uptake in neuronal cells, IPS-derived neurons and in ex vivo murine brain slices.114 In contrast, a recent paper has clearly shown that in AD brains there is a substantial increase of a specific 3-O-sulfatedHS domain, designated as Tetra-1, and that this modification is used in Tau internalization.115 Moreover, overexpression of the HS3ST1 gene, which encodes the enzyme responsible for the synthesis of the specific 3-O-sulfated Tetra-1, could indeed represent an AD-associated genetic risk factor.115 These studies suggest that the 3-O-sulfated HS may serve as a new target for modifying AD and as a biomarker for early diagnosis.115 Collectively, the studies summarized previously propose that a valuable therapeutic for AD patients could be a pharmacological targeting of HSPG/Tau interactions. As HSPGs can be novel AD biomarkers,116 we need to define more precisely the spatiotemporal components of HS structural domains and their diversity in both normal and AD brains.
Proteoglycans in infection: Novel roles in SARS-CoV-2
Over the past two decades, a major role for various proteoglycans in innate immunity has been established.117,118,119,120 It is well known that viruses are obligate intracellular parasites and, consequently, viruses need to first bind a cell surface receptor and then enter permissive host cells ultimately hijacking their host intracellular machinery. It is also established that viruses can act as passive and metabolically inactive entities with incomplete available strategies to cross the plasma membrane of host cells. However, most mammalian cells are dynamic and offer several structures and processes allowing the uptake of macromolecular assemblies. The COVID-19 pandemic initially caused by an outbreak of SARS-CoV-2 in Wuhan, China has perturbed all facets of life globally since January 2020. As of February 2023, the virus has infected over 755 million people causing the death of >6.8 million people (https://covid19.who.int/). Although vaccinations and antiviral agents have been established, the virus continues to mutate, spike in incidence, and perturb society and the economy.121 It is integral to understanding the mechanism by which viral infection occurs to further develop pharmaceuticals to minimize the risk of severe consequences of COVID-19 infection. As discussed in detail in the following, proteoglycans and specially cell surface HSPGs, are directly involved in SARS-CoV-2 infectivity and cellular invasion. Recent seminal studies have discovered that SARS-CoV-2 infection is dependent not only on angiotensin-converting enzyme 2 (ACE2) but also on HS proteoglycans, especially SDC1 and SDC4, and these are required for virus binding and infection.122,123 SDC1 was shown to act as a co-receptor for SARS-CoV-2 binding.123,124 It is also established that HS proteoglycans act as internalizing receptors for extracellular vesicles and SARS-CoV-2 lipid-enclosed particles.125 The similarities in their biogenesis, biophysical property, size, and lipid composition could elucidate a shared dependence on HS proteoglycans for effective cell-surface attachment and uptake.125
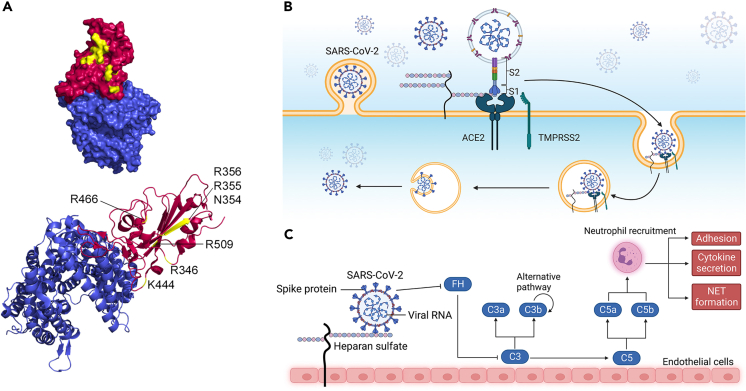
Cell surface HS interacts with specific residues in the receptor-binding domain (RBD) region of the S1 subunit of the spike protein.122 Molecular modeling highlights specific positively charged residues within the RBD region that likely bind to heparin which are highly acidic: R346, R355, K444, R466, and R509122 (Figure 5A). Notably, the region containing these residues is inactive during the “closed” conformation of the RBD and fully exposed when “open.” SARS-CoV-2 infection relies heavily on HSPG tissue expression. The SARS-CoV-2 S protein is cleaved into two subunits by pro-protein convertases in virus-producer cells. While the S1 subunit binds to ACE2, the S2 subunit consists of a fusion peptide that allows for immediate membrane fusion126 (Figure 5B). This site contains an additional internal site dubbed the S2′ site which is exposed after ACE2 engagement. After ACE2-mediated endocytosis, S2′ is subsequently cleaved by transmembrane protease serine 2 (TMPRSS2) in the endosomal compartment127 (Figure 5B). This causes a release of the fusion peptide which initiates fusion pore formation – a step that is vital for viral access to host cell machinery.128 Moreover, cell surface HS interacts with the ectodomain of the SARS-CoV-2 spike proteins through the RBD in the S1 subunit of the spike protein. This in term shifts the structure of the protein to favor the RBD open conformation that in turn binds to ACE2. Additional findings also indicate HS polymers likely recruit endocytic ligands that further promote viral uptake.129 HS interaction with the RBD to favor the “open” conformation is so essential in receptor accessibility and subsequent viral entry125 that more recently, findings do suggest that HS could solely trigger or facilitate internalization, as SARS-CoV-2 infection is possible in the presence of HS and absence of ACE2.130
Figure 5.
SARS-CoV-2 binding to ACE2 is enhanced with heparan sulfate acting as an adhesion receptor
(A) PyMOL rendering of the crystal structure of SARS-CoV-2 spike including the receptor-binding domain bound to ACE2 receptor. Heparin binding sites are highlighted in yellow in the RBD region of SARS-CoV-2. (PDB ID: 6M0J).
(B) Graphical depiction of the binding and internalization of SARS-CoV-2.
(C) Graphical depiction of proposed activation of the complement cascade. Viral uptake results in activation of the canonical and alternative complement pathways which contribute to the pathology of viral infection in various tissues. For additional details, please refer to the text. Panels (B and C) were generated with BioRender.com.
As a linear, highly acidic polysaccharide, HS can form weak ionic bonds with basic residues on viral capsid proteins which allows for augmentation of binding likelihood.131 Though ACE2 is the functional receptor for SARS-CoV-2 viral entry—allowing for viral entry of the cell via endocytosis, HS acts as an adhesion receptor that is essential for SAR-CoV-2 viral binding and replication.132 While ACE2 can indeed bind to the SARS-CoV-2 spike protein independently, the binding is enhanced significantly by HS.122
Heparin-protein interactions are heavily favored toward positive electrostatic surfaces. Molecular modeling has demonstrated that positively charged amino acids R346, R355, K444, R466, and R509 within the RBD region adjacent to the ACE2 binding domain likely interact directly with heparin. Notably, the site is inactive in the RBD “closed” conformation but fully exposed in the “open” conformation.122 With the establishment of HS as an essential factor for viral entry and replication, pharmaceutical use of HS mimetics has been proposed to both reduce the severity and incidence of infection.129,131,133,134 Exogenous heparin has also been proposed to compete with cell surface heparan interaction with viral proteins135 as well as lactoferrin binding to HS proteoglycans in SARS pseudovirus infection.136 More broadly, this could prove relevant for other similar exogenous sulfated polysaccharides.124
The complement pathway plays an essential role in the severity of respiratory symptoms caused by SARS-CoV-2. The virus competes with C3b-regulatory factor H (FH) binding to HS.137 This removes the FH-mediated inhibition of C3 which further downstream activates the alternative pathway137 (Figure 5C). Thus, the virus is a non-canonical alternative pathway activator which gradually evokes complement activation through interactions with HS.138 In addition to complement activation via the alternative pathway, SARS-CoV-2 induces complement activation via the classical and mannose-binding lectin pathways simply as a pathogen. Overall, complement activation causes cleavage of C3 and C5 complexes into bioactive components C3a, C3b, C5a, and C5b139 (Figure 5C). Cytokines are released, neutrophils are activated, and inflammation at the infection site is subsequently generated. Targeting the complement system activation appears to be key to lessening the risk of severe COVID-19 symptoms and HS offers a target of interest for future therapeutics.
As the field of corona viruses is constantly expanding, we should point out that other co-regulators have been recently identified, including the transmembrane leucine-rich repeat-containing protein 15 (LRRC15)140,141,142 and Neuropilin-1.143,144 Notably, LRRC15, which is often expressed in various solid tumors, can inhibit adenoviral infection145 and can hinder SARS-CoV-2 cellular entry in trans.146 In agreement with this anti-viral activity, decreasing LRRC15 plasma levels in infected patients are associated with a more severe clinical outcome.147 It is not yet established whether HS is a co-factor for LRRC15 or neuorpilin-1, but we should point out that neuropilin-1 is a HS-binding protein. Specifically, neuropilin-1 binds to a rare modification of HS, the 3-O-sulfate groups,148 and by doing so it enhances its biological activity.149
Proteoglycans and heparanase as proautophagic secreted factors
Autophagy is an essential modulator of both tumor suppression and promotion; currently, rapamycin and chloroquine, two potent autophagic modulators, are frequently used to regulate autophagy in anticancer therapy. In terms of cancer development, autophagy is also important in prevention in both cell autonomous and non-cell autonomous methods.150 Notably, the extracellular matrix contains several constituents that can affect intracellular digestive machinery as well as macroautophagy.151,152,152,152,153,154,155,156,157
Decorin is a well-studied small leucine-rich proteoglycan (SLRP) with a complex genomic structure and complex promoter region regulated by various growth factors158,159,160,161 Decorin has been implicated for a long time in the regulation of collagen fibrillogenesis,162,163,164,165,166 cartilage and tendon homeostasis,165,166,167,168 regulation of TGFβ activity, wound healing and fibrosis,169,170,171,172,173,174,175,176,177 angiogenesis,154,171,178,179,180 Lyme disease,181 and ocular pathophysiology.166,175,182,183,184 Soluble decorin inhibits cancer growth primarily by acting as a pan-RTK inhibitor185,186; it binds to EGFR and downregulates its biological activity187,188,189,190 while elevating cytosolic Ca2+ in A431 carcinoma cells.191 Decorin suppresses the growth of various tumors with different histopathological backgrounds,192,193,194,195,196,197,198,199,200,201,202,203,204 but also plays a role in developmental and genetic diseases.205,206,207,208,209,210,211,212 Notably, reduced expression of stromal decorin is linked to a poor outcome in invasive breast cancer patients.213,214,215 Thus, evaluation of stromal decorin may be a good biomarker to predict breast cancer patient outcome.214,215 One way through which decorin may affect prostate cancer growth and metastatic potential is via MEIS transcription factors that regulate HOX genes. Indeed, MEIS1 expression hinders proliferation of prostate cancer cells in vitro and in murine xenografts by evoking an HOXB13-mediated upregulation of decorin.216
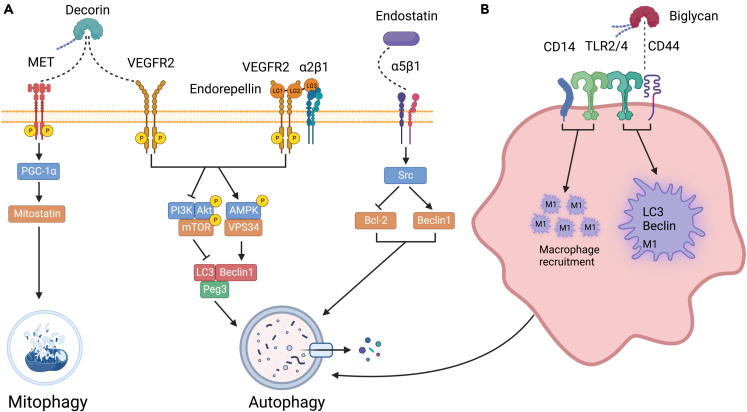
Decorin evokes Peg3-dependent autophagy in endothelial cells of various genetic lineages including human, mouse, and porcine endothelial cells.151,217,218 We have previously shown using wild-type mice starved for 2 days that decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagic progression in various organs.219 Moreover, Dcn−/− mice display an aberrant response to fasting compared to wild-type mice.219 Decorin can also promote autophagy by chronic suppression of Met bioactivity in trophoblasts.220 Decorin binds to vascular endothelial growth factor receptor 2 (VEGFR2) resulting in the phosphorylation of AMPK at Thr172 which among its regulation of a plethora of catabolic processes, is capable of initiating autophagy221,222,223 (Figure 6A). Concurrently, AMPK phosphorylation results in an attenuation of the mTOR axis, an anti-autophagic coordinator of anabolic pathways.224 The combination of these signaling cascades causes an accumulation of Peg3 which co-localizes with Beclin 1 and LC3 to associate with autophagosomes in both microvascular and macrovascular endothelial cells. More recently, decorin has been characterized to interact via the MET/mitostatin/Parkin pathway to sustain tumor cell mitophagy225 (Figure 6A).
Figure 6.
Schematic representation of the various proteoglycans and proteoglycan fragments evoking autophagy
(A) Decorin and endorepellin share VEGFR2 to target endothelial cells. However, decorin uses the MET receptor to evoke mitophagy in cancer cells. Instead, endorepellin requires the co-receptor α2β1 integrin to evoke autophagy in endothelial cells sharing similar pathways. Endostatin acts via the α5β1 integrin in endothelial cells.
(B) Effects of biglycan on macrophage autophagy are mediated by a complex of receptors including TOLL2/4, CD14, and CD44. For additional details, please refer to the text. The graphic was created with BioRender.com.
Of interest, there is a link between decorin-evoked autophagy and cancer as decorin suppresses glioma cell invasion by inducing autophagy via the Met/Akt/mTOR axis.226 Decorin can also induce prolonged respiratory complex turnover and mitochondrial DNA depletion in triple negative breast cancer cells.227 The beneficiary effect on “cellular protection” is also shown by the finding that decorin protects intestinal cells via its pro-autophagic action in inflammatory bowel disease228 and seemingly protects retinal pigment epithelial cells from oxidative stress and apoptosis via AMPK-mTOR-regulated autophagy.229 Moreover, decorin evokes reversible mitochondrial depolarization in carcinoma and vascular endothelial cells.230 This pathway is novel and requires further elucidation; decorin-evoked mitophagy requires TCHP/mitostatin mRNA interaction with PGC-1α which results in accumulation of mitostatin.
It has been known for a long time that large modular proteoglycans, such as perlecan and collagen XVIII, can be processed by various proteases to generate bioactive fragments such as endostatin and endorepellin.231,232,233,234 Endorepellin is the C-terminal domain of perlecan HSPG2, a very large HSPG with a multifaceted gene organization235 and a complex promoter region.236,237 Endorepellin has been known for two decades now as a key inhibitor of angiogenesis, in both developmental and cancer associated angiogenesis.29,60,238,239,240,241 In contrast, the parent proteoglycan perlecan is required for vascularization and is potently pro-angiogenic, a function mainly mediated by its N-terminus cluster of three HS chains.242,243,244,245,246,247,248,249,250 One of the main features of perlecan is its ability to interact with various extracellular matrix proteins, growth factors and surface receptors via either the HS chains or the modular protein core251,252,253,254,255,256,257,258,259 (see the following text).
Endorepellin potently induces autophagy via VEGFR2 in a similar fashion to decorin. We found that endorepellin interacts with both the α2β1 integrin and VEGFR2 (Figure 6A), and we introduced the concept of dual receptor antagonism,260 We further discovered that endorepellin by interacting with the ectodomain of VEGFR2 induces significant phosphorylation of AMPK, which leads to an increase in the autophagic markers Beclin 1 and LC3-II.261 Like decorin, concurrent inhibition of the anti-autophagic mTOR pathway via phosphorylation of mTOR at Ser2448 further drives autophagy.262 Notably, differing from decorin-mediated autophagy via VEGFR2, the kinetics of phosphorylation is markedly slower for endorepellin: AMPK phosphorylation peaks at 4 h of treatment when compared to the 30 min of decorin. More recently we have shown that conditional expression of endorepellin in the tumor vasculature attenuates breast cancer growth, angiogenesis and hyaluronan deposition.263 The proposed mechanism occurs through an endorepellin evoked downregulation of HAS2, the main hyaluronan synthase of most cancer and endothelial cells.264 Thus, angiostatic cues from the matrix link endothelial cell autophagy to hyaluronan biology and its role as a pro-angiogenic and pro-inflammatory polysaccharide.28,265
Another matrix component that has been involved in autophagy in vascular endothelial cells is endostatin, the C-terminal monomeric fragment of type VIII collagen which is a hybrid HS proteoglycan. One of endostatin primary targets is integrin α5β1 (Figure 6A). Because endostatin has been characterized to induce autophagy in a nutrient-stress independent manner in endothelial cells, downstream signaling from α5β1 integrin binding is hypothesized to reduce Bcl-2 levels via Src tyrosine kinase signaling which concomitantly results in an increase in Beclin 1.266 The physiological disruption of the Bcl-2/Beclin 1 ratio causes an induction of autophagy.266
Biglycan, another versatile SLRP, in addition to being a structural component of the extracellular matrix, is a multifaceted signaling molecule regulating a plethora of cellular functions including innate immunity and inflammation. Furthermore, soluble biglycan is a damage-associated molecular pattern267 that promotes M1 macrophage recruitment and inflammation via Toll-like receptor (TLR) 2 and 4 together with the coreceptor cluster of differentiation 14 (CD14)268,269,270,271 (Figure 6B). Transgenic biglycan promotes C-X-C motif chemokine ligand (CXCL)1, CXCL2, CCL2, and CCL5-mediated neutrophil and macrophage infiltration via (MyD)88/TRIF pathways to trigger a potent pro-inflammatory milieu in the renal parenchyma.272 Moreover, soluble biglycan is a potentially useful biomarker for inflammatory renal diseases.273 Collectively, the ability of biglycan to modulate and aggravate inflammatory signaling through various receptors makes it a novel therapeutic target for inflammation associated diseases.
Biglycan was first hypothesized to modulate autophagy as mice stably overexpressing soluble biglycan were found to have a significant increase in autophagic macrophages.272 Upon further investigation, it was discovered that biglycan TLR2/4 as well as co-receptors CD44/CD14 which initiates inflammatory response.270 CD44 binding affinity is significantly higher for biglycan when compared to CD14.274 CD14 interaction induces recruitment of M1 macrophages. Biglycan induces Beclin 1 expression along with LC3-II and p62 accumulation by engaging CD44 and TLR4 in these M1 macrophages, which directly drives phagophore/autophagosome formation and assembly.274 Biglycan also exhibits protective effects toward doxorubicin in osteosarcoma cells by activating the Wnt/β-catenin pathway and suppressing autophagy.275 Enhancing chemotherapeutic efficacy is also mediated by an ability of biglycan to bind and activate insulin-like growth factor 1 receptor (IGF-IR) signaling.276
In addition to its role in inflammation and autophagy, biglycan signaling carries clinical implications in the modulation of tumorigenesis.271,277,278 For example, targeted suppression of stromal biglycan inhibits metastasis, impairs tumor angiogenesis, and enhances tumor CD8+ T cell infiltration, thereby improving chemotherapeutic efficacy in breast cancer.279 The multitude and diversity of tumors where biglycan serves as a protumorigenic/prognostic marker and as a promising therapeutic target indicate its potential role in cancer biology.278,280
In addition to proteoglycans, enzymes involved in the catabolism and modification of HS chains such as heparanase,281,282 can be involved in regulation of autophagy. Heparanase is a multifunctional molecule harboring both enzymatic and non-enzymatic functions.283,284 Through its endo-β-glucuronidase activity, heparanase cleaves HS chains in various HSPGs releasing relatively large HS fragments of 4–7 kDa fragments that are biologically active.285 Heparanase also releases HS-bound growth factors, cytokines, and chemokines stored within the microenvironment of remodeling tissues and growing cancers.286,287 Moreover, heparanase can mediate tumor-host crosstalk enhancing pro-tumorigenic gene transcription ultimately activating pro-survival signaling pathways and the formation of exosomes.288 Together, the enzymatic and non-enzymatic biological activities of heparanase establish this unique enzyme as a multifunctional molecule that increases the aggressiveness of tumor cells.288,289 Notably, heparanase has been recently shown to evoke autophagy in cancer cells, leading to tumor growth and chemo-resistance.281 Specifically, heparanase localizes to autophagosomes and concurrently possesses the ability to positively regulate the autophagic process.281 Notably, along with increasing absolute autophagosome number, heparanase overexpression also causes an increase in the number of vesicles released from the cancer cells.281 It is highly likely that these vesicles are indeed exosomes as heparanase can evoke exosome secretion, composition, and function of tumor cell-derived exosomes which could amplify tumor development.290 In human cancer cells of various histogenetic backgrounds, including myeloma, lymphoblastic, and breast cancer cells, when expression of heparanase is enhanced or when tumor cells are exposed to exogenous heparanase, exosome secretion is dramatically increased.290 Exosomes evoked by hypoxic conditions, often a hallmark of cancer, transport heparanase and enhance macrophage migration, endothelial tube generation and cancer cell stemness.291 Thus, targeting heparanase in tumors using synthetic or chemically modified inhibitors,292 seems a reasonable approach to combat cancer. Collectively, these observations provide a potential mechanism for heparanase to increase tumor growth through the induction of autophagy, and further propose a functional link between heparanase activity, autophagy, and exosome secretion, culminating in cancer progression. In conclusion, proteoglycans, together with bioactive proteolytic fragments of their protein cores and processed GAGs by heparanase, and perhaps other lyases, are emerging as potent regulators of autophagy and could become even more relevant as potential therapeutics for cancer, neurodegenerative diseases, and inflammatory processes.
Heparan sulfate proteoglycans as antiautophagic factors
In contrast to the proautophagic proteoglycans described previously, decorin and biglycan, and C-terminal unglycanated fragments of HSPHs, perlecan and collagen XVIII, the parent molecule, perlecan/Hspg2, functions as a suppressor of autophagy. An elegant genetic study has utilized Hspg2−/− mice,293 which are usually embryonic lethal,239,294 by rescuing them via transgenic expression of Hspg2 into the cartilage (Hspg2−/−Tg). This rescue is based on the high expression of Hspg2 in cartilage and its loss causes early respiratory failure.294 Genetic ablation of Hspg2 resulted in tenotomy-induced atrophy of the soleus muscle while concurrently increased autophagy vis-à-vis wild-type mice that had undergone the same procedure.293 In addition to a decrease in autophagic substrates such as p62 and an increase in LC3-II levels, perlecan deficiency caused a reduction in phosphorylated p70S6k and Akt, while concurrently inducing phosphorylation of AMPKα, suggesting that Hspg2 inhibits autophagy in through the mTORC1 pathway.293 The main conclusion of this study posits perlecan as a necessary factor for maintaining muscle mass through proper regulation of autophagy, a concept also supported by zebrafish studies where perlecan morphants show a severe myopathy characterized by abnormal actin filaments and disorganized sarcomeres.246
It is well established that neuromuscular junctions display developmental and activity dependent plasticity that can be directly affected by HSPGs. Notably, in Drosophila, muscle-specific knockdown of HS biosynthetic machinery increased autophagy at the neuromuscular junctions.295 Moreover, HS production was also essential for maintaining normal autophagic processes in the fat body, the principal energy storage and nutritional sensing organ of Drosophila. These data are further supported by an elegant study using Drosophila Parkin mutants where globally suppressing HS biosynthesis led to increased lifespan and resistance to oxidative stress.296 Collectively, these findings demonstrate that HS is a critical regulator of on autophagy, a biological process essential for natural assembly of postsynaptic membrane specializations.295 The mechanism by which HS inhibits autophagy has yet to be fully elucidated and we do not know which HSPG is primarily responsible for this activity. Moreover, how these extracellular polysaccharides influence intracellular catabolic events and the cell surface receptors and signaling molecules at play is an interesting question, and its answer would benefit both the proteoglycan and autophagy fields.265
Limitations of the study
In this review we have not covered the expanding field of hyaluronan science due to space limitations. Although not a true proteoglycan, hyaluronan is mostly bound to proteins such as the cell surface receptors CD44 and RHAMM and, in tissues, hyaluronan is non-covalently linked to the G1 domains of the hyaluronan- and lectin-binding proteoglycans known as hyalectans297 which include aggrecan, versican, brevican, and neurocan.5 The hyaluronan field has expanded enormously in the past two decades primarily because of its involvement in cancer, angiogenesis, inflammation, and innate immunity. We refer the readers to several recent reviews covering this subject.298,299,300,301,302 Other topics not covered here are the proteoglycan roles in cartilage and bone diseases, and their functions during development and organogenesis. There are several excellent reviews covering these important topics as well.303,304,305
Unanswered questions and future challenges
The current proteoglycan arena is exciting, powerful, and promising regarding both diagnostic and therapeutic applications. Nevertheless, several outstanding questions regarding the molecular mechanisms and machinery regulating proteoglycan/growth factor interactions and their role in development and diseases remain to be addressed in the future.
Another key question is how can we exploit high-throughput sequencing, such as single-cell RNAseq, to attain an improved understanding of proteoglycan heterogeneity and detect new mutations/isoforms of various proteoglycan genes in patients for precision medicine?
On a more practical and achievable level, what is the present and future role of biomarkers that could be used to identify patients that could benefit from proteoglycan-targeted therapy? How will distinct combinatorial therapies be established, and which treatment will act synergistically with therapies targeting tumor-specific antigens?
What is the role of the immune system in regulating proteoglycan activity and vice versa? How could we activate the immune system against specific proteoglycans such as glypican, for example,42 to penetrate the highly immunosuppressive tumor microenvironment and eliminate solid tumors?
Another important bioactivity that needs further attention is on the role of protease-evoked release of source proteoglycans and their dual roles as true and decoy receptors. Unraveling the complex mechanism of action and the potential commitment of pro-inflammatory signaling pathways triggered by these shed proteoglycans is a clear gap in our knowledge.
Will recombinant decorin be used as an oncosuppressive therapeutic and could it be utilized as effective adjuvant therapy for human malignancies? The fact that decorin affects both epithelial and bone marrow-derived malignancies,306,307,308 and its role in evoking epithelial mesenchymal transition,309 infers a more general role for this proteoglycan in curtailing mammalian malignancies.
Another intriguing recent technological advance is the ENCODE4 RNAseq that can read long transcripts, thereby discovering numerous transcripts and many alternative splice variants.310 Thus, an important question is how many previously unrecognized transcripts will be found in the proteoglycan encoding genes? And, if so, how many new biological roles will be uncovered in the future? Given the global impact of proteoglycan science on human physiology and diseases, the future of proteoglycans is so bright “ …. we will have to wear shades” (Pat MacDonald).
Acknowledgments
We are indebted to Drs. Ralph Sanderson and Lianchun Wang for valuable comments on the figures and text, and Gabriel Pascal for help with the graphical abstract. We are indebted to all the past and present members of our laboratories and apologize for not referencing many valuable contributions to the field due to space limitation.
Grants: The original research was supported, in part, by National Institutes of Health Grants RO1 CA245311 and RO3 CA270830 (to R.V.I.) and by the Deutsche Forschungsgemeinschaft DFG, German Research Foundation) SFB 1177, and Project E02-ID 259130777 SFB 1039, Project B02 (to L.S.).
Author contributions
R.V.I. and C.X. were responsible for conceptualization, drafting, and editing of the manuscript, as well as the design and generation of all the figures. L.S. contributed to writing and editing. R.V.I. was responsible for the final editing and proofing of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Gubbiotti M.A., Vallet S.D., Ricard-Blum S., Iozzo R.V. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol. 2016;55:7–21. doi: 10.1016/j.matbio.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallet S.D., Berthollier C., Ricard-Blum S. The glycosaminoglycan interactome 2.0. Am. J. Physiol. Cell Physiol. 2022;322:C1271–C1278. doi: 10.1152/ajpcell.00095.2022. [DOI] [PubMed] [Google Scholar]
- 3.Basu A., Patel N.G., Nicholson E.D., Weiss R.J. Spatiotemporal diversity and regulation of glycosaminoglycans in cell homeostasis and human disease. Am. J. Physiol. Cell Physiol. 2022;322:C849–C864. doi: 10.1152/ajpcell.00085.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handel T.M., Dyer D.P. Perspectives on the biological role of chemokine:Glycosaminoglycan interactions. J. Histochem. Cytochem. 2021;69:87–91. doi: 10.1369/0022155420977971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iozzo R.V., Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez S., Makshakova O., Angulo J., Bedini E., Bisio A., de Paz J.L., Fadda E., Guerrini M., Hricovini M., Hricovini M., et al. Glycosaminoglycans: What remains to be deciphered? JACS Au. 2023;3:628–656. doi: 10.1021/jacsau.2c00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karamanos N.K., Piperigkou Z., Theocharis A.D., Watanabe H., Franchi M., Baud S., Brézillon S., Götte M., Passi A., Vigetti D., et al. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem. Rev. 2018;118:9152–9232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 8.Esko J.D., Selleck S.B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 9.Esko J.D., Lindahl U. Molecular diversity of heparan sulfate. J. Clin. Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitelock J.M., Iozzo R.V. Heparan sulfate: a complex polymer charged with biological activity. Chem. Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 11.Noborn F., Gomez Toledo A., Green A., Nasir W., Sihlbom C., Nilsson J., Larson G. Site-specific identification of heparan and chondroitin sulfate glycosaminoglycans in hybrid proteoglycans. Sci. Rep. 2016;6 doi: 10.1038/srep34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noborn F., Nilsson J., Larson G. Site-specific glycosylation of proteoglycans: A revisited frontier in proteoglycan research. Matrix Biol. 2022;111:289–306. doi: 10.1016/j.matbio.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Song Y., Zhang F., Linhardt R.J. Analysis of the glycosaminoglycan chains of proteoglycans. J. Histochem. Cytochem. 2021;69:121–135. doi: 10.1369/0022155420937154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallet S.D., Annaval T., Vives R.R., Richard E., Hénault J., Le Narvor C., Bonnaffé D., Priem B., Wild R., Lortat-Jacob H. Functional and structural insights into human N-deacetylase/N-sulfotransferase activities. Proteoglycan Res. 2023;1 [Google Scholar]
- 15.McMillan I.O., Li J.P., Wang L. Heparan sulfate proteoglycan in Alzheimer's disease: aberrant expression and functions in molecular pathways related to amyloid-Î2 metabolism. Am. J. Physiol. Cell Physiol. 2023;324:C893–C909. doi: 10.1152/ajpcell.00247.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop J.R., Schuksz M., Esko J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 17.Gude F., Froese J., Steffes G., Grobe K. The role of glycosaminoglycan modification in Hedgehog regulated tissue morphogenesis. Biochem. Soc. Trans. 2023;51:983–993. doi: 10.1042/BST20220719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdiaki A., Neagu M., Spyridaki I., Kuskov A., Perez S., Nikitovic D. Hyaluronan and reactive oxygen species signaling-novel cues from the matrix? Antioxidants. 2023;12:824. doi: 10.3390/antiox12040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffrin A.M., Hsieh-Wilson L.C. Photoaffinity probes for the Identification of sequence-specific glycosaminoglycan-binding proteins. J. Am. Chem. Soc. 2020;142:13672–13676. doi: 10.1021/jacs.0c06046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ori A., Wilkinson M.C., Fernig D.G. The heparanome and regulation of cell function: structures, functions and challenges. Front. Biosci. 2008;13:4309–4338. doi: 10.2741/3007. [DOI] [PubMed] [Google Scholar]
- 21.Ricard-Blum S., Perez S. Glycosaminoglycan interaction networks and databases. Curr. Opin. Struct. Biol. 2022;74 doi: 10.1016/j.sbi.2022.102355. [DOI] [PubMed] [Google Scholar]
- 22.Vallet S.D., Clerc O., Ricard-Blum S. Glycosaminoglycan-protein interactions: The first draft of the glycosaminoglycan interactome. J. Histochem. Cytochem. 2021;69:93–104. doi: 10.1369/0022155420946403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Chapla D., Amos R.A., Ramiah A., Moremen K.W., Li H. Structural basis for heparan sulfate co-polymerase action by the EXT1-2 complex. Nat. Chem. Biol. 2023;19:565–574. doi: 10.1038/s41589-022-01220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Pedersen L.C. Emerging chemical and biochemical tools for studying 3-O-sulfated heparan sulfate. Am. J. Physiol. Cell Physiol. 2022;322:C1166–C1175. doi: 10.1152/ajpcell.00110.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Critcher M., Huang M.L. Excavating proteoglycan structure-function relationships: modern approaches to capture the interactions of ancient biomolecules. Am. J. Physiol. Cell Physiol. 2022;323:C415–C422. doi: 10.1152/ajpcell.00222.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead T.J., Bhutada S., Martin D.R., Apte S.S. Proteolysis: a key post-translational modification regulating proteoglycans. Am. J. Physiol. Cell Physiol. 2022;323:C651–C665. doi: 10.1152/ajpcell.00215.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivet R., Rao R.M., Nizet P., Belloy N., Huber L., Dauchez M., Ramont L., Baud S., Brézillon S. Differential MMP-14 targeting by biglycan, decorin, fibromodulin and lumican unraveled by In silico approach. Am. J. Physiol. Cell Physiol. 2023;324:C353–C365. doi: 10.1152/ajpcell.00429.2022. [DOI] [PubMed] [Google Scholar]
- 28.Chen C.G., Iozzo R.V. Angiostatic cues from the matrix: endothelial cell autophagy meets hyaluronan biology. J. Biol. Chem. 2020;295:16797–16812. doi: 10.1074/jbc.REV120.014391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poluzzi C., Iozzo R.V., Schaefer L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv. Drug Deliv. Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filmus J., Selleck S.B. Glypicans: proteoglycans with a surprise. J. Clin. Invest. 2001;108:497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filmus J. Glypicans, 35 years later. Proteglycan Res. 2023;1:e5. [Google Scholar]
- 32.Filmus J., Capurro M., Rast J. Genome Biol. 2008;9:224. doi: 10.1186/gb-2008-9-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Cat B., Muyldermans S.-Y., Coomans C., Degeest G., Vanderschueren B., Creemers J., Biemar F., Peers B., David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 2003;163:625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capurro M., Shi W., Izumikawa T., Kitagawa H., Filmus J. Processing by convertases is required for Glypican-3-induced inhibition of Hedgehog signaling. J. Biol. Chem. 2015;290:7576–7585. doi: 10.1074/jbc.M114.612705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuda M., Kamimura K., Nakato H., Archer M., Staatz W., Fox B., Humphrey M., Olson S., Futch T., Kaluza V., et al. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature. 1999;400:276–280. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- 36.Lin X., Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature. 1999;400:281–284. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- 37.McGough I.J., Vecchia L., Bishop B., Malinauskas T., Beckett K., Joshi D., O'Reilly N., Siebold C., Jones E.Y., Vincent J.P. Glypicans shield the Wnt lipid moiety to enable signalling at a distance. Nature. 2020;585:85–90. doi: 10.1038/s41586-020-2498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grobe K., Guerrero I. Dally-like is unlike Dally in assisting Wingless spread. Dev. Cell. 2020;54:572–573. doi: 10.1016/j.devcel.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Filmus J. The function of glypicans in the mammalian embryo. Am. J. Physiol. Cell Physiol. 2022;322:C694–C698. doi: 10.1152/ajpcell.00045.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultheis N., Becker R., Berhanu G., Kapral A., Roseman M., Shah S., Connell A., Selleck S. Regulation of autophagy, lipid metabolism, and neurodegenerative pathology by heparan sulfate proteoglycans. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1012706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S., Qiu Y., Bai B. The expression, regulation, and biomarker potential of Glypican-1 in cancer. Front. Oncol. 2019;9:614. doi: 10.3389/fonc.2019.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N., Gao W., Zhang Y.F., Ho M. Glypicans as cancer therapeutic targets. Trends Cancer. 2018;4:741–754. doi: 10.1016/j.trecan.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filmus J., Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014;35:248–252. doi: 10.1016/j.matbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Pan J., Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am. J. Physiol. Cell Physiol. 2021;321:C846–C858. doi: 10.1152/ajpcell.00290.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traister A., Shi W., Filmus J. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem. J. 2008;410:503–511. doi: 10.1042/BJ20070511. [DOI] [PubMed] [Google Scholar]
- 46.Kakugawa S., Langton P.F., Zebisch M., Howell S., Chang T.H., Liu Y., Feizi T., Bineva G., O'Reilly N., Snijders A.P., et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng F., Hansson V.C., Georgolopoulos G., Mani K. Attenuation of cancer proliferation by suppression of glypican-1 and its pleiotropic effects in neoplastic behavior. Oncotarget. 2023;14:219–235. doi: 10.18632/oncotarget.28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudnicka L., Varga J., Christiano A.M., Iozzo R.V., Jimenez S.A., Uitto J. Elevated expression of type VII collagen in the skin of patients with systemic sclerosis. J. Clin. Invest. 1994;93:1709–1715. doi: 10.1172/JCI117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodge G.R., Kovalszky I., Hassell J.R., Iozzo R.V. Transforming growth factor β alters the expression of heparan sulfate proteoglycan in human colon carcinoma cells. J. Biol. Chem. 1990;265:18023–18029. [PubMed] [Google Scholar]
- 50.Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 51.Ryynänen M., Ryynänen J., Sollberg S., Iozzo R.V., Knowlton R.G., Uitto J. Genetic linkage of Type VII collagen (COL7A1) to dominant dystrophic epidermolysis bullosa in families with abnormal anchoring fibrils. J. Clin. Invest. 1992;89:974–980. doi: 10.1172/JCI115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capurro M.I., Xiang Y.Y., Lobe C., Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 53.Capurro M., Wanless I.R., Sherman M., Deboer G., Shi W., Miyoshi E., Filmus J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 54.Mauro M., Ugo P., Walton Z., Ali S., Rastellini C., Cicalese L. Glypican-3 (GPC-3) structural analysis and cargo in serum small extracellular vesicles of hepatocellular carcinoma patients. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241310922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparn C., Dimou E., Meyer A., Saleppico R., Wegehingel S., Gerstner M., Klaus S., Ewers H., Nickel W. Glypican-1 drives unconventional secretion of fibroblast growth factor 2. Elife. 2022;11 doi: 10.7554/eLife.75545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparn C., Meyer A., Saleppico R., Nickel W. Unconventional secretion mediated by direct protein self-translocation across the plasma membranes of mammalian cells. Trends Biochem. Sci. 2022;47:699–709. doi: 10.1016/j.tibs.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Gopal S., Arokiasamy S., Pataki C., Whiteford J.R., Couchman J.R. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11 doi: 10.1098/rsob.200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maszota-Zieleniak M., Liwo A., Ricard-Blum S., Samsonov S.A. Interplay of heparan sulfate chains with the core proteins of syndecan 2 and 4. Proteglycan Res. 2023;1:e10. [Google Scholar]
- 59.Iozzo R.V. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J. Cell Biol. 1984;99:403–417. doi: 10.1083/jcb.99.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bix G., Fu J., Gonzalez E.M., Macro L., Barker A., Campbell S., Zutter M.M., Santoro S.A., Kim J.K., Höök M., et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J. Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bix G., Iozzo R.A., Woodall B., Burrows M., McQuillan A., Campbell S., Fields G.B., Iozzo R.V. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1 integrin receptor. Blood. 2007;109:3745–3748. doi: 10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodall B.P., Nyström A., Iozzo R.A., Eble J.A., Niland S., Krieg T., Eckes B., Pozzi A., Iozzo R.V. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 63.Stewart M.D., Ramani V.C., Sanderson R.D. Shed Syndecan-1 Translocates to the Nucleus of Cells Delivering Growth Factors and Inhibiting Histone Acetylation: A Novel Mechanism of tumor-host crosstalk. J. Biol. Chem. 2015;290:941–949. doi: 10.1074/jbc.M114.608455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart M.D., Sanderson R.D. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol. 2014;35:56–59. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kovalszky I., Hjerpe A., Dobra K. Nuclear translocation of heparan sulfate proteoglycans and their functional significance. Biochim. Biophys. Acta. 2014;1840:2491–2497. doi: 10.1016/j.bbagen.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 66.Ramani V.C., Yang Y., Ren Y., Nan L., Sanderson R.D. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J. Biol. Chem. 2011;286:6490–6499. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iozzo R.V., Sanderson R.D. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corti F., Wang Y., Rhodes J.M., Atri D., Archer-Hartmann S., Zhang J., Zhuang Z.W., Chen D., Wang T., Wang Z., et al. N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat. Commun. 2019;10:1562. doi: 10.1038/s41467-019-09605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corti F., Ristori E., Rivera-Molina F., Toomre D., Zhang J., Mihailovic J., Zhuang Z.W., Simons M. Syndecan-2 selectively regulates VEGF-induced vascular permeability. Nat. Cardiovasc. Res. 2022;1:518–528. doi: 10.1038/s44161-022-00064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramani V.C., Sanderson R.D. Chemotherapy stimulates syndecan-1 shedding: a potentially negative effect of treatment that may promote tumor relapse. Matrix Biol. 2014;35:215–222. doi: 10.1016/j.matbio.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Afratis N.A., Nikitovic D., Multhaupt H.A.B., Theocharis A.D., Couchman J.R., Karamanos N.K. Syndecans: key regulators of cell signaling and biological functions. FEBS J. 2017;284:27–41. doi: 10.1111/febs.13940. [DOI] [PubMed] [Google Scholar]
- 72.Hayashida K., Aquino R.S., Park P.W. Coreceptor functions of cell surface heparan sulfate proteoglycans. Am. J. Physiol. Cell Physiol. 2022;322:C896–C912. doi: 10.1152/ajpcell.00050.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derksen P.W.B., Keehnen R.M.J., Evers L.M., van Oers M.H.J., Spaargaren M., Pals S.T. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood. 2002;99:1405–1410. doi: 10.1182/blood.v99.4.1405. [DOI] [PubMed] [Google Scholar]
- 74.Ren Z., van Andel H., de Lau W., Hartholt R.B., Maurice M.M., Clevers H., Kersten M.J., Spaargaren M., Pals S.T. Syndecan-1 promotes Wnt/β-catenin signaling in multiple myeloma by presenting Wnts and R-spondins. Blood. 2018;131:982–994. doi: 10.1182/blood-2017-07-797050. [DOI] [PubMed] [Google Scholar]
- 75.Ren Z., Spaargaren M., Pals S.T. Syndecan-1 and stromal heparan sulfate proteoglycans: key moderators of plasma cell biology and myeloma pathogenesis. Blood. 2021;137:1713–1718. doi: 10.1182/blood.2020008188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibrahim S.A., Gadalla R., El-Ghonaimy E.A., Samir O., Mohamed H.T., Hassan H., Greve B., El-Shinawi M., Mohamed M.M., Götte M. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol. Cancer. 2017;16:57. doi: 10.1186/s12943-017-0621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reszegi A., Tátrai P., Regős E., Kovalszky I., Baghy K. Syndecan-1 in liver pathophysiology. Am. J. Physiol. Cell Physiol. 2022;323:C289–C294. doi: 10.1152/ajpcell.00039.2022. [DOI] [PubMed] [Google Scholar]
- 78.Hwang J., Park E., Choi Y.W., Min S., Oh E.S. Emerging role of syndecans in maintaining homeostasis of colon epithelium during inflammation. Am. J. Physiol. Cell Physiol. 2022;322:C960–C966. doi: 10.1152/ajpcell.00048.2022. [DOI] [PubMed] [Google Scholar]
- 79.Onyeisi J.O.S., Lopes C.C., Götte M. Role of syndecan-4 in breast cancer pathophysiology. Am. J. Physiol. Cell Physiol. 2022;323:C1345–C1354. doi: 10.1152/ajpcell.00152.2022. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Yaccoby S., Liu W., Langford J.K., Pumphrey C.Y., Theus A., Epstein J., Sanderson R.D. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- 81.Hayashida A., Saeed H.N., Zhang F., Song Y., Liu J., Parks W.C., Bispo P.J.M., Park P.W. Sulfated motifs in heparan sulfate inhibit Streptococcus pneumoniae adhesion onto fibronectin and attenuate corneal infection. Proteoglycan Res. 2023;1:e9. doi: 10.1002/pgr2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Green L.R., Issa R., Albaldi F., Urwin L., Thompson R., Khalid H., Turner C.E., Ciani B., Partridge L.J., Monk P.N. CD9 co-operation with syndecan-1 is required for a major staphylococcal adhesion pathway. mBio. 2023;14 doi: 10.1128/mbio.01482-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gopal S. Syndecans in inflammation at a glance. Front. Immunol. 2020;11:227. doi: 10.3389/fimmu.2020.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beauvais D.M., Nelson S.E., Adams K.M., Stueven N.A., Jung O., Rapraeger A.C. Plasma membrane proteoglycans syndecan-2 and syndecan-4 engage with EGFR and RON kinase to sustain carcinoma cell cycle progression. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beauvais D.M., Jung O., Yang Y., Sanderson R.D., Rapraeger A.C. Syndecan-1 (CD138) Suppresses Apoptosis in Multiple Myeloma by Activating IGF1 Receptor: Prevention by SynstatinIGF1R Inhibits Tumor Growth. Cancer Res. 2016;76:4981–4993. doi: 10.1158/0008-5472.CAN-16-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poças J., Marques C., Gomes C., Otake A.H., Pinto F., Ferreira M., Silva T., Faria-Ramos I., Matos R., Ribeiro A.R., et al. Syndecan-4 is a maestro of gastric cancer cell invasion and communication that underscores poor survival. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2214853120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fuki I.V., Kuhn K.M., Lomazov I.R., Rothman V.L., Tuszynski G.P., Iozzo R.V., Swenson T.L., Fisher E.A., Williams K.J. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J. Clin. Invest. 1997;100:1611–1622. doi: 10.1172/JCI119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stanford K.I., Bishop J.R., Foley E.M., Gonzales J.C., Niesman I.R., Witztum J.L., Esko J.D. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y., Foley E.M., Gonzales J.C., Gordts P.L., Li Y., Esko J.D. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology. 2012;55:277–286. doi: 10.1002/hep.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Nardo W., Miotto P.M., Bayliss J., Nie S., Keenan S.N., Montgomery M.K., Watt M.J. Proteomic analysis reveals exercise training induced remodelling of hepatokine secretion and uncovers syndecan-4 as a regulator of hepatic lipid metabolism. Mol. Metabol. 2022;60 doi: 10.1016/j.molmet.2022.101491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson K.G., Ghose A., Epstein E., Lincecum J., O'Connor M.B., Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Rawson J.M., Dimitroff B., Johnson K.G., Rawson J.M., Ge X., Van Vactor D., Selleck S.B. The heparan sulfate proteoglycans Dally-like and Syndecan have distinct functions in axon guidance and visual-system assembly in Drosophila. Curr. Biol. 2005;15:833–838. doi: 10.1016/j.cub.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 93.Blanchette C.R., Thackeray A., Perrat P.N., Hekimi S., Bénard C.Y. Functional requirements for heparan sulfate biosynthesis in morphogenesis and nervous system development in C. elegans. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schwieterman A.A., Steves A.N., Yee V., Donelson C.J., Bentley M.R., Santorella E.M., Mehlenbacher T.V., Pital A., Howard A.M., Wilson M.R., et al. The Caenorhabditis elegans Ephrin EFN-4 functions non-cell autonomously with heparan sulfate proteoglycans to promote axon outgrowth and branching. Genetics. 2016;202:639–660. doi: 10.1534/genetics.115.185298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lázaro-Peña M.I., Díaz-Balzac C.A., Bülow H.E., Emmons S.W. Synaptogenesis Is modulated by heparan sulfate in Caenorhabditis elegans. Genetics. 2018;209:195–208. doi: 10.1534/genetics.118.300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van Horssen J., Wesseling P., van den Heuvel L.P.W.J., de Waal R.M.W., Verbeek M.M. Heparan sulphate proteoglycans in Alzheimer's disease and amyloid-related disorders. Lancet Neurol. 2003;2:482–492. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 97.Cui H., Freeman C., Jacobson G.A., Small D.H. Proteoglycans in the central nervous system: role in development, neural repair, and Alzheimer's disease. IUBMB Life. 2013;65:108–120. doi: 10.1002/iub.1118. [DOI] [PubMed] [Google Scholar]
- 98.Snow A.D., Cummings J.A., Lake T. The unifying hypothesis of Alzheimer's disease: Heparan sulfate proteoglycans/glycosaminoglycans are key as first hypothesized over 30 years ago. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.710683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Y., Gandy L., Zhang F., Liu J., Wang C., Blair L.J., Linhardt R.J., Wang L. Heparan sulfate proteoglycans in Tauopathy. Biomolecules. 2022;12 doi: 10.3390/biom12121792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jendresen C.B., Cui H., Zhang X., Vlodavsky I., Nilsson L.N.G., Li J.P. Overexpression of heparanase lowers the amyloid burden in amyloid-β precursor protein transgenic mice. J. Biol. Chem. 2015;290:5053–5064. doi: 10.1074/jbc.M114.600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.García B., Martín C., García-Suárez O., Muñiz-Alonso B., Ordiales H., Fernández-Menéndez S., Santos-Juanes J., Lorente-Gea L., Castañón S., Vicente-Etxenausia I., et al. Upregulated expression of heparanase and heparanase 2 in the brains of Alzheimer's disease. J. Alzheimers Dis. 2017;58:185–192. doi: 10.3233/JAD-161298. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X., O'Callaghan P., Li H., Tan Y., Zhang G., Barash U., Wang X., Lannfelt L., Vlodavsky I., Lindahl U., Li J.P. Heparanase overexpression impedes perivascular clearance of amyloid-β from murine brain: relevance to Alzheimer's disease. Acta Neuropathol. Commun. 2021;9:84. doi: 10.1186/s40478-021-01182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holmes B.B., DeVos S.L., Kfoury N., Li M., Jacks R., Yanamandra K., Ouidja M.O., Brodsky F.M., Marasa J., Bagchi D.P., et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mah D., Zhao J., Liu X., Zhang F., Liu J., Wang L., Linhardt R., Wang C. The sulfation code of Tauopathies: Heparan sulfate proteoglycans in the prion like spread of Tau pathology. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.671458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song L., Oseid D.E., Wells E.A., Coaston T., Robinson A.S. Heparan sulfate proteoglycans (HSPGs) serve as the mediators between monomeric Tau and its subsequent intracellular ERK1/2 pathway activation. J. Mol. Neurosci. 2022;72:772–791. doi: 10.1007/s12031-021-01943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu C.C., Zhao N., Yamaguchi Y., Cirrito J.R., Kanekiyo T., Holtzman D.M., Bu G. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-β clearance and aggregation in Alzheimer's disease. Sci. Transl. Med. 2016;8:332ra44. doi: 10.1126/scitranslmed.aad3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saroja S.R., Gorbachev K., Julia T., Goate A.M., Pereira A.C. Astrocyte-secreted glypican-4 drives APOE4-dependent tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2108870119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hudák A., Kusz E., Domonkos I., Jósvay K., Kodamullil A.T., Szilák L., Hofmann-Apitius M., Letoha T. Contribution of syndecans to cellular uptake and fibrillation of α-synuclein and tau. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-53038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng F., Fransson L.Å., Mani K. Interplay between APP and glypican-1 processing and α-synuclein aggregation in undifferentiated and differentiated human neural progenitor cells. Glycobiology. 2023;33:325–341. doi: 10.1093/glycob/cwad013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng F., Fransson L.Å., Mani K. Complex modulation of cytokine-induced α-synuclein aggregation by glypican-1-derived heparan sulfate in neural cells. Glycobiology. 2022;32:333–342. doi: 10.1093/glycob/cwab126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stopschinski B.E., Holmes B.B., Miller G.M., Manon V.A., Vaquer-Alicea J., Prueitt W.L., Hsieh-Wilson L.C., Diamond M.I. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. J. Biol. Chem. 2018;293:10826–10840. doi: 10.1074/jbc.RA117.000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen M., Vincent J., Ezeanii A., Wakade S., Yerigenahally S., Mor D.E. Heparan sulfate proteoglycans mediate prion-like α-synuclein toxicity in Parkinson's in vivo models. Life Sci. Alliance. 2022;5 doi: 10.26508/lsa.202201366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huynh M.B., Ouidja M.O., Chantepie S., Carpentier G., Maïza A., Zhang G., Vilares J., Raisman-Vozari R., Papy-Garcia D. Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauch J.N., Chen J.J., Sorum A.W., Miller G.M., Sharf T., See S.K., Hsieh-Wilson L.C., Kampmann M., Kosik K.S. Tau internalization is regulated by 6-O sulfation on heparan sulfate proteoglycans (HSPGs) Sci. Rep. 2018;8:6382. doi: 10.1038/s41598-018-24904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z., Patel V.N., Song X., Xu Y., Kaminski A.M., Doan V.U., Su G., Liao Y., Mah D., Zhang F., et al. Increased 3-O-sulfated heparan sulfate in Alzheimer's disease brain is associated with genetic risk gene HS3ST1. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hudák A., Letoha A., Vizler C., Letoha T. Syndecan-3 as a novel biomarker in Alzheimer's disease. Int. J. Mol. Sci. 2022;23:3407. doi: 10.3390/ijms23063407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaefer L., Babelova A., Kiss E., Hausser H.-J., Baliova M., Krzyzankova M., Marsche G., Young M.F., Mihalik D., Götte M., et al. The matrix component biglycan is proinflammatory and signals through toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Babelova A., Moreth K., Tsalastra-Greul W., Zeng-Brouwers J., Eickelberg O., Young M.F., Bruckner P., Pfeilschifter J., Schaefer R.M., Gröne H.J., Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via Toll-like and P2X receptors. J. Biol. Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bertolin G., Ferrando-Miguel R., Jacoupy M., Traver S., Grenier K., Greene A.W., Dauphin A., Waharte F., Bayot A., Salamero J., et al. The TOMM machinery is a molecular switch in PINK1 and PARK2/PARKIN-dependent mitochondrial clearance. Autophagy. 2013;9:1801–1817. doi: 10.4161/auto.25884. [DOI] [PubMed] [Google Scholar]
- 120.Merline R., Moreth K., Beckmann J., Nastase M.V., Zeng-Brouwers J., Tralhão J.G., Lemarchand P., Pfeilschifter J., Schaefer R.M., Iozzo R.V., Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci. Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nugent M.A. The future of the COVID-19 pandemic: How good (or bad) can the SARS-CoV2 spike protein get? Cells. 2022;11 doi: 10.3390/cells11050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., et al. SARS-CoV-2 Infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183:1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bermejo-Jambrina M., Eder J., Kaptein T.M., van Hamme J.L., Helgers L.C., Vlaming K.E., Brouwer P.J.M., van Nuenen A.C., Spaargaren M., de Bree G.J., et al. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J. 2021;40 doi: 10.15252/embj.2020106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kearns F.L., Sandoval D.R., Casalino L., Clausen T.M., Rosenfeld M.A., Spliid C.B., Amaro R.E., Esko J.D. Spike-heparan sulfate interactions in SARS-CoV-2 infection. Curr. Opin. Struct. Biol. 2022;76 doi: 10.1016/j.sbi.2022.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cerezo-Magaña M., Bång-Rudenstam A., Belting M. Proteoglycans: a common portal for SARS-CoV-2 and extracellular vesicle uptake. Am. J. Physiol. Cell Physiol. 2023;324:C76–C84. doi: 10.1152/ajpcell.00453.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Q., Chen C.Z., Swaroop M., Xu M., Wang L., Lee J., Wang A.Q., Pradhan M., Hagen N., Chen L., et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020;6:80. doi: 10.1038/s41421-020-00222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puray-Chavez M., LaPak K.M., Schrank T.P., Elliott J.L., Bhatt D.P., Agajanian M.J., Jasuja R., Lawson D.Q., Davis K., Rothlauf P.W., et al. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jiang L., Zhang T., Lu H., Li S., Lv K., Tuffour A., Zhang L., Ding K., Li J.P., Li H., Liu X. Heparin mimetics as potential intervention for COVID-19 and their bio-manufacturing. Synth. Syst. Biotechnol. 2023;8:11–19. doi: 10.1016/j.synbio.2022.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milewska A., Zarebski M., Nowak P., Stozek K., Potempa J., Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014;88:13221–13230. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ling J., Li J., Khan A., Lundkvist Å., Li J.P. Is heparan sulfate a target for inhibition of RNA virus infection? Am. J. Physiol. Cell Physiol. 2022;322:C605–C613. doi: 10.1152/ajpcell.00028.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guimond S.E., Mycroft-West C.J., Gandhi N.S., Tree J.A., Le T.T., Spalluto C.M., Humbert M.V., Buttigieg K.R., Coombes N., Elmore M.J., et al. Synthetic Heparan Sulfate Mimetic Pixatimod (PG545) Potently Inhibits SARS-CoV-2 by Disrupting the Spike-ACE2 Interaction. ACS Cent. Sci. 2022;8:527–545. doi: 10.1021/acscentsci.1c01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mycroft-West C.J., Su D., Pagani I., Rudd T.R., Elli S., Gandhi N.S., Guimond S.E., Miller G.J., Meneghetti M.C.Z., Nader H.B., et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemostasis. 2020;120:1700–1715. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lang J., Yang N., Deng J., Liu K., Yang P., Zhang G., Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Afzali B., Noris M., Lambrecht B.N., Kemper C. The state of complement in COVID-19. Nat. Rev. Immunol. 2022;22:77–84. doi: 10.1038/s41577-021-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lo M.W., Amarilla A.A., Lee J.D., Albornoz E.A., Modhiran N., Clark R.J., Ferro V., Chhabra M., Khromykh A.A., Watterson D., Woodruff T.M. SARS-CoV-2 triggers complement activation through interactions with heparan sulfate. Clin. Transl. Immunology. 2022;11:e1413. doi: 10.1002/cti2.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lim E.H.T., van Amstel R.B.E., de Boer V.V., van Vught L.A., de Bruin S., Brouwer M.C., Vlaar A.P.J., van de Beek D. Complement activation in COVID-19 and targeted therapeutic options: A scoping review. Blood Rev. 2023;57 doi: 10.1016/j.blre.2022.100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong W., Montano M., Corley M.J., Helmy E., Kobayashi H., Kinisu M., Suryawanshi R., Luo X., Royer L.A., Roan N.R., et al. Neuropilin-1 mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio. 2022;13 doi: 10.1128/mbio.02308-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shilts J., Crozier T.W.M., Teixeira-Silva A., Gabaev I., Gerber P.P., Greenwood E.J.D., Watson S.J., Ortmann B.M., Gawden-Bone C.M., Pauzaite T., et al. LRRC15 mediates an accessory interaction with the SARS-CoV-2 spike protein. PLoS Biol. 2023;21 doi: 10.1371/journal.pbio.3001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loo L., Waller M.A., Moreno C.L., Cole A.J., Stella A.O., Pop O.T., Jochum A.K., Ali O.H., Denes C.E., Hamoudi Z., et al. Fibroblast-expressed LRRC15 is a receptor for SARS-CoV-2 spike and controls antiviral and antifibrotic transcriptional programs. PLoS Biol. 2023;21 doi: 10.1371/journal.pbio.3001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daly J.L., Simonetti B., Klein K., Chen K.E., Williamson M.K., Antón-Plágaro C., Shoemark D.K., Simón-Gracia L., Bauer M., Hollandi R., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O'Prey J., Wilkinson S., Ryan K.M. Tumor antigen LRRC15 impedes adenoviral infection: implications for virus-based cancer therapy. J. Virol. 2008;82:5933–5939. doi: 10.1128/JVI.02273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Song J., Chow R.D., Peña-Hernández M.A., Zhang L., Loeb S.A., So E.Y., Liang O.D., Ren P., Chen S., Wilen C.B., Lee S. LRRC15 inhibits SARS-CoV-2 cellular entry in trans. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gisby J.S., Buang N.B., Papadaki A., Clarke C.L., Malik T.H., Medjeral-Thomas N., Pinheiro D., Mortimer P.M., Lewis S., Sandhu E., et al. Multi-omics identify falling LRRC15 as a COVID-19 severity marker and persistent pro-thrombotic signals in convalescence. Nat. Commun. 2022;13:7775. doi: 10.1038/s41467-022-35454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thacker B.E., Xu D., Lawrence R., Esko J.D. Heparan sulfate 3-O-sulfation: a rare modification in search of a function. Matrix Biol. 2014;35:60–72. doi: 10.1016/j.matbio.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thacker B.E., Seamen E., Lawrence R., Parker M.W., Xu Y., Liu J., Vander Kooi C.W., Esko J.D. Expanding the 3-O-sulfate proteome--Enhanced binding of neuropilin-1 to 3-O-sulfated heparan sulfate modulates Its cctivity. ACS Chem. Biol. 2016;11:971–980. doi: 10.1021/acschembio.5b00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Levine B., Kroemer G. Biological functions of autophagy genes: A disease perspective. Cell. 2019;176:11–42. doi: 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Buraschi S., Neill T., Goyal A., Poluzzi C., Smythies J., Owens R.T., Schaefer L., Torres A., Iozzo R.V. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. USA. 2013;110:E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Buraschi S., Neill T., Iozzo R.V. Decorin is a devouring proteoglycan: Remodeling of intracellular catabolism via autophagy and mitophagy. Matrix Biol. 2019;75–76:260–270. doi: 10.1016/j.matbio.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gubbiotti M.A., Iozzo R.V. Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept. Matrix Biol. 2015;48:6–13. doi: 10.1016/j.matbio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gubbiotti M.A., Buraschi S., Kapoor A., Iozzo R.V. Proteoglycan signaling in tumor angiogenesis and endothelial cell autophagy. Semin. Cancer Biol. 2020;62:1–8. doi: 10.1016/j.semcancer.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schaefer L., Tredup C., Gubbiotti M.A., Iozzo R.V. Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology. FEBS J. 2017;284:10–26. doi: 10.1111/febs.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sylakowski K., Wells A. ECM-regulation of autophagy: The yin and the yang of autophagy during wound healing. Matrix Biol. 2021;100–101:197–206. doi: 10.1016/j.matbio.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neill T., Kapoor A., Xie C., Buraschi S., Iozzo R.V. A functional outside-in signaling network of proteoglycans and matrix molecules regulating autophagy. Matrix Biol. 2021;100–101:118–149. doi: 10.1016/j.matbio.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Danielson K.G., Fazzio A., Cohen I., Cannizzaro L.A., Eichstetter I., Iozzo R.V. The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5' untranslated region, and mapping of the gene to chromosome 12q23. Genomics. 1993;15:146–160. doi: 10.1006/geno.1993.1022. [DOI] [PubMed] [Google Scholar]
- 159.Mauviel A., Santra M., Chen Y.Q., Uitto J., Iozzo R.V. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-α. J. Biol. Chem. 1995;270:11692–11700. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- 160.Iozzo R.V., Danielson K.G. Transcriptional and post-transcriptional control of proteoglycan gene expression. Prog. Nucleic Acid Res. Mol. Biol. 1999;62:19–53. doi: 10.1016/s0079-6603(08)60504-8. [DOI] [PubMed] [Google Scholar]
- 161.Mauviel A., Korang K., Santra M., Tewari D., Uitto J., Iozzo R.V. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. J. Biol. Chem. 1996;271:24824–24829. doi: 10.1074/jbc.271.40.24824. [DOI] [PubMed] [Google Scholar]
- 162.Vogel K.G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem. J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Danielson K.G., Baribault H., Holmes D.F., Graham H., Kadler K.E., Iozzo R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reed C.C., Iozzo R.V. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 165.Zhang G., Ezura Y., Chervoneva I., Robinson P.S., Beason D.P., Carine E.T., Soslowsky L.J., Iozzo R.V., Birk D.E. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J. Cell. Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 166.Zhang G., Chen S., Goldoni S., Calder B.W., Simpson H.C., Owens R.T., McQuillan D.J., Young M.F., Iozzo R.V., Birk D.E. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Han B., Li Q., Wang C., Patel P., Adams S.M., Doyran B., Nia H.T., Oftadeh R., Zhou S., Li C.Y., et al. Decorin regulates the aggrecan network integrity and biomechanical functions of cartilage extracellular matrix. ACS Nano. 2019;13:11320–11333. doi: 10.1021/acsnano.9b04477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Q., Han B., Wang C., Tong W., Wei Y., Tseng W.J., Han L.H., Liu X.S., Enomoto-Iwamoto M., Mauck R.L., et al. Mediation of cartilage matrix degeneration and fibrillation by decorin in post-traumatic osteoarthritis. Arthritis Rheumatol. 2020;72:1266–1277. doi: 10.1002/art.41254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yamaguchi Y., Mann D.M., Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 170.Schaefer L., Macakova K., Raslik I., Micegova M., Gröne H.J., Schönherr E., Robenek H., Echtermeyer F.G., Grässel S., Bruckner P., et al. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am. J. Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Järveläinen H., Puolakkainen P., Pakkanen S., Brown E.L., Höök M., Iozzo R.V., Sage E.H., Wight T.N. A role for decorin in cutaneous wound healing and angiogenesis. Wound Rep.Reg. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 172.Baghy K., Dezsó K., László V., Fullár A., Péterfia B., Paku S., Nagy P., Schaff Z., Iozzo R.V., Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab. Invest. 2011;91:439–451. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baghy K., Iozzo R.V., Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem. 2012;60:262–268. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Weis S.M., Zimmerman S.D., Shah M., Covell J.W., Omens J.H., Ross J., Jr., Dalton N., Jones Y., Reed C.C., Iozzo R.V., McCulloch A.D. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005;24:313–324. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 175.Gupta S., Buyank F., Sinha N.R., Grant D.G., Sinha P.R., Iozzo R.V., Chaurasia S.S., Mohan R.R. Decorin regulates collagen fibrillogenesis during corneal wound healing in mouse in vivo. Exp. Eye Res. 2022;216 doi: 10.1016/j.exer.2022.108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Järvinen T.A.H., Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc. Natl. Acad. Sci. USA. 2010;107:21671–21676. doi: 10.1073/pnas.1016233107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Järvinen T.A.H., Pemmari T. Systemically administered, target-specific, multi-functional therapeutic recombinant proteins in regenerative medicine. Nanomaterials. 2020;10 doi: 10.3390/nano10020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grant D.S., Yenisey C., Rose R.W., Tootell M., Santra M., Iozzo R.V. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 179.Järveläinen H., Sainio A., Wight T.N. Pivotal role for decorin in angiogenesis. Matrix Biol. 2015;43:15–26. doi: 10.1016/j.matbio.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Neill T., Painter H., Buraschi S., Owens R.T., Lisanti M.P., Schaefer L., Iozzo R.V. Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3. J. Biol. Chem. 2012;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brown E.L., Wooten R.M., Johnson B.J., Iozzo R.V., Smith A., Dolan M.C., Guo B.P., Weis J.J., Höök M. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Invest. 2001;107:845–852. doi: 10.1172/JCI11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Balne P.K., Gupta S., Zhang J., Bristow D., Faubion M., Heil S.D., Sinha P.R., Green S.L., Iozzo R.V., Mohan R.R. The functional role of decorin in corneal neovascularization in vivo. Exp. Eye Res. 2021;207 doi: 10.1016/j.exer.2021.108610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schneider M., Pawlak R., Weber G.R., Dillinger A.E., Kuespert S., Iozzo R.V., Quigley H.A., Ohlmann A., Tamm E.R., Fuchshofer R. A novel ocular function for decorin in the aqueous humor outflow. Matrix Biol. 2021;97:1–19. doi: 10.1016/j.matbio.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 184.Schneider M., Dillinger A.E., Ohlmann A., Iozzo R.V., Fuchshofer R. Decorin-An antagonist of TGFβ in astrocytes of the optic nerve. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22147660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Neill T., Schaefer L., Iozzo R.V. An oncosuppressive role for decorin. Mol. Cell. Oncol. 2015;2 doi: 10.4161/23723556.2014.975645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Iozzo R.V., Buraschi S., Genua M., Xu S.-Q., Solomides C.C., Peiper S.C., Gomella L.G., Owens R.C., Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Iozzo R.V., Moscatello D.K., McQuillan D.J., Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 188.Csordás G., Santra M., Reed C.C., Eichstetter I., McQuillan D.J., Gross D., Nugent M.A., Hajnóczky G., Iozzo R.V. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J. Biol. Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 189.Zhu J.-X., Goldoni S., Bix G., Owens R.T., McQuillan D.J., Reed C.C., Iozzo R.V. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis. J. Biol. Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 190.Seidler D.G., Goldoni S., Agnew C., Cardi C., Thakur M.L., Owens R.T., McQuillan D.J., Iozzo R.V. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J. Biol. Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 191.Patel S., Santra M., McQuillan D.J., Iozzo R.V., Thomas A.P. Decorin activates the epidermal growth factor receptor and elevates cytosolic Ca2+ in A431 cells. J. Biol. Chem. 1998;273:3121–3124. doi: 10.1074/jbc.273.6.3121. [DOI] [PubMed] [Google Scholar]
- 192.Iozzo R.V., Cohen I. Altered proteoglycan gene expression and the tumor stroma. Experientia. 1993;49:447–455. doi: 10.1007/BF01923588. [DOI] [PubMed] [Google Scholar]
- 193.Bi X., Tong C., Dockendorff A., Bancroft L., Gallagher L., Guzman G., Iozzo R.V., Augenlicht L.H., Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Buraschi S., Pal N., Tyler-Rubinstein N., Owens R.T., Neill T., Iozzo R.V. Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels. J. Biol. Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baghy K., Horváth Z., Regős E., Kiss K., Schaff Z., Iozzo R.V., Kovalszky I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013;280:2150–2164. doi: 10.1111/febs.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie C., Mondal D.K., Ulas M., Neill T., Iozzo R.V. Oncosuppressive roles of decorin through regulation of multiple receptors and diverse signaling pathways. Am. J. Physiol. Cell Physiol. 2022;322:C554–C566. doi: 10.1152/ajpcell.00016.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li Y., Gan L., Lu M., Zhang X., Tong X., Qi D., Zhao Y., Ye X. HBx downregulated decorin and decorin-derived peptides inhibit the proliferation and tumorigenicity of hepatocellular carcinoma cells. Faseb. J. 2023;37 doi: 10.1096/fj.202200999RR. [DOI] [PubMed] [Google Scholar]
- 198.Tralhão J.G., Schaefer L., Micegova M., Evaristo C., Schönherr E., Kayal S., Veiga-Fernandes H., Danel C., Iozzo R.V., Kresse H., Lemarchand P. In vivo selective and distant killing of cancer cells using adenovirus-mediated decorin gene transfer. Faseb. J. 2003;17:464–466. doi: 10.1096/fj.02-0534fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Neill T., Schaefer L., Iozzo R.V. Decorin as a multivalent therapeutic agent against cancer. Adv. Drug Deliv. Rev. 2016;97:174–185. doi: 10.1016/j.addr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Goldoni S., Seidler D.G., Heath J., Fassan M., Baffa R., Thakur M.L., Owens R.T., McQuillan D.J., Iozzo R.V. An anti-metastatic role for decorin in breast cancer. Am. J. Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu Y., Xia Q., Rao Q., Shi S., Shi Q., Ma H., Lu Z., Chen H., Zhou X. DCN deficiency promotes renal cell carcinoma growth and metastasis through downregulation of P21 and E-cadherin. Tumour Biol. 2016;37:5171–5183. doi: 10.1007/s13277-015-4160-1. [DOI] [PubMed] [Google Scholar]
- 202.Nyman M.C., Jokilammi A.B., Boström P.C., Kurki S.H., Sainio A.O., Grenman S.E., Orte K.J., Hietanen S.H., Elenius K., Järveläinen H.T. Decorin expression in human vulva carcinoma: Oncosuppressive effect of decorin cDNA transduction on carcinoma cells. J. Histochem. Cytochem. 2019;67:511–522. doi: 10.1369/0022155419845373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Buraschi S., Neill T., Owens R.T., Iniguez L.A., Purkins G., Vadigepalli R., Evans B., Schaefer L., Peiper S.C., Wang Z.X., Iozzo R.V. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mavrogonatou E., Papadopoulou A., Fotopoulou A., Tsimelis S., Bassiony H., Yiacoumettis A.M., Panagiotou P.N., Pratsinis H., Kletsas D. Down-regulation of the proteoglycan decorin fills in the tumor-promoting phenotype of ionizing radiation-induced senescent human breast dtromal fibroblasts. Cancers. 2021;13 doi: 10.3390/cancers13081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zoeller J.J., Pimtong W., Corby H., Goldoni S., Iozzo A.E., Owens R.T., Ho S.-Y., Iozzo R.V. A central role for decorin during vertebrate convergent extension. J. Biol. Chem. 2009;284:11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P.P. Clathrin-mediated intermalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 207.Bredrup C., Stang E., Bruland O., Palka B.P., Young R.D., Haavik J., Knappskog P.M., Rødahl E. Decorin accumulation contributes to the stromal opacities found in congenital stromal corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2010;51:5578–5582. doi: 10.1167/iovs.09-4933. [DOI] [PubMed] [Google Scholar]
- 208.Bi X., Pohl N.M., Qian Z., Yang G.R., Gou Y., Guzman G., Kajdacsy-Balla A., Iozzo R.V., Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–330. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fetting J.L., Guay J.A., Karolak M.J., Iozzo R.V., Adams D.C., Maridas D.E., Brown A.C., Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen S., Sun M., Meng X., Iozzo R.V., Kao W.W.Y., Birk D.E. Pathophysiological mechanisms of autosomal dominant congenital stromal corneal dystrophy. C-terminal-truncated decorin results in abnormal matrix assembly and altered expression of small leucine-rich proteoglycans. Am. J. Pathol. 2011;179:2409–2419. doi: 10.1016/j.ajpath.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nikitovic D., Aggelidakis J., Young M.F., Iozzo R.V., Karamanos N.K., Tzanakakis G.N. The biology of small leucine-rich proteoglycans in bone pathophysiology. J. Biol. Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen S., Sun M., Iozzo R.V., Kao W.W.Y., Birk D.E. Intracellularly-retained decorin lacking the C-terminal ear repeat causes ER stress: a cell-based etiological mechanism for congenital stromal corneal dystrophy. Am. J. Pathol. 2013;183:247–256. doi: 10.1016/j.ajpath.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Troup S., Njue C., Kliewer E.V., Parisien M., Roskelley C., Chakravarti S., Roughley P.J., Murphy L.C., Watson P.H. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin. Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 214.Oda G., Sato T., Ishikawa T., Kawachi H., Nakagawa T., Kuwayama T., Ishiguro M., Iida S., Uetake H., Sugihara K. Significance of stromal decorin expression during the progression of breast cancer. Oncol. Rep. 2012;28:2003–2008. doi: 10.3892/or.2012.2040. [DOI] [PubMed] [Google Scholar]
- 215.Li S.J., Chen D.L., Zhang W.B., Shen C., Che G.W. Prognostic value of stromal decorin expression in patients with breast cancer: a meta-analysis. J. Thorac. Dis. 2015;7:1939–1950. doi: 10.3978/j.issn.2072-1439.2015.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.VanOpstall C., Perike S., Brechka H., Gillard M., Lamperis S., Zhu B., Brown R., Bhanvadia R., Vander Griend D.J. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. Elife. 2020;9 doi: 10.7554/eLife.53600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Torres A., Gubbiotti M.A., Iozzo R.V. Decorin-inducible Peg3 evokes Beclin 1-mediated autophagy and Thrombospondin 1-mediated angiostasis. J. Biol. Chem. 2017;292:5055–5069. doi: 10.1074/jbc.M116.753632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Neill T., Sharpe C., Owens R.T., Iozzo R.V. Decorin-evoked paternally expressed gene 3 (PEG3) is an upstream regulator of the transcription factor EB (TFEB) in endothelial cell autophagy. J. Biol. Chem. 2017;292:16211–16220. doi: 10.1074/jbc.M116.769950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gubbiotti M.A., Neill T., Frey H., Schaefer L., Iozzo R.V. Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 2015;48:14–25. doi: 10.1016/j.matbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Y., Zhang H., Zhang Y., Li X., Hu X., Wang X. Decorin promotes apoptosis and autophagy via suppressing c-Met in HTR-8 trophoblasts. Reproduction. 2020;159:669–677. doi: 10.1530/REP-19-0458. [DOI] [PubMed] [Google Scholar]
- 221.Neill T., Schaefer L., Iozzo R.V. Instructive roles of extracellular matrix on autophagy. Am. J. Pathol. 2014;184:2146–2153. doi: 10.1016/j.ajpath.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Urbanczyk M., Jeyagaran A., Zbinden A., Lu C.E., Marzi J., Kuhlburger L., Nahnsen S., Layland S.L., Duffy G., Schenke-Layland K. Decorin improves human pancreatic β-cell function and regulates ECM expression in vitro. Matrix Biol. 2023;115:160–183. doi: 10.1016/j.matbio.2022.12.005. [DOI] [PubMed] [Google Scholar]
- 223.Whitworth A.J., Pallanck L.J. PINK1/Parkin mitophagy and neurodegeneration-what do we really know in vivo? Curr. Opin. Genet. Dev. 2017;44:47–53. doi: 10.1016/j.gde.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 224.Goyal A., Neill T., Owens R.T., Schaefer L., Iozzo R.V. Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol. 2014;34:46–54. doi: 10.1016/j.matbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Neill T., Buraschi S., Kapoor A., Iozzo R.V. Proteoglycan-driven autophagy: A nutrient-independent mechanism to control intracellular catabolism. J. Histochem. Cytochem. 2020;68:733–746. doi: 10.1369/0022155420937370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jia Y., Feng Q., Tang B., Luo X., Yang Q., Yang H., Li Q. Decorin suppresses invasion and EMT phenotype of glioma by Inducing autophagy via c-Met/Akt/mTOR axis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.659353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Neill T., Torres A., Buraschi S., Owens R.T., Hoek J.B., Baffa R., Iozzo R.V. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin. J. Biol. Chem. 2014;289:4952–4968. doi: 10.1074/jbc.M113.512566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhao H., Xi H., Wei B., Cai A., Wang T., Wang Y., Zhao X., Song Y., Chen L. Expression of decorin in intestinal tissues of mice with inflammatory bowel disease and its correlation with autophagy. Exp. Ther. Med. 2016;12:3885–3892. doi: 10.3892/etm.2016.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xie X., Li D., Cui Y., Xie T., Cai J., Yao Y. Decorin protects retinal pigment epithelium cells from oxidative stress and apoptosis via AMPK-mTOR-regulated autophagy. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3955748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Neill T., Xie C., Iozzo R.V. Decorin evokes reversible mitochondrial depolarization in carcinoma and vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2022;323:C1355–C1373. doi: 10.1152/ajpcell.00325.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.O'Reilly M.S., Holmgren L., Shing Y., Chen C., Rosenthal R.A., Moses M., Lane W.S., Cao Y., Sage E.H., Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 232.Whitelock J.M., Murdoch A.D., Iozzo R.V., Underwood P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 233.Gonzalez E.M., Reed C.C., Bix G., Fu J., Zhang Y., Gopalakrishnan B., Greenspan D.S., Iozzo R.V. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 234.Cailhier J.-F., Sirois I., Laplante P., Lepage S., Raymond M.A., Brassard N., Prat A., Iozzo R.V., Pshezhetsky A.V., Hébert M.J. Caspase-3 activation triggers extracellular release of cathepsin L and endorepellin proteolysis. J. Biol. Chem. 2008;283:27220–27229. doi: 10.1074/jbc.M801164200. [DOI] [PubMed] [Google Scholar]
- 235.Cohen I.R., Grässel S., Murdoch A.D., Iozzo R.V. Structural characterization of the complete human perlecan gene and its promoter. Proc. Natl. Acad. Sci. USA. 1993;90:10404–10408. doi: 10.1073/pnas.90.21.10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Iozzo R.V., Cohen I.R., Grässel S., Murdoch A.D. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem. J. 1994;302:625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Iozzo R.V., Pillarisetti J., Sharma B., Murdoch A.D., Danielson K.G., Uitto J., Mauviel A. Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming factor-β via a nuclear factor 1-binding element. J. Biol. Chem. 1997;272:5219–5228. doi: 10.1074/jbc.272.8.5219. [DOI] [PubMed] [Google Scholar]
- 238.Mongiat M., Sweeney S.M., San Antonio J.D., Fu J., Iozzo R.V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 239.Costell M., Gustafsson E., Aszódi A., Mörgelin M., Bloch W., Hunziker E., Addicks K., Timpl R., Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bix G., Castello R., Burrows M., Zoeller J.J., Weech M., Iozzo R.A., Cardi C., Thakur M.L., Barker C.A., Camphausen K., Iozzo R.V. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J. Natl. Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 241.Mongiat M., Buraschi S., Andreuzzi E., Neill T., Iozzo R.V. Extracellular matrix: the gatekeeper of tumor angiogenesis. Biochem. Soc. Trans. 2019;47:1543–1555. doi: 10.1042/BST20190653. [DOI] [PubMed] [Google Scholar]
- 242.Aviezer D., Hecht D., Safran M., Eisinger M., David G., Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 243.Aviezer D., Iozzo R.V., Noonan D.M., Yayon A. Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol. Cell Biol. 1997;17:1938–1946. doi: 10.1128/mcb.17.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Handler M., Yurchenco P.D., Iozzo R.V. Developmental expression of perlecan during murine embryogenesis. Dev. Dynam. 1997;210:130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 245.Cohen I.R., Murdoch A.D., Naso M.F., Marchetti D., Berd D., Iozzo R.V. Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 1994;54:5771–5774. [PubMed] [Google Scholar]
- 246.Zoeller J.J., McQuillan A., Whitelock J., Ho S.-Y., Iozzo R.V. A central function for perlecan in skeletal muscle and cardiovascular development. J. Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zoeller J.J., Whitelock J.M., Iozzo R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009;28:284–291. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chuang C.Y., Lord M.S., Melrose J., Rees M.D., Knox S.M., Freeman C., Iozzo R.V., Whitelock J.M. Heparan sulfate-dependent signaling of fibroblast growth growth factor 18 by chondrocyte-derived perlecan. Biochemistry. 2010;49:5524–5532. doi: 10.1021/bi1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lord M.S., Chuang C.Y., Melrose J., Davies M.J., Iozzo R.V., Whitelock J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Iozzo R.V. Basement membrane proteoglycans: from cellar to ceiling. Nat. Rev. Mol. Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 251.Göhring W., Sasaki T., Heldin C.-H., Timpl R. Mapping of the binding of platelet-derived growth factor to distinct domains of the basement membrane proteins BM-40 and perlecan and distinction from the BM-40 collagen-binding epitope. Eur. J. Biochem. 1998;255:60–66. doi: 10.1046/j.1432-1327.1998.2550060.x. [DOI] [PubMed] [Google Scholar]
- 252.Mongiat M., Otto J., Oldershaw R., Ferrer F., Sato J.D., Iozzo R.V. Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J. Biol. Chem. 2001;276:10263–10271. doi: 10.1074/jbc.M011493200. [DOI] [PubMed] [Google Scholar]
- 253.Fuki I., Iozzo R.V., Williams K.J. Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J. Biol. Chem. 2000;275:31554. [PubMed] [Google Scholar]
- 254.Mongiat M., Fu J., Oldershaw R., Greenhalgh R., Gown A.M., Iozzo R.V. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J. Biol. Chem. 2003;278:17491–17499. doi: 10.1074/jbc.M210529200. [DOI] [PubMed] [Google Scholar]
- 255.Gonzalez E.M., Mongiat M., Slater S.J., Baffa R., Iozzo R.V. A novel interaction between perlecan protein core and progranulin: Potential effects on tumor growth. J. Biol. Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 256.Bix G., Iozzo R.V. Matrix revolutions: "tails" of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 257.Whitelock J.M., Melrose J., Iozzo R.V. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Neill T., Schaefer L., Iozzo R.V. Decoding the matrix: Instructive roles of proteoglycan receptors. Biochemistry. 2015;54:4583–4598. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gubbiotti M.A., Neill T., Iozzo R.V. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017;57–58:285–298. doi: 10.1016/j.matbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Goyal A., Pal N., Concannon M., Paul M., Doran M., Poluzzi C., Sekiguchi K., Whitelock J.M., Neill T., Iozzo R.V. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) J. Biol. Chem. 2011;286:25947–25962. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goyal A., Gubbiotti M.A., Chery D.R., Han L., Iozzo R.V. Endorepellin-evoked autophagy contributes to angiostasis. J. Biol. Chem. 2016;291:19245–19256. doi: 10.1074/jbc.M116.740266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Goyal A., Poluzzi C., Willis C.D., Smythies J., Shellard A., Neill T., Iozzo R.V. Endorepellin affects angiogenesis by antagonizing diverse VEGFR2- evoked signaling pathways: transcriptional repression of HIF-1α and VEGFA and concurrent inhibition of NFAT1 activation. J. Biol. Chem. 2012;287:43543–43556. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chen C.G., Kapoor A., Xie C., Moss A., Vadigepalli R., Ricard-Blum S., Iozzo R.V. Conditional expression of endorepellin in the tumor vasculature attenuates breast cancer growth, angiogenesis and hyaluronan deposition. Matrix Biol. 2023;118:92–109. doi: 10.1016/j.matbio.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chen C.G., Gubbiotti M.A., Kapoor A., Han X., Yu Y., Linhardt R.J., Iozzo R.V. Autophagic degradation of HAS2 in endothelial cells: A novel mechanism to regulate angiogenesis. Matrix Biol. 2020;90:1–19. doi: 10.1016/j.matbio.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 265.Chen C.G., Iozzo R.V. Extracellular matrix guidance of autophagy: a mechanism regulating cancer growth. Open Biol. 2022;12 doi: 10.1098/rsob.210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nguyen T.M.B., Subramanian I.V., Xiao X., Ghosh G., Nguyen P., Kelekar A., Ramakrishnan S. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels. J. Cell Mol. Med. 2009;13:3687–3698. doi: 10.1111/j.1582-4934.2009.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Moreth K., Iozzo R.V., Schaefer L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle. 2012;11:2084–2091. doi: 10.4161/cc.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Moreth K., Frey H., Hubo M., Zeng-Brouwers J., Nastase M.V., Hsieh L.T.H., Haceni R., Pfeilschifter J., Iozzo R.V., Schaefer L. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014;35:143–151. doi: 10.1016/j.matbio.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Poluzzi C., Nastase M.V., Zeng-Brouwers J., Roedig H., Hsieh L.T.H., Michaelis J.B., Buhl E.M., Rezende F., Manavski Y., Bleich A., et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019;95:540–562. doi: 10.1016/j.kint.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 271.Diehl V., Huber L.S., Trebicka J., Wygrecka M., Iozzo R.V., Schaefer L. The role of decorin and biglycan signaling in tumorigenesis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.801801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zeng-Brouwers J., Beckmann J., Nastase M.V., Iozzo R.V., Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2014;35:132–142. doi: 10.1016/j.matbio.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hsieh L.T.H., Nastase M.V., Zeng-Brouwers J., Iozzo R.V., Schaefer L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int. J. Biochem. Cell Biol. 2014;54:223–235. doi: 10.1016/j.biocel.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Roedig H., Nastase M.V., Frey H., Moreth K., Zeng-Brouwers J., Poluzzi C., Hsieh L.T.H., Brandts C., Fulda S., Wygrecka M., Schaefer L. Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol. 2019;77:4–22. doi: 10.1016/j.matbio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 275.Giatagana E.M., Berdiaki A., Gaardløs M., Tsatsakis A.M., Samsonov S.A., Nikitovic D. Rapamycin-induced autophagy in osteosarcoma cells is mediated via the biglycan/Wnt/β-catenin signaling axis. Am. J. Physiol. Cell Physiol. 2022;323:C1740–C1756. doi: 10.1152/ajpcell.00368.2022. [DOI] [PubMed] [Google Scholar]
- 276.Giatagana E.M., Berdiaki A., Gaardløs M., Samsonov S.A., Tzanakakis G.N., Nikitovic D. Biglycan Interacts with type I Insulin-like receptor (IGF-IR) signaling pathway to regulate osteosarcoma cell growth and response to chemotherapy. Cancers. 2022;14 doi: 10.3390/cancers14051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Berdiaki A., Giatagana E.M., Tzanakakis G., Nikitovic D. The landscape of small leucine-rich proteoglycan impact on cancer pathogenesis with a focus on biglycan and lumican. Cancers. 2023;15 doi: 10.3390/cancers15143549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yu L., Maishi N., Matsuda A., Hida K. The functional network of biglycan: A new frontier in tumor progression. Proteoglycan Res. 2023;1:e11. [Google Scholar]
- 279.Cong L., Maishi N., Annan D.A., Young M.F., Morimoto H., Morimoto M., Nam J.M., Hida Y., Hida K. Inhibition of stromal biglycan promotes normalization of the tumor microenvironment and enhances chemotherapeutic efficacy. Breast Cancer Res. 2021;23:51. doi: 10.1186/s13058-021-01423-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhao S.F., Yin X.J., Zhao W.J., Liu L.C., Wang Z.P. Biglycan as a potential diagnostic and prognostic biomarker in multiple human cancers. Oncol. Lett. 2020;19:1673–1682. doi: 10.3892/ol.2020.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shteingauz A., Boyango I., Naroditsky I., Hammond E., Gruber M., Doweck I., Ilan N., Vlodavsky I. Heparanase enhances tumor growth and chemoresistance by promoting autophagy. Cancer Res. 2015;75:3946–3957. doi: 10.1158/0008-5472.CAN-15-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ilan N., Shteingauz A., Vlodavsky I. Function from within: Autophagy induction by HPSE/heparanase--new possibilities for intervention. Autophagy. 2015;11:2387–2389. doi: 10.1080/15548627.2015.1115174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Vlodavsky I., Kayal Y., Hilwi M., Soboh S., Sanderson R.D., Ilan N. Heparanase - A sigle protein with multiple enzymatic and nonenzymatic functions. Proteoglycan. 2023;1:e6. doi: 10.1002/pgr2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Masola V., Bellin G., Gambaro G., Onisto M. Heparanase: A multitasking protein involved in extracellular matrix (ECM) remodeling and intracellular events. Cells. 2018;7:236. doi: 10.3390/cells7120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Vlodavsky I., Iozzo R.V., Sanderson R.D. Heparanase: multiple functions in inflammation, diabetes and atherosclerosis. Matrix Biol. 2013;32:220–222. doi: 10.1016/j.matbio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 286.Bianchi R., Russo E., Bachmann S.B., Proulx S.T., Sesartic M., Smaadahl N., Watson S.P., Buckley C.D., Halin C., Detmar M. Postnatal deletion of podoplanin in lymphatic endothelium results in blood filling of the lymphatic system and impairs dendritic cell migration to lymph nodes. Arterioscler. Thromb. Vasc. Biol. 2017;37:108–117. doi: 10.1161/ATVBAHA.116.308020. [DOI] [PubMed] [Google Scholar]
- 287.Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75–76:1–11. doi: 10.1016/j.matbio.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Masola V., Zaza G., Gambaro G., Franchi M., Onisto M. Role of heparanase in tumor progression: Molecular aspects and therapeutic options. Semin. Cancer Biol. 2020;62:86–98. doi: 10.1016/j.semcancer.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 289.Chen X., Chang J., Deng Q., Xu J., Nguyen T.A., Martens L.H., Cenik B., Taylor G., Hudson K.F., Chung J., et al. Progranulin does not bind tumor necrosis factor (TNF) receptors and is not a direct regulator of TNF-dependent signaling or bioactivity in immune or neuronal cells. J. Neurosci. 2013;33:9202–9213. doi: 10.1523/JNEUROSCI.5336-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Thompson C.A., Purushothaman A., Ramani V.C., Vlodavsky I., Sanderson R.D. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tripathi K., Bandari S.K., Sanderson R.D. Extracellular vesicles released during hypoxia transport heparanase and enhance macrophage migration, endothelial tube formation and cancer cell stemness. Proteoglycan Res. 2023;1:e1. doi: 10.1002/pgr2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mohan C.D., Hari S., Preetham H.D., Rangappa S., Barash U., Ilan N., Nayak S.C., Gupta V.K., Basappa, Vlodavsky I., Vlodavsky I., Rangappa K.S. Targeting heparanase in cancer: Inhibition by synthetic, chemically modified, and natural compounds. iScience. 2019;15:360–390. doi: 10.1016/j.isci.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ning L., Xu Z., Furuya N., Nonaka R., Yamada Y., Arikawa-Hirasawa E. Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle. Matrix Biol. 2015;48:26–35. doi: 10.1016/j.matbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 294.Arikawa-Hirasawa E., Watanabe H., Takami H., Hassell J.R., Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 295.Reynolds-Peterson C.E., Zhao N., Xu J., Serman T.M., Xu J., Selleck S.B. Heparan sulfate proteoglycans regulate autophagy in Drosophila. Autophagy. 2017;13:1262–1279. doi: 10.1080/15548627.2017.1304867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Reynolds-Peterson C., Xu J., Zhao N., Cruse C., Yonel B., Trasorras C., Toyoda H., Kinoshita-Toyoda A., Dobson J., Schultheis N., et al. Heparan sulfate structure affects autophagy, lifespan, responses to oxidative stress, and cell degeneration in Drosophila parkin mutants. G3. 2020;10:129–141. doi: 10.1534/g3.119.400730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Iozzo R.V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 298.Garantziotis S., Savani R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019;78–79:1–10. doi: 10.1016/j.matbio.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Cowman M.K., Turley E.A. Functional organization of extracellular hyaluronan, CD44, and RHAMM. Proteoglycan Res. 2023;1:e4. [Google Scholar]
- 300.Passi A., Vigetti D. Hyaluronan as tunable drug delivery system. Adv. Drug Deliv. Rev. 2019;146:83–96. doi: 10.1016/j.addr.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 301.Garantziotis S., Savani R.C. Proteoglycans in Toll-like receptor responses and innate immunity. Am. J. Physiol. Cell Physiol. 2022;323:C202–C214. doi: 10.1152/ajpcell.00088.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Passi A., Vigetti D., Buraschi S., Iozzo R.V. Dissecting the role of hyaluronan synthases in the tumor microenvironment. FEBS J. 2019;286:2937–2949. doi: 10.1111/febs.14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Krishnan Y., Grodzinsky A.J. Cartilage diseases. Matrix Biol. 2018;71–72:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nakato H., Li J.P. Functions of heparan sulfate proteoglycans in development: Insights from Drosophila models. Int. Rev. Cell Mol. Biol. 2016;325:275–293. doi: 10.1016/bs.ircmb.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 305.Schwartz N.B., Domowicz M.S. Proteoglycans in brain development and pathogenesis. FEBS Lett. 2018;592:3791–3805. doi: 10.1002/1873-3468.13026. [DOI] [PubMed] [Google Scholar]
- 306.Iozzo R.V., Chakrani F., Perrotti D., McQuillan D.J., Skorski T., Calabretta B., Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc.Natl.Acad.Sci.USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Li X., Pennisi A., Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Goldoni S., Iozzo R.V. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int. J. Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 309.Mao L., Yang J., Yue J., Chen Y., Zhou H., Fan D., Zhang Q., Buraschi S., Iozzo R.V., Bi X. Decorin deficiency promotes epithelial-mesenchymal transition and colon cancer metastasis. Matrix Biol. 2021;95:1–14. doi: 10.1016/j.matbio.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Reese F., Williams B., Balderrama-Gutierrez G., Wyman D., Çelik M.H., Rebboah E., Rezaie N., Trout D., Razavi-Mohseni M., Jiang Y., et al. The ENCODE4 long-read RNA-seq collection reveals distinct classes of transcript structure diversity. bioRxiv. 2023 Preprint at. [Google Scholar]
- 311.Vigetti D., Karousou E., Viola M., Deleonibus S., De Luca G., Passi A. Hyaluronan: biosynthesis and signaling. Biochim. Biophys. Acta. 2014;1840:2452–2459. doi: 10.1016/j.bbagen.2014.02.001. [DOI] [PubMed] [Google Scholar]