Cytotoxic CD8+ T cells (CTLs) can kill any virus-infected cell or tumor cell, providing they display antigenic peptides on major histocompatibility complex class I (MHC-I) molecules. However, tumor cells or virus-infected cells cannot present antigens in a sufficiently stimulating manner to also prime naïve CD8+ T cells to become CTLs. This is the role of conventional dendritic cells (cDCs), which bridge innate and adaptive immunity by capturing exogenous cell-associated antigens on MHC-I molecules and presenting them to naïve CD8+ T cells, while also providing co-stimulation (1). This process is called cross-presentation (2). Despite the importance of cross-presentation for antitumor and antiviral immunity and vaccine design, the mechanisms remain unclear. On page 1258 of this issue, Rodríguez-Silvestre et al. (3) report that endosomal perforin-2 is a channel-forming protein that allows the release of internalized exogenous cell-associated antigens to the cytosol of cross-presenting cDCs.
A fundamental yet unresolved question is how exogenous antigens, which enter the cross-presenting DCs (referred to as cDC1) through endosomal pathways, are trimmed into peptides in the cytosol and then access MHC-I, which is primarily loaded with peptides in the endoplasmic reticulum (ER). One theory posits that all processing and loading might occur within vacuoles when MHC-I molecules are recycled, thus circumventing the problem of exogenous antigen transport from phagosome to cytosol (P2C). Another, more widely supported theory proposes P2C shuttling of antigens, followed by proteasomal protein processing in the cytosol and transporter associated with antigen processing (TAP)–dependent entry of peptides into the ER for final processing and loading on MHC-I (4, 5). The P2C pathway has been shown to depend either on phagosome-ER fusion and recruitment of ER retrotranslocation channel machinery, or highly localized endosomal vesicle damage and rupture (4, 5).
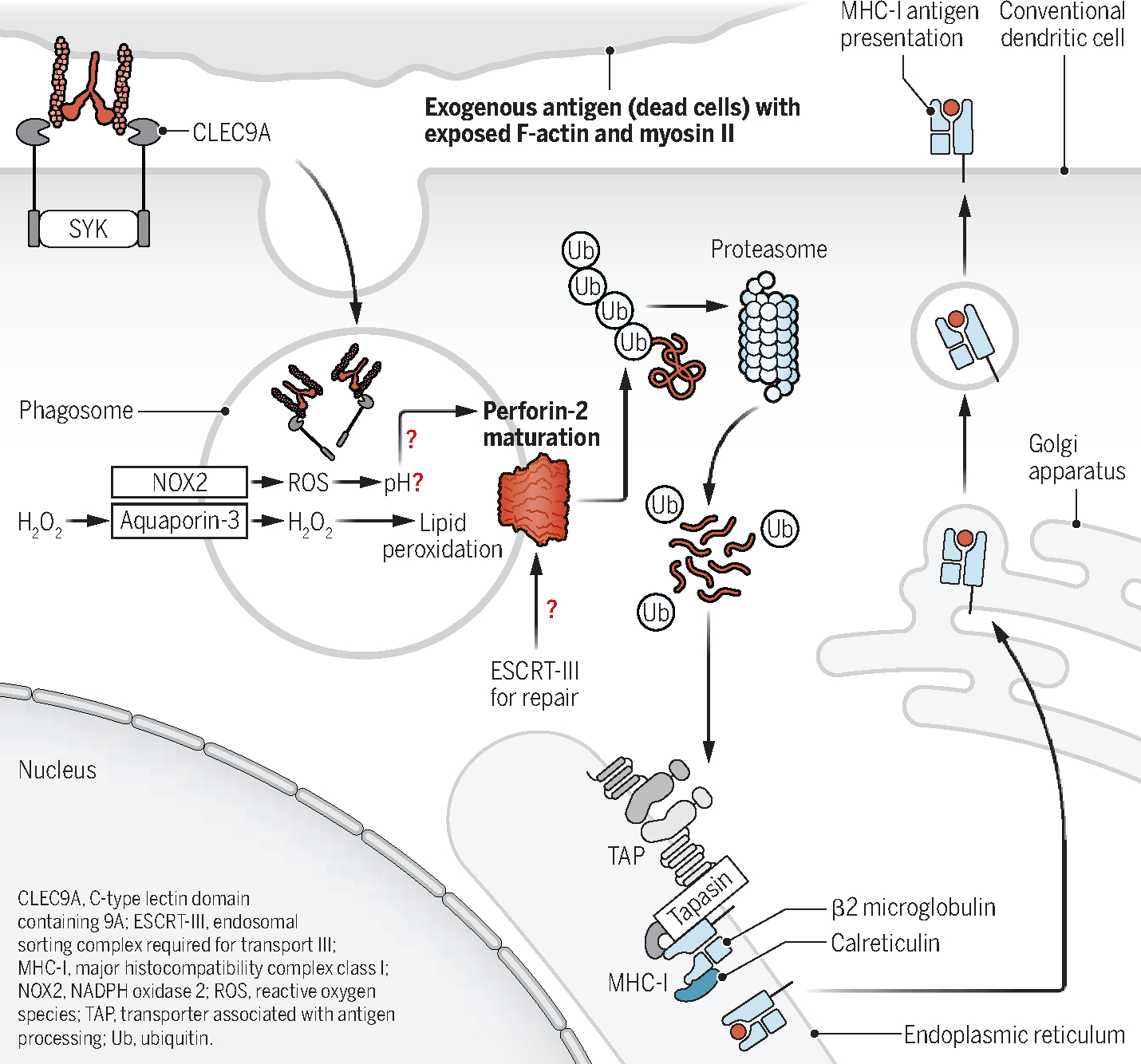
Perforin-2 is a pore-forming antibacterial protein that is essential in innate immunity. Perforin-2 shares similarities with the pore-forming membrane attack complex (MAC) of complement and with perforin-1 (6). Perforin-1, which is released by CTLs, targets the outer cellular membrane, resulting in cytotoxicity. In macrophages, perforin-2 can lead to pH-dependent pore formation, which kills bacteria in phagolysosomes. Rodríguez-Silvestre et al. show that perforin-2 pore formation also occurs in phagosomal membranes in cDC1, allowing the regulated release of exogenous antigens into the cytosol while preserving the integrity of the vesicle (see the figure). Their findings indicated that the absence of perforin-2 in cDC1 resulted in a partial decrease in cross-presentation of cell-associated antigens, whereas no significant difference was observed in cross-presentation of soluble antigens. The decreased presentation of cell-associated antigens by perforin-2–deficient cDC1s resulted from impaired antigen shuttling to the cytosol rather than a failure in antigen uptake.
The mechanism by which perforin-2 elicits antigen endocytic escape in cDC1s differs from previously described mechanisms in the P2C pathway. However, because Rodríguez-Silvestre et al. used dead cells as an exogenous antigen source in their study, it becomes intriguing to further explore the interplay between perforin-2 and the cDC1-specific protein C-type lectin domain containing 9A (CLEC9A), which plays a role in the uptake and subsequent signaling of necrotic cells in cDC1.
Rodríguez-Silvestre et al. demonstrate that perforin-2 maturation is required for pore formation on the endosomal membrane and is pH dependent. However, the pH of cDC1 phagosomes is mostly neutral, so additional mediators are required to regulate pH in a timely manner and prevent antigen degradation after uptake. Perhaps perforin-2 maturation depends on delivery of cargo by CLEC9A, which recognizes F-actin and associated myosin on necrotic cells (7). Additionally, NADPH oxidase 2 (NOX2) is triggered by CLEC9A, which generates reactive oxygen species (ROS) and regulates phagosomal pH. Hence, it is critical to comprehend the timing of the pH shift that enables perforin-2 maturation and the impact on antigens in the endosomes. The authors report no loss in endosome membrane integrity after perforin-2 activation, so another mediator potentially involved in maintaining phagosomal membrane integrity could be endosomal sorting complex required for transport III (ESCRT-III), which facilitates the primary repair system for intracellular membranes associated with cDC1 and cross-presentation (8).
A study found that Nox2-deficient mice display no defect in cross-presentation (9), raising the question of whether there are alternative mechanisms for the endosomal lipid peroxidation in cDC1. An alternative to NOX2 could be aquaporin-3. Mice lacking this channel exhibit a reduced ability to mount antiviral responses owing to decreased cross-presentation. Aquaporin-3 directly transports hydrogen peroxide into endosomes, which could contribute to endosomal lipid peroxidation. However, a maladaptive side effect is compromised membrane integrity and the release of endosomal cargo (9). How aquaporin-3 influences the maturation of perforin-2, along with the other mediators, will require further investigation.
Toll-like receptor (TLR) ligation enhances cross-presentation and promotes fusion events between phagosomes and MHC-I–containing recycling endosomes (10, 11). Rodríguez-Silvestre et al. demonstrate that TLR stimulation, particularly TLR9 and TLR11, enhanced perforin-2 maturation, outlining another potential mechanism by which TLR enhances cross-presentation. Together, it is possible that TLR agonists boost perforin-2 proteolytic maturation and function through the induction of lipid peroxidation, resulting in increased P2C antigen release and cross-presentation. If this newly discovered P2C molecular pathway is important, viruses might have found ways to undermine it. In the future, it will be intriguing to delve into the role of perforin-2 in disease models, particularly in the context of cancer or viral infections. Additionally, investigating whether other antigen-presenting cells, such as TLR7-stimulated cDC2 and monocytes, use perforin-2 for cross-presentation could reveal conserved function (12, 13). Lastly, exploring how the nature of antigens affects the mechanisms of perforin-2–mediated functions has the potential to reveal missing links within the P2C pathway. Such investigations could greatly enhance our understanding of immune responses and make valuable contributions to developing personalized vaccines. ■
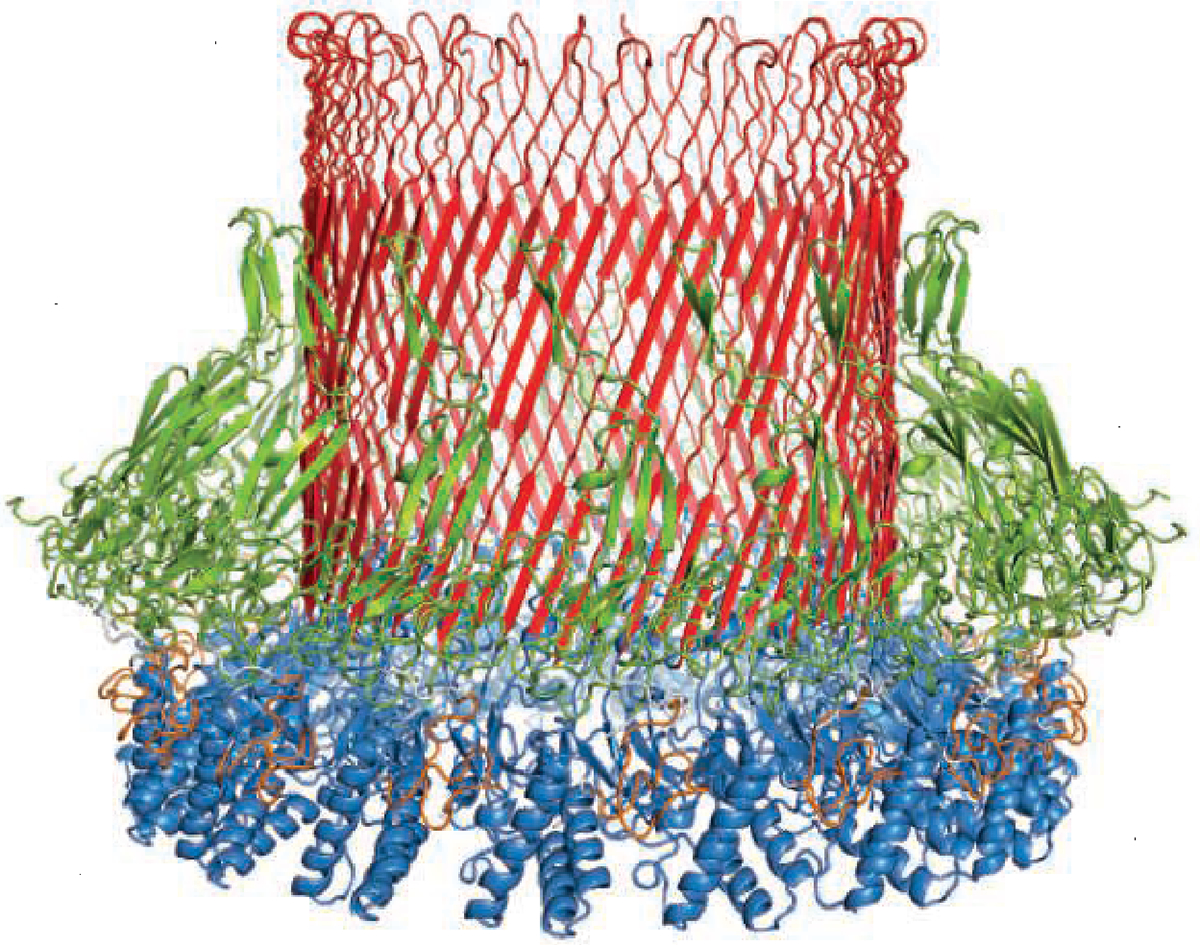
Schematic of a hexadecameric perforin-2 pore, which forms in phagosome membranes to facilitate the transportation of antigens to the cytosol.
Coordinating phagocytosis and antigen presentation.
In cross-presenting conventional dendritic cells (cDC1s), exogenous cell-associated antigens are recognized by CLEC9A. Upon phagocytosis, pH modulation by NOX2, ROS production, and aquaporin-3 facilitate perforin-2 maturation and phagosome-to-cytosol antigen transport. This leads to perforin-2–mediated localized rupture of the phagosome membrane through lipid peroxidation; ESCRT-III may repair ruptured phagosomes. Cytosolic antigens use classical MHC-I pathway molecules for cross-presentation (ubiquitination, proteasome degradation, and TAP transport into the endoplasmic reticulum).
REFEREN CES AND NOTES
- 1.den Haan JM et al. , J. Exp. Med. 192, 1685 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacsovics-Bankowski M, Rock KL, Science 267, 243 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Silvestre P et al. , Science 380, 1258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohara RA, Murphy KM, Semin. Immunol. 66, 101711 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz FM et al. , Semin. Immunol. 66, 101729 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack RM et al. , eLife 4, e06508 (2015).26402460 [Google Scholar]
- 7.Sancho D et al. , Nature 458, 899 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gros M et al. , Cell Rep. 40, 111205 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalle SC et al. , PLOS ONE 15, e0238484 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair-Gupta P et al. , Cell 158, 506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zehner M et al. , Immunity 42, 850 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Desch AN et al. , Nat. Commun. 5, 4674 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson SR et al. , Cell Death Differ. 23, 997 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


