Abstract
The application of laser technology in the field of assisted reproductive technology (ART) has experienced rapid growth over the past decades owing to revolutionary techniques such as intracytoplasmic sperm injection (ICSI), preimplantation genetic testing (PGT), and in vitro manipulation of gametes and embryos. For male gametes, in vitro manipulation techniques include spermatozoa selection, sorting, immobilization, and quality assessment. A number of studies have been conducted to investigate the application of different laser technologies in the manipulation of human spermatozoa. However, there is a lack of a unified understanding of laser application in the in vitro manipulation of sperm and safety considerations in ART and, subsequently, the inability to make clear and accurate decisions on the clinical value of these laser technologies. This review summarizes the advancements and improvements of laser technologies in the manipulation of human spermatozoa, such as photobiomodulation therapy, laser trap systems for sperm analysis and sorting, laser-assisted selection of immotile sperm and laser-assisted immobilization of sperm prior to ICSI. The safety of those technologies used in ART is also discussed. This review will provide helpful and comprehensive insight into the applications of laser technology in the manipulation of human spermatozoa.
Keywords: Infrared lasers, Photobiomodulation therapy, Laser optical trap, Laser-assisted selection, Laser-assisted immobilization, Sperm motility, Male infertility, Assisted reproductive technology (ART)
Background
The term laser is an acronym for light amplification by the stimulated emission of radiation. Since the first laser was developed in 1960, the role that lasers play in various fields, including biology, chemistry, and medicine, has increased steadily [1]. Many procedures have only become possible with the use of lasers. Currently, more than forty different types of lasers have been found in medicine [2]. In the early 1970s, lasers were introduced into the field of gynecology for surgery, excision and ablation of tissue [3–5]. In the 1980 and 1990 s, lasers began to be used in infertility treatment of endometriosis, tubal surgery, ectopic pregnancy and polycystic ovarian syndrome through operative microscopes and laparoscopes [6–8]. Since the successful establishment of assisted reproductive technology (ART), in vitro manipulation of gametes, zygotes and embryos has been an essential integral part of ARTs. Laser technology was introduced into the field of ART as a valuable tool to replace many mechanical and chemical procedures used in the in vitro manipulation of gametes, zygotes and embryos and to optimize the procedural efficiency of techniques such as intracytoplasmic sperm injection (ICSI), assisted hatching, and embryo biopsy [9–11].
For male gametes, in vitro manipulations include techniques for sperm selection, sorting, immobilization and other incubation procedures. The first use of lasers to manipulate human gametes can be traced to 1984 [12]. Sato et al. were the first to report the use of lasers to manipulate human sperm and explore the effects of laser exposure on sperm motility and velocity in vitro [12]. Over the next few years, near-infrared (NIR) laser beams with wavelengths ranging from 700 to 1200 nm were used as optical traps (laser tweezers) in sperm micromanipulation [13–15]. Further development of laser applications was the introduction of an infrared diode laser emitting at a wavelength of 1480 nm, which is far from the absorption peak of DNA (260 nm) [16]. The system allows laser beams along the microscope’s optical axis to the target with minimal absorption by the culture dish and the water molecules [9, 17]. This 1480 nm laser is used across a variety of applications, including sperm selection, sorting, immobilization prior to ICSI, and viability assessment of immotile sperm.
Many studies have been performed to investigate the different laser technologies in in vitro manipulations of human spermatozoa. However, there is a lack of a unified understanding of laser application in the manipulation of spermatozoa and, subsequently, the inability to make clear and accurate decisions on the clinical value of these laser technologies. The present review aims to summarize the advancements and improvements of laser technologies applied in the manipulation of human spermatozoa, such as photobiomodulation, sperm sorting, selection, and immobilization prior to ICSI. We also evaluate the potential value of these laser technologies in the treatment of male infertility and safety considerations for clinical application.
Photobiomodulation therapy on human spermatozoa
Photobiomodulation (PBM) therapy, previously termed low-level laser therapy (LLLT), generally employs light at red and NIR wavelengths to modulate biological activity [18]. Several studies were conducted in vitro on human spermatozoa through the employment of low-level laser therapy, demonstrating a positive effect on sperm function. PBM parameters have been mostly reported within the red and NIR wavelength range of 600–1100 nm, with an energy density of between 5 and 200 mW/cm2. PBM laser devices commonly include Krypton Laser, gallium aluminum arsenide (GaAlAs), neodymium-doped yttrium aluminum garnet, and indium gallium aluminum phosphide (InGaAlP) diode lasers. Table 1 presents a summary of the results.
Table 1.
Effect of low-level laser therapy (LLLT) on human sperm parameters
| Study | Source of sperm | Type | Device | Type of light and wavelength | Intensity/duration of exposure | Main findings and results | References |
|---|---|---|---|---|---|---|---|
| Sato et al. (1984) | Normal sperm | Fresh | Krypton Laser | Red light (647 nm) | 0.5, 1.0, 2.0, 4.0, 8.0, and 32 J/cm2/80 and 160 s | Total sperm motility increased after Laser irradiation at 4 J/cm2, 8 J/cm2, and 32 J/cm2 respectively compared with control. | [12] |
| Lenzi et al. (1989) | Normal sperm | Fresh | LM infrared laser | Infrared laser | 5–30 mW/120 s | Laser irradiation had a positive effect on sperm motility. | [22] |
| Singer et al. (1991) | Normal and abnormal sperm | Fresh | BioBeam instrument | Infrared laser (940 nm) | Maximal intensity 20 mW/cm2/4 min | Light exposure significantly increased the percentages of motile, viable, and morphologically normal sperm. | [31] |
| Firestone et al. (2012) | Normospermia, oligospermia, and asthenospermia | Fresh | Theralaser TLC-1000 | Laser light (905 nm) | 50 mW/cm2/30 s | Low-level laser light had a positive short-term effect on the motility of sperm and did not cause any increase in DNA damage measured at 2 h. | [26] |
| Salman Yazdi et al. (2014) | Asthenzoospermia | Fresh | GaAlAs laser | GaAlAs laser (830 nm) | 4, 6, and 10 J/cm2/0, 30, 45, and 60 min | Irradiating human sperms with low-level 830-nm diode lasers can improve their progressive motility depending on both laser density and postexposure time. | [25] |
| Preece et al. (2017) | Healthy men | Frozen-thawed | Monochromatic coherent laser (Intense 7404) | Red laser light (633 nm) | 5.66 mW/cm2/35 min; 31mW/cm2/30 min | Red light improved sperm motility and did not induce oxidative DNA damage. | [19] |
| Gabel et al. (2018) | Human | Fresh and frozen-thawed | GaAlAs | GaAlAs single laser (810 nm) and an LED cluster (660 and 850 nm) |
GaAlAs single laser: 200 mW/10, 20, and 40 s for frozen sperm, and 15,20, and 300 s for fresh sperm LED cluster: total power 2 W/25, 50, and 75 s for frozen sperm, and 50,100, 200, and 400 s for fresh sperm |
The sperm motility index and total functional sperm count increased up to fourfold compared to controls. The motility modification was dependent upon beam irradiance and irradiation time as well as the condition of the sample. |
[20] |
| Highland et al. (2018) | Normal sperm | Fresh | NA | NIR radiation (750–1100 nm) | 87 lux/15 min | NIR radiation resulted in a loss of viability and membrane function, increased free radical formation, and induced sperm apoptosis. | [23] |
| Safian et al. (2020) | Normal sperm | Fresh |
Diode laser probes (NILTVIR202 Noura Instruments) |
NIR light: 810 nm | 0.6 J/cm2/NA | PBM treatment before cryopreservation significantly increased the percentages of viable sperm, sperm with high membrane potential, and high mitochondrial activity. | [21] |
| Safian et al. (2021) | Normal sperm | Fresh |
Diode laser (NILTVIR202 Noura Instruments) |
Red light (630 nm), NIR (810 nm), or red + NIR (630 + 810 nm) | 0.6, 1.2, and 2.4 J/cm2/15, 30, and 60 min | The NIR laser at 0.6 J/cm2 energy density significantly increased sperm motility and viability and decreased the DNA fragmentation index compared with the red and red + NIR protocols. | [27] |
| Espey et al. (2022) | Asthenozoospermia and normozoospermia | Fresh | Pulsed laser-probe (Reimers & Jansen) | Pulsed laser-probe (655 nm) | 4, 6, and 10 J/cm2/0, 30, 60, 90, and 120 min | Exposure to laser energy doses of 4 and 6 J/cm² improved sperm motility and velocity in asthenozoospermic patients. | [24] |
| Safian et al. 2022 | Normal sperm | Fresh | Diode laser (NILTVIR202 Noura Instruments | NIR light: 810 nm | 0.6 J/cm2/NA | PBM therapy before cryopreservation significantly improved the quality of post-thawed human sperm. | [28] |
NA: not available; LED: light emitting diodes; NIR: near-infrared; PBM: photobiomodulation
Effects of PBM on the parameters of human spermatozoa
Sperm motility
Sperm motility is one of the most important characteristics associated with fertility. Almost all studies have examined the impact of PBM therapy on the motility and other kinematic parameters of human sperm. Sato et al. reported that normal sperm samples were exposed to red laser at different dosages, 0.5, 1.0, 2.0, 4.0, 8.0, and 32 J/cm2, for 160 s or 80 s and found that total sperm motility significantly increased at 4.0, 8.0 and 32 J/cm2 [12]. However, there was no stimulating effect on sperm velocity [12]. A study by Preece et al. assessed the effect of a 633 nm red laser at a power density of 5.66 mW/cm2 on frozen human sperm and indicated that the swimming speed improved within 35 min of irradiation [19]. In another work, human fresh and frozen sperm were exposed to light from a GaAlAs single laser beam (810 nm, 200 mW) and an LED cluster (660 and 850 nm, total power 2 W) for different irradiation times [20]. The results showed that the change in sperm motility was dependent upon the stimulatory dose, exposure time, and condition of the sample [20]. Concerning the effects of red and infrared laser irradiation on the motility of sperm, Safian et al. performed three different energy densities of two wavelengths of laser [21]. The results concluded that the NIR laser at 0.6 J/cm2 density was superior to the other irradiation protocols in stimulating the effect on motility [21]. The results from the study of Lenzi et al. indicated that the increase in progressive sperm motility after laser irradiation (647 nm) was related to the fast consumption of sperm ATP contents, suggesting that laser irradiation may have an “energetic modulation effect” on normal sperm [22]. However, one study reported by Highland et al. found that NIR irradiation resulted in a damaging effect on sperm viability and a diminished membrane function of sperm [23]. In addition, several studies have used laser beams to stimulate abnormal sperm, including sperm from oligospermia and asthenospermic patients, and showed that irradiation significantly increased sperm motility and velocity [24–26]. In two studies by Safian et al., PBM therapy before human sperm cryopreservation dramatically increased the percentage of live spermatozoa [27, 28].
DNA integrity
Sperm DNA integrity is critical for the success of fertilization, embryo development, and implantation and is therefore considered a predictive factor for the clinical outcomes of patients undergoing ART. The potential effect of laser light on sperm DNA integrity has been a focus of attention by investigators. Most published studies have confirmed that red and infrared light does not induce DNA damage. Firestone et al. reported that infrared laser irradiation did not cause any increase in DNA damage at 2 h after exposure in normospermic, oligospermic, and asthenospermic samples [26]. Similarly, exposure to a pulsed-wave laser had no significant effect on the DNA fragmentation level in sperm from asthenozoospermic patients [24]. 830-nm laser irradiation can slightly increase the level of DNA fragmentation in sperm from asthenospermic patients, but it was not statistically significant [25]. The results from the study of Preece et al. showed that red light exposure could not produce sufficiently high levels of reactive oxygen species (ROS) to cause significant oxidative damage in normal sperm DNA [19]. Consistent with previous research, Gabel et al. also found no damage to sperm DNA integrity by light irradiation at a very high-density dose [20]. Interestingly, Safian et al. studied the effects of red and NIR ranges of PBM with a diode laser alone and together on the DNA fragmentation index (DFI) of fresh human sperm and observed that compared with the control, both the red + NIR and red lasers significantly increased DFI, while the NIR range of PBM did not result in a detectable increase in DNA damage [21].
Mechanism of PBM
Although the underlying pathways of PBM therapy are not well established and may vary among different sperm states (fresh versus frozen, normal versus abnormal), laboratory and clinical studies suggest that PBM significantly improves sperm motility and does not damage DNA. It has become increasingly clear that the biological effects of PBM are closely associated with dosage and irradiation time as well as sperm condition. The lack of consistency in study conditions has always been a confounding factor in the interpretation of PBM mechanisms. Nonetheless, several studies have addressed probable mechanisms regarding the interaction of laser light and spermatozoa.
At present, at least four mechanisms are believed to be related to the response of sperm to PBM. The first hypothesis suggests that the effects of irradiation are driven by changes in mitochondrial function. Current data indicate that PBM mainly acts on cytochrome c oxidase (CcO) in the mitochondrial respiratory chain [29]. Photonic energy in red or NIR light is absorbed by CcO, resulting in an increased transmembrane proton gradient that drives the production of adenosine triphosphate (ATP), thus increasing sperm motility [19, 20, 30, 31]. ATP is the universal energy source in sperm cells essential for the maintenance of motility driven by flagellar dyneins. An increase in ATP synthesis leads to increased activity of all ATP-driven carriers for ions, such as Na+/K+ ATPase and Ca2+ pumps [32, 33]. Ca2+ is a key regulator of sperm motility. Furthermore, since ATP is the substrate of adenylate cyclase, ATP levels control the level of cAMP [34]. Both Ca2+ and cAMP are very important second messengers [35, 36]. The second mechanism considers the generation and/or release of reactive oxygen species (ROS) from sperm cells after laser light irradiation. ROS are very small molecules, including oxygen ions, free radicals, and hydrogen peroxide. Previous studies have shown that exposure of sperm to light increases hyperactivated motility mediated by mitochondrial ROS production and the cAMP/PKA pathway [37, 38]. Using the electron paramagnetic resonance (EPR) spin-trapping technique, Lavi et al. followed light-induced hydroxyl radicals in sperm cells and found that the concentration of hydroxyl radicals increased with illumination time and that ROS were produced in both the membrane and cytoplasm [39]. According to a study by Shahar et al., light-stimulated hyperactivated motility was increased through ROS-dependent activation of the epidermal growth factor receptor (EGFR) [38]. The third mechanism for PBM involves nitric oxide (NO), which is an important biological messenger that plays a crucial role in the regulation of energy production and mitochondrial biogenesis [40]. Previous studies revealed that light can induce NO formation by increasing the activity of nitric oxide synthase (NOS) [41–43]. NO is important in sperm motility and capacitation [44, 45]. The fourth hypothesis is linked to the interaction between light and specific receptors of the opsin family, which are coupled to G-proteins in sperm [46]. There is evidence that sperm opsins, specifically rhodopsin, play a role in the response of sperm to light [47]. At least seven opsin proteins are present in human sperm, of which the contents of encephalopsin and neuropsin are the most abundant [48]. The opsins, as light transducers, work through the light activation of photosensitive molecules linked to opsins [47]. The exact function of these opsins in the response of sperm to light still requires further study. Schematic diagrams of four possible mechanisms of PBM therapy on sperm are presented in Fig. 1.
Fig. 1.
Diagram illustrating four possible mechanisms of PBM therapy on human sperm. Photonic energy in low level light is absorbed by the enzyme cytochrome c oxidase (CcO) located in the mitochondrial respiratory chain. The activated enzyme leads to a proton gradient. Consequently, the levels of reactive oxygen species (ROS) and adenosine triphosphate (ATP) are increased. On the other hand, the application of low level light activates specific receptors of the opsin family coupled to G-proteins in sperm. Phosphatidylinositol 4,5-diphosphate (PIP2) is catalyzed by hydrolysis to produce inositol 1,4,5-triphosphate (IP3). All of these activities activate light-sensitive ion channels and increase the levels of calcium ions (Ca2+). Soluble adenylyl cyclase (sAC) is activated by Ca2+. The increased sAC activity activates 3’,5’-cyclic adenosine monophosphate (cAMP)/ protein kinase A (PKA) pathway, thereby promoting sperm motility. In addition, exposure to low level light induces nitric oxide (NO) production by nitric oxide synthase (NOS), which activates cyclic guanosine monophosphate (cGMP)-dependent protein kinases production from soluble guanylate cyclase (sGC). Sperm motility is promoted by activation of protein kinase G (PKG).
PBM therapy for male infertility
PBM therapy is a fast-growing technology and provides a promising tool for improving male infertility status [49]. Currently, only a small number of studies have been carried out to evaluate the effect of PBM on the functional capacity of sperm from oligo- and astheno-zoospermia patients [24–26, 50]. Due to a variety of protocol parameters, it is difficult to compare directly between different studies. However, the results of these studies reveal a similar trend; PBM positively affects sperm motility and velocity without causing any damage to the DNA in samples of oligo- and astheno-zoospermia patients [24–26]. In addition, PBM therapy prior to human sperm cryopreservation plays a significant role in improving the quality of postthawed sperm and preventing cryo-damage [21, 28]. However, few studies have conducted assessments of human sperm function, such as acrosome reaction, hyperactivation, and fertilization ability. Recent studies evaluated the effect of PBM therapy on the improvement of spermatogenesis in hyperthermia-induced azoospermia mouse models and found that the spermatogenesis process is significantly improved by PBM therapy [51, 52]. In addition, accumulated evidence from animal studies suggested that PBM therapy improved sperm capacitation and fertilizing ability, as well as reproductive performance [53, 54]. It is postulated that PBM therapy can be considered a promising first-line medical intervention in the treatment of male infertility in the future [30, 31].
Given a lack of guidelines and a limited evidence base for PBM treatment on human sperm, the issues outlined below have been highlighted and addressed for consideration. First, the mechanisms responsible for the beneficial effect on sperm reported by PBM therapy need to be completely elucidated. Theoretically, sperm cells are not exposed to any type of light, so it can be speculated that both normal and abnormal sperm can be sensitive to exogenous bright stimuli. Although different hypotheses have been proposed to explain the effects of light irradiation on sperm, future research is needed to clarify which mechanism plays an important role. Further understanding of the mechanisms is necessary for optimizing clinical treatment. Second, PBM treatment protocols need to be optimized for each type of sperm, such as normal sperm and sperm with mild, moderate, and severe asthenozoospermia. There is now a wide and increasing array of laser equipment to choose from. The effectiveness of PBM therapy is likely to depend on specific laser characteristics. To achieve a desirable clinical outcome, the proper wavelength, pulse duration and energy density must be tailored to the clinical indication. Finally, to evaluate the suitability of PBM for routine clinical use, it is necessary to further study whether PBM has potential genotoxic effects on sperm. DNA integrity is considered a fundamental factor for the fertilization and transmission of paternal genetic information to offspring. Further research on the effects of low-level lasers on sperm cells is imperative.
Taken together, several general conclusions can be drawn: (i) The mechanical basis of PBM therapy is associated with several intracellular metabolism pathways that regulate sperm motility. (ii) The overall results from preclinical and clinical studies suggest that PBM therapy holds promise as a non-invasive treatment for male infertility disorders, such as asthenospermia and oligospermia, by enhancing sperm motility and quality. (iii) PBM beneficial effects depend on wavelengths, exposure time, stimulatory dose, irradiated area, and other treatment parameters (e.g., condition of the sample). Tailoring PBM treatment protocols for specific sperm conditions is essential.
Micromanipulation of human sperm using a laser optical trap
An optical trap is a noninvasive biophotonic tool that has been studied for practical applications in a variety of fields, including physics, chemistry, biology, and medical science [55, 56]. A laser optical trap was first reported to manipulate single cells in 1987 [57]. The authors achieved damage-free trapping and manipulation of suspensions of single cells and organelles located within individual living cells [57]. Berns et al. first showed that a laser trap could be used to move the chromosomes inside mitotic cells in vitro [58]. Not long afterward, single-point laser traps were used as a tool to manipulate individual sperm cells and analyze the interaction between laser and sperm and sperm motility by measuring sperm swimming forces in the late 1980s [13, 59]. Several studies have demonstrated that laser optical trapping and micromanipulation of sperm cells using a NIR beam is technically feasible [14, 15, 60–70].
Laser trapping optical system
The optical trapping system is designed as a biomedical tool to study the physiological and biomechanical properties of cells. This system introduces NIR laser light into an inverted microscope, creating a single-point and three-dimensional gradient laser trap at the focal point of the microscope. Trapping provides a noninvasive method for analyzing and classifying sperm based on sperm swimming speed and swimming force [13–15, 60, 65–67, 70] and studying the effects of light radiation [63], oxidative phosphorylation inhibitors [64], freezing [62], drugs [64], and other factors [61, 68, 69].
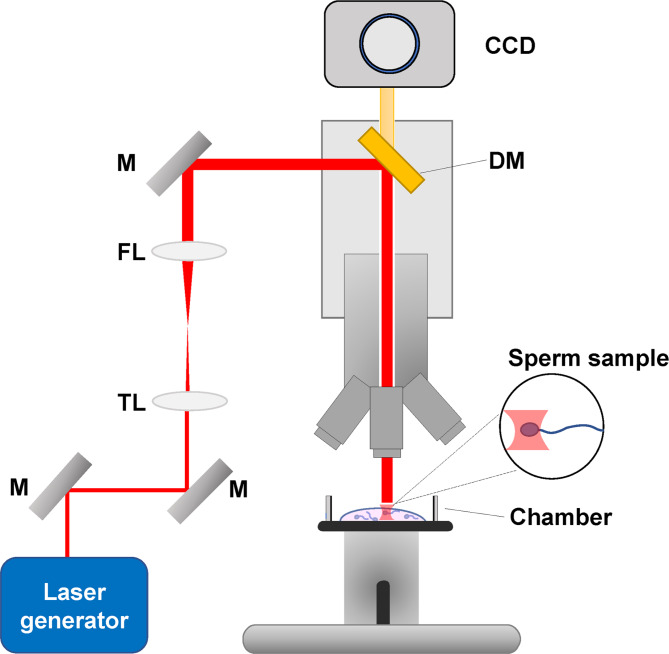
The laser-induced optical trap reported in the literature consisted of a laser operating in a continuous wave at a wavelength of 760–1070 nm, which traveled through a series of mirrors and lenses into the microscope, as shown in Fig. 2. Laser devices generally included the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, titanium-sapphire laser, and CW ytterbium fiber laser. In the described automatic microscope system, real-time tracking and trapping were described, which provided a user-friendly robotic interface. Sperm cells were continuously tracked, and the changes in sperm swimming behavior were dynamically monitored. To improve the accuracy of sperm analysis and sorting, automatic annular laser trapping was developed [67]. Table 2 presents a summary of the laser traps used for sperm analysis and sorting.
Fig. 2.
Schematic diagram of the laser system for sperm trapping. M: mirrors; TL: telescope lens; FL: focusing lens; DM: dichroic mirror; CCD: charge-coupled device
Table 2.
Summary of laser traps (optical tweezers) used for sperm analysis and sorting
| Study | Source of sperm | Type | Type of laser | Wavelength | Trapping power | Exposure time | Total number of sperm trapped | Analysis parameters | References |
|---|---|---|---|---|---|---|---|---|---|
| Tadir et al., 1989 | Donor | Fresh | Neodymium: Yttrium-Aluminum-Garnet (Nd:YAG) laser | 1.06 μm | 1 W | 15, 30, 45, 60, 90, and 120 s | 514 | Linear velocity, actual distance traveled, maximum lateral head displacement and motility patterns (before, during, and after exposure) | [13] |
| Tadir et al., 1990 | Donor/normal | Fresh | Neodymium: Yttrium-Aluminum- Garnet (Nd:YAG) laser | 1.06 μm | 10–150 mW | < 10 s | 705 | Linear velocity, motility patterns | [14] |
| Colon et al., 1992 | Donor/normal | Fresh | Neodymium-doped yttrium aluminum garnet (Nd:YAG) laser | 1.06 μm | 25–96 mW | 30, 60, 120, and 180 s | NA | Sperm velocity | [60] |
| Westphal et al., 1993 | Donor/normal | Fresh | Titanium-sapphire laser | 760 nm | < 300 mW | < 10 s | 892 | Linear motility, hyperactivated motility, and cumulus-related motility | [61] |
| Dantas et al., 1995 | Normal | Fresh | Titanium-sapphire laser | 800 nm | 0-300 mW | NA | 2130 | Relative escape force of sperm | [62] |
| Liu et al., 1996 | Donor/normal | Fresh | Nd:YAG laser | 1064 nm | < 500 mW | < 10 min | NA |
Sperm cell temperature, DNA structure, viability, and pH |
[15] |
| König et al., 1996 | Donor/normal | Fresh | Tuneable cw Ti: Sapphire ring laser, | UVA: 320–400 nm; NIR: 760 nm, 800 nm |
1.5 mW (UVA), 105 mW (NIR) |
2 min | 580 | Cytotoxic effect | [63] |
| Patrizio et al., 2000 | Normozoospermic and asthenozoospermic donors | Fresh | Nd:YAG laser | 1064 nm | < 500 mW | NA | ≥ 80 per patient | Relative escape force of sperm | [64] |
| Shao et al., 2007 | Donor | Frozen | CW Ytterbium fiber laser | 1070 nm | 0–25 mW | NA | 93 |
Curvilinear velocity , smooth path velocity, amplitude of lateral head displacement |
[65] |
| Nascimento et al., 2008 | Donor | Fresh and frozen | NA | 1064 nm | NA | NA | NA | Curvilinear velocity, escape force | [66] |
| Shi et al., 2009 | Donor | Frozen | CW Ytterbium fiber laser | 1070 nm | < 24 mW | NA | 264 | Curvilinear velocity, smooth path velocity, amplitude of lateral head displacement | [67] |
| Hyun et al., 2012 | Normal | Frozen | Nd:YVO4 laser | 1064 nm | 450 mW | NA | NA | Curvilinear velocity, minimum laser power | [68] |
| Auka et al., 2019 | Donor | Frozen | CrystaLaser | 1064 nm | 700 mW | NA | 479 | DNA analysis | [69] |
| Zhong et al., 2022 | Infertility patients | Fresh | Fiber laser | NA | ~ 400 mW | < 20 s | NA | Longitudinal rolling dynamics of single sperm | [70] |
NA: not available; NIR: near-infrared; UVA: ultraviolet A
Effects of laser trapping on sperm quality
Early studies on sperm quality parameters using the force generated by infrared laser beams mainly focused on the power and swimming force loaded onto the trapped sperm when it escaped out of the optical trap. Single-spot, gradient-force laser tweezers were first used to trap human sperm cells to study velocity in the late 1980s [13]. Sperm velocity was not significantly affected by the trap after short periods of exposure, but prolonged exposure time led to a decrease in sperm velocity [13]. Tadir and coworkers later found that there existed a positive correlation between sperm velocity and laser power, and the average trapping power varied depending on sperm linear velocities [14]. Based on previous studies, Colon et al. developed a three-dimensional laser optical trap to explore the effect of manipulation time on sperm velocity [60]. The results from Liu et al. demonstrated that it was feasible to monitor sperm cell physiology in situ during continuous wave and pulsed laser trapping [15]. In addition, the authors also found that optical tweezers and microbeams interfered with the levels of cell metabolic activity and sperm viability [15]. Although single-point laser traps provided a way to assess the motility and quality of individual sperm, they also had some drawbacks, among which the biggest problem was low throughput. Soon thereafter, a 3-D annular laser trap was developed and applied to study many aspects of sperm cellular physiology [66] and provided the possibility of multilevel, high-throughput sorting and analysis of human sperm based on motility [65, 67]. Recently, Zhong et al. used an optical trap to determine the chirality and frequency of longitudinal rolling of human sperm, which was expected to achieve automatic evaluation and separation of single sperm in the future [70].
Optical traps as a tool for studying the impact of the external environment on sperm motility
The motility generated by sperm swimming is attributed to flagellar movement, which is not only a fundamental expression of sperm viability but also essential for evaluating fertilization ability. Because the change in momentum of the photons produces a force acting on an object, optical traps can confine and manipulate sperm cells. Sperm motility was studied by Tadir et al. using force generated by a laser-generated optical trap [13]. The swimming force of sperm is qualified by measuring the minimum trapping force required to keep sperm in traps, which is proportional to the applied laser power. This value is also defined as the relative escape force.
In a study by Westphal et al., a laser optical trap was used to investigate the relative force generated by human sperm displaying different motility patterns [61]. The results showed that the relative force was associated with the motility patterns (linear, hyperactivated, and cumulus-related), and exposure of sperm to the cumulus mass resulted in the greatest relative force [61]. Araujo et al. used a laser trap to compare the relative escape force of human epididymal sperm with that of human ejaculated sperm and concluded that the average relative escape force of epididymal sperm was 60% weaker than that of ejaculated sperm [71]. Dantas et al. studied the effect of freezing on sperm escape force and found that there was no significant difference in the overall mean relative escape forces between fresh and frozen-thawed sperm [62]. Patrizio et al. investigated the effect of pentoxifylline on the swimming forces of human sperm and demonstrated that pentoxifylline significantly increased sperm intrinsic relative force in sperm from normozoospermic and asthenozoospermic samples [64]. Hyun et al. reported the effect of media with different viscosities on sperm motility and found that the sperm swimming force increased with increasing viscosity [68]. Chow et al. found that 633 nm red light irradiation significantly increased the mean squared displacement, which is related to an increase in the swimming force [72].
In total, it can be concluded that 3-D annular laser traps have more advantages than single-beam traps for high-throughput sorting and analysis of sperm quality, including motility, swimming velocity, and force. Moreover, optical traps, as a noninvasive tool, may also play a unique role in assessing the impact of the external environment on sperm motility.
Laser-assisted selection of viable but immotile sperm
Viable sperm are necessary for successful ICSI. Generally, sperm motility is the main sign used to determine sperm viability. However, the challenge faced by embryologists is how to judge whether the sperm is dead or viable when encountering sperm samples without obvious vitality. The absence of motile spermatozoa is often seen in absolute asthenozoospermia samples, epididymal sperm aspirations and testicular biopsy specimens [73, 74]. It is necessary to establish a fast and simple method to distinguish between dead and viable but immotile sperm. Several testing methods have been developed to select viable but immotile sperm for ICSI, including a hypo-osmotic swelling test (HOST), chemical substances for induction of tail movements, mechanical touch technique and laser-assisted immotile sperm selection [75–79]. Table 3 shows the advantages and disadvantages of different immotile sperm selection techniques.
Table 3.
Advantages and disadvantages of immotile sperm selection techniques
| Procedures | Principle | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Hypo-osmotic swelling test (HOST) | HOST is based on the semi-permeability of intact cell membranes. The tail of viable sperm curves or swells after exposure to a hypo-osmotic medium. |
• Simple and economical • Repeatable and reliable • Recommended in the WHO laboratory manual |
• Need for prior treatment • Time-consuming • Not suitable for cryopreserved sperm |
[75–79] |
| Pharmacological stimulation | The mechanism of drug-induced motility is mainly to activate signaling pathways related to sperm motility. |
• Relatively easy to use • Easy identification of viable sperm |
• Need for pharmacological agents • Need for prior treatment • Possible side effects |
[111–113] |
| Mechanical testing | The ICSI pipette is used to test the elasticity of the sperm tail. If sperm exhibiting elastic tails is presumed to be viable. |
• Simple and economical • No need for additional reagents and equipments |
• Highly dependent on practical experiences • Not easy to identify viable sperm • A relatively small number of relevant literatures |
[73, 114] |
| Laser-assisted selection | The end of the sperm tail was targeted with a laser pulse. Those sperm that presented with curling of the tails were regarded as viable. |
• Time-saving • Easy to perform • Noncontact • Can be performed during ICSI |
• Need for laser equipment • Still not standardized |
[79, 80, 83, 84, 86–89] |
WHO: World Health Organization; ICSI: intracytoplasmic sperm injection
The introduction of laser-assisted immotile sperm selection is an advancement in the use of lasers in the treatment of male infertility. The laser can help discriminate between viable sperm and dead sperm in cases of completely immotile sperm. The mechanism may be related to sperm membrane integrity [80]. Viable sperm treated by laser exhibit tail curling due to the instantaneous opening of the membrane. Dead sperm show no reaction, as the membrane integrity has been undermined. Laser-assisted selection is superior and most suitable for routine application in ART laboratories compared with the other testing methods [79]. The main advantage of this method is that it is convenient and reliable [81, 82]. It does not require chemical compounds to induce sperm motility, so it does not produce side effects. Most importantly, this technique is suitable for the different types of immotile sperm [79]. Frozen sperm spontaneously develop tail swelling, which may bias the results. Laser-assisted selection is especially used for sperm that have been frozen and then thawed. The main results are reported in Table 4.
Table 4.
Clinical applications of diode lasers in the selection of immotile but viable spermatozoa before ICSI
| Study | Source of spermatozoa | Type | Intensity of laser | duration of exposure | No. cycles of ART | No. oocytes fertilized/Total oocytes | No. pregnancies | No. live births | References |
|---|---|---|---|---|---|---|---|---|---|
| Aktan et al. (2004) | 24 patients with complete asthenozoospermia (complete immotile spermatozoa) and 21 patients with either obstructive or nonobstructive azoospermia (testicular spermatozoa) | Fresh | 120 µJ | 1.2 ms |
24 (ejaculate) 21 (testicular) |
64.2% (NA) 45.4% (NA) |
33.3%(8/24) 25.0%(5/20) |
28.0%(7/25) 19.0%(4/21) |
[80] |
| Gerber et al. (2008) | A patient with Primary cilia dyskinesia | Fresh | 400 µJ | 5 ms | 1 | 57.1% (4/7) | 1 | NA | [83] |
| Nordhoff et al. (2013) | 48 patients with testicular biopsy | Fresh | NA | 6-7ms |
58 TESE-ICSI cycles 65 control cycles |
52.7% (292/554) 42.1% (216/617) |
NA | NA | [85] |
| Chen et al. (2015) | Two patients with obstructive azoospermia and severe asthenospermia | Fresh | NA | 2 ms | 2 | 83.3%(10/12); 100%(6/6) | 2 | NA | [86] |
| Chen et al. (2017a) | A patient with nonobstructive azoospermia (testicular spermatozoa) | Frozen-thawed | 200 µJ | 2 ms | 1 | 80% (4/5) | 1 | NA | [87] |
| Chen et al. (2017b) | 7 patients with severe asthenozoospermia (complete immotile spermatozoa) and 25 patients with azoospermia (testicular spermatozoa) | Fresh and frozen-thawed | 120 µJ | 1.2 ms | NS |
78.7% (37/47) (ejaculate) 80.3% (139/173)(testicular) |
NA | NA | [88] |
| Ozkavukcu et al. (2018) | A patient with Kartagener’s syndrome | Fresh | NA | 350 µs | 1 | 45.5% (10/22) | 1 | 1 | [84] |
| Chen et al. (2021) | 33 TESA-ICSI cycles/99 controls | Fresh and frozen-thawed | 200 µJ | 2 ms |
33 (test group) 99 (control group) |
78.2%(283/362) 80.5% (763/948) |
61.2% (30/49) 47.5% (76/160) |
69.7% (23/33) 60.6% (60/99) |
[89] |
NA: not available; TESA: testicular aspiration; ICSI: intracytoplasmic sperm injection
The use of lasers for the selection of viable but immotile sperm was first introduced by Aktan et al., who performed a direct laser shot of 129 µJ for approximately 1.2 ms to the tip of the sperm tail [80]. Sperm that showed curling or coiling of the tail were regarded as viable. Since nonviable sperm had no such change, this method helped to identify sperm with membrane functional integrity [80]. Laser selection of sperm from ejaculated absolute asthenozoospermic samples and testicular biopsies showed a similar percentage of viable sperm compared with the hypo-osmotic swelling test. Gerber et al. and Ozkavukcu et al. separately reported a case with primary cilia dyskinesia (PCD) who achieved a normal pregnancy and live birth by laser-assisted viability assessment of immobile sperm during ICSI [83, 84]. In a large sample study, Nordhoff et al. compared sperm selection with light microscopy with laser-assisted selection and found that the fertilization rate improved significantly when applying laser selection [85]. Chen et al. reported that two successful pregnancies were achieved by using laser selection of viable but immotile sperm from two cases of obstructive azoospermia and severe asthenospermia [86]. The team of Chen et al. first reported a successful pregnancy using completely immotile frozen-thawed testicular spermatozoa selected by laser [87]. The team of Chen et al. continued to report the results of cryopreservation of completely immotile testicular sperm or ejaculate sperm of 32 patients by laser-assisted selection and showed similar rates of fertilization, cleavage and good-quality embryos compared to routine fresh immotile sperm [88]. This study confirmed that combined with laser-assisted selection of viable sperm, completely immotile sperm can be frozen for fertility preservation [88]. Recently, a retrospective comparative study by Chen et al. demonstrated that no negative effect on perinatal and neonatal outcomes was observed in patients with immotile sperm by using laser-assisted selection for ICSI [89].
In summary, laser-assisted immotile sperm selection, an innovative technique used in ART, is simple and effective and does not require the use of additional substances to either induce motility or cause sperm flagellum curling, consequently there is no accompanying damage to sperm or embryos. Furthermore, several clinical studies have shown promising results in male infertility treatment and fertility preservation, with a focus on safety and positive reproductive outcomes.
Laser-assisted immobilization of sperm
Sperm immobilization prior to ICSI is necessary for successful fertilization to occur [90, 91]. Mechanical immobilization is achieved by pressing the sperm tail to the bottom of a culture dish using the tip of an ICSI injection needle [92–96]. This method has been regarded as a classic method for many years. However, an alternative method of sperm immobilization was described in which a noncontact diode laser system was used [97–104]. Immobilization of sperm was performed by a single or double laser shot [97–100, 102–104]. Table 5 shows the advantages and disadvantages between laser-assisted and mechanical immobilized sperm. Laser-assisted immobilization has two advantages: first, it avoids the negative impact of polyvinylpyrrolidone (PVP) on sperm during mechanical immobilization; second, it simplifies the operation process and shortens the manipulation time. Sperm immobilization with this method was found to yield comparable results in the rates of fertilization [98–100, 104], cleavage [98, 104], and good-quality embryos [100, 104] compared with mechanical immobilization. Table 6 presents a summary of laser-assisted immobilization of sperm.
Table 5.
Comparison of advantages and disadvantages between laser-assisted and mechanical immobilized sperm
| Procedures | Principle | Advantage | Disadvantage | References |
|---|---|---|---|---|
| Mechanical immobilization | Immobilization is performed by touching the tail of the sperm with ICSI pipette. |
• No need for additional equipment • Easy to standardize and adapt in clinical scenarios |
• Resulting in sperm membrane damages • Require PVP to facilitate sperm capture |
[92–97] |
| Laser-assisted immobilization | Sperm is immobilized with 1.48 μm wavelength diode laser. |
• Simple and time-saving • Retaining membrane integrity • Noncontact • Avoiding the harmful effects of PVP on sperm and embryos |
• Need for laser equipment • Literature insufficiency on the percentage of live birth |
[79, 97–99, 101, 102] |
ICSI: intracytoplasmic sperm injection; PVP: polyvinylpyrrolidone
Table 6.
Summary of laser-assisted immobilization of sperm for intracytoplasmic sperm injection
| Study | Source of sperm | Strategy for immobilization of sperm | Target regions for immobilization | Intensity of laser | Duration of exposure | No. oocytes fertilized/Total MII oocytes) | No. good-quality embryos/Total MII oocytes | No. pregnancies | No. live births | Main findings | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Montag et al. (2000) | Fresh ejaculated sperm | Single or double laser shot | The middle region of the sperm tail | 0.25 mJ, 0.5 mJ, 1.0 mJ, 2.0 mJ, 2.5 mJ, 3.0 mJ | NA | NA | NA | NA | NA | Laser can be used for immobilization and membrane permeabilization of the sperm tail. | [102] |
| Ebner et al. (2001) | Fresh ejaculated sperm | Double laser shot | The middle and end regions of the sperm tail | 1.5 mJ, 1.0 mJ | 15 ms, 10 ms | 174/262 (66.4%) | 58/262 (22.1%) | NA | NA | There were no significant differences in rates of fertilization, early cleavage, or blastocyst formation between laser-assisted immobilization and mechanical immobilization groups. | [100] |
| Eroglu et al. 2002 | Fresh ejaculated sperm | NA | The middle region of the sperm tail | NA | NA | 13/78 (17%) | NA | NA | NA | Laser-assisted immobilization of sperm may be useful in ICSI procedures. | [101] |
| Ebner et al. (2002) | Fresh ejaculated sperm | Double laser shot irradiations | The middle and end regions of the sperm tail | 1.5 mJ, 1.0 mJ | 15 ms, 10 ms | 3/4 (75%) | NA | 1 | 1 | Successful birth was achieved after transfer of embryos derived from laser-immobilized sperm. | [99] |
| Debrock et al. (2003) | Fresh ejaculated sperm | Double laser shot | Aside of the middle of the sperm tail followed by a second shot directly to the middle of the sperm tail | NA | NA | 89/179 (49.7%) | NA | 3/10 (30%) | 2/10 (20%) | No difference was observed in fertilization rate, cleavage, or blastocyst formation when oocytes were injected with sperm immobilized with laser compared to sperm immobilized with mechanical method. | [98] |
| Li et al. (2004) | Fresh sperm from testicular biopsies | Single laser shot | Behind the middle section of the sperm tail | NA | 1 ms | 68/95 (71.6%) | 51/95 (53.7%) | NA | NA | There were no significant differences in rates of fertilization, cleavage, and good-quality embryos between laser immobilization and mechanical immobilization groups. | [104] |
| Xu et al. (2007) | Fresh ejaculated sperm | Single laser shot | Behind the middle section of the sperm tail | NA | 0.45 ms | NA | NA | NA | NA | Laser-assisted sperm immobilization did not cause direct damage to the sperm DNA. | [103] |
| Chan et al. (2017) | Fresh ejaculated sperm | NA |
Head and midpiece region; principal piece Region; end piece region |
NA | NA | NA | NA | NA | NA | Laser-assisted immobilization did not result in any external membrane damages besides morphological changes at SEM level. | [97] |
NA: not available; SEM: scanning electronic microscopy; ICSI: intracytoplasmic sperm injection
The first study of 1.48 μm wavelength laser-assisted immobilization of human sperm for ICSI was reported in the early 2000s. Montag et al. evaluated two strategies for the immobilization of human sperm using a laser: a single laser shot and a double shot. After injecting human laser-assisted immobilized sperm, mouse oocytes were successfully activated and formed pronuclei [102]. A prospective self-controlled study by Ebner et al. found no significant differences in the rates of fertilization, early cleavage, or blastocyst formation between laser-assisted immobilization and mechanical immobilization groups [100]. These results were confirmed by the data from two studies by Debrock et al. [98] and Li et al. [104] reporting no difference in fertilization rate, cleavage, or blastocyst formation of oocytes injected with sperm that were immobilized with laser compared to sperm immobilized with the mechanical method. Ebner et al. first reported a live birth achieved with sperm that were immobilized by using two successive laser shots [99]. Xu et al. studied the effect of laser-assisted immobilization on sperm DNA and demonstrated that sperm immobilization by laser did not cause direct damage to the sperm DNA [103]. Chan DYL and Li TC explored the impact of the different methods of immobilization on the external morphology and function of sperm at the scanning electronic microscopy (SEM) level [97]. The results showed that laser-assisted immobilization did not lead to any observable external membrane damage besides distinctive morphological changes at the SEM level.
In short, laser-assisted immobilization of sperm prior to ICSI is a potentially useful alternative to the conventional mechanical method. Laser-assisted immobilization is effective in achieving successful fertilization and does not harm sperm DNA. More research is needed to focus on the relevance and safety of clinical outcomes.
Safety considerations for clinical application
In the past 3 decades, technological advances and the use of lasers in the assisted reproductive technology laboratory have developed rapidly. Safety has always been an important aspect of any laser application in the in vitro manipulation of human sperm. Most concerns focus on the effects on sperm DNA integrity, a basic factor for fertilization and transmission of genomic information from the father to the offspring [105, 106].
The wavelength, pulse duration, and energy density are all factors that must be taken into consideration when evaluating the safety of lasers [10, 107]. The emitted wavelength of a laser usually covers the entire light spectrum from infrared to ultraviolet (UV). UV lasers have been reported to induce potential genetic damage to gametes, which is believed to be the result of UV photoabsorption by DNA and RNA, as well as photooxidation reactions [63, 108]. Consequently, most lasers that are used in the ART laboratory generate light within the visible (400–760 nm) and NIR (760-1,400 nm) ranges. To minimize the damage caused by thermal effects, the ideal pulse duration should be lower than the thermal relaxation time of the target chromophore [109]. The pulse durations employed in ART vary from 20 ms down to the femtosecond level [109]. The appropriate energy density, delivered by the laser beam, needs to be determined based on their intended use.
For PBM therapy on spermatozoa, low-level laser light is applied to trigger sperm cell biostimulation, which leads to photochemical and photophysical reactions and avoids heating or thermal effects [110]. Several studies have demonstrated that low-level red light exposure does not induce a statistically significant effect on DNA damage [19–21, 24–26, 28]. In contrast, investigating the sensitivity of human sperm to NIR radiation showed that exposure to NIR for 15 min severely damaged sperm membrane permeability and function and led to reduced sperm viability [23]. The authors found significant increases in the percentages of disrupted DNA-protamine toroid assembly and sperm with chromatin dispersion after exposure to NIR, suggesting a profound detrimental effect on DNA integrity when exposed to NIR radiation [23]. Research on PBM therapy on human sperm has made significant progress, but there is still a distance from clinical application. Further studies will be necessary to exclude potential genotoxic effects on sperm and to clarify the suitability of PBM therapy for clinical application.
A further expansion in laser-assisted immobilization of sperm was the development of a technique for the use of lasers for the selection of viable but immotile sperm. Both energy levels of the laser pulse are slightly different. A single shot of 120–400 µJ is sufficient to detect viable but immotile sperm [80, 83, 87–89]. For the immobilization of sperm using a double shot strategy, due to splitting up energy into two separate pulses, the total energy applied to the sperm should be higher compared to the selection of immotile sperm. However, both applications of laser shots are aimed near the end of the sperm, which is far from the sperm head containing the genetic material. Xu et al. did not find any direct damage to sperm DNA when a laser was applied to immobilize sperm, even though the author used two methods to detect sperm DNA damage [103]. A study by Chan DYL and Li TC showed that laser-assisted immobilization did not result in any external membrane damage at the scanning electronic microscopy level [97].
To summarize, there are now increasingly more available laser technologies for the in vitro manipulation of human sperm. In all cases described thus far, the application of laser technologies has been proven to be useful and effective. Proper laser selection for indication and treatment requires a deep understanding of the laser principles and sperm structure and function. This will help to reduce the risk of damage to sperm DNA and allow researchers to obtain better results.
Conclusions
The laser system is now being applied across ART laboratories and provides a promising tool for the diagnosis and treatment of subfertility and male factor infertility. Some of the laser technologies described in this review are already mature and routinely applied in daily work. Studies have confirmed that laser-assisted operation in the selection of immotile sperm and sperm immobilization did not appear to increase the risk of adverse neonatal outcomes. However, some laser technologies, such as laser tweezer and PBM therapy, have still only had proof-of-principle demonstrations. The research data are not yet abundant. Although the use of lasers in the manipulation of human sperm offers several benefits. However, there are several drawbacks and challenges that will be faced.
Standardization is lacking. In laser-based sperm manipulation research, laser parameters should be united including wavelength, energy density, and exposure time. Standardized protocols and methodologies are needed for various sperm conditions, along with safety assessments. Standardized reporting guidelines should be adopted for detailing laser parameters, samples, and outcomes. Without standardization, comparison of results and determination of optimal settings may be hindered. Therefore, developing standardization for laser-based sperm manipulation should be a priority in future research efforts to enhance consistency.
Safety assessment is limited. Considering the potential damaging effects of different laser procedures and treatments described in this review on sperm DNA integrity, many critical treatment phases may potentially affect epigenetic, genetic, or chromosomal errors during embryo development. Thus, the ideal paradigm for introducing a new approach would be first to experiment on animals and then research should be conducted on donated human gametes for appropriate risk assessment. Additionally, sufficiently powered randomized controlled trials (RCTs) need to demonstrate the clinical benefit and safety of the different laser approaches, where appropriate, follow-up of newborns.
This review provides current knowledge on some laser techniques applied in the manipulation of human sperm. Undoubtedly, this field seems very promising because the techniques are new and beneficial. Meanwhile, there are still some challenges that need to be faced because the technologies encompass sophisticated equipment and complex experimental procedures. Therefore, a deeper comprehension of the mechanism of laser action on sperm cells is required for the optimization of the protocols in clinical practice, and the refinements based on evidence-based medicine are also a priority for future research.
Acknowledgements
Not applicable.
Abbreviations
- ART
Assisted reproductive technology
- ICSI
Intracytoplasmic sperm injection
- PGT
Preimplantation genetic testing
- PBM
Photobiomodulation
- LLLT
Low-level laser therapy
- NIR
Near-infrared
- GaAlAs
Gallium aluminum arsenide
- InGaAlP
Indium gallium aluminum phosphide
- DFI
DNA fragmentation index
- CcO
Cytochrome c oxidase
- ROS
Reactive oxygen species
- EPR
Electron paramagnetic resonance
- EGFR
Epidermal growth factor receptor
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- Nd:YAG
Neodymium: Yttrium-Aluminum-Garnet
- PCD
Primary cilia dyskinesia
- PVP
Polyvinylpyrrolidone
- SEM
Scanning electronic microscopy
- UV
Ultraviolet
- WHO
World Health Organization
- HOST
Hypo-osmotic swelling test
Authors’ contributions
Y. M. X. and K. L. collected the literature and drafted the original manuscript. Y. P. X. and X. H. C. discussed and revised the manuscript. Y. M. X. and K. L. designed, revised, and edited the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (The year 2018), and the Zhejiang Provincial Natural Science Foundation (LY22H040011).
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schneckenburger H. Laser application in Life sciences. Int J Mol Sci. 2023; 24. [DOI] [PMC free article] [PubMed]
- 2.Nappi L, Sorrentino F, Angioni S, Pontis A, Greco P. The use of laser in hysteroscopic Surgery. Minerva Ginecol. 2016;68:722–6. [PubMed] [Google Scholar]
- 3.Kaplan I, Goldman J, Ger R. The treatment of erosions of the uterine cervix by means of the CO 2 laser. Obstet Gynecol. 1973;41:795–6. [PubMed] [Google Scholar]
- 4.Wheeler JM. Clinical research of lasers in gynecology. Aspects of study design and statistical analysis. Obstet Gynecol Clin North Am. 1991;18:667–76. [PubMed] [Google Scholar]
- 5.Phillips C, Hillard T, Salvatore S, Toozs-Hobson P, Cardozo L. Lasers in gynaecology. Eur J Obstet Gynecol Reprod Biol. 2020;251:146–55. doi: 10.1016/j.ejogrb.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Karu TI. Lasers in infertility treatment: irradiation of oocytes and spermatozoa. Photomed Laser Surg. 2012;30:239–41. doi: 10.1089/pho.2012.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutton C. Lasers in infertility. Hum Reprod. 1993;8:133–46. doi: 10.1093/oxfordjournals.humrep.a137863. [DOI] [PubMed] [Google Scholar]
- 8.Daniell JF. The role of lasers in infertility Surgery. Fertil Steril. 1984;42:815–23. doi: 10.1016/s0015-0282(16)48249-x. [DOI] [PubMed] [Google Scholar]
- 9.Ebner T, Moser M, Tews G. Possible applications of a non-contact 1.48 microm wavelength diode laser in assisted reproduction technologies. Hum Reprod Update. 2005;11:425–35. doi: 10.1093/humupd/dmi009. [DOI] [PubMed] [Google Scholar]
- 10.Davidson LM, Liu Y, Griffiths T, Jones C, Coward K. Laser technology in the ART laboratory: a narrative review. Reprod Biomed Online. 2019;38:725–39. doi: 10.1016/j.rbmo.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Schopper B, Ludwig M, Edenfeld J, Al-Hasani S, Diedrich K. Possible applications of lasers in assisted reproductive technologies. Hum Reprod. 1999;14(Suppl 1):186–93. doi: 10.1093/humrep/14.suppl_1.186. [DOI] [PubMed] [Google Scholar]
- 12.Sato H, Landthaler M, Haina D, Schill WB. The effects of laser light on sperm motility and velocity in vitro. Andrologia. 1984;16:23–5. doi: 10.1111/j.1439-0272.1984.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 13.Tadir Y, Wright WH, Vafa O, Ord T, Asch RH, Berns MW. Micromanipulation of sperm by a laser generated optical trap. Fertil Steril. 1989;52:870–3. [PubMed] [Google Scholar]
- 14.Tadir Y, Wright WH, Vafa O, Ord T, Asch RH, Berns MW. Force generated by human sperm correlated to velocity and determined using a laser generated optical trap. Fertil Steril. 1990;53:944–7. doi: 10.1016/s0015-0282(16)53539-0. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Sonek GJ, Berns MW, Tromberg BJ. Physiological monitoring of optically trapped cells: assessing the effects of confinement by 1064-nm laser tweezers using microfluorometry. Biophys J. 1996;71:2158–67. doi: 10.1016/S0006-3495(96)79417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germond M, Nocera D, Senn A, Rink K, Delacretaz G, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48-microns diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–11. doi: 10.1016/s0015-0282(16)57800-5. [DOI] [PubMed] [Google Scholar]
- 17.Rink K, Delacretaz G, Salathe RP, Senn A, Nocera D, Germond M, et al. Non-contact microdrilling of mouse zona pellucida with an objective-delivered 1.48-microns diode laser. Lasers Surg Med. 1996;18:52–62. doi: 10.1002/(SICI)1096-9101(1996)18:1<52::AID-LSM7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Tsai SR, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B. 2017;170:197–207. doi: 10.1016/j.jphotobiol.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preece D, Chow KW, Gomez-Godinez V, Gustafson K, Esener S, Ravida N, et al. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci Rep. 2017;7:46480. doi: 10.1038/srep46480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabel CP, Carroll J, Harrison K. Sperm motility is enhanced by low level laser and light emitting diode photobiomodulation with a dose-dependent response and differential effects in fresh and frozen samples. Laser Ther. 2018;27:131–6. doi: 10.5978/islsm.18-OR-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safian F, Ghaffari Novin M, Karimi M, Kazemi M, Zare F, Ghoreishi SK, et al. Photobiomodulation with 810 nm wavelengths improves human sperms’ motility and viability in Vitro. Photobiomodul Photomed Laser Surg. 2020;38:222–31. doi: 10.1089/photob.2019.4773. [DOI] [PubMed] [Google Scholar]
- 22.Lenzi A, Claroni F, Gandini L, Lombardo F, Barbieri C, Lino A, et al. Laser radiation and motility patterns of human sperm. Arch Androl. 1989;23:229–34. doi: 10.3109/01485018908986845. [DOI] [PubMed] [Google Scholar]
- 23.Highland H, Rajput N, Sharma R, George LB. Differential sensitivity of the human sperm cell to near infrared radiation. J Photochem Photobiol B. 2018;183:119–26. doi: 10.1016/j.jphotobiol.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Espey BT, Kielwein K, van der Ven H, Steger K, Allam JP, Paradowska-Dogan A, et al. Effects of pulsed-Wave Photobiomodulation Therapy on Human Spermatozoa. Lasers Surg Med. 2022;54:540–53. doi: 10.1002/lsm.23399. [DOI] [PubMed] [Google Scholar]
- 25.Salman Yazdi R, Bakhshi S, Jannat Alipoor F, Akhoond MR, Borhani S, Farrahi F, et al. Effect of 830-nm diode laser irradiation on human sperm motility. Lasers Med Sci. 2014;29:97–104. doi: 10.1007/s10103-013-1276-7. [DOI] [PubMed] [Google Scholar]
- 26.Firestone RS, Esfandiari N, Moskovtsev SI, Burstein E, Videna GT, Librach C, et al. The effects of low-level laser light exposure on sperm motion characteristics and DNA damage. J Androl. 2012;33:469–73. doi: 10.2164/jandrol.111.013458. [DOI] [PubMed] [Google Scholar]
- 27.Safian F, Ghaffari Novin M, Nazarian H, Shams Mofarahe Z, Abdollahifar MA, Jajarmi V, et al. Photobiomodulation preconditioned human semen protects sperm cells against detrimental effects of cryopreservation. Cryobiology. 2021;98:239–44. doi: 10.1016/j.cryobiol.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Safian F, Bayat M, Jajarmi V, Abdollahifar MA, Nazarian H, Mofarahe ZS, et al. Comparative effect of Photobiomodulation on human semen samples pre- and post-cryopreservation. Reprod Sci. 2022;29:1463–70. doi: 10.1007/s43032-021-00805-x. [DOI] [PubMed] [Google Scholar]
- 29.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5:58–62. [PMC free article] [PubMed] [Google Scholar]
- 30.Zupin L, Pascolo L, Luppi S, Ottaviani G, Crovella S, Ricci G. Photobiomodulation therapy for male infertility. Lasers Med Sci. 2020;35:1671–80. doi: 10.1007/s10103-020-03042-x. [DOI] [PubMed] [Google Scholar]
- 31.Singer R, Sagiv M, Barnet M, Levinsky H, Segenreich E, Fuchs Y, et al. Low energy narrow band non-coherent infrared illumination of human semen and isolated sperm. Andrologia. 1991;23:181–4. doi: 10.1111/j.1439-0272.1991.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 32.Jimenez T, Sanchez G, Blanco G. Activity of the Na,K-ATPase alpha4 isoform is regulated during sperm capacitation to support sperm motility. J Androl. 2012;33:1047–57. doi: 10.2164/jandrol.111.015545. [DOI] [PubMed] [Google Scholar]
- 33.Syeda SS, Sanchez G, McDermott JP, Hong KH, Blanco G, Georg GI. The na + and K + transport system of sperm (ATP1A4) is essential for male fertility and an attractive target for male contraceptiondagger. Biol Reprod. 2020;103:343–56. doi: 10.1093/biolre/ioaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellezza I, Minelli A. Adenosine in sperm physiology. Mol Aspects Med. 2017;55:102–9. doi: 10.1016/j.mam.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Boisen IM, Rehfeld A, Mos I, Poulsen NN, Nielsen JE, Schwarz P, et al. The calcium-sensing receptor is essential for calcium and bicarbonate sensitivity in human spermatozoa. J Clin Endocrinol Metab. 2021;106:e1775–92. doi: 10.1210/clinem/dgaa937. [DOI] [PubMed] [Google Scholar]
- 36.Di Benedetto G, Pendin D, Greotti E, Pizzo P, Pozzan T. Ca2 + and cAMP cross-talk in mitochondria. J Physiol. 2014;592:305–12. doi: 10.1113/jphysiol.2013.259135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahar S, Wiser A, Ickowicz D, Lubart R, Shulman A, Breitbart H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum Reprod. 2011;26:2274–82. doi: 10.1093/humrep/der232. [DOI] [PubMed] [Google Scholar]
- 38.Shahar S, Hillman P, Lubart R, Ickowicz D, Breitbart H. Activation of sperm EGFR by light irradiation is mediated by reactive oxygen species. Photochem Photobiol. 2014;90:1077–83. doi: 10.1111/php.12281. [DOI] [PubMed] [Google Scholar]
- 39.Lavi R, Shainberg A, Shneyvays V, Hochauser E, Isaac A, Zinman T, et al. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg Med. 2010;42:473–80. doi: 10.1002/lsm.20919. [DOI] [PubMed] [Google Scholar]
- 40.Brown GC. Cell biology. NO says yes to mitochondria. Science. 2003;299:838–9. doi: 10.1126/science.1082028. [DOI] [PubMed] [Google Scholar]
- 41.Ankri R, Friedman H, Savion N, Kotev-Emeth S, Breitbart H, Lubart R. Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg Med. 2010;42:348–52. doi: 10.1002/lsm.20849. [DOI] [PubMed] [Google Scholar]
- 42.Cohen N, Lubart R, Rubinstein S, Breitbart H. Light irradiation of mouse spermatozoa: stimulation of in vitro fertilization and calcium signals. Photochem Photobiol. 1998;68:407–13. [PubMed] [Google Scholar]
- 43.Zhu Q, Yu W, Yang X, Hicks GL, Lanzafame RJ, Wang T. Photo-irradiation improved functional preservation of the isolated rat heart. Lasers Surg Med. 1997;20:332–9. doi: 10.1002/(sici)1096-9101(1997)20:3<332::aid-lsm12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, He Q, Yan X, Cai Y, Chen J. Effect of exogenous nitric oxide on sperm motility in vitro. Biol Res. 2014;47:44. doi: 10.1186/0717-6287-47-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadlec M, Ros-Santaella JL, Pintus E. The roles of NO and H(2)S in sperm Biology: recent advances and new perspectives. Int J Mol Sci. 2020; 21. [DOI] [PMC free article] [PubMed]
- 46.Blanco-Prieto O, Catalan J, Trujillo-Rojas L, Pena A, Rivera Del Alamo MM, Llavanera M et al. Red LED Light acts on the mitochondrial Electron chain of mammalian sperm via light-time exposure-dependent mechanisms. Cells. 2020; 9. [DOI] [PMC free article] [PubMed]
- 47.Rodriguez-Gil JE. Photostimulation and Thermotaxis of sperm: overview and practical implications in porcine reproduction. Theriogenology. 2019;137:8–14. doi: 10.1016/j.theriogenology.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, Kiss V, et al. Involvement of opsins in mammalian sperm thermotaxis. Sci Rep. 2015;5:16146. doi: 10.1038/srep16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poorhassan M, Gholaminejhad M, Ahmadi H, Mehboudi L, Chahar Kameh M, Pirani M, et al. Preclinical and clinical applications of Photobiomodulation Therapy in sperm motility: a narrative review. J Lasers Med Sci. 2022;13:e75. doi: 10.34172/jlms.2022.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ban Frangez H, Frangez I, Verdenik I, Jansa V, Virant Klun I. Photobiomodulation with light-emitting diodes improves sperm motility in men with asthenozoospermia. Lasers Med Sci. 2015;30:235–40. doi: 10.1007/s10103-014-1653-x. [DOI] [PubMed] [Google Scholar]
- 51.Ziaeipour S, Norouzian M, Abbaszadeh HA, Aliaghaei A, Nazarian H, Karamian A, et al. Photobiomodulation therapy reverses spermatogenesis arrest in hyperthermia-induced azoospermia mouse model. Lasers Med Sci. 2023;38:114. doi: 10.1007/s10103-023-03780-8. [DOI] [PubMed] [Google Scholar]
- 52.Hasani A, Khosravi A, Rahimi K, Afshar A, Fadaei-Fathabadi F, Raoofi A, et al. Photobiomodulation restores spermatogenesis in the transient scrotal hyperthermia-induced mice. Life Sci. 2020;254:117767. doi: 10.1016/j.lfs.2020.117767. [DOI] [PubMed] [Google Scholar]
- 53.Yeste M, Codony F, Estrada E, Lleonart M, Balasch S, Pena A, et al. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci Rep. 2016;6:22569. doi: 10.1038/srep22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zan-Bar T, Bartoov B, Segal R, Yehuda R, Lavi R, Lubart R, et al. Influence of visible light and ultraviolet irradiation on motility and fertility of mammalian and fish sperm. Photomed Laser Surg. 2005;23:549–55. doi: 10.1089/pho.2005.23.549. [DOI] [PubMed] [Google Scholar]
- 55.Moffitt JR, Chemla YR, Smith SB, Bustamante C. Recent advances in optical tweezers. Annu Rev Biochem. 2008;77:205–28. doi: 10.1146/annurev.biochem.77.043007.090225. [DOI] [PubMed] [Google Scholar]
- 56.Choudhary D, Mossa A, Jadhav M, Cecconi C. Bio-molecular applications of recent developments in Optical tweezers. Biomolecules. 2019; 9. [DOI] [PMC free article] [PubMed]
- 57.Ashkin A, Dziedzic JM, Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987;330:769–71. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- 58.Berns MW, Wright WH, Tromberg BJ, Profeta GA, Andrews JJ, Walter RJ. Use of a laser-induced optical force trap to study chromosome movement on the mitotic spindle. Proc Natl Acad Sci U S A. 1989;86:4539–43. doi: 10.1073/pnas.86.12.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi LZ, Nascimento J, Botvinick E, Durrant B, Berns MW. An interdisciplinary systems approach to study sperm physiology and evolution. Wiley Interdiscip Rev Syst Biol Med. 2011;3:36–47. doi: 10.1002/wsbm.106. [DOI] [PubMed] [Google Scholar]
- 60.Colon JM, Sarosi P, McGovern PG, Askin A, Dziedzic JM, Skurnick J, et al. Controlled micromanipulation of human sperm in three dimensions with an infrared laser optical trap: effect on sperm velocity. Fertil Steril. 1992;57:695–8. doi: 10.1016/s0015-0282(16)54926-7. [DOI] [PubMed] [Google Scholar]
- 61.Westphal LM, el Dansasouri I, Shimizu S, Tadir Y, Berns MW. Exposure of human spermatozoa to the cumulus oophorus results in increased relative force as measured by a 760 nm laser optical trap. Hum Reprod. 1993;8:1083–6. doi: 10.1093/oxfordjournals.humrep.a138197. [DOI] [PubMed] [Google Scholar]
- 62.Dantas ZN, Araujo E, Jr, Tadir Y, Berns MW, Schell MJ, Stone SC. Effect of freezing on the relative Escape force of sperm as measured by a laser optical trap. Fertil Steril. 1995;63:185–8. [PubMed] [Google Scholar]
- 63.Konig K, Tadir Y, Patrizio P, Berns MW, Tromberg BJ. Effects of ultraviolet exposure and near infrared laser tweezers on human spermatozoa. Hum Reprod. 1996;11:2162–4. doi: 10.1093/oxfordjournals.humrep.a019069. [DOI] [PubMed] [Google Scholar]
- 64.Patrizio P, Liu Y, Sonek GJ, Berns MW, Tadir Y. Effect of pentoxifylline on the intrinsic swimming forces of human sperm assessed by optical tweezers. J Androl. 2000;21:753–6. [PubMed] [Google Scholar]
- 65.Shao B, Shi LZ, Nascimento JM, Botvinick EL, Ozkan M, Berns MW, et al. High-throughput sorting and analysis of human sperm with a ring-shaped laser trap. Biomed Microdevices. 2007;9:361–9. doi: 10.1007/s10544-006-9041-3. [DOI] [PubMed] [Google Scholar]
- 66.Nascimento JM, Shi LZ, Meyers S, Gagneux P, Loskutoff NM, Botvinick EL, et al. The use of optical tweezers to study sperm competition and motility in primates. J R Soc Interface. 2008;5:297–302. doi: 10.1098/rsif.2007.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi L, Shao B, Chen T, Berns M. Automatic annular laser trapping: a system for high-throughput sperm analysis and sorting. J Biophotonics. 2009;2:167–77. doi: 10.1002/jbio.200810053. [DOI] [PubMed] [Google Scholar]
- 68.Hyun N, Chandsawangbhuwana C, Zhu Q, Shi LZ, Yang-Wong C, Berns MW. Effects of viscosity on sperm motility studied with optical tweezers. J Biomed Opt. 2012;17:025005. doi: 10.1117/1.JBO.17.2.025005. [DOI] [PubMed] [Google Scholar]
- 69.Auka N, Valle M, Cox BD, Wilkerson PD, Dawson Cruz T, Reiner JE, et al. Optical tweezers as an effective tool for spermatozoa isolation from mixed forensic samples. PLoS ONE. 2019;14:e0211810. doi: 10.1371/journal.pone.0211810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong Z, Zhang C, Liu R, He J, Yang H, Cheng Z, et al. Chirality and frequency measurement of longitudinal rolling of human sperm using optical trap. Front Bioeng Biotechnol. 2022;10:1028857. doi: 10.3389/fbioe.2022.1028857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Araujo E, Jr, Tadir Y, Patrizio P, Ord T, Silber S, Berns MW, et al. Relative force of human epididymal sperm. Fertil Steril. 1994;62:585–90. [PubMed] [Google Scholar]
- 72.Chow KW, Preece D, Berns MW. Effect of red light on optically trapped spermatozoa. Biomed Opt Express. 2017;8:4200–5. doi: 10.1364/BOE.8.004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Oliveira NM, Vaca Sanchez R, Rodriguez Fiesta S, Lopez Salgado T, Rodriguez R, Bethencourt JC, et al. Pregnancy with frozen-thawed and fresh testicular biopsy after motile and immotile sperm microinjection, using the mechanical touch technique to assess viability. Hum Reprod. 2004;19:262–5. doi: 10.1093/humrep/deh083. [DOI] [PubMed] [Google Scholar]
- 74.Baldini D, Ferri D, Baldini GM, Lot D, Catino A, Vizziello D, et al. Sperm selection for ICSI: do we have a winner? Cells. 2021. 10. [DOI] [PMC free article] [PubMed]
- 75.Mangoli V, Mangoli R, Dandekar S, Suri K, Desai S. Selection of viable spermatozoa from testicular biopsies: a comparative study between pentoxifylline and hypoosmotic swelling test. Fertil Steril. 2011;95:631–4. doi: 10.1016/j.fertnstert.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl. 2006;27:45–52. doi: 10.2164/jandrol.05079. [DOI] [PubMed] [Google Scholar]
- 77.Ibis E, Hayme S, Baysal E, Gul N, Ozkavukcu S. Efficacy and safety of papaverine as an in vitro motility enhancer on human spermatozoa. J Assist Reprod Genet. 2021;38:1523–37. doi: 10.1007/s10815-021-02160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ortega C, Verheyen G, Raick D, Camus M, Devroey P, Tournaye H. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17:684–92. doi: 10.1093/humupd/dmr018. [DOI] [PubMed] [Google Scholar]
- 79.Nordhoff V. How to select immotile but viable spermatozoa on the day of intracytoplasmic sperm injection? An embryologist’s view. Andrology. 2015;3:156–62. doi: 10.1111/andr.286. [DOI] [PubMed] [Google Scholar]
- 80.Aktan TM, Montag M, Duman S, Gorkemli H, Rink K, Yurdakul T. Use of a laser to detect viable but immotile spermatozoa. Andrologia. 2004;36:366–9. doi: 10.1111/j.1439-0272.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 81.Rubino P, Vigano P, Luddi A, Piomboni P. The ICSI procedure from past to future: a systematic review of the more controversial aspects. Hum Reprod Update. 2016;22:194–227. doi: 10.1093/humupd/dmv050. [DOI] [PubMed] [Google Scholar]
- 82.Simopoulou M, Gkoles L, Bakas P, Giannelou P, Kalampokas T, Pantos K, et al. Improving ICSI: a review from the spermatozoon perspective. Syst Biol Reprod Med. 2016;62:359–71. doi: 10.1080/19396368.2016.1229365. [DOI] [PubMed] [Google Scholar]
- 83.Gerber PA, Kruse R, Hirchenhain J, Krussel JS, Neumann NJ. Pregnancy after laser-assisted selection of viable spermatozoa before intracytoplasmatic sperm injection in a couple with male primary cilia dyskinesia. Fertil Steril. 2008;89:1826e1829–1812. doi: 10.1016/j.fertnstert.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Ozkavukcu S, Celik-Ozenci C, Konuk E, Atabekoglu C. Live birth after laser assisted viability Assessment (LAVA) to detect pentoxifylline resistant ejaculated immotile spermatozoa during ICSI in a couple with male Kartagener’s syndrome. Reprod Biol Endocrinol. 2018;16:10. doi: 10.1186/s12958-018-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nordhoff V, Schuring AN, Krallmann C, Zitzmann M, Schlatt S, Kiesel L, et al. Optimizing TESE-ICSI by laser-assisted selection of immotile spermatozoa and polarization microscopy for selection of oocytes. Andrology. 2013;1:67–74. doi: 10.1111/j.2047-2927.2012.00020.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen HH, Feng GX, Zhang B, Shu JH, Gan XY, Zhou H, et al. [Successful pregnancy following laser-assisted selection of viable but immotile spermatozoa for intracytoplasmic sperm injection: a report of 2 cases] Zhonghua Nan Ke Xue. 2015;21:988–91. [PubMed] [Google Scholar]
- 87.Chen H, Feng G, Zhang B, Zhou H, Shu J, Gan X. A successful pregnancy using completely immotile but viable frozen-thawed spermatozoa selected by laser. Clin Exp Reprod Med. 2017;44:52–5. doi: 10.5653/cerm.2017.44.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H, Feng G, Zhang B, Zhou H, Wang C, Shu J, et al. A new insight into male fertility preservation for patients with completely immotile spermatozoa. Reprod Biol Endocrinol. 2017;15:74. doi: 10.1186/s12958-017-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Wang C, Zhou H, Shu J, Gan X, Xu K, et al. Laser-assisted selection of immotile spermatozoa has no effect on obstetric and neonatal outcomes of TESA-ICSI pregnancies. Reprod Biol Endocrinol. 2021;19:159. doi: 10.1186/s12958-021-00835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanderzwalmen P, Bertin G, Lejeune B, Nijs M, Vandamme B, Schoysman R. Two essential steps for a successful intracytoplasmic sperm injection: injection of immobilized spermatozoa after rupture of the oolema. Hum Reprod. 1996;11:540–7. doi: 10.1093/humrep/11.3.540. [DOI] [PubMed] [Google Scholar]
- 91.Yanagida K, Katayose H, Hirata S, Yazawa H, Hayashi S, Sato A. Influence of sperm immobilization on onset of ca(2+) oscillations after ICSI. Hum Reprod. 2001;16:148–52. doi: 10.1093/humrep/16.1.148. [DOI] [PubMed] [Google Scholar]
- 92.Dozortsev D, Rybouchkin A, De Sutter P, Dhont M. Sperm plasma membrane damage prior to intracytoplasmic sperm injection: a necessary condition for sperm nucleus decondensation. Hum Reprod. 1995;10:2960–4. doi: 10.1093/oxfordjournals.humrep.a135829. [DOI] [PubMed] [Google Scholar]
- 93.Fishel S, Lisi F, Rinaldi L, Green S, Hunter A, Dowell K, et al. Systematic examination of immobilizing spermatozoa before intracytoplasmic sperm injection in the human. Hum Reprod. 1995;10:497–500. doi: 10.1093/oxfordjournals.humrep.a135974. [DOI] [PubMed] [Google Scholar]
- 94.Gerris J, Mangelschots K, Van Royen E, Joostens M, Eestermans W, Ryckaert G. ICSI and severe male-factor infertility: breaking the sperm tail prior to injection. Hum Reprod. 1995;10:484–6. doi: 10.1093/oxfordjournals.humrep.a135968. [DOI] [PubMed] [Google Scholar]
- 95.Palermo G, Joris H, Derde MP, Camus M, Devroey P, Van Steirteghem A. Sperm characteristics and outcome of human assisted fertilization by subzonal insemination and intracytoplasmic sperm injection. Fertil Steril. 1993;59:826–35. doi: 10.1016/s0015-0282(16)55867-1. [DOI] [PubMed] [Google Scholar]
- 96.Palermo GD, Cohen J, Alikani M, Adler A, Rosenwaks Z. Intracytoplasmic sperm injection: a novel treatment for all forms of male factor infertility. Fertil Steril. 1995;63:1231–40. doi: 10.1016/s0015-0282(16)57603-1. [DOI] [PubMed] [Google Scholar]
- 97.Chan DYL, Li TC. Comparison of the external physical damages between laser-assisted and mechanical immobilized human sperm using scanning electronic microscopy. PLoS ONE. 2017;12:e0188504. doi: 10.1371/journal.pone.0188504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Debrock S, Spiessens C, Afschrift H, Van der Auwera I, D’Hooghe TM. Application of the fertilase-laser system versus the conventional mechanical method to immobilize spermatozoa for intracytoplasmic sperm injection. A randomized controlled trial. Gynecol Obstet Invest. 2003;56:102–5. doi: 10.1159/000072995. [DOI] [PubMed] [Google Scholar]
- 99.Ebner T, Moser M, Yaman C, Sommergruber M, Tews G. Successful birth after laser assisted immobilization of spermatozoa before intracytoplasmic injection. Fertil Steril. 2002;78:417–8. doi: 10.1016/s0015-0282(02)03208-9. [DOI] [PubMed] [Google Scholar]
- 100.Ebner T, Yaman C, Moser M, Sommergruber M, Hartl J, Tews G. Laser assisted immobilization of spermatozoa prior to intracytoplasmic sperm injection in humans. Hum Reprod. 2001;16:2628–31. doi: 10.1093/humrep/16.12.2628. [DOI] [PubMed] [Google Scholar]
- 101.Eroglu A, Nahum RT, Isaacson K, Toth TL. Laser-assisted intracytoplasmic sperm injection in human oocytes. J Reprod Med. 2002;47:199–203. [PubMed] [Google Scholar]
- 102.Montag M, Rink K, Delacretaz G, van der Ven H. Laser-induced immobilization and plasma membrane permeabilization in human spermatozoa. Hum Reprod. 2000;15:846–52. doi: 10.1093/humrep/15.4.846. [DOI] [PubMed] [Google Scholar]
- 103.Xu ZP, Sun HX, Hu YL, Zhang NY, Zhao X. [Laser-assisted immobilization causes no direct damage to sperm DNA] Zhonghua Nan Ke Xue. 2007;13:216–8. [PubMed] [Google Scholar]
- 104.Li PX, Li SW, Peng EL, Li L, Ma QH, Huang ZY, et al. The application of laser assisted immobilization before intracytoplasmic sperm injection of testicular spermatozoa in human (in Chinese) Laser J. 2004;25:75–6. [Google Scholar]
- 105.Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol. 2017;6:761–S764. doi: 10.21037/tau.2017.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haddock L, Gordon S, Lewis SEM, Larsen P, Shehata A, Shehata H. Sperm DNA fragmentation is a novel biomarker for early pregnancy loss. Reprod Biomed Online. 2021;42:175–84. doi: 10.1016/j.rbmo.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 107.Ilina I, Sitnikov D. From Zygote to Blastocyst: Application of Ultrashort Lasers in the Field of Assisted Reproduction and Developmental Biology. Diagnostics (Basel). 2021; 11. [DOI] [PMC free article] [PubMed]
- 108.Richa, Sinha RP, Hader DP. Physiological aspects of UV-excitation of DNA. Top Curr Chem. 2015;356:203–48. doi: 10.1007/128_2014_531. [DOI] [PubMed] [Google Scholar]
- 109.Bogdan Allemann I, Kaufman J. Laser principles. Curr Probl Dermatol. 2011;42:7–23. doi: 10.1159/000328236. [DOI] [PubMed] [Google Scholar]
- 110.Plavskii VY, Barulin NV, Mikulich AV, Tretyakova AI, Ananich TS, Plavskaya LG, et al. Effect of continuous wave, quasi-continuous wave and pulsed laser radiation on functional characteristics of fish spermatozoa. J Photochem Photobiol B. 2021;216:112112. doi: 10.1016/j.jphotobiol.2020.112112. [DOI] [PubMed] [Google Scholar]
- 111.Ebner T, Tews G, Mayer RB, Ziehr S, Arzt W, Costamoling W, et al. Pharmacological stimulation of sperm motility in frozen and thawed testicular sperm using the dimethylxanthine theophylline. Fertil Steril. 2011;96:1331–6. doi: 10.1016/j.fertnstert.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 112.Tasdemir I, Tasdemir M, Tavukcuoglu S. Effect of pentoxifylline on immotile testicular spermatozoa. J Assist Reprod Genet. 1998;15:90–2. doi: 10.1007/BF02766832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, et al. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000;17:194–9. doi: 10.1023/A:1009435732258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Soares JB, Glina S, Antunes N, Jr, Wonchockier R, Galuppo AG, Mizrahi FE. Sperm tail flexibility test: a simple test for selecting viable spermatozoa for intracytoplasmic sperm injection from semen samples without motile spermatozoa. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:250–3. doi: 10.1590/s0041-87812003000500003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.