Abstract
Fifteen female rhesus monkeys (Macaca mulatta), ranging in age from 8 to 34 years, were studied for one year to characterize the endocrine and menstrual changes associated with menopause in this species. Five monkeys were premenopausal; these younger monkeys, ages 8–11 years, menstruated and showed cyclic ovarian activity during the 12-month study period, as evidenced by menses and periodic elevations of serum estradiol (E2) and luteinizing hormone (LH) concentrations. Four females, ages 24–26 years, were in transition to menopause. Two of these perimenopausal females menstruated and secreted E2 and LH in a periodic fashion; the other two females showed elevated LH concentrations, consistently low E2 levels, and no evidence of menstruation. Six females, ages 27–34 years, were clearly postmenopausal; LH concentrations were high, whereas E2 concentrations were uniformly low. There was a significant inverse correlation between basal E2 concentrations and age, and a significant positive correlation between age and LH concentrations across all 15 animals. Hormonal changes indicative of ovulation, when they occurred, were generally restricted to the winter and early spring months. Histological analysis of ovaries from four postmenopausal females revealed little or no evidence of active folliculogenesis. These data indicate that menopause in female rhesus monkeys does not occur until the second half of the third decade of life.
Keywords: Rhesus monkey, Macaca mulatta, menopause, estradiol, luteinizing hormone, aging
INTRODUCTION
In rhesus monkeys (Macaca mulatta) the endocrine correlates of reproductive events such as puberty [Foster, 1977; Rapsidara et al., 1983; Terasawa et al., 1984a,b; Terasawa, 1985], menstrual cyclicity [Neill et al., 1969; Johansson et al., 1968], pregnancy [Atkinson et al., 1975; Hodgen et al., 1975; Challis et al., 1980], and seasonal breeding [Wilson et al., 1982, 1983; Walker et al., 1983], have been well characterized. However, relatively little attention has been given to the occurrence of menopause in this species, in large part because of the paucity of aged female monkeys. In the 1970s, Van Wagenen first described menopause in rhesus monkeys [Van Wagenen, 1970, 1972]. Based on observation of menses in two monkeys, she concluded that the climacteric in this species occurred between 25 and 30 years of age. A later study by Hodgen et al. [1977] described the endocrine correlates of monkeys of uncertain age, but who were described as being at least 22 years of age. These monkeys exhibited much variability in the frequency and pattern of menstrual bleeding and endocrine secretions. Only four of the 17 monkeys studied showed elevated gonadotropin concentrations and basal estradiol (E2) and progesterone (P4) levels characteristic of postmenopausal human females [Sherman et al., 1976]. However, other animals appeared to ovulate, as characterized by a midcycle peak in luteinizing hormone (LH), follicle stimulating hormone (FSH), and E2, followed by a sustained elevation of P4. Further data from a single female rhesus monkey estimated to be more than 20 years of age depict an endocrine profile of elevated FSH and low E2 concentrations [Dierschke, 1985]. Taken together, these data illustrate the variability in endocrine function in a sample of pre-, peri-, and postmenopausal female monkeys.
One of the limitations of describing the occurrence and timing of reproductive events in a controlled laboratory environment is the natural occurrence of anovulatory cycles [Hartman, 1932; Van Wagenen, 1945; Keverne & Michael, 1970; Riesen et al., 1971] and ovulations with abnormal luteal phases [Daily & Neill, 1981; Hutz et al., 1985] during the summer months. Because rhesus monkeys breed seasonally when housed outdoors [Lindburg, 1971; Vandenbergh, 1973; Neville, 1978; Gordon, 1981; Walker et al., 1984], there appears to be some vestige of this seasonality in the laboratory. Consequently, abnormal gonadotropin secretion or failure to ovulate, particularly if studied during the non-breeding season, could be misleading. For example, Hodgen et al. [1977], collected daily endocrine samples from May until July, a period corresponding to both seasonal acyclicity in outdoor-housed females and cycle irregularities in indoor-housed females [Hartman, 1932; Van Wagenen, 1945; Keverne & Michael, 1970; Riesen et al., 1971]. By monitoring pituitary-ovarian function throughout the year, any seasonal changes in endocrine function can be assessed.
The present study sought to elucidate more fully the endocrine events associated with menopause in aged female rhesus monkeys. To dissociate the potential effects of seasonal acyclicity from those of the climacteric on endocrine function, these females were monitored weekly for 52 consecutive weeks. The results indicate that rhesus monkeys reach menopause at around 27 years of age, and the endocrine profile of climacteric is similar to that in humans.
METHODS
Subjects.
Fifteen female rhesus monkeys (Macaca rnulatta) served as subjects. Animals ranged in age from 8 to 34 years at the onset of the study. The animals were obtained from a number of outside sources and maintained as part of a colony of aged primates by the Neuropathology Laboratory at The Johns Hopkins University School of Medicine. Birth records were available for all animals except for three females who were obtained from the Wisconsin Regional Primate Research Center. These animals were feral-born, and their ages are estimated based on veterinary evaluation undertaken upon their arrival at the Wisconsin Center. The ages of the females are given in Table 1.
Table 1.
The Ages at the Beginning of the Study, the Origin of the Animals, the Occurrences of Menses (Yes/No) and Ovulations (Yes/No) in the 15 Females Throughout One Year
| Female | Age (years) | Origin | Menses (yes/no) | Inferred ovulation (yes/no) |
|---|---|---|---|---|
| 38X | 8 | Captivity | Yes | Yes |
| 33X | 8 | Captivity | Yes | Yes |
| 24X | 8 | Captivity | Yes | Yes |
| P25U | 11 | Captivity | Yes | Yes |
| N265U | 11 | Captivity | Yes | No (?) |
| 1419 | 24 | Feral | No | Yes |
| H97 | 24 | Captivity | No | Yes |
| 1422 | 24 | Feral | Yes | Yes |
| 4953 | 26 | Captivity | Yes | Yes |
| 4605 | 27 | Captivity | No | No |
| 4514 | 27 | Captivity | No | No |
| 4948 | 27 | Captivity | No | Yes |
| 816 | 29 | Captivity | No | No |
| 1089 | 30 | Feral | No | No |
| 735 | 34 | Captivity | No | No |
The ovaries from six monkeys were examined histologically. Ovaries were collected at autopsy from postmenopausal females 735, 1089, 4514, and 816 following the 12-month study period. The other ovaries were collected as part of routine post-mortem examination from a 7-year-old and a 9-year-old female who were not a part of the present endocrine study.
All animals were individually housed in cages measuring 1.0 × 1.5 × 1.5 m in a temperature- (23oC), light- (12:12, light:dark), and humidity- (50%) controlled room. Food (Purina Monkey Chow) and water were available ad libitum. The animals’ diet was supplemented weekly with fresh fruit.
Procedure.
Data collection began in January, 1991, and continued through December, 1991. Menstrual cycle data were obtained by monitoring the presence of vaginal bleeding. Caretakers observed and recorded the daily presence of blood in the pans beneath the animals’ cages. Weekly, the perineal area was closely examined by vaginal swab to detect menstrual bleeding. These markers provided a crude basis for determining the occurrence of menses.
Weekly blood samples were obtained in the afternoon between 1300 and 1500 h. Blood (5 cc) was drawn from the saphenous vein of unanesthetized animals in their home cages. The animals were trained to extend a leg for venipuncture, as described previously [Walker et al., 1982]. Blood samples were allowed to clot before centrifugation. Serum was harvested and stored at −70°C until assay.
Sera were analyzed for 17β-estradiol (E2) by radioimmunoassay (RIA) using a modified Diagnostic Products Corporation kit (Los Angeles, CA). Intra- and interassay coefficients of variation (CV) were <10.0 and 13.8%, respectively. Serum LH was determined by RIA [Monroe et al., 1970] using rabbit antiserum against human chorionic gonadotropin and radioiodinated macaque LH. The intra- and interassay CV’s were <10.0 and 12.7%, respectively.
The ovaries that were studied histologically were fixed in 10% neutral-buffered formalin, embedded in paraffin, and serially sectioned at 6–10 μm in thickness. Sections were stained with hematoxylin and eosin (H&E) for microscopic examination.
Data were analyzed using a Pearson product-moment correlation (r), a point biserial correlation (r), Chi square (χ2), and two-way ANOVA. Data are expressed as mean ± standard error (Mean ± SE).
RESULTS
Endocrine Data.
The endocrine profiles of the five younger females, illustrating E2 and LH concentrations for one year, are depicted in Figure 1. In younger females, basal (non-ovulatory) E2 levels typically were <120 pg/ml and LH levels were <50 ng/ml. Ovulation was possible in these females (except in female N265U, whose LH concentrations were low but whose E2 concentrations were erratic) as evidenced by simultaneous peaks in both E2 and LH concentrations. None of the younger females exhibited a pattern of sustained LH elevations.
Figure 1.
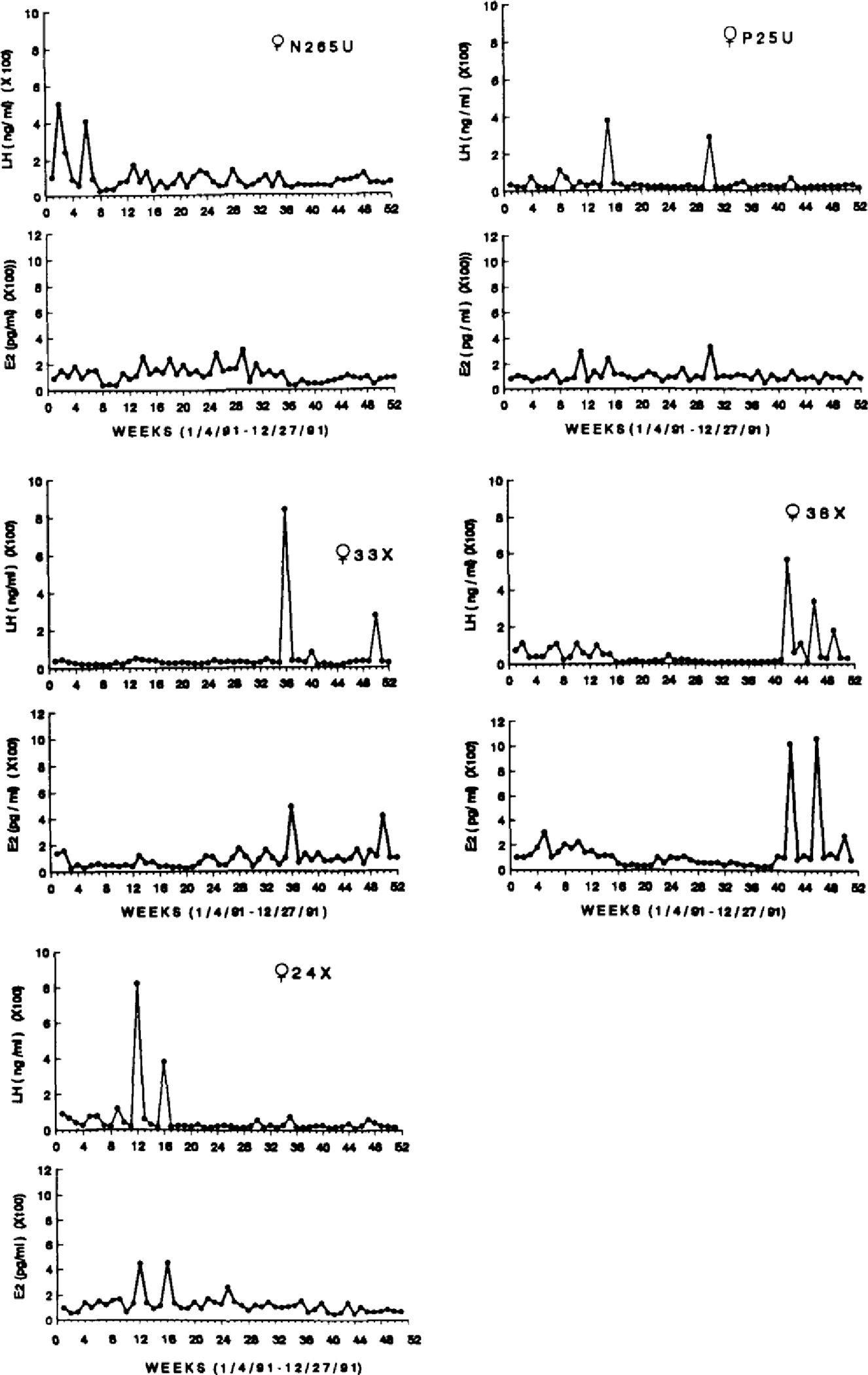
Weekly E2 (pg/ml) and LH (ng/ml) concentrations for five premenopausal females, ages 8–11 years, over one year.
The E2 and LH concentrations of females who appear to be in transition to menopause are depicted in Figure 2. These females (n = 4) ranged in age from 24–26 years. Female 1422, who was 24 years old, appears to have ovulated at least once during the year. However, her LH levels were consistently high (Mean = 132.66 ± 21.60 ng/ml), and conversely, her E2 levels were consistently low (Mean = 55.96 ± 4.49 pg/ml). Female 1419 showed a similar pattern. She possibly ovulated at least once, but her LH concentrations remained high (Mean = 91.58 ± 6.39 ng/ml) while her E2 concentrations were low (Mean = 43.08 ± 11.29 pg/ml). Nevertheless, two other females, ages 24 and 26 years, exhibited similar endocrine profiles to the younger females. Each exhibited periodic fluctuation in E2 and LH, and at other times E2 and LH concentrations were basal.
Figure 2.
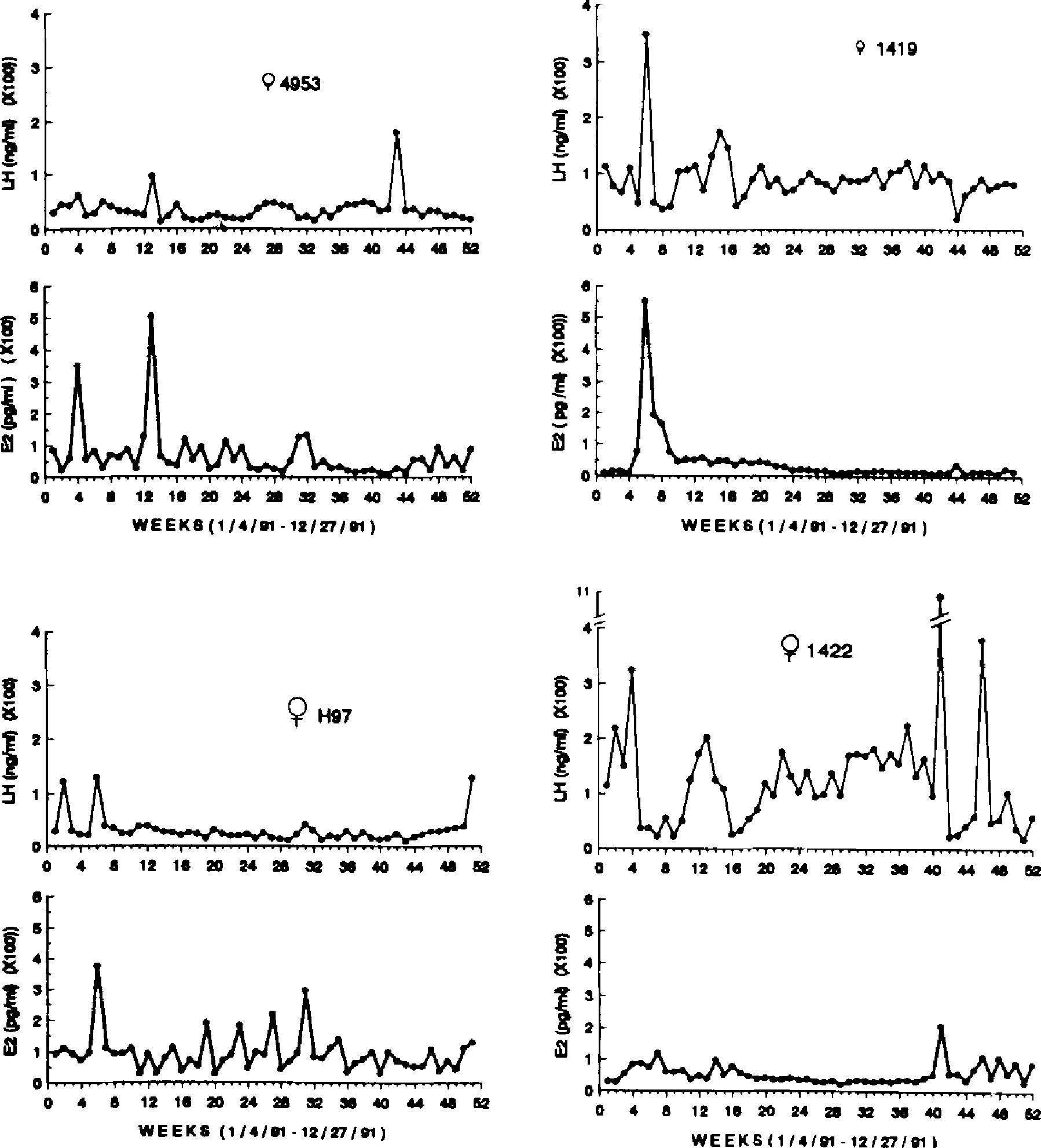
Weekly E2 (pg/ml) and LH (ng/ml) concentrations for four perimenopausal females, ages 24–26 years, over one year.
All females who were 27 years and older (n = 6) showed similar LH and E2 patterns throughout the year, as illustrated in Figures 3 and 4. LH concentrations were high in all females (Mean = 294.93 ± 63.2 ng/ml), whereas E2 concentrations were uniformly low (Mean = 43.98 ± 4.49 pg/ml). In only one case was there any evidence of ovulation, that being a singular peak in both LH and E2 in female 4948 (see Fig. 4). Female 816 exhibited a single sustained rise in E2 coincident with a drop in LH concentrations which lasted for several weeks.
Figure 3.
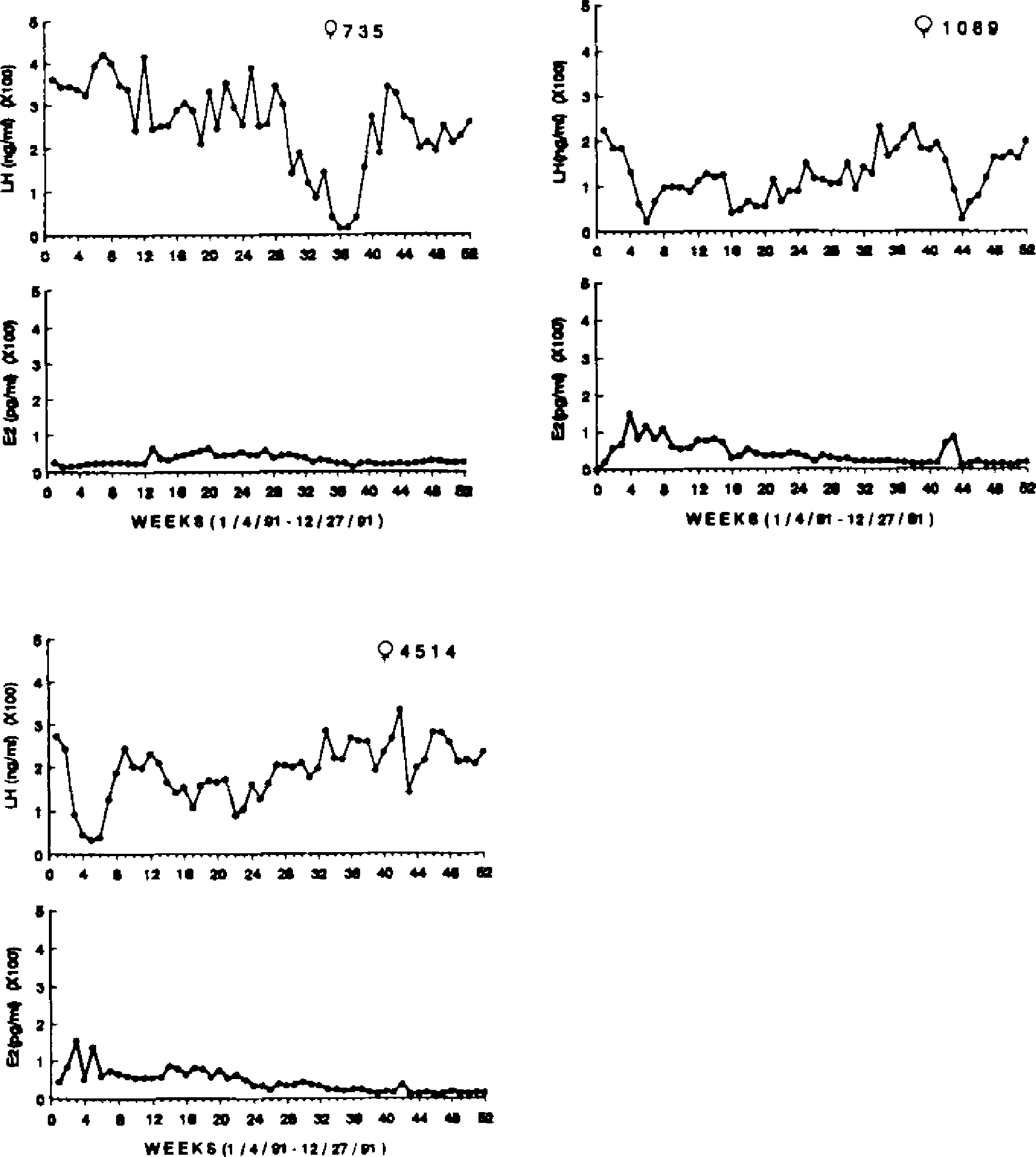
Weekly E2 (pg/ml) and LH (ng/ml) concentrations for three postmenopausal females, ages 27–34 years, over one year.
Figure 4.
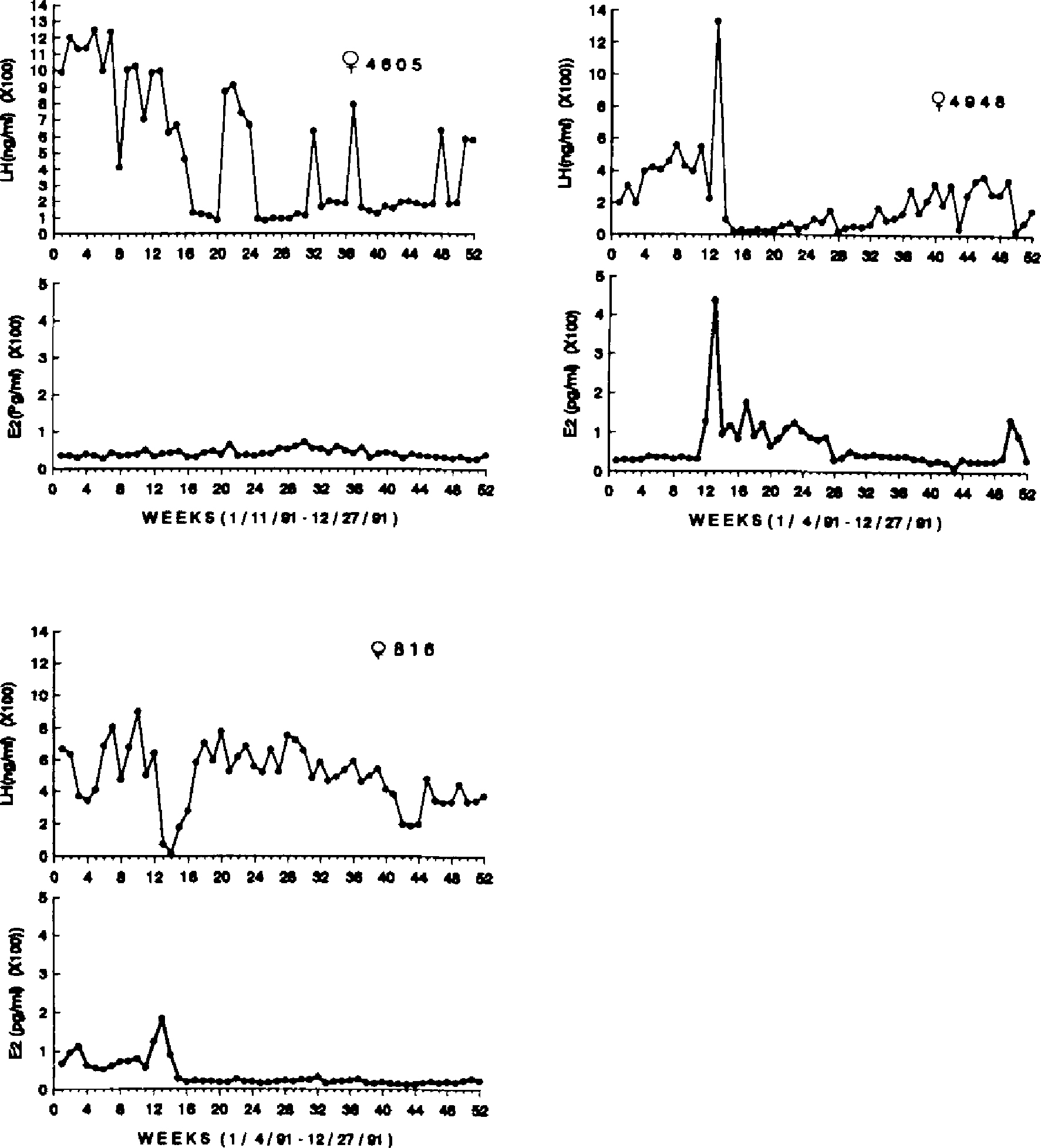
Weekly E2 (pg/ml) and LH (ng/ml) concentrations for three postmenopausal females, ages 27–29 years, over one year.
The mean concentrations of E2 and LH for the premenopausal, perimenopausal and postmenopausal females are depicted in Figure 5. The top panel shows that LH concentrations were significantly elevated in the postmenopausal females as compared to the pre- and perimenopausal females (F(2,12) = 9.36, P = .003). Concentrations of E2 were significantly lower in the postmenopausal females, as compared to the other two groups (F(2,12) = 27.88, P <.0001).
Figure 5.
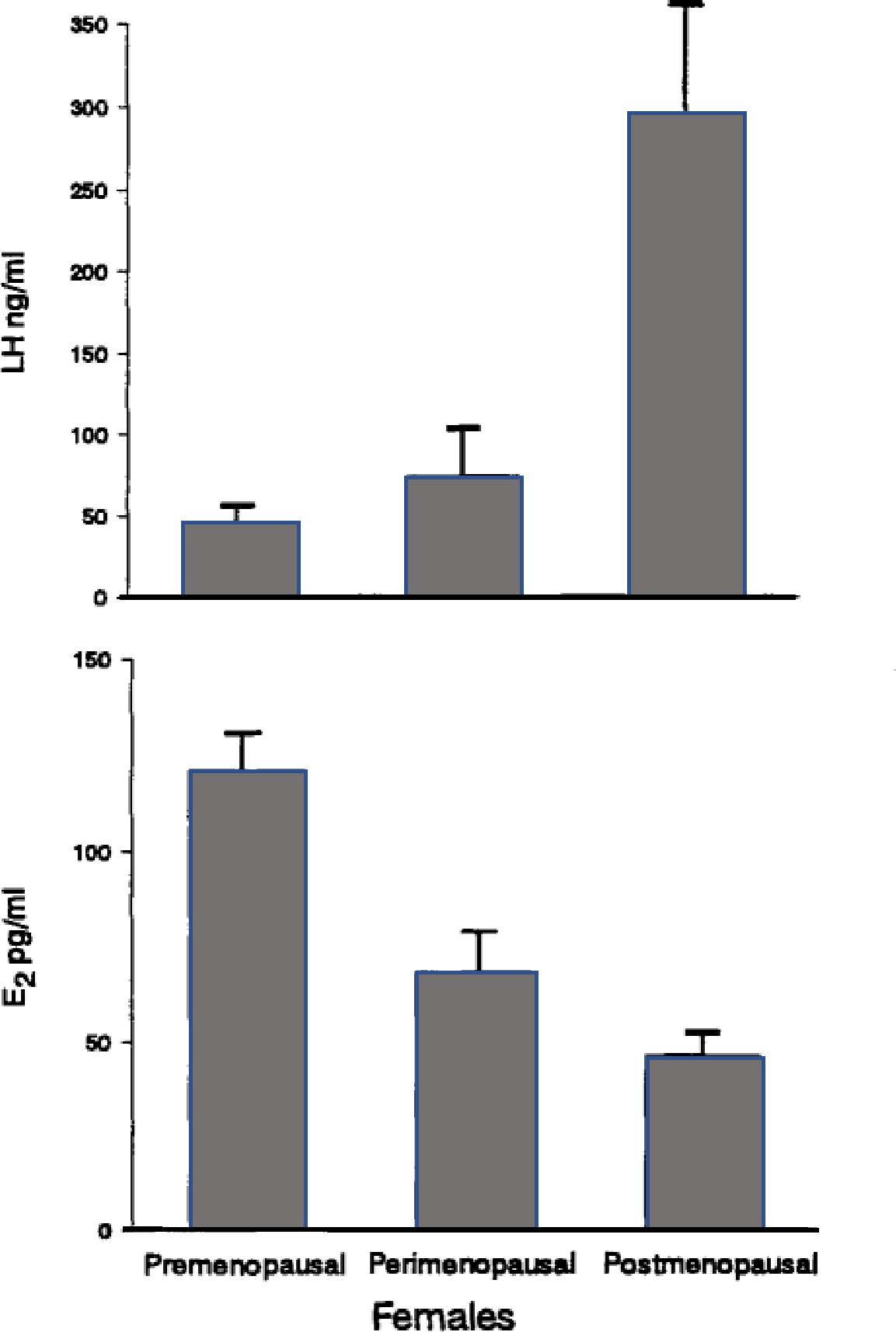
Mean ± SE E2 (pg/ml) and LH (ng/ml) concentrations for the premenopausal, perimenopausal, and postmenopausal females.
A correlation analysis was performed between age and mean E2 concentrations for all females. As expected, E2 concentrations were inversely related to age, r = −.91, P <.00001. A significant positive correlation was found between age and mean LH concentrations, r = .58, P = .02.
Of the 17 presumptive ovulations that were observed (simultaneous peaks of LH and E2) on the basis of weekly hormone samples, five occurred in the spring (March-May), one occurred in the summer (June-Aug.), two in the fall (Sept.-Nov.), and nine occurred during the winter (Dec.-Feb.) months. This seasonal difference in frequency of ovulation was significant, χ2(3) = 9.75, P <.05. There was also a significant negative correlation between the number of ovulations and age, r = −.68, P <.005.
Menstrual Data.
The occurrence of vaginal bleeding in the females over the 12-month study period is depicted in Table 1. The younger females (8–11 years) showed evidence of menses at least once during the year. Of the older females (24–34 years), 80% failed to exhibit any sign of menstrual bleeding throughout the year. Two of the four females aged 24–26 years menstruated, whereas none of the females aged 27 years and older menstruated. A point biserial correlation revealed that age was inversely, but weakly, related to the occurrence of menses, r = −.45, P =.09.
Histological Data.
Photomicrographs of ovaries from two representative females are depicted in Figure 6. The ovary from a female, age 7 years, showed ongoing folliculogenesis, as evidenced by follicles in various stages of development. There was little or no evidence of active folliculogenesis in the four postmenopausal ovaries that were examined histologically, although a small number of atretic follicles were seen. The histological data are congruent with the endocrine evidence for acyclicity in these postmenopausal females.
Fig. 6.
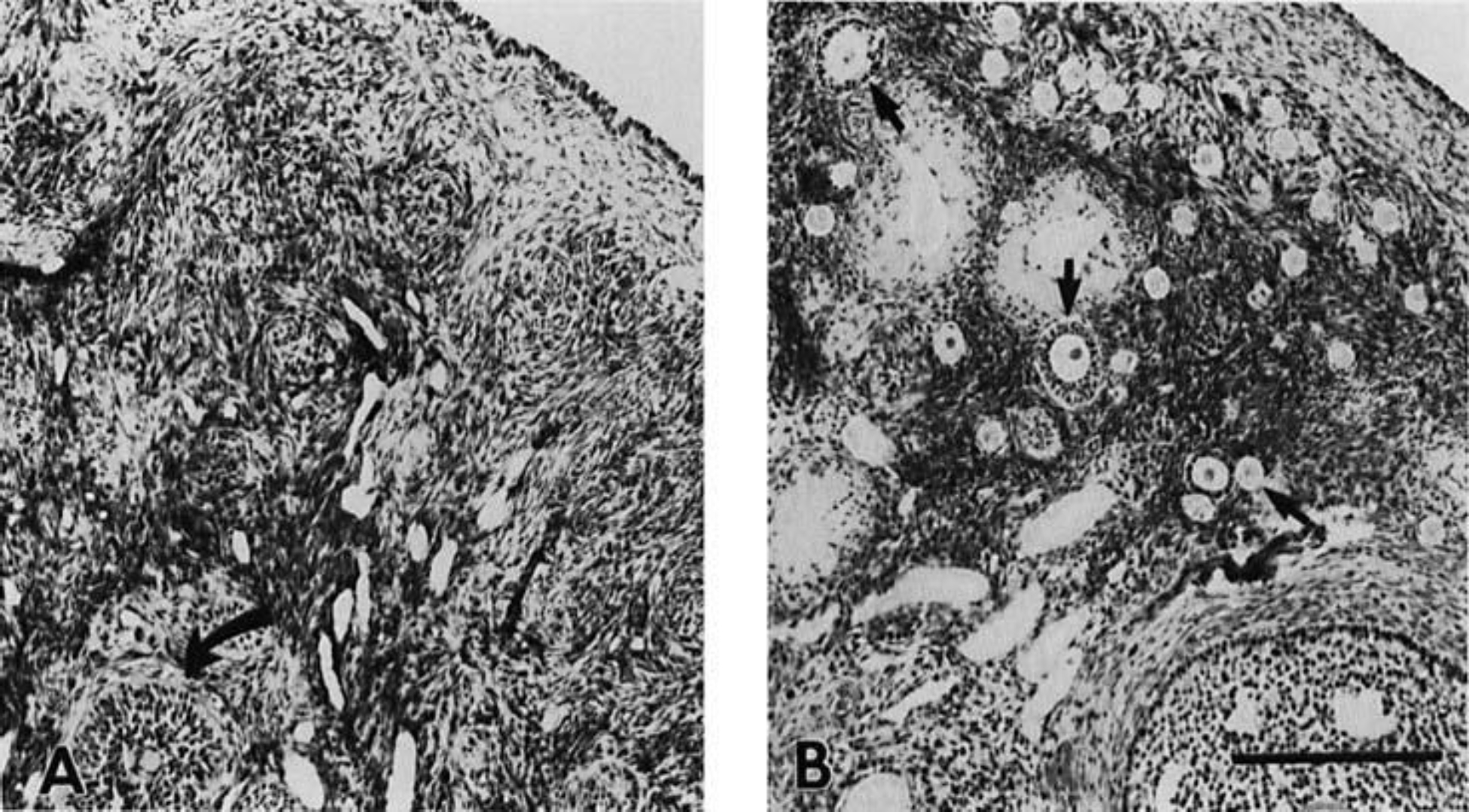
Photomicrographs of hematoxylin and eosin-stained sections from the ovaries of two females. A: ovary from a 34-year-old female showing no developing follicles and one atretic follicle (curved arrow). B: ovary from a 7-year-old female showing numerous follicles in various stages of development (3 follicles are indicated by straight arrows). Magnification bar = 250 μm.
DISCUSSION
The aged female rhesus monkeys in this study exhibited an endocrine secretory pattern similar to that reported in postmenopausal humans [Sherman et al., 1976]. All monkeys aged 27 years and older averaged E2 concentrations that were similar to those reported for humans [<30 pg/ml; Sherman et al., 1976]. Similarly, LH concentrations were elevated in postmenopausal monkeys. In the presence of diminished ovarian follicular activity, the hypothalamic-pituitary system is released from ovarian steroid negative feedback, and the pituitary secretion of LH is thereby enhanced. These data indicate that female rhesus monkeys who live long enough attain menopause that is, hormonally, similar to that of human females.
In an earlier study by Hodgen et al. [1977] the transition to menopause was studied in female rhesus monkeys who were estimated to be at least 22 years of age. They reported an irregular pattern of menses in some of these females. In the present study, the method used to record menstrual data precludes drawing conclusions regarding the detailed pattern of menstrual bleeding throughout the year. However, all of the younger females (8–11 years) menstruated during the year. Of the perimenopausal females, on the other hand, only two of the four showed evidence of menstrual bleeding. Moreover, no postmenopausal females exhibited menses. The cessation of menstruation, historically the technical definition of menopause, has been previously reported for two monkeys aged 27 and 28 years [Van Wagenen, 1970] and for colony data (occurring around 26 years) from the Wisconsin Regional Primate Research Center [Uno & Walker, 1993]. Based on these data, menopause (i.e., failure to menstruate) occurs by approximately 27 years of age in this species. This can be compared to the upper limit at which menopause is reached in humans [52–56 years; Treolar et al., 1967]. Thus, to observe menopause in rhesus monkeys, it is necessary to select animals who are at least 25 years of age. Significantly, the lifespan of rhesus monkeys (>35 years) is greater than was previously estimated [Bowden & Williams, 1984; Tigges et al., 1988].
Although progesterone concentrations were not measured in this study, the sampling schedule was frequent enough to detect fluctuations in E2 and LH concentrations. The younger females, ages 8–11 years, showed evidence of ovulatory cycles. Only female N265U exhibited a hormonal profile which was difficult to interpret. Nevertheless, her LH concentrations were basal and therefore distinctly different from the pattern seen in the older females. Therefore, in large part, these “younger” females appear to be reproductively normal.
Of the four perimenopausal females, all exhibited simultaneous peaks in E2 and LH concentrations, suggestive of ovulation. Two of these females, H97 and 4953, showed LH and E2 patterns similar to the younger females. LH concentrations were not markedly elevated and E2 levels were basal. Therefore, although these females were 24 and 26 years old, respectively, they were clearly not postmenopausal, nor did they appear to exhibit the serum hormone profiles described for menopausal women [Sherman et al., 1976].
The postmenopausal females, ages 27–34 years, failed to exhibit evidence of ovulatory cycles, except for female 4948. This female appears to have ovulated at least once, although the LH response was exaggerated (>1,300 ng/ml). The occurrence of ovulatory episodes, despite aberrant hormonal secretions, has also been found in human females [Sherman & Korenman, 1975]. A single sustained increase in E2 concentrations coupled with a decline in LH were seen in female 816. It is unclear why this occurred, although it seems unrelated to any apparent ovulatory activity. Secretion of LH and E2 in monkey 4948 was also erratic throughout the study period, in a manner similar to that reported for humans [Sherman et al., 1976].
Because a seasonal suppression of ovarian activity might have been confused with a menopausal decline in E2 secretion, endocrine data were collected throughout a one-year period in this study. Of 17 inferred ovulations in the colony, the majority was clustered during the winter and early spring months, whereas few ovulations occurred during the summer months. This observation supports earlier studies of seasonal anovulation in laboratory-housed rhesus monkeys [Hartman, 1932; Van Wagenen, 1945; Keverne & Michael, 1970; Riesen et al., 1971]. However, unlike the endocrine pattern characteristic of menopause (i.e., elevated LH, basal E2), these anovulatory cycles were characterized by diminished E2 and LH secretions. In contrast, Hutz et al. [1985] reported elevated LH concentrations in young females housed in a controlled laboratory environment during the summer months. The present study further illustrates the tendency toward the natural occurrence of seasonal anovulation even in the absence of any obvious environmental zeitgeber.
Examination of the ovaries of four aged female monkeys revealed histological changes similar to those associated with menopause in human females [Hertig, 1944; Tervila, 1958]. The ovaries from the postmenopausal female monkeys were irregular in size and cellular composition, supporting an earlier observation by Collins et al. [1983], who found that older ovaries have fewer follicles and compacted interstitial tissue. There was little or no evidence of folliculogenesis despite an abundance of gonadotropin stimulation in these animals.
This study suggests that the climacteric in female rhesus monkeys is hormonally similar to that of human females. Furthermore, it illustrates the necessity to re-evaluate the reproductive life span of rhesus females. On the basis of the age at which menopause occurs, further support is lent to the premise that the term “aged” be applied only to monkeys older than approximately 25 years of age.
CONCLUSIONS
Females ranging in age from 24–26 years showed endocrine and menstrual evidence indicative of transition to menopause.
Females ranging in age from 27–34 years were clearly postmenopausal; they exhibited elevated LH and basal E2 concentrations.
Concurrent E2 and LH peaks, which may indicate ovulations in the group of females as a whole, were mostly restricted to the winter and early spring months.
The ovaries of postmenopausal female monkeys exhibited histological changes similar to those found in postmenopausal humans.
Based on our current knowledge of the lifespan of rhesus monkeys, the timing of menopause in female rhesus monkeys more closely approximates that found in human females in relation to life span than was previously thought.
Female rhesus monkeys are an excellent model for the study of the human climacteric.
ACKNOWLEDGMENTS
The author gratefully acknowledges both Bryan Devan and Grant Blank for their devotion to the project and their technical assistance. I also thank Drs. Linda C. Cork, Mary Lou Voytko, Lary C. Walker, and Donald L. Price for helpful discussions, and for allowing access to the aged primate colony in the Neuropathology Laboratory at The Johns Hopkins University School of Medicine. The monkey colony is supported by US. Public Health Service grant NS20471 to Dr. Price. I also thank Carolyn Westbrook for her help with the artwork. This research was supported, in part, by a Faculty Research Grant awarded to the author by Towson State University. Thanks also go to Dean Laurence Boucher who generously underwrote the assay costs. The assays were performed by the RIA Laboratory at the Yerkes Regional Primate Research Center, Atlanta, GA. This research was approved by the Institutional Animal Care and Use Committees at Towson State University and The Johns Hopkins University School of Medicine, and the work was conducted in accordance with the Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, and Public Law 89–544, “The Animal Welfare Act,” August 24, 1966, and its amendments.
REFERENCES
- Atkinson LE; Hotchkiss J; Gritz GR; Surve AH; Neill JD; Knobil E Circulating levels of steroid and chorionic gonadotropin during pregnancy in the rhesus monkey, with special attention to the rescue of the corpus luteum in early pregnancy. BIOLOGY OF REPRODUCTION 12:335–345, 1975. [DOI] [PubMed] [Google Scholar]
- Bowden DM: Williams DD Aging. Pp 305–341 in ADVANCES IN VETERINARY SCIENCE AND COMPARATIVE MEDICINE, Vol. 28. New York, Academic Press, Inc., 1984. [DOI] [PubMed] [Google Scholar]
- Challis JRG; Socol M; Murata Y; Manning FA: Martin, C.B. Diurnal variations in maternal and fetal steroids in pregnant rhesus monkeys. ENDOCRINOLOGY 106:1283–1288, 1980. [PubMed] [Google Scholar]
- Collins K; Uno H; Dierschke DJ Ovarian follicle populations in peri- and post- menopausal rhesus monkeys. FEDERATION PROCEEDINGS 42:316, 1983. [Google Scholar]
- Daily RA; Neill JD Seasonal variation in reproductive hormones of rhesus monkeys: Anovulatory and short luteal phase menstrual cycles. BIOLOGY OF REPRODUCTION 25:560–567, 1981. [DOI] [PubMed] [Google Scholar]
- Dierschke DJ Temperature changes suggestive of hot flushes in rhesus monkeys: Preliminary observations. JOURNAL OF MEDICAL PRIMATOLOGY 14:271–280, 1985. [PubMed] [Google Scholar]
- Foster DL Luteinizing hormone and progesterone secretion during sexual maturation of the rhesus monkey: Short luteal phases during initial menstrual cycles. BIOLOGY OF REPRODUCTION 17:584–590, 1977. [DOI] [PubMed] [Google Scholar]
- Gordon TP Reproductive behavior in the rhesus monkey: Social and endocrine variables. AMERICAN ZOOLOGIST 21:185–195, 1981. [Google Scholar]
- Hartman CG Studies in the reproduction of the monkey Macacus (Pithecus) rhesus, with special references to menstruation and pregnancy. CONTRIBUTIONS TO EMBRYOLOGY 134:3–160, 1932. [Google Scholar]
- Hertig AT The aging ovary: A preliminary note. JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM 4: 581–582. 1944. [Google Scholar]
- Hodgen G; Niemann W; Tullner W Duration of chorionic gonadotropin production by the placenta of the rhesus monkey. ENDOCRINOLOGY 96:789–791, 1975. [DOI] [PubMed] [Google Scholar]
- Hodgen GD; Goodman AL; O’Connor A,; Johnson, D.K. Menopause in rhesus monkeys: Model for study of disorders in the human climacteric. AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOLGY 127:581–584, 1977. [DOI] [PubMed] [Google Scholar]
- Hutz RJ; Dierschke DJ; Wolf RC Seasonal effects on ovarian folliculogenesis in rhesus monkeys. BIOLOGY OF REPRODUCTION 33:653–659, 1985. [DOI] [PubMed] [Google Scholar]
- Johansson EDB; Neill JD; Knobil E Periovulatory progesterone concentration in the peripheral plasma of the rhesus monkey with a methodologic note on the detection of ovulation. ENDOCRINOLOGY 82:143–148, 1968. [DOI] [PubMed] [Google Scholar]
- Keverne EB; Michael RP Annual changes in menstruation of rhesus monkeys. JOURNAL OF ENDOCRINOLOGY 48:669–670, 1970. [DOI] [PubMed] [Google Scholar]
- Lindburg DG The rhesus monkey in north India: An ecological and behavioral study. PRIMATE BEHAVIOR 2:1–106, Rosenblum LA, ed. New York, Academic Press, 1971. [Google Scholar]
- Monroe SE; Peckman WD; Neill JD; Knobil E A radioimmunoassay for rhesus monkey luteinizing hormone (RhLH). ENDOCRINOLOGY 86:1012–1018, 1970. [DOI] [PubMed] [Google Scholar]
- Neill JD; Johansson EDB; Knobil E Patterns of circulating progesterone concentrations during the fertile menstrual cycle and the remainder of gestation in the rhesus monkey. ENDOCRINOLOGY 84: 45–48, 1969. [DOI] [PubMed] [Google Scholar]
- Neville MK Ecology and activity of Himalayan foothill rhesus monkeys (Macaca mulatta). ECOLOGY 49:110–123, 1978. [Google Scholar]
- Rapisarda JJ; Bergman KS; Steiner RA; Foster DL Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. ENDOCRINOLOGY 112:1172–1179,1983. [DOI] [PubMed] [Google Scholar]
- Riesen JW; Meyer RK; Wolf RC The effect of season on the occurrence of ovulation in the rhesus monkey. BIOLOGY OF REPRODUCTION 5:111–114, 1971. [DOI] [PubMed] [Google Scholar]
- Sherman BM; Korenman SG Hormonal characteristics of the human menstrual cycle throughout reproductive life. JOURNAL OF CLINICAL INVESTIGATION 55:699–706,1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BM; West JH; Korenman SG The menopausal transition: Analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM 42:629–636, 1976. [DOI] [PubMed] [Google Scholar]
- Terasawa E Developmental changes in the positive feedback effect of estrogen on luteinizing hormone release in ovariectomized female rhesus monkeys. ENDOCRINOLOGY 117:2490–2497, 1985. [DOI] [PubMed] [Google Scholar]
- Terasawa E; Bridson WE; Nass TE; Noonan JJ; Dierschke DJ Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: Comparison between gonadally intact and ovariectomized animals. ENDOCRINOLOGY 115:2233–2240, 1984a. [DOI] [PubMed] [Google Scholar]
- Terasawa E; Noonan JJ; Nass TE; Loose MD Posterior hypothalamic lesions advance the onset of puberty in the female rhesus monkey. ENDOCRINOLOGY 115:2241–2250, 1984b. [DOI] [PubMed] [Google Scholar]
- Tervila L The weight of the ovaries after stress ending in death. ANNALES CHIRURGIAE ET GYNEAECOLOGIAE FENNIAE 47:232–244, 1958. [PubMed] [Google Scholar]
- Tigges J; Gordon TP; McClure HM; Hall EC; Peters A Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. AMERICAN JOURNAL OF PRIMATOLOGY 15:263–273, 1988. [DOI] [PubMed] [Google Scholar]
- Treolar AE; Boynton RE; Behn BG; Brown BW Variation of the human menstrual cycle through reproductive life. INTERNATIONAL JOURNAL OF FERTILITY 12:77–126, 1967. [PubMed] [Google Scholar]
- Uno H; Walker LC The age of biosenescence and the incidence of cerebral β-amyloidosis in aged captive rhesus monkeys. ANNALS OF THE NEW YORK ACADEMY OF SCIENCES 695:232–235, 1993. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG Environmental influences on breeding in rhesus monkeys. Pp.1–19 in SYMPOSIUM OF THE IVTH INTERNATIONAL CONGRESS OF PRIMATOLOGY: PRIMATE REPRODUCTIVE BEHAVIOR. Basel, Switzerland, S. Karger, 1973. [Google Scholar]
- Van Wagenen G Optimal mating time for pregnancy in the monkey. ENDOCRINOLOGY 34:307–311, 1945. [DOI] [PubMed] [Google Scholar]
- Van Wagenen G Menopause in a subhuman primate. ANATOMICAL RECORD 166: 392,1970. [Google Scholar]
- Van Wagenen G Vital statistics from a breeding colony. JOURNAL OF MEDICAL PRIMATOLOGY 1:3–28, 1972. [PubMed] [Google Scholar]
- Walker ML; Gordon TP; Wilson ME Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta). JOURNAL OF MEDICAL PRIMATOLOGY 11:291–302, 1982. [PubMed] [Google Scholar]
- Walker ML; Gordon TP; Wilson ME Menstrual cycle characteristics of seasonally breeding rhesus monkeys. BIOLOGY OF REPRODUCTION 29:841–848, 1983. [DOI] [PubMed] [Google Scholar]
- Walker ML; Wilson ME; Gordon TP Endocrine control of the seasonal occurrence of ovulation in rhesus monkeys housed outdoors. ENDOCRINOLOGY 114:1074–1081, 1984. [DOI] [PubMed] [Google Scholar]
- Wilson ME; Gordon TP; Collins DC Variation in ovarian steroids associated with the annual mating period in female rhesus monkeys (Macaca mulatta). BIOLOGY OF REPRODUCTION 27:530–539, 1982. [DOI] [PubMed] [Google Scholar]
- Wilson ME; Walker ML; Gordon TP Natural fertility and reproductive dysfunction: Common hormonal mechanisms. AMERICAN JOURNAL OF PRIMATOLOGY 4:325, 1983. [Google Scholar]


