Abstract
Sex and reproductive status of the host have a major impact on the immune response against infection. Our aim was to understand their impact on host tolerance or resistance in the systemic Mycobacterium marinum infection of Drosophila melanogaster. We measured host survival and bacillary load at time of death, as well as expression by quantitative real-time polymerase chain reaction of immune genes (diptericin and drosomycin). We also assessed the impact of metabolic and hormonal regulation in the protection against infection by measuring expression of upd3, impl2 and ecR. Our data showed increased resistance in actively mating flies and in mated females, while reducing their tolerance to infection. Data suggests that Toll and immune deficiency (Imd) pathways determine tolerance and resistance, respectively, while higher basal levels of ecR favours the stimulation of the Imd pathway. A dual role has been found for upd3 expression, linked to increased/decreased mycobacterial load at the beginning and later in infection, respectively. Finally, impl2 expression has been related to increased resistance in non-actively mating males. These results allow further assessment on the differences between sexes and highlights the role of the reproductive status in D. melanogaster to face infections, demonstrating their importance to determine resistance and tolerance against M. marinum infection.
Keywords: Drosophila melanogaster, immunity, sexual dimorphism, reproductive status, mycobacterial infections, Mycobacterium marinum
1. Introduction
Reproduction and immunity are resource-intensive traits in their deployment and maintenance, thus, the existence of a trade-off between the two biological processes has been widely questioned in both mammals and non-mammals, especially when the resources are limited [1–5]. Its study is important to shed light on the impact of directional selection and evolution in the maintenance or the variation of these traits, thus helping to better characterize the relationship between these two physiological mechanisms [6].
In this regard, Drosophila melanogaster is a powerful model organism to study the interaction between reproduction and immunity [7]. Sexual dimorphism as well as effects in mating and reproduction have been extensively reported in D. melanogaster studies [8–10]. This, in addition to its well-characterized immune system [11] and to the great benefits in terms of cost, time-consumption and genetic tools availability [12], make D. melanogaster a good model to study a possible trade-off between the two physiological aspects. Immunity in D. melanogaster relies solely on innate immune response. Nevertheless, as in mammals, this immune response is also divided into humoral and cellular arms, and have Nuclear factor kappa B(NF-κB) family transcription factors and signal transduction pathways [11]. The humoral response is mainly based on the synthesis of antimicrobial peptides (AMPs) in the fat body of the fly regulated by two signalling pathways: the Toll and the immune deficiency (Imd) pathways, which induce the production of Drosomycin and Diptericin (as the main AMP of each pathway), respectively [13, 14]. Flies also have a simpler but complete Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway [15, 16]. Regarding cellular immunity in Drosophila, it is mediated by haemocytes. When a septic injury, tissue damage, or exposure to lipopolysaccharides occurs, haemocytes trigger the expression of Unpaired (Upd) 3, which activates the JAK/STAT pathway in the fat body and contributes to the humoral response activation and the upregulation of immune genes [15, 17].
It is well known that hormones and metabolism, as well as circadian cycles, have central roles in the regulation of systemic physiology, including immunity, in both insects and mammals [18–20]. The metabolic homeostasis in insects is mainly regulated by the insulin/insulin-like signalling (IIS) pathway. When there is an immune stimulus, the immune pathways interact with the IIS pathway in the fat body resulting in loss of energy storage and suppression of host growth [21–23]. This fact has been recently described as the ‘selfish immune system theory’, which is based on the fact that activated phagocytes release signalling molecules (selfish immune factors, SIFs) that regulate host energy to steal resources from other non-immune tissues [24–27]. Authors propose the insulin/insulin-like growth factor (IGF) antagonist Imaginal morphogenesis protein late 2 (Impl2)—which has been identified as a cancer-derived cachectic factor in flies—and Upd3 as potential SIFs in Drosophila [28–30].
Hormones also play an important role in the regulation of immunity [18]. In both mammals and insects it has been described that a hormonal-driven sexual dimorphism might exist affecting immunity (survival, pathology, bacterial load and activity in response to infections) at basal conditions, after a pathogenic challenge, and even upon ageing [31, 32]. Specifically in D. melanogaster, some studies on the subject revealed that these differences might be mediated by different immune players depending on the pathogen and the route of infection. For example, males appeared to be more resistant to systemic infections by Providencia species, and Enterococcus faecalis, while females seemed to be more resistant to Pseudomonas aeruginosa, Staphylococcus aureus and Serratia infections [9, 33, 34]. Recently, a role for some Toll receptors in mediating sex dimorphisms has arisen with the finding that the loss of Toll-7 reduces resistance to P. aeruginosa and E. faecalis in males but not in females in the Toll-7g1–5/Cyo mutant line in comparison with WT w1118 flies [35], although these results could be influenced by the different genetic background of the compared strains. In mammals, steroid hormones have been linked with these sex differences, and interactions between these type of hormones and the innate immune system of Drosophila have also been described [36, 37]. The steroid hormone Ecdysone is the main regulator of the insect life cycle [38]. In adult flies, Ecdysone is produced in the ovaries after mating, thus showing higher levels in females than in males [39–41]. Its signalling through the receptor complex formed by the Ecdysone receptor (EcR) and Ultrapiracle (Usp) is required for the proper expression of the pathogen-sensing receptor peptidoglycan recognition protein LC(PGRP-LC) and, thus, the production of Imd-dependent AMPs [42] and the cellular immunity [37, 43]. At the same time, Ecdysone levels are regulated by stress signals and it has been related to age-related immune changes, as its depletion increases Drosophila’s lifespan [44, 45].
It is not only sex that affects immunity, but also reproductive status. In Drosophila, mated females showed decreased survival, higher pathogen loads and reduced AMPs production after pathogenic infections [46–48], which did not occur when the agent was non-pathogenic [4, 49, 50]. However, when mated female flies are unable to generate eggs, they do not have this reduction in immunity after mating [48]. This makes evident the trade-off between the reproductive factor of producing eggs and immunity due to reproduction costs. On the other side, immunity has also shown to take a reproductive toll by reducing egg viability in mated females [51]. However, if females were fed yeast ad libitum, both fecundity and resistance to infection were improved, suggesting, altogether, that reproduction and immunity compete for energy resources [48, 51, 52]. Along the same lines, males exposed to a higher number of females also showed increased susceptibility to bacterial infections [53]. Conversely, it has been also demonstrated that, despite the significant metabolic cost of infection, in infected wild-type Canton S (CS) mated male and female flies the pre-copulatory reproductive behaviours are preserved [7, 54]. Likewise, mated males have shown better survival curves against Pseudomonas entomophila infection as well as a better bacterial clearance ability against Providencia rettgeri than virgin ones, showing absence of reproduction-immunity trade-off [6].
In humans, sexual dimorphism has been also observed in the pathogenesis of some infectious diseases such as tuberculosis [55] which has been the leading cause of death due to a single pathogen (Mycobacterium tuberculosis, Mtb), surpassing the second deadliest bacterial infection: the plague (caused by Yersinia pestis). In the last years, it has only being exceeded by the viral COVID-19 pandemic on 2020 [56]. Mycobacterium marinum is the most common pathogenic mycobacteria used for studying host-pathogen interactions in non-mammal animal models because it is a natural pathogen of ectotherms causing a granulomatous infection that highly resembles tuberculosi (TB) in humans [57–59]. The infection of this mycobacterium in the D. melanogaster model has also been well characterized. The microorganism is able to kill flies even with very low initial doses, at the early stages of the infection, as it is able to replicate inside Drosophila haemocytes [60]. It has also been described that the formation of lipid droplets inside phagocytic cells derived from the production of Upd3 in these cells benefits the intracellular growth of the pathogen, thus resembling the formation of foamy macrophages in humans. As the infection evolves, M. marinum causes a progressive loss in energy storage inducing a cachexia-like process that eventually, accompanied by widespread tissue damage, is responsible for killing the flies. This wasting process is mediated by the disruption of the IIS pathway [61].
Some studies have addressed the possibility of a trade-off between reproduction and immunity in D. melanogaster analysing how the interaction between sex of the fly (male, female), its reproductive status (virgin and mated kept alone or together) and a specific infection affect the immunity of the fly [8–10]. However, this is the first study that does it with mycobacteria, specifically with the model that is currently used in Drosophila to study tuberculosis. In this study, we have assessed the role of sexual dimorphism and reproductive status on the response to the infection by M. marinum in D. melanogaster. We have also assessed the differences that these features have on the innate immune response triggered by the pathogen by measuring the expression of Toll- and Imd-dependent AMPs, drosomycin and diptericin, respectively. In addition, due to the importance that metabolism has on the M. marinum infection in flies [61], we have assessed the expression levels after the infection of upd3 and impl2 based on previous studies that described the role that these molecules play in the metabolic regulation of the host during infections [25, 62]. While doing this, we reinforced the fact that mycobacterial infections, such as tuberculosis, should be tackled considering all these parameters.
2. Materials and methods
2.1. Fly stocks and husbandry
Oregon-R-C wild-type flies were obtained from the Bloomington Drosophila Stock Centre (Indiana University) and they belong to stock number #5. Flies were raised on a standard cornmeal medium (Nutri-Fly® Bloomington Formulation, Genesee Scientific) prepared following manufacturer’s instructions and maintained at 25°C, 65–70% humidity with a 12 h light/dark cycle. Male and female flies were aged for 3–5 days post-eclosion before experimentation.
2.2. Mycobacterial stock preparation and infection
Mycobacterium marinum E11 strain resistant to kanamycin (a kind gift from Wilbert Bitter, Vrije Universiteit Amsterdam) was used [63]. The mycobacterial strain was cultured in 7H9 complete medium complemented with 20 µg/ml of antibiotic and placed at 30°C with constant agitation (170 rpm) for 10 days (until an OD600nm of 1.5). The cultures were centrifuged for 5 min at 4000g, resuspended in phosphate-buffered saline (PBS) with 0.2% Tween 80 and centrifuged again for 5 min at 500g to remove clumps. Supernatants were transferred to a new tub, centrifuged for 5 min at 4000g and resuspended in 1ml of 7H9 with 15% glycerol. Mycobacterial cultures were then aliquoted and frozen at −80°C. Each stock was tittered after being frozen at least overnight.
For systemic infections, an aliquot was defrosted and centrifuged at 15 000g for 5 min. Pellet was rinsed twice with sterile PBS and diluted to the proper concentration. For the mycobacterial infection, inoculation doses of 50, 500, 5000, 50 000 colony forming units(CFUs) per fly were used. Flies injected with sterile PBS were used as uninfected control. Fourteen nanolitres of each solution were injected systemically employing a nano-injector (Nanoject II, Drummond) into the abdomen of anaesthetized flies.
2.3. Experimental design
Flies were divided into three experimental groups: virgin flies, mated flies that were separated by sex after the infection and flies that were allowed to mate throughout the infection (actively mating). Virgin flies were collected at the 2–3 h post-eclosion, and kept in same-sex groups before and after experimentation. In all experiments, male and female flies were kept in groups of 30 individuals, either only one sex or equally distributed between males and females, and were all infected 3–5 days post-eclosion. Survival, bacterial load upon death (BLUD) and gene expression were measured for each experimental group.
Overall, the experiment consisted of 30 male and 30 female flies for each experimental group (each reproductive status) to track survival and BLUD. Flies’ survival was tracked for a month, as all infected flies at all inoculation doses were already dead at least 1 week or 10 days before. Dead flies were collected twice a day, washed with 70% ethanol, and rinsed twice with PBS. Each fly was then mechanically homogenized into 200 μl of sterile PBS, diluted and plated into 7H10 plates complemented with kanamycin, and incubated for 10 days at 30°C. We performed all experiments in triplicate with a total of 90 males and 90 females per group. In the first replicate, we assessed for differences between flies collected first in the morning (8 am) and in the afternoon (5 pm). No statistically significant differences were observed among bacillary loads depending on the time dead flies were collected (Supplementary Fig. 1). For gene expression analysis, additional males and females for each condition were infected and three pools of three flies were analysed per each group and time point. Experiments were also performed in triplicate.
2.4. Tolerance and resistance
Previous studies have defined three defensive strategies against infections: qualitative resistance, as the ability to remain uninfected upon pathogen exposure; quantitative resistance, as the ability to reduce the pathogen load; and tolerance, as the ability of the host to maintain the fitness, measured by the survival to the infection in our study, given a certain pathogen load [64, 65]. Given our model of study in which the minimum lethal dose of the pathogen is 1, meaning that a single mycobacterium is able to kill the host, we did not assess qualitative resistance but focussed on tolerance and quantitative resistance.
Tolerance was defined by the slope of the regression between the host fitness against the pathogen burden [65–70], with more tolerant groups presenting less steep slopes. We used flies’ survival rates as an indicator of host fitness and inoculation dose as the pathogen burden, because it highly correlates with both survival times and BLUD. Uninfected flies injected with sterile PBS were included in the analysis to account for the fitness of each group in the absence of infection, commonly referred to as general vigour [65]. On the other hand, resistance was measured as the Y-intercept of the regression line between the BLUD and the inoculation dose. When slopes are equal, the lower the Y-intercept the more resistant the group is [65, 67, 68].
2.5. Gene expression by quantitative real-time PCR
The differential gene expression was assessed at 24 h, 5 days and 10 days post-infection. Overall, a total of nine pools of three flies infected with 500 CFUs each were analysed per sex, group and time point, obtained from the three independent experiments. All flies were preserved in RNA later (Fisher Scientific, S.L.) and stored at −80°C before RNA extraction. Total RNA was extracted from flies using the MasterPure™ Complete DNA and RNA Purification Kit (Lucigen) and 600 μg of RNA were converted to cDNA with the PrimeScript RT Master Mix (Takara), following the instructions of the kit’s manufacturers for both procedures. cDNA was stored at −20°C until used.
Quantitative real-time polymerase chain reaction (rt-qPCR) was carried out using the KAPA SYBR® FAST Mix (Sigma) on a LightCycler 480 (Roche Diagnostics) system. RT-qPCR conditions were 95°C for 5min followed by 40 cycles of 95°C for 10 s and 60°C or 62°C for 20 s. The specificity of each pair of primers was checked by melting curve analysis (95°C for 5 s, 65°C for 1 min and a continuous rise in temperature to 97°C at 2.5°C/s ramp rate followed by 97°C for 30 s ). To check reproducibility, each assay was performed with technical triplicates for each biological sample. The relative transcripts levels of target genes were calculated using the 2-ΔΔCT method [71] with rpl32 used as the reference gene for normalization of target gene abundance. The oligonucleotides used are detailed in Table 1.
Table 1.
Oligonucleotide sequences used for real-time PCR
| Gene | Primer forward (5ʹ–3ʹ) | Primer reverse (5ʹ–3ʹ) |
|---|---|---|
| Rpl32 | ACAGGCCCAAGATCGTGAAG | TCGACAATCTCCTTGCGCTT |
| Diptericin | GGCTTATCCGATGCCCGACG | TCTGTAGGTGTAGGTGCTTCC |
| Drosomycin | CCAAGCTCCGTGAGAACCTT | CAGGTCTCGTTGTCCCAGAC |
| Upd3 | GCAAGAAACGCCAAAGGA | CTTGTCCGCATTGGTGGT |
| Impl2 | GCCGATACCTTCGTGTATCC | TTTCCGTCGTCAATCCAATAG |
| Ecdysone receptor | CAACAGCTCGGACTCAATATTCTT | GTTCTCCTCCTGGGTAATCTGAA |
2.6. Statistical analysis
Tolerance and resistance analyses were carried out in GraphPad Prism (version 9.0.0). Survival, CFU counts and gene expression data were checked for outliers with the Robust regression and Outlier removal(ROUT) method (Q = 1%) and for normality with the Shapiro–Wilk normality test. For multiple comparisons we performed Tukey’s test for normally distributed data and Dunn’s test for non-normally distributed data. For comparisons between two groups we performed unpaired t test for normally distributed data and Mann–Whitney test for non-normally distributed data. A linear regression model was used to analyse the tolerance and resistance. Slopes and Y-intercepts were analysed for statistically significant differences with the Extra sum-of-squares F test. The principal component analysis (PCA) was performed with the Factoextra package in R (version 4.0.1). We have analysed each replicate separately to ensure the consistency of the outcomes prior to analyse them together.
3. Results
3.1. The effect of reproduction in tolerance and resistance to M. marinum infections is sex-dependent and reproductive status-dependent
To study tolerance and resistance to the infection by M. marinum, flies were injected systemically with increasing doses of the pathogen (50, 500, 5000 and 50 000 cfu/fly) or with PBS as control, and kept at 25°C throughout the experiment.
Our study revealed no significant differences in the general vigour (understood as the survival of non-infected flies for each group) in males, independently of their reproductive status. Instead, when looking to results of infection, they showed that those males kept together with females were less tolerant to the infection by M. marinum compared to males kept alone and virgin males (P = 0.0026; P = 0.0447, respectively), but more resistant (P < 0.0001) (Fig. 1a; Table 2). Altogether results suggest that males reproductively active (males kept together) are also more immune activated in response to infection, rather than those having previously being mated or not.
Figure 1.
Tolerance and resistance of D. melanogaster to M. marinum infections depending on the reproductive status or the sex of the flies. (a) Males that were not in presence of females (virgins and alone) were more tolerant but less resistant than those kept together with females. (b) In females, virgins were more tolerant but less resistant than mated females, independently of the presence or absence of males. When comparing both sexes for each reproductive status, we observed that males were more resistant when virgins or together with females, although no differences in tolerance were found in these groups (c and e). When flies were kept alone after infection, females showed higher general vigour and resistance, but lower tolerance to infection (d). Lines represent the regression lines fitted for each group and each circle represents an individual. Both survival and bacillary load between the groups were analysed independently for each inoculation dose and were tested for normality. Statistically significant differences were represented as follow: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001 (Kruskal–Wallis test).
Table 2.
Analysis of tolerance and resistance to M. marinum infections depending on the reproductive status
| Males | |||
|---|---|---|---|
| Tolerance | |||
| Group | Slope | Comparison | P value |
| Virgins | −3.149 | Alone | 0.3492 |
| Alone | −3.014 | Together | 0.0026** |
| Together | −3.447 | Virgins | 0.0447* |
| Resistance | |||
|---|---|---|---|
| Group | Y-inter | Comparison | P value |
| Virgins | 5.138 | Alone | 0.0325* |
| Alone | 5.196 | Together | <0.0001**** |
| Together | 4.913 | Virgins | <0.0001**** |
| Females | |||
|---|---|---|---|
| Tolerance | |||
| Group | Slope | Comparison | P value |
| Virgins | −3.165 | Alone | 0.0175* |
| Alone | −3.536 | Together | 0.6273 |
| Together | −3.465 | Virgins | 0.0484* |
| Resistance | |||
|---|---|---|---|
| Group | Y-inter | Comparison | P value |
| Virgins | 5.366 | Alone | <0.0001**** |
| Alone | 4.947 | Together | 0.2354 |
| Together | 5.100 | Virgins | 0.0067* |
Significance (P < 0.05) is indicated by asterisks. Differences in resistance’s slopes were not statistically significant between groups.
On the other hand, the general vigour of PBS-injected virgin females was significantly lower when compared with both mated groups. In fact, if we look to females’ response to infection, we saw that virgin females were more tolerant (P = 0.0175; P = 0.0484) but less resistant (P < 0.0001; P = 0.0067) to the infection, compared to both mated groups (mated kept alone and mated kept together, respectively) (Fig. 1b; Table 2). Contrary to males, these results suggested that the immune phenotype to the infection in female flies was more conditioned by whether they have been mated, regardless of whether they were reproductively active at the time of infection or not.
When we compared tolerance and resistance of males and females for each reproductive status independently, data showed that virgin flies differed neither in general vigour nor in overall tolerance to the infection, but males were significantly more resistant (P < 0.0001) (Fig. 1c; Table 3). The same pattern was observed in flies that were kept together in equal proportions throughout the procedure: both males and females had the same general vigour and the same tolerance levels, but males showed up to be more resistant (P < 0.0001) (Fig. 1e; Table 3). On the contrary, when flies had mated but were kept separated by sex after the infection, females showed increased general vigour and resistance (P = 0.0288), but reduced tolerance to the infection with M. marinum compared with males (P = 0.0008) (Fig. 1d; Table 3).
Table 3.
Analysis of tolerance and resistance to M. marinum infections depending on the sex
| Tolerance | |||
|---|---|---|---|
| Group | Slope males | Slope females | P value |
| Virgins | −3.171 | −3.142 | >0.9999 |
| Alone | −3.014 | −3.505 | 0.0008*** |
| Together | −3.447 | −3.351 | 0.5099 |
| Resistance | |||
|---|---|---|---|
| Group | Y-inter. males | Y-inter. females | P value |
| Virgins | 5.138 | 5.366 | <0.0001**** |
| Alone | 5.196 | 4.947 | 0.0288* |
| Together | 4.913 | 5.196 | <0.0001**** |
Significance (P < 0.05) is indicated by asterisks. Differences in resistance’s slopes were not statistically significant between groups.
All these results allow us to classify from tolerant or resistant to the M. marinum infection each fly according to its sex and each reproductive status. Being more tolerant and less resistant: virgin males, mated males kept alone and virgin females. And more resistant and less tolerant: mated and kept alone males and females, and females kept together.
3.2. The humoral innate immune response against M. marinum infection depends on the reproductive status of the host
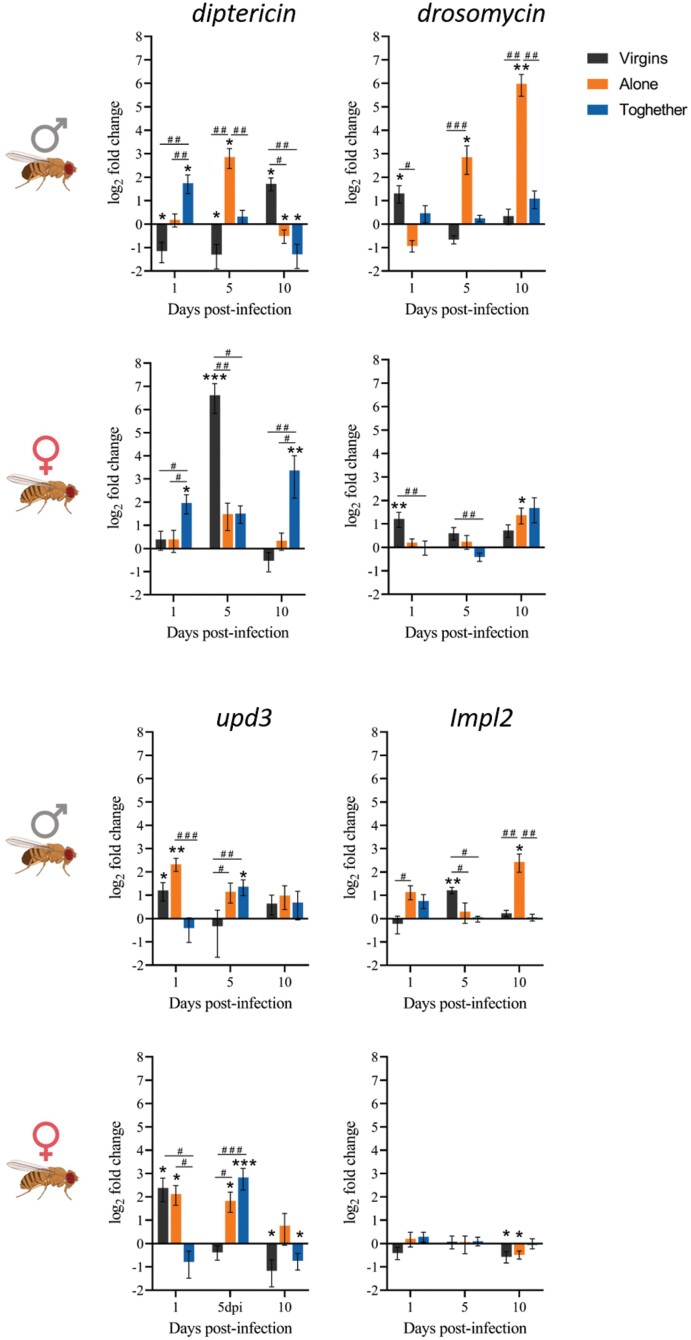
The differences in the innate immune response triggered by the infection in each reproductive status were measured by the expression of the antimicrobial peptides Diptericin and Drosomycin in flies infected with 500 CFU of M. marinum at different time points (Fig. 2a). These AMPs are regulated by the Imd and the Toll pathways, respectively. In males, only those kept together with females showed immediate significant production of Diptericin, while the other groups showed a delayed production of this AMP. Moreover, virgin males presented significantly increased production of Drosomycin immediately after the infection, while in males alone this production was delayed, and no significant changes were observed in males kept together with females. In females, we observed similar expression profiles as males for each reproductive status: females kept together with males had immediate production of Diptericin, but not Drosomycin; virgin females had delayed production of Diptericin, but early production of Drosomycin; females alone showed delayed or null production of both AMPs.
Figure 2.
Expression of innate immunity (a) and metabolic (b) genes during the infection in males and females depending on their reproductive status. Gene expression relative to the internal control gene rpl32 was quantified in nine replicate pools of three males or females exposed to the infection with M. marinum relative to their expression in uninfected controls. Each group at each time point was compared to its relative control independently (the line in each graph represents the controls’ relative expression with a log2 fold change of 0). Data was analysed for normality and significant differences were represented as follow: *P ≤ 0.05, **P ≤ 0.01 (Welch’s corrections for normally distributed data and Mann–Whitney test for not normally distributed data). Differences among the different groups were also analysed for each time point independently following the same approach. Differences were represented as follow: #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001, and ####P ≤ 0.0001.
These results for the expression levels showed a link with the phenotypic results obtained previously. We found that the activation of the Toll pathway (production of Drosomycin) is associated with more tolerant but less resistant phenotypes in both males (virgins and mated kept alone) and females (virgins). In addition, the more sustained this activation was (mated males kept alone) the lower resistance was observed. On the other hand, we also observed that while early activation of the Imd pathway was associated with the more resistant groups (males and females together), late or null activation of this pathway seemed to be linked with reduced resistance with the exception of females alone. These interactions were also observed when comparing the expression levels between males and females for each reproductive status (Supplementary Fig. 2a). We found that the less resistant groups (virgin females, females kept together with males, and males kept alone) showed significantly increased production of both AMPs late in the infection, while the more tolerant group (males kept alone) had increased production of Drosomycin. Finally, results also suggest that females kept alone after mating may acquire their resistance through a mechanism that does not involve the humoral innate immunity, as they did not show any activation of the Imd pathway throughout the infection.
3.3. The metabolic regulation during infection is altered by the reproductive status of the host
The link with the metabolism, especially with the IIS pathway, together with the suggested role for Upd3 and Impl2 as ‘SIFs’, prompted us to investigate whether a differential expression of these molecules within the different reproductive status of the host might also influence the tolerance and resistance levels to the infection by M. marinum (Fig. 2b).
In males, both virgins and those kept alone showed significant early overexpression of upd3, which was not sustained later in the infection. On the other hand, those males that were kept together with females during the whole procedure presented a significant overexpression of this gene later in the infection. In females, virgins showed a significant early but not sustained overexpression of upd3, while females that have mated presented a significantly increased expression of this gene later in the infection whether they were in the presence or absence of males. Overall, these results suggest a dual role for upd3, as its early expression correlated with more tolerant but less resistant groups while its expression later in the infection correlated with more resistant and less tolerant groups.
Finally, assessing the expression of impl2 during the M. marinum infection we observed no significant differences among females depending on their reproductive status. On the other hand, both virgin males and mated males kept alone showed a significant increase in the expression of this gene later in the infection, but not sustained. These results suggested that, in males, the punctual expression of impl2 might increase tolerance to the infection with M. marinum, but not in females.
We also compared the expression levels of both genes between males and females for each reproductive status independently (Supplementary Fig. 2b). We found that females have an overall increased expression of upd3 compared to males, while impl2 seemed to only play a role in males with its late production correlating with increased tolerance (males kept alone).
3.4. Induction of the ecdysone receptor after infection correlated with basal levels prior to the infection in males, but not in females
We also evaluated the expression levels of the ecdysone receptor (EcR) due to the tight relationship of the Ecdysone pathway with immunity and reproduction. We measured the relative expression of this gene after the infection and the basal expression levels when injected with PBS for each reproductive status (Fig. 3c).
Figure 3.
Expression of the ecdysone receptor (EcR) in males (up) and females (down) depending on their reproductive status after the infection (a). Gene expression relative to the internal control gene rpl32 was quantified in nine replicate pools of three males and three females each exposed to the infection with M. marinum relative to their expression in uninfected controls. Each group at each time point was compared to its relative control independently (the line in each graph represents the controls’ relative expression with a log2 fold change of 0). Data was analysed for normality and significant differences were represented as follow: *P ≤ 0.05, **P ≤ 0.01 (Welch’s corrections for normally distributed data and Mann–Whitney test for not normally distributed data). Differences among the different groups were also analysed for each time point independently following the same approach. Differences were represented as follow: #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001, and ####P ≤ 0.0001. Basal expression levels of the EcR gene in uninfected flies (b) were calculated using the 2-ΔCT method with the rpl32 gene for normalization (all values were multiplied by 104 for more visual results). Groups were compared independently for each time point. Data was analysed for normality and significant differences were represented as follow: *P ≤ 0.05, **P ≤ 0.01 (Kruskal–Wallis test). Finally, the correlation between the basal levels and the fold change after infection was performed using the nonparametric spearman correlation test (c).
We found that virgin males significantly induced the expression of the receptor later in the infection, while males kept alone did it immediately after. On the other hand, when males were kept together with females, the infection triggered a significant repression of the receptor (Fig. 3a). We also found that males presented a significant correlation between the basal level of EcR and the induction of this receptor post-infection, in which lower basal levels translated into higher activation of this gene post-infection (Fig. 3b and c). In females, virgins showed no changes neither in the basal levels nor in the induction of EcR after the infection. Females that had mated showed similar patterns as their males’ counterparts, while females kept alone showed decreasing induction of EcR when infected, but no significant changes on the basal levels (Fig. 3a and b). However, the correlation between basal levels and induction of EcR after the infection was not statistically significant in females (Fig. 3c).
The results also showed a link between the basal expression levels of EcR and the resistance to the infection. Those groups with higher basal expression levels (males and females kept together and females alone) were more resistant to the infection, while those groups with lower basal levels (virgin flies and males alone) were less resistant.
When comparing both basal and induction levels between males and females (Supplementary Fig. 3a), we observed that virgin males presented lower basal levels but increased production of EcR after the infection, while flies separated after the infection showed no significant different basal levels but females increased its production after the infection. Finally, when flies were kept together throughout the procedure, no differences at basal levels among sexes were found, but a late induction in females.
3.5. Gene expression upon infection in D. melanogaster might be influenced by sexual dimorphism
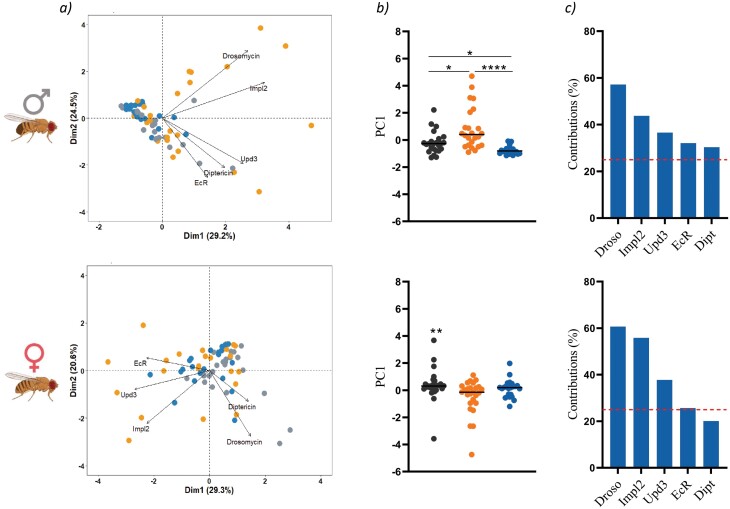
Finally, we assessed the overall correlation between all the genes studied. The PCA (Fig. 4a) performed with the relative expression levels of infected flies including all time points for each condition revealed that, in males, all three groups presented significantly different gene expression profiles (Fig. 4b top), which linked with all three groups presenting different tolerance and resistance profiles as well. In females, only virgins presented a significantly different gene expression profile (Fig. 4b bottom), also linking with the phenotypic results. Finally, the analysis of the variable contribution (Fig. 4c) for dimension 1 (PC1) and 2 (PC2), revealed that, in both sexes, the differences among reproductive status were mainly driven by the differential expression of Drosomycin, Impl2 and Upd3. The differential expression of EcR and Diptericin, turned out to play a less significant role in both sexes, especially in females.
Figure 4.
Heterogeneity of gene expression among flies with different reproductive status. PCA based on expression of genes of interest in males and females infected with M. marinum at all time points (a), PC1 scores (b) and variable contribution to PC1 and PC2 (c). Each circle represents an individual and lines are means. Statistically significant differences were represented as follow: *P ≤ 0.05, ****P ≤ 0.0001 (Kruskal–Wallis test).
We performed the same analysis but taking into consideration each time point separately in order to identify the chronological importance of the expression of each gene (Supplementary Fig. 4). Results showed several common patterns between males and females. First, flies kept alone presented an expression profile significantly different from the other two groups at 24 h post-infection whereas virgins were those significantly different later in the infection. When observing which genes were relevant to differentiate between groups, we observed that Drosomycin was crucial at all time points whereas Diptericin was only significant later in the infection. In addition, we found that Upd3 was relevant at the beginning (24 h) and at late stages (10 days) of the infection, and EcR was crucial at the beginning of the infection but not 10 days post-infection. On the other hand, we also showed that Impl2 was more relevant among males than in females.
4. Discussion and conclusions
In this study, we have been able to decipher whether reproductive status of D. melanogaster has a cost in immunity against infection and whether those flies display a specific phenotype for each of these reproductive status group in terms of tolerance and resistance to that infection. Several laboratory studies have revealed a significant ‘cost of mating’ to Drosophila females in the form of reduced longevity [72–75]. However, other studies have previously reported contrary results in both wild-caught [75] and laboratory-reared [76] flies. As we have observed in our study, they also showed no cost of mating in mated females flies compared to virgins when uninfected. These discrepancies are mostly driven by both the genetic background and the environment. Aspects as dietary and/or seasonal husbandry, even within the same laboratory, can substantially affect the study of mating costs and sex differences [75, 76]. However, in our study all experiments were performed independently and per triplicate. We performed all infections and other manipulations of flies at the same time slot, to not disrupt their circadian rhythm or create new possible variables for the results obtained. In addition, the wild-type D. melanogaster Oregon-R-C, has demonstrated to have a genetic background that does not affect its fertility, on contrary to other D. melanogaster crosses [77, 78]. One hypothesis that might explain this phenomenon we observed in our study is that non-infected flies have been exposed to a sterile injection. This sterile wounding induces an immune response that triggers the melanization of the damaged tissue via activation of the phenoloxidase cascade [79], and the production of reactive oxygen species derived from melanization has been shown to correlate with reduced longevity in flies [80]. It has been described that the seminal fluids induce the production of Juvenile hormone in mated females, which reduces the production of phenoloxidase [36, 81, 82]. Thus, these differences in the survival of uninfected female flies might be explained by the reduction in the oxidative response after a sterile damage in mated females, which is not altered in mated males.
Regarding the response to infections, a vast majority of studies described that both males and females reduce their resistance to some infections after mating [48, 53, 83]. Yet, some others reported no changes or even an increase in the resistance to infections after mating [6, 49, 54, 84]. In contrast, data presented herein shows that the opposite happens in both sexes during infection with M. marinum. Males increase their resistance to the infection after mating when they are together with females, although at the expense of a reduced survival, while females increase their resistance to M. marinum infection after mating, independently of the presence or absence of males. These sex-dependant results suggest that the benefit of mating might depend on the time elapsed since the cohabitation in males, but not in females.
Overall, phenotypic results from this study show that the sexual dimorphism observed in D. melanogaster in the outcome of M. marinum infection is highly related to the reproductive status of the host. In addition, a negative correlation between tolerance and resistance has been shown in our study, probably because the energy required to reduce the bacillary load directly affects other physiological mechanisms and this results in reduced survival. Our results together with previous published studies seem to indicate that the existence of a trade-off between immunity and reproduction is specific for each host-pathogen system studied, and thus, when assessing the differences in the tolerance and/or resistance of D. melanogaster to other infections, the hosts’ reproductive situation needs to be characterized.
Regarding the hormonal levels and their relation with the immune response, our data show that certain EcR levels are required for the flies after the infection, as flies with higher basal levels presented lower induction or repression of the gene post-infection. However, this correlation was only statistically significant in males. Previous studies showed that ecdysone induces the expression of the receptor PGRP-LC and, thus, modulates the Imd pathway [42]. Other studies had related the Imd pathway in the control of resistance to infections [85], while its negative regulation mediates tolerance to infections [86], although any of these were performed in mycobacterial infections. Our data support these findings, as those flies with prior higher basal levels of EcR showed higher expression levels of Diptericin immediately after infection and correlate with the more resistant phenotypes, while flies that did not show early production of this AMP were more tolerant to the infection.
Several studies performed with a wide range of pathogens have described the Toll pathway as key in determining resistance to infections [35, 87, 88], and even to be essential for resistance in males, but not in females [9]. However, no role in increasing tolerance to infection has been described for this pathway previously. In our study, we showed that production of the Toll-dependant AMP, Drosomycin, early in the infection was linked with increased tolerance but reduced resistance to M. marinum infection in flies, while their activation in later stages of the infection correlated with increased resistance. Altogether, these data suggest a role for the Toll pathway in determining both tolerance and resistance to M. marinum infections. Recent studies have described a Toll-dependent metabolic switch that directs fatty acids from neutral cellular storage toward phospholipid biosynthesis [89], which might reduce the lipid droplet accumulation inside phagocytic cells and, thus, hindered the intracellular replication of M. marinum.
This study also suggests a dual role for Upd3 in M. marinum infections. Our data correlate with previous studies that linked Upd3-deficient flies with a reduction of lipid droplet accumulation within cells and reduced bacillary loads [62], showing that flies with increased expression of Upd3 early in the infection presented higher bacillary loads. Yet, this study also suggests a second role for Upd3, as its production late in the infection correlate with increased resistance, probably due to its role in increasing the metabolism of phagocytic cells [27]. In addition, our data also suggest that the increased resistance in females alone might be driven by Upd3 rather than by innate immune pathways. Finally, the expression of Impl2 during M. marinum infections seems to be only relevant in males that are not actively mating and to be related to increased bacillary loads, although further studies should be performed to determine its role during mycobacterial infections in D. melanogaster.
In this study, we have focussed on characterizing the phenotypic immune profile of a population of D. melanogaster individuals (differing by their reproductive status) in response to a M. marinum infection. Lastly, to be able to correlate these findings with their evolutionary implications (e.g. changes in egg investment or genetic changes among individuals in a population among generations) it would be necessary to study how D. melanogaster evolve to these traits (reproductive status selection regimens and infection to M. marinum) along several generations [6]. Only by doing this, we will be able to shed light, not only in the phenotypical but in the importance of this findings in the evolution of both species.
5. Concluding remarks
It has been extensively studied that males and females respond differently to the same infections. Many of these studies have focussed on establishing the physiological basis for these differences, but very few have studied how the reproductive status of the host affects males and females and the role it plays in the response to infection. This study aimed to show that the intrinsic differences observed between males and females during the infection by M. marinum were tightly related to the reproductive status of the host and that reproduction affects males and females differently. Here we show that being actively mating (kept together) increased the resistance but reduced the tolerance to the infection in males. The same phenotype was found in mated females regardless if they were kept alone or together after mating. All of this demonstrates that when assessing the sexual dimorphism of D. melanogaster, the hosts’ reproductive situation needs to be characterized.
The results obtained in this study also suggest a possible role for the Toll pathway in determining tolerance to infection, while it appears that the Imd pathway is associated with increased resistance. Results also showed a correlation between basal levels of EcR and its induction after the infection, suggesting that a certain amount of this receptor is required upon infection. This phenomenon was observed in both males and females in all reproductive statuses, although only in males was statistically significant. In addition, basal levels of EcR also correlated with higher expression levels of Diptericin.
Finally, we have also proposed a dual role for Upd3 upon infection with M. marinum. Our results show that an early production of this molecule correlate with higher bacillary loads. This might be linked with the Upd3-mediated lipid droplets accumulation inside phagocytic cells, which favours mycobacterial replication. However, the late production of this molecule is associated with lower bacillary loads, most likely due to its role in increasing phagocytic cells metabolism. Finally, we could only validate the role for Impl2 in increasing the tolerance in non-virgin non-actively mating males.
Supplementary Material
Contributor Information
Marta Arch, Tuberculosis Research Unit, Germans Trias i Pujol Research Institute (IGTP), Badalona, Catalonia, Spain; Comparative Medicine and Bioimage Centre of Catalonia (CMCiB), Germans Trias i Pujol Research Institute (IGTP), 08916 Badalona, Catalonia, Spain.
Maria Vidal, Tuberculosis Research Unit, Germans Trias i Pujol Research Institute (IGTP), Badalona, Catalonia, Spain; Genetics and Microbiology Department, Universitat Autònoma de Barcelona, Bellaterra, Catalonia, Spain.
Esther Fuentes, Tuberculosis Research Unit, Germans Trias i Pujol Research Institute (IGTP), Badalona, Catalonia, Spain; Comparative Medicine and Bioimage Centre of Catalonia (CMCiB), Germans Trias i Pujol Research Institute (IGTP), 08916 Badalona, Catalonia, Spain; Microbiology Department, Laboratori Clínic Metropolitana Nord, Germans Trias i Pujol University Hospital, 08916 Badalona, Catalonia, Spain.
Anmaw Shite Abat, Tuberculosis Research Unit, Germans Trias i Pujol Research Institute (IGTP), Badalona, Catalonia, Spain; Department of Veterinary Paraclinical Studies, University of Gondar, Gondar, Ethiopia.
Pere-Joan Cardona, Tuberculosis Research Unit, Germans Trias i Pujol Research Institute (IGTP), Badalona, Catalonia, Spain; Comparative Medicine and Bioimage Centre of Catalonia (CMCiB), Germans Trias i Pujol Research Institute (IGTP), 08916 Badalona, Catalonia, Spain; Microbiology Department, Laboratori Clínic Metropolitana Nord, Germans Trias i Pujol University Hospital, 08916 Badalona, Catalonia, Spain; Genetics and Microbiology Department, Universitat Autònoma de Barcelona, Bellaterra, Catalonia, Spain; Centro de Investigación Biomédica en Red en Enfermedades Respiratorias (CIBERES), Instituto de Salud Carlos III (ISCIII), Madrid, Spain.
Author contributions
Marta Arch (Conceptualization [equal], Data curation [equal], Investigation [equal], Methodology [equal], Software [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Maria Vidal (Investigation [equal], Methodology [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Esther Fuentes (Investigation [equal], Methodology [equal], Validation [equal], Writing—review & editing [equal]), Anmaw Shite Abat (Investigation [equal], Methodology [equal], Writing—review & editing [equal]), and Pere-Joan Cardona (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Resources [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]).
Funding
This research has been funded by ‘La Caixa’ Foundation (ID 100010434), under agreement LCF/PR/GN16/10290002, Spanish Government-FEDER Funds through PI20/01431 grant, AGAUR 2017 SGR 00500, 2021 SGR 00920 and 2021 SGR 00934. Anmaw Shite Abat was granted by the EMJMD LIVE (Erasmus + Mundus Joint Master Degree Leading International Vaccinology Education), co-funded by the EACEA (Education, Audiovisual and Culture Executive Agency, award 2018-1484) of the European commission, receives a scholarship from the EACEA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1. Stearns SC. Trade-offs in life-history evolution. Funct Ecol 1989;3:259. doi: 10.2307/2389364. [DOI] [Google Scholar]
- 2. Mills SC, Grapputo A, Jokinen Iet al. Fitness trade-offs mediated by immunosuppression costs in a small mammal. Evolution 2010;64:166–79. doi: 10.1111/j.1558-5646.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 3. Abrams ET, Miller EM.. The roles of the immune system in women’s reproduction: evolutionary constraints and life history trade-offs. Am J Phys Anthropol 2011;146:134–54. doi: 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- 4. Schwenke RA, Lazzaro BP, Wolfner MF.. Reproduction–immunity trade-offs in insects. Annu Rev Entomol 2016;61:239–56. doi: 10.1146/annurev-ento-010715-023924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albery GF, Watt KA, Keith Ret al. Reproduction has different costs for immunity and parasitism in a wild mammal. Funct Ecol 2020;34:229–39. doi: 10.1111/1365-2435.13475. [DOI] [Google Scholar]
- 6. Syed ZA, Gupta V, Arun MGet al. Absence of reproduction-immunity trade-off in male Drosophila melanogaster evolving under differential sexual selection. BMC Evol Biol 2020;20:1–10. doi: 10.1186/s12862-019-1574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rose S, Beckwith EJ, Burmester Cet al. Pre-copulatory reproductive behaviours are preserved in Drosophila melanogaster infected with bacteria. Proc R Soc B 2022;289:1–9. doi: 10.1098/rspb.2022.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belmonte RL, Corbally MK, Duneau DFet al. Sexual dimorphisms in innate immunity and responses to infection in Drosophila melanogaster. Front Immunol 2020;10:3075. doi: 10.3389/fimmu.2019.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duneau DF, Kondolf HC, Im JHet al. The Toll pathway underlies host sexual dimorphism in resistance to both gram-negative and gram-positive bacteria in mated Drosophila. BMC Biol 2017;15:1–17. doi: 10.1186/s12915-017-0466-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon KE, Wolfner MF, Lazzaro BP.. A single mating is sufficient to induce persistent reduction of immune defense in mated female Drosophila melanogaster. J Insect Physiol 2022;140:1–10. doi: 10.1016/j.jinsphys.2022.104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buchon N, Silverman N, Cherry S.. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Younes S, Al-Sulaiti A, Nasser EAAet al. Drosophila as a model organism in host–pathogen interaction studies. Front Cell Infect Microbiol 2020;10:214. doi: 10.3389/fcimb.2020.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Gregorio E, Spellman P, Rubin Get al. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A 2001;98:12590–5. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanji T, Hu X, Weber ANRet al. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol 2007;27:4578–88. doi: 10.1128/mcb.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agaisse H, Petersen U, Boutros Met al. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell 2003;5:441–50. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 16. Arbouzova N, Zeidler M.. JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 2006;133:2605–16. doi: 10.1242/dev.02411. [DOI] [PubMed] [Google Scholar]
- 17. Lagueux M, Perrodou E, Levashina Eet al. Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci U S A 2000;97:11427–32. doi: 10.1073/pnas.97.21.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sternberg E. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 2006;6:318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee J, Edery I.. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol 2008;18:195–9. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotamisligil G, Erbay E.. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DiAngelo J, Bland M, Bambina Set al. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A 2009;106:20853–8. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzawa M, Muhammad N, Joseph Bet al. The toll signaling pathway targets the insulin-like peptide Dilp6 to inhibit growth in Drosophila. Cell Rep 2019;28:1439–1446.e5. doi: 10.1016/j.celrep.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 23. Agrawal N, Delanoue R, Mauri Aet al. The Drosophila TNF eiger is an adipokine that acts on insulin-producing cells to mediate nutrient response. Cell Metab 2016;23:675–84. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 24. Bajgar A, Kucerova K, Jonatova Let al. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol 2015;13:1–23. doi: 10.1371/journal.pbio.1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Almajwal A, Alam I, Zeb Fet al. Energy metabolism and allocation in selfish immune system and brain: a beneficial role of insulin resistance in aging. Food Nutr Sci 2018;10:64–80. doi: 10.4236/fns.2019.101006. [DOI] [Google Scholar]
- 26. Dolezal T, Krejcova G, Bajgar Aet al. Molecular regulations of metabolism during immune response in insects. Insect Biochem Mol Biol 2019;109:31–42. doi: 10.1016/j.ibmb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 27. Bajgar A, Krejčová G, Doležal T.. Polarization of macrophages in insects: opening gates for immuno-metabolic research. Front Cell Dev Biol 2021;9:103. doi: 10.3389/fcell.2021.629238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krejčová G, Bajgar A, Nedbalová Pet al. Macrophage-derived insulin/IGF antagonist ImpL2 regulates systemic metabolism for mounting an effective acute immune response in Drosophila. bioRxiv 2021. doi: 10.1101/2020.09.24.311670. [DOI] [Google Scholar]
- 29. Chakrabarti S, Dudzic J, Li Xet al. Remote control of intestinal stem cell activity by haemocytes in and Drosophila. PLoS Genet 2016;12:1–24. doi: 10.1371/journal.pgen.1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin M, Cha N, Koranteng Fet al. Subpopulation of macrophage-like plasmatocytes attenuates systemic growth via JAK/STAT in the Drosophila fat body. Front Immunol 2020;11:63. doi: 10.3389/fimmu.2020.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kopp A, Duncan I, Carroll SB.. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 2000;408:553–9. doi: 10.1038/35046017. [DOI] [PubMed] [Google Scholar]
- 32. Millington JW, Rideout EJ.. Sex differences in Drosophila development and physiology. Curr Opin Physiol 2018;6:46–56. doi: 10.1016/j.cophys.2018.04.002. [DOI] [Google Scholar]
- 33. Kutch IC, Fedorka KM.. A test for Y-linked additive and epistatic effects on surviving bacterial infections in Drosophila melanogaster. J Evol Biol 2017;30:1400–8. doi: 10.1111/jeb.13118. [DOI] [PubMed] [Google Scholar]
- 34. Leech T, Evison SEF, Armitage SAOet al. Interactive effects of social environment, age and sex on immune responses in Drosophila melanogaster. J Evol Biol 2019;32:1082–92. doi: 10.1111/jeb.13509. [DOI] [PubMed] [Google Scholar]
- 35. Chowdhury M, Li C-F, He Zet al. Toll family members bind multiple Spätzle proteins and activate antimicrobial peptide gene expression in Drosophila. J Biol Chem 2019;294:10172–81. doi: 10.1074/jbc.ra118.006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flatt T, Heyland A, Rus Fet al. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol 2008;211:2712–24. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Regan JC, Brandão AS, Leitão ABet al. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS Pathog 2013;9:e1003720. doi: 10.1371/journal.ppat.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Di Cara F, King-Jones K.. How clocks and hormones act in concert to control the timing of insect development. Curr Top Dev Biol 2013;105:1–36. doi: 10.1016/B978-0-12-396968-2.00001-4. [DOI] [PubMed] [Google Scholar]
- 39. Schwedes CC, Carney GE.. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol 2012;58:293–302. doi: 10.1016/j.jinsphys.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 40. Sieber MH, Spradling AC.. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr Biol 2015;25:993–1004. doi: 10.1016/j.cub.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ameku T, Niwa R.. Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet 2016;12:e1006123. doi: 10.1371/journal.pgen.1006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rus F, Flatt T, Tong Met al. Ecdysone triggered PGRP-LC expression controls Drosophila innate immunity. EMBO J 2013;32:1626–38. doi: 10.1038/emboj.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sorrentino RP, Carton Y, Govind S.. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- 44. Hirashima A, Rauschenbach I, Sukhanova M.. Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Biosci Biotechnol Biochem 2000;64:2657–62. doi: 10.1271/bbb.64.2657. [DOI] [PubMed] [Google Scholar]
- 45. Simon A, Shih C, Mack Aet al. Steroid control of longevity in Drosophila melanogaster. Science 2003;299:1407–10. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 46. Fedorka K, Linder J, Winterhalter Wet al. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc Biol Sci 2007;274:1211–7. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Short S, Lazzaro B.. Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proc Biol Sci 2010;277:3649–57. doi: 10.1098/rspb.2010.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Short SM, Wolfner MF, Lazzaro BP.. Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. J Insect Physiol 2012;58:1192–201. doi: 10.1016/j.jinsphys.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McKean KA, Nunney L.. Bateman’s principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution 2005;59:1510–7. doi: 10.1554/04-657. [DOI] [PubMed] [Google Scholar]
- 50. Barnes A, Wigby S, Boone Jet al. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc Biol Sci 2008;275:1675–83. doi: 10.1098/rspb.2008.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ye Y, Chenoweth S, McGraw E.. Effective but costly, evolved mechanisms of defense against a virulent opportunistic pathogen in Drosophila melanogaster. PLoS Pathog 2009;5:e1000385. doi: 10.1371/journal.ppat.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McKean K, Yourth C, Lazzaro Bet al. The evolutionary costs of immunological maintenance and deployment. BMC Evol Biol 2008;8:1–19. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McKean KA, Nunney L.. Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc Natl Acad Sci U S A 2001;98:7904–9. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gupta V, Ali ZS, Prasad NG.. Sexual activity increases resistance against Pseudomonas entomophila in male Drosophila melanogaster. BMC Evol Biol 2013;13:1–11. doi: 10.1186/1471-2148-13-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neyrolles O, Quintana-Murci L.. Sexual inequality in tuberculosis. PLoS Med 2009;6:e1000199. doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Global Tuberculosis Report 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (9 Aug 2022, date last accessed).
- 57. Prouty MG, Correa NE, Barker LPet al. Zebrafish-Mycobacterium marinum model for mycobacterial pathogenesis. FEMS Microbiol Lett 2003;225:177–82. doi: 10.1016/S0378-1097(03)00446-4. [DOI] [PubMed] [Google Scholar]
- 58. Bouley DM, Ghori N, Mercer KLet al. Dynamic nature of host-pathogen interactions in Mycobacterium marinum granulomas. Infect Immun 2001;69:7820–31. doi: 10.1128/iai.69.12.7820-7831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tobin DM, Ramakrishnan L.. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell Microbiol 2008;10:1027–39. doi: 10.1111/J.1462-5822.2008.01133.X. [DOI] [PubMed] [Google Scholar]
- 60. Dionne MS, Ghori N, Schneider DS.. Drosophila melanogaster is a genetically tractable model host for Mycobacterium marinum. Infect Immun 2003;71:3540–50. doi: 10.1128/iai.71.6.3540-3550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dionne M, Pham L, Shirasu-Hiza Met al. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol 2006;16:1977–85. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 62. Péan CB, Schiebler M, Tan SWSet al. Regulation of phagocyte triglyceride by a STAT-ATG2 pathway controls mycobacterial infection. Nat Commun 2017;8:1–11. doi: 10.1038/ncomms14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weerdenburg EM, Abdallah AM, Rangkuti Fet al. Genome-wide transposon mutagenesis indicates that Mycobacterium marinum customizes its virulence mechanisms for survival and replication in different hosts. Infect Immun 2015;83:1778–88. doi: 10.1128/iai.03050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gandon S, Michalakis Y.. Evolution of parasite virulence against qualitative or quantitative host resistance. Proc R Soc London Ser B Biol Sci 2000;267:985–90. doi: 10.1098/rspb.2000.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lefèvre T, Williams AJ, De Roode JC.. Genetic variation in resistance, but not tolerance, to a protozoan parasite in the monarch butterfly. Proc R Soc B Biol Sci 2011;278:751–9. doi: 10.1098/rspb.2010.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Simms EL, Triplett J.. Costs and benefits of plant responses to disease: resistance and tolerance. Evolution 1994;48:1973–85. doi: 10.2307/2410521. [DOI] [PubMed] [Google Scholar]
- 67. Kover PX, Schaal BA.. Genetic variation for disease resistance and tolerance among Arabidopsis thaliana accessions. Proc Natl Acad Sci U S A 2002;99:11270–4. doi: 10.1073/pnas.102288999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Råberg L, Sim D, Read AF.. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007;318:812–4. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 69. Blanchet S, Rey O, Loot G.. Evidence for host variation in parasite tolerance in a wild fish population. Evol Ecol 2010;24:1129–39. doi: 10.1007/s10682-010-9353-x. [DOI] [Google Scholar]
- 70. Howick VM, Lazzaro BP.. The genetic architecture of defence as resistance to and tolerance of bacterial infection in Drosophila melanogaster. Mol Ecol 2017;26:1533–46. doi: 10.1111/mec.14017. [DOI] [PubMed] [Google Scholar]
- 71. Livak KJ, Schmittgen TD.. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001;25:402–8. doi: 10.1006/METH.2001.1262. [DOI] [PubMed] [Google Scholar]
- 72. Chapman T, Hutchings J, Partridge L.. No reduction in the cost of mating for Drosophila melanogaster females mating with spermless males. Proc R Soc London Ser B Biol Sci 1993;253:211–7. doi: 10.1098/rspb.1993.0105. [DOI] [PubMed] [Google Scholar]
- 73. Liddle LF, Chapman T, Partridge Let al. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 1995;373:241–4. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 74. Ueyama M, Fuyama Y.. Enhanced cost of mating in female sterile mutants of Drosophila melanogaster. Genes Genet Syst 2003;78:29–36. doi: 10.1266/ggs.78.29. [DOI] [PubMed] [Google Scholar]
- 75. Markow TA. “Cost” of virginity in wild Drosophila melanogaster females. Ecol Evol 2011;1:596–600. doi: 10.1002/ece3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoffman JM, Dudeck SK, Patterson HKet al. Sex, mating and repeatability of Drosophila melanogaster longevity. R Soc Open Sci 2021;8:210273. doi: 10.1098/rsos.210273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Aruna S, Flores HA, Barbash DA.. Reduced fertility of Drosophila melanogaster hybrid male rescue (Hmr) mutant females is partially complemented by Hmr orthologs from sibling species. Genetics 2009;181:1437–50. doi: 10.1534/genetics.108.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Massey JH, Chung D, Siwanowicz Iet al. The yellow gene influences drosophila male mating success through sex comb melanization. Elife 2019;8:1–20. doi: 10.7554/elife.49388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Márkus R, Kurucz E, Rus Fet al. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett 2005;101:108–11. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 80. Shields HJ, Traa A, Van Raamsdonk JM.. Beneficial and detrimental effects of reactive oxygen species on lifespan: a comprehensive review of comparative and experimental studies. Front Cell Dev Biol 2021;9:628157. doi: 10.3389/fcell.2021.628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rolff J, Siva-Jothy MT.. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc Natl Acad Sci U S A 2002;99:9916–8. doi: 10.1073/pnas.152271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rantala MJ, Vainikka A, Kortet R.. The role of juvenile hormone in immune function and pheromone production trade-offs: a test of the immunocompetence handicap principle. Proc R Soc B Biol Sci 2003;270:2257–61. doi: 10.1098/rspb.2003.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fan Y, Rafaeli A, Moshitzky Pet al. Common functional elements of Drosophila melanogaster seminal peptides involved in reproduction of Drosophila melanogaster and Helicoverpa armigera females. Insect Biochem Mol Biol 2000;30:805–12. doi: 10.1016/S0965-1748(00)00052-7. [DOI] [PubMed] [Google Scholar]
- 84. Wigby S, Domanitskaya EV, Choffat Yet al. The effect of mating on immunity can be masked by experimental piercing in female Drosophila melanogaster. J Insect Physiol 2008;54:414–20. doi: 10.1016/j.jinsphys.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nelson B, Freisinger T, Ishii Ket al. Activation of Imd pathway in hemocyte confers infection resistance through humoral response in Drosophila. Biochem Biophys Res Commun 2013;430:1120–5. doi: 10.1016/j.bbrc.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 86. Prakash A, Monteith KM, Vale PF.. Negative regulation of IMD contributes to disease tolerance during systemic bacterial infection in Drosophila. bioRxiv 2021. doi: 10.1101/2021.09.23.461574. [DOI] [Google Scholar]
- 87. Lau GW, Goumnerov BC, Walendziewicz CLet al. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa. Infect Immun 2003;71:4059–66. doi: 10.1128/IAI.71.7.4059-4066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ferreira AG, Naylor H, Esteves SSet al. The toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog 2014;10:e1004507. doi: 10.1371/journal.ppat.1004507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Martínez BA, Hoyle RG, Yeudall Set al. Innate immune signaling in Drosophila shifts anabolic lipid metabolism from triglyceride storage to phospholipid synthesis to support immune function. PLoS Genet 2020;16:e1009192. doi: 10.1371/journal.pgen.1009192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.