Abstract
Purpose:
Long-term lithium therapy (LTLT) has been associated with chronic kidney disease (CKD). We investigated changes in clinical characteristics, pharmacotherapeutic treatments for medical/psychiatric disorders, and outcomes among patients with bipolar disorder (BD) and CKD on LTLT in a 2-year mirror-image study design.
Methods:
Adult BD patients on LTLT for ≥1 year who enrolled in the Mayo Clinic Bipolar Disorder Biobank, and developed CKD (stage 3) were included, and our study was approved by the Mayo Clinic Institutional Review Board. The primary outcome was the time to the first mood episode after CKD diagnosis among the lithium (Li) continuers and discontinuers. Cox proportional hazards models were used to estimate the time to the first mood episode. We tested for differences in other medication changes between the Li continuers and discontinuers group using Mantel-Haenszel X2 tests (linear associations).
Results:
Of 38 BD patients who developed CKD, 18 (47%) discontinued Li, and the remainder continued (n=20). The median age of the cohort was 56 years (interquartile range [IQR], 48–67 years), 63.2% were females, and 97.4% were White. As compared to continuers, discontinuers had more psychotropic medication trials (6, [IQR, 4—6] vs 3 [IQR, 2—5], P = 0.02), a higher rate of 1 or more mood episodes (61% vs 10%, P = 0.002), and a higher risk of a mood episode after CKD diagnoses (Hazard Ratio, 8.38; 95% confidence interval, 1.85—38.0 [log-rank P = 0.001]).
Conclusions:
Bipolar Disorder patients on LTLT who discontinued Li had a higher risk for relapse and a shorter time to the first mood episode, suggesting a need for more thorough discussion before Li discontinuation after the CKD diagnosis.
INTRODUCTION
Lithium (Li) is a gold-standard treatment for bipolar disorder (BD). Still, it remains under-prescribed,1 in part due to concerns regarding the association of long-term lithium therapy (LTLT) with kidney dysfunction.2–4 This is while the nature of this association remains unclear.2–4 Patients with BD have a higher prevalence of hypertension and diabetes mellitus5,6, which are independent risk factors for chronic kidney disease (CKD).7 A prior historical cohort study of BD patients on LTLT suggested diabetes mellitus was one of the main predictors for CKD development.4 Almost 50% of patients discontinued Li after they were diagnosed with CKD Stage 3 (estimated glomerular filtration rate [eGFR] between 30 and 59),8 but a short-term longitudinal follow-up did not reveal any significant differences in the progression of CKD between those who continued Li (continuers) versus those who discontinued Li (discontinuers).4 These results were consistent with a large population-based study from Denmark,9 finding that Li use alone may not contribute to CKD progression. A recent study from Korea reported no significant difference in the prevalence of CKD development among patients treated with lithium compared to valproate.10 In addition, a study conducted by Kessing et al11 identified that patients who continued Li, despite the CKD diagnosis, did not have an increased end-stage kidney disease rate compared with patients who discontinued Li and switched to a different mood stabilizer. Additional studies have shown successful use of Li in BD patients with hemodialysis12–15 and suggested restarting Li use in cases of mood destabilization and when mental health deteriorates with alternative psychiatric treatments like mood stabilizing anticonvulsants (MSACs) and second-generation atypical antipsychotics (SGAs). However, at the same time, some authors have suggested discontinuing Li in patients with CKD stages 4 and 5 because of fear of worsening kidney function or Li toxicity.16 Despite research for more than the last five to six decades, the controversy continues to linger.17
Several pathological changes have been reported among patients on LTLT, including tubulointerstitial and glomerular damage and kidney microcyst formation.18,19 However, the antiapoptotic effects of Li, which results in the formation of kidney microcysts, have been hypothesized as one of the primary mechanisms for a significant reduction in eGFR.18 Mood stabilizing anticonvulsants such as valproic acid, carbamazepine, and lamotrigine, have been advocated as Li alternatives. However, MSACs such as valproic acid have been shown to possess antiapoptotic properties and may also increase the risk of kidney microcyst formation.20 This could be the reason why, in the study conducted by Kessing et al,11 shifting from Li to an anticonvulsant rather than continuing Li was not advantageous among BD patients with CKD. Thus, valproic acid or other anticonvulsants may not provide any added benefit in terms of kidney function. On the other hand, lamotrigine, the only U.S Food and Drug Administration—approved mood stabilizer besides Li for maintenance therapy, is less effective than Li in preventing manic recurrences.21 Second-generation atypical antipsychotics are one of the frontline alternatives for BD management.22 However, recent data from large studies suggested the role of SGAs in CKD development23 with a high risk of acute kidney injury in older patients24. This would further limit the effective pharmacopeia for this unique population.
These controversies have created a clinical conundrum for treating physicians/psychiatrists in managing such BD patients with CKD. There is a lack of real-world clinical practice data to guide clinicians regarding the change in practices following CKD diagnosis in BD patients receiving LTLT. Most patients on LTLT are stable and at a high risk of decompensation in the context of Li discontinuation.25,26 Thus, it is essential to make an informed decision, especially regarding continuing/discontinuing Li.
Therefore, we conducted a two-year mirror-image study in a real-world clinical practice among BD patients on LTLT who developed CKD to further explore differences in clinical outcomes among continuers and discontinuers. We hypothesized that Li discontinuers have a higher risk of mood episodes than continuers and discontinuers have more psychotropic drug usage than continuers after the CKD diagnosis.
MATERIALS AND METHODS
This was a historical cohort study of adult patients (≥18 years) with BD who developed CKD while on LTLT for ≥1 year. We studied subjects from the Mayo Clinic Bipolar Disorder Biobank, Rochester, Minnesota.4,27 Details regarding the Mayo Clinic Bipolar Biobank have been published previously.27 All the patients fulfilled DSM-IV-TR criteria and diagnostic confirmation of BD-I/BD-II or schizoaffective BD with the Structured Clinical Interview for DSM-IV. Only patients who provided consent for the use of their medical records for research were included and this study was approved by the Mayo Clinic Institutional Review Board. The diagnosis of CKD was defined by either Kidney Disease Outcomes Quality Initiative clinical practice guidelines for CKD,8,28 or the documented clinical diagnosis of CKD in the electronic medical records. CKD stage ≥3 was assessed based on the eGFR of <60 ml/min/1.73 m2.
A mirror-image study design is a unique method to compare a previous treatment period with a subsequent treatment period.26,29 Mirror-image approaches have utility to examine the impact of different treatment modalities/events where the outcomes of those treatments/events are evaluated for the same duration of time, both before and after the treatment/event. By doing so, each patient becomes their own control, minimizing confounding at an individual level.29,30 The index event for our mirror-image study was the CKD stage 3 diagnosis. Mirror periods were defined as 2 years pre-CKD and post-CKD among BD patients on LTLT. The median time to stop Li after the CKD diagnosis in discontinuers was 0.2 years (interquartile range [IQR], 0—0.9 years).4
Mood disorder fellows psychiatrists/trained post-doctoral physicians (RK, NAN, MP, BJ) extracted demographic, clinical characteristics, medical comorbidities,31 treatment interventions (antihypertensives: angiotensin-converting enzyme inhibitors [ACEI], angiotensin II receptor blockers [ARBs], beta-blockers, calcium channel blockers, diuretics; antidepressants; mood stabilizers; antipsychotics; stimulants; benzodiazepines; and other psychotropics), and treatment outcomes (mood episodes) from electronic health records. Estimated GFR was calculated with the CKD Epidemiology Collaboration equation using the standardized creatinine values.32
STATISTICAL ANALYSIS
Descriptive statistics for categorical and continuous variables were summarized as frequencies and median (IQR), respectively. Wilcoxon rank sum and Fisher exact tests were used to compare continuous and categorical outcomes, respectively. For each subject, we determined whether they had increased, no change, or decreased their use of each specific type of medication. Next, we tested for differences in demographics, clinical characteristics, and medication variables between the groups using exact Mantel-Haenszel X2 tests (linear associations) and Fisher exact tests after subsetting to only those who had increased or decreased. Finally, we tested for differences in increased versus reduced medication use between groups using exact McNemar tests.
Our primary outcome was time to the first mood episode after CKD diagnosis, which was compared between the continuers and discontinuers. Cox proportional hazards model was used to estimate the hazard ratio and median time to the first mood episode among the continuers and discontinuers post-CKD diagnoses. Kaplan–Meier plots were used to show the time from CKD diagnosis to the first mood episode among continuers and discontinuers. Analyses were performed in SAS Studio 3.81 (SAS Institute, Cary NC). P-value < .05 was considered significant.
RESULTS
Of 41 patients who developed CKD, follow-up data were unavailable for 2 patients, and 1 patient discontinued Li after 2 years. Thus, after excluding 3 patients, we included 38 patients in the primary analysis. The median age of the cohort was 56 years (IQR, 48–67 yrs.), 63% were females, and 97% were White (Table 1). There were no significant differences in demographic and clinical variables (age, sex, antihypertensives, diuretics, statins, and creatinine or eGFR) between continuers and discontinuers at the time of CKD diagnoses (all P > 0.1). As compared to continuers, discontinuers were on a greater number of psychotropic medications and more likely to be on lamotrigine at the time of CKD (both P = 0.04).
Table 1.
Clinical and Demographic Characteristics of Patients at the Time of CKD and Comparison Between Patients Who Discontinued Li (Discontinuer Group) Versus Who Continued Li (Continuer Group)
| Characteristics | Total Sample (N=38) | Li Discontinuers (N=18) | Li Continuers (N=20) | P-value |
|---|---|---|---|---|
| Age, median (IQR), y | 56 (48, 67) | 52 (48, 63) | 59 (48, 69) | 0.24 |
| Race | ||||
| • White, n (%) | 37 (97%) | 17 (94%) | 20 (100%) | 0.47 |
| • Other, n (%) | 1 (3%) | 1 (6%) | 0 (0%) | |
| Female, n (%) | 24 (63%) | 12 (67%) | 12 (60%) | 0.74 |
| Age at Li start | 0.87 | |||
| • Median (Q1, Q3) | 42.85 (28.42,51.90) | 41.77 (31.55,52.37) | 42.80(28.41,51.85) | |
| Total duration on lithium, y | 0.07 | |||
| • Median (Q1, Q3) | 14.57 (9.66,23.99) | 14.45 (9.46, 23.99) | 8.46 (6.27, 15.26) | |
| Comorbidities | ||||
| Anxiety Disorders | 18 (47.4%) | 9 (50.0%) | 9 (45.0%) | 1.00 |
| ADHD | 3 (0.8%) | 1 (5.6%) | 2 (10.0%) | 1.00 |
| History of psychosis | 21 (55.2%) | 10 (55.6%) | 11 (55.0%) | 1.00 |
| Hypertension | 28 (73.7%) | 13 (72.2%) | 15 (75.0%) | 1.00 |
| Medications | ||||
| A) Anti-HTN Drugs, n (%) | ||||
| • ACEIs-ARBs | 6 (15.8%) | 3 (16.7%) | 3 (15.0%) | 1.00 |
| • ACEIs | 3 (7.9%) | 2 (11.1%) | 1 (5.0%) | 0.60 |
| • ARBs | 3 (7.9%) | 1 (5.6%) | 2 (10.0%) | 1.00 |
| • CCB | 11 (28.9%) | 6 (33.3%) | 5 (25.0%) | 0.72 |
| • Beta-Blockers | 16 (42.1%) | 9 (50.0%) | 7 (35.0%) | 0.51 |
| B) Diuretics, n (%) | ||||
| • Hydrochlorothiazide | 2 (5.3%) | 1 (5.6%) | 1 (5.0%) | 1.00 |
| • Furosemide | 6 (15.8%) | 4 (22.2%) | 2 (10.0%) | 0.40 |
| • Other Diuretics (like K+ sparing diuretics) | 1 (2.7%) | 1 (5.9%) | 0 (0.0%) | 0.46 |
| C) Aspirin | 11 (28.9%) | 5 (27.8%) | 6 (30.0%) | 1.00 |
| D) Statins | 13 (34.2%) | 8 (44.4%) | 5 (25.0%) | 0.30 |
| Current number of Psychotropics in Median (Q1, Q3) | 3.00 (2.0, 4.0) | 3.0(3.0, 4.0) | 2.5 (1.0, 3.0) | 0.04 |
| • SSRI | 5 (13.2%) | 4 (22.2%) | 1 (5.0%) | 0.17 |
| • SNRI | 1 (2.6%) | 0 (0.0%) | 1 (5.0%) | 1.00 |
| • Bupropion | 2 (5.3%) | 2 (11.1%) | 0 (0.0%) | 0.22 |
| • Buspirone | 1 (2.6%) | 1 (5.6%) | 0 (0.0%) | 0.47 |
| Anti-Psychotics | 19 (50%) | 11 (61.1%) | 8 (40.0%) | 0.33 |
| MSACs | ||||
| • (Valproate/CBZ/OXC) | 7 (18.4%) | 5 (27.8%) | 2 (10.0%) | 0.22 |
| • Lamotrigine | 7 (18.4%) | 6 (33.3%) | 1 (5.0%) | 0.04 |
| • Gabapentin | 3 (7.9%) | 3 (16.7%) | 0 (0.0%) | 0.09 |
| Stimulants | 2 (5.3%) | 1 (5.6%) | 1 (5.0%) | 1.0 |
| Benzodiazepines | 18 (47.4%) | 11 (61.1%) | 7 (35.0%) | 0.19 |
| Z drugs | 4 (10.5%) | 2 (11.1%) | 2 (10.0%) | 1.00 |
| Creatinine, Median (IQR) | 1.30 (1.10, 1.41) | 1.30 (1.10, 1.80) | 1.25 (1.05, 1.41) | 0.28 |
| eGFR, Median (IQR) | 51.9 (47.4, 59.8) | 50.9 (45.3, 54.8) | 57.2 (48.0, 60.7) | 0.16 |
Appendix: ACEIs- Angiotensin-converting enzyme inhibitors; ADHD, attention deficit/hyperactivity disorder; ARB- Angiotensin II receptor blockers; CCB- Calcium channel blockers; CBZ- Carbamazepine; MSACs – Mood stabilizing anticonvulsants, OXC- Oxcarbazepine, SSRI- Selective Serotonin Reuptake Inhibitor, SNRI- Serotonin-Norepinephrine Reuptake Inhibitor.
Discontinuers had more psychotropic medication trials as compared to continuers in the 2 years post-CKD (median IQR, 6 [4—6] vs 3 [2—5], P = 0.02). Discontinuers were more likely to have mood episodes as compared to continuers (61% [11 of 18] vs 10% [2 of 20], P = 0.002) in the 2 years post-CKD diagnoses. Compared to continuers, discontinuers had a higher risk for a first mood episode after CKD diagnosis (Hazard Ratio, 8.38; 95% confidence interval, 1.85—38.0; log-rank P = 0.001) (Fig. 1). The median time to first mood episode for discontinuers was 17.6 months. Among discontinuers who had a mood episode (n = 11), 36% (n = 4 of 11) had a manic/mixed episode, whereas 64% (7 of 11) had a depressive episode, whereas all continuers with a mood episode had depression (n = 2). Among discontinuers with a mood episode, the time to recurrence for those whose episode was manic/mixed (mean 5.8 months; median, 4.2 months; IQR, 2.0—8.0 months) seemed to occur faster than for those whose episode was depressive (mean, 9.7 months; median, 4.1, IQR 3.3—17.6 months), however, this difference was not statistically significant. (Fig. 2). Six patients (33%) discontinued Li rapidly (within < 2 weeks), 5 patients (28%) discontinued Li gradually (over > 1 month), whereas duration of discontinuation details were missing for 7 patients (39%). Because of small sample sizes, we did not pursue subset analysis based on the rate of Li discontinuation.
Figure 1.
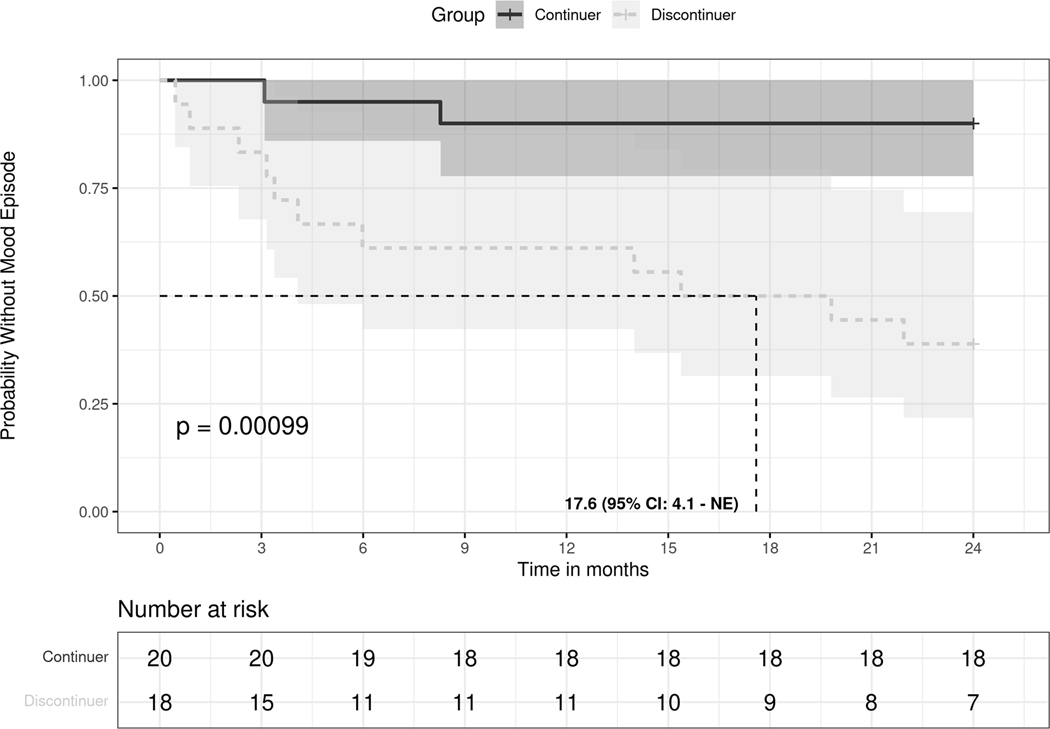
Probability without mood episode between continuers and discontinuers of long-term lithium therapy groups after CKD.
A solid black line indicates those continuing long-term lithium therapy, and a dashed grey line indicates those discontinuing long-term lithium therapy. The P value is estimated based on a log-rank test. Shaded areas represent a 95% CI range. The vertical dashed line indicates, among those who discontinued, the median time to the first mood episode. NE, non-existent.
Figure 2.
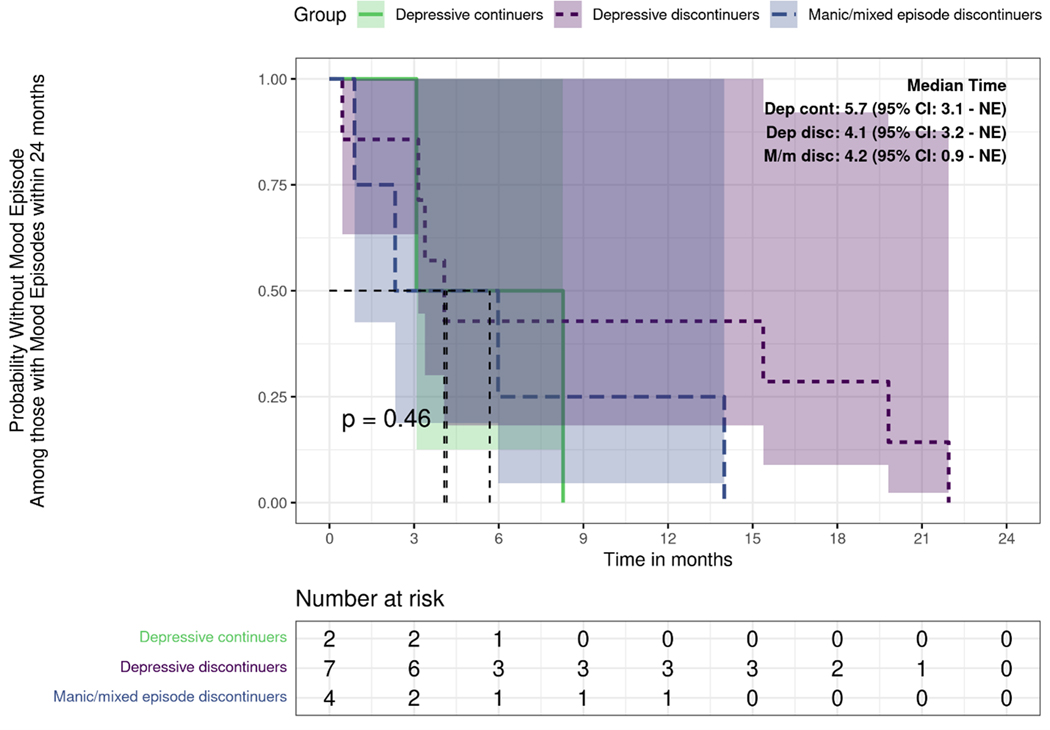
Probability without mood episodes among those with mood episodes between continuers and discontinuers of long-term lithium therapy after CKD within 24 months.
Changes in Psychotropic Medications
Supplementary Table 1, summarizes the prescription rates for psychotropic medications at different time points for both groups. In the analyzed 2 years of the study, discontinuers either discontinued (38%) or did not change (62%) SSRI prescriptions, while continuers tended not to change (94%) or increase (6%) (p=0.006). Furthermore, there were no significant differences in other pharmacotherapeutic interventions or changes in the number of psychotropics between groups (continuers vs discontinuers), both among all study subjects and among only those who did change (all P > 0.1). In addition, there were no significant differences in prescribed medications between the continuers and discontinuers in the CKD to 2 years post analysis (all P ≥ 0.05). In the CKD to 2 years postanalysis, across both groups, if patients had changed trazodone usage, they were significantly more likely to not have it at 2 years post-CKD than to have it (P = 0.007). There were no other significant differences in pharmacotherapeutic interventions or a number of psychotropics across both groups between 2 years pre-CKD to 2-years post (all P > 0.05) (Supplementary Table 2).
Changes in Medical drugs
Supplementalary Table 1 summarizes the prescription rates for antihypertensive medications (ACEIs/ARB, calcium channel blockers, diuretics, beta-blockers) at different time points for both groups. The only change in the prescription rate of beta-blockers from CKD to 2 years post-CKD suggested a difference between the group (P = 0.053), where continuers had a slight increase (5%) and primarily no change (95%) compared to discontinuers among whom 33% discontinued and 60% had no change.
Changes in kidney functions
There were no differences between the groups in creatinine or eGFR changes from pre-CKD to post-CKD or from CKD diagnosis to post (all P ≥ 0.39). Overall, creatinine was lower pre-CKD compared to post-CKD (median difference = −0.13; P = 0.001), and eGFR was higher pre-CKD than post-CKD (median, 9.2; P < 0.001) (Supplementary Table 2). Otherwise, no differences in eGFR were found between CKD diagnosis to post CKD (both P > 0.1).
DISCUSSION
Using a mirror-image design, this study examined real-world practices/ implications among BD patients with CKD on LTLT. Differences in outcomes among patients who continued or discontinued Li are underscored. Discontinuation of Li after CKD diagnoses led to a significant increase in the likelihood of a mood episode. In addition, the time to the first mood episode from CKD (stage 3) diagnosis was significantly shorter among discontinuers than continuers. This is consistent with prior studies showing an increased risk of recurrence following Li discontinuation in BD patients. For example, a study by Suppes et al25 showed early recurrence of mania.
In the 2-year post-CKD analysis, we did not observe significant differences in the pharmacotherapeutic interventions between groups (Li continuers vs discontinuers). However, there was a trend towards SSRI and beta-blocker discontinuation in the discontinuer group. Beta-blockers were one of the most common antihypertensives, especially among the continuers. Beta-blockers are also used to treat essential tremors, a common dose-related adverse effect with LTLT.33 In Li discontinuers with proteinuric kidney disease, ACEIs and ARBs can exert their kidney-protective effects by reducing filtration pressure.34 Thus, cautious use of ACEIs/ARBs is not uncommon in CKD patients. In our cohort, prescription rates for ACEIs-ARBs fluctuated between 16 to 26%.
In this cohort, 29% of patients were prescribed low-dose aspirin (similar among the two groups) to prevent cardiovascular disease (CVD). There was no significant difference in aspirin prescription rates across groups between 2 years pre-CKD and 2 years to 2-years post. Although guidelines no longer recommend aspirin for primary prevention of CVD/colorectal cancer, there is grade C evidence to initiate low dose aspirin for the prevention of CVD in adults aged 40 to 59 who have a 10% or greater CVD risk.35,36 In our study, aspirin was mostly prescribed for patients with comorbid hyperlipidemia, hypertension, diabetes, and higher CVD burden. This could have contributed to renal insufficiency for some patients. Fifty percent of patients had hyperlipidemia, and 34% were prescribed statins at the time of CKD diagnosis. Statins are often used as a first-line treatment for the prevention of CVD in patients with elevated cholesterol, diabetes, and 40–75 years of age.37
Studies using mathematical modeling techniques have identified Li as the treatment of choice for BD.38 A decision analysis modeling study advocated continuing Li unless the chances of progression of ESKD exceed 41% or if an MSAC outperforms Li in relapse prevention.39 Several studies40,41 have highlighted strategies to minimize Li’s adverse kidney effects by avoiding other nephrotoxins, maintaining low serum Li levels, regular monitoring of kidney function, and administering Li on the once-a-day dosing scheme. We noticed similar practices in the continuers in this study cohort. This could have contributed to a safer kidney profile in this cohort, as published earlier.4 Li continuers had a lower risk of mood episodes without showing a decline in kidney function than discontinuers. However, the fact that kidney function, at least in the short-term, remained similar in both groups, despite switching to MSACs or SGAs, highlights the need for careful consideration before Li discontinuation because of the high risk of mood decompensation. However, we would like to acknowledge that a 2-year follow-up for the potential evolution of CKD is rather short. It is possible that a 5- or 10-year follow-up might show very different results in the evolution of CKD in lithium continuers vs discontinuers.
Strengths and limitations
Using a mirror-image study design allowed us to conduct a quasi-experimental observational study with Li discontinuation as the intervention in patients with BD and CKD. The before and after mirror-image design minimizes confounding because individuals act as their controls.26,42 In addition, our study is based on real-world clinical data at symptom and treatment levels for the given period. The major limitations of our study include the small sample size and our study’s noninterventional and non-randomized design. In addition, there was an absence of a control group, which means we cannot conclude with certainty that Li discontinuation is superior or inferior in patients with CKD compared with prescribing any other mood stabilizer or antipsychotic drug. This is an observational cohort study with an inherent risk of confounding.
CONCLUSION
Bipolar disorder patients with CKD on LTLT who discontinued lithium therapy had a higher risk for relapse and a shorter time to the first mood episode, suggesting a need for more thorough discussion and probably a higher threshold before Li discontinuation after the CKD diagnosis.
Supplementary Material
AUTHOR DISCLOSURE INFORMATION
Nicolas Nuñez reports research support by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008685. Paul Croarkin has received research support from Neuronetics, Inc., NeoSync, Inc., and Pfizer. He has received grant-in-kind (equipment and laboratory support for research studies) from Assurex Health, Neuronetics, Inc., and MagVenture, Inc. He has served as a consultant for Engrail Therapeutics, Myriad Neuroscience, Procter & Gamble, and Sunovion. Balwinder Singh received grant support from Mayo Clinic. Susan McElroy is or has been a consultant to or member of the scientific advisory boards of Avanir, Allergan (now AbbVie), Bracket (now Signant Health), Naurex, Idorsia, Intra-Cellular Therapies, Inc., Shire (now Takeda), Sunovion, and Takeda. She is or has been a principal or co-investigator on studies sponsored by the Agency for Healthcare Research & Quality (AHRQ), Avenir, AstraZeneca, Cephalon, Forest, Marriott Foundation, Medibio, National Institute of Mental Health, Orexigen Therapeutics, Inc., Jazz, Shire (now Takeda), Sunovian, and Takeda Pharmaceutical Company Ltd. She is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent’s assignee, the University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent. Mark Frye Support from Assurex Health, Mayo Foundation, Medibio. Remaining authors declare no conflicts of interest. This research was supported by the Marriott Family Foundation.
Data Availability Statement:
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to restrictions-their containing information that could compromise the privacy of the research participant.
References:
- 1.Nivoli AM, Murru A, Vieta E. Lithium: still a cornerstone in the long-term treatment in bipolar disorder? Neuropsychobiology. 2010;62:27–35. [DOI] [PubMed] [Google Scholar]
- 2.Singh B, Nunez NA, Schock L, et al. Lithium continuation for a patient with bipolar disorder and chronic kidney disease. Bipolar Disord. 2020;22:316–318. [DOI] [PubMed] [Google Scholar]
- 3.Pahwa M, Singh B. Lithium therapy in patients with chronic kidney disease-A clinical dilemma? Eur Neuropsychopharmacol. 2020;34:87. [DOI] [PubMed] [Google Scholar]
- 4.Pahwa M, Joseph B, Nunez NA, et al. Long-term lithium therapy and risk of chronic kidney disease in bipolar disorder: A historical cohort study. Bipolar Disord. 2021;23:715–723. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen L, Strudsholm U, Foldager L, et al. Increased risk of hypertension in patients with bipolar disorder and patients with anxiety compared to background population and patients with schizophrenia. J Affect Disord. 2006;95:13–17. [DOI] [PubMed] [Google Scholar]
- 6.Calkin CV, Gardner DM, Ransom T, et al. The relationship between bipolar disorder and type 2 diabetes: more than just co-morbid disorders. Ann Med. 2013;45:171–181. [DOI] [PubMed] [Google Scholar]
- 7.Abboud H, Henrich WL. Clinical practice. Stage IV chronic kidney disease. N Engl J Med. 2010;362:56–65. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 9.Kessing LV, Gerds TA, Feldt-Rasmussen B, et al. Use of Lithium and Anticonvulsants and the Rate of Chronic Kidney Disease: A Nationwide Population-Based Study. JAMA Psychiatry. 2015;72:1182–1191. [DOI] [PubMed] [Google Scholar]
- 10.Cho Y, Lee D, Baek JH, et al. Estimated glomerular filtration rate in Korean patients exposed to long-term lithium maintenance therapy. Int J Bipolar Disord. 2022;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136:615–622. [DOI] [PubMed] [Google Scholar]
- 12.Knebel RJ, Rosenlicht N, Colllins L. Lithium carbonate maintenance therapy in a hemodialysis patient with end-stage renal disease. Am J Psychiatry. 2010;167:1409–1410. [DOI] [PubMed] [Google Scholar]
- 13.Chang CWL, Ho CSH. Lithium Use in a Patient With Bipolar Disorder and End-Stage Kidney Disease on Hemodialysis: A Case Report. Front Psychiatry. 2020;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruner JF, Dennert J, Schafer G. Lithium treatment in maintenance dialysis. Review of the literature and report of a new case on hemodialysis. Pharmacopsychiatry. 1991;24:13–16. [DOI] [PubMed] [Google Scholar]
- 15.Bjarnason NH, Munkner R, Kampmann JP, et al. Optimizing lithium dosing in hemodialysis. Ther Drug Monit. 2006;28:262–266. [DOI] [PubMed] [Google Scholar]
- 16.Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. [DOI] [PubMed] [Google Scholar]
- 17.Pahwa M, Singh B. Lithium In Patients With Chronic Kidney Disease - To Continue Vs Discontinue? Bipolar Disord. 2022;24:549–550 [DOI] [PubMed] [Google Scholar]
- 18.Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50:290–298. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11:1439–1448. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Yatham LN, Kusumakar V, Calabrese JR, et al. Third generation anticonvulsants in bipolar disorder: a review of efficacy and summary of clinical recommendations. J Clin Psychiatry. 2002;63:275–283. [DOI] [PubMed] [Google Scholar]
- 22.Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of psychiatrists clinical practice guidelines for mood disorders: Bipolar disorder summary. Bipolar Disord. 2020;22:805–821. [DOI] [PubMed] [Google Scholar]
- 23.Hojlund M, Lund LC, Herping JLE, et al. Second-generation antipsychotics and the risk of chronic kidney disease: a population-based case-control study. BMJ Open. 2020;10:e038247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study. Ann Intern Med. 2014;161:242–248. [DOI] [PubMed] [Google Scholar]
- 25.Suppes T, Baldessarini RJ, Faedda GL, et al. Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry. 1991;48:1082–1088. [DOI] [PubMed] [Google Scholar]
- 26.Ohlund L, Ott M, Bergqvist M, et al. Clinical course and need for hospital admission after lithium discontinuation in patients with bipolar disorder type I or II: mirror-image study based on the LiSIE retrospective cohort. BJPsych Open. 2019;5:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye MA, McElroy SL, Fuentes M, et al. Development of a bipolar disorder biobank: differential phenotyping for subsequent biomarker analyses. Int J Bipolar Disord. 2015;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS. A decade after the KDOQI CKD guidelines. Am J Kidney Dis. 2012;60:683–685. [DOI] [PubMed] [Google Scholar]
- 29.Vincent PD, Demers MF, Doyon-Kemp V, et al. One year mirror-image study using paliperidone palmitate for relapse prevention of schizophrenia in four university hospitals in Canada. Schizophr Res. 2017;185:96–100. [DOI] [PubMed] [Google Scholar]
- 30.Miura G, Misawa F, Kawade Y, et al. Long-Acting Injectables Versus Oral Antipsychotics: A Retrospective Bidirectional Mirror-Image Study. J Clin Psychopharmacol. 2019;39:441–445. [DOI] [PubMed] [Google Scholar]
- 31.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vestergaard P, Poulstrup I, Schou M. Prospective studies on a lithium cohort. 3. Tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand. 1988;78:434–441. [DOI] [PubMed] [Google Scholar]
- 34.Navis G, Faber HJ, de Zeeuw D, et al. ACE inhibitors and the kidney. A risk-benefit assessment. Drug Saf. 1996;15:200–211. [DOI] [PubMed] [Google Scholar]
- 35.Mahase E. US taskforce advises against low dose aspirin for primary prevention of cardiovascular disease. BMJ. 2021;375:n2521. [DOI] [PubMed] [Google Scholar]
- 36.McNeil JJ, Wolfe R, Woods RL, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med. 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126:186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gitlin M. Lithium and the kidney: an updated review. Drug Saf. 1999;20:231–243. [DOI] [PubMed] [Google Scholar]
- 40.Kovvuru K, Kanduri SR, Thongprayoon C, et al. Lithium and nephrotoxicity: Nephrology’s perspectives. Bipolar Disord. 2020;22:331–333. [DOI] [PubMed] [Google Scholar]
- 41.Pahwa M, Singh B. Lithium in patients with chronic kidney disease - To continue versus discontinue? Bipolar Disord. 2022;24:549–550 [DOI] [PubMed] [Google Scholar]
- 42.Taylor DM, Sparshatt A, Amin F, et al. Aripiprazole long-acting injection - a mirror image study of its effects on hospitalisation at one year. J Psychopharmacol. 2017;31:1564–1569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to restrictions-their containing information that could compromise the privacy of the research participant.


