Abstract
This study explores a new approach for antimicrobial therapy with light activation of targeted poly-l-lysine (pL)–chlorin e6 (ce6) conjugates. The goal was to test the hypothesis that these conjugates between pL and ce6 would efficiently target photodestruction towards gram-positive (Actinomyces viscosus) and gram-negative (Porphyromonas gingivalis) oral species while sparing an oral epithelial cell line (HCPC-1). Conjugates of ce6 with pL (average molecular weight, 2,000) having a positive, neutral, or negative charge were prepared. Illumination with red light (λmax = 671 nm) from a diode array produced a dose-dependent loss of CFU from the bacteria, under conditions that did not affect the viability of the epithelial cells. For P. gingivalis, the cationic conjugate produced 99% killing, while the neutral conjugate killed 91% and the anionic conjugate killed 76% after 1 min of incubation and exposure to red light for 10 min. For A. viscosus, the cationic conjugate produced >99.99% killing while HCPC-1 cells remained intact. The importance of the positive charge was shown by the effectiveness of ce6-monoethylenediamine monoamide (a monocationic derivative of ce6) in killing both bacteria. The clinically employed benzoporphyrin derivative under the same conditions killed epithelial cells while leaving P. gingivalis relatively unharmed. A mixture of ce6 with pL did not show phototoxicity comparable with that of the cationic conjugate. These results were explained by the selective uptake of the conjugates by bacteria (20- to 100-fold) compared to that by mammalian cells, while free ce6 showed much less selectivity for bacteria (5- to 20-fold). The data suggest that the cationic pL-ce6 conjugate may have an application for the photodynamic therapy of periodontal disease.
Photodynamic therapy is based on the concept that a certain photoactivatable compound, or photosensitizer (PS), can be preferentially localized in certain tissues and subsequently activated by light of the appropriate wavelength to generate singlet oxygen and free radicals that are cytotoxic to cells of the target tissue (9). Bacteria also can be killed by visible light after their treatment with an appropriate PS (3). Several studies have demonstrated that gram-positive bacteria are susceptible to photoinactivation (1, 3, 13). Although lethal photosensitization of a wide range of gram-negative bacteria has been reported elsewhere (5, 17, 33), other studies have shown that gram-negative bacteria have been resistant to photodynamic action (3, 23), unless the permeability of their outer membrane is modified (2, 22).
Periodontal disease results from the accumulation of bacterial biofilms (plaques) on tooth surfaces. Mechanical removal of the biofilms is the current method of treatment. Antiseptics and antibiotics are also used, but development of resistance in the target organisms and disruption of the oral microflora are problems associated with the use of such agents. In addition to these antibacterial therapies, the use of a PS to cause lethal photodestruction of oral bacteria has also been demonstrated elsewhere (34), indicating that photodynamic therapy could be a useful alternative in eliminating periodontopathogenic bacteria. The main problem to be overcome is devising a strategy to target the PS to the bacteria while leaving the host gingival tissue unharmed.
Many naturally occurring antibacterial peptides which all have in common inter alia a pronounced polycationic charge have been discovered (7). This is thought to be the initial factor which allows them to bind to negatively charged bacteria (30). We hypothesized that a polycationic macromolecule might effectively deliver the PS chlorin e6 (ce6) to both- gram-positive and gram-negative bacteria and allow their selective photodestruction while sparing host epithelial cells. The backbone chosen was poly-l-lysine (pL) (average molecular mass, 2 kDa). In order to investigate the effect of the charge borne by these conjugates we compared polycationic (pL-ce6), neutral (pL-ce6-ac), and polyanionic (pL-ce6-succ) conjugates. Uptake and phototoxicity were examined in an oral epithelial cell line (HCPC-1) and two oral bacterial species: Porphyromonas gingivalis, a key gram-negative periodontal pathogen (26), and Actinomyces viscosus, a gram-positive bacterium also found in the subgingival plaque (8). In order to establish the combined role of charge and covalent conjugation, the three conjugates were compared to unconjugated ce6, ce6-monoethylenediamine monoamide (CMA) (a more cationic derivative of ce6), and a mixture of pL and ce6. A widely used clinical PS, benzoporphyrin derivative monoacid ring A (BPD), which is known to efficiently kill mammalian cells, was also tested.
MATERIALS AND METHODS
Preparation of pL-ce6 conjugates of varying charges.
All reactions were carried out in the dark at room temperature and have been described previously (27). Briefly, N-hydroxysuccinimide ester of ce6 (Porphyrin Products, Logan, Utah) was prepared by reacting 1.5 equivalents of dicyclohexylcarbodiimide and 1.5 equivalents of N-hydroxysuccinimide with 1 equivalent of ce6 in dry dimethyl sulfoxide (DMSO) for 24 h and was frozen in aliquots for further use. pL hydrobromide (Sigma, St. Louis, Mo.) (average molecular weight = 2,000; 50 mg) was dissolved in dry DMSO (50 ml), and N-ethylmorpholine (1 ml) was added, followed by ce6–N-hydroxysuccinimide ester (25 mg), and reacted for 24 h. After exhaustive dialysis, this gave the purified cationic conjugate (pL-ce6). The DMSO solution of pL-ce6 was treated with an excess of acetic anhydride (100 mg dissolved in 0.5 ml of dry DMSO) to produce a neutrally charged conjugate (pL-ce6-ac). In a similar fashion, the pL-ce6 in DMSO was treated with an excess of succinic anhydride (100 mg dissolved in 0.5 ml of dry DMSO), which converted the positively charged amino groups to negatively charged carboxylic acid groups (pL-ce6-succ). The resulting solutions of conjugates had a concentration equivalent to 0.5 to 1 mM ce6 and were checked for purity by thin-layer chromatography (1:1 mixture of methanol and 10% aqueous NH4Cl; silica gel on polyester thin-layer chromatography plates [Aldrich Chemical Co., Milwaukee, Wis.]). On this system, noncovalently bound ce6 has an Rf value of approximately 0.5 while the conjugates remain at the origin.
Other PSs.
Free ce6 and CMA (Porphyrin Products) were dissolved in a small amount of 0.1 M NaOH solution before neutralization in phosphate-buffered saline (PBS; pH 7.4). BPD (nonliposomal) (a kind gift from QLT Phototherapeutics, Vancouver, Canada) was dissolved in a small amount of DMSO, which was diluted to give less than 0.1% DMSO in incubation medium. A 1:1 molar mixture of ce6 and pL (degree of polymerization = 20) in PBS was also prepared.
Microorganisms.
The organisms used in this study were P. gingivalis 381 (S. S. Socransky, Forsyth Dental Center, Boston, Mass.) and A. viscosus T14 (Forsyth Dental Center). Cultures were maintained by weekly subculture in Trypticase soy agar (Baltimore Biological Laboratories, Cockeysville, Md.) with 5 μg of hemin per ml, 0.3 μg of vitamin K per ml, and 5% sheep blood for P. gingivalis and in Trypticase soy agar with 5% sheep blood for A. viscosus. For experimental purposes, the organisms were grown anaerobically in a chamber with 80% N2, 10% H2, and 10% CO2 at 35°C for 48 h; harvested by centrifugation; and resuspended in Trypticase soy broth with 5 μg of hemin per ml and 0.3 μg of vitamin K per ml. Cells were dispersed by sonication and repeated passage through Pasteur pipettes. Cell numbers were measured in a spectrophotometer (wavelength, 600 nm; 1 optical density unit equals approximately 109 cells/ml) in 1-ml tubes.
Oral epithelial cell line.
A hamster cheek pouch cell carcinoma line (HCPC-1 [J. Schwartz, Harvard Dental School, Boston, Mass.]) was used for comparison studies (24). Cells were grown in Dulbecco’s modified Eagle’s medium with high glucose (Gibco, Grand Island, N.Y.) supplemented with heat-inactivated 10% fetal calf serum (Gibco), 100 U of penicillin G per ml, and 100 μg of streptomycin (Sigma) per ml. The medium was changed every 2 to 3 days, and cells were passaged weekly with trypsin-EDTA. All cells were maintained in 10-cm-diameter petri dishes with 12 ml of growth medium and kept at 37°C in a humidified, 95% air–5% CO2 atmosphere.
Uptake studies. (i) Bacteria.
Suspensions of the microorganisms (109 cells/ml) were incubated in the dark at room temperature for 1 min with 1, 5, and 10 μM ce6 equivalent (final concentration in Trypticase soy broth) and the same concentrations for free ce6 and CMA in triplicate. The cell suspension was centrifuged (9,000 × g for 1 min), the PS solution was aspirated, and bacteria were washed once by resuspending the cell pellet in 1 ml of sterile PBS and centrifuging as described above. Finally, the cell pellet was dissolved by digesting it in 1.5 ml of 0.1 M NaOH–1% sodium dodecyl sulfate for at least 24 h to give a homogeneous solution. The fluorescence of the cell extract was measured on a spectrofluorimeter (model FluoroMax; SPEX Industries, Edison, N.J.). The excitation wavelength was 400 nm, and the emission spectra of the cell suspensions were recorded from 580 to 700 nm. The protein content of the entire cell extract was then determined by a modified Lowry method (14) with bovine serum albumin dissolved in 0.1 M NaOH–1% sodium dodecyl sulfate to construct calibration curves. Results were expressed as nanomoles of ce6 per milligram of cell protein.
(ii) Epithelial cells.
Cells were trypsinized in the exponential growth phase and counted with a hemocytometer. One hundred thousand cells in 1 ml of growth medium with 10% serum were seeded into each well of 24-well culture plates. These were incubated overnight to allow cells to attach and resume exponential growth, after which time PS was added. The medium was removed and replaced with medium with 10% serum containing the conjugates, ce6, and CMA at the concentrations described above in triplicate for 1 min. The drug solution was then aspirated from the wells, and cells were washed once with 1 ml of sterile PBS and incubated with 1 ml of trypsin-EDTA for 10 min. The resulting cell suspension was then centrifuged, the trypsin supernatant was aspirated, and the pellets were dissolved in 0.1 M NaOH–1% sodium dodecyl sulfate. The measurement of the fluorescence of the cell extract as well as the determination of the protein content was performed as described above.
Light source.
The irradiation source was a 4- by 4-cm (nominal) array of 52 light-emitting diodes (Hewlett-Packard, San Jose, Calif.) mounted on a printed circuit board. The emission spectrum was determined with a spectrophotometer which had been corrected for optical spectral response. The emission peak was at 671 nm, the full-width half-maximum value of the emission was 24 nm, and 98% of the emitted light was between 635 and 695 nm, which covered the absorbance of all the PSs. The entire array was immersed in an aluminum box filled with silicone oil to provide thermal conduction to box walls, which were in turn cooled by a small fan. The maximum power available from the array was 305 mW, but the array was generally operated at about 230 mW of total power. The actual irradiance incident on the cells was determined by placing a power meter (model 200; Coherent, Palo Alto, Calif.) at the plane of the cells, measuring the power, and dividing by the active area of the meter.
For the determination of the fraction of the light falling within the absorption band of each PS, the following procedure was used. The area under the emission spectrum of the light-emitting diode array was determined numerically with 10-nm-wide steps. The absorption spectra of the PSs were measured, and the absorption maxima and the full-width half-maximum of the peaks were determined. The fraction of the light that could be absorbed by each PS was then determined by calculating the degree of overlap between the emission peak and the longest wavelength peak of the PS.
Photodynamic therapy studies. (i) Bacteria.
Suspensions of bacteria (108/ml) were incubated in the dark at room temperature for 1 min with 5 μM ce6 equivalent from each conjugate, the mixture of ce6 and pL, free ce6, and CMA and the same concentration for BPD in triplicate. Cell suspensions were centrifuged, cells were washed once with sterile PBS, and 1 ml of fresh medium was added. The bacterial suspensions were then placed in the wells of 12-well plates. All wells were exposed to light from the light-emitting diode array in the dark at room temperature. The light exposure was from below with fluences ranging from 0 to 15 J/cm2 at an irradiance of 25 mW/cm2. Bacteria incubated with CMA, BPD, and the mixture were exposed to only 15 J of incident light per cm2. All plates were kept covered during the illumination in order to maintain the sterility of the culture. After illumination of the appropriate wells, serial dilutions of the contents of each well were prepared in Trypticase soy broth, and 100-μl aliquots were spread over the surfaces of blood agar plates. Survival fractions in each well were calculated by counting the colonies on the plates and dividing by the number of colonies from dark controls incubated with a PS and kept at room temperature for periods equal to irradiation times. Other controls were bacteria untreated with a PS or light but kept in plates at room temperature covered with aluminum foil during irradiation and cells exposed to light in the absence of a PS.
(ii) Epithelial cells.
HCPC-1 cells (2 × 104) in 0.1 ml of growth medium with 10% serum were seeded in 96-well plates and cultured for 24 h until they were 70% confluent. Six wells from each plate were treated with the same concentrations of PSs as described above. After illumination, cells were incubated with fresh medium at 37°C for 24 h. Controls were the same as described above. Cell viability was determined 24 h after irradiation by the 3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide microculture tetrazolium assay (20), a method of assessing the amount of dehydrogenase activity in the mitochondria of living cells. The percentage of cells surviving was determined by dividing mean 550-nm absorbances of formazan produced from treated cells by the mean absorbances from dark controls incubated with a PS and kept at room temperature for periods equal to irradiation times.
Statistics.
Differences between means were analyzed for statistical significance by Student’s t test.
RESULTS
Preparation of conjugates.
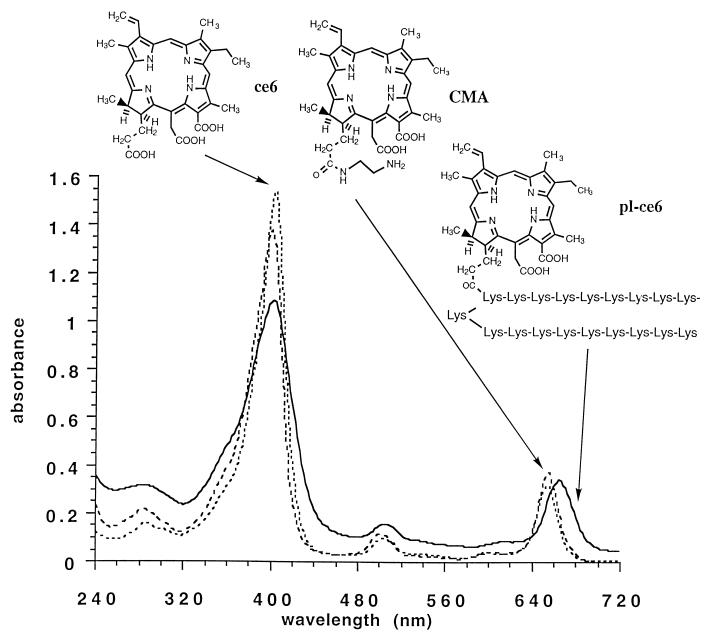
The pL chains employed had an average molecular weight of 2,000, i.e., an average degree of polymerization of 20 lysine residues. Assuming quantitative recovery from dialysis, the spectrophotometric measurements showed that an average of one ce6 molecule was present per pL chain, representing a substitution ratio of 5%. This meant that the average molecular weights of the conjugates were as follows: pL-ce6, 2,600; pL-ce6-succ, 4,500; pL-ce6-ac, 3,400. The absorption spectra in 0.1 M NaOH–1% (wt/vol) sodium dodecyl sulfate and chemical structures of ce6, CMA, and pL-ce6 are shown in Fig. 1. The absorption spectra of conjugates were characterized by a Soret band maximum at 404 nm and a long-wavelength maximum at 664 to 666 nm. For free ce6 and CMA, the long-wavelength maximum was 654 nm.
FIG. 1.
UV-visible absorption spectra and chemical structures of ce6, CMA, and pL-ce6 in 0.1 M NaOH–1% sodium dodecyl sulfate.
Uptake studies.
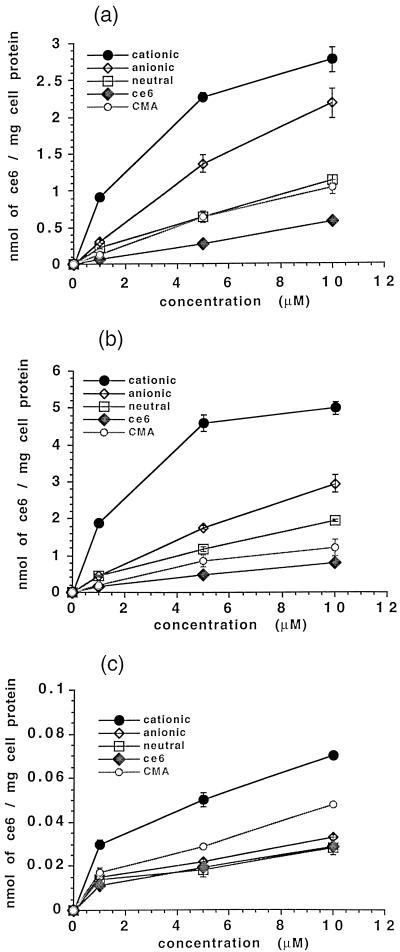
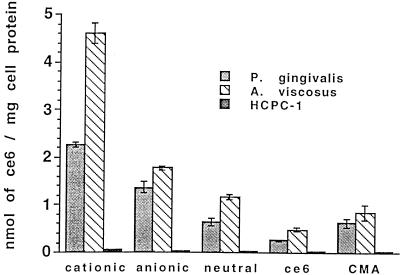
The uptake of ce6 from the conjugates and unconjugated ce6 was increased on a dose-dependent basis, as Fig. 2a shows for P. gingivalis. Selectivity was pronounced for the cationic conjugate, which gave the highest uptake, followed by the anionic and neutral conjugates, CMA, and free ce6 (Fig. 2a). Similar results were obtained for A. viscosus (Fig. 2b). At all concentrations, bacteria took up two to four times more ce6 from the cationic conjugate than that delivered by the anionic or neutral conjugate (P < 0.001). Both bacteria took up significantly more ce6 from all the conjugates than from the unconjugated molecules CMA and ce6. A. viscosus took up 1.3 to 2 times more ce6 from the three conjugates and free ce6 than did P. gingivalis (Fig. 3). The uptake of ce6 from the cationic conjugate by A. viscosus was almost double that of P. gingivalis (P < 0.001) at all concentrations. At all concentrations of the three charged pL conjugates, bacteria took up 20 to 100 times more ce6 than did HCPC-1 cells (Fig. 3).
FIG. 2.
Uptake of pL-ce6 conjugates, free ce6, and CMA by P. gingivalis (a), A. viscosus (b), and HCPC-1 cells (c). Bacteria and epithelial cells were incubated for 1 min with 1, 5, and 10 μM ce6 equivalent of each conjugate, and ce6 uptake was expressed as nanomoles of ce6 equivalent per milligram of cell protein. Each bar is the mean of values obtained from three tubes, and data are representative of duplicate experiments. Values are expressed as means ± standard errors of the means.
FIG. 3.
Uptake of pL-ce6 conjugates, free ce6 and CMA by P. gingivalis, A. viscosus, and HCPC-1 cells. Bacteria and epithelial cells were incubated for 1 min with 5 μM ce6 equivalent of each conjugate. Each bar is the mean of values obtained from three tubes, and data are representative of duplicate experiments. Values are expressed as means ± standard errors of the means.
For HCPC-1 cells, the cationic conjugate gave the highest uptake, followed by CMA, the anionic and neutral conjugates, and ce6 (Fig. 2c). The uptake of ce6 from the cationic conjugate was 2 to 2.5 times higher than that delivered from the other PSs at all concentrations (P < 0.001).
Light absorbed by the PS.
Table 1 gives the overlap between the absorption spectra of the PSs and the emission spectrum of the light source, which shows the percentage of the light absorbed by each PS during irradiation. The conjugates showed the highest relative absorbed light dose, followed by BPD, ce6, and CMA.
TABLE 1.
Overlap between the absorption spectra of the PSs and the emission spectrum of the diode array
| PS | Absorption peak (half peak width) (nm) | Extinction coefficienta | % Overlap with light emission | Light doseb |
|---|---|---|---|---|
| ce6c | 658 (22) | 30,000 | 35 | 79.5 |
| CMA | 658 (24) | 28,000 | 37 | 78 |
| Conjugate | 662 (28) | 20,000 | 66 | 100 |
| BPD | 687 (19) | 50,000 | 24 | 91 |
Measured in 0.1 M NaOH–1% sodium dodecyl sulfate.
The relative effective light dose absorbed by each PS is defined as extinction coefficient × overlap with diode emission in percent and was expressed as percentage of that absorbed by the conjugates.
No difference in the spectrum was observed when ce6 was mixed with pL.
Photodynamic treatment.
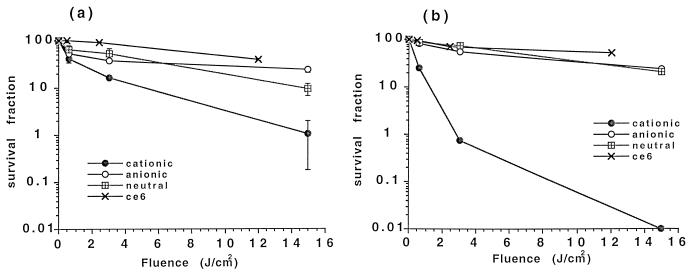
Both P. gingivalis and A. viscosus were unaffected (reduction of viability was less than 15%) after 15 J of illumination per cm2 alone or by 1 min of incubation with a 5 μM concentration of all PSs in the dark, with the exception of BPD, which showed a remarkable dark toxicity of 72% on A. viscosus. There was no reduction in the viability of HCPC-1 cells after their exposure to light alone or after their incubation with a 5 μM concentration of any of the PSs in the dark. The fluence values in the phototoxicity experiments have been corrected for the relative effective absorption of photons by the long-wavelength peak of the PS. The phototoxic effects of the three pL-ce6 conjugates and free ce6 on P. gingivalis and A. viscosus for increasing light doses are shown in Fig. 4. The phototoxicity was fluence dependent. The cationic conjugate gave the highest phototoxicity towards both bacteria, followed by the neutral conjugate. The anionic conjugate and free ce6 gave intermediate levels of phototoxicity. Table 2 shows the photodynamic effects of all PSs on P. gingivalis, A. viscosus, and HCPC-1 cells. The cationic conjugate was the most potent of these PSs by reducing the viability of P. gingivalis and A. viscosus by 99 and >99.99%, respectively. Epithelial cells remained intact after photodynamic therapy with all PSs, except for BPD, which reduced their viability about 90% (Table 2).
FIG. 4.
Phototoxicity of P. gingivalis (a) and A. viscosus (b) after incubation with 5 μM ce6 equivalent from each conjugate and free ce6 for 1 min followed by treatment with red light and colony-forming assay. Each point is the mean of values obtained from three wells, and data are representative of duplicate experiments. Values are expressed as means ± standard errors of the means.
TABLE 2.
Photoinactivation of P. gingivalis, A. viscosus, and HCPC-1 cellsa
| PS | % Survival for organism or cell line
|
||
|---|---|---|---|
| P. gingivalis | A. viscosus | HCPC-1 | |
| Cationic conjugate | 1.1 ± 0.9 | <0.01 | 102 ± 2.1 |
| Anionic conjugate | 23.9 ± 3.3 | 24.3 ± 3.4 | 127 ± 2.9 |
| Neutral conjugate | 9.3 ± 2.6 | 21.2 ± 1.5 | 108 ± 4.2 |
| Free ce6 | 39.6 ± 2.3 | 52.9 ± 5.9 | 126 ± 2.7 |
| CMA | 8.1 ± 1.8 | 2 ± 0.1 | 100 ± 2.5 |
| BPD | 81.1 ± 9.4 | 7.6 ± 7.6 | 8.8 ± 0.7 |
| Mixture (pL and ce6) | 8.1 ± 1.8 | 2 ± 0.1 | 100 ± 2.5 |
The percent survival of bacteria was assayed by the colony-forming assay following 1 min of treatment with PS and exposure to 15 J/cm2. The viability of HCPC-1 cells was assayed by the microculture tetrazolium assay. Surviving bacteria and epithelial cells were expressed as a percentage of controls receiving PS in the dark out of the incubator. Values for bacteria are the means from three wells, whereas for HCPC-1 cells values are means from six wells, and data are representative of duplicate experiments (± standard errors of the means).
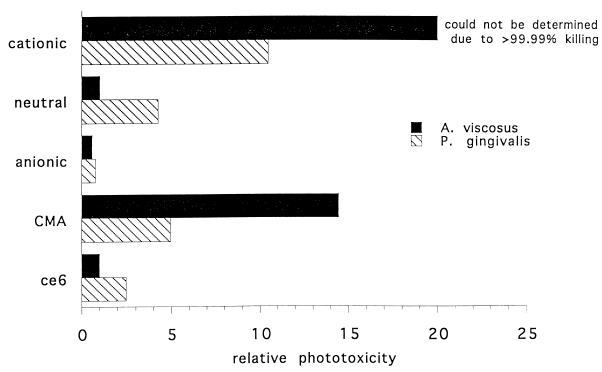
The relative phototoxicities per mole of ce6 taken up per cell for P. gingivalis and A. viscosus are shown in Fig. 5. These numbers were calculated by taking the reciprocal of the survival fraction (Table 2) and dividing it by the number of nanomoles of ce6 taken up per milligram of cell protein. They are expressed relative to the values for A. viscosus and free ce6. It can be seen that the cationic conjugate was the most potent PS, followed by CMA. The exact value for the phototoxicity of the cationic conjugate towards A. viscosus could not be estimated but was >99.99%.
FIG. 5.
Relative phototoxicity (at 15 J/cm2) of ce6 delivered by the conjugates, free ce6 and CMA per mole of ce6 taken up per cell for P. gingivalis and A. viscosus. The phototoxicity is expressed relative to the values for A. viscosus and free ce6.
DISCUSSION
This study tested the hypothesis that polymeric conjugates between pL and ce6 might selectively target bacteria for photodestruction. The effect of varying the charge of the conjugates from cationic through neutral to anionic on the selectivity they exhibited towards two oral bacteria of the subgingival plaque and an oral epithelial cell line was studied. We specifically wished to determine whether a polycationic conjugate which emulated well-known natural cationic antibacterial peptides would be effective for delivering PS to gram-negative bacteria.
Conjugation of ce6 molecules to pL promotes the uptake of ce6 by oral bacteria and HCPC-1 cells, and this increased uptake occurs in a concentration-dependent manner. The cationic conjugate showed the highest uptake for both P. gingivalis and A. viscosus. Neither species of oral bacteria accumulated significantly more ce6 from the cationic conjugate at 10 μM than at 5 μM. This similarity of uptake suggested a saturation effect, and therefore the 5 μM concentration was used in subsequent photosensitization studies. The enhanced uptake of ce6 from the cationic conjugate could be due to the binding of the positively charged pL-ce6 to the outer membrane of P. gingivalis as well as to the phosphate groups of the wall teichoic acids of A. viscosus, both of which bear a strong negative charge (29). HCPC-1 cells accumulated 30 to 100 times less ce6 from the cationic conjugate than did bacteria, although the plasma membrane of malignant epithelial cells also bears an overall net negative charge, which is greater than that of normal epithelial cells due to overexpression of polysialic acid residues (11).
An increasing array of small naturally occurring antibacterial peptides have been discovered (7). These have been found in mammals, fish, insects, and invertebrates (6). Examples of this class include histatins, defensins, magainins, cecropins, and tachyplasins. The mechanism of action of these peptides is being intensively investigated, but a common factor in all the structures is the polycationic charge due to lysine, histidine, and arginine residues in the amino acid sequence (7). Although tertiary structures such the alpha-helix are thought to play a role in their selective cytotoxicity towards bacteria (10), the polycationic charge borne by these polypeptides is probably responsible for their initial binding to bacteria.
Photosensitization of microorganisms was carried out in complete culture medium after exposure to the PS for only 60 s; effective killing is more challenging under these conditions than under conditions of incubation in PBS in the presence of the PS. The use of solutions such as PBS for both the incubation and the illumination of bacteria allows much easier photoinactivation than do the protective effects of complete medium. For the removal of the weakly bound PS prior to illumination, only one washing in PBS was used, as extensive washings can damage the membrane barrier of gram-negative bacteria (21). Consistent with this concept, it has been shown that only one washing was enough to remove more than 60% of the originally bound cationic water-soluble zinc phthalocyanine from Escherichia coli (18). The amount of the PS removed after four washings was 80% (18). The same authors showed that there was no substantial decrease in the degree of bacterial killing with light after several washings, suggesting that it was the tightly bound PS rather than the weakly bound PS which was involved in the photosensitization.
The pronounced photodynamic effect of the cationic conjugate on A. viscosus may be due to the electrostatic attraction between the conjugate and the negatively charged membrane of the bacterium. This hypothesis is supported by data regarding the phototoxicity of a mixture of pL (positively charged) and ce6 as well as of free ce6 on A. viscosus. A reduction of 60% in survival of A. viscosus was achieved after treatment with visible light of 12 J/cm2 with free ce6 (Table 2). However, ce6 did not have any photodynamic effect when the mixture was used (Table 2). It is likely that pL interacts with the negatively charged cell wall and blocks the interaction of ce6 with bacteria, so that after washing most of the PS has been removed.
We have previously shown that both epithelial and endothelial cells accumulate sufficient ce6 from charged pL conjugates to enable efficient photoinactivation to take place (27). However, in mammalian cells this process of uptake is time dependent due to the necessity of these macromolecules being actively internalized, and the sparing of HCPC-1 cells in the present study is due mainly to the very short incubation time. This short incubation time reduced the total uptake of the HCPC-1 cells and also meant that the amount of ce6 which did bind to the cells was likely to be at the plasma membrane rather than internalized. If the PS is localized at the plasma membrane, then it will absorb light and transfer its energy to molecular oxygen, forming singlet oxygen, which has a mean diffusion distance of 100 to 200 nm (19). Because mammalian epithelial cells have a volume often 2,000 times greater than that of bacteria (29), active oxygen species generated at the surface are very much less likely to be able to diffuse to a more sensitive intracellular location than those in the case of bacteria. We have previously shown that in epithelial cells after 6 h of incubation the cationic conjugate had the lowest relative phototoxicity compared to neutral and anionic conjugates (27). This situation is in complete contrast to the present study, where the cationic conjugate has by far the highest relative phototoxicity in bacteria after 1 min of incubation.
In the case of P. gingivalis, other mechanisms may operate, as this interaction was weaker and the structure of the outer membrane is more complex. The entire problem of photodynamic inactivation of gram-negative bacteria has been comprehensively reviewed elsewhere (12), and the structure of the lipid layers of the outer membrane is thought to be responsible for their remarkable resistance to conventional anionic PS (22). Antibacterial photodynamic activity on gram-negative species has been achieved in the presence of membrane-disorganizing polycations such as polymyxin B nonapeptide (21). pLs are among the polycations which bind to the anionic sites of lipopolysaccharide. This binding may weaken the intermolecular interactions of the lipopolysaccharide constituents, disorganize the structure, and render it permeable to drugs (30) by enabling them to cross the outer membrane. The effect of a pL with 20 lysine residues (similar to that used in this study) on smooth Salmonella typhimurium after a short treatment (10 min) was a rapid release of 20 to 30% of the lipopolysaccharide from the outer membrane and the subsequent sensitization of the bacteria to the anionic sodium dodecyl detergents (31). The same authors also showed that this polymer sensitized smooth E. coli and S. typhimurium strains to hydrophobic antibiotics by a factor of 100 or more (32). In both studies, the polymer was not found to be bactericidal. It is possible that the cationic conjugate begins its action by electrostatic interaction with the negatively charged groups in the lipopolysaccharide of P. gingivalis and then subsequently alters the permeability of the outer membrane, and therefore the conjugate gains access to internal targets. Furthermore, the photodynamic effect of ce6 of P. gingivalis became more pronounced when the mixture with pL was used (Table 2). This suggests that the positively charged pL altered the outer membrane barrier function and helped ce6 to penetrate the cell. In the case of the neutral conjugate, pL seems to have the same role as above. Although the uptake of ce6 from the neutral conjugate by A. viscosus was twice that of P. gingivalis, the phototoxicity of the conjugate was much greater on P. gingivalis (Table 2). Recently, it has also been suggested that the resistance of gram-negative bacteria to photoinactivation could be related to the charge on the PS (15, 18). The gram-negative bacteria E. coli and Pseudomonas aeruginosa could be photoinactivated by illumination in the presence of a cationic water-soluble zinc pyridinium phthalocyanine for 30 min but not by illumination in the presence of a neutral tetradiethanolamine phthalocyanine or a negatively charged tetrasulfonated phthalocyanine (18). Meso-substituted cationic porphyrins were also efficient PSs of gram-negative bacteria such as Vibrio anguillarum and E. coli after incubation for 5 min (15). In another study, the authors suggested that it was the positive charge that promoted the binding of the porphyrin to the outer membrane, inducing a limited damage which favored the penetration of the PS (16). They also showed that the photosensitizing activity of cationic porphyrins toward gram-negative bacteria was inhibited by their incorporation into liposomes (16).
The uptake and photosensitization of both A. viscosus and P. gingivalis by the anionic conjugate were almost the same, and this is consistent with electrostatic repulsion between the charged sensitizer molecule and the cell membrane. CMA was the second most potent PS, after the cationic conjugate, for both bacteria (Fig. 5). This is not surprising, considering that CMA has a carboxyl group replaced by a primary amino group, thus markedly increasing its cationic character. Although it is not polycationic and cannot disrupt the gram-negative bacterial outer membrane, its small size may still allow it to penetrate the outer surface of both bacteria. BPD, a hydrophobic chlorin-like porphyrin derivative used in clinical trials, was very phototoxic on A. viscosus and on HCPC-1 cells (Table 2). The very strong dark cytotoxic effect of BPD on A. viscosus (72% reduction of survival) was a surprising finding. Because of its hydrophobicity, BPD may penetrate the cellular membrane quickly and localize in an intracellular site that is very sensitive to photodamage. The more complex structure of the outer membrane of P. gingivalis may prevent the diffusion of BPD into the bacterial cell. No PS except BPD led to any phototoxicity on HCPC-1 cells at any concentration tested in this study, suggesting that there is a therapeutic window whereby bacteria could be killed without damaging epithelial cells.
A number of studies have shown that oral bacteria (including P. gingivalis) could be killed by red light after sensitization with the cationic PS toluidine blue in pure cultures of the organisms (35), in biofilms (5), or as a mixed population present in subgingival plaque from patients with chronic periodontitis (25). P. gingivalis could be photoinactivated after sensitization with toluidine blue under various environmental and physiological conditions (4). Lethal photosensitization of Streptococcus sanguis, a major plaque-forming organism, was also possible with toluidine blue at sensitizer concentrations and light doses that did not affect the viability of oral keratinocytes and oral fibroblasts (28). The disadvantage of this approach is the dark blue staining of the oral mucosa obtained with this dye.
As a whole, our results suggest that the increased uptake and the pronounced phototoxicity of the cationic ce6 conjugate on P. gingivalis and A. viscosus depend on the charge borne by the conjugate. Based on the data in the present study, we propose that the disorganizing action of the 20-residue pL chain leads to increased penetration of the PS through the outer membrane of gram-negative bacteria, which significantly contributes to the enhanced photodynamic effect of the cationic conjugate on P. gingivalis. The results clearly showed that the viability of oral malignant keratinocytes was not affected after their incubation with the cationic conjugate for 60 s and exposure to visible light. Further studies in an animal model of periodontitis, where the PS will be introduced into the dental pocket, followed by red light illumination via an optical fiber, will show whether the cationic conjugate will have an application for therapy of periodontal disease.
ACKNOWLEDGMENTS
We thank Ana Paola Colombo, Department of Periodontology, Forsyth Dental Center, for her assistance in performing the uptake studies; J. Max Goodson, Department of Periodontology, Forsyth Dental Center, for critically reading the manuscript; Thomas Deutsch, Wellman Laboratories of Photomedicine, Massachusetts General Hospital, for useful advice and suggestions; and John Scarangello, Hewlett-Packard Company, Optoelectronics Division, San Jose, Calif., for kindly donating the diode array.
This work was supported in part by the Department of Defense Medical Free Electron Laser Program (N00014-94-1-0927) and by Periodontix, Inc., Watertown, Mass. Laurie Ann Ximenez-Fyvie was funded by the DGAPA, UNAM, Mexico.
REFERENCES
- 1.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier J E. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 2.Bertoloni G, Rossi F, Valduga G, Jori G, van Lier J. Photosensitizing activity of water- and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol Lett. 1990;59:149–155. doi: 10.1111/j.1574-6968.1990.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 3.Bertoloni G, Salvato B, Dall’Acqua M, Vazzoler M, Jori G. Hematoporphyrin-sensitized photoinactivation of Streptococcus faecalis. Photochem Photobiol. 1984;39:811–816. doi: 10.1111/j.1751-1097.1984.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M. Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem Photobiol. 1997;65:1026–1031. doi: 10.1111/j.1751-1097.1997.tb07964.x. [DOI] [PubMed] [Google Scholar]
- 5.Dobson J, Wilson M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch Oral Biol. 1992;37:883–887. doi: 10.1016/0003-9969(92)90058-g. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Biosynthesis of defensins and other antimicrobial peptides. Ciba Found Symp. 1994;186:62–71. doi: 10.1002/9780470514658.ch4. [DOI] [PubMed] [Google Scholar]
- 7.Groisman E A. How bacteria resist killing by host-defense peptides. Trends Microbiol. 1994;2:444–449. doi: 10.1016/0966-842x(94)90802-8. [DOI] [PubMed] [Google Scholar]
- 8.Haffajee A D, Cugini M A, Dibart S, Smith C, Kent R L, Jr, Socransky S S. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–334. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 9.Hasan T, Parrish J A. Photodynamic therapy of cancer. In: Holland J F, Frei E I, Bast R C J, Kufe D W, Morton D L, Weichselbaum R R, editors. Cancer medicine. Baltimore, Md: Williams & Wilkins; 1996. pp. 739–751. [Google Scholar]
- 10.Hirsh D J, Hammer J, Maloy W L, Blazyk J, Schaefer J. Secondary structure and location of a magainin analogue in synthetic phospholipid bilayers. Biochemistry. 1996;35:12733–12741. doi: 10.1021/bi961468a. [DOI] [PubMed] [Google Scholar]
- 11.Kornguth S E, Kalinke T, Robins H I, Cohen J D, Turski P. Preferential binding of radiolabeled poly-L-lysines to C6 and U87 MG glioblastomas compared with endothelial cells in vitro. Cancer Res. 1989;49:6390–6395. [PubMed] [Google Scholar]
- 12.Malik Z, Ladan H, Nitzan Y. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J Photochem Photobiol B. 1992;14:262–266. doi: 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- 13.Malik Z, Ladan H, Nitzan Y, Ehrenberg B. The bactericidal activity of a deuteroporphyrin-hemin mixture on gram-positive bacteria. A microbiological and spectroscopic study. J Photochem Photobiol B. 1990;6:419–430. doi: 10.1016/1011-1344(90)85115-d. [DOI] [PubMed] [Google Scholar]
- 14.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 15.Merchat M, Bertolini G, Giacomini P, Villanueva A, Jori G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1996;32:153–157. doi: 10.1016/1011-1344(95)07147-4. [DOI] [PubMed] [Google Scholar]
- 16.Merchat M, Spikes J D, Bertoloni G, Jori G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J Photochem Photobiol B. 1996;35:149–157. doi: 10.1016/s1011-1344(96)07321-6. [DOI] [PubMed] [Google Scholar]
- 17.Millson C E, Wilson M, MacRobert A J, Bedwell J, Bown S G. The killing of Helicobacter pylori by low-power laser light in the presence of a photosensitiser. J Med Microbiol. 1996;44:245–252. doi: 10.1099/00222615-44-4-245. [DOI] [PubMed] [Google Scholar]
- 18.Minnock A, Vernon D I, Schofield J, Griffiths J, Parish J H, Brown S B. Photoinactivation of bacteria. Use of a cationic water-soluble zinc phthalocyanine to photoinactivate both gram-negative and gram-positive bacteria. J Photochem Photobiol B. 1996;32:159–164. doi: 10.1016/1011-1344(95)07148-2. [DOI] [PubMed] [Google Scholar]
- 19.Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53:549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Nitzan Y, Dror R, Ladan H, Malik Z, Kimel S, Gottfried V. Structure-activity relationship of porphines for photoinactivation of bacteria. Photochem Photobiol. 1995;62:342–347. doi: 10.1111/j.1751-1097.1995.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 22.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 23.Nitzan Y, Shainberg B, Malik Z. Photodynamic effects of deuteroporphyrin on Gram positive bacteria. Curr Microbiol. 1987;15:251–258. [Google Scholar]
- 24.Odukoya O, Schwartz J, Weichselbaum R, Shklar G. An epidermoid carcinoma cell line derived from hamster 7,12-dimethylbenz[a]anthracene-induced buccal pouch tumors. J Natl Cancer Inst. 1983;71:1253–1258. [PubMed] [Google Scholar]
- 25.Sarkar S, Wilson M. Lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. J Periodontal Res. 1993;28:204–210. doi: 10.1111/j.1600-0765.1993.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 26.Socransky S S, Haffajee A D. The nature of periodontal diseases. Ann Periodontol. 1997;2:3–10. doi: 10.1902/annals.1997.2.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Soukos N S, Hamblin M R, Hasan T. The effect of charge on cellular uptake and phototoxicity of polylysine chlorin e6 conjugates. Photochem Photobiol. 1997;65:723–729. doi: 10.1111/j.1751-1097.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 28.Soukos N S, Wilson M, Burns T, Speight P M. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg Med. 1996;18:253–259. doi: 10.1002/(SICI)1096-9101(1996)18:3<253::AID-LSM6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Tortora G J, Funke B R, Case C L. Microbiology: an introduction. Redwood City, Calif: The Benjamin/Cummings Publishing Company Inc.; 1992. [Google Scholar]
- 30.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaara M, Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983;24:114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaara M, Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983;24:107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J Appl Bacteriol. 1993;75:299–306. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181–189. [PubMed] [Google Scholar]
- 35.Wilson M, Dobson J, Harvey W. Sensitisation of oral bacteria to killing by low-power laser irradiation. Curr Microbiol. 1992;25:77–81. doi: 10.1007/BF01570963. [DOI] [PubMed] [Google Scholar]