Abstract
Background
After the implementation of the universal two-child policy in China, it was more frequent to have long interpregnancy intervals (IPIs) and advanced maternal age. However, the interactions between long IPIs and advanced maternal age on neonatal outcomes are unknown.
Methods
The study subjects of this historical cohort study were multiparas with singleton live births between October 1st, 2015, and October 31st, 2020. IPI was defined as the interval between delivery and conception of the subsequent pregnancy. Logistic regression models were used to calculate adjusted odds ratios (aORs) and 95% confidence intervals (CIs) of the risks of preterm birth (PTB), low birth weight (LBW), small for gestation age, and 1-min Apgar score ≤ 7 in different IPI groups. Relative excess risk due to interaction (RERI) was used to evaluate the additive interaction between long IPIs and advanced maternal age.
Results
Compared with the 24 ≤ IPI ≤ 59 months group, the long IPI group (IPI ≥ 60 months) was associated with a higher risk of PTB (aOR, 1.27; 95% CI: 1.07–1.50), LBW (aOR, 1.32; 95% CI 1.08–1.61), and one-minute Apgar score ≤ 7 (aOR, 1.46; 95% CI 1.07–1.98). Negative additive interactions (all RERIs < 0) existed between long IPIs and advanced maternal age for these neonatal outcomes. Meanwhile, IPI < 12 months was also associated with PTB (aOR, 1.51; 95% CI 1.13–2.01), LBW (aOR, 1.50; 95% CI 1.09–2.07), and 1-min Apgar score ≤ 7 (aOR, 1.93; 95% CI 1.23–3.04).
Conclusions
Both short and long IPIs are associated with an increased risk of adverse neonatal outcomes. Appropriate IPI should be recommended to women planning to become pregnant again. In addition, better antenatal care might be taken to balance the inferiority of advanced maternal age and to improve neonatal outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-023-00728-4.
Keywords: Interaction, Interpregnancy interval, Low birth weight, Maternal age, Preterm birth
Introduction
Birth outcomes are critical indicators for predicting infant health [1]. Adverse birth outcomes are related to health problems later in life and are important public health problems globally [2]. Interpregnancy intervals (IPIs) were identified as a key and potentially modifiable risk factor for adverse maternal and neonatal outcomes [3–6]. Previous studies revealed a J-shaped relationship between IPIs and adverse perinatal outcomes; that is, both short and long IPIs were associated with adverse perinatal outcomes [7–9]. Many previous studies [10–16] observed that a short IPI was a risk factor for adverse neonatal outcomes, including preterm birth (PTB), low birth weight (LBW), and small-for-gestational-age (SGA). Nevertheless, only a few studies have focused on the relationship between a long IPI and neonatal outcomes [10, 16].
Since the family planning policy was implemented in the 1980s in China, the one-child policy has been successively implemented in the past few decades. However, to maintain the growth rate of the population, the two-child policy was universally carried out in 2015, which allows all families to have a second child [17]. From the one-child policy to the universal two-child policy, many couples in China with only one child gave birth to their second child, which resulted in a significant increase in pregnant women with long IPIs. It provides a unique opportunity to study the association between long IPIs and neonatal outcomes during the period of family planning updates in China.
Long IPIs are frequently combined with advanced maternal age (≥ 35 years), which is also associated with increased risks of adverse neonatal outcomes [18, 19]. Accompanied by the implementation of the universal two-child policy in 2015 in China, the rate of pregnant women with advanced maternal age increased significantly [20]. In general, advanced maternal age was also associated with a higher rate of maternal complications, which may be an intermediary for the increased adverse neonatal outcomes (Fig. 1). In previous studies on the association between IPI and perinatal outcomes, maternal complications were used as the outcomes, and the influence of maternal complications on neonatal outcomes was ignored [21, 22].
Fig. 1.
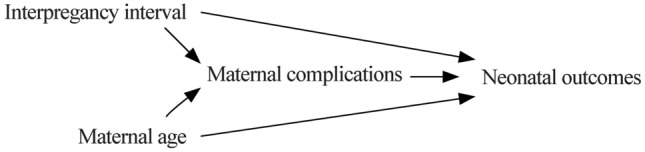
Directed acyclic graph (DAG)
After the implementation of the universal two-child policy in China, more antenatal care and examinations have been strengthened to reduce adverse neonatal outcomes [17]. However, as potentially modifiable risk factors, it is essential to determine the mechanism of IPI and maternal age on neonatal outcomes. Thus, the present study aimed to reveal the comprehensive associations between IPI and maternal age with adverse neonatal outcomes and to provide epidemiological evidence for the formulation of public health policies and prepregnancy consultation for reproductive women.
Methods
The data were collected from the Maternity and Child Registration System in the present historical cohort study. It was provided by the Health Commission of Luzhou City and used for tracking and managing pregnant women and their fetuses/newborns who visited and gave birth in all hospitals in Luzhou district. After obtaining electronic authorization, the Maternity and Child Registration System could scrape the rough data from the Hospital Information System. The outlier data were filtered automatically, and one of our authors checked it in the original data.
A total of 18,605 births were recorded from October 1st, 2015, to October 31st, 2020. The inclusion criterion was multiparas with two or more pregnancies. Primipara (n = 9586) was excluded first. Pregnant women with twins or multiple births (n = 394), severe diseases (such as cancer, n = 8), received assisted reproductive technology (n = 787) during the current pregnancy, and data missing on IPI (n = 77) or birth weight (n = 84) were excluded. A total of 7669 singleton live births were finally eligible for analysis. This was a historical cohort study, and the protocol was reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Southwest Medical University (No. KY2021264). As the data were collected anonymously, informed consent was not required by the patients.
IPI was defined as the interval between delivery and conception of the subsequent pregnancy, which was calculated in months from the date of the last birth to the date of the present birth, minus the gestational age, and a long IPI was defined as ≥ 60 months [10, 23]. PTB was delivered before the 37th completed week of gestation. LBW was defined as birthweight < 2500 g, and macrosomia was birthweight > 4000 g. SGA and large for gestation age (LGA) were birthweight less than the 10th percentile and more than the 90th percentile according to sex-age based on Chinese national growth curves, respectively [24].
The factors that potentially influence the associations between exposure and outcomes were adjusted in the analysis, including maternal age at the first delivery (< 25, 25–29, or ≥ 30), maternal age at the current delivery (< 25, 25–29, 30–34, or ≥ 35), gravidity (2, 3, or more than 3), parity (2 or more), body mass index (BMI) at admission for current delivery (< 25, 25–29.9, or ≥ 30), methods of the last delivery (vaginal delivery or cesarean section), abortion history (yes or no), gestational diabetes mellitus (GDM, yes or no) and pregnancy-induced hypertension (PIH, yes or no). BMI was calculated as weight (kg)/height (m)2.
The WHO recommends that the IPI should not be less than 24 months [25] and the American College of Obstetricians and Gynecologists recommends that the optimal IPI is 18 months to 5 years [26]. According to these recommendations, the IPI groups were classified as < 12 months, 12–23 months, 24–59 months, and 60 months or greater, and the IPI of 24–59 months was set as the reference group. The outcomes were described as categorical variables, and the chi-squared test (χ2) was used to compare the baseline characteristics and outcomes among the groups with different IPIs.
A series of logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) of the outcomes. The model fitness was checked using the Hosmer and Lemeshow goodness of fit. In Model 1, the crude OR of each outcome for the IPI was calculated by an unadjusted logistic regression. After checking multicollinearity for the data, maternal age at the first delivery, gravidity, parity, BMI, mode of the last delivery and abortion history were adjusted in a multivariable logistic regression (Model 2). Two other logistic models were used to explore the role of maternal age and maternal complications at the present delivery in the association of IPI and neonatal outcomes.
We applied “relative excess risk due to interaction” (RERI) to evaluate the additive interaction between a long IPI and advanced maternal age. The RERI is defined as RERI = OR11 − OR10 − OR01 + 1. The OR11 is in group exposure to both long IPI (1 = exposed, 0 = unexposed) and advanced maternal age (1 = exposed, 0 = unexposed), OR10 is in group exposure to long IPI, and OR01 is in group exposure to advanced maternal age, compared to the doubly unexposed group, respectively. RERI > 0 and RERI < 0 were regarded as significant positive and negative additive interactions, respectively [27]. Furthermore, to interpret the role of maternal age profoundly, a logistic regression analysis was performed to test the association between maternal age and adverse neonatal outcomes. Statistical analyses were conducted with SAS software 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
The differences in maternal characteristics among subgroups with different IPIs are displayed in Table 1. A total of 49.9% of the women delivered after long IPIs (≥ 60 months), and 46.8% of the women with long IPIs were ≥ 35 years old. The pregnant women with different IPIs had significant differences in maternal age at the current delivery (P < 0.001) and at the first delivery (P < 0.001), gravidity (P < 0.001), parity (P < 0.001), BMI at admission for delivery (P < 0.001), methods of the last delivery (P < 0.001), abortion history (P < 0.001), GDM (P < 0.001) and PIH (P < 0.001). Pregnant women with long IPIs were older at delivery, had a more frequent pregnancy history, had a higher BMI, had higher risks of GDM and PIH, and had fewer previous abortion history.
Table 1.
Differences in baseline characteristics among pregnant women with different interpregnancy intervals
| Variables | Total (N = 7669) | Interpregnancy interval (mon) | P | |||
|---|---|---|---|---|---|---|
| < 12 (n = 350) | 12–23 (n = 945) | 24–59 (n = 2544) | ≥ 60 (n = 3830) | |||
| Maternal age at current delivery (y) | < 0.001 | |||||
| < 25 | 602 (7.9) | 102 (29.1) | 213 (22.5) | 258 (10.1) | 29 (0.8) | |
| 25–29 | 2070 (27.0) | 152 (43.4) | 387 (41.0) | 969 (38.1) | 562 (14.7) | |
| 30–34 | 2831 (36.9) | 63 (18.0) | 286 (30.3) | 1036 (40.7) | 1446 (37.8) | |
| ≥ 35 | 2166 (28.2) | 33 (9.4) | 59 (6.2) | 281 (11.1) | 1793 (46.8) | |
| Maternal age at first delivery(y) | < 0.001 | |||||
| < 25 | 4123 (53.8) | 197 (56.3) | 479 (50.7) | 1222 (48.0) | 2225 (58.1) | |
| 25–29 | 2982 (38.9) | 108 (30.9) | 356 (37.7) | 1081 (42.5) | 1437 (37.5) | |
| ≥ 30 | 564 (7.3) | 45 (12.9) | 110 (11.6) | 241 (9.5) | 168 (4.4) | |
| Gravidity | < 0.001 | |||||
| 2 | 1909 (24.9) | 171 (48.9) | 360 (38.1) | 726 (28.5) | 652 (17.0) | |
| 3 | 2156 (28.1) | 96 (27.4) | 296 (31.3) | 800 (31.5) | 964 (25.2) | |
| > 3 | 3604 (47.0) | 83 (23.7) | 289 (30.6) | 1018 (40.0) | 2214 (57.8) | |
| Parity | < 0.001 | |||||
| 2 | 6055 (78.9) | 255 (72.9) | 688 (72.8) | 1894 (74.4) | 3218 (84.0) | |
| > 2 | 1614 (21.1) | 95 (27.1) | 257 (27.2) | 650 (25.6) | 612 (16.0) | |
| Body mass index at admission for delivery | < 0.001 | |||||
| < 25 | 1948 (25.4) | 132 (37.7) | 311 (32.9) | 653 (25.7) | 852 (22.2) | |
| 25–29.9 | 3890 (50.7) | 149 (42.6) | 433 (45.8) | 1317 (51.8) | 1991 (52.0) | |
| ≥ 30 | 1411 (18.4) | 46 (13.1) | 139 (14.7) | 442 (17.3) | 784 (20.5) | |
| Missing | 420 (5.5) | 23 (6.6) | 62 (6.6) | 132 (5.2) | 203 (5.3) | |
| Last cesarean section | < 0.001 | |||||
| Yes | 4884 (63.7) | 163 (46.5) | 545 (57.7) | 1702 (66.9) | 2474 (64.6) | |
| No | 2749 (35.8) | 185 (52.9) | 393 (41.6) | 829 (32.6) | 1342 (35.0) | |
| Missing | 36 (0.5) | 2 (0.6) | 7 (0.7) | 13 (0.5) | 14 (0.4) | |
| Previous abortion history | < 0.001 | |||||
| Yes | 5299 (69.1) | 133 (38.0) | 483 (51.1) | 1625 (63.9) | 3058 (79.8) | |
| No | 2370 (30.9) | 217 (62.0) | 462 (48.9) | 919 (36.1) | 772 (20.2) | |
| Gestational diabetes mellitus | < 0.001 | |||||
| Yes | 924 (12.1) | 32 (9.1) | 65 (6.9) | 235 (9.2) | 592 (15.5) | |
| No | 6745 (87.9) | 318 (90.9) | 880 (93.1) | 2309 (90.8) | 3238 (84.5) | |
| Pregnancy-induced hypertension | < 0.001 | |||||
| Yes | 534 (7.0) | 14 (4.0) | 45 (4.8) | 126 (5.0) | 349 (9.1) | |
| No | 7135 (93.0) | 336 (96.0) | 900 (95.2) | 2418 (95.0) | 3481 (90.9) | |
Table 2 shows the differences in neonatal outcomes in different subgroups, and there were significant differences in PTB (P < 0.001), LBW (P < 0.001), and one-minute Apgar score ≤ 7 (P < 0.001). Higher rates of PTB, LBW, and one-minute Apgar score ≤ 7 were observed in infants born to mothers with IPI < 12 months or IPI ≥ 60 months.
Table 2.
Differences in adverse neonatal outcomes among groups with different interpregnancy intervals
| Variables | Total | Interpregnancy interval (mon) | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| < 12 | 12–23 | 24–59 | ≥ 60 | ||||
| Preterm birth | 1294 (16.9) | 82 (23.4) | 161 (17.0) | 371 (14.6) | 680 (17.8) | 22.3 | < 0.001 |
| Low birth weight | 930 (12.1) | 64 (18.3) | 114 (12.1) | 261 (10.3) | 491 (12.8) | 22.5 | < 0.001 |
| Macrosomia | 357 (4.7) | 13 (3.7) | 50 (5.3) | 118 (4.6) | 176 (4.6) | 1.5 | 0.661 |
| Small for gestational age | 419 (5.5) | 25 (7.1) | 44 (4.7) | 128 (5.0) | 222 (5.8) | 4.8 | 0.184 |
| Large for gestational age | 1373 (17.9) | 53 (15.1) | 156 (16.5) | 470 (18.5) | 694 (18.1) | 3.7 | 0.289 |
| 1-min Apgar score ≤ 7 | 342 (4.5) | 29 (8.3) | 41 (4.3) | 92 (3.6) | 180 (4.7) | 16.8 | 0.001 |
In Table 3 Model 2, compared with the reference group (IPI at 24–59 months), the long IPI group (IPI ≥ 60 months) was associated with a higher risk of PTB (adjusted OR, 1.15; 95% CI 1.00–1.34) and LBW (adjusted OR, 1.19; 95% CI 1.00–1.41). Model 3 showed that while entering maternal age at the current delivery, the ORs in long IPIs for PTB, LBW, and one-minute Apgar score ≤ 7 increased. In addition, negative interaction effects were observed between a long IPI and advanced maternal age for PTB (RERI = − 0.62), LBW (RERI = − 0.78), and one-minute Apgar score ≤ 7 (RERI = − 1.35). When maternal age at the current delivery and maternal complications were entered simultaneously in Model 4, the long IPI group was still associated with a higher risk of PTB (adjusted OR, 1.24; 95% CI: 1.04–1.47), LBW (adjusted OR, 1.29; 95% CI 1.05–1.58), and 1-min Apgar score ≤ 7 (adjusted OR, 1.42; 95% CI 1.04–1.94). This indicates that a long IPI is an independent risk factor for PTB, LBW and a 1-min Apgar score ≤ 7.
Table 3.
Crude and adjusted odds ratios for adverse neonatal outcomes in groups with interpregnancy intervals
| Variables | Model 1a crude OR (95% CI) | Model 2b adjusted OR (95% CI) | Model 3c adjusted OR (95% CI) | Model 4d adjusted OR (95% CI) |
|---|---|---|---|---|
| Preterm birth | ||||
| IPI < 12 mon | 1.79 (1.37–2.35) | 1.65 (1.25–2.19) | 1.51 (1.13–2.01) | 1.51 (1.13–2.02) |
| IPI 12–23 mon | 1.20 (0.98–1.47) | 1.13 (0.92–1.39) | 1.06 (0.85–1.31) | 1.06 (0.85–1.31) |
| IPI 24–59 mon | Reference | Reference | Reference | Reference |
| IPI ≥ 60 mon | 1.26 (1.10–1.45) | 1.15 (1.00–1.34) | 1.27 (1.07–1.50) | 1.24 (1.04–1.47) |
| Low birth weight | ||||
| IPI < 12 mon | 1.96 (1.45–2.64) | 1.67 (1.22–2.28) | 1.50 (1.09–2.07) | 1.52 (1.10–2.11) |
| IPI 12–23 mon | 1.20 (0.95–1.52) | 1.08 (0.85–1.37) | 1.00 (0.78–1.27) | 1.00 (0.78–1.28) |
| IPI 24–59 mon | Reference | Reference | Reference | Reference |
| IPI ≥ 60 mon | 1.29 (1.10–1.51) | 1.19 (1.00–1.41) | 1.32 (1.08–1.61) | 1.29 (1.05–1.58) |
| Small for gestation age | ||||
| IPI < 12 mon | 1.45 (0.93–2.27) | 1.23 (0.78–1.93) | 1.13 (0.71–1.79) | 1.15 (0.71–1.84) |
| IPI 12–23 mon | 0.92 (0.65–1.31) | 0.83 (0.59–1.19) | 0.78 (0.54–1.12) | 0.77 (0.53–1.11) |
| IPI 24–59 mon | Reference | Reference | Reference | Reference |
| IPI ≥ 60 mon | 1.16 (0.93–1.45) | 1.16 (0.92–1.47) | 1.21 (0.92–1.59) | 1.18 (0.89–1.56) |
| 1-min Apgar score ≤ 7 | ||||
| IPI < 12 mon | 2.41 (1.56–3.71) | 2.13 (1.36–3.32) | 1.93 (1.23–3.04) | 1.93 (1.22–3.04) |
| IPI 12–23 mon | 1.21 (0.83–1.76) | 1.12 (0.76–1.63) | 1.03 (0.70–1.52) | 1.03 (0.70–1.52) |
| IPI 24–59 mon | Reference | Reference | Reference | Reference |
| IPI ≥ 60 mon | 1.31 (1.02–1.70) | 1.26 (0.97–1.65) | 1.46 (1.07–1.98) | 1.42 (1.04–1.94) |
Model 1: crude OR
Model 2: adjusted for maternal age at first delivery, gravidity, parity, BMI at admission for delivery, last cesarean section, and previous abortion history
Model 3: adjusted for maternal age at first delivery, gravidity, parity, BMI at admission for delivery, last cesarean section, previous abortion history, and maternal age at current delivery
Model 4: adjusted for maternal age at first delivery, gravidity, parity, BMI at admission for delivery, last cesarean section, previous abortion history, gestational diabetes mellitus, pregnancy-induced hypertension, and maternal age at current delivery
OR odds ratio, CI confidence interval, IPI interpregnancy intervals, BMI body mass index
The short IPI group (IPI < 12 months) was also associated with an increased risk of PTB (adjusted OR, 1.51; 95% CI 1.13–2.01), LBW (adjusted OR, 1.50; 95% CI 1.09–2.07), and 1 min Apgar score ≤ 7 (adjusted OR, 1.93; 95% CI 1.23–3.04) (Table 3 Model 3).
Discussion
Our findings showed that short and long IPIs are associated with an increased risk of PTB, LBW and a 1-min Apgar score ≤ 7. In addition, negative interactions exist between a long IPI and advanced maternal age for these neonatal outcomes.
Consistent with previous studies [10, 16, 28–30], the present historical cohort study indicates that a long IPI is an independent risk factor for adverse neonatal outcomes. Many previous studies [11, 15, 31] examined the association between the IPI and neonatal outcomes, focusing on a short IPI. Unfortunately, few studies [10, 16, 28–30] have examined the association between a long IPI and neonatal outcomes. The specific potential mechanism between long IPI and adverse neonatal outcomes is still not clear. Physiological regression hypothesis was proposed in a previous study that pregnancy helps women obtain the capacity of growth support, and the benefit may gradually be lost after delivery if another pregnancy occurs with long IPIs [32].
With the implementation of the universal two-child policy in China in 2015, the number of pregnant women with a long IPI increased with a higher rate of pregnant women with advanced maternal age [20]. In the present study population, almost half of the pregnant women with long IPIs (≥ 60 months) were 35 years or older. Many previous studies [17, 18, 33] have reported that advanced maternal age increases the risk of adverse neonatal outcomes. Interestingly, advanced maternal age was not associated with an increased risk of adverse neonatal outcomes in the present study (Supplementary Table 1), consistent with the report of Qin et al. in 2017 [34]. This phenomenon may be attributed to the self-selection of pregnant women and their family support. In this initial stage of the universal two-child policy implementation, women with an advanced age who would like to bear a second baby might have a better socioeconomic and health status and may even have improved health care during pregnancy.
In contrast to previous studies, the present study aimed to interpret the interactions between a long IPI and advanced maternal age on adverse neonatal outcomes. When maternal age at the current delivery was included in the model, ORs were increased in the long IPI group for PTB, LBW and one-minute Apgar score ≤ 7 (Table 3, Model 3) compared with before adjustment. Furthermore, negative interaction effects were observed between a long IPI and advanced maternal age in the RERI model. Both results support that advanced maternal age does not increase the risk of adverse neonatal outcomes in our study subjects. Thus, we conclude that a long IPI is an independent risk factor for adverse neonatal outcomes, but advanced age does not strengthen this effect in this initial stage of universal two-child policy implementation in China.
In some previous studies, both a long IPI and advanced maternal age were associated with a higher incidence of maternal complications [21, 22, 35, 36], and maternal complications were also proven to correlate with adverse neonatal outcomes [37, 38]. Thus, maternal complications should be considered the intermediate variable in the pathway between a long IPI and neonatal outcomes (Fig. 1). Based on this directed acyclic graph (DAG) and some well-known paradoxes in epidemiology studies [39, 40], an overadjustment bias is generated by adjusting for an intermediate variable [41]. In contrast to maternal age, with controlling for maternal complications, the real associations between a long IPI and neonatal outcomes cannot be consistently estimated. In the present study, the ORs of long IPIs for neonatal outcomes decreased after adjusting for maternal complications (Table 3, Model 4). Thus, the real associations between IPIs and neonatal outcomes can be estimated from Model 3 (Table 3). We should strictly distinguish the interaction and intermediate effect in future studies.
In the present study, a short IPI was also associated with increased risks of PTB, LBW, and a 1-min Apgar score ≤ 7, which is consistent with previous studies [12, 42]. A short interval between successive pregnancies may worsen maternal nutritional status by reducing the time to recover from delivery. Additionally, breastfeeding can enhance maternal malnutrition, leading to insufficient placental function [32]. In the clinical consultation, we should also advise women to avoid pregnancy after a short IPI.
Our study has several limitations. We did not address some residual confounding factors, such as maternal smoking, alcohol, pregnancy intention, maternal illness, and fertility issues. Additionally, we did not include pregnancies induced by assisted reproductive technology. These women have worse fertility and may have an increased risk of adverse neonatal outcomes. Although we included both live births and stillbirths after at least 28 gestational weeks, we did not address pregnancy loss before 28 weeks. Furthermore, we performed a single-center study with the advantage of excluding the bias of different therapeutic approaches in obstetrics that could affect neonatal outcomes; thus, the results may not be generalized to the whole Chinese cohorts.
In conclusion, our data showed that short and long IPIs are associated with an increased risk of adverse neonatal outcomes after implementing the universal two-child policy in China. While planning to give birth to another baby, an appropriate IPI should be recommended to reduce the risks of adverse neonatal outcomes. In addition, better antenatal care might be taken to balance the inferiority of advanced maternal age and to improve neonatal outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Yu-Xin Xiang, Dong Lei, Jia-Lin Cheng, and Ke-Ru Hou, the undergraduate students from School of Pediatrics, Southwest Medical University for collecting data.
Author contributions
MY and FH contributed equally to this paper. LXP: conceptualization, writing–review and editing. MY and FH: formal analysis, writing–original draft. LY, BZR, and DWB: data curation and writing–review and editing. MY and FH contributed equally to the study. All authors approved the final version to be published.
Funding
Funding for this project was provided by the Sichuan Science and Technology Program, grant No. 2019YJ0696 and the Science and Technology Strategic Cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University, Grant No. 2021LZXNYD-J21 to Xiaoping Lei. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
From the publication date, upon reasonable request to the corresponding author (researchers who provide a methodologically sound proposal and assuming use of the data to meet the goals of this proposal), individual participant data that underlie the results reported in this article can be made available after de-identification.
Declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
The protocol of this historical cohort study has been reviewed and approved by the Medical Ethics Committee of the Affiliated Hospital of Southwest Medical University (No. KY2021264). As the data were collected anonymously, the informed consents were not required to sign by patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 SDG Collaborators Measuring progress and projecting attainment on the basis of past trends of the health-related sustainable development goals in 188 countries: an analysis from the global burden of disease study 2016. Lancet. 2017;390:1423–1459. doi: 10.1016/S0140-6736(17)32336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaminathan A, Fell D, Regan A, Walker M, Corsi D. Association between interpregnancy interval and subsequent stillbirth in 58 low-income and middle-income countries: a retrospective analysis using demographic and health surveys. Lancet Glob Health. 2020;8:e113–e122. doi: 10.1016/S2214-109X(19)30458-9. [DOI] [PubMed] [Google Scholar]
- 4.Garg B, Darney B, Pilliod RA, Caughey AB. Long and short interpregnancy intervals increase severe maternal morbidity. Am J Obstet Gynecol. 2021;225(331):e1–8. doi: 10.1016/j.ajog.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhu B, Rolfs R, Nangle B, Horan J. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589–594. doi: 10.1056/NEJM199902253400801. [DOI] [PubMed] [Google Scholar]
- 6.Coo H, Brownell M, Ruth C, Flavin M, Au W, Day A. Interpregnancy interval and adverse perinatal outcomes: a record-linkage study using the Manitoba population research data repository. J Obstet Gynaecol Can. 2017;39:420–433. doi: 10.1016/j.jogc.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynaecol Obstet. 2005;89:S25–33. doi: 10.1016/j.ijgo.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Shi G, Zhang B, Kang Y, Dang S, Yan H. Association of short and long interpregnancy intervals with adverse birth outcomes: evidence from a cross-sectional study in Northwest China. Int J Gen Med. 2021;14:2871–2881. doi: 10.2147/IJGM.S315827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon S, Lazo-Escalante M, Villaran M, Li C. Relationship between interpregnancy interval and birth defects in Washington State. J Perinatol. 2012;32:45–50. doi: 10.1038/jp.2011.49. [DOI] [PubMed] [Google Scholar]
- 10.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta A. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809–1823. doi: 10.1001/jama.295.15.1809. [DOI] [PubMed] [Google Scholar]
- 11.Lonhart JA, Mayo JA, Padula AM, Wise PH, Stevenson DK, Shaw GM. Short interpregnancy interval as a risk factor for preterm birth in non-hispanic black and white women in California. J Perinatol. 2019;39:1175–1181. doi: 10.1038/s41372-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shachar B, Mayo J, Lyell D, Baer R, Jeliffe-Pawlowski L, Stevenson D, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG. 2016;123:2009–2017. doi: 10.1111/1471-0528.14165. [DOI] [PubMed] [Google Scholar]
- 13.Koullali B, Kamphuis EI, Hof MH, Robertson SA, Pajkrt E, de Groot CJ, et al. The effect of interpregnancy interval on the recurrence rate of spontaneous preterm birth: a retrospective cohort study. Am J Perinatol. 2017;34:174–182. doi: 10.1055/s-0036-1584896. [DOI] [PubMed] [Google Scholar]
- 14.Mahande MJ, Obure J. Effect of interpregnancy interval on adverse pregnancy outcomes in northern Tanzania: a registry-based retrospective cohort study. BMC Pregnancy Childbirth. 2016;16:140. doi: 10.1186/s12884-016-0929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cofer F, Fridman M, Lawton E, Korst L, Nicholas L, Gregory K. Interpregnancy interval and childbirth outcomes in California, 2007–2009. Matern Child Health J. 2016;20:43–51. doi: 10.1007/s10995-016-2180-0. [DOI] [PubMed] [Google Scholar]
- 16.Xu T, Miao H, Chen Y, Luo L, Guo P, Zhu Y. Association of interpregnancy interval with adverse birth outcomes. JAMA Netw Open. 2022;5:e2216658. doi: 10.1001/jamanetworkopen.2022.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Editorial Department Interpretation of the universal two-child policy in China. China Population Today. 2015;32:11–21. [Google Scholar]
- 18.Koshida S, Arima H, Fujii T, Ito Y, Murakami T, Takahashi K. Impact of advanced maternal age on adverse infant outcomes: a Japanese population-based study. Eur J Obstet Gynecol Reprod Biol. 2019;242:178–181. doi: 10.1016/j.ejogrb.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Vandekerckhove M, Guignard M, Civadier M, Benachi A, Bouyer J. Impact of maternal age on obstetric and neonatal morbidity: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21:732. doi: 10.1186/s12884-021-04177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HT, Xue M, Hellerstein S, Cai Y, Gao Y, Zhang Y, et al. Association of China’s universal two child policy with changes in births and birth related health factors: national, descriptive comparative study. BMJ. 2019;366:l4680. doi: 10.1136/bmj.l4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Liu H, Wu DD, Hu HT, Wang HH, Zhou CL, et al. Long interpregnancy interval and adverse perinatal outcomes: A retrospective cohort study. Sci China Life Sci. 2020;63:898–904. doi: 10.1007/s11427-018-9593-8. [DOI] [PubMed] [Google Scholar]
- 22.Mignini LE, Carroli G, Betran AP, Fescina R, Cuesta C, Campodonico L, et al. Interpregnancy interval and perinatal outcomes across Latin America from 1990 to 2009: a large multi-country study. BJOG. 2016;123:730–737. doi: 10.1111/1471-0528.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta A. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297–308. doi: 10.1016/j.ajog.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Zhang R, Zhang S, Shi W, Yan W, Wang X, et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua Er Ke Za Zhi. 2015;53:97–103. [PubMed] [Google Scholar]
- 25.Marston C. Report of a who technical consultation on Birth spacing, Geneva, Switzerland, 13–15 June 2005. Geneva, Switzerland, World Health Organizat WHO. 2006;2006(50):137. [Google Scholar]
- 26.Committee Opinion No 666: optimizing postpartum care. Obstet Gynecol. 2016;127:e187–e192. doi: 10.1097/AOG.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 27.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 28.Tessema G, Marinovich M, Håberg S, Gissler M, Mayo JA, Nassar N, et al. Interpregnancy intervals and adverse birth outcomes in high-income countries: an international cohort study. PLoS ONE. 2021;16:e0255000. doi: 10.1371/journal.pone.0255000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Class Q, Rickert M, Oberg A, Sujan AC, Almqvist C, Larsson H, et al. Within-family analysis of interpregnancy interval and adverse birth outcomes. Obstet Gynecol. 2017;130:1304–1311. doi: 10.1097/AOG.0000000000002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Shen S, He J, Chan F, Lu J, Li W, et al. Effect of interpregnancy interval on adverse perinatal outcomes in southern china: a retrospective cohort study, 2000–2015. Paediatr Perinat Epidemiol. 2018;32:131–140. doi: 10.1111/ppe.12432. [DOI] [PubMed] [Google Scholar]
- 31.de Weger FJ, Hukkelhoven CW, Serroyen J, te Velde ER, Smits LJ. Advanced maternal age, short interpregnancy interval, and perinatal outcome. Am J Obstet Gynecol. 2011;204(421):e1–9. doi: 10.1016/j.ajog.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Conde-Agudelo A, Rosas-Bermudez A, Norton FCOH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43:93–114. doi: 10.1111/j.1728-4465.2012.00308.x. [DOI] [PubMed] [Google Scholar]
- 33.Sohn K. The trend in the relationship of advanced maternal age to preterm birth and low birthweight. Eur J Contracept Reprod Health Care. 2017;22:363–368. doi: 10.1080/13625187.2017.1372569. [DOI] [PubMed] [Google Scholar]
- 34.Qin C, Mi C, Xia A, Chen WT, Chen C, Li Y, et al. A first look at the effects of long inter-pregnancy interval and advanced maternal age on perinatal outcomes: a retrospective cohort study. Birth. 2017;44:230–237. doi: 10.1111/birt.12289. [DOI] [PubMed] [Google Scholar]
- 35.Mehari M, Maeruf H, Robles C, Woldemariam S, Adhena T, Mulugeta M, et al. Advanced maternal age pregnancy and its adverse obstetrical and perinatal outcomes in Ayder comprehensive specialized hospital, Northern Ethiopia, 2017: a comparative cross-sectional study. BMC Pregnancy Childbirth. 2020;20:60. doi: 10.1186/s12884-020-2740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kahveci B, Melekoglu R, Evruke I, Cetin C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth. 2018;18:343. doi: 10.1186/s12884-018-1984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Zhang X, Zhou M, Juan J, Wang X. Association of gestational diabetes mellitus with adverse pregnancy outcomes and its interaction with maternal age in Chinese urban women. J Diabetes Res. 2021;2021:5516937. doi: 10.1155/2021/5516937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Y, Lin M, Pai L, Fang J, Mou C, Sung F, et al. Population-based study on birth outcomes among women with hypertensive disorders of pregnancy and gestational diabetes mellitus. Sci Rep. 2021;11:17391. doi: 10.1038/s41598-021-96345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buekens P, Notzon F, Kotelchuck M, Wilcox A. Why do Mexican Americans give birth to few low-birth-weight infants? Am J Epidemiol. 2000;152:347–351. doi: 10.1093/aje/152.4.347. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox A. Birth weight and perinatal mortality: the effect of maternal smoking. Am J Epidemiol. 1993;137:1098–1104. doi: 10.1093/oxfordjournals.aje.a116613. [DOI] [PubMed] [Google Scholar]
- 41.Ananth C, Schisterman E. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167–175. doi: 10.1016/j.ajog.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendt A, Gibbs C, Peters S, Hogue C. Impact of increasing inter-pregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239–258. doi: 10.1111/j.1365-3016.2012.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
From the publication date, upon reasonable request to the corresponding author (researchers who provide a methodologically sound proposal and assuming use of the data to meet the goals of this proposal), individual participant data that underlie the results reported in this article can be made available after de-identification.


