Abstract
Background
Panic disorder is common in the general population. It is often associated with other psychiatric disorders, such as drug dependence, major depression, bipolar disorder, social phobia, specific phobia and generalised anxiety disorder. Azapirones are a class of drugs used as anxiolytics. They are associated with less drowsiness, psychomotor impairment, alcohol potentiation and potential for addiction or abuse than benzodiazepines. However, azapirones are not widely used in the treatment of panic disorder and evidence for their efficacy is unclear. It is important to find out if azapirones are effective and acceptable in the treatment of panic disorder.
Objectives
To assess the effects of azapirones on panic disorder in adults, specifically:
1. to determine the efficacy of azapirones in alleviating symptoms of panic disorder, with or without agoraphobia, in comparison with placebo; 2. to review the acceptability of azapirones in panic disorder, with or without agoraphobia, in comparison with placebo; and 3. to investigate adverse effects of azapirones in panic disorder with or without agoraphobia, including general prevalence of adverse effects, compared with placebo.
Search methods
We searched the Cochrane Depression Anxiety and Neurosis Group Trials Specialised Register (CCDANCTR, search date: 10th January 2014), which includes relevant randomised controlled trials from The Cochrane Library (all years), MEDLINE (1950‐), EMBASE (1974‐), and PsycINFO (1967‐).
Selection criteria
Randomised controlled trials that compared azapirones with placebo for panic disorder in adults.
Data collection and analysis
Three review authors independently identified studies, assessed trial quality and extracted data. We contacted study authors for additional information.
Main results
Three studies involving 170 participants compared the azapirone buspirone with placebo. No study provided enough usable information on our primary efficacy outcome (response). For our primary acceptability outcome, moderate‐quality evidence indicated that azapirones had lower acceptability than placebo: risk ratio (RR) for dropouts for any reason 2.13 (95% confidence interval (CI) 1.11 to 4.07; 3 studies, 170 participants. Evidence for secondary efficacy outcomes were of low quality. Results on efficacy between azapirone and placebo in terms of agoraphobia (standardised mean difference (SMD) ‐0.01, 95% CI ‐0.56 to 0.53; 1 study, 52 participants), general anxiety (mean difference (MD) ‐2.20, 95% CI ‐5.45 to 1.06; 2 studies, 115 participants) and depression (MD ‐1.80, 95% CI ‐5.60 to 2.00; 1 study, 52 participants) were uncertain. None of the studies provided information for the assessment of allocation concealment or sequence generation. Conflicts of interest were not explicitly expressed. The risk of attrition bias was rated high for all three studies. Information on adverse effects other than dropouts for any reason was insufficient to include in the analyses.
Authors' conclusions
The efficacy of azapirones is uncertain due to the lack of meta‐analysable data for the primary outcome and low‐quality evidence for secondary efficacy outcomes. A small amount of moderate‐quality evidence suggested that the acceptability of azapirones for panic disorder was lower than for placebo. However, only trials of one azapirone (namely buspirone) were included in this review; this, combined with the small sample size, limits our conclusions. If further research is to be conducted, studies with larger sample sizes, with different azapirones and with less risk of bias are necessary to draw firm conclusions regarding azapirones for panic disorder.
Plain language summary
Azapirones versus placebo for panic disorder in adults
Why is this review important?
Panic disorder is common in the general population and is often associated with various psychiatric disorders. Azapirones are a class of drugs occasionally used in the treatment of panic disorder, although none has been approved by a regulatory agency for this purpose. They are associated with less drowsiness, psychomotor impairment, alcohol potentiation and potential for addiction or abuse. However, azapirones are not widely used for panic disorder. Evidence for their efficacy in treating panic disorder is unclear. It is important to find out if azapirones are effective and acceptable in the treatment of panic disorder.
Who will be interested in this review?
Patients and general practitioners.
What questions does this review aim to answer?
This review aims to answer the following questions for panic disorder in adults:
1. Are azapirones more effective than placebo? 2. Are azapirones more acceptable than placebo? 3. What kind of adverse effects do azapirones have compared with placebo?
Which studies were included in the review?
We searched electronic database to find all relevant studies published between 1950 and January 2014. To be included in the review, studies had to be randomised controlled trials that compared azapirone with placebo for panic disorder in adults. We include three studies involving a total of 170 people in the review. All three looked at the same type of azapirone, a drug called buspirone. The review authors rated the quality of the evidence as 'low' to moderate'.
What does the evidence from the review tell us?
There was not enough information to work out whether azapirones are any more or less effective than placebo in causing substantial improvements in panic disorder overall.
A small amount of moderate‐quality evidence suggests that the acceptability of azapirones for panic disorder is lower than for placebo.
There was not enough information to compare any differences in adverse effects caused by azapirones and placebo.
What should happen next?
Studies with larger sample sizes and fewer risks of bias should be carried out. Studies should report how participants were allocated to each treatment, and whether the trials were financially sponsored by the manufacturer of the drug. Study protocols should be registered to avoid selective reporting of outcomes by authors.
Trials need to test azapirones other than buspirone to determine their effectiveness.
Remission or response should be reported as the efficacy outcome and longer‐term outcomes need to be addressed to establish whether the effect is transient or durable.
Trials should better report any harms experienced by participants during the trial.
Summary of findings
Summary of findings for the main comparison. Azapirone compared to placebo for panic disorder in adults.
| Azapirones compared to placebo for panic disorder in adults | ||||||
| Patient or population: adults with panic disorder Settings: outpatient Intervention: Azapirones Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Azapirone | |||||
|
Rate of 'response' Follow‐up: 8 weeks |
See comment | See comment | See comment | See comment | See comment | None of the studies reported on this outcome |
| Dropouts for any reason Follow‐up: 8 weeks | Study population | RR 2.13 (1.11 to 4.07) | 170 (3 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 131 per 1000 | 279 per 1000 (145 to 533) | |||||
|
General anxiety Hamilton rating scale for anxiety Follow‐up: 8 weeks |
The mean general anxiety in the intervention groups was 2.20 lower (5.45 lower to 1.06 higher) | 115 (2 studies) | ⊕⊕⊝⊝ low1,2 | |||
|
Agoraphobia Phobia scale Follow‐up: 8 weeks |
The mean agoraphobia in the intervention groups was 0.01 standard deviations lower (0.56 lower to 0.53 higher) | 52 (1 study) | ⊕⊕⊝⊝ low1,2 | A standard deviation of 0.01 represents a small difference between groups | ||
|
Depression Hamilton rating scale for depression Follow‐up: 8 weeks |
The mean depression in the intervention groups was 1.80 lower (5.60 lower to 2.00 higher) | 52 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Confidence interval was wide. 2All the included studies had high attrition rate.
The results on frequency of panic attacs could not be pooled.
None of the studies reported on the outcome of rate of 'remission' and quality of life.
Background
Description of the condition
A panic attack is a discrete period of fear or anxiety that has a rapid onset and reaches a peak within 10 minutes, during which at least four of 13 characteristic symptoms are experienced. Many of these symptoms involve bodily systems, such as racing heart, chest pain, sweating, shaking, dizziness, flushing, stomach churning, faintness and breathlessness. Further recognised panic attack symptoms involve fearful cognitions, such as fears of collapse, going mad or dying and derealisation (APA 1994).
Panic disorder first entered diagnostic classification systems in 1980 with the publication of the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM‐III), after it was observed that people with panic attacks responded to treatment with the tricyclic antidepressant (TCA) imipramine (Klein 1964). For a diagnosis of panic disorder, further conditions must be met related to the frequency of attacks, the need for some attacks to come on ‘out of the blue’ rather than in a predictable externally‐triggered situation, and exclusions wherein attacks are attributable solely to medical causes or panic‐inducing substances, notably caffeine. DSM‐IV requires additionally that at least one attack has been followed by (a) persistent concern about having additional attacks, (b) worry about the implications of the attack or its consequences, or (c) a significant change in behaviour related to the attacks (APA 1994).
Panic disorder is common in the general population, with a lifetime prevalence of 1% to 4% (Eaton 1994; Bijl 1998). In primary care settings, panic syndromes have been reported to have a prevalence of around 10% (King 2008). The origin of panic disorder is not fully understood and is probably heterogeneous. Biological theories incorporate faulty triggering of a built‐in anxiety response, possibly a suffocation alarm. Evidence for this comes from biological challenge tests (lactate and carbon dioxide trigger panic in those with the disorder) and from animal experiments and neuroimaging studies in humans that show activation of fear circuits, such as that involving the periaqueductal grey matter (Gorman 2000).
Agoraphobia is anxiety about being in places or situations from which escape might be difficult or embarrassing, or in which help may not be available, in the event of a panic attack (APA 1994). Agoraphobia can occur with panic disorder (APA 1994). About one‐fourth of people suffering from panic disorder also have agoraphobia (Kessler 2006). The presence of agoraphobia is associated with increased severity and worse outcome (Kessler 2006). Several risk factors predict the development of agoraphobia in people suffering from panic disorder: female gender, more severe dizziness during panic attacks, cognitive factors, dependent personality traits and social anxiety disorder (Starcevic 2009).
Panic disorder, with or without agoraphobia, is highly comorbid with other psychiatric disorders, such as drug dependence, major depression, bipolar I disorder, social phobia, specific phobia and generalised anxiety disorder (Grant 2006). It is estimated that generalised anxiety disorder co‐occurs in 68% of people with panic disorder, whilst major depression has a prevalence of 24% to 88% among people with panic disorder (Starcevic 2009).
Description of the intervention
Treatment of panic disorder includes psychological and pharmacological interventions, often used in combination (Watanabe 2009). Historically, pharmacological interventions for panic disorder have been based on the use of monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs) (Bruce 2003; Stein 2010; Batelaan 2011). However, MAOIs and TCAs are burdened by severe adverse effects, such as dietary restrictions (to avoid hypertensive crisis) for MAOIs and anticholinergic, arrhythmogenic and overall poor tolerability for TCAs (Wade 1999). Benzodiazepines, particularly high‐potency ones, have been used as an alternative to MAOIs and TCAs in panic disorder (Stein 2010). Recent guidelines (British Association for Psychopharmacology (BAP) guideline: BAP 2005; American Psychiatric Association (APA) guideline: APA 2009; National Institute for Health and Clinical Excellence (NICE) guideline: NICE 2011) consider antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs), as first‐line pharmacological treatment for panic disorder because of their more favourable adverse effect profile over MAOIs and TCAs. Azapirones are a class of drugs used as anxiolytics. They seem to be associated with less drowsiness, psychomotor impairment, alcohol potentiation and potential for addiction or abuse than benzodiazepines (Napoliello 1991). Examples include alnespirone, binospirone, buspirone, enilospirone, eptapirone, gepirone, ipsapirone, revospirone, tandospirone and zalospirone, all of which are serotonin 1A (5‐HT1A) receptor partial agonists. Recommended initial dose of buspirone is 15 mg per day and the maximum daily dosage should not exceed 60 mg per day according to the US Food and Drug Administration. Other properties include 5‐HT2A and α1‐ and α2‐adrenergic receptor antagonism, which differ between individual drugs (Kishi 2013).
How the intervention might work
The exact mechanism of action of azapirones in anxiety disorders has not been established, but they are known to be partial agonists at the serotonin 5‐HT1A receptor. In some brain areas such as the raphe nuclei, this is an autoreceptor that when fully activated may act in a homeostatic capacity, exerting an inhibitory effect to oppose the otherwise beneficial effects of increasing serotonin availability.
Evidence suggests that under‐activity of 5‐HT1A receptors is associated with anxiety. In animal studies, knockout mice lacking the 5‐HT1A receptor in the cortex and limbic system show more anxiety behaviours than those who have the receptor intact (Parks 1998). In humans, 5‐HT1A receptor binding availability in the brain correlates inversely with anxiety in normal volunteers (Tauscher 2001). In adults with untreated panic disorder, evidence derived from positron emission tomography (PET) studies shows 5‐HT1A receptor abnormalities in specific brain regions (Nash 2008), with lower 5‐HT1A receptor availability in the raphe and amygdala, which are implicated in anxiety, as well as in the anterior medial and lateral temporal lobes and the orbitofrontal cortex. It is conceivable therefore that as partial agonists, azapirones may generate sufficient 5‐HT1A receptor activation in key brain areas to counteract anxiety, but not to the extent that inhibitory homeostatic autoreceptor processes are invoked, as would be the case if only serotonin were binding to the receptor unopposed. Over longer treatment periods, azapirones can down‐regulate 5‐HT1A receptor numbers, which may contribute to the anxiolytic effect by removing the potential for unhelpful homeostatic effects by activated 5‐HT1A receptors. The 5‐HT1A responsivity by ipsapirone, one of the azapirones, was shown to be different in trial participants with panic disorder (Lesch 1992; Broocks 2002), so that 5‐HT1A receptor–related serotonergic dysfunction is said to be linked to the pathophysiology of panic disorder (Lesch 1992; Neumeister 2004).
Recent evidence has suggested an alternative mechanism of action for azapirones based on the dopamine system. Buspirone is an antagonist at nigrostriatal dopamine D2 and D3 receptors. In vitro data indicate that affinity for D3 is considerably greater than that for D2 and is slightly greater than that for 5‐HT1A receptors. Evidence from animal studies shows that dopamine D3 receptor function may have an effect on anxiety behaviours (Diaz 2011). Although the dopaminergic theory of azapirone action remains unproven, evidence in humans is emerging to confirm that standard therapeutic doses of azapirones such as buspirone can achieve significant D3 receptor occupancy (Payer 2013).
Finally, azapirone inhibition of α2‐adrenoceptors on serotonergic neurons may also play a role in the anxiolytic effects of buspirone. As these receptors moderate serotonin release, azapirone may be instrumental in increasing overall serotonin availability through this mechanism.
Why it is important to do this review
Azapirones are not widely used in panic disorder. The evidence for their efficacy in panic disorder is unclear, though they might be associated with fewer adverse effects than benzodiazepines, which are often used as an alternative to MAOIs and TCAs. To our knowledge, no systematic study on azapirones in panic disorder has been recently conducted. It would be important to understand how treatment can assist in recovery from panic disorder and what agents are most effective in the treatment of panic. A review is needed to help prescribers identify the effect size of active treatment compared with placebo in treating panic disorder, so they can be better guided in selecting the most appropriate pharmacological agent. Another two Cochrane reviews on antidepressants versus placebo in panic disorder and benzodiazepines versus placebo in panic disorder are in progress; findings reported in these reviews will further help clinicians in identifying effective pharmacological treatments for panic disorder (Guaiana 2013a; Guaiana 2013b). This review will add to the Cochrane reviews on combination therapy for panic disorder (specifically, psychotherapy plus benzodiazepines (Watanabe 2009) and psychological therapies for panic disorder (Pompoli 2013)).
Objectives
To assess the effects of azapirones on panic disorder in adults, specifically:
to determine the efficacy of azapirones in alleviating symptoms of panic disorder, with or without agoraphobia, in comparison with placebo;
to review the acceptability of azapirones in panic disorder, with or without agoraphobia, in comparison with placebo; and
to investigate the adverse effects of azapirones in panic disorder with or without agoraphobia, including general prevalence of adverse effects, compared with placebo.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, controlled trials using a parallel‐group design that compare azapirones with placebo as monotherapy.
We included cross‐over trials, randomised placebo‐controlled trials with more than two arms and cluster‐randomised placebo‐controlled trials.
We excluded quasi‐randomised trials, such as those in which allocation is performed by using alternate days of the week.
Types of participants
Participants were 18 years of age or older with a primary diagnosis of panic disorder, with or without agoraphobia, diagnosed according to any of the following criteria: Feighner criteria, Research Diagnostic Criteria, Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM‐III), DSM‐III‐R, DSM‐IV or International Classification of Diseases, 10th Edition (ICD‐10). In case study eligibility focused on agoraphobia rather than panic disorder, studies were to be included if operationally diagnosed according to the above‐named criteria and when it could be safely assumed that some of the participants were suffering from panic disorder as defined by those criteria. In other words, we would have excluded studies that focused on agoraphobia without panic disorder. There is evidence that over 95% of people with agoraphobia seen clinically suffer from panic disorder as well (Goisman 1995). However, there were no such studies and we did not examine the effect of the inclusion of these studies in a sensitivity analysis.
We excluded studies focused on participants with serious comorbid physical disorders (e.g. myocardial infarction, chronic obstructive pulmonary disorder, uncontrolled diabetes, electrolyte disturbances), as they may confound treatment effectiveness and tolerability.
We included participants with comorbid mental disorders, but the effect of including these participants was examined in sensitivity analyses.
Types of interventions
Any trial comparing azapirones (buspirone, gepirone, tandospirone, ipsapirone or lesopitron) as monotherapy with placebo in the treatment of panic disorder, with or without agoraphobia. We included only acute treatment studies treating participants for less than six months. We excluded relapse prevention studies. If a study treated participants for more than six months but reported outcome data within six months, we include the data.
We applied no restriction on dose, frequency, intensity, route of administration or duration.
We also excluded studies administering psychosocial therapies targeted at panic disorder concurrently.
Types of outcome measures
Primary outcomes
1. Rate of 'response' (i.e. substantial improvement from baseline as defined by the original investigators). Examples include 'very much or much improved' according to the Clinical Global Impression Change Scale, more than 40% reduction on the Panic Disorder Severity Scale and more than 50% reduction on the Fear Questionnaire Agoraphobia Subscale.
2. Total number of dropouts for any reason as a proxy measure of treatment acceptability.
Secondary outcomes
3. 'Remission' (i.e. satisfactory end‐state as defined by global judgement of the original investigators). Examples include 'panic free' and 'no or minimal symptom' according to the Clinical Global Impression Severity Scale.
4. Panic symptom scales and global judgement on a continuous scale. Examples include Panic Disorder Severity Scale total score (0 to 28), Clinical Global Impression Severity Scale (1 to 7), Clinical Global Impression Change Scale (1 to 7), etc. When multiple measures were used, we prioritised them in the order listed above, with preference given to panic symptoms scales. The actual measure entered into meta‐analysis are indicated at the top of the listings in the Characteristics of included studies table.
5. Frequency of panic attacks, as recorded, for example, by a panic diary.
6. Agoraphobia, as measured, for example, by Fear Questionnaire, Mobility Inventory, behavioural avoidance test, etc.
7. General anxiety, as measured, for example, by Hamilton Rating Scale for Anxiety, Beck Anxiety Inventory, State‐Trait Anxiety Index, Sheehan Patient‐Rated Anxiety Scale, Anxiety Subscale of Symptom Checklist (SCL)‐90‐R, etc.
8. Depression, as measured, for example, by Hamilton Rating Scale for Depression, Beck Depression Inventory, Depression Subscale of SCL‐90‐R, etc.
9. Social functioning, as measured, for example, by Sheehan Disability Scale, Global Assessment Scale, Social Adjustment Scale‐Self Report, etc.
10.Quality of life, as measured, for example, by Short Form (SF)‐36, SF‐12, etc.
11. Participant satisfaction with treatment.
12. Economic costs.
13. Number of dropouts due to adverse effects.
14. Number of participants experiencing at least one adverse effect.
Timing of outcome assessment
All outcomes are short‐term, which we define as acute phase treatment that would normally last two to six months.
When studies report response rates at different time points within two to six months, we preferred the time point closest to 12 weeks.
Search methods for identification of studies
Electronic searches
The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at its editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 35,000 reports of RCTs in depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual, using a controlled vocabulary; please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), EMBASE (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers via the World Health Organization's trials portal (the International Clinical Trials Registry Platform (ICTRP)), pharmaceutical companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies can be found on the Group's website.
We searched the CCDAN registers using the following terms.
Search 1: Azapirones and Panic
CCDANCTR‐Studies
Diagnosis = (panic) and Intervention = ((azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or *piron*) and placebo*))
CCDANCTR‐References
We searched the References Register using the following free‐text terms to identify additional untagged references: (panic or agoraphobi*) and (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or *piron*)
We screened abstracts for azapirone trials and retrieved full‐text articles to check for placebo controls.
Search 2: Azapirones and Anxiety Disorders not otherwise specified (ADNOS)
We carried out a further search of the CCDANCTR to identify reports of azapirone trials for ‘Anxiety Disorders Not Otherwise Specified’ (ADNOS).
CCDANCTR‐Studies
Condition = (anxiety or anxious) and Intervention = ((azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or *piron*) and placebo*)
CCDANCTR‐ References
We searched the References Register using the following free‐text terms to identify additional untagged references: ((anxiety or anxious or ADNOS) and (azapirone or alnespirone or binospirone or buspirone or enilospirone or eptapirone or gepirone or ipsapirone or revospirone or tandospirone or zalospirone or *piron*)) and not (agoraphobi* or panic or (social NEAR (anxi* or phobi*)) or generalised or generalized or obsessive or compulsive or OCD or PTSD or post‐trauma* or “post trauma*” or posttrauma*)
We screened abstracts and retrieved full‐text articles to check for appropriate placebo controls.
We placed no restrictions on date, language or publication status.
National and International Trials Registers
We conducted complementary searches on the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov.
Searching other resources
Review authors checked the reference lists of all included studies, non‐Cochrane systematic reviews and major textbooks of affective disorders (written in English), for published reports and citations of unpublished research. We also conducted a citation search via the Web of Science (included studies only) to identify additional works. We contacted experts in the field.
Data collection and analysis
Selection of studies
Two review authors (from HI, TA and PC) independently selected trials for inclusion in this systematic review.
HI, TA and PC inspected the search hits by reading the titles and the abstracts to see whether they met the criteria. We resolved possible doubts by consultation with the review co‐authors. We obtained each potentially relevant study located in the search as a full‐text article; three review authors independently assessed them for inclusion, and in the case of discordance sought resolution by discussion between the review authors. We calculated the discordance in the selection of studies using Cohen's kappa (k) (Cohen 1960), a more robust measure than a simple percentage agreement calculation because it takes into account the agreement between review authors that occurs by chance. When it was not possible to evaluate the study because of language problems or missing information, we classified the study as 'study awaiting assessment' until we could obtain a translation or further information. Reasons for the exclusion of trials are reported in the Characteristics of excluded studies table.
We recorded all decisions made during the selection process, along with numbers of studies and references, and present them in a PRISMA flow diagram (Moher 2009) at the end of the review.
Data extraction and management
Three review authors used a data extraction form to independently extract the data from included studies concerning participant characteristics (age, sex, severity of panic disorder, study setting), intervention details (dosage, duration of study, sponsorship), study characteristics (blinding, allocation, etc.) and outcome measures of interest. We planned to pilot the data extraction sheet on a sample of 10% of the included studies, but were unable to do this as the number of included studies was too small. Again, we resolved any disagreement by consensus or by consultation with the third member of the review team. If necessary, we contacted authors of studies to obtain clarification.
Main comparison
Azapirones as a whole versus placebo.
Assessment of risk of bias in included studies
Three review authors independently assessed risks of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome assessment, the completeness of outcome data, selective reporting and other potential biases. We also considered sponsorship bias.
We assessed and categorised the risk of bias, in each domain and overall, as:
low risk of bias: plausible bias unlikely to seriously alter the results;
high risk of bias: plausible bias that seriously weakens confidence in the results;
unclear risk of bias: plausible bias that raises some doubt about the results.
If the assessors disagreed, we made the final rating by consensus or with the involvement of another member of the review group. When inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies to obtain further information. We also report non‐concurrence in quality assessment.
Measures of treatment effect
The main outcome result was reduction of severity of panic and agoraphobia symptoms. Improvement was usually presented as a change on a panic disorder scale(s) (mean and standard deviation), as dichotomous outcomes (responder or non‐responder, remitted or not‐remitted), or as both.
Binary or dichotomous data
For binary outcomes, we calculated a standard estimation of the random‐effects model risk ratio (RR) and its 95% confidence interval (CI). It has been shown that a random‐effects model has good generalisability (Furukawa 2002) and that the RR is more intuitive (Boissel 1999) than the odds ratio. Furthermore, odds ratios tend to be interpreted as RRs by clinicians (Deeks 2000). This may lead to an overestimation of the impression of the effect (Higgins 2011). For all primary outcomes, we calculated the number needed to treat for an additional beneficial outcome or the number needed to treat for an additional harmful outcome statistic (NNTB or NNTH) and its 95% confidence interval (CI) using Visual Rx (www.nntonline.net/), while taking account of the event rate in the control group.
Continuous data
1. Summary statistics
We used standardised mean difference (SMD) as originally planned in the protocol, when the studies used an idiosyncratic scale that is seldom or never used elsewhere (e.g. Phobia Scale for Agoraphobia). However, when all the included studies used the same standard scales such as Hamilton Rating Scale for Anxiety and Hamilton Rating Scale for Depression, we used mean differences (MDs).
2. Endpoint versus change data
Trials usually report results using endpoint means and standard deviations of scales or using change in mean values from baseline of assessment rating scales. We prefer to use scale endpoint data, which typically cannot have negative values and are easier to interpret from a clinical point of view. If endpoint data were unavailable, we planned to use the change data in separate analyses. In cases where we used MDs, we planned to pool results based on change data and endpoint data in the same analysis. However, there were no studies using change data.
Unit of analysis issues
Cross‐over trials
Cross‐over trials are those in which all participants receive both the control and the intervention treatment but in a different order. The major problem is a carry‐over effect from the first phase to the second phase of the study, especially if the condition of interest is unstable (Elbourne 2002). As this is the case with panic disorder, randomised cross‐over studies were eligible, but we planned to use only data up to the point of first cross‐over. However, there were no cross‐over trials included in our review.
Studies with multiple treatment groups
When a study involves more than two treatment arms, especially two appropriate dose groups of the same drug, we had planned to pool the different dose arms and to treat them as one group, but no such trials were included. If the trial involved one placebo arm and two or more arms of antidepressants of different classes, we compared each arm with placebo separately. In this case, a unit of analysis error can occur because of the unaddressed differences between the estimated intervention effects from multiple comparisons (Higgins 2011), resulting in double‐counting. To avoid this, we planned to include each pair‐wise comparison separately, according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.5.4 (Higgins 2011). If the variable was dichotomous, we planned to divide the shared interventions group evenly among the comparisons. If the variable was continuous, only the total number of participants would be divided up, leaving means and standard deviations unchanged. However, there were no studies to which this analysis was applicable.
Cluster‐randomised trials
In cluster‐randomised trials, groups of individuals rather than separate individuals are randomly assigned to different interventions. If we had identified cluster placebo‐controlled randomised trials, we planned to use the generic inverse variance technique, provided such trials had been appropriately analysed, while taking into account intraclass correlation coefficients to adjust for cluster effects. If trialists had not adjusted for the effects of clustering, we planned to do this by obtaining an intracluster correlation coefficient and then following the guidance given in Chapter 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, there were no cluster‐randomised trials included in our review.
Dealing with missing data
We tried to contact the study authors to obtain all relevant missing data.
1. Dichotomous outcomes
We planned to calculate response, or remission on treatment, using an intention‐to‐treat (ITT) analysis. We followed the principle 'once randomised always analysed'. When participants left the study before the intended endpoint, we assumed that they would have experienced the negative outcome. We tested the validity of the above assumption by sensitivity analysis, with application of 'worst‐case' and 'best‐case' scenarios. When dichotomous outcomes were not reported but the baseline mean and standard deviation on a panic disorder scale were reported, we calculated the number of responding or remitted participants according to a validated imputation method (Furukawa 2005). We checked the validity of the above approach by sensitivity analysis. If necessary, we contacted authors of studies to obtain data or clarification, or both.
2. Continuous outcomes
For continuous data, the Cochrane Handbook for Systematic Reviews of Interventions recommends avoiding imputations, and suggests that data should be used as presented by the original authors. When ITT data were available, we preferred them to 'per protocol' analysis'. If necessary, we contacted authors of studies to obtain data or clarification, or both.
3. Skewed data
We presented skewed or qualitative data descriptively.
We considered several strategies for skewed data. If papers report a mean and a standard deviation, and an absolute minimum possible value is also available for the outcome, we divided the mean by the standard deviation. If the value obtained was less than two, we concluded that some skewness is indicated. If the value obtained was less than one (i.e. the standard deviation is larger than the mean), skewness is almost certain. If papers did not report the skewness and simply reported means, standard deviations and sample sizes, we used these numbers. Because these data may not have been properly analysed and can be misleading, we conducted analyses with and without these studies. If the data had been log‐transformed for analysis, and geometric means were reported, skewness would be reduced. This is the recommended method of analysis for skewed data (Higgins 2011). If papers used non‐parametric tests and described averages using medians, they could not be formally pooled in the analysis. We reported the results of these studies in the text. This means that the data are not lost from the review, and that we can consider the results when drawing conclusions, even if they cannot be formally pooled in the analyses.
4. Missing statistics
When only P or standard error (SE) values are reported, we calculated standard deviations (SDs) (Altman 1996). In the absence of supplementary data after we had requested them from the authors, we calculated the SDs according to a validated imputation method (Furukawa 2006). We examined the validity of these imputations in the sensitivity analyses.
Assessment of heterogeneity
In accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, we quantified heterogeneity by the I² statistic. The Cochrane Handbook for Systematic Reviews of Interventions recommends overlapping intervals for I² interpretation (Section 9.5.2, Higgins 2011) as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: may represent considerable heterogeneity.
We also used the Chi² test and its P value to determine the direction and magnitude of the treatment effects. In a meta‐analysis of few trials, Chi² will be underpowered to detect heterogeneity, if it exists. We used P = 0.10 as a threshold of statistical significance.
Assessment of reporting biases
Reporting biases arise when dissemination of research findings is influenced by the nature and direction of the results. These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A funnel plot is usually used to investigate publication bias. However, it has a limited role when only a few studies of similar size are identified. Also, asymmetry of a funnel plot does not always reflect publication bias. We planned to use visual inspection of funnel plots to assess publication bias, as well as a statistical test for funnel plot asymmetry, as proposed by Eggers or Rücker (Higgins 2011). However, we did not use funnel plots for outcomes because we identified fewer than the recommended minimum of 10 studies.
Data synthesis
We used a random‐effects model to calculate treatment effects. We prefer the random‐effects model, as it takes into account differences between studies, even when we found no evidence of statistical heterogeneity. It gives a more conservative estimate than the fixed‐effect model. We note that the random‐effects model gives added weight to the findings of small studies, which can either increase or decrease the effect size. We applied a fixed‐effect model to primary outcomes only to see whether this markedly changed the effect size.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses are often exploratory in nature and should be interpreted cautiously, because they often involve multiple analyses and can lead to false‐positive results. While keeping in mind the above reservations, we planned to perform the following subgroup analyses:
Participants with and without agoraphobia, because the same treatment may have differential effectiveness with regard to panic and agoraphobia.
If groups within any of the subgroups were found to be significantly different from one another, we ran meta‐regression for exploratory analyses of additive or multiplicative influences of the variables in question.
Acute‐phase treatment studies lasting less than four months versus acute‐phase treatment studies lasting four months or longer.
However, we found no study to be relevant to these subgroup analyses.
Sensitivity analysis
We conducted the following sensitivity analyses a priori. We examined whether the results changed and checked for the robustness of observed findings by:
Excluding trials at high risk of bias (i.e. trials with inadequate allocation concealment and blinding, with incomplete data reporting and/or with high probability of selective reporting);
Excluding trials with dropout rates greater than 20%;
Excluding studies funded by the pharmaceutical company marketing each azapirone. This sensitivity analysis is particularly important (a) because repeated findings indicate that funding strongly affects outcomes of research studies (Als‐Nielsen 2003; Lexchin 2003; Bhandari 2004), and (b) because industry sponsorship and authorship of clinical trial reports have increased over the past 20 years (Buchkowsky 2004);
Excluding studies in which participants clearly have significant psychiatric comorbidities, including primary or secondary depressive disorders; and
Excluding studies where SDs were imputed.
Our routine application of random‐effects and fixed‐effect models, as well as our secondary outcomes of remission rates and continuous severity measures, might be considered additional forms of sensitivity analyses.
Summary of findings table
We present our results using a Table 1, in which we assess the quality of the evidence according to the GRADE approach (Higgins 2011).
Results
Description of studies
Results of the search
The number of references identified by searches to January 2014 was 129, of which 128 remained after de‐duplication. We excluded 88 studies after assessment of the titles and abstracts. We retrieved 40 full‐text papers for full inspection. Of these, we excluded 35 studies. Finally, we included five reports, representing three studies, in the review (there were two additional reports of the Robinson 1989 study (Sheehan 1988; Sheehan 1990)). Cohen's kappa among three authors for the selection from 40 full‐text papers was 0.92, which means a very high level of agreement. We contacted authors of the three studies for additional information, but none of them responded. See Figure 1 for a PRISMA flow diagram (Moher 2009) depicting the study selection process.
1.
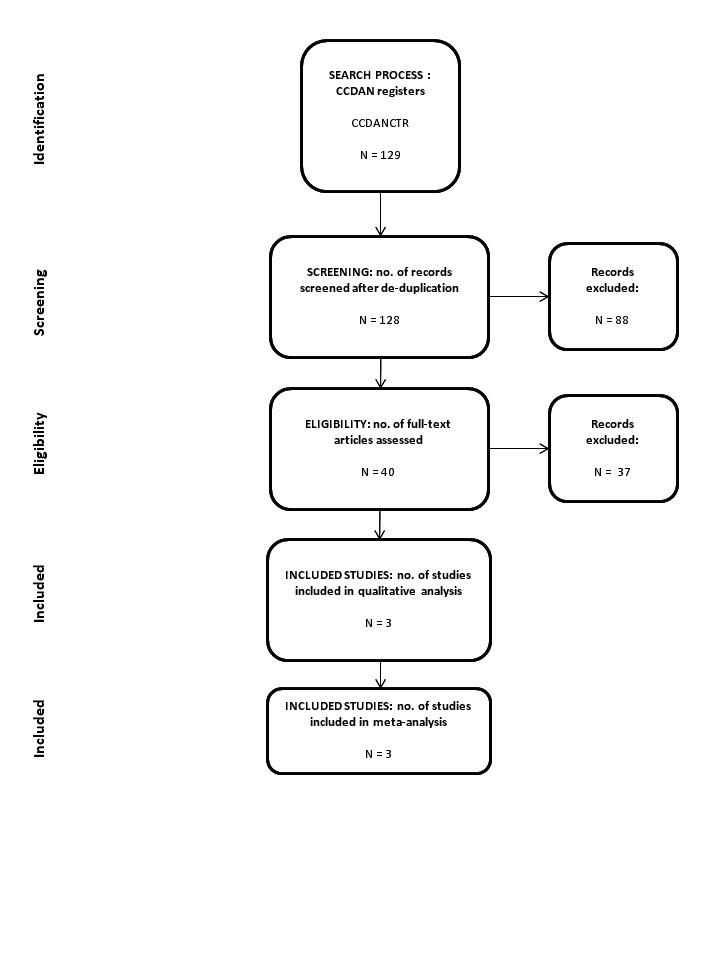
Included studies
We include three studies (Pohl 1989; Robinson 1989; Sheehan 1993) in this review, with characteristics as follows (see also Characteristics of included studies).
Design
All the included studies were parallel‐group, individually‐randomised controlled trials.
Sample sizes
The sample sizes per arm were small, consisting of between 18 and 34 participants in each arm.
Setting
All trials were conducted in the outpatient setting. All were carried out in the USA.
Participants
The proportion of women ranged from 47% to 72%. Mean age ranged from 31.3 to 36.9 years.
Interventions
All of the studies were three‐armed and used buspirone as the azapirone to be tested. Two of the studies included an imipramine arm (Pohl 1989; Robinson 1989) and one study included an alprazolam arm (Sheehan 1993), in addition to a placebo arm. Mean delivered dose of buspirone ranged from 29.5 to 61 mg/day. Duration of the intervention was 8 weeks.
Outcomes
None of the studies reported on response and remission. The number of dropouts for any reason was reported in all the studies. The frequency of panic attacks was used in all the studies, but without reporting standard deviations (SDs). The Hamilton Rating Scale for Anxiety (HAM‐A) was used in two studies (Robinson 1989; Sheehan 1993), but with no reported SD in one study (Robinson 1989). The Hamilton Rating Scale for Depression and Phobia Scale/Agoraphobia was reported in one study (Sheehan 1993). We imputed missing SDs by using data from the systematic review with largest number of participants for panic disorder (Furukawa 2007).
Excluded studies
We excluded 31 studies. Participants in 22 studies were not diagnosed with panic disorder; participants in three studies were diagnosed with anxiety disorders but not stratified; the intervention in three studies was too short; the intervention in two studies was combined therapy; and participants in one study were too young to be included. See also Characteristics of excluded studies.
Ongoing studies
Our searches identified no ongoing studies.
Studies awaiting classification
We identified no studies awaiting classification.
Risk of bias in included studies
For details of the 'Risk of bias' judgements for each study, see Characteristics of included studies. Graphical representations of the overall risk of bias in the included studies are presented in Figure 2 and Figure 3.
2.
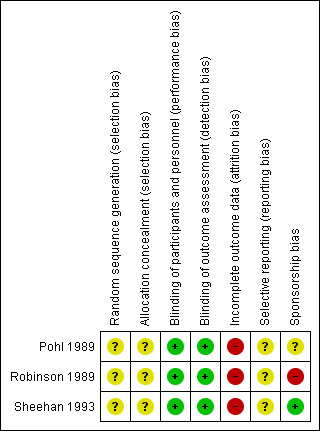
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
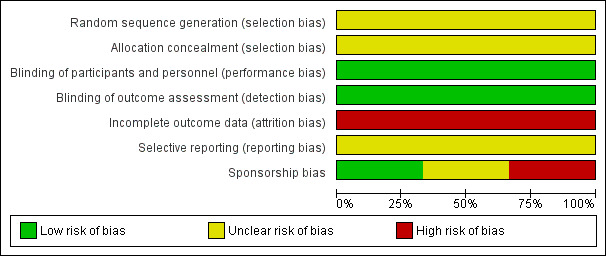
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged the risk of selection bias in all the studies to be unclear, as none of the studies provided information about their method of allocation.
Blinding
Blinding of participants and personnel
We judged the blinding of participants and personnel to be adequate, as all of the studies used an identical capsule as the placebo, although the success of blinding was not tested.
Blinding of outcome assessment
We judged the blinding of participants and personnel to be adequate, so all were assessed as having a low risk of bias for this domain.
Incomplete outcome data
We rated the attrition bias of all the studies as high risk, as they analysed only completers, and the attrition rate was high (ranging from 24% to 39%).
Selective reporting
As no protocol was available for any of the studies, the risk of reporting bias was judged to be unclear.
Spnsorship bias
We rated one study at high risk of bias, because study authors were employees of the company marketing buspirone (Robinson 1989). Another study did not report the relevant information regarding sponsorship (Pohl 1989). We judged the third study to be at low risk of bias, as it was funded by a pharmaceutical company which did not market buspirone (Sheehan 1993).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
Comparison : Azapirones versus placebo
Three studies including 170 participants contributed data to this comparison. See also: Table 1.
Primary outcomes
1.1 Response
Two studies reported the 21‐point Clinician‐rated Global Improvement (CGI) (Pohl 1989; Sheehan 1993). However the response rate could not be imputed, as both of the studies lacked SDs and one study lacked the number of participants measured (Pohl 1989). Pohl 1989 reported CGI severity, but this also lacked SDs. Both studies reported no significant difference in improvement in CGI between buspirone and placebo at post‐treatment.
1.2 Dropouts for any reason
There was moderate‐quality evidence that treatment acceptability was lower for azapirones than for placebo, as expressed in dropouts for any reason: risk ratio (RR) 2.13 (95% confidence interval (CI) 1.11 to 4.07; 3 studies, 170 participants) (Analysis 1.1). There was no heterogeneity (I² = 0%).
1.1. Analysis.
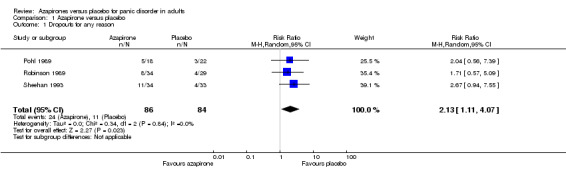
Comparison 1 Azapirone versus placebo, Outcome 1 Dropouts for any reason.
Secondary outcomes
1.3 Remission
The data were not available for the same reason as 1.1. Response.
1.4 Panic symptoms
The data were not available for the same reason as 1.1. Response.
1.5 Frequency of panic attacks
All three studies reported the frequency of panic attacks, but the results could not be pooled, as they were presented in different ways: Pohl 1989 reported the mean number of panic attacks with last observation carried forward (LOCF) imputation: mean difference (MD) ‐2.00 (95% CI ‐5.21 to 1.21; 44 participants) and with completers' data: MD 7.00 (95% CI 4.26 to 9.74; 31 participants), whereas Robinson 1989 reported it with completers' data only: MD ‐3.80 (95% CI ‐5.56 to ‐2.04; 75 participants), and Sheehan 1993 reported the median of the number of panic attacks (buspirone, median = 1 (n = 23); placebo, median = 2 (n = 29)). The former two studies also lacked SDs, and we therefore imputed them by using all the available data from a previous review (Furukawa 2007).
1.6 Agoraphobia
One study evaluated agoraphobia by Phobia scale (Sheehan 1993), detecting no significant difference in effectiveness between buspirone and placebo: SMD ‐0.01 (95% CI ‐0.56 to 0.53; 1 study, 52 participants) (Analysis 1.2). We reported SMD instead of MD because the Phobia scale is seldom used in panic studies.
1.2. Analysis.
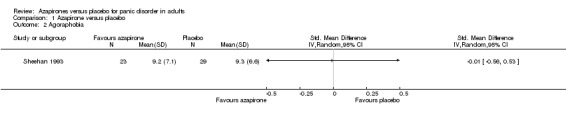
Comparison 1 Azapirone versus placebo, Outcome 2 Agoraphobia.
1.7 General anxiety
Two studies (Robinson 1989; Sheehan 1993) reported general anxiety as measured by the Hamilton Rating Scale for Anxiety (HAM‐A). As Robinson 1989 lacked SDs, we imputed them using all the available data from a previous review (Furukawa 2007). There was no significant difference in effectiveness between buspirone and placebo: MD ‐2.20 (95% CI ‐5.45 to 1.06; 2 studies, 115 participants) (Analysis 1.3). We found no heterogeneity (I² = 0%).
1.3. Analysis.
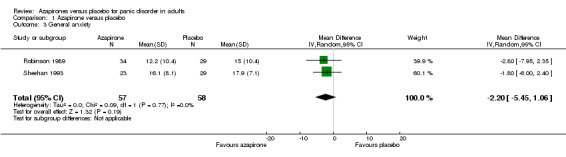
Comparison 1 Azapirone versus placebo, Outcome 3 General anxiety.
1.8 Depression
One study evaluated depression as measured by the Hamilton Rating Scale for Depression (HAM‐D) (Sheehan 1993), which detected no significant difference in effectiveness between buspirone and placebo: MD ‐1.80 (95% CI ‐5.60 to 2.00; 1 study, 52 participants) (Analysis 1.4).
1.4. Analysis.
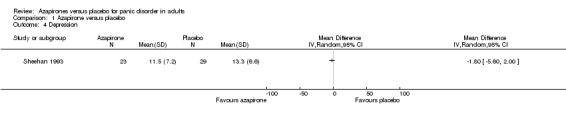
Comparison 1 Azapirone versus placebo, Outcome 4 Depression.
1.9 Social functioning
No relevant data were reported.
1.10 Quality of life
No relevant data were reported.
1.11 Participant satisfaction with treatment
No relevant data were reported.
1.12 Economic costs
No relevant data were reported.
1.13 Dropouts for adverse effects
No relevant data were reported.
1.14 Number of participants experiencing at least one adverse effect
No relevant data were reported.
Subgroup analyses
We could not conduct any of the preplanned subgroup analyses, as no relevant data were available.
Sensitivity analyses
1. Excluding trials with high risk of bias
We were unable to conduct this sensitivity analysis, because all the studies were at the same risk of bias except for sponsorship bias.
2. Excluding trials with dropout rates greater than 20%
We were unable to conduct this sensitivity analysis, because the dropout rates in the intervention arm of all the studies were greater than 20%.
3. Excluding studies funded by the pharmaceutical company marketing each azapirone
Dropouts for any reason
We include two studies in this sensitivity analysis (Pohl 1989; Sheehan 1993). Treatment acceptability was lower for azapirones than for placebo as expressed in dropouts for any reason: RR 2.40 (95% CI 1.07 to 5.39; 2 studies, 107 participants) (Analysis 2.1). We found no heterogeneity (I² = 0%). This result was in accordance with the overall results.
2.1. Analysis.
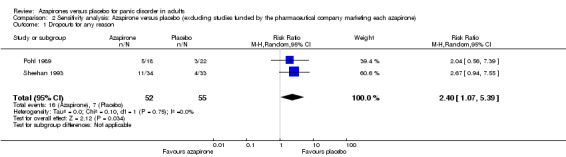
Comparison 2 Sensitivity analysis: Azapirone versus placebo (excluding studies funded by the pharmaceutical company marketing each azapirone), Outcome 1 Dropouts for any reason.
General anxiety
One study (Sheehan 1993) was left after the exclusion. There was no significant difference in effectiveness in reducing general anxiety between buspirone and placebo: MD ‐1.80 (95% CI ‐6.00 to 2.40; 1 study, 52 participants) (Analysis 2.2). This result was in accordance with those without exclusion of the study.
2.2. Analysis.
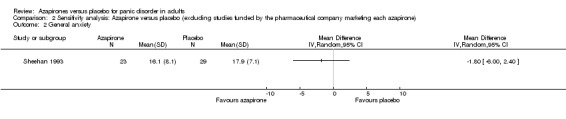
Comparison 2 Sensitivity analysis: Azapirone versus placebo (excluding studies funded by the pharmaceutical company marketing each azapirone), Outcome 2 General anxiety.
4. Excluding studies whose participants clearly have significant psychiatric comorbidities
Dropouts for any reason
Two studies were included in the sensitivity analysis (Pohl 1989; Robinson 1989). RR for dropouts for any reason was no longer statistically significant: RR 1.84 (95% CI 0.80 to 4.23; 2 studies, 103 participants) (Analysis 3.1) but was in general agreement with the overall results
3.1. Analysis.
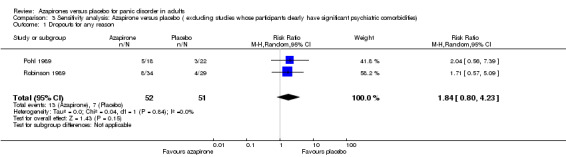
Comparison 3 Sensitivity analysis: Azapirone versus placebo ( excluding studies whose participants clearly have significant psychiatric comorbidities), Outcome 1 Dropouts for any reason.
General anxiety
One study (Robinson 1989) was left after the exclusion. There was no significant difference in effectiveness between buspirone and placebo: MD ‐2.80 (95% CI ‐7.95 to 2.35; 1 study; 63 participants) (Analysis 3.2). This result was in accordance with those without exclusion of the study.
3.2. Analysis.
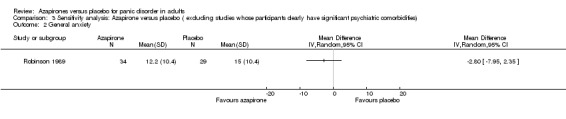
Comparison 3 Sensitivity analysis: Azapirone versus placebo ( excluding studies whose participants clearly have significant psychiatric comorbidities), Outcome 2 General anxiety.
5. Excluding studies with imputed values
General anxiety
One study (Sheehan 1993) was left after the exclusion. There was no significant difference in effectiveness between buspirone and placebo: MD ‐1.80 (95% CI ‐6.00 to 2.40; 1 study; 62 participants) (Analysis 4.1). This result was in accordance with that which included the studies with the imputed SDs.
4.1. Analysis.
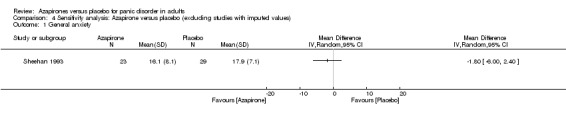
Comparison 4 Sensitivity analysis: Azapirone versus placebo (excluding studies with imputed values), Outcome 1 General anxiety.
Reporting Bias
We could not assess the reporting bias, as only three studies were included in the review.
Discussion
Summary of main results
This review assessed the efficacy and acceptability of azapirones compared with placebo (See Table 1). No study provided enough information contributing to our primary efficacy outcome (response). Our primary harm outcome (dropouts for any reason as a surrogate measure of overall treatment acceptability) was assessed, although we could not assess secondary outcomes related to adverse effects because of lack of information.
Moderate‐quality evidence suggests that azapirones were less acceptable than placebo in terms of dropouts for any reason (3 studies, 170 participants). The result did not change in the sensitivity analysis excluding studies funded by the pharmaceutical company marketing each azapirone.
Low‐quality evidence suggests that there is no significant difference between azapirones and placebo in terms of efficacy as evaluated by agoraphobia (1 study, 52 participants), general anxiety (2 studies, 115 participants) or depression (1 study, 52 participants). The results did not change in the sensitivity analysis excluding studies funded by the pharmaceutical company marketing each azapirone or in the sensitivity analysis excluding studies whose participants clearly had significant psychiatric comorbidities.
Overall completeness and applicability of evidence
The small number of studies and insufficient information in each included study severely limit the completeness and applicability of evidence in this review.
Overall completeness of evidence is poor in the present review. We evaluated efficacy by secondary continuous outcomes and not by the primary outcome, because of lack of information in included studies. Neither could we evaluate information on adverse effects because of lack of information, although data on the primary outcome for acceptability (dropouts for any reason) were available.
Applicability of evidence is also limited. The number of participants assessed was 170 at most from three efficacy trials with selected participants. Pohl 1989 excluded people with fewer than four panic attacks during the past month or no attacks in the week prior to the intervention. Sheehan 1993 required participants to have had at least one panic attack each week for the three weeks prior to baseline and at least three unexpected panic attacks during the history of the disorder to be included in the study. Sensitivity analyses did not change the overall results.
After careful consideration, we judged Robinson 1989 to be the primary report (composite data) of a two‐site study (sponsored by Bristol‐Myers Pharmaceutical Company), incorporating Sheehan 1990. Preliminary results of Sheehan’s trial, conducted at the University of South Florida site, were also reported in Sheehan 1988. It is interesting that Sheehan does not appear to acknowledge the two‐site study and was also not a co‐author of Robinson’s composite report (including data from the Lafayette Clinic, Detroit). We tried to contact Drs David Sheehan and Donald Robinson for clarification on this issue, but neither responded to our query.
Quality of the evidence
The evidence for outcomes on efficacy were of low quality. All the included studies were at high risk of attrition bias: over 20% of data in the intervention arm were not reported. They are also imprecise: the included participants in outcomes on efficacy amounted to 115 at most and the confidence interval was large. The standard deviations (SDs) of the Hamilton Rating Scale for Anxiety (HAM‐A) in Robinson 1989 and the number of panic attacks of Pohl 1989 and Robinson 1989 were imputed from another systematic review with the largest number of participants for panic disorder (Furukawa 2007).
The evidence for the outcome on dropouts for any reason was of moderate quality. However, only three included studies with a total of 170 participants contributed to this outcome.
The risk of biases may make the quality of evidence unstable, as all of the included studies were at unclear risks of bias. None of the studies reported random sequence generation or allocation concealment. Protocols of the studies were not available to judge selective outcome reporting. Conflicts of interest were not explicitly expressed, and our judgements were based only on the authors' reported affiliations. We did not find any possible factor which would upgrade the quality of the evidence.
Potential biases in the review process
We could not evaluate study publication bias or outcome reporting bias due to the small number of included studies and due to the unavailability of the study protocols. Although we searched extensively for relevant trials, it is possible that unpublished trials remain unknown to us.
Imputation of SDs might have affected the results. Imputed SDs of HAM‐A (SD = 10.4) for one of the studies in this analysis (Robinson 1989) were a little larger than the SDs reported in the other included study (8.1 in buspirone and 7.1 in placebo) (Sheehan 1993). This would have widened the confidence interval for the study, reducing its weight in the analysis and underestimating the overall treatment effect.
The included studies date back to around 1990 and the quality of reporting was suboptimal. For example, all the studies lacked information on sequence generation, allocation concealment and conflicts of interest.
Agreements and disagreements with other studies or reviews
As far as we know, this is the first and only systematic review comparing azapirones with placebo for panic disorder. Among the anxiety disorders, one systematic review compared azapirones with other treatment for generalised anxiety disorder (Chessick 2006). The result for the efficacy of azapirones for generalised anxiety disorder was inconclusive. It indicated that azapirones are more effective than placebo in terms of HAM‐A score reduction: MD ‐4.48 (95% CI ‐6.86 to ‐2.10; 4 studies, 135 participants), but no significant improvement was observed in azapriones compared with placebo in terms of clinician‐rated global improvement (CGI): RR 2.35 (95% CI 0.72 to 7.72; 2 studies, 187 participants). When buspirone was compared with placebo, buspirone showed no significantly greater effect than placebo for HAM‐A score: MD 0.40 (95% CI ‐5.62 to 6.42; 1 study, 21 participants). However, significant improvement was observed with buspirone compared with placebo in terms of CGI: RR 1.48 (95% CI 1.01 to 2.17; 1 study, 162 participants). These results provide no additional information to help determine the efficacy of azapirone for panic disorder.
On the other hand, the result for acceptability of azapirones for generalised anxiety disorder was definitive. It indicated that the number of dropouts in azapirones was less than in placebo: RR 0.64 (95% CI 0.47 to 0.87; 8 studies, 629 participants); and the results were similar when comparison was limited to buspirone and placebo: RR 0.68 (95% CI 0.49 to 0.94, 6 studies, 584 participants). These results conflict with those of our review, but the reasons for this disparity are not clear.
Authors' conclusions
Implications for practice.
The efficacy of azapirones compared with placebo for panic disorder is uncertain: no meta‐analysable data were available for our primary efficacy outcome of response, and the available evidence showed no significant difference in efficacy between azapirone and placebo in terms of several secondary outcomes. Moreover the evidence was judged to be of low quality and limited applicability.
Moderate‐quality evidence suggests that the acceptability of azapirones for panic disorder is lower than for placebo. However the number of studies and participants was small.
Based on this single comprehensive review of randomised evidence for the efficacy of azapirones for panic disorder, clinicians who wish to prescribe buspirone, let alone other compounds in this class, to people with panic disorder must do so with a clear awareness of this meagre evidence base and with explicit informed and shared consent from their patients.
Implications for research.
If further research is to be conducted, we recommend the following: first, studies with larger sample size and fewer biases should be carried out. In particular, information on selection, detection and sponsorship is necessary. Study protocols should be registered to avoid selective outcome reporting. Secondly, trials with azapirones other than buspirone are necessary, to determine if the efficacy is due to a specific effect of buspirone or to the class effect of 5‐HT1A agonists. Thirdly, measurement and full reporting of panic disorder‐specific outcomes are necessary. Preferably, remission and response, judged according to a priori standardised definitions, should be reported for comparability between studies. The longer‐term outcomes also need to be addressed, to establish whether any effect is transient or durable.
Notes
This review is one of several separate reviews examining the efficacy and tolerability of pharmacological and non‐pharmacological treatments for panic disorders. These individual reviews will be combined in a network meta‐analysis (protocol to be published in The Cochrane Library).
Acknowledgements
CRG Funding Acknowledgement The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Depression, Anxiety and Neurosis Group.
Disclaimer The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS) or the Department of Health.
Data and analyses
Comparison 1. Azapirone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts for any reason | 3 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.11, 4.07] |
| 2 Agoraphobia | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 General anxiety | 2 | 115 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐5.45, 1.06] |
| 4 Depression | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Sensitivity analysis: Azapirone versus placebo (excluding studies funded by the pharmaceutical company marketing each azapirone).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts for any reason | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.07, 5.39] |
| 2 General anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Sensitivity analysis: Azapirone versus placebo ( excluding studies whose participants clearly have significant psychiatric comorbidities).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts for any reason | 2 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.80, 4.23] |
| 2 General anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 4. Sensitivity analysis: Azapirone versus placebo (excluding studies with imputed values).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General anxiety | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pohl 1989.
| Methods | Study design: Randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III Panic disorder or agoraphobia with panic attacks Method of Diagnosis: Not stated Age: for buspirone, M = 31.1 (SD = 2.1); for placebo, M = 31.6 (SD = 2.2); for imipramine, M = 29.2 (SD = 2.2) Sex: for buspirone, 44% women, 56% men; for placebo 50% women, 50% men Location: United States of America Co‐morbidities: none Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: (1) Buspirone arm (n = 18) Duration: 8 weeks Treatment Protocol: flexible dosage; range = 10 ‐ 60 mg, M = 29.5 (SD = 4.0) (2) Placebo arm (n = 22) Duration: 8 weeks Treatment Protocol: Flexible (3) Imipramine arm (n = 20) Duration: 8 weeks Treatment Protocol: flexible dosage; range = 50 ‐ 300 mg, M = 140 (SD = 17.5) |
|
| Outcomes |
Timepoints for assessment: weekly for the first 4 weeks, and biweekly for the last 4 weeks Outcomes:
|
|
| Notes |
Date of study: Not stated Funding source: Not stated Declarations of interest among the primary researchers: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) | Low risk | This is a double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Sponsorship bias | Unclear risk | No information provided |
Robinson 1989.
| Methods | Study design: Randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III Panic disorder Method of Diagnosis: Not stated Age: for buspirone, M = 34.4 (SD = 1.8); for placebo, M = 33.1 (SD = 1.9); for imipramine, M = 30.1 (SD = 1.0) Sex: for buspirone, 64% women, 36% men; for placebo 62% women, 38% men; imipramine 75% women, 25% men Location: United States of America Co‐morbidities: unclear Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: (1) Buspirone arm (n = 34) Duration: 8 weeks Treatment Protocol: flexible dosage; range = not stated, M = 43 (SD = 3) (2) Placebo arm (n = 29) Duration: 8 weeks Treatment Protocol: Flexible (3) Imipramine arm (n = 28) Duration: 8 weeks Treatment Protocol: flexible dosage; range = not stated, M = 221 (SD = 18) |
|
| Outcomes |
Timepoints for assessment: at 0, 2, 4, 6, 7, 8 weeks Outcomes:
|
|
| Notes |
Date of study: Not stated Funding source: Not stated Declarations of interest among the primary researchers: One of the authors belonged to Bristol‐Myers Company Pharmaceutical Research and Development Division. The authors were advised by employees from Bristol‐Myers Company. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) | Low risk | This is a double‐blind trial. |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Sponsorship bias | High risk | All the authors were employed by the drug company marketing the drug. |
Sheehan 1993.
| Methods | Study design: Randomised controlled trial | |
| Participants |
Diagnosis: DSM‐III‐R Panic disorder with extensive phobic avoidance (agoraphobia with panic attacks) or panic disorder with limited phobic avoidance Method of Diagnosis: Structured Clinical Interview for DSM‐III Age(data were available for participants at week 3 only) : for buspirone (n = 27), M = 36.6 (SD = 9.4); for placebo (n = 31), M = 37.2 (SD = 10.9); for alprazolam (n = 34), M = 36.4 (SD = 8.8) Sex(data were available for participants at week 3 only): for buspirone (n = 27), 67% women, 33% men; for placebo (n = 31) 77% women, 23% men; for alprazolam (n = 34) 76% women, 24% men Location: United States of America Co‐morbidities: Social phobia: for buspirone, 15%; for placebo 23%; for alprazolam 26%. Major depressive disorder: for buspirone, 4%; for placebo 23%; for alprazolam 32%. Rescue medication: none |
|
| Interventions | Participants were randomly assigned to either: (1) Buspirone arm (randomised n = 34) Duration: 8 weeks Treatment Protocol: flexible dosage; range = 15 ‐ 100, M = 61 (SD = 26.5) at study completion (n = 23) (2) Placebo arm (randomised n = 33) Duration: 8 weeks Treatment Protocol: flexible dosage at study completion (n = 29) (3) Alprazolam arm (randomised n = 34) Duration: 8 weeks Treatment Protocol: flexible dosage; range = 1.5 ‐ 10, M = 5.2 (SD = 2.6) at study completion (n = 33) |
|
| Outcomes |
Timepoints for assessment: at baseline and weekly for 8 weeks Outcomes:
|
|
| Notes | There were 9 dropouts in the first 2 weeks of treatment, 7 on buspirone and 2 on placebo; 92 participants completed at least 3 weeks of treatment, with further dropouts before completion of the trial at 8 weeks (Buspirone = 23, Placebo = 29, Alprazolam = 33) Date of study: Not stated Funding source: The study was supported in part by grant from Upjohn Pharmaceutical Company Declarations of interest among the primary researchers: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Identical capsules were used. |
| Blinding of outcome assessment (detection bias) | Low risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Sponsorship bias | Low risk | The study was sponsored by a drug company other than the company marketing the drug. |
M: mean
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bohm 1990a | Participants were not diagnosed with panic disorder. |
| Bohm 1990b | Participants were not diagnosed with panic disorder. |
| Bouvard 1997 | This is a combined therapy with cognitive behaviour therapy. |
| Broocks 2002 | Intervention was one day. |
| Broocks 2003 | Intervention was one day. |
| Bueno 1988 | Participants were not diagnosed with panic disorder. |
| Cottraux 1995 | This is a combined therapy with cognitive behaviour therapy. |
| Gershon 1982 | Participants were not diagnosed with panic disorder. |
| Goldberg 1979a | Participants were not diagnosed with panic disorder. |
| Goldberg 1979b | Participants were not diagnosed with panic disorder. |
| Goldberg 1982 | Participants were not diagnosed with panic disorder. |
| Heller 1990 | Participants were not diagnosed with panic disorder. |
| Hongbo 1994 | Participants were not diagnosed with panic disorder. |
| Hua 2002 | Participants were diagnosed with anxiety disorders, but were not stratified by it, including panic disorder. |
| Kai 1992 | Participants were not diagnosed with panic disorder. |
| Klaassen 2002 | Participants were diagnosed with anxiety disorders, but were not stratified by it, including panic disorder. |
| Lee 2005 | Participants were not diagnosed with panic disorder. |
| Lesch 1990 | Participants were not diagnosed with panic disorder. |
| Lesch 1992 | Intervention was one day. |
| Mavissakalian 1986 | Interevention did not include azapirone. |
| Miura 1992 | Participants were not diagnosed with panic disorder. |
| Murasaki 1992 | Participants were not diagnosed with panic disorder. |
| Newton 1982 | Participants were not diagnosed with panic disorder. |
| Rackel 1990 | Participants were not diagnosed with panic disorder. |
| Rakel 1987 | Participants were not diagnosed with panic disorder. |
| Rickels 1981 | Participants were not diagnosed with panic disorder. |
| Rickels 2000 | Participants were not diagnosed with panic disorder. |
| Simeon 1994 | Participants were less than 14 years old. |
| Tyrer 1984 | Participants were diagnosed with anxiety disorders, but were not stratified by it. |
| Van Laar 1992 | Participants were not diagnosed with panic disorder. |
| Zelvelder 1990 | Participants were not diagnosed with panic disorder. |
Differences between protocol and review
We reported standardised mean difference (SMD) instead of mean difference (MD), as originally planned in the protocol, when the studies used an idiosyncratic scale that is seldom or never used elsewhere (e.g. Phobia Scale for Agoraphobia). We continue to use MD for standard rating scales such as Hamilton Rating Scale for Anxiety and Hamilton Rating Scale for Depression.
In the protocol, we stated that where study eligibility focused on agoraphobia rather than panic disorder, we would include studies when it could be safely assumed that 30% of participants were suffering from panic disorder. We have removed this 30% threshold and replaced it with 'some'; this has made no difference to the studies included in this version of the review.
Contributions of authors
GG devised the idea. GG, CB and AC worked on the first draft of the protocol. MK, SJCD, HI and DC provided suggestions and input. All authors reviewed and approved the final version of the protocol.
Sources of support
Internal sources
-
NIHR, UK.
Andrea Cipriani is supported by the NIHR Oxford Cognitive Health Clinical Research Facility
External sources
None, Other.
Declarations of interest
Hissei Imai: none known.
Aran Tajika: Aran Tajika has received honoraria for speaking at a meeting sponsored by Eli Lilly.
Peiyao Chen: none known.
Giuseppe Guaiana: none known.
Mariasole Castellazzi: none known.
Irene Bighelli: none known.
Francesca Girlanda: none known.
Corrado Barbui: none known.
Markus Koesters: none known.
Andrea Cipriani:none known.
Toshi A Furukawa:Toshi A Furukawa has received honoraria for speaking at CME meetings sponsored by Eli Lilly, Meiji, Mochida, MSD, Pfizer and Tanabe‐Mitsubishi. He is diplomate of the Academy of Cognitive Therapy. He has received royalties from Igaku‐Shoin, Seiwa‐Shoten and Nihon Bunka Kagaku‐sha. He is on advisory board for Sekisui Chemicals and Takeda Science Foundation. The Japanese Ministry of Education, Science, and Technology, the Japanese Ministry of Health, Labor and Welfare, and the Japan Foundation for Neuroscience and Mental Health have funded his research projects.
New
References
References to studies included in this review
Pohl 1989 {published data only}
- Pohl R, Balon R, Yeragani YK, Gershon S. Serotonergic anxiolytics in the treatment of panic disorder: a controlled study with buspirone. Psychopathology 1989;22(Suppl 1):60‐7. [DOI] [PubMed] [Google Scholar]
Robinson 1989 {published data only}
- Robinson D, Shrotriya RC, Alms DR, Messina M, Andary J. Treatment of panic disorder: nonbenzodiazepine anxiolytics, including buspirone. Psychopharmacology Bulletin 1989;25(1):21‐6. [PubMed] [Google Scholar]
- Sheehan D, Raj AB, Sheehan H, Soto S. The relative efficacy of buspirone, imipramine and placebo in panic disorder: a preliminary report. Pharmacology, Biochemistry, and Behavior 1988;29(4):815‐7. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Raj AB, Sheehan KH, Soto S. Is buspirone effective for panic disorder?. Journal of Clinical Psychopharmacology 1990;10(1):3‐11. [PubMed] [Google Scholar]
Sheehan 1993 {published data only}
- Sheehan DV, Raj AB, Harnett‐Sheehan K, Soto S, Knapp E. The relative efficacy of high‐dose buspirone and alprazolam in the treatment of panic disorder: a double‐blind placebo‐controlled study. Acta Psychiatrica Scandinavica 1993;88(1):1‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bohm 1990a {published data only}
- Bohm C, Robinson DS, Gammans RE, Shrotriya RC, Alms D, Leroy A, et al. Buspirone therapy in anxious elderly patients: a controlled clinical trial. Journal of Clinical Psychopharmacology 1990;10(3 Suppl):47S‐51S. [DOI] [PubMed] [Google Scholar]
Bohm 1990b {published data only}
- Bohm C, Placchi M, Stallone F, Gammans RE, Alms DR, Shrotriya RC, et al. A double‐blind comparison of buspirone, clobazam, and placebo in patients with anxiety treated in a general practice setting. Journal of Clinical Psychopharmacology 1990;10(3 Suppl):38S‐42S. [DOI] [PubMed] [Google Scholar]
Bouvard 1997 {published data only}
- Bouvard M, Mollard E, Guerin J, Cottraux J. Study and course of the psychological profile in 77 patients experiencing panic disorder with agoraphobia after cognitive behaviour therapy with or without buspirone. Psychotherapy and Psychosomatics 1997;66(1):27‐32. [DOI] [PubMed] [Google Scholar]
Broocks 2002 {published data only}
- Broocks A, Bandelow B, Koch K, Bartmann KU, Kinkelbur J, Schweiger U, et al. Smoking modulates neuroendocrine responses to ipsapirone in patients with panic disorder. Neuropsychopharmacology 2002;27(2):270‐8. [DOI] [PubMed] [Google Scholar]
Broocks 2003 {published data only}
- Broocks A, Meyer T, Opitz M, Bartmann U, Hillmer‐Vogel U, George A, et al. 5‐HT1A responsivity in patients with panic disorder before and after treatment with aerobic exercise, clomipramine or placebo. European Neuropsychopharmacology 2003;13(3):153‐64. [DOI] [PubMed] [Google Scholar]
Bueno 1988 {published data only}
- Bueno JR, Laks J. [Atividade ansiolitica da buspirona estudo comparativo com placebo e diazepam]. Journal Brasileiro de Psiquiatria 1988;37(2):97‐9. [Google Scholar]
Cottraux 1995 {published data only}
- Cottraux J, Note ID, Cungi C, Légeron P, Heim F, Chneiweiss L, et al. A controlled study of cognitive behaviour therapy with buspirone or placebo in panic disorder with agoraphobia. British Journal of Psychiatry 1995;167(5):635‐41. [DOI] [PubMed] [Google Scholar]
Gershon 1982 {published data only}
- Gershon S. Drug interactions in controlled clinical trials. Journal of Clinical Psychiatry 1982;43(12):95‐8. [PubMed] [Google Scholar]
Goldberg 1979a {published data only}
- Goldberg HL. Buspirone ‐ a new antianxiety agent not chemically related to any presently marketed drugs. Psychopharmacology Bulletin 1979;15(2):90‐2. [PubMed] [Google Scholar]
Goldberg 1979b {published data only}
- Goldberg HL, Finnerty R. The comparative efficacy of buspirone and diazepam in the treatment of anxiety. American Journal of Psychiatry 1979;136(9):1184‐7. [DOI] [PubMed] [Google Scholar]
Goldberg 1982 {published data only}
- Goldberg HL, Finnerty R. Comparison of buspirone in two separate studies. Journal of Clinical Psychiatry 1982;12(12 Pt 2):87‐91. [PubMed] [Google Scholar]
Heller 1990 {published data only}
- Heller AH, Beneke M, Kuemmel B, Spencer D, Kurtz NM. Ipsapirone: evidence for efficacy in depression. Psychopharmacology Bulletin 1990;26(2):219‐22. [PubMed] [Google Scholar]
Hongbo 1994 {published data only}
- Hongbo Z. A clinical comparative trial of buspirone in the treatment of anxiety neurosis. Chinese Journal of Nervous and Mental Disease 1994;20(5):277‐9. [Google Scholar]
Hua 2002 {published data only}
- Hua H, Huaquing M. A comparative study in psychotherapy and drug in teatment of anxiety disorders. Chinese Journal of Nervous and Mental Disorder 1994;28(2):85‐7. [Google Scholar]
Kai 1992 {published data only}
- Kai S, Mizuki Y, Kajimura N, Suetsugi M, Yamada M. Pharmaco‐EEG studies on buspirone, a new anxiolytic drug, in high‐ and low‐anxious humans. Clinical Neuropharmacology 1992;15(Suppl 1):5398. [Google Scholar]
Klaassen 2002 {published data only}
- Klaassen T, Riedel WJ, Praag HM, Menheere P, Griez E. Neuroendocrine response to meta‐chlorophenylpiperazine and ipsapirone in relation to anxiety and aggression. Psychiatry Research 2002;113(1‐2):29‐40. [DOI] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee ST, Park JH, Kim M. Eficacy of the 5‐HT1A agonist, buspirone hydrochloride, in migraineurs with anxiety: a randomized, prospective, parallel group, double‐blind, placebo‐controlled study. Headache 2005;45(8):1004‐11. [DOI] [PubMed] [Google Scholar]
Lesch 1990 {published data only}
- Lesch KP, Mayer S, Disselkamp‐Tietze J, Hoh A, Schoellnhammer G, Schulte HM. Subsensitivity of the 5‐hydroxytryptamine1A (5‐HT1A) receptor‐mediated hypothermic response to ipsapirone in unipolar depression. Life Science 1990;46(18):1271‐7. [DOI] [PubMed] [Google Scholar]
Lesch 1992 {published data only}
- Lesch KP, Wiesmann M, Hoh A, Muller T, Disselkamp‐Tietze J, Osterheider M, Schulte HM. 5‐HT1A receptor‐effector system responsivity in panic disorder. Psychopharmacology 1992;106:111‐117. [DOI] [PubMed] [Google Scholar]
Mavissakalian 1986 {published data only}
- Mavissakalian M, Michelson L. Agoraphobia: relative and combined effectiveness of therapist‐assisted in vivo exposure and imipramine. Journal of Clinical Psychiatry 1986;47(3):117‐22. [PubMed] [Google Scholar]
Miura 1992 {published data only}
- Miura S, Asai M, Ito K, Endo S, Ogura C, Kazamatsuri H, et al. Clinical study of buspirone on neurosis ‐ a comparative double‐blind trial with placebo. Clinical Evaluation 1992;19:447‐75. [Google Scholar]
Murasaki 1992 {published data only}
- Murasaki M, Mori A, Endo S, Takemasa K, Hasegawa K, Kamijima K, et al. Late phase III study of a new anxiolytic SM‐3997 (tandospirone) on neurosis. Clinical Evaluation 1992;20:259‐293. [Google Scholar]
Newton 1982 {published data only}
- Newton RE, Casten GP, Alms DR, Benes CO, Marunycz JD. The side effect profile of buspirone in comparison to active controls and placebo. journal of Clinical Psychiatry 1982;43(12 Pt 2):100‐2. [PubMed] [Google Scholar]
Rackel 1990 {published data only}
- Rakel RE. Long‐term buspirone therapy for chronic anxiety: a multicenter international study to determine safety. Southern Medical Journal 1990;83(2):194‐7. [DOI] [PubMed] [Google Scholar]
Rakel 1987 {published data only}
- Rakel R. Assessing the efficacy of antianxiety agents. The American Journal of Medicine 1987;82:1‐6. [DOI] [PubMed] [Google Scholar]
Rickels 1981 {published data only}
- Rickels K. Recent advances in anxiolytic therapy. Journal of Clinical Psychiatry 1981;42(11):40‐4. [PubMed] [Google Scholar]
Rickels 2000 {published data only}
- Rickels K, DeMartinis N, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Rynn M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long‐term benzodiazepine therapy. American Journal of Psychiatry 2000;157(12):1973‐9. [DOI] [PubMed] [Google Scholar]
Simeon 1994 {published data only}
- Simeon J, Knott VJ, Dubois C, Wiggins D, Geraets I, Thatte S, et al. Buspirone therapy of mixed anxiety disorders in childhood and adolescence: a pilot study. Journal of Child and Adolescent Psychopharmacology 1994;4(3):159‐70. [Google Scholar]
Tyrer 1984 {published data only}
- Tyrer P, Owen R. Anxiety in primary care: is short‐term drug treatment appropriate?. Journal of Psychiatry Research 1984;18(1):73‐8. [DOI] [PubMed] [Google Scholar]
Van Laar 1992 {published data only}
- Laar MW, Volkerts ER, Willigenburg AP. Therapeutic effects and effects on actual driving performance of chronically administered buspirone and diazepam in anxious outpatients. Journal of Clinical Psychopharmacology 1992;12(2):86‐95. [PubMed] [Google Scholar]
Zelvelder 1990 {published data only}
- Zelvelder WG. Buspirone (Buspar), a double‐blind comparative study of two dosing schedules [Buspirone (Busper); een dubbelblind vergelijkend onderzoek van twee doserings‐schema's]. Journal for Drug Therapy and Research 1990;15(4):112‐8. [Google Scholar]
Additional references
Als‐Nielsen 2003
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. JAMA 2003;290(7):921‐8. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland MJ. Detecting skewness for summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Arlington, VA: American Psychiatric Publishing, 1994. [Google Scholar]
APA 2009
- American Psychiatric Association. American Psychiatric Association Practice Guidelines for the Treatment of Panic Disorder. American Psychiatric Association Practice Guideline for the Treatment of Panic Disorder. Arlington, VA: American Psychiatric Publishing, 2009. [Google Scholar]
BAP 2005
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence‐based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2005;19(6):567‐96. [DOI] [PubMed] [Google Scholar]
Batelaan 2011
- Batelaan NM, Balkom AJ, Stein DJ. Evidence‐based pharmacotherapy of panic disorder: an update. International Journal of Neuropsychopharmacology 2011;15(3):403‐15. [DOI: 10.1017/S1461145711000800] [DOI] [PubMed] [Google Scholar]
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PMC free article] [PubMed] [Google Scholar]
Bijl 1998
- Bijl RV, Ravelli A, Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 1998;33(12):587‐95. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [in French]. Therapie 1999;54(4):405‐11. [PubMed] [Google Scholar]
Bruce 2003
- Bruce SE, Vasile RG, Goisman RM, Salzman C, Spencer M, Machan JT, et al. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia?. American Journal of Psychiatry 2003;160(8):1432‐8. [DOI] [PubMed] [Google Scholar]
Buchkowsky 2004
- Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Annals of Pharmacotherapy 2004;38(4):579‐85. [DOI] [PubMed] [Google Scholar]
Chessick 2006
- Chessick CA, Allen MH, Thase ME, Batista Miralha da Cunha AABC, Kapczinski FFK, Silva de Lima M, et al. Azapirones for generalized anxiety disorder. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 October 25‐28.
Diaz 2011
- Diaz MR, Chappell AM, Christian DT, Anderson NJ, McCool BA. Dopamine D3‐like receptors modulate anxiety‐like behavior and regulate GABAergic transmission in the rat lateral/basolateral amygdala. Neuropsychopharmacology 2011;36(5):1090‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eaton 1994
- Eaton WW, Kessler RC, Wittchen HU, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry 1994;151(3):413‐20. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [DOI] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta‐analysis. International Clinical Psychopharmacology 2005;20(1):49‐52. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2000;59(1):7‐10. [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Churchil R. Combined psychotherapy plus antidepressants for panic disorder with or without agoraphobia. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goisman 1995
- Goisman RM, Warshaw MG, Steketee GS, Fierman EJ, Rogers MP, Goldenberg I, et al. DSM‐IV and the disappearance of agoraphobia without a history of panic disorder: new data on a controversial diagnosis. American Journal of Psychiatry 1995;152(10):1438‐43. [DOI] [PubMed] [Google Scholar]
Gorman 2000
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. American Journal of Psychiatry 2000;157(4):493‐505. [DOI] [PubMed] [Google Scholar]
Grant 2006
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, et al. The epidemiology of DSM‐IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2006;67(3):363‐74. [DOI] [PubMed] [Google Scholar]
Guaiana 2013a
- Guaiana G, Barbui C, Chiodo D, Cipriani A, Davies SJC, Koesters M. Antidepressants versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010676] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guaiana 2013b
- Guaiana G, Barbui C, Chiodo D, Cipriani A, Davies SJC, Koesters M. Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010677] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. J.
Kessler 2006
- Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey Replication. Archives of General Psychiatry 2006;63(4):415‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2008
- King M, Nazareth I, Levy G, Walker C, Morris R, Weich S, et al. Prevalence of common mental disorders in general practice attendees across Europe. British Journal of Psychiatry 2008;192(5):362‐7. [DOI] [PubMed] [Google Scholar]
Kishi 2013
- Kishi T, Meltzer Y, Iwata N. Augmentation of antipsychotic drug action by azapirone 5‐HT1A receptor partial agonists: a meta‐analysis. International Journal of Neuropsychopharmacology 2013;16(6):1259‐66. [DOI] [PubMed] [Google Scholar]
Klein 1964
- Klein DF. Delineation of two drug‐responsive anxiety syndromes. Psychopharmacologia 1964;5:397‐408. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. British Medical Journal 2003;326(7400):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLOS Medicine 2009;6(6):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Napoliello 1991
- Napoliello MJ, Domantay AG. Buspirone: a worldwide update. British Journal of Psychiatry 1991;159(Suppl 12):40‐4. [PubMed] [Google Scholar]
Nash 2008
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, et al. Serotonin 5‐HT1A receptor binding in people with panic disorder: positron emission tomography study. British Journal of Psychiatry 2008;193(3):229‐34. [DOI] [PubMed] [Google Scholar]
Neumeister 2004
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, et al. Reduced serotonin type 1A receptor binding in panic disorder. Journal of Neuroscience 2004;24(3):589‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Generalised Anxiety Disorder and Panic Disorder (With or Without Agoraphobia) in Adults [CG113]. London: National Institute for Health and Clinical Excellence, 2011. [Google Scholar]
Parks 1998
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proceedings of the National Academy of Sciences of the United States of America 1998;95(18):10734–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Payer 2013
- Payer D, Bolleau I, Guranda M, Graff A, Nakajima S, Meyer J, et al. Buspirone occupancy of D2/3 dopamine receptors in humans measured by positron emission tomography. Proceedings of the College on Problems of Drug Dependence, 75th Annual Scientific Meeting, San Diego, CA. 15‐20 June 2013.
Pompoli 2013
- Pompoli A, Furukawa TA, Imai H, Tajika A, Efthimiou O, Salanti G. Psychological therapies for panic disorder with or without agoraphobia in adults. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD011004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheehan 1988
- Sheehan D, Raj AB, Sheehan H, Soto S. The relative efficacy of buspirone, imipramine and placebo in panic disorder: a preliminary report. Pharmacology, Biochemistry, and Behavior 1988;29(4):815‐7. [DOI] [PubMed] [Google Scholar]
Sheehan 1990
- Sheehan DV, Raj AB, Sheehan KH, Soto S. Is buspirone effective for panic disorder?. Journal of Clinical Psychopharmacology 1990;10(1):3‐11. [PubMed] [Google Scholar]
Starcevic 2009
- Starcevic V. Anxiety Disorders in Adults: A Clinical Guide. Oxford, UK: Oxford University Press, 2009. [Google Scholar]
Stein 2010
- Stein M, Steckler T, Lightfoot JD, Hay E, Goddard AW. Pharmacologic treatment of panic disorder. Current Topics in Behavioral Neurosciences 2010;2:469‐85. [DOI] [PubMed] [Google Scholar]
Tauscher 2001
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5‐HT(1A) receptor binding and anxiety: a [(11)C]WAY‐100635 PET investigation in healthy volunteers. American Journal of Psychiatry 2001;158(8):1326‐8. [DOI] [PubMed] [Google Scholar]
Wade 1999
- Wade AG. Antidepressants in panic disorder. International Clinical Psychopharmacology 1999;14(Suppl 2):13‐7. [PubMed] [Google Scholar]
Watanabe 2009
- Watanabe N, Churchill R, Furukawa TA. Combined psychotherapy plus benzodiazepines for panic disorder. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005335.pub2] [DOI] [PubMed] [Google Scholar]


