Abstract
The mechanism of intestinal secretion of the difluorinated quinolone sparfloxacin was investigated with the epithelial cell line Caco-2 and was compared to that of the P-glycoprotein (P-gp) substrate vinblastine. The P-gp inhibitors verapamil and progesterone significantly increased the epithelial cell accumulation of both vinblastine and sparfloxacin. This increase is likely to result from an inhibition of drug secretion since both vinblastine uptake and sparfloxacin uptake are known to proceed through a passive transmembrane diffusion. The unidirectional fluxes across cell monlayers grown on permeable filters indicated that a net secretion of sparfloxacin and vinblastine occurred across Caco-2 cells. These secretions were significantly inhibited by the MDR-reversing agent verapamil. We conclude that the P-gp is likely to be involved in the intestinal elimination of the difluorinated quinolone sparfloxacin.
Sparfloxacin (Fig. 1) is a difluorinated quinolone antibiotic with a broad spectrum of activity against gram-positive and gram-negative bacteria (16). This fluoroquinolone exhibits very good bioavailability following oral administration, with values of about 80% for rats (14), dogs (16), and humans (15). Fluoroquinolones are only slightly metabolized in the organism, and extrarenal routes have been considered important for their elimination (10, 22, 24). In this regard, the fraction of fluoroquinolones eliminated in the intestine following oral administration was estimated to range between 10 and 25% of the dose ingested in humans, rats, and rabbits (20, 21, 24). Since biliary secretion of fluoroquinolones accounts for the secretion of only 1 to 3% of the oral dose, active transepithelial elimination may be responsible for most of the drug excretion in the feces.
FIG. 1.
Chemical structure of sparfloxacin (∗, radiolabelled carbon).
To date, the only secretory pathway that has been unequivocally characterized in the intestinal epithelium involves the P glycoprotein (P-gp) (6, 27). The multidrug transporter P-gp is a 170-kDa membrane glycoprotein which mediates the active transmembrane transport of a variety of lipophilic substrates, including Vinca alkaloids, steroids, anthracyclines, and quinine derivatives, and is involved in the multidrug resistance (MDR) that occurs during cancer chemotherapy (5). In the epithelial cells of the gastrointestinal tract, as well as in other epithelia, expression of the P-gp has been shown to be restricted to the apical membranes of epithelial cells (2). This location is consistent with a role of P-gp in the elimination of cytotoxic drugs and other hydrophobic drugs into the intestinal lumen (5, 6). Reports of two different studies have recently indicated that the MDR-reversing agent verapamil effectively reduces the transepithelial secretion of some quinolones across the rat small intestine and Caco-2 cell monolayers, thus suggesting that P-gp may be involved in the intestinal elimination of these drugs (7, 19).
The aim of the present study was to investigate whether P-gp is involved in the secretion of sparfloxacin across the intestinal epithelium. For this purpose, we have used the human colon carcinoma cell-line Caco-2, which has been shown to exhibit the morphological and functional characteristics of normal enterocytes (9, 17) and to express P-gp at the apical membrane (12). The secretion and intracellular accumulation of sparfloxacin were compared to those of the typical P-gp substrate vinblastine. The effects of MDR-reversing agents on sparfloxacin accumulation were also evaluated in differentiated Caco-2 cells. Undifferentiated Caco-2 cells and the undifferentiated IEC-17 intestinal cell line were also used as control cells that lacked MDR expression (18).
MATERIALS AND METHODS
Chemicals.
Sparfloxacin was kindly provided by Rhône Poulenc-Rorer (Antony, France), and [14C]sparfloxacin (16.4 mCi/mmol) was custom synthesized by Dainippon Pharmaceutical Laboratory (Osaka, Japan). [3H]vinblastine (11 Ci/mmol) was obtained from Amersham (Les Ulis, France). Cell culture media and reagents were purchased from Gibco BRL (Cergy-Pontoise, France), and all other reagents were from Sigma (La Verpillière, France).
Cell culture.
Caco-2 cells were a gift from G. Trugnan (Institut National de la Santé et de la Recherche Médicale, Unité 410, Paris, France) and were used between passages 30 and 65. Cells were maintained routinely at 37°C in an O2-CO2 atmosphere (95:5) and were grown as monolayer cultures in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal calf serum, 1% nonessential amino acids, 6 mM l-glutamine, and 200 μg of gentamicin per ml, and the medium was replaced every 2 days. The confluence was reached 7 days after seeding, and the uptake experiments were conducted with the monolayers on days 11 to 13 postconfluence. In selected experiments, Caco-2 cells were used at 2 days postseeding as undifferentiated control cells.
The rat IEC-17 cell line was kindly provided by M. Kedinger (Institut National de la Santé et de la Recherche Médicale, Strasbourg, France) and was used between passages 21 and 40. The cells were cultured at 37°C in humidified atmosphere with 5% CO2 and were maintained in DMEM containing 4.5 g of glucose per liter, 200 μg of gentamicin per ml, and 4 μg of insulin per ml and supplemented with 5% fetal calf serum. IEC-17 cells were seeded at 2 × 104 cells/cm2, were grown in 75-cm2 flasks, and reached confluence in 4 to 5 days.
Measurement of the uptake and accumulation of [14C]sparfloxacin and [3H]vinblastine.
The uptake measurements were performed with confluent epithelial monolayers grown on 24-well plates as reported previously (2) after incubation with 10 μM [14C]sparfloxacin or 10 nM [3H]vinblastine as the substrate. Briefly, the culture medium was renewed 3 h before the start of the experiment. The culture medium was discarded and cells were preincubated for 15 min in HEPES-buffered saline (pH 7.4) containing 100 mM glucose (HSG). The intracellular accumulation of sparfloxacin and vinblastine was measured at 37°C in HSG at times ranging from 20 s (initial rate of uptake) to 60 min (accumulation). At the end of the incubation, the monolayers were washed three times with ice-cold HSG (pH 7.4) and the cells were scraped into 0.5 ml of 0.1 M NaOH. The amounts of [14C]sparfloxacin and [3H]vinblastine that accumulated in the cells were determined by liquid scintillation counting and were expressed as the number of picomoles per milligram of protein. The protein concentration was determined by the method of Smith et al. (23), with bovine serum albumin used as a standard. In most experiments, cells were used at 11 to 13 days postconfluence. As a negative control, we measured the effect of P-gp inhibitors on [14C]sparfloxacin and [3H]vinblastine accumulation in undifferentiated Caco-2 cells (2 days postseeding) and in IEC-17 cells.
Efflux and intracellular retention of [14C]sparfloxacin.
For efflux measurement, Caco-2 cells were grown in 24-well plates and were used on days 11 to 13. The monolayers were washed before the loading of 10 μM [14C]sparfloxacin (0.16 μCi/ml) in uncomplemented DMEM and incubation for 60 min at 37°C. After two further washings of the monolayers with HSG, the efflux was monitored at 37°C for up to 40 min in 500 μl of HSG (pH 7.4). The amount of radiolabelled quinolone recovered in aliquots collected at the indicated time points was evaluated by scintillation counting and was expressed as the number of picomoles per well. At each time point, the cell monolayers were washed twice with cold HSG and were scraped into 0.5 ml of 0.1 M NaOH. The amount of [14C]sparfloxacin retained in the cells was then quantified with a 250-μl aliquot by liquid scintillation counting and was expressed as the number of picomoles per milligram of protein.
Measurement of bidirectional transepithelial sparfloxacin and vinblastine fluxes.
For the measurement of bidirectional fluxes, Caco-2 cells were grown as epithelial cell monolayers by seeding them onto permeable polyethylene terephtalate filters (0.25 × 106 cells/4.91-cm2 Falcon filter), and the monolayers were used on days 11 to 13 postconfluence. The resistance of the epithelial cell monolayers was measured before the assays to evaluate their integrity. Preliminary experiments have shown that higher concentrations of radiolabelled sparfloxacin were required in order to obtain a significant signal in bidirectional flux assays. Therefore, for these assays the sparfloxacin and vinblastine concentrations were increased to 40 μM and 40 nM, respectively. Transport experiments were run at 37°C in phosphate-buffered saline (PBS; pH 7.4) containing 10 mM glucose. The epithelial layers were washed twice with 2 ml of PBS prewarmed to 37°C. Thereafter, 2.5 ml of PBS containing [14C]sparfloxacin (40 μM) or [3H]vinblastine (40 nM) was added to either the apical or the basolateral compartment, with the same amount of unlabelled molecule being added simultaneously to the contralateral compartment. The amount of radiolabelled material present in a 200-μl aliquot from the contralateral compartment was measured every 30 min for 2 h. Steady-state apical-to-basal (Jab) and basal-to-apical (Jba) fluxes were calculated from the slope of [14C]sparfloxacin or [3H]vinblastine accumulation in the basal and apical compartments, respectively. The net flux (Jnet) was calculated as the difference between Jab and Jba. Positive values of Jnet indicate absorption of the drug, whereas negative values represent secretion of the drug.
Effect of pharmacological agents and ATP depletion.
Verapamil and progesterone were used as P-gp inhibitors for accumulation and flux measurements. Stock solutions of these inhibitors were prepared in dimethyl sulfoxide (DMSO), resulting in the presence of 0.01% DMSO in the experiments. Control wells consisting of 0.01% DMSO in HSG were included in each experiment. ATP depletion was achieved with a 15-min pretreatment of Caco-2 cells with 15 mM sodium azoture and 50 mM 2-deoxyglucose.
Statistical analysis.
Data are presented as means ± standard deviations (SDs) of n experiments. Student’s unpaired t test was used to test the significance of the difference between two mean values. A P value of < 0.05 was considered statistically significant.
RESULTS
Uptake, efflux, and cellular retention of sparfloxacin through the apical membrane of Caco-2 cells.
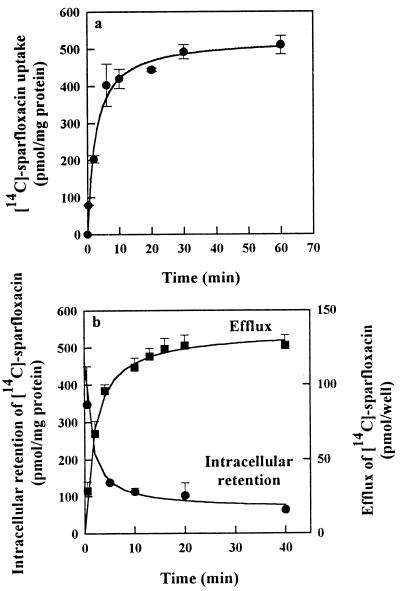
Figure 2a reports the uptake of [14C]sparfloxacin during the loading period preceding the measurement of efflux and intracellular retention. The uptake of [14C]sparfloxacin by the Caco-2 cell monolayers increased sharply, and a plateau was reached after 30 min. On the other hand, the efflux of radiolabelled sparfloxacin measured during a 40-min period is reported in Fig. 2b. The efflux of [14C]sparfloxacin measured thereafter was extremely rapid and reached a plateau within 20 min. At this time point, no further loss of drug could be detected and about 20% of the initial amount of [14C]sparfloxacin was retained in the cells.
FIG. 2.
Uptake, efflux, and intracellular retention of [14C]sparfloxacin from the apical membrane of Caco-2 cells. (a) For the uptake, the cells were incubated with 10 μM [14C]sparfloxacin for incubation times ranging from 20 s to 60 min. After 1 h, the cells were washed with ice-cold incubation medium and 500 μl of buffer was added. (b) The efflux was measured as described in Materials and Methods by taking an aliquot at the indicated time points, and the cells were washed and scraped to determine the intracellular retention of [14C]-sparfloxacin. Values are means ± SDs of three experiments.
Effect of P-gp substrate and inhibitors on the accumulation of sparfloxacin and vinblastine in epithelial cells.
The addition of 100 μM verapamil or progesterone during the 1-h incubation significantly increased the level of accumulation of [14C]sparfloxacin in differentiated Caco-2 cells (days 11 to 13 postconfluence) by 30 and 60%, respectively. These two inhibitors also induced four- and sixfold increases in the level of accumulation of [3H]vinblastine, respectively (Table 1), suggesting that common mechanisms are involved in the secretion of [14C]sparfloxacin and [3H]vinblastine. We also observed that a 10-fold excess of vinblastine caused a small increase (30%; P < 0.05) in the level of sparfloxacin accumulation, whereas a 100-fold excess of sparfloxacin failed to modify the retention of vinblastine in the Caco-2 cell line, suggesting differences in the affinity for the transporter. The ATP depletion also resulted in different effects on sparfloxacin and vinblastine accumulation. In fact, while only 70% ATP depletion was achieved, this treatment significantly increased (3.5-fold) the level of accumulation of vinblastine (Table 1). In contrast to the results for vinblastine, ATP depletion did not enhance the accumulation of sparfloxacin by differentiated Caco-2 cells (Table 1).
TABLE 1.
Effects of inhibitors of P-gp, ATP depletion, and the fluoroquinolone sparfloxacin on [14C]sparfloxacin (10 μM) and [3H]vinblastine (10 nM) accumulation after incubation for 1 h at 37°C with differentiated Caco-2 cellsa
| Inhibitor | Vinblastine accumulation (% of control) | Sparfloxacin accumulation (% of control) |
|---|---|---|
| Control | 100 ± 10 | 100 ± 7 |
| Verapamil (100 μM) | 357 ± 65** | 129 ± 10* |
| Progesterone (100 μM) | 601 ± 198** | 159 ± 21** |
| Vinblastine (100 μM) | 131 ± 11* | |
| Sparfloxacin (100 μM) | 109 ± 5 | |
| ATP depletion | 368 ± 23** | 108 ± 8 |
Values are the means ± SDs of three experiments. A statistically significant difference from the level of accumulation for the control is indicated as follows: *, P < 0.01; **, P < 0.001.
In order to further determine the involvement of P-gp, the effect of P-gp inhibitors on sparfloxacin accumulation were tested with undifferentiated Caco-2 cells and IEC-17 cells. Neither 100 μM progesterone nor 100 μM verapamil affected the accumulation of [14C]sparfloxacin in undifferentiated Caco-2 cells (day 2 postseeding) and in the crypt-like cell line IEC-17 (Table 2). The same results were observed for [3H]vinblastine, except that the addition of progesterone increased the level of vinblastine accumulation in IEC-17 cells. The latter effect was not observed in undifferentiated Caco-2 cells.
TABLE 2.
Effects of P-gp inhibitors on [14C]sparfloxacin (10 μM) and [3H]vinblastine (10 nM) accumulation after incubation for 1 h at 37°C with either IEC-17 cells or undifferentiated Caco-2 cells at 2 days postseedinga
| Cell and inhibitor | Vinblastine accumulation (% of control) | Sparfloxacin accumulation (% of control) |
|---|---|---|
| IEC-17 cells | ||
| Control | 100 ± 3 | 100 ± 7 |
| With verapamil (100 μM) | 89 ± 4 | 89 ± 5 |
| With progesterone (100 μM) | 139 ± 6* | 90 ± 13 |
| Caco-2 cells | ||
| Control | 100 ± 6 | 100 ± 32 |
| With progesterone (100 μM) | 114 ± 9 | 115 ± 35 |
Values are means ± SDs of three experiments. *, P < 0.05.
Transepithelial sparfloxacin and vinblastine fluxes across Caco-2 cell monolayers.
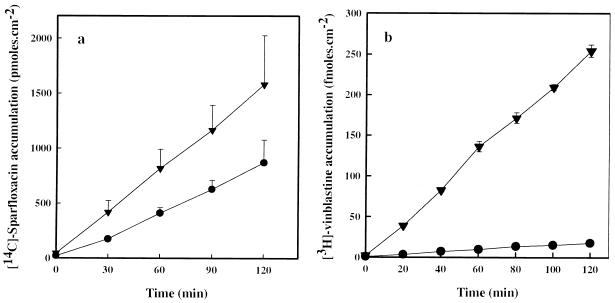
Sparfloxacin and vinblastine exhibited comparable patterns of transepithelial fluxes, with Jba fluxes being greater than Jab fluxes, indicating that both drugs were secreted by the epithelial cells (Fig. 3). In agreement with the well-known involvement of the P-gp in the secretion of vinblastine, pretreatment of Caco-2 cell monolayers with 100 μM verapamil significantly reduced (∼50%) the Jnet flux of vinblastine (Table 3). Significantly increased sparfloxacin Jab fluxes with a subsequent reduction (−82%; P < 0.05) of the net secretion of this fluoroquinolone were observed in verapamil-treated monolayers.
FIG. 3.
Time dependence of [14C]sparfloxacin (40 μM) (a) and [3H]vinblastine (40 nM) (b) flux across Caco-2 cell monolayers. Apical-to-basal (•) and basal-to-apical (▾) transport were determined by measuring the level of accumulation of the radiolabelled drug in the contralateral compartment. Values are means ± SDs of three to five experiments.
TABLE 3.
Unidirectional (Jab and Jba) and net (Jnet) fluxes of [14C]sparfloxacin (40 μM) and [3H]vinblastine (40 nM) across Caco-2 cell monolayers and effect of the P-gp inhibitor verapamil on fluxesa
| Inhibitor | Jab | Jba | Jnet |
|---|---|---|---|
| Sparfloxacin (pmol · h−1 · cm−2) | 376 ± 51 | 740 ± 55 | −363 ± 43 |
| Sparfloxacin + 100 μM verapamil | 552 ± 89* | 616 ± 74 | −63 ± 15* |
| Vinblastine (fmol · h−1 · cm−2) | 8 ± 1 | 126 ± 4 | −118 ± 4 |
| Vinblastine + 100 μM verapamil | 44 ± 1* | 98 ± 6* | −54 ± 6* |
Values are means ± SDs of three to five experiments. *, a statistically significant difference (P < 0.05) from the control flux.
DISCUSSION
The gastrointestinal secretion of fluoroquinolones represents a quantitatively important route for the clearance of these antibiotics (20, 24). However, the mechanism involved in this secretion is still a matter of debate. Griffiths and coworkers (8) have recently proposed the existence of a carrier-mediated process specifically located at the basolateral membrane of the enterocyte-like Caco-2 cells. This carrier would be responsible for an accumulative transport of fluoroquinolones including norfloxacin and ciprofloxacin, with a diffusive leak of these fluoroquinolones across the apical membrane. However, a potential role of the P-gp in this secretion was also suggested by the report that the net secretion of ciprofloxacin across the membranes of cells of this cell line was partly inhibited by the P-gp inhibitor verapamil (−38%) (7). Although the latter hypothesis is supported by a recent observation made by in situ perfusion that the P-gp inhibitors inhibited the secretion of ofloxacin in rat intestinal segments (19), there is so far no formal proof of either mechanism. In this report, we show that P-gp inhibitors interfere with the secretion of sparfloxacin across Caco-2 cell monolayers and provide a possible explanation for the actual controversy in this matter.
The P-gp has been shown to be expressed in the apical membranes of differentiated Caco-2 cells (12) and to be responsible for the net secretion of many drugs including Vinca alkaloids, cyclosporin A, anthracyclines, and celiprolol (5, 11, 27). Our results provide evidence for the involvement of P-gp in the secretion of sparfloxacin across differentiated Caco-2 cell monolayers as well. Indeed, in our study the net secretory flux of vinblastine across differentiated Caco-2 cells was inhibited by the MDR-reversing agent verapamil. As observed with vinblastine, a significant inhibition of sparfloxacin net secretion across differentiated Caco-2 monolayers was achieved with 100 μM verapamil. While verapamil significantly reduced the Jba flux of vinblastine, only a marginal reduction of this flux was observed for sparfloxacin. This observation suggests that P-gp is more efficient at preventing the apical uptake of sparfloxacin than at preventing its release from cells. In fact, a P-gp inhibitor can affect Jab fluxes by favoring the entry of molecules and by inhibiting the release of molecules, while for Jba fluxes, such an inhibitor can affect only the release. On the other hand, we have previously shown that sparfloxacin strongly binds to the brush-border membranes (4). Therefore, it is possible that the low level of decrease in sparfloxacin Jba flux in the presence of verapamil mirrors the ability of P-gp to release this molecule from the intracellular compartment. This hypothesis is consistent with our previous report that sparfloxacin strongly binds to cell membranes (3, 4). Thus, epithelial cells cultured on a filter were not used for further investigations of P-gp involvement in sparfloxacin secretion.
The comparison of sparfloxacin influx and efflux revealed very similar patterns with a very high initial rate and with a plateau being reached after 20 min. Our results are consistent with a fast and labile binding of sparfloxacin to membrane components, which can bias the direct measurement of its efflux. However, it is possible to estimate the extent of sparfloxacin secretion by measuring its level of accumulation at the plateau. Indeed, this accumulation depends on both uptake and secretion, and we have previously shown that sparfloxacin uptake occurs through a passive transmembrane diffusion (3). A 30% increase in the level of sparfloxacin accumulation was observed in Caco-2 cells treated with verapamil or vinblastine; verapamil is a P-gp inhibitor and vinblastine is a P-gp substrate. These results are consistent with the observation by others that an excess of vinblastine inhibits the MDR-mediated secretion of celiprolol across Caco-2 cell monolayers (13) and strongly suggest a role for P-gp in sparfloxacin secretion. Interestingly, a 60% increase in the level of sparfloxacin accumulation was induced by progesterone, which is the most potent inhibitor of P-gp function among steroids (5). Furthermore, consistent with the low level of metabolism of sparfloxacin, we have previously reported the absence of metabolites after 1 h of incubation of sparfloxacin with Caco-2 cells (3). Taken together, these results support the existence of common pathways of sparfloxacin and vinblastine secretion and that these pathways involve P-gp.
It is surprising that ATP depletion did not affect sparfloxacin accumulation in differentiated Caco-2 cells since this treatment was reported to influence the secretion of fluoroquinolones by Caco-2 cell monolayers cultured on filters (6). This discrepancy in the effect of ATP depletion could result from the incomplete depletion in our experiments (30% residual ATP). However, since vinblastine accumulation was enhanced by the same treatment, one might speculate that other secretion systems exist for sparfloxacin. Another possible explanation would be that the fraction of sparfloxacin that binds to the membranes was larger than that secreted via the P-gp. The absence of an increased level of sparfloxacin accumulation in response to progesterone observed in undifferentiated Caco-2 cells or IEC-17 cells provides additional support for a role of P-gp in the secretion of sparfloxacin. In this regard, the expression of the P-gp transporter is restricted to the brush-border membrane of the intestinal epithelium and increases as cells differentiate (2, 26). However, progesterone treatment induced a 39% increase in the level of vinblastine accumulation by IEC-17 cells but not by undifferentiated Caco-2 cells. The latter result points out the potential differences between these two cell lines and the factors influencing the choice of which system to use as an experimental system.
In conclusion, the interaction between sparfloxacin and the P-gp inhibitors reported here support the hypothesis of an active involvement of P-gp in the secretion of this fluoroquinolone. The structural, chemical, and uptake characteristics of sparfloxacin are in accordance with those of most of the P-gp substrates (5): (i) sparfloxacin is a polycyclic molecule, (ii) sparfloxacin possesses the highest level of liposolubility among the fluoroquinolones (25, 28), and (iii) we have previously demonstrated that uptake occurs by passive diffusion through the apical membrane of Caco-2 cells (3). However, the measurement of sparfloxacin accumulation does not allow us to completely rule out the involvement of a second secretory mechanism located in the basolateral membrane that is involved in the elimination of fluoroquinolones (8). In this regard, a 4,4′-diigothiocyanostilbene-2,2′-disulfonic acid- and verapamil-sensitive system has recently been suggested to be responsible for the secretion of ciprofloxacin by Caco-2 cells (1). Since fluoroquinolones are barely metabolized (15), their potential to interact with P-gp inhibitors may have significant consequences in vivo. Thus, coadministration of sparfloxacin with another P-gp substrate or inhibitor could modify the elimination and thus both the efficacy and the local toxicity of one of these drugs.
ACKNOWLEDGMENTS
Estelle Cormet-Boyaka and Agnès Mordrelle were supported by grants 93157 and 94231, respectively, from the French Ministry of University and Research.
REFERENCES
- 1.Cavet M W, Simmoms N L. Fluoroquinolone (ciprofloxacin) secretion by human intestinal epithelial (Caco-2) cells. Br J Pharmacol. 1997;121:1567–1578. doi: 10.1038/sj.bjp.0701302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordon-Cardo C, O’Brien J P, Boccia J, Casals D, Bertino J R, Melamed M R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- 3.Cormet E, Huneau J F, Bouras M, Carbon C, Rubinstein E, Tome D. Evidence for a passive diffusion mechanism for sparfloxacin uptake at the brush-border membrane of the human intestinal cell-line Caco-2. J Pharm Sci. 1997;86:33–36. doi: 10.1021/js960262s. [DOI] [PubMed] [Google Scholar]
- 4.Cormet E, Huneau J F, Tome D. Sparfloxacin binds to rabbit intestinal brush-border membrane vesicles by ionic interactions. Int J Pharm. 1997;159:127–130. [Google Scholar]
- 5.Ford J M, Hait W. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 6.Gan L S L, Thakker D R. Application of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv Drug Deliv Rev. 1997;23:77–98. [Google Scholar]
- 7.Griffiths N M, Hirst B H, Simmons N L. Active secretion of the fluoroquinolone ciprofloxacin by human intestinal epithelial Caco-2 cell layers. Br J Pharmacol. 1993;108:575–576. doi: 10.1111/j.1476-5381.1993.tb12844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths N M, Hirst B H, Simmons N L. Active intestinal secretion of the fluoroquinolones antibacterials ciprofloxacin, norfloxacin and pefloxacin: a common secretory pathway? J Pharmacol Exp Ther. 1994;269:496–502. [PubMed] [Google Scholar]
- 9.Hidalgo I J, Raub T J, Brochardt R T. Characterization of the human colon carcinoma cell-line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 10.Huneau J F, Tome D, Ramon J, Carbon C, Rubinstein E. Absorption and secretion of sparfloxacin in the isolated rabbit intestinal tract. Drugs. 1993;45:251. [Google Scholar]
- 11.Hunter J, Hirst B H, Simmons N L. Drug absorption limited by P-glycoprotein-mediated secretory drug transport in human intestinal epithelial Caco-2 cell layers. Pharm Res. 1993;10:743–749. doi: 10.1023/a:1018972102702. [DOI] [PubMed] [Google Scholar]
- 12.Hunter J, Jepson M A, Tsuruo T, Simmons N L, Hirst B H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. J Biol Chem. 1993;268:14991–14997. [PubMed] [Google Scholar]
- 13.Karlsson J, Kuo S M, Ziemniak J, Artursson P. Transport of celiprolol across human intestinal epithelial (Caco-2) cells: mediation by multiple transporters including P-glycoprotein. Br J Pharmacol. 1993;110:1009–1016. doi: 10.1111/j.1476-5381.1993.tb13914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsunaga Y, Miyazaki H, Oh-e Y, Nambu K, Furukawa H, Yoshida K, Hashimoto M. Disposition and metabolism of [14C]-sparfloxacin in the rat. Arzneim-Forsch/Drug Res. 1991;41:747–759. [PubMed] [Google Scholar]
- 15.Montay G, Vergniol J C, Ebmeier M, Le Roux Y, Guimart C, Frydman A, Chassard D, Thebault J J. Pharmacokinetics of sparfloxacin in humans after single oral administration at doses of 200, 400, 600, and 800 mg. J Clin Pharmacol. 1994;34:1071–1076. doi: 10.1002/j.1552-4604.1994.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Kurobe N, Hashimoto M, Shimizu M. Pharmacokinetics of a novel quinolone, AT-4140, in animals. Antimicrob Agents Chemother. 1990;34:89–93. doi: 10.1128/aac.34.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell-line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 18.Quaroni A, Isselbacher K J. Cytotoxic effects and metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in duodenal and ileal epithelial cell cultures. J Natl Cancer Inst. 1981;67:1353–1362. [PubMed] [Google Scholar]
- 19.Rabbaa L, Dautrey S, Colaslinhart N, Carbon C, Farinotti R. Intestinal elimination of ofloxacin enantiomers in the rat: evidence of a carrier-mediated process. Antimicrob Agents Chemother. 1996;40:2126–2130. doi: 10.1128/aac.40.9.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohwedder R, Bergan T, Thorsteinson S B, Scholl H. Transintestinal elimination of ciprofloxacin. Chemotherapy (Basel) 1990;36:77–84. doi: 10.1159/000238751. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein E, St. Julien L, Ramon J, Dautrey S, Farinotti R, Huneau J F, Carbon C. The intestinal elimination of ciprofloxacin in the rat. J Infect Dis. 1994;169:218–221. doi: 10.1093/infdis/169.1.218. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein E, Dautrey S, Farinotti R, St. Julien L, Ramon J, Carbon C. Intestinal elimination of sparfloxacin, fleroxacin, and ciprofloxacin in rats. Antimicrob Agents Chemother. 1995;39:99–102. doi: 10.1128/aac.39.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Geoke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Sorgel F, Nager K G, Jaehde U, Reiter A, Seelmann R, Sigl G. Brief report: gastrointestinal secretion of ciprofloxacin, evaluation of the charcoal model for investigation in healthy volunteers. Am J Med. 1989;87:62S–65S. doi: 10.1016/0002-9343(89)90025-9. [DOI] [PubMed] [Google Scholar]
- 25.Sorgel, F., and M. Kinzig. 1993. Pharmacokinetics of gyrase inhibitor. Part 1. Basic chemistry and gastrointestinal disposition. Am. J. Med. 94(Suppl. 3A):44S-51S. [PubMed]
- 26.Trezise A E O, Romano P R, Gill D R, Hyde S C, Sepulveda F V, Buchwald M, Higgins C F. The multidrug resistance and cystic fibrosis gene have complementary patterns of epithelial expression. EMBO J. 1992;11:4291–4303. doi: 10.1002/j.1460-2075.1992.tb05528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res. 1996;13:963–977. doi: 10.1023/a:1016086003070. [DOI] [PubMed] [Google Scholar]
- 28.Zabinski R A, Walker K J, Larsson A J, Moody J A, Kaatz G W, Rotschafer J C. Effect of aerobic and anaerobic environments on antistaphylococcal activities of five fluoroquinolones. Antimicrob Agents Chemother. 1995;39:507–512. doi: 10.1128/aac.39.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]