Abstract
Acute myeloid leukemia (AML) is a heterogeneous and aggressive form of blood cancer characterized by the uncontrolled proliferation of myeloid precursor cells in the bone marrow. It affects individuals of all ages, with incidence increasing notably in those over 65 years old. Despite advancements in treatment, overall survival rates remain unsatisfactory, underscoring the need for a deeper understanding of the disease. Among the various genetic alterations implicated in AML pathogenesis, mutations in the FLT3 (Fms-like tyrosine kinase 3) gene have emerged as significant contributors to leukemogenesis. The FLT3 gene encodes a type III receptor tyrosine kinase crucial in regulating normal hematopoiesis. Approximately one-third of AML patients carry FLT3 mutations, making it one of the most frequently mutated genes in the disease. FLT3 mutations can be classified into internal tandem duplications (ITDs) and point mutations in the tyrosine kinase domain (TKD). FLT3 mutations are associated with adverse clinical features and are independent prognostic factors for poor overall survival and decreased remission rates in AML patients. Understanding the molecular mechanisms underlying FLT3 mutations in AML is critical for improving risk stratification, prognosis assessment, and the development of targeted therapies. By reviewing the current literature, this study aims to elucidate the functional consequences of FLT3 mutations in AML pathogenesis, explore the interaction of FLT3 signaling with other oncogenic pathways, and assess the prognostic significance of FLT3 mutations in clinical practice, providing information that can guide future research directions and facilitate the development of more effective therapeutic strategies.
Keywords: acute myeloid leukemia (aml), flt3 mutations, flt3 signaling, targeted therapies, therapeutic resistance
Introduction and background
Background of acute myeloid leukemia (AML)
AML represents a heterogeneous and aggressive hematologic malignancy characterized by the uncontrolled proliferation of myeloid precursor cells within the bone marrow. Despite therapeutic advancements, AML continues to exhibit a dismal prognosis, underscoring the imperative for enhanced comprehension of its pathogenesis and treatment modalities [1].
Importance of FLT3 mutations in AML
Among the various genetic alterations implicated in AML pathogenesis, mutations in the FLT3 (Fms-like tyrosine kinase 3) gene have emerged as a significant contributor to leukemogenesis. The FLT3 gene encodes a receptor tyrosine kinase that plays a crucial role in regulating normal hematopoiesis, including the proliferation, survival, and differentiation of hematopoietic stem and progenitor cells [2]. Approximately one-third of AML patients carry FLT3 mutations, making it one of the most frequently mutated genes in this disease [3]. FLT3 mutations can be broadly classified into two main types: internal tandem duplications (ITDs) and point mutations within the tyrosine kinase domain (TKD) [4]. These mutations result in constitutive activation of FLT3 signaling, leading to aberrant proliferation, impaired differentiation, and increased survival of leukemic cells. FLT3 mutations are associated with adverse clinical features, including higher white blood cell counts, increased blast percentage, and a higher likelihood of relapse [5]. They have also been identified as independent prognostic factors for poor overall survival and decreased remission rates in AML patients [6]. Therefore, understanding the molecular mechanisms underlying FLT3-mutated AML is crucial for improving risk stratification, prognosis assessment, and development of targeted therapies.
Purpose of the research paper
The purpose of this research paper is to provide a comprehensive analysis of FLT3 mutations in AML, focusing on their molecular mechanisms and implications for targeted therapies. By reviewing the current literature, we aim to elucidate the functional consequences of FLT3 mutations in AML pathogenesis, explore the interaction of FLT3 signaling with other oncogenic pathways, and evaluate the prognostic significance of FLT3 mutations in clinical practice. Furthermore, we will discuss the recent advances in targeted therapies against FLT3-mutated AML, such as first- and second-generation FLT3 inhibitors, and highlight the challenges associated with their use, including the development of resistance mechanisms. Additionally, we will explore emerging therapeutic strategies, including third-generation inhibitors and combination approaches, as well as the potential role of immunotherapy in the management of FLT3-mutated AML.
By examining the latest research findings and discussing the clinical implications, this paper aims to contribute to a better understanding of FLT3 mutations in AML and provide insights that may guide future research directions and facilitate the development of more effective therapeutic strategies. Moreover, a recent study conducted in 2021 revealed the clonal evolution of AML with FLT3-ITD mutation under treatment with midostaurin, showcasing the dynamic nature of FLT3 mutations and their potential implications for treatment response [7]. The findings of this study underscore the importance of gaining a deeper understanding of FLT3 mutations and their influence on treatment outcomes, further highlighting the significance of investigating FLT3 mutations in AML.
Review
FLT3 gene and its role in hematopoiesis
Overview of the FLT3 Gene
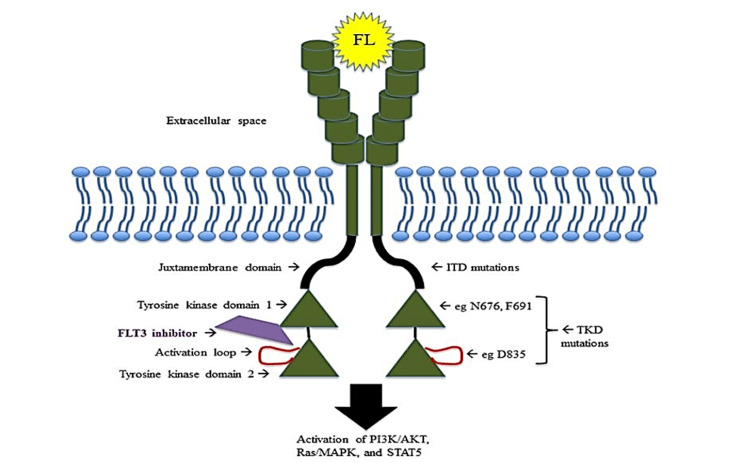
The FLT3 gene, also known as CD135, is situated on chromosome 13q12 and encodes a type III receptor tyrosine kinase. Comprising 24 exons, the FLT3 gene undergoes alternative splicing, giving rise to several isoforms. Notably, the primary isoform, FLT3-ITD, frequently associated with AML, is distinguished by the insertion of tandem duplications of varying lengths within the gene's coding region, particularly within the juxtamembrane domain [8,9]. This genetic alteration at the DNA level has significant implications for the function and activation of the encoded protein, influencing its role in AML pathogenesis [8,9]. To better understand the structural alterations caused by FLT3 mutations, Figure 1 provides a schematic representation of FLT3 and highlights the positions of FLT3-ITD and FLT3-TKD mutations within the receptor. FLT3-ITD mutations involve tandem duplications in the juxtamembrane domain, while FLT3-TKD mutations result in substitutions in the TKD.
Figure 1. Schematic representation of FLT3 structure and mutations.
Schematic representation of FLT3 structure and the location of FLT3-ITD and FLT3-TKD mutations within the receptor. FLT3-ITD mutations result in tandem duplications in the juxtamembrane domain, while FLT3-TKD mutations involve substitutions in the TKD.
Source: [10].
FLT3, Fms-like tyrosine kinase 3; FL, FLT3 ligand; TKD, tyrosine kinase domain; ITD, internal tandem duplication
Normal Function of FLT3 in Hematopoiesis
In normal hematopoiesis, FLT3 plays a pivotal role in the development and maintenance of hematopoietic stem and progenitor cells (HSPCs). Binding of its ligand, FLT3 ligand (FL), leads to dimerization and autophosphorylation of the FLT3 receptor, initiating downstream signaling cascades [11]. Activation of FLT3 promotes the survival, proliferation, and differentiation of HSPCs, thereby regulating the production of mature blood cells [12].
Signaling Pathways Activated by FLT3
Upon activation, FLT3 triggers several signaling pathways that contribute to its role in hematopoiesis. One of the key pathways is the RAS/mitogen-activated protein kinase (MAPK) pathway, which regulates cell proliferation and survival [13]. FLT3 activation also leads to the activation of phosphatidylinositol 3-kinase (PI3K)/AKT signaling, which promotes cell survival and inhibits apoptosis [14]. Additionally, the signal transducer and activator of transcription (STAT) pathway are activated downstream of FLT3, playing a role in cell survival, differentiation, and proliferation [15].
Importance of FLT3 Mutations in Disturbing Hematopoiesis
FLT3 mutations, particularly ITDs and TKD mutations, have a profound impact on hematopoiesis. FLT3-ITD mutations lead to the constitutive activation of FLT3 signaling, resulting in dysregulated proliferation, impaired differentiation, and enhanced survival of leukemic cells [11]. These mutations disrupt the balance of hematopoietic cell populations, leading to the expansion of undifferentiated blasts and the inhibition of normal hematopoietic cell production. FLT3 mutations contribute to leukemogenesis by conferring a growth advantage to leukemic cells, enabling them to bypass normal regulatory mechanisms and promote the expansion of malignant clones. The altered signaling pathways downstream of mutated FLT3 dysregulate key cellular processes, disrupting the normal hematopoietic hierarchy and contributing to the development of AML [15]. The presence of FLT3 mutations, particularly FLT3-ITD, is associated with adverse clinical features, such as higher leukocyte counts, increased risk of relapse, and reduced overall survival in AML patients [8]. Understanding the impact of FLT3 mutations on hematopoiesis is essential for unraveling the mechanisms underlying leukemogenesis and developing targeted therapies that specifically inhibit aberrant FLT3 signaling to restore normal hematopoiesis.
Types and prevalence of FLT3 mutations in AML
The types and prevalence of FLT3 mutations in AML are summarized in Table 1. FLT3-ITD mutations, occurring in the juxtamembrane domain and resulting in tandem duplications, are the most common FLT3 mutation, accounting for approximately 25% to 30% of AML cases [8]. Point mutations in the TKD of FLT3 are less common, occurring in approximately 5% to 10% of AML cases [9].
Table 1. Types and prevalence of FLT3 mutations in AML.
FLT3, Fms-like tyrosine kinase 3; TKD, tyrosine kinase domain; ITD, internal tandem duplication; AML, acute myeloid leukemia
| Mutation type | Definition and prevalence |
| ITD | FLT3-ITD mutations occur in the juxtamembrane domain and result in the insertion of tandem duplications of variable lengths. They are the most common FLT3 mutation in AML, accounting for approximately 25%-30% of cases. |
| Point mutations (TKD mutations) | FLT3-TKD mutations involve substitutions within the TKD of FLT3. They are less common than FLT3-ITD mutations and occur in approximately 5%-10% of AML cases. |
ITD Mutations
Definition and prevalence: ITD mutations in the FLT3 gene involve the insertion of tandem duplications of varying lengths within the juxtamembrane domain. These insertions result in a constitutively activated FLT3 receptor and dysregulated signaling [2]. ITD mutations are the most common FLT3 mutations in AML, accounting for approximately 20% to 30% of cases [16].
Impact on the FLT3 function: FLT3-ITD mutations lead to ligand-independent activation of the receptor and increased autophosphorylation, leading to the activation of downstream signaling pathways. This results in aberrant cell proliferation, impaired differentiation, and enhanced cell survival [2]. The length and location of ITD mutations may influence their impact on FLT3 signaling and clinical outcomes.
Prognostic significance in AML patients: AML patients with FLT3-ITD mutations often present with adverse clinical features, including higher leukocyte counts, increased blast percentage, and a higher likelihood of relapse [6]. FLT3-ITD mutations are associated with a decreased complete remission rate and poor overall survival, particularly in patients with a high allelic ratio or the presence of additional genetic abnormalities [17]. Furthermore, FLT3-ITD status has been incorporated into risk stratification systems for AML, aiding in treatment decisions and prognosis assessment.
Furthermore, it has been reported that specific FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene expression signature in younger adults with de novo cytogenetically normal AML lacking FLT3 ITDs [2]. This finding highlights the relevance of considering different FLT3 mutation types and their impact on AML outcomes [18].
Point Mutations (TKD Mutations)
Definition and prevalence: Point mutations in the TKD of the FLT3 gene involve single amino acid substitutions within critical regions of the receptor. These mutations confer constitutive FLT3 activation and are distinct from ITD mutations in terms of their molecular mechanisms and clinical implications. TKD mutations occur in approximately 7% to 10% of AML cases [4].
Consequences for FLT3 activity and clinical outcomes: FLT3-TKD mutations result in an increased kinase activity of the receptor, leading to constitutive activation of downstream signaling pathways [2]. Although TKD mutations are generally associated with a milder phenotype compared to FLT3-ITD mutations, their prognostic impact in AML remains controversial. Some studies suggest an adverse effect on clinical outcomes, such as decreased overall survival and increased relapse rates [8], while others report no significant association with prognosis [17]. Further investigations are needed to clarify the prognostic relevance of FLT3-TKD mutations.
Co-occurrence of FLT3 Mutations With Other Genetic Abnormalities in AML
FLT3 mutations often co-occur with other genetic abnormalities in AML, contributing to disease heterogeneity and influencing clinical outcomes. Common co-occurring genetic alterations include NPM1 mutations, DNMT3A mutations, and mutations in genes involved in chromatin modification (such as ASXL1 and IDH1/2) [8]. The presence of FLT3 mutations, particularly FLT3-ITD, in combination with other genetic abnormalities, may confer additional adverse prognostic implications and impact treatment strategies.
Understanding the prevalence and implications of different FLT3 mutations in AML is essential for risk stratification, treatment decisions, and the development of targeted therapies aimed at specifically inhibiting FLT3 signaling pathways.
Molecular mechanisms of FLT3 mutations in AML pathogenesis
Constitutive Activation of FLT3 Signaling Pathways
Impact on cell proliferation and survival: FLT3 mutations result in the constitutive activation of FLT3 signaling pathways, leading to dysregulated cell proliferation and enhanced cell survival in AML. The aberrant activation of FLT3 signaling promotes the proliferation of leukemic cells by driving cell cycle progression and overcoming normal growth regulatory mechanisms [19]. This uncontrolled cell proliferation contributes to the expansion of leukemic blasts in the bone marrow and peripheral blood.
Moreover, FLT3 mutations confer a survival advantage to leukemic cells by inhibiting apoptosis, the programmed cell death process. Constitutively active FLT3 signaling activates anti-apoptotic pathways, such as the PI3K/AKT and RAS/MAPK pathways, leading to the suppression of cell death signals [20]. Consequently, leukemic cells with FLT3 mutations exhibit increased resistance to apoptosis, contributing to their prolonged survival.
Influence on differentiation and self-renewal of hematopoietic stem/progenitor cells: FLT3 mutations also disrupt the normal differentiation and self-renewal processes of HSPCs. In normal hematopoiesis, FLT3 signaling plays a crucial role in regulating the balance between self-renewal and differentiation of HSPCs [12]. However, FLT3 mutations disturb this balance, promoting the expansion of undifferentiated progenitor cells at the expense of mature blood cell production.
FLT3-ITD mutations, in particular, have been associated with a blockage of myeloid differentiation, leading to the accumulation of undifferentiated myeloblasts in AML [2]. This differentiation block contributes to the aggressive phenotype of FLT3-ITD AML, as immature blasts are less responsive to differentiation-inducing agents and are associated with poor treatment outcomes.
Interaction With Other Oncogenic Pathways
Crosstalk with RAS and JAK-STAT signaling: FLT3 signaling pathways interact and crosstalk with other oncogenic pathways, amplifying the leukemogenic effects of FLT3 mutations. One important interaction is between FLT3 and the RAS pathway. Activated FLT3 can stimulate RAS signaling, leading to further enhancement of cell proliferation and survival [15]. This crosstalk between FLT3 and RAS pathways contributes to the aggressive nature of FLT3-mutated AML.
Additionally, FLT3 mutations can activate the JAK-STAT signaling pathway. Activation of STAT proteins downstream of FLT3 promotes cell survival, proliferation, and resistance to apoptosis [13]. The interplay between FLT3 and JAK-STAT signaling pathways further supports the leukemogenic potential of FLT3 mutations.
Synergistic effects on leukemogenesis: FLT3 mutations can also act synergistically with other genetic abnormalities in AML, leading to an increased leukemogenic potential. Co-occurrence of FLT3 mutations with mutations in genes such as NPM1, DNMT3A, or IDH1/2 can have additive or synergistic effects on leukemogenesis [2]. These interactions may involve shared downstream signaling pathways or cooperativity in disrupting normal hematopoietic processes.
Microenvironmental Factors and FLT3 Mutations
The microenvironment of the bone marrow plays a critical role in AML pathogenesis, and FLT3 mutations can influence the interactions between leukemic cells and their surrounding niche. The bone marrow microenvironment provides signals that support the survival and growth of leukemic cells, and FLT3 mutations can modulate the responsiveness of leukemic cells to these signals [14]. Additionally, factors such as cytokines, stromal cells, and extracellular matrix components in the microenvironment can further activate FLT3 signaling or contribute to drug resistance in FLT3-mutated AML.
Understanding the molecular mechanisms underlying FLT3 mutations in AML pathogenesis is essential for developing targeted therapies and improving treatment outcomes for patients with FLT3-mutated AML.
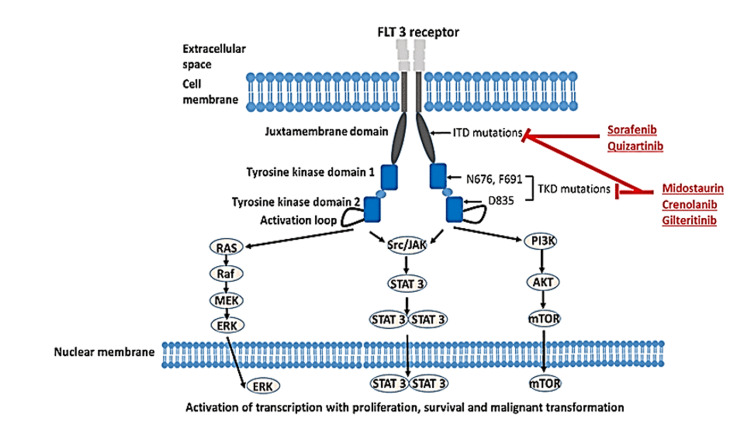
To elucidate the molecular mechanisms underlying the pathogenesis of AML, Figure 2 depicts the normal FLT3 signaling pathways involved in hematopoiesis and the dysregulated signaling pathways caused by FLT3 mutations in AML. The figure illustrates how FLT3 mutations result in constitutive activation of downstream signaling pathways, leading to aberrant cell proliferation, enhanced survival, and impaired differentiation.
Figure 2. FLT3 Signaling pathways and their dysregulation in AML.
Illustration of normal FLT3 signaling pathways involved in hematopoiesis and the dysregulated signaling pathways caused by FLT3 mutations in AML. FLT3 mutations lead to constitutive activation of downstream signaling pathways, promoting cell proliferation, survival, and impaired differentiation.
Source: [21].
FLT3, Fms-like tyrosine kinase 3; TKD, tyrosine kinase domain; ITD, internal tandem duplication; STAT, signal transducer and activator of transcription; mTOR, mammalian target of rapamycin; ERK, extracellular signal-regulated kinase; PI3K, phosphoinositide 3-kinase
Clinical implications and targeted therapies
Prognostic Value of FLT3 Mutations in AML
Impact on overall survival and relapse rates: FLT3 mutations have significant prognostic implications in AML patients. Several studies have consistently shown that AML patients with FLT3-ITD mutations have poorer overall survival compared to those without FLT3 mutations [6]. FLT3-ITD mutations are associated with higher relapse rates, shorter duration of remission, and increased risk of treatment failure [17]. The presence of FLT3-ITD mutations, particularly with high allelic ratio and additional adverse cytogenetic abnormalities, often indicates a more aggressive disease course.
Risk stratification in clinical practice: Due to the prognostic significance of FLT3 mutations, they are incorporated into risk stratification systems to guide treatment decisions in AML. The European LeukemiaNet (ELN) and other guidelines consider FLT3-ITD mutations as an adverse risk factor and classify patients accordingly [6]. Risk stratification helps identify patients who may benefit from more intensive therapies or targeted agents.
Overview of FLT3 Inhibitors
First-generation inhibitor midostaurin: Midostaurin is a multi-targeted kinase inhibitor that was the first FLT3 inhibitor approved by regulatory authorities for the treatment of FLT3-mutated AML. It inhibits both FLT3-ITD and FLT3-TKD mutations, as well as other kinases. Clinical trials have shown that the addition of midostaurin to standard chemotherapy improves overall survival and increases the rate of complete remission in newly diagnosed FLT3-mutated AML patients [22]. Midostaurin is typically used in combination with chemotherapy and has become a standard of care for this patient population.
Second-generation inhibitors gilteritinib and quizartinib: Second-generation FLT3 inhibitors, such as gilteritinib and quizartinib, have been developed to overcome the limitations of first-generation inhibitors. These agents specifically target FLT3 and exhibit higher potency and selectivity for FLT3 mutations. Gilteritinib and quizartinib have shown promising clinical activity as single agents in relapsed or refractory FLT3-mutated AML patients [23]. They have demonstrated high response rates and significant improvements in overall survival in this patient population. Ongoing studies are evaluating their efficacy in combination with chemotherapy or other targeted agents.
Challenges and Limitations in FLT3-Targeted Therapy
Resistance mechanisms and relapse: Despite the initial responses observed with FLT3 inhibitors, the development of resistance remains a significant challenge in FLT3-mutated AML. Resistance mechanisms include the acquisition of secondary FLT3 mutations, activation of alternative signaling pathways, and alterations in the bone marrow microenvironment [24]. These resistance mechanisms can lead to relapse or incomplete response to targeted therapy.
Combination strategies and future directions: To address the challenge of resistance and improve outcomes in FLT3-mutated AML, combination strategies are being explored. Combinations of FLT3 inhibitors with chemotherapy, other targeted agents, or immune-based therapies are being investigated in clinical trials [20]. Additionally, novel FLT3 inhibitors with enhanced potency and selectivity are being developed to overcome resistance and improve treatment outcomes. Furthermore, the identification of additional molecular targets and understanding of the complex interplay between signaling pathways hold promise for future therapeutic interventions in FLT3-mutated AML.
FLT3 mutations in AML have important clinical implications, affecting prognosis, risk stratification, and treatment decisions. FLT3 inhibitors, including first-generation inhibitor midostaurin and second-generation inhibitors gilteritinib and quizartinib, have shown efficacy in treating FLT3-mutated AML. However, challenges such as resistance mechanisms and relapse necessitate further research and the development of combination strategies. Future directions include the exploration of novel agents, combination therapies, and a deeper understanding of the underlying biology of FLT3-mutated AML.
Table 2 provides an overview of FLT3 inhibitors and their associated clinical trials. Midostaurin, a multi-targeted kinase inhibitor, showed improved overall survival in combination with standard chemotherapy in a phase III trial (RATIFY) conducted in newly diagnosed FLT3-mutated AML patients [20]. Gilteritinib, a selective FLT3 inhibitor, demonstrated superior overall survival compared to salvage chemotherapy in a phase III trial (ADMIRAL) involving relapsed/refractory FLT3-mutated AML patients [21]. Quizartinib, another selective FLT3 inhibitor, was evaluated in a phase III trial (QuANTUM-First) to assess its efficacy compared to chemotherapy in newly diagnosed FLT3-ITD AML patients [25].
Table 2. FLT3 inhibitors and clinical trials.
FLT3, Fms-like tyrosine kinase 3; ITD, internal tandem duplication; AML, acute myeloid leukemia
| Inhibitor | Mechanism of action | Clinical trials |
| Midostaurin | Multi-targeted kinase inhibitor | Phase III trial (RATIFY) showed improved overall survival when combined with standard chemotherapy in newly diagnosed FLT3-mutated AML patients. |
| Gilteritinib | Selective FLT3 inhibitor | Phase III trial (ADMIRAL) demonstrated superior overall survival compared to salvage chemotherapy in relapsed/refractory FLT3-mutated AML patients. |
| Quizartinib | Selective FLT3 inhibitor | Phase III trial (QuANTUM-First) evaluated the efficacy of quizartinib compared to chemotherapy in newly diagnosed FLT3-ITD AML patients. |
Conclusions
Advancements in comprehending these mutations offer potential benefits for AML diagnosis and treatment. Molecular testing for FLT3 mutations aids in accurate risk assessment and treatment guidance, with ongoing research focusing on overcoming treatment resistance and enhancing outcomes for FLT3-mutated AML. The identification of novel therapeutic targets and innovative strategies further expands the therapeutic landscape. Overall, the investigation of FLT3 mutations in AML unveils key molecular mechanisms, impacting diagnosis, risk assessment, and treatment decisions. Persistent research efforts have the potential to enhance patient outcomes and foster more personalized therapies for FLT3-mutated AML.
The authors have declared that no competing interests exist.
References
- 1.Acute myeloid leukemia. Döhner H, Weisdorf DJ, Bloomfield CD. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 2.A review of FLT3 inhibitors in acute myeloid leukemia. Zhao JC, Agarwal S, Ahmad H, Amin K, Bewersdorf JP, Zeidan AM. Blood Rev. 2022;52:100905. doi: 10.1016/j.blre.2021.100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. Paschka P, Schlenk RF, Gaidzik VI, et al. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 4.FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Levis M. Hematol Am Soc Hematol Educ Program. 2013;2013:220–226. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frequency of FLT3 internal tandem duplications in adult Syrian patients with acute myeloid leukemia and normal karyotype. Al-Arbeed IF, Wafa A, Moassass F, Al-Halabi B, Al-Achkar W, Abou-Khamis I. Asian Pac J Cancer Prev. 2021;22:3245–3251. doi: 10.31557/APJCP.2021.22.10.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Döhner H, Estey E, Grimwade D, et al. https://ashpublications.org/blood/article/129/4/424/36196/Diagnosis-and-management-of-AML-in-adults-2017-ELN. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clonal evolution of acute myeloid leukemia with FLT3-ITD mutation under treatment with midostaurin. Schmalbrock LK, Dolnik A, Cocciardi S, et al. Blood. 2021;137:3093–3104. doi: 10.1182/blood.2020007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The role of small molecule Flt3 receptor protein-tyrosine kinase inhibitors in the treatment of Flt3-positive acute myelogenous leukemias. Roskoski R Jr. Pharmacol Res. 2020;155:104725. doi: 10.1016/j.phrs.2020.104725. [DOI] [PubMed] [Google Scholar]
- 9.De novo adult acute myeloid leukemia with two new mutations in juxtatransmembrane domain of the FLT3 gene: a case report. Alarbeed IF, Wafa A, Moassass F, Al-Halabi B, Al-Achkar W, Liehr T, Aboukhamis I. https://ashpublications.org/blood/article/101/8/3164/16684/Suppression-of-myeloid-transcription-factors-and. J Med Case Rep. 2021;15:22. doi: 10.1186/s13256-020-02587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The biology and targeting of FLT3 in pediatric leukemia. Annesley CE, Brown P. Front Oncol. 2014;4:263. doi: 10.3389/fonc.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. Fischer T, Stone RM, Deangelo DJ, et al. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. McKenna HJ, Stocking KL, Miller RE, et al. https://ashpublications.org/blood/article/95/11/3489/253344/Mice-lacking-flt3-ligand-have-deficient. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 13.Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD and promotes apoptosis in myeloid leukemias. Zhao S, Konopleva M, Cabreira-Hansen M, et al. Leukemia. 2004;18:267–275. doi: 10.1038/sj.leu.2403220. [DOI] [PubMed] [Google Scholar]
- 14.Activation of Akt and MAPK pathways enhances the tumorigenicity of CD133+ primary colon cancer cells. Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG, Zheng S, Huang J. Carcinogenesis. 2010;31:1376–1380. doi: 10.1093/carcin/bgq120. [DOI] [PubMed] [Google Scholar]
- 15.FLT3: ITDoes matter in leukemia. Levis M, Small D. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 16.Internal tandem duplication of FLT3 associated with leukocytosis in acute promyelocytic leukemia. Leukemia Study Group of the Ministry of Health and Welfare (Kohseisho) Kiyoi H, Naoe T, Yokota S, et al. Leukemia. 1997;11:1447–1452. doi: 10.1038/sj.leu.2400756. [DOI] [PubMed] [Google Scholar]
- 17.Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. Schlenk RF, Döhner K, Krauter J, et al. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 18.FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Whitman SP, Ruppert AS, Radmacher MD, et al. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FLT3 mutations in acute myeloid leukemia: key concepts and emerging controversies. Kennedy VE, Smith CC. Front Oncol. 2020;10:612880. doi: 10.3389/fonc.2020.612880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Activation mechanisms of STAT5 by oncogenic Flt3-ITD. Choudhary C, Brandts C, Schwable J, et al. Blood. 2007;110:370–374. doi: 10.1182/blood-2006-05-024018. [DOI] [PubMed] [Google Scholar]
- 21.Molecular targeting in acute myeloid leukemia. Lim SH, Dubielecka PM, Raghunathan VM. https://link.springer.com/article/10.1186/s12967-017-1281-x. J Transl Med. 2017;15:183. doi: 10.1186/s12967-017-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. Stone RM, Mandrekar SJ, Sanford BL, et al. N Engl J Med. 2017;377:454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Erba HP, Montesinos P, Kim HJ, et al. https://pubmed.ncbi.nlm.nih.gov/37116523/ Lancet. 2023;401:1571–1583. doi: 10.1016/S0140-6736(23)00464-6. [DOI] [PubMed] [Google Scholar]
- 24.Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Perl AE, Altman JK, Cortes J, et al. Lancet Oncol. 2017;18:1061–1075. doi: 10.1016/S1470-2045(17)30416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Cortes J, Perl AE, Döhner H, et al. Lancet Oncol. 2018;19:889–903. doi: 10.1016/S1470-2045(18)30240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]