Abstract
Background
Early neurological deterioration after endovascular thrombectomy (EVT) is associated with poor prognosis. National Institutes of Health Stroke Scale (NIHSS) score measured at 24 h after EVT may be a better outcome predictor than other methods that focus on changes in NIHSS. Nevertheless, clinical fluctuations in ischemic stroke patients during the immediate phase after symptoms onset are well recognized. Therefore, a delayed NIHSS evaluation may improve prognostic accuracy. We evaluate the 7-day NIHSS in predicting long-term patient outcomes after EVT.
Methods
This was a multi-center retrospective cohort study of 300 consecutive ischemic stroke patients with large vessel occlusion who underwent EVT at three-stroke centers in China from August 2018 to March 2022. NIHSS was recorded on admission, pre-EVT, 24 h, and 7 days after EVT.
Results
A total of 236 eligible patients were subdivided into two groups: 7-day NIHSS ≤6 and NIHSS >6 post-EVT. 88.29% achieved a favorable outcome (modified Rankin Scale 0–2) in the NIHSS ≤6 group compared to 15.20% in the NIHSS >6 group at 90 days, and an improved favorable outcome in the former group was observed after adjusting for potential confounding factors (adjusted odds ratio 39.7, 95% confidence interval, 17.5–89.7, p < 0.001).
Conclusion
The 7-day NIHSS score may be a reliable predictor of 90-day stroke patient outcome after EVT.
Keywords: stroke, prognosis, mechanical thrombectomy, endovascular thrombectomy, NIHSS
1. Introduction
Mechanical endovascular thrombectomy (EVT) has been demonstrated to improve outcomes in patients with large vessel occlusion ischemic stroke when performed within 6 h of symptom onset or up to 24 h in selected patients [1], leading to its establishment as the current standard of care [2,3,4]. Identifying early prognostic markers that accurately predict long-term outcomes in patients after EVT is paramount in facilitating medical decision-making and can be utilized as surrogate endpoints in clinical trials [4].
Early neurological deterioration following EVT has been associated with poor 90-day outcomes [5], indicating that short-term neurological examinations may have value in predicting long-term patient prognosis. The National Institute of Health Stroke Scale (NIHSS) enables quantification of stroke severity in patients with a higher score, reflecting more impairment. Accordingly, early neurological recovery characterized by a greater than 2 point NIHSS improvement at 2 h or four points at 24 h is associated with good outcomes at 3 months [6], with other reports finding similar results [4,7]. Importantly, a recent study found an NIHSS of 8 or less at 24 h after EVT to be the best predictor of favorable outcomes compared to other methods that measured changes in NIHSS [8]. Clinical fluctuations in stroke patients, in particular during the immediate phase after symptom onset, are well recognized [9,10], and a delayed NIHSS evaluation may improve the accuracy of the neurological examination and provide early prognostication.
In this study, we evaluated the efficacy of the 7-day NIHSS in predicting long-term patient outcomes after EVT. To our knowledge, this is the first study to use 7-day NIHSS in post-EVT to predict ischemic patients’ outcomes.
2. Materials and methods
2.1. Patient selection
This is a retrospective analysis of prospectively collected data from consecutive ischemic stroke patients who underwent EVT from August 2018 to March 2022 at three academic comprehensive stroke centers in China. The data were derived from the Big Data Observatory Platform for stroke in China and from the hospital data platform.
Inclusion criteria were as follows: (1) patients who underwent emergency endovascular therapy, (2) age ≥18 years old, and (3) presented within 24 h of stroke onset. The exclusion criteria were as follows: (1) patients with pre-EVT NIHSS ≤5, (2) missing follow-up data, and (3) missing 7 ± 2-day NIHSS after EVT
2.2. Data collection
We collected the following data: age, sex, risk factors of cerebrovascular disease (hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation (AF), prior stroke, hyperlipemia, coronary kidney disease, smoking status), initial premorbid modified Rankin Scale (mRS), The Alberta stroke program early CT score, door-to-needle time (DNT), onset-to-needle time (ONT), door to puncture time (DPT), last known normal-to-puncture time, door-to-recanalization time (DRT), and modified thrombolysis in cerebral infarction (mTICI) post-thrombectomy. Successful reperfusion was defined as mTICI ≥2b.
Attending neurologists or neurosurgeons measured and recorded the NIHSS of consecutive patients and entered the data into the database prospectively. NIHSS was recorded on admission, pre-EVT, 24 h after EVT, and 7 days after EVT.
2.3. Outcome measures
The primary outcome was mRS 3 months after EVT. The follow-up mRS was measured routinely by specialized stroke nurses and doctors by telephone or in-person appointments during outpatient follow-up. A favorable outcome was defined as mRS of 0–2 at 90 days. Walking independence was defined as mRS of 3. The secondary outcome was 90-day mortality.
2.4. Statistical analysis
Patients with 7-day NIHSS >6 were compared to those with NIHSS ≤6. The non-parametric Mann–Whitney U test was performed using the IBM SPSS version 26 (IBM-Armonk, NY) to analyze non-normally distributed continuous data, reported as medians along with the interquartile range. Normally distributed data were reported as means with corresponding standard deviations (SD) and compared using the student’s t-test. Results were considered statistically significant if the p-value was less than 0.05.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee. The study was approved by The Foshan Sanshui District People’s Hospital review board (No. SRY-EC-2022-051) and the Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine Hospital review board (No. 2022-085).
Informed consent: Informed consent has been obtained from all individuals included in this study.
3. Results
In total, 300 EVT patients were evaluated. There were four patients excluded for missing follow-up data, 58 patients excluded due to death or discharge before seven days, thus missing 7-day NIHSS, and another two patients excluded for NIHSS pre-EVT of less than 6. This resulted in a cohort of 236 patients. The patients were divided into two groups according to 7-day NIHSS ≤6 (n = 111) and NIHSS >6 after EVT (n = 125). The baseline characteristics of patients are demonstrated in Table 1.
Table 1.
Comparison of baseline characteristics of patients with NIHSS ≤6 and NIHSS >6 on day 7
| NIHSS ≤6 | NIHSS >6 | X 2/t/z | P | |
|---|---|---|---|---|
| Number | 111 | 125 | ||
| Age, mean ± SD | 61.69 ± 13.33 | 66.88 ± 12.18 | 3.124 | 0.002 |
| Male, n (%) | 73 (65.77) | 89 (71.20) | 0.807 | 0.369 |
| Hypertension, n (%) | 59 (53.15) | 82 (65.60) | 3.787 | 0.052 |
| Diabetes mellitus, n (%) | 16 (14.41) | 32 (25.60) | 4.540 | 0.033 |
| CAD, n (%) | 14 (12.61) | 25 (20.00) | 2.326 | 0.127 |
| AF, n (%) | 38 (34.23) | 45 (36.00) | 0.080 | 0.777 |
| Prior stroke, n (%) | 20 (18.02) | 28 (22.40) | 0.697 | 0.404 |
| Hyperlipemia, n (%) | 24 (21.62) | 18 (14.40) | 2.096 | 0.148 |
| CKD, n (%) | 8 (7.21) | 9 (7.20) | 0.001 | 0.998 |
| Smoker, n (%) | 26 (23.42) | 23 (18.40) | 0.902 | 0.342 |
| NIHSS pre-EVT (IQR) | 13.00 (10.00, 17.00) | 16.00 (12.00, 20.00) | −4.116 | <0.001 |
| ASPECTS pre-treatment (IQR) | 8.00 (8.00, 9.00) | 8.00 (8.00, 9.00) | −0.967 | 0.334 |
| mRS pre-premorbid (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | −1.679 | 0.093 |
| Occluded vessel | ||||
| Distal/terminal ICA, n (%) | 15 (13.51) | 26 (20.80) | 6.833 | 0.337 |
| MCA-M1, n (%) | 57 (51.35) | 48 (38.40) | ||
| MCA-M2, n (%) | 7 (6.31) | 8 (6.40) | ||
| MCA-M3, n (%) | 0 (0.00) | 1 (0.80) | ||
| Tandem, n (%) | 16 (14.41) | 15 (12.00) | ||
| Basilar, n (%) | 12 (10.81) | 20 (16.00) | ||
| Others, n (%) | 4 (3.60) | 7 (5.60) | ||
| IV thrombolysis, n (%) | 50 (45.05) | 39 (31.20) | 4.798 | 0.028 |
| DNT | 41.00 (28.50, 51.50) | 45.00 (33.00, 65.00) | −1.434 | 0.152 |
| ONT | 129.00 (95.00, 178.50) | 113.00 (89.00, 146.00) | −1.063 | 0.288 |
| Median DPT (IQR), min | 126.00 (100.00, 190.00) | 150.00 (113.50, 205.00) | −2.156 | 0.031 |
| Median DRT (IQR), min | 205.00 (153.00, 258.00) | 245.00 (186.50, 311.00) | −3.113 | 0.002 |
| Median PRT (IQR), min | 53.00 (35.00, 80.00) | 73.00 (47.50, 110.00) | −3.585 | <0.001 |
| Median LKNPT (IQR), min | 290.00 (195.00, 446.00) | 305.00 (190.00, 480.50) | −0.731 | 0.465 |
| mTICI post ≥2b, n (%) | 23 (82.14) | 102 (49.04) | 10.856 | 0.001 |
| sICH, n (%) | 0 (0.00) | 16 (12.80) | 15.241 | <0.001 |
Abbreviations: AF; atrial fibrillation; ASPECTS, The Alberta stroke program early CT score; CE, cardioembolic; CKD, chronic kidney disease; CAD, coronary artery disease; DM, diabetes mellitus; DPT, door-to-puncture time; DRT, door-to-recanalization time; EVT, endovascular thrombectomy; IQR, interquartile range; IV, intravenous; LKNPT, last-known normal-to-puncture time; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Compared to the 7-day NIHSS >6 group, the NIHSS ≤6 group was younger (61.69, SD 13.33 years vs 66.88, SD 12.18 years, p = 0.002) and had a lower prevalence of diabetes mellitus (14.41 vs 25.60%, p = 0.03) and lower median NIHSS pre-EVT (13.00 vs 16.00, p < 0.001). There were no significant differences in sex, coronary artery disease (CAD), AF, prior stroke, hypertension, hyperlipidemia, smoking history, and occluded vessels. Compared to the NIHSS >6 group, a greater number of patients in the NIHSS ≤6 group received IV thrombolysis (45.05 vs 31.20%, p = 0.028) (Table 1).
In terms of the time metrics of EVT, patients with 7-day NIHSS ≤6 had significantly shorter median DPT (126.00 vs 150.00 min, p = 0.031), median DRT (205.00 vs 245.00 min, p = 0.002), and median PRT (53.00 vs 73.00 min, p < 0.001) than patients with NIHSS >6. In addition, none of the NIHSS ≤6 group had symptomatic intracerebral hemorrhage (sICH), compared to 12.80% of the NIHSS >6 group (p < 0.001) (Table 1).
3.1. Primary outcome: 90-day mRS
The median mRS discharge was lower in the NIHSS ≤6 group compared to those with NIHSS >6 (1.00 vs 4.00, p <0.001) (Table 2).
Table 2.
Comparison of 90-day clinical outcomes in patients with 7-day NIHSS ≤6 compared to NIHSS >6
| 7-day NIHSS ≤6 | 7-day NIHSS >6 | X 2/z | p | |
|---|---|---|---|---|
| Median mRS discharge (IQR) | 1.00 (1.00, 2.00) | 4.00 (4.00, 5.00) | −11.33 | <0.001 |
| 90-Day favorable outcome, n (%) | 98 (88.29) | 19 (15.20) | 125.63 | <0.001 |
| 90-Day mortality, n (%) | 4 (3.60) | 32 (25.60) | 22.01 | <0.001 |
Favorable outcome: mRS (0–2) at 3 months. Poor outcome: mRS ≥3. Abbreviations: IQR, interquartile range; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
At 90 days, 88.29% achieved a favorable outcome (mRS 0–2) in the NIHSS ≤6 group while only 15.20% of patients achieved a 90-day favorable outcome in the NIHSS >6 group (p < 0.001). Favorable outcome at 90 days remained significantly higher in patients with NIHSS ≤6 compared to NIHSS >6 even after adjustment for age, hypertension, diabetes mellitus, pre-EVT NIHSS, and pre-morbid mRS (adjusted odds ratio [OR] 39.7, 95% confidence interval [CI] 17.5–89.7, p < 0.001). The adjusted analysis of 90-day outcomes is shown in Tables 3 and 4.
Table 3.
Comparison of 90-day outcomes of patients with 7-day NIHSS ≤6 versus NIHSS >6 after adjusting for baseline relevant factors
| Adjusted OR* | 95% CI | p | |
|---|---|---|---|
| 90-Day favorable outcome (mRS 0–2), n (%) | 39.666 | 17.548–89.662 | <0.001 |
| 90-Day poor outcome (mRS ≥3), n (%) | 0.025 | 0.011–0.057 | <0.001 |
| 90-Day mortality, n (%) | 0.156 | 0.051–0.474 | <0.001 |
Model: *Adjusted for age, hypertension, diabetes, pre-EVT NIHSS, and pre-morbid mRS. Abbreviation: mRS, modified Rankin scale.
Table 4.
Comparison of 90-day outcomes of patients with 7-day NIHSS ≤6 versus NIHSS >6 after adjusting for relevant factors
| Adjusted OR* | 95% CI | p | |
|---|---|---|---|
| 90-Day favorable outcome (mRS 0–2), n (%) | 33.822 | 13.834–82.685 | <0.001 |
| 90-Day poor outcome (mRS ≥3), n (%) | 0.030 | 0.012–0.072 | <0.001 |
| 90-Day mortality, n (%) | 0.223 | 0.067–0.740 | 0.014 |
Model: *Adjusted for age, hypertension, diabetes, pre-EVT NIHSS and pre-morbid mRS, MCA-M2, IV thrombolysis, DRT, sICH, mTICI post ≥2b. Abbreviation: mRS, modified Rankin scale.
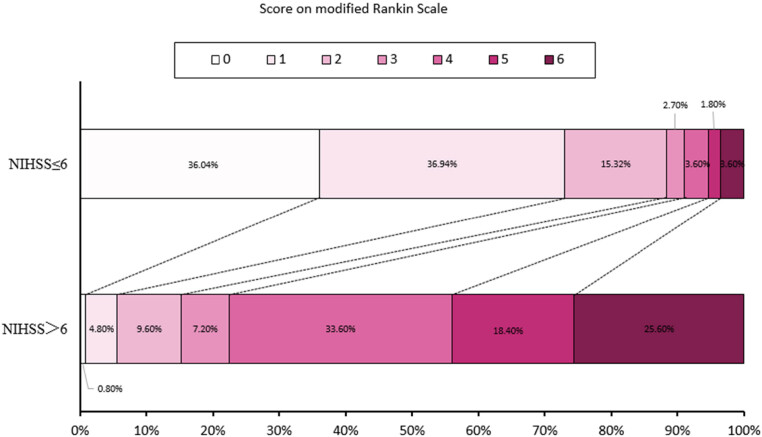
The distribution of 90-day mRS in patients with NIHSS ≤6 and NIHSS >6 on day 7 is shown in Figure 1 and Table S1.
Figure 1.
Distribution of 90-day mRS in patients with NIHSS ≤6 and NIHSS >6 on day 7.
The optimal cut-off of 7-day NIHSS 6.50 had a specificity of 0.891 and a sensitivity of 0.838 for the prediction of favorable functional outcome at 90 days (area under the curve [AUC] 0.909, 95% CI 0.871–0.947, p <0.001) (Figure 2).
Figure 2.
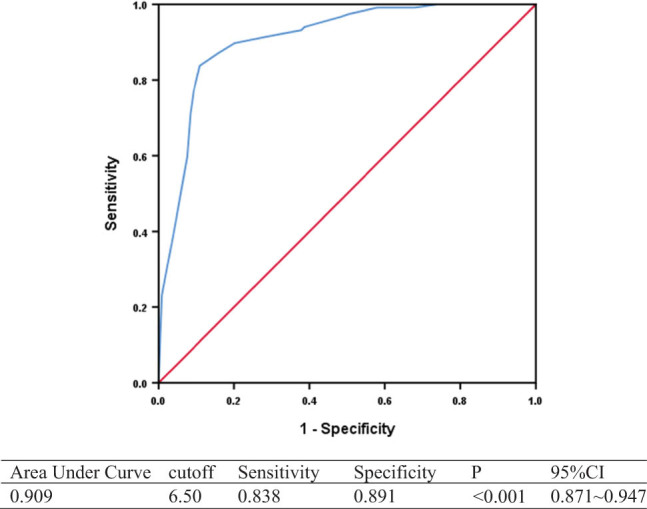
ROC of 7-day NIHSS predicting 90-day favorable outcome (mRS 0–2).
3.2. Secondary outcome: 90-day mortality
Mortality at 90 days was significantly lower in patients with 7-day NIHSS ≤6 compared to NIHSS >6 on univariable analysis (25.60 vs 3.60%, p < 0.001) (Table 2) and on adjusted analysis (adjusted OR 0.156, 95% CI 0.051–0.474, p = 0.001) (Table 3). The optimal cut-off of 7-day NIHSS 13.5 had a specificity of 0.810 and a sensitivity of 0.667 for the prediction of mortality at 90 days (AUC 0.815, 95% CI 0.740–0.891, p <0.001) (Figure 3).
Figure 3.
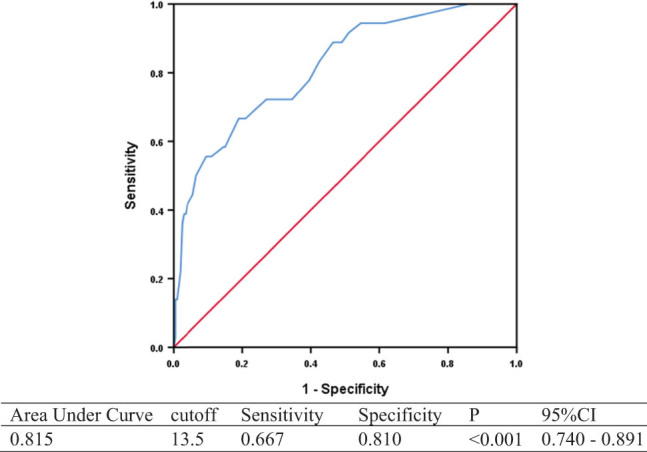
ROC of 7-day NIHSS predicting 90-day mortality.
Furthermore, we calculated the mRS at discharge as shown in Figure 4.
Figure 4.
ROC of discharge mRS.
4. Discussion
Our study found that NIHSS on day 7 is a predictor of 90-day clinical outcomes post-EVT, including favorable functional outcomes on mRS and mortality.
Stroke severity measured by baseline NIHSS on presentation is well established to be predictive of outcomes in ischemic stroke [11]. As such, the 2019 American Heart Association/American Stroke Association guidelines recommend that baseline NIHSS should be assessed, and stroke outcomes measures are strongly influenced by baseline stroke severity on NIHSS [12]. Given the strong association of NIHSS with 90-day outcomes, change in NIHSS or early NIHSS at 24 h has become an attractive surrogate for outcomes in clinical trials, particularly in patients who may be considered non-compliant with long-term follow-up. For example, the Blood Pressure after Endovascular Therapy for Ischemic Stroke study and the randomized controlled trial by the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group used a 24-h change in NIHSS as a surrogate outcome for early neurological recovery [13,14]. However, the optimal surrogate markers for early neurological recovery are not established, and a post hoc analysis of a prospective cohort study on EVT-treated patients at 12 stroke centers in the US found that the absolute 24-h NIHSS adjusted for baseline NIHSS on multivariable analysis had the highest predictive power compared to 24-h change in NIHSS and percentage change in NIHSS [7]. The 24-h NIHSS of ≤7 had a sensitivity of 80.1% and a specificity of 80.4% for predicting 90-day mRS score of 0–2 [7], but the predictive value of NIHSS scores beyond 24 h in post-EVT stroke patients requires further study.
Consistent with multiple studies that have reported that 7-day NIHSS is a sensitive measure for acute stroke outcomes [15,16,17], we found in our study that the 7-day NIHSS of >6 predicted 90-day mRS 0–2 with a sensitivity of 83.8% and a specificity of 89.1%. This is comparable to a previous study on serial NIHSS post-acute symptomatic middle cerebral artery territory infarcts, which found that 7-day NIHSS of ≥6 predicted poor outcomes with a sensitivity of 84% and a specificity of 94%, and had the highest AUC ROC for poor outcomes among baseline, day 3, and day 14 NIHSS scores [18]. In a random sample of 10,000 patients who underwent recombinant tissue-type plasminogen activator (rtPA), out of 4 clinical endpoints of mRS at 30 and 90 days, and NIHSS at 7 and 90 days, 7-day NIHSS required the smallest sample size to generate statistical power of >80% to detect a treatment effect of alteplase [15] and may be a sensitive endpoint for clinical trials. We also observed that 7-day NIHSS was predictive of 90-day mortality, after adjustment for baseline NIHSS, age, hypertension, diabetes, and pre-morbid mRS, and a cut-off of 13.5 had a specificity of 81.0% and a sensitivity of 66.7%. Therefore, the 7-day NIHSS may be a feasible and sensitive surrogate marker of outcomes post-EVT, such as 90-day mRS and mortality, but further validation in prospective studies and RCTs is needed.
Previous studies have shown that age is a significant risk factor for the outcome of stroke patients [19–21]. Patients in the NIHSS >6 group tended to be older, compared to the NIHSS ≤6 group (p = 0.002), which can be considered a confounding factor. To remove the possibility of such confounding factors influencing our conclusion, we demonstrated that the reported favorable outcome at 90 days remained significantly higher even after adjustment for age in this cohort.
The NIHSS quantifies the extent of neurological deficits present after acute ischemic stroke and represents the severity of stroke. While baseline NIHSS identifies patients presenting with severe strokes, NIHSS after 1 week can identify a subset of patients with poor neurological recovery, which may be more strongly associated with longer-term outcomes. Early neurological recovery, measured by improvement in NIHSS by 10 points or absolute score of ≤4 within 2 h (OR 2.4), and continuous neurological recovery, or improvement in NIHSS by 8 points at 2–24 h (OR 7.3), were independent predictors of good functional outcomes at 3 months in a prospective cohort study [14]. The recovery process post-stroke is time dependent, and the most significant improvements occur within the first 3 months when surviving neurons in peri-infarct areas enlarge their dendritic trees and sprout new axons to form new synaptic connections in local and distant brain areas [22,23]. Therefore, the 7-day NIHSS may better predict the efficacy of this neurological recovery process compared to baseline NIHSS and hence serve as a better surrogate for functional recovery post-stroke. Furthermore, workflow optimization and identifying parameters, such as recanalization status, can play significant roles in shortening DNT and DPT, thus improving patient outcomes and reducing mortality [24–26].
There are several limitations to this study. First, this is a small-to-moderate size retrospective cohort study and hence may be susceptible to confounding factors; therefore, a causative relationship cannot be determined. However, we have adjusted for clinical factors most predictive of functional outcomes, such as age, hypertension, diabetes, and baseline NIHSS [27]. The biological feasibility and temporal association of 7-day NIHSS and 90-day functional outcome also increases the reliability of the observed relationship. Due to the lack of 7-day NIHSS in patients who died or were discharged before 7 days, there may be a selection bias in the cohort included in our study, as the extremes of patient presentations were excluded (those with very severe presentation who died before 7 days, and those with very mild presentation who were discharged before 7 days). In addition, patients with basilar occlusion were included in the study with 20 basilar occlusions in the NIHSS >6 group versus 12 in the NIHSS ≤6 group. Although there were no statistically significant differences (p = 0.245) between the two groups in our study, and previous studies have demonstrated the effectiveness of EVT in basilar artery occlusion [28,29], such variations may serve as an additional cofounder which needs to be addressed in future studies. Exclusion criteria resulted in the removal of 64 patients (21.3%) from the final analysis. This is a substantial number, and future large-scale studies are required to address such limitations. Despite such limitations, these results can prompt further studies at other stroke centers. Further large prospective studies and RCTs are needed to validate the use of 7-day NIHSS to predict outcomes post-EVT and its use as a surrogate marker in clinical trials. Compared to 90-day outcomes that require longer-term follow-up and are more resource intensive, shorter-term outcomes like 7-day NIHSS may have the potential to be both cost-effective and pragmatic, but this needs to be investigated in further studies.
5. Conclusions
In this retrospective analysis of a prospectively maintained database, a 7-day NIHSS score of ≤6 was a predictor of 90-day functional outcomes (mRS 0–2) and mortality. Further prospective studies and RCTs are needed to validate the use of 7-day NIHSS to predict outcomes post-EVT and as a pragmatic, surrogate marker in clinical trials.
Supplementary Material
Acknowledgments
The authors would like to thank all colleagues for data collection and patient contribution.
Footnotes
Funding information: This study was supported by Foshan 14th Five-Year-Plan High Level Medical Discipline Priority Construction Project; Foshan Nanhai District 14th 5-Year-Plan High Level Medical Discipline Priority Construction Project; and Foshan 14th 5-Year Plan Key Discipline Foundation, Guangdong provincial TCM Bureau Key Discipline Foundation, Foshan Brain Heart co-therapy Foundation.
Author contributions: All authors contributed to this manuscript. All authors have read and approved the final version of the manuscript.
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Yimin Chen, Email: drymchen@126.com.
Sijie Zhou, Email: zhousijie91@163.com.
References
- [1].Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017;378:11–21. [DOI] [PubMed]
- [2].Campbell BCV, Nguyen TN. Advances in stroke: Treatments-interventional. Stroke. 2022;53:264–7. [DOI] [PubMed]
- [3].Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. [DOI] [PubMed]
- [4].Heitsch L, Ibanez L, Carrera C, Binkley MM, Strbian D, Tatlisumak T, et al. Early neurological change after ischemic stroke is associated with 90-day outcome. Stroke. 2021;52:132–41. [DOI] [PMC free article] [PubMed]
- [5].Zhang M, Xing P, Tang J, Shi L, Yang P, Zhang Y, et al. Predictors and outcome of early neurological deterioration after endovascular thrombectomy: A secondary analysis of the DIRECT-MT trial. J Neurointerv Surg. 2022;15:9–16. [DOI] [PubMed]
- [6].Soize S, Fabre G, Gawlitza M, Serre I, Bakchine S, Manceau PF, et al. Can early neurological improvement after mechanical thrombectomy be used as a surrogate for final stroke outcome? J Neurointerv Surg. 2019;11:450–4. [DOI] [PubMed]
- [7].Mistry EA, Yeatts S, de Havenon A, Mehta T, Arora N, De Los Rios La Rosa F, et al. Predicting 90-day outcome after thrombectomy: Baseline-adjusted 24-hour NIHSS is more powerful than NIHSS score change. Stroke. 2021;52:2547–53. [DOI] [PMC free article] [PubMed]
- [8].Hendrix P, Melamed I, Collins M, Lieberman N, Sharma V, Goren O, et al. NIHSS 24 h after mechanical thrombectomy predicts 90-day functional outcome. Clin Neuroradiol. 2022;32:401–6. [DOI] [PubMed]
- [9].Romano JG, Gardener H, Smith EE, Campo-Bustillo I, Khan Y, Tai S, et al. Frequency and prognostic significance of clinical fluctuations before hospital arrival in stroke. Stroke. 2022;53:482–7. [DOI] [PubMed]
- [10].Vahidy FS, Hicks WJ, 2nd, Acosta I, Hallevi H, Peng H, Pandurengan R, et al. Neurofluctuation in patients with subcortical ischemic stroke. Neurology. 2014;83:398–405. [DOI] [PMC free article] [PubMed]
- [11].Ospel JM, Brown S, Kappelhof M, van Zwam W, Jovin T, Roy D, et al. Comparing the prognostic impact of age and baseline national institutes of health stroke scale in acute stroke due to large vessel occlusion. Stroke. 2021;52:2839–45. [DOI] [PubMed]
- [12].Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. [DOI] [PubMed]
- [13].National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–8. [DOI] [PubMed]
- [14].Mistry EA, Sucharew H, Mistry AM, Mehta T, Arora N, Starosciak AK, et al. Blood pressure after endovascular therapy for ischemic stroke (BEST): A multicenter prospective Cohort Study. Stroke. 2019;50:3449–55. [DOI] [PMC free article] [PubMed]
- [15].Kerr DM, Fulton RL, Lees KR. Seven-day NIHSS is a sensitive outcome measure for exploratory clinical trials in acute stroke: Evidence from the Virtual International Stroke Trials Archive. Stroke. 2012;43(5):1401–3. [DOI] [PubMed]
- [16].Tudor R, Iovanescu G, Reisz D, Cornea A, Potre-Oncu C, Tutelca A, et al. Additional factors to corelate with a more than 30% NIHSS score improvement in patients 7 days after fibrinolytic and/or endovascular treatment for ischemic stroke. BMC Neurol. 2020;20(1):417. [DOI] [PMC free article] [PubMed]
- [17].Bai QK, Zhao ZG, Lu LJ, Shen J, Zhang JY, Sui HJ, et al. Treating ischaemic stroke with intravenous tPA beyond 4.5 hours under the guidance of a MRI DWI/T2WI mismatch was safe and effective. Stroke Vasc Neurol. 2019;4(1):8–13. [DOI] [PMC free article] [PubMed]
- [18].Bang OY, Park HY, Yoon JH, Yeo SH, Kim JW, Lee MA, et al. Predicting the long-term outcome after subacute stroke within the middle cerebral artery territory. J Clin Neurol. 2005;1:148–58. [DOI] [PMC free article] [PubMed]
- [19].Lui SK, Nguyen MH. Elderly stroke rehabilitation: Overcoming the complications and its associated challenges. Curr Gerontol Geriatr Res. 2018;2018:9853837. [DOI] [PMC free article] [PubMed]
- [20].Roy-O’Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159(8):3120–31. [DOI] [PMC free article] [PubMed]
- [21].Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, et al. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121(7):879–91. [DOI] [PubMed]
- [22].Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Wakerley B, et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:e590–6. [DOI] [PubMed]
- [23].Grefkes C, Fink GR. Recovery from stroke: Current concepts and future perspectives. Neurol Res Pract. 2020;2:17. [DOI] [PMC free article] [PubMed]
- [24].Yang S, Yao W, Siegler JE, Mofatteh M, Wellington J, Wu J, et al. Shortening door-to-puncture time and improving patient outcome with workflow optimization in patients with acute ischemic stroke associated with large vessel occlusion. BMC Emerg Med. 2022;22(1):136. [DOI] [PMC free article] [PubMed]
- [25].Chen Y, Nguyen TN, Wellington J, Mofatteh M, Yao W, Hu Z, et al. Shortening Door-to-Needle time by multidisciplinary collaboration and workflow optimization during the COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2022;31(1):106179. [DOI] [PMC free article] [PubMed]
- [26].Chen Y, Zhou S, Yang S, Mofatteh M, Hu Y, Wei H, et al. Developing and predicting of early mortality after endovascular thrombectomy in patients with acute ischemic stroke. Front Neurosci. 2022;16:1034472. [DOI] [PMC free article] [PubMed]
- [27].Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31:1192–6. [DOI] [PMC free article] [PubMed]
- [28].Tao C, Nogueira RG, Zhu Y, Sun J, Han H, Yuan G, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med. 2022;387(15):1361–72. [DOI] [PubMed]
- [29].Jovin TG, Li C, Wu L, Wu C, Chen J, Jiang C, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med. 2022;387(15):1373–84. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.