Abstract
Context
Paragangliomas located within the pericardium represent a rare yet challenging clinical situation.
Objective
The current analysis aimed to describe the clinical characteristics of cardiac paragangliomas, with emphasis on the diagnostic approach, genetic background, and multidisciplinary management.
Methods
Twenty-four patients diagnosed with cardiac paraganglioma (PGL) in Peking Union Medical College Hospital, Beijing, China, between 2003 and 2021 were identified. Clinical data was collected from medical record. Genetic screening and succinate dehydrogenase subunit B immunohistochemistry were performed in 22 patients.
Results
The median age at diagnosis was 38 years (range 11-51 years), 8 patients (33%) were females, and 4 (17%) had familial history. Hypertension and/or symptoms related to catecholamine secretion were present in 22 (92%) patients. Excess levels of catecholamines and/or metanephrines were detected in 22 (96%) of the 23 patients who have completed biochemical testing. Cardiac PGLs were localized with 131I-metaiodobenzylguanidine scintigraphy in 11/22 (50%), and 99mTc-hydrazinonicotinyl-tyr3-octreotide scintigraphy in 24/24 (100%) patients. Genetic testing identified germline SDHx mutations in 13/22 (59%) patients, while immunohistochemistry revealed succinate dehydrogenase (SDH) deficiency in tumors from 17/22 (77%) patients. All patients were managed by a multidisciplinary team through medical preparation, surgery, and follow-up. Twenty-three patients received surgical treatment and perioperative death occurred in 2 cases. Overall, 21 patients were alive at follow-up (median 7.0 years, range 0.6-18 years). Local recurrence or metastasis developed in 3 patients, all of whom had SDH-deficient tumors.
Conclusion
Cardiac PGLs can be diagnosed based on clinical manifestations, biochemical tests, and appropriate imaging studies. Genetic screening, multidisciplinary approach, and long-term follow-up are crucial in the management of this disease.
Keywords: cardiac paraganglioma, succinate dehydrogenase, catecholamines, cardiac imaging techniques, germline mutation
Paragangliomas (PGLs) are rare neuroendocrine tumors derived from extra-adrenal chromaffin cells. Symptoms of PGLs (eg, hypertension, headaches, palpitations, sweating), if present, result from excessive synthesis and secretion of catecholamines [1]. The pathogenesis of PGLs is strongly genetically driven. To date, up to 40% of patients with PGL are identified with germline mutations, while an additional 30% carry somatic variants [2, 3]. These PGLs can be categorized into 3 main molecular clusters with distinct phenotypes and clinical behavior [4]. In the era of personalized medicine, genetic testing layout is the foundation for diagnostic work-up, management and disease surveillance [4, 5].
Most primary PGLs arise along the sympathetic trunk or from parasympathetic ganglia in the head and neck region. Cardiac PGLs are particularly unusual, with approximately 200 cases reported in English literature. Fed by the coronary arteries, these tumors are biochemically active, highly vascular, and proximal to vital structures [6-8]. With such features, cardiac PGLs present management challenges for endocrinologists, cardiologists, surgeons, and anesthesiologists. The genetic background of cardiac PGLs remains largely unclear. Limited genetically screened cases suggest a high prevalence of hereditary PGL syndromes associated with germline mutations in genes encoding the succinate dehydrogenase (SDH) subunits [9, 10]. The SDH complex, localized on the inner mitochondrial membrane, plays key roles in both the tricarboxylic acid cycle and aerobic respiration [11]. Loss of heterozygosity in patients carrying germline inactivating mutations of the SDHx genes gives rise to hereditary PGL syndromes 1-5, and increases the risk for renal cell carcinoma, gastrointestinal stromal tumor, and pituitary adenoma [12-16]. We hereby report our 2-decade experience with cardiac PGLs, focusing on the diagnostic approach, genetic background, and multidisciplinary team (MDT) management.
Materials and Methods
Subjects
Patients diagnosed with PGLs within the pericardium at the Peking Union Medical College Hospital (PUMCH), Beijing, China, from January 1, 2003, to December 31, 2021, were enrolled in this retrospective observational study. Following the Endocrine Society guideline and the Chinese Society of Endocrinology consensus statement [4, 17], PGLs were diagnosed based on clinical manifestations, biochemical testing, imaging studies, and confirmed by postsurgical pathological examination. Metastasis was diagnosed with nuclear imaging evidence of PGL in locations where chromaffin cells are not usually present (eg, lungs, bones, lymph nodes). Surgical preparations and intraoperative techniques were reported previously [7]. Medical records were retrieved from an electronic hospital information system. Informed consent was obtained from all patients. The study was approved by the ethics committee of PUMCH.
Genetic Screening
Peripheral blood samples of patients were collected for germline mutation screening. The screening strategy and method had evolved to cover the major predisposing genes known at the time. For patients diagnosed between 2003 and 2008 (patients nos. 1-5), polymerase chain reaction–based sequencing was performed on the exons of SDHB and SDHD. For patients diagnosed between 2009 and 2016 (patients nos. 6-18), Sanger sequencing was performed to cover exons of SDHB, SDHC, SDHD, VHL, and RET. In addition, the NF1 gene was sequenced in presence of classical signs of the disease. For patients diagnosed between 2017 and 2021 (patients nos. 19, 21, 22, 24), next-generation sequencing among 18 predisposing genes (SDHA, SDHB, SDHC, SDHD, SDHAF2, VHL, RET, NF1, MAX, TMEM127, FH, KIF1B, BAP1, CDKN2A, KMT2D, MEN1, EGLN1, MERTK) was performed. Large deletions were tested with the multiplex ligation-dependent probe amplification technique. Three patients (patients nos. 4, 8, 11), without identified mutation in the initial screening, later received DNA microarray analysis of 20 predisposing genes (18 above-mentioned genes plus MET and MDH2). All sequencing and microarray analyses were completed in the Macro & Micro-test Bio-Tech (Beijing, China) laboratory. The sequence data that support the findings of this study have been deposited into CNGB Sequence Archive of China National GeneBank DataBase (CNGBdb) [18] with accession number CNP0004323.
SDHB Immunohistochemistry
Formalin-fixed and paraffin-embedded specimens were retrieved from the pathology archive of PUMCH. The detailed immunohistochemistry method has been reported previously [19]. In brief, immunohistochemical stain of SDHB (catalog # ab14714, RRID:AB_301432;1:400; Abcam, Cambridge, UK) was applied to sections after deparaffinization, rehydration, and heat-mediated antigen retrieval. Slides were scored positive in the presence of granular cytoplasmic staining of tumor cells. Slides showing negative staining for tumor cells and positive staining for stromal cells were considered true negative.
Data Analysis
Quantitative data are expressed as median with range. Qualitative data are expressed as exact counts with percentages. SPSS V.22.0 (IBM, Armonk, NY, USA) was used for statistical analysis. The Mann–Whitney test was applied for comparison of quantitative data. Fisher's exact test was applied for the comparison of qualitative data. P < .05 was considered to be statistically significant.
Results
A total of 24 patients were identified with cardiac PGLs. The median age at diagnosis was 38 years (range 11-51 years) and 3 (13%) patients were diagnosed under the age of 18. Eight patients (33%) were females. Hypertension (n = 22, 92%) was the most common clinical manifestation, followed by palpitation (n = 12, 50%), perspiration (n = 11, 46%), and headache (n = 10, 42%) (Table 1). Local symptoms including chest pain and shortness of breath were present in 4 (17%) patients. All the cases reported were identified as sporadic. Four (17%) patients had a family history of either pheochromocytoma or noncardiac PGL. There were no reports of cardiac PGLs in other family members. Comorbid nonchromaffin neoplasms including pituitary adenoma, meningioma, thyroid carcinoma, nonsmall cell lung carcinoma, and leukemia were recorded in 5 (21%) patients.
Table 1.
Clinical characteristics of patients with cardiac paraganglioma
| Patient no. | Gender | Age at Dx (years) | Year of Dx | Presentation | Family history | Multiple PPGLs | Nonchromaffin tumors | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTN | Headache | Palpitation | Perspiration | Chest pain | SOB | |||||||
| 1 | M | 16 | 2003 | + | + | − | − | − | − | − | − | − |
| 2 | M | 39 | 2003 | + | + | + | + | + | − | − | − | − |
| 3 | F | 35 | 2004 | + | + | + | + | − | − | − | HNPGLs | − |
| 4 | M | 22 | 2005 | + | + | + | + | − | − | − | − | − |
| 5 | M | 24 | 2007 | + | − | + | + | − | − | Metastatic PGL in 1 brother, PGL in daughter | Multiple CPGLs, bilateral PCCs, RPGLs | − |
| 6 | M | 49 | 2009 | + | − | − | − | − | − | − | HNPGL | papillary thyroid carcinoma, NSCLC |
| 7 | M | 44 | 2009 | + | − | − | − | − | − | Metastatic PCC in a first cousin, PGL in another first cousin | Multiple CPGLs, HNPGL, PCC, RPGL | pituitary microadenoma |
| 8 | M | 37 | 2010 | + | + | + | + | − | − | − | − | − |
| 9 | F | 48 | 2012 | + | + | − | + | − | − | − | − | − |
| 10 | F | 23 | 2012 | + | + | + | + | − | − | − | − | − |
| 11 | M | 11 | 2013 | + | − | + | + | − | − | − | − | − |
| 12 | M | 34 | 2014 | + | − | + | − | + | + | Metastatic PCC in 1 sister | HNPGL, RPGL | − |
| 13 | F | 50 | 2015 | + | − | + | − | − | − | − | − | − |
| 14 | F | 48 | 2015 | + | − | − | − | − | − | − | − | − |
| 15 | F | 32 | 2015 | − | − | − | − | − | − | − | HNPGLs | − |
| 16 | M | 48 | 2015 | + | + | + | + | − | − | − | HNPGLs, RPGL | − |
| 17 | M | 13 | 2015 | + | − | − | + | − | − | − | — | − |
| 18 | F | 21 | 2016 | + | + | − | − | − | − | − | RPGL | − |
| 19 | M | 44 | 2018 | + | + | + | − | − | − | − | RPGL, bladder PGL | − |
| 20 | M | 45 | 2019 | + | − | − | − | + | − | − | − | Pituitary microadenoma, chronic leukemia |
| 21 | M | 45 | 2019 | + | − | − | − | − | − | PPGLs in a first cousin once removed and a second cousin | Multiple CPGLs, bilateral PCCs | − |
| 22 | F | 32 | 2019 | − | − | − | − | + | − | − | − | Meningioma |
| 23 | M | 51 | 2020 | + | − | − | − | − | − | − | PCC, HNPGL, RPGL | − |
| 24 | M | 42 | 2021 | + | − | − | − | − | − | − | − | Thyroid nodule, pituitary microadenoma |
Abbreviations: CPGL, cardiac paraganglioma; Dx, diagnosis; F, female; HNPGL, head and neck paraganglioma; HTN, hypertension; M, male; NSCLC, nonsmall cell lung carcinoma; PCC, pheochromocytoma; PPGL, pheochromocytomas and paraganglioma; RGPL, retroperitoneal paraganglioma; SOB, shortness of breath.
Patients underwent a series of biochemical and imaging work-ups before the diagnosis of cardiac PGL was reached. Among the 23 patients who undertook 24-hour urinary catecholamines and/or plasma/urinary metanephrine tests, only 1 patient (4%) had a biochemically silent tumor (Table 2). Norepinephrine secretion was predominant in all of 22 patients with functional pheochromocytoma and paraganglioma (PPGLs), and additional excess secretion of epinephrine and dopamine was present in 7 (32%) and 14 (64%) patients, respectively. Chest computed tomography (CT) with enhancement, cardiac magnetic resonance imaging, and transthoracic echocardiography were performed to locate the cardiac PGLs (Fig. 1). Successful identification of cardiac PGLs was achieved in 20/20 (100%) cases for CT, 8/8 (100%) cases for magnetic resonance imaging, and 17/21 (81%) cases for transthoracic echocardiography. The cardiac PGLs showed abnormal uptake of 131I-metaiodobenzylguanidine (131I-MIBG) and 99mTc-hydrazinonicotinyl-tyr3-octreotide on scintigraphy in 11/22 (50%) and 24/24 (100%) cases, respectively (Fig. 2). Twelve patients underwent 18F-fluorodeoxyglucose (FDG) positron emission tomography with CT (PET/CT), all had positive uptake for the cardiac PGLs. In addition, the nuclear imaging studies recognized multifocal PPGLs in 11 (46%) patients. Coronary angiography was performed in 20 patients prior to surgery and successfully elucidated the blood supply in 19 (95%) cases.
Table 2.
Preoperative biochemical findings of patients with cardiac paraganglioma
| Urinea | Plasmab | ||||||
|---|---|---|---|---|---|---|---|
| Patient no. | NE (μg/24 hours) (16.69-40.65) | E (μg/24 hours) (1.74-6.42) | DA (μg/24 hours) (120.93-330.59) | NMN (μg/24 hours) (<1464) | MN (μg/24 hours) (<394) | NMN (nmol/L) (<0.9) | MN (nmol/L) (<0.5) |
| 1 | 973.92 | 1.84 | 1020.46 | NA | NA | NA | NA |
| 2 | 100.56 | 2.86 | 326.76 | NA | NA | NA | NA |
| 3 | 1243.96 | 16.03 | 926.89 | NA | NA | NA | NA |
| 4 | 2347.71 | 2.83 | 1023.94 | NA | NA | NA | NA |
| 5 | 226.29 | 10.25 | 451.76 | NA | NA | NA | NA |
| 6 | 297.92 | 4.26 | 450.68 | NA | NA | NA | NA |
| 7 | 127.68 | 0.78 | 364.96 | NA | NA | NA | NA |
| 8 | 294.58 | 9.9 | 332.54 | NA | NA | NA | NA |
| 9 | 939.6 | 5.55 | 742.89 | NA | NA | NA | NA |
| 10 | 721.29 | 5.09 | 879.43 | NA | NA | NA | NA |
| 11 | 231.94 | 1.98 | 492.17 | NA | NA | NA | NA |
| 12 | 259.2 | 2.88 | 362.88 | NA | NA | NA | NA |
| 13 | 35.55 | 3.56 | 327.06 | 310 | 57 | NA | NA |
| 14 | 90.68 | 3.49 | 60.68 | NA | NA | NA | NA |
| 15 | 117.09 | 5.85 | 189.69 | 3195 | 238 | NA | NA |
| 16 | 98.39 | 6.75 | 160.24 | 672 | 364 | NA | NA |
| 17 | 2180.38 | 6.77 | 2645.78 | NA | NA | NA | NA |
| 18 | 839.76 | 3.15 | 226.45 | NA | NA | NA | NA |
| 19 | 91.45 | 2.54 | 203.23 | 1171 | 184 | NA | NA |
| 20 | 243.18 | 9.35 | 374.12 | NA | NA | 2.62 | 0.26 |
| 21 | 566.09 | 4.04 | 323.48 | NA | NA | 4.29 | 0.12 |
| 22 | NA | NA | NA | NA | NA | NA | NA |
| 23 | 161.18 | 2.91 | 260.37 | NA | NA | 2.95 | 0.12 |
| 24b | 808.1 | 16 | 1499.9 | NA | NA | 5.29 | 0.15 |
Abbreviations: DA, dopamine; E, epinephrine; LC-MS/MS, liquid chromatography tandem mass spectrometry; MN, metanephrine; NA, not available; NE, norepinephrine; NMN, normetanephrine; ref, reference.
24-hour urinary catecholamines were measured by high-performance liquid chromatography-electrochemical detection (patients nos. 1 to 23) or LC-MS/MS (patient no. 24).
The reference ranges for 24-hour urinary catecholamines measured by LC-MS/MS are NE <76.9 μg, E < 11.0 μg, DA < 459.9 μg.
Figure 1.
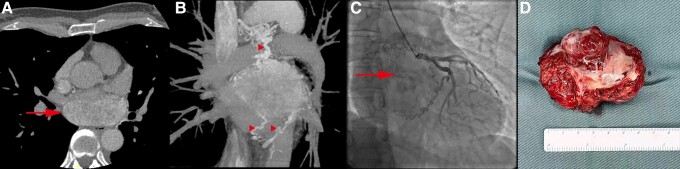
Computed tomography, coronary angiography, and surgical specimen of a cardiac paraganglioma. (A, B) chest computed tomography with contrast. (A) axial plane illustrates a mass (arrow) within the left atrium. (B) reconstruction imaging demonstrates the adjacency to pulmonary veins, surrounding tortuous vascularization (arrowheads), and central necrosis. (C) right anterior oblique view on coronary angiography: the tumor (arrow) receives a rich blood supply from branches of the left circumflex artery. (D) gross image of the resected tumor specimen: the tumor has a dusky appearance and part of the posterior wall of the left atrium was resected simultaneously.
Figure 2.
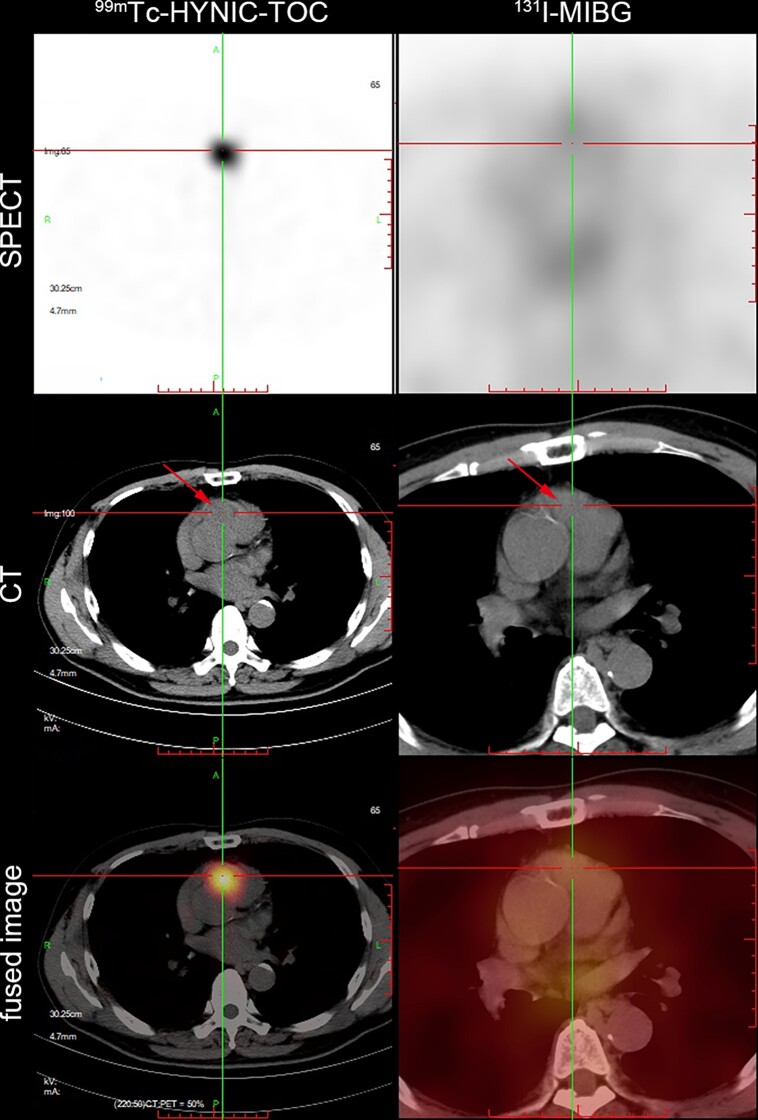
99mTc-hydrazinonicotinyl-tyr3-octreotide (99mTc-HYNIC-TOC) and 131I-metaiodobenzylguanidine (131I-MIBG) single photon emission computed tomography (SPECT)/computed tomography (CT) in a patient with cardiac paraganglioma. Intense 99mTc-HYNIC-TOC, but not 131I-MIBG uptake correlated with the soft-tissue mediastinal mass (arrow) at the root of aorta and pulmonary trunk.
Initially, multiple endocrine neoplasia was suspected when comorbid pituitary adenoma or thyroid nodule was identified. With the popularization of genetic screening, most cases with family history and/or suspected multiple endocrine neoplasia were later identified as hereditary PGL syndromes. Eventually, a total of 22 patients underwent sequencing of the major predisposing genes known at the time. Hereditary PGL syndromes were identified in 13 (59%) patients (Table 3). Among them, 4 were diagnosed with PGL syndrome type 1 (SDHD mutation), 2 with PGL syndrome type 3 (SDHC mutation), 5 with PGL syndrome type 4 (SDHB mutation), and 2 with PGL syndrome type 5 (SDHA mutation). No pathological variants were identified in other major predisposing genes. Clinical characteristics except for the presence of family history were not significantly different between SDHx mutation carriers and patients without identified mutations (Table 4).
Table 3.
The genetics, pathology, and outcome of patients with cardiac paraganglioma
| Patient no. | Germline mutation | Tumor location | Tumor size (cm) | Ki-67 (%) | SDHB IHC | Follow-up (years) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | SDHB c.757delT | Root of aorta | 4.5 × 3.1 | 4.2 | − | 18 | Recurrence in POY 17, received a second operation |
| 2 | SDHB c. 170delA | Interatrial septum | 6.0 × 7.0 × 4.0 | 2 | − | 14 | Incomplete resection in first operation, received a second operation in POY 12 |
| 3 | NI | Root of aorta and MPA | 4.5 × 5.0 × 4.2 | 1.4 | NA | 10 | Alive without disease |
| 4 | NI | Root of aorta | 6.7 × 5.5 | >3 | − | 15 | Recurrence and metastasis in POY 4 |
| 5 | SDHD c.128G>A | Right AVG | 4.8 × 4.8 | 1.7 | − | 10 | Alive without disease |
| Root of aorta and MPA | 4.5 × 3.0 | ||||||
| 6 | SDHC c. 497T>G | IAG | 4.0 × 3.4 × 3.0 | 2.9 | − | 11 | Alive without disease |
| 7 | SDHD c.177_181delCAAGG | Root of aorta | 8.0 × 5.5 | 1 | − | 9 | Alive without disease |
| Right AVG | 6.0 × 4.0 | ||||||
| 8 | SDHA c.2T > C | Root of aorta and MPA | 5.0 × 4.3 × 3.5 | 10 | − | 10 | Alive without disease |
| 9 | NI | Root of aorta and MPA | 3.0 × 3.0 | 1 | − | 10 | Alive without disease |
| 10 | SDHC c. 107_108delAA | Right AVG | 7.5 × 6.5 | <1 | − | 9 | Alive without disease |
| 11 | SDHA c.508C>A | Root of aorta | 7.4 × 6.1 | 2 | − | 7 | Alive without disease |
| 12 | SDHB c. 689G>A | Root of aorta and MPA | 5.5 × 3.4 | 3 | − | 6 | Recurrence and metastasis in POY 6, deceased due to pneumonia |
| 13 | NI | Root of aorta and MPA | 7.5 × 6.4 | 10 | − | 7 | alive without disease |
| 14 | NI | Root of aorta | 5.0 × 4.0 | 2 | − | Not applicable | Deceased due to hemorrhage on POD 1 |
| 15a | SDHD c.277_279delT | RA | 2.5 × 2.0 × 3.8 | NA | − | 6 | Alive with unresected CPGL |
| 16 | NI | Root of MPA | 4.0 × 3.0 | <1 | + | 2 | alive without disease |
| 17 | NI | Root of aorta and MPA | 6.5 × 4.1 × 5.1 | 3 | − | 4 | alive without disease |
| 18 | NI | Right AVG | 4.0 × 3.0 | 3 | − | 1 | Alive without disease |
| 19 | SDHB c.649C>T | LA | 3.5 × 2.5 × 2.0 | 2 | − | 2 | Alive with unresected RPGL |
| 20 | NA | Left AVG | 2.5 × 2 × 1.8 | 1 | + | 1 | alive without disease |
| 21 | SDHD c.278_280delATT | Root of aorta | 7.0 × 6.5 × 3.8 | 5 | + | 0.6 | Alive without disease |
| RVOT | 5.5 × 3 × 2.8 | ||||||
| 22 | SDHB c.175C>T | Root of aorta | 6.5 × 5.5 × 3.2 | 20 | NA | 1 | Alive without disease |
| 23 | NA | Root of aorta and MPA | 4.7 × 2.5 × 2.6 | 2 | + | 1.8 | Alive without disease |
| 24 | NI | LA | 6.0 × 4.5 × 3.0 | 3 | + | Not applicable | Deceased due to LA thrombosis in POM 3 |
Abbreviations: AVG, atrioventricular groove; CPGL: cardiac paraganglioma; IAG, interatrial groove; IHC, immunohistochemistry; LA, left atrium; MPA, main pulmonary artery; NA, not available; NI, Not identified; POD, postoperation day; POM, postoperation month; POY, postoperation year; RA, right atrium; RGPL, retroperitoneal paraganglioma; RVOT, right ventricular outflow tract; SDHA, succinate dehydrogenase subunit A; SDHB, succinate dehydrogenase subunit B; SDHC, succinate dehydrogenase subunit C; SDHD, succinate dehydrogenase subunit D.
Patient no. 15 did not receive resection of the cardiac PGL, the location and size of the tumor were identified by imaging studies, SDHB IHC was performed on her head and neck paraganglioma specimen.
Table 4.
Characteristics of cardiac paragangliomas in patients with or without germline SDHx mutation
| Germline mutation | |||
|---|---|---|---|
| SDHx (n = 13) | NI (n = 9) | P | |
| Gender, n (%) | |||
| Female | 3 (23) | 5 (56) | .187 |
| Age (median, range) | 34 (11-49) | 42 (13-50) | .471 |
| Presentation, n (%) | |||
| HTN | 11 (85) | 9 (100) | .494 |
| Headache | 5 (39) | 5 (56) | 0.666 |
| Palpitation | 8 (62) | 4 (44) | 0.666 |
| Perspiration | 6 (46) | 5 (56) | 1 |
| Chest pain | 3 (23) | 0 (0) | .24 |
| Multiple PPGLs, n (%) | 7 (54) | 3 (33) | .415 |
| Metastasis, n (%) | 1 (8) | 1 (11) | 1 |
| Family history, n (%) | 4 (31) | 0 (0) | .002 |
| Urinary CA elevationa, n (%) | |||
| Norepinephrine | 12 (100) | 8 (89) | .429 |
| Epinephrine | 2 (17) | 4 (44) | .331 |
| Dopamine | 8 (67) | 5 (56) | .673 |
| MTD (cm) (median, range) | 5.5 (3.5-8.0) | 5.0 (3.0-7.5) | .601 |
| Ki-67 index ≥ 3%a, n (%) | 5 (42) | 5 (56) | .67 |
Abbreviations: CA, catecholamine; HTN, hypertension; MTD, maximum tumor diameter; NI, not identified; PPGLs, pheochromocytomas and paragangliomas; SDHx, succinate dehydrogenase subunits.
Urinary CA and Ki-67 index were measured in 12 patients with SDHx mutation.
After diagnosis and clinical evaluation, an MDT discussion was held in each case to decide the management plan. The MDT regularly consisted of clinicians specializing in endocrinology, cardiology, cardiac surgery, anesthesiology, radiology, intensive care, etc. Ear, nose, and throat surgeons, vascular surgeons, and urologists were also involved when faced with multifocal PPGLs. Hormone secretion, anatomical interrelationship, and blood supply of the cardiac PGL, as well as the basic condition, cardiac function, and comorbidities of the patient were discussed in detail. Thoughts and concerns from the patient him/herself were also heard by the team, before making an integrated plan covering medical preparation, surgical approach, anesthesiologic precautions, and postoperation care.
Surgical resections of cardiac PGLs were performed in 23 (96%) patients. Patient no. 15, who had multiple head and neck PGLs and a cardiac PGL, received surgery for a carotid body tumor and a glomus jugulare tumor and postponed her cardiac surgery for personal reasons. Surgical preparation involved administration of an alpha blocker (phenoxybenzamine, final dose 0.5-1 mg/kg·day, n = 22) with or without beta blockers (metoprolol or bisoprolol, n = 9) or ivabradine (n = 1). Concomitant resection of the great vessels and coronary artery bypass grafting was necessitated in 14 (61%) and 9 (39%) patients, respectively. Cardiac autotransplantation was performed for 1 patient (patient no. 24) with left atrium PGL due to the posterior location and difficulty of surgical exposure.
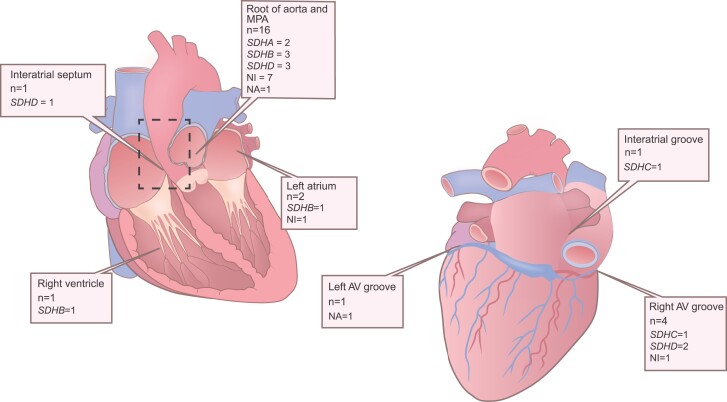
Among the 27 cardiac PGLs from the 24 patients, the majority (n = 16, 59%) were located at the root of the aorta and/or pulmonary trunk (Table 3 and Fig. 3). The median maximum tumor diameter was 5.0 cm (range 2.5-8.0 cm). All cardiac PGL specimens stained positive for chromogranin A. Ki-67 index was 3% or above in tumor samples from 9/23 (39%) patients. Expression of SDHB was assessed in specimens from 22 patients (Fig. 4). Loss of SDHB expression within the tumor cells was identified in 11/12 patients with SDHx mutation, 6/8 patients without identified mutation, and 0/2 patients with unknown genetic backgrounds. The total prevalence of negative SDHB immunostaining was 77%.
Figure 3.
Distribution of cardiac paraganglioma. AV, atrioventricular; MPA, main pulmonary artery; NA, not available; NI, not identified; SDHA, succinate dehydrogenase subunit A; SDHB, succinate dehydrogenase subunit B; SDHC, succinate dehydrogenase subunit C; SDHD, succinate dehydrogenase subunit D.
Figure 4.
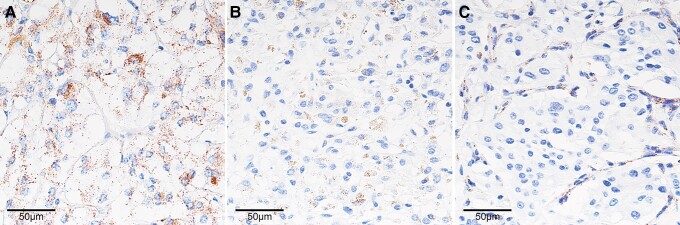
Succinate dehydrogenase subunit B (SDHB) immunohistochemistry in cardiac paragangliomas. (A) Normal adrenal medulla (positive control) showing cytoplasmic granular staining. (B) SDHB-positive cardiac paraganglioma: similar cytoplasmic granular staining within tumor cells. (C) SDHB-negative cardiac paraganglioma: SDHB staining lost within tumor cells but preserved in the stromal cells. Original magnification: ×400.
Surgical complications led to the death of 2 (9%) patients: patient no. 13 died on postoperational day 1 due to massive hemorrhage despite emergent surgical exploration; patient no. 24 died 3 months after surgery due to left atrial thrombosis despite oral anticoagulant therapy. Among the remaining 22 patients, 21 were alive at follow-up (median 7.0 years, range 0.6-18 years). After surgery, symptoms of the classic triad, as well as chest pain and shortness of breath, were eliminated in 15/16 (94%) patients, while the hypertensive state persisted in 4/20 (20%) patients. Levels of catecholamines and their metabolites normalized in 15/19 (79%) and improved in 4/19 (21%) patients.
Redo operations were performed in 2 patients: patient no. 1 for local recurrence 17 years after initial surgery, and patient no. 2 for growth of residual tumor 12 years after initial surgery. Metastasis to bone and/or lungs developed in patients no. 4 and 12, 4 and 6 years after tumor resection, respectively. As both patients had negative results for MIBG scintigraphy, targeted radiotherapy was not feasible. Patient no. 4 received an alpha blocker for medical therapy. He was free of clinical symptoms and had normal blood pressure at the most recent follow-up. Patient no. 12 died due to pulmonary infection before discussion on a further management plan.
Discussion
As cardiac PGLs are particularly rare, current knowledge of these tumors is largely based on summarization of single case reports [10, 20]. Case series of the disease are few and have reported only limited number of patients, many of whom were diagnosed before the maturation of genetic screening [21, 22]. We hereby report our single center experience on the evaluation and management of the disease learned from 24 patients diagnosed in the past 2 decades.
Over half of patients with cardiac PGLs in the current study presented with classical symptoms of excess release of catecholamines. The remaining patients were generally discovered during investigation of suspected secondary hypertension. Notably, accompanying chest pain and/or dyspnea, when present, might help to locate the tumor. In rare cases, these local symptoms can be the sole manifestation, leading to possible detours in the diagnostic process [23].
PGLs derived from sympathetic nerve system are generally biochemically active and predominantly secret norepinephrine and its metabolites. As expected, all but 1 patient who underwent biochemical analysis in this cohort had an elevated level of norepinephrine and/or normetanephrine. Remarkably, cosecretion of dopamine was detected in over 60% of the patients. Such a biochemical profile has often been documented in patients with SDHx mutations [24].
Various imaging methods are available for the initial localization of cardiac PGLs. Thorax CT with or without contrast may identify cardiac PGLs in most cases; however, the tumor might be missed if only the abdominal and pelvic region is scanned. In such cases, functional imaging studies may provide guidance for subsequent localization. As the likelihood of multiplicity is high, the value of functional imaging modalities also lay in their simultaneous recognition of multifocal PPGLs throughout the body. In the current study, the octreotide scan and FDG PET/CT have shown remarkable advantages over the conventional MIBG scan in terms of sensitivity. This finding should be viewed in the context of the study population, as poor performance of MIBG scintigraphy has been reported among SDHx variation carriers [25]. Nevertheless, the MIBG scan still provides useful information regarding radioactive therapy options with metastatic disease. The more sensitive octreotide scintigraphy or FDG-PET/CT is suggested in cases when the MIBG scan provides a negative result.
When a PGL has been located within the pericardium, further imaging work-up is usually required to determine the precise location of the tumor, the adjacency to cardiac structures and great vessels, and the feeding vessel. CT with contrast provides excellent spatial resolution, and the reconstruction techniques help delineate the mass on multiple planes. Cardiac magnetic resonance imaging has an advantage for tissue characterization and provides hemodynamic information. Transthoracic echocardiography also gives understanding of the physiology, but with its limited field of view might miss PGLs located at the root of the aorta and pulmonary trunk [26]. An experienced examiner knowing of the possibility of pericardial masses and using a transesophageal scan may reduce the false negative rate for echocardiography [10]. As most of the cardiac PGLs derive blood supply from the coronary arteries, and surgical revascularization is frequently required, we now routinely perform coronary angiography for suspected cardiac PGLs.
The current study further strengthened the proposed association between cardiac PGLs and germline SDHx mutations [9, 27]. Genetic screening identified SDHx mutations in over 60% of the patients, and SDHB immunohistochemistry demonstrated SDH deficiency in approximately three-quarters. These rates were considerably higher than the prevalence of SDHx mutations among nonselected patients with PPGLs (20-30%) [4, 28], or the prevalence of SDH deficiency among noncardiac PGLs in our center (40%) [19].
As expected, SDHB and SDHD variations were most frequently encountered among our patients. Interestingly, the relatively rare SDHA and SDHC mutations were also identified. SDHA-related tumors were recognized in only 3% of patients with sporadic PPGLs, and were not associated with tumor location or biological behavior [28]. A recent long-term observation raises concern, as 3/6 of the SDHA-related PPGLs developed metastasis or local invasion years after surgery, suggesting the need for close follow-up [29]. The SDHC variations were mostly recognized in patients with head and neck PGLs and seldomly associated with pheochromocytomas [30]. The current study adds 2 more cases to the 9 SDHC-related cardiac PGLs reported in the literature [9, 31-36]. Considering the limited total number of cardiac PGL cases reported with genetic background [20], the presence of SDHC variations might not be uncommon with this specific location. Based on the high prevalence and potential navigational value for management and follow-up, we recommend direct genetic sequencing for the SDHx genes or a stepwise screen strategy guided by SDHB immunostaining in all cardiac PGL cases.
Complete surgical resection remains the cornerstone of the treatment of cardiac PGLs. In our series, we experienced a 30-day mortality of 1/23 (4%), considerably lower than the reported mortality of 10% to 14% in the contemporary era [8, 37]. As has been pointed out, preoperative medical preparation, intraoperative anesthesia management, and postoperative intensive care set the stage for these delicate yet often challenging surgical procedures [37]. Our experience suggests the MDT model helps to coordinate specialties in the management plan and is particularly valuable for patients with (1) synchronously diagnosed multiple functional PPGLs; (2) local recurrence or previous incomplete resection; and (3) cardiac insufficiency due to underlying disease or catecholamine induced cardiomyopathy.
Three adolescent patients were included in the current study, with the youngest diagnosed at the age of 11. In the literature, PPGLs detected before the age of 18 years constitute 10% to 20% of all cases, and have been associated with cluster 1 (VHL, SDHx, FH, etc.) mutations and higher prevalence of recurrence and metastasis [38, 39]. Apart from concerns over genetic counseling and life-long follow-up, we suggest cardiac PGLs in the pediatric setting should be handled with greater care as (1) the spectrum of primary cardiac tumors is different from that in adults and may complicate the differential diagnosis; (2) trade-off between complete tumor resection and preservation of vital structures require wisdom and courage; and (3) in addition to medical preparation, psychological preparation is often needed both for the child and the parents.
The clinical outcome of patients surviving the perioperative period is generally satisfactory. Recurrence and metastasis years after the initial surgery emphasize the need for life-long biochemical and imaging surveillance [40]. The hypertensive state might also persist after normalization of catecholamine levels, therefore requiring medical treatment and monitoring. For patients with germline SDHx mutations, comorbid renal cell carcinoma and gastrointestinal stromal tumor should be screened, and family members should be referred to genetic counselors [41].
The rarity of cardiac PGLs has hindered development of consensus over management protocol. The current study represents an attempt to gather comprehensive clinical information from by far the largest cohort from a single center. Our cohort is further characterized by low hospital mortality, long-term follow-up, and covering of complicated and juvenile cases. Findings from our series suggest that for patients suspected of PGL who have negative results on routine imaging work-up, cardiac localization should be incorporated in the following evaluation. The high prevalence of hereditary PGL syndromes marks the value of genetic testing and SDH immunohistochemistry, as such information allows personalized strategy for tumor screening and postsurgical follow-up. The MDT approach helps improve treatment efficiency and patient care, especially in complicated cases. With total resection and appropriate perioperative management, patients may expect favorable outcomes.
Acknowledgments
The authors thank Ms. Jun Jiang for technical assistance regarding genetic screening.
Abbreviations
- CT
computed tomography
- FDG
fluorodeoxyglucose
- MDT
multidisciplinary team
- MIBG
metaiodobenzylguanidine
- PET/CT
positron emission tomography with CT
- PGL
paraganglioma
- PPGL
pheochromocytoma and paraganglioma
- PUMCH
Peking Union Medical College Hospital
- SDH
succinate dehydrogenase
- SDHA
succinate dehydrogenase subunit A
- SDHAF2
succinate dehydrogenase assembly factor 2
- SDHB
succinate dehydrogenase subunit B
- SDHC
succinate dehydrogenase subunit C
- SDHD
succinate dehydrogenase subunit D
Contributor Information
Chuan Shi, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China; Department of Internal Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Jian-Zhou Liu, Department of Cardiac Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Zheng-Pei Zeng, Email: zengzhengpei@aliyun.com, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Qi Miao, Email: miaoqipumc@hotmail.com, Department of Cardiac Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Li-Gang Fang, Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Shi Chen, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Fan Ping, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Hao Sun, Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Lin Lu, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Li-Bo Chen, Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Yong Fu, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Da-Chun Zhao, Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Chun-Hua Yu, Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Rui-Zhi JiaJue, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Xi Wang, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Xing-Rong Liu, Department of Cardiac Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Guo-Tao Ma, Department of Cardiac Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Chao-Ji Zhang, Department of Cardiac Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Hui Pan, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Hong-Bo Yang, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Yi-Ning Wang, Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Ming Li, Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Fang Li, Department of Nuclear Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Zhu-Jun Shen, Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Zhi-Yong Liang, Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Xiao-Ping Xing, Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Wen-Ling Zhu, Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China.
Funding
This work was supported by the National Key Research and Development Program of China during the “10th Five-Year Plan” (grant number 2004BA720A29).
Disclosures
The authors declare that they have no conflict of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Neumann HPH, Young WF, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381(6):552‐565. [DOI] [PubMed] [Google Scholar]
- 2. Favier J, Amar L, Gimenez-Roqueplo A-P. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101‐111. [DOI] [PubMed] [Google Scholar]
- 3. Fishbein L, Leshchiner I, Walter V, et al. . Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nölting S, Bechmann N, Taieb D, et al. . Personalized management of pheochromocytoma and paraganglioma. Endocr Rev. 2022;43(2):199‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lenders JWM, Duh Q-Y, Eisenhofer G, et al. . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915‐1942. [DOI] [PubMed] [Google Scholar]
- 6. Khan MF, Datta S, Chisti MM, Movahed MR. Cardiac paraganglioma: clinical presentation, diagnostic approach and factors affecting short and long-term outcomes. Int J Cardiol. 2013;166(2):315‐320. [DOI] [PubMed] [Google Scholar]
- 7. Liu XP, Miao Q, Liu XR, Zhang CJ, Ma GT, Liu JZ. Outcomes of surgery for functional cardiac paragangliomas: a single-center experience of 17 patients. J Thorac Cardiovasc Surg. 2019;157(4):1556‐1564. [DOI] [PubMed] [Google Scholar]
- 8. Chan EY, Ali A, Umana JP, et al. . Management of primary cardiac paraganglioma. J Thorac Cardiovasc Surg. 2020;164(1):158‐166. [DOI] [PubMed] [Google Scholar]
- 9. Martucci VL, Emaminia A, del Rivero J, et al. . Succinate dehydrogenase gene mutations in cardiac paragangliomas. Am J Cardiol. 2015;115(12):1753‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang JG, Han J, Jiang T, Li YJ. Cardiac paragangliomas. J Card Surg. 2015;30(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 11. Sun F, Huo X, Zhai Y, et al. . Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121(7):1043‐1057. [DOI] [PubMed] [Google Scholar]
- 12. Baysal BE, Ferrell RE, Willett-Brozick JE, et al. . Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287(5454):848‐851. [DOI] [PubMed] [Google Scholar]
- 13. Hao H-X, Khalimonchuk O, Schraders M, et al. . SDH5, A gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325(5944):1139‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26(3):268‐270. [DOI] [PubMed] [Google Scholar]
- 15. Astuti D, Latif F, Dallol A, et al. . Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69(1):49‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burnichon N, Brière J-J, Libé R, et al. . SDHA Is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19(15):3011‐3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adrenal group, Chinese Society of Endocrinology . Expert consensus on the diagnosis and treatment of pheochromocytoma and paraganglioma. Chin J Endocrinol Metab. 2016;32(3):181‐187. [Google Scholar]
- 18. Chen FZ, You LJ, Yang F, et al. . CNGBdb: China national GeneBank DataBase. Hereditas. 2020;42(08):799‐809. [DOI] [PubMed] [Google Scholar]
- 19. Shi C, Zeng ZP, Zhao DC, et al. . Application of SDHB and SDHC immunohistochemistry in the differentiation of malignant and benign pheochromocytoma and paraganglioma. Chin J Endocrinol Metab. 2018;34(6):472‐478. [Google Scholar]
- 20. Tella SH, Jha A, Taïeb D, Horvath KA, Pacak K. Comprehensive review of evaluation and management of cardiac paragangliomas. Heart. 2020;106(16):1202‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamilton BH, Francis IR, Gross BH, et al. . Intrapericardial paragangliomas (pheochromocytomas): imaging features. AJR Am J Roentgenol. 1997;168(1):109‐113. [DOI] [PubMed] [Google Scholar]
- 22. Brown ML, Zayas GE, Abel MD, Young WF, Schaff HV. Mediastinal paragangliomas: the Mayo Clinic experience. Ann Thorac Surg. 2008;86(3):946‐951. [DOI] [PubMed] [Google Scholar]
- 23. Yadav PK, Baquero GA, Malysz J, Kelleman J, Gilchrist IC. Cardiac paraganglioma. Circ Cardiovasc Interv. 2014;7(6):851‐856. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhofer G, Lenders JWM, Timmers H, et al. . Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57(3):411‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gimenez-Roqueplo A-P, Caumont-Prim A, Houzard C, et al. . Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.EVA investigators. J Clin Endocrinol Metab. 2013;98(1):E162‐E173. [DOI] [PubMed] [Google Scholar]
- 26. Li L, Zhu W, Fang L, et al. . Transthoracic echocardiographic features of cardiac pheochromocytoma: a single-institution experience. Echocardiography. 2012;29(2):153‐157. [DOI] [PubMed] [Google Scholar]
- 27. Ghayee HK, Havekes B, Corssmit EPM, et al. . Mediastinal paragangliomas: association with mutations in the succinate dehydrogenase genes and aggressive behavior. Endocr Relat Cancer. 2009;16(1):291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korpershoek E, Favier J, Gaal J, et al. . SDHA Immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96(9):E1472‐E1476. [DOI] [PubMed] [Google Scholar]
- 29. Tufton N, Ghelani R, Srirangalingam U, et al. . Mutated paragangliomas may be at high risk of metastasis. Endocr Relat Cancer. 2017;24(7):L43‐L49. [DOI] [PubMed] [Google Scholar]
- 30. Schiavi F, Boedeker CC, Bausch B, et al. . Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294(16):2057‐2063. [DOI] [PubMed] [Google Scholar]
- 31. Illouz F, Pinaud F, De Brux J-L, Mirebeau-Prunier D, Rodien P. Long-delayed localization of a cardiac functional paraganglioma with SDHC mutation. Ann Intern Med. 2012;157(3):222‐223. [DOI] [PubMed] [Google Scholar]
- 32. Tracy JC, Wein RO. Intrapericardial paraganglioma associated with succinate dehydrogenase complex subunit C mutation syndrome. Head Neck. 2013;35(8):E251‐E253. [DOI] [PubMed] [Google Scholar]
- 33. Millar AC, Mete O, Cusimano RJ, et al. . Functional cardiac paraganglioma associated with a rare SDHC mutation. Endocr Pathol. 2014;25(3):315‐320. [DOI] [PubMed] [Google Scholar]
- 34. Bickmann JK, Sollfrank S, Schad A, et al. . Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: lessons from three unrelated cases with an identical germline mutation (p.Arg133*). J Clin Endocrinol Metab. 2014;99(3):E489‐E496. [DOI] [PubMed] [Google Scholar]
- 35. Farquhar HE, Wong M, Puri G, Sinha A. A series of two patients with cardiac paragangliomas. AACE Clin Case Rep. 2020;6(4):e174‐e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guenthart BA, Trope W, Keeyapaj W, et al. . Intracardiac paragangliomas: surgical approach and perioperative management. Gen Thorac Cardiovasc Surg. 2021;69(3):555‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramlawi B, David EA, Kim MP, et al. . Contemporary surgical management of cardiac paragangliomas. Ann Thorac Surg. 2012;93(6):1972‐1976. [DOI] [PubMed] [Google Scholar]
- 38. Pamporaki C, Hamplova B, Peitzsch M, et al. . Characteristics of pediatric vs adult pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2017;102(4):1122‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Tersant M, Généré L, Freyçon C, et al. . Pheochromocytoma and paraganglioma in children and adolescents: experience of the French Society of Pediatric Oncology (SFCE). J Endocr Soc. 2020;4(5):bvaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamidi O, Young WF, Iñiguez-Ariza NM, et al. . Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. 2017;102(9):3296‐3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amar L, Pacak K, Steichen O, et al. . International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat Rev Endocrinol. 2021;17(7):435‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.