Abstract
Chronic liver disease (CLD) entails elevated risk of COVID-19 severity and mortality. The effectiveness of the booster dose of inactivated SARS-CoV-2 vaccine in stimulating antibody response in CLD patients is unclear. Therefore, we conducted a cross-sectional study involving 237 adult CLD patients and 170 healthy controls (HC) to analyze neutralizing antibodies (NAbs) against SARS-CoV-2 prototype and BA.4/5 variant, anti-receptor binding domain (RBD) IgG, and total anti-SARS-CoV-2 antibodies. Serum levels of the total anti-SARS-CoV-2 antibodies, anti-RBD IgG and inhibition efficacy of NAbs were significantly elevated in CLD patients after the booster dose compared with the pre-booster dose, but were relatively lower than those of HCs. Induced humoral responses decreased over time after booster vaccination. The neutralization efficiency of the serum against BA.4/5 increased but remained below the inhibition threshold. All four SARS-CoV-2 antibodies, including total anti-SARS-CoV-2 antibodies, anti-RBD IgG and NAbs against prototype and BA.4/5, were lower in patients with severe CLD than those with non-severe CLD. After booster shot, age and time after the last vaccine were the risk factors for seropositivity of NAb against BA.4/5 in CLD patients. Additionally, white blood cell counts and hepatitis B core antibodies were the protective factors, and severe liver disease was the risk factor associated with seropositivity of total anti-SARS-CoV-2 antibodies. Overall, our data uncovered that antibody responses were improved in CLD patients and peaked at 120 days after the booster vaccines. All antibodies excepting total anti-SARS-CoV-2 antibodies declined after peak. CLD patients exhibited impaired immunologic responses to vaccination and weakened NAbs against BA.4/5, which hindered the protective effect of the booster shot against Omicron prevalence. Cellular immune responses should be further evaluated to determine the optimal vaccine regimen for CLD patients.
Keywords: Chronic liver disease (CLD), SARS-CoV-2 inactivated vaccines, Booster vaccination, Antibody response, Immune response
Highlights
-
•
Chronic liver disease (CLD) patients exhibit good immune responses to booster inactivated SARS-CoV-2 vaccine.
-
•
Omicron BA.4/5 can evade the immune response elicited by the booster shot.
-
•
HBsAg levels positively correlate with neutralizing antibodies and anti-RBD IgG.
-
•
Decreased levels of B and NK cells may explain lowered antibodies in CLD patients.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused serious challenges to public health. More evidence revealed that individuals with chronic liver disease (CLD) are at an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and are vulnerable to hospitalization, severe diseases and death after infection (Mistry et al., 2021). Severe liver diseases (SLD) patients, such as those with cirrhosis, hepatobiliary malignancies, and those who have undergone liver transplantation, are more likely to develop adverse outcomes (Cornberg et al., 2021).
SARS-CoV-2 vaccination establishes herd immunity and reduces severe disease rates and mortality risk by triggering the humoral and cellular immune responses against the virus (Bar-On et al., 2021; Liu et al., 2021). According to the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver expert consensus statement, CLD patients are among the priority group to receive SARS-CoV-2 vaccines (Cornberg et al., 2021; Fix et al., 2021). Researchers have proved that the liver-related adverse effects associated with the vaccines, such as autoimmune hepatitis or other liver injuries, were very rare, and the vaccination was safe for patients with CLD. The overall benefits of vaccination outweigh the risks of these rare side effects (Bril et al., 2021; Chow et al., 2022; Efe et al., 2022; Lleo et al., 2022; Palla et al., 2022). Several studies focusing on the immunogenicity of SARS-CoV-2 vaccination in CLD patients reported that the CLD patients developed a lower protective immune response after two doses of inactivated, mRNA or vector-based SARS-CoV-2 vaccines, compared to healthy individuals (Ai et al., 2022; Bakasis et al., 2022; Duengelhoef et al., 2022; Ruether et al., 2022; Thuluvath et al., 2021; Toutoudaki et al., 2023; Xiang et al., 2021). After two shots of the inactivated vaccine, the seropositivity rates of neutralizing antibodies were higher in CLD patients with Child-Pugh A classification than those with B and C (Wang et al., 2022). However, no significant difference in the humoral or cellular responses was observed in patients with or without cirrhosis after a standardized inoculation schedule of mRNA, adenovirus or inactivated vaccine (Ai et al., 2022; He et al., 2022; Thuluvath et al., 2021). Inactivated SARS-CoV-2 vaccine is widely used in China and other countries worldwide. However, there are few reports on the humoral immunological landscape following the administration of the booster inactivated vaccine and the risk factors associated with poor immune responses among CLD patients in China.
The SARS-CoV-2 variants of concern (VOC) resulted in several waves of infections due to their increased mutations and transmissibility (Mistry et al., 2021), with Omicron variants BA.4/5 being the current dominant epidemic lineage (Shrestha et al., 2022). The BA.4/5 variants can evade the neutralizing antibody, resulting in breakthrough infections in healthy volunteers receiving three doses of AstraZeneca or Pfizer vaccines or convalescents infected with other Omicron subvariants (Hachmann et al., 2022; Tuekprakhon et al., 2022). The current SARS-CoV-2 vaccines are designed to elicit neutralizing antibodies against the spike protein from the SARS-CoV-2 prototype strain (Mistry et al., 2021). For example, the CoronaVac vaccine (an inactivated vaccine widely used in China) targets 10 representative strains of SARS-CoV-2, two of which (CN1 and OS1) are closely associated with the original strains (2019-nCoV-BetaCoV/Wuhan/WIV04/2019 and EPI_ISL_412973) (Gao et al., 2020). The mutations in the spike protein receptor binding domain (RBD) increase the transmission and virulence of VOC, enabling them to evade the neutralizing antibodies induced by SARS-CoV-2 infections or vaccine shots, thus reducing the efficacy of current vaccines (Mistry et al., 2021). There is insufficient evidence about the immunogenicity of inactivated SARS-CoV-2 vaccines against Omicron subvariants in CLD patients. Thus, this study, aimed to analyze the immune responses induced by the prime-boost inactivated vaccine against SARS-CoV-2 and the evasive subvariants BA.4/5 among CLD patients in China.
2. Material and methods
2.1. Participants
This study, included all patients (>18 years) with either clinically or pathologically diagnosed CLD who visited the Fifth Hospital of Shijiazhuang from 8th July to 29th August 2022, received two or three doses of inactivated vaccines, and volunteered to participate in this study were included. A booster vaccination is a third dose of the same vaccine administered to people who have completed two doses of inactivated vaccine six months after the second dose. The inactivated SARS-CoV-2 vaccines include BBIBP-CorV (Beijing Institute of Biological Products Co., Ltd., China), CoronaVac (Sinovac Life Sciences Co., Ltd., China), and WIBP-CorV (Beijing Institute of Biological Products Co.,Ltd., China). In this study, CLD included viral hepatitis B/C, nonalcoholic fatty liver disease, alcoholic liver disease, autoimmune hepatitis, primary biliary cholangitis, and liver cirrhosis. The SLD included compensated cirrhosis, decompensated cirrhosis and liver cancer. Patients who were pregnant or previously tested positive (by reverse-transcription polymerase chain reaction tests) for SARS-CoV-2 infections were excluded. Healthy volunteers with normal serological liver function parameters and without a history of liver disease, who visited the Health Examination Center of Peking Union Medical College Hospital between 13th January and 29th August 2022, and volunteered to participate in this study, were included. CLD patients were divided into these four groups: at least 180 days after the second dose of the inactivated vaccine (n = 24), utmost 120 days after the booster dose (n = 22), 121–180 days after the booster dose (n = 38), and at least 180 days after the booster dose (n = 153). Serum samples were collected, numbered, and stored at −80 °C. All blood samples for antibody testing were frozen and thawed only once for the analysis.
For laboratory dimensions, B cells, CD4+ and CD8+ lymphocytes, and natural killer (NK) cells were analyzed by Flow CytoMetry on the BD FACSCanto II (Becton, Dickinson and Company, USA). Lymphocytes counts were measured using Sysmex XN-000 Pure (Sysmex Corporation, Japan), whereas serum liver parameters were detected on the TOSHIBA-FX8 analyzer (TOSHIBA Corporation, Japan). For hepatitis B serologic tests, hepatitis B surface antigen (HBsAg) and antibody (HBsAb), hepatitis B envelope antigen (HBeAg) and antibody (HBeAb), and hepatitis B core antibody (HBcAb) were measured using ARCHITECT i2000SR (Abbott Laboratories, USA), and HBV DNA was detected on Cobas Taqman48 (F. Hoffmann-La Roche, Ltd, Switzerland). Since HBsAg, HBsAb, and HBV DNA are quantitative indicators, their positive cut-off values are defined as >0.05 IU/mL, >10 mIU/mL, and >200 IU/mL, respectively. The HBeAg, HBeAb, and HBcAb are semi-quantitative indicators, and their positive cut-off values are defined as >1.0 S/CO. The clinical characteristics information of CLD patients, including age, sex, types of CLD, current therapeutic regimen, and time of each inactivated vaccine dose, were collected from Hospital Information Systems.
2.2. Total anti-SARS-CoV-2 antibodies test
Double antigen sandwich enzyme-linked immunosorbent assays (ELISA, Beijing Wantai Biological Pharmacy Enterprise Co.,Ltd., China) were used to detect total anti-SARS-CoV-2 antibodies (including IgM, IgG, and IgA). Microplates were pre-coated with the SARS-CoV-2 antigen (the RBD region of the S1 subunit), and 100 μL of serum samples were added. The plates were incubated at 37 °C for 30 min. After washing, 100 μL of SARS-CoV-2 antigens (the RBD region of the S1 subunit) labeled with horseradish peroxidase (HRP) were added, and the plates were then incubated for 30 min at 37 °C, forming a “coated antigen-antibody-labeled antigen” complex. Thereafter, 50 μL of chromogenic agents A (≥0.3 g/L peroxide) and B (≥0.2 g/L tetramethylbenzidine, TMB) was added for coloration. HRP attached to the complex catalyzed a TMB reaction, resulting in blue coloration. Subsequent termination of the reaction by sulfuric acid resulted in yellow coloration. The absorbance value was detected at a wavelength of 450 nm. Total anti-SARS-CoV-2 antibodies were semi-quantitative, and the optical density (OD) value of the sample at 450 nm was used as the measurement unit. After analyzing 133 negative and 52 positive SARS-CoV-2 samples using the receiver operating characteristic (ROC) curve analysis, the cut-off value was determined as 0.16 plus the mean absorbance value of the negative control wells (if the negative control well was lower than 0.03, it was calculated as 0.03). Samples with absorbance values greater than or equal to the cut-off value were considered positive. Detailed information on the total antibody detection is provided in the Supplementary Data.
2.3. Anti-SARS-CoV-2 spike receptor binding domain (RBD) IgG test
Indirect ELISA (Hangzhou Proprium Biotech Co, Ltd., China) was performed to detect anti-RBD IgG. The SARS-CoV-2 RBD protein was pre-coated onto the microplate and sequentially conjugated to anti-RBD antibodies in 100 μL of the serum samples (diluted 30 times) or calibrators for 1 h at room temperature, Thereafter, 100 μL of anti-human IgG-HRP was added, and the plate was incubated for 30 min at room temperature to form a “coated antigen-antibody-labeled antibody” complex. Finally, 100 μL of TMB substrate solution was added into the microwells. Color developed proportionally to the amount of IgG anti-RBD antibodies, and the OD value at 450 nm was measured. The anti-RBD IgG concentration [binding antibody units (BAU)/mL] was calculated from a standard curve with a range of 10–1000 BAU/mL range. The sample concentration calculated based on the standard curve was multiplied by 30 to obtain the final concentration of the IgG anti-RBD antibodies before sample dilution. Samples with concentrations greater or equal to 10 BAU/mL were considered positive according to the manufacturer's instructions. As indicated by the manufacturer, the assay shows a performance of specificity of 99.8%, a sensitivity of 97.8% and coefficient of variation (CV) ≤ 10%.
2.4. Neutralizing antibody against SARS-CoV-2 prototype and Omicron BA.4/5 subvariant tests
The SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) assay (GenScript cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit, USA) was used to detect neutralizing antibodies (NAbs) against the prototype and Omicron variants BA.4/5. The technology is based on the ELISA principle and mimics the interaction between the virus and the host cells. The samples and controls were pre-incubated with the HRP-RBD (in a volume ratio 1:1) for 30 min at 37 °C to allow binding of the circulating NAbs to HRP-RBD. After that, 100 μL of the mixture was added to the capture plates pre-coated with the human angiotensin-converting enzyme 2 receptor proteins and incubated for 15 min at 37 °C. The unbound HRP-RBD and any HRP-RBD bound to non-NAb were captured on the plate, whereas the circulating NAb-RBD (-HRP) complexes remained in the supernatant and were subsequently washed away. After adding 100 μL of TMB and 50 μL of the stop solution, the absorbance of the plate was immediately read at 450 nm. The OD value of the sample was inversely dependent on the titer of NAb. The inhibition rate of NAb was calculated according to the formula: inhibition rate = (1 − OD value of sample/OD value of negative control) × 100%. Samples with inhibition rates above or equal to 30% were considered positive. Detailed information on the neutralizing antibody test is provided in the Supplementary Data.
2.5. Statistical analysis
Statistical analysis was performed using R version 4.1.3 software, IBM SPSS Statistics version 26.0 (IBM Corp, USA), and GraphPad Prism 8.0 (GraphPad, San Diego, California, USA). The Shapiro-Wilk test was performed to establish the normal distribution of the values. Independent sample t-test or one-way analysis of variance (ANOVA) were respectively applied to compare two groups or multiple groups for values with normal distribution; otherwise, the Wilcoxon rank sum test or Kruskal-Wallis test was performed. The χ2 test were used to compare the differences between categorical variable data. The mean (standard deviation) and median (interquartile range) values were determined for quantitative data that followed normal and non-normal distributions, respectively. Categorical variables were presented as numbers and percentages. SPSS software was used to perform binary logistic regression analyses. For the multivariable logistic regression analyses, the variance inflation factor less than 3 and tolerance greater than 0.1 indicated no multicollinearity. Correlation analysis of the non-normally distributed data was done by determining the Spearman's correlation coefficients. P < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of participants
A total of 237 CLD patients and 170 healthy controls (HCs) of similar age and post-vaccination days were enrolled in the study. The demographic and clinical characteristics of the CLD patients at different times after COVID-19 vaccination are shown in Table 1 and Supplementary Table S1. CLD patients included in this study had a median age of 48 years with a male-to-female gender ratio of 1.85: 1. The four groups of CLD patients did not significantly differ in age and gender. Age and days after vaccination of the HCs were similar to those of the CLD patients (Supplementary Table S2).
Table 1.
Demographic and clinical characteristics of CLD patients.
| Total patients with CLD (n = 237) | Above 180 days after the second dose (n = 24) | Below 120 days after booster dose (n = 22) | 121–180 days after booster dose (n = 38) | Above 180 days after booster dose (n = 153) | P value | |
|---|---|---|---|---|---|---|
| Age (year) | 47.01 (12.00) | 48.00 (12.51) | 41.32 (10.81) | 46.84 (14.64) | 47.84 (11.23) | 0.119 |
| Male/female, n | 154/83 | 16/8 | 12/10 | 27/11 | 99/54 | 0.636 |
| Type of CLD, n (%) | ||||||
| Hepatitis Ba | 144 (60.76) | 11 (45.83) | 12 (54.55) | 23 (60.53) | 98 (64.05) | 0.348 |
| Hepatitis Ca | 10 (4.22) | 1 (4.17) | 1 (4.55) | 1 (2.63) | 7 (4.58) | 0.962 |
| Cirrhosis | 53 (22.36) | 5 (20.83) | 5 (22.73) | 10 (26.32) | 33 (21.57) | 0.934 |
| Otherb | 30 (12.66) | 7 (29.17) | 4 (18.18) | 4 (10.53) | 15 (9.80) | 0.050 |
| Treatments, n (%) | 187 (78.90) | 18 (75.00) | 16 (72.73) | 29 (76.32) | 124 (81.05) | 0.730 |
| Antiviral treatmentc, n (%) | 163 (68.78) | 16 (66.67) | 14 (63.64) | 27 (71.05) | 106 (69.28) | 0.934 |
| Chinese patent medicinesd, n (%) | 53 (22.36) | 6 (25.00) | 10 (45.45) | 10 (26.32) | 27 (17.65) | 0.027 |
| Hepatitis B parameters | ||||||
| HBsAg (IU/mL) | 1370 (266.8, 3960) | 291.2 (11.51, 4166) | 957.7 (96.92, 4647) | 792.7 (95.83, 4595) | 1822 (357,3926) | 0.715 |
| HBsAg (+), n (%) | 119 (50.21) | 8 (33.33) | 14 (63.64) | 20 (52.63) | 77 (50.33) | 0.220 |
| HBsAg (mIU/mL) | 0.89 (0.5, 3.05) | 0.5 (0.06, 1.60) | 0.53 (0.5, 2.08) | 1.79 (0.5, 5.49) | 1 (0.5, 3.12) | 0.128 |
| HBsAb (+), n (%) | 9 (3.80) | 0 (0.00) | 2 (9.09) | 1 (2.63) | 6 (3.92) | 0.426 |
| HBeAg (+), n (%) | 45 (18.99) | 3 (12.50) | 6 (27.27) | 9 (23.68) | 27 (17.65) | 0.501 |
| HBeAb (+), n (%) | 64 (27.00) | 7 (29.17) | 8 (36.36) | 11 (28.95) | 38 (24.84) | 0.689 |
| HBcAb (+), n (%) | 107 (45.15) | 9 (37.50) | 14 (63.64) | 16 (42.11) | 68 (44.44) | 0.287 |
| HBV DNA (+), n (%) | 46 (19.41) | 2 (8.33) | 7 (31.82) | 7 (18.42) | 30 (19.61) | 0.253 |
| Liver function parameters | ||||||
| Alanine aminotransferase (U/L) | 23.60 (17.20, 36.35) | 22.50 (16.10, 31.45) | 27.90 (18.80, 55.43) | 23.95 (18.03, 37.18) | 23.90 (17.28, 35.70) | 0.516 |
| Aspartate aminotransferase (U/L) | 22.70 (17.70, 29.15) | 23.70 (15.80, 31.70) | 23.45 (18.50, 32.85) | 21.15 (16.95, 25.28) | 22.40 (17.58, 29.25) | 0.601 |
| γ-glutamyl transferas (U/L) | 20.80 (15.25, 33.25) | 25.90 (16.55, 53.85) | 24.75 (16.18, 46.10) | 17.70 (11.60, 27.58) | 20.80 (15.38, 32.23) | 0.090 |
| Alkaline phosphatase (U/L) | 82 (69, 102) | 81 (64, 110.5) | 87 (72.75, 108) | 76 (66.25, 90.75) | 83 (69, 104) | 0.264 |
| Total bilirubin (μmol/L) | 14.20 (11.30, 19.95) | 12.80 (11.25, 22.55) | 14.30 (11.68, 20.75) | 14.75 (12.43, 19.88) | 14.10 (10.58, 19.85) | 0.575 |
| Direct bilirubin (μmol/L) | 4.40 (3.10, 6.05) | 4.10 (3.50, 7.95) | 4.60 (3.35, 6.65) | 4.55 (3.90, 7.05) | 4.35 (3.00, 6.00) | 0.437 |
| Total biliary acid (μmol/L) | 3.40 (2.30, 9.30) | 2.90 (1.80, 7.00) | 3.10 (2.30, 16.00) | 3.40 (2.35, 53.45) | 3.50 (1.70, 8.70) | 0.724 |
| Total protein (g/L) | 75.70 (72.60, 77.80) | 72.20 (67.90, 78.10) | 76.15 (74.18, 79.03) | 74.10 (71.45, 77.73) | 76.05 (73.05, 77.80) | 0.112 |
| Albumin (g/L) | 46.20 (43.90, 48.1) | 44.40 (38.00, 47.10) | 46.65 (44.58, 47.65) | 46.00 (43.50, 48.10) | 46.40 (44.25, 48.33) | 0.256 |
| Prealbumin (mg/L) | 200.30 (84.37) | 216.30 (58.67) | 235.80 (96.76) | 240.70 (144.80) | 187.60 (72.46) | 0.361 |
| Lactate dehydrogenase (U/L) | 177.5 (157.3, 200) | 190 (158.3, 210.3) | 172 (157, 209) | 177 (160, 212) | 178 (156.3, 196) | 0.785 |
| Cholinesterase (U/L) | 8301 (4784, 10207) | 7526 (5291, 10213) | 9432 (5234, 11183) | 7453 (4235, 10585) | 8511 (4617, 9908) | 0.715 |
| Blood cell counts | ||||||
| White blood cells ( × 109/L) | 5.12 (4.19, 6.44) | 6.19 (4.31, 7.48) | 5.13 (3.97, 6.26) | 4.92 (3.97, 6.14) | 5.07 (4.21, 6.5) | 0.485 |
| Lymphocytes ( × 109/L) | 1.75 (1.25, 2.10) | 1.76 (1.54, 2.67) | 1.89 (1.43, 2.16) | 1.68 (1.23, 1.96) | 1.70 (2.07, 2.07) | 0.621 |
Normally distributed data are shown as mean (standard deviation), non-normally distributed data are shown as median (Q1, Q3).
HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBeAg, hepatitis B envelope antigen; HBeAb, hepatitis B envelope antibody; HBcAb, hepatitis B core antibody.
Viral hepatitis has not yet progress to cirrhosis.
Other CLD included primary biliary cholangitis, alcoholic hepatitis, fatty liver, toxic hepatitis, liver cancer.
Antiviral drugs include entecavir, tenofovir disoproxil fumarate, tenofovir disoproxil fumarate, sofosbuvir velpatasvir, elbavir glarevir, tenofovir ami, pegylated interferon.
Chinese patent medicines include silymarin, Ruangan Huajian Granules, Fuzheng Huayu Preparation, Dahuang Zhechong Pills, Chaihuangyigan Granule, compound Yiganling, Biejiajianwan.
3.2. Total anti-SARS-CoV-2 and IgG anti-RBD antibodies increase at the post-booster dose shot in CLD patients
Before booster vaccinations, the level of total anti-SARS-CoV-2 antibodies was lower in the CLD than in HCs groups [1.00 (0.47, 2.91) vs. 2.99 (1.70, 3.19), P = 0.060], while increased and remained at high titers in CLD patients after the booster shots. Total antibody titers in CLD patients were slightly lower than HCs [utmost 120 days after booster dose: 3.11 (3.05, 3,19) vs.3.11 (3.05, 3.17), P = 0.830; 121–180 days after booster dose: 3.11 (3.05, 3.14) vs. 3.19 (3.05, 3.23), P = 0.007; at least 180 days after booster dose: 3.16 (3.06, 3.21) vs. 3.24 (3.16, 3.33), P < 0.001] (Fig. 1A and B, Supplementary Table S3). However, the seroconversion rates of total anti-SARS-CoV-2 antibodies were similar in CLD and HCs groups (Fig. 1C, Supplementary Table S3).
Fig. 1.
Total anti-SARS-CoV-2 antibodies are elevated following booster dose of inactivated vaccines among CLD patients. Levels (A) and kinetics (B) of total anti-SARS-CoV-2 antibodies (OD value) in CLD patients (n = 237) and HC (n = 170) before and after booster shot. C Seropositivity (orange) of total anti-SARS-CoV-2 antibodies in CLD (n = 237) comparing with HC (n = 170) groups. D Comparison of levels of total anti-SARS-CoV-2 antibodies between SLD patients (n = 67), NSLD patients (n = 59), and HC (n = 65) after booster shot. Booster shot refers to the third dose of the inactivated vaccine. The OD values of total anti-SARS-CoV-2 antibodies were measured by ELISA at 450 nm wavelength. OD values ≥ 0.19 were regarded as positive, provided by kit. Data were shown as median with interquartile range (A, D), median (B) or percentage (C). Statistical analysis was performed by Wilcoxon rank sum test (A, D) and χ2 test (C). ∗∗P < 0.01, ∗∗∗P < 0.001. CLD, chronic liver disease; SLD, severe liver disease; NSLD, non-severe liver disease; HC, healthy controls.
Anti-SARS-CoV-2 spike RBD IgG antibodies increased in CLD patients within 120 days post-booster shot [at least 180 days after the second dose: 0.00 BAU/mL (0.00, 28.65) vs. utmost 120 days after the booster dose: 321.60 BAU/mL (136.50, 595.50), P < 0.001], but significantly decreased after 120 days [119.30 BAU/mL (56.67, 463.70), P = 0.099] and 180 days [107.10 BAU/mL (34.97, 242.10), P < 0.001] post-booster shot. The concentrations of anti-RBD IgG in all CLD subgroups were lower than those in HCs [utmost 120 days after the booster dose: 321.60 BAU/mL (136.50, 595.50) vs. 431.00 BAU/mL (181.90, 602.80), P = 0.012; 121–180 days after booster dose: 119.30 BAU/mL (56.67, 463.70) vs. 452.30 BAU/mL (206.80, 601.70), P = 0.028; at least 180 days after the booster dose: 107.10 BAU/mL (34.97, 242.10) vs. 266.20 BAU/mL (106.40, 554.50), P < 0.001] (Fig. 2A and B, Supplementary Table S3). The seroconversion rates in three CLD subgroups did not differ from corresponding HCs after booster vaccination (P = 0.488, P = 0.615, and P = 0.480) (Fig. 2C, Supplementary Table S3).
Fig. 2.
Anti-RBD IgG was raised after the booster dose of inactivated vaccines among CLD patients. Levels (A) and kinetics (B) of anti-RBD IgG (BAU/mL) in CLD patients (n = 237) and HC (n = 170) before and after booster shot. C Seropositivity (orange) of anti-RBD IgG in CLD (n = 237) comparing with HC (n = 170). D Comparison of anti-RBD IgG levels among SLD patients (n = 67), NSLD patients (n = 59), and HC (n = 65) after booster shot. Booster shot refers to the third dose of the inactivated vaccine. The OD values of anti-RBD IgG were measured by ELISA at 450 nm wavelength. The antibody concentration was calculated from a standard curve with a range of 10–1000 BAU/mL range. Concentrations ≥10 BAU/mL were regarded as positive. Data were shown as median with interquartile range (A, D), median (B) or percentage (C). Statistical analysis was performed by Wilcoxon rank sum test (A, D) and χ2 test (B). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. CLD, chronic liver disease; SLD, severe liver disease; NSLD, non-severe liver disease; HC, healthy controls; RBD, receptor binding domain.
3.3. Humoral responses against SARS-CoV-2 VOC wane in CLD and HC groups after booster vaccination
The inhibition effect (%) of the booster inoculation on the NAbs against SARS-CoV-2 prototype varied in CLD and HCs [utmost 120 days after the booster dose: 49.37% (14.99, 89.46) vs. 90.23% (59.00, 97.22), P = 0.009; 121–180 days after the booster dose: 26.32% (4.17, 57.45) vs. 68.48% (37.04, 89.54), P = 0.006; at least 180 days after the booster dose: 25.17% (9.04, 48.80) vs. 49.17% (21.23, 84.30), P < 0.001] (Fig. 3A, Supplementary Table S3). CLD patients had an increased inhibition rate (%) of NAb against the prototype after receiving the booster dose vaccine, but the inhibition rate significantly declined over time (Fig. 3B). Furthermore, the positive rate of NAb against the prototype was quite different between the CLD and HC groups (utmost 120 days after the booster dose: 72.73% vs. 100%, P = 0.021; 121–180 days after the booster dose: 44.74% vs. 79.41%, P = 0.003; at least 180 days after the booster dose: 41.83% vs. 65.98%, P < 0.001) (Fig. 3C, Supplementary Table S3).
Fig. 3.
Neutralizing antibodies against SARS-CoV-2 prototype (NAb-prototype) are increased after the booster dose of inactivated vaccines among CLD patients. Inhibition rate (A) and kinetics (B) of NAb-prototype in CLD patients (n = 237) and HC (n = 170) before and after booster shot. C Seropositivity (orange) of NAb-prototype in CLD (n = 237) comparing with HC (n = 170). D Comparison of NAb-prototype levels (inhibition rate) among SLD patients (n = 67), NSLD patients (n = 59), and HC (n = 65) after booster shot. Booster shot refers to the third dose of the inactivated vaccine. The OD values of NAb-prototype were measured by ELISA at 450 nm wavelength. The inhibition rate of NAb was calculated as (1 − OD value of sample/OD value of negative control) × 100%, which ≥30% was considered positive, provided by kit. Data were shown as median with interquartile range (A, D), median (B) or percentage (C). Statistical analysis was performed by Wilcoxon rank sum test (A, D) and χ2 test (B). ∗∗P < 0.01, ∗∗∗P < 0.001. CLD, chronic liver disease; SLD, severe liver disease; NSLD, non-severe liver disease; HC, healthy controls; sVNT, surrogate virus neutralization test.
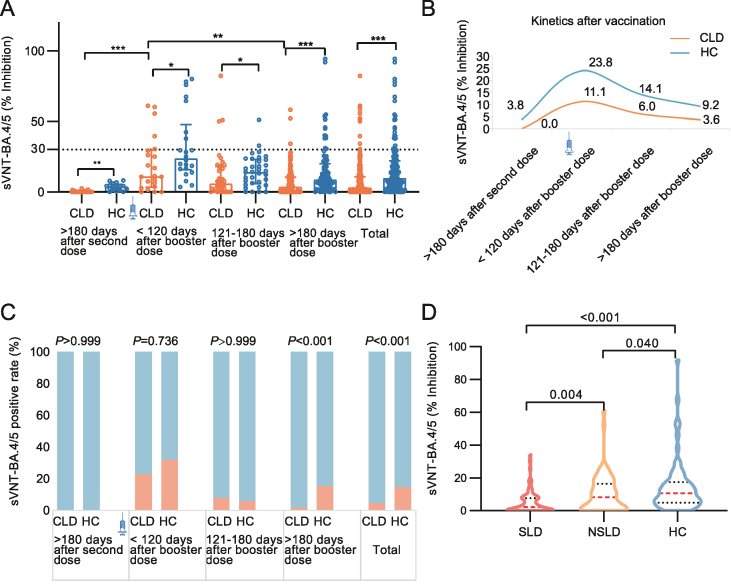
Neutralization responses to Omicron BA.4/5 variants (most prevalent VOCs currently) were also assessed. The inhibition rate of NAb against BA.4/5 variants was two times higher in HCs than in CLD patients [utmost 120 days after the booster dose: 23.84% (15.70, 47.91) vs. 11.08% (3.44, 29.63), P = 0.033, 121–180 days after the booster dose: 14.11% (5.98, 23.04) vs. 6.01% (0.53, 6.01), P = 0.035, at least 180 days after booster dose: 8.82% (4.19, 20.03) vs. 3.63% (0.00, 10.42), P < 0.001] (Fig. 4A, Supplementary Table S3). Moreover, the NAb seropositivity against BA.4/5 variants was significantly decreased in CLD compared to HCs (4.64% vs. 14.12%, P < 0.001) (Fig. 4C, Supplementary Table S3). Notably, the waning of humoral responses of NAb against BA.4/5 variants was observed in both the CLD and HC groups after the booster dose (Fig. 4B).
Fig. 4.
Neutralizing antibodies against SARS-CoV-2 variant BA.4/5 (NAb-BA.4/5) are enhanced after the booster dose of inactivated vaccines among CLD patients. Inhibition rate (A) and kinetics (B) of NAb-BA.4/5 in CLD patients (n = 237) and HC (n = 170) before and after booster shot. C Seropositivity (orange) of NAb-BA.4/5 in CLD (n = 237) comparing with HC (n = 170). D Comparison of NAb-BA.4/5 levels (inhibition rate) among SLD patients (n = 67), NSLD patients (n = 59), and HC (n = 65) after booster shot. Booster shot refers to the third dose of the inactivated vaccine. The OD values of NAb-BA.4/5 were measured by ELISA at 450 nm wavelength. The inhibition rate of NAb was calculated as (1 − OD value of sample/OD value of negative control) × 100%, which ≥30% was considered positive, provided by kit. Data were shown as median with interquartile range (A, D), median (B) or percentage (C). Statistical analysis was performed by Wilcoxon rank sum test (A, D) and χ2 test (B). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. CLD, chronic liver disease; SLD, severe liver disease; NSLD, non-severe liver disease; HC, healthy controls; sVNT, surrogate virus neutralization test.
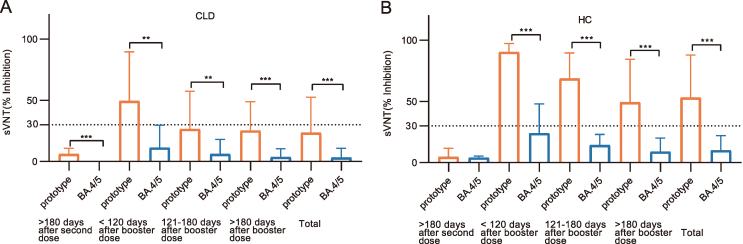
Compared with the SARS-CoV-2 prototype, the neutralization rate against BA.4/5 variants was reduced in CLD patients post-booster vaccination [utmost 120 days after the booster dose: 11.08% (3.44, 29.63) vs. 49.37% (14.99, 89.46), P = 0.001, 121–180 days after the booster dose: 6.01% (0.53, 6.01) vs. 26.32% (4.17, 57.45), P = 0.001, at least 180 days after the booster dose: 3.63% (0.00, 10.42) vs. 25.17% (9.04, 48.80), P < 0.001] (Fig. 5A, Supplementary Table S4). The median inhibition rates of BA.4/5-NAbs were less than 30% in CLD patients. A similar weaker neutralization against BA.4/5 compared to the SARS-CoV-2 prototype was observed in the HC group (Fig. 5B, Supplementary Table S4). These findings suggested that booster vaccination might not invigorate the neutralization against BA.4/5 variants.
Fig. 5.
Low neutralizing responses against Omicron BA.4/5 after booster vaccination in both CLD patients and HC. Comparison of neutralizing capacity of NAb against BA.4/5 (blue) and the prototype (orange) in CLD patients (n = 237) (A) and HC (n = 170) (B). Booster shot refers to the third dose of the inactivated vaccine. The OD values of NAb were measured by ELISA at 450 nm wavelength. The inhibition rate of NAb was calculated as (1 − OD value of sample/OD value of negative control) × 100%, which≥ 30% was considered positive, provided by kit. Data were shown as median. Statistical analysis was performed by Wilcoxon rank sum test. ∗∗P < 0.01, ∗∗∗P < 0.001. CLD, chronic liver disease; HC, healthy controls; sVNT, surrogate virus neutralization test, NAb, neutralizing antibody.
3.4. Antibody responses to booster shot reduce in SLD patients
There were 67 SLD patients in the CLD cohort. The immune effects of total antibodies against SARS-CoV-2, anti-RBD IgG antibodies, and the inhibition rate of NAb against SARS-CoV-2 prototype and BA.4/5 variants after booster vaccination between SLD patients, sex-, age- and days after vaccination-matched 59 NSLD patients and 65 HCs were compared. We found that SLD patients had the lower antibodies levels than the NSLD patients [total antibodies: 3.105 (2.897, 3.175) vs. 3.150 (3.098, 3.200), P = 0.015; anti-RBD IgG: 88.62 BAU/mL (27.2, 278.4) vs. 150.2 BAU/mL (56.82, 464.5), P = 0.025; NAb-prototype: 21% (5.303, 50.09) vs. 35.75% (15.73, 60.19), P = 0.009; NAb-BA.4/5: 2.12% (0, 7.649) vs. 8.20% (4.872, 17.42), P = 0.004] (Figs. 1D, 2D and 3D, 4D). These results indicated that the humoral immune responses were weakened in SLD patients.
However, further analysis of the humoral immune responses after booster vaccination showed that the levels of total anti-SARS-CoV-2 antibodies [3.11 (3.05, 3.20) vs. 3.13 (3.09, 3.17), P = 0.800], the concentrations of anti-RBD IgG antibodies [102.4 BAU/mL (43.23, 327) vs. 142 BAU/mL (66.34, 409.4), P = 0.656], and the inhibition rates of neutralizing antibodies against the prototype [23.84% (11.29, 45.8) vs. 37.9% (6.79, 68.35), P = 0.637] and Omicron BA.4/5 variants [2.79% (0, 8.69) vs. 4.84% (0, 8.27), P = 0.741] were comparable between 28 compensated cirrhosis patients and 20 sex-, age- and days after vaccination-matched decompensated cirrhosis patients. Lymphocyte counts (P = 0.095), CD3+ T cell (P = 0.413), CD4+ T cell (P = 0.428), CD8+ T cell (P = 0.292), B cell (P = 0.056), and NK cell (P = 0.104) counts also exhibited no statistical difference between patients with compensated and decompensated cirrhosis (Supplementary Fig. S1).
3.5. Correlation between antibody levels after booster vaccinations and clinical factors
Spearman's correlation analyses of the association between the levels of the four anti-SARS-CoV-2 antibodies and clinical factors in CLD patients are summarized in Fig. 6A and Supplementary Table S5. After booster shots, the inhibition rate of NAbs-prototype was positively correlated with that of NAbs-BA.4/5 (r = 0.66, P < 0.001) (Fig. 6B) and the anti-RBD IgG concentration (r = 0.83, P < 0.001) (Fig. 6C). NAbs-BA.4/5 was positively correlated with anti-RBD IgG (r = 0.69, P < 0.001) (Fig. 6D). These results suggested the excellent correlation between anti-RBD IgG and NAb against SARS-CoV-2 prototype. Notably, there were positive correlations between HBsAg and NAbs-prototype (r = 0.29, P = 0.001), NAbs-BA.4/5 (r = 0.27, P = 0.003), and anti-RBD IgG (r = 0.28, P = 0.002) (Fig. 6E–G), and HBcAb was positively correlated with anti-RBD IgG (r = 0.32, P = 0.0037) (Fig. 6H). Besides, age, time after the last vaccine and some liver indexes (total bilirubin, direct bilirubin, and γ-glutamyl transferase) were negatively correlated with the antibodies (Supplementary Table S5). Additionally, we also analyzed the correlation between above indicators and the seroconversion rate of the SARS-CoV-2 antibodies after booster vaccination. The results showed that the levels of HBsAg (r = 0.29, P = 0.006) and HBcAb (r = 0.21, P = 0.016) were positively correlated with the positive rate of NAb-prototype, while age (r = −0.22, P = 0.002) was negatively correlated with the positive rate of NAb-BA.4/5. HBcAb was a positive factor (r = 0.27, P = 0.018), while SLD was a negative factor (r = −0.15, P = 0.028) for the total anti-SARS-CoV-2 antibody seropositivity (Supplementary Table S6).
Fig. 6.
Correlations of SARS-CoV-2 specific antibodies and clinical factors in patients with CLD. A The heatmap showed the factors correlated with antibody levels after booster vaccination. B–D The correlation between NAb-prototype (% inhibition), NAb-BA.4/5 (% inhibition), and levels of anti-RBD IgG (BAU/mL). E–G The correlations between levels of HBsAg (IU/mL) and NAb-prototype (% inhibition) (E), NAb-BA.4/5 (% inhibition) (F), and levels of anti-RBD IgG (BAU/mL) (G). H The correlations between levels of HBcAb (S/CO) and anti-RBD IgG (BAU/mL). The points show the distribution of samples, the lines represent the regression curves, and the gray intervals are the confidence intervals for the curve fitting. Booster shot refers to the third dose of the inactivated vaccine. Statistical analysis was performed by Spearman's correlation. CLD, chronic liver disease; NAb-prototype, neutralizing antibody against SARS-CoV-2 prototype; NAb-BA.4/5, neutralizing antibody against Omicron BA.4/5; RBD, receptor binding domain; HBsAg, hepatitis B surface antigen; HBcAb, hepatitis B core antibody.
3.6. Factors associated with seroconversion of SARS-CoV-2 antibodies after booster vaccination
Furthermore, to determine the factors affecting seroconversion rates of SARS-CoV-2 antibody, we analyzed the demographic, clinical and immunological characteristics using univariate logistic regression. Age (OR = 1.096, 95% CI: 1.031, 1.166, P = 0.003) and time after the last vaccine (OR = 1.016, 95% CI: 1.006, 1.026, P = 0.001) were the risk factors for NAb seropositivity against Omicron BA.4/5. The white blood cell counts (OR = 0.497, 95% CI: 0.251, 0.982, P = 0.044), HBcAb (OR = 0.654, 95% CI: 0.466, 0.918, P = 0.014) and SLD (OR = 5.667, 95% CI: 1.009, 31.82, P = 0.049) were the dominant effectors for seropositivity of the total antibodies against SARS-CoV-2. Protective and risk factors with statistically significant differences in the univariate logistic regression were further analyzed in multivariate logistic regression. Old age (OR = 1.014, 95% CI: 1.004, 1.024, P = 0.006) and several days after booster vaccine (OR = 1.085, 95% CI: 1.018, 1.156, P = 0.012) were the risk factors for negative NAb against Omicron BA.4/5; however, no significant factor was observed for the total antibody seropositivity (Table 2, Supplementary Table S7). This might be due to the small number of included samples or the lack of some data, resulting in skewed analysis results.
Table 2.
Factors associated with negative SARS-CoV-2 antibodies in CLD patients with booster vaccination.
| Categories | Risk factors | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| NAbs against BA.4/5 | Age (years) | 1.096 | 1.031–1.166 | 0.003 | 1.014 | 1.004–1.024 | 0.006 |
| Male, n | 1.571 | 0.463–5.332 | 0.468 | / | |||
| Time after last vaccine (days) | 1.016 | 1.006–1.026 | 0.001 | 1.085 | 1.018–1.156 | 0.012 | |
| Total anti-SARS-CoV-2 antibodies | Age (years) | 1.056 | 0.985–1.132 | 0.124 | / | ||
| Male, n | 2.782 | 0.319–24.262 | 0.354 | / | |||
| White cell count ( × 109/L) | 0.497 | 0.251–0.982 | 0.044 | 0.631 | 0.354–1.122 | 0.117 | |
| HBcAb (S/CO) | 0.654 | 0.466–0.918 | 0.014 | 0.842 | 0.637–1113 | 0.227 | |
| Severe liver disease, n | 5.667 | 1.009–31.82 | 0.049 | 0.856 | 0.154–4.753 | 0.859 | |
NAb, neutralizing antibody; HBcAb, hepatitis B core antibody; OR, odds ratio.
3.7. Changes in lymphocyte subsets after booster vaccination
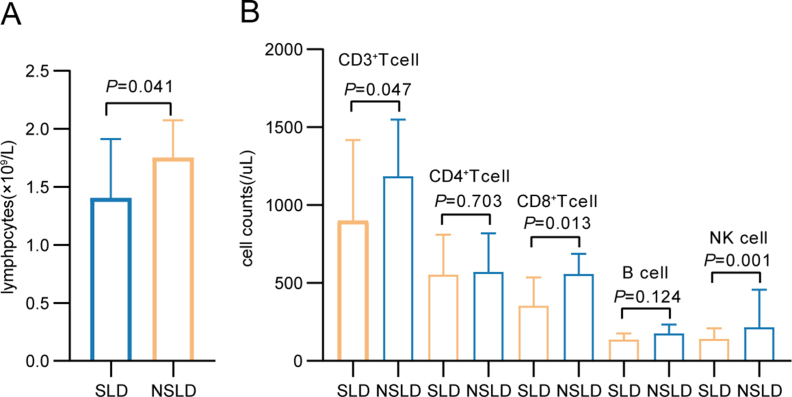
The changes in B cells, CD4+ T or CD8+ T cells, and NK cells were also analyzed after booster vaccination. Although CLD patients and HCs had similar level of lymphocyte counts (Supplementary Fig. S2A), the median counts of B and NK cells were both lower in CLD patients than in HCs (P = 0.013 and P = 0.015, respectively) (Supplementary Fig. S2B). However, the count and percentage of the T cell subsets (CD3+ T cell, CD4+ T cell, and CD8+ T cell), which were associated with cellular immunity, were unaffected in the CLD samples (Supplementary Figs. S2D and S2E; Supplementary Table S8).
Since decreased antibody response against SARS-CoV-2 was observed in SLD patients, we compared the differences in lymphocyte subsets between patients with and without SLD after booster vaccination. We found the counts of lymphocytes (P = 0.041), CD3+ T cells (P = 0.047), CD8+ T cells (P = 0.013), and NK cells (P = 0.001), and the percentage of CD8+ T (P = 0.019) cells, were lower in SLD patients than in NSLD ones (Supplementary Fig. S3).
4. Discussion
With over 90% vaccination rate of the Chinese population and lower disease severity caused by the Omicron variant, the Chinese government lifted epidemic control measures and implemented the gradual reopening policy in December 2022. This was to maximize the protection of people's lives and health while minimizing the impact of the epidemic on socioeconomic development. However, the reopening policy resulted in a higher risk of infection and severe disease in vulnerable populations. Nationwide vaccination is critical to establish herd immunity and reduce severe cases and mortality. In addition, measuring B cell immunity and antibody cross-response is crucial in monitoring and controlling the transmission of the SARS-CoV-2 variant. Thus, this study focused on vaccine efficacy among vulnerable populations, which might have directive implications for optimizing post-reopening managements. To our knowledge, our research is the first to focus on the immunological alterations of pre- and post-booster doses of inactivated vaccines in CLD individuals. The levels of total anti-SARS-CoV-2 antibodies were lower in CLD patients compared with HCs at least 180 days after the second vaccine but were subsequently upregulated to a comparable level with HCs after the booster shot. Similarly, elevated levels of anti-RBD IgG antibodies and NAbs against the SARS-CoV-2 prototype and Omicron BA.4/5 induced by the booster dose strengthened the humoral responses in CLD patients, but their seropositivity was still lower than that of HCs. The booster vaccine could not prevent BA.4/5 lineage evasion from the immune response in CLD patients and HCs due to the minimal inhibition rate of NAb-BA.4/5.
Many studies have demonstrated the improved immune responses against SARS-CoV-2 among CLD patients receiving two-dose vaccines (Ai et al., 2022; He et al., 2022; Thuluvath et al., 2021; Xiang et al., 2021) but the vaccine protection is waning six months after administration (Chemaitelly et al., 2021; Goldberg et al., 2021; Levin et al., 2021), consistent with our findings that the antibodies were extremely down-regulated at 181 days post-second doses. The low SARS-CoV-2-specific IgG antibody and NAb levels could be mitigated by triple-dose vaccines since the antibody responses would reach a new peak after the booster dose (Belik et al., 2022). Therefore, it's critical to implement the booster-dose immunization regimen to enhance the humoral immune responses, especially for vulnerable populations such as CLD patients. A significant improvement in humoral and cellular immune responses was observed among 62 liver transplant recipients in Israel after the booster dose of the BNT162b2 mRNA vaccine (Davidov et al., 2022). Moreover, booster mRNA vaccines can overcome vaccine hyporesponsiveness among the cirrhosis population (John et al., 2022). Our study demonstrated that the seropositivity of anti-RBD IgG and NAb against the SARS-CoV-2 prototype and Omicron subvariants was enhanced among CLD patients after the booster shot of inactivated vaccine. However, the humoral immune responses in CLD were lower than in HCs. The effect of the booster inactivated vaccine reached its peak within 120 days after vaccination but subsequently declined at a faster rate in CLD patients than in HCs, resulting insufficient immunological protection among CLD patients after the booster intramuscular vaccine. Moreover, time after the booster vaccine was an independent risk factor affecting NAb positivity against Omicron BA.4/5, consistent with the results of a randomized controlled trial of healthy adults receiving four booster vaccines (including ChAdTS-S, RQ3013, ZR202-CoV and CoronaVac). In the randomized controlled trial, the anti-RBD-specific IgG increased at day 7, peaked at day 14, slightly waned at day 28, and remained constant at day 90. However, the NAb against different Omicron subvariants decreased drastically from day 14 to day 90 (Zhang et al., 2023). Booster shots were proven to prolong the decay time of the neutralizing antibody, total anti-RBD antibodies and anti-RBD IgG antibodies, and anti-Spike IgG (Goel et al., 2022; Liang et al., 2022); however, the half-lives of the antibodies remained unsatisfactory in terms of immunity persistence. A fourth dose or annual revaccination might help to prevent decay. Furthermore, we also found that the cell counts of B and NK cells declined in CLD patients compared to HCs, consistent with low antibody titers. This was consistent with the previous studies that anti-RBD IgG was associated with SARS-CoV-2-specific B cells in patients with chronic hepatitis B virus infections after two doses of inactivated vaccine (He et al., 2022). Positive humoral response against SARS-CoV-2 was observed in solid organ transplant patients with high lymphocyte counts (Abravanel et al., 2022) and chronic lymphocytic leukemia patients with elevated NK cells (Molica et al., 2022).
Risk factors in CLD patients also affected the seropositivity response after the boost vaccination against SARS-CoV-2. Age was negatively related to the levels of NAb against prototype, Omicron BA.4/5 and anti-RBD IgG, and serve as a risk element for the positivity of NAb against BA.4/5 variants in the multivariate logistic analyses. Several study studies reported that the elderly showed lower neutralization potency against the prototype and other VOCs (including B.1.1.7, B.1.351 and P.1.) than the younger individuals after one, two or three doses of vaccines (Collier et al., 2021; Kawasuji et al., 2021; Liang et al., 2022; Newman et al., 2022; Tuekprakhon et al., 2022; Xu et al., 2021). The poor neutralizing responses, including lower levels of antibodies (quantity) and lower-affinity antibodies (quality), in the elderly individuals might be explained by reduced T-cell response (Tober-Lau et al., 2021), lowered spike-specific IgG+IgM−CD19+ memory B cells, decreased somatic hypermutation in the BCR gene, or less enrichment for public BCR clonotypes associated with neutralization (Collier et al., 2021).
Furthermore, the univariate logistic regression analysis revealed that SLD was an adverse factor, while the white cell count and HBcAb titers were advantageous factors for the negative seroconversion of the total anti-SARS-CoV-2 antibodies. SLD is characterized by immune dysfunction and exhaustion of T cell subsets, which might have contributed to the observed susceptibility to infection had worse prognosis (Albillos et al., 2022; Barsch et al., 2022). SLD patients who received two doses of inactivated vaccines have worse antibody responses and more impaired RBD-specific memory B cells responses than patients with general chronic liver diseases, such as nonalcoholic fatty liver disease and chronic hepatitis B (Chen et al., 2022). Lower levels of anti-spike antibodies after two doses of the mRNA vaccine and a single dose of the Johnson & Johnson vaccine were reported in 61% of the liver transplant recipients and 24% of the NSLD patients (Thuluvath et al., 2021). Our results reported a sharp decrease in the total antibodies against SARS-CoV-2, anti-RBD IgG antibody titers and NAbs inhibition rate against the prototype and BA.4/5 variants in SLD patients after the booster shot. Advanced cirrhosis was associated with inferior SARS-CoV-2-specific T-cell reactivity in the SLD population who received the second mRNA vaccine (Al-Dury et al., 2022). Consistently, our findings showed lower counts of lymphocytes, CD3+ T cells, CD8+ T cells, and NK cells in SLD patients compared to NSLD patients, reflecting an inevitable waning of the antibody responses after COVID-19 vaccination. Vincenzo et al. reported that slower and suboptimal humoral and cellular responses in cirrhosis patients caused by the lack of prompt T cell response and B cell dysfunction recovered comparably to that of healthy individuals after the booster BNT162b2 vaccine (Giambra et al., 2022), inconsistent with our results. Currently, only a few studies have assessed the cellular responses towards different types of vaccines after the booster shots among CLD patients; thus, we could not elaborate on exact association between SARS-CoV-2-specific cells and the corresponding antibody responses. Besides, HBcAb and white blood cell counts were the protective factors for total antibodies against SARS-CoV-2. HBsAb and HBcAb were positively associated with total antibodies against SARS-CoV-2 and anti-RBD IgG, respectively. Higher antibody levels against hepatitis B virus (HBV) and immune cells represent the available immune function, which benefits antibody production. Considering the poor clinical outcomes of CLD patients infected with COVID-19, the low immune response of CLD patients (especially in SLD patients) to vaccination, and the waning of antibodies levels against SARS-CoV-2 over time and with age, it is imperative to focus on the cellular immune responses after booster shots and to develop the optimal vaccination regimens to improve the efficacy of vaccines.
In addition, correlation analyses suggested that HBsAg was positively correlated with the levels of NAbs-prototype, NAbs-BA.4/5, and anti-RBD IgG. Sondes et al. found that PGTNTSN peptide chains in HBsAg of HBV corresponded to an exposed site in the S protein of SARS-CoV-2 (Haddad-Boubaker et al., 2021). An S protein segment containing PGTNTSN peptide chains induced a positive B-cell reaction in convalescent COVID-19 patients (Zhang et al., 2020). Neil et al. developed a vaccine candidate comprising a SARS-CoV-2 RBD-hepatitis B surface antigen virus-like particle which elicited protective immunity in cynomolgus macaques (Dalvie et al., 2022), demonstrating the possible adjuvant potential of HBsAg for vaccines against SARS-CoV-2.
The Omicron BA.4/5 variants have new mutations in their RBD, such as R493Q, L452R and F486V, indicating possible evolution from BA.2 (Liang et al., 2022). In this study, BA.4/5 variants had 6.10-fold reduced neutralization in CLD patients and 5.11-fold in HCs when compared with the prototype, consistent with the result of a previous mRNA booster vaccination cohort (Tuekprakhon et al., 2022). Despite receiving the booster shot, the inhibition rates of NAb against BA.4/5 variants in CLD patients were still below the positive threshold value, which might have accelerated the evasion and breakthrough infections of Omicron variants. This is because all vaccines used are against the ancestral Wuhan strain and are insufficient to protect people from the VOCs infection. Using a bivalent Omicron-containing mRNA vaccine against ancestral Wuhan-Hu-1 and Omicron BA.1 spike as a second booster dose effectively elicited NAb responses against Omicron (Chalkias et al., 2022). Thus, a better vaccination strategy must be formulated to protect the vulnerable population from Omicron sublineage infections.
Despite the findings, this study had limitations. First, although age and time after vaccination matched between CLD patients and HCs, gender discrepancies were not eliminated in groups vaccinated with booster shot after six months. This might have led to biased interpretations of the results. Second, this research was a cross-sectional study, and thus, we did not longitudinally follow up the dynamic immune response of CLD patients. Our study also included a relatively small sample size of the cirrhosis subgroup. Studies with larger sample sizes are required to explore how various liver function statuses influence on SARS-CoV-2 vaccine immunogenicity. Third, owing to the limited blood sample volume, we did not evaluate the responses of SARS-CoV-2-specific immune cells to booster shots, thus impeding the further exploration of the association between cellular and humoral immune responses.
5. Conclusions
In summary, booster SARS-CoV-2 vaccination enhanced humoral immune response among CLD patients; however, the antibody responses were lower in CLD patients than in HCs and reduced with time post-inoculation. The lowered inhibition rate of NAbs against Omicron BA.4/5 enabled the SARS-CoV-2 variants to escape from the immunity induced by vaccination. Age, time after the last vaccine, white blood cell counts, levels of HBcAb, and incidence of SLD were independent factors for the seropositivity of SARS-CoV-2 antibodies. Further studies on the cellular immune response after booster shots are needed to identify immunocompromised patients, which could help them develop optimal vaccination regimens and maximize the benefit of additional shot.
Data availability
The data will be available upon requested.
Ethics statement
The study was approved by the Ethics Committee of Peking Union Medical College Hospital (I-22PJ147 and I-22PJ250) and the Fifth Hospital of Shijiazhuang (2022-017-1). Informed consent was obtained from all participants.
Author contributions
Yongmei Liu: investigation, methodology, project administration, validation, visualization, writing-original draft, review and editing. Jianhua Lu: data curation, investigation, methodology, project administration, validation. Haoting Zhan: investigation, methodology, project administration, validation, writing-review and editing. Wenfang Yuan: conceptualization, data curation, investigation, project administration, resources, writing-review and editing. Xiaomeng Li: investigation, methodology, supervision, writing-review and editing. Haiyan Kang: data curation, investigation, methodology. Haolong Li: methodology, project administration, validation, writing-review and editing. Yongliang Chen: investigation, writing-review and editing. Linlin Cheng: investigation, writing-review and editing. Xingli Sun: investigation, writing-review and editing. Haojie Zheng: investigation, writing-review and editing. Wei Wang: investigation, writing-review and editing. Erhei Dai: funding acquisition, methodology, project administration, supervision, writing-review and editing. Yongzhe Li: data curation, funding acquisition, methodology, project administration, supervision, writing-review and editing.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgments
This work was supported by the Beijing Natural Science Foundation (M23008), the National Key Research and Development Program of China (2018YFE0207300), Beijing Municipal Science & Technology Commission (Z211100002521021), the National High Level Hospital Clinical Research Funding (2022-PUMCH-B-124), and Key Research and Development Plan of Hebei Province, Special Health Innovation Project (22377744D).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2023.07.005.
Contributor Information
Erhei Dai, Email: daieh2008@126.com.
Yongzhe Li, Email: yongzhelipumch@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
References
- Abravanel F., Marion O., Del B.A., Beunon T., Romieu-Mourez R., Couat C., Pucelle M., Staes L., Guitard J., Esposito L., Faguer S., Kamar N., Izopet J. Humoral and cellular immune responses of solid organ transplant patients on belatacept to three doses of mRNA-based anti-SARS-CoV-2 vaccine. Vaccines (Basel) 2022;10:354. doi: 10.3390/vaccines10030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J., Wang J., Liu D., Xiang H., Guo Y., Lv J., Zhang Q., Li J., Zhang X., Li Q., Liang J., Guo X., Feng Y., Liu L., Zhang X., Qin W., Wang X., Rao W., Zhang Q., Tian Q., Zhang Y., Xie F., Jiang S., Yan Y., Qiu Y., Wu H., Hou Z., Zhang N., Zhang A., Ji J., Yang J., Huang J., Zhao Z., Gu Y., Bian L., Zhang Z., Zou S., Ji H., Ge G., Du X., Hou A., Zhu Y., Cong Q., Xu J., Zu H., Wang Y., Yan Z., Yan X., BianBa Y., Ci Q., Zhang L., Yang S., Gao X., Zhong L., He S., Liu C., Huang Y., Liu Y., Xu D., Zhu Q., Xu X., Lv M., Zhang W., Qi X. Safety and immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin. Gastroenterol. Hepatol. 2022;20:1516–1524. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A., Martin-Mateos R., Van der Merwe S., Wiest R., Jalan R., Alvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat. Rev. Gastroenterol. Hepatol. 2022;19:112–134. doi: 10.1038/s41575-021-00520-7. [DOI] [PubMed] [Google Scholar]
- Al-Dury S., Waern J., Waldenstrom J., Alavanja M., Saed H.H., Tornell A., Arabpour M., Wiktorin H.G., Einarsdottir S., Ringlander J., Ringstrom G., Hellstrand K., Martner A., Lagging M. Impaired SARS-CoV-2-specific T-cell reactivity in patients with cirrhosis following mRNA COVID-19 vaccination. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2022.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakasis A.D., Bitzogli K., Mouziouras D., Pouliakis A., Roumpoutsou M., Goules A.V., Androutsakos T. Antibody responses after SARS-CoV-2 vaccination in patients with liver diseases. Viruses. 2022;14:207. doi: 10.3390/v14020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., Huppert A. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsch M., Salie H., Schlaak A.E., Zhang Z., Hess M., Mayer L.S., Tauber C., Otto-Mora P., Ohtani T., Nilsson T., Wischer L., Winkler F., Manne S., Rech A., Schmitt-Graeff A., Bronsert P., Hofmann M., Neumann-Haefelin C., Boettler T., Fichtner-Feigl S., van Boemmel F., Berg T., Rimassa L., Di Tommaso L., Saeed A., D'Alessio A., Pinato D.J., Bettinger D., Binder H., John W.E., Schultheiss M., Thimme R., Bengsch B. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022;77:397–409. doi: 10.1016/j.jhep.2022.02.032. [DOI] [PubMed] [Google Scholar]
- Belik M., Jalkanen P., Lundberg R., Reinholm A., Laine L., Vaisanen E., Skon M., Tahtinen P.A., Ivaska L., Pakkanen S.H., Hakkinen H.K., Ortamo E., Pasternack A., Ritvos M.A., Naves R.A., Miettinen S., Sironen T., Vapalahti O., Ritvos O., Osterlund P., Kantele A., Lempainen J., Kakkola L., Kolehmainen P., Julkunen I. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat. Commun. 2022;13:2476. doi: 10.1038/s41467-022-30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril F., Al D.S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkias S., Harper C., Vrbicky K., Walsh S.R., Essink B., Brosz A., McGhee N., Tomassini J.E., Chen X., Chang Y., Sutherland A., Montefiori D.C., Girard B., Edwards D.K., Feng J., Zhou H., Baden L.R., Miller J.M., Das R. A bivalent omicron-containing booster vaccine against covid-19. N. Engl. J. Med. 2022;387:1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., Al K.H., Coyle P., Ayoub H.H., Al K.Z., Al K.E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul R.H., Nasrallah G.K., Al K.M., Al R.H., Butt A.A., Al-Thani M.H., Al K.A., Bertollini R., Abu-Raddad L.J. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang Y., Song R., Wang L., Hu X., Li H., Cai D., Hu P., Shi X., Ren H. Waning humoral immune responses to inactivated SARS-CoV-2 vaccines in patients with severe liver disease. Signal Transduct. Targeted Ther. 2022;7:174. doi: 10.1038/s41392-022-01032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K.W., Pham N.V., Ibrahim B.M., Hong K., Saab S. Autoimmune hepatitis-like syndrome following COVID-19 vaccination: a systematic review of the literature. Dig. Dis. Sci. 2022;67:4574–4580. doi: 10.1007/s10620-022-07504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D.A., Ferreira I., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., Meng B., Abdullahi A., Elmer A., Kingston N., Graves B., Le Gresley E., Caputo D., Bergamaschi L., Smith K., Bradley J.R., Ceron-Gutierrez L., Cortes-Acevedo P., Barcenas-Morales G., Linterman M.A., McCoy L.E., Davis C., Thomson E., Lyons P.A., McKinney E., Doffinger R., Wills M., Gupta R.K. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipient. J. Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie N.C., Tostanoski L.H., Rodriguez-Aponte S.A., Kaur K., Bajoria S., Kumru O.S., Martinot A.J., Chandrashekar A., McMahan K., Mercado N.B., Yu J., Chang A., Giffin V.M., Nampanya F., Patel S., Bowman L., Naranjo C.A., Yun D., Flinchbaugh Z., Pessaint L., Brown R., Velasco J., Teow E., Cook A., Andersen H., Lewis M.G., Camp D.L., Silverman J.M., Nagar G.S., Rao H.D., Lothe R.R., Chandrasekharan R., Rajurkar M.P., Shaligram U.S., Kleanthous H., Joshi S.B., Volkin D.B., Biswas S., Love J.C., Barouch D.H. SARS-CoV-2 receptor binding domain displayed on HBsAg virus-like particles elicits protective immunity in macaques. Sci. Adv. 2022;8:l6015. doi: 10.1126/sciadv.abl6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidov Y., Indenbaum V., Tsaraf K., Cohen-Ezra O., Likhter M., Ben Y.G., Halperin R., Levy I., Mor O., Agmon-Levin N., Afek A., Rahav G., Lustig Y., Ben A.Z. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J. Hepatol. 2022;77:702–709. doi: 10.1016/j.jhep.2022.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duengelhoef P., Hartl J., Ruther D., Steinmann S., Brehm T.T., Weltzsch J.P., Glaser F., Schaub G.M., Sterneck M., Sebode M., Weiler-Normann C., Addo M.M., Lutgehetmann M., Haag F., Schramm C., Schulze Z.W.J., Lohse A.W. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease. United Eur. Gastroenterol. J. 2022;10:319–329. doi: 10.1002/ueg2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe C., Kulkarni A.V., Terziroli B.B., Magro B., Stattermayer A., Cengiz M., Clayton-Chubb D., Lammert C., Bernsmeier C., Gul O., la Tijera F.H., Anders M., Lytvyak E., Akin M., Purnak T., Liberal R., Peralta M., Ebik B., Duman S., Demir N., Balaban Y., Urzua A., Contreras F., Venturelli M.G., Bilgic Y., Medina A., Girala M., Gunsar F., Londono M.C., Androutsakos T., Kisch A., Yurci A., Guzelbulut F., Cagin Y.F., Avci E., Akyildiz M., Dindar-Demiray E.K., Harputluoglu M., Kumar R., Satapathy S.K., Mendizabal M., Silva M., Fagiuoli S., Roberts S.K., Soylu N.K., Idilman R., Yoshida E.M., Montano-Loza A.J., Dalekos G.N., Ridruejo E., Schiano T.D., Wahlin S. Liver injury after SARS-CoV-2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76:1576–1586. doi: 10.1002/hep.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix O.K., Blumberg E.A., Chang K.M., Chu J., Chung R.T., Goacher E.K., Hameed B., Kaul D.R., Kulik L.M., Kwok R.M., McGuire B.M., Mulligan D.C., Price J.C., Reau N.S., Reddy K.R., Reynolds A., Rosen H.R., Russo M.W., Schilsky M.L., Verna E.C., Ward J.W., Fontana R.J. American association for the study of liver diseases expert panel consensus statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., Gao H., Ge X., Kan B., Hu Y., Liu J., Cai F., Jiang D., Yin Y., Qin C., Li J., Gong X., Lou X., Shi W., Wu D., Zhang H., Zhu L., Deng W., Li Y., Lu J., Li C., Wang X., Yin W., Zhang Y., Qin C. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra V., Piazzolla A.V., Cocomazzi G., Squillante M.M., De Santis E., Totti B., Cavorsi C., Giuliani F., Serra N., Mangia A. Effectiveness of booster dose of anti SARS-CoV-2 BNT162b2 in cirrhosis: longitudinal evaluation of humoral and cellular response. Vaccines (Basel) 2022;10:1281. doi: 10.3390/vaccines10081281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R.R., Painter M.M., Lundgreen K.A., Apostolidis S.A., Baxter A.E., Giles J.R., Mathew D., Pattekar A., Reynaldi A., Khoury D.S., Gouma S., Hicks P., Dysinger S., Hicks A., Sharma H., Herring S., Korte S., Kc W., Oldridge D.A., Erickson R.I., Weirick M.E., McAllister C.M., Awofolaju M., Tanenbaum N., Dougherty J., Long S., D'Andrea K., Hamilton J.T., McLaughlin M., Williams J.C., Adamski S., Kuthuru O., Drapeau E.M., Davenport M.P., Hensley S.E., Bates P., Greenplate A.R., Wherry E.J. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185:1875–1887. doi: 10.1016/j.cell.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachmann N.P., Miller J., Collier A.Y., Ventura J.D., Yu J., Rowe M., Bondzie E.A., Powers O., Surve N., Hall K., Barouch D.H. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad-Boubaker S., Othman H., Touati R., Ayouni K., Lakhal M., Ben M.I., Ghedira K., Kharrat M., Triki H. In silico comparative study of SARS-CoV-2 proteins and antigenic proteins in BCG, OPV, MMR and other vaccines: evidence of a possible putative protective effect. BMC Bioinf. 2021;22:163. doi: 10.1186/s12859-021-04045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T., Zhou Y., Xu P., Ling N., Chen M., Huang T., Zhang B., Yang Z., Ao L., Li H., Chen Z., Zhang D., Shi X., Lei Y., Wang Z., Zeng W., Hu P., Lan Y., Zhou Z., Kang J., Huang Y., Shi T., Pan Q., Zhu Q., Ran X., Zhang Y., Song R., Xiang D., Xiao S., Zhang G., Shen W., Peng M., Cai D., Ren H. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;42:1287–1296. doi: 10.1111/liv.15173. [DOI] [PubMed] [Google Scholar]
- John B.V., Ferreira R.D., Doshi A., Kaplan D.E., Taddei T.H., Spector S.A., Paulus E., Deng Y., Bastaich D., Dahman B. Third dose of COVID-19 mRNA vaccine appears to overcome vaccine hyporesponsiveness in patients with cirrhosis. J. Hepatol. 2022;77:1349–1358. doi: 10.1016/j.jhep.2022.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasuji H., Morinaga Y., Tani H., Saga Y., Kaneda M., Murai Y., Ueno A., Miyajima Y., Fukui Y., Nagaoka K., Ono C., Matsuura Y., Niimi H., Yamamoto Y. Age-dependent reduction in neutralization against alpha and beta variants of BNT162b2 SARS-CoV-2 vaccine-induced immunity. Microbiol. Spectr. 2021;9 doi: 10.1128/Spectrum.00561-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., Rubin C., Freedman L., Kreiss Y., Regev-Yochay G. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Zhang G., Huang M., Wang L., Hong W., Li A., Liang Y., Wang T., Lu J., Ou M., Ren Z., Lu H., Zheng R., Cai X., Pan X., Xia J., Ke C. Progress of the COVID-19: persistence, effectiveness, and immune escape of the neutralizing antibody in convalescent serum. Pathogens. 2022;11:1531. doi: 10.3390/pathogens11121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.M., Xu Q.Y., Jia Z.J., Wu M.J., Liu Y.Y., Lin L.R., Liu L.L., Yang T.C. A third dose of an inactivated vaccine dramatically increased the levels and decay times of anti-SARS-CoV-2 antibodies, but disappointingly declined again: a prospective, longitudinal, cohort study at 18 serial time points over 368 days. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.876037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Qin C., Liu M., Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect. Dis. Pov. 2021;10:132. doi: 10.1186/s40249-021-00915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A., Cazzagon N., Rigamonti C., Cabibbo G., Lai Q., Muratori L., Carbone M. Clinical update on risks and efficacy of anti-SARS-CoV-2 vaccines in patients with autoimmune hepatitis and summary of reports on post-vaccination liver injury. Dig. Liver Dis. 2022;54:722–726. doi: 10.1016/j.dld.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., James W., Gordon S., Pepper M.S. SARS-CoV-2 variants, vaccines, and host immunity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molica S., Giannarelli D., Lentini M., Zappala D., Mannella A., Loiacono D., Gianfelici V., Panduri G., Gariani I., Minchella P., Talarico F., Levato L. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia: a serologic and cellular study. Chemotherapy. 2022;67:91–95. doi: 10.1159/000521229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Thakur N., Peacock T.P., Bialy D., Elrefaey A., Bogaardt C., Horton D.L., Ho S., Kankeyan T., Carr C., Hoschler K., Barclay W.S., Amirthalingam G., Brown K.E., Charleston B., Bailey D. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 2022;7:1180–1188. doi: 10.1038/s41564-022-01163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination: a rare adverse effect? Hepatology. 2022;75:489–490. doi: 10.1002/hep.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., Wehmeyer M., Jahnke-Triankowski J., Mayer L., Hoffmann A., Fischer L., Addo M.M., Lutgehetmann M., Lohse A.W., Schulze Z.W.J., Sterneck M. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin. Gastroenterol. Hepatol. 2022;20:162–172. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha L.B., Foster C., Rawlinson W., Tedla N., Bull R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission. Rev. Med. Virol. 2022;32 doi: 10.1002/rmv.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuluvath P.J., Robarts P., Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J. Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober-Lau P., Schwarz T., Vanshylla K., Hillus D., Gruell H., Suttorp N., Landgraf I., Kappert K., Seybold J., Drosten C., Klein F., Kurth F., Sander L.E., Corman V.M. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir. Med. 2021;9:e104–e105. doi: 10.1016/S2213-2600(21)00456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutoudaki K., Dimakakou M., Androutsakos T. Efficacy, safety and immunogenicity of anti-SARS-CoV-2 vaccines in patients with cirrhosis: a narrative review. Vaccines (Basel) 2023;11:452. doi: 10.3390/vaccines11020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H., Das R., Skelly D., Ritter T.G., Amini A., Bibi S., Adele S., Johnson S.A., Constantinides B., Webster H., Temperton N., Klenerman P., Barnes E., Dunachie S.J., Crook D., Pollard A.J., Lambe T., Goulder P., Paterson N.G., Williams M.A., Hall D.R., Fry E.E., Huo J., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhang Q., Ai J., Liu D., Liu C., Xiang H., Gu Y., Guo Y., Lv J., Huang Y., Liu Y., Xu D., Chen S., Li J., Li Q., Liang J., Bian L., Zhang Z., Guo X., Feng Y., Liu L., Zhang X., Zhang Y., Xie F., Jiang S., Qin W., Wang X., Rao W., Zhang Q., Tian Q., Zhu Y., Cong Q., Xu J., Hou Z., Zhang N., Zhang A., Zu H., Wang Y., Yan Z., Du X., Hou A., Yan Y., Qiu Y., Wu H., Hu S., Deng Y., Ji J., Yang J., Huang J., Zhao Z., Zou S., Ji H., Ge G., Zhong L., He S., Yan X., Yangzhen B.B., Qu C., Zhang L., Yang S., Gao X., Lv M., Zhu Q., Xu X., Zeng Q.L., Qi X., Zhang W. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol. Int. 2022;16:691–701. doi: 10.1007/s12072-022-10332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T., Liang B., Wang H., Quan X., He S., Zhou H., He Y., Yang D., Wang B., Zheng X. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell. Mol. Immunol. 2021;18:2679–2681. doi: 10.1038/s41423-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.Y., Xue J.H., Xiao Y., Jia Z.J., Wu M.J., Liu Y.Y., Li W.L., Liang X.M., Yang T.C. Response and duration of serum anti-SARS-CoV-2 antibodies after inactivated vaccination within 160 days. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.786554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.Z., Hu Y.F., Chen L.L., Yau T., Tong Y.G., Hu J.C., Cai J.P., Chan K.H., Dou Y., Deng J., Wang X.L., Hung I.F., To K.K., Yuen K.Y., Huang J.D. Mining of epitopes on spike protein of SARS-CoV-2 from COVID-19 patients. Cell Res. 2020;30:702–704. doi: 10.1038/s41422-020-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Luo M.T., Wu Q., Wang Y.X., Ma X., Yan G., Zhang S.H., Chen Y., Wan N., Zhang L., You D., Wei J., Zhang Z., Zhou T.C. Long-term and effective neutralization against omicron sublineages elicited by four platform COVID-19 vaccines as a booster dose. Cell Discov. 2023;9:17. doi: 10.1038/s41421-023-00518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available upon requested.