Abstract
Sarcomas are rare and heterogeneous mesenchymal neoplasms originating from the bone or soft tissues, which pose significant treatment challenges. The current standard treatment for sarcomas consists of surgical resection, often combined with chemo- and radiotherapy; however, local recurrence and metastasis remain significant concerns. Although immunotherapy has demonstrated promise in improving long-term survival rates for certain cancers, sarcomas are generally considered to be relatively less immunogenic than other tumors, presenting substantial challenges for effective immunotherapy. In this review, we examine the possible opportunities for sarcoma immunotherapy, noting cancer testis antigens expressed in sarcomas. We then cover the current status of immunotherapies in sarcomas, including progress in cancer vaccines, immune checkpoint inhibitors, and adoptive cellular therapy and their potential in combating these tumors. Furthermore, we discuss the limitations of immunotherapies in sarcomas, including a low tumor mutation burden and immunosuppressive tumor microenvironment, and explore potential strategies to tackle the immunosuppressive barriers in therapeutic interventions, shedding light on the development of effective and personalized treatments for sarcomas. Overall, this review provides a comprehensive overview of the current status and potential of immunotherapies in sarcoma treatment, highlighting the challenges and opportunities for developing effective therapies to improve the outcomes of patients with these rare malignancies.
Keywords: bone cancer, immunotherapy, sarcomas, soft tissue sarcoma, tumor microenviorment
INTRODUCTION
Sarcomas represent a heterogeneous group of mesenchymal neoplasms originating from the bone or soft tissues such as the cartilage, muscle, and other connective tissues. Sarcomas account for 15% of childhood and 1% of all adult malignancies. Despite being a rare malignancy, approximately 13,190 patients in the United States were diagnosed with sarcoma in 2022, with 5,130 fatalities (Siegel et al., 2022). Patients with sarcomas experience a high probability of local recurrence (5-year local recurrence rate of 19%-51%) (Pawlik et al., 2006; Thorkildsen et al., 2021; Xie et al., 2015; Zhao et al., 2018), and repeated relapses eventually accompany distant metastasis (Panagi et al., 2022). Sarcomas are classified into soft tissue sarcomas (STSs), including rhabdomyosarcoma and liposarcoma, and bone sarcomas (BSs), including osteosarcoma and chondrosarcoma, encompassing more than 70 distinct subtypes (Hui, 2016). The main treatment challenge to overcome is the propensity for local recurrence or eventual metastatic spread despite extensive adjuvant therapy (Goorin et al., 1984; Patel et al., 2017; von Konow et al., 2021), which is related to the substantial heterogeneity of these tumors, leading to varied symptoms, treatment responses, and prognoses among patients (Du et al., 2020; Grunewald et al., 2020). Delayed diagnosis is common, with symptoms in the pediatric population often misdiagnosed as growing pains, further narrowing the available treatment options (Lodhia et al., 2022).
Surgical removal is the standard treatment approach for localized, clinically resectable STSs and BSs (Blasius et al., 2022; Gronchi, 2021). Radiotherapy can also be administered in neoadjuvant, adjuvant, or definitive settings (Hoefkens et al., 2016), although the optimal approach varies depending on individual cases. Chemotherapy is recommended for patients with high-grade STSs following resection, but has failed to demonstrate distinct improvements in terms of relapse-free survival or overall survival (OS) (Squires et al., 2020). Although BS subtypes generally do not respond well to chemotherapy, multidrug chemotherapy has shown moderate treatment efficacy for osteosarcoma and Ewing sarcoma in combination with surgery (Ingley et al., 2022). Chondrosarcoma, another common BS subtype, is generally refractory to conventional chemotherapy and radiation therapy, with no supporting efficacy data available (Gelderblom et al., 2008; Monga et al., 2020; Riedel et al., 2009). Metastatic sarcomas exhibit substantial resistance to chemo- and radiotherapy (Olivier et al., 2015). Although the exact mechanisms underlying chemotherapy resistance remain elusive, emerging evidence implicates epigenetic (Rytlewski et al., 2022) and genetic modifications (Kim et al., 2020), and epithelial-mesenchymal transition (EMT) plasticity (Sannino et al., 2017), and the tumor microenvironment (TME) (Tu et al., 2016) as contributory factors. Sarcomas exhibit radioresistance, often attributed to epigenetic changes (de Jong et al., 2019), non-coding RNAs (Chen et al., 2022), and slow cell division rates (Chen et al., 2022; Li et al., 2014). Collectively, current adjuvant chemo- and radiotherapies are insufficient in preventing the metastasis and recurrence of sarcomas, and there remains uncertainty regarding the best resection margins and appropriate use of chemo- and radiotherapy (Hoefkens et al., 2016; Zhang et al., 2022).
Immunotherapy, a strategy that modulates the immune system to target cancer cells, has demonstrated promise for several cancer types (Tan et al., 2020; Waldman et al., 2020), with significantly improved long-term survival rates in melanoma and lymphomas, eliciting less cytotoxicity compared to traditional chemotherapies (Abramson et al., 2020; Tan et al., 2020). Notably, immunotherapy can achieve simultaneous local and distant control while adapting to changes in antigen expression over time, which is significant for preventing recurrent and metastatic tumors (Finkelstein et al., 2012). Cancer immunotherapy relies on two main immune mechanisms: immune surveillance and immune editing. Immune surveillance involves the detection and elimination of tumors by the immune system (Zitvogel et al., 2006), whereas immune editing modifies tumor immunogenicity to either strengthen the immune system’s capacity to eliminate tumor cells or prevent the emergence of immune-resistant tumor variants (O'Donnell et al., 2019). By selectively manipulating the anti-tumor immune mechanism, immunotherapy has potential to prevent cancer metastasis and recurrence (Edwards et al., 2021).
Several successful cancer immunotherapies have emerged recently utilizing cancer vaccines, adoptive transfer therapy, or cytokine therapy (Zhang and Zhang, 2020). However, sarcomas are considered to be relatively less immunogenic than other cancers (Rytlewski et al., 2021; Weng et al., 2022; Zhu et al., 2020). Specifically, sarcomas lack well-established antigens and often display an immunosuppressive TME. Therefore, understanding the immune escape mechanisms and immunosuppressive TME in sarcoma is essential for developing effective sarcoma immunotherapy. In this review, we provide an update of the current status of sarcoma immunotherapy, focusing on the needs, emerging strategies, and challenges.
IMMUNOTHERAPIES: ADDRESSING UNMET NEEDS FOR SARCOMA TREATMENT
Cancer testis antigen-based vaccine therapy
Sarcomas typically express cancer testis antigens (CTAs), a group of tumor-associated antigens found predominantly in male germ cells in the testis but not in the adult somatic tissue. CTAs are generally quiescent in healthy tissues, but their expression is induced in various malignancies (Whitehurst, 2014). The epitopes of CTAs can be recognized by T cells, facilitating immune activation (Juretic et al., 2003).
Among the several CTAs identified in sarcomas to date, New York esophageal squamous cell carcinoma 1 (NY-ESO-1), melanoma-associated antigen (MAGE), and preferentially expressed antigen of melanoma (PRAME) are the most prevalent (Kakimoto et al., 2019; Wei et al., 2020). NY-ESO-1 is detected in various sarcoma subtypes, including myxoid liposarcomas, osteosarcomas, and synovial sarcomas (Hashimoto et al., 2022; Jungbluth et al., 2001; Kakimoto et al., 2019). MAGE expression has been observed in osteosarcoma, synovial sarcoma, and myxoid/round cell liposarcoma, with distinct MAGE subtypes exhibiting variable expression levels. For instance, osteosarcoma exhibited high expression of MAGE-A1, -A2, and -A3, but low expression of MAGE-A12 (Zou et al., 2012). Synovial sarcoma and myxoid/round cell liposarcoma displayed elevated levels of MAGE-A4 (Hemminger et al., 2014; Iura et al., 2017). PRAME expression is elevated in osteosarcoma, synovial sarcoma, and myxoid/round cell liposarcoma (Epping et al., 2005; Wei et al., 2020).
The potential of CTAs as tumor vaccines has been explored with the aim of enhancing immunological recognition and improving immune responses against tumors (Fig. 1A). Tumor vaccines can be classified into two distinct categories: peptide-based vaccines, which involve the direct delivery of antigens, and dendritic cell (DC)-based vaccines, which employ antigen-presenting DCs for targeted immunotherapy (Saxena et al., 2021; Tagliamonte et al., 2014). NY-ESO-1 peptide was successfully delivered using a Salmonella typhimurium vaccine strain, eliciting NY-ESO-1-specific CD8+ and CD4+ T cells in peripheral blood mononuclear cells from patients (Nishikawa et al., 2006). Treatment of another NY-ESO-1 vaccine, CDX-1401, induced both humoral and cellular immunity to NY-ESO-1 in 45 patients with multiple malignant tumors, including sarcomas, 13 of whom experienced disease stabilization and two of whom exhibited tumor regression (Dhodapkar et al., 2014). This first-in-human study of DC-based vaccines showed promise in treating sarcomas. However, the application of CTA-targeting vaccines in sarcomas has yielded inadequate results compared to that for other malignant tumors. Possible explanations for this discrepancy include low T cell functional avidity, high regulatory T cell (Treg) activity, and higher expression of immune checkpoints such as programmed cell death 1 (PD-1) in sarcomas (Wei et al., 2020).
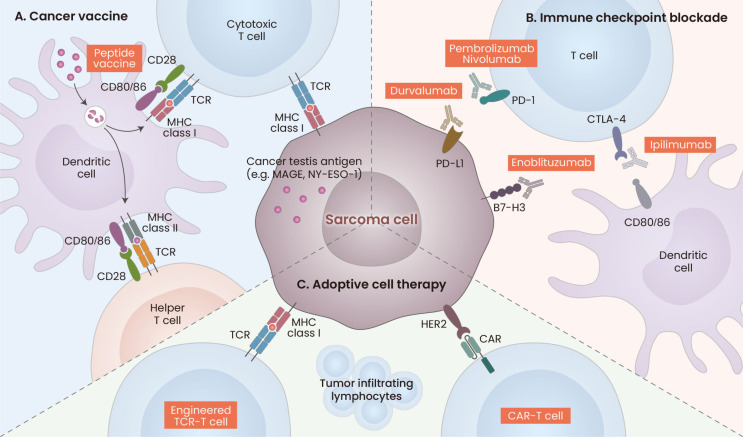
Fig. 1. Current immunotherapies used for sarcoma treatment.
(A) Cancer vaccine: Dendritic cells present sarcoma-derived antigen or neoantigen peptides through MHC class II to T cell receptor (TCR), activating T cells with CD28-CD80/86 co-stimulation. MHC class I presents tumor-specific antigens such as MAGE-A1 or NY-ESO-1, leading to cytotoxic T-lymphocyte (CTL)-mediated killing of sarcoma cells. (B) Immune checkpoint blockade: Sarcoma cells evade T cell-mediated cytotoxicity by expressing immune checkpoint ligands such as programmed cell death ligand 1 (PD-L1), which suppresses T cell activation by binding with complementary receptors on T cells such as programmed cell death 1 (PD-1). Immune checkpoint blockade antibodies targeting PD-L1 or PD-1 interfere with this interaction. Similarly, blocking the cytotoxic T lymphocyte associated antigen 4 (CTLA-4) receptor binding with CD80/86 complex prevents CTL suppression. (C) Adoptive cell therapy: Engineered TCR-T and chimeric antigen receptor (CAR)-T cells specifically engage with tumor cells that express the target antigen on their surface. CAR-T cells are designed to bind with sarcoma cells independent of MHC class I complex. MAGE, melanoma-associated antigen; NY-ESO-1, New York esophageal squamous cell carcinoma 1; HER2, human epidermal growth factor receptor 2.
Immune checkpoint inhibitors
The expression of immune checkpoint proteins such as PD-1 and its ligand (PD-L1), as well as cytotoxic T lymphocyte associated antigen 4 (CTLA-4), is a key immune evasion mechanism of tumors (Beatty and Gladney, 2015; Lee et al., 2021b). Large cohort studies demonstrated the presence of PD-1 (43% of sarcoma cases) (Kim et al., 2016) and CTLA-4 (32% of sarcoma cases) on T cells, as well as PD-L1 (23% of cases) on tumor cells in various STSs and BSs (Paydas et al., 2016; Samiei et al., 2022; Zheng et al., 2015). These findings underscore the potential of these checkpoint proteins as emerging therapeutic targets for immunotherapies in sarcoma treatment (Fig. 1B).
To date, clinical trials evaluating the efficacy of immune checkpoint inhibitors (ICIs) in patients with STSs have demonstrated modest results, with varying responses depending on treatment regimens or histological subtypes. Pembrolizumab, a PD-1 monoclonal antibody, was assessed in a phase II trial involving STSs and BSs, yielding meaningful results in undifferentiated pleomorphic sarcoma (objective response in 40% of patients) and liposarcoma (objective response in 20% of patients), while demonstrating limited efficacy in BS (Tawbi et al., 2017). Additionally, pembrolizumab exhibited high-level, prolonged anti-tumor activity in select sarcoma histotypes such as alveolar soft part sarcoma and chordoma, with 6-month OS rates of 100% and 75%, respectively (NCT03012620). Ipilimumab, a CTLA-4 antibody, and nivolumab, a PD-1 antibody, are two other notable immune checkpoint blockade antibodies promoting anti-tumor immunity in sarcoma (Tawbi et al., 2017; Weber, 2007). Nivolumab resulted in a complete or partial response in 5% of patients with advanced sarcoma when delivered as a monotherapy, whereas ipilimumab monotherapy resulted in a 7% partial response rate; their combination displayed promising efficacy with three times higher confirmed objective responses (16%) in multiple sarcoma subtypes (D'Angelo et al., 2018). A recent clinical trial demonstrated a progression-free survival (PFS) rate of 49% at 12 weeks in patients with various advanced or metastatic sarcomas that received combined therapy with durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) (Somaiah et al., 2022).
Adoptive cellular therapy
Immune evasion in cancer can arise from a lack of neo-antigens or defects in antigen presentation. Adoptive cellular therapy (ACT), or cellular immunotherapy, has potential to surmount these challenges by modifying patient-specific T cells to enhance the targeting of tumor-specific antigens. ACT is a personalized treatment approach involving the isolation, ex vivo modification, expansion, and reinfusion of a patient’s own immune cells, thus bypassing antigen presentation defects (Zhao and Cao, 2019). Various forms of ACT exist, including engineered T cell receptor (TCR) and chimeric antigen receptor (CAR) T cell therapy (June et al., 2018) (Fig. 1C).
TCR therapies using Afamitresgene autoleucel (afami-cel) and Letetresgene autoleucel (lete-cel) are currently under assessment in a phase I/II trial to induce anti-tumor responses in patients with synovial sarcoma and myxoid/round cell liposarcoma. These therapies consist of autologous CD4+ and CD8+ T cells that are genetically modified to express a TCR recognizing MAGE-A4 (afami-cel) or NY-ESO1 (lete-cel) bound to human leukocyte antigen A02. Pooled data from 69 patients (phase I, n = 18; phase II, n = 51) showed an investigator-assessed objective response rate of 36.2% (40.7% in synovial sarcoma; 10.0% in myxoid/round cell liposarcoma) (D'Angelo et al., 2022).
Human epidermal growth factor receptor 2 (HER2) is a potent antigen identified in various sarcomas, albeit with low expression (Ahmed et al., 2009; Yan et al., 2015). CAR-T therapy targeting HER2 exhibited tumor regression in both localized and metastatic lesions, with prolonged survival in mice inoculated even with the low-HER2-expressing osteosarcoma cell line (Ahmed et al., 2009). In a clinical phase I/II study of HER2 CAR-T cells to treat HER2-positive osteosarcoma, Ewing sarcoma, neuroectodermal tumor, and desmoplastic small round cell tumor, 4 of the 17 patients experienced stable disease for 12 weeks to 14 months and three patients had their residual tumors removed without additional therapy, with one specimen achieving ≥90% tumor necrosis (Ahmed et al., 2015). Combination of this therapy with lympho-depleting chemotherapy showed further improvement in outcomes (Navai et al., 2019). The ganglioside antigen GD2 is expressed in Ewing sarcoma and osteosarcoma (Kailayangiri et al., 2012; Roth et al., 2014). GD2-specific CAR-T cells in combination with AMG102, a human growth factor receptor neutralizing antibody, inhibited the primary tumor growth and metastasis of Ewing sarcoma (Charan et al., 2020).
TACKLING IMMUNOSUPPRESSIVE BARRIERS IN THERAPEUTIC INTERVENTIONS FOR SARCOMA
Immunotherapy has shown beneficial results in treating various cancers, yet its efficacy in STSs and BSs remains restricted to specific subtypes (Ayodele and Razak, 2020; Panagi et al., 2022), necessitating further strategies to improve efficacy (Klemen et al., 2021). Some combined immunotherapies, in conjunction with chemotherapy, radiotherapy, and targeted therapies, have demonstrated improved prognosis in sarcomas (D'Angelo et al., 2018; Husain et al., 2023; Kelly et al., 2020; Tang et al., 2021). However, challenges persist in treating immunologically “cold” sarcomas. In this section, we discuss key mechanisms that might contribute to these challenges and the approaches developed to potentially overcome them (Fig. 2).
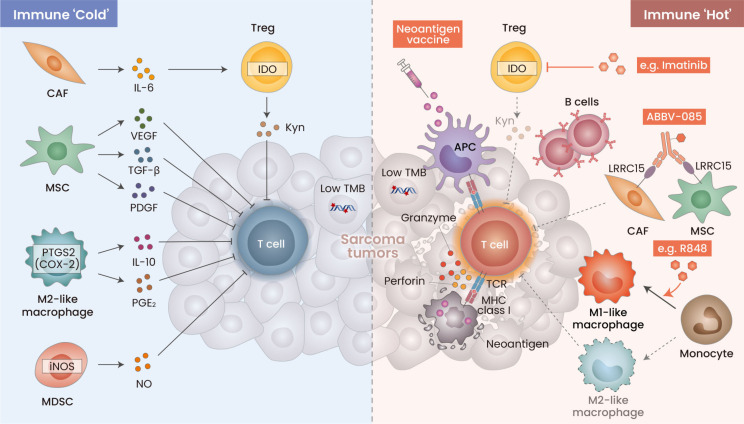
Fig. 2. Barriers to immunotherapy in sarcomas and strategies for modulating their tumor microenvironment.
Sarcomas are primarily recognized by their low tumor mutation burden, which classifies them as tumors displaying limited immune reactivity. The immunosuppressive features characterizing the tumor microenvironment (TME) of sarcomas are correlated with the existence of regulatory T cells, tumor-associated macrophages (TAM), and myeloid-derived suppressor cells (MDSCs) (left). These components present prospective targets for crafting immunotherapy approaches with enhanced efficacy in tackling sarcomas (right). A personalized cancer vacccine addresses low tumor mutation burden by utilizing neoantigens to activate precise cytotoxic T cells targeting sarcoma tumors. TAMs can be regulated by inhibiting M2-like TAMs or by stimulating M1-like macrophages to amplify anti-tumor immune responses. Approaches that combine imatinib with CTLA-4 blockade or employ anti-CD25 antibodies alongside PD-1 blockade can control regulatory T cells (Tregs) and improve treatment outcomes. CCR4 inhibitors have shown potential in reducing Treg infiltration. Cancer-associated fibroblasts (CAFs) expressing LRRC15 have emerged as promising targets, with the antibody-drug conjugate ABBV-085, which targets LRRC15, displaying notable anti-tumor activity in clinical trials. These strategies seek to overcome the immunologically cold TME in sarcomas, enhancing the efficacy of immunotherapy. MSCs, mesenchymal stem cells; IDO, indoleamine 2,3-dioxygenase; TMB, tumor mutation burden; TCR, T cell receptor.
Low tumor mutation burden
Tumor mutation burden (TMB), reflecting the number of mutations harbored by tumors, is a valuable biomarker positively related to the immunotherapy response (Strickler et al., 2021). Among 151 immunotherapy-treated patients, a higher TMB correlated with better outcomes, including response rate, PFS, and OS (Goodman et al., 2017). This favorable response is attributed to a positive correlation between TMB and immunogenic neoantigens (Lagos et al., 2020).
The U.S. Food and Drug Administration approved pembrolizumab for cancers with a TMB ≥ 10 mutations/Mb, indicating therapeutic implications based on qualitative next-generation sequencing; however, no patients with sarcoma were enrolled in this study (Marabelle et al., 2020). According to a comprehensive mutational analysis pipeline that incorporated clinical data, it was found that among 206 cases of STS, patients exhibited an average TMB of 1.06 mutations/Mb (Cancer Genome Atlas Research Network, 2017). In the case of BSs, TMB ranged from 0 to 7 mutations/Mb among 31 osteosarcoma patients. The 3rd quartile of TMB in this patient cohort was determined to be 2.565 mutations/Mb (Xie et al., 2021). Collectively, sarcomas are primarily characterized by low TMB (compared to melanoma and lung cancers, with 20% of patients meeting the TMB cutoff of ≥10 mutations/Mb for pembrolizumab), rendering them immunologically cold tumors (Doig et al., 2022). This poses a considerable challenge for the development of effective immunotherapies for sarcoma patients.
To overcome the challenge of low TMB, recent strides in cancer immunotherapy have explored the capacity of neoantigens to stimulate cytotoxic T cells, targeting sarcoma tumor cells with distinct specificity (Gubin et al., 2014; Yang et al., 2019). Originating from somatic mutations or chromosomal rearrangements, neoantigens are frequently present in sarcomas. In synovial sarcomas, peptides from the typical SYT-SSX fusion have been effective in generating specific CTLs for synovial sarcoma (Kawaguchi et al., 2012; Sato et al., 2002). The implementation of a customized immunotherapy strategy, tailored to the distinctive tumor traits of each individual, presents exciting new opportunities for effectively treating sarcomas with low TMB.
Immunosuppressive TME
The TME plays a pivotal role in shaping the immune response to cancer cells; thus, understanding its composition is essential for developing effective immunotherapies. Petitprez et al. (2020) demonstrated that STSs with immune-deprived TMEs displayed low expression of immune checkpoint-related genes (PD-1, PD-L2, CTLA-4, and TIM3). In contrast, B-cell-rich tertiary lymphoid structures, predominantly observed in immune-rich classes, were positively correlated with improved survival and higher response rates to PD-1 blockade. The immune cell distribution within the TME critically influences the immune response in sarcomas. Tight-junction protein 1 (TJP1) may play a role in shaping the immunosuppressive landscape of TME in leiomyosarcoma. TJP1 contributes to tumor growth by modulating cell-cell aggregation and cytokine-mediated communication in TME. Notably, TJP1 exhibits a negative correlation with expression of colony stimulating factor 1 (CSF1), which typically fosters an immunosuppressive TME through macrophage regulation (Lee et al., 2021a). Only 22% and 12% of 50 sarcoma specimens exhibited PD-1 and PD-L1 expression, respectively, whereas 75% of the specimens contained FOXP3+ Tregs in the immunological milieu (D'Angelo et al., 2015). Single-cell transcriptomic profiling of osteosarcomas and their TMEs identified 11 main cell clusters such as osteoblastic cells, myeloid cells, mesenchymal stem cells (MSCs), fibroblasts, and tumor-infiltrating lymphocytes (TILs). Notably, the TILs cluster displayed high expression of T cell exhaustion inhibitory receptors, including TIGIT and LAG3, and blocking TIGIT enhanced the cytotoxic effects of CD3+ T cells against osteosarcoma cells (Zhou et al., 2020).
Antigen presentation by tumor-associated macrophages (TAMs) contributes to anti-tumor immune responses. M1-like macrophages exhibit anti-tumoral functions, whereas M2-like macrophages exhibit pro-tumoral functions. Immunohistochemistry of STS samples demonstrated the widespread presence of macrophages in the TME, which were polarized toward the M2-like phenotype. Concurrent expression of the M2-like macrophage marker CD163, along with IL-10 and PTGS2—indicators of an immunosuppressive TME—was also observed. Notably, the favorable prognostic role of MS4A1-expressing CD20-positive B cells was not apparent in tumors with high expression of IL-10 and CD163. This finding underscores the rationale for targeting M2-polarized macrophages in STS (Tsagozis et al., 2019), which can be achieved by either inhibiting M2-like TAMs or activating M1-like macrophages.
The TMEs of multiple murine sarcoma models induced tumors to produce retinoic acid (RA), which promoted the differentiation to M2-like macrophages and suppressed DCs. Reducing tumor RA production and inhibiting RA-receptor signaling enhanced anti-tumor immunity. Human sarcomas such as leiomyosarcoma, dedifferentiated liposarcoma, myxofibrosarcoma, undifferentiated pleomorphic sarcoma, and synovial sarcoma, also exhibited RA-mediated immunosuppression (Devalaraja et al., 2020). Inhibition of TREM2, an oncogenic marker found in tumor-infiltrating macrophages, increased the responsiveness to anti-PD-1 immunotherapy in sarcoma model mice. Deletion of TREM2 or administering anti-TREM2 treatments resulted in decreased tumor infiltration of CX3CR1+/MRC1+ TAMs and expansion of myeloid subsets that express immunostimulatory molecules improving T cell responses (Molgora et al., 2020). Moreover, various strategies investigated in other types of cancer may also hold promise for sarcoma treatment. Dual-targeted nanoparticles containing small interfering RNA against colony-stimulating factor-1 receptor depleted M2-like macrophages, increasing pro-inflammatory cytokines and decreasing immunosuppressive cytokines (Qian et al., 2017). R848, an agonist of Toll-like receptors 7 and 8, was identified as a potent driver of the M1 phenotype, and its nanoparticle-mediated delivery significantly shifted TAMs toward the M1 phenotype, leading to inhibited tumor growth (Rodell et al., 2018).
Myeloid-derived suppressor cells (MDSCs) encompass a diverse range of immature and developed myeloid cells that play crucial roles in cancer-associated immunosuppression (Nakamura and Smyth, 2020). MDSCs are divided into monocytic (M-MDSC) and granulocytic (G-MDSC) subgroups. Highfill et al. (2014) showed that G-MDSCs could suppress T cells in rhabdomyosarcoma. G-MDSCs are recruited to tumors via the CXCR2 signaling pathway, leading to immunosuppressive consequences and unfavorable outcomes for anti-PD-1 therapies in patients with rhabdomyosarcoma.
Stromal regions in the TME contain blood and lymphatic endothelial cells, MSCs, cancer-associated fibroblasts (CAFs), pericytes, and other cell types (Turley et al., 2015). CAFs are predominant in the TME, expressing α-smooth actin and fibroblast activation protein. The accumulation of CAFs in the TME is associated with a poor prognosis in many cancers; CAFs diminish anti-tumor immune responses by recruiting Tregs (Liu et al., 2019; Quante et al., 2011) and higher Treg levels have been associated with an increased risk of local recurrence in patients with STS (Smolle et al., 2021). Various strategies have been proposed to control Tregs as a therapy for sarcoma. Combining imatinib with CTLA-4 blockade showed synergistic effects in gastrointestinal stromal tumor (a subtype of STS). The intracellular enzyme indoleamine 2,3-dioxygenase (IDO) participates in immune escape pathways as a major regulator of the tryptophan catabolism pathway, with kynurenine being a crucial metabolite. Kynurenine fosters immune tolerance by selectively expanding Tregs (Holmgaard et al., 2013). IDO1 expression was observed in 39.1% of human sarcoma cases and its blockade led to a significant decrease in both the plasmatic kynurenine-to-tryptophan ratio and tumoral kynurenine level (Nafia et al., 2020). IDO1-deficient mice showed an enhanced response to immunotherapies utilizing ICIs targeting CTLA4, PD-1, and PD-L1 (Holmgaard et al., 2013). These findings suggest IDO1 as a potential target to increase the immunogenicity of sarcomas and improve the efficacy of immunotherapies. Imatinib inhibits IDO and suppresses the number and activity of Tregs (Balachandran et al., 2011). The use of anti-CD25 antibodies in combination with PD-1 blockade resulted in the synergistic elimination of tumors in various cancer models, including a fibrosarcoma model (Arce Vargas et al., 2017). As the CCR4–CCL22 axis assists in the migration of Tregs to tumor sites, CCR4 inhibitors have been considered to inhibit Treg infiltration. In a clinical study of mogamulizumab, an anti-CCR4 antibody, sarcoma patients exhibited improved prognosis when treated with durvalumab, an anti-PD-1 antibody (Zamarin et al., 2020).
MSCs, which are sources of fibroblasts and pericytes, are also well-known immune modulators that contribute to anti-tumor immunity suppression (Li et al., 2022). MSCs and CAFs have been identified in the TME of osteosarcoma (Zhou et al., 2020). Based on single-cell RNA sequencing and bioinformatic analysis, CAFs exhibit higher infiltration levels in recurrent osteosarcoma, correlating with enrichment of the EMT pathway (Huang et al., 2022). MSCs secrete factors relevant to osteosarcoma growth and immunosuppression, such as angiogenesis factors (vascular endothelial growth factor and platelet-derived growth factor) and tumor growth factor-β, which attenuate anti-tumor immune activity (Ohm et al., 2003; Zheng et al., 2018). Stromal cells have also been investigated as therapeutic targets to overcome immunosuppression. Various tumors, including osteosarcoma and undifferentiated pleomorphic sarcoma, displayed high expression of leucine-rich repeat-containing 15 (LRRC15) on CAFs and stromal cells, impeding CD8+ T cell function (Krishnamurty et al., 2022; Purcell et al., 2018). A first-in-human phase I clinical trial of the antibody-drug conjugate ABBV-085, which targets LRRC15, demonstrated the ability to treat tumors by targeting stromal cells, exhibiting anti-tumor activity in patients with osteosarcoma and undifferentiated sarcoma (Demetri et al., 2021).
SUMMARY AND PROSPECTS
Despite advancements in various chemotherapy and radiotherapy options beyond conventional surgical treatment for sarcomas, significant treatment challenges remain. The promise of immunotherapy in various solid cancers has inspired numerous studies investigating its potential in sarcomas, including cancer vaccines, immune checkpoint blockade, and ACT. However, sarcomas are often immunologically cold due to both intrinsic and extrinsic factors. Intrinsically, sarcomas may lack appropriate antigens for immune cell recognition or may induce T cell exhaustion through continuous expression of inhibitory signal. Extrinsic factors such as the TME can contribute to an immunosuppressive environment, compromising immune infiltration or activation. Despite progress in understanding the components of the immunosuppressive TMEs, many immune-resistance mechanisms remain unknown, and sarcoma TMEs can vary widely across subtypes. Therefore, a deeper understanding of TMEs in sarcomas is essential for the development of advanced immunotherapy strategies that can effectively overcome these barriers and provide more personalized and effective treatments.
Funding Statement
ACKNOWLEDGMENTS This work was supported by grants from the National Research Foundation of Korea (NRF-2023R1A2C3003864 and RS-2023-00213119); Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2023-00217266); the Institute for Basic Science from the Ministry of Science, ICT, and Future Planning of Korea (IBS-R008-D1); the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (HI21C1218); and Suh Kyungbae foundation (18010068).
Footnotes
AUTHOR CONTRIBUTIONS
S.J., S.A., H.G.K., Y.-J.K., and J.-H.K. contributed to the literature search and the final manuscript. H.G.K., Y.-J.K., and J.-H. K. discussed and designed the frame of the manuscript. S.J., S.A., H.G.K., Y.-J.K., and J.-H.K. wrote the first draft of this manuscript and generated figures. S.J., S.A., D.K., J.N., J.S., J.H.K., H.J.Y., H.G.K., Y.-J.K., and J.-H.K. reviewed, revised, and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Abramson J.S., Palomba M.L., Gordon L.I., Lunning M.A., Wang M., Arnason J., Mehta A., Purev E., Maloney D.G., Andreadis C., et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- Ahmed N., Brawley V.S., Hegde M., Robertson C., Ghazi A., Gerken C., Liu E., Dakhova O., Ashoori A., Corder A., et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015;33:1688–1696. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N., Salsman V.S., Yvon E., Louis C.U., Perlaky L., Wels W.S., Dishop M.K., Kleinerman E.E., Pule M., Rooney C.M., et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce Vargas F., Furness A.J.S., Solomon I., Joshi K., Mekkaoui L., Lesko M.H., Miranda Rota E., Dahan R., Georgiou A., Sledzinska A., et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayodele O., Razak A.R.A. Immunotherapy in soft-tissue sarcoma. Curr. Oncol. 2020;27(Suppl 1):17–23. doi: 10.3747/co.27.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran V.P., Cavnar M.J., Zeng S., Bamboat Z.M., Ocuin L.M., Obaid H., Sorenson E.C., Popow R., Ariyan C., Rossi F., et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G.L., Gladney W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius F., Delbruck H., Hildebrand F., Hofmann U.K. Surgical treatment of bone sarcoma. Cancers (Basel) 2022;14:2694. doi: 10.3390/cancers14112694.ac4782518cd94eb0b6677e25450cb4c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–965.e28. doi: 10.1016/j.cell.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan M., Dravid P., Cam M., Audino A., Gross A.C., Arnold M.A., Roberts R.D., Cripe T.P., Pertsemlidis A., Houghton P.J., et al. GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma. Int. J. Cancer. 2020;146:3184–3195. doi: 10.1002/ijc.32743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Zhang T.N., Zhang F.Y., Zhang T. Non-coding RNAs in drug and radiation resistance of bone and soft-tissue sarcoma: a systematic review. Elife. 2022;11:e79655. doi: 10.7554/eLife.79655.sa2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo S.P., Attia S., Blay J.Y., Strauss S.J., Morales C.M.V., Razak A.R.A., Winkle E.V., Annareddy T., Sattigari C., Diamantopoulos E., et al. Identification of response stratification factors from pooled efficacy analyses of afamitresgene autoleucel ("Afami-cel" [Formerly ADP-A2M4]) in metastatic synovial sarcoma and myxoid/round cell liposarcoma phase 1 and phase 2 trials. J. Clin. Oncol. 2022;40(16 Suppl):11562. doi: 10.1200/JCO.2022.40.16_suppl.11562. [DOI] [Google Scholar]
- D'Angelo S.P., Mahoney M.R., Van Tine B.A., Atkins J., Milhem M.M., Jahagirdar B.N., Antonescu C.R., Horvath E., Tap W.D., Schwartz G.K., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19:416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo S.P., Shoushtari A.N., Agaram N.P., Kuk D., Qin L.X., Carvajal R.D., Dickson M.A., Gounder M., Keohan M.L., Schwartz G.K., et al. Prevalence of tumor-infiltrating lymphocytes and PD-L1 expression in the soft tissue sarcoma microenvironment. Hum. Pathol. 2015;46:357–365. doi: 10.1016/j.humpath.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong Y., Ingola M., Briaire-de Bruijn I.H., Kruisselbrink A.B., Venneker S., Palubeckaite I., Heijs B., Cleton-Jansen A.M., Haas R.L.M., Bovee J. Radiotherapy resistance in chondrosarcoma cells; a possible correlation with alterations in cell cycle related genes. Clin. Sarcoma Res. 2019;9:9. doi: 10.1186/s13569-019-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G.D., Luke J.J., Hollebecque A., Powderly J.D., 2nd, Spira A.I., 2nd, Subbiah V., 2nd, Naumovski L., 2nd, Chen C., 2nd, Fang H., 2nd, Lai D.W., 2nd, et al. First-in-human phase I study of ABBV-085, an antibody-drug conjugate targeting LRRC15, in sarcomas and other advanced solid tumors. Clin. Cancer Res. 2021;27:3556–3566. doi: 10.1158/1078-0432.CCR-20-4513. [DOI] [PubMed] [Google Scholar]
- Devalaraja S., To T.K.J., Folkert I.W., Natesan R., Alam M.Z., Li M., Tada Y., Budagyan K., Dang M.T., Zhai L., et al. Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell. 2020;180:1098–1114.e16. doi: 10.1016/j.cell.2020.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar M.V., Sznol M., Zhao B., Wang D., Carvajal R.D., Keohan M.L., Chuang E., Sanborn R.E., Lutzky J., Powderly J., et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014;6:232ra51. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig K.D., Fellowes A., Scott P., Fox S.B. Tumour mutational burden: an overview for pathologists. Pathology. 2022;54:249–253. doi: 10.1016/j.pathol.2021.11.008. [DOI] [PubMed] [Google Scholar]
- Du X.H., Wei H., Zhang P., Yao W.T., Cai Q.Q. Heterogeneity of soft tissue sarcomas and its implications in targeted therapy. Front. Oncol. 2020;10:564852. doi: 10.3389/fonc.2020.564852.66333db584304cf3a0621857393cf643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S.C., Hoevenaar W.H.M., Coffelt S.B. Emerging immunotherapies for metastasis. Br. J. Cancer. 2021;124:37–48. doi: 10.1038/s41416-020-01160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping M.T., Wang L., Edel M.J., Carlee L., Hernandez M., Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Finkelstein S.E., Fishman M., Conley A.P., Gabrilovich D., Antonia S., Chiappori A. Cellular immunotherapy for soft tissue sarcomas. Immunotherapy. 2012;4:283–290. doi: 10.2217/imt.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H., Hogendoorn P.C., Dijkstra S.D., van Rijswijk C.S., Krol A.D., Taminiau A.H., Bovee J.V. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–329. doi: 10.1634/theoncologist.2007-0237. [DOI] [PubMed] [Google Scholar]
- Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorin A.M., Delorey M.J., Lack E.E., Gelber R.D., Price K., Cassady J.R., Levey R., Tapper D., Jaffe N., Link M. Prognostic significance of complete surgical resection of pulmonary metastases in patients with osteogenic sarcoma: analysis of 32 patients. J. Clin. Oncol. 1984;2:425–431. doi: 10.1200/JCO.1984.2.5.425. [DOI] [PubMed] [Google Scholar]
- Gronchi A. Surgery in soft tissue sarcoma: the thin line between a surgical or more conservative approach. Future Oncol. 2021;17(21s):3–6. doi: 10.2217/fon-2021-0449. [DOI] [PubMed] [Google Scholar]
- Grunewald T.G., Alonso M., Avnet S., Banito A., Burdach S., Cidre-Aranaz F., Di Pompo G., Distel M., Dorado-Garcia H., Garcia-Castro J., et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020;12:e11131. doi: 10.15252/emmm.201911131.32421af97ff64f339c73c8bfd6f57409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J., et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nishimura S., Ito T., Oka N., Kakinoki R., Akagi M. Clinicopathological assessment of cancer/testis antigens NY-ESO-1 and MAGE-A4 in osteosarcoma. Eur. J. Histochem. 2022;66:3377. doi: 10.4081/ejh.2022.3377.84a941f546f3495fa10b8624d54c6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminger J.A., Toland A.E., Scharschmidt T.J., Mayerson J.L., Guttridge D.C., Iwenofu O.H. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod. Pathol. 2014;27:1238–1245. doi: 10.1038/modpathol.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfill S.L., Cui Y., Giles A.J., Smith J.P., Zhang H., Morse E., Kaplan R.N., Mackall C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefkens F., Dehandschutter C., Somville J., Meijnders P., Van Gestel D. Soft tissue sarcoma of the extremities: pending questions on surgery and radiotherapy. Radiat. Oncol. 2016;11:136. doi: 10.1186/s13014-016-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard R.B., Zamarin D., Munn D.H., Wolchok J.D., Allison J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Wang L., Guo H., Zhang W., Shao Z. Single-cell transcriptomics reveals the regulative roles of cancer associated fibroblasts in tumor immune microenvironment of recurrent osteosarcoma. Theranostics. 2022;12:5877–5887. doi: 10.7150/thno.73714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J.Y. Epidemiology and etiology of sarcomas. Surg. Clin. North Am. 2016;96:901–914. doi: 10.1016/j.suc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Husain M., Quiroga D., Kim H.G., Lenobel S., Xu M., Iwenofu H., Chen J.L., Verschraegen C., Liebner D., Tinoco G. Clinical markers of immunotherapy outcomes in advanced sarcoma. BMC Cancer. 2023;23:326. doi: 10.1186/s12885-023-10758-w.a313bc60a4274fc19fc0bd6444b4595b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingley K.M., Maleddu A., Grange F.L., Gerrand C., Bleyer A., Yasmin E., Whelan J., Strauss S.J. Current approaches to management of bone sarcoma in adolescent and young adult patients. Pediatr. Blood Cancer. 2022;69:e29442. doi: 10.1002/pbc.29442. [DOI] [PubMed] [Google Scholar]
- Iura K., Kohashi K., Ishii T., Maekawa A., Bekki H., Otsuka H., Yamada Y., Yamamoto H., Matsumoto Y., Iwamoto Y., et al. MAGEA4 expression in bone and soft tissue tumors: its utility as a target for immunotherapy and diagnostic marker combined with NY-ESO-1. Virchows Arch. 2017;471:383–392. doi: 10.1007/s00428-017-2206-z. [DOI] [PubMed] [Google Scholar]
- June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- Jungbluth A.A., Antonescu C.R., Busam K.J., Iversen K., Kolb D., Coplan K., Chen Y.T., Stockert E., Ladanyi M., Old L.J. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int. J. Cancer. 2001;94:252–256. doi: 10.1002/ijc.1451. [DOI] [PubMed] [Google Scholar]
- Juretic A., Spagnoli G.C., Schultz-Thater E., Sarcevic B. Cancer/testis tumour-associated antigens: immunohistochemical detection with monoclonal antibodies. Lancet Oncol. 2003;4:104–109. doi: 10.1016/S1470-2045(03)00982-3. [DOI] [PubMed] [Google Scholar]
- Kailayangiri S., Altvater B., Meltzer J., Pscherer S., Luecke A., Dierkes C., Titze U., Leuchte K., Landmeier S., Hotfilder M., et al. The ganglioside antigen G(D2) is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br. J. Cancer. 2012;106:1123–1133. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T., Matsumine A., Kageyama S., Asanuma K., Matsubara T., Nakamura T., Iino T., Ikeda H., Shiku H., Sudo A. Immunohistochemical expression and clinicopathological assessment of the cancer testis antigens NY-ESO-1 and MAGE-A4 in high-grade soft-tissue sarcoma. Oncol. Lett. 2019;17:3937–3943. doi: 10.3892/ol.2019.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S., Tsukahara T., Ida K., Kimura S., Murase M., Kano M., Emori M., Nagoya S., Kaya M., Torigoe T., et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci. 2012;103:1625–1630. doi: 10.1111/j.1349-7006.2012.02370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C.M., Antonescu C.R., Bowler T., Munhoz R., Chi P., Dickson M.A., Gounder M.M., Keohan M.L., Movva S., Dholakia R., et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol. 2020;6:402–408. doi: 10.1001/jamaoncol.2019.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Kim E.K., Jung H., Chon H.J., Han J.W., Shin K.H., Hu H., Kim K.S., Choi Y.D., Kim S., et al. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434. doi: 10.1186/s12885-016-2451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Cho Y., Kim H.S., Kang D., Cheon D., Kim Y.J., Chang M.J., Lee K.M., Chang C.B., Kang S.B., et al. A system-level approach identifies HIF-2alpha as a critical regulator of chondrosarcoma progression. Nat. Commun. 2020;11:5023. doi: 10.1038/s41467-020-18817-7.433db9da38794f3ca4269f360c059b88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemen N.D., Kelly C.M., Bartlett E.K. The emerging role of immunotherapy for the treatment of sarcoma. J. Surg. Oncol. 2021;123:730–738. doi: 10.1002/jso.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurty A.T., Shyer J.A., Thai M., Gandham V., Buechler M.B., Yang Y.A., Pradhan R.N., Wang A.W., Sanchez P.L., Qu Y., et al. LRRC15(+) myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature. 2022;611:148–154. doi: 10.1038/s41586-022-05272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos G.G., Izar B., Rizvi N.A. Beyond tumor PD-L1: emerging genomic biomarkers for checkpoint inhibitor immunotherapy. Am. Soc. Clin. Oncol. Educ. Book. 2020;40:1–11. doi: 10.1200/EDBK_289967. [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Kim M., Choi B.K., Kim D.H., Choi I., You H.J. TJP1 contributes to tumor progression through supporting cell-cell aggregation and communicating with tumor microenvironment in leiomyosarcoma. Mol. Cells. 2021a;44:784–794. doi: 10.14348/molcells.2021.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee J.B., Ha S.J., Kim H.R. Clinical perspectives to overcome acquired resistance to anti-programmed death-1 and anti-programmed death ligand-1 therapy in non-small cell lung cancer. Mol. Cells. 2021b;44:363–373. doi: 10.14348/molcells.2021.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Fan Q., Peng X., Yang S., Wei S., Liu J., Yang L., Li H. Mesenchymal/stromal stem cells: necessary factors in tumour progression. Cell Death Discov. 2022;8:333. doi: 10.1038/s41420-022-01107-0.2ec4daf3d489405993858eeb4d1e9687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Geng P., Jiang W., Wang Y., Yao J., Lin X., Liu J., Huang L., Su B., Chen H. Enhancement of radiosensitivity by 5-Aza-CdR through activation of G2/M checkpoint response and apoptosis in osteosarcoma cells. Tumour Biol. 2014;35:4831–4839. doi: 10.1007/s13277-014-1634-5. [DOI] [PubMed] [Google Scholar]
- Liu T., Han C., Wang S., Fang P., Ma Z., Xu L., Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1.779213d6b2824f0fbadfe5efccb405cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhia J., Goodluck G., Tendai J., Urassa E., Nkya G., Mremi A. Case series of high-grade soft tissue sarcoma of the lower limb with delayed diagnosis: experience at a tertiary hospital in northern Tanzania. Int. J. Surg. Case Rep. 2022;97:107475. doi: 10.1016/j.ijscr.2022.107475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W.H., Jr., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- Molgora M., Esaulova E., Vermi W., Hou J., Chen Y., Luo J., Brioschi S., Bugatti M., Omodei A.S., Ricci B., et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182:886–900.e17. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga V., Mani H., Hirbe A., Milhem M. Non-conventional treatments for conventional chondrosarcoma. Cancers (Basel) 2020;12:1962. doi: 10.3390/cancers12071962.916f2455de7749ef870a8d760072001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafia I., Toulmonde M., Bortolotto D., Chaibi A., Bodet D., Rey C., Velasco V., Larmonier C.B., Cerf L., Adam J., et al. IDO targeting in sarcoma: biological and clinical implications. Front. Immunol. 2020;11:274. doi: 10.3389/fimmu.2020.00274.938b0aa849da43e2911a5a9d4a708446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Smyth M.J. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell. Mol. Immunol. 2020;17:1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navai S.A., Derenzo C., Joseph S., Sanber K., Byrd T., Zhang H., Mata M., Gerken C., Shree A., Mathew P.R., et al. Abstract LB-147: Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 2019;79(13 Suppl):LB-147. doi: 10.1158/1538-7445.AM2019-LB-147. [DOI] [Google Scholar]
- Nishikawa H., Sato E., Briones G., Chen L.M., Matsuo M., Nagata Y., Ritter G., Jager E., Nomura H., Kondo S., et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- Ohm J.E., Gabrilovich D.I., Sempowski G.D., Kisseleva E., Parman K.S., Nadaf S., Carbone D.P. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- Olivier T., Pop D., Chouiter Djebaili A., Falk A.T., Iannessi A., Saada E., Nettekoven W., Blay J.Y., Baque P., Cupissol D., et al. Treating metastatic sarcomas locally: a paradoxe, a rationale, an evidence? Crit. Rev. Oncol. Hematol. 2015;95:62–77. doi: 10.1016/j.critrevonc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Panagi M., Pilavaki P., Constantinidou A., Stylianopoulos T. Immunotherapy in soft tissue and bone sarcoma: unraveling the barriers to effectiveness. Theranostics. 2022;12:6106–6129. doi: 10.7150/thno.72800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.A., Royce T.J., Barysauskas C.M., Thornton K.A., Raut C.P., Baldini E.H. Surveillance imaging patterns and outcomes following radiation therapy and radical resection for localized extremity and trunk soft tissue sarcoma. Ann. Surg. Oncol. 2017;24:1588–1595. doi: 10.1245/s10434-016-5755-5. [DOI] [PubMed] [Google Scholar]
- Pawlik T.M., Pisters P.W., Mikula L., Feig B.W., Hunt K.K., Cormier J.N., Ballo M.T., Catton C.N., Jones J.J., O'Sullivan B., et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann. Surg. Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Paydas S., Bagir E.K., Deveci M.A., Gonlusen G. Clinical and prognostic significance of PD-1 and PD-L1 expression in sarcomas. Med. Oncol. 2016;33:93. doi: 10.1007/s12032-016-0807-z. [DOI] [PubMed] [Google Scholar]
- Petitprez F., de Reynies A., Keung E.Z., Chen T.W., Sun C.M., Calderaro J., Jeng Y.M., Hsiao L.P., Lacroix L., Bougouin A., et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- Purcell J.W., Tanlimco S.G., Hickson J., Fox M., Sho M., Durkin L., Uziel T., Powers R., Foster K., McGonigal T., et al. LRRC15 is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res. 2018;78:4059–4072. doi: 10.1158/0008-5472.CAN-18-0327. [DOI] [PubMed] [Google Scholar]
- Qian Y., Qiao S., Dai Y., Xu G., Dai B., Lu L., Yu X., Luo Q., Zhang Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S., et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel R.F., Larrier N., Dodd L., Kirsch D., Martinez S., Brigman B.E. The clinical management of chondrosarcoma. Curr. Treat. Options Oncol. 2009;10:94–106. doi: 10.1007/s11864-009-0088-2. [DOI] [PubMed] [Google Scholar]
- Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Li R., Ahmed M.S., Kohler R.H., Pittet M.J., Weissleder R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Linkowski M., Tarim J., Piperdi S., Sowers R., Geller D., Gill J., Gorlick R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. 2014;120:548–554. doi: 10.1002/cncr.28461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytlewski J., Brockman Q.R., Dodd R.D., Milhem M., Monga V. Epigenetic modulation in sensitizing metastatic sarcomas to therapies and overcoming resistance. Cancer Drug Resist. 2022;5:25–35. doi: 10.20517/cdr.2021.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytlewski J., Milhem M.M., Monga V. Turning 'Cold' tumors 'Hot': immunotherapies in sarcoma. Ann. Transl. Med. 2021;9:1039. doi: 10.21037/atm-20-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiei A., Gjertson D.W., Memarzadeh S., Konecny G.E., Moatamed N.A. Expression of immune checkpoint regulators, programmed death-ligand 1 (PD-L1/PD-1), cytotoxic T lymphocyte antigen 4 (CTLA-4), and indolaimine-2, 3-deoxygenase (IDO) in uterine mesenchymal tumors. Diagn. Pathol. 2022;17:70. doi: 10.1186/s13000-022-01251-2.dac3aa54a46b4d54849bb4eaab27335e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino G., Marchetto A., Kirchner T., Grunewald T.G.P. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transition in mesenchymal tumors: a paradox in sarcomas? Cancer Res. 2017;77:4556–4561. doi: 10.1158/0008-5472.CAN-17-0032. [DOI] [PubMed] [Google Scholar]
- Sato Y., Nabeta Y., Tsukahara T., Hirohashi Y., Syunsui R., Maeda A., Sahara H., Ikeda H., Torigoe T., Ichimiya S., et al. Detection and induction of CTLs specific for SYT-SSX-derived peptides in HLA-A24(+) patients with synovial sarcoma. J. Immunol. 2002;169:1611–1618. doi: 10.4049/jimmunol.169.3.1611. [DOI] [PubMed] [Google Scholar]
- Saxena M., van der Burg S.H., Melief C.J.M., Bhardwaj N. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;21:360–378. doi: 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- Smolle M.A., Herbsthofer L., Granegger B., Goda M., Brcic I., Bergovec M., Scheipl S., Prietl B., Pichler M., Gerger A., et al. T-regulatory cells predict clinical outcome in soft tissue sarcoma patients: a clinico-pathological study. Br. J. Cancer. 2021;125:717–724. doi: 10.1038/s41416-021-01456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaiah N., Conley A.P., Parra E.R., Lin H., Amini B., Solis Soto L., Salazar R., Barreto C., Chen H., Gite S., et al. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: a single-centre phase 2 trial. Lancet Oncol. 2022;23:1156–1166. doi: 10.1016/S1470-2045(22)00392-8. [DOI] [PubMed] [Google Scholar]
- Squires M.H., Ethun C.G., Suarez-Kelly L.P., Yu P.Y., Hughes T.M., Shelby R.D., Tran T.B., Poultsides G., Charlson J., Gamblin T.C., et al. Trends in the use of adjuvant chemotherapy for high-grade truncal and extremity soft tissue sarcomas. J. Surg. Res. 2020;245:577–586. doi: 10.1016/j.jss.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Strickler J.H., Hanks B.A., Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin. Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliamonte M., Petrizzo A., Tornesello M.L., Buonaguro F.M., Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum. Vaccin. Immunother. 2014;10:3332–3346. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Li D., Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed. Pharmacother. 2020;124:109821. doi: 10.1016/j.biopha.2020.109821. [DOI] [PubMed] [Google Scholar]
- Tang F., Tie Y., Wei Y.Q., Tu C.Q., Wei X.W. Targeted and immuno-based therapies in sarcoma: mechanisms and advances in clinical trials. Biochim. Biophys. Acta Rev. Cancer. 2021;1876:188606. doi: 10.1016/j.bbcan.2021.188606. [DOI] [PubMed] [Google Scholar]
- Tawbi H.A., Burgess M., Bolejack V., Van Tine B.A., Schuetze S.M., Hu J., D'Angelo S., Attia S., Riedel R.F., Priebat D.A., et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18:1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorkildsen J., Norum O.J., Myklebust T.A., Zaikova O. Chondrosarcoma local recurrence in the Cancer Registry of Norway cohort (1990-2013): patterns and impact. J. Surg. Oncol. 2021;123:510–520. doi: 10.1002/jso.26308. [DOI] [PubMed] [Google Scholar]
- Tsagozis P., Augsten M., Zhang Y., Li T., Hesla A., Bergh J., Haglund F., Tobin N.P., Ehnman M. An immunosuppressive macrophage profile attenuates the prognostic impact of CD20-positive B cells in human soft tissue sarcoma. Cancer Immunol. Immunother. 2019;68:927–936. doi: 10.1007/s00262-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B., Zhu J., Liu S., Wang L., Fan Q., Hao Y., Fan C., Tang T.T. Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3. Oncotarget. 2016;7:48296–48308. doi: 10.18632/oncotarget.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- von Konow A., Ghanei I., Styring E., Vult, von Steyern F. Late local recurrence and metastasis in soft tissue sarcoma of the extremities and trunk wall: better outcome after treatment of late events compared with early. Ann. Surg. Oncol. 2021;28:7891–7902. doi: 10.1245/s10434-021-09942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- Wei R., Dean D.C., Thanindratarn P., Hornicek F.J., Guo W., Duan Z. Cancer testis antigens in sarcoma: expression, function and immunotherapeutic application. Cancer Lett. 2020;479:54–60. doi: 10.1016/j.canlet.2019.10.024. [DOI] [PubMed] [Google Scholar]
- Weng W., Yu L., Li Z., Tan C., Lv J., Lao I.W., Hu W., Deng Z., Liu Z., Wang J., et al. The immune subtypes and landscape of sarcomas. BMC Immunol. 2022;23:46. doi: 10.1186/s12865-022-00522-3.98921edd194b4016bddf353ada5aec4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehurst A.W. Cause and consequence of cancer/testis antigen activation in cancer. Annu. Rev. Pharmacol. Toxicol. 2014;54:251–272. doi: 10.1146/annurev-pharmtox-011112-140326. [DOI] [PubMed] [Google Scholar]
- Xie C., Whalley N., Adasonla K., Grimer R., Jeys L. Can local recurrence of a sacral chordoma be treated by further surgery? Bone Joint J. 2015;97-B:711–715. doi: 10.1302/0301-620X.97B5.35131. [DOI] [PubMed] [Google Scholar]
- Xie L., Yang Y., Guo W., Che D., Xu J., Sun X., Liu K., Ren T., Liu X., Yang Y., et al. The clinical implications of tumor mutational burden in osteosarcoma. Front. Oncol. 2021;10:595527. doi: 10.3389/fonc.2020.595527.392628cf119d4d639fcafb348f5826db [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Schwaederle M., Arguello D., Millis S.Z., Gatalica Z., Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Lee K.W., Srivastava R.M., Kuo F., Krishna C., Chowell D., Makarov V., Hoen D., Dalin M.G., Wexler L., et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat. Med. 2019;25:767–775. doi: 10.1038/s41591-019-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin D., Hamid O., Nayak-Kapoor A., Sahebjam S., Sznol M., Collaku A., Fox F.E., Marshall M.A., Hong D.S. Mogamulizumab in combination with durvalumab or tremelimumab in patients with advanced solid tumors: a phase I study. Clin. Cancer Res. 2020;26:4531–4541. doi: 10.1158/1078-0432.CCR-20-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Huang W., Feng Q., Sun W., Yan W., Wang C., Zhang J., Huang K., Yu L., Qu X., et al. Clinical significance and risk factors of local recurrence in synovial sarcoma: a retrospective analysis of 171 cases. Front. Surg. 2022;8:736146. doi: 10.3389/fsurg.2021.736146.cd19b6e19bd54df289d57e55891da95a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020;17:807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Cao Y.J. Engineered T cell therapy for cancer in the clinic. Front. Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250.1885fa40dea34a94a49d04103d9673fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Yu X., Feng Y., Yang Z., Chen X., Wand J., Ma S., Zhang Z., Guo X. Local recurrence is correlated with decreased overall survival in patients with intermediate high-grade localized primary soft tissue sarcoma of extremity and abdominothoracic wall. Asia Pac. J. Clin. Oncol. 2018;14:e109–e115. doi: 10.1111/ajco.12807. [DOI] [PubMed] [Google Scholar]
- Zheng W., Xiao H., Liu H., Zhou Y. Expression of programmed death 1 is correlated with progression of osteosarcoma. APMIS. 2015;123:102–107. doi: 10.1111/apm.12311. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wang G., Chen R., Hua Y., Cai Z. Mesenchymal stem cells in the osteosarcoma microenvironment: their biological properties, influence on tumor growth, and therapeutic implications. Stem Cell Res. Ther. 2018;9:22. doi: 10.1186/s13287-018-0780-x.236a62233d8c4b9ca98936be91df4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang D., Yang Q., Lv X., Huang W., Zhou Z., Wang Y., Zhang Z., Yuan T., Ding X., et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat. Commun. 2020;11:6322. doi: 10.1038/s41467-020-20059-6.41de5804a75d4d98bfa174ff84c5c9b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.M.T., Shenasa E., Nielsen T.O. Sarcomas: immune biomarker expression and checkpoint inhibitor trials. Cancer Treat. Rev. 2020;91:102115. doi: 10.1016/j.ctrv.2020.102115. [DOI] [PubMed] [Google Scholar]
- Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- Zou C., Shen J., Tang Q., Yang Z., Yin J., Li Z., Xie X., Huang G., Lev D., Wang J. Cancer-testis antigens expressed in osteosarcoma identified by gene microarray correlate with a poor patient prognosis. Cancer. 2012;118:1845–1855. doi: 10.1002/cncr.26486. [DOI] [PubMed] [Google Scholar]