Summary
Background
Autoimmune hepatitis (AIH) varies significantly in incidence and prevalence across countries and regions. We aimed to examine global, regional, and national trends in incidence and prevalence of AIH from 1970 to 2022.
Methods
We conducted a thorough search of the PubMed/MEDLINE, Embase, CINAHL, Google Scholar, and Cochrane databases from database inception to August 9, 2023, using the search term “autoimmune hepatitis” in combination with “incidence,” “prevalence,” or “trend.” Only general population-based observational studies with larger samples sizes were considered for inclusion. Studies that recruited convenience samples, and those with fewer than 50 participants were excluded. Summary data were extracted from published reports. A random effects model was used and pooled estimates with 95% CI were used to calculate the incidence and prevalence of AIH. Heterogeneity was evaluated using the I2 statistic. The study protocol was registered with PROSPERO, CRD42023430138.
Findings
A total of 37 eligible studies, encompassing more than 239 million participants and 55,839 patients with AIH from 18 countries across five continents, were included in the analysis. Global pooled incidence and prevalence of AIH were found to be 1.28 cases per 100,000 inhabitant-years (95% CI, 1.01–1.63, I2 = 99·51%; number of studies, 33; sample population, 220,673,674) and 15.65 cases per 100,000 inhabitants (95% CI, 13.42–18.24, I2 = 99·75%; number of studies, 26; sample population, 217,178,684), respectively. The incidence of AIH was greater in countries with high Human Development Index (>0.92), in North America and Oceania (compared with Asia), among females, adults (compared with children), and high latitude (>45°). Similar patterns in AIH prevalence were observed. Pooled AIH prevalence increased gradually from 1970 to 2019 (1970–1999; 9.95 [4.77–15.13], I2 = 95·58% versus 2015–2022; 27.91 [24.86–30.96], I2 = 99·32%; cases per 100,000 inhabitants). The overall incidence and prevalence of AIH, as well as some subgroup analyses of the studies, displayed asymmetry in the funnel plots, suggesting potential evidence of publication bias.
Interpretation
AIH incidence and prevalence have increased significantly and exhibit substantial variation across regions worldwide. Further research is required to assess the incidence and prevalence of AIH, specifically in South America and Africa.
Funding
National Research Foundation of Korea.
Keywords: Autoimmune hepatitis, Incidence, Prevalence, Global trend, Systematic review and meta-analysis
Research in context.
Evidence before this study
A thorough search of PubMed/MEDLINE, Embase, CINAHL, Google Scholar, and Cochrane databases, from inception to August 9, 2023, was performed to gather information on AIH incidence and prevalence without language restrictions. The primary search terms were “autoimmune hepatitis” combined with “incidence,” “prevalence,” or “trend” using the “AND” operator. We found several studies on AIH incidence and prevalence published since the previous meta-analysis. Although there was a meta-analysis study conducted in the year 2019, it is important to note that certain large-scale studies were excluded from this review, and it did not provide detailed information on income, age, data sources, or specific subtypes of AIH. Furthermore, no global or temporal trend of AIH was included in the analysis.
Added value of this study
This systematic review and meta-analysis examined the incidence and prevalence of AIH from 1970 to 2022, incorporating data from 34 studies with a total of 239,345,726 participants and 55,839 patients with AIH across 18 countries on five continents. Global pooled incidence and prevalence of AIH were found to be 1.28 cases per 100,000 inhabitant-years (95% CI, 1.01–1.63) and 15.65 cases per 100,000 inhabitants (95% CI, 13.42–18.24), respectively. The incidence of AIH was greater in countries with high HDI (>0.92), in North America and Oceania (compared with Asia), among females, adults (compared with children), and high latitude (>45°). Pooled AIH incidence increased gradually from 1970 to 2022. Similar patterns in the prevalence of AIH were observed.
Implications of all the available evidence
Our study provides insights into the incidence and prevalence of AIH across various factors such as income, sex, age, geographical region, geographic latitude, diagnostic criteria, data source, and disease type. These findings highlight the consistent increase in the incidence and prevalence of AIH, suggesting that it may become a significant global health concern. Further studies focusing on South America and Africa are required to better understand the incidence and prevalence of the disease in these regions.
Introduction
Autoimmune hepatitis (AIH) is a chronic immune-mediated liver disease, characterized by circulating autoantibodies, elevated serum IgG levels, and histologic interface hepatitis.1 The disease is believed to lead to a loss of immunologic tolerance, triggered by environmental factors in genetically predisposed individuals.2 While AIH predominantly occurs in women, it is seen in individuals of all races and age groups worldwide.3 Clinically, the disease exhibits phenotypes varying from asymptomatic to severe liver failure.4 Diagnosis is typically based on clinical, biochemical, serologic, and histological findings, using the diagnostic scoring system provided by the International Autoimmune Hepatitis Group (IAIHG).5, 6, 7
A recent systematic review reported higher incidence and prevalence of AIH in women than in men and higher prevalence in European and American populations than in Asian populations.8 However, this study included only 22 studies on AIH and did not investigate factors including income, age, or data source or comprehensively analyse global trends in AIH.
In light of the increasing number of AIH cases9,10 contributing to a growing disease burden, a systematic review and meta-analysis offering precise estimations of the incidence and prevalence of AIH is urgently needed. Therefore, our study aimed to comprehensively analyse global, regional, and national trends in the incidence and prevalence of AIH from 1970 to 2022. We examined incidence and prevalence of AIH in the general population, and conducted subgroup analyses based on income, sex, age, geographical area, geographic latitude, diagnostic criteria, data source, and disease type.
Methods
Search strategy and selection criteria
This systematic literature review and meta-analysis was conducted to evaluate global trends in the incidence and prevalence of AIH. This systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,11 and the protocol was registered with PROSPERO (registration no. CRD42023430138).
PubMed/MEDLINE, Embase, CINAHL, Google Scholar, and Cochrane databases were searched from their inception dates to August 9, 2023, to find studies reporting the incidence or prevalence of AIH (according to appropriate diagnostic guidelines) in any age group. Studies including participants from the general population or community-based datasets were considered eligible.
Several exclusion criteria were applied to ensure validity of the results. First, studies that did not accurately calculate prevalence or incidence were excluded. Second, studies that recruited convenience samples, such as individuals attending health checkups at screening clinics, employees at institutions, or university students were excluded. Third, studies with <50 participants were excluded. As no interventional studies were available, we included general population-based observational studies with larger sample sizes, published in English. In addition, studies published in a language apart from English with abstracts written in English were included.
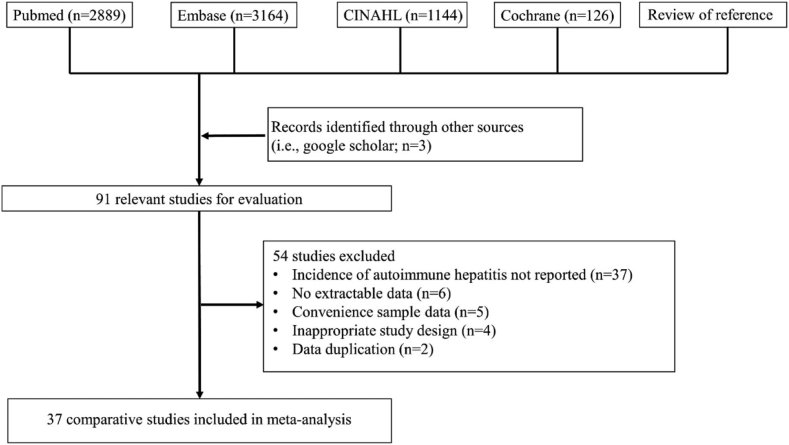
The search strategy involved conducting a database search using the terms “AIH” or “autoimmune hepatitis,” combined with the free text terms “incidence,” “prevalence,” or “trend,” using the “AND” set operator. Initially, 7326 studies were screened; subsequently, titles and abstracts of all selected studies were rescreened to identify potential suitability. After the initial screening, non-eligiable studies were excluded (Table E1 and Fig. 1). A recursive search was conducted using the bibliographies of all eligible papers. Two investigators (JWH and DKY) independently evaluated eligibility, and discrepancies were resolved through discussion with a third investigator (JSK).
Fig. 1.
Flow diagram of study design.
Data analysis
Data extraction was conducted independently by two investigators (JWH and DKY) using Microsoft Excel version 2013 (Redmond, WA, USA). The following data were collected for each study: first author, publication year, country, patient identification, diagnostic criteria for AIH, study design (retrospective or prospective), study duration, patient age, total number of samples providing data, and number of patients with AIH. All studies were assessed for risk of bias (RoB) using the criteria developed by Hoy et al.12 Based on an evaluation of nine items, a study was deemed to have low RoB if three or fewer items were relevant, and high RoB if seven or more were applicable.
A comprehensive subgroup analysis was conducted based on several variables including income, sex, age, geographical area, diagnostic scoring system, data source, and disease type. The countries in the study were categorized based on Human Development Index (HDI).13 Countries with an HDI >0.92, including Canada, Denmark, Finland, Germany, Iceland, Japan, New Zealand, Norway, Singapore, South Korea, Sweden, Taiwan, the Netherlands, the United Kingdom, and the United States, were classified in one group. Countries with an HDI <0.92, such as Argentina, Brunei, Israel, and Spain, were classified into another group. In this study, countries were categorized into two groups based on latitude: countries with a latitude of 45° or higher, and countries with a latitude of less than 45°. Countries with a latitude of 45° or higher included Canada, Denmark, Finland, Germany, Iceland, Japan, Norway, Sweden, Netherlands, United Kingdom, and the United States. Countries with a latitude of less than 45° included Argentina, Brazil, Israel, New Zealand, Singapore, South Korea, Spain, and Taiwan.
Diagnostic scoring systems for AIH were introduced by the IAIHG in 1993,7 1999,6 and 2008.5 The 1993 scoring system included multiple parameters such as liver histology, serum biochemistry, serum immunoglobulins, serum autoantibodies, viral markers, other etiological factors, and response to therapy.7 Comparatively, the 1999 scoring system had several modifications, including changes to the alkaline phosphatase (ALP):aspartate aminotransferase (AST) (or alanine transaminase [ALT]) ratio, drug history, liver histology, response to therapy, and exclusion of markers of infection with hepatotropic viruses other than hepatitis A, B, and C.6 The 2008 scoring system was modified to be a practical tool for routine clinical practice, based on four parameters: liver histology, autoantibody titers, gamma-globulin levels, and absence of viral hepatitis.5 Overlap syndrome (overlap of AIH with primary biliary cirrhosis [PBC] or primary sclerosing cholangitis [PSC]) was diagnosed based on the IAIHG study.14
Subgroup analysis was conducted on studies without researcher validation or explicit diagnostic criteria for AIH, such as code-based studies (k = 10), as well as on strict studies with researcher validation or explicit diagnostic scoring systems for AIH (k = 21).
A random-effects model was used to merge the proportions of patients with AIH from each included study to determine the global incidence and prevalence of AIH. Subgroup differences in incidence and prevalence were analyzed using mean differences with 95% CIs. Heterogeneity was evaluated using the I2 statistic,15 which measures heterogeneity among studies, with higher values indicating greater heterogeneity. Pooled estimates with 95% CIs were used to compare the global incidence and prevalence of AIH. The meta-analysis is based on a log transformed scale to address the sparse data issue. Egger's test and funnel plots were used to identify publication bias. Bayesian statistics were used to generate a 95% prediction interval (PI) and assess robustness and uncertainty of the main findings.16 Additionally, for analyses where publication bias was detected, sensitivity analyses using the trim-and-fill method were conducted to assess the robustness of the results. Pooling analysis by the Mantel-Haenszel method was used to calculate the pooled odds ratios for sex and age, with 95% CIs. Pearson correlation analysis was performed to examine the relationships between country-specific HDI values and the incidence and prevalence of AIH, as well as between country-specific latitude and AIH incidence and prevalence. P value for trends was calculated using proportion trend test to investigate time trends. The main results were calculated using Microsoft Excel and R software (version 3.1.1; R Foundation, Vienna, Austria), and all tables were generated using these tools. Statistical significance was set at a two-sided P value <0.05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors (JSK and DKY) has full access to all the data in the study and has final responsibility for the decision of submission to the journal for publication.
Results
Initially, 7326 studies were identified using the search strategy. After evaluating 91 studies, 54 were excluded for reasons such as insufficient data on disease incidence, convenience sampling data, inappropriate study design, lack of population-based studies, and data duplication. Eventually, 37 studies (k = 379,10,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52; researcher-validated studies, k = 219,21,26, 27, 28,30,31,34, 35, 36, 37, 38,40, 41, 42, 43, 44, 45, 46,48,49) were deemed eligible, involving 239,345,726 participants (approximately 2.9% of the global population) and 55,839 patients with AIH from 18 different countries (Argentina, Canada, Denmark, Finland, France, Iceland, Israel, Japan, New Zealand, Norway, Singapore, South Korea, Spain, Sweden, Taiwan, the Netherlands, the United Kingdom, and the United States [by country, 10.9% of the global population]) across five continents. Search strategy results are summarized in Fig. 1. All studies were conducted in a single country and included population-based studies on patients with AIH from 1970 to 2022. Twenty studies included both children and adults,9,10,18, 19, 20, 21,23,25,26,30,32,35, 36, 37, 38,42,45, 46, 47,51 14 studies included only adults,17,27,28,31,34,39, 40, 41,43,44,48, 49, 50 and 4 studies included only children.24,29,33,52 Table 1 shows detailed characteristics of all the studies that met the inclusion criteria.
Table 1.
Characteristics of previous studies assessing the incidence and/or prevalence of AIH included in our systematic review.
| First author, publication year | Country | Patient identification | Diagnosis criteria | Study design | Period | Age of patients | Total sample | Patients with AIH |
|---|---|---|---|---|---|---|---|---|
| Bjarnason et al., 1982 | Iceland | Liver biopsy | Clinical, biochemical, and histological features | Retrospective | 1970–1979 | Adults & Children | 225,549 | 18 |
| Hodges et al., 1982 | United Kingdom | Hospital database + liver biopsy | Clinical, biochemical, and histological features | Retrospective | 1976–1980 | Adults | 404,000 | 14 |
| Ritland et al., 1985 | Norway | Hospital database + liver biopsy | Clinical, biochemical, and histological features | Retrospective | 1981 | Adults | 4,117,647a | 49 |
| Homberg et al., 1987 | France | Hospital | Clinical, biochemical, and histological features | Retrospective | 1975–1984 | Adults & Children | 10,761,625 | 47 |
| Tanner et al., 1989 | United Kingdom | Hospital database + liver biopsy | Clinical, biochemical, and histological features | Retrospective | 1971–1987 | Adults & Children | 210,000 | 15 |
| Byron et al., 1996 | Canada | Hospital | Clinical, biochemical, and histological features | Retrospective | 1987–1993 | Adults & Children | 650,000 | 39 |
| Boberg et al., 1998 | Norway | Hospital | Original IAIHG | Prospective, Cohort | 1986–1995 | Adults | 130,000 | 25 |
| Lee et al., 2001 | Singapore | Hospital | Original IAIHG | Retrospective | 1990–1996 | Adults | 567,685 | 24 |
| Hurlburt et al., 2002 | United States | Hospital | Revised IAIHG | Retrospective | 1984–2000 | Adults & Children | 114,219 | 49 |
| Primo et al., 2004 | Spain | Hospital | Revised IAIHG | Prospective, Cross-sectional | 1990–2003 | Adults | 112,003 | 13 |
| Koay et al., 2006 | Taiwan | Hospital | Revised IAIHG | Retrospective, Cohort | 2000–2004 | Adults | 1,846,464 | 48 |
| Whalley et al., 2007 | United Kingdom | Hospital | Code-based definition | Retrospective, Cross-sectional | 2003–2004 | Adults | 200,000 | 6 |
| Werner et al., 2008 | Sweden | Hospital | Revised IAIHG | Retrospective | 1990–2003 | Adults & Children | 715,000 | 473 |
| Primo et al., 2009 | Spain | Hospital | Revised or Simplified IAIHG | Prospective, Cohort | 2003 | Adults & Children | 1,774,736 | 19 |
| Ngu et al., 2010 | New Zealand | Hospital | Revised IAIHG | Retrospective, Prospective | 2001–2007 | Adults & Children | 494,170 | 121 |
| Delgado et al., 2013 | Israel | Hospital | Simplified IAIHG | Retrospective | 1995–2010 | Adults | 995,024a | 109 |
| Deneau et al., 2013 | United States | Hospital | Simplified IAIHG | Retrospective, Cohort | 2005–2011 | Children | 1,466,667a | 44 |
| Gronbaek et al., 2014 | Denmark | Hospital | Code-based definition | Retrospective | 1994–2012 | Adults & Children | 5,648,536a | 1350 |
| Van Gerven et al., 2014 | The Netherlands | Hospital | Revised or Simplified IAIHG | Retrospective | 2000–2010 | Adults | 799,000 | 146 |
| Chong et al., 2015 | Brunei Darussalam | Hospital | Simplified IAIHG | Retrospective | 2011 | Adults & Children | 459,016a | 28 |
| Jimenez et al., 2015 | Canada | Hospital | Revised IAIHG | Retrospective, Cohort | 2000–2009 | Children (0–18 years) | 70,277,839 | 159 |
| Yoshizawa et al., 2016 | Japan | Hospital (Questionnaire) | Revised IAIHG | Retrospective, Prospective | 2004–2014 | Adults | 187,205 | 48 |
| Danielsson et al., 2017 | Sweden | Hospital | Revised IAIHG | Retrospective | 1990–2009 | Adults & Children | 3,664,740a | 634 |
| Kim et al., 2017 | South Korea | National Health Insurance Service database | Code-based definition | Retrospective | 2009–2013 | Adults & Children | 50,008,453 | 12,447 |
| Sharma et al., 2017 | United States | Medical records | Clinical, biochemical, and histological features | Retrospective | 1990–2015 | Adults | 97,222a | 70 |
| Costaguta et al., 2018 | Argentina | Hospital | Simplified IAIHG | Retrospective, Cohort | 2003–2013 | Children (0–18 years) | 10,017,106 | 56 |
| Puustinen et al., 2019 | Finland | Social insurance database | Code-based definition | Retrospective | 2008–2015 | Adults & Children | 6,202,797a | 887 |
| Valgeirsson et al., 2019 | Iceland | Hospital | Simplified IAIHG | Retrospective | 2006–2015 | Adults & Children | 342,007a | 92 |
| Gronbaek et al., 2020 | England | Hospital | Code-based definition | Retrospective, Cohort | 1997–2015 | Adults & Children | 3,591,476a | 691 |
| Sebode et al., 2020 | Germany | Health insurance data | Code-based definition | Retrospective | 2011–2014 | Adults & Children | 8,100,000 | 1700 |
| Lamba et al., 2021 | New Zealand | Hospital | Simplified or Revised IAIHG | Prospective, Cohort | 2008–2016 | Adults & Children | 599,900 | 165 |
| Tunio et al., 2021 | United States | Electronic Health Record data | Code-based definition | Retrospective | 2014–2019 | Adults & Children | 37,161,280 | 11,600 |
| Webb et al., 2021 | United Kingdom | Primary care records | Code-based definition | Retrospective, Cohort | 2002–2016 | Adults & Children | 3,278,496a | 1116 |
| Bittermann et al., 2022 | United States | Administrative claims data | Code-based definition | Retrospective, Cohort | 2009–2018 | Adults | 18,103,568 | 3386 |
| Sutton et al., 2022 | United Kingdom | Hospital | Clinical, biochemical and histological features | Retrospective | 2013–2018 | Children (0–16 years) | 918,000 | 38 |
| Nielsen et al., 2023 | Denmark | Hospital | Simplified IAIHG | Retrospective, Cohort | 2004–2021 | Adults & Children | 48,000 | 45 |
| Hitawala et al., 2023 | United States | Electronic Health Record data | Code-based definition | Retrospective | 1999–2022 | Adults | 70,352,325 | 20,550 |
AIH: autoimmune hepatitis; IAIHG: international autoimmune hepatitis group.
These results were calculated and estimated using original data.
In the 33 studies meeting the inclusion criteria, the global pooled incidence of AIH was found to be 1.28 cases per 100,000 inhabitant-years (95% CI, 1.01–1.63; I2 = 99.51%), with a 95% prediction interval of 0.35–4.70 (Table 2).9,10,17, 18, 19,21,23, 24, 25, 26, 27, 28, 29,31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46,48,49,51,52
Table 2.
Global pooled incidence of AIH included in our systematic review.
| Number of studies | Number of participants | Pooled estimates (95% CI)a | 95% prediction interval | I2 (%) | P value for I2 | Egger's P value | |
|---|---|---|---|---|---|---|---|
| Overall incidence | 33 | 220,673,674 | 1.28 (1.01–1.63) | 0.35–4.70 | 99.51 | <0.0001 | <0.001 |
| Income | |||||||
| HDI >0.92 | 26 | 143,009,677 | 1.63 (1.28–2.07) | 0.51–5.24 | 99.50 | <0.0001 | 0.0040 |
| HDI <0.92 | 4 | 12,898,869 | 0.73 (0.49–1.08) | 0.17–3.12 | 49.28 | 0.12 | 0.59 |
| Sex | |||||||
| Male | 17 | 61,144,540 | 0.90 (0.65–1.24) | 0.25–3.23 | 97.73 | <0.0001 | 0.0090 |
| Female | 17 | 66,436,411 | 2.79 (2.14–3.64) | 0.90–8.68 | 99.27 | <0.0001 | 0.0050 |
| Age group | |||||||
| Children | 8 | 100,035,200 | 0.35 (0.24–0.52) | 0.09–1.38 | 90.64 | <0.0001 | 0.77 |
| Adults aged under 65 years | 17 | 87,144,548 | 1.45 (1.22–1.73) | 0.55–3.83 | 98.51 | <0.0001 | <0.001 |
| Adults aged over 65 years | 8 | 14,770,970 | 3.59 (2.76–4.68) | 1.04–12.36 | 97.51 | <0.0001 | <0.001 |
| Geographical areas | |||||||
| North America | 5 | 56,129,062 | 3.35 (2.71–4.13) | 1.63–6.89 | 98.79 | <0.0001 | 0.37 |
| Europe | 18 | 28,118,418 | 1.38 (1.18–1.61) | 0.81–2.34 | 90.60 | <0.0001 | 0.073 |
| Asia | 5 | 53,604,831 | 0.99 (0.60–1.64) | 0.16–6.01 | 97.97 | <0.0001 | 0.41 |
| Oceania | 3 | 1,558,288 | 1.90 (1.59–2.28) | 0.58–6.20 | 0.00 | 0.70 | 0.60 |
| Geographic latitude | |||||||
| Above 45° | 21 | 89,490,232 | 1.83 (1.43–2.35) | 0.60–5.54 | 99.51 | <0.0001 | 0.013 |
| Below 45° | 9 | 66,874,557 | 0.93 (0.67–1.31) | 0.32–2.71 | 87.72 | <0.0001 | 0.48 |
| Diagnostic scoring system for AIHa | |||||||
| Before IAIHG | 5 | 5,598,514 | 1.10 (0.85–1.41) | 0.74–1.65 | 0.00 | 0.71 | 0.0070 |
| Original IAIHG | 2 | 697,685 | 1.03 (0.33–3.19) | NA | 49.08 | 0.16 | NA |
| Revised IAIHG | 9 | 75,340,696 | 1.04 (0.52–2.09) | 0.08–13.23 | 98.98 | <0.0001 | 0.30 |
| Simplified IAIHG | 3 | 1,385,031 | 1.83 (0.72–4.69) | NA | 81.51 | 0.0040 | 0.84 |
| Data source | |||||||
| Researcher validated | 20 | 15,415,852 | 1.29 (1.02–1.63) | 0.51–3.25 | 90.41 | <0.0001 | 0.011 |
| Code-based | 8 | 122,530,210 | 2.17 (1.48–3.17) | 0.54–8.71 | 99.82 | <0.0001 | 0.041 |
| Type | |||||||
| Type I AIH | 8 | 6,533,380 | 1.00 (0.55–1.82) | 0.13–7.54 | 91.04 | <0.0001 | 0.027 |
| Type II AIH | 8 | 96,780,945 | 0.03 (0.02–0.04) | 0.02–0.04 | 0.00 | 0.70 | 0.65 |
| Seronegative AIH | 3 | 71,842,811 | 0.05 (0.00–0.83) | NA | 91.21 | <0.0001 | 0.61 |
| Overlap syndrome | |||||||
| AIH-PBC | 5 | 2,046,594 | 0.17 (0.09–0.32) | 0.06–0.47 | 0.00 | 0.59 | 0.95 |
| AIH-PSC | 1 | 319,403 | 0.03 (0.00–0.22) | NA | NA | NA | NA |
| Score | |||||||
| Definite AIH | 6 | 2,705,442 | 1.06 (0.49–2.30) | 0.08–14.99 | 91.85 | <0.0001 | 0.64 |
| Probable and definite AIH | 6 | 2,705,442 | 1.67 (0.95–2.95) | 0.22–12.59 | 95.20 | <0.0001 | 0.95 |
AIH, autoimmune hepatitis; HDI, human development index; IAIHG, international autoimmune hepatitis group; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; NA, not available.
These results were calculated and estimated using original data.
Table E6 shows the subgroup differences in incidence of AIH in this study. The pooled incidence of AIH was higher in countries with HDI >0.929,10,17, 18, 19, 20, 21,23,25, 26, 27, 28,31,32,35,37, 38, 39, 40,42, 43, 44, 45, 46,48,49,51,52 compared to countries with HDI <0.92,24,34,36,41 with 1.63 (95% CI, 1.28–2.07) and 0.73 (95% CI, 0.49–1.08) cases per 100,000 inhabitant-years, respectively. The incidence of AIH in females9,10,17, 18, 19,21,23,25, 26, 27, 28,32,34,36,41 was higher than that in males,9,10,17, 18, 19,21,23,25, 26, 27, 28,32,34,36,41 with 2.79 (95% CI, 2.14–3.64) and 0.90 (95% CI, 0.65–1.24) cases per 100,000 inhabitant-years, respectively. The odds ratio (OR) for AIH in females compared with males was 3.10 (95% CI, 2.04–4.71). The incidence of AIH was highest in adults aged over 65 years,10,17, 18, 19,25,28,36,41 at 3.59 (95% CI, 2.76–4.68) cases per 100,000 inhabitant-years, followed by adults aged under 65 years10,17, 18, 19,25,27,28,31,34,36,39, 40, 41,43,44,48,49 at 1.45 (95% CI, 1.22–1.73) cases per 100,000 inhabitant-years, and lowest in children10,18,19,24,25,29,33,52 at 0.35 (95% CI, 0.24–0.52) cases per 100,000 inhabitant-years. The OR for AIH in adults aged under 65 years compared with children was 4.14 (95% CI, 2.71–6.33). The incidence of AIH was highest in North America17,19,27,42,45 at 3.35 (95% CI, 2.71–4.13) cases per 100,000 inhabitant-years, followed by Oceania9,35 at 1.90 (95% CI, 1.59–2.28) cases per 100,000 inhabitant-years, and lowest in Asia25,28,34,40,43 at 0.99 (95% CI, 0.60–1.64) cases per 100,000 inhabitant-years. Countries with a geographical latitude above 45°17, 18, 19,21,23,26, 27, 28,31,32,37, 38, 39,42,44, 45, 46,48,49,51 exhibited an incidence of AIH at 1.83 (95% CI, 1.43–2.35) cases per 100,000 inhabitant-years, while countries with a latitude below 45°9,24,25,34, 35, 36,40,41,43 had an incidence of AIH at 0.93 (95% CI, 0.67–1.31) cases per 100,000 inhabitant-years. Compared with code-based studies,10,17, 18, 19,23,25,32,39 the pooled incidence of AIH was lower in researcher validation case studies,9,21,26, 27, 28,31,34, 35, 36, 37, 38,40, 41, 42, 43, 44, 45, 46,48,49 at 1.29 (95% CI, 1.02–1.63) versus 2.17 (95% CI, 1.48–3.17) cases per 100,000 inhabitant-years.
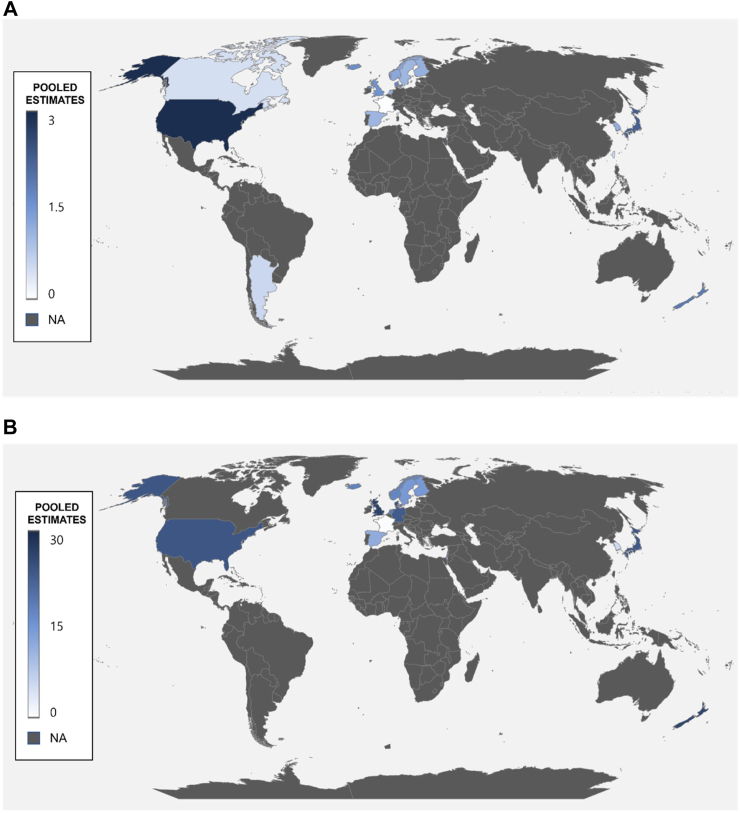
Table E2 and Fig. 2(A) show national pooled incidences of AIH in each country. The highest incidence was observed in the United States (2.94 cases per 100,000 inhabitant-years; 95% CI, 1.64–4.23),17,19,27,33,42 while the lowest incidence was observed in Canada (0.42 cases per 100,000 inhabitant-years; 95% CI, 0.12–1.51).29,45
Fig. 2.
Global incidence (A) and prevalence (B) of autoimmune hepatitis, 1970–2022. Pooled estimates, cases per 100,000 inhabitant-years for incidence, and cases per 100,000 inhabitants for prevalence.
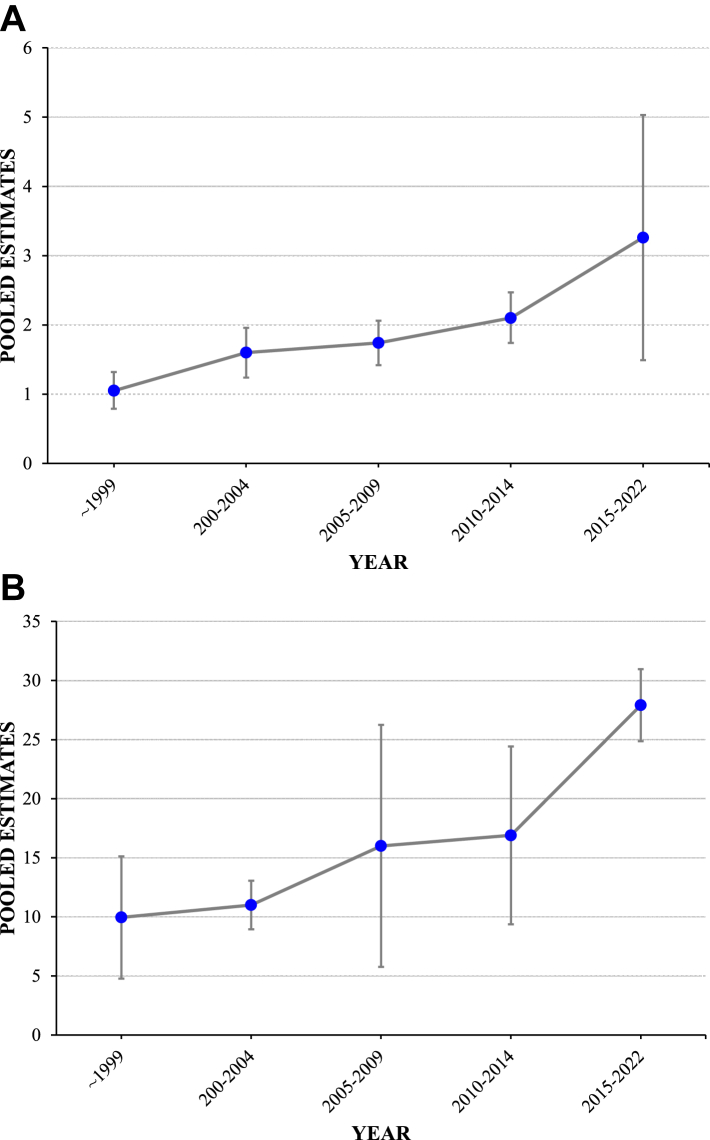
The incidence of AIH over time was reported in 65 subgroup studies divided into 5-year intervals. We found that the incidence of AIH increased over time, with the highest incidence reported from 2015 to 2022. Pooled incidence before 1999 was 1.05 (95% CI, 0.79–1.32) cases per 100,000 inhabitant-years,10,32,37,41, 42, 43, 44, 45, 46,48,49 while from 2015 to 2022 it was 3.26 (95% CI, 1.49–5.03) cases per 100,000 inhabitant-years.9,10,18,19,21 Details of time trends in pooled incidence of AIH are provided in Table E4. Our systematic review showed a significant increase in the incidence of AIH over time (P trend < 0.001), as shown in Fig. 3. In addition, temporal trends in the incidence rates of AIH within the longitudinal cohort studies included in our systematic review are presented in Figure E1.
Fig. 3.
Time trends of incidence (A) and prevalence (B) of autoimmune hepatitis, 1970–2022. Pooled estimates, cases per 100,000 inhabitant-years for incidence, and cases per 100,000 inhabitants for prevalence.
A total of 26 studies were included in our systematic review to estimate the global pooled prevalence of AIH, found to be 15.65 cases per 100,000 inhabitants (95% CI, 13.42–18.24; I2 = 99.75%) with a 95% prediction interval of 7.00–35.00 (Table 3).9,10,17, 18, 19, 20, 21,23,25,26,28,30, 31, 32, 33, 34, 35,37,38,41, 42, 43, 44,50, 51, 52 Table E7 shows the subgroup differences in prevalence of AIH in this study. The pooled prevalence of AIH was higher in countries with HDI >0.929,10,17, 18, 19, 20, 21,23,25,26,28,31,32,35,37,38,42, 43, 44,50,51 compared with countries with HDI <0.92,30,34,41 with 19.97 (95% CI, 17.00–23.44) versus 9.15 (95% CI, 6.11–13.72) cases per 100,000 inhabitants, respectively. The positive correlation was shown between increasing HDI and the prevalence of AIH (Pearson's R = 0.341, P < 0.05) (Figure E2). The pooled prevalence of AIH was higher in females17, 18, 19,23,25,26,28,32,34,35,38,41,50 than in males17, 18, 19,23,25,26,28,32,34,35,38,41,50 (28.04 [95% CI, 21.31–36.89] versus 8.05 [95% CI, 5.87–11.04] cases per 100,000 inhabitants). The OR for AIH in females compared with males was 3.48 (95% CI, 2.29–5.29). The pooled prevalence of AIH was highest in adults aged over 65 years17, 18, 19,25,50 (44.05 [95% CI, 33.65–57.67]), followed by Adults aged under 65 years17, 18, 19,25,28,30,31,34,41,43,44,50 (16.05 [95% CI, 13.01–19.80]), and lowest in children18,19,25,30,33,52 (2.04 [95% CI, 1.01–4.14] per 100,000 inhabitants). The OR for AIH in adults aged under 65 years compared with children was 7.87 (95% CI, 3.77–16.42). The pooled prevalence of AIH was highest in North America (29.57 [95% CI, 27.84–31.40]),17,19,42,50 followed by Oceania (26.22 [95% CI, 23.32–29.47]),17,19,42 and lowest in Asia (8.06 [95% CI, 3.39–19.16] per 100,000 inhabitants).25,28,30,34,43 Countries with a geographical latitude above 45°17, 18, 19, 20, 21,23,26,28,31,32,37,38,42,44,50,51 exhibited a prevalence of AIH at 23.09 (95% CI, 21.33–25.00) cases per 100,000 inhabitants, while countries with a latitude below 45°9,25,30,34,35,41,43 had a prevalence of AIH at 10.19 (95% CI, 4.99–20.79) cases per 100,000 inhabitants. The positive correlation was shown between increasing latitude and the prevalence of AIH (Pearson's R = 0.447, P < 0.05) (Figure E3)” (Results).
Table 3.
Global pooled prevalence of AIH included in our systematic review.
| Number of studies | Number of participants | Pooled estimates (95% CI)a | 95% prediction interval | I2 (%) | P value for I2 | Egger's P value | |
|---|---|---|---|---|---|---|---|
| Overall prevalence | 26 | 217,178,684 | 15.65 (13.42–18.24) | 7.00–35.00 | 99.75 | <0.0001 | 0.030 |
| Income | |||||||
| HDI >0.92 | 21 | 213,227,973 | 19.97 (17.00–23.44) | 9.19–43.42 | 99.77 | <0.0001 | 0.12 |
| HDI <0.92 | 3 | 1,566,043 | 9.15 (6.11–13.72) | NA | 75.00 | 0.018 | 0.72 |
| Sex | |||||||
| Male | 13 | 93,583,856 | 8.05 (5.87–11.04) | 2.31–28.07 | 99.40 | <0.0001 | 0.17 |
| Female | 13 | 106,521,438 | 28.04 (21.31–36.89) | 9.04–86.99 | 99.81 | <0.0001 | 0.22 |
| Age group | |||||||
| Children | 6 | 19,443,377 | 2.04 (1.01–4.14) | 0.15–26.92 | 97.33 | <0.0001 | 0.96 |
| Adults aged under 65 years | 12 | 126,351,940 | 16.05 (13.01–19.80) | 6.50–39.62 | 99.75 | <0.0001 | 0.11 |
| Adults aged over 65 years | 5 | 35,575,631 | 44.05 (33.65–57.67) | 12.59–154.13 | 99.48 | <0.0001 | 0.72 |
| Geographical areas | |||||||
| North America | 4 | 125,734,165 | 29.57 (27.84–31.40) | 22.57–38.74 | 97.91 | <0.0001 | 0.36 |
| Europe | 14 | 36,666,398 | 16.56 (13.31–20.61) | 6.72–40.82 | 98.90 | <0.0001 | 0.25 |
| Asia | 5 | 52,217,383 | 8.06 (3.39–19.16) | NA | 99.66 | <0.0001 | 0.58 |
| Oceania | 2 | 1,094,070 | 26.22 (23.32–29.47) | NA | 0.00 | 0.34 | NA |
| Geographic latitude | |||||||
| Above 45° | 17 | 161,554,992 | 23.09 (21.33–25.00) | 16.64–32.05 | 98.86 | <0.0001 | 0.053 |
| Below 45° | 7 | 53,236,251 | 10.19 (4.99–20.79) | 0.75–139.18 | 99.20 | <0.0001 | 0.15 |
| Diagnostic scoring system for AIHa | |||||||
| Before IAIHG | 1 | 212,500 | 8.00 (4.97–12.87) | NA | NA | NA | NA |
| Original IAIHG | 2 | 697,685 | 8.45 (2.17–32.87) | NA | 95.46 | 0.0010 | NA |
| Revised IAIHG | 7 | 9,361,665 | 16.57 (12.63–21.75) | 6.46–42.50 | 95.00 | <0.0001 | 0.48 |
| Simplified IAIHG | 3 | 1,385,031 | 27.43 (10.46–71.92) | NA | 98.08 | <0.0001 | 0.33 |
| Data source | |||||||
| Researcher validated | 14 | 6,774,799 | 15.53 (12.94–18.64) | 7.55–31.96 | 94.90 | <0.0001 | 0.24 |
| Code-based | 9 | 201,738,235 | 20.55 (16.21–26.06) | 8.33–50.67 | 99.90 | <0.0001 | 0.21 |
| Type | |||||||
| Type I AIH | 7 | 6,346,713 | 11.00 (6.74–17.95) | 1.89–64.13 | 98.00 | <0.0001 | 0.40 |
| Type II AIH | 3 | 12,791,557 | 0.43 (0.33–0.56) | 0.08–2.38 | 0.00 | 0.61 | 0.74 |
| Seronegative AIH | 2 | 1,130,950 | 1.44 (0.11–19.25) | NA | 92.49 | <0.0001 | NA |
| Overlap syndrome | |||||||
| AIH-PBC | 4 | 8,669,763 | 1.22 (1.01–1.48) | 0.80–1.86 | 0.00 | 0.66 | 0.87 |
| AIH-PSC | 3 | 8,555,544 | 0.46 (0.23–0.91) | NA | 71.35 | 0.03 | 0.92 |
| Score | |||||||
| Definite AIH | 6 | 10,704,115 | 8.30 (4.87–14.13) | 1.19–58.04 | 98.04 | <0.0001 | 0.90 |
| Probable and definite AIH | 6 | 10,704,115 | 11.64 (7.28–18.62) | 2.07–65.39 | 98.28 | <0.0001 | 0.70 |
AIH, autoimmune hepatitis; HDI, human development index; IAIHG, international autoimmune hepatitis group; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; NA, not available.
These results were calculated and estimated using original data.
Table E3 and Fig. 2(B) provide information regarding the nationwide pooled prevalence of AIH in individual countries. The highest prevalence was found in the United States (29.55 cases per 100,000 inhabitants; 95% CI, 27.82–31.28),17,19,33,42,50 while the lowest prevalence was observed in Singapore (4.23 cases per 100,000 inhabitants; 95% CI, 2.83–6.31).43
The prevalence of AIH over time was reported in 26 subgroup studies, which were divided into 5-year intervals. The prevalence of AIH increased over time. The pooled prevalence was highest in 2015–2022 with 27.91 (95% CI, 24.86–30.96) cases per 100,000 inhabitants,9,17, 18, 19,21,23,50,51 compared with a prevalence of 9.95 (95% CI, 4.77–15.13) cases per 100,000 inhabitants prior to 1999.37,42, 43, 44,47 Table E5 provides details of time-trend characteristics in the pooled prevalence of AIH. Our systematic review revealed a significant increase in the prevalence of AIH over time (P trend < 0.001) (Fig. 3).
Asymmetry of the funnel plots was assessed to determine the risk of publication bias, which was found to be high for both the overall incidence (P < 0.001) and prevalence (P = 0.030) of AIH based on Egger's test. In addition, some subgroup analyses of the incidence and prevalence studies showed asymmetry in the funnel plots, indicating potential evidence of publication bias. Detailed funnel plots for the incidence and prevalence studies are shown in Figures E4–E55. A sensitivity analysis was conducted using the trim-and-fill method. The results showed that there was no statistically significant difference in the overall incidence of AIH (1.72, 95% CI, 1.38–2.14) and the overall prevalence of AIH (14.31, 95% CI, 12.32–16.63) when compared to the unadjusted analysis results (P = 0.079, P = 0.42, respectively) (Table E9). In the subgroup analysis of adults aged under 65 years, the adjusted analysis result showed an incidence of 1.99 (95% CI, 1.70–2.34), which was statistically significant when compared to the unadjusted analysis result (P < 0.05). However, no statistical significance was observed in other subgroups (Table E9). The adjusted funnel plot using the trim-and-fill method is presented in Figures E56–E67.
A total of 38 studies were assessed to determine RoB, out of which one study was excluded due to a high RoB.22 Of the remaining studies, 11 were considered to have a moderate RoB,18,24,27,28,33,34,37,39,46,48,49 and 26 were evaluated as having a low RoB.9,10,17,19, 20, 21,23,25,26,29, 30, 31, 32,35,36,38,40, 41, 42, 43, 44, 45,47,50, 51, 52 Among the questions used to evaluate the risk in most studies, those that were most frequently evaluated as posing a risk included “Were data collected directly from the patients (as opposed to a proxy)?” (applicable for 24 studies)10,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30,32, 33, 34,37, 38, 39,46,48,49 and “Was some form of random selection used to select the sample or was a census undertaken?” (applicable for 21 studies).9,24,27,28,30,33, 34, 35, 36, 37,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 Details of RoB for prevalence studies are provided in Table E8.
Discussion
In this systematic review and meta-analysis, data were collected from 37 studies with a total sample size of 239,345,726 participants and 55,839 patients from 18 countries across five continents. Incidence and prevalence of AIH from 1970 to 2022 were reported. The global pooled incidence of AIH was found to be 1.28 cases per 100,000 inhabitant-years, whereas the global pooled prevalence was 15.65 cases per 100,000 inhabitants. Moreover, the incidence and prevalence of AIH were higher in populations with high HDI (>0.92), females, adults aged over 65 years, North American populations (versus Europe, Asia, and Oceania) and high latitude (>45°). The pooled prevalence of AIH also increased gradually over time, from 9.95 cases per 100,000 inhabitants from 1970 to 1999 to 27.91 cases per 100,000 inhabitants from 2015 to 2022.
Previous studies have examined the incidence and prevalence of AIH in the general population.8,53 A recent systematic review published in 2019 examined 22 studies on AIH,8 reporting that its overall incidence was 1.37 cases per 100,000 inhabitant-years, and overall prevalence was 17.44 cases per 100,000 inhabitants. Subgroup analysis showed that European and American populations had a higher prevalence than Asian populations; in addition, a higher prevalence was observed in women and adults aged over 65 years. However, some studies were excluded from the review due to insufficient inclusion of electronic medical databases, resulting in only 22 studies being included. In addition, the review did not provide data on income, age, data source, or specific types of AIH. Furthermore, no global or temporal trend of AIH was included in the analysis. This highlights the importance of conducting research reflecting current trends, as demonstrated in our study.
The incidence and prevalence of AIH in our study showed 3.1-fold and 2.8-fold increases, respectively, compared with rates prior to the 2000s. There are several potential reasons for this increase. First, it may be associated with a higher incidence of other autoimmune diseases; age- and sex-standardized incidence rates of various autoimmune diseases have shown an increase in recent years.54 A significant percentage of patients with AIH also exhibit concomitant extrahepatic autoimmune disorders, particularly Hashimoto's thyroiditis, type I diabetes mellitus, and inflammatory bowel disease (IBD).55 In addition, the prevalence of obesity, a risk factor for autoimmune diseases, has also increased significantly.56,57 Hence, the observed global rise in the incidence of autoimmune diseases might be interconnected. Secondly, there may be an influence of changing environmental exposures. Pathogenesis of AIH is believed to occur when genetically predisposed individuals are exposed to environmental triggers.58 Antibiotics are considered an independent risk factor for the development of AIH,59 and nitrofurantoin and minocycline have historically been associated with drug-induced AIH.60 Recently, biologic agents such as infliximab, adalimumab, and natalizumab have also been reported to cause drug-induced AIH.61 Considering the continued increase in IBD prevalence,62 the increasing prevalence of AIH may be caused by these factors. Thirdly, changes in diagnostic criteria for AIH may also be a contributing factor. The IAIHG scoring system for AIH has undergone several modifications over time. While the 1993, 1999 system included multiple parameters,6,7 the 2008 system was based on four parameters and designed for routine clinical practice5; Due to their convenience of use in clinical practice, the simplified criteria have gained popularity as a primary diagnostic tool in AIH. The advancement of diagnostic tools leading to an increase in disease incidence is supported by examples such as the introduction of pancreatic enzyme testing, which resulted in a more than 60% increase in acute pancreatitis cases.63 In this study, the incidence of AIH using the revised IAIHG criteria was 1.04 (95% CI, 0.52–2.09), while using the simplified IAIHG criteria, the incidence was 1.83 (95% CI, 0.72–4.69). Therefore, the adoption of simplified criteria is associated with improved disease awareness, enhanced diagnosis, and a notable increase in AIH diagnoses.
Several potential factors contribute to the regional and national variations found in the incidence and prevalence of AIH. First, the distribution of predisposing human leukocyte antigen (HLA) genes varies by ethnicity and geographical region. Genetic factors are believed to have a significant impact on development of AIH. Specifically, certain HLA genes, such as DR3 and DR4, are more prevalent in European and North American populations.2,64 These genetic variations contribute to the observed regional and national differences in the incidence and prevalence of AIH. However, unlike North America, where the majority of studies were conducted after 2017,17,19,27,42,45,50 Europe has consistently reported studies on the incidence and prevalence of AIH since 1982. Considering the gradual increase in the incidence and prevalence of the disease, the lower incidence and prevalence in Europe compared to North America could be attributed to the inclusion of earlier studies. Second, the impact of environmental factors differs across regions. Several studies have reported microbiome alterations in patients with AIH2 as well as an association between the enrichment of Veillonella dispar and disease severity.65 Factors such as living conditions, lifestyle habits, and diet may influence the intestinal microbiome and contribute to the development of AIH.66 In addition, a recent study in Iceland revealed an association between the use of biologics and increased incidence of AIH.21 These differences in environmental factors may have contributed to regional variations in the incidence and prevalence of AIH. Thirdly, this could also be explained by the hygiene hypothesis. Epidemiological studies have reported a decrease in the incidence of infectious diseases and an increase in allergic/autoimmune diseases in developed countries.67,68 In this study, a higher incidence of AIH in countries with higher HDI was observed compared to countries with lower HDI. HDI represents the average of health, knowledge, and standard of living in a country, indicating its level of development. This aligns with the hygiene hypothesis. Fourthly, this could be due to differences in the availability of advanced healthcare services. For AIH, diagnostic procedures such as liver biopsy and autoimmune serological testing are essential. But in regions with underdeveloped healthcare services, these tests may not be conducted, leading to an underreporting of the true incidence or prevalence of AIH. This could potentially exaggerate the disparity in disease rates between countries with high and low HDI. Studies on the Healthcare Access and Quality (HAQ) index have also reported differences in HAQ index scores, particularly between low and high socio-demographic index groups.69
Our study shows a gradual increase in the incidence and prevalence of AIH, with the highest rates observed in adults aged over 65 years. Approximately 25% of patients with AIH are asymptomatic,56,57 however, the disease progresses in a similar manner regardless of symptoms.4 Moreover, AIH is associated with a two-fold increased risk of death70 and can contribute to acute-on-chronic liver failure,71 indicating a significant health burden. Consequently, early detection and treatment of asymptomatic patients is crucial. Additionally, adults aged over 65 years tend to have a milder disease course and better treatment response, leading to overall improved prognosis.72 Therefore, conducting diagnostic testing and providing active treatment for AIH in adults aged over 65 years is extremely important.
This study has several limitations. First, the available data on the incidence and prevalence of AIH lacks sufficient representation of South American and African patients. We investigated the incidence and prevalence of AIH in the general population, focusing primarily on North America, Europe, Asia, and Oceania. There is insufficient relevant literature available for South America and Africa (except for Argentina), making it challenging to establish a comprehensive understanding of regional differences in the incidence or prevalence of AIH. To address this limitation, we aimed to present an additional analysis by country to express regional differences. Second, the studies included in our analysis exhibited significant heterogeneity, warranting a cautious interpretation of the results. Several factors contributed to this heterogeneity, including differences in genetic predisposition, environmental factors, characteristics of prevalence studies, changes in diagnostic criteria for AIH, and demographic or cultural variations among study populations. To address this, we conducted subgroup analyses to elucidate the sources of heterogeneity. Despite the observed heterogeneity, our summary data provide valuable insights into the global perspective of the incidence and prevalence of AIH. Third, nonvalidated code-based studies were included in the data analysis. Considering the importance of study validation for data analysis, we conducted case validation assessments for all included studies; ten code-based studies lacked validation. In addition, Bittermann et al. reported that relying solely on ICD codes may not allow for reliable identification of true AIH cases using administrative data.73 However, excluding code-based studies made it challenging to capture recent trends of incidence and prevalence of AIH, as seven out of the eleven studies published after 2019 were code-based.10,17, 18, 19, 20,23,50, 51, 52 Additionally, other systematic reviews and meta-analyses on gastrointestinal diseases have used ICD-10 codes to diagnose the disease.63 When conducting subgroup analysis using researcher-validated cases, a lower incidence of AIH was observed compared with code-based studies (1.29 [95% CI, 1.02–1.63] versus 2.17 [95% CI, 1.48–3.17] per 100,000 inhabitant-years). This suggests that a code-based approach may lead to overestimation of the incidence of AIH, emphasizing the need for cautious interpretation. Fourth, the temporal trend analysis presented in this study should be interpreted with caution. For instance, prior to 2008, there was no specific ICD code for AIH, and the transition from ICD-9 to ICD-10, advancements in diagnostic techniques, and improved access to high-quality healthcare services could have impacted the results. To address this issue, we conducted analyses stratified by code-based and researcher-validated study designs. However, the potential for milder cases to be more accurately diagnosed due to advancements in diagnostic technology and improved access to high-quality healthcare services still remains, warranting caution in the interpretation of the results. Fifth, the range of HDI values covered in this study was relatively narrow. Subgroup analysis of AIH incidence and prevalence was conducted based on HDI categories. The HDI classification designates 0.8 and above as “very high,” and all 18 countries included in this study had HDI values of 0.8 or higher. This aspect should be interpreted with caution. Sixth, the interpretation of the association between geographic latitude and the incidence and prevalence of AIH. While the study revealed that countries with a geographic latitude above 45° exhibited significantly higher incidence and prevalence of AIH compared to countries below 45°. Notably, countries such as Iceland, Sweden, Finland, Norway, and Canada, with latitudes above 66°, did not show correspondingly high incidence and prevalence rates. This suggests that the interpretation of a linear relationship between latitude increase and AIH incidence and prevalence should be approached with caution. Due to the diagnostic complexity of AIH, in regions with limited healthcare access, there may be underreporting of the actual incidence or prevalence of AIH. Moreover, in areas with a high prevalence of viral and toxic liver diseases, or among elderly men, AIH is frequently not included in the initial diagnostic considerations. Consequently, the possibility of an unknown rate of undiagnosed cases in the incidence and prevalence of AIH exists.
Despite these limitations, this study had several strengths. A comprehensive search of multiple databases was conducted to identify relevant studies on AIH. Furthermore, the study presented a temporal trend in AIH incidence and prevalence, visually illustrating its increasing global burden. Compared with a previous study conducted in 2019,8 this analysis provided a more in-depth examination of AIH by considering factors such as income, age, data source, and specific AIH types. This study also introduces global maps depicting the incidence and prevalence of AIH. In addition, by providing prediction intervals, advanced statistical techniques can be used to further investigate the incidence and prevalence of AIH. Finally, the analysis focused exclusively on studies involving the general population with AIH, ensuring a comprehensive representation of the disease.
In conclusion, our study provides insights into the incidence and prevalence of AIH across various factors such as income, sex, age, geographical region, diagnostic criteria, data source, and disease type. These findings highlight the consistent increase in the incidence and prevalence of AIH, suggesting that it may become a significant global health concern. Further studies focusing on South America and Africa are required to better understand the incidence and prevalence of the disease in these regions. Moreover, the substantial variation observed in the incidence and prevalence of AIH worldwide emphasizes the potential influence of genetic and environmental factors on this disease. Continued efforts to explore these factors are essential to develop a comprehensive understanding of AIH as well as targeted interventions for this disease.
Contributors
DKY was responsible for the conceptualization and design of the study. JWH and DKY were responsible for acquisition, analysis and interpretation of data. JWH, DKY and JSK were responsible for data verification. JWH and DKY were responsible for writing the original draft of the manuscript. JSK and DKY were responsible for review and editing of the manuscript. All authors commented on drafts of the manuscript. DKY is a senior author. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
All data relevant to the study are included in the article or uploaded as online supplemental information. The full search strategy and key results used to generate data that inform the conclusions of this systematic review can be found in online supplemental material.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This research was supported by grants from the National Research Foundation of Korea (NRF; RS-2023-00248157), the Ministry of Food and Drug Safety (grant number: 21153MFDS601) in 2023, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HE23C002800). Patients and/or the public were not involved in the design, or conduct, or reporting. However, we plan on disseminating the results of this study to any of the study participants or wider relevant communities on request.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102280.
Contributor Information
Jae Sung Ko, Email: kojs@snu.ac.kr.
Dong Keon Yon, Email: yonkkang@gmail.com.
Appendix A. Supplementary data
References
- 1.European Association for the Study of the L EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Muratori L., Lohse A.W., Lenzi M. Diagnosis and management of autoimmune hepatitis. BMJ. 2023;380 doi: 10.1136/bmj-2022-070201. [DOI] [PubMed] [Google Scholar]
- 3.Mack C.L., Adams D., Assis D.N., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 4.Muratori P., Lalanne C., Barbato E., et al. Features and progression of asymptomatic autoimmune hepatitis in Italy. Clin Gastroenterol Hepatol. 2016;14:139–146. doi: 10.1016/j.cgh.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Hennes E.M., Zeniya M., Czaja A.J., et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez F., Berg P.A., Bianchi F.B., et al. International autoimmune hepatitis group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 7.Johnson P.J., McFarlane I.G. Meeting report: international autoimmune hepatitis group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 8.Lv T., Li M., Zeng N., et al. Systematic review and meta-analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J Gastroenterol Hepatol. 2019;34:1676–1684. doi: 10.1111/jgh.14746. [DOI] [PubMed] [Google Scholar]
- 9.Lamba M., Ngu J.H., Stedman C.A.M. Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2021;19:573–579.e1. doi: 10.1016/j.cgh.2020.05.061. [DOI] [PubMed] [Google Scholar]
- 10.Gronbaek L., Otete H., Ban L., et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997-2015. A population-based cohort study. Liver Int. 2020;40:1634–1644. doi: 10.1111/liv.14480. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.W., Koo M.J. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle Committee Recommendations. Life Cycle. 2022;2 [Google Scholar]
- 12.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Morgan E., Arnold M., Gini A., et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–344. doi: 10.1136/gutjnl-2022-327736. [DOI] [PubMed] [Google Scholar]
- 14.Boberg K.M., Chapman R.W., Hirschfield G.M., et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54:374–385. doi: 10.1016/j.jhep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J., et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J.S., Lee Y.A., Shin C.H., et al. Long-term health outcomes of early menarche in women: an umbrella review. QJM. 2022;115:837–847. doi: 10.1093/qjmed/hcac187. [DOI] [PubMed] [Google Scholar]
- 17.Bittermann T., Lewis J.D., Levy C., et al. Sociodemographic and geographic differences in the US epidemiology of autoimmune hepatitis with and without cirrhosis. Hepatology. 2023;77:367–378. doi: 10.1002/hep.32653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb G.J., Ryan R.P., Marshall T.P., et al. The epidemiology of UK autoimmune liver disease varies with geographic latitude. Clin Gastroenterol Hepatol. 2021;19:2587–2596. doi: 10.1016/j.cgh.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunio N.A., Mansoor E., Sheriff M.Z., et al. Epidemiology of autoimmune hepatitis (AIH) in the United States between 2014 and 2019: a population-based national study. J Clin Gastroenterol. 2021;55:903–910. doi: 10.1097/MCG.0000000000001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebode M., Kloppenburg A., Aigner A., et al. Population-based study of autoimmune hepatitis and primary biliary cholangitis in Germany: rising prevalences based on ICD codes, yet deficits in medical treatment. Z Gastroenterol. 2020;58:431–438. doi: 10.1055/a-1135-9306. [DOI] [PubMed] [Google Scholar]
- 21.Valgeirsson K.B., Hreinsson J.P., Bjornsson E.S. Increased incidence of autoimmune hepatitis is associated with wider use of biological drugs. Liver Int. 2019;39:2341–2349. doi: 10.1111/liv.14224. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka A., Mori M., Matsumoto K., et al. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol Res. 2019;49:881–889. doi: 10.1111/hepr.13342. [DOI] [PubMed] [Google Scholar]
- 23.Puustinen L., Barner-Rasmussen N., Pukkala E., et al. Incidence, prevalence, and causes of death of patients with autoimmune hepatitis: a nationwide register-based cohort study in Finland. Dig Liver Dis. 2019;51:1294–1299. doi: 10.1016/j.dld.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Costaguta A., Gonzalez A., Pochettino S., et al. Incidence and clinical features of autoimmune hepatitis in the province of Santa Fe (Argentina) J Pediatr Gastroenterol Nutr. 2018;67:e107–e110. doi: 10.1097/MPG.0000000000002122. [DOI] [PubMed] [Google Scholar]
- 25.Kim B.H., Choi H.Y., Ki M., et al. Population-based prevalence, incidence, and disease burden of autoimmune hepatitis in South Korea. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danielsson Borssen A., Marschall H.U., Bergquist A., et al. Epidemiology and causes of death in a Swedish cohort of patients with autoimmune hepatitis. Scand J Gastroenterol. 2017;52:1022–1028. doi: 10.1080/00365521.2017.1335772. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A., Mukewar S., Czaja A. Epidemiology, clinical characteristics and outcomes of autoimmune hepatitis in olmsted county: a population-based study. Am J Gastroenterol. 2017;112:S511. [Google Scholar]
- 28.Yoshizawa K., Joshita S., Matsumoto A., et al. Incidence and prevalence of autoimmune hepatitis in the Ueda area, Japan. Hepatol Res. 2016;46:878–883. doi: 10.1111/hepr.12639. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Rivera C., Ling S.C., Ahmed N., et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics. 2015;136:e1237–e1248. doi: 10.1542/peds.2015-0578. [DOI] [PubMed] [Google Scholar]
- 30.Chong V.H., Jalihal A., Telisinghe P.U. Autoimmune hepatitis in adult and pediatric patients: is there any difference? Indian J Gastroenterol. 2015;34:264–265. doi: 10.1007/s12664-015-0564-5. [DOI] [PubMed] [Google Scholar]
- 31.van Gerven N.M., Verwer B.J., Witte B.I., et al. Epidemiology and clinical characteristics of autoimmune hepatitis in The Netherlands. Scand J Gastroenterol. 2014;49:1245–1254. doi: 10.3109/00365521.2014.946083. [DOI] [PubMed] [Google Scholar]
- 32.Gronbaek L., Vilstrup H., Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Deneau M., Jensen M.K., Holmen J., et al. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013;58:1392–1400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 34.Delgado J.S., Vodonos A., Malnick S., et al. Autoimmune hepatitis in southern Israel: a 15-year multicenter study. J Dig Dis. 2013;14:611–618. doi: 10.1111/1751-2980.12085. [DOI] [PubMed] [Google Scholar]
- 35.Ngu J.H., Bechly K., Chapman B.A., et al. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010;25:1681–1686. doi: 10.1111/j.1440-1746.2010.06384.x. [DOI] [PubMed] [Google Scholar]
- 36.Primo J., Maroto N., Martinez M., et al. Incidence of adult form of autoimmune hepatitis in Valencia (Spain) Acta Gastroenterol Belg. 2009;72:402–406. [PubMed] [Google Scholar]
- 37.Bjarnason I., Magnusson B., Bjornsson S. Idiopathic chronic active hepatitis in Iceland. Acta Med Scand. 2009;211:305–307. doi: 10.1111/j.0954-6820.1982.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 38.Werner M., Prytz H., Ohlsson B., et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232–1240. doi: 10.1080/00365520802130183. [DOI] [PubMed] [Google Scholar]
- 39.Whalley S., Puvanachandra P., Desai A., et al. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med. 2007;7:119–124. doi: 10.7861/clinmedicine.7-2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koay L.B., Lin C.Y., Tsai S.L., et al. Type 1 autoimmune hepatitis in Taiwan: diagnosis using the revised criteria of the International Autoimmune Hepatitis Group. Dig Dis Sci. 2006;51:1978–1984. doi: 10.1007/s10620-005-9068-y. [DOI] [PubMed] [Google Scholar]
- 41.Primo J., Merino C., Fernandez J., et al. [Incidence and prevalence of autoimmune hepatitis in the area of the Hospital de Sagunto (Spain)] Gastroenterol Hepatol. 2004;27:239–243. doi: 10.1016/s0210-5705(03)70452-x. [DOI] [PubMed] [Google Scholar]
- 42.Hurlburt K.J., McMahon B.J., Deubner H., et al. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterol. 2002;97:2402–2407. doi: 10.1111/j.1572-0241.2002.06019.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee Y.M., Teo E.K., Ng T.M., et al. Autoimmune hepatitis in Singapore: a rare syndrome affecting middle-aged women. J Gastroenterol Hepatol. 2001;16:1384–1389. doi: 10.1046/j.1440-1746.2001.02646.x. [DOI] [PubMed] [Google Scholar]
- 44.Boberg K.M., Aadland E., Jahnsen J., et al. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 45.Byron D., Minuk G.Y. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813–815. doi: 10.1002/hep.510240410. [DOI] [PubMed] [Google Scholar]
- 46.Tanner A.R., Dellipiani A.W. Chronic active hepatitis: a sixteen year survey at a district general hospital. Postgrad Med J. 1989;65:725–728. doi: 10.1136/pgmj.65.768.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homberg J.C., Abuaf N., Bernard O., et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987;7:1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- 48.Ritland S. The incidence of chronic active hepatitis in Norway. A retrospective study. Scand J Gastroenterol Suppl. 1985;107:58–60. doi: 10.3109/00365528509099753. [DOI] [PubMed] [Google Scholar]
- 49.Hodges J.R., Millward-Sadler G.H., Wright R. Chronic active hepatitis: the spectrum of disease. Lancet. 1982;1:550–552. doi: 10.1016/s0140-6736(82)92056-6. [DOI] [PubMed] [Google Scholar]
- 50.Hitawala A.A., Almomani A., Onwuzo S., et al. Prevalence of autoimmune, cholestatic and nonalcoholic fatty liver disease in celiac disease. Eur J Gastroenterol Hepatol. 2023;35:1030–1036. doi: 10.1097/MEG.0000000000002599. [DOI] [PubMed] [Google Scholar]
- 51.Nielsen K.R., Midjord J., Johannesen H.L., et al. A nationwide study of autoimmune liver diseases in the Faroe Islands: incidence, prevalence, and causes of death 2004 - 2021. Int J Circumpolar Health. 2023;82 doi: 10.1080/22423982.2023.2221368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutton H., Tayler R., Chalmers I., et al. The epidemiology of pediatric autoimmune hepatitis in scotland: a national cohort study. JPGN Rep. 2022;3:e223. doi: 10.1097/PG9.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jepsen P., Gronbaek L., Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(Suppl 2):2–12. doi: 10.1159/000440705. [DOI] [PubMed] [Google Scholar]
- 54.Conrad N., Misra S., Verbakel J.Y., et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–1890. doi: 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]
- 55.Gronbaek L., Vilstrup H., Pedersen L., et al. Extrahepatic autoimmune diseases in patients with autoimmune hepatitis and their relatives: a Danish nationwide cohort study. Liver Int. 2019;39:205–214. doi: 10.1111/liv.13963. [DOI] [PubMed] [Google Scholar]
- 56.Collaboration NCDRF Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collaboration NCDRF Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trivedi P.J., Hirschfield G.M. Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut. 2021;70:1989–2003. doi: 10.1136/gutjnl-2020-322362. [DOI] [PubMed] [Google Scholar]
- 59.Ngu J.H., Gearry R.B., Frampton C.M., et al. Autoimmune hepatitis: the role of environmental risk factors: a population-based study. Hepatol Int. 2013;7:869–875. doi: 10.1007/s12072-013-9448-x. [DOI] [PubMed] [Google Scholar]
- 60.Bjornsson E., Talwalkar J., Treeprasertsuk S., et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 61.de Boer Y.S., Kosinski A.S., Urban T.J., et al. Features of autoimmune hepatitis in patients with drug-induced liver injury. Clin Gastroenterol Hepatol. 2017;15:103–112.e2. doi: 10.1016/j.cgh.2016.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collaborators GBDIBD The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iannuzzi J.P., King J.A., Leong J.H., et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162:122–134. doi: 10.1053/j.gastro.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 64.Czaja A.J., Souto E.O., Bittencourt P.L., et al. Clinical distinctions and pathogenic implications of type 1 autoimmune hepatitis in Brazil and the United States. J Hepatol. 2002;37:302–308. doi: 10.1016/s0168-8278(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 65.Wei Y., Li Y., Yan L., et al. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569–577. doi: 10.1136/gutjnl-2018-317836. [DOI] [PubMed] [Google Scholar]
- 66.Liwinski T., Heinemann M., Schramm C. The intestinal and biliary microbiome in autoimmune liver disease-current evidence and concepts. Semin Immunopathol. 2022;44:485–507. doi: 10.1007/s00281-022-00936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bach J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 68.Bach J.F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18:105–120. doi: 10.1038/nri.2017.111. [DOI] [PubMed] [Google Scholar]
- 69.Access GBDH, Quality C Assessing performance of the Healthcare Access and Quality Index, overall and by select age groups, for 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Glob Health. 2022;10:e1715–e1743. doi: 10.1016/S2214-109X(22)00429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma R., Verna E.C., Soderling J., et al. Increased mortality risk in autoimmune hepatitis: a nationwide population-based cohort study with histopathology. Clin Gastroenterol Hepatol. 2021;19:2636–2647.e13. doi: 10.1016/j.cgh.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anand L., Choudhury A., Bihari C., et al. Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology. 2019;70:587–596. doi: 10.1002/hep.30205. [DOI] [PubMed] [Google Scholar]
- 72.Al-Chalabi T., Boccato S., Portmann B.C., et al. Autoimmune hepatitis (AIH) in the elderly: a systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. J Hepatol. 2006;45:575–583. doi: 10.1016/j.jhep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Bittermann T., Mahmud N., Lewis J.D., et al. Validating a novel algorithm to identify patients with autoimmune hepatitis in an administrative database. Pharmacoepidemiol Drug Saf. 2021;30:1168–1174. doi: 10.1002/pds.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.