Summary
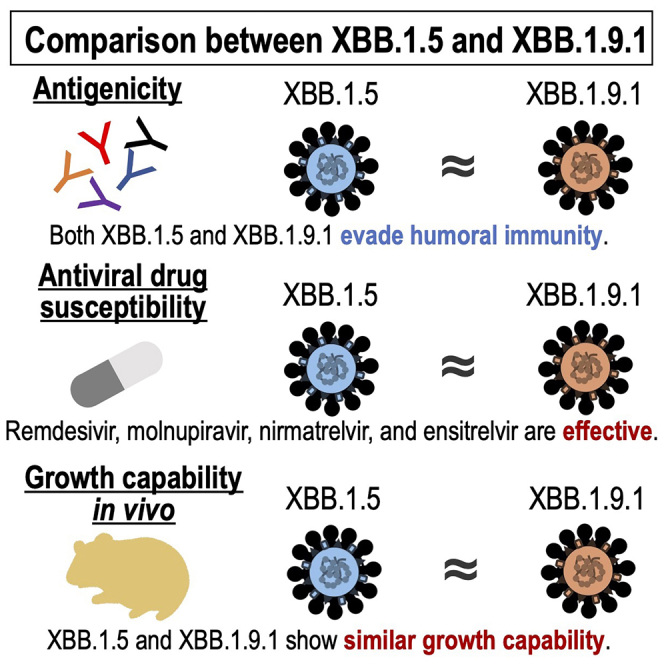
The emergence and spread of new SARS-CoV-2 variants with mutations in the spike protein, such as the XBB.1.5 and XBB.1.9.1 sublineages, raise concerns about the efficacy of current COVID-19 vaccines and therapeutic monoclonal antibodies (mAbs). In this study, none of the mAbs we tested neutralized XBB.1.9.1 or XBB.1.5, even at the highest concentration used. We also found that the bivalent mRNA vaccine could enhance humoral immunity against XBB.1.9.1, but that XBB.1.9.1 and XBB.1.5 still evaded humoral immunity induced by vaccination or infection. Moreover, the susceptibility of XBB.1.9.1 to remdesivir, molnupiravir, nirmatrelvir, and ensitrelvir was similar to that of the ancestral strain and the XBB.1.5 isolate in vitro. Finally, we found the replicative fitness of XBB.1.9.1 to be similar to that of XBB.1.5 in hamsters. Our results suggest that XBB.1.9.1 and XBB.1.5 have similar antigenicity and replicative ability, and that the currently available COVID-19 antivirals remain effective against XBB.1.9.1.
Subject areas: Virology, Clinical microbiology
Graphical abstract
Highlights
-
•
The antigenicity of XBB.1.9.1 is similar to that of XBB.1.5
-
•
XBB.1.9.1 remains susceptible to antiviral drugs
-
•
The replicative ability of XBB.1.9.1 is comparable to that of XBB.1.5
Virology; Clinical microbiology
Introduction
As of April 2023, XBB.1.5, a recombinant sublineage of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 (omicron) XBB subvariant, is currently dominant around the world. However, the prevalence of the XBB.1.9.1 sublineage is increasing in the United States (https://covid.cdc.gov/covid-data-tracker/#variant-proportions).
We and other groups have shown that XBB.1.5 is resistant to several therapeutic monoclonal antibodies and effectively evades humoral immunity elicited by natural infection or COVID-19 vaccinations.1,2,3,4,5 In contrast, XBB.1.5 and the ancestral strain show similar susceptibility to drugs such as remdesivir (an RNA-dependent RNA polymerase (RdRp) inhibitor), molnupiravir (an RdRp inhibitor), and nirmatrelvir (a main protease inhibitor), which have all been authorized by the US Food and Drug Administration (FDA) for the treatment of COVID-19, and to ensitrelvir (a main protease inhibitor), which has been approved for emergency use in Japan for COVID-19 treatment.3
Although XBB.1.5 and XBB.1.9.1 possess the same amino acid substitutions in the spike protein, RdRp and main protease (Figure S1), there are additional amino acid substitutions in other virus proteins that may affect infectivity, viral replication, transmissibility and/or pathogenicity (Figure S1). Accordingly, we investigated vaccine efficacy against a clinical isolate of XBB.1.9.1, as well as the antiviral susceptibility and replicative fitness of the XBB.1.9.1 isolate in vitro or in vivo.
Results and discussion
Efficacy of monoclonal antibodies against XBB.1.9.1 in vero E6-TMPRSS2-T2A-ACE2 cells
As expected, due to the spike protein substitutions, we found that the reactivity of COVID-19 therapeutic monoclonal antibodies (mAbs) against XBB.1.9.1 (hCoV-19/Japan/TY41-951/2023) and XBB.1.5 (hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022) patient isolates was similar. None of the tested mAbs, including LYCoV1404 (marketed as bebtelovimab), REGN10987 (known as imdevimab), REGN10933 (known as casirivimab), COV2-2196 (known as tixagevimab), COV2-2130 (known as cilgavimab), and S309 (known as the precursor of sotrovimab), neutralized the XBB.1.9.1 or XBB.1.5 isolate even at the highest concentration (>50,000 ng/mL) in Vero E6-TMPRSS2-T2A-ACE2 cells (Table 1).
Table 1.
Efficacy of monoclonal antibodies against omicron subvariants in vero E6-TMPRSS2-T2A-ACE2 cellsa
| WHO label (Pango lineage) | Virus | Neutralization activity of monoclonal antibody — ng/mlb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| REGN10987, imdevimab | REGN10933, casirivimab | COV2-2196, tixagevimab | COV2-2130, cilgavimab | S309, sotrovimab precursor | LYCoV1404, bebtelovimab | REGN10987 plus REGN10933 | COV2-2196 plus COV2-2130 | ||
| (A) | SARS-CoV-2/UT-NC002-1T/Human/2020/Tokyo | 89 | 64 | 64 | 152 | 5127 | 48 | 72 | 73 |
| Omicron (XBB) | hCoV-19/Japan/TY41-795/2022 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| Omicron (XBB.1.5) | hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| Omicron (XBB.1.9.1) | hCoV-19/Japan/TY41-951/2023 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
The antibodies used in this analysis were produced in the authors’ laboratories and are not identical to the commercially available products. Severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) variants are denoted according to the World Health Organization labels for the Pango lineage.
The individual monoclonal antibodies were tested at a starting concentration of 50,000 ng/mL as a 50% focus reduction neutralization test (FRNT50) titer.
Neutralizing activity of plasma from vaccinees and patients against XBB.1.9.1
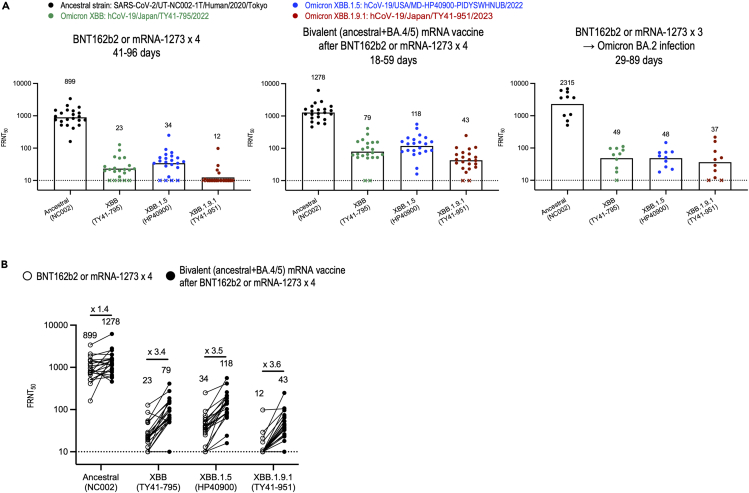
We also assessed neutralization of the XBB.1.9.1 isolate by using plasma obtained from three cohorts: (1) individuals who received a fourth dose of the monovalent mRNA vaccine, (2) individuals who received the bivalent mRNA vaccine as a fifth vaccine, and (3) vaccinees with BA.2 breakthrough infection after a third dose of the mRNA vaccine. The focus reduction neutralization test (FRNT50) geometric mean titers against XBB.1.9.1 were lower than those against the ancestral strain in plasma from all three cohorts (Figure 1A and Tables S1–S3). Consistent with our previous study, most samples of plasma from vaccinees with BA.2 breakthrough infection or from individuals who received the bivalent vaccine administered as a fifth dose had neutralizing activity and, samples from those who received a booster shot of the bivalent vaccine had increased neutralizing activity against XBB.1.9.1 (Figure 1B). This finding suggests that the bivalent vaccine can enhance humoral immunity, although XBB.1.9.1, as well as XBB.1.5, evades humoral immunity induced by mRNA vaccines or natural infection.
Figure 1.
In vitro neutralizing activity of plasma against SARS-CoV-2 omicron variants
(A) Neutralizing titers of plasma samples obtained from individuals who had received four doses of BNT162b2 or mRNA-1273 vaccine (n = 22), individuals immunized with the bivalent (ancestral and BA.4/5) vaccine as a fifth dose (n = 22), and patients who were infected with the omicron BA.2 subvariant after receiving either the BNT162b2 or mRNA-1273 vaccine (n = 10). Detailed information about the participants is provided in Tables S1–S3. FRNT50 values were determined in Vero E6-TMPRSS2-T2A-ACE2 cells. Each dot represents data from one individual. The lower limit of detection (value = 10) is indicated by the horizontal dashed line. Samples under the detection limit (<10-fold dilution) were assigned an FRNT50 of 10 and are represented by X. Geometric mean titers are shown.
(B) Neutralizing titers of plasma samples from the same individuals (n = 22) after receiving four doses of BNT162b2 or mRNA-1273 vaccine compared with those after receiving the bivalent (ancestral and BA.4/5) vaccine as a fifth dose. Geometric mean titers are shown. Each line represents data from one individual.
Efficacy of antiviral drugs against XBB.1.9.1 in vero E6-TMPRSS2-T2A-ACE2 cells
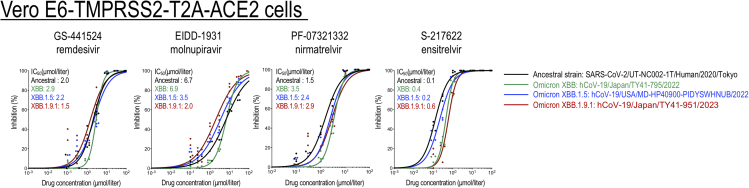
Next, we examined the antiviral efficacy of four drugs, specifically, remdesivir, molnupiravir, nirmatrelvir, and ensitrelvir. We determined their in vitro 50% inhibitory concentration (IC50) values against the XBB.1.9.1 isolate. The susceptibilities of XBB.1.9.1 to these four antivirals were similar to those of the ancestral strain and those of the XBB.1.5 isolate (Figure 2). These results demonstrate that remdesivir, molnupiravir, nirmatrelvir, and ensitrelvir are effective against XBB.1.9.1 in vitro.
Figure 2.
In vitro inhibitory activity of antiviral drugs against Omicron subvariants
The in vitro 50% inhibitory concentration (IC50) values were determined in Vero E6-TMPRSS2-T2A-ACE2 cells. GS-441524 (the main metabolite of remdesivir) and EIDD-1931 (the active form of molnupiravir) are RNA-dependent RNA polymerase inhibitors. PF-07321332 (nirmatrelvir) and S-217622 (ensitrelvir) are inhibitors of Mpro (also called 3CLpro). Data are the mean values for triplicate experiments. Statistical analysis of the data was not performed.
Replicative fitness of XBB.1.9.1 compared with that of XBB.1.5 in hamsters
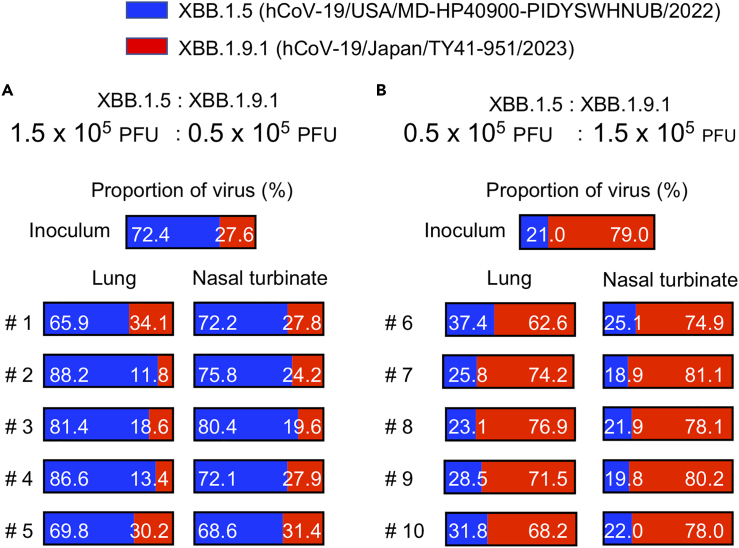
To further compare the replicative fitness of XBB.1.9.1 and XBB.1.5, we compared the growth of XBB.1.9.1 with that of XBB.1.5 in wild-type hamsters (Figure 3). Wild-type hamsters were intranasally inoculated with 2 × 105 PFU of a mixture of XBB.1.9.1 and XBB.1.5 at ratios of 3:1 or 1:3. At 4 days post-infection, the proportion of each virus in the nasal turbinates and lungs of the infected hamsters was determined by using next-generation sequencing (NGS). NGS analysis revealed that the proportion of XBB.1.9.1 was similar in both the nasal turbinates and lungs of all infected animals to that in each inoculum for both ratios (Figure 3). Taken together, these results suggest that the antigenicity and replicative ability of XBB.1.9.1 are comparable to those of XBB.1.5.
Figure 3.
Replicative fitness of XBB.1.9.1 compared with that of XBB.1.5 in hamsters
XBB.1.5 and XBB.1.9.1 were mixed at 3:1 (A) or 1:3 (B) ratios on the basis of their infectious titer, and the virus mixture (total 2 × 105 PFU in 60 μL) was intranasally inoculated into wild-type hamsters (n = 5 per group). Nasal turbinates and lungs were collected from the infected animals at 4 dpi and analyzed by using NGS. The proportions of XBB.1.9.1 and XBB.1.5 were calculated from the four nucleotide differences in the ORF1a/b gene between the two viruses. Shown are the relative proportions of XBB.1.9.1 and XBB.1.5 in the infected animals.
Overall, our data suggest that bivalent mRNA vaccine boosters can enhance humoral immunity against the omicron sublineage XBB.1.9.1 and that remdesivir, molnupiravir, nirmatrelvir, and ensitrelvir remain effective in vitro. Considering the similar antigenicity and replicative ability of XBB.1.9.1 compared with XBB.1.5, it is likely that factors other than viral factors are responsible for the rising prevalence of XBB.1.9.1.
Limitations of the study
We found that XBB.1.9.1 and XBB.1.5 have comparable replicative ability in naive wild-type hamsters. However, in the human population, many people possess SARS-CoV-2-specific adaptive immunity through natural infection and/or vaccination. Therefore, it remains uncertain whether the replicative ability of XBB.1.9.1 is comparable to that of XBB.1.5 in animals or humans with immunity to SARS-CoV-2. Given this uncertainty, it would be informative to compare the viral replicative ability of XBB.1.9.1 and XBB.1.5 in primary human airway/bronchial epithelial cells or airway organoids.1,6
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Tixagevimab | Takashita et al.7 | NA |
| Casirivimab | Takashita et al.7 | NA |
| Cilgavimab | Takashita et al.7 | NA |
| Imdevimab | Takashita et al.7 | NA |
| S309 | Takashita et al.7 | NA |
| bebtelovimab | Takashita et al.8 | NA |
| SARS-CoV-2 nucleoprotein (clone N45) | TAUNS Laboratories, Inc. | NA |
| Peroxidase AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson ImmunoResearch Laboratories Inc. | Cat#115-035-003; RRID:AB_10015289 |
| Bacterial and virus strains | ||
| hCoV-19/Japan/TY41-951/2023 | This study | N/A |
| hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022 | Uraki et al.3 | N/A |
| hCoV-19/Japan/TY41-795/2022 | Imai et al.9 | N/A |
| SARS-CoV-2/UT-NC002-1T/Human/2020/Tokyo | Takashita et al.7 | N/A |
| Biological samples | ||
| Human sera | Uraki et al.10 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco's modified Eagle's medium (DMEM) | SIGMA | Cat #D5796 |
| Fetal Calf Serum (FCS) | gibco | Cat #10437-028 |
| penicillin–streptomycin | FUJIFILM Wako Pure Chemical Corporation | Cat #168-23191 |
| Puromycin | InvivoGen | Cat # ant-pr-1 |
| Geneticin | InvivoGen | Cat # ant-gn-5 |
| plasmocin prophylactic | InvivoGen | Cat #ant-mpp |
| Expi293 expression medium | Thermo Fisher Scientific | Cat # A1435101 |
| GS-441524 | MedChemExpress | Cat # HY-103586 |
| EIDD-1931 | MedChemExpress | Cat # HY-125033 |
| PF-07321332 | MedChemExpress | Cat # HY-138687 |
| S-217622 | Shionogi & Co., Ltd. | N/A |
| Methyl Cellulose 400 | FUJIFILM Wako Pure Chemical Corporation | Cat # 132-05055 |
| Deposited data | ||
| the variable region of the heavy and light chains of tixagevimab | PDB | QLI33947 and QLI33948 |
| the variable region of the heavy and light chains of casirivimab | PDB | 6XDG_B and 6XDG_D |
| the variable region of the heavy and light chains of cilgavimab | PDB | QKY76296 and QKY75909 |
| the variable region of the heavy and light chains of imdevimab | PDB | 6XDG_A and 6XDG_A |
| the variable region of the heavy and light chains of S309 | PDB | 6WS6_A and 6WS6_F |
| the variable region of the heavy and light chains of bebtelovimab | PDB | 7MMO_D and 7MMO_E |
| the constant gamma heavy chain coding sequences | UniProtKB/Swiss-Prot | P01857 |
| the constant kappa light chain coding sequences | UniProtKB/Swiss-Prot | P01834 |
| the constant lambda light chain coding sequences | UniProtKB/Swiss-Prot | P0DOY2 |
| hCoV-19/Japan/TY41-951/2023 (XBB.1.9.1) sequence | the Global Initiative on Sharing All Influenza Data (GISAID) database | EPI_ISL_17482300 |
| Wuhan/Hu-1/2019 sequence | GenBank | MN908947 |
| Experimental models: Cell lines | ||
| VeroE6/TMPRSS2 cells | JCRB Cell Bank | JCRB1819 |
| Vero E6-TMPRSS2-T2A-ACE2 cells | Graham laboratory | NA |
| Chinese hamster ovary (CHO) cells | GenScript | NA |
| Expi293F cells | Thermo Fisher Scientific | Cat# A14527 |
| Experimental models: Organisms/strains | ||
| Slc:Syrian hamsters (male, 6 weeks old) | Japan SLC Inc. | http://www.jslc.co.jp/pdf/data/2013/syrian2013.pdf |
| Software and algorithms | ||
| GraphPad Prism 9.3.0 | GraphPad Software, Inc. | https://www.graphpad.com/scientific-software/prism/ |
| BioSpot software | Cellular Technology | https://immunospot.com/plaque-colony-counting |
| Other | ||
| QIAamp Viral RNA Mini Kit | QIAGEN | Cat# 52926 |
| LunarScript RT SuperMix Kit | New England BioLabs | Cat# E3010 |
| Q5 High-Fidelity DNA polymerase | New England BioLabs | Cat# M0491 |
| Q5 Hot Start DNA polymerase | New England BioLabs | Cat# M0493 |
| QIAseq FX DNA Library Kit | QIAGEN | Cat# 180477 |
Resource availability
Lead contact
Further information or requests should be directed to and will be fulfilled by the Lead Contact, Yoshihiro Kawaoka (yoshihiro.kawaoka@wisc.edu).
Materials availability
All materials can be obtained directly from the authors or through commercially available sources, with the exception of clinical specimens. Due to the extremely limited availability of these clinical specimens, we are unable to provide them.
Experimental model and study participant details
Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval number PA19-75). Virus inoculations were performed under isoflurane, and all efforts were made to minimize animal suffering.
To collect and use clinical specimens, the research protocol was approved by the Research Ethics Review Committee of the Institute of Medical Science of the University of Tokyo (approval numbers: 2019–71–0201 and 2020-740226). After informed consent was obtained, plasma specimens were collected from COVID-19 convalescent individuals and vaccinees.
Method details
Cells
Vero E6-TMPRSS2-T2A-ACE2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% Fetal Calf Serum (FCS), 100 U/mL penicillin–streptomycin, and 10 μg/mL puromycin. VeroE6/TMPRSS2 (JCRB 1819) cells11,12(Imai et al., 2021; Matsuyama et al., 2020)(Matsuyama, Nao et al. 2020, Imai, Halfmann et al. 2021)(Imai et al., 2021; Matsuyama et al., 2020)(Imai et al., 2021; Matsuyama et al., 2020)(Imai et al., 2021; Matsuyama et al., 2020) were propagated in the presence of 1 mg/ml geneticin (G418; Invivogen) and 5 μg/ml plasmocin prophylactic (Invivogen) in DMEM containing 10% FCS. Vero E6-TMPRSS2-T2A-ACE2 and VeroE6/TMPRSS2 cells were maintained at 37°C with 5% CO2. Chinese hamster ovary (CHO) cells were maintained in DMEM containing 10% FCS and antibiotics at 37°C with 5% CO2. Expi293F cells (Thermo Fisher Scientific) were maintained in Expi293 expression medium (Thermo Fisher Scientific) at 37°C under 8% CO2. The cells were regularly tested for mycoplasma contamination by using PCR, and confirmed to be mycoplasma-free.
Viruses
The SARS-CoV-2 viruses hCoV-19/Japan/TY41-951/2023 (Omicron XBB.1.9.1; isolated using Vero E6-TMPRSS2-T2A-ACE2 cells), hCoV-19/USA/MD-HP40900-PIDYSWHNUB/2022 (Omicron XBB.1.5)3, hCoV-19/Japan/TY41-795/2022 (Omicron XBB),9 and SARS-CoV-2/UT-NC002-1T/Human/2020/Tokyo7 (ancestral strain) were propagated in VeroE6/TMPRSS2 cells.
All experiments with SARS-CoV-2 were performed in enhanced biosafety level 3 containment laboratories at the University of Tokyo and the National Institute of Infectious Diseases, Japan, which are approved for such use by the Ministry of Agriculture, Forestry, and Fisheries, Japan.
Antibodies
Amino acid sequences for the variable region of the heavy and light chains of the following human monoclonal antibodies against the S protein were used for gene synthesis: clones tixagevimab (COV2-2196/AZD8895; GenBank accession numbers QLI33947 and QLI33948), casirivimab (REGN10933; PDB accession numbers 6XDG_B and 6XDG_D), cilgavimab (COV2-2130/AZD1061; GenBank accession numbers QKY76296 and QKY75909), imdevimab (REGN10987; PDB accession numbers 6XDG_A and 6XDG_A), S309 (PDB accession numbers 6WS6_A and 6WS6_F), and bebtelovimab (LYCoV1404; PDB accession numbers 7MMO_D and 7MMO_E). An artificial signal sequence and the constant gamma heavy (IgG1, UniProtKB/Swiss-Prot accession number P01857) and kappa (UniProtKB/Swiss-Prot accession number P01834) or lambda (UniProtKB/Swiss-Prot accession number P0DOY2) light chain coding sequences were added before and after each variable region. Codon usage was optimized for expression in CHO cells. The synthesized genes were cloned into a plasmid for protein expression and transfected into CHO cells. Cell culture media were harvested after incubation for 10–14 days at 37°C. Monoclonal antibodies were purified by using MabSelect SuRe LX (Cytiva) or a protein A column. Purity was confirmed by SDS-PAGE and/or HPLC before use. The reactivities of these antibodies against SARS-CoV-2, including the Alpha, Beta, Delta, Gamma, and Omicron variants, have been tested previously.7
Antiviral compounds
Active components of remdesivir and molnupiravir (i.e., GS-441524 and EIDD-1931), and nirmatrelvir (PF-07321332) were purchased from MedChemExpress. Ensitrelvir (S-217622) was kindly provided by Shionogi & Co., Ltd. All compounds were dissolved in dimethyl sulfoxide.
Focus reduction neutralization test (FRNT)
Neutralization activities of monoclonal antibodies and human plasma were determined by using a focus reduction neutralization test as previously described.13 Serially diluted antibodies (starting concentration, 50,000 ng/ml) were mixed with 100–400 focus-forming units (FFU) of virus/well and incubated for 1 h at 37°C. The antibody-virus mixture (50μl) was then inoculated onto Vero E6-TMPRSS2-T2A-ACE2 cells in 96-well plates in triplicate. After a 1-h incubation at 37°C, 100μl of 1.5% Methyl Cellulose 400 (FUJIFILM Wako Pure Chemical Corporation, Japan) in culture medium was added to each well. The cells were incubated for 14–18 h at 37°C and then fixed with formalin. For human plasma, the samples were first incubated at 56°C for 1 h. Then, the treated plasma samples were serially diluted five-fold with DMEM containing 2% FCS in 96-well plates and mixed with 100–400 FFU of virus/well, followed by incubation at 37°C for 1 h. The plasma-virus mixture was inoculated onto Vero E6-TMPRSS2-T2A-ACE2 cells in 96-well plates in duplicate. After a 1-h incubation at 37°C, 100μl of 1.5% Methyl Cellulose 400 (FUJIFILM Wako Pure Chemical Corporation) in culture medium was then added to each well. The cells were incubated for 14–18 h at 37°C and then fixed with formalin.
After the formalin was removed, the cells were immunostained with a mouse monoclonal antibody against SARS-CoV-2 nucleoprotein [N45 (TAUNS Laboratories, Inc., Japan)], followed by a horseradish peroxidase-labeled goat anti-mouse immunoglobulin (Jackson ImmunoResearch Laboratories Inc.). The infected cells were stained with TrueBlue Substrate (SeraCare Life Sciences) and then washed with distilled water. After cell drying, the focus numbers were quantified by using an ImmunoSpot S6 Analyzer, ImmunoCapture software, and BioSpot software (Cellular Technology). The results are expressed as the 50% focus reduction neutralization titer (FRNT50). The FRNT50 values were calculated by using GraphPad Prism (GraphPad Software). Samples under the detection limit (<10-fold dilution) were assigned an FRNT50 value of 10.
Inhibitory effect of compounds against SARS-CoV-2 in vitro
Antiviral susceptibilities of SARS-CoV-2 were determined by applying a focus reduction assay as previously described.9,14 Vero E6-TMPRSS2-T2A-ACE2 cells in 96-well plates were infected with 100–400 FFU of virus/well. Virus adsorption was carried out for 1 h at 37°C and then the inoculum was removed and 1% Methyl Cellulose 400 (FUJIFILM Wako Pure Chemical Corporation) in culture medium containing serial dilutions of antiviral compounds was added to each well in triplicate. The cells were incubated for 18 h at 37°C and then fixed with formalin. After the formalin was removed, immunostaining was performed as described for the FRNT. The results are expressed as the 50% inhibitory concentration (IC50). The IC50 values were calculated by using GraphPad Prism (GraphPad Software).
Experimental infection of Syrian hamsters
XBB.1.9.1 was mixed with XBB.1.5 at a 1:3 or 3:1 ratio on the basis of their titers, and each virus mixture (total 2 × 105 PFU in 60 μL) was inoculated into five six-week-old male wild-type hamsters. At 4 days post-infection, five animals were euthanized and nasal turbinates and lungs were collected.
Whole genome sequencing
Viral RNA was extracted by using a QIAamp Viral RNA Mini Kit (QIAGEN). The whole genome of SARS-CoV-2 was amplified by using a modified ARTIC network protocol in which some primers were replaced or added. Briefly, viral cDNA was synthesized from the extracted RNA by using a LunarScript RT SuperMix Kit (New England BioLabs). The DNA was amplified by performing a multiplexed PCR in two pools using the ARTIC-N5 primers and the Q5 High-Fidelity DNA polymerase or Q5 Hot Start DNA polymerase (New England BioLabs). The DNA libraries for Illumina NGS were prepared from pooled amplicons by using a QIAseq FX DNA Library Kit (QIAGEN) and were then analyzed by using the MiSeq (Illumina) or iSeq 100 System (Illumina). To determine the virus sequences, the reads were assembled by CLC Genomics Workbench (version 22, Qiagen) with the Wuhan/Hu-1/2019 sequence (GenBank accession no. MN908947) as a reference. The sequences of XBB.1.9.1 (hCoV-19/Japan/TY41-951/2023) was deposited in the Global Initiative on Sharing All Influenza Data (GISAID) database with accession ID: EPI_ISL_17482300.
For the analysis of the XBB.1.9.1:XBB.1.5 ratio after co-infection, the ratio of XBB.1.9.1 to XBB.1.5 was calculated from the four nucleotide differences in the ORF1a/b gene between the two viruses. Samples with more than 1000 read-depths were analyzed.
Quantification and statistical analysis
We used GraphPad Prism 9.3.0 for data visualization to determine the FRNT50 and IC50 values.
Acknowledgments
We thank Susan Watson for scientific editing. We also thank Mashiho Yanagi, Kyoko Yokota, Kyoko Tada, Tomoka Nagashima, Naoko Mizutani, Rie Onoue, and Madoka Yoshikawa for technical assistance. Vero E6-TMPRSS2-T2A-ACE2 cells were provided by Dr. Barney Graham, NIAID Vaccine Research Center.
This study was supported by grants from the Center for Research on Influenza Pathogenesis and Transmission (75N93021C00014) by the National Institute of Allergy and Infectious Diseases, and a research program on emerging and re-emerging infectious diseases (JP21fk0108552), the Japan Program for Infectious Diseases Research and Infrastructure (JP23wm0125002), and the Japan Initiative for World-leading Vaccine Research and Development Centers (JP233fa627001) from the Japan Agency for Medical Research and Development. The funders had no role in the study design, data collection, data analysis, interpretation, or writing of the paper.
Author contributions
R.U.: conceptualization, formal analysis, validation, visualization, and writing of the first draft. M. Ito, M. Kiso: data curation, formal analysis, and methodology. S. Yamayoshi: conceptualization, data curation, formal analysis, and methodology. K.I-H.: resources and validation. Y.S-T., M. Imai, M. Koga, S. Yamamoto, E.A., M.S., T.T, A.O., T.K., H.Y.: resources. S.F., S.W., T.S., and K.M.: virus isolation and data curation. Y. Kawaoka: conceptualization, supervision, writing (review and editing), and funding acquisition. R.U., M. Ito, M. Kiso, and S. Yamayoshi contributed equally.
Declaration of interests
Y. Kawaoka has received unrelated funding support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. LTD, Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio. T.K.is employed by Nihon Sumo Kyokai.
Published: October 4, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108147.
Supplemental information
Data and code availability
-
•
All data used in this paper are available in the main text, in the supplemental information, or the sources have been clearly stated.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Chen J.J., Li L.B., Peng H.H., Tian S., Ji B., Shi C., Qian C., Jiang W.G., Liu M.C., Li T.T., et al. Neutralization against XBB.1 and XBB.1.5 after omicron subvariants breakthrough infection or reinfection. Lancet Reg. Health. West. Pac. 2023;33:100759. doi: 10.1016/j.lanwpc.2023.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu P., Faraone J.N., Evans J.P., Zheng Y.M., Carlin C., Anghelina M., Stevens P., Fernandez S., Jones D., Panchal A.R., et al. Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants. Cell Rep. 2023;42:112443. doi: 10.1016/j.celrep.2023.112443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uraki R., Ito M., Kiso M., Yamayoshi S., Iwatsuki-Horimoto K., Furusawa Y., Sakai-Tagawa Y., Imai M., Koga M., Yamamoto S., et al. Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate. Lancet Infect. Dis. 2023;23:402–403. doi: 10.1016/S1473-3099(23)00070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uriu K., Ito J., Zahradnik J., Fujita S., Kosugi Y., Schreiber G., Sato K., Genotype to Phenotype Japan G2P-Japan Consortium Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023;23:280–281. doi: 10.1016/S1473-3099(23)00051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue C., Song W., Wang L., Jian F., Chen X., Gao F., Shen Z., Wang Y., Wang X., Cao Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 2023;23:278–280. doi: 10.1016/S1473-3099(23)00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muramoto Y., Takahashi S., Halfmann P.J., Gotoh S., Noda T., Kawaoka Y. Replicative capacity of SARS-CoV-2 omicron variants BA.5 and BQ.1.1 at elevated temperatures. Lancet. Microbe. 2023;4:e486. doi: 10.1016/S2666-5247(23)00100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., Iwatsuki-Horimoto K., Chiba S., Halfmann P., Nagai H., et al. Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant. N. Engl. J. Med. 2022;386:995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takashita E., Yamayoshi S., Simon V., van Bakel H., Sordillo E.M., Pekosz A., Fukushi S., Suzuki T., Maeda K., Halfmann P., et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022;387:468–470. doi: 10.1056/NEJMc2207519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai M., Ito M., Kiso M., Yamayoshi S., Uraki R., Fukushi S., Watanabe S., Suzuki T., Maeda K., Sakai-Tagawa Y., et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023;388:89–91. doi: 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uraki R., Ito M., Kiso M., Yamayoshi S., Iwatsuki-Horimoto K., Sakai-Tagawa Y., Imai M., Koga M., Yamamoto S., Adachi E., et al. Efficacy of antivirals and mRNA vaccination against an XBF clinical isolate. Lancet Reg. Health. West. Pac. 2023;34:100777. doi: 10.1016/j.lanwpc.2023.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai M., Halfmann P.J., Yamayoshi S., Iwatsuki-Horimoto K., Chiba S., Watanabe T., Nakajima N., Ito M., Kuroda M., Kiso M., et al. Characterization of a new SARS-CoV-2 variant that emerged in Brazil. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2106535118. e2106535118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderheiden A., Edara V.V., Floyd K., Kauffman R.C., Mantus G., Anderson E., Rouphael N., Edupuganti S., Shi P.Y., Menachery V.D., et al. Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies. Curr. Protoc. Immunol. 2020;131:e116. doi: 10.1002/cpim.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashita E., Morita H., Ogawa R., Nakamura K., Fujisaki S., Shirakura M., Kuwahara T., Kishida N., Watanabe S., Odagiri T. Susceptibility of Influenza Viruses to the Novel Cap-Dependent Endonuclease Inhibitor Baloxavir Marboxil. Front. Microbiol. 2018;9:3026. doi: 10.3389/fmicb.2018.03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data used in this paper are available in the main text, in the supplemental information, or the sources have been clearly stated.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.