Abstract
Background
Empirical use of antibiotics was reported throughout the coronavirus disease of 2019 (COVID-19) pandemic; however, evidence of bacterial coinfection or secondary bacterial infection among COVID-19 patients was sparse. Antibiotic overprescription for COVID-19 patients without confirmed bacterial coinfection can increase antimicrobial resistance (AMR). The objective of this study is to assess the appropriateness of antibiotic use during COVID-19 by summarizing the frequency of antibiotic use among hospitalized COVID-19 and the frequency of antibiotic use in patients with COVID-19.
Methods
A systematic search was conducted of the Embase, Medline, Web of Science, and Cochrane Library databases by generating search terms using the concepts of “COVID-19,” “Bacterial Coinfection,” “Secondary bacterial infection,” and “Antimicrobial resistance” to identify studies reporting antibiotic prescription for hospitalized COVID-19 patients with or without bacterial coinfection. We excluded studies on outpatients, studies informed infection due to mechanical ventilation, and randomized controlled trials. The pooled estimate of the percentage of the total and confirmed appropriate antibiotic prescriptions provided to hospitalized COVID-19 patients was generated using a random effect meta-analysis with inverse variance weighting. The study protocol registration DOI is osf.io/d3fpm.
Results
Of 157,623 participants from 29 studies (11 countries, 45 % women) included in our review, antibiotics were prescribed to 67 % of participants (CI 64 %–71 %, P < 0·001), of which 80 % (CI 76 %–83 %, P < 0·001) of prescriptions were for COVID-19 patients without confirmed bacterial coinfections. Antibiotic overprescription varied during different periods of the pandemic and between High-Income and Upper and Lower Middle-Income Countries. We found heterogeneity among the studies (I2 = 100 %). The risk of bias analysis showed that 100 % of the included studies had the proper sample framing, and we are at low risk of bias due to sampling.
Discussion
We find greater than expected use of antibiotics to treat hospitalized COVID-19 patients without bacterial coinfections, which may contribute to AMR globally. Concrete guidelines for using antibiotics to treat COVID-19 patients, strict monitoring, and administering Antimicrobial Stewardship are needed to prevent overprescription.
Highlights
-
•
We summarized 29 studies of antibiotic use (11 countries; 45 % female) in COVID-19
-
•
Antibiotics were prescribed to 67 % of hospitalized COVID-19 patients
-
•
80 % of these were without confirmed bacterial coinfections.
-
•
Antibiotic overprescription varied between high and low-income countries.
-
•
Unnecessary antibiotics to treat COVID-19 contributes to Antimicrobial resistance.
1. Introduction
Antimicrobials-including antibiotics, antivirals, antifungals, and antiparasitics – are medicines used to prevent and treat infections in humans, animals, and plants [1]. Globally, the use of antibiotics has increased remarkably [1]. The ongoing COVID-19 pandemic has significantly contributed to the changing landscape of antibiotic use in patient care. The World Health Organization (WHO) and other expert advisory groups suggested not initiating antibiotic therapy for suspected, probable, or confirmed mild COVID-19. [2,3], While the reporting of bacterial coinfections ranged from 1·2 % to 46·38 % and secondary bacterial infections ranged from 1·56 % to 32·3 % in hospitalized patients with COVID-19 infection [4,5], antibiotic prescription for these patients ranged from 1·3 %–100 % [6,7].
The unregulated use of antibiotics can lead to antimicrobial resistance (AMR), a global health emergency that kills around 700,000 people in a year [8]. A predictive statistical model by Murray calculated that 4·95 million deaths were related to bacterial AMR in 2019 and that AMR was the direct cause of 1·27 million deaths in the same year [9]. The World Health Assembly acknowledged the threat of AMR and endorsed a Global Action Plan in 2015 to “optimize the use of antimicrobial medicines” as one of five objectives to ensure Antimicrobial Stewardship (AMS) [10]. AMS is a comprehensive set of actions to “promote the responsible use of antimicrobials” [10], that is, “the right antibiotic for the right patient at the right time with the right dose and the right route causing the least harm to the patient and the future patients [11]." Experts have a growing concern that excessive use of antibiotics during the pandemic may increase the risk of antimicrobial resistance [12].
Different studies indicated increased antimicrobial consumption during the pandemic period, especially at the beginning of the pandemic [[13], [14], [15]]. Annual antimicrobial consumption for different classifications of antimicrobial drugs increased by up to 26 % in 2020 compared to the previous years (2011–2019) [14,15]. Furthermore, increased prescribing increases the risk of Clostridioids difficile, which carries significant morbidity, mortality, and infection control implications [16]. Increased prescribing also leads to additional healthcare costs, particularly in low-income countries, where the burden is on the health system or individual patients (depending on public/private funding) [17].
It is essential to collect data regarding the usage of antibiotics in the context of the COVID-19 pandemic and assess the contribution of novel prescribing patterns to AMR. This systematic review aimed to summarize the frequency of antibiotic use among hospitalized COVID-19 and the frequency of antibiotic use in patients with COVID-19, and these data may contribute to assessing the appropriateness of antibiotic use during COVID-19.
2. Material and methods
2.1. Overview
For this systematic review and meta-analysis, we searched OVID. Medline, EMBASE, Web of Science, and Cochrane Library. We used to generate keywords related to the concepts of: “coronavirus,” “COVID-19,” “SARS-COV-2,” “secondary bacterial infection,” “bacterial coinfection,” “Antibiotic prescription,” “Antimicrobial resistance,” “Antibiotic resistance.” We also used Canadian Agency for Drugs and Technologies in Health (CADTH.) COVID-19 search strings-generated search vocabularies for searching COVID-9-related literature in our search strategy for OVID. Medline and EMBASE databases [18]. After finalizing the search terms, we conducted our final search on March 5, 2022. We used “.ti = title, .ab = abstract, .kw = author-provided keyword exact, .kf = word in author provided” in Medline and used “.mp = multipurpose” in Embase for Textword searching. We conducted a text search for “ALL = All Fields” for the Web of Science. We combined all the search terms within a concept with the “OR” Boolean operator and then used the “AND” Boolean operator to combine the concepts. Reference lists of the included articles were also checked.
2.2. Inclusion and exclusion criteria
The inclusion criteria consist of the following-
-
•
Language: We limited our search to English-language articles to ensure consistent interpretation and analysis.
-
•
Study Design: We considered primary research studies that presented original data, including observational studies, retrospective studies, and prospective studies.
-
•
Target Population: We focused exclusively on hospitalized COVID-19 patients.
-
•
Outcome Measures: We sought articles that reported the frequency of secondary bacterial infections or bacterial coinfections, specifically among hospitalized COVID-19 patients. These articles were expected to present data on the number of cases with confirmed bacterial infections among hospitalized COVID-19 patients.
-
•
Antibiotic Prescription Data: Additionally, we aimed to collect information on the frequency of antibiotic prescriptions in the context of secondary bacterial infections or bacterial coinfections among hospitalized COVID-19 patients.
We excluded studies that were not conducted on humans or on hospitalized COVID-19 patients, studies on outpatient department patients, and studies that focused on nosocomial infections due to mechanical ventilation. We did not include any case study/report for individual patients, opinions, commentary articles, or randomized controlled trials.Articles that did not report the prescription rate of antibiotics among COVID-19 patients were excluded. However, if groups were presented separately and allowed the calculation of the required data, data from the study were included (Detail process and example in appendix).
2.3. Data management and synthesis
We used Covidence, a web-based collaboration software platform [19], to screen titles and abstracts, full-text articles, and extract data (Extraction 2.0, a data extraction tool by Covidence). All retrieved titles and abstracts were first screened for duplicates, and unique abstracts were screened by a single reviewer, Fazle Rabbi (FR). The full texts of all the abstracts that passed the screening were reviewed independently by two reviewers, FR and Mehnaz Munir (MM). Any conflict on articles’ inclusion/exclusion was resolved by discussion between two reviewers (FR and MM).
Data were extracted independently and duplicated by FR and MM, and disagreements were resolved after discussing them in detail. Where necessary, a senior investigator, Russell J. de Souza (RJdS), was consulted to resolve disagreements. From each study, we extracted 1) the number of patients with COVID-19 who were prescribed antibiotics and 2) the number of patients with confirmed bacterial coinfection/secondary coinfection. We made the following assumptions regarding data extraction:
For studies in which it is not clear whether the patients suffered from secondary bacterial infection or bacterial coinfection, we counted this bacterial infection under the category of “bacterial coinfection.”
For our analysis, we included articles containing all the necessary data and some missing data that could be assumed based on the range of confidence intervals (CI). Specifically, we included articles where both the highest and lowest values of the CI could be assumed. To analyze the data, we first used the articles containing all necessary data and the articles where the missing data were assumed to be at the lower end of the CI range. After that, we repeated the analysis using the articles with available data and the articles where the missing data were assumed to be at the higher end of the CI range. We included articles with complete or partially assumed data in our analysis and then conducted separate analyses using the available data and the assumed values for missing data.
The outcomes we extracted were the prevalence of antibiotic prescription (%), and the prevalence of antibiotic prescriptions in COVID-19 patients without confirmed bacterial co-infections (%). We used the Wilson Score Interval method to calculate the confidence interval surrounding the prevalence estimates:
In cases where at least two studies provided combinable data, a DerSimonian and Laird's random effect meta-analysis was performed, which yields conservative confidence intervals (CI) around the prevalence estimates in the presence of heterogeneity [20]. Heterogeneity was detected using Cochran's Q test (significant at P < 0.10) and quantified using the I2 statistic (ranging from 0 to 100 %). The pooled estimate of the percentage of the total and confirmed appropriate antibiotic prescriptions provided to hospitalized COVID-19 patients was generated using a random effect meta-analysis with inverse variance weighting. All analyses were completed using Review Manager 5.4.1 (The Cochrane Collaboration, 2020) [21].
We performed a pre-specified subgroup analysis according to publication date, classified as either pre-immunosuppressive (1 December 2019 to before 16 June 2020) or post-immunosuppressive (After 17 June but before 30 November 2021) to observe the antibiotic prescription pattern before and after the announcement of dexamethasone as the treatment for COVID-19 [22].
2.4. Risk of bias assessment
The Joanna Briggs Institute (JBI) critical appraisal tool for systematic reviews of prevalence studies was used to assess the study risk of bias [23]. It is a tool designed to assess the quality of systematic reviews of prevalence studies. It is a standardized method that helps to ensure that the review process is thorough and unbiased. The tool covers several vital areas, including the study design, sample size, data collection methods, and analysis.
Role of the funding source
There was no funding for the study.
3. Results
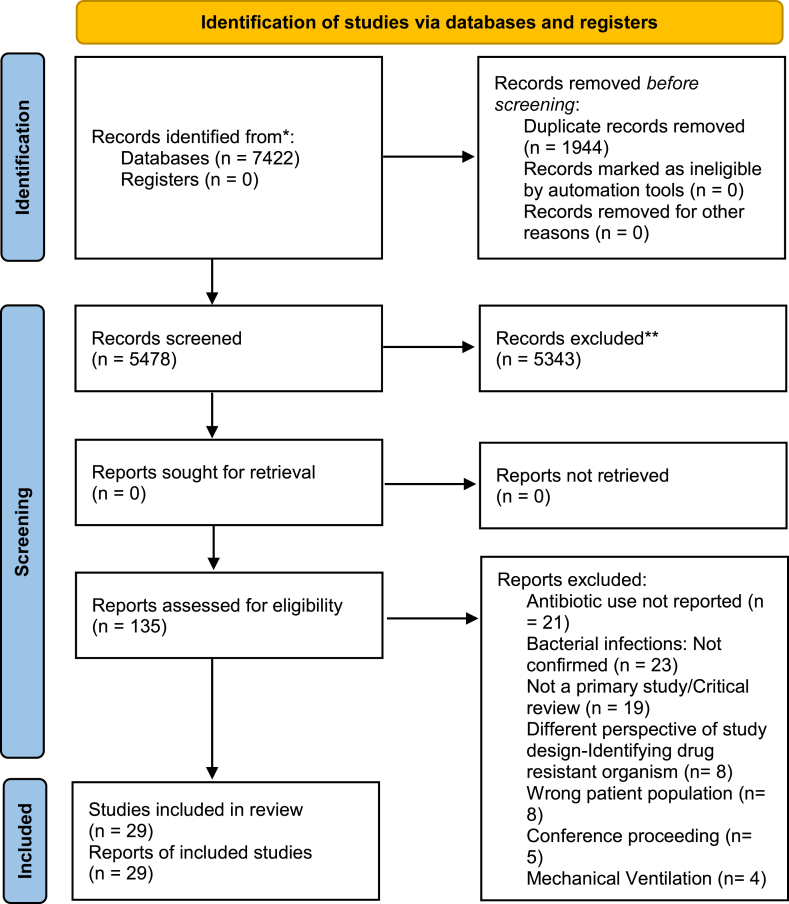
A total of 7422 abstracts from four different databases (OVID MEDLINE, EMBASE, Web of Science, and Cochrane Library) were identified and imported for screening. After removing duplicates, 5474 citations were reviewed, and 125 studies for full-text review were selected. Of these, 29 articles were included in this review. The total number of participants contributing data from the selected studies was 157,623, approximately 56 % of whom were male. Fig. 1 shows the PRISMA flowchart for the study selection process, and Table 1 summarizes the selected articles.
Fig. 1.
PRISMA flowchart for the systematic review.
Table 1.
Summary table for the selected articles.
| Summary Table for the Selected Articles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | Study ID | Country of the research | Study design | Study period ended | Sample Size | Age | # of Male | % of Patient with Antibiotics | % of Pt with Bacterial CI |
| 1 | Vaughn 2020 | USA | Cohort study | 1 December 2019 to before 16 June 2020 | 1705 | 64·7 | 885 | 56·6 | 3·5 |
| 2 | Petty 2021 | USA | Cohort study | After 17 June 2020 but before 30 November 2021 | 2205 | 64·9 | 1154 | 62·9 | 6·4 |
| 3 | Lehmann 2021 | USA | Cohort study | 1 December 2019 to before 16 June 2020 | 321 | 60 | 155 | 69 | 2.2 |
| 4 | Nori 2021 | USA | Cohort study | 1 December 2019 to before 16 June 2020 | 152 | 62 | 89 | 79 | 40·13 |
| 5 | Elabbadi 2021 | France | Cohort study | 1 December 2019 to before 16 June 2020 | 101 | 61 | 79 | 57·4 | 19·81 |
| 6 | Asmarawati 2021 | Indonesia | Cohort study | After 17 Jun 2020 but before 30 Nov 2021 | 218 | 52·45 | 120 | 75·2 | 19·72 |
| 7 | Sharma 2021 | India | Cohort study | After 17 June 2020 but before 30 November 2021 | 1844 | 48 | NA | 75 | 17·9 |
| 8 | VanLaethem 2022 | Belgium | Cohort study | 1 December 2019 to before 16 June 2020 | 429 | 64 | 245 | 39 | 5 |
| 9 | Papst 2022 | Croatia, Italy, Serbia and Slovenia | Cross sectional study | After 17 June 2020 but before 30 November 2021 | 988 | NA | NA | 52·7 | 1·9 |
| 10 | Martin 2021 | USA | Cohort study | 1 December 2019 to before 16 June 2020 | 208 | 69 | 105 | 83 | 8 |
| 11 | Angell 2021 | USA | Cohort study | After 17 June 2020 but before 30 November 2021 | 296 | NA | NA | 35·47 | 16·89 |
| 12 | Baghdadi 2021 | USA | Cohort study | After 17 June 2020 but before 30 November 2021 | 64961 | 18 to >70 | 34370 | 76·3 | 18·5 |
| 13 | Townsend 2020 | Ireland | Cohort study | 1 December 2019 to before 16 June 2020 | 117 | 66 | 74 | 81 | 12·82 |
| 14 | Cheng 2020 | Hong Kong | Cohort study | 1 December 2019 to before 16 June 2020 | 147 | 36 | 85 | 35 | 8.2 |
| 15 | Milas 2021 | Belgium | Cohort study | 1 December 2019 to before 16 June 2020 | 164 | 60·5 | 81 | 61 | 17·1 |
| 16 | Karaba 2021 | USA | Cross sectional study | 1 December 2019 to before 16 June 2020 | 1016 | 62 | 543 | 71 | 5·2 |
| 17 | SEMI-COVID-19Network 2021 | Spain | Cohort study | After 17 June 2020 but before 30 November 2021 | 13932 | 56·5–77·2 | NA. | 78·13 | 10·9 |
| 18 | Grasselli 2021 | Italy | Cohort study | 1 December 2019 to before 16 June 2020 | 774 | 62 | 597 | 69 | 46·38 |
| 19 | Karami 2021 | Netherlands | Cohort study | 1 December 2019 to before 16 June 2020 | 925 | 70 | 591 | 72·32 | 1·6 |
| 20 | Wang 2021 | UK. | Cohort study | 1 December 2019 to before 16 June 2020 | 1396 | 67·4 | 903 | 98 | 1·2 |
| 21 | Hughes 2021 | UK. | Cohort study | After 17 June 2020 but before 30 November 2021 | 624 | 40·2–79·5 | NA. | 49·7 | 2·7 |
| 22 | Estrada 2021 | Spain | Cohort study | 1 December 2019 to before 16 June 2020 | 13932 | 69 | 7819 | 87·8 | 10·8 |
| 23 | Soto 2021 | Peru | Cohort study | After 17 June 2020 but before 30 November 2021 | 93 | 61·7 | 66 | 81·7 | 40·86 |
| 24 | Pink 2021 | Germany | Cohort study | After 17 June 2020 but before 30 November 2021 | 99 | 57 | 72 | 68·7 | 12·121 |
| 25 | Martinez-Guerra 2021 | Mexico | Cohort study | 1 December 2019 to before 16 June 2020 | 794 | 52 | 489 | 92 | 3.65 |
| 26 | ISARIC4CInvestigators 2021 | UK. | Cohort study | 1 December 2019 to before 16 June 2020 | 48902 | 74 | 27979 | 85.2 | 3·97 |
| 27 | Coenen 2021 | Netherlands | Cohort study | 1 December 2019 to before 16 June 2020 | 384 | 61·1 | 157 | 81 | 2·86 |
| 28 | Stevens 2021 | USA | Cohort study | After 17 June 2020 but before 30 Nov 2021 | 654 | 63·6 | 365 | 85·1 | 7·49 |
| 29 | Neto 2021 | USA | Cohort study | 1 Dec 2019 to before 16 Jun 2020 | 242 | 66 | 123 | 67 | 19 |
Only 13 of the 29 (44·23 %) identified studies reported discrete information on antibiotic prescription among patients without bacterial infection and/or coinfections. Only five articles reported secondary bacterial infection, the prevalence of which ranged from 1·56 % to 32·3 % (Mean: 10·3 %).
Among the included studies, 93 % (27) were cohort studies, and 7 % were cross-sectional studies (Table 1, study # 9, 16). Studies from Upper and Lower Middle-Income Countries (U&LMICs, using the World Bank Country and Lending Groups) [24] were rare, and we only found 4 (14 %) studies (only 2 % of the total study population) from U&LMICs that met our eligibility criteria, while 86 % of studies (representing 98 % of the study population) were from High-Income Countries (HICs). Most studies were conducted in the USA (10, 34 %), followed by the UK (3, 10 %), Belgium (2, 7 %), Netherlands (2, 7 %), Spain (2, 7 %), and 1 (3 %) study from each of the following countries- France, Germany, Hong Kong, India, Indonesia, Ireland, Italy, Mexico, Peru. One multinational study was conducted in Croatia, Italy, Serbia, and Slovenia. A total of 18 studies (62 %) were conducted during the pre-immunosuppressive period and 11 (38 %) during the post-immunosuppressive period.
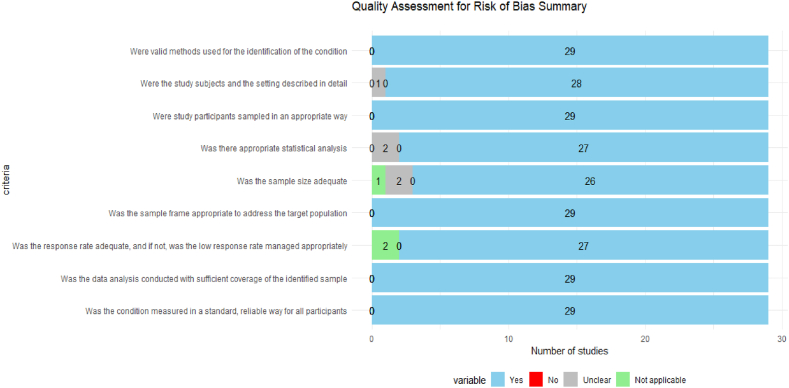
Fig. 2 presents the summary of the JBI. Critical Appraisal checklist. 100 % of the included studies had the proper sample framing and followed the sampling method. Also, all the studies used valid methods to identify the patients' conditions. Data analysis covered sufficient participants for all the studies. Six studies presented some risk of bias across at least one domain. For two studies(Table 1, study#5, 13), the statistical analysis approach was unclear. One study (Table 1, study#11) did not describe the study setting in detail, and it was unclear in another study (Table 1, study#9). The adequacy of the sample size was unclear for two studies (Table 1, study#14, 24). These six studies contributed only 1 % (n = 1748/157623) of the total population of the review.
Fig. 2.
Quality assessment for risk of bias summary.
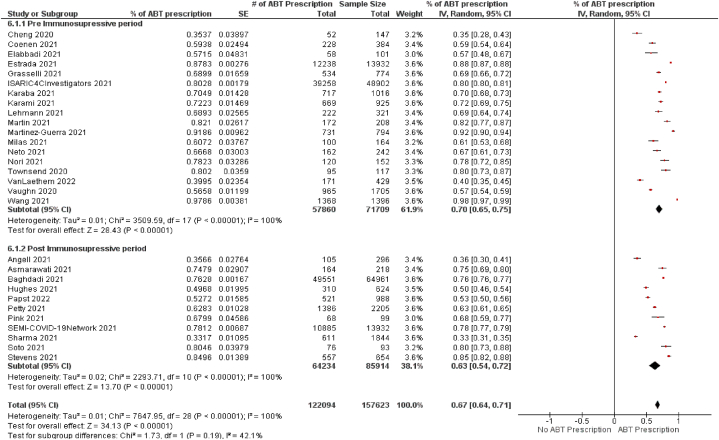
Fig. 3 shows the prevalence of antibiotic prescriptions among the total population. Only four studies reported an antibiotic prescription percentage lower than 50 %, and the highest percentage was observed by Wang (2021) at 98 % (CI 97 %–99 %). The pooled estimate of the prevalence of confirmed bacterial coinfection was 12 %, ranging from 1.2 % [4] to 46.38 % [5], which was available from 157,623 participants in 29 studies.
Fig. 3.
The figure portrays the overall use of antibiotics among the total population before and after the immunosuppressive period of the pandemic. Although the difference was statistically insignificant between the two subgroups, antibiotic prescriptions were more frequent before the dexamethasone announcement as the treatment for COVID-19.
The pooled percentage of antibiotic prescriptions was 67 % (CI 64 %–71 %, P < 0·00001), which was 70 % (CI 65 %–75 %, P < 0·00001) during the pre-immunosuppressive period, and 63 % (CI 54 %–72 %, P < 0·00001) during the post-immunosuppressive period (Fig. 3). Although there was a 7 % difference in the overall antibiotic prescription rate between before and after the immunosuppressive period, it was not statistically significant (P < 0·19).
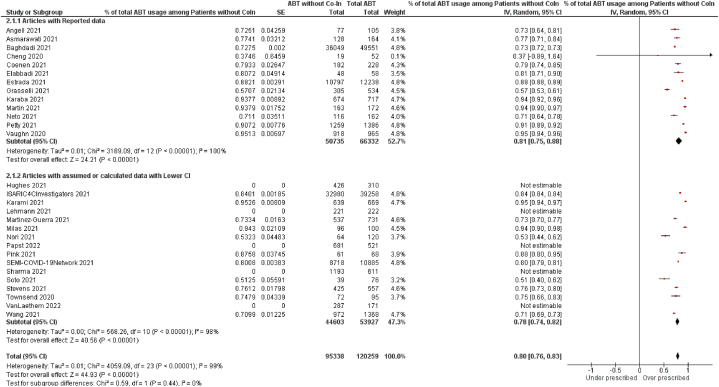
If used appropriately, the percentage of patients prescribed antibiotics in the absence of a confirmed bacterial co-infection or secondary infection should be 0 %, consistent with the definition of good antimicrobial stewardship [25]. Of total antibiotic prescriptions, 81 % were to patients without bacterial coinfections (CI 75 %–88 %, P < 0·00001, # of studies = 13, n = 50,735/66,332) when limited to only those articles that provided direct estimates of both. This value was 78 % (CI 74 %–82 %, P < 0·00001, # of studies = 11, n = 44,603/53,927), and when calculated only for the articles with data imputed as the lowest value of CI. When both data types are pooled, this value is 80 % (CI 76 %–83 %, P < 0·00001, # of studies = 24, antibiotic prescriptions without bacterial coinfections = 95338, total antibiotic prescriptions = 12025). (Fig. 4 for the Lowest value of CI, and Appendix Fig. 5 for the highest value of CI). Thus, the values we have obtained are consistent with over-prescription. Of total antibiotic prescriptions for COVID-19 patients, 79 % (CI 75 %–83 %, P < 0·00001) did not have any bacterial coinfection. This was based on data from 11 studies (for which we imputed data from the highest value of the CI), with a total of 47,855 antibiotic prescriptions without bacterial coinfections out of 55,762 total prescriptions. In the analysis of 24 studies (including 11 with imputed data and 13 with available data), we found that 80 % (CI 76 %–83 %, P < 0·00001) of antibiotic prescriptions for COVID-19 patients did not have any bacterial coinfection. This was based on a total of 98,590 antibiotic prescriptions without bacterial coinfections out of 122,094 total prescriptions.
Fig. 4.
Percentages of Antibiotic prescription among the COVID-19 patients without bacterial coinfections for the articles with available data and the articles with assumed data for the lowest value of CI.
In subgroup analyses comparing the pre-immunosuppressive and post-immunosuppressive periods, we found 4 % higher antibiotic overprescriptions (AOPs) during the pre-immunosuppressive period. The 4 % higher AOPs were constant during the pre-immunosuppressive period than the post-immunosuppressive period for both analyses. However, it was not statistically significant (P = 0·19 and 0·21) (Appendix Fig. 6 and Appendix Fig. 7), indicating AOPs were not reduced remarkably after the announcement of dexamethasone as the COVID-19 treatment. These values were not remarkably different in sensitivity analyses using wider confidence limit estimates. (Appendix Fig. 7).
The antibiotic over-prescription rate was higher in High-income Countries (HICs) than the Upper and Lower Middle-income Countries (U&LMICs). In HICs, the percentage was 81 % (CI 78 %–85 %, P < 0·00001), compared with 69 % (CI 59 %–79 %, P < 0·00001) in U&LMICs (p-value for subgroup differences = 0·03; Appendix Figs. 8 and 9). However, U&LMICs comprised only 2 % of the study population for our meta-analysis.
4. Discussion
Our systematic review and meta-analysis found that more than one-third of the study population were prescribed antibiotics, and four out of five antibiotic prescriptions were given to patients without bacterial infection. The rates were higher in HIC than in U&LMIC but were robust to several approaches to handling missing data. In contrast, bacterial coinfection was rare among hospitalized COVID-19 patients, and only one out of ten had bacterial coinfection. Critically ill patients were more likely to be affected by bacterial coinfections.
Limited testing facilities, a lack of awareness and proper monitoring systems, an overwhelming situation due to skewed patient load and severity during the pandemic, and a lack of experience with such emergencies are vital factors that contributed to the overuse of antibiotics. Effective and regular training programs for health workers for emergency preparedness can improve their skills to deal with future health emergencies and community awareness programs can help improve the general population's health literacy. An effective monitoring system for antibiotic purchases is needed, especially for the U&LMICs, where these crucial bacterial-resistant drugs are easily accessible even from street vendors, without any doctors' prescriptions.
A meta-analysis by Langford et al. (2021) revealed a similar result, with three-quarters of COVID-19 patients being treated with antibiotics, although bacterial coinfections were reported in only 6·1 %–8 % [26]. In another rapid living review and meta-analysis, Langford et al. (2020) found 3.5 % bacterial coinfections, 14·3 % secondary bacterial infection, and 6·9 % bacterial infection among COVID-19 and critically ill patients [27]. However, the antibiotic prescription rate was 71·9 % among COVID-19 patients. In our meta-analysis study [5] on hospitalized critical COVID-9 patients reported the highest bacterial coinfection percentage (46·38 %).
In most cases, the patients were empirically given antibiotics during admission without any pathological test, blood, urine, or sputum culture. Multiple studies [27,28] suggested that the initial reason for prescribing antibiotics was suspected bacterial infection, despite the viral characteristics of the disease. Abelenda-Alonso & Carratala (2020) supported the lack of information, emergency preparedness, and testing facilities as the immediate result of increased antibiotic prescription [29].
Several studies reported increased antibiotic prescriptions during the pandemic, especially at the beginning [13,14]. Khouja et at. (2022) also reported higher consumption of antibiotics in developed countries than the developing countries, which likely reflects greater accessibility [30]. We found a higher antibiotic prescriptions rate in HICs than in the U&LMICs. However, it was expected that HICs would be more compliant with judicial antibiotic prescription due to the wide and evident implication of the Antimicrobial Stewardship (AMS) program in High-income settings [31]. The availability of blood, urine, or sputum culture tests in high-resource settings should positively impact judicial antibiotic prescriptions. However, the underlying factor for the higher consumption of antibiotics in HICs could be the negative impact of COVID-19 on AMS programs.
A study in the UK reported a significant negative impact of COVID-19 on the ongoing national AMS program [32]. Our review included only a small population from U&LMICs countries. A study by Molla et al. (2021) reported a 100 % antibiotic prescriptions rate in a dedicated COVID-19 ward in Dhaka Medical College Hospitals in Bangladesh [7]. Drug-resistant microorganisms, specifically multi-drug-resistant bacteria, are likely to spread globally if unchecked. Robust data from U&LMICs is needed to understand the impact of the ongoing pandemic on AMR In this era of globalization, worsening AMR in U&LMICs is likely to spread resistant strains rapidly. High-income countries with established mechanisms to control unregulated antibiotic use can support U&LMICs to adapt and introduce these mechanisms.
Our review also found differences in antibiotic prescription rates between the pre-immunosuppressive period (before the announcement of dexamethasone as the treatment for COVID-19) and post-immunosuppressive period, although the result was not significant (P < 0·19). Dexamethasone was the first proven drug that showed positive outcomes in reducing the mortality of COVID-19 patients [33]. It was a significant breakthrough for COVID-19 treatment, and we expected it to reduce non-judicial antibiotic prescriptions. However, research on this issue is rare, and we did not find any studies that explicitly compared the antibiotics usage rate for those periods.
Self-medication for COVID-19 treatment was a critical concern during the pandemic, although self-medicating with antibiotics has always been a contributor to worsening antimicrobial resistance. An online cross-sectional survey in Dhaka City (Bangladesh) revealed that self-medication during the pandemic of COVID-19 was 88·33 %. A lack of a proper monitoring system makes tracking non-prescription drug purchases challenging, especially in Lower and Middle-Income Countries (LMICs). Zhang et al. (2021) highlighted “COVID-19 pandemic-induced psychological distress” as one of the significant factors related to increased self-medication. A knowledge gap about antibiotics, inappropriate antibiotic prescription practices, the qualities of the patient-doctor relationship, and demographic factors might also cause the prophylactic use of antibiotics.
Our study had certain limitations in finding the appropriate data to answer our research question. We aimed to compare bacterial coinfection and antibiotic prescription frequencies among the same population; however, not many studies performed this comparison. Furthermore, few studies specifically presented data comparing antibiotic prescriptions between COVID-19 patients with and without bacterial coinfections. We had to rely on the studies with available data to calculate the percentages for the remaining included articles. We also did not conduct an analysis by sex or gender, or by COVID-19 severity, possible effect modifiers, but outside the scope of our research question. Data from LMICs was sparse, which might limit the generalizability of the review.
Our study had specific strengths. We strictly followed a robust literature search strategy with the help of our health science librarian (Information System specialist) to find out the maximum number of relevant articles. We adhered to strict inclusion and exclusion criteria, reviewed full-text articles, and extracted data independently and in duplicate, as per best practices, to ensure high-quality data. We presented the results separately to compare both outcomes for the available and assumed/calculated data (for the lowest and the highest confidence intervals).
This systematic review holds significant scientific and clinical importance within the context of COVID-19 research. Our review addresses a critical aspect of patient management by systematically synthesizing available evidence pertaining to secondary bacterial infections and bacterial coinfections among hospitalized COVID-19 patients, along with the frequency of antibiotic prescriptions. Understanding bacterial infection prevalence and optimal management in this specific patient population is pivotal for guiding diagnostic and therapeutic decision-making. Moreover, in light of the global concern surrounding antibiotic resistance, evaluating the appropriateness of antibiotic utilization in the context of COVID-19-associated bacterial infections is imperative. The findings of this rigorous systematic review offer valuable insights to healthcare professionals, policymakers, and researchers, facilitating the formulation of evidence-based guidelines and strategies to enhance patient outcomes and combat antibiotic resistance during the ongoing COVID-19 pandemic.
The findings of this review demonstrate the overprescription of antibiotics in the setting of hospitalized COVID-19. Robust data collection for both HICs and U&LMICs is critical to answering whether COVID-19 patients are being overtreated with antibiotics. Data on the types and indications for antibiotics used to treat COVID-19 patients without bacterial infections and COVID-19 patients with bacterial coinfections is urgently required.
Ethical approval statement
There was no need for ethical approval for the study as it was a systematic review and meta-analysis.
Author contribution statement
Fazle Rabbi; Russell Jude de Souza: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Laura Banfield: Conceived and designed the experiments; Wrote the paper.
Mehnaz Munir: Performed the experiments; Wrote the paper.
Zain Chagla: Analyzed and interpreted the data; Wrote the paper.
Alexandra Mayhew: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Preregistration
The study protocol was registered with the Open Science Framework (OSF) (DOI:osf.io/d3fpm).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Z. Chagla has advised and/or received speaker from Merck, Pfizer, Moderna, AstraZeneca, Gilead, GSK, Roche, and Avir. He has received research grants from Merck, Gilead, and Roche.R. J. de Souza has served as an external resource person to the World Health Organization's Nutrition Guidelines Advisory Group on trans fats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012 to 2017 to present and discuss this work. He has presented updates of this work to the WHO in 2022. He has also done contract research for the Canadian Institutes of Health Research's Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has received speaker's fees from the University of Toronto, and McMaster Children's Hospital. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada), co-chair of the Method working group of the ADA/EASD Precision Medicine in Diabetes group, and is a co-opted member of the Scientific Advisory Committee on Nutrition (SACN) Subgroup on the Framework for the Evaluation of Evidence (Public Health England). He has held grants from the Canadian Institutes of Health Research, Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator, and is a co-investigator on several funded team grants from the Canadian Institutes of Health Research.M. Munir, F. Rabbi, A. Mayhew, and L. Banfield report no competing interests.
Acknowledgments
Department of Global Health, McMaster University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20563.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO . 2021. “Antimicrobial Resistance,” World Health Organization.https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Google Scholar]
- 2.WHO . World Health Organization; 2022. Therapeutics and COVID-19 : Living Guideline.https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.3 [Online]. Available: [PubMed] [Google Scholar]
- 3.NIH . National Institutes of Health; 2022. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 4.Wang L., et al. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2021;76(3):796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160(2):454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hadidi S.H., et al. The spectrum of antibiotic prescribing during COVID-19 pandemic: a systematic literature review. Microb. Drug Resist. 2021;27(12):1705–1725. doi: 10.1089/mdr.2020.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molla M.M.A., et al. Antibiotic prescribing patterns at COVID-19 dedicated wards in Bangladesh: findings from a single center study. Infect. Prev. Pract. 2021;3(2) doi: 10.1016/j.infpip.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; 2019. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis.https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis [Google Scholar]
- 9.Murray C.J., et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Antimicrobial stewardship programmes in health-care facilities in low- and middle-income countries. a WHO practical toolkit. 2019;1(3) doi: 10.1093/jacamr/dlz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.BSAC . 2018. Antimicrobial Stewardship from Principles to Practice. [Google Scholar]
- 12.Hsu J. “How covid-19 is accelerating the threat of antimicrobial resistance,”. 2020;1983:18–19. doi: 10.1136/bmj.m1983. May. [DOI] [PubMed] [Google Scholar]
- 13.Grau S., et al. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiot. (Basel, Switzerland) 2021;10(no. 2) doi: 10.3390/antibiotics10020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-azzam S., et al. “An Assessment of the Impact of Coronavirus Disease (COVID-19) Pandemic on National Antimicrobial Consumption in Jordan,”. 2021:1–12. doi: 10.3390/antibiotics10060690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Lopes A., et al. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the COVID-19 pandemic. Antibiot. (Basel, Switzerland) 2021;10(7) doi: 10.3390/antibiotics10070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavrielatou E., Temperikidis Prodromos, Tsimaras M., magira Eleni. “707. Hospital-onset clostridioides difficile infection rates during COVID-19 pandemic in the ICU patients,”. Open Forum Infect. Dis. 2021;8 doi: 10.1093/ofid/ofab466.904. Supplement_1, pp. S453–S453. [DOI] [Google Scholar]
- 17.Nandi A., Pecetta S., Bloom D.E., Bill F., Foundation M.G. “Articles Global antibiotic use during the COVID-19 pandemic : analysis of pharmaceutical sales data from 71 countries , 2020 – 2022,”. eClinicalMedicine. 2023;57 doi: 10.1016/j.eclinm.2023.101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CADTH “CADTH COVID-19 search strings.”. 2022. https://covid.cadth.ca/literature-searching-tools/cadth-covid-19-search-strings/
- 19.Covidence, “Covidence”. https://support.covidence.org/help/how-can-i-cite-covidence [Online]. Available:
- 20.Dersimonian R., Laird N. vol. 188. 1986. pp. 177–188. (Meta-Analysis in Clinical Trials). [DOI] [PubMed] [Google Scholar]
- 21.TheCochraneCollaboration . The Cochrane Collaboration; 2020. “Review Manager.”. [Google Scholar]
- 22.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. https://www.nature.com/articles/d41586-020-01824-5 (Accessed 21 July 2022) [DOI] [PubMed] [Google Scholar]
- 23.JoannaBriggsInstitute . 2017. “Checklist for Prevalence Studies,”.https://jbi.global/critical-appraisal-tools [Online]. Available: [Google Scholar]
- 24.WorldBank, “World Bank . The World Bank; 2022. Country and Lending Groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Google Scholar]
- 25.Llor C., Bjerrum L. 2014. Antimicrobial Resistance : Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem; pp. 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford B.J., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27(4):520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford B.J., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawson T.M., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing Timothy. Clin. Infect. Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abelenda-alonso G., Carratalà J. 2020. Antibiotic Prescription during the COVID-19 Pandemic : A Biphasic Pattern; pp. 1371–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khouja T., Mitsantisuk K., Tadrous M., Suda K.J. 2022. Global Consumption of Antimicrobials : Impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 Coronavirus Pandemic; pp. 1491–1499. February. [DOI] [PubMed] [Google Scholar]
- 31.Kpokiri E.E., Taylor D.G., Smith F.J. “Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries : A Mixed-Methods Study in Nigerian Hospitals,”. 2020:1–11. doi: 10.3390/antibiotics9040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashiru-oredope D., et al. 2021. Assessing the Impact of COVID-19 on Antimicrobial Stewardship Activities/Programs in the United Kingdom; pp. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim W.S., et al. 2021. Dexamethasone in Hospitalized Patients with Covid-19; pp. 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.