Abstract
Interoception plays an important role in homeostatic regulation of energy intake and metabolism. Major interoceptive pathways include gut-to-brain and adipose tissue-to brain signaling via vagal sensory nerves and hormones, such as leptin. However, signaling via spinal sensory neurons is rapidly emerging as an additional important signaling pathway. Here we provide an in-depth review of the known anatomy and functions of spinal sensory pathways and discuss potential mechanisms relevant for energy balance homeostasis in health and disease. Because sensory innervation by dorsal root ganglia (DRG) neurons goes far beyond vagally innervated viscera and includes adipose tissue, skeletal muscle, and skin, it is in a position to provide much more complete metabolic information to the brain. Molecular and anatomical identification of function specific DRG neurons will be important steps in designing pharmacological and neuromodulation approaches to affect energy balance regulation in disease states such as obesity, diabetes, and cancer.
Keywords: Interoception, Food intake, Energy expenditure, Obesity, Diabetes, Gut-brain communication, Interorgan communication, Spinal cord, Sensory nerves
Graphical abstract
1. Introduction
Despite the availability of improved treatment options, obesity, and related metabolic disorders continue to rise globally Obesity and overweight (who.int). It is generally acknowledged that environmental pressures of modern life, leading to hyperalimentation and lack of physical activity in an interactive manner with genetic predisposition, are the main drivers of common obesity [[1], [2], [3]]. Although prevention of obesity should be our priority, research has shifted to understand and prevent weight regain after dietary, pharmacological, and surgical interventions [[4], [5], [6], [7], [8]]. Because the elevated body weight in most patients with obesity is becoming their new, actively defended setpoint, weight regain should be considered as a normal physiological response to weight loss [6,9]. These powerful responses such as increased hunger and reduced metabolism lessen the effectiveness of most weight loss treatments unless they directly impact the elusive setpoint mechanism.
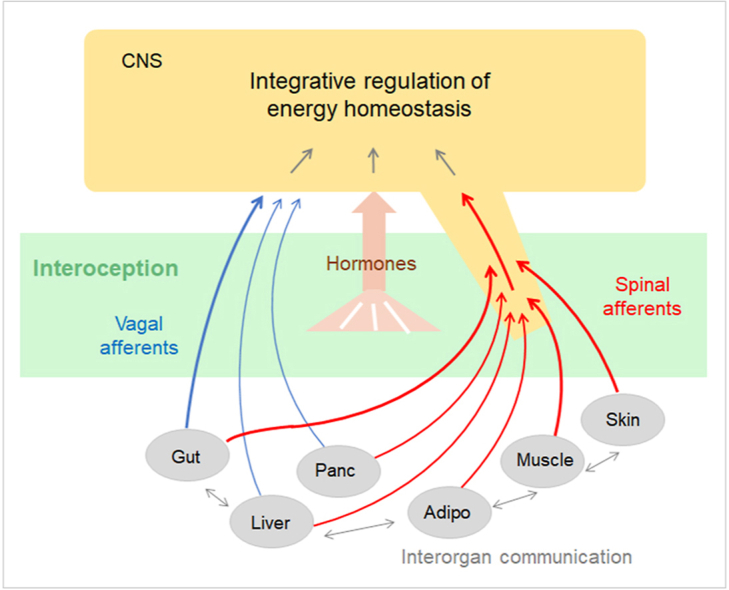
Current concepts and models of biological factors on energy balance and body weight regulation assume that large numbers of exteroceptive and interoceptive signals are integrated in the brain and lead to coordinated changes in energy intake and expenditure through behavioral, autonomic, and endocrine effector pathways [10,11] (Figure 1). The hypothalamus has historically been a prime candidate for the control of energy intake and expenditure and the regulation of a body weight set point [12,13] and research with modern neurobiological tools is fully confirmatory [14]. However, the key molecular mechanisms and pathways to and within the hypothalamus underlying the defense of a healthy body weight are still not well-defined.
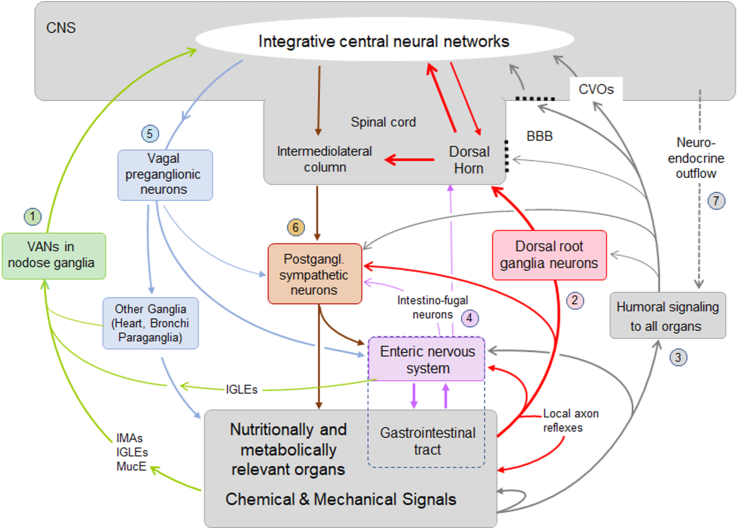
Figure 1.
Schematic diagram showing signaling pathways between the brain and peripheral organs potentially contributing to the regulation of energy balance. Communication in both directions is accomplished by three major pathways, vagal, spinal, and humoral. Communication from specific peripheral organs/tissues such as the gut and other associated visceral organs (but not cutaneous and subcutaneous tissues such as subcutaneous fat) to the brain, is accomplished by vagal afferent neurons (VANs, 1). All peripheral organs/tissues communicate with the brain through dorsal root ganglia neurons and the spinal cord (2), and through the general blood circulation (3). Signals generated in DRGs as well as humoral signals can also communicate with the enteric nervous system, postganglionic neurons, and the spinal cord via short loops. In addition to DRGs, the gut can also communicate to the spinal cord and postganglionic sympathetic neurons via intestinofugal neurons in the enteric nervous system (4). Communication from the brain to the periphery is accomplished by vagal and lumbo-sacral parasympathetic (not shown) efferents (5), and by sympathetic nervous system (6) and neuroendocrine (7) outflow. Note that specific signals generated in the periphery can be mediated by multiple and mixed pathways to the brain and other peripheral organs. Abbreviations: BBB, blood–brain barrier; CVOs, circumventricular organs with missing blood brain barrier; IGLEs. Intraganglionic laminar vagal afferent endings; IMA, intramuscular array vagal afferent endings; MucE, mucosal vagal afferent endings.
As illustrated in Figure 1, important signals used by the brain originate from relevant organs in the periphery such as the gastrointestinal tract, liver, pancreas, adipose tissue, and skeletal muscle. These signals may consist of nutrients, hormones, cytokines, neurotransmitters, miRNAs (and other molecules contained in exosomes) and act via neural, humoral, or mixed pathways. Considerable research has been dedicated to sensory neurons traveling in the vagus nerve (vagal afferent neurons, VANs) that prominently supply the gastrointestinal tract and pancreas, but not subcutaneous adipose tissue and skeletal muscle [15]. Signaling through the spinal cord is accomplished by dorsal root ganglion (DRG) afferents innervating all organs as well as some intestinofugal neurons in the enteric nervous system and projection neurons in the dorsal horn of the spinal cord. Finally, humoral signaling via the general circulation represents the third major signaling pathway. These sensory pathways are contrasted by motor outflow from the brain, accomplished through vagal and lumbo-sacral parasympathetic neurons, the sympathetic nervous system, and neuroendocrine humoral outflow. Importantly, a given sensory signal may reach the brain through one, two, or all three pathways, and the three main signaling systems interact at several levels (Figure 1).
Because the peripheral nervous system is much easier accessible for manipulation, and the medical device-industry made significant progress in biomedical electronics, the National Institute of Health started a large research program (Stimulation of Peripheral Activity to Relieve Conditions, SPARC; www.commonfund.nih.gov/sparc/highlights) and a smaller initiative for interoception (https://neuroscienceblueprint.nih.gov/; RFA-AT-21-003) to develop new strategies in the fight against obesity and other diseases. If we knew enough details about the location, structure, and function of peripheral nerves we could manipulate them in a way that is healing a specific health condition.
The purpose of this non-systematic review is to highlight the potential contribution of spinal sensory pathways and discuss available evidence for their role in energy balance regulation, including the control of food intake. Past metabolic research has been focused on vagal sensory and motor pathways and on the sympathetic nervous system, with less attention paid to dorsal root afferents. A major reason for this likely is the complexity of their organization and relative inaccessibility of functionally specific components. There is, however, considerable recent literature focusing on the role of spinal afferents in mechanisms of pain, particularly visceral pain that accompanies the many gastrointestinal diseases such as irritable bowel syndrome, inflammatory bowel disease, constipation, and intestinal motility disorders [[16], [17], [18]].
Here we first contrast anatomy and function of the spinal sensory system with the sympathetic nervous (outflow) system, including DRG afferent connectivity in the spinal cord and the basics of their brain projections. After briefly reviewing available methodology for selective manipulation of specific components of spinal afferents, we then critically review the literature and formulate hypotheses as to how DRGs innervating various tissues may contribute to the regulation of energy balance homeostasis in health and disease.
2. Functional anatomy of afferent spinal pathways with focus on visceral functions
Over the centuries, knowledge about the autonomic nervous system developed in parallel to the available methods of its analysis – from dissection and the microscope via neural tract tracing, all the way to the latest genetics-guided techniques of neuronal identification. An informative and entertaining history of this development culminating in a precise description of the current view of the autonomic nervous system can be found in the recent comprehensive review by Jaenig [19].
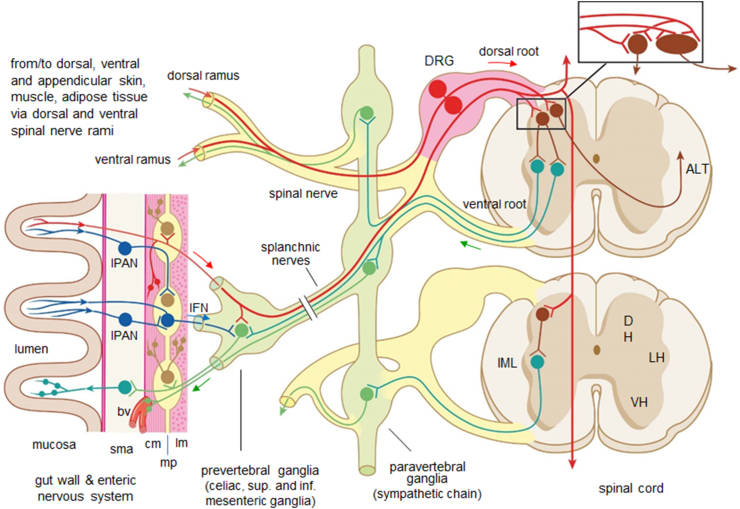
The great majority of spinal afferents (>95 %) innervates the skin and skeletal muscle, while only about 2 % innervate the viscera (visceral afferent neurons) such as the GI-tract, liver, and pancreas [19] that are particularly relevant to this discussion. Visceral afferent neurons are mostly small and medium-sized pseudounipolar sensory neurons located in the DRG, with peripheral processes projecting to the viscera, and central processes projecting to the dorsal horn of the spinal cord via the dorsal roots (Figure 2). Most of the visceral DRG afferents belong to the small, myelinated Aδ and unmyelinated C classes. Very few are larger diameter Aβ neurons connected to Pacinian corpuscles in mesenteries [20]. Peripheral axons of visceral DRG afferents travel along with autonomic efferents in thoracic and lumbar splanchnic “sympathetic” and pelvic “parasympathetic” nerves, thereby traversing autonomic ganglia and eventually giving off collaterals to neurons in prevertebral ganglia such as the celiac and superior mesenteric ganglia. DRG afferents can trigger reflexes mediated by these autonomic efferent systems, however, terming them “sympathetic” or “parasympathetic” afferents, respectively, is misleading and should be avoided [19]. The term “sympathetic” is reserved for thoracolumbar pre- and postganglionics located in the spinal cord and sympathetic chain, prevertebral and pelvic ganglia, respectively. Visceral pre-ganglionic axons travel through the ventral roots, white communicating rami, and splanchnic nerves to paravertebral and prevertebral ganglia as well as hypogastric nerves to pelvic ganglia, where they synapse on postganglionic neurons that send projections into the various tissues (Figure 2). Some preganglionics located in the sacral spinal cord traveling through the pelvic nerves and projecting to pelvic ganglia are conventionally considered part of the parasympathetic system (but see [21] for discussion of the “sacral sympathetic outflow”). Projections to the pelvic ganglia contain thus mixed sympathetic and parasympathetic preganglionics [22,23]. Sensory information from the gastrointestinal tract can also be mediated to the spinal cord by enteric neurons projecting to prevertebral ganglia (intestinofugal neurons) and to the spinal cord (rectospinal neurons), as discussed in Section 3 below.
Figure 2.
Relationship between dorsal root afferents, sympathetic outflow, and the enteric nervous system. Dorsal root afferent neurons (red) pick up information in the gut, skin, muscle, and adipose tissue and conduct it to the dorsal horn (DH) of the spinal cord, where they synapse on spinal large projection neurons and small interneurons (brown) and project via collaterals rostro-caudally over several segments. Projection neurons ascend through anterolateral tracts (ALT) mainly contralaterally to supraspinal targets. Sensory information carried by DRG neurons can also be passed to enteric motor and inter neurons (light brown) in the myenteric plexus, to sympathetic postganglionic neurons in prevertebral ganglia (green) for short-loop reflex actions and possibly also to smooth muscle via axon reflexes. In addition to DRGs, the gut can also communicate to postganglionic sympathetic neurons and spinal cord (not shown) via intrinsic primary afferent neurons (IPANs, blue) and intestinofugal neurons (IF, blue) in the enteric nervous system. These sensory pathways are partly overlapping with the sympathetic nervous outflow system (green). Other abbreviations: bv, blood vessel; DRG, dorsal root ganglion; IML, intermediolateral column; cm, circular muscle; lm, longitudinal muscle mp, myenteric plexus; sma, submucosa; LH and VH, lateral and ventral horn, respectively.
In addition to spinal afferent neurons from the viscera, we will also consider spinal afferent neurons from skin, subcutaneous adipose tissue and from deep somatic structures (skeletal muscle and fasciae, as they are also able to elicit autonomic and behavioral responses relevant for the regulation of energy homeostasis.
2.1. Innervation territories and segmental distribution
Anterograde and retrograde tracing, retrograde trans-synaptic tracing, and immuno-histochemical detection of specific marker proteins/peptides are the methods of choice to characterize the peripheral and central processes as well as the segmental distribution of DRG neurons. Anterograde tracing after DRG injections of suitable dyes (DiI, various HRP conjugates, dextran-biotin) or Cre-dependent viral constructs in corresponding Cre-mouse lines is much more challenging and time-consuming, but it is highly selective. Anterograde tracing has therefore only been accomplished from a few select DRGs out of the total 32 ganglia on each side (Table 1). An additional limitation is the typically incomplete labeling of all neurons in a given ganglion. Retrograde tracing with suitable tracers from specific visceral tissues are the methods of choice to map the location of DRG neuron cell bodies that are linked to specific organs and physiological stimuli (Table 1 for references). Yet, to show functional activation of DRG neurons following peripheral organ stimuli would require activity recordings of DRGs for maximal specificity as recently established by measuring gut induced calcium dynamics in DRGs [24]. The early response marker cFos has been an excellent immunohistochemical marker for neuronal activation in the CNS (brain and spinal cord), but cFos induction it is not reliable in ganglia [25] and thus should be avoided. Also, because of potential spread of tracer to other than intended tissues, great caution must be exerted in interpreting the results with retrograde tracing. This is particularly true for tissues within the abdominal cavity such as the GI-tract, pancreas, and liver, which constantly rub against each other, and where the many potential targets of sensory innervation are difficult to selectively infiltrate [26]. A careful approach avoiding these problems is to exteriorize the organs before tracer injection [27] or to apply the tracer to cut nerves (Table 1), but for obvious reasons these technically rather demanding approaches cannot be applied to every viscus. It seems less of a problem for subcutaneous fat tissues and skeletal muscle, although such injections could easily infiltrate the overlying skin. Finally, immunohistochemistry has been widely used to visualize DRG neuron cell bodies and their peripheral and central terminals expressing Calcitonin-gene-related peptide (CGRP), substance P (SP), and other peptides [28]. However, immunohistochemistry requires controls to rule out nonspecific labeling and to verify DRG origin when tissues are innervated by nerve fibers of vagal or enteric origin expressing the same markers.
Table 1.
Overview of various methods for mapping segmental and peripheral distribution of spinal afferent neurons.
|
Retrograde and transganglionic tracing Organ injections |
Rat stomach (HRP): T4-L1 (T8-10) | [139] |
| Mouse dist. colon (fast blue): T8-L1, L6/S1 | [140] | |
| Mouse jejunum (cholera toxin B): T9-T13 | [141] | |
| Mouse dist. colon (cholera toxin B): T9-L1, L4-S2 | [141] | |
| Mouse dist. colon (cholera toxin B): T10-L1, L6/S1 | [45] | |
| Rat liver (HRP): T7 – 11 | [142] | |
| Mouse hepatoportal vein: T8-13 | [107] | |
| Rat pancreas (true blue): T6-L2 (T9-13) | [143] | |
| Mouse pancreas (cholera toxin B): T5-13 (T9-12) | [29] | |
| Hamster iBAT (PRV): T1-3 | [126] | |
| Mouse iBAT (PRV): T2-5 | [62] | |
| Hamster iWAT (fluorogold): T11-L3 | [144] | |
| Mouse iWAT (cholera toxin B): T11-L3 | [145] | |
| Mouse iWAT (PRV): T11-13 | [63] | |
| Rat stomach (HSV-1 H129): T8-9 | [146] | |
|
Retrograde and transganglionic tracing Cut nerve applications |
Cat greater splanchnic nerve (HRP): T7-11 | [147,148] |
| Rat greater splanchnic nerve (HRP): T3-13 (T8-12) | [149] | |
| Rat lumbar splanchnic nerves (HRP): T11-L2 | [40] | |
| Cat lumbar colonic nerves (HRP): T13-L5 (L3-L4) | [150] | |
| Cat hypogastric nerve (HRP): T12-L5 | [151] | |
| Cat pelvic nerve (HRP): S1-3 | [152] | |
| Anterograde tracing from DRG to organs or CNS | C2-4 (WGA-HRP): to rat sternomastoid muscle | [153] |
| T8-13 (CTB-HRP): to cat lower esophageal sphincter | [154,155] | |
| T8-12 (dextran biotin): to mouse upper GIT | [156] | |
| L6-S1 (dextran biotin): to mouse colon | [[157], [158], [159]] | |
| T9-10 (dextran biotin: to WBN/Kob rat pancreas | [160] | |
| T13-L1 (AAV-FP): to mouse iWAT | [145] | |
| T7-10 (HSV-LSL-TK-tomato): to mouse CNS | [50] | |
| Neuronal activity markers | Gastric stimulation | [161] |
| Colorectal distention | [162] | |
| Inflammatory bowel disease | [163] | |
| Fiber photometry | vGlut2-expressing neurons n DRGs 11 and 12 responding to gastric infusion of diverse osmolar stimuli | [24] |
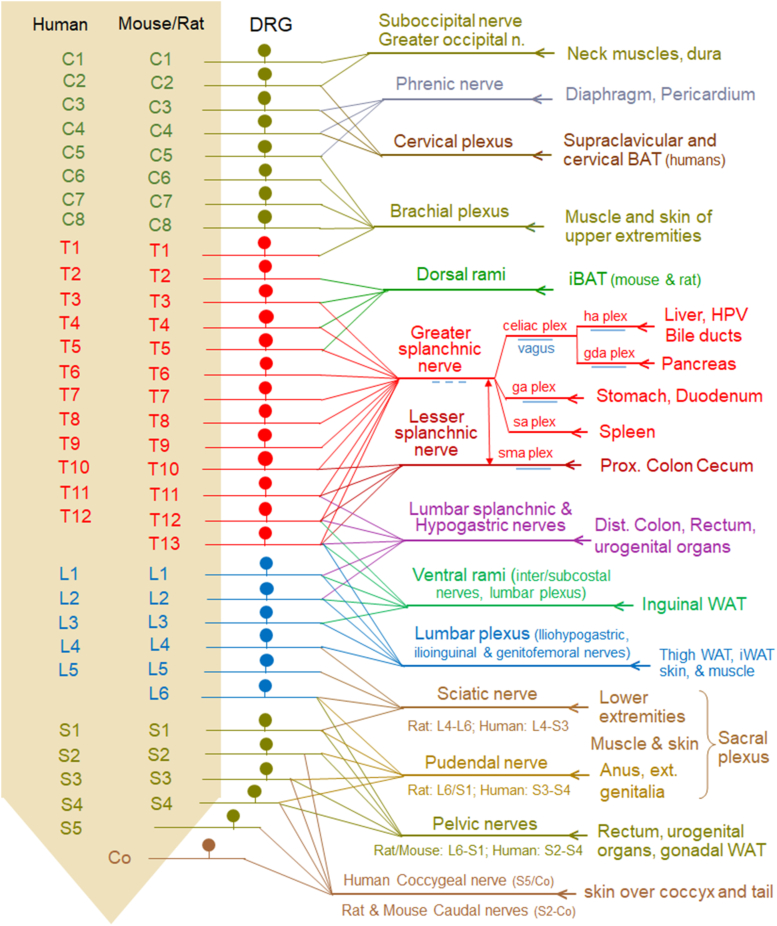
Collectively, these studies reveal the approximate innervation territories of the nutritionally and metabolically most relevant tissues by individual DRGs and the corresponding nerves as shown in Figure 3 and Table 1. The upper gastrointestinal tract and the embryologically associated liver and pancreas are mainly innervated through the greater splanchnic nerve and DRG between thoracic segments 3–13, peaking at mid-to lower levels. The distal colon and rectum are innervated by lower thoracic, upper lumbar (T9 – L1), and lumbo-sacral (L5 – S2, peak at L6 and S1) DRGs. iBAT receives innervation from upper thoracic DRG neurons, while inguinal WAT is innervated by DRG neurons at T12-L3 via branches from lower intercostal and lumbar plexus nerves. Skeletal muscle and skin are innervated by all DRGs in a rostro-caudal segmental fashion.
Figure 3.
Innervation territories of DRG at different spinal levels with emphasis on nutritional and metabolic relevance. The 32 DRGs on each side, grouped by their location relative to the cervical, thoracic, lumbar, and sacral spinal cord and numbered from rostral to caudal in each group, project through 14 major nerves/plexuses to their specific visceral and somatic destinations in an overlapping fashion. Peripheral axons of DRGs from up to 12 segments (greater splanchnic nerve) converge in each nerve that can innervate large tissue territories. As shown in Figure 2, the DRG axons are typically intermingled with preganglionic sympathetic neurons in most of these nerves, with intestinofugal neuron projections in the splanchnic nerves and subsidiaries including the celiac plexus (ce plex), superior mesenteric plexus (sma plex), hepatic artery plexus (ha plex), gastric artery plexus (ga plex), gastroduodenal artery plexus (gda plex), and splenic artery plexus (sa plex), as well as the hypogastric, and pelvic nerves, and with vagal efferent and afferent fibers in parts of the splanchnic nerve and all subsidiaries. Furthermore, DRGs innervating subcutaneous white and brown adipose tissues are comingled with DRGs innervating overlaying muscle and skin. Note that innervation of skin and skeletal muscle for much of the thorax and abdomen is not shown.
Most studies do not allow conclusions regarding innervation density because anterograde tracers were only injected into a limited number of DRGs or retrograde tracer injections did not completely infiltrate target areas or were not analyzed throughout the length of the spinal cord. In a well-controlled retrograde cholera-toxin B tracer study of the mouse pancreas, a total number of about 100 labeled DRG neurons distributed bilaterally over spinal segments T5-T13 were identified [29]. The maximal number of pancreas-projecting neurons in a single DRG was roughly ten (T9-T12), and there was no significant difference between left and right. Thus, assuming the total number of neurons per DRG is about 1000, only a very small percentage innervates the pancreas, and an even smaller percentage likely projects to a specific target such as the β-cell. Similarly, retrograde tracing from iWAT with cholera-toxin B tracer resulted in tracing of 10–50 labelled cells per DRG (T11-L3).
The exceedingly complex innervation pattern shown in Figure 3 makes selective manipulation of function-specific populations of DRG neurons difficult if not impossible. For example, to selectively silence all DRG neurons signaling from the liver using TetTox viral constructs, up to 10 DRGs would have to be injected bilaterally. In the case of skeletal muscle or skin, such an approach is even less feasible. A more systematic discussion of deafferentation procedures is provided in Section 5 below.
2.2. Distribution, architecture, and sensory modality of peripheral endings
Anterograde tracing of DRG neurons has revealed the architecture of sensory endings in the gastrointestinal tract, pancreas, iWAT, and skeletal muscle (Table 1). Using dextran-amine as an anterograde tracer from DRGs (T8-T12) in mice, extensively ramifying intraganglionic varicose endings were found in the myenteric plexus of the upper GI-tract, with less frequent and simpler endings in the circular muscle, and mucosa. Tracing from lumbosacral DRGs (L6-S1) revealed no less than thirteen distinct types of mostly CGRP-positive endings in all layers of the large intestine and rectum, sometimes originating as collaterals from a single afferent parent axon. At the ultrastructural level direct contacts of anterogradely labeled DRG afferents with myenteric ganglion neurons in the cat lower esophageal sphincter were documented. These sensory endings supposedly detect mechanical, chemical, and noxious stimuli and may also release CGRP in a local-effector manner mediating axon reflexes (for critical discussion see [18]. In Wistar Bonn/Kobori rats, a model for spontaneous chronic pancreatitis, an increased number of anterogradely traced DRG endings characterizes their pancreas compared to wildtype rats. In mouse inguinal WAT, DRG afferents were found around blood vessels and adipocytes. In the rat sternomastoid muscle, anterogradely labeled thin caliber DRG afferents were shown along small blood vessels and in the intramuscular connective tissue, compatible with CGRP immunohistochemistry [28].
Studies with anterograde tracing of spinal afferents to the small intestine, liver, hepatic portal vein, and iBAT are lacking. Distribution and architecture of DRG afferents in these tissues has been inferred from CGRP and substance P immunohistochemistry. In the liver, CGRP-positive simple endings are present around blood vessels and bile ducts in periportal fields but not between hepatocytes [30]. In rat iBAT and perirenal BAT perivascular and “intraparenchymal” substance P and CGRP positive fibers were found [31].
2.3. Dorsal horn connectivity
The central processes of small and medium-sized DRG neurons terminate exclusively in dorsal horn layers of the spinal cord, and they typically divide upon entry into the spinal cord and supply terminals to several spinal cord segments (Figure 4). Thus, individual DRG neurons can address many second order neurons (divergence of signaling) but, at the same time, individual second order neurons can receive input from many different DRG neurons (convergence of signaling). DRG afferents synapse on both projection neurons and the much more numerous interneurons. Interneurons constitute a complex network of excitatory and inhibitory neurons which are getting more and more functionally, chemically and genetically characterized [32,33]. Using an intersectional genetic approach, specific populations of excitatory dorsal horn interneurons could be linked to various sensorimotor reflexes [34]. Of potential relevance for processing of spinal visceral afferents are GRPR-, calretinin- and calbindin-expressing interneurons in laminae I and outer lamina II where most of these DRG afferents terminate (see below). Rare projection neurons relay the final output of dorsal horn activity to the brain [35]. Importantly, the sensitivity of DRG afferent input can be modulated pre-synaptically through axo-axonal contacts with descending projections from the medulla and higher brain areas and interneurons [36,37].
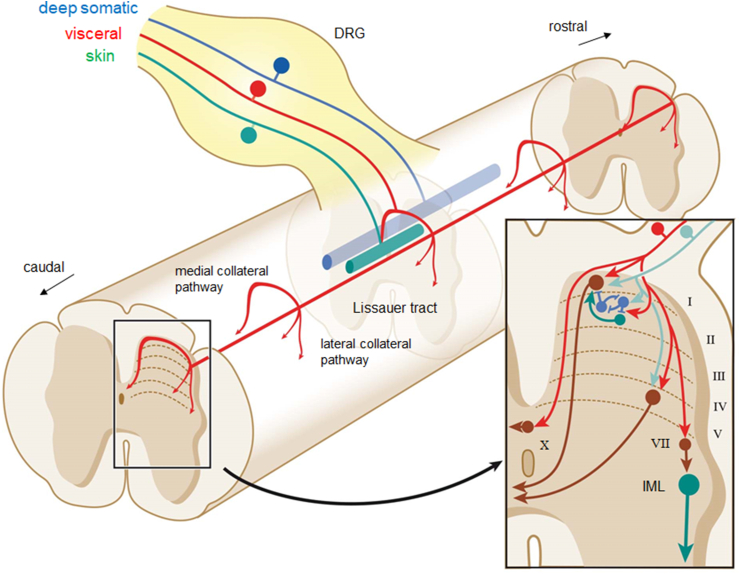
Figure 4.
Dorsal horn circuitry of DRG sensory input. Upon entry into the spinal cord, DRG axons split into ascending and descending branches coursing in Lissauer's tract (LT) issuing collaterals to synapse on neurons at multiple segmental levels of the dorsal horn. DRG with visceral input (red) spread across the largest rostro-caudal extent with up to 10 segments giving off sparse wide spaced collaterals, DRG with input from skin (green) spread the least terminating as dense clusters, while afferents from deep somatic structures (muscles, joint capsules, fasciae; blue) spread less densely over an intermediate range. At a given level, DRG axon collaterals with input from viscera, skin, and deep somatic structures tend to innervate neurons in different lamina of the dorsal horn (inset). Viscera-related DRG axons preferentially terminate in laminae I and outer II, with fewer terminations in lamina V and X. The preganglionic intermediolateral nucleus (IML) may be reached via interneurons in lamina VII (brown). In laminae I, II and V, visceral DRG axons (red) can converge on single projection neurons (brown), on inhibitory (blue) and excitatory (green) interneurons, or on both. Many projection neurons and interneurons receive at the same time DRG neuron inputs from skin and deep somatic structures (turquoise). Abbreviations: MCP, LCP, medial and lateral, respectively, collateral pathways.
While much of this insight was derived from studying nociceptive afferents from the skin, there are several morphological and functional studies dealing with visceral afferents, many of them traveling via the splanchnic nerves. HRP applications onto cut splanchnic, hypogastric, and pelvic nerves (Table 1) followed by transganglionic bulk labeling of spinal terminals and tracer injections into single splanchnic C-afferents established a distinct termination pattern of afferents from abdominal and pelvic organs mainly in spinal laminae I, outer lamina II, and laminae V and X, but avoiding inner lamina II and laminae III and IV. The DRG collateral pathways reaching into laminae V and X are thereby termed lateral and medial collateral pathways (LCP and MCP, respectively) along the contours of the dorsal horn (Figure 4). Axons in the LPC are coming close to dorsally directed dendrites of the intermediolateral nucleus and related interneurons in lamina VII at thoracolumbar, and the sacral preganglionic nucleus, at lumbosacral levels thus allowing short reflex loops from spinal afferents to sympathetic and sacral parasympathetic circuits. Phaseolus vulgaris leucoagglutinin (PHAL) injections into single identified splanchnic C-afferents confirmed this general pattern [38].
In contrast, thin caliber cutaneous afferents distribute also to the above-mentioned laminae but in addition, densely to inner lamina II which is almost avoided by visceral afferents ([38] #473; [164] #785}. Laminae III and IV (core of the dorsal horn, nucleus proprius) harbor the dense termination arbors of thick caliber cutaneous afferents [39]. The few splanchnic Aβ afferents ascend in the dorsal column and terminate in dorsal column nuclei [20,40].
Moreover, visceral afferents span over a much greater rostro-caudal distance than cutaneous afferents, with single splanchnic C-afferents extending almost over half the thoracic spinal cord and likely responsible for the rather diffuse and not well localized sensation of GI pain. In contrast, cutaneous C-afferent terminals are almost restricted to their segment of entry [38], consistent with our ability to precisely localize the origin of skin sensation of touch or pain. Recordings in the spinal cord upon electrical stimulation of the cat splanchnic nerve demonstrated activation mainly in Lamina I and V but also in deeper laminae VII and VIII [41,42]. A significant proportion of these neurons projected through the contralateral anterolateral funiculus to supraspinal targets [43,44]. Remarkably, almost all spinal neurons that are activated by splanchnic afferent stimulation had convergent cutaneous input, which has been suggested as an explanation for referred pain where the site of perceived pain (e.g., back pain) is different from the site where pain is induced (e.g., viscera). Indeed, second order neurons specifically processing afferent signals from viscera apparently are rare or do not exist at all [43] or were hitherto overlooked.
Transganglionic tracing from the mouse distal colon using two cholera toxin B (CTB) retrograde tracers with differently conjugated fluorophores demonstrated the same visceroafferent spinal terminal distribution [45]. Wall injections resulted in labeling of mainly lamina I, V and X (dorsal grey commissure) of spinal segments T13-L1 via the lumbar splanchnic nerves and L6-S1 via the pelvic nerve, while injections into the lumen resulted additionally in labeling in lamina I, III and X in segments L6-S1 via the pelvic nerve. In the same study, expression of p-ERK (a measure of dorsal horn neuron activation) was assessed after innocuous or noxious colorectal distension (CRD; [45]. Noxious CRD activated a considerable number of neurons in laminae I and V of the thoracolumbar cord as well as laminae I, II, and X in the lumbo-sacral cord, while innocuous CRD activated only very few neurons in lamina I of the thoracolumbar cord but many more in lamina I and deeper layers of the lumbosacral cord. Both lamina I projection neurons and interneurons in laminae II and III (mainly excitatory, few inhibitory) were activated. There was also activation in the area of the intermediolateral nucleus at thoracolumbar levels and the sacral preganglionic nucleus at L6-S1 although it is unclear if interneurons or preganglionic neurons were concerned.
There are no transganglionic tracing studies of spinal terminations of DRG afferents from segments of the upper GI-tract as well as the liver, portal hepatic vein, and pancreas, but it is reasonable to assume that they follow the general pattern as described above.
Thin afferents from skeletal muscle and other deep somatic tissues terminate predominantly in lamina I and IV/V and somewhat more than visceral afferents also in Lamina II [[46], [47], [48], [49]]. Single deep somatic class III and IV afferents extend over about 2–3 segments, i.e., their rostro-caudal range lies between cutaneous and visceral afferents [38,46,47].
Although it is tempting to assume that all single thin caliber afferents from a given origin, i.e., visceral, muscular or cutaneous, follow the general termination pattern as described above, subpopulations subserving specific functions may target only one or two of the spinal gray laminae and the second order neurons therein. Remarkably, spinal afferents innervating the mouse stomach appeared to terminate only in lamina X and even more ventrally, sparing laminae I and V [50] and some high threshold muscular afferents terminated selectively in lamina I while others branched also into laminae IV and V [46]. It remains to be elucidated if these differences have more methodological reasons or represent true anatomical differences with functional consequences.
2.4. Central connectivity
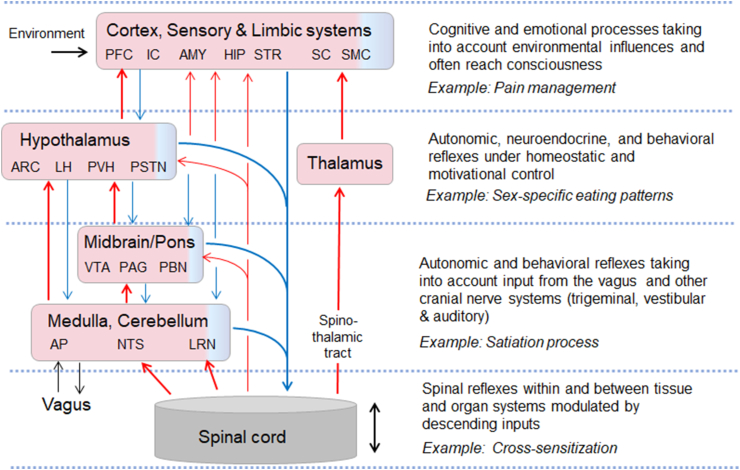
An in-depth general description of ascending and descending pathways in the spinal cord of the rat can be found in Chapter 5 of “The Rat Autonomic Nervous System”, edited by George Paxinos [51] and of human in Chapter 7 of “The human Nervous System”, edited by Jürgen Mai and Georges Paxinos [52]. There are direct projections to the medulla (e.g., the lateral reticular nucleus [LRN] and nucleus tractus solitarii [NTS], cerebellum, pons (e.g., lateral parabrachial nucleus [LPBN]), midbrain (e.g., reticular formation and central gray), diencephalon (thalamus and hypothalamus), and, probably mostly indirect, to the telencephalon (e.g. amygdala, septum, BNST, nucleus accumbens, infralimbic and orbital cortex) (Figure 5).
Figure 5.
Highly schematic diagram of ascending and descending neural pathways from the spinal cord to the brain and potential integrative hierarchy in the brain most relevant for energy homeostasis regulation. Among the ascending connections (red), the spinothalamic tract, which underlies touch and pain sensation, is by far the best investigated pathway. Ascending pathways most relevant for energy balance regulation are indirect connections via medulla, pons, or hypothalamus to the forebrain. Similarly, descending connections (blue) arise from all major areas of the brain, some of them direct and some indirect. See text for details.
Sensory-specific transneuronal tracing with the H129 strain of HSV-1 virus has provided important initial information about central nervous feedback regulation of adipose tissue function [53]. Subsequent functional studies used recordings from WAT spinal afferents and showed that they are activated in response to lipolysis inducing stimuli [54]. Also, selective denervation of iWAT spinal afferents (iWAT injection of high dose capsaicin, see also later for method details) significantly suppressed sympathetic tone in the denervated iWAT, but also modulated sympathetic activity in other WAT depots and in iBAT with intact innervation [55]. These data are consistent with a role of WAT spinal afferents in afferent-sympathetic reflex loops. Furthermore, given the strong connectivity of the spinal cord with the cerebellum, together with increasing appreciation of cerebellar functions relevant for feeding behaviors [56,57], we speculate that DRG afferents are involved in this circuitry. Splanchnic afferents to the cerebellum, probably relayed via the lateral reticular nucleus, have been demonstrated [58,59].
Many of the targets of direct input from the spinal cord are among the “usual suspects” in the neurobiology of food intake control and energy balance regulation. For example, pathways from the dorsal vagal complex in the medulla via the LPBN to the hypothalamus and limbic system are considered crucial for the controls of food intake and regulation of body weight. However, while these pathways can only be taken as a general blueprint, central connectivity maps for functionally specific spinal cord sensory inputs need to be established using modern neurobiological tools. A recent study using intersectional genetic strategies for disentangling two somatosensory spino-parabrachial pathways which subserve distinct sensory modalities provided an example how such issues could be tackled also in the spinal visceroafferent area [60].
Descending pathways to the spinal cord originate in all major brain regions, including prominent projections from the medulla, pons, hypothalamus, and cerebral cortex [51,52] (Figure 5). Commensurate with the title of this review, we will mainly focus on descending inputs that directly impinge on sensory processing within the spinal cord. Descending inputs that drive sympathetic and/or parasympathetic preganglionics have been summarized by others [19,[61], [62], [63], [64]]. Regarding the regulation of energy balance, several direct, long projections are of particular interest. Anterograde tracing with PHAL from undisclosed PVH neurons has identified axons coursing through separate pathways to reach spinal laminae I and X in the intermediolateral column of the spinal cord of rats [65]. Conversely, retrograde tracing from the spinal cord has revealed participation of oxytocin, vasopressin, dynorphin, enkephalin, and Nos1-expressing neurons in different parts of the PVH [[66], [67], [68]]. Similarly, orexin-expressing neurons in the lateral hypothalamus densely innervate laminae I and X, and the IML all the way down to the sacral spinal cord [69]. In addition, the pontine locus coeruleus, parabrachial nuclei [70], the noradrenergic group A5 and nucleus raphe magnus have been identified to directly innervate the dorsal horn.
Together, these findings suggest that descending projections from the hypothalamus and other brain areas to the spinal cord represent crucial effector pathways for the regulation of energy balance. These projections not only drive thoraco-lumbar sympathetic and lumbo-sacral parasympathetic outflow to visceral organs, adipose subcutaneous WAT, iBAT, skeletal muscle, and skin, but they also modulate sensory input related to autonomic tone, temperature, and pain perception.
Overall, these extensive interconnections of the spinal cord with brain areas involved in the controls of food intake and metabolism suggest that DRG afferents play an important role. However, the central circuitry as well as the behavioral and physiologic consequences underlying specific DRG-mediated sensory signals and potential plasticity of these circuits in metabolic diseases is largely unknown. These are challenging studies that require recording of neural activity from multiple brain sites combined with highly selective interruption/silencing of suspected pathways in behaving animals.
3. Other sensory pathways from the gastrointestinal tract
Besides DRG afferents, the gastrointestinal tract can broadcast sensory information to the spinal cord and prevertebral ganglia via neurons in the enteric nervous system (Figures 1 and 2). Recto-spinal neurons located in the myenteric plexus of rectum have been shown to project directly to the sacral dorsal horn using retrograde tracing [71]. Neurons in the myenteric plexus of the distal small intestine and colon (intestinofugal neurons) project to celiac, superior mesenteric, and inferior mesenteric ganglia as demonstrated in guinea pigs and rats [[72], [73], [74], [75]] (for review see [76], where they can modulate sympathetic outflow to other viscera and for example affect glucose homeostasis and food intake [77]. These neurons can directly detect mechanical and chemical stimuli via dendrites in the mucosa or receive mechanosensory and chemosensory input indirectly via synapses from multipolar intrinsic primary afferent neurons (IPANs) located in the myenteric and submucosal plexus (Figure 1, Figure 2). Enteroendocrine and enterochromaffin cells are specialized excitable cells in the intestinal epithelium that transduce relevant luminal information into hormonal and electrical signals used by IPANs and intestinofugal neurons. While enteroendocrine cells are primarily involved in the transport and signaling of nutrients, enterochromaffin cells detect irritants and microbial metabolites [78].
4. Methods for deafferentation and manipulation of DRG
If we embrace the notion that DRG neurons serve many sensory- and organ/tissue-specific functions, we should ideally be aiming at selectively manipulating a single population, while leaving all other DRG neurons with different functions intact. In addition, we should not interfere with any other autonomic/peripheral nervous system component such as sympathetic outflow or vagal functions. Such a high degree of selectivity has not yet been achieved for DRG afferents and only non-selective surgical and chemical approaches have been used in the past to functionally interrupt or eliminate DRG afferents (Table 2).
Table 2.
Overview of currently available and future DRG denervation techniques.
| Approaches | Time and effort | Selectivity | Collateral damage | References |
|---|---|---|---|---|
| Surgical | ||||
| DRG removal | medium to high | medium | DRG neuron function non-specific | [79] |
| Splanchnic nerve transection: | low | low | DRG neuron function non-specific plus SNS and a few vagal fibers | [80] |
| Celiac/sup. mesent. ganglionectomy: | medium | lowest | DRG neuron function non-specific plus SNS, intestinofugal, and vagal fibers | [81,82] |
| Chemical | ||||
| Capsaicin | low to medium | medium | DRG neuron function non-specific plus vagal afferents and ENS | [[85], [86], [87]] |
| Systemic or local | ||||
| Resiniferatoxin intrathecal | medium | medium | DRG neuron function non-specific | [75,89] |
| Genetic | ||||
| Local virus injections (used for vagal afferents) | high | highest | minimal | [90,92,93] |
| Local saporin injections (used for vagal afferents) | medium | high | receptor-specific | [98] |
| Intersectional | highest | medium to high | Cre-expression-dependent | ([97,99,136,137] |
Surgical approaches include DRG removal, splanchnic nerve transection, and celiac/superior mesenteric ganglion extirpation in decreasing order of selectivity. One of the most selective of these procedures is DRG removal [79], as it does not interfere with any other nerves, such as sympathetic outflow. However, if the specific sensory function in question stretches over several spinal segments, the technique can be labor-intensive and indiscriminately affects all DRG neurons in the removed ganglia, independent of function. Splanchnic nerve transection, particularly when done acutely, can be a simple and useful first approach for bulk elimination of DRG afferents from the upper abdominal viscera [80]. It is, however, accompanied by extensive loss of sympathetic innervation of the upper viscera (Figure 3), which in the chronic case will likely have major implications, and it is not function-specific. Finally, celiac/superior mesenteric ganglionectomy is by far the crudest approach [81,82]. Besides the same collateral damage as incurred with splanchnic nerve transection, it additionally interferes with several other autonomic and peripheral nervous system functions and in our opinion should no longer be used.
Chemical approaches include capsaicin and resinifera toxin due to their high affinity for the transient receptor potential-v1 (trpv1) ion channel, expressed in a majority of small DRG neurons, leading to overexcitation and apoptosis [83,84]. Capsaicin has been administered systemically or locally for bulk elimination of all trpv1-expressing primary afferents (DRG and vagal) [85] or selectively ablate sensory fibers in mixed nerves such as the sensory nerves to the hepatic portal vein and liver [86], specific organs such as the pancreas [87] or subcutaneous adipose tissue [88] (Table 2). Resinifera toxin has been administered intrathecally to infiltrate the termination site of DRG neurons in the dorsal horn and selectively eliminate all trpv1-expressing DRG neurons, no matter what function they serve [75,89].
Genetically guided approaches include viral-transfection and intersectional techniques with the aim to manipulate only sub-populations of DRG afferents that serve a specific function, while leaving other populations intact. This strategy became widely accessible after molecularly fingerprinting vagal afferents with scRNAseq [90,91] and has since been successfully used for functional and behavioral studies on vagal afferents [90,92,93] (Table 2).
Since DRG neurons have also been recently molecularly fingerprinted [91,[94], [95], [96]], they should also be accessible for these selective approaches. The approach typically consists of injecting a cre-inducible viral construct carrying either TetTox or Caspase into the DRG of choice in mice that express cre-recombinase in the gene of choice. Up to now, there has not been any report of such selective DRG ablation, but it has led to the identification of two separate vagal afferent neuron populations specifically mediating effects of intestinal glucose and fat to the brain [92]. However, a very recent study using combined morphological and intersectional genetic characterization and functional approaches including optogenetics and diphtheria toxin ablation identified five subtypes of distal colonic DRG afferents displaying high or low threshold mechanoreceptive properties [97]. These methodologies may serve as a blueprint for elucidating also metabolic roles of spinal visceral afferents. In the absence of transgenic mouse cre-lines or in the rat, injections of a saporin-conjugated peptide into DRGs should also be a feasible approach, as saporin-CCK injected into the nodose ganglia of mice and rats selectively ablates vagal afferent neurons expressing CCKA receptors [98]. Of note, DRG injection is typically a major surgical undertaking due to the need for bilateral injection of several DRG pairs. Also, generally upper thoracic DRGs are less accessible for injections compared to lower thoracic and lumbar DRGs and all studies using survival surgeries for DRG injections or calcium dynamics have been performed on lower thoracic or lumbar DRGs.
If the DRG injection technique is not available or not feasible, intersectional approaches in which two [99], or even three transgenic mouse lines are crossed can also be used. Such intersectional approaches could also be used for selective ablation, stimulation, and inhibition of selective and functionally specific populations of DRG afferents. However, as with all genetic models, care needs to be taken to ensure that gene expression is not found in other unexpected cells. Also, intrathecal injections may allow a simpler way to reach several adjacent DRGs and can be combined with intersectional approaches.
5. Evidence for roles of DRG afferents in nutrient sensing and metabolic regulation
By far the most articles published on DRG afferents have focused on pain, somatosensory physiology, peripheral nerve, and spinal cord injury. However, presentation of a “metabolic” phenotype in mice with whole body deletion of TRPV1 or CGRP, two markers strongly expressed in DRG afferents, has suggested involvement in energy homeostasis for some time. Specifically, TRPV1-KO mice which are insensitive to capsaicin show decreased metabolism and increased weight gain [100], and CGRP-KO mice, which have problems with heat-induced vasodilation, show less weight gain on a high-fat diet compared to wildtype mice [101]. More specific roles in metabolic regulation and diseases, particularly for DRG afferents innervating the viscera such as the GI-tract, liver, and pancreas, adipose tissue such as subcutaneous WAT and iBAT, as well as the large skeletal muscles, has only recently emerged. Here we critically review this literature and formulate hypotheses as to how DRG innervating various tissues may contribute to the regulation of energy balance homeostasis in health and disease.
5.1. DRG afferents from the GI-tract
Gut-brain communication has been strongly implicated in the control of food intake and regulation of energy homeostasis [102]. Communication by vagal afferents and the blood circulation have received the bulk of attention in the past and communication by DRG afferents has not received much attention. The traditional view that vagal afferents mediate signals for normal physiological regulation, while spinal afferents mediate signals associated with pathological conditions such as painful over-distension [103,104] left little room for a role of spinal afferents in signaling nutritionally relevant information. In addition, there has been a focus on the more distal gut, as these regions were associated with the painful symptoms of inflammatory bowel disease and irritable bowel syndrome.
However, the early observation that surgically severing the greater splanchnic nerve abolishes the compensatory ingestion of food removed from the stomach that occurs in normal rats, but leaves normal ingestion unaffected, suggested a role for splanchnic/spinal afferents in the satiation process [80]. But note that a more comprehensive study found a large suppression of food intake and body weight after vagotomy, but no effect after splanchnicectomy in rats [105]. Furthermore, flavor preference learning enabled by intragastric or intraduodenal nutrient infusion in rats was found not to be abolished after non-selective subdiaphragmatic or selective sensory abdominal vagotomy, but significantly attenuated by celiac/sup. mesenteric ganglionectomy by Sclafani and colleagues [82,106], leaving room for a contributing role of splanchnic/spinal afferents in this important nutrient signaling system. Indeed, spinal afferents have been suggested to mediate the intestinal glucose (via the hepatic portal vein, see below) but not fat signal to basomedial AGRP neurons, considered to be master energy sensors and orchestrators of energy intake [81]. Using a more selective DRG de-afferentation approach, namely intrathecal resinifera toxin injections, a crucial role for DRG afferents innervating the stomach in gastric distension/bloating-induced suppression of food intake was demonstrated [75]. Another highly specific approach was successfully used to measure single cell calcium dynamics in glutamatergic DRG neurons (with gradient index (GRIN) lenses) and demonstrated that osmolarity sensing (acetic acid, water, NaCl or ensure) from the gut is only sporadic via glutamatergic DRG neurons, but robustly stimulus-timed in glutamatergic vagal afferent neurons [24]. However, global DRG silencing techniques can still only be the first step and more selective DRG ablation/silencing, stimulation and single cell calcium dynamic techniques are exciting and necessary avenues for future studies. Nevertheless, these techniques have been only successfully performed in lower thoracic or lumbar DRGs that are located distal to the spinal cord, while upper thoracic DRGs in close proximity to the spinal cord will remain difficult to access experimentally.
5.2. DRG afferents from the liver, hepatic portal vein, and bile ducts
The hepatic portal vein and liver are in a strategically compelling position to monitor fluxes of absorbed nutrients and some of their metabolic derivatives [86], and both are clearly innervated by DRG afferents that also connect with hindbrain neurons in the NTS or even the dorsal motor complex for potential spinal-vagal reflexes [107,108]. While the hepatic portal vein and bile ducts are also clearly innervated, the hepatic parenchyma is only sparsely innervated by vagal afferents, at least in the rat [109].
DRG afferents originating in the hepatic portal vein were speculated to be mediating the suppressive effects of intragastric glucose on basomedial hypothalamic AGRP neuron activity and subsequent effects on food intake based on celiac/sup. mesenteric ganglionectomy in mice [81]and CGRP-expressing DRG afferents innervating the hepatic portal vein were found to be necessary for detection of hypoglycemia [86].
DRG afferents originating in the liver triggered by activation of extracellular regulated kinase (ERK), and vagal efferents to the pancreas via a central nervous relay pathway, were partially implicated in the regulation of pancreatic β-cell mass in the rat based on splanchnic and vagal nerve transections [110]. However, none of these studies demonstrated activation of DRG afferents and specifically identified DRG afferent neuron innervation of HPV and liver, and the projection neurons in the spinal cord. Furthermore, because celiac/sup. mesenteric ganglionectomy and splanchnic nerve transection are far from selectively eliminating DRG afferents to the hepatic portal space, these studies provide interesting working hypotheses at best. Splanchnic nerve transection also interrupts sympathetic outflow to most of the upper GI-tract, liver, pancreas, spleen, and retroperitoneal fat tissue, as well as all DRG afferents from these tissues. Celiac/sup. mesenteric ganglionectomy additionally interrupts intestinofugal signaling from the upper GI-tract, as well as vagal efferents and afferents coursing in the celiac branches of the vagus [111,112]. As for the GI-tract, methods need to be developed to manipulate function and/or tissue-specific select populations of DRG afferents.
5.3. DRG afferents from pancreas
The pancreas, particularly the pancreatic islets with their secretion of insulin, glucagon, and amylin play a prominent role in metabolic regulation and food intake, yet relatively little is known about the role of their sensory innervation. Both vagal and DRG afferents have been demonstrated in or near pancreatic islets in rats and humans [113,114]. Combined retrograde tracing and CGRP-IHC has clearly shown that the pancreas, including pancreatic islets, is innervated by T8-T11 DRG in the rat [[115], [116], [117]]. Local ablation of TRPV1-expressing DRG neurons by pancreas intra-ductal injections of capsaicin increased insulin release upon glucose stimulation and lowered post-absorptive glucose levels in male, but not female mice [118]. A recent study also suggested that pancreatic β-cells communicate with vagal sensory neurons via serotonin-signaling and may convey information about the “secretory state” of β-cells to the brain [119]. This brings up a number of questions. Why would the brain monitor a co-secreted transmitter rather than insulin itself? Are there similar monitoring systems for the other islet hormones? And what would be the purpose of such monitoring systems? Alternatively, sensory neurons may monitor the inputs to the islets rather than their outputs, or the sensory innervation may be used for the brain to generate a map of the body as part of “the conscient self” and consciousness [120,121].
A role in pathophysiology of autoimmune diabetes for insulin-responsive capsaicin-sensitive TRPV1 afferent neurons of possible DRG origin was proposed [122]. However, no other physiological role for pancreas DRGs other than driving inflammation and increased pain perception in pancreatitis has been suggested (e.g., [123].
5.4. DRG afferents from WAT and BAT
In rodents, both subcutaneous WAT and iBAT are exclusively innervated by spinal afferents and not by vagal afferents [124]. The late Timothy Bartness and his colleagues suggested that DRG afferents in subcutaneous WAT and BAT have roles in regulating adipose tissue mass, lipolysis, and thermogenesis. They first demonstrated that selective denervation (with localized capsaicin injection) of epididymal WAT mimicked lipectomy-induced compensatory increases in inguinal WAT observed previously. On the basis of these observations, they suggested that information related to lipid stores in different fat pads is conveyed via DRG to the brain to orchestrate compensation [125]. Follow-up experiments suggested that spinal afferents sense the level of lipolysis in iWAT and via a central neural network consisting of specific nuclei in the medulla, midbrain/pons, and hypothalamus, change iBAT thermogenesis [54,126,127]. It will be important to determine the molecular mechanisms responsible for detection of lipolysis and identify the crucial neural reflex pathway with modern neuroscience techniques. The possibility that WAT and BAT innervating DRG sense other tissue parameters, such as cytokines, temperature, and mechanical forces should also be considered.
5.5. DRG afferents from skeletal muscle
Skeletal muscle represents the largest metabolically active tissue mass. It has an enormous capacity to expend energy when active and just like cold exposure, exercise can powerfully stimulate energy intake [128,129]. Together with the realization that lean mass may be an important regulator of energy balance [4,128], also came a new appreciation of skeletal muscle signaling to other organs, including the brain [130]. Current evidence suggests that IL-6 secreted from exercising muscle may modulate appetite by acting directly on the brain or via changes in gut hormone secretion. GDF-15 is another myokine that can strongly suppress appetite. GDF-15 is largely responsible for cancer-induced appetite suppression (cancer cachexia), as administration of neutralizing monoclonal antibody to GDF-15 abrogates cancer-induced hypophagia and weight loss [131]. Mitochondrial stress induced by blocking fatty acid β-oxidation in skeletal muscle CPT-1 knockout mice increases circulating GDF-15 levels and significantly attenuates high-fat diet-induced obesity [132].
There is presently no evidence for a role of DRG muscle afferents in ingestive behavior and metabolic regulation. However, because IL-6 secreted from exercising muscle [133] can activate unmyelinated muscle afferents [46], it could be speculated that this leads to metabolic adaptations and/or changes in food intake. However, the more common explanation is that IL-6 from exercising muscle stimulates gut hormone secretion such as GLP-1 and ghrelin, which in turn affect appetite and metabolism.
5.6. DRG afferents from skin
The skin and integumentary system is not normally regarded as an organ important for metabolic regulation, although it has the second largest surface area (∼1.5–2 m2) and represents up to 10 % of body mass in humans and 20% in furry mammals. Similar to the immune defensive functions of the gastrointestinal mucosa, the skin plays an important role in immunity to external pathogens [134]. The skin is also important for temperature regulation and vitamin D homeostasis, which obviously are relevant for metabolic regulation and homeostasis. Heat dissipation through the tail is an important thermoregulatory mechanism in rodents [135]. With more than 100 nerve endings per cm2, the human skin is also very densely innervated and most of these endings are from DRG neurons sensitive to touch, pressure, and temperature. Temperature sensation by the skin has direct implications for energy balance regulation. Perhaps the most striking demonstration for a role of DRG in body weight/adiposity regulation comes from a recent study using a selective, inducible knockout model of CGRPα expressing DRG neurons [136]. Loss of these neurons, many of which are heat-sensitive results in an attenuation of high-fat diet-induced body weight gain of about 60 % over 16 weeks by engaging energy dissipation processes including lipid fuel utilization and cutaneous vasodilation leading to increased energy expenditure but no changes in food intake [136]. Heat sensing DRG neurons have been shown to tonically inhibit cold-sensing DRG neurons at the level of the spinal cord [137]. Since the effects of heat sensing DRG neuron ablation were not associated with reduced core temperature [136], the mechanism likely involved heat conservation and production pathways, but the exact neural pathway is unclear.
Another interesting aspect is possible convergence of somatic (e.g., cutaneous) and visceral afferent input on secondary dorsal horn neurons. Virtually all spinal visceral afferents converge with somatic afferents on secondary neurons, so that there are very few specific viscero-sensory spinal second order neurons [41]. DRG neurons described in the paper by Makwana et al. [136] could thus well be cutaneous thermo-sensors which may interact with visceral afferents.
6. Relationship to vagal pathways
While the focus of this review is on spinal afferent pathways, the importance of abdominal vagal afferents in food intake control and metabolic regulation cannot be overstated (e.g. [138]. Recent progress in dissecting the functional roles of specific vagal afferent neuron populations with cutting-edge tools has both, reinforced a critical role in generating satiety, and substantiated a new role in food reward and food choice [90,92]. Clearly, this modern toolbox now needs to be applied to spinal afferent pathways.
At the anatomical level, vagal and dorsal root afferents are not completely separated. Many organs and tissues that are supplied by DRG afferents are innervated by mixed nerves, not only containing sympathetic postganglionic fibers but also vagal afferents and efferents. This is particularly true for the gastrointestinal tract and associated organs, which are innervated via mixed nerve plexuses along blood vessels (Fig. 3). A typical example is the sumptuous nerve plexus surrounding the celiac artery and its offshoots, the left gastric, hepatic, and splenic arteries, where vagal afferent and efferent axons are intimately intermingled with DRG and postganglionic sympathetic axons from the celiac and superior mesenteric ganglia [109]. At this anatomical level any attempt for selective manipulation such as transection or electrical stimulation is extremely difficult if not impossible (see also discussion in Section 4).
In the brain, DRG and vagal afferent pathways converge in many areas, although it is not known whether they directly interact. The NTS is the main hub of direct vagal afferent input, and it also receives Information from certain DRG neurons via the spinosolitary tract. From the NTS, vagal afferent information is relayed to many midbrain and forebrain areas that also receive direct or indirect input from DRG afferents (Fig. 5). Many of these brain areas are known to play a role in food intake, energy expenditure, and body weight regulation. However, specific interactions between vagal and DRG afferent inputs in these areas of convergence have not been studied.
7. Conclusions and future directions
Understanding the mechanisms of energy balance regulation is important for the prevention and treatment of obesity and associated metabolic diseases, which continue to rise and increasingly affect children and adolescents. Throughout its history, this research field has struggled with the integration of peripheral and central/neural mechanisms. Is the primary defect in obesity to be found in faulty processing by peripheral tissues such as adipose tissue, liver, and gut, or faulty processing by the brain? The question appeared to be answered in favor of the brain by the seminal studies on the role of the hypothalamus that started in the mid 1900's and lasted into our modern era of neuroscience. However, the brain is not acting in isolation, but uses both external and internal information to make decisions in a learning-dependent fashion. While this seminal role of central neural integration may be true for external factors such as environmental stimuli and incentives, it is much less clear for stimuli and inputs from the internal milieu. In other words, decisions regarding energy balance made by the brain are strongly influenced by interoception and depend on the quality of this information.
Interoception is accomplished by both humoral and neural communication between the periphery and the brain, with each route having specific advantages. The strength of neural communication are speed and spatial discreteness. Information from very specific locations in the periphery can reach the brain in milliseconds via sensory/afferent nerve pathways and the brain can reach specific peripheral targets equally fast via motor/efferent nerve pathways. On the sensory side, the important role in energy balance regulation by vagal afferents supplying the abdominal viscera has been widely acknowledged. The potential role of spinal afferents has just now started to be of interest but is still largely unexplored. Here we provided an in-depth look at the anatomy and known functions of dorsal root/spinal afferents which potentially affect energy balance regulation. Because information derived from DRG afferents can elicit sympathetic nervous system actions via long and short spinal reflexes (Fig. 4), DRG neurons are often erroneously called “sympathetic afferents”. However, because functional implications of DRG afferent information as laid out in this review go far beyond such reflexes we should refrain from this terminology.
In contrast to vagal afferents, DRG/spinal afferents innervate not only abdominal organs but also subcutaneous white and brown adipose tissue, skeletal muscle, and skin. They are, therefore, in a position to provide more complete sensory information from organs and tissues that are key players in energy balance regulation. The crucial role of subcutaneous adipose tissue in energy homeostasis is well known, and its communication via spinal afferents and the sympathetic nervous system has already been recognized. The roles of skeletal muscle and skin and its DRG innervation are less obvious and have received little attention. However, the large metabolically active mass together with temperature sensing and heat dissipation/conservation mechanisms, muscle and skin have a huge potential to influence energy homeostasis. Therefore, it will be of great interest to identify the pathways and mechanisms by which muscle and skin communicate with other organs including the brain. Clearly, circulating myokines, hormones and other factors are part of this communication. However, humoral communication signals lack speed and discreteness of origin and destination, which can only be provided with neural communication such as spinal afferents.
The fact that spinal afferent involvement in energy homeostasis has received less attention is undoubtedly anchored in the inherent complexity of its circuitry. Surgical and traditional chemical approaches, which can only be used to target bulk innervation and typically have unintended effects on a host of other pathways of the peripheral nervous system, have often led to erroneous conclusions. For deciphering the functional specificity of subpopulations of DRG afferents (as well as SNS preganglionics and postganglionics), modern neurobiological techniques are sorely needed and may allow selective neuromodulation approaches to treat or improve metabolic disease or associated illnesses.
Funding
Research by the authors was supported by National Institutes of Health Grants R01DK47348 (HRB); 2R01DK092587, 1R01AT011683 (HM).
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Heike Muenzberg reports financial support was provided by National Institute of Health. Hans-Rudolf Berthoud reports financial support was provided by National Institute of Health.
Acknowledgments
Research by the authors was supported by National Institutes of Health Grants R01DK47348 (HRB); 2R01DK092587, 1R01AT011683 (HM). We thank Dr. med. Katja Dalkowski for help with the figures.
Contributor Information
Heike Münzberg, Email: Heike.Munzberg@pbrc.edu.
Hans-Rudolf Berthoud, Email: berthohr@pbrc.edu.
Winfried L. Neuhuber, Email: Winfried.Neuhuber@fau.de.
Data availability
No data was used for the research described in the article.
References
- 1.Bray G.A., Kim K.K., Wilding J.P.H., World Obesity F. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–723. doi: 10.1111/obr.12551. [DOI] [PubMed] [Google Scholar]
- 2.Pillon N.J., Loos R.J.F., Marshall S.M., Zierath J.R. Metabolic consequences of obesity and type 2 diabetes: balancing genes and environment for personalized care. Cell. 2021;184:1530–1544. doi: 10.1016/j.cell.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qasim A., Turcotte M., de Souza R.J., Samaan M.C., Champredon D., Dushoff J., et al. On the origin of obesity: identifying the biological, environmental and cultural drivers of genetic risk among human populations. Obes Rev. 2018;19:121–149. doi: 10.1111/obr.12625. [DOI] [PubMed] [Google Scholar]
- 4.Dulloo A.G. Physiology of weight regain: lessons from the classic Minnesota Starvation Experiment on human body composition regulation. Obes Rev. 2021;22(Suppl 2) doi: 10.1111/obr.13189. [DOI] [PubMed] [Google Scholar]
- 5.El Ansari W., Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg. 2021;31:1755–1766. doi: 10.1007/s11695-020-05160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvey W.T. Is obesity or adiposity-based chronic disease curable: the set point theory, the environment, and second-generation medications. Endocr Pract. 2022;28:214–222. doi: 10.1016/j.eprac.2021.11.082. [DOI] [PubMed] [Google Scholar]
- 7.Hall K.D., Jordan P.N. Modeling weight-loss maintenance to help prevent body weight regain. Am J Clin Nutr. 2008;88:1495–1503. doi: 10.3945/ajcn.2008.26333. [DOI] [PubMed] [Google Scholar]
- 8.MacLean P.S., Wing R.R., Davidson T., Epstein L., Goodpaster B., Hall K.D., et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity. 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud H.R., Seeley R.J., Roberts S.B. Physiology of energy intake in the weight-reduced state. Obesity. 2021;29(Suppl 1):S25–S30. doi: 10.1002/oby.23080. [DOI] [PubMed] [Google Scholar]
- 10.Berthoud H.R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz M.W., Seeley R.J., Zeltser L.M., Drewnowski A., Ravussin E., Redman L.M., et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev. 2017;38:267–296. doi: 10.1210/er.2017-00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand B.K., Brobeck J.R. Localization of a "feeding center" in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- 13.Hoebel B.G., Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 14.Sternson S.M., Eiselt A.K. Three pillars for the neural control of appetite. Annu Rev Physiol. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 15.Berthoud H.R., Neuhuber W.L. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah N., Defaye M., Altier C. Neural control of gut homeostasis. Am J Physiol Gastrointest Liver Physiol. 2020;319:G718–G732. doi: 10.1152/ajpgi.00293.2020. [DOI] [PubMed] [Google Scholar]
- 17.Gershon M.D., Margolis K.G. The gut, its microbiome, and the brain: connections and communications. J Clin Invest. 2021;131 doi: 10.1172/JCI143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith-Edwards K.M., Najjar S.A., Edwards B.S., Howard M.J., Albers K.M., Davis B.M. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology. 2019;157:522–536 e522. doi: 10.1053/j.gastro.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaenig W. Cambridge University Press; 2022. The integrative action of the autonomic nerveous system. [Google Scholar]
- 20.Rigamonti D.D., Hancock M.B., Kapur S.P. Visceral afferent projections to the dorsal column nuclei. Brain Res. 1978;150:408–412. doi: 10.1016/0006-8993(78)90292-5. [DOI] [PubMed] [Google Scholar]
- 21.Ernsberger U., Rohrer H. Sympathetic tales: subdivisons of the autonomic nervous system and the impact of developmental studies. Neural Dev. 2018;13:20. doi: 10.1186/s13064-018-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix B., Catalin D., Miolan J.P., Niel J.P. Integrative properties of the major pelvic ganglion in the rat. J Auton Nerv Syst. 1998;69:6–11. doi: 10.1016/s0165-1838(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 23.Keast J.R. Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience. 1995;66:655–662. doi: 10.1016/0306-4522(94)00595-v. [DOI] [PubMed] [Google Scholar]
- 24.Ichiki T., Wang T., Kennedy A., Pool A.H., Ebisu H., Anderson D.J., et al. Sensory representation and detection mechanisms of gut osmolality change. Nature. 2022;602:468–474. doi: 10.1038/s41586-021-04359-5. [DOI] [PubMed] [Google Scholar]
- 25.Hunt S.P., Pini A., Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 26.Fox E.A., Powley T.L. False-positive artifacts of tracer strategies distort autonomic connectivity maps. Brain Res Brain Res Rev. 1989;14:53–77. doi: 10.1016/0165-0173(89)90009-x. [DOI] [PubMed] [Google Scholar]
- 27.Yu W.H. Uptake sites of horseradish peroxidase after injection into peritoneal structures: defining some pitfalls. J Neurosci Methods. 1980;2:123–133. doi: 10.1016/0165-0270(80)90054-0. [DOI] [PubMed] [Google Scholar]
- 28.Ohlen A., Lindbom L., Staines W., Hokfelt T., Cuello A.C., Fischer J.A., et al. Substance P and calcitonin gene-related peptide: immunohistochemical localisation and microvascular effects in rabbit skeletal muscle. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;336:87–93. doi: 10.1007/BF00177756. [DOI] [PubMed] [Google Scholar]
- 29.Fasanella K.E., Christianson J.A., Chanthaphavong R.S., Davis B.M. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiegs G., Bang R., Neuhuber W.L. Requirement of peptidergic sensory innervation for disease activity in murine models of immune hepatitis and protection by beta-adrenergic stimulation. J Neuroimmunol. 1999;96:131–143. doi: 10.1016/s0165-5728(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 31.Norman D., Mukherjee S., Symons D., Jung R.T., Lever J.D. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J Neurocytol. 1988;17:305–311. doi: 10.1007/BF01187853. [DOI] [PubMed] [Google Scholar]
- 32.Haring M., Zeisel A., Hochgerner H., Rinwa P., Jakobsson J.E.T., Lonnerberg P., et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- 33.Todd A.J. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol Pain. 2017;13 doi: 10.1177/1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatto G., Bourane S., Ren X., Di Costanzo S., Fenton P.K., Halder P., et al. A functional topographic map for spinal sensorimotor reflexes. Neuron. 2021;109:91–104 e105. doi: 10.1016/j.neuron.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browne T.J., Smith K.M., Gradwell M.A., Iredale J.A., Dayas C.V., Callister R.J., et al. Spinoparabrachial projection neurons form distinct classes in the mouse dorsal horn. Pain. 2021;162:1977–1994. doi: 10.1097/j.pain.0000000000002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francois A., Low S.A., Sypek E.I., Christensen A.J., Sotoudeh C., Beier K.T., et al. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron. 2017;93:822–839 e826. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Zhao S., Rodriguez E., Takatoh J., Han B.X., Zhou X., et al. Identifying local and descending inputs for primary sensory neurons. J Clin Invest. 2015;125:3782–3794. doi: 10.1172/JCI81156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura Y., Terui N., Hosoya Y., Tonosaki Y., Nishiyama K., Honda T. Quantitative analysis of central terminal projections of visceral and somatic unmyelinated (C) primary afferent fibers in the Guinea pig. J Comp Neurol. 1993;332:315–325. doi: 10.1002/cne.903320305. [DOI] [PubMed] [Google Scholar]
- 39.Brown A.G., Fyffe R.E., Rose P.K., Snow P.J. Spinal cord collaterals from axons of type II slowly adapting units in the cat. J Physiol. 1981;316:469–480. doi: 10.1113/jphysiol.1981.sp013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhuber W. The central projections of visceral primary afferent neurons of the inferior mesenteric plexus and hypogastric nerve and the location of the related sensory and preganglionic sympathetic cell bodies in the rat. Anat Embryol. 1982;164:413–425. doi: 10.1007/BF00315762. [DOI] [PubMed] [Google Scholar]
- 41.Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pomeranz B., Wall P.D., Weber W.V. Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol. 1968;199:511–532. doi: 10.1113/jphysiol.1968.sp008666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervero F. Supraspinal connections of neurones in the thoracic spinal cord of the cat: ascending projections and effects of descending impulses. Brain Res. 1983;275:251–261. doi: 10.1016/0006-8993(83)90986-1. [DOI] [PubMed] [Google Scholar]
- 44.Cervero F., Tattersall J.E. Somatic and visceral inputs to the thoracic spinal cord of the cat: marginal zone (lamina I) of the dorsal horn. J Physiol. 1987;388:383–395. doi: 10.1113/jphysiol.1987.sp016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrington A.M., Caraballo S.G., Maddern J.E., Grundy L., Castro J., Brierley S.M. Colonic afferent input and dorsal horn neuron activation differs between the thoracolumbar and lumbosacral spinal cord. Am J Physiol Gastrointest Liver Physiol. 2019;317:G285–G303. doi: 10.1152/ajpgi.00013.2019. [DOI] [PubMed] [Google Scholar]
- 46.Hoheisel U., Lehmann-Willenbrock E., Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28:495–507. doi: 10.1016/0306-4522(89)90195-4. [DOI] [PubMed] [Google Scholar]
- 47.Ling L.J., Honda T., Shimada Y., Ozaki N., Shiraishi Y., Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the Guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- 48.Mense S., Craig A.D., Jr. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience. 1988;26:1023–1035. doi: 10.1016/0306-4522(88)90117-0. [DOI] [PubMed] [Google Scholar]
- 49.Neuhuber W.L., Zenker W. Central distribution of cervical primary afferents in the rat, with emphasis on proprioceptive projections to vestibular, perihypoglossal, and upper thoracic spinal nuclei. J Comp Neurol. 1989;280:231–253. doi: 10.1002/cne.902800206. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L., Han W., Lin C., Li F., de Araujo I.E. Sugar metabolism regulates flavor preferences and portal glucose sensing. Front Integr Neurosci. 2018;12:57. doi: 10.3389/fnint.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tracey D.J. In: The rat nervous system. Paxinos G., editor. Academic Press; New York: 1995. Ascending and descending pathways in the spinal cord; pp. 67–80. [Google Scholar]
- 52.Sengul G.W.,C. In: The human nervous system. Paxinos J.M.a.G., editor. Academic Press; New York: 2012. Spinal cord: connections; pp. 233–258. In The Human Nervous System. [Google Scholar]
- 53.Ryu V., Bartness T.J. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R886–R900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garretson J.T., Szymanski L.A., Schwartz G.J., Xue B., Ryu V., Bartness T.J. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metabol. 2016;5:626–634. doi: 10.1016/j.molmet.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen N.L., Barr C.L., Ryu V., Cao Q., Xue B., Bartness T.J. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am J Physiol Regul Integr Comp Physiol. 2017;312:R132–R145. doi: 10.1152/ajpregu.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iosif C.I., Bashir Z.I., Apps R., Pickford J. Cerebellum; 2022. Cerebellar prediction and feeding behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Low A.Y.T., Goldstein N., Gaunt J.R., Huang K.P., Zainolabidin N., Yip A.K.K., et al. Reverse-translational identification of a cerebellar satiation network. Nature. 2021;600:269–273. doi: 10.1038/s41586-021-04143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman P.P., Paul D.H. The projection of splanchnic afferents on the cerebellum of the cat. J Physiol. 1969;202:223–227. doi: 10.1113/jphysiol.1969.sp008806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perrin J., Crousillat J. Splanchnic afferent input to the lateral reticular nucleus of the cat. J Auton Nerv Syst. 1983;8:383–393. doi: 10.1016/0165-1838(83)90032-2. [DOI] [PubMed] [Google Scholar]
- 60.Choi S., Hachisuka J., Brett M.A., Magee A.R., Omori Y., Iqbal N.U., et al. Parallel ascending spinal pathways for affective touch and pain. Nature. 2020;587:258–263. doi: 10.1038/s41586-020-2860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Francois M., Torres H., Huesing C., Zhang R., Saurage C., Lee N., et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann N Y Acad Sci. 2019;1454:3–13. doi: 10.1111/nyas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huesing C., Qualls-Creekmore E., Lee N., Francois M., Torres H., Zhang R., et al. Sympathetic innervation of inguinal white adipose tissue in the mouse. J Comp Neurol. 2021;529:1465–1485. doi: 10.1002/cne.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huesing C., Zhang R., Gummadi S., Lee N., Qualls-Creekmore E., Yu S., et al. Organization of sympathetic innervation of interscapular brown adipose tissue in the mouse. J Comp Neurol. 2022;530:1363–1378. doi: 10.1002/cne.25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng J.Q., Seki M., Hayakawa T., Ito H., Zyo K. Descending projections from the paraventricular hypothalamic nucleus to the spinal cord: anterograde tracing study in the rat. Okajimas Folia Anat Jpn. 1995;72:119–135. doi: 10.2535/ofaj1936.72.2-3_119. [DOI] [PubMed] [Google Scholar]
- 66.Hallbeck M., Larhammar D., Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 67.Sawchenko P.E., Swanson L.W. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 68.Sutton A.K., Pei H., Burnett K.H., Myers M.G., Jr., Rhodes C.J., Olson D.P. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34:15306–15318. doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van den Pol A.N. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshida A., Chen K., Moritani M., Yabuta N.H., Nagase Y., Takemura M., et al. Organization of the descending projections from the parabrachial nucleus to the trigeminal sensory nuclear complex and spinal dorsal horn in the rat. J Comp Neurol. 1997;383:94–111. [PubMed] [Google Scholar]
- 71.Neuhuber W.L., Appelt M., Polak J.M., Baier-Kustermann W., Abelli L., Ferri G.L. Rectospinal neurons: cell bodies, pathways, immunocytochemistry and ultrastructure. Neuroscience. 1993;56:367–378. doi: 10.1016/0306-4522(93)90338-g. [DOI] [PubMed] [Google Scholar]
- 72.Costa M., Furness J.B. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the Guinea-pig small intestine. Neuroscience. 1983;8:665–676. doi: 10.1016/0306-4522(83)90002-7. [DOI] [PubMed] [Google Scholar]
- 73.Furness J.B., Koopmans H.S., Robbins H.L., Lin H.C. Identification of intestinofugal neurons projecting to the coeliac and superior mesenteric ganglia in the rat. Auton Neurosci. 2000;83:81–85. doi: 10.1016/S0165-1838(00)00159-4. [DOI] [PubMed] [Google Scholar]
- 74.Mann P.T., Furness J.B., Pompolo S., Mader M. Chemical coding of neurons that project from different regions of intestine to the coeliac ganglion of the Guinea pig. J Auton Nerv Syst. 1995;56:15–25. doi: 10.1016/0165-1838(95)00053-1. [DOI] [PubMed] [Google Scholar]
- 75.Zhang T., Perkins M.H., Chang H., Han W., de Araujo I.E. An inter-organ neural circuit for appetite suppression. Cell. 2022;185:2478–2494 e2428. doi: 10.1016/j.cell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furness J.B., Jones C., Nurgali K., Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol. 2004;72:143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Muller P.A., Matheis F., Schneeberger M., Kerner Z., Jove V., Mucida D. Microbiota-modulated CART(+) enteric neurons autonomously regulate blood glucose. Science. 2020;370:314–321. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O'Donnell T.A., et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–198 e116. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kyloh M.A., Hibberd T.J., Castro J., Harrington A.M., Travis L., Dodds K.N., et al. Disengaging spinal afferent nerve communication with the brain in live mice. Commun Biol. 2022;5:915. doi: 10.1038/s42003-022-03876-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deutsch J.A., Jang Ahn S. The splanchnic nerve and food intake regulation. Behav Neural Biol. 1986;45:43–47. doi: 10.1016/s0163-1047(86)80004-8. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein N., McKnight A.D., Carty J.R.E., Arnold M., Betley J.N., Alhadeff A.L. Hypothalamic detection of macronutrients via multiple gut-brain pathways. Cell Metabol. 2021;33:676–687 e675. doi: 10.1016/j.cmet.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sclafani A., Ackroff K., Schwartz G.J. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78:285–294. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- 83.Karai L., Brown D.C., Mannes A.J., Connelly S.T., Brown J., Gandal M., et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pecze L., Josvay K., Blum W., Petrovics G., Vizler C., Olah Z., et al. Activation of endogenous TRPV1 fails to induce overstimulation-based cytotoxicity in breast and prostate cancer cells but not in pain-sensing neurons. Biochim Biophys Acta. 2016;1863:2054–2064. doi: 10.1016/j.bbamcr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Acs G., Palkovits M., Blumberg P.M. Comparison of [3H]resiniferatoxin binding by the vanilloid (capsaicin) receptor in dorsal root ganglia, spinal cord, dorsal vagal complex, sciatic and vagal nerve and urinary bladder of the rat. Life Sci. 1994;55:1017–1026. doi: 10.1016/0024-3205(94)00636-9. [DOI] [PubMed] [Google Scholar]
- 86.Fujita S., Bohland M., Sanchez-Watts G., Watts A.G., Donovan C.M. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol Endocrinol Metab. 2007;293:E96–E101. doi: 10.1152/ajpendo.00415.2006. [DOI] [PubMed] [Google Scholar]
- 87.Papazoglou I., Lee J.H., Cui Z., Li C., Fulgenzi G., Bahn Y.J., et al. A distinct hypothalamus-to-beta cell circuit modulates insulin secretion. Cell Metabol. 2022;34:285–298 e287. doi: 10.1016/j.cmet.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi H., Song C.K., Giordano A., Cinti S., Bartness T.J. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–R1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 89.Bai L., Sivakumar N., Yu S., Mesgarzadeh S., Ding T., Ly T., et al. Enteroendocrine cell types that drive food reward and aversion. Elife. 2022;11 doi: 10.7554/eLife.74964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai L., Mesgarzadeh S., Ramesh K.S., Huey E.L., Liu Y., Gray L.A., et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179:1129–1143 e1123. doi: 10.1016/j.cell.2019.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kupari J., Haring M., Agirre E., Castelo-Branco G., Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27:2508–2523 e2504. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M., Tan H.E., Lu Z., Tsang K.S., Chung A.J., Zuker C.S. Gut-brain circuits for fat preference. Nature. 2022;610:722–730. doi: 10.1038/s41586-022-05266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan H.E., Sisti A.C., Jin H., Vignovich M., Villavicencio M., Tsang K.S., et al. The gut-brain axis mediates sugar preference. Nature. 2020;580:511–516. doi: 10.1038/s41586-020-2199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hockley J.R.F., Taylor T.S., Callejo G., Wilbrey A.L., Gutteridge A., Bach K., et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut. 2019;68:633–644. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma N., Flaherty K., Lezgiyeva K., Wagner D.E., Klein A.M., Ginty D.D. The emergence of transcriptional identity in somatosensory neurons. Nature. 2020;577:392–398. doi: 10.1038/s41586-019-1900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Usoskin D., Furlan A., Islam S., Abdo H., Lonnerberg P., Lou D., et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 97.Wolfson R.L., Abdelaziz A., Rankin G., Kushner S., Qi L., Mazor O., et al. DRG afferents that mediate physiologic and pathologic mechanosensation from the distal colon. Cell. 2023;186:3368–3385 e3318. doi: 10.1016/j.cell.2023.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diepenbroek C., Quinn D., Stephens R., Zollinger B., Anderson S., Pan A., et al. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol. 2017;313:G342–G352. doi: 10.1152/ajpgi.00095.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borgmann D., Ciglieri E., Biglari N., Brandt C., Cremer A.L., Backes H., et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metabol. 2021;33:1466–1482 e1467. doi: 10.1016/j.cmet.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garami A., Pakai E., Oliveira D.L., Steiner A.A., Wanner S.P., Almeida M.C., et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walker C.S., Li X., Whiting L., Glyn-Jones S., Zhang S., Hickey A.J., et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology. 2010;151:4257–4269. doi: 10.1210/en.2010-0284. [DOI] [PubMed] [Google Scholar]
- 102.Clemmensen C., Muller T.D., Woods S.C., Berthoud H.R., Seeley R.J., Tschop M.H. Gut-brain cross-talk in metabolic control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grundy D. What activates visceral afferents? Gut. 2004;53(Suppl 2):ii5–ii8. doi: 10.1136/gut.2003.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80. doi: 10.1016/j.autneu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 105.Furness J.B., Koopmans H.S., Robbins H.L., Clerc N., Tobin J.M., Morris M.J. Effects of vagal and splanchnic section on food intake, weight, serum leptin and hypothalamic neuropeptide Y in rat. Auton Neurosci. 2001;92:28–36. doi: 10.1016/S1566-0702(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 106.Sclafani A., Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav. 1996;60:447–453. doi: 10.1016/s0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 107.Garcia-Luna C., Sanchez-Watts G., Arnold M., de Lartigue G., DeWalt N., Langhans W., et al. The medullary targets of neurally conveyed sensory information from the rat hepatic portal and superior mesenteric veins. eNeuro. 2021;8 doi: 10.1523/ENEURO.0419-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sasaki Y., Hayashi N., Kasahara A., Matsuda H., Fusamoto H., Sato N., et al. Calcitonin gene-related peptide in the hepatic and splanchnic vascular systems of the rat. Hepatology. 1986;6:676–681. doi: 10.1002/hep.1840060423. [DOI] [PubMed] [Google Scholar]
- 109.Berthoud H.R., Kressel M., Neuhuber W.L. An anterograde tracing study of the vagal innervation of rat liver, portal vein and biliary system. Anat Embryol. 1992;186:431–442. doi: 10.1007/BF00185458. [DOI] [PubMed] [Google Scholar]
- 110.Imai J., Katagiri H., Yamada T., Ishigaki Y., Suzuki T., Kudo H., et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 111.Berthoud H.R., Powley T.L. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 112.Wang F.B., Powley T.L. Vagal innervation of intestines: afferent pathways mapped with new en bloc horseradish peroxidase adaptation. Cell Tissue Res. 2007;329:221–230. doi: 10.1007/s00441-007-0413-7. [DOI] [PubMed] [Google Scholar]
- 113.Makhmutova M., Caicedo A. Optical imaging of pancreatic innervation. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.663022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neuhuber W.L. Vagal afferent fibers almost exclusively innervate islets in the rat pancreas as demonstrated by anterograde tracing. J Auton Nerv Syst. 1989;29:13–18. doi: 10.1016/0165-1838(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 115.Korsgren O., Jansson L., Ekblad E., Sundler F. Reinnervation of syngeneic pancreatico-duodenal grafts in rats. Transplantation. 2001;71:8–13. doi: 10.1097/00007890-200101150-00002. [DOI] [PubMed] [Google Scholar]
- 116.McEwan S., Kwon H., Tahiri A., Shanmugarajah N., Cai W., Ke J., et al. Deconstructing the origins of sexual dimorphism in sensory modulation of pancreatic beta cells. Mol Metabol. 2021;53 doi: 10.1016/j.molmet.2021.101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Su H.C., Bishop A.E., Power R.F., Hamada Y., Polak J.M. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J Neurosci. 1987;7:2674–2687. doi: 10.1523/JNEUROSCI.07-09-02674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bou Karam J., Cai W., Mohamed R., Huang T., Meng L., Homan E.P., et al. TRPV1 neurons regulate beta-cell function in a sex-dependent manner. Mol Metabol. 2018;18:60–67. doi: 10.1016/j.molmet.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Makhmutova M., Weitz J., Tamayo A., Pereira E., Boulina M., Almaca J., et al. Pancreatic beta-cells communicate with vagal sensory neurons. Gastroenterology. 2021;160:875–888 e811. doi: 10.1053/j.gastro.2020.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Craig A.D. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 121.Craig A.D. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 122.Razavi R., Chan Y., Afifiyan F.N., Liu X.J., Wan X., Yantha J., et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 123.Nathan J.D., Peng R.Y., Wang Y., McVey D.C., Vigna S.R., Liddle R.A. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G938–G946. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 124.Giordano A., Song C.K., Bowers R.R., Ehlen J.C., Frontini A., Cinti S., et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 125.Shi H., Bartness T.J. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R514–R520. doi: 10.1152/ajpregu.00036.2005. [DOI] [PubMed] [Google Scholar]
- 126.Ryu V., Watts A.G., Xue B., Bartness T.J. Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2017;312:R324–R337. doi: 10.1152/ajpregu.00456.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song C.K., Schwartz G.J., Bartness T.J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blundell J.E., Gibbons C., Beaulieu K., Casanova N., Duarte C., Finlayson G., et al. The drive to eat in homo sapiens: energy expenditure drives energy intake. Physiol Behav. 2020;219 doi: 10.1016/j.physbeh.2020.112846. [DOI] [PubMed] [Google Scholar]
- 129.Mayer J., Roy P., Mitra K.P. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr. 1956;4:169–175. doi: 10.1093/ajcn/4.2.169. [DOI] [PubMed] [Google Scholar]
- 130.Grannell A., De Vito G., Murphy J.C., le Roux C.W. The influence of skeletal muscle on appetite regulation. Expet Rev Endocrinol Metabol. 2019;14:267–282. doi: 10.1080/17446651.2019.1618185. [DOI] [PubMed] [Google Scholar]
- 131.Kim-Muller J.Y., Song L., LaCarubba Paulhus B., Pashos E., Li X., Rinaldi A., et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2022.111947. [DOI] [PubMed] [Google Scholar]
- 132.Vandanmagsar B., Warfel J.D., Wicks S.E., Ghosh S., Salbaum J.M., Burk D., et al. Impaired mitochondrial fat oxidation induces FGF21 in muscle. Cell Rep. 2016;15:1686–1699. doi: 10.1016/j.celrep.2016.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gonzalez-Gil A.M., Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020;12 doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Streilein J.W., Alard P., Niizeki H. A new concept of skin-associated lymphoid tissue (SALT): UVB light impaired cutaneous immunity reveals a prominent role for cutaneous nerves. Keio J Med. 1999;48:22–27. doi: 10.2302/kjm.48.22. [DOI] [PubMed] [Google Scholar]
- 135.Tanaka M., McKinley M.J., McAllen R.M. Preoptic-raphe connections for thermoregulatory vasomotor control. J Neurosci. 2011;31:5078–5088. doi: 10.1523/JNEUROSCI.6433-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Makwana K., Chodavarapu H., Morones N., Chi J., Barr W., Novinbakht E., et al. Sensory neurons expressing calcitonin gene-related peptide alpha regulate adaptive thermogenesis and diet-induced obesity. Mol Metabol. 2021;45 doi: 10.1016/j.molmet.2021.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCoy E.S., Taylor-Blake B., Street S.E., Pribisko A.L., Zheng J., Zylka M.J. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78:138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wachsmuth H.R., Weninger S.N., Duca F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022;54:377–392. doi: 10.1038/s12276-021-00677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Neuhuber W., Niederle B. Spinal ganglion cells innervating the stomach of the rat as demonstrated by somatopetal transport of horseradish peroxidase (HRP) Anat Embryol. 1979;155:355–362. doi: 10.1007/BF00317648. [DOI] [PubMed] [Google Scholar]
- 140.Robinson D.R., McNaughton P.A., Evans M.L., Hicks G.A. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neuro Gastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 141.Tan L.L., Bornstein J.C., Anderson C.R. Distinct chemical classes of medium-sized transient receptor potential channel vanilloid 1-immunoreactive dorsal root ganglion neurons innervate the adult mouse jejunum and colon. Neuroscience. 2008;156:334–343. doi: 10.1016/j.neuroscience.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 142.Magni F., Carobi C. The afferent and preganglionic parasympathetic innervation of the rat liver, demonstrated by the retrograde transport of horseradish peroxidase. J Auton Nerv Syst. 1983;8:237–260. doi: 10.1016/0165-1838(83)90108-x. [DOI] [PubMed] [Google Scholar]
- 143.Sharkey K.A., Williams R.G., Dockray G.J. Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology. 1984;87:914–921. [PubMed] [Google Scholar]
- 144.Murphy K.T., Schwartz G.J., Nguyen N.L., Mendez J.M., Ryu V., Bartness T.J. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–E1347. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y., Scarneo S.A., Kim S.H., Zhang X., Chen J., Yang K.W., et al. Expression of ectopic heat shock protein 90 in male and female primary afferent nociceptors regulates inflammatory pain. Pain. 2022;163:1091–1101. doi: 10.1097/j.pain.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rinaman L., Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cervero F., Connell L.A. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- 148.Cervero F., Connell L.A., Lawson S.N. Somatic and visceral primary afferents in the lower thoracic dorsal root ganglia of the cat. J Comp Neurol. 1984;228:422–431. doi: 10.1002/cne.902280309. [DOI] [PubMed] [Google Scholar]
- 149.Neuhuber W.L., Sandoz P.A., Fryscak T. The central projections of primary afferent neurons of greater splanchnic and intercostal nerves in the rat. A horseradish peroxidase study. Anat Embryol. 1986;174:123–144. doi: 10.1007/BF00318344. [DOI] [PubMed] [Google Scholar]
- 150.Baron R., Janig W., McLachlan E.M. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. III. The colonic nerves, incorporating an analysis of all components of the lumbar prevertebral outflow. J Comp Neurol. 1985;238:158–168. doi: 10.1002/cne.902380204. [DOI] [PubMed] [Google Scholar]
- 151.Morgan C., deGroat W.C., Nadelhaft I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol. 1986;243:23–40. doi: 10.1002/cne.902430104. [DOI] [PubMed] [Google Scholar]
- 152.Morgan C., Nadelhaft I., de Groat W.C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- 153.Zenker W., Sandoz P.A., Neuhuber W. The distribution of anterogradely labeled I--IV primary afferents in histochemically defined compartments of the rat's sternomastoid muscle. Anat Embryol. 1988;177:235–243. doi: 10.1007/BF00321134. [DOI] [PubMed] [Google Scholar]
- 154.Clerc N., Mazzia C. Morphological relationships of choleragenoid horseradish peroxidase-labeled spinal primary afferents with myenteric ganglia and mucosal associated lymphoid tissue in the cat esophagogastric junction. J Comp Neurol. 1994;347:171–186. doi: 10.1002/cne.903470203. [DOI] [PubMed] [Google Scholar]
- 155.Mazzia C., Clerc N. Ultrastructural relationships of spinal primary afferent fibres with neuronal and non-neuronal cells in the myenteric plexus of the cat oesophago-gastric junction. Neuroscience. 1997;80:925–937. doi: 10.1016/s0306-4522(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 156.Spencer N.J., Kyloh M., Beckett E.A., Brookes S., Hibberd T. Different types of spinal afferent nerve endings in stomach and esophagus identified by anterograde tracing from dorsal root ganglia. J Comp Neurol. 2016;524:3064–3083. doi: 10.1002/cne.24006. [DOI] [PubMed] [Google Scholar]
- 157.Kyloh M., Spencer N.J. A novel anterograde neuronal tracing technique to selectively label spinal afferent nerve endings that encode noxious and innocuous stimuli in visceral organs. Neuro Gastroenterol Motil. 2014;26:440–444. doi: 10.1111/nmo.12266. [DOI] [PubMed] [Google Scholar]
- 158.Spencer N.J., Kyloh M.A., Travis L., Dodds K.N. Identification of spinal afferent nerve endings in the colonic mucosa and submucosa that communicate directly with the spinal cord: the gut-brain axis. J Comp Neurol. 2020;528:1742–1753. doi: 10.1002/cne.24854. [DOI] [PubMed] [Google Scholar]
- 159.Spencer N.J., Kyloh M.A., Travis L., Dodds K.N. Sensory nerve endings arising from single spinal afferent neurons that innervate both circular muscle and myenteric ganglia in mouse colon: colon-brain axis. Cell Tissue Res. 2020;381:25–34. doi: 10.1007/s00441-020-03192-y. [DOI] [PubMed] [Google Scholar]
- 160.Takamido S., Kataoka Y., Tanano A., Cui Y., Ikeura T., Shimatani M., et al. Intrapancreatic axonal hyperbranching of dorsal root ganglia neurons in chronic pancreatitis model rats and its relation to pancreatic pain. Pancreas. 2006;33:268–279. doi: 10.1097/01.mpa.0000240600.72946.23. [DOI] [PubMed] [Google Scholar]
- 161.Ouelaa W., Ghouzali I., Langlois L., Fetissov S., Dechelotte P., Ducrotte P., et al. Gastric electrical stimulation decreases gastric distension-induced central nociception response through direct action on primary afferents. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Landrum L.M., Jones S.L., Blair R.W. The expression of Fos-labeled spinal neurons in response to colorectal distension is enhanced after chronic spinal cord transection in the rat. Neuroscience. 2002;110:569–578. doi: 10.1016/s0306-4522(01)00548-6. [DOI] [PubMed] [Google Scholar]
- 163.Nishida K., Matsumura S., Kobayashi T. Involvement of Brn3a-positive spinal dorsal horn neurons in the transmission of visceral pain in inflammatory bowel disease model mice. Front Pain Res (Lausanne) 2022;3 doi: 10.3389/fpain.2022.979038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Light A.R., Perl E.R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.