Abstract
The present review summarizes the beneficial and detrimental roles of reactive oxygen species in myocardial ischemia/reperfusion injury and cardioprotection. In the first part, the continued need for cardioprotection beyond that by rapid reperfusion of acute myocardial infarction is emphasized. Then, pathomechanisms of myocardial ischemia/reperfusion to the myocardium and the coronary circulation and the different modes of cell death in myocardial infarction are characterized. Different mechanical and pharmacological interventions to protect the ischemic/reperfused myocardium in elective percutaneous coronary interventions and coronary artery bypass grafting, in acute myocardial infarction and in cardiotoxicity from cancer therapy are detailed. The second part keeps the focus on ROS providing a comprehensive overview of molecular and cellular mechanisms involved in ischemia/reperfusion injury. Starting from mitochondria as the main sources and targets of ROS in ischemic/reperfused myocardium, a complex network of cellular and extracellular processes is discussed, including relationships with Ca2+ homeostasis, thiol group redox balance, hydrogen sulfide modulation, cross-talk with NAPDH oxidases, exosomes, cytokines and growth factors. While mechanistic insights are needed to improve our current therapeutic approaches, advancements in knowledge of ROS-mediated processes indicate that detrimental facets of oxidative stress are opposed by ROS requirement for physiological and protective reactions. This inevitable contrast is likely to underlie unsuccessful clinical trials and limits the development of novel cardioprotective interventions simply based upon ROS removal.
Keywords: Cardioprotection, Infarct size, Ischemic conditioning, Mitochondrion, Myocardial ischemia, Myocardial infarction, Reperfusion
Graphical abstract
Highlights
-
•
Ischemic heart disease is the most frequent cause of death worldwide.
-
•
Ischemia/reperfusion is the substrate of ischemic heart disease.
-
•
Cardioprotection reduces infarct size and coronary microvascular obstruction.
-
•
ROS are signals of cardioprotection and executors of injury.
-
•
Translation of cardioprotection to clinical benefits has been difficult so far.
-
•
The role of ROS in cardioprotection remains ambivalent.
Abbreviations
- Δp
proton motive force
- ABCB8
ATP binding cassette protein 8
- ADP
adenosine diphosphate
- ADSCs
adipose-derived stem cells
- AIF
apoptosis initiating factor
- Akt
protein kinase B
- ALG-CHO
partially oxidized alginate
- ALRs
absent in melanoma-2-like receptors
- AMI
acute myocardial infarction
- AMPK
adenosine monophosphate-activated protein kinase
- Apaf-1
apoptotic protease activating factor 1
- ATG
autophagy-related gene protein
- ATP
adenosine triphosphate
- Bax/Bak
Bcl-2 associated X protein/Bcl-2 homologous antagonist
- BMP
bone morphogenetic protein
- CABG
coronary artery bypass grafting
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CCS
chronic coronary syndrome
- CMR
cardiac magnetic resonance
- CoQ
coenzyme Q
- CPB
cardiopulmonary bypass
- CPK
creatine phosphokinase
- CSE
cystathionine γ-lyase
- CTO
chronic total occlusion
- cTn
cardiac troponin
- CypD
cyclophilin D
- DAMP
damage-associated molecular pattern
- DIC
dicarboxylate transporter
- DCM
diabetic cardiomyopathy
- EC
excitation-contraction
- ET-1
endothelin-1
- EV
extracellular vesicle
- FGF-2
fibroblast growth factor-2
- GFAT1
glutamine-fructose-6-phosphate transaminase 1
- GLUT
glucose transporter
- GPCR
G-protein coupled receptor
- GPX4
glutathione peroxidase-4
- GSDMD
gasdermin-D
- GSK3β
glycogen synthase kinase 3β
- GSSG
oxidized glutathione
- HBP
hexosamine biosynthetic pathway
- HGF
hepatocyte growth factor
- HIF-1
hypoxia-inducible factor 1
- HNO
Nitroxyl
- IGF-1R
insulin-like growth factor 1 receptor
- IMAC
inner mitochondrial anion channels
- IPC
ischemic preconditioning
- I/R
ischemia/reperfusion
- ISR
integrated stress response
- LC3
light chain 3
- LV
left ventricular
- NLR
leucine-rich repeat
- MACE
major adverse cardiovascular events
- MAM
mitochondrial-associated membrane
- MAO
monoamine oxidases
- MAPKs
mitogen-activated protein kinases
- MCT1
monocarboxylate transporter 1
- MCU
mitochondrial Ca2+ uniporter
- MIF
migration inhibitory factor
- MitoPQ
mito paraquat
- MLKL
mixed-lineage kinase domain-like pseudokinase
- MYDGF
myeloid-derived growth factor
- mTOR
mammalian target of rapamycin
- NaHS
sodium hydrosulfide
- Na2S
sodium sulfide
- NCX
Na+/Ca2+ exchanger
- NFkB
nuclear factor kappa B
- NHE
Na+/H+ exchanger
- NNT
nicotinamide nucleotide transhydrogenase
- NOX
NADPH oxidase
- NRF2
nuclear factor erythroid 2 related factor 2
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- PAMP
pathogen associated molecular pattern
- PCI
percutaneous coronary intervention
- PCr
phosphocreatine
- PI3K
phosphatidylinositol-3-kinase
- PMI
peri-procedural myocardial injury
- PostC
postconditioning
- PP1
protein phosphatase 1
- PPCI
primary percutaneous coronary intervention
- PRAS40
proline-rich Akt substrate of 40 kDa
- PTP
permeability transition pore
- Raptor
regulator-associated protein of mTOR
- RET
reverse electron transport
- RIC
remote ischemic conditioning
- RIPK
receptor-interacting serine/threonine-protein kinase
- RISK
reperfusion injury salvage kinases
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- SERCA
sarcoplasmic Ca2+ ATPase
- sEV
small EVs
- SGLT
sodium-glucose -linked transporter
- SOD
superoxide dismutase
- SPECT
single photon emission computed tomography
- SPRC
S-propyl-l-cysteine
- SR
sarcoplasmic reticulum
- SSH
S-sulfhydration
- STAT3
signal transducer and activator of transcription 3
- STEMI
ST segment elevation myocardial infarction
- STS
sodium thiosulfate
- SUCNR1
succinate receptor 1
- TCA
tricarboxylic acid
- TGF-β
transforming growth factor-β
- TRP
transient receptor potential
- TRADD
tumor necrosis factor receptor 1 -associated death domain protein
- TRAILR
tumor necrosis factor-related apoptosis inducing ligand receptor
- UCPs
uncoupling proteins
- UDMI
universal definition of myocardial infarction
- ULK-1
Unc-51-like kinase
- URL
upper reference limit
- VEGF
vascular endothelial growth factor
1. Cardioprotection – attenuation of myocardial ischemia/reperfusion injury
1.1. Cardioprotection - what does it mean?
Cardioprotection has been defined as “all mechanisms and means that contribute to the preservation of the heart by reducing or even preventing myocardial damage” [1]. This is a very broad definition which encompasses primary and secondary prevention, non-pharmacological and pharmacological conservative therapy, and interventional and surgical invasive therapy of all cardiac diseases from arrhythmias to coronary artery disease to valve disease and finally heart failure. While this definition is reasonable, it is conceptually very broad and pragmatically not very helpful. In a stricter sense, cardioprotection is therefore defined as prevention or reduction of myocardial injury from myocardial ischemia/reperfusion (I/R) [2]. Myocardial I/R is the pathophysiological substrate of ischemic heart disease which is still the most frequent cause of death worldwide [3]. In this sense, cardioprotection comprises the reduction of I/R injury not only to cardiomyocytes, but also to other cellular compartments, notably the coronary circulation. Also, cardioprotection may not only refer to reduction of acute injury to cardiomyocytes and the coronary circulation, i.e., infarct size and coronary microvascular obstruction, but also to inflammation, healing, repair and remodeling after such myocardial I/R injury, in particular when clinical outcome, notably mortality and heart failure, after follow-up is considered as endpoint. There is in fact a compelling medical need to develop therapies that protect the heart from cardiomyocyte loss. Death of cardiomyocytes is dramatic after myocardial infarction, when it can affect up to 25% of the approximately 4 billion cells in the left ventricle [4], but also accompanies most other cardiac conditions. These include disorders of cardiac overload (such as hypertension [5] or aortic stenosis [6]), viral myocarditis [7], Takotsubo syndrome [8] and peri-partum cardiomyopathy [9]. Cardiomyocyte death also accompanies virtually all forms of inherited cardiomyopathies, including Duchenne muscular dystrophy [10], Danon [11] and desmin [12] cardiomyopathies. There is evidence of cardiomyocyte loss in both dilated and hypertrophic cardiomyopathy [13] and in arrhythmogenic cardiomyopathy [14]. Finally, cardiac cell loss occurs during perioperative myocardial injury and reperfusion [15] and following cancer therapy, in particular using anthracyclines [16].
1.2. The clinical need for cardioprotection
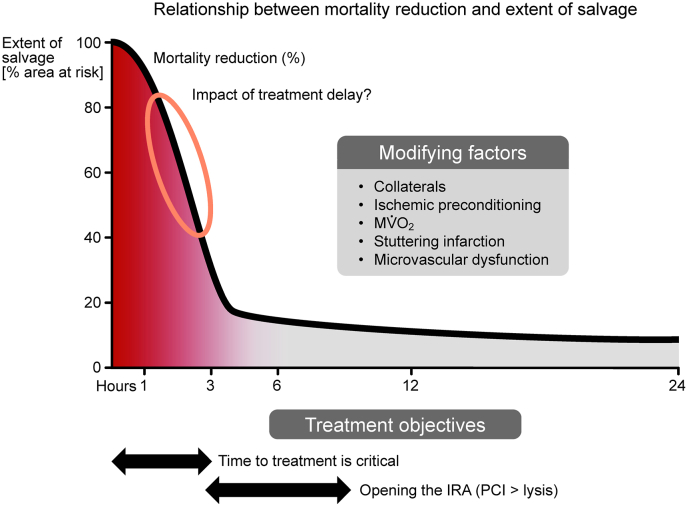
Cardiovascular disease is still the greatest health burden worldwide, and ischemic heart disease is still the most frequent cause of death worldwide [17]. The mortality in the first year following an acute myocardial infarction (AMI) remains at 15–21% in large European registries [18,19]. The modern era of reperfusion therapy, propelled in part by the seminal studies of Ross et al. [20,21] and Reimer and Jennings [22,23], demonstrating infarct size reduction by timely reperfusion and illustrating the gradual progression of necrosis after coronary occlusion in dogs, has resulted in a therapeutic revolution. Subsequent experimental studies demonstrated the significant benefits of standard reperfusion therapy on myocardial salvage but only an incremental benefit from the addition of physical interventions and pharmacologic agents which limit reperfusion injury [24]. A hypothetical construct illustrating the relationship between mortality reduction, myocardial salvage, and the duration of ischemia prior to reperfusion emphasized the narrow time window in which restoration of reperfusion could achieve significant salvage [25] (Fig. 1). This relationship between the slope of the curve and the extent of salvage could be altered by modifying factors such as ischemic conditioning interventions, the extent of the collateral circulation, myocardial oxygen consumption, stuttering infarction and microvascular dysfunction. A subsequent meta-analysis of 10 randomized control trials comprising 2632 patients emphasized the critical importance of infarct size for one-year prognosis after primary percutaneous coronary intervention (PPCI) [26].
Fig. 1.
Time course of myocardial salvage. With short duration of ischemia, reperfusion alone salvages almost all myocardium at risk. With a long duration of ischemia and late reperfusion, there remains little myocardium to be salvaged. There is a narrow time window for cardioprotective interventions beyond reperfusion (encircled). The time scale as such is influenced by a number of intervening variables (inserted box). IRA, infarct-related artery. From [25].
In regard to the blueprint for optimal care of the patients with ST segment elevation myocardial infarction (STEMI), we know what to do and the process begins with the establishment of a treatment protocol and a network [27]. Nonetheless there are many variables than can have a major impact upon the outcome, from delivery of reperfusion therapy to as many people and as quickly as possible. These include physical constraints such as geography, travel distance, weather, and access to ambulance transport as well as the coordination and availability of services both institutionally and regionally [28]. Despite a widespread understanding of the pivotal importance of time-to-reperfusion on the extent of myocardial salvage and the relationship between final infarct size and clinical outcomes the delivery of reperfusion therapy is subject to limitations and as such there remains an ongoing need for cardioprotection [29]. Several recent studies have drawn attention to difficulties in adhering to guidelines-directed times of treatment and the detrimental impact of treatment delay upon clinical outcomes [30,31]. A large study from a USA National Cardiovascular Data Registry of 22481 patients undergoing transfer to a PPCI-capable hospital with the median estimated inter-hospital drive time of 57 min emphasized that the majority had a first door-to-balloon time much longer than the guideline-recommended goal of less than or equal to 120 min, and the use of fibrinolytics as a pharmaco-invasive strategy in patients with longer drive times was disappointingly low [30]. These studies in high-income countries emphasize the logistical constraints imposed by geography, weather, and socio-economic disparities in healthcare. When these issues are faced in the context of low-income countries like Sub-Saharan Africa, the magnitude of the task and the obstacles to optimal reperfusion therapy are exceptionally challenging, and potentially the role of the cardioprotection is much greater [32] in that the cardioprotective effects of the pharmacological and non-pharmacological approaches may increase with the duration of ischemia. An example of the dramatic adverse impact of treatment delay on the outcomes of an invasive strategy for STEMI was highlighted by a prospective registry of patients in 55 interventional centers primarily in the United Kingdom and Europe during the initial days of the COVID pandemic [33].
From the perspective of cardioprotection, the road from the experimental laboratory to the clinical arena has been difficult [34,35]. Although the agenda for both pharmacologic and non-pharmacologic approaches has been extensive, the results overall have been unimpressive. The pathophysiology of I/R injury and microvascular dysfunction is complex and involves both cardiomyocyte and coronary vascular compartments [36]. Multiple molecular pathways are involved, and what is encouraging is the plethora of potential therapeutic targets, but a theoretical disadvantage is the potential for redundancy and alternative pathways. Only one clinical outcome trial (RIC-STEMI) has achieved its primary endpoint of reduced mortality and hospitalization for heart failure [37] although some studies have demonstrated positive signals in regard to surrogate endpoints including cardiac magnetic resonance (CMR) measurements of infarct size and microvascular function [2].
Obviously, there are differences between the experimental models and clinical studies [35]. Nonetheless, we believe we need to look further into the changing natural and unnatural history of STEMI and its impact on hard clinical endpoints. One example is provided by recent trials of remote ischemic conditioning (RIC). The basic science is well studied, logical and indeed exciting and cell-signaling pathways have been well defined as has evidence for multi-organ protection during cardiac and non-cardiac surgery [[38], [39], [40]]. Surrogate endpoints such as ST segment resolution, creatine phosphokinase (CPK) and cardiac troponin (cTn) levels, CMR estimates of infarct size, salvage and myocardial edema, N-terminal pro-brain natriuretic peptide (NT-proBNP) and admissions for heart failure have been positive in favor of RIC. This led to the large CONDI 2-ERIC/PPCI trial of 4637 patients which was neutral in regard to the 12-month outcome of cardiac death or heart failure hospitalization (9.4% with RIC vs. 8.6% in controls p = 0.32) [41]. This well-conducted trial was in a way a victim of its own success and provides an impressive example of what can be achieved in high-income countries in the contemporary era of PPCI. Cardiovascular mortality was less than 3%, and 96% of patients had no signs or symptoms of heart failure. This was an excellent trial, but it is easy to understand that irrespective of whether RIC improves myocardial salvage, the magnitude of the impact of this intervention in this population with a very low event rate is probably insufficient to change prognosis [28].
Nonetheless, there remains an unmet need for adjunctive cardioprotection, and particularly in sicker patients with hemodynamic complications, i.e. higher Killip classes, and less than optimal reperfusion and in patients in low- and middle-income countries among whom delayed presentation to hospital is frequent, access to invasive care is limited and there is a high incidence of untreated associated comorbidities. In this setting, RIC remains a highly promising, innovative, and biologically plausible strategy which needs to be tested in different clinical settings. Much needed trials and ongoing trials include the RIP-HIGH trial in patients with Killip class 2 or higher in Germany (NCT 04844931) and the RIC-AFRICA trial in South Africa, Sudan, Uganda, and Kenya [42].
Fig. 1 illustrates the critical interactions between the duration of ischemia and time to reperfusion and emphasizes that the window of opportunity for an intervention to exert a clinically significant prognostic impact is limited. For patients treated early in the course of AMI, it will be difficult to demonstrate a prognostic difference, and if treated late on the “flat” part of the curve it will be too late to make a difference. Perhaps ischemic conditioning can move the graph to the right and widen the window. The concepts are sound, the need for cardioprotection remains in many different clinical settings but the logistical constraints are formidable [43]. Ongoing trials will hopefully resolve many unanswered questions.
1.3. Cardiomyocyte ischemia/reperfusion injury – pathophysiology and targets for cardioprotection
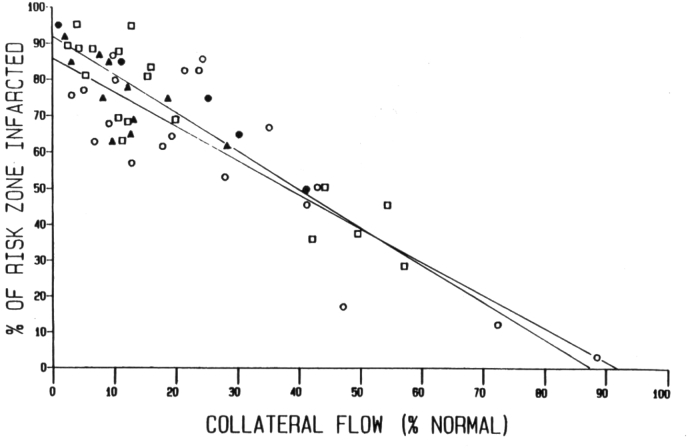
The myocardium is elegantly designed to deal with cyclical transient hypoxia of short duration that occurs with high intra-myocardial pressure during the systolic phase of the cardiac cycle. However, prolonged myocardial ischemia over many tens of minutes is non-physiological, for which there is no evolutionary adaptation in the mammalian heart. Four factors effectively define the final infarct size after coronary occlusion. First is the anatomical location of the coronary occlusion. Proximal left coronary occlusion is associated with both large myocardial infarct size and high lethality if left untreated. Second is the presence or absence of collateralization: the greater the collateralization of the ischemic zone, the more resistant the myocardium will be to coronary occlusion (Fig. 2). Third is the severity of the coronary occlusion: critical myocardial ischemia may occur even when there is some residual coronary flow (Thrombolysis in Myocardial Infarction (TIMI) flow greater than 0). And fourth, the ischemic time before restoration of coronary flow to the ischemic myocardium. Without reperfusion, the ischemic zone will progress to irreversible death, but reperfusion itself is not a benign process [2,36,44]. Up to 50% of the myocardial injury that occurs following reperfusion may be secondary to the reperfusion process itself [45]. Reperfusion can be divided temporally into three phases: (1) hyper-acute (the first 10–15 min), (2) acute (first 24 h) and (3) subacute (first 3 days). The hyper-acute phase of I/R injury is key to the final injury: the smaller the initial reperfusion injury, the less there will be of consequent acute and subacute inflammatory pathways.
Fig. 2.
Inverse relationship between infarct size and ischemic myocardial blood flow. Open symbols depict infarct sizes in dog hearts following 24 (circles) or 48 h (squares) of coronary artery branch occlusion. Closed symbols are from published studies by Reimer and Jennings with 96 h (triangles) or 4 h (circles) of ischemia. Infarct size varied widely because of a high degree of variability in collateral flow among the canine hearts [329].
Classically, myocardial infarction is described as unregulated cellular degradation and hypercontracture, that has disastrous consequences upon adjoining cells. Classically, necrosis is considered as a non-regulated form of cellular death, and this view is still true except for necroptosis which is described separately below. Contraction band necrosis will drive up local intramyocardial pressure, which in combination with myocyte swelling, will impact local coronary perfusion within the ischemic zone, serving to further intensify local myocardial ischemia. Concomitantly, necrotic cellular rupture will release cellular proteins into the extracellular milieu to propagate cell death via receptor-mediated pathways. However, necrotic cell death is not the sole mechanism of cellular injury response, but rather one bookend of cell damage that ranges from reversible injury through to unregulated necrotic cell death. The ischemic myocardium has a gradient of injury, dominated by necrosis at its core. The necrotic core is surrounded by a mantle of cells undergoing a stress response that may lead to a programmed form of cell death, with or without inflammatory consequences, intended to facilitate tissue repair. The pathophysiology of AMI is thus characterized by repurposed physiological processes designed to deal with cellular stress, trauma and pathogen exposure.
1.3.1. Ischemic stress signaling-enzymatic and mitochondrial
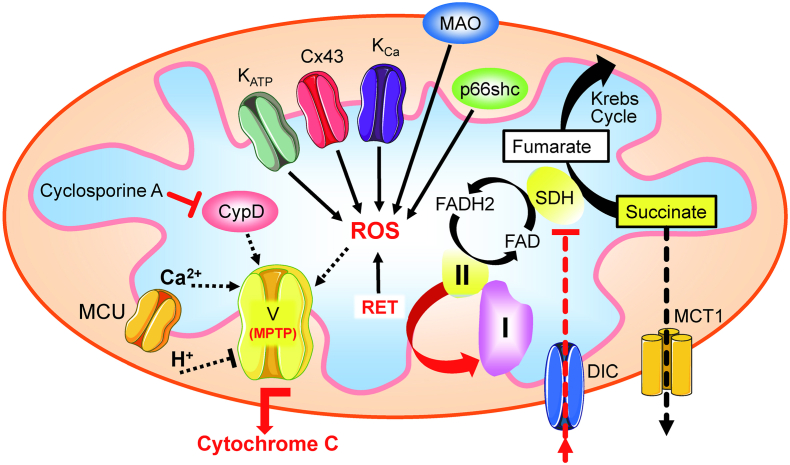
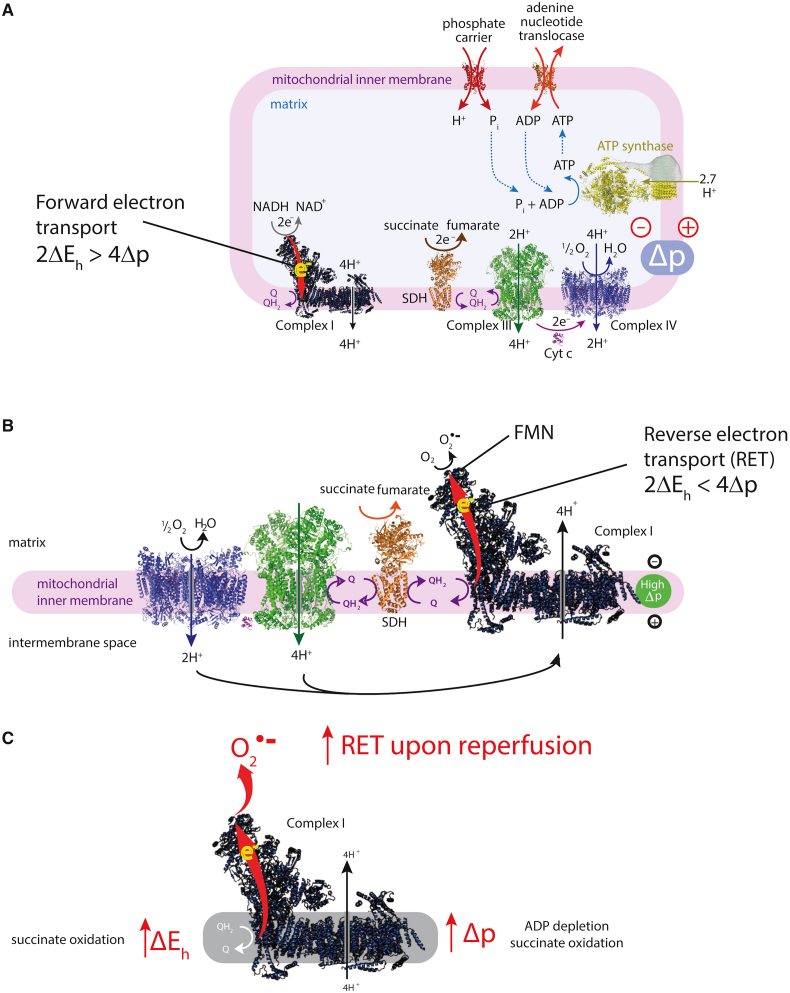
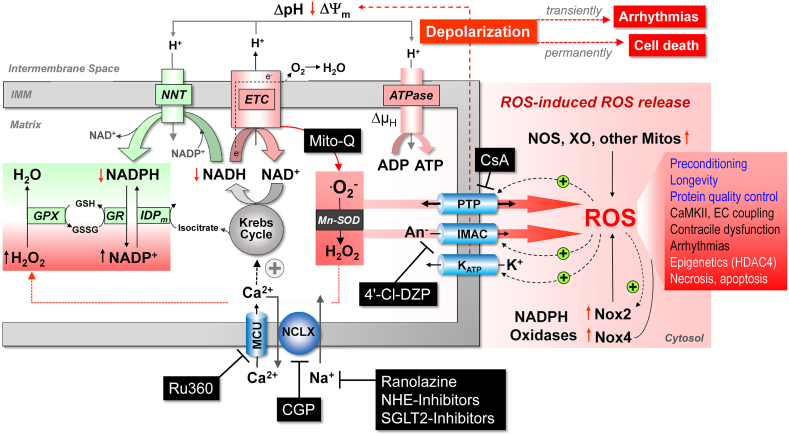
Following acute coronary occlusion, the myocardium is subjected initially to hypoxia and recruitment of acute stress responses that are initially cardioprotective. Hypoxia-inducible factor 1 (HIF-1) is the prototypical sensor of hypoxia that has both acute responses through promoting glycolytic and anaerobic metabolic changes that assist to reduce mitochondrial stress [46,47] and more chronically, differential gene expression through nuclear factor kappa B (NFkB). Another hypoxia/metabolic sensor is adenosine monophosphate-activated protein kinase (AMPK), which responds primarily to the prevailing AMP:ATP ratio but also to the intracellular calcium concentration via calcium calmodulin kinase kinase 2 (CaMKK2) [48]. There is cross -talk between AMPK and HIF-1 pathways that promote gene transcription of glucose transport via glucose transporters (GLUT) [49] and sodium-glucose -linked transporter 1 (SGLT1) [50], both of which are beneficial as the myocardium switches towards glucose metabolism. Such changes would be highly beneficial in the border zone of the ischemic myocardium, where hypoxia likely persists rather than progressing through to necrosis as found towards the center of the ischemic zone. As ischemic duration increases, more maladaptive processes will occur, through the generation of reactive oxygen species (ROS), either from enzyme systems such as NADPH oxidase (NOX) [51] or from mitochondria, such as reversed mitochondrial electron transport following succinate accumulation during ischemia, leading to ROS generation via complex I [52]. In addition to being an important source of ROS, the mitochondria are particularly vulnerable to ischemic stress, leading to organelle swelling and rupture and mediated through ROS- and mitochondrial calcium-triggered opening of the mitochondrial permeability transition pore (PTP). Rupture of the outer mitochondrial membrane then releases cytochrome-c into the cytosol. Mitochondrial fission may be an important step in the acute management of the hypoxic/ischemic stress response, breaking up mitochondrial tubule networks within cardiomyocytes to prevent rapid progression of mitochondrial rupture throughout a whole chain of coupled mitochondria [53]. Mitochondrial release of cytochrome-c is a key step in apoptotic cell signaling. Once wholesale cellular homeostasis collapses and cytosolic membranes are ruptured, the release of cellular contents into the extracellular milieu are recognized by adjacent cells as damage-associated molecular patterns (DAMPs) – that in turn may activate cell death pathways, apoptosis and pyroptosis.
Targeting these initiators of the hyperacute I/R response may therefore provide useful targets for promoting protection against I/R injury, such has adaptive signaling activation through HIF-1 α or AMPK [54] or though inhibiting injury processes, such as ROS formation from enzymatic sources and mitochondria [55], or through metabolic modification to prevent succinate accumulation and mitochondrial ROS generation [52]. Prevention of PTP formation is also a key final pathway to cellular death, with close interaction with programmed cell death pathways, and cyclophilin-D is one regulatory target to prevent PTP opening [56]. However, a multimodal approach may be more appropriate to optimize cellular survival, by regulating down-stream programmed forms of cellular injury [57].
1.3.2. Cell-stress response and cell-death mechanisms
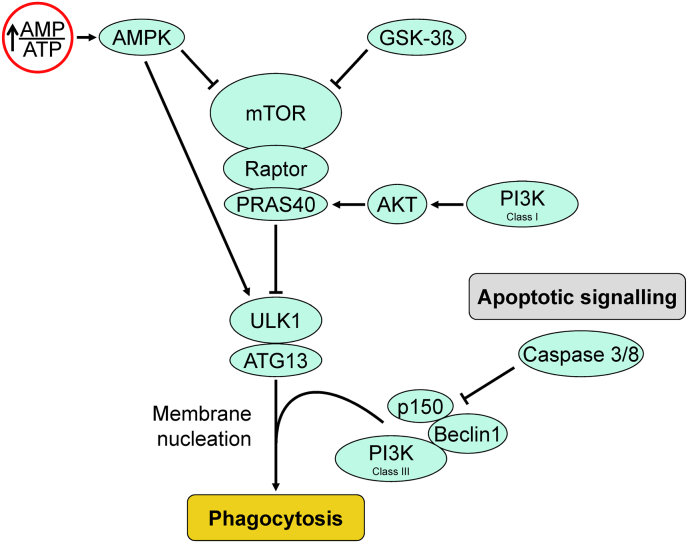
Autophagy is a component of cellular homeostasis and maintenance, recycling degraded proteins and cytoplasmic organelles, performing cellular hygiene tasks. Following an ischemic insult, autophagy is initiated through the inhibition of mammalian target of rapamycin (mTOR), which-in turn-is inhibited through phosphorylation by AMPK and glycogen synthase kinase 3β (GSK3β). Inhibition of mTOR releases its brake on the autophagic signal transduction and activates autophagy [58]. Downstream signaling leads to formation of double-membrane autophagic vesicles. Breakdown of the autophagic vesicles is through recruitment of lysosomal activity and forms complexes with beclin-1 and autophagy-related gene protein (ATG) and lead to P62 degradation (Fig. 3). This process is largely anti-inflammatory, safely compartmentalizing and removing damaged proteins and organelles such as mitochondria, before they activate cell death pathways (for example with cytochrome-c release and activation of the apoptosome through caspase-8 and apoptotic protease activating factor 1 (Apaf-1)) [2,59].
Fig. 3.
Autophagic signaling cascade. The autophagic process is predominantly regulated by the Unc-51-like kinase-1 (ULK1) and is facilitated through phosphorylation via class III PI3K and Beclin-1. Mammalian target of rapamycin (mTOR) inhibits autophagy and is itself inhibited by adenosine monophosphate kinase (AMPK) and glycogen synthase kinase 3β (GSK3β). PRAS40 = proline-rich Akt (protein kinase B) substrate of 40 kDa. ATG13 = autophagy related gene protein 13.
Autophagy, however, can be a double-edged sword. Excessive autophagy will lead to unnecessary self-digestion and cell death [60]. Vice versa, autophagic inhibition, either by apoptotic or necroptotic signaling (Fig. 3) or increased lysosomal zinc during I/R [61] may commit a potentially reversibly injured cell to an untimely programmed cell death. Thus the challenge is to optimize autophagic activity to balance the benefit of damaged organelle and protein removal against excessive premature removal that endangers the survival of a recoverable cell. Autophagy is a homeostatic mechanism to remove and recycle senescent cellular material and contributes to infarction only when excessive (autosis) [62].
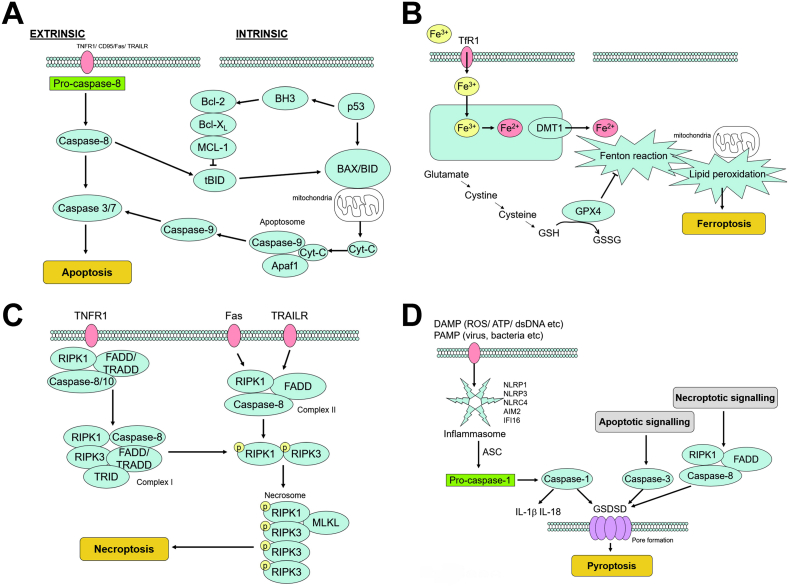
Apoptosis is characterized by cell shrinkage, chromatin condensation, plasma membrane blebbing without rupture, formation of apoptotic bodies and cytoskeletal disintegration. The apoptotic pathway can be initiated either extrinsically, through activation of cell surface receptors, tumor necrosis factor receptor 1 (TNFR1), CD95/FS-7-associated surface (Fas) antigen receptor and tumor necrosis factor-related apoptosis inducing ligand receptor (TRAILR), through cleavage of pro-caspase-8 and activation of caspase 3 (Fig. 4 A). The intrinsic pathway is initiated by the assembly of the apoptosome – a protein complex consisting of caspase-9, Apaf-1 and mitochondrial released cytochrome-c. This leads to the activation of the distal caspases in the final common pathway of apoptosis: caspase 3 (Fig. 4 A). The apoptotic pathway is itself regulated by the Bcl-2 family of proteins, that alternately lead to activation of apoptosis, through permeabilization of the mitochondrial outer membrane by oligomerization of Bax/Bak [63]. Anti-apoptotic Bcl-2 family members are up-regulated through their phosphorylation by salvage kinases, such as PI3K and Akt [64]. Apoptotic initiator protein p53 is another target for phosphorylation, through the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK1/2) pathway, which can also function to attenuate downstream caspase activation pivotal to apoptotic signaling; this signaling pathway is complex [65] and depending on the stress that the cell has been subjected to can be either pro- or anti-apoptotic.
Fig. 4.
A: Apoptosis signaling. Apoptosis can be triggered by either via either extrinsic or intrinsic pathways. The former is typically triggered by the tumor necrosis factor receptor (TNFR), CD95/Fas or the tumor necrosis factor-related apoptosis inducing ligand receptor (TRAILR). The intrinsic pathway utilizes the tumor suppressing protein, p53, that may activate pro-apoptotic Bcl-2 family members, such as Bax and Bid. B: Ferroptosis signaling. Ferroptosis in myocardial ischemia-reperfusion is a consequence of ROS generation resulting from the Fenton reaction of ferric iron and mediated through down-regulation of glutathione peroxidase-4 (GPX4) during ischemia and reperfusion. The resulting lipid peroxidation leads to severe mitochondrial damage. C: Necroptosis signaling. Extrinsic activation of necroptotic signaling pathway occurs via similar receptors that also result in apoptosis: TNFR, Fas and TRAILR. However, the key difference is the recruitment of receptor-interacting serine/threonine-protein kinase (RIPK) RIPK1 and RIPK3 and facilitated through mixed lineage kinase domain like pseudo kinase (MLKL). D: Pyroptosis signaling. Pyroptosis is a response to damage associated molecular patterns (DAMPs) and pathogen associated molecular patterns (PAMPs) that signal to lead to the formation of the inflammasome that consists of sensor proteins and through association with apoptosis-associated spec-like protein containing a caspase activation and recruitment domain (ASC) to form a focus within the cell, and then recruit and activate caspase-1, ultimately leading to cytosolic perforation by gasdermin-D (GSDMD). Interestingly, apoptosis, through caspase-3, and necroptosis, through complex II (RIPK1/FADD/caspase-8), also lead to oligomerization of GSDM.
Importantly, apoptosis is largely “non-inflammatory” – the apoptotic cell is presented for phagocytosis by macrophages without inducing an inflammatory response, a process that contrasts with the pro-inflammatory phagocytosis of necrotic cells [66]. However, apoptotic signaling may interfere with helpful autophagy and commit a potentially reversibly injured cell to unnecessary death. Given this mixed picture it is useful to note that broad caspase inhibition attenuates I/R injury [67]. Thus targeting caspase may be beneficial in attenuating apoptosis and other cell death pathways, such as pyroptosis, to promote survival. Apoptosis is a “silent” form of cell death without an inflammatory reaction.
Ferroptosis is a relatively novel mechanism of cell death that is distinct from the other modes of cell death discussed here, leading to a unique histological sequence of mitochondrial shrinkage, increased mitochondria membrane density, cristae destruction and outer mitochondrial membrane rupture. These changes however occur in the absence of nuclear morphological change, whereas mitochondrial rupture is likely to lead to apoptotic or pyroptotic signaling activation. In I/R injury, ferroptosis is largely driven by down-regulation of glutathione peroxidase-4 (GPX4) [68], oxidizing glutathione (GSH) and generating ferric (Fe2+) iron from the Fenton reaction. This will lead to lipid peroxidation and ferroptotic mitochondrial injury (Fig. 4 B). Ferroptotic ROS generation may, therefore, contribute to myocardial cell death, and interestingly, iron chelators have been shown to improve myocardial survival [69]. Thus, cardiac iron may be a target for novel cardioprotective strategies, attenuating ROS burden of I/R injury [70]. Ferroptosis derives its name from iron-catalyzed reactions but is essentially characterized by a defect of glutathione peroxidase-4 and consequent lipid peroxidation.
Necroptosis has some signaling similarities with apoptosis, but the cellular fate is quite different, leading to cytoplasmic and organelle swelling, formation of the necrosome, plasma membrane rupture and release of cellular constituent histological ends that appear more similar to necrotic cell death. The involvement of death receptor activation (TNFR1, Fas and TAILR) leads to activation of receptor-interacting serine/threonine-protein kinase (RIPK) RIPK1, FAS-associated death domain protein (FADD)/TNF receptor type 1-associated death domain protein (TRADD) and caspases 8/10. This protein complex in turn leads to formation of the necrosome complex to induce necroptosis (Fig. 4C) [71]. Necroptosis is characterized by ROS formation, random degradation of DNA and DAMP release. Class 1A isoforms of PI3K are likely involved in RIPK1/RIPK3 signaling pathway activation [72], but the specific isoforms involved with this signaling are not yet known. In addition to the canonical activation pathway, necroptosis can also be instigated by the recruitment of Ca2+/calmodulin-dependent protein kinase II (CaMKII) that itself may be a target for phosphorylation and activation by RIPK3 [73].
Necroptosis is etymologically a cellular response to microbial infection and intended to drive a host immune response, but within the context of the pathophysiology of I/R injury, this may be a less helpful response; the release of DAMPs will extend the wave of cell death within the necrotic core of the ischemic zone. Inhibition of RIP1 signaling by necrostatin-1 has been shown to attenuate I/R injury [74], and phase 2 safety and efficacy trials in chronic inflammation have been undertaken of a small-molecule RIP1/RIP1K inhibitor, GSK2982772 [75] – but thus far, this inhibitor has not tested in the cardiovascular system. Necroptosis shares most features with necrosis but is regulated with involvement of RIP1 and can be specifically inhibited.
Pyroptosis is the prototypical response to DAMP and pathogen associated molecular pattern (PAMP) signaling, characterized histologically by cytoplasmic swelling, formation of pyroptotic bodies, plasma membrane rupture, release of cell contents and without loss of mitochondrial integrity. The pyroptotic inflammasome is a heterodimer of sensor molecules, that cleaves pro-caspase-1 (Fig. 4 D). Caspase-1 leads to the release of inflammatory interleukins IL-1β and IL-18, by polymerizing gasdermin-D (GSDMD) to form a pyroptotic perforating pore in the cytoplasm, leading to cellular lysis [76]. The pyroptotic GSDMD pore can also be induced through alternate pathways, involving capase-3 activity and via RIPK1/FADD/capase-8 that are involved in apoptotic and pyroptotic pathways, respectively. These proteolytic pathways illustrate the interplay of death pathways during I/R injury, and like necroptosis, pyroptosis is a pathogen response pathway to microbial infection that is not ideal in the context of I/R injury. Inhibiting proteases and the formation of the GSDMD pore are the most obvious targets for therapeutic intervention and indeed, GSDMD knockout has been shown to reduce I/R injury in mouse models [77]. Pyroptosis in an inflammatory form of cell death and interacts with inflammatory cells possibly to progressively extend the borders of the infarcting myocardium.
1.3.3. Targets for intervention
Identifying the characteristics of cell death during the hyperacute phase of I/R injury is a vital step in identifying potential targets for therapeutic intervention. To date, much of the research interest has concentrated on the canonical conditioning pathway, via the reperfusion injury salvage kinase (RISK) group of signaling pathways that include PI3K/Akt, MAPK/ERK(1/2) and downstream targets inclusive of mTOR and the mitochondria, specifically the mitochondrial PTP via cyclophilin-D regulation [78]. The survivor activating factor enhancement (SAFE) pathway involving the transcription factor, signal transducer and activator of transcription 3 (STAT3) is an alternate pathway that also targets mitochondrial viability [[79], [80], [81], [82], [83], [84], [85]].
Traditional single occasion RIC administered by 4 times of 5 min intermittent limb occlusion acts through the RISK pathway, which includes the PI3K-Akt-PKC-ERK signalling cascade, as documented in a variety of species, including rodents and pigs [86,87] and even with transferability across species [82]. Studies in isolated mice hearts have demonstrated that RIC also initiates autophagy [88]. The initial activation of the autophagosome during the first window of protection alleviates the damage of I/R injury and supports cell function by clearing damaged protein aggregates, by removing damaged ROS-producing mitochondria and through the recycling of macromolecules for use in cell repair [88]. A study of exosomes from rats has shown that this response may appear as long as 48 h after the RIC stimulus [89]. Preservation of post-ischemic cardiac function, measured by post-ischemic LV end-diastolic and developed pressure, is similar by acute, delayed and repeated RIC in experimental studies of rats and mice [88,90,91].
PI3K is an obvious upstream target for cardioprotection, involved in cellular survival pathways. However, its role is complex: class I PI3K is intrinsically linked to activation of pyroptotic mechanisms, whereas class III PI3K is associated with autophagy. The key to unlocking the potential of PI3K as a target for cardioprotection is the recognition of not only the different classes of PI3K, but also the different isoforms within each class. For example, there are a number of PI3K class I p110 subunit isoforms (α, β, ꝩ), which have the potential for differential regulation of cell-survival pathways. The α isoform has been linked to acute cardioprotection [92], and Gong et al. have reported on the discovery of UCL-TRO-1938, a small molecule activator of the PI3Kα isoform that provides significant cardioprotection against I/R injury [93]. Similarly, ROS are intrinsic to hyperacute injury, and managing ROS generation from enzymatic processes, such as from NOX, mitochondrial complex I or from dysregulated ferric iron Fenton reactions will also represent important therapeutic targets for cardioprotection. Beyond these initiator processes, targeting and modifying programmed cell death provide additional targets for protection. Pyroptotic cell death and cells destined for necroptosis are likely to be key in these instances – thus, preventing the formation of the GSDMD pore or inhibiting RIPK1/RIPK3 activation are likely key for the attenuation of acute inflammatory myocardial injury.
However, it may be too simplistic to target just one pathway and expect this to translate to significantly reduced myocardial injury in man given human heterogeneity, comorbidities and concomitant drug use [57,94]. However, a multi-modal approach to inhibit cellular rupture pathways and promote cellular survival in both the hyperacute phase and through secondary acute genomic transcription of pro-survival proteins would seem to be the optimal approach to future clinical translation.
1.3.4. Confounders of myocardial ischemia/reperfusion injury and cardioprotection
Confounders of myocardial I/R injury and cardioprotection have just recently been reviewed in great detail [95], therefore only briefly in here. Aging [96] and the classical risk factors of hypertension [97], hyperlipidemia [98] and diabetes [99] not only predispose to the development and progression of coronary atherosclerosis, but also sensitize the myocardium to I/R injury and interfere with cardioprotective signal transduction. Female sex before menopause appears to protect from I/R injury in most rodent models [100], but in pigs there is no difference in I/R injury per se and in protection by ischemic preconditioning (IPC) [101]. Patients with coronary artery disease and acute myocardial I/R typically have a number of medications which can interfere with cardioprotection; some of them protect per se and may limit the potential for further protection, e.g. nitroglycerine, morphine, P2Y12 inhibitors [102], some of them interfere with cardioprotective signaling, e.g. sulfonylureas [103]. Whether or not such interference with cardioprotection is really of clinical importance, is still largely uncertain [104].
1.4. The coronary circulation as culprit and target of myocardial ischemia/reperfusion injury
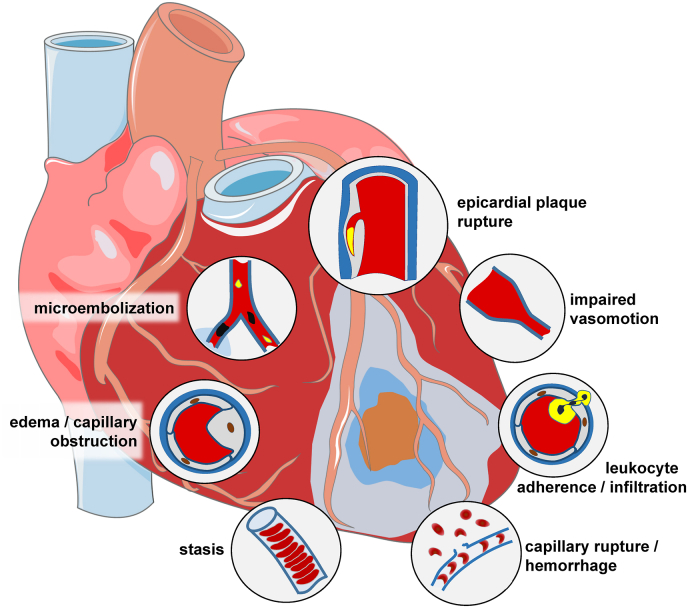
Atherosclerosis of the coronary circulation is the cause of myocardial ischemia which is best defined as a critical reduction of coronary blood flow such that the physiological electrical and contractile processes and ultimately cellular integrity and viability are no longer maintained [105]. The coronary circulation is both a culprit and a target of myocardial I/R. In acute myocardial infarction, the rupture of an epicardial coronary atherosclerotic plaque initiates immediate intravascular thrombosis and occlusion of the affected coronary artery at the atherosclerotic lesion site [106]. More recently, particularly with the increasing use of statins, plaque erosion is becoming more frequent [107], and plaque erosion more often causes coronary microembolization [108] and non-STEMI [109]. With underlying atherosclerosis and acute plaque rupture or erosion, the epicardial coronary circulation is clearly a culprit of myocardial ischemia. More recently, awareness has increased that also coronary microvascular disease in the absence of significant epicardial coronary obstruction can initiate acute myocardial infarction, and this occurs more frequently in women than in men [110,111]. Such coronary microvascular disease is characterized by endothelial dysfunction, enhanced vasoconstrictor responsiveness and reduced coronary dilator reserve on an adenosine challenge, and it is diagnosed by a combination of imaging procedures [111,112]. The role of the coronary circulation as a victim of myocardial I/R has long been neglected but is now clear and receiving increasing attention [113,114]. I/R injury to the coronary circulation affects predominantly the microcirculation, both endothelial and vascular smooth muscle cells, and has multiple mechanisms. Preclinical experimental studies have elaborated on increased vascular permeability and edema formation [115], impaired vasomotion notably as a consequence of endothelial dysfunction [116] and release of soluble vasoconstrictor substances from epicardial coronary lesions [117], adherence of platelets and leukocytes to the endothelium [118], formation of platelet aggregates [119] and erythrocyte stasis [120], microembolization of particulate atherosclerotic and thrombotic material from the epicardial coronary lesion [108], and ultimately capillary rupture [121] with hemorrhage into the interstitium [122]. The manifestations of coronary microvascular injury can be reversible (edema, impaired vasomotion, intravascular cell aggregates) or irreversible (particulate embolization, capillary rupture, hemorrhage) (Fig. 5). Clinically, the impairment of the coronary circulation during reperfusion following myocardial ischemia is known for a long time from interventional procedures for stable coronary artery disease and from thrombolytic and interventional reperfusion for AMI. The diagnosis and quantification of coronary vascular impairment in these settings has traditionally been made from angiographic indices (TIMI flow grade, TIMI frame count, myocardial blush grade) or intravascular Doppler flow velocity and flow velocity reserve [123,124]. The reference standard for the assessment of coronary microvascular impairment is now CMR, which – along with infarct size and contractile function – can quantify magnitude and spatial extent of edema, no-reflow, and hemorrhage [125]. It is now evident, that in fact coronary microvascular obstruction – beyond infarct size – is a major determinant of the prognosis for patients with reperfused AMI [126], as is intramyocardial hemorrhage [127]. These recent data make coronary microvascular obstruction a worthwhile target for clinical attempts of cardioprotection.
Fig. 5.
Schematic presentation of mechanisms contributing to coronary microvascular injury with ischemia/reperfusion. From [113].
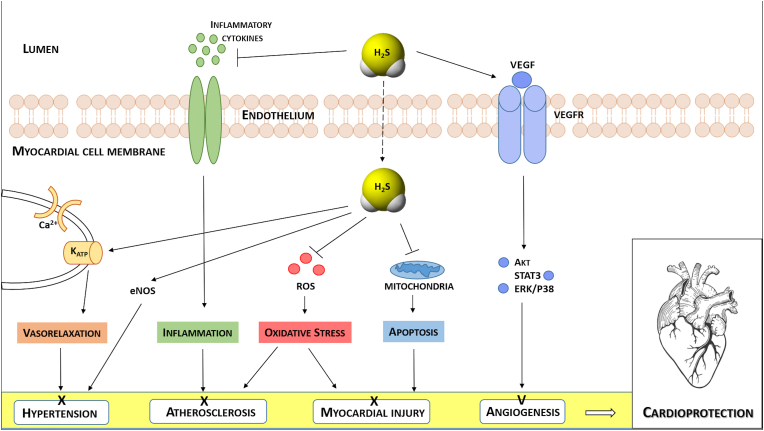
The role of ROS in the coronary circulation displays the typical concentration-dependent ambivalence - signaling function at lower concentrations and injury at excessive concentrations [128,129]. ROS, notably hydrogen peroxide (H2O2), are released from human coronary arterioles and mediate flow-dependent dilation [130], thus exhibiting a physiological function; NOX and endothelial mitochondria contribute to this ROS release [131,132]. However, in myocardial I/R ROS contribute to coronary microvascular injury [133]. The specific contribution of ROS to coronary vascular vs. cardiomyocyte injury from I/R cannot be distinguished at this point [113]. Specific targeting of mitochondrial ROS formation by use of transgenic mice with endothelial manganese-dependent superoxide dismutase improved angiogenesis, decreased scar size and preserved left ventricular (LV) function after permanent coronary occlusion [134].
In preclinical experiments, all of the above mechanisms of coronary microvascular injury could be successfully targeted, and the respective injury could be attenuated, typically by mechanical interventions of ischemic pre- and post-conditioning and RIC, but also by drugs relating to the signal transduction of ischemic conditioning procedures [2,135]. In fact, IPC reduced edema, endothelial dysfunction, leukocyte adherence and coronary microvascular obstruction, and it improved coronary vasomotion. Ischemic postconditioning attenuated edema and endothelial dysfunction, and it reduced no-reflow. RIC reduced no-reflow along with infarct size [101]. Some drugs also attenuated no-reflow along with infarct size, and angiopoietin-like peptide 4 appears to have a specific protective action on the coronary microcirculation [136]. Ischemic preconditioning is not feasible in patients with AMI, but a number of studies using CMR in patients with reperfused AMI have demonstrated that ischemic postconditioning [[137], [138], [139]] and RIC [140,141] attenuated edema, no-reflow and intramyocardial hemorrhage, sometimes along with infarct size reduction, but sometimes not [114]. In a post-hoc analysis of METOCARD, metoprolol also reduced coronary microvascular obstruction, as assessed by CMR, in 106 vs. 114 control patients with reperfused STEMI [142]. It has been suggested that the consideration of the coronary microcirculation as a site of I/R injury and consequently as a target of cardioprotection will improve the translation from preclinical studies to clinical practice [2,36,143]. The remodeling process following reperfusion of myocardial infarction and eventually leading to heart failure [24] also involves the coronary circulation, notably through angiogenesis [144], and coronary blood flow again impacts on heart failure and vice versa [145].
1.5. Percutaneous coronary intervention in chronic coronary syndrome
For many patients with obstructive coronary artery disease, percutaneous coronary intervention (PCI) remains the major revascularization strategy of choice, with an estimated 5 million procedures performed worldwide each year [146]. A substantial number of chronic coronary syndrome (CCS) patients undergoing elective PCI experience procedural-related myocardial injury and infarction, the occurrences of which are associated with an increased risk of future major adverse cardiovascular events (MACE) such as death, re-infarction, and revascularization [147,148]. As such, PCI-related myocardial injury and infarction are important targets for cardioprotection especially for those undergoing complex higher-risk PCI procedures.
PCI-related myocardial infarction or type 4a MI has been defined by the Fourth Universal Definition of MI (UDMI) [149] as a post-PCI elevation of cTn >5 × 99th percentile upper reference limit (URL) within 48 h of the PCI procedure in patients with normal baseline (pre-PCI) values and associated ECG/imaging/angiographic evidence of new myocardial ischemia. However, post-PCI elevations in cTn in the absence of new evidence of myocardial ischemia are indicative of peri-procedural myocardial injury (PMI). The Fourth UDMI has defined PMI as any post-PCI elevation of cTn >1 × 99th URL in patients with normal baseline values [149]. The prognostic relevance of such definition has been questioned, although recent data suggest that a post-PCI cTn cut-off elevation of >5 × 99th percentile URL is the optimum threshold for independently predicting all-cause mortality at one year in terms of sensitivity and specificity [147].
The cause of PMI and type 4a MI is multifactorial and may be due to side-branch occlusions, distal coronary embolization of intracoronary thrombus and atheromatous material [108], coronary vasospasm due to neuro-hormonal activation, and other PCI-related factors such as pre-dilation, partially occlusive devices (such as catheter extension devices, retrograde chronic total occlusion (CTO) procedures, atherectomy devices), which can result in prolonged total vessel occlusion times [147].
Several cardioprotective strategies have been evaluated for their ability to reduce PMI and type 4a MI in CCS patients when administered prior to PCI although the endpoints for cardioprotection which have been used have varied from study to study (Table 1, Table 4) [[150], [151], [152], [153]]. One of the most promising interventions are high-dose statins (e.g., atorvastatin 80 mg or rosuvastatin 40 mg) which when administered prior to PCI have been reported to reduce the risk of PMI, type 4a MI, and MACE in CCS patients [[154], [155], [156], [157], [158]], although not all studies have been positive [159,160]. The mechanisms of cardioprotection as identified from pre-clinical animal studies appear to be pleiotropic, involving the upregulation of cytoprotective pathways including RISK, decreased inflammation, inhibition of platelet aggregation, improvement of endothelial function, and plaque stabilization [161,162].
Table 1.
Therapeutic strategies to prevent periprocedural myocardial injury and type 4a myocardial infarction in chronic coronary syndrome patients.
| Agent | Timing of administration | Potential mechanism of action | Findings |
|---|---|---|---|
| High-dose Statins | Pre-PCI | Pleiotropic effect on inflammation [161,162] Production of endothelial progenitor cells [162] |
Several RCTs have reported ↓ incidence of periprocedural myocardial injury and type 4a MI [[154], [155], [156], [157]]. A meta-analysis of 14 trials reported ↓ incidence of periprocedural myocardial injury, type 4a MI and MACE (death, re-infarction and revascularization [158]). However, neutral effects in some studies [159,160]. |
| Cangrelor | At the time of PCI (intravenous) | Intravenous P2Y12 platelet inhibitor | Large multicentre RCT (CHAMPION PHOENIX) of 11,145 CCS patients reported ↓ incidence of periprocedural myocardial injury and type 4a MI when compared to clopidogrel [150] |
| Remote ischemic conditioning | Pre-PCI (3–4 5-min cycles of limb ischemia/reperfusion) | Reduces acute myocardial ischemia-reperfusion injury | Mixed results with some positive studies reporting ↓ incidence of periprocedural myocardial injury and type 4a MI [[163], [164], [165]], with other studies showing no reduction in PMI [166] One follow-up RCT of 225 CCS patients showing ↓ incidence of MACE (not powered for clinical outcomes) [167] Meta-analysis of 11 studies showed no overall benefit with RIC [168] |
| Vitamin C | Pre-PCI (IV infusion) | Anti-oxidant effects | RCT of 532 CCS patients showing ↓incidence of periprocedural myocardial injury [151] RCT of 56 CCS patients showed ↑microcirculatory reperfusion [152]. |
| Enalaprilat | At the time of PCI (intracoronary) | Endothelium-dependent epicardial coronary vasodilation mediated by endogenous bradykinin activity | RCT of 40 CCS patients showing ↓ incidence of periprocedural myocardial injury [153] |
| Colchicine | At the time of PCI | Anti-inflammatory effects | RCT [172] of 400 CCS did not show any impact on PCI-related myocardial injury. RCT of 5545 CCS patients showed 31% reduction in cardiovascular death, spontaneous (nonprocedural) myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization. However, the incidence of death from noncardiovascular causes was higher in the colchicine group than in the placebo group (HR 1.51) [171]. |
Table 4.
Clinical trial acronyms.
| AMI HOT I, II [[263], [264]] | Acute myocardial infarction with hyperoxemic therapy |
| ASSAIL-MI [242] | Assessing the effect of anti-IL6 treatment in myocardial infarction |
| CHAMPION PHOENIX [150] | A clinical trial comparing cangrelor to clopidogrel standard of care therapy in subjects who require PCI |
| CHILL-MI [257] | Efficacy of endovascular catheter cooling combined with cold saline for treatment of acute myocardial infarction |
| CIRCUS [232] | Cyclosporine A in reperfused acute myocardial infarction |
| CONDI 1 [219] | Effect of remote ischemic conditioning during evolving ST-elevation myocardial infarction |
| CONDI 2 ERIC/PPCI [41] | Effect of remote ischemic conditioning on clinical outcome in patients with acute myocardial infarction |
| COOL AMI [259] | Trial to assess cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction |
| CYCLE [233] | Cyclosporine A in reperfused acute myocardial infarction |
| EARLY-BAMI [245] | Early intravenous beta blocker in patients with ST-segment elevation myocardial infarction before PPCI |
| EMBRACE-STEMI [234] | Trial to evaluate safety, tolerability and efficacy of intravenous bendavia on reperfusion injury in patients treated with standard therapy including PCI and stenting for STEMI |
| ERICCA [193] | Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing CABG surgery |
| GIPS-IV (NCT 02899364) | Groningen intervention study for the preservation of cardiac function with sodium thiosulfate after STEMI |
| LIPSIA-CONDITIONING [222] | Leipzig cardioprotection by combined intrahospital remote ischemic preconditioning and postconditioning in ST-elevation myocardial infarction |
| LIPSIA-N-ACC [239] | Leipzig immediate PCI acute myocardial infarction N-ACC trial |
| METOCARD-CNIC [244] | Effect of metoprolol on infarct size in ST-segment elevation myocardial infarction undergoing PPCI |
| MITOCARE [235] | Mitochondrial care in acute myocardial infarction |
| NACIAM [240] | N-acetylcysteine in acute myocardial infarction |
| NIAMI [236] | Intravenous sodium nitrite in acute ST-elevation myocardial infarction |
| NOMI [238] | Nitric oxide inhalation in ST-elevation myocardial infarction |
| PROTECTION-AMI [231] | Inhibition of delta protein kinase C for the reduction of infarct size in acute myocardial infarction |
| RAPID-MI-ICE [258] | Rapid intravascular cooling in myocardial infarction as adjunct to PCI |
| RESILIENCE (NCT05223413) | Remote ischemic conditioning in lymphoma patients receiving anthracyclines |
| RIC-AFRICA [42] | Remote ischemic conditioning in African patients with myocardial infarction |
| RIC-STEMI [37] | Remote ischemic conditioning in ST-segment elevation myocardial infarction as adjuvant to primary angioplasty |
| RIP-HEART [198] | Remote ischemic preconditioning in heart surgery |
| RIP-HIGH (NCT04844931) | Remote ischemic preconditioning in high risk myocardial infarction |
| SHOCK-COOL [261] | Mild hypothermia in cardiogenic shock complicating myocardial infarction |
| STEMI-DTU (NCT03947619) | Door to unloading with Impella system in acute myocardial infarction |
| STOP-CA (NCT 02943590) | Statins to prevent the cardiotoxicity from anthracyclines |
Other cardioprotective interventions which have been investigated in CCS patients undergoing PCI include RIC although the results have been mixed [[163], [164], [165], [166], [167]], and a recent meta-analysis of 11 studies found no overall beneficial effects in terms of reduced PMI as assessed by elevated circulating cTn levels [168]. A small clinical study has investigated the combined effects of high-dose atorvastatin and RIC and reported additive cardioprotective effects with reduced PMI when compared to high-dose statin alone [169]. A recent study has reported beneficial effects with RIC reducing PMI in patients undergoing PCI with drug-coated balloons, an intervention associated with prolonged angioplasty inflation times and reduced risk of restenosis [170].
More recently, low-dose treatment with the anti-inflammatory agent, colchicine, has been reported to reduce mainly ischemia-driven clinical events in CCS patients [171]. However, pre-treatment of CCS patients with high-dose colchicine prior to PCI failed to reduce the incidence of PMI, type 4a MI, or Society of CardiovascuIar Angiography and Intervention-defined PMI, when compared to placebo [172]. Whether or not post-PCI treatment with low-dose colchicine can reduce major adverse cardiovascular events in CCS patients experiencing type 4a MI post-PCI is not known. It must be noted that the incidence of death from non-cardiovascular causes was higher in the low-dose colchicine group than in the placebo group (hazard ratio 1.51) [171].
Since the occurrences of PCI-related myocardial injury and type 4a MI in CCS patients are associated with worse clinical outcomes, this form of injury is an obvious therapeutic target for cardioprotection and improving patient outcomes especially in patients undergoing complex PCI procedures. However, the multi-factorial nature of PCI-related myocardial injury and type 4a MI may, in part, explain why it has been challenging to demonstrate effective cardioprotection against this form of injury.
1.6. Cardioprotection in coronary artery bypass grafting
With the advent of the cardiopulmonary bypass (CPB) [173], surgeons were able to perform more complex operations such as those on the aorta and coronary artery bypass grafting (CABG). Surgeons generally need to arrest the heart in order to perform the operation. Cardiac arrest is performed with the delivery of a hyperkalemic solution (cardioplegia) to reduce its metabolic rate after commencing CPB in order to maintain perfusion to the body while the heart and lungs are not moving. Despite its routine use and safety in most cases, cardioplegia and CPB are not without potential adverse consequences. Cardioplegia protects the heart from ischemic injury, reperfusion injury and inflammation, but myocardial injury or even infarction occur in some cases despite the surgeon's best efforts to prevent it. CPB and cardioplegia do not lessen surgical trauma and do not prevent graft failure, valve repair failure, intraoperative aortic dissection or other catastrophic events that sometimes occur and carry a poor outcome.
CPB and cardioplegia and the subsequent myocardial reperfusion have been associated with intraoperative and postoperative myocardial and microvascular dysfunction [[174], [175], [176]]. Hypothermia was introduced to further decrease myocardial metabolism during cardiac arrest and further improve outcomes [173]. Numerous studies in animal models and subsequent clinical trials have validated the principles of depolarizing hypothermic potassium cardioplegia [[177], [178], [179], [180]].
Since its invention [181], there have been many refinements of CPB to accompany cardioplegic arrest, including coatings of the bypass circuit to reduce cellular activation and thrombosis, filters, and more efficient oxygenators. Today, hypothermic, hyperkalemic cardioplegia under conditions of CPB is a routine, integral tool for cardiac surgeons performing a variety of cardiovascular operations. Despite its effective use in most cases, myocardial stunning [182] and injury [183,184] occur to some extent in most cardiovascular operations. The administration of hyperkalemic cardioplegia solution decreases myocardial oxidative stress [180]. Increased oxidative stress by myocardial ischemia, cardioplegia and CPB may affect levels of nitric oxide and other oxidative moieties and affect tissue integrity and enzyme function [[184], [185], [186]]. The restoration of blood flow to ischemic tissue after termination of cardioplegia and CPB further stimulates pathways involving oxidative processes, leading to the further generation of ROS [[184], [185], [186]].
Despite variability in composition, delivery and temperature, most cardioplegic solutions in use today involve some level of potassium chloride as the main inducer of cardiac arrest, along with ions such as magnesium, low-dose calcium and a pH buffer such as bicarbonate. After an initial delivery to arrest the heart, the cardioplegia solution is then given every 15–20 min due to “wash out” of the solution. Blood is often used as a buffer in a 4:1 blood to cardioplegia ratio, but the optimal cardioplegia remains debated [187,188]. The standard “modern” cardioplegic solution consists of a hyperkalemic (15–25 mmol/l), hypothermic (4–8 °C) crystalloid solution mixed in a 1:4 ratio with blood from the patient. A low concentration of magnesium is often added to limit calcium sequestration in the myocytes. The hemoglobin probably does not provide much oxygen delivery to the heart during arrest, but may act as a buffer to acidosis. Despite the theoretical advantage of adding blood or another buffer to the cardioplegic solution, few studies have definitively demonstrated a significant clinical benefit of using one solution over another [187,188].
1.6.1. Adjuvants to cardioplegia for myocardial protection
There have been many attempts to improve myocardial protection during cardiac surgery including drugs to reduce oxidative stress, neutrophil infiltration and sequestration, and complement activation, but none of these adjuvant drugs have been found to provide a clinically significant improvement in outcomes after surgery. IPC initially provided much enthusiasm for the development of novel methods to diminish the effects of myocardial ischemia during coronary occlusion and cardiac surgery. Intermittent aortic cross-clamping has been used clinically, especially prior to the refinement of methods of cardioplegic arrest. Improved cardioprotection has been demonstrated in animal models with lessened release of biomarkers [189]. Similar protection was seen in patients undergoing coronary artery bypass grafting with aortic cross-clamping [190,191] or a modified preconditioning protocol by intermittent hypoxic perfusion of the unloaded heart [192]. RIC has been examined in several clinical trials and the results have been mixed [[193], [194], [195], [196], [197], [198], [199], [200]]. A study by Thielmann et al. [201] found improved biomarker evidence of cardioprotection and early outcomes including a slight mortality benefit and less repeat revascularization with RIC. However, two prospective, phase III trials [ERICCA [193] and RIPHeart [198]] of RIC in patients undergoing cardiovascular surgery found no difference in cTn release or clinical outcome after 1 year [199]. In both studies, patients were anesthetized with propofol, which may diminish the effects of RIC (Table 2, Table 4) [[202], [203], [204]]. In a meta-analysis of 15 clinical trials, inhalation agents and beta-blockers were also found to attenuate the effects of RIC [205]. Interestingly, in this meta-analysis, valve surgery patients seemed to derive more benefit than CABG patients from RIC.
Table 2.
Selected clinical trials of ischemic conditioning in cardiac surgery.
| Study | n | Population/Treatment | Findings |
|---|---|---|---|
| Hausenloy et al. [193] | 57 | CABG patients, randomized to RIPC vs. control | Less troponin release with RIPC |
| Xie JJ et al. [194] | 73 | Patients with valve surgery, randomized to RIPC vs control | Less troponin release and better cardiac function with RIPC |
| Kottenberg et al. [203] | 27 230 |
CABG, diabetic patients treated with sulphonylureas vs. non-diabetics, RIPC |
Non-diabetic patients had less troponin release with RIPC, diabetic patients treated with sulphonylureas had no change in troponin with RIPC. |
| Thielmann et al. [201] | 329 | CABG patients, randomized to RIPC vs. sham | Less troponin release, less MACCE |
| Zhou et al. [205] | Meta-analysis of 15 randomized trials | Benefit of RIPC shown but β-blockers and volatile anesthetics attenuated benefits of RIPC in adult cardiac surgery | |
| Zhang et al. [196] | Meta-analysis of 9 randomized trials, RIPC vs. control in CABG patients |
No benefit of RIPC on troponin release or clinical outcomes | |
| Hong et al. [195] | 1280 | Cardiac surgery patients, randomized to RIPC with RIPostC vs. control | No effect on clinical outcome |
| Zangrillo et al. [204] | Meta-analysis of 55 randomized trials in cardiac surgery of RIPC with and without volatile anesthetics | Both RIPC and volatile anesthetics improved outcome, combination was best. | |
| Kurapeev et al. [192] | 90 | CABG patients, preconditioning induced by ischemia and reperfusion on CPB prior to CP, perfusion alone or control. All patients had blood CP. |
Less troponin release and better clinical outcome with preconditioning |
| Hausenloy et al. [197] | 1612 | Multi-center randomized trial, randomized to RIPC vs. sham (anesthesia not controlled) | No difference in combined endpoint of MI, stroke, CV death, revascularization, kidney injury at 12 months, no early differences in troponin release, acute kidney injury or quality of life |
| Meybohm et al. [198] | 1403 | Cardiac surgery patients, RIPC or sham (propofol used) |
No benefit of RIPC demonstrated in composite outcome of death, MI, stroke, renal failure early or at 90 days follow up |
| Kleinbongard et al. [199] | 329 | CABG patients, randomized to RIPC vs. control | Improved short term recovery and survival, persistent benefit for up to 9 years |
| Moscarelli et al. [200] | 124 | 2 center randomized trial with heart surgery patients (CABG and AVR) RIPC vs. sham control |
No difference in troponin release or release of inflammatory cytokines, serum creatinine or lactate, myocardial ATP |
AVR, aortic valve replacement; CABG, coronary artery bypass grafting; CP, cardioplegia; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; RIPC, remote ischemic preconditioning; RIPostC= Remote ischemic postconditioning.
Because several clinical trials demonstrated little if any clinically significant benefit of ischemic conditioning, it is currently rarely used as an adjuvant to hypothermic cardioplegia. In fact, the act of providing intermittent coronary or peripheral vascular occlusion is time consuming, awkward at times, and disrupts the flow of the time-sensitive operation. An improvement in myocardial contractile function with ischemic conditioning is generally not as clearly demonstrated in larger animal models such as the pig or human [206]. This may in part explain the improvement in cTn release without a benefit in cardiac function or other clinical outcomes in clinical trials.
As opposed to when a patient arrives to the emergency room with an AMI requiring emergent treatment, the condition of a patient undergoing elective or even urgent cardiac surgery can often be optimized prior to the operation. This may include controlling blood glucose and hypertension, treating infections, inflammatory disorders and renal insufficiency and optimizing preoperative cardiac function. These interventions can have a marked effect on improving the outcomes of cardiac operations. Patients with coronary artery disease have pre-existing endothelial dysfunction which contributes to postoperative microvascular dysfunction. Microvascular changes in reactivity and permeability are well documented after cardiac surgery in the coronary circulation and in the circulation of brain, skeletal muscle and many other vascular beds [184]. In clinical studies, poorly controlled hypertension [176] or diabetes [[207], [208], [209]] are associated with a marked increase in post-operative changes in both vascular reactivity and permeability. Preoperative hypercholesterolemia has been shown to increase microvascular endothelial injury, oxidative stress and infarct size in a porcine model of acute myocardial I/R [210]. In order to best understand the effects of cardioprotective procedures in the human heart, it may be prudent to examine human myocardial tissue [176,[207], [208], [209],211,212]. Cardiovascular operations are the ideal source for this human atrial and ventricular myocardium and other tissues such as skeletal muscle.
1.7. Myocardial infarction and cardioprotection
The implementation of PPCI resulted in a marked decrease of morbidity and mortality in STEMI patients [[213], [214], [215]]. Improvements of clinical outcome are closely linked to a reduction of infarct size [26]. However, during the last years mortality rates have plateaued [18]. Therefore, additional approaches are needed to further improve clinical outcome. Over the past 3 to 4 decades, many cardioprotective strategies against myocardial I/R injury have been proposed in AMI. In general, these can be divided into several categories based on the protective modality, time of application, cellular and also the intracellular target.
1.7.1. Ischemic conditioning
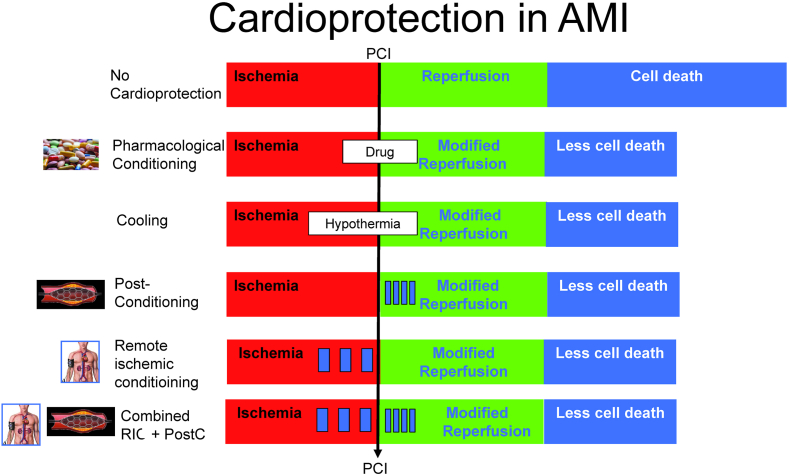
The best studied cardioprotective modalities that have garnered significant attention are RIC and local ischemic postconditioning (PostC). These techniques aim to protect the heart against I/R injury through distinct mechanisms and offer potential benefits in terms of reduced infarct size with possible subsequent improvement in cardiac function and clinical outcome such as reduced mortality or reduction in heart failure hospitalization. The most studied intervention is RIC. In the majority of trials RIC has been induced by 4 alternating cycles of 5 min inflation to 200 mmHg followed by 5 min deflation of an upper arm blood pressure cuff (Fig. 6) [41]. Numerous trials showed a reduction of enzymatic infarct size with RIC in STEMI patients. All these studies used remote ischemic per- or postconditioning by means of limb ischemia using a pneumatic cuff [140,141,[216], [217], [218]]. Other studies, including the large CONDI-2/ERIC-PPCI trial, failed to show a significant reduction of enzymatic infarct size with RIC [41,219,220]. White et al. reported a reduction of CMR imaging-derived infarct size with RIC, whereas other studies did not show a significant effect on CMR-derived infarct size [141,220]. Botker et al. demonstrated improved myocardial salvage index, measured by single photon emission computed tomography (SPECT), with RIC compared to standard PCI [219]. PostC involves the application of brief cycles of myocardial ischemia and reperfusion immediately after the restoration of blood flow following sustained ischemia. Usually, repeated balloon inflation is used [217,221]. Within 1 min of re-opening of the infarct-related artery the angioplasty balloon is positioned at the site of the index lesion and re-inflated 4 times at 4–6 atm. Each inflation usually lasts 1 min followed by 1 min of reperfusion (Fig. 6). To ensure re-occlusion of the coronary artery a small dose of contrast agent is usually injected during balloon inflation. In case of incomplete occlusion an increase of inflation pressure is recommended. Experimental studies have provided compelling evidence for the cardioprotective effects of PostC, with reduced infarct size, improved myocardial function, and preservation of endothelial function observed in animal models. However, clinical studies investigating PostC in STEMI have yielded mixed results, with some trials reporting positive effects on infarct size while others have shown no significant benefit [57,202]. The LIPSIA CONDITIONING trial showed higher CMR-derived myocardial salvage index with a combined strategy of RIC and PostC which is currently the only clinical trial testing this combined approach [222]. Another study showed reduced CMR-determined myocardial edema following remote ischemic postconditioning, whereas no significant differences in other CMR parameters were detected [137].
Fig. 6.
Schematic diagram of ischemic conditioning interventions and their impact on infarct size.
Data supporting a clinical benefit with RIC in STEMI patients are scarce. Post-hoc analyses from the LIPSIA-CONDITIONING trial suggested improved long-term prognosis with combined RIC and PostC compared to conventional treatment [223]. This finding was driven by a significant reduction of new congestive heart failure at long-term follow-up. The long-term outcome analysis of the CONDI 1 trial suggested a lower rate of the combined endpoint as well as all-cause mortality in the RIC group [224].
The implementation of STEMI networks during the last two decades resulted in a marked decrease of door-to-balloon time in STEMI patients [225,226]. Consequently, STEMI patients present in improved hemodynamic condition on hospital admission, which is associated with improved outcome. In the recent European CONDI-2/ERIC-PPCI trial approximately 96% of patients presented with no hemodynamic complications, i.e. Killip class I, resulting in low cardiac mortality rates of about 3% at 1 year [41]. Not surprisingly, in such an ideal setting of patient care a potential further reduction of myocardial damage by ischemic conditioning interventions will not translate in additional prognostic benefit [28].
However, RIC and PostC might be of clinical benefit in higher risk STEMI patients with hemodynamic complications, i.e. Killip class ≥2, where mortality rates are much higher, offering more space for potential prognostic benefit [227]. Indeed, the cardioprotective effects of RIC increase with ischemia time in STEMI patients [228]. In the LIPSIA-CONDITIONING trial, a trend towards improved myocardial salvage index with RIC plus PostC compared to conventional treatment was observed in patients with Killip class ≥2 [222]. Furthermore, a recent observational study showed improved clinical outcome with RIC at 90 days in STEMI presenting with cardiogenic shock or cardiac arrest [229].
Accordingly, ischemic conditioning might be of particular benefit in the setting of less well developed STEMI networks, where transport delays are longer and reperfusion therapy is often not optimal [28]. Therefore, the effect of ischemic conditioning should finally be tested in a randomized controlled trial in a high-risk STEMI population [230]. Currently, the ongoing RIP-HIGH randomized multi-center trial in Germany and Austria investigates the effect of combined RIC plus PostC compared to standard treatment on clinical outcome and myocardial damage in a high-risk STEMI population. The trial uses an adaptive design, and currently >100 patients have been randomized. Trial results are expected by the end of 2024 (clinicaltrials.gov: NCT 04844931).
1.7.2. Pharmacological protection
A variety of pharmacological approaches to cardioprotection have been tried, many of them attempting to recruit signalling steps of local or remote ischemic pre-, per-, and postconditioning. The results are for the most part disappointing. They can all be summarized in that most experimental studies which had shown a benefit could also be transferred into human studies with a reduction in infarct size. However, subsequent large-scale clinical randomized trials all failed to show a benefit in clinical endpoints.
Selected studies on cardioprotection by pharmacological agents can be found in Table 3, Table 4 and have been summarized in multiple reviews [36,44,57], including cyclosporine A [[231], [232], [233]] and other mitochondria-targeting agents [234,235], nitrite [236,237], inhaled nitric oxide [238], the ROS scavenger acetylcysteine without [239] or with nitroglycerine [240], anti-inflammatory interventions [241,242], inhibition of the protein kinase C delta isoform with delcasertib [243], and beta blockade with metoprolol [244,245]. Cyclosporine reduced infarct size, as measured by CPK release and CMR in a small trial [231], but in a subsequent larger trial neither reduced CPK release nor improved clinical outcome after 1 year [232]. Other trials targeting the mitochondria also did not report reduced infarct size or better clinical outcome [246]. Reflecting lack of robust preclinical data [247], initial promising results with metoprolol in the METOCARD trial [244] were not confirmed in the much larger EARLY-BAMI trial [245]. Other agents which have reduced infarct size but did not result in improved clinical outcome include adenosine [248], atrial natriuretic peptide [249], and exenatide [250].
Table 3.
Selected clinical trials of pharmacological cardioprotection in patients with reperfused acute myocardial infarction.
| Study | n | Patient criteria | Treatment protocol | Main outcome |
|---|---|---|---|---|
| Cyclosporin-A | ||||
| Piot et al. [231] | 58 | All STEMI | Intravenous bolus of CsA administered 10 min prior to PPCI | Reduced MI size assessed by AUC CK. No difference in troponin I. Subset of 37 patient reduce MI size on MRI at day 5 post-PPCI |
| Cung et al. [232] CIRCUS |
970 | Anterior STEMI Pre-PPCI TIMI 0/1 |
Intravenous bolus of CsA administered prior to PPCI | No difference in primary outcome worsening in-pt heart failure, HHF, or adverse LV remodeling at 1 year |
| Ottani et al. [233] CYCLE |
410 | All STEMI | Intravenous bolus of CsA administered prior to PPCI | No difference in primary endpoint of ≥70% ST-segment resolution 60 min After TIMI flow grade 3, or MI size (day 4 hs-cTnT) or LV remodeling at 6 months |
| MTP-131 | ||||
| Gibson et al. [234] EMBRACE-STEMI |
118 | Anterior STEMI Pre-PPCI TIMI 0/1 |
Intravenous 60 min infusion of MTP-131 started prior to PPCI | No difference in primary endpoint of MI size (72 h AUC CK). No difference in MI size or LV remodeling on MRI at 4 ad 30 days |
| TRO40303 | ||||
| Atar et al. [235] MITOCARE |
163 | All STEMI within 6 h chest pain Pre-PPCI TIMI 0/1 |
Intravenous bolus of TRO40303 administered prior to PPCI | No difference in primary endpoint of MI size (72 h AUC CK or hs-cTnI), significant increase in major adverse events |
| Nitrite | ||||
| Siddiqi et al. [236] NIAMI |
229 | All STEMI TIMI 0/1 |
Intravenous bolus of nitrite administered prior to PPCI | No difference in primary endpoint of MI size on MRI at day 6–8. No difference in LV remodeling or MI size by (72 h AUC CK or cTnI) |
| Jones et al. [237] | 198 | All STEMI | Intracoronary bolus of nitrite administered prior to PPCI | No difference in primary endpoint of MI size (72 h AUC CK or hs-cTnI) |
| N-acetylcysteine | ||||
| Thiele et al. [239] LIPSIA-N-ACC |
251 | All STEMI | 2 × 1200 mg/day N-ACC for 48 h | No difference in myocardial salvage index measured by CMR at day 3–4 (43.5; IQR 25.4 to 71.9 vs. 51.5; IQR 29.5 to 75.3; p = 0.36) |
| N-acetylcysteine + nitroglycerin | ||||
| Pasupathy et al. [240] NACIAM |
75 | All STEMI | Intravenous infusion of NAC for 48 h initiated prior to PPCI. On background of IV GTN infusion. | Reduction (by 33%) in primary endpoint of MI size by CMR at day 2–3 post-PPCI |
| Inhaled nitric oxide | ||||
| Janssens et al. [238] NOMI |
250 | All STEMI | Inhaled oxygen with NO started 10 min prior to PPCI and continued for 4 h. | No difference in primary endpoint of MI size by MRI at day 2–3 |
| Kleveland et al. [241] | 117 | NSTEMI | Intravenous 60 min infusion started prior to PPCI | Reduced hsCRP levels. Reduced median AUC for hs-cTnT by 30% |
| Broch et al. [242] ASSAIL-MI |
199 | All STEMI | Intravenous 60 min infusion started during PPCI | Increased myocardial salvage by 5.6% on CMR (2–7 days) and less MVO but no difference in MI size |
| PKC-δ inhibition (delcasertib) | ||||
| Lincoff et al. [191] PROTECION-AMI |
1010 166 |
Anterior STEMI Inferior STEMI |
Intravenous infusion prior to PPCI for 2.5 h | No difference in CK-MB, troponin, and ST segment resolution |
| β-blocker (metoprolol) | ||||
| Ibanez et al. [244] METOCARD-CNIC |
220 | Anterior STEMI Killip ≤2 | Intravenous infusion before PPCI | Infarct size by CMR reduced from 32 to 25.6 g |
| Roolvink et al. [245] EARLY-BAMI |
342 | STEMI Killip ≤2 |
Intravenous infusion before PPCI | Infarct size by CMR not reduced |
AUC, area under curve; CMR: cardiac magnetic resonance; CK-MB, creatine kinase MB isoenzyme; GTN, glyceryl trinitrate; hs-cTnT/I, high-sensitive cardiac troponin T/I; HHF, hospitalization for heart failure; LAD, left anterior descending coronary artery; MI, myocardial infarction; MVO, microvascular obstruction; NAC, N-acetylcysteine; NSTEMI, non-ST-segment elevation myocardial infarction; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
1.7.3. Physical intervention
Physical interventions are numerous and even aspiration thrombectomy targeting thrombus and possible peripheral embolization and reperfusion injury may be summarized under physical interventions [108,251]. However, in general, physical interventions aiming at reducing the classical reperfusion injury such as LV unloading, hypothermia or hyperoxemia are considered treatment strategies. LV unloading reduced infarct size in a pig model of myocardial infarction and in preliminary studies in STEMI patients, even when the duration of ischemia was prolonged by the unloading procedure [252], but the mechanism [253] and clinical benefit from LV unloading are still unclear; the STEMI-DTU (NCT03947619) will provide further answers. Experimental studies in different animal species have consistently shown that mild hypothermia, induced prior or during reperfusion in acute MI, reduces infarct size [254]. Cooling before reperfusion therefore appears to be a promising adjunct to PPCI in STEMI patients. Several randomized clinical trials have shown mixed results, although intravascular cooling appeared to be safe and well tolerated [[255], [256], [257]]. A combined analysis of RAPID MI-ICE and CHILL MI revealed a significant reduction in infarct size in a subgroup of early presenters with anterior STEMI who were cooled below 35 °C prior to reperfusion [258].
A similar favorable signal was also observed in the COOL AMI pilot randomized trial, which enrolled 50 patients with anterior STEMI and resulted in a numerical reduction in infarct size (23.8%–16.7% of the left ventricle; p = 0.31) [259]. In the COOL AMI EU Pivotal Trial, powered to demonstrate a clinically meaningful reduction in infarct size using rapid intravascular systemic hypothermia prior to PPCI compared to standard treatment with PPCI alone, hypothermia resulted in a longer ischemic delay and did not reduce infarct size. There was also a concern with associated increased adverse events [260]. In the randomized SHOCK-COOL trial, moderate hypothermia in cardiogenic shock patients with AMI did not result in improved hemodynamics in comparison to control. However, hypothermia was associated with higher lactate levels, possibly even indicating harm in this setting of cardiogenic shock [261]. Randomized clinical trials powered to show differences in clinical outcome are not available for hypothermia in AMI. Data on hyperoxemic reperfusion are controversial [262]. Final infarct size as measured by SPECT 2 weeks after PPCI in STEMI patients was not reduced in the AMIHOT I trial [263]. In contrast, infarct size was reduced in the AMIHOT II trial [264]. However, clinical outcome was not affected by hyperoxemic reperfusion.
In summary, the quest for novel strategies to protect the myocardium against I/R injury in acute MI continues to drive scientific research. Rigorous large-scale randomized controlled trials are needed to evaluate the safety, efficacy, and optimal protocols for these cardioprotective strategies. Investigations into the combination of these approaches with existing therapies and identification of specific patient populations that may benefit the most are also warranted.
1.8. Heart failure and cardioprotection
Although interventional approaches for coronary reperfusion and improvement in procedures and logistics have reduced the incidence of post-infarction heart failure [265], heart failure following AMI continues to develop with cumulative incidence rates between 12 and 22% after 2.9 and 3 years, respectively [266,267]. This adverse condition remains associated with a two to three times increased risk of subsequent death or hospital admissions [267]. Most cardioprotective interventions to improve survival and prevent subsequent chronic heart failure have focused on reducing the immediate I/R injury during AMI by mechanical ischemic conditioning strategies or pharmacological interventions. A more recent approach has aimed to explore the potential of applying repeated daily conditioning strategies as an adjunct to the standard recommended medication that modifies post-infarction remodeling in order to reverse the physical limitation in patients, when heart failure has developed [268,269]. The approach takes advantage of the unique feature of a biphasic pattern of cardioprotection by mechanical conditioning strategies such as RIC. The first window of protection initiates instantaneously and lasts for ∼2–3 h. Following a period without protection, the second window of protection reappears between 12 and 24 h after the original stimulus and lasts for up to 72–96 h [[270], [271], [272]]. Because RIC is easily and non-invasively applied, the intervention can be administered repeatedly for extended periods, such as once daily for weeks. Experimental studies have demonstrated that RIC repeated daily for 28 days after AMI protected against adverse left ventricular remodeling and increased survival in a rat model even though infarct size was not reduced further compared with the single-occasion treatment [90]. The improvement in LV chamber size, LV function and hemodynamic changes after AMI was largest in the group that received repeated RIC every day for 28 days compared to a control group and two groups receiving RIC either during ischemia or every third day following AMI [90]. A similar beneficial effect on remodeling was obtained even when RIC treatment in rats was commenced as long as 4 weeks after AMI, thus documenting favorable influence of repeated daily RIC in a more chronic phase of AMI [91]. Hence, the benefit appears to go beyond the initial improvement by reduction in infarct size, suggesting that a distinct mechanism of cardioprotection may act directly on remodeling.
The mechanisms underlying RIC involve an initial reduction in oxidative stress and blunting of the inflammatory response that downgrades cytokine signaling and reduces levels of neutrophil and macrophage infiltration in the myocardium in an in-vivo rat model of myocardial infarction [90]. Repeated RIC adds an attenuation of the expression of genes associated with fibrosis and hypertrophy [90]. In the rat study initiating repeated RIC 4 weeks after AMI, an association between reduced systemic oxidative stress and attenuation of AMI-induced left ventricular interstitial fibrosis in the boundary region of the infarct [91] provided direct mechanistic evidence for modulation of remodeling after infarct healing by delayed repeated RIC.
Beyond the interfering patterns of kinase and autophagy activity by acute single occasion RIC, repeated RIC in rats adds modulation of separate phagosomes that involve downregulation of mTOR and subsequent upregulation of pro-autophagy proteins [88], supporting that separate mechanisms mediate distinct benefits on LV remodeling as compared to a single occasion RIC stimulus given at the time of the infarction. Inter-organ signaling by single occasion RIC was initially shown to involve neuronal as well as humoral mediators [87]. More recent studies have shown that extracellular vesicles and exosomes [273], containing proteins, lipids, mRNAs and microRNAs, mediate cardioprotective inter-organ communication in rodents [274,275], and that this mediation is transferable between species [276]. In repeated RIC, the effects on the heart also involve mediation by exosomes as microRNA-29a (miR-29a), a key regulator for reduction of tissue fibrosis, is highly expressed in the exosomes and in the infarct border zone in the RIC group, when RIC is initiated in rats 4 weeks after AMI [91]. Moreover, insulin-like growth factor 1 receptor (IGF-1R) is highly expressed in the exosomes and remote non-infarcted myocardium of the RIC group [91]. A role for exosome mediation is supported by studies in mice, demonstrating that RIC induces release of skeletal muscle exosomes, in which proteome analyses related ∼20 dominating proteins to signaling pathways responsible for the synthesis, contraction, and relaxation of cardiac muscle [277].
So, experimental findings point towards a beneficial effect of repeated daily RIC on adverse remodeling during the phase of infarct healing and beyond. It remains to be determined how autophagy signaling specifically contributes to the remodeling process and to which extent remodeling can be modulated in a chronic condition of completed infarct healing. An additional limitation of the experimental studies is that studies so far have been conducted only in healthy animals that were not treated with the post-infarction medication usually administered to humans with heart failure and known to interfere with RIC efficacy [95].
In humans, repeated RIC initiated on day 3 following AMI in patients treated with PPCI and given once daily for 4 weeks did not affect LV ejection fraction, infarct size or left ventricular end-diastolic and systolic volumes after 4 month [278]. Reduction of infarct size achieved by single occasion RIC immediately prior to PPCI is a major determinant for improvement in LV ejection fraction and remodeling in particular among patients with extensive area at risk [279]. Even though a critical period, when inflammation and oxidative stress contribute to adverse LV remodeling, may be within the first 3 days post-infarction, a subsequent more comprehensive CMR study of the same patients demonstrated that repeated RIC, commenced 3 days after infarction, initiated improvement in cardiac remodeling after 4 months [280]. A similar beneficial modulation of cardiac remodeling, measured as global longitudinal shortening, by repeated RIC once daily for 4 weeks has been demonstrated among the most severely compromised patients with stable chronic ischemic congestive heart failure as defined by NT-proBNP plasma levels above the geometric mean of 372 ng/l [268]. Also, blood pressure and circulating NT-proBNP were lowered by RIC. Overall, LV ejection fraction, peak cardiopulmonary exercise capacity and disease-related quality of life did not significantly change in this outcome-assessor blinded study [268]. RIC applied twice daily for 7 days on both arms increased coronary flow reserve modestly in patients with chronic congestive heart failure due to ischemic or dilated cardiomyopathy [281]. An impressive effect was demonstrated in a single-blinded randomized trial without sham control that revealed improved LV ejection fraction (from 39.2% to 43.4%, assessed by echocardiography), increased exercise capacity (assessed by 6-min walk test), reduced NYHA class, and lower levels of plasma BNP with repeated RIC given as 4 × 5 min cycles of upper arm cuff inflation and deflation twice daily for 6 weeks in patients with chronic ischemic heart failure randomized to RIC or standard therapy [269]. Overall, the findings may indicate a potential for modifying further adverse cardiac remodeling in established heart failure.
The mechanisms underlying the beneficial effects of repeated RIC on NT-proBNP and remodeling are not clear but may relate to less myocardial wall stress, caused by reduction in afterload consistent with lowering of systemic blood pressure by repeated RIC. This may be mediated by release of known vasodilator mediators of RIC such as adenosine and nitric oxide [268] and a mild anti-inflammatory effect with modest reductions in C-reactive protein and calprotectin [282] and reduced neutrophil adhesion [283]. The upregulation of cardioprotective microRNAs, nitric oxide production, and oxidative stress reduction that facilitate reverse LV remodeling in the post-infarction healing period [284] may be analogous mechanisms in chronic heart failure patients. Also, the beneficial effects of repeated RIC in heart failure patients have been associated with correction of cardiac autonomic dysfunction by increased parasympathetic and reduced sympathetic activity, as assessed by heart rate variability [269]. Other specific signaling pathways have not yet been investigated specifically in heart failure patients. Exosome mediation of cardioprotection may be involved as translational studies indicate that labelled exosomes in plasma from healthy volunteers following single occasion RIC accumulate more intensely in the infarct area than in sham hearts, when transferred to a Langendorff rat model [276].
Beyond the effect on the heart, repeated RIC seems to enhance skeletal muscle strength in heart failure patients, so that muscle waste, known as an inherent component of heart failure, may be prevented and disease-related quality of life improved [268]. This is consistent with findings in trained athletes and non-athletes, in whom RIC improves physical performance [285]. Blood flow restricted exercise (BFRE) may represent a more potent performance-enhancing corollary to RIC in heart failure patients [286]. The concept may be of aid in optimizing physical rehabilitation in populations that are not be able to perform exercise practice at intensity levels required to promote optimal outcome through mechanisms mobilizing endogenous protective like by RIC [287]. Such mechanisms involve secretion of myokines, growth factors [288] and muscle stem cells [289,290] mediated by micro-RNAs delivered to remote locations via exosome transport [291].
1.9. Cardio-oncology and cardioprotection
Significant advances in cancer therapy have greatly reduced mortality, with non-malignant comorbid conditions becoming important determinants of their quality of life and overall survival. Among this heterogeneous group of comorbid conditions, cardiovascular diseases are major contributors to overall morbidity and mortality in cancer survivors [292,293]. Cardiovascular diseases and cancer share common risk factors in both the aged and the pediatric populations and are further linked through toxicities in the cardiovascular system effects of contemporary cancer treatment.
The first anticancer agents widely used were anthracyclines. Anthracyclines are a non-specific agent with highly effective anti-tumoral capacity. Still today, anthracyclines remain first line treatment (alone or in combination with other therapies) for many cancer types, including lymphomas, leukemias, sarcomas and breast cancer. The principal limitation to use of high-dose anthracyclines is their established cardiotoxic effect [294]. One in every 3 patients receiving anthracyclines develops some form of cardiotoxicity [295]. Fortunately, this cardiotoxicity is mild and transient most of the times, but in more than 5% of patients who receive anthracyclines, irreversible cardiac dysfunction with associated chronic heart failure occurs [295]. Given the high number of patients receiving anthracyclines in Europe every year, the prevalence of chronic heart failure in cancer survivors directly related to anthracycline cardiotoxicity is estimated in 1 million EU citizens. For cancer survivors, the trade-off between cancer and chronic heart failure is of massive psychological burden. For healthcare systems, the growing incidence of chronic heart failure has devastating economic consequences. Treatment for cancer has massively evolved in recent decades, and now there are new highly specific (targeted) therapies for specific types of cancers. These include human epidermal growth factor receptor 2 (HER2)-targeted interventions, tyrosine kinase inhibitors, immune checkpoint inhibitors, proteasome inhibitors, androgen deprivation therapy, and chimeric antigen receptor T cells. These therapies are used most often as part of combos, which include anthracyclines. The use of targeted therapies is not free from cardiovascular adverse events, and the development of cardiac dysfunction and heart failure are common side effects of several targeted therapies. Targeted therapies have also been associated with increased incidence of cardiovascular side effects different from cardiac dysfunction, such as myocarditis, accelerated atherosclerosis and arterial hypertension [294,296,297]. From all the above, it is obvious that there is a need to identify cardioprotective strategies that, when used in combination with anti-cancer therapies, can prevent the development of these unwanted cardiovascular side effects [297]. One major limitation for the identification of cardioprotective therapies in this setting is the incomplete understanding of the mechanisms leading to cardiovascular toxicities of these anticancer treatments. Despite considerable inter-individual vulnerability, anthracycline cardiotoxicity is strongly associated with the cumulative dose received throughout life. These figures are the basis for the recommendation for a maximum lifetime cumulative dose of 400 mg/m2 for all patients receiving anthracyclines [298]. The maximum recommended dose also depends on whether or not mediastinal radiation therapy is given, which increases the risk of cardiotoxicity. Patients with previous cardiovascular comorbidities (hypertension, diabetes, alcohol consumption, etc.) are at higher risk for developing cardiotoxicity [293]. There are important similarities between the mechanisms leading to cardiac injury from I/R and from anthracycline cardiotoxicity. These include mitochondrial damage with massive ROS production [299], and microvascular injury [300]. Early detection of anthracycline cardiotoxicity can be made by using multiparametric CMR imaging, since this can detect intra-cardiomyocyte edema as a result of mitochondrial vacuolization as part of quality control during injury [301].
Given its high clinical relevance, therapies that can prevent the development of anthracycline cardiotoxicity have been sought for several years at the experimental and clinical levels [293]. Several interventions have been tested with inconsistent results [294]. Most of the clinical trials testing cardioprotective strategies for this condition have included a very limited number of patients, precluding final conclusions. Beta-blockers [[302], [303], [304]], renin-angiotensin-aldosterone-system inhibitors [[305], [306], [307]], and statins [[308], [309], [310], [311]] are among the most frequently tested therapies. The STOP-CA trial compared atorvastatin 40 mg against placebo in 300 patients with lymphoma receiving moderately high dose anthracycline regime. The incidence of cardiotoxicity events (defined according to a pre-specified decline in LV systolic function) was significantly reduced in patients allocated to statin preventive treatment. In contrast, in another (smaller) recent trial including patients with several cancer types, atorvastatin 40 mg did not show cardioprotection as evaluated by CMR imaging [311,312].
Given the similarities between the mechanisms of damage of I/R injury and anthracycline cardiotoxicity, it is intuitive to argue that RIC is an ideal intervention. The fact that the exposure to the injury agent (anthracyclines) is a programmed intervention (administration of chemotherapy in a controlled hospital environment), it is in theory the ideal clinical setting to test RIC. By using a highly translatable pig model of anthracycline cardiotoxicity within long-term serial examinations by state-of-the-art CMR imaging, Galan-Arriola et al. recently demonstrated the strong cardioprotective effects of RIC when applied before each anthracycline administration [299]. Pigs randomized to RIC before each anthracycline injection displayed a significant preservation of cardiac systolic function. This was associated with structural and functional preservation of mitochondria, less ROS production, and attenuation of dysregulated autophagy. (Fig. 7). The effect of RIC to prevent anthracycline cardiotoxicity has been tested in 2 very recent very small pilot trials, one in an adult population [313], and another in a pediatric one [314]. Both trials were neutral for the primary endpoint (release of cTn), however cardiotoxicity (as defined by clinical practice guidelines [293] was not observed in patients in any of the trials. Of note, in the small trial in adult patients RIC increased cancer and total mortality over placebo [313], raising the concern that RIC might induce a systemic survival signal which benefits not only the heart, but also the cancer [315]. These considerations highlight that future trials should enroll patients at risk for anthracycline cardiotoxicity and get away from the all-comers design [316]. The ongoing ‘REmote iSchemic condItioning in Lymphoma PatIents REceiving ANthraCyclinEs’ (RESILIENCE) trial (NCT05223413) is testing the benefits of RIC in a population with one or more high-risk features for cardiotoxicity. In this European Commission-funded trial, 608 patients will be randomized to weekly RIC (4 × 5 min cycles) or sham during the period on chemotherapy (approx. 4 months). Primary outcome measure will be based on serial CMR imaging.
Fig. 7.
Effect of remote ischemic preconditioning (RIPC) on left ventricular ejection fraction (LVEF) and mitochondrial structure in a pig model of anthracycline cardiotoxicity. Pigs received five biweekly intracoronary doxorubicin injections with RIPC of control applied before each injection. Serial cardiac magnetic resonance imaging studies were performed during 4 months follow-up. Anthracycline-induced systolic dysfunction was significantly attenuated in pigs receiving RIPC. At the ultrastructural level, mitochondria were fragmented in pigs receiving doxorubicin since subclinical stages (i.e. a time where cardiac function was still normal). At late stages (i.e. when severe cardiac dysfunction was present), mitochondria were severely damaged (electrodense and ultra-fragmented). Mitochondria from pigs undergoing RIPC before each anthracycline administration were much more preserved at every timepoint. Figure adapted from [299].
2. Sources and actions of reactive oxygen species
2.1. Reactive oxygen species in myocardial ischemia/reperfusion - the early days
A role for ROS in biology originated more than a century ago when investigators asked why obligate anaerobic bacteria were killed by the presence of oxygen. In 1907 it was found that oxygen toxicity in bacteria was correlated with the absence of catalase which was known to scavenge H2O2, a highly toxic oxygen derivative. Catalase was present in most aerobic bacteria tested and absent in all obligate anaerobes [317]. This led to the hypothesis that oxygen caused formation of H2O2, a ROS, in bacteria that was lethal if they lacked the scavenger catalase. But where did the H2O2 come from? In 1968 Joe McCord and Irwin Fridovich [318] were studying the reduction of cytochrome c by milk xanthine oxidase. They found this reaction could be blocked by carbonic anhydrase even though it does not bind to cytochrome c. They subsequently found that the xanthine oxidase was generating the free radical superoxide which was reducing the cytochrome c and that there was a contaminant in their carbonic anhydrase that was dismutating superoxide to H2O2 thus halting the reduction reaction. In a follow-up study they purified the contaminant and named it superoxide dismutase (SOD). They found that it, like catalase, is widely distributed among all mammalian species tested, including man [319].
Three ROS received particular attention in the 1980s: superoxide radical (O2*-), H2O2, and hydroxyl radical (*OH). Radicals are molecules with unpaired electrons in one of the shells which make them highly reactive. H2O2, while not a radical is still very reactive. All three of these ROS have the potential to disrupt vital molecules like enzymes and nucleic acids through redox reactions. H2O2 produced by SOD is reduced to water by catalase. H2O2 in the presence of free Fe2+ or Cu2+ can be catalyzed into the highly reactive hydroxyl radical. Thus a combination of SOD plus catalase should eliminate all three ROS.
Following the discovery of SOD and the apparent importance of the body's ROS scavenging systems, ROS quickly moved to the forefront of scientific research. Popular culture also picked up on the ROS hypothesis. Foods were advertised as being chock-full of anti-oxidants. Even James Bond, in a conversation with miss Moneypenny, likened members of his arch enemy SPECTOR to “free radicals” (Never Say Never Again, 1983). Among the proposed ROS targets was the ischemic heart. It seemed a likely setting for such a ROS disaster.
In 1973 David Hearse and colleagues [320] looked at the effect of hypoxia-reoxygenation in isolated rat hearts and noted that reoxygenation was accompanied by an abrupt release of cytosolic enzymes into the venous effluent, a marker of cell death by membrane failure. This was termed the “oxygen paradox”. The reason why oxygen, which is obviously needed for the hypoxic heart to recover, also appeared to causes a reoxygenation injury was unknown, but ROS were mentioned among other possible explanations. The first paper claiming that SOD could reduce infarct size appeared in 1984 using canine hearts experiencing I/R [321]. SOD and catalase are both large proteins that would not easily enter cells. However, it was known that the reperfused myocardium is soon flooded with neutrophils. It was proposed that neutrophils inappropriately directed a ROS attack on injured but still viable cardiomyocytes and that SOD in the extracellular space prevented that attack [322].
What followed was a cascade of studies in which infarct size was measured in animal hearts subjected to an ischemic insult followed by reperfusion. A search of PubMed today for SOD and infarct size brings up more than 1000 hits. SOD with or without catalase was tested in these models, but the results were very mixed. Some labs reported robust protection while others saw little or none. Except for a very early canine study [323], Downey's lab reported mostly negative SOD results [324,325]. In 1989 two seminal papers were published on the role of ROS in the ischemic heart, one by Keith Reimer [326] and the other by Robert Kloner [327]. Central concepts and open questions at that time are shown in Fig. 8.
Fig. 8.
The view in 1989 on pathomechanisms of I/R injury and the open questions on the role of ROS in it. From Ref. [327] with permission.
Both reviews took a critical look at the many published reports and came to the conclusion that the more sophisticated the animal models became the less protective the SOD was. They also seriously challenged the neutrophil hypothesis. Today most investigators think neutrophils act to debride the infarcted myocardium to promote healing rather that contribute to its infarction, since infarct size is amazingly similar in blood-free Langendorff hearts and in situ hearts [328].
Looking back, many of the positive reports on SOD's protection were probably the result of artifacts in the infarct size measurement itself. Unfortunately, there are numerous potential pitfalls in the methods used to measure infarct size in animal hearts that can lead to erroneous findings. These include poor histological techniques for identifying infarcted tissue and failure to accurately measure the infarct's volume. Failure to accurately account for the factors that affect infarct size including risk zone size, collateral flow and temperature of the heart during ischemia can all lead to uncontrolled variability in the measurement of infarct size. Most of the early studies used dogs which were very expensive so group sizes were usually small. Miura et al. [329] reported a study of 54 untreated dogs subjected to 4, 24, 48 or 96 h of permanent coronary occlusion. Fig. 2 reveals that infarct size ranged from 4% to 95% of the risk zone and was inversely related to the level of collateral flow to the ischemic zone which ranged from as high as 88% of the pre-occlusion flow to as low as 2%. Thus without measuring collateral flow, as was the case in many of the early canine studies, it would be virtually impossible to detect any effect of an intervention on infarct size because of the extreme variability in collateralization among canine hearts. Note that the terminal infarct size was already apparent after 4 h of ischemia since further prolongation of the ischemic time did not increase the infarct size.
Accordingly, in those early canine studies significance often hinged on the presence of one or two key “responders” or on the exclusion of one or two key “outliers”. Additionally, failure to maintain the temperature of the heart during ischemia can introduce large variations of infarct size in isolated, small animal hearts. Van Winkle's group found the infarct size following I/R increases by 7% per degree C during ischemia [330]. Immersing the isolated hearts in 38ᵒC Krebs solution during ischemia greatly reduced variability in infarct size. Today the infarct size model can be quite powerful and robust, but only if all of the factors that affect infarct size are controlled and sufficient numbers of hearts are studied.
Much research was directed at the source of ROS in the reperfused heart. Neil Granger found that xanthine oxidase was responsible for superoxide-induced injury in the reperfused intestine [331], and Downey's lab found similar results in canine hearts. However, unlike canine hearts, human hearts lack xanthine oxidase as do rabbit hearts where xanthine oxidase inhibitors have no effect on infarction [332]. Finally, ROS are not always injurious. IPC was the first intervention found to unambiguously protect the ischemic heart. Its protection involves a complex signal transduction cascade that includes one step that uses a ROS signal. Blocking it eliminates preconditioning's protection [333].
Although SOD failed to salvage the ischemic myocardium, ROS still are thought to be involved in myocardial infarction as in the case of ferroptosis where iron toxicity causes formation of hydroxides and hydroxyl radicals through the reaction of Fe2+ and hydrogen peroxide [334]. The toxic ROS are also active in micro-compartments such as the mitochondria [52] that would be unreachable by an extracellular scavenger like SOD or catalase.
2.2. Mitochondrial ROS and cardioprotection
As discussed in the section above, ROS play a crucial role in cardiac injury induced by I/R. The question is then where ROS are generated and which are the most relevant targets of oxidative stress in the ischemic heart. Even limiting the discussion just to cardiomyocytes, the question of sources and targets keeps generating a steady flow of debate and controversies, yet is far from being conclusively solved.
Regarding intracellular sites, a consensus exists that mitochondria are responsible for the largest amount of ROS generated especially during post-ischemic reperfusion [335]. Therefore, ROS accumulation adds to other detrimental processes, such as a decrease in ATP synthesis and an increase in [Ca2+], by which mitochondria made dysfunctional by the lack of oxygen change from vital organelles into the main executioners of I/R injury. The involvement of mitochondria in I/R injury was introduced more than 50 years ago [336] by associating the loss of cardiomyocyte viability with calcium accumulation within the mitochondrial matrix. Soon thereafter, mitochondrial function was shown to be required for cell death upon reperfusion, since both inhibition of respiratory chain and uncoupling of oxidative phosphorylation decreased enzyme release [337,338]. These seminal observations created a tight link between mitochondrial alterations and necrosis that was extended to apoptosis during the nineties [339]. Furthermore, two elements required for contraction and viability, such as Ca2+ and oxygen, were established as main determinants of the loss of function and viability in hearts with I/R [340]. This concept was further elaborated by demonstrating that both Ca2+ and ROS accumulation promote the opening of the mitochondrial PTP in a synergistic manner [341]. On this basis, it is hardly surprising that protective strategies were developed to prevent I/R-induced increases in Ca2+ and/or ROS, or inhibit PTP opening. However, although in controlled experimental protocols of severe and prolonged ischemia this rationale might prove valid, the failure of attempts to translate that approach into clinical settings [246] is likely to depend on the notion that mitochondrial Ca2+ and ROS overload, as well as PTP opening, are involved also in self-defense mechanisms, such as those underlying conditioning-induced cardioprotection [333,342]. The elucidation of mechanistic issues, some of which are discussed below, should allow the improvement of therapeutic strategies.
2.2.1. Pathways of mitochondrial ROS formation and removal
The fact that mitochondria are accepeted as the prevailing cellular site for ROS formation does not tell us which mitochondrial sources are relevant in I/R, which one of them is involved in injury or protection, and which targets are more relevant. The answer to those relevant questions is complicated by the involvement of several mitochondrial processes which generate ROS as a role alternative to their vital functions [335]. This is especially the case with the electron transport chain subunits or flavin-containing dehyrogenases. Genetic or pharmacological interventions on those enzymes would affect not only ROS formation, but also bioenergetics and cell viability. Nevertheless, the fact that mitochondria generate ROS in vivo is proven unambiguously by enzymes that generate ROS as their natural products and are not involved directly in bioenergetics. In this respect, the study of monoamine oxidases (MAO) and p66Shc provided a clear demonstration of the detrimental role of ROS generated within mitochondria in I/R, as well as in various cardiac pathologies [343,344] (Fig. 9). Indeed, MAO and p66Shc inhibition or downregulation elicit a high degree of protection against cardiomyocyte and vascular abnormalities [344,345]. Notably, the protective effects were not increased by combining MAO and p66Shc inhibition [346]. A major advantage with MAO inhibition is that it is obtained with clinically available compounds, a feature that is not shared by any other mitochondrial reaction generating ROS.
Fig. 9.
Schematic diagram of ROS-generating systems in mitochondria. Big calcium-activated potassium channel (KCa); connexin 43 (Cx43); cyclophilin D (Cyp D); dicarboxylate carrier (DIC); mitochondrial calcium (Ca2+) uniporter (MCU); mitochondrial ATP-dependent potassium channel (KATP); mitochondrial permeability transition pore (MPTP); monoamino oxidase (MAO); p66shc, p66 Src homologous and collagen; reverse electron transport (RET); sarcolemmal monocarboxylate transporter 1 (MCT1); succinate dehydrogenase (SDH). Modified from [379].
Besides H2O2, the oxidative deamination catalyzed by MAO generates ammonia and aldehydes that are likely to synergize in causing mitochondrial and cellular alterations. Of note, the decrease in I/R injury elicited by stimulation of aldehyde dehydrogenase 2 [347], another mitochondrial enzyme, demonstrates the high toxicity of aldehyde moieties, especially those generated from aromatic amines. Indeed, aldehydes that are generated also by means of lipid peroxidation might play a deleterious role similar to or larger than that caused directly by ROS.
Pathways involved in ROS removal lend a further support to the deleterious role of oxidative stress generated in mitochondria. Indeed, SOD down-regulation exacerbated I/R injury [348], whereas cardioprotection was obtained by expressing catalase in mitochondria [349]. Furthermore, mitochondrial membrane potential is related directly to antioxidant defenses by means of uncoupling proteins (UCPs) and nicotinamide nucleotide transhydrogenase (NNT). Overexpression of UCP2, the most abundant cardiac isoform, resulted in cardioprotection, although the underlying mechanism remains controversial [350]. On the other hand, NNT-catalyzed conversion of NADH(H+) into NADPH(H+) is necessary to maintain peroxidase activities [351].
2.2.2. Mitochondrial ROS and cytosolic processes, back and forth
It is worth pointing out that mitochondrial ROS formation can be triggered by cytosolic events generated in response to physiological or pathological stimuli, such as NOX stimulation or an elevation in intracellular [Ca2+]. NOX-induced ROS formation has been suggested to represent an initial trigger for a more substantial ROS generation in mitochondria [352]. Regarding Ca2+, although linking mechanisms are still matter of debate [353], an increase in cytosolic and/or mitochondrial [Ca2+], such as that associated with an increased beating rate, is paralleled by ROS accumulation [354]. The link could result from Ca2+-dependent stimulation of various enzymes. For instance, arachidonic acid generated by Ca2+-activated phospholipase A2 is a powerful agonist of PTP opening also associated with mitochondrial ROS formation [355]. More recently, as a possible mechanism underlying arrhythmogenic cardiomyopathy, Ca2+-activated calpain has been shown to cause mitochondrial ROS formation leading to oxiation of cleaved apoptosis initiating factor that upon its translocation into nuclei triggers cell death [356].
Since mitochondrial ROS formation is mostly the consequence of an initial alteration, such as an elevation in intracellular [Ca2+], uncertainities might remain on whether injury is caused by ROS or by a triggering event. The question whether a primary increase in mitochondrial ROS can affect cardiomyocyte function and viability was addressed by using the mitochondria-targeted compound MitoParaquat (MitoPQ) [357]. Acting as a redox cycler at the level of complex I, MitoPQ generates superoxide directly into mitochondria. Submicromolar concentrations of MitoPQ caused mitochondrial dysfunction due to PTP opening and loss of cell viability. At concentrations <0.1 mM viability was slightly affected, but intracellular Ca2+ transients were profoundly altered, suggesting that oxidative stress produced in mitochondria can disrupt Ca2+ homeostasis and consequently contractile function. Interestingly, at concentrations <10 nM both in vitro and in vivo, MitoPQ elicited a robust protection against I/R injury. The similarity with preconditioning-induced protection was supported by showing that an antioxidant abrogated MitoPQ-induced maintenance of cell viability. While these findings indicate that mitochondrial ROS formation is sufficient to generate the entire spectrum of cardiomyocyte responses to I/R, the mitohormesis elicited by MitoPQ appears to further explain the lack of success of antioxidant treatment in clinical settings.
2.3. Mitochondrial metabolism and cardioprotection
It is widely accepted that the mitochondrial tricarboxyl acid (TCA) cycle metabolite succinate significantly accumulates during tissue ischemia and is now seen as a hallmark of ischemia [52]. This phenomenon is not limited to acute myocardial infarction in the heart; it is observed in various conditions characterized by I/R injury as a core mechanism of tissue damage, including organ transplantation [358], stroke [359], and resuscitation [360]. Furthermore, succinate accumulation is not exclusive to ischemic conditions but also occurs during less specific stressors like exercise, hypothermia, and inflammation [[361], [362], [363]]. Therefore, mitochondrial metabolites are central to pathophysiology and represent a potentially useful therapeutic target.
The lack of oxygen, the terminal electron acceptor, coupled with the low tissue pH in ischemic conditions, leads to a reduced coenzyme Q (CoQ) pool and the generation of large amounts of succinate. During ischemia, succinate dehydrogenase (SDH) operates in reverse mode, utilizing electrons from the CoQ pool to reduce fumarate into succinate [364,365]. The fumarate may be generated by multiple sources including the degradation of AMP, which accumulates during ischemic events and is provided via the purine nucleotide cycle in the cytosol [366]. However, there is no identified transporter of fumarate, thus it is likely that the fumarate is first converted to malate and transported into mitochondria by the dicarboxylate transporter (DIC). Furthermore, cytosolic malate may also be generated by the transamination of aspartate [367]. To achieve high tissue succinate levels, succinate is transported into the cytosol by the DIC, where it can exchange for malate, bringing in further substrates to maintain the production of succinate [367]. The detailed sequence of events is displayed in Fig. 10.
Fig. 10.
Superoxide Production by Complex I during Reperfusion Injury. (A) Operation of complex I in the forward direction oxidizing NADH in order to generate a protonmotive force (Δp) to be used to synthesize ATP. For forward electron transport to occur the difference in reduction potential between the NAD+/NADH and the Coenzyme Q (CoQ) pool across complex I (ΔEh) has to be sufficient to pump protons across the mitochondrial inner membrane against the Δp. As four protons are pumped for every two electrons that pass through complex I, 2ΔEh > 4Δp is the requirement for the forward reaction to occur. The red arrow in complex I indicates forward electron transport. SDH, succinate dehydrogenase. (B) RET by complex I. When the Δp is large and/or the ΔEh across complex I is low such that 4Δp > 2ΔEh, electrons can be driven backward from the CoQ pool onto the FMN of complex I, reducing the FMN which can donate a pair of electrons to NAD+ to form NADH, or pass one electron to oxygen to generate superoxide. The red arrow in complex I indicates RET. (C) The factors that favor RET at complex I during reperfusion. The condition to be met for RET to occur is that 4Δp > 2ΔEh. The rapid oxidation of the succinate that accumulates during ischemia favors reduction of the CoQ pool, thereby maintaining a large ΔEh. The reduced CoQ pool also favors proton pumping by complexes III and IV helping maintain a large Δp upon reperfusion. In addition, the degradation of adenine nucleotides during ischemia limits ADP availability upon reperfusion that would otherwise diminish Δp by stimulating ATP synthesis. From Ref. [367] with permission.
When reperfusion is initiated by unblocking the culprit artery through interventions such as primary angioplasty in AMI or mechanical thrombectomy in stroke, the accumulated succinate is rapidly transported from the cytosol into the mitochondria by the DIC and oxidized by SDH and reaches normoxic levels within a few minutes [52,359]. Simultaneously, the electron transport chain resumes its function, and together with succinate oxidation maintaining a reduced CoQ pool, the initiation of proton pumping by complexes III and IV generates a high proton motive force (Δp) [368]. These two conditions, the reduced CoQ pool and high Δp, give rise to a phenomenon known as reverse electron transport (RET). RET is known to produce the proximal ROS superoxide at the flavin site of mitochondrial complex I [369]. The mechanism of RET produces the highest amount of superoxide in mitochondria. Although RET was initially believed to occur only in vitro, the drastic conditions in ischemic tissue enable its occurrence in vivo, establishing RET as the primary source of mitochondrial ROS production during I/R injury (Fig. 10).
Downstream, likely in conjunction with increased calcium levels, ROS are ultimately responsible for tissue damage through the mitochondrial PTP. When the PTP modulator Cyclosporin A is administered in vivo, the succinate increase during ischemia remains unaffected, suggesting that the RET/ROS mechanism at complex I indeed precedes the detrimental PTP opening [370]. Therefore, ischemia-accumulated succinate drives superoxide production by RET upon reperfusion which initiates the damage in I/R injury.
Blocking various stages of the aforementioned mechanism has shown profound protection against I/R injury in various models, diseases, and species. Inhibiting superoxide production by RET by targeting complex I with rotenone or MitoSNO has demonstrated cardioprotective effects [371,372]. However, prolonged inhibition of complex I disrupts electron flow through the electron transport chain, and studies have shown that mice with genetically reduced complex I activity develop severe cardiomyopathy [373].
Therefore, a more promising approach is to target succinate metabolism [374]. By inhibiting SDH, this can prevent succinate accumulation during ischemia or its oxidation during reperfusion. Malonate, a potent, naturally occurring competitive SDH inhibitor, can effectively block succinate accumulation when administered before or during the ischemic event [375]. While this is less useful in AMI where the ischemic window is unpredictable, this may be particular useful in situations such as organ transplantation or elective surgery where the onset of ischemia is known. More relevant for cardioprotection, malonate can also be given on reperfusion where it slows succinate oxidation. By slowing succinate oxidation, this prevents succinate maintaining a reduced CoQ pool and removes a driving force for RET to occur, preventing I/R injury [52].
In addition to there being sufficient succinate oxidized to produce superoxide by RET on reperfusion, some of the accumulated succinate effluxes from cardiomyocytes. In the heart, approximately 50% of the accumulated succinate is released into the circulation [366]. Patients with STEMI exhibit a significant increase in succinate release from the heart immediately upon reopening of the culprit artery through primary angioplasty [376].
Succinate export into the circulation occurs through the monocarboxylate transporter 1 (MCT1) [377]. Intriguingly, MCT1 is the same transporter responsible for the uptake of exogenous malonate when used as a cardioprotective agent during reperfusion. While malonate is a dicarboxylate at physiological pH and thus poorly diffuses across cell membranes, its monocarboxylic form is favored under low pH conditions [378,379]. As heart tissue reaches pH as low as pH 6 during ischemia, malonate uptake via MCT1 is highly efficient under these conditions. Additionally, an acidic formulation can further enhance this uptake, making acidic malonate an ideal candidate for cardioprotection against I/R injury at reperfusion [378]. In fact, infarct size reduction by intracoronary malonate has been demonstrated in pigs [380,381].
Once in the circulation, succinate represents the intriguing possibility of mitochondrial metabolites acting as a signal of ischemic events. Furthermore, the succinate may be involved in the subsequent mechanisms downstream or distal from the injury site, such as inflammation, or even remote organs. The precise function of the succinate receptor (SUCNR1) is not yet fully understood but may serve as the missing link in understanding these effects [382,383].
Thus, the example of succinate during I/R injury highlights the significance of mitochondrial metabolites as regulators of tissue damage and protection. Further research is necessary to unravel the regulation of mitochondrial metabolites in cardioprotection and to identify promising drug targets.
2.4. Mitochondrial reactive oxygen species, calcium and cardioprotection
2.4.1. Mechanisms
The redox state of cardiac mitochondria, that is, the ratio of reduced over oxidized pyridine nucleotides in the mitochondrial matrix, is continuously modulated by the availability of substrates and oxygen for oxidative phosphorylation, intracellular ion movements, and the intrinsic fitness of the organelle. Reducing equivalents derived from the oxidation of glucose and fatty acids via the TCA cycle maintain the proton-motive force that drives ATP production and sustain the regeneration of mitochondrial antioxidant enzymes required for ROS elimination (Fig. 11). Under physiological conditions, these processes are tightly coupled with the ATP demand imposed by excitation-contraction (EC) coupling via calcium (Ca2+) and adenosine diphosphate (ADP), which act in parallel to stimulate the TCA cycle and oxidative phosphorylation, respectively, thereby continuously adapting the rate of oxidative metabolism to the rate of ATP turnover in the cytosol [384]. In particular, mitochondrial Ca2+ uptake via the mitochondrial Ca2+ uniporter (MCU) complex is essential for matching energy supply and demand in cardiac myocytes, since Ca2+ activates rate-limiting dehydrogenases, i.e., pyruvate-, isocitrate- and α-ketoglutarate dehydrogenases of the TCA cycle (Fig. 11).
Fig. 11.
Regulation of mitochondrial respiration and redox state by ion handling. The Krebs cycle is stimulated by Ca2+ that enters mitochondria via the mitochondrial Ca2+ uniporter (MCU) and is exported by the mitochondrial Na+/Ca2+-exchanger (NCLX). The Krebs cycle produces NADH, which donates electrons to the electron transport chain (ETC). Sequential redox reactions along the ETC establish a proton gradient (ΔpH) across the inner mitochondrial membrane (IMM) which together with the electrical potential (ΔΨm) constitutes the proton motive force (ΔμH), which is harnessed by the F1/Fo-ATP synthase (ATPase) to regenerate ATP via oxidative phosphorylation of ADP. During respiration, superoxide (O2−) is generated at complexes I and III, which are dismutated to hydrogen peroxide (H2O2) by the Mn2+-dependent superoxide dismutase (MnSOD). H2O2 is then eliminated by glutathione peroxidase (GPX) and the thioredoxin/peroxiredoxin system (not shown). GPX is regenerated by reduced glutathione (GSH), which in turn is reduced by the glutathione reductase (GR), which uses NADPH that is produced by NADP+-dependent isocitrate dehydrogenase (IDPm) and the nicotinamide nucleotide transhydrogenase (NNT). Reactive oxygen species (ROS) from NADPH oxidases (Nox) 2 and 4, but also xanthine/xanthine oxidase (XO), nitric oxide synthase (NOS) or other mitochondria (Mitos) can activate redox-sensitive ion channels in the IMM, such as the permeability transition pore (PTP), the inner mitochondrial membrane anion channel (IMAC) or the ATP-sensitive K+-channel (KATP). Opening of these channels dissipates ΔΨm, requiring accelerated electron flux along the ETC to maintain ΔΨm. This oxidizes NADH and (via reverse-mode NNT) NADPH and thereby, the antioxidative capacity, limiting H2O2 elimination. ROS can leave mitochondria through the IMAC or PTP and trigger ROS release from neighboring mitochondria. Depending on the concentrations and durations of ROS elevations, ROS can serve protective roles, such as ischemic preconditioning, longevity and/or protein quality control, but at higher concentrations can deteriorate excitation-contraction coupling and induce epigenetic signaling, apoptosis and/or necrosis. When ΔΨm (transiently or permanently) dissipates, ATP production ceases, which activates sarcolemmal KATP channels, making the cell inexcitable. Heterogeneities of ΔΨm in different cardiac myocytes within the myocardium resemble “metabolic sinks” which can induce re-entry mechanisms to induce arrhythmias. In heart failure, elevated cytosolic [Na+]i accelerates mitochondrial Ca2+ extrusion, which can be ameliorated by inhibiting the NCLX with CGP-37157 (CGP) or lowering [Na+]i by inhibitors of the Na+/H+-exchanger (NHE), of late Na+ current (i.e., ranolazine) and as observed for Sodium/Glucose Co-transporter 2 (SGLT2)-inhibitors via inhibiting NHE and/or late Na+ current. CsA, cyclosporine A. GSSG, oxidized glutathione. CaMKII, Ca2+/calmodulin-dependent protein kinase II; HDAC4, histone deacetylase 4; EC coupling, excitation-contraction coupling. Modified from [398].
During ischemia, interruption of the supply of nutrients and oxygen to cardiac myocytes stops oxidative metabolism. The consequent shortage of ATP and phosphocreatine (PCr) hinders cellular ion handling and EC coupling. Hydrolysis of ATP derived from glycolysis leads to accumulation of protons (H+) and intracellular acidosis [385], which decreases myofilament Ca2+ affinity [386]. The acidic intracellular pH is partly compensated by extrusion of H+ in the form of weak acids (lactate) and via the sarcolemmal sodium (Na+)/H+ exchanger (NHE) [387]) but this, together with the decreased activity of the sarcolemmal Na+/K+ ATPase and Na+ influx via non-inactivating Na+ channels, elevates intracellular Na+ concentration ([Na+]i) [388]. In turn, high [Na+]i increases cytosolic Ca2+ concentrations ([Ca2+]c) because of the decreased trans-sarcolemmal Na + gradient that drives Ca2+ extrusion via the Na+/Ca2+ exchanger (NCX) [389]. Further, elevation of [Ca2+]c can be attributed to Ca2+ entry via L-type Ca2+ channels and impaired Ca2+ reuptake into the sarcoplasmic reticulum (SR) due to decreased activity of the SR Ca2+ ATPase (SERCA) [390]. Upon reperfusion, physiological pH is restored within a few minutes as the NHE and Na+-dependent bicarbonate exchange extrude excess H+ to the extracellular space. The increased NHE activity during the first seconds of reperfusion might further aggravate [Na+]i overload [391] and lead to even more Ca2+ influx via the reverse mode of the NCX.
Mitochondrial Ca2+ overload is considered one of the main mechanisms of irreversible mitochondrial damage and subsequent cardiac myocyte death on myocardial reperfusion [392,393]. It is still debated whether Ca2+ overload is mediated by Ca2+ uptake via the MCU or by the mitochondrial NCX, which represents the primary Ca2+ efflux pathway under physiological conditions (Fig. 11), operating in reverse mode during ischemia and the early phase of reperfusion [394]. Mitochondrial Ca2+ overload partly dissipates the proton motive force and, above a certain threshold, triggers PTP opening, in particular in combination with oxidative stress [392,395]. Mitochondrial permeability transition is inhibited during ischemia by the acidic cytosolic environment; therefore, PTP opening happens in the early phase of reperfusion, when the intracellular pH is restored [387]. On these grounds, the cardioprotective effects of PostC can be traced to the delayed recovery of intracellular pH [396].
Reperfusion is accompanied by burst-like release of ROS from mitochondria, which is considered an early driver of cardiac myocyte death. Breakdown of purine nucleotides during ischemia produces fumarate, which is converted to succinate by the reversal of SDH (complex II of the electron transport chain) reaction. Upon reperfusion, succinate is rapidly oxidized by SDH, driving reverse electron transport and extensive superoxide production at complex I [52]. Mitochondrial ROS production induces mitochondrial membrane instability and sensitizes to PTP opening [394]. Furthermore, the burst of ROS during reperfusion of ischemic myocardium causes cytosolic Ca2+ overload and myocardial “stunning”, which is characterized by diastolic and systolic dysfunction that is - at least to some extent – reversible [182,397].
2.4.2. Redox-optimized ROS balance
Mitochondrial superoxide is efficiently dismutated to H2O2 by the Mn2+-dependent SOD (MnSOD), while elimination of H2O2 is governed by glutathione peroxidase and the thioredoxin/peroxiredoxin systems, which all require NADPH for regeneration (Fig. 11) [398]. NADPH, in turn, is produced by enzymes that derive their substrates from the TCA cycle, i.e., malic enzyme, isocitrate dehydrogenase and NNT. Therefore, during physiological increases in cardiac workload, mitochondrial Ca2+ uptake is required not only to maintain NADH for ATP production, but also of NADPH to detoxify ROS (Fig. 11) [384]. While the primary formation of superoxide is highest when the mitochondrial redox state (and therefore, the ETC) is highly reduced [399] elimination of ROS is compromised when the mitochondrial redox state (and in particular, of NADPH) is strongly oxidized. Therefore, in working cardiac myocytes, the lowest net H2O2 emission occurs at an intermediate redox state, as expressed by the concept of “redox-optimized ROS balance” [400].
2.4.3. ROS-induced ROS release
A feedforward mechanism to boost mitochondrial ROS emission is the concept of “ROS-induced ROS-release”, where ROS activate redox-sensitive ion channels in the inner mitochondrial membrane, such as the PTP [401], inner mitochondrial anion channels (IMAC) [402,403] and ATP-dependent K+ channels (mKATP) [404,405] of the same or neighboring mitochondria(Fig. 11). Activation of these channels dissipates the mitochondrial membrane potential (ΔΨm) and thereby accelerates oxidation of NADH and consequently, of NADPH through the reverse-mode of the NNT [406], depleting the anti-oxidative capacity and provoking ROS emission (Fig. 11). Such ROS-induced ROS release with IMAC activation underlies synchronous oscillations of the mitochondrial membrane potential, creating “metabolic sinks” which makes those areas of the myocardium unexcitable and thereby provides a substrate for re-entrant arrhythmias during cardiac I/R [403]. While opening of the IMAC occurs at low amounts of oxidative stress and is often reversible, activation of the PTP typically occurs at higher amounts of ROS and is rather irreversible, often inducing necrotic cell death [407]. ROS-induced ROS release was also observed through communication between different sources of ROS, such that ROS from NOX2 can increase mitochondrial ROS [405,408,409] and vice versa, mitochondrial ROS can increase NOX2-related ROS (Fig. 11) [410].
2.4.4. ROS and mitochondrial KATP channels
Cardioprotection through IPC requires low amounts of mitochondrial ROS, which can be induced by pharmacological opening of mKATP channels [333,[411], [412], [413]], Many signaling pathways that induce preconditioning converge onto mKATP channels [414]. These ROS then trigger protective pathways that eventually reduce the amounts of mitochondrial ROS released during a larger I/R injury [411]. The mere existence and the molecular composition of the mKATP have been enigmatic for decades; recently, CCDC51 was identified to form a channel with mKATP-like properties when associating with the ATP Binding Cassette protein 8 (ABCB8) [415], which had already been shown earlier to modulate mKATP activity [416]. Alternatively, mKATP channel activity has been proposed for F1F0-ATP synthase based upon its K+ conductance [417]. Notably F1F0-ATPase has been also described as a structural component of the PTP [392,395]. Additional studies are necessary to clarify the role of F1F0-ATPase in cardioprotection related to mKATP and PTP (Fig. 11) [418].
Also beyond I/R, mitochondria are considered the major source of ROS in heart failure [384]. In failing hearts, superoxide is generated at complex I and is converted to hydroxyl radical [419,420]. Furthermore, dysregulated cytosolic Ca2+ and Na+ handling deteriorate mitochondrial Ca2+ accumulation and thereby, regeneration of NADH and NADPH in failing cardiac myocytes, which contributes to energetic deficit, oxidative stress, contractile dysfunction and arrhythmias [384]. Furthermore, the mere increase in afterload, which is typical for heart failure, drains anti-oxidative NADPH towards NADH and ATP production, but at the cost of the anti-oxidative capacity, thereby further increasing the emission of H2O2 from mitochondria [406]. This increase in ROS is causal for the development of necrosis, progressive heart failure and death [404,406,421,422].
2.4.5. Interventions
Based on this mechanistic framework, several therapeutic strategies have been developed and tested in animal models and, in some instances, clinical trials to mitigate cardiac I/R injury. Mitochondrial permeability transition is considered the final common pathway through which dysfunctional mitochondria induce cardiac myocyte death; indeed, PTP inhibition with cyclosporine or genetic ablation of cyclophilin D substantially reduce the sensitivity to I/R injury in rodents [423]. Despite these promising results in animal models and in one phase 2 trial [231], intravenous infusion of cyclosporine immediately prior to reperfusion did not improve 1-year cardiovascular outcomes nor affected left ventricular remodeling in patients with anterior STEMI in a phase 3 randomized controlled trial [232].
Therapeutic strategies targeting processes upstream of PTP opening, i.e. preventing mitochondrial Ca2+ overload or dampening mitochondrial ROS, yielded conflicting results. While antioxidant therapy after reperfusion with mitochondria-targeted coenzyme Q (Mito-Q, Fig. 11) reduced infarct size [424], also the inhibition of respiratory chain complexes I [425] or II [426] as well as mild mitochondrial uncoupling [427] were cardioprotective in I/R injury by inhibiting succinate-mediated ROS production. Inhibiting IMAC during I/R with 4′-chlorodiazepam prevented postischemic ventricular arrhythmias [403].
In principle, interventions that reduce the rise in cytosolic Ca2+ during I/R also reduce the amount of myocardial injury [428]. Genetic deletion or pharmacological inhibition of the NHE reduced infarct size in mice [429], but did not improve outcome in patients with myocardial infarction [430]. Sodium-glucose transporter 2 (SGLT2) inhibitors reduce hospitalization for heart failure and cardiovascular death in patients with diabetes, chronic kidney disease and heart failure [431,432]. Since the benefits in patients with heart failure are independent of the presence of diabetes, it has been suggested that SGLT2-inibitors directly target the heart. In fact, SGLT2-inhibitors block the NHE [433,434], but also the late sodium current (Fig. 11) [[435], [436], [437]], which is considered the main source of elevated [Na+]i in heart failure [438]. In support of such concepts, SGLT2-inhibitors reduced infarct size in vivo in mice with genetic global deletion of SGLT2 [439], which may be related to lowering of [Na+]i and consequently, cytosolic Ca2+ to prevent mitochondrial Ca2+ overload (Fig. 11).
In mice, cardiac myocyte-specific, inducible (but not global constitutive [440] knock-out of the MCU protected against mitochondrial Ca2+ overload and PTP opening, reduced infarct size and improved postischemic cardiac function [441]. Also in rats, pharmacological inhibition of the MCU with Ru360 (Fig. 11) improved cardiac post-ischemic functional recovery [442], however, it is unclear whether this effect is indeed related to a reduction in mitochondrial Ca2+ accumulation. Since the main uptake of Ca2+ into mitochondria during I/R is still controversial, it is relevant to mention that inhibiting the NCX did not reduce infarct size, but efficiently prevented arrhythmias in pigs after myocardial I/R. In guinea pigs with heart failure, NCX inhibition protected from contractile dysfunction, cardiac remodeling and ventricular arrhythmias [443].
2.5. Thiol-oxidant signaling in myocardial health and disease
2.5.1. Oxidants are produced by cells and are implicated in disease progression
Cells produce a great variety of molecules capable of oxidatively modifying other components in the system. Although these oxidant molecules are conveniently grouped together under the umbrella terms ROS or nitrogen species (RNS), this is not always helpful as it does not consider the enormously variable chemistry between these entities. This is important to remember because these biochemical reactions underlie the cellular impact of these species, both in the context of oxidative damage as well as the sensing and signalling they can initiate. This illustrates the importance of defining the species of oxidant that is measured and using absolute quantitation, which cannot be readily achieved with fluorescence reporter probes [444]. Measuring specific oxidants is notoriously difficult, not least because their reactivity often makes their existence fleeting, although their lifetime varies enormously between molecular species, cellular location and environmental conditions. Nevertheless, the anticipated high reactivity of oxidants is a cornerstone of the paradigm that they causally mediate disease by oxidizing and thus damaging the fabric of the cell. Of course, as we will come to below, oxidation events may not be solely synonymous with damage and instead may represent passive or indeed oxidant sensing events that mediate homeostatic signal transduction.
A common scenario is for a study to report that ROS, RNS or markers of their presence are increased in samples from patients or disease models compared with healthy controls. However, these measurements of oxidant abundance or indirect markers of oxidative stress could simply reflect epiphenomena that are not causative in the pathogenesis. Indeed, it has been questioned how useful it is for our community to keep measuring oxidants levels [445], especially when it is often done in a way that does not define the molecular species and does not use absolute quantification with reference standards. Although oxidants can mediate homeostatic or protective signaling, they continue to be routinely implicated as important, causal mediators of all manner of diseases, including those of the cardiovascular system. Causality has mostly been established through the use of antioxidants, with a plethora of preclinical studies showing these interventions alleviate both the oxidative stress and improve function. Studies with transgenic models in which oxidant producing or scavenging enzymes were modulated provided more evidence that oxidants were pathogenic. Furthermore, tool compounds or drugs that inhibit oxidant-generating enzymes have proven therapeutic. Thus, it seems abundantly clear that ROS or RNS can causally mediate cardiovascular disease at multiple levels, including inflammation, atherosclerosis, hypertension and heart failure [446]. Cardiac ischemia and hypoxia are often associated with increased ROS, but there is complexity and controversy as there is evidence that the decrease in oxygen availability decreases oxidant levels [447]. This may relate to reductive stress, which is recognized in the pathogenesis of cardiovascular pathologies. The pentose phosphate pathway generates the reductant NADPH used by multiple antioxidant enzyme systems to scavenge oxidants, but it is also used for anabolic membrane and nucleic acid synthesis and is causatively upregulated during pathological cardiac hypertrophy [448].
ROS contribute to LV dysfunction [449] in mice with transgenic overexpression of ROS formation [450], in rabbits with pacing-induced heart failure [451], in pigs with coronary microembolization [452] and in dogs with postischemic contractile dysfunction (stunning) [453,454]. Post-ischemic reperfusion, which is essential for tissue survival, resupplies oxygen and is associated with oxidant-induced reperfusion injury that in countless pre-clinical studies has been attenuated by antioxidants.
2.5.2. Sources of ROS and the susceptibility of sulfur containing targets to oxidation
ROS and RNS-induced damage were often considered indiscriminate because they oxidise most biomolecules in their path. However, we now understand oxidant production can be localized - constrained to specific organelles or signalling complexes. Sources of oxidants include, but are not limited to, mitochondria [52], NOX [455], xanthine oxidoreductase, MAOs or uncoupled nitric oxide synthase (NOS) or inducible NOS generating nitrosating entities [446]. Localized production is anticipated to constrain and therefore limit the number of targets that are oxidatively modified, a scenario that is more compatible with regulated oxidant signalling. Further potential for regulation comes from some targets being preferentially susceptible to oxidation, which together with the selective reactivity of oxidants enables fidelity and regulation.
Many amino acids in proteins can be oxidized, but the sulfur containing amino acids methionine and especially cysteine are preferentially susceptible. The cysteine thiol (−SH) is not intrinsically reactive with oxidants. However, in some proteins the proton can be donated to an acceptor (e.g., the side chain of lysine, arginine or histidine) to electrostatically stabilize the thiolate (-S-) state, which is very reactive and enables them to form oxidative post-translational modifications. There is substantial diversity in the post-translational modifications that may occur on protein thiols, varying with the oxidant encountered and its abundance and duration of exposure.
2.5.3. Redox sensitive proteins cysteines in cardiac disease and protection
Vast numbers of proteins are now known to be regulated by various oxidative post-translational modifications of cysteines, which can impact cellular homeostasis and disease pathogenesis. Indeed, the Oximouse study reported tens of thousands of reversibly modified cysteines with significant oxidation stoichiometry that was enabled by their proximal amino acids [456]. Ageing, as with major diseases, has been repeatedly touted to be mediated by oxidative damage. However, the Oximouse study found no widespread increase in protein cysteine oxidation with age and notably, the sites with a high modification stoichiometry were more reduced to the thiol state in older mice. This finding resonates with a continually emerging literature, together with the considerations above, that oxidants are not simple perpetrators of disease but also facilitate signalling that enable homeostasis during health or enable protective adaptation during disease.
Whilst oxidation of proteins, including their sensitive cysteine residues, has been implicated in I/R injury [457], it is notable that oxidants are important triggers of IPC and this cardioprotective process is blocked by antioxidants [458]. In addition to ROS or RNS production during the protection phase initiating signaling that limits injury, they induce S-nitrosation [459] as well as disulfides [458] of protein cysteines that may protect them from irreversible, harmful over-oxidations during subsequent longer damaging periods of I/R. S-nitrosation of mitochondrial complex I at a specific cysteine has been shown to be cardioprotective against IR by decreasing their production of ROS [425]. The redox state of specific cysteine residues in proteins including protein kinase A RIα, protein kinase GIα, soluble epoxide hydrolase and optic atrophy 1 impact broadly on cardiovascular physiology and disease outcomes [457,460,461].
2.5.4. Cardiovascular protection by pro-oxidants and electrophilic drugs
It is clear that the redox state of cardiovascular tissues is perturbed both during health and disease-related scenarios. Although most of the focus has been on oxidants causing pathologies, it is notable as considered above, in some scenarios, tissues instead become more reduced, that antioxidants are not therapeutic against human cardiovascular disease and that IPC protection is associated with induction of protein oxidation. Furthermore, relatively unselective pro-oxidant interventions that are anticipated to induce widespread oxidation of protein thiols can attenuate infarction during IR [372,458]. Nitroxyl (HNO), a reduced derivative of nitric oxide, is a potent unselective thiol oxidizer generated endogenously and donors of which are showing therapeutic promise against heart failure, improving systolic and diastolic function, and as an anti-hypertensive [460]. This cardioprotection afforded by a drug that donates an RNS is a notable example of how such species do not simply mediate dysfunction. However, the complexity is highlighted by endogenous S-nitrosating species derived from inducible NOS causing heart failure with preserved ejection fraction by inducing the S-nitrosation of endonuclease inositol-requiring protein 1α [446]. Endogenous production of hydrogen peroxide by NOX4 protects the heart from hypertrophic growth [455]. There are other classes of electrophilic drugs that induce protein cysteine oxidation that are used clinically (e.g., dimethyl fumarate) or have regulatory approval for human clinical trials such as the stabilized sulforaphane derivative and SFX-01 and nitro-alkenes, consistent with a growth and significant advances in the development of drugs that target and covalently modify cysteines in proteins [462].
How to reconcile protection from these oxidant interventions from the robust contradictory evidence that they mediate disease is not straight forward. However, if we consider there are tens of thousands of redox active protein cysteines [456]. We can understand that the net outcome of an oxidative stress will be scenario-specific, with different profiles and outcomes from protein oxidation depending on the source and species of oxidant, as well as their abundance and duration of supply. Some protein oxidation events will be neutral, some beneficial, and others detrimental, and the net outcome will have to be considered on a case-by-case basis. Early studies with pan-specific kinase inhibitors, have now given way to molecules with high target precision and we do not typically consider global tissues phosphorylation or dephosphorylation to be ‘good or bad’, whereas historically oxidation is mostly synonymous with ‘bad’. We understand phosphorylation to be an important and complex regulatory mechanism that depending on the specific situation can mediate both health maintenance as well as disease progression, and the same can be applied to oxidative post-translational modifications. Clearly, we already have electrophilic drugs, mentioned above, in clinical use or development that are not very selective and modify many targets, including those that do not mediate the therapy and may cause side effects. In the same way as highly specific kinase inhibitors have been developed that overcome the off-target effects of broad-spectrum drugs, this can be achieved for specific cysteine residues in a protein [462]. Indeed, FMK was engineered to selectively inactivate p90 RSK1/2, a kinase that can mediate myocardial dysfunction, by covalently adducting a conserved cysteine thiol in their C-terminal kinase domain to prevent ATP utilization [463]. Furthermore, thiol-reactive drug G1 was developed that selectively induces the oxidation of protein kinase G Iα cysteine 42, targeting and activating the kinase to lower blood pressure in hypertensive mice [464]. It is anticipated that a variety of new drugs will eventually emerge that selectively target a specific regulatory cysteine residue in a protein to provide new therapies, including for diseases of the cardiovascular system.
2.6. Hydrogen sulfide and cardioprotection
Hydrogen sulfide (H2S), an endogenous signaling molecule, has been recognized, in the last decades as an important endogenous gasotransmitter that influences several important (patho-)physiological processes related to cardiovascular health and disease [465,466]. Endogenous H2S is as an important regulator of the cardiovascular system, particularly of myocardial function [467] and its upregulation may reduce ischemic injury [468]. Findings on the endogenous synthesis of H2S and its protective role in cellular necrosis, apoptosis, oxidative stress and inflammation in myocardial I/R injury have encouraged interest in the development of new therapies based on facilitation of endogenous H2S for cardioprotection [469].
H2S is a second messenger implicated in protection from oxidative stress by direct scavenging of ROS and indirect antioxidant effects [470]. H2S is generated mainly by cystathionine γ-lyase (CSE) in cardiovascular tissues [468]. In CSE knockout rats subjected to I/R injury, oxidative stress was aggravated, whereas increased expression of H2S and CSE in aortic tissues resulted in alleviation of oxidative stress accompanied by reduced expression of apoptosis-related proteins [471]. H2S produced by CSE improves contractile function in heart failure, and treatment with S-propyl-l-cysteine (SPRC) or sodium hydrosulfide (NaHS), modulators of blood H2S levels, attenuated the development of heart failure, reduced lipid peroxidation, and preserved mitochondrial function in mouse models [472]. However, CSE gene deletion does not substantially exacerbate the long-term response to myocardial infarction, and the H2S donor GYY4137 when administered after the onset of myocardial infarction preserved cardiac function and protected against adverse cardiac remodeling in both WT and CSE-deficient mice [468].
Conditioning-like infarct limitation by enhanced level of H2S has been demonstrated in many animal models of myocardial I/R injury in vivo [473]. Numerous studies have shown that H2S has a significant protective role in myocardial I/R injury and is considered an important mediator of IPC, through different mechanisms which include: i) activation of mitochondrial potassium channels, ii) reduction of oxidative stress and activation of endogenous antioxidant mechanisms, iii) limitation of inflammatory responses, iv) preservation of mitochondrial function, v) angiogenic actions, vi) interaction with NO, and vii) S-sulfhydration [467,[474], [475], [476]]. H2S is also involved in postconditioning-induced cardioprotection through multiple mechanisms, including redox-based post-translational modification on protein cysteine residue(s), i.e. S-sulfhydration (SSH) and preservation of mitochondria [477,478]. Since endogenous concentrations of H2S are generally low, the necessity for an exogenous source of H2S provides a unique challenge for the development of chemical tools that facilitate the study of H2S under biological conditions. H2S donors include a wide variety of functional groups and delivery systems, some of which mimic the tightly controlled endogenous production in response to specific, biologically relevant conditions [reviewed in] [479]. The most common class of H2S donors employed in biological studies are the sulfide salts, sodium hydrosulfide (NaSH) and sodium sulfide (Na2S) which in many in vivo studies reduced myocardial infarct size and conferred cardioprotection when administered prior or during reperfusion [470,[480], [481], [482]]. A mitochondria-specific H2S compound, AP39, protects against I/R injury, an effect mediated through inhibition of PTP opening via a cyclophilin D-independent mechanism [483]. Τhioester-based H2S donors release H2S in a slow and controllable manner that could mimic its slow-releasing process in vivo. The donor 5e reduced myocardial infarct size and cardiomyocyte apoptosis in mice [484]. A macromolecular H2S prodrug grafts 2-aminopyridine-5-thiocarboxamide (a small-molecule H2S donor) on partially oxidized alginate (ALG-CHO). This drug formulation then mimics the slow and continuous release of endogenous H2S and tetra-aniline (a conductive oligomer) and adipose-derived stem cells (ADSCs) and forms a stem cell-loaded conductive H2S-releasing hydrogel. After myocardial injection, longer ADSCs retention and elevated sulfide concentration in rat myocardium were demonstrated, accompanied an increased ejection fraction and smaller infarct size [485]. Isothiocyanate-based H2S-releasing drugs decreased I/R-induced tissue injury in an in vivo model of acute myocardial infarction in rats [467]. The newly designed H2S-releasing ibuprofen derivative, BM-88, reduced infarct size in the ischemic/reperfused myocardium in isolated rat hearts [486]. Furthermore, reactive sulfur species, including RSSH and polysulfides, exhibit cardioprotective actions [487].
Since multitarget cardioprotective therapies can possibly more effectively translate cardioprotection to patients, there are some hybrid molecules that may have 2 or more structural domains and act as 2 distinct pharmacophores to provide additive cardioprotection [57]. A hybrid compound that combines the adenine nucleus with a moiety that slowly releases H2S induced additive infarct size reduction in anesthetized rabbits [488]. H2S and NO share a wide range of physical properties and physiological functions that not only affect each other's biosynthesis but also produce novel species through chemical interaction and play a regulatory role in the cardiovascular system through similar signaling mechanisms or molecular targets [489]. Based on the above, therapeutic strategies that increase both H2S and NO have emerged such as ZYZ-803, a hybrid molecule of a dual donor for H2S and NO which preserved cardiac function and reduced infarct size significantly after 24 h left coronary artery ligation through reversing an H2S and NO imbalance in mice [490].
Multiple targets for H2S-releasing drugs have been identified for pre- and postconditioning, such as PTP, KATP, PKC, eNOS/cGMP, ERK/GSK3β, VEGF/JAK2/STAT-3/iNOS signaling, attenuation of ROS, SSH on protein cysteine residue(s) [477], and mitochondrial preservation, indicating that H2S may attenuate irreversible I/R injury [491,492]. A summary of these major cardioprotective pathways initiated by H2S is provided in Fig. 12. In a meta-analysis, preconditioning with H2S caused an infarct limitation of - 20-25% and postconditioning limited infarct size by - 21-61% and this infarct-sparing effect was robust and consistent when H2S was applied before ischemia or at reperfusion, independently of animal species or sulfide source [473].
Fig. 12.
Schematic illustration of the effects of H2S in different heart diseases and the molecular mechanisms underlying H2S-induced cardioprotection. From Ref. [492] with permission.
However, most of the studies investigating the cardioprotective effects of H2S have been performed in small animal I/R injury models. For translation to the clinical situation of large animal I/R injury models are important [493], but only few studies have evaluated the cardioprotective effects of H2S in large animal models. H2S infusion initiated at the onset of ischemia and continued into the reperfusion period, but not bolus administration over 10 s at the start of reperfusion markedly reduced myocardial infarct size, improved regional left ventricular function, as well as endothelium-dependent and endothelium-independent microvascular reactivity in Yorkshire pigs [494]. Treatment with sodium sulfide 10 min prior to the onset of reperfusion improved myocardial function, reduced infarct size and improved coronary microvascular reactivity potentially through anti-inflammatory properties in Yorkshire pigs [495]. Zofenopril, a sulfhydrylated angiotensin-converting enzyme inhibitor increased H2S and NO bioavailability via bradykinin-dependent signaling, reduced myocardial infarct size and cardiac troponin I levels after IRI when administered 7 days before 75 min ischemia and 48 h reperfusion in pigs [496].
More recently, several studies also focused on the cardioprotective effects of H2S in the presence of co-morbidities. H2S ameliorated I/R injury in different animal models of diabetic cardiomyopathy (DM) through different pathways that regulate apoptotic, autophagic, necroptotic, and pyroptotic cell death [497,498]. Preconditioning with H2S reduced infarct size in isolated rat hearts with diabetes and DM [499]. Divergent results exist on H2S postconditioning in diabetes; H2S reduced infarct size in isolated diabetic rat hearts [500], whereas in other studies H2S post-conditioning failed to induce cardioprotection in diabetic rat hearts, mainly due to their altered myocardial architecture along with exacerbated oxidative stress and mitochondrial dysfunction [500]. Obesity increases the risk of developing diabetes and subsequently DM. Reduced cardioprotective and antioxidant H2S and increased pyroptotic cell death contribute to adverse cardiac remodeling and DM, and exercise training prevented the development of DM possibly by promoting H₂S-mediated cardioprotection and attenuating pyroptosis in mice [501]. Cardioprotection is effective in young hearts but is lost in aged hearts [96]. Exogenous H2S restored postconditioning-induced cardioprotection through decreasing infarct size and apoptosis, improving cardiac function, and increasing cell viability and autophagy in aged hearts and cardiomyocytes. Additional mechanisms involved up-regulation of HB-EGF/EGFR signaling, which activates the ERK1/2-c-myc and PI3K-Akt- GSK-3β pathways in aged cardiomyocytes of rats and in H9C2 cardiomyocytes [502,503].
All the above observations have inspired the rapid development of H2S-releasing compounds for clinical translation to patients with cardiovascular disease. The H2S prodrug SG-1002 has been evaluated in a phase I clinical trial [ID: NCT01989208] in healthy and heart failure subjects, showing attenuation in the increases in BNP in heart failure patients [504], but it is not used in the treatment of heart failure patients because of its lack of a stable, controllable H2S booster [505]. Despite its promises from preclinical studies, H2S has not been evaluated in humans. The Groningen Intervention study for the Preservation of cardiac function with sodium thiosulfate (STS) after STEMI (GIPS-IV) used a double-blind, randomized, placebo-controlled, multicenter trial design and enrolled patients with a first STEMI who received the H2S-donor sodium thiosulfate in addition to standard care immediately at arrival in the catheterization laboratory. Unfortunately, this trial was not completed according to the original power-analysis based study design, and all results including the primary endpoint infarct size after 4 months by CMR were neutral [506].
In summary, H2S has emerged as an important cardioprotective molecule with potential for clinical applications. H2S-donors as pro-drugs able to generate exogenous H2S, are viewed as promising therapeutic agents for a number of cardiovascular diseases. However, the influence of co-morbidities and co-medication has not been really studied in most preclinical studies, and data from humans are limited or absent.
2.7. Protective effects of NADPH oxidases on the heart
NOXs are a family of multi-subunit transmembrane enzymes that generate ROS (either superoxide anion [*O2−] and/or [H2O2] through NADPH-dependent reduction of molecular oxygen. ROS generation is a primary function of the NOXs, in contrast to most biological enzymes (e.g. the complexes of the mitochondrial electron transport chain or nitric oxide synthases) which have other primary functions. NOXs are now recognized to be especially important in mediating specific redox signaling in diverse physiological and pathophysiological contexts, a property that relates to NOX-mediated ROS production typically being physiologically regulated and spatially compartmentalized within or outside cells [[507], [508], [509]]. The combination of finely regulated ROS production (on a temporal and quantitative level) and proximity between ROS source and target(s) underpins highly specific molecular signaling in many cases. Of particular relevance to cardioprotection, NOXs have significant potential to mediate adaptive or protective responses through such signaling, in stark contrast to non-specific detrimental effects associated with high level ROS production and oxidative stress. The NOX family of enzymes comprises 7 isoforms (NOX1-5 and DUOX1-2) among which NOX2 and NOX4 are significantly expressed in the heart and are the focus of this section.
NOX2 was initially identified more than 30 years ago as the phagocytic oxidase responsible for high-level ROS production (the “oxidative burst”) during neutrophil phagocytosis. It is a complex enzyme that when inactive is formed of a membrane-bound heterodimer between the catalytic subunit NOX2 (also known as gp91phox) and a smaller p22phox subunit. Activation of the enzyme involves post-translational modifications of several cytosolic subunits (p67phox, p47phox, Rac1 or Rac2, and p40phox) induced by intracellular signaling cascades, followed by their translocation to the membrane to associate with the NOX2-p22phox heterodimer and the triggering of electron transfer from NADPH bound to NOX2 to molecular oxygen on the other side of the membrane. We now know that NOX2 is expressed in numerous non-phagocytic cells including cardiomyocytes, endothelial cells and fibroblasts where it is involved in intracellular redox signaling [[510], [511], [512]]. Cardiomyocyte NOX2 is located predominantly on the plasma membrane and T-tubules and is activated by G-protein coupled receptor (GPCR) agonists (such as angiotensin II, endothelin-1), cytokines, growth factors and mechanical forces [513]. NOX2-mediated redox signaling in cardiomyocytes (and other non-phagocytic cells) appears to be underpinned by endosomal signaling and a much lower level of ROS production than in neutrophils.
NOX4 is a deceptively simple isoform that like NOX2 exists as a membrane-bound heterodimer with a p22phox subunit but exhibits constitutive low-level ROS production and does not require cytosolic subunits for activation. Other striking differences from NOX2 are its intracellular location at the endoplasmic reticulum (ER) and the production of H2O2 rather than *O2− as its main ROS product [514]. NOX4 has a very wide (possibly ubiquitous) tissue distribution and appears to be regulated predominantly by its protein abundance [507]. It is highly inducible by numerous stressful stimuli such as ischemic, metabolic, neurohumoral and mechanical cues, secondary to both transcriptional activation (notably by ATF4) and post-transcriptional mechanisms [513,515]. NOX4 has attracted considerable attention because of its substantial potential to mediate adaptive (protective) signaling in the heart and elsewhere, underpinned by its involvement in several fundamental stress pathways [513] A summary of these adaptive and maladaptive pathways initiated by NOX isoforms is provided in Fig. 13 [516].
Fig. 13.
Roles of NADPH oxidases (NOX) in physiology and pathophysiology. Both NOX2 and NOX4, the main NOX isoforms in cardiomyocytes, can regulate intermediary metabolism in response to a variety of stresses. Several mechanisms are involved, including activation of transcription factors or in the case of NOX4 location at the MAM, targeted ROS signaling to influence mitochondrial function and cell viability. ATF4, activating transcription factor 4; NRF2, nuclear factor erythroid factor 2-related factor 2; HIF1α, hypoxia-inducible factor 1-alpha; ISR, integrated stress response. From Ref. [516] under the Creative Commons license.
2.7.1. NOX2-dependent adaptive and maladaptive pathways
The vast majority of studies on NOX2 in the heart have reported that it augments pathological cardiac remodeling in response to stresses such as chronic neurohumoral activation, chronic hemodynamic overload and acute myocardial infarction [513]. Many different components of the pathological remodeling phenotype are enhanced by NOX2, including cardiomyocyte hypertrophy, cardiomyocyte cell death, abnormal excitation-contraction coupling, arrhythmia, contractile dysfunction, inflammation, interstitial fibrosis and ventricular dilatation. These effects typically involve the NOX/redox-mediated amplification of responses such as kinase signaling, transcription factor activation, aberrant Ca2+ release from the SR, and/or matrix metalloprotease activation. Genetic studies that employed cell-specific manipulation of NOX2 levels in mice have also established that different NOX2-expressing cells in the heart – including cardiomyocytes, endothelial cells, fibroblasts and immune/inflammatory cells – make distinct contributions to these responses [513].
More recently, it has become evident that NOX2 also has a number of physiological actions. Prosser et al. [517] showed that cardiomyocyte NOX2 physiologically tunes stretch-induced release of Ca2+ from the SR and thereby optimizes length-dependent augmentation of contractile performance. Physiological increases in contractile performance in response to acute angiotensin II stimulation are also amplified by NOX2, this time through an enhancement of SR Ca2+ uptake [518]. Another adaptive role for NOX2 is reported to be an involvement in myocardial ischemic preconditioning which was found to be blunted in mice globally deficient in NOX2 [519]. NOX2 is capable of activating hypoxia-inducible factor 1α (HIF1α) [520], which might also contribute to adaptive responses although direct investigation of this remains limited.
2.7.2. NOX4-dependent adaptive pathways
Initial studies that investigated the role of NOX4 in the heart using gene-modified mouse models generated controversy because opposite effects (protective or detrimental) in the setting of chronic pressure overload were reported by the 2 laboratories undertaking independent studies [521,522]. With time, however, compelling evidence has accumulated that NOX4 mediates protective effects in the heart under diverse stress situations; even the group that initially reported detrimental effects subsequently observed protective features [513,523]. Moreover, protective effects of NOX4 against disease stress are also observed in many other tissues. It has become evident that part of the explanation for detrimental effects of NOX4 in some disease models relates to very high levels of induced NOX4 overexpression and/or the disruption of coupling to signaling pathways essential for its protective effects [524].
2.7.2.1. NOX4-dependent activation of HIF1α
Studies employing global and cardiomyocyte-targeted mouse models of NOX4 deletion or NOX4 overexpression revealed that an increase in NOX4 levels during chronic pressure overload was beneficial by reducing the magnitude of cardiomyocyte hypertrophy, contractile dysfunction, interstitial fibrosis and chamber dilatation [521]. An increase in endogenous NOX4 levels was found to be essential for activation of a HIF1α -vascular endothelial growth factor (VEGF) axis that preserves myocardial capillary density and cardiac contractile function during chronic hemodynamic overload, consistent with prior reports of the importance of this axis in this setting. NOX4 both in cardiomyocytes and endothelial cells appears to contribute to these effects [525]. It is suggested that the mechanism of HIF1α activation by NOX4 involves a ROS-dependent inhibition of the prolyl hydroxylase that regulates HIF1α stability. NOX4-dependent HIF1 activation has also been reported to contribute to cardioprotective effects during ischemia but the mechanisms downstream of HIF1α that mediate protection require more study. Interestingly, protective effects of NOX4-dependent HIF1α activation are also reported in the peripheral vasculature and kidneys.
2.7.2.2. NOX4-dependent activation of NRF2
Nuclear factor erythroid 2 related factor 2 (NRF2) is well known to be activated by oxidative or electrophilic stress – which result in the oxidation and targeting of the NRF2 inhibitor, KEAP1, for proteosomal degradation – but recent studies indicate a unique obligatory role for endogenous NOX4 in the activation of NRF2 in the heart in several settings. NRF2 upregulation in response to hemodynamic overload was abrogated in global NOX4 knockout mice and contributed to the detrimental phenotype in these animals [526]. In cultured cardiomyocytes, phenylephrine-induced increases in NRF2 levels were dependent on NOX4 but not NOX2. An even more striking finding is that exercise-induced physiological induction of NRF2 in the heart in mice is absolutely dependent on NOX4. Using several different genetic models including cardiomyocyte-specific NOX4 or NRF2 knockout mice, it was definitively demonstrated that endogenous NOX4 in cardiomyocytes activates NRF2 during physiological free-running wheel exercise and plays a critical role in optimizing cardiac contractile function and maximizing exercise capacity [527]. The effects downstream of NRF2 that mediate these beneficial effects both during physiological and pathological stress are of considerable interest. NRF2 is well known to be a master regulator of antioxidant and cytoprotective responses by inducing diverse genes that regulate glutathione biosynthesis, intracellular redox state, phase II antioxidant enzymes and a wide variety of metabolic pathways. Studies to date indicate an important role for NRF2 in maintaining optimal cytosolic and mitochondrial redox state during stress [526,527]. In addition, metabolic effects consequent to NRF2 induction may also be important in the protective effects. At a conceptual level, it is notable that there appears to be an integral reciprocal relationship between NOX4-dependent NRF2 signaling and redox state (akin to the concept of hormesis), opposite to the conventional paradigm that ROS production associates with oxidative stress. How NOX4 mediates this specific role requires further study but it may be noted that NOX4-mediated activation of NRF2 is observed in several other cell types and organs [455].
2.7.2.3. Regulation of ATF4 and the integrated stress response by NOX4
The integrated stress response (ISR) is an evolutionarily conserved adaptive pathway that responds to multiple stresses including amino acid and glucose deprivation, hypoxia, oxidative stress and ER stress. The central component of this pathway is the phosphorylation of the α-subunit of the eukaryotic initiation factor 2 (eIF2α) by one of four kinases, each responsive to different stress stimuli, resulting in a global inhibition of protein translation but enhanced cap-independent translation of a subset of mRNAs including the transcription factor ATF4. ATF4 activation induces multiple genes involved in amino acid import and metabolism, redox balance, ER chaperone functions, autophagy and intermediary metabolism, a repertoire which usually mediates adaptive functions in response to stress [528]. ATF4 activation is subject to negative regulation via the dephosphorylation of eIF2α by a sub-fraction of protein phosphatase 1 (PP1), which is targeted to the ER by a PP1-targeting subunit GADD34. NOX4 specifically enhances eIF2α phosphorylation and ATF4 translation during stress, secondary to an interaction with GADD34 and redox inhibition of PP1 at the ER [515]. NOX4-mediated enhancement of ATF4 induces strong protective effects against acute ischemic cardiac injury as well as in a model of acute renal tubular necrosis. Moreover, NOX4 itself is a transcriptional target of ATF4 [515], resulting in an additional positive feedback mechanism, and probably explaining why so many stress stimuli increase NOX4 levels.
The specific mechanisms downstream of NOX4-dependent enhancement of ATF4 signaling that mediate protection against ischemia or other stresses remain to be fully elucidated. An enhancement of cardiomyocyte autophagy is suggested to be important in the NOX4-ATF4-dependent protective response to cardiac ischemia [523]. NOX4-dependent protection against hemodynamic overload-induced contractile dysfunction may in part involve an alteration in cardiac substrate utilization. Cardiac-specific NOX4 overexpression leads to a reduction in myocardial glucose oxidation rate, an increase in fatty acid oxidation rate and a well-preserved energetic state during chronic pressure overload [529]. The mechanism underlying the increase in fatty acid oxidation appears to be the post-translational O-GlcNAcylation (addition of N-acetylglucosamine) of the fatty acid membrane transporter CD36, which regulates fatty acid uptake by the myocardium. The increase in O-GlcNAcylation is driven by an ATF4-dependent increase in expression of glutamine-fructose-6-phosphate transaminase 1 (GFAT1), the rate-limiting enzyme in the hexosamine biosynthetic pathway (HBP) – a glycolytic branch pathway that mediates protein O-GlcNAcylation. Therefore, NOX4-dependent upregulation of ATF4 links increased flux into the HBP with an increase in fatty acid oxidation to preserve energetic balance.
2.7.2.4. NOX4-mediated inhibition of Ca2+ transfer from ER to mitochondria
A final protective mechanism involving NOX4 that was recently discovered is the regulation of ER-to-mitochondrial Ca2+ transfer during severe stress. It is well established that severe stresses (such as ischemia) may induce a significant transfer of Ca2+ from ER to mitochondria, resulting in a triggering of the mitochondrial PTP and cell necrosis [530]. NOX4 is highly concentrated at the junctions between ER and mitochondria (mitochondrial-associated membrane, MAM) in multiple tissues and its levels are significantly increased at this location during severe cell stress [531]. NOX4 enhances Akt-dependent phosphorylation of inositol 1,4,5-trisphosphate receptors (InsP3R) at the MAM by inhibiting PP2A (which normally dephosphorylates InsP3R). The consequence of increased InsP3R phosphorylation at the MAM is an inhibition of stress-induced Ca2+ release via these channels to mitochondria and an inhibition of PTP-dependent cell necrosis. This mechanism was found to be strongly protective against cardiac I/R injury [531].
These studies indicate that NOX4 mediates adaptive effects in the heart in response to diverse stresses and involving several different redox-regulated mechanisms. Elucidation of the detailed signalling underpinning these pathways may lead to novel therapeutic options, involving an enhancement of such adaptive pathways.
2.8. Crosstalk between sources of reactive oxygen species
Since mitochondria and NADPH oxidases are both involved in ROS formation during I/R, there is a vital crosstalk between these ROS sources and one can stimulate the other, as reported for a broad set of disease and cellular stress conditions [532]. The concept of “ROS-triggered ROS formation” was first reported for self-amplified mitochondrial ROS formation, visualized by waves of enhanced ROS levels along mitochondrial networks [401]. According to this hypothesis damaged mitochondria produce ROS that initiate ROS formation by neighboring mitochondria. Later, similar crosstalk was also reported for interaction of NOX and mitochondria in angiotensin II (AT-II) mediated preconditioning [533]. Protection by AT–II–mediated preconditioning was blocked by the NOX inhibitor apocynin, and blockade of the mtKATP in cardiac myocytes by 5-hydroxydecanoate (5-HD). In an editorial to this original paper, Brandes proposed that cytosolic ROS generated by NOX can stimulate mitochondrial ROS formation [534]. In general, the concept of “kindling radicals” (or also “bonfire” hypothesis) explains the activation of secondary ROS sources and functional damage of redox-regulated enzymes such as eNOS but also redox-driven conversion of xanthine dehydrogenase to its oxidase form [535]. There, the kindling radicals (most likely from NADPH oxidases or mitochondria) cause oxidative conversion of eNOS or xanthine dehydrogenase to ROS-producing source enzymes (see “redox switches” in Fig. 14) [352]. The concept of the interaction (crosstalk) of different ROS sources was developed to explain the observation that pharmacological inhibition or genetic deletion of one specific ROS source is in many disease models enough to confer a complete normalization of the disease phenotype (see numerous examples for hypertension and AMI in reference) [352].
Fig. 14.
Crosstalk between different sources of RONS: mitochondria, NADPH oxidase (NOX), xanthine oxidase (XO) and uncoupled NOS. XO originates from oxidative stress-mediated conversion of the xanthine dehydrogenase via oxidation of critical thiols in cysteine535/992. NOS (mainly eNOS) are uncoupled upon oxidative depletion of BH4, S-glutathionylation (-SSG), adverse phosphorylation by protein kinase C (PKC) and other redox switches [535]. Mitochondrial O2•‾/H2O2 formation is triggered by oxidative stress from all ROS sources (including other damaged/activated mitochondria) via redox-activation of PKC, mitogen-activated protein kinases (MAPK), other kinase pathways and potential involvement of redox-sensitive mtKATP with subsequent p66Shc, monoamine oxidase (MAO), respiratory complex activation or impairment of mitochondrial antioxidant defense [352]. Mitochondrial O2•‾/H2O2 is released to the cytosol via mitochondrial pores and channels (e.g. redox-sensitive mPTP, inner membrane anion channel (IMAC) or aquaporins) or by diffusion due to increased mitochondrial permeability under pro-inflammatory conditions. In the cytosol these species (along with released calcium) cause activation of redox-sensitive PKC and tyrosine kinases (cSrc) with subsequent activation of NOX and amplification of the cellular oxidative stress [410]. Adapted from Ref. [352] with permission.
Whereas the majority of reports for this redox crosstalk was published for the NOX2/mitochondrial axis in the setting of hypertension [410,536,537], it was also observed in nitrate tolerance, a nitroglycerin-induced oxidative stress condition [538] and the aging process [410,[538], [539], [540]]. Especially the role of CypD, a small redox sensitive regulator of the PTP, in the crosstalk of mitochondrial ROS and NOX2-dependent ROS formation is meanwhile well established in AT-II induced hypertension, by prevention of most adverse effects in CypD knockout mice [410,541]. Cysteine 203 in CypD determines the activity of the PTP regulator CypD, and therefore represents a redox switch of PTP which confers higher opening probability of the pore under oxidative stress conditions [542]. In contrast, S-nitros(yl)ation of cysteine 203 prevented H2O2-induced PTP opening, identifying nitric oxide as an antagonist of ROS in this redox process, which may be the process of nitroglycerine-mediated cardioprotection that was lost on cyclophilin D knockout mice [543].
I/R damage is based on mitochondrial ROS formation as a central pathophysiological mechanism [[544], [545], [546]]. Rathore et al. reported a mechanism by which mitochondrial ROS activate PKCε (prevented by chelerythrine and PKCε deletion) with subsequent increase in NOX activity (prevented by apocynin and p47phox deletion) in the setting of hypoxia as a model of I/R damage (e.g. as observed in AMI or stroke) [547]. The authors showed that hypoxia activates most likely NOX1 isoform in pulmonary arteries, as documented by translocation of p47phox to the plasma membrane. The involvement of mitochondrial ROS formation in this process was proven by lower NADPH activity in GPx-1 overexpressing mice and higher NADPH activity in GPx-1 knockout mice. The crosstalk concept was meanwhile extended to all common ROS sources to explain reperfusion damage [548]. Oxidative stress in general and this crosstalk in particular have also large impact on cellular calcium homeostasis and mitochondrial function in the diabetic heart [549], similar to the Ca2+/ROS crosstalk previously described in cancer development and progression [550] and cellular function per se [551].
2.9. Cardioprotective exosomes/extracellular vesicles – is there a role for redox mechanisms?
Exosomes are a type of small extracellular vesicle (EV) that have been determined to have multiple beneficial effects on recipient cells and organs [552]. Like other EVs, exosomes are surrounded by a lipid bilayer, and contain proteins, microRNAs (miRNAs) and other non-coding RNAs originating from the cytosol. All cell types appear to have the machinery to produce and release exosomes, although the extent to which they do so appears to vary both on cell type and physiological state [552]. Most cells can also produce microvesicles, which are somewhat larger EVs with different characteristics from exosomes. However, since it is challenging to isolate highly pure exosomes, they are usually referred to experimentally as small EVs (sEV). The bulk of sEVs in the blood originate from erythrocytes and platelets, although sEVs originating from the endothelium and other organs can also be identified. Several studies have demonstrated the potential for plasma sEVs to protect the heart from I/R injury [552]. However, the bulk of interest has focused on stem and progenitor cells cultured in vitro as a more practical source of potential cardioprotective sEVs. Irrespective of the type of stem or progenitor cell, the sEVs they produce appear to be protective both in the acute setting where they can reduce the size of infarct following I/R, and in the chronic setting, where they are able to improve cardiac remodeling, reduce fibrosis, increase angiogenesis, and improve cardiac contractile function in the weeks following I/R or chronic myocardial ischemia [552]. As yet, there is no consensus as to the mechanism of cardioprotection by sEVs, as it also depends on their cargo molecules that includes proteins as well as coding and non-coding RNAs. Many studies interested in the chronic setting of infarction and heart failure are interested in examining the role of miRNAs, since these have the potential to remodel transcriptional and translational networks over that time scale. With regards to acute cardioprotection, where the majority of the infarct is believed to be formed acutely after reperfusion, it seems more likely that sEVs activate one of the known cardioprotective kinase signalling pathways in the heart via interaction with surface receptors, or alternatively by delivery of cytoprotective proteins from sEVs into cardiac cells. Cellular calcium overload and oxidative stress are well established as major mediators of I/R injury. One might therefore hypothesize that sEVs exert part of their cardioprotective capabilities via antioxidant mechanisms. Surprisingly, the redox activity of sEVs has not received a great deal of attention. Nevertheless, there are several relevant studies suggesting that sEVs are redox-active. A summary of the cellular sources and their cardioprotective effects is provided in Fig. 15 [553].
Fig. 15.
Effects and sources of exosomes. (upper part) Some of the major effects that have been reported of exosomes that are relevant to the ischemic heart, and the cell types that have been reported to be involved in the effect. See text for details. (lower part) The different potential sources of exosomes discussed in this review. Although each type of cell has certain unique characteristics, the exosomes they produce are notable for the consistent array of effects they induce. From Ref. [553] with permission.
2.9.1. EVs and redox modulation of endogenous cardioprotective mechanisms
IPC increases the rate of release of sEVs from isolated perfused rat hearts and hypoxia/reoxygenation similarly stimulates EV release from cultured endothelial cells. When these sEVs are added to cardiomyocytes in vitro, they increase their resistance to injury [552]. Furthermore, preconditioned hearts released sEVs that protected naïve hearts from I/R injury when transferred via the perfusate [552]. This suggested that sEVs may mediate the inter-organ signalling pathway of RIC. A subsequent study found that in both rats and humans, RIC increased the number of sEVs in the blood, although unexpectedly, plasma sEVs isolated from either naive or preconditioned individuals were found to be similarly protective when administered intravenously to rats undergoing IR [552]. In contrast, Minghua et al. found that RIC-induced exosomes attenuated infarct size and improved heart function when administered intramyocardially, with evidence that EV-delivery of miR-24 was able to reduce oxidative stress, at least in H2O2-treated H9c2 cells in vitro [552].
Another stimulus known to improve cardiovascular health and limit injury following I/R is exercise training. Exercise alters the cargo of circulating sEVs, increasing their antioxidant function by stimulating glutathione reductase and catalase activities. Furthermore, the exercise-induced sEVs can reduce oxidative damage in human iPS-derived cardiomyocytes subject to oxidative stress [554]. sEVs derived from ticagrelor-pretreated H9c2 significantly decreased hyperglycemia-stimulated ROS production and prevented apoptosis [555].
Oxidative stress has been implicated in the pathogenesis of metabolic disease-induced (diabetes or hyperlipidemia) cardiovascular complications [556]. Many cardioprotective modalities including IPC and RIC are impaired or ineffective in the setting of diabetes [556]. RIC was ineffective in diabetic Zucker fatty rats, and an exosome-rich sample from the diabetic rats was unable to protect cardiomyocytes from hypoxia/reoxygenation-induced death [557]. Similarly, sEVs from diabetic rats had lost the ability to protect cardiomyocytes. Importantly, however, protection could be restored by using exosomes from a healthy, non-diabetic source [558].
In certain circumstances such as sepsis, sEVs from platelets may even be detrimental. Exposure of platelets to NO or lipopolysaccharides caused the release of redox-active exosomes containing nNOS and NOX, which generated superoxide and NO. This caused endothelial cell apoptosis via formation of peroxynitrite [559], and induced myocardial dysfunction in isolated rabbit heart and papillary muscle preparations [560]. On the other hand, seemingly toxic sEVs are not always necessarily damaging. Energetically stressed adipocytes released EVs containing respiration-competent, but oxidatively damaged mitochondrial particles. These were taken up by cardiomyocytes, where they triggered a burst of ROS resulting in compensatory antioxidant signalling that protected cardiomyocytes from subsequent acute oxidative stress – somewhat reminiscent of preconditioning [561].
2.9.2. Exogenous EVs and redox modulation of cardioprotective mechanisms
The first report that MSC-derived exosomes could reduce infarct size in mice subject to I/R was by Lai et al., in 2010 [562]. In a subsequent publication they showed that increased myocardial viability and cardiac function in this model corresponded with increased levels and ATP and NADH, decreased oxidative stress and activated PI3K/Akt pathway in the heart [552].
Since then, sEVs from many different stem and progenitor cell sources have been shown to be cardioprotective, but MSC remain of particular interest due to their apparent beneficial immunomodulatory effects of the sEVs the produce [552]. There is some evidence that they have redox-mediated effects. For example, bone marrow-derived MSC decreased ROS production and apoptosis in cardiac stem cells after oxidative stress injury [563]. Interestingly, the effect was greater with those EV isolated from MSC cultured in hypoxic conditions. The mechanism was proposed to be via transfer of miRNA-214 to suppress its target, CaMKII.
In a proteomic analysis, sEVs from human vascular endothelial cells were found to contain proteins associated with redox state, calcium handling and cellular metabolism. Importantly, analysis of human cardiomyocytes after exposure to the sEVs revealed corresponding changes in these proteins, suggesting their cargo was delivered within the cells. Treatment with the sEVs increased the respiratory capacity of normoxic cardiomyocytes and reduced tissue damage in a human heart-on-a-chip I/R injury model [564].
EVs may modulate redox signalling between cells of the heart. For example, treatment of cardiomyocytes with Tongxinluo (a traditional Chinese medicine approved in 1996 by the State Food and Drug Administration of China for treating angina pectoris and ischemic stroke), causes them to release sEVs containing the lncRNA Linc-ROR. When the sEVs are taken up by cardiac endothelial cells, linc-ROR downregulates miR-145–5p leading to activation of eNOS. The NO increases survival in both the endothelial cells and cardiomyocytes [565].
Another type of cardiac injury involving ROS is chemotherapy-induced cardiotoxicity. For example, doxorubicin/trastuzumab-induced cardiac toxicity increases ROS in cardiomyocytes. Intravenous administration of cardiac progenitor cell-derived exosomes prevents ROS and protects against doxorubicin/trastuzumab-induced cardiac toxicity [552]. In this case, the sEVs were highly enriched in miR-146a-5p and suppressed target genes in doxorubicin-treated cells, including NOX4 and myeloperoxidase, both major ROS-producing enzymes [552]. Interestingly, sEVs are no longer beneficial when they are obtained from inflamed hearts. Instead, they increase ROS production by NOX, thereby reducing NO bioavailability in treated cardiac endothelial cells [566]. Thus, the functional effects of sEVs can depend greatly on the health of the cells of origin.
Ageing-associated vascular dysfunction involves oxidative stress. With aging, primary cells undergo senescence, during which they cease proliferation, become pro-inflammatory and produce higher levels of ROS. This increases the production of sEVs [567]. sEVs from endothelial progenitor cells down-regulated the NOX2/ROS pathway by delivery of miR-18a, thereby protecting them from hypoxia and reoxygenation injury [567]. Interestingly, a population of microvesicles from endothelial cells contained enzymes and substrates of the pentose phosphate pathway leading to their ability to synthesize NADPH, which is a key metabolite in antioxidative pathways. Thus, they may act as ROS scavengers [567]. This pathway was even more active in senescent endothelial cells. On the other hand, when human umbilical vein endothelial cells were subject to hypoxia/reoxygenation, the microvesicles they produced induced the phosphorylation of p38 and JNK1/2 and promoted apoptosis and oxidative stress in H9c2 cardiomyocytes [567].
The cargo of sEVs is rich in small non-coding RNAs such as miRNAs. miRNAs have been associated with both cardioprotection and also with regulation of redox signalling of the heart. In a recent study of miRNA-mRNA interaction of I/R and oxidative stress-induced alterations followed by microRNA-mRNA target interaction, network analysis revealed that microRNAs and their mRNA targets that may play a role in cardioprotection via alterations in redox signalling [568].
In summary, there remains great interest in sEVs as cardioprotective agents, and there is suggestive evidence in the literature that part of their benefit may be mediated through redox-regulated pathways. Although there is no consensus as to their main mechanism of action, this may be a reflection of the multi-factorial nature of the sEVs, which contain an assortment of proteins, lipids and miRNAs that may all contribute to the overall benefit. This is in line with the proposal that a multi-target strategy may be required for successful clinical translation of cardioprotection [57].
2.10. Cardioprotective cytokines and growth factors
One of the problems that underlies many cardiac disorders is the incapacity of the heart to undergo regeneration after damage. Measurements by 14C-carbon dating [569], imaging mass spectrometry [570] and analysis of DNA synthesis [571] are concordant in showing a minimal regenerative capacity of the adult mammalian heart, in the order of 1% cardiomyocyte renewal per year. This is well below what would be required to compensate for pathological loss. Thus, the goal of developing treatments that protect cardiomyocytes from death after acute I/R injury or more chronically in other forms of cardiac disease has paramount importance.
Studies performed over 2 decades ago have already shown that various growth factors, including insulin-like growth factor-1 (IGF-1), hepatocyte growth factor (HGF), endothelin-1 (ET-1), fibroblast growth factor-2 (FGF-2), and transforming growth factor-β (TGF-β) can protect the heart against oxidative stress (reviewed in Ref. [572]). These growth factors stimulate intracellular signal transduction pathways that converge on the activation of protective kinases, in particular PI3K/AKT and MAPKs, which eventually protect cardiomyocytes from oxidative stress-induced apoptosis [572]. Other cardioprotective cytokines against I/R injury include macrophage migration inhibitory factor (MIF), which contributes to the regulation of cardioprotective AMPK (reviewed in Ref. [573]), IL-6 [574,575], irisin (a myokine produced by FNDC5 cleavage) [576,577] and follistatin like-1 (FSTL-1) [578,579]. Several of these cytokines are physiologically expressed by the skeletal muscle or heart after injury and are part of the endogenous protective mechanism against I/R injury, which can be enhanced for therapeutic purposes [580]. Other cytokines are produced by different leukocytes populations. Among these, myeloid-derived growth factor (MYDGF) is highly upregulated in both human and mouse infarcts and is predominantly expressed by CXCR4+ monocytes and macrophages [581]. Treatment with this recombinant cytokine was shown to reduce scar size and contractile dysfunction after AMI by inducing AKT activation followed by reduction of apoptosis, while also promoting cardiac endothelial cell proliferation [581].
Recent work performed by the Giacca laboratory has tackled the issue of identifying cardioprotective secreted molecules in a systematic, unbiased manner and independent of whether these cytokines and growth factors are physiologically expressed in the heart after injury. Through an AAV-based method that functionally selects for tissue protective molecules [582,583] and the use of a cDNA library corresponding to the mouse secretome, this work systemically ranked a collection of about 1200 cDNAs coding for secreted factors for their cardioprotective efficacy [584]. After two rounds of iterative, in vivo selection, the most effective cytokines in this functional screening were Chrdl1, an inhibitor of bone morphogenic protein (BMP) [585], Fam3c/ILEI, a metabolic regulator that takes part in various biological functions [[586], [587], [588]] and Famb3b/PANDER, which is normally expressed in the pancreas and participates in the regulation of glucose homeostasis and β-cell function [[589], [590], [591]]. None of these three cytokines are known to take part in the normal cardiac response to injury but are effective when pharmacologically administered to the heart.
Several of the above mentioned cardioprotective cytokines act by blunting oxidative damage after myocardial infarction, and their effect can thus be scored by visualizing viable myocardium immediately after I/R. However, several others also act by different mechanisms that are equally essential to determine longer term cardiomyocyte survival and cardiac function. These mechanisms include, among others, the extent of perfusion and collateral flow induction, efficiency in removal of dysfunctional mitochondria, blunting of excessive inflammation, and activation of intracellular metabolic pathways. These protective mechanisms are activated several hours after the acute ischemic events, and thus cannot be properly assessed immediately after reperfusion, as they act on a vast number of cardiomyocytes in the so called “area-at-risk” [592], which is significantly larger than the area that eventually becomes infarcted [593]. A more straightforward manner to assess the efficacy of cardioprotection, which also considers cardiomyocyte survival in these areas of uncertain fate after reperfusion, is by analyzing the effects of treatments over time. For this purpose, CMR not only provides information on the extent of early damage after ischemia reperfusion [594], but, also and most notably, on the evolution of myocardial infarction and cardiac function at later time points.
In terms of the mechanisms underlying cardioprotection, multiple studies have shown that autophagy and, more specifically, the autophagic recycling of malfunctional mitochondria (mitophagy) exert an essential protective effect after ischemia/reperfusion to prevent later pathological remodeling and the development of heart failure [595,596]. Aberrant mitochondrial function not only results in inadequate energy production but, more relevant, augments the generation of reactive oxidative species [597]. Originally identified in yeast [598], mitophagy is a universal autophagic response that specifically targets these potentially cytotoxic mitochondria (reviewed in Refs. [599,600]). Not surprisingly, several treatments that reduce cardiac damage, including after I/R, also modulate mitophagy (reviewed in Ref. [601]). In a consistent manner, all the three cytokines identified in the above mentioned, systematic, AAV functional selection screening are also powerful inducers of autophagy [584], as it is ghrelin, a 28 amino acid peptide that was previously identified as protective for skeletal muscle and heart by a similar in vivo selection approach [583].
In light of the information obtained over the last years on the effect of molecules that act extracellularly to protect cardiomyocytes from death, the development of an injectable cytokine as a therapeutic molecule for acute or chronic cardioprotection appears to be an attainable goal.
3. Conclusions and perspectives
Rapid reperfusion is mandatory to salvage myocardium from I/R injury but there is a continued need for cardioprotection beyond that by rapid reperfusion. A number of mechanical and pharmacological interventions have reduced infarct size and coronary microvascular obstruction in preclinical and clinical proof-of-concept studies but their translation to better clinical outcome of patients has been largely disappointing such that further research on mechanisms of I/R injury and its attenuation is needed [35]. ROS play a decisive role on I/R injury and its attenuation, both as damaging agents and as cardioprotective signals (Fig. 16). There continues to be significant advances in understanding mechanisms of ROS production during I/R [52] and how these mediate dysfunction [457]. Perhaps these new insights may offer opportunities for therapies that are more successful than the antioxidant interventions that failed to translate, as will now be discussed in the next section. Given the multitude of preclinical studies showing antioxidants are protective, including against cardiovascular conditions, it seems obvious that their administration to patients should be therapeutic. Whilst some clinical trials have demonstrated antioxidants protect against cardiovascular disease, this has not been widely replicated with the outcomes of large-scale clinical trials showing supplementation is not beneficial. Indeed, supplementing cardiovascular disease patients with antioxidants worsens outcomes in terms of all-cause mortality, as it has in other diseases such as cancers [460]. In the same way that ROS and RNS are a diverse array of molecules with often markedly different chemistry, the same is true for antioxidants, with differing abilities to react with and so neutralize the supposedly damaging oxidants (Fig. 16). Little consideration is typically given to the fact that cells are replete with antioxidant systems and whether supplementation of a human with small tablets of antioxidant can significantly or sufficiently bolster the endogenous defenses against oxidant species. In addition, oxidation of antioxidants may in some cases generate pro-oxidant molecule that initiate biological actions, including those that may be beneficial to the cardiovascular system [602,603]. Furthermore, as antioxidants are electron donors, they can potentially generate oxidant species to counterintuitively induce oxidative stress.
Fig. 16.
ROS and RNS are commonly thought to induce injury by oxidizing (i.e., damaging) the cellular fabric, including proteins, lipids and nucleotides. There is significant pre-clinical evidence that oxidants are damaging based on protection by antioxidants, but this has not translated though to human studies with large-scale clinical trials often showing no benefit or sometimes adverse outcomes. This could be because antioxidants scavenge oxidants that otherwise initiate protective signaling events. As electron donors, antioxidants have the potential to fuel oxidants generation as shown by the dotted line, which adds further complexity. Another consideration is that oxidized antioxidants can exert biological actions via their pro-oxidant chemistry.
The concept that diseases caused by oxidative stress can be corrected with antioxidants or inhibitors of the enzymes that synthesize these perceived perpetrators, is perhaps attractive because of the seeming simplicity of the paradigm for which there is so much pre-clinical evidence. Furthermore, natural antioxidants are often considered safe, perhaps because they are present in our diets, and they are also readily available. The oxidative damage concept is arguably widely grasped by the lay public with advertisements for antioxidant supplements for aging and all manner of conditions enabling a multibillion-dollar annual market. However, whether the lay public are aware of the evidence against the use of antioxidants in disease and significantly understand the basic principles of redox processes is questionable.
The dual role of ROS, beneficial and detrimental, is shared by other processes, such as intracellular [Ca2+] elevation and PTP opening. Indeed, preconditioning-like protection has been obtained by increasing extracellular Ca2+ [604], while CypD deletion abolished IPC-induced protection [605]. Furthermore, translating experimental acute vs prolonged effects should be considered carefully. For instance, suppression of mitochondrial ROS generation by mitochondrial-targeted catalase hampered autophagy worsening outcome in a model of heart failure [606], Similarly, the transition from hypertrophy to failure was shown to be exacerbated by PTP inhibition [607]. Therefore, the impetuous flow of knowledge in basic science will provide real benefits in clinical settings only if all aspects are considered thoroughly.
Funding
GH is supported by the German Research Foundation (CRC 1116, B8) and the European Union (COST ACTION CARDIOPROTECTION CA 16225).
IA is supported by by the Operational Program “Competitiveness, Entrepreneurship and Innovation"2014–2020 under the call “RESEARCH – CREATE - INNOVATE” (project codes: 03427 and 5048539) and the European Union (COST ACTION CARDIOPROTECTION CA 16225).
HEB was supported by Independent Research Fund Denmark (0134–00294B), Novo Nordisk Foundation (NNF19OC0058543, NNF18OC0052789) and the European Union (COST ACTION CARDIOPROTECTION CA 16225).
SMD acknowledges the support of the British Heart Foundation (BHF PG/16/85/32471, PG/18/44/33790, PG/19/51/34493, PG/21/10798).
PE is supported by The Barts Charity Cardiovascular Programme Award G00913 and programme grants from the British Heart Foundation and the Medical Research Council.
PF was supported by the National Research, Development and Innovation Office of Hungary (Research Excellence Program TKP within the framework of the Therapeutic Development thematic program of the Semmelweis University; National Heart Laboratory (RRF-2.3.1-21-2022-00003). PF is a vice chair of the COST CIG (IG16225) and an MC member of the COST CardioRNA project (CA17129).
MG was supported by the European Research Council (ERC) Advanced Grant 787971 “CuRE”; British Heart Foundation (BHF) Programme Grant RG/19/11/34633; King's College London BHF Centre of Research Excellence grant RE/18/2/34213; grants 825670 “CardioReGenix” and 874764 “REANIMA” from the European Commission Horizon 2020 programme.
DH is supported by the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research Investigator Award (MOH-STaR21jun-0003), Centre Grant scheme (NMRC CG21APR1006), and Collaborative Centre Grant scheme (NMRC/CG21APRC006). This article is based upon work supported by the COST Action EU-CARDIOPROTECTION IG16225 supported by COST (European Cooperation in Science and Technology).
BI is supported by the European Commission (ERC-CoG 819775, and H2020-HEALTH 945118), by the Spanish Ministry of Science and Innovation (PID2019‐110369RB‐I00), and by the Comunidad de Madrid (P2022/BMD-7403, RENIM-CM).
CM is funded by the Deutsche Forschungsgemeinschaft (DFG; SFB-1525 project # 453989101, and Ma 2528/8–1).
RS is supported by Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) [Project number 268555672—SFB 1213, Project B05].
FS was supported by R01HL46716 and R01HL128831 from the National Heart Lung and Blood Institute.
AMS is supported by the British Heart Foundation (CH/1999001/11735, RE/18/2/34213) and a Foundation Leducq Transatlantic Network of Excellence award (17CVD04).
Declaration of competing interest
PF is the founder and CEO of Pharmahungary Group, a group of R&D companies. AMS is an adviser to Forcefield Therapeutics and CYTE – Global Network for Clinical Research and sits on the Board of Heqet Therapeutics.
CM served as an advisor to Amgen, Boehringer Ingelheim, Bristol Myers Squibb, NovoNordisk and Servier and received speaker honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Berlin Chemie, Novartis and NovoNordisk.
No other author had an interest to declare.
Acknowledgement
We are grateful to Professor Dr. Andreas Daiber for his intellectual and organisatory impact on this article.
Contributor Information
Gerd Heusch, Email: gerd.heusch@uk-essen.de.
Fabio Di Lisa, Email: fabio.dilisa@gmail.com.
Data availability
No data was used for the research described in the article.
References
- 1.Kübler W., Haass M. Cardioprotection: definition, classification, and fundamental principles. Heart. 1996;75:330–333. doi: 10.1136/hrt.75.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y. [DOI] [PubMed] [Google Scholar]
- 3.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murry C.E., Reinecke H., Pabon L.M. Regeneration gaps: observations on stem cells and cardiac repair. J. Am. Coll. Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez A., Fortuno M.A., Querejeta R., Ravassa S., Lopez B., Lopez N., Diez J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc. Res. 2003;59:549–562. doi: 10.1016/s0008-6363(03)00498-x. [DOI] [PubMed] [Google Scholar]
- 6.Hein S., Arnon E., Kostin S., Schonburg M., Elsasser A., Polyakova V., et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 7.Kyto V., Saraste A., Saukko P., Henn V., Pulkki K., Vuorinen T., Voipio-Pulkki L.M. Apoptotic cardiomyocyte death in fatal myocarditis. Am. J. Cardiol. 2004;94:746–750. doi: 10.1016/j.amjcard.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Nef H.M., Mollmann H., Hilpert P., Troidl C., Voss S., Rolf A., et al. Activated cell survival cascade protects cardiomyocytes from cell death in Tako-Tsubo cardiomyopathy. Eur. J. Heart Fail. 2009;11:758–764. doi: 10.1093/eurjhf/hfp076. [DOI] [PubMed] [Google Scholar]
- 9.Stapel B., Kohlhaas M., Ricke-Hoch M., Haghikia A., Erschow S., Knuuti J., et al. Low STAT3 expression sensitizes to toxic effects of beta-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur. Heart J. 2017;38:349–361. doi: 10.1093/eurheartj/ehw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend D., Yasuda S., McNally E., Metzger J.M. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. Faseb. J. 2011;25:3106–3114. doi: 10.1096/fj.10-178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashem S.I., Perry C.N., Bauer M., Han S., Clegg S.D., Ouyang K., et al. Brief Report: oxidative stress mediates cardiomyocyte apoptosis in a human model of Danon disease and heart failure. Stem Cell. 2015;33:2343–2350. doi: 10.1002/stem.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloyan A., Sanbe A., Osinska H., Westfall M., Robinson D., Imahashi K., et al. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation. 2005;112:3451–3461. doi: 10.1161/CIRCULATIONAHA.105.572552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn G.W. 2nd, Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc. Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji K., Fujimoto S., Ikeda Y., Masuda K., Nakamura S., Saito Y., Yutani C. Apoptotic myocardial cell death in the setting of arrhythmogenic right ventricular cardiomyopathy. Acta Cardiol. 2005;60:465–470. doi: 10.2143/AC.60.5.2004965. [DOI] [PubMed] [Google Scholar]
- 15.Shernan S.K. Perioperative myocardial ischemia reperfusion injury. Anesthesiol. Clin. N. Am. 2003;21:465–485. doi: 10.1016/s0889-8537(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 16.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Diseases Injuries Collaborators G.B.D. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szummer K., Wallentin L., Lindhagen L., Alfredsson J., Erlinge D., Held C., et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995-2014. Eur. Heart J. 2017;38:3056–3065. doi: 10.1093/eurheartj/ehx515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blöndal M., Ainla T., Eha J., Löiveke P., Marandi T., Saar A., et al. Comparison of management and outcomes of ST-segment elevation myocardial infarction patients in Estonia, Hungary, Norway, and Sweden according to national ongoing registries. Eur. Heart J. Qual. Care Clin. Outcomes. 2022;8:307–314. doi: 10.1093/ehjqcco/qcaa098. [DOI] [PubMed] [Google Scholar]
- 20.Maroko P.R., Libby P., Ginks W.R., Bloor C.M., Shell W.E., Sobel B.E., Ross J., Jr. Coronary artery reperfusion. I. Early effects on local myocardial function and the extent of myocardial necrosis. J. Clin. Invest. 1972;51:2710–2716. doi: 10.1172/JCI107090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginks W.R., Sybers H.D., Maroko P.R., Covell J.W., Sobel B.E., Ross J., Jr. Coronary artery reperfusion. II. Reduction of myocardial infarct size at 1 week after the coronary occlusion. J. Clin. Invest. 1972;51:2717–2723. doi: 10.1172/JCI107091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reimer K.A., Lowe J.E., Rasmussen M.M., Jennings R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.CIR.56.5.786. [DOI] [PubMed] [Google Scholar]
- 23.Reimer K.A., Jennings R.B. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab. Invest. 1979;40:633–644. [PubMed] [Google Scholar]
- 24.Heusch G., Libby P., Gersh B., Yellon D., Böhm M., Lopaschuk G., Opie L. Cardiovascular remodeling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gersh B.J., Stone G.W., White H.D., Holmes D.R., Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293:979–986. doi: 10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 26.Stone G.W., Selker H.P., Thiele H., Patel M.R., Udelson J.E., Ohman E.M., et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol. 2016;67:1674–1683. doi: 10.1016/j.jacc.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 27.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2017;39(2018):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 28.Heusch G., Gersh B.J. Is cardioprotection salvageable? Circulation. 2020;141:415–417. doi: 10.1161/CIRCULATIONAHA.119.044176. [DOI] [PubMed] [Google Scholar]
- 29.Jollis J.G., Granger C.B., Zegre-Hemsey J.K., Henry T.D., Goyal A., Tamis-Holland J.E., et al. Treatment time and in-hospital mortality among patients with ST-segment elevation myocardial infarction, 2018-2021. JAMA. 2022;328:2033–2040. doi: 10.1001/jama.2022.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vora A.N., Holmes D.N., Rokos I., Roe M.T., Granger C.B., French W.J., et al. Fibrinolysis use among patients requiring interhospital transfer for ST-segment elevation myocardial infarction care: a report from the US National Cardiovascular Data Registry. JAMA Intern. Med. 2015;175:207–215. doi: 10.1001/jamainternmed.2014.6573. [DOI] [PubMed] [Google Scholar]
- 31.Redfors B., Mohebi R., Giustino G., Chen S., Selker H.P., Thiele H., et al. Time delay, infarct size, and microvascular obstruction after primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009879. [DOI] [PubMed] [Google Scholar]
- 32.Lukhna K., Hausenloy D.J., Ali A.S., Bajaber A., Calver A., Mutyaba A., et al. Remote ischaemic conditioning in STEMI patients in sub-Saharan AFRICA: rationale and study design for the RIC-AFRICA trial. Cardiovasc. Drugs Ther. 2021;37:299–305. doi: 10.1007/s10557-021-07283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kite T.A., Ludman P.F., Gale C.P., Wu J., Caixeta A., Mansourati J., et al. International prospective registry of acute coronary syndromes in patients with COVID-19. J. Am. Coll. Cardiol. 2021;77:2466–2476. doi: 10.1016/j.jacc.2021.03.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heusch G. Critical issues for the translation of cardioprotection. Circ. Res. 2017;120:1477–1486. doi: 10.1161/CIRCRESAHA.117.310820. [DOI] [PubMed] [Google Scholar]
- 35.Heusch G. Cardioprotection and its translation: a need for new paradigms? Or for new pragmatism? An opinionated retro- and perspective. J. Cardiovasc. Pharmacol. Therapeut. 2023 doi: 10.1177/10742484231179613. [DOI] [PubMed] [Google Scholar]
- 36.Heusch G., Gersh B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 2017;38:774–784. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 37.Gaspar A., Lourenço A.P., Álvares Pereira M., Azevedo P., Roncon-Albuquerque R., Jr., Marques J., Leite-Moreira A.F. Randomized controlled trial of remote ischemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI) Basic Res. Cardiol. 2018;113:14. doi: 10.1007/s00395-018-0672-3. [DOI] [PubMed] [Google Scholar]
- 38.Kharbanda R.K., Nielsen T.T., Redington A.N. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 39.Lieder H.R., Skyschally A., Heusch G., Kleinbongard P. Plasma from remotely conditioned pigs reduces infarct size when given before or after ischemia to isolated perfused rat hearts. Pflügers Arch. - Eur. J. Physiol. 2019;471:1371–1379. doi: 10.1007/s00424-019-02314-y. [DOI] [PubMed] [Google Scholar]
- 40.Bell R.M., Basalay M., Bøtker H.E., Beikoghli Kalkhoran S., Carr R.D., Cunningham J., et al. Remote ischaemic conditioning: defining critical criteria for success-report from the 11th Hatter Cardiovascular Workshop. Basic Res. Cardiol. 2022;117:39. doi: 10.1007/s00395-022-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausenloy D.J., Kharbanda R.K., Møller U.K., Ramlall M., Aarøe J., Butler R., et al. Effect of remote ischemic conditioning on clinical outcomes at 12 months in acute myocardial infarction patients: the CONDI-2/ERIC-PPCI trial. Lancet. 2019;394:1415–1424. doi: 10.1016/S0140-6736(19)32039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukhna K., Hausenloy D.J., Ali A.S., Bajaber A., Calver A., Mutyaba A., et al. Remote ischaemic conditioning in STEMI patients in sub-Saharan AFRICA: rationale and study design for the RIC-AFRICA trial. Cardiovasc. Drugs Ther. 2023;37:299–305. doi: 10.1007/s10557-021-07283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heusch G., Bøtker E.H., Ferdinandy P., Schulz R. Primordial non-responsiveness – a neglected obstacle to cardioprotection. Eur. Heart J. 2023;44:1687–1689. doi: 10.1093/eurheartj/ehad160. [DOI] [PubMed] [Google Scholar]
- 44.Hausenloy D.J., Bøtker H.E., Engstrøm T., Erlinge D., Heusch G., Ibanez B., et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur. Heart J. 2017;38:935–941. doi: 10.1093/eurheartj/ehw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong S.G., Lee W.H., Theodorou L., Kodo K., Lim S.Y., Shukla D.H., et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 2014;104:24–36. doi: 10.1093/cvr/cvu172. [DOI] [PubMed] [Google Scholar]
- 47.Sousa Fialho M.D.L., Abd Jamil A.H., Stannard G.A., Heather L.C. Hypoxia-inducible factor 1 signalling, metabolism and its therapeutic potential in cardiovascular disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865:831–843. doi: 10.1016/j.bbadis.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 48.Dengler F. Activation of AMPK under hypoxia: many roads leading to Rome. Int. J. Mol. Sci. 2020;21:2428. doi: 10.3390/ijms21072428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webster I., Friedrich S.O., Lochner A., Huisamen B. AMP kinase activation and glut4 translocation in isolated cardiomyocytes. Cardiovasc. J. Afr. 2010;21:72–78. [PMC free article] [PubMed] [Google Scholar]
- 50.Banerjee S.K., Wang D.W., Alzamora R., Huang X.N., Pastor-Soler N.M., Hallows K.R., et al. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J. Mol. Cell. Cardiol. 2010;49:683–692. doi: 10.1016/j.yjmcc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsushima S., Tsutsui H., Sadoshima J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 2014;24:202–205. doi: 10.1016/j.tcm.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott I., Youle R.J. Mitochondrial fission and fusion. Essays Biochem. 2010;47:85–98. doi: 10.1042/bse0470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paiva M.A., Goncalves L.M., Providencia L.A., Davidson S.M., Yellon D.M., Mocanu M.M. Transitory activation of AMPK at reperfusion protects the ischaemic-reperfused rat myocardium against infarction. Cardiovasc. Drugs Ther. 2010;24:25–32. doi: 10.1007/s10557-010-6222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 56.Lim S.Y., Davidson S.M., Hausenloy D.J., Yellon D.M. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc. Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson S.M., Ferdinandy P., Andreadou I., Bøtker H.E., Heusch G., Ibanez B., et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]
- 58.Sciarretta S., Forte M., Frati G., Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 2018;122:489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra P.K., Adameova A., Hill J.A., Baines C.P., Kang P.M., Downey J.M., et al. Guidelines for evaluating myocardial cell death. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H891–H922. doi: 10.1152/ajpheart.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sciarretta S., Hariharan N., Monden Y., Zablocki D., Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr. Cardiol. 2011;32:275–281. doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing Y., Sui Z., Liu Y., Wang M.M., Wei X., Lu Q., et al. Blunting TRPML1 channels protects myocardial ischemia/reperfusion injury by restoring impaired cardiomyocyte autophagy. Basic Res. Cardiol. 2022;117:20. doi: 10.1007/s00395-022-00930-x. [DOI] [PubMed] [Google Scholar]
- 62.Nah J., Zhai P., Huang C.Y., Fernandez A.F., Mareedu S., Levine B., Sadoshima J. Upregulation of Rubicon promotes autosis during myocardial ischemia/reperfusion injury. J. Clin. Invest. 2020;130:2978–2991. doi: 10.1172/JCI132366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kale J., Osterlund E.J., Andrews D.W. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng Y., Wang W., Kang J., Wang X., Sun L. Role of the PI3K/AKT signalling pathway in apoptotic cell death in the cerebral cortex of streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017;13:2417–2422. doi: 10.3892/etm.2017.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yue J., Lopez J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gordon S., Pluddemann A. Macrophage clearance of apoptotic cells: a critical assessment. Front. Immunol. 2018;9:127. doi: 10.3389/fimmu.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mocanu M.M., Baxter G.F., Yellon D.M. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br. J. Pharmacol. 2000;130:197–200. doi: 10.1038/sj.bjp.0703336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park T.J., Park J.H., Lee G.S., Lee J.Y., Shin J.H., Kim M.W., et al. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10:835. doi: 10.1038/s41419-019-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeBoer D.A., Clark R.E. Iron chelation in myocardial preservation after ischemia-reperfusion injury: the importance of pretreatment and toxicity. Ann. Thorac. Surg. 1992;53:412–418. doi: 10.1016/0003-4975(92)90260-b. [DOI] [PubMed] [Google Scholar]
- 70.Ravingerova T., Kindernay L., Bartekova M., Ferko M., Adameova A., Zohdi V., et al. The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21217889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo X., Chen Y., Liu Q. Necroptosis in heart disease: molecular mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2022;169:74–83. doi: 10.1016/j.yjmcc.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu S., Chang X., Zhu H., Wang D., Chen G. PI3K mediates tumor necrosis factor induced-necroptosis through initiating RIP1-RIP3-MLKL signaling pathway activation. Cytokine. 2020;129 doi: 10.1016/j.cyto.2020.155046. [DOI] [PubMed] [Google Scholar]
- 73.Zhang T., Zhang Y., Cui M., Jin L., Wang Y., Lv F., et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat. Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 74.Smith C.C., Davidson S.M., Lim S.Y., Simpkin J.C., Hothersall J.S., Yellon D.M. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc. Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 75.Weisel K., Scott N., Berger S., Wang S., Brown K., Powell M., et al. A randomised, placebo-controlled study of RIPK1 inhibitor GSK2982772 in patients with active ulcerative colitis. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2021-000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao Z., Zhu Y., Yang P., Chen Z., Xia Y., Qiao C., et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310–4329. doi: 10.7150/thno.71086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi H., Gao Y., Dong Z., Yang J., Gao R., Li X., et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res. 2021;129:383–396. doi: 10.1161/CIRCRESAHA.120.318629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yellon D.M., Beikoghli Kalkhoran S., Davidson S.M. The RISK pathway leading to mitochondria and cardioprotection: how everything started. Basic Res. Cardiol. 2023;118:22. doi: 10.1007/s00395-023-00992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadebe N., Cour M., Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res. Cardiol. 2018;113:9. doi: 10.1007/s00395-018-0670-5. [DOI] [PubMed] [Google Scholar]
- 80.Heusch G., Musiolik J., Gedik N., Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ. Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 81.Boengler K., Hilfiker-Kleiner D., Heusch G., Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skyschally A., Gent S., Amanakis G., Schulte C., Kleinbongard P., Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ. Res. 2015;117:279–288. doi: 10.1161/CIRCRESAHA.117.306878. [DOI] [PubMed] [Google Scholar]
- 83.Gent S., Skyschally A., Kleinbongard P., Heusch G. Ischemic preconditioning in pigs: a causal role for signal transducer and activator of transcription 3. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H478–H484. doi: 10.1152/ajpheart.00749.2016. [DOI] [PubMed] [Google Scholar]
- 84.Kleinbongard P., Amanakis G., Skyschally A., Heusch G. Reflection of cardioprotection by remote ischemic perconditioning in attenuated ST-segment elevation during ongoing coronary occlusion in pigs: evidence for cardioprotection from ischemic injury. Circ. Res. 2018;122:1102–1108. doi: 10.1161/CIRCRESAHA.118.312784. [DOI] [PubMed] [Google Scholar]
- 85.Kleinbongard P. Perspective: mitochondrial STAT3 in cardioprotection. Basic Res. Cardiol. 2023;118:32. doi: 10.1007/s00395-023-01003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hausenloy D.J., Iliodromitis E.K., Andreadou I., Papalois A., Gritsopoulos G., Anastasiou-Nana M., et al. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc. Drugs Ther. 2012;26:87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 87.Heusch G., Bøtker H.E., Przyklenk K., Redington A., Yellon D.M. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rohailla S., Clarizia N., Sourour M., Sourour W., Gelber N., Wei C., et al. Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D.N., Zhao Y., Zhang C.Y., Wang F., Zhou Y., Jin S.Q. Plasma exosomes at the late phase of remote ischemic pre-conditioning attenuate myocardial ischemia-reperfusion injury through transferring miR-126a-3p. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.736226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei M., Xin P., Li S., Tao J., Li Y., Li J., et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ. Res. 2011;108:1220–1225. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi T., Izumi Y., Nakamura Y., Yamazaki T., Shiota M., Sano S., et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int. J. Cardiol. 2015;178:239–246. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 92.Rossello X., Riquelme J.A., He Z., Taferner S., Vanhaesebroeck B., Davidson S.M., Yellon D.M. The role of PI3Kalpha isoform in cardioprotection. Basic Res. Cardiol. 2017;112:66. doi: 10.1007/s00395-017-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gong G.Q., Bilanges B., Allsop B., Masson G.R., Roberton V., Askwith T., et al. A small-molecule PI3Kalpha activator for cardioprotection and neuroregeneration. Nature. 2023;618:159–168. doi: 10.1038/s41586-023-05972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rossello X., Yellon D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2018;113:2. doi: 10.1007/s00395-017-0662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ferdinandy P., Andreadou I., Baxter G.F., Bøtker H.E., Davidson S.M., Dobrev D., et al. Interaction of cardiovascular nonmodifiable risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by pharmacological treatments and ischemic conditioning. Pharmacol. Rev. 2023;75:159–216. doi: 10.1124/pharmrev.121.000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boengler K., Schulz R., Heusch G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 97.Pagliaro P., Penna C. Hypertension, hypertrophy, and reperfusion injury. J. Cardiovasc. Med. 2017;18:131–135. doi: 10.2459/JCM.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 98.Andreadou I., Schulz R., Badimon L., Adameova A., Kleinbongard P., Lecour S., et al. Hyperlipidaemia and cardioprotection: animal models for translational studies. Br. J. Pharmacol. 2020;177:5287–5311. doi: 10.1111/bph.14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Penna C., Andreadou I., Aragno M., Beauloye C., Bertrand L., Lazou A., et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020;177:5312–5335. doi: 10.1111/bph.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perrino C., Ferdinandy P., Bøtker H.E., Brundel B., Collins P., Davidson S.M., et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: position paper and recommendations of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2021;117:367–385. doi: 10.1093/cvr/cvaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kleinbongard P., Lieder H., Skyschally A., Heusch G. No sex-related differences in infarct size, no-reflow and protection by ischaemic preconditioning in Göttingen minipigs. Cardiovasc. Res. 2023;119:561–570. doi: 10.1093/cvr/cvac062. [DOI] [PubMed] [Google Scholar]
- 102.Kleinbongard P., Andreadou I., Vilahur G. The platelet paradox of injury versus protection in myocardial infarction-has it been overlooked? Basic Res. Cardiol. 2021;116:37. doi: 10.1007/s00395-021-00876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kottenberg E., Thielmann M., Kleinbongard P., Frey U.H., Heine T., Jakob H., et al. Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol. Scand. 2014;58:453–462. doi: 10.1111/aas.12278. [DOI] [PubMed] [Google Scholar]
- 104.Kleinbongard P., Bøtker H.E., Ovize M., Hausenloy D.J., Heusch G. Co-morbidities and co-medications as confounders of cardioprotection - does it matter in the clinical setting? Br. J. Pharmacol. 2020;177:5252–5269. doi: 10.1111/bph.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heusch G. Myocardial ischemia: lack of coronary blood flow, myocardial oxygen supply-demand imbalance, or what? Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1439–H1446. doi: 10.1152/ajpheart.00139.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Falk E. Plaque rupture with severe pre-existing stenosis precipitating coronary thrombosis. Characteristics of coronary atherosclerotic plaques underlying fatal occlusive thrombi. Br. Heart J. 1983;50:127–134. doi: 10.1136/hrt.50.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 108.Kleinbongard P., Heusch G. A fresh look at coronary microembolization. Nat. Rev. Cardiol. 2022;19:265–280. doi: 10.1038/s41569-021-00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puymirat E., Simon T., Cayla G., Cottin Y., Elbaz M., Coste P., et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French registry of acute ST-elevation or non-ST-elevation myocardial infarction) 1995 to 2015. Circulation. 2017;14:1908–1919. doi: 10.1161/CIRCULATIONAHA.117.030798. [DOI] [PubMed] [Google Scholar]
- 110.Bairey Merz C.N., Pepine C.J., Walsh M.N., Fleg J.L. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reynolds H.R., Maehara A., Kwong R.Y., Sedlak T., Saw J., Smilowitz N.R., et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parwani P., Kang N., Safaeipour M., Mamas M.A., Wei J., Gulati M., et al. Contemporary diagnosis and management of patients with MINOCA. Curr. Cardiol. Rep. 2023;25:561–570. doi: 10.1007/s11886-023-01874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heusch G. The coronary circulation as a target of cardioprotection. Circ. Res. 2016;118:1643–1658. doi: 10.1161/CIRCRESAHA.116.308640. [DOI] [PubMed] [Google Scholar]
- 114.Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res. Cardiol. 2019;114:45. doi: 10.1007/s00395-019-0756-8. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez-Jimenez R., Garcia-Prieto J., Sanchez-Gonzalez J., Aguero J., Lopez-Martin G.J., Galan-Arriola C., et al. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. J. Am. Coll. Cardiol. 2015;66:816–828. doi: 10.1016/j.jacc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 116.Ehring T., Krajcar M., Baumgart D., Kompa S., Hümmelgen M., Heusch G. Cholinergic and a-adrenergic coronary vasomotion with increasing ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 1995;268:H886–H894. doi: 10.1152/ajpheart.1995.268.2.H886. [DOI] [PubMed] [Google Scholar]
- 117.Kleinbongard P., Boese D., Baars T., Moehlenkamp S., Konorza T., Schoener S., et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ. Res. 2011;108:344–352. doi: 10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 118.Kupatt C., Wichels R., Horstkotte J., Krombach F., Habazettl H., Boekstegers P. Molecular mechanisms of platelet-mediated leukocyte recruitment during myocardial reperfusion. J. Leukoc. Biol. 2002;72:455–461. doi: 10.1189/jlb.72.3.455. [DOI] [PubMed] [Google Scholar]
- 119.Folts J.D. Deleterious hemodynamic effects of thrombotic/embolic materials on the distal myocardial vasculature. Cardiovasc. Res. 1999;42:6–7. doi: 10.1016/S0008-6363(99)00028-0. [DOI] [PubMed] [Google Scholar]
- 120.Driesen R.B., Zalewski J., Vanden Driessche N., Vermeulen K., Bogaert J., Sipido K.R., et al. Histological correlate of a cardiac magnetic resonance imaged microvascular obstruction in a porcine model of ischemia-reperfusion. Cardiovasc. Pathol. 2012;21:129–131. doi: 10.1016/j.carpath.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 121.Kloner R.A., Ganote C.E., Jennings R.B. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Higginson L.A., White F., Heggtveit H.A., Sanders T.M., Bloor C.M., Covell J.W. Determinants of myocardial hemorrhage after coronary reperfusion in the anesthetized dog. Circulation. 1982;65:62–69. doi: 10.1161/01.cir.65.1.62. [DOI] [PubMed] [Google Scholar]
- 123.Baumgart D., Haude M., Goerge G., Ge J., Vetter S., Dagres N., et al. Improved assessment of coronary stenosis severity using the relative flow velocity reserve. Circulation. 1998;98:40–46. doi: 10.1161/01.cir.98.1.40. [DOI] [PubMed] [Google Scholar]
- 124.Herrmann J., Haude M., Lerman A., Schulz R., Volbracht L., Ge J., et al. Abnormal coronary flow velocity reserve following coronary intervention is associated with cardiac marker elevation. Circulation. 2001;103:2339–2345. doi: 10.1161/01.cir.103.19.2339. [DOI] [PubMed] [Google Scholar]
- 125.Ibanez B., Aletras A.H., Arai A.E., Arheden H., Bax J., Berry C., et al. Cardiac MRI endpoints in myocardial infarction experimental and clinical trials. JACC scientific expert panel JACC scientific expert panel. J. Am. Coll. Cardiol. 2019;74:238–256. doi: 10.1016/j.jacc.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Waha S., Patel M.R., Granger C.B., Ohman E.M., Maehara A., Eitel I., et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017;38:3502–3510. doi: 10.1093/eurheartj/ehx414. [DOI] [PubMed] [Google Scholar]
- 127.Reinstadler S.J., Stiermaier T., Reindl M., Feistritzer H.J., Fuernau G., Eitel C., et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imag. 2019;20:138–146. doi: 10.1093/ehjci/jey101. [DOI] [PubMed] [Google Scholar]
- 128.Sies H. Findings in redox biology: from H2O2 to oxidative stress. J. Biol. Chem. 2020;295:13458–13473. doi: 10.1074/jbc.X120.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23:499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 130.Liu Y., Bubolz A.H., Mendoza S., Zhang D.X., Gutterman D.D. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ. Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Larsen B.T., Bubolz A.H., Mendoza S.A., Pritchard K.A., Jr., Gutterman D.D. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2009;29:739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bubolz A.H., Mendoza S.A., Zheng X., Zinkevich N.S., Li R., Gutterman D.D., Zhang D.X. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H634–H642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hori M., Gotoh K., Kitakaze M., Iwai K., Iwakura K., Sato H., et al. Role of oxygen-derived free radicals in myocardial edema and ischemia in coronary microvascular embolization. Circulation. 1991;84:828–840. doi: 10.1161/01.cir.84.2.828. [DOI] [PubMed] [Google Scholar]
- 134.Teixeira R.B., Pfeiffer M., Zhang P., Shafique E., Rayta B., Karbasiafshar C., et al. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic Res. Cardiol. 2023;118:3. doi: 10.1007/s00395-022-00976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hausenloy D.J., Chilian W., Crea F., Davidson S.M., Ferdinandy P., Garcia-Dorado D., et al. The coronary circulation in acute myocardial ischaemia/reperfusion injury - a target for cardioprotection. Cardiovasc. Res. 2019;115:1143–1155. doi: 10.1093/cvr/cvy286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Galaup A., Gomez E., Souktani R., Durand M., Cazes A., Monnot C., et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 2012;125:140–149. doi: 10.1161/CIRCULATIONAHA.111.049072. [DOI] [PubMed] [Google Scholar]
- 137.Thuny F., Lairez O., Roubille F., Mewton N., Rioufol G., Sportouch C., et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012;59:2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 138.Mewton N., Thibault H., Roubille F., Lairez O., Rioufol G., Sportouch C., et al. Postconditioning attenuates no-reflow in STEMI patients. Basic Res. Cardiol. 2013;108:383. doi: 10.1007/s00395-013-0383-8. [DOI] [PubMed] [Google Scholar]
- 139.Traverse J.H., Swingen C.M., Henry T.D., Fox J., Wang Y.L., Chavez I.J., et al. NHLBI-sponsored randomized trial of postconditioning during primary percutaneous coronary Iintervention for ST-elevation myocardial infarction. Circ. Res. 2019;124:769–778. doi: 10.1161/CIRCRESAHA.118.314060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crimi G., Pica S., Raineri C., Bramucci E., De Ferrari G.M., Klersy C., et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2013;6:1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 141.White S.K., Froehlich G.M., Sado D.M., Maestrini V., Fontana M., Treibel T.A., et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 142.Garcia-Prieto J., Villena-Gutierrez R., Gomez M., Bernardo E., Pun-Garcia A., Garcia-Lunar I., et al. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017;8 doi: 10.1038/ncomms14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Niccoli G., Montone R., Ibanez B., Thiele H., Crea F., Heusch G., et al. Optimized treatment of ST-elevation myocardial infarction: the unmet need to target coronary microvascular obstruction as primary treatment goal to further improve prognosis. Circ. Res. 2019;125:245–258. doi: 10.1161/CIRCRESAHA.119.315344. [DOI] [PubMed] [Google Scholar]
- 144.van der Laan A.M., Piek J.J., van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat. Rev. Cardiol. 2009;6:515–523. doi: 10.1038/nrcardio.2009.103. [DOI] [PubMed] [Google Scholar]
- 145.Heusch G. Coronary blood flow in heart failure: cause, consequence and bystander. Basic Res. Cardiol. 2022;117:1. doi: 10.1007/s00395-022-00909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Faxon D.P., Leopold J.A., Abbott J.D., McElhinney D.B., Williams D.O. Circulation: cardiovascular interventions: the first 10 years. Circ. Cardiovasc. Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006901. [DOI] [PubMed] [Google Scholar]
- 147.Bulluck H., Paradies V., Barbato E., Baumbach A., Bøtker H.E., Capodanno D., et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: a consensus document of the ESC working group on cellular biology of the heart and European association of percutaneous cardiovascular interventions (EAPCI) Eur. Heart J. 2021;42:2630–2642. doi: 10.1093/eurheartj/ehab271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Silvain J., Zeitouni M., Paradies V., Zheng H.L., Ndrepepa G., Cavallini C., et al. Procedural myocardial injury, infarction and mortality in patients undergoing elective PCI: a pooled analysis of patient-level data. Eur. Heart J. 2021;42:323–334. doi: 10.1093/eurheartj/ehaa885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., et al. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 150.Cavender M.A., Bhatt D.L., Stone G.W., White H.D., Steg P.G., Gibson C.M., et al. Consistent reduction in periprocedural myocardial infarction with cangrelor as assessed by multiple definitions: findings from CHAMPION PHOENIX (cangrelor versus standard therapy to achieve optimal management of platelet inhibition) Circulation. 2016;134:723–733. doi: 10.1161/CIRCULATIONAHA.115.020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Z.J., Hu W.K., Liu Y.Y., Shi D.M., Cheng W.J., Guo Y.H., et al. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can. J. Cardiol. 2014;30:96–101. doi: 10.1016/j.cjca.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 152.Basili S., Tanzilli G., Mangieri E., Raparelli V., Di Santo S., Pignatelli P., Violi F. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovasc. Interv. 2010;3:221–229. doi: 10.1016/j.jcin.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 153.Mangiacapra F., Peace A.J., Di Serafino L., Pyxaras S.A., Bartunek J., Wyffels E., et al. Intracoronary enalaprilat to reduce microvascular damage during percutaneous coronary intervention (ProMicro) study. J. Am. Coll. Cardiol. 2013;61:615–621. doi: 10.1016/j.jacc.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 154.Mulukutla S.R., Marroquin O.C., Smith C., Varghese R., Anderson W.D., Lee J.S., et al. Effect of statin therapy prior to elective percutaneous coronary intervention on frequency of periprocedural myocardial injury. Am. J. Cardiol. 2004;94:1363–1366. doi: 10.1016/j.amjcard.2004.07.140. [DOI] [PubMed] [Google Scholar]
- 155.Pasceri V., Patti G., Nusca A., Pristipino C., Richichi G., Di Sciascio G. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention. Results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) Study. Circulation. 2004;110:674–678. doi: 10.1161/01.CIR.0000137828.06205.87. [DOI] [PubMed] [Google Scholar]
- 156.Briguori C., Visconti G., Focaccio A., Golia B., Chieffo A., Castelli A., et al. Novel approaches for preventing or limiting events (Naples) II trial impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. J. Am. Coll. Cardiol. 2009;54:2157–2163. doi: 10.1016/j.jacc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 157.Sardella G., Lucisano L., Mancone M., Conti G., Calcagno S., Stio R.E., et al. Comparison of high reloading ROsuvastatin and Atorvastatin pretreatment in patients undergoing elective PCI to reduce the incidence of MyocArdial periprocedural necrosis. The ROMA II trial. Int. J. Cardiol. 2013;168:3715–3720. doi: 10.1016/j.ijcard.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 158.Pan Y., Tan Y., Li B., Li X. Efficacy of high-dose rosuvastatin preloading in patients undergoing percutaneous coronary intervention: a meta-analysis of fourteen randomized controlled trials. Lipids Health Dis. 2015;14:97. doi: 10.1186/s12944-015-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Veselka J., Hajek P., Tomasov P., Tesar D., Bruhova H., Matejovic M., et al. Effect of rosuvastatin therapy on troponin I release following percutaneous coronary intervention in nonemergency patients (from the TIP 3 study) Am. J. Cardiol. 2014;113:446–451. doi: 10.1016/j.amjcard.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 160.Zemanek D., Branny M., Martinkovicova L., Hajek P., Maly M., Tesar D., et al. Effect of seven-day atorvastatin pretreatment on the incidence of periprocedural myocardial infarction following percutaneous coronary intervention in patients receiving long-term statin therapy. A randomized study. Int. J. Cardiol. 2013;168:2494–2497. doi: 10.1016/j.ijcard.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 161.Patti G., Chello M., Pasceri V., Colonna D., Nusca A., Miglionico M., et al. Protection from procedural myocardial injury by atorvastatin is associated with lower levels of adhesion molecules after percutaneous coronary intervention: results from the ARMYDA-CAMs (Atorvastatin for Reduction of MYocardial Damage during Angioplasty-Cell Adhesion Molecules) substudy. J. Am. Coll. Cardiol. 2006;48:1560–1566. doi: 10.1016/j.jacc.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 162.Ye H., He F., Fei X., Lou Y., Wang S., Yang R., et al. High-dose atorvastatin reloading before percutaneous coronary intervention increased circulating endothelial progenitor cells and reduced inflammatory cytokine expression during the perioperative period. J. Cardiovasc. Pharmacol. Therapeut. 2014;19:290–295. doi: 10.1177/1074248413513500. [DOI] [PubMed] [Google Scholar]
- 163.Hoole S.P., Heck P.M., Sharples L., Khan S.N., Duehmke R., Densem C.G., et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 164.Luo S.J., Zhou Y.J., Shi D.M., Ge H.L., Wang J.L., Liu R.F. Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can. J. Cardiol. 2013;29:1084–1089. doi: 10.1016/j.cjca.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 165.Pei H., Wu Y., Wei Y., Yang Y., Teng S., Zhang H. Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta-analysis of 11 randomized trials. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prasad A., Gossl M., Hoyt J., Lennon R.J., Polk L., Simari R., et al. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Cathet. Cardiovasc. Interv. 2013;81:930–936. doi: 10.1002/ccd.24443. [DOI] [PubMed] [Google Scholar]
- 167.Davies W.R., Brown A.J., Watson W., McCormick L.M., West N.E., Dutka D.P., Hoole S.P. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ. Cardiovasc. Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 168.Haller P.M., Vargas K.G., Haller M.C., Piackova E., Wojta J., Gyongyosi M., et al. Remote ischaemic conditioning for myocardial infarction or elective PCI: systematic review and meta-analyses of randomised trials. Eur. Heart J. Acute Cardiovasc. Care. 2020;9:82–92. doi: 10.1177/2048872618784150. [DOI] [PubMed] [Google Scholar]
- 169.El Desoky E.S., Hassan A.K.M., Salem S.Y., Fadil S.A., Taha A.F. Cardioprotective effect of atorvastatin alone or in combination with remote ischemic preconditioning on the biochemical changes induced by ischemic/reperfusion injury in a mutual prospective study with a clinical and experimental animal arm. Int. J. Cardiol. 2016;222:866–873. doi: 10.1016/j.ijcard.2016.07.178. [DOI] [PubMed] [Google Scholar]
- 170.Zhao Z., Shi Q., Guo Q., Peng L., Li X., Rao L., Li M. Remote ischemic preconditioning can extend the tolerance to extended drug-coated balloon inflation time by reducing myocardial damage during percutaneous coronary intervention. Int. J. Cardiol. 2022;353:3–8. doi: 10.1016/j.ijcard.2022.01.049. [DOI] [PubMed] [Google Scholar]
- 171.Nidorf S.M., Fiolet A.T.L., Mosterd A., Eikelboom J.W., Schut A., Opstal T.S.J., et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 172.Shah B., Pillinger M., Zhong H., Cronstein B., Xia Y., Lorin J.D., et al. Effects of ccute colchicine administration prior to percutaneous coronary intervention: COLCHICINE-PCI Randomized Trial. Circ. Cardiovasc. Interv. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.119.008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.DeWall R.A. The origins of open heart surgery at the University of Minnesota 1951 to 1956. J. Thorac. Cardiovasc. Surg. 2011;142:267–269. doi: 10.1016/j.jtcvs.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 174.Sellke F.W., Shafique T., Ely D.L., Weintraub R.M. Coronary endothelial injury after cardiopulmonary bypass and ischemic cardioplegia is mediated by oxygen-derived free radicals. Circulation. 1993;88:II395–400. [PubMed] [Google Scholar]
- 175.Sabe S.A., Feng J., Sellke F.W., Abid M.R. Mechanisms and clinical implications of endothelium-dependent vasomotor dysfunction in coronary microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2022;322:H819–H841. doi: 10.1152/ajpheart.00603.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sabe S.A., Kononov M.A., Bellam K.G., Sodha N., Ehsan A., Jackson W.F., et al. Poorly controlled hypertension is associated with increased coronary myogenic tone in patients undergoing cardiac surgery with cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 2023;165:e256–e267. doi: 10.1016/j.jtcvs.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hearse D.J., Stewart D.A., Braimbridge M.V. Hypothermic arrest and potassium arrest.Metabolic and myocardial protection during elective cardiac arrest. Circ. Res. 1975;36:481–489. doi: 10.1161/01.res.36.4.481. [DOI] [PubMed] [Google Scholar]
- 178.Mankad P.S., Chester A.H., Yacoub M.H. Role of potassium concentration in cardioplegic solutions in mediating endothelial damage. Ann. Thorac. Surg. 1991;51:89–93. doi: 10.1016/0003-4975(91)90457-2. [DOI] [PubMed] [Google Scholar]
- 179.Dobson G.P., Faggian G., Onorati F., Vinten-Johansen J. Hyperkalemic cardioplegia for adult and pediatric surgery: end of an era? Front. Physiol. 2013;4:228. doi: 10.3389/fphys.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Diao H., Gu H., Chen Q.M. Hyperkalemic or low potassium cardioplegia protects against reduction of energy metabolism by oxidative stress. Antioxidants (Basel) 2023 doi: 10.3390/antiox12020452. 10.3390/antiox12020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lena T., Amabile A., Morrison A., Torregrossa G., Geirsson A., Tesler U.F. John H. Gibbon and the development of the heart-lung machine: the beginnings of open cardiac surgery. J. Card. Surg. 2022;37:4199–4201. doi: 10.1111/jocs.17067. [DOI] [PubMed] [Google Scholar]
- 182.Heusch G. Myocardial stunning and hibernation revisited. Nat. Rev. Cardiol. 2021;18:522–536. doi: 10.1038/s41569-021-00506-7. [DOI] [PubMed] [Google Scholar]
- 183.Baikoussis N.G., Papakonstantinou N.A., Verra C., Kakouris G., Chounti M., Hountis P., et al. Mechanisms of oxidative stress and myocardial protection during open-heart surgery. Ann. Card Anaesth. 2015;18:555–564. doi: 10.4103/0971-9784.166465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kant S., Banerjee D., Sabe S.A., Sellke F., Feng J. Microvascular dysfunction following cardiopulmonary bypass plays a central role in postoperative organ dysfunction. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suleiman M.S., Zacharowski K., Angelini G.D. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br. J. Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zakkar M., Guida G., Suleiman M.S., Angelini G.D. Cardiopulmonary bypass and oxidative stress. Oxid. Med. Cell Longev. 2015. 2015 doi: 10.1155/2015/189863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jacob S., Kallikourdis A., Sellke F., Dunning J. Is blood cardioplegia superior to crystalloid cardioplegia? Interact. Cardiovasc. Thorac. Surg. 2008;7:491–498. doi: 10.1510/icvts.2008.178343. [DOI] [PubMed] [Google Scholar]
- 188.Sanetra K., Pawlak I., Cisowski M. Del Nido cardioplegia - what is the current evidence? Kardiochir Torakochirurgia Pol. 2018;15:114–118. doi: 10.5114/kitp.2018.76477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Abd-Elfattah A.S., Ding M., Wechsler A.S. Intermittend aortic crossclamping prevents cumulative adenosine triphosphate depletion, ventricular fibrillation, and dysfunction (stunning): is it preconditioning? J. Thorac. Cardiovasc. Surg. 1995;110:328–339. doi: 10.1016/S0022-5223(95)70228-8. [DOI] [PubMed] [Google Scholar]
- 190.Yellon D.M., Alkhulaifi A.M., Pugsley W.B. Preconditioning the human myocardium. Lancet. 1993;342:276–277. doi: 10.1016/0140-6736(93)91819-8. [DOI] [PubMed] [Google Scholar]
- 191.Alkhulaifi A.M., Yellon D.M., Pugsley W.B. Preconditioning the human heart during aorto-coronary bypass surgery. Eur. J. Cardio. Thorac. Surg. 1994;8:270–275. doi: 10.1016/1010-7940(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 192.Kurapeev D.I., Kabanov V.O., Grebennik V.K., Sheshurina T.A., Dorofeykov V.V., Galagudza M.M., Shlyakhto E.V. New technique of local ischemic preconditioning induction without repetitive aortic cross-clamping in cardiac surgery. J. Cardiothorac. Surg. 2015;10:9. doi: 10.1186/s13019-015-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hausenloy D.J., Mwamure P.K., Venugopal V., Harris J., Barnard M., Grundy E., et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 194.Xie J.J., Liao X.L., Chen W.G., Huang D.D., Chang F.J., Chen W., et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart. 2012;98:384–388. doi: 10.1136/heartjnl-2011-300860. [DOI] [PubMed] [Google Scholar]
- 195.Hong D.M., Lee E.H., Kim H.J., Min J.J., Chin J.H., Choi D.K., et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur. Heart J. 2014;35:176–183. doi: 10.1093/eurheartj/eht346. [DOI] [PubMed] [Google Scholar]
- 196.Zhang B., Zhou J., Li H., Zhou M., Chen A., Zhao Q. Remote ischemic preconditioning does not improve the clinical outcomes in patients undergoing coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Int. J. Cardiol. 2014;172:e36–e38. doi: 10.1016/j.ijcard.2013.12.086. [DOI] [PubMed] [Google Scholar]
- 197.Hausenloy D.J., Candilio L., Evans R., Ariti C., Jenkins D.P., Kolvekar S., et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N. Engl. J. Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 198.Meybohm P., Bein B., Brosteanu O., Cremer J., Gruenewald M., Stoppe C., et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 199.Kleinbongard P., Peters J., Jakob H., Heusch G., Thielmann M. Persistent survival benefit from remote ischemic preconditioning in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2018;71:251–262. doi: 10.1016/j.jacc.2017.10.083. [DOI] [PubMed] [Google Scholar]
- 200.Moscarelli M., Fiorentino F., Suleiman M.S., Emanueli C., Reeves B.C., Punjabi P.P., Angelini G.D. Remote ischaemic preconditioning in isolated aortic valve and coronary artery bypass surgery: a randomized trial. Eur. J. Cardio. Thorac. Surg. 2019;55:905–912. doi: 10.1093/ejcts/ezy404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thielmann M., Kottenberg E., Kleinbongard P., Wendt D., Gedik N., Pasa S., et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 202.Heusch G., Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ. Res. 2016;119:676–695. doi: 10.1161/CIRCRESAHA.116.308736. [DOI] [PubMed] [Google Scholar]
- 203.Kottenberg E., Thielmann M., Bergmann L., Heine T., Jakob H., Heusch G., Peters J. Protection by remote ischaemic preconditioning during coronary artery bypass grafting with isoflurane but not with propofol anesthesia - a clinical trial. Acta Anaesthesiol. Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 204.Zangrillo A., Musu M., Greco T., Di Prima A.L., Matteazzi A., Testa V., et al. Additive effect on survival of anaesthetic cardiac protection and remote ischemic preconditioning in cardiac surgery: a Bayesian network meta-analysis of randomized trials. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhou C., Liu Y., Yao Y., Zhou S., Fang N., Wang W., Li L. Beta-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J. Cardiothorac. Vasc. Anesth. 2013;27:305–311. doi: 10.1053/j.jvca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 206.Tofukuji M., Metais C., Li J., Hariawala M.D., Franklin A., Vassileva C., et al. Effects of ischemic preconditioning on myocardial perfusion, function, and microvascular regulation. Circulation. 1998;98:II–197. -II-205. [PubMed] [Google Scholar]
- 207.Emani S., Ramlawi B., Sodha N.R., Li J., Bianchi C., Sellke F.W. Increased vascular permeability after cardiopulmonary bypass in patients with diabetes is associated with increased expression of vascular endothelial growth factor and hepatocyte growth factor. J. Thorac. Cardiovasc. Surg. 2009;138:185–191. doi: 10.1016/j.jtcvs.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Feng J., Liu Y., Chu L.M., Singh A.K., Dobrilovic N., Fingleton J.G., et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sellke N., Kuczmarski A., Lawandy I., Cole V.L., Ehsan A., Singh A.K., et al. Enhanced coronary arteriolar contraction to vasopressin in patients with diabetes after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2018;156:2098–2107. doi: 10.1016/j.jtcvs.2018.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Osipov R.M., Bianchi C., Feng J., Clements R.T., Liu Y., Robich M.P., et al. Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation. 2009;120:S22–S30. doi: 10.1161/CIRCULATIONAHA.108.842724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heusch G., Musiolik J., Kottenberg E., Peters J., Jakob H., Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ. Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 212.Ale-Agha N., Jakobs P., Goy C., Zurek M., Rosen J., Dyballa-Rukes N., et al. Mitochondrial telomerase reverse transcriptase protects from myocardial ischemia/reperfusion injury by improving complex I composition and function. Circulation. 2021;144:1876–1890. doi: 10.1161/CIRCULATIONAHA.120.051923. [DOI] [PubMed] [Google Scholar]
- 213.Jernberg T., Johanson P., Held C., Svennblad B., Lindback J., Wallentin L., Swedeheart/Riks H.I.A. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–1684. doi: 10.1001/jama.2011.522. [DOI] [PubMed] [Google Scholar]
- 214.Puymirat E., Simon T., Steg P.G., Schiele F., Gueret P., Blanchard D., et al. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308:998–1006. doi: 10.1001/2012.jama.11348. [DOI] [PubMed] [Google Scholar]
- 215.Kristensen S.D., Laut K.G., Fajadet J., Kaifoszova Z., Kala P., Di Mario C., et al. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur. Heart J. 2014;35:1957–1970. doi: 10.1093/eurheartj/eht529. [DOI] [PubMed] [Google Scholar]
- 216.Rentoukas I., Giannopoulos G., Kaoukis A., Kossyvakis C., Raisakis K., Driva M., et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 217.Prunier F., Angoulvant D., Saint Etienne C., Vermes E., Gilard M., Piot C., et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res. Cardiol. 2014;109:400. doi: 10.1007/s00395-013-0400-y. [DOI] [PubMed] [Google Scholar]
- 218.Yellon D.M., Ackbarkhan A.K., Balgobin V., Bulluck H., Deelchand A., Dhuny M.R., et al. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J. Am. Coll. Cardiol. 2015;65:2764–2765. doi: 10.1016/j.jacc.2015.02.082. [DOI] [PubMed] [Google Scholar]
- 219.Bøtker H.E., Kharbanda R., Schmidt M.R., Bøttcher M., Kaltoft A.K., Terkelsen C.J., et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 220.Verouhis D., Sorensson P., Gourine A., Henareh L., Persson J., Saleh N., et al. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am. Heart J. 2016;181:66–73. doi: 10.1016/j.ahj.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 221.Staat P., Rioufol G., Piot C., Cottin Y., Cung T.T., L'Huillier I., et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 222.Eitel I., Stiermaier T., Rommel K.P., Fuernau G., Sandri M., Mangner N., et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur. Heart J. 2015;36:3049–3057. doi: 10.1093/eurheartj/ehv463. [DOI] [PubMed] [Google Scholar]
- 223.Stiermaier T., Jensen J.O., Rommel K.P., de Waha-Thiele S., Fuernau G., Desch S., et al. Combined intrahospital remote ischemic perconditioning and postconditioning improves clinical outcome in ST-elevation myocardial infarction. Circ. Res. 2019;124:1482–1491. doi: 10.1161/CIRCRESAHA.118.314500. [DOI] [PubMed] [Google Scholar]
- 224.Sloth A.D., Schmidt M.R., Munk K., Kharbanda R.K., Redington A.N., Schmidt M., et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur. Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 225.Terkelsen C.J., Sorensen J.T., Maeng M., Jensen L.O., Tilsted H.H., Trautner S., et al. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA. 2010;304:763–771. doi: 10.1001/jama.2010.1139. [DOI] [PubMed] [Google Scholar]
- 226.Huber K., Gersh B.J., Goldstein P., Granger C.B., Armstrong P.W. The organization, function, and outcomes of ST-elevation myocardial infarction networks worldwide: current state, unmet needs and future directions. Eur. Heart J. 2014;35:1526–1532. doi: 10.1093/eurheartj/ehu125. [DOI] [PubMed] [Google Scholar]
- 227.Morrow D.A., Antman E.M., Charlesworth A., Cairns R., Murphy S.A., de Lemos J.A., et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 228.Pryds K., Terkelsen C.J., Sloth A.D., Munk K., Nielsen S.S., Schmidt M.R., et al. Remote ischaemic conditioning and healthcare system delay in patients with ST-segment elevation myocardial infarction. Heart. 2016;102:1023–1028. doi: 10.1136/heartjnl-2015-308980. [DOI] [PubMed] [Google Scholar]
- 229.Cheskes S., Koh M., Turner L., Heslegrave R., Verbeek R., Dorian P., et al. Field implementation of remote ischemic conditioning in ST-segment-elevation myocardial infarction: the FIRST Study. Can. J. Cardiol. 2020;36:1278–1288. doi: 10.1016/j.cjca.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 230.Hausenloy D.J., Ntsekhe M., Yellon D.M. A future for remote ischaemic conditioning in high-risk patients. Basic Res. Cardiol. 2020;115:35. doi: 10.1007/s00395-020-0794-2. [DOI] [PubMed] [Google Scholar]
- 231.Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 232.Cung T.T., Morel O., Cayla G., Rioufol G., Garcia-Dorado D., Angoulvant D., et al. Cyclosporine before PCI in patients with acute myocardial infarction. N. Engl. J. Med. 2015;373:1021–1023. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 233.Ottani F., Latini R., Staszewsky L., La Vecchia L., Locuratolo N., Sicuro M., et al. Cyclosporine A in reperfused myocardial infarction. Themulticenter, contolled, open-label CYCLE Trial. J. Am. Coll. Cardiol. 2016;67:365–374. doi: 10.1016/j.jacc.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 234.Gibson C.M., Giugliano R.P., Kloner R.A., Bode C., Tendera M., Janosi A., et al. EMBRACE STEMI study: a phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur. Heart J. 2016;37:1296–1303. doi: 10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]
- 235.Atar D., Arheden H., Berdeaux A., Bonnet J.L., Carlsson M., Clemmensen P., et al. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur. Heart J. 2015;36:112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- 236.Siddiqi N., Neil C., Bruce M., Maclennan G., Cotton S., Papadopoulou S., et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur. Heart J. 2014;35:1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jones D.A., Pellaton C., Velmurugan S., Rathod K.S., Andiapen M., Antoniou S., et al. Randomized phase 2 trial of intracoronary nitrite during acute myocardial infarction. Circ. Res. 2015;116:437–447. doi: 10.1161/CIRCRESAHA.116.305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Janssens S.P., Bogaert J., Zalewski J., Toth A., Adriaenssens T., Belmans A., et al. Nitric oxide for inhalation in ST-elevation myocardial infarction (NOMI): a multicentre, double-blind, randomized controlled trial. Eur. Heart J. 2018;39:2717–2725. doi: 10.1093/eurheartj/ehy232. [DOI] [PubMed] [Google Scholar]
- 239.Thiele H., Hildebrand L., Schirdewahn C., Eitel I., Adams V., Fuernau G., et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J. Am. Coll. Cardiol. 2010;55:2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 240.Pasupathy S., Tavella R., Grover S., Raman B., Procter N.E.K., Du Y.T., et al. Early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]) Circulation. 2017;136:894–903. doi: 10.1161/CIRCULATIONAHA.117.027575. [DOI] [PubMed] [Google Scholar]
- 241.Kleveland O., Kunszt G., Bratlie M., Ueland T., Broch K., Holte E., et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016;37:2406–2413. doi: 10.1093/eurheartj/ehw171. [DOI] [PubMed] [Google Scholar]
- 242.Broch K., Anstensrud A.K., Woxholt S., Sharma K., Tollefsen I.M., Bendz B., et al. Randomized trial of interleukin-6 receptor Inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049. [DOI] [PubMed] [Google Scholar]
- 243.Lincoff A.M., Roe M., Aylward P., Galla J., Rynkiewicz A., Guetta V., et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014;35:2516–2523. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- 244.Ibanez B., Macaya C., Sanchez-Brunete V., Pizarro G., Fernandez-Friera L., Mateos A., et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the effect of metoprolol in cardioprotection during an acute myocardial infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 245.Roolvink V., Ibanez B., Ottervanger J.P., Pizarro G., van Royen N., Mateos A., et al. Early administration of intravenous beta blockers in patients with ST-elevation myocardial infarction before primary PCI. J. Am. Coll. Cardiol. 2016;67:2705–2715. doi: 10.1016/j.jacc.2016.03.522. [DOI] [PubMed] [Google Scholar]
- 246.Bøtker H.E., Cabrera-Fuentes H.A., Ruiz-Meana M., Heusch G., Ovize M. Translational issues for mitoprotective agents as adjunct to reperfusion therapy in patients with ST-segment elevation myocardial infarction. J. Cell Mol. Med. 2020;24:2717–2729. doi: 10.1111/jcmm.14953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kleinbongard P., Lieder H., Skyschally A., Heusch G. No robust reduction of infarct size and no-reflow by metoprolol pretreatment in adult Göttingen minipigs. Basic Res. Cardiol. 2023;118:23. doi: 10.1007/s00395-023-00993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bulluck H., Sirker A., Loke Y.K., Garcia-Dorado D., Hausenloy D.J. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: an updated meta-analysis of randomized controlled trials. Int. J. Cardiol. 2016;202:228–237. doi: 10.1016/j.ijcard.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kitakaze M., Asakura M., Kim J., Shintani Y., Asanuma H., Hamasaki T., et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 250.Garcia Del Blanco B., Otaegui I., Rodriguez-Palomares J.F., Bayes-Genis A., Fernandez-Nofrerias E., Vilalta Del Olmo V., et al. Effect of COMBinAtion therapy with remote ischemic conditioning and exenatide on the myocardial infarct size: a two-by-two factorial randomized trial (COMBAT-MI) Basic Res. Cardiol. 2021;116:4. doi: 10.1007/s00395-021-00842-2. [DOI] [PubMed] [Google Scholar]
- 251.Ndrepepa G., Kastrati A. Mechanical strategies to enhance myocardial salvage during primary percutaneous coronary intervention in patients with STEMI. EuroIntervention. 2016;12:319–328. doi: 10.4244/EIJV12I3A52. [DOI] [PubMed] [Google Scholar]
- 252.Swain L., Reyelt L., Bhave S., Qiao X., Thomas C.J., Zweck E., et al. Transvalvular ventricular unloading before reperfusion in acute myocardial infarction. J. Am. Coll. Cardiol. 2020;76:684–699. doi: 10.1016/j.jacc.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Heusch G., Rassaf T. Left ventricular unloading in myocardial infarction: gentle reperfusion through the backdoor? J. Am. Coll. Cardiol. 2020;76:700–702. doi: 10.1016/j.jacc.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 254.Tissier R., Ghaleh B., Cohen M.V., Downey J.M., Berdeaux A. Myocardial protection with mild hypothermia. Cardiovasc. Res. 2012;94:217–225. doi: 10.1093/cvr/cvr315. [DOI] [PubMed] [Google Scholar]
- 255.Dixon S.R., Whitbourn R.J., Dae M.W., Grube E., Sherman W., Schaer G.L., et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J. Am. Coll. Cardiol. 2002;40:1928–1934. doi: 10.1016/S0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 256.Götberg M., Olivecrona G.K., Koul S., Carlsson M., Engblom H., Ugander M., et al. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2010;3:400–407. doi: 10.1161/CIRCINTERVENTIONS.110.957902. [DOI] [PubMed] [Google Scholar]
- 257.Erlinge D., Gotberg M., Lang I., Holzer M., Noc M., Clemmensen P., et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction (The CHILL-MI trial) J. Am. Coll. Cardiol. 2014;63:1857–1865. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 258.Erlinge D., Gotberg M., Noc M., Lang I., Holzer M., Clemmensen P., et al. Therapeutic hypothermia for the treatment of acute myocardial infarction-combined analysis of the RAPID MI-ICE and the CHILL-MI trials. Ther. Hypothermia Temp. Manag. 2015;5:77–84. doi: 10.1089/ther.2015.0009. [DOI] [PubMed] [Google Scholar]
- 259.Noc M., Erlinge D., Neskovic A.N., Kafedzic S., Merkely B., Zima E., et al. COOL AMI EU pilot trial: a multicentre, prospective, randomised controlled trial to assess cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction. EuroIntervention. 2017;13:e531–e539. doi: 10.4244/EIJ-D-17-00279. [DOI] [PubMed] [Google Scholar]
- 260.Noc M., Laanmets P., Neskovic A.N., Petrovic M., Stanetic B., Aradi D., et al. A multicentre, prospective, randomised controlled trial to assess the safety and effectiveness of cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction: the COOL AMI EU Pivotal Trial. EuroIntervention. 2021;17:466–473. doi: 10.4244/EIJ-D-21-00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fuernau G., Beck J., Desch S., Eitel I., Jung C., Erbs S., et al. Mild hypothermia in cardiogenic shock complicating myocardial infarction: the randomized SHOCK-COOL Trial. Circulation. 2018;139:448–457. doi: 10.1161/CIRCULATIONAHA.117.032722. [DOI] [PubMed] [Google Scholar]
- 262.Kloner R.A., Creech J.L., Stone G.W., O'Neill W.W., Burkhoff D., Spears J.R. Update on cardioprotective strategies for STEMI: focus on supersaturated oxygen delivery. JACC Basic Transl. Sci. 2021;6:1021–1033. doi: 10.1016/j.jacbts.2021.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.O'Neill W.W., Martin J.L., Dixon S.R., Bartorelli A.L., Trabattoni D., Oemrawsingh P.V., et al. Acute myocardial infarction with hyperoxemic therapy (AMIHOT): a prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007;50:397–405. doi: 10.1016/j.jacc.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 264.Stone G.W., Martin J.L., de Boer M.J., Margheri M., Bramucci E., Blankenship J.C., et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009;2:366–375. doi: 10.1161/CIRCINTERVENTIONS.108.840066. [DOI] [PubMed] [Google Scholar]
- 265.Desta L., Jernberg T., Lofman I., Hofman-Bang C., Hagerman I., Spaak J., Persson H. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. 2015;3:234–242. doi: 10.1016/j.jchf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 266.De Filippo O., D'Ascenzo F., Wanha W., Leonardi S., Raposeiras Roubin S., Fabris E., et al. IncidenCe and predictOrs of heaRt fAiLure after acute coronarY Syndrome: the CORALYS registry. Int. J. Cardiol. 2023;370:35–42. doi: 10.1016/j.ijcard.2022.10.146. [DOI] [PubMed] [Google Scholar]
- 267.Hamilton E., Desta L., Lundberg A., Alfredsson J., Christersson C., Erlinge D., et al. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail. 2023;10:1347–1357. doi: 10.1002/ehf2.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pryds K., Nielsen R.R., Jorsal A., Hansen M.S., Ringgaard S., Refsgaard J., et al. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res. Cardiol. 2017;112:67. doi: 10.1007/s00395-017-0658-6. [DOI] [PubMed] [Google Scholar]
- 269.Chen L., Zhou Q., Jin H., Zhu K., Zhi H., Chen Z., Ma G. Effects of remote ischaemic conditioning on heart rate variability and cardiac function in patients with mild ischaemic heart failure. Heart Lung Circ. 2017;4:477–483. doi: 10.1016/j.hlc.2017.03.164. [DOI] [PubMed] [Google Scholar]
- 270.Kuzuya T., Hoshida S., Yamashita N., Fuji H., Oe H., Hori M., et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ. Res. 1993;72:1293–1299. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 271.Marber M.S., Latchman D.S., Walker J.M., Yellon D.M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 272.Loubani M., Hassouna A., Galinanes M. Delayed preconditioning of the human myocardium: signal transduction and clinical implications. Cardiovasc. Res. 2004;61:600–609. doi: 10.1016/j.cardiores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 273.Davidson S.M., Boulanger C.M., Aikawa E., Badimon L., Barile L., Binder C.J., et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles. Cardiovasc. Res. 2023;119:45–63. doi: 10.1093/cvr/cvac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Giricz Z., Varga Z.V., Baranyai T., Sipos P., Paloczi K., Kittel A., et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cell. Cardiol. 2014;68:75–78. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 275.Vicencio J.M., Yellon D.M., Sivaraman V., Das D., Boi-Doku C., Arjun S., et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015;65:1525–1536. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 276.Lassen T.R., Just J., Hjortbak M.V., Jespersen N.R., Stenz K.T., Gu T., et al. Cardioprotection by remote ischemic conditioning is transferable by plasma and mediated by extracellular vesicles. Basic Res. Cardiol. 2021;116:16. doi: 10.1007/s00395-021-00856-w. [DOI] [PubMed] [Google Scholar]
- 277.Homme R.P., Zheng Y., Smolenkova I., Singh M., Tyagi S.C. Remote hind-limb ischemia mechanism of preserved ejection fraction during heart failure. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.745328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vanezis A.P., Arnold J.R., Rodrigo G., Lai F.Y., Debiec R., Nazir S., et al. Daily remote ischaemic conditioning following acute myocardial infarction: a randomised controlled trial. Heart. 2018;104:1955–1962. doi: 10.1136/heartjnl-2018-313091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Munk K., Andersen N.H., Schmidt M.R., Nielsen S.S., Terkelsen C.J., Sloth E., et al. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ. Cardiovasc. Imag. 2010;3:656–662. doi: 10.1161/CIRCIMAGING.110.957340. [DOI] [PubMed] [Google Scholar]
- 280.Arnold J.R., Vanezis A.P., Rodrigo G.C., Lai F.Y., Kanagala P., Nazir S., et al. Effects of late, repetitive remote ischaemic conditioning on myocardial strain in patients with acute myocardial infarction. Basic Res. Cardiol. 2022;117:23. doi: 10.1007/s00395-022-00926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kono Y., Fukuda S., Hanatani A., Nakanishi K., Otsuka K., Taguchi H., Shimada K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des. Dev. Ther. 2014;8:1175–1181. doi: 10.2147/DDDT.S68715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pryds K., Schmidt M.R., Bjerre M., Thiel S., Refsgaard J., Bøtker H.E., et al. Effect of long-term remote ischemic conditioning on inflammation and cardiac remodeling. Scand. Cardiovasc. J. 2019:1–26. doi: 10.1080/14017431.2019.1622770. [DOI] [PubMed] [Google Scholar]
- 283.Shimizu M., Saxena P., Konstantinov I.E., Cherepanov V., Cheung M.M., Wearden P., et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J. Surg. Res. 2010;158:155–161. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 284.Ikonomidis I., Vlastos D., Andreadou I., Gazouli M., Efentakis P., Varoudi M., et al. Vascular conditioning prevents adverse left ventricular remodelling after acute myocardial infarction: a randomised remote conditioning study. Basic Res. Cardiol. 2021;116:9. doi: 10.1007/s00395-021-00851-1. [DOI] [PubMed] [Google Scholar]
- 285.Jean-St-Michel E., Manlhiot C., Li J., Tropak M., Michelsen M.M., Schmidt M.R., et al. Remote preconditioning improves maximal performance in highly trained athletes. Med. Sci. Sports Exerc. 2011;43:1280–1286. doi: 10.1249/MSS.0b013e318206845d. [DOI] [PubMed] [Google Scholar]
- 286.Groennebaek T., Sieljacks P., Nielsen R., Pryds K., Jespersen N.R., Wang J., et al. Effect of blood flow restricted resistance exercise and remote ischemic conditioning on functional capacity and myocellular adaptations in patients with heart failure. Circ. Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006427. [DOI] [PubMed] [Google Scholar]
- 287.Pearson S.J., Hussain S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 288.Cornish S.M., Bugera E.M., Duhamel T.A., Peeler J.D., Anderson J.E. A focused review of myokines as a potential contributor to muscle hypertrophy from resistance-based exercise. Eur. J. Appl. Physiol. 2020;120:941–959. doi: 10.1007/s00421-020-04337-1. [DOI] [PubMed] [Google Scholar]
- 289.Nielsen J.L., Aagaard P., Bech R.D., Nygaard T., Hvid L.G., Wernbom M., et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J. Physiol. 2012;590:4351–4361. doi: 10.1113/jphysiol.2012.237008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Aguayo D., Mueller S.M., Boutellier U., Auer M., Jung H.H., Fluck M., Toigo M. One bout of vibration exercise with vascular occlusion activates satellite cells. Exp. Physiol. 2016;101:295–307. doi: 10.1113/EP085330. [DOI] [PubMed] [Google Scholar]
- 291.Gu T., Just J., Stenz K.T., Yan Y., Sieljacks P., Wang J., et al. The role of plasma extracellular vesicles in remote ischemic conditioning and exercise-induced ischemic tolerance. Int. J. Mol. Sci. 2022;23:3334. doi: 10.3390/ijms23063334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Barac A., Murtagh G., Carver J.R., Chen M.H., Freeman A.M., Herrmann J., et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J. Am. Coll. Cardiol. 2015;65:2739–2746. doi: 10.1016/j.jacc.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lyon A.R., Lopez-Fernandez T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., et al. ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS) Eur. Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. 2022. [DOI] [PubMed] [Google Scholar]
- 294.Omland T., Heck S.L., Gulati G. The role of cardioprotection in cancer therapy cardiotoxicity: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022;4:19–37. doi: 10.1016/j.jaccao.2022.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lopez-Sendon J., Alvarez-Ortega C., Zamora Aunon P., Buno Soto A., Lyon A.R., Farmakis D., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur. Heart J. 2020;41:1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 296.Hahn V.S., Zhang K.W., Sun L., Narayan V., Lenihan D.J., Ky B. Heart failure with targeted cancer therapies: mechanisms and cardioprotection. Circ. Res. 2021;128:1576–1593. doi: 10.1161/CIRCRESAHA.121.318223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Totzeck M., Schuler M., Stuschke M., Heusch G., Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 298.Zamorano J.L., Lancellotti P., Rodriguez Munoz D., Aboyans V., Asteggiano R., Galderisi M., et al. ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. 2016. [DOI] [PubMed] [Google Scholar]
- 299.Galan-Arriola C., Villena-Gutierrez R., Higuero-Verdejo M.I., Diaz-Rengifo I.A., Pizarro G., Lopez G.J., et al. Remote ischaemic preconditioning ameliorates anthracycline-induced cardiotoxicity and preserves mitochondrial integrity. Cardiovasc. Res. 2021;117:1132–1143. doi: 10.1093/cvr/cvaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Galan-Arriola C., Vilchez-Tschischke J.P., Lobo M., Lopez G.J., de Molina-Iracheta A., Perez-Martinez C., et al. Coronary microcirculation damage in anthracycline cardiotoxicity. Cardiovasc. Res. 2021;118:531–541. doi: 10.1093/cvr/cvab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Galan-Arriola C., Lobo M., Vilchez-Tschischke J.P., Lopez G.J., de Molina-Iracheta A., Perez-Martinez C., et al. Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J. Am. Coll. Cardiol. 2019;73:779–791. doi: 10.1016/j.jacc.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 302.Bosch X., Rovira M., Sitges M., Domenech A., Ortiz-Perez J.T., de Caralt T.M., et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J. Am. Coll. Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 303.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., Jr., das Dores Cruz F., Goncalves Brandao S.M., Rigaud V.O.C., et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY Trial. J. Am. Coll. Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 304.Huang S., Zhao Q., Yang Z.G., Diao K.Y., He Y., Shi K., et al. Protective role of beta-blockers in chemotherapy-induced cardiotoxicity-a systematic review and meta-analysis of carvedilol. Heart Fail. Rev. 2019;24:325–333. doi: 10.1007/s10741-018-9755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Akpek M., Ozdogru I., Sahin O., Inanc M., Dogan A., Yazici C., et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015;17:81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 306.Gulati G., Heck S.L., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37:1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Cardinale D., Ciceri F., Latini R., Franzosi M.G., Sandri M.T., Civelli M., et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International CardioOncology Society-one trial. Eur. J. Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 308.Acar Z., Kale A., Turgut M., Demircan S., Durna K., Demir S., et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2011;58:988–989. doi: 10.1016/j.jacc.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 309.Nabati M., Janbabai G., Esmailian J., Yazdani J. Effect of rosuvastatin in preventing chemotherapy-induced cardiotoxicity in women with breast cancer: a randomized, single-blind, placebo-controlled trial. J. Cardiovasc. Pharmacol. Therapeut. 2019;24:233–241. doi: 10.1177/1074248418821721. [DOI] [PubMed] [Google Scholar]
- 310.Hundley W.G., D'Agostino R., Jr., Crotts T., Craver K., Hackney M.H., Jordan J.H., et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2023;1 doi: 10.1056/evidoa2200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Thavendiranathan P., Houbois C., Marwick T.H., Kei T., Saha S., Runeckles K., et al. Statins to prevent early cardiac dysfunction in cancer patients at increased cardiotoxicity risk receiving anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023 doi: 10.1093/ehjcvp/pvad031. 10.1093/ehjcvp/pvad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Neilan T.G., Quinaglia T., Onoue T., Mahmood S.S., Drobni Z.D., Gilman H.K., et al. Atorvastatin for anthracycline-associated cardiac dysfunction: the STOP-CA randomized clinical trial. JAMA. 2023;330:528–536. doi: 10.1001/jama.2023.11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mallouppas M., Chung R., Ghosh A.K., Macklin A., Yellon D.M., Walker J.M. Anthracyclines and biomarkers of myocardial injury: the effect of remote ischemic conditioning (RIC) JACC CardioOncol. 2023;5:343–355. doi: 10.1016/j.jaccao.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Cheung Y.-F., Wing-yi Li V., Kam-fung So E., Wai-tsoi Cheng F., Ping-wa Yau J., Chiu S.Y., et al. Remote ischemic conditioning in pediatric cancer patients receiving anthracycline chemotherapy: a sham-controlled single-blind randomized trial. JACC CardioOncol. 2023;5:332–342. doi: 10.1016/j.jaccao.2022.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Heusch G. Cardioprotection in cardio-oncology – a case for concern? Cardiovasc. Res. 2023;10 doi: 10.1093/cvr/cvad111. 1093/cvr/cvad111. [DOI] [PubMed] [Google Scholar]
- 316.Ibanez B., Gomes-Silva M. Remote ischemic conditioning for anthracycline cardiotoxicity: the need to protect the most vulnerable. JACC CardioOncol. 2023;5:356–359. doi: 10.1016/j.jaccao.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rywalsch D., Rywalsch M. Über die Katalyse des H202 durch Bakterien. Zentralbl Bakt und Parasit. 1907;44:295–298. [Google Scholar]
- 318.McCord J.M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968;243:5753–5760. doi: 10.1016/S0021-9258(18)91929-0. [DOI] [PubMed] [Google Scholar]
- 319.McCord J.M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969;244:6056–6063. doi: 10.1016/S0021-9258(18)63505-7. [DOI] [PubMed] [Google Scholar]
- 320.Hearse D.J., Humphrey S.M., Chain E.B. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J. Mol. Cell. Cardiol. 1973;5:395–407. doi: 10.1016/0022-2828(73)90030-8. [DOI] [PubMed] [Google Scholar]
- 321.Jolly S.R., Kane W.J., Bailie M.B., Abrams G.D., Lucchesi B.R. Canine myocardial reperfusion injury its reduction by the combined administration of superoxide dismutase and catalase. Circ. Res. 1984;54:277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- 322.Werns S.W., Lucchesi B.R. Oxygen Radicals in the Pathophysiology of Heart Disease. 1988. The role of the polymorphonuclear leukocyte in mediating myocardial reperfusion injury; pp. 123–144. [Google Scholar]
- 323.Chambers D.E., Parks D.A., Patterson G., Roy R., McCord J.M., Yoshida S., et al. Xanthine oxidase as a source of free radical damage in myocardial ischemia. J. Mol. Cell. Cardiol. 1985;17:145–152. doi: 10.1016/s0022-2828(85)80017-1. [DOI] [PubMed] [Google Scholar]
- 324.Miura T., Downey J.M., Hotta D., Iimura O. Effect of superoxide dismutase plus catalase on myocardial infarct size in rabbits. Can. J. Cardiol. 1988;4:407–411. [PubMed] [Google Scholar]
- 325.Ooiwa H., Stanley A., Felaneous-Bylund A.C., Wilborn W., Downey J.M. Superoxide dismutase conjugated to polyethylene glycol fails to limit myocardial infarct size after 30 min ischemia followed by 72 h of reperfusion in the rabbit. J. Mol. Cell. Cardiol. 1991;23:119–125. doi: 10.1016/0022-2828(91)90099-8. [DOI] [PubMed] [Google Scholar]
- 326.Reimer K.A., Murry C.E., Richard V.J. The role of neutrophils and free radicals in the ischemic-reperfused heart: why the confusion and controversy? J. Mol. Cell. Cardiol. 1989;21:1225–1239. doi: 10.1016/0022-2828(89)90669-x. [DOI] [PubMed] [Google Scholar]
- 327.Kloner R.A., Przyklenk K., Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Circulation. 1989;80:1115–1127. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 328.Ytrehus K., Liu Y., Tsuchida A., Miura T., Liu G.S., Yang X.-M., et al. Rat and rabbit heart infarction: effects of aneasthesia, perfusate, risk zone, and method of infarct sizing. Am. J. Physiol. Heart Circ. Physiol. 1994;267:H2383–H2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]
- 329.Miura T., Yellon D.M., Hearse D.J., Downey J.M. Determinants of infarct size during permanent occlusion of a coronary artery in the closed chest dog. J. Am. Coll. Cardiol. 1987;9:647–654. doi: 10.1016/s0735-1097(87)80060-8. [DOI] [PubMed] [Google Scholar]
- 330.Chien G.L., Wolff R.A., Davis R.F., van Winkle D.M. "Normothermic range" temperature affects myocardial infarct size. Cardiovasc. Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- 331.Grisham M.B., Hernandez L.A., Granger D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 332.Eddy L.J., Stewart J.R., Jones H.P., Engerson T.D., McCord J.M., Downey J.M. Free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am. J. Physiol. 1987;253:H709–H711. doi: 10.1152/ajpheart.1987.253.3.H709. [DOI] [PubMed] [Google Scholar]
- 333.Pain T., Yang X.M., Critz S.D., Yue Y., Nakano A., Liu G.S., et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 334.Nakamura T., Naguro I., Ichijo H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1398–1409. doi: 10.1016/j.bbagen.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 335.Murphy E., Ardehali H., Balaban R.S., DiLisa F., Dorn G.W., 2nd, Kitsis R.N., et al. Mitochondrial function, biology, and role in disease: a scientific statement from the American heart association. Circ. Res. 2016;118:1960–1991. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Shen C., Jennings R.B. Myocardial calcium and magnesium in acute ischemic injury. Am. J. Pathol. 1972;67:417–440. [PMC free article] [PubMed] [Google Scholar]
- 337.Ganote C.E., Worstell J., Kaltenbach J.P. Oxygen-induced enzyme release after irreversible myocardial injury. Effects of cyanide in perfused rat hearts. Am. J. Pathol. 1976;84:327–350. [PMC free article] [PubMed] [Google Scholar]
- 338.Elz J.S., Nayler W.G. Calcium gain during postischemic reperfusion. The effect of 2,4-dinitrophenol. Am. J. Pathol. 1988;131:137–145. [PMC free article] [PubMed] [Google Scholar]
- 339.Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssiere J.L., Petit P.X., Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hearse D.J., Humphrey S.M., Bullock G.R. The oxygen paradox and the calcium paradox: two facts of the same problem? J. Mol. Cell. Cardiol. 1978;10:641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- 341.Bernardi P., Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Penna C., Mancardi D., Rastaldo R., Pagliaro P. Cardioprotection: a radical view free radicals in pre- and postconditioning. Biochim. Biophys. Acta. 2009;1787:781–793. doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 343.Kaludercic N., Carpi A., Nagayama T., Sivakumaran V., Zhu G., Lai E.W., et al. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxidants Redox Signal. 2014;20:267–280. doi: 10.1089/ars.2012.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Di Lisa F., Giorgio M., Ferdinandy P., Schulz R. New aspects of p66Shc in ischemia reperfusion injury and cardiovascular diseases. Br. J. Pharmacol. 2017;174:1690–1703. doi: 10.1111/bph.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Heger J., Hirschhauser C., Bornbaum J., Sydykov A., Dempfle A., Schneider A., et al. Cardiomyocytes-specific deletion of monoamine oxidase B reduces irreversible myocardial ischemia/reperfusion injury. Free Radic. Biol. Med. 2021;165:14–23. doi: 10.1016/j.freeradbiomed.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 346.Carpi A., Menabo R., Kaludercic N., Pelicci P., Di L.F., Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim. Biophys. Acta. 2009;1787:744–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 347.Chen C.H., Budas G.R., Churchill E.N., Disatnik M.H., Hurley T.D., Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Asimakis G.K., Lick S., Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/-) but not SOD1(+/-) mouse hearts. Circulation. 2002;105:981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- 349.Zaha V.G., Qi D., Su K.N., Palmeri M., Lee H.Y., Hu X., et al. AMPK is critical for mitochondrial function during reperfusion after myocardial ischemia. J. Mol. Cell. Cardiol. 2016;91:104–113. doi: 10.1016/j.yjmcc.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Schulz R., Schlüter K.D. Importance of mitochondria in cardiac pathologies: focus on uncoupling proteins and monoamine oxidases. Int. J. Mol. Sci. 2023;24:6459. doi: 10.3390/ijms24076459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kaludercic N., Di Lisa F. The energetic cost of NNT-dependent ROS removal. J. Biol. Chem. 2020;295:16217–16218. doi: 10.1074/jbc.H120.016368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Daiber A., Di Lisa F., Oelze M., Kröller-Schön S., Steven S., Schulz E., Münzel T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017;174:1670–1689. doi: 10.1111/bph.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Murphy E., Liu J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023;119:1105–1116. doi: 10.1093/cvr/cvac134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Heinzel F.R., Luo Y., Dodoni G., Boengler K., Petrat F., Di Lisa F., et al. Formation of reactive oyxgen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc. Res. 2006;71:374–382. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 355.Penzo D., Petronilli V., Angelin A., Cusan C., Colonna R., Scorrano L., et al. Arachidonic acid released by phospholipase A(2) activation triggers Ca(2+)-dependent apoptosis through the mitochondrial pathway. J. Biol. Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 356.Chelko S.P., Keceli G., Carpi A., Doti N., Agrimi J., Asimaki A., et al. Exercise triggers CAPN1-mediated AIF truncation, inducing myocyte cell death in arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abf0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Antonucci S., Mulvey J.F., Burger N., Di Sante M., Hall A.R., Hinchy E.C., et al. Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radic. Biol. Med. 2019;134:678–687. doi: 10.1016/j.freeradbiomed.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Martin J.L., Costa A.S.H., Gruszczyk A.V., Beach T.E., Allen F.M., Prag H.A., et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019;1:966–974. doi: 10.1038/s42255-019-0115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mottahedin A., Prag H.A., Dannhorn A., Mair R., Schmidt C., Yang M., et al. Targeting succinate metabolism to decrease brain injury upon mechanical thrombectomy treatment of ischemic stroke. Redox Biol. 2023;59 doi: 10.1016/j.redox.2023.102600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Taghavi S., Abdullah S., Toraih E., Packer J., Drury R.H., Aras O.A.Z., et al. Dimethyl malonate slows succinate accumulation and preserves cardiac function in a swine model of hemorrhagic shock. J. Trauma Acute Care Surg. 2022;93:13–20. doi: 10.1097/TA.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Reddy A., Bozi L.H.M., Yaghi O.K., Mills E.L., Xiao H., Nicholson H.E., et al. pH-Gated succinate secretion regulates muscle remodeling in response to exercise. Cell. 2020;183:62–75 e17. doi: 10.1016/j.cell.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Mills E.L., Pierce K.A., Jedrychowski M.P., Garrity R., Winther S., Vidoni S., et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560:102–106. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Mills E.L., Kelly B., Logan A., Costa A.S., Varma M., Bryant C.E., et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470 e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Pryde K.R., Hirst J. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 2011;286:18056–18065. doi: 10.1074/jbc.M110.186841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Pell V.R., Chouchani E.T., Murphy M.P., Brookes P.S., Krieg T. Moving forwards by blocking back-flow: the Yin and Yang of MI therapy. Circ. Res. 2016;118:898–906. doi: 10.1161/CIRCRESAHA.115.306569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Zhang J., Wang Y.T., Miller J.H., Day M.M., Munger J.C., Brookes P.S. Accumulation of succinate in cardiac ischemia primarily occurs via canonical Krebs cycle activity. Cell Rep. 2018;23:2617–2628. doi: 10.1016/j.celrep.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-Parsy K., Frezza C., et al. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabol. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 368.Vujic A., Koo A.N.M., Prag H.A., Krieg T. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free Radic. Biol. Med. 2021;166:287–296. doi: 10.1016/j.freeradbiomed.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 369.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C., et al. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293:9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Prag H.A., Kula-Alwar D., Bernardi P., Di Lisa F., Murphy M.P., Krieg T. Cyclophilin D knockout mice do not accumulate succinate during cardiac ischemia. J. Mol. Cell. Cardiol. 2022;173:73–74. doi: 10.1016/j.yjmcc.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 371.Chen Q., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J. Pharmacol. Exp. Therapeut. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 372.Methner C., Chouchani E.T., Buonincontri G., Pell V.R., Sawiak S.J., Murphy M.P., Krieg T. Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts. Eur. J. Heart Fail. 2014;16:712–717. doi: 10.1002/ejhf.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Chouchani E.T., Methner C., Buonincontri G., Hu C.H., Logan A., Sawiak S.J., et al. Complex I deficiency due to selective loss of Ndufs4 in the mouse heart results in severe hypertrophic cardiomyopathy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Prag H.A., Murphy M.P., Krieg T. Preventing mitochondrial reverse electron transport as a strategy for cardioprotection. Basic Res. Cardiol. 2023;118:34. doi: 10.1007/s00395-023-01002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kula-Alwar D., Prag H.A., Krieg T. Targeting succinate metabolism in ischemia/reperfusion injury. Circulation. 2019;140:1968–1970. doi: 10.1161/CIRCULATIONAHA.119.042791. [DOI] [PubMed] [Google Scholar]
- 376.Kohlhauer M., Dawkins S., Costa A.S.H., Lee R., Young T., Pell V.R., et al. Metabolomic profiling in acute ST-segment-elevation myocardial infarction identifies succinate as an early marker of human ischemia-reperfusion injury. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Prag H.A., Gruszczyk A.V., Huang M.M., Beach T.E., Young T., Tronci L., et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res. 2021;117:1188–1201. doi: 10.1093/cvr/cvaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Prag H.A., Aksentijevic D., Dannhorn A., Giles A.V., Mulvey J.F., Sauchanka O., et al. Ischemia-selective cardioprotection by malonate for ischemia/reperfusion injury. Circ. Res. 2022;131:528–541. doi: 10.1161/CIRCRESAHA.121.320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Schulz R., Heusch G. Targeted mito- and cardioprotection by malonate. Circ. Res. 2022;131:542–544. doi: 10.1161/CIRCRESAHA.122.321582. [DOI] [PubMed] [Google Scholar]
- 380.Valls-Lacalle L., Barba I., Miro-Casas E., Alburquerque-Bejar J.J., Ruiz-Meana M., Fuertes-Agudo M., et al. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc. Res. 2016;109:374–384. doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- 381.Valls-Lacalle L., Barba I., Miro-Casas E., Ruiz-Meana M., Rodriguez-Sinovas A., Garcia-Dorado D. Selective inhibition of succinate dehydrogenase in reperfused myocardium with intracoronary malonate reduces infarct size. Sci. Rep. 2018;8:2442. doi: 10.1038/s41598-018-20866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.He W., Miao F.J., Lin D.C., Schwandner R.T., Wang Z., Gao J., et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 383.Murphy M.P., O'Neill L.A.J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174:780–784. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 384.Bertero E., Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ. Res. 2018;122:1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- 385.Smith G.L., Donoso P., Bauer C.J., Eisner D.A. Relationship between intracellular pH and metabolite concentrations during metabolic inhibition in isolated ferret heart. J. Physiol. 1993;472:11–22. doi: 10.1113/jphysiol.1993.sp019932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J. Physiol. 1978;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hartmann M., Decking U.K.M. Blocking Na+ -H+ exchange by cariporide reduces Na+ -overload in ischemia and is cardioprotective. J. Mol. Cell. Cardiol. 1999;31:1985–1995. doi: 10.1006/jmcc.1999.1029. [DOI] [PubMed] [Google Scholar]
- 388.Pike M.M., Kitakaze M., Marban E. 23Na-NMR measurements of intracellular sodium in intact perfused ferret hearts during ischemia and reperfusion. Am. J. Physiol. 1990;259:H1767–H1773. doi: 10.1152/ajpheart.1990.259.6.H1767. [DOI] [PubMed] [Google Scholar]
- 389.Murphy E., Steenbergen C. Ion transport and energetics during cell death and protection. Physiology (Bethesda) 2008;23:115–123. doi: 10.1152/physiol.00044.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sun J., Picht E., Ginsburg K.S., Bers D.M., Steenbergen C., Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ. Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018. [DOI] [PubMed] [Google Scholar]
- 391.Imahashi K., Kusuoka H., Hashimoto K., Yoshioka J., Yamaguchi H., Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ. Res. 1999;84:1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 392.Halestrap A.P. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 393.Finkel T., Menazza S., Holmstrom K.M., Parks R.J., Liu J., Sun J., et al. The ins and outs of mitochondrial calcium. Circ. Res. 2015;116:1810–1819. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ashok D., Papanicolaou K., Sidor A., Wang M., Solhjoo S., Liu T., O'Rourke B. Mitochondrial membrane potential instability on reperfusion after ischemia does not depend on mitochondrial Ca(2+) uptake. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Bernardi P., Carraro M., Lippe G. The mitochondrial permeability transition: recent progress and open questions. FEBS J. 2022;289:7051–7074. doi: 10.1111/febs.16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Cohen M.V., Yang X.M., Downey J.M. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation. 2007;115:1895–1903. doi: 10.1161/CIRCULATIONAHA.106.675710. [DOI] [PubMed] [Google Scholar]
- 397.Bolli R., Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;70:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 398.Nickel A., Kohlhaas M., Maack C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014;73:26–33. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 399.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 400.Aon M.A., Cortassa S., O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Aon M.A., Cortassa S., Marban E., O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 403.Akar F.G., Aon M.A., Tomaselli G., O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J. Clin. Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Dai D.F., Johnson S.C., Villarin J.J., Chin M.T., Nieves-Cintron M., Chen T., et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and G{alpha}q overexpression-induced heart failure. Circ. Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Kimura S., Zhang G., Nishiyama A., Shokoji T., Yao L., Fan Y., et al. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 406.Nickel A.G., von Hardenberg A., Hohl M., Loffler J.R., Kohlhaas M., Becker J., et al. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metabol. 2015;22:472–484. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 407.Aon M.A., Cortassa S., Maack C., O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J. Biol. Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Maack C., Böhm M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J. Am. Coll. Cardiol. 2011;58:83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 409.Dai D.F., Chen T., Szeto H., Nieves-Cintron M., Kutyavin V., Santana L.F., Rabinovitch P.S. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kröller-Schön S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxidants Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Ardehali H., O'Rourke B. Mitochondrial KATP channels in cell survival and death. J. Mol. Cell. Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Cohen M.V., Yang X.-M., Liu G.S., Heusch G., Downey J.M. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ. Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 413.Heinzel F.R., Luo Y., Li X., Boengler K., Buechert A., García-Dorado D., et al. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ. Res. 2005;97:583–586. doi: 10.1161/01.RES.0000181171.65293.65. [DOI] [PubMed] [Google Scholar]
- 414.Juhaszova M., Zorov D.B., Kim S.-H., Pepe S., Fu Q., Fishbein K.W., et al. Glycogen synthase kinase-3ß mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi: 10.1172/JCI200419906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Paggio A., Checchetto V., Campo A., Menabo R., Di Marco G., Di Lisa F., et al. Identification of an ATP-sensitive potassium channel in mitochondria. Nature. 2019;572:609–613. doi: 10.1038/s41586-019-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Ardehali H., O'Rourke B., Marban E. Cardioprotective role of the mitochondrial ATP-binding cassette protein 1. Circ. Res. 2005;97:740–742. doi: 10.1161/01.RES.0000186277.12336.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Juhaszova M., Kobrinsky E., Zorov D.B., Nuss H.B., Yaniv Y., Fishbein K.W., et al. ATP Synthase K(+)- and H(+)-fluxes drive ATP synthesis and enable mitochondrial K(+)-"uniporter" function: II. Ion and ATP synthase flux regulation. Function (Oxf) 2022;3 doi: 10.1093/function/zqac001. zqac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Bertero E., Maack C. Rethinking Mitchell's chemiosmotic theory: potassium dominates over proton flux to drive mitochondrial F1Fo-ATP synthase. Function (Oxf) 2022;3 doi: 10.1093/function/zqac012. zqac012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ide T., Tsutsui H., Kinugawa S., Utsumi H., Kang D., Hattori N., et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 420.Ide T., Tsutsui H., Kinugawa S., Suematsu N., Hayashidani S., Ichikawa K., et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ. Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 421.Dey S., DeMazumder D., Sidor A., Foster D.B., O'Rourke B. Mitochondrial ROS drive sudden cardiac death and chronic proteome remodeling in heart failure. Circ. Res. 2018;123:356–371. doi: 10.1161/CIRCRESAHA.118.312708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Dai D.F., Hsieh E.J., Liu Y., Chen T., Beyer R.P., Chin M.T., et al. Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovasc. Res. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 424.Adlam V.J., Harrison J.C., Porteous C.M., James A.M., Smith R.A., Murphy M.P., Sammut I.A. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb. J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 425.Chouchani E.T., Methner C., Nadtochiy S.M., Logan A., Pell V.R., Ding S., et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wojtovich A.P., Smith C.O., Haynes C.M., Nehrke K.W., Brookes P.S. Physiological consequences of complex II inhibition for aging, disease, and the mK(ATP) channel. Biochim. Biophys. Acta. 2013;1827:598–611. doi: 10.1016/j.bbabio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Brennan J.P., Southworth R., Medina R.A., Davidson S.M., Duchen M.R., Shattock M.J. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of K(ATP) channel activation. Cardiovasc. Res. 2006;72:313–321. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 428.Hebert-Chatelain E. Src kinases are important regulators of mitochondrial functions. Int. J. Biochem. Cell Biol. 2013;45:90–98. doi: 10.1016/j.biocel.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 429.Wang Y., Meyer J.W., Ashraf M., Shull G.E. Mice with a null mutation in the NHE1 Na+ -H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ. Res. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774. [DOI] [PubMed] [Google Scholar]
- 430.Theroux P., Chaitman B.R., Danchin N., Erhardt L., Meinertz T., Schroeder J.S., et al. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Circulation. 2000;102:3032–3038. doi: 10.1161/01.CIR.102.25.3032. [DOI] [PubMed] [Google Scholar]
- 431.Butler J., Packer M., Filippatos G., Ferreira J.P., Zeller C., Schnee J., et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Jhund P.S., Kondo T., Butt J.H., Docherty K.F., Claggett B.L., Desai A.S., et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Baartscheer A., Schumacher C.A., Wust R.C., Fiolet J.W., Stienen G.J., Coronel R., Zuurbier C.J. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60:568–573. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Uthman L., Baartscheer A., Bleijlevens B., Schumacher C.A., Fiolet J.W.T., Koeman A., et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722–726. doi: 10.1007/s00125-017-4509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Mustroph J., Baier M.J., Pabel S., Stehle T., Trum M., Provaznik Z., et al. Empagliflozin inhibits cardiac late sodium current by Ca/Calmodulin-dependent kinase II. Circulation. 2022;146:1259–1261. doi: 10.1161/CIRCULATIONAHA.122.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Hegyi B., Mira Hernandez J., Shen E.Y., Habibi N.R., Bossuyt J., Bers D.M. Empagliflozin reverses late Na(+) current enhancement and cardiomyocyte proarrhythmia in a translational murine model of heart failure with preserved ejection fraction. Circulation. 2022;145:1029–1031. doi: 10.1161/CIRCULATIONAHA.121.057237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Philippaert K., Kalyaanamoorthy S., Fatehi M., Long W., Soni S., Byrne N.J., et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143:2188–2204. doi: 10.1161/CIRCULATIONAHA.121.053350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Clancy C.E., Chen-Izu Y., Bers D.M., Belardinelli L., Boyden P.A., Csernoch L., et al. Deranged sodium to sudden death. J. Physiol. 2015;593:1331–1345. doi: 10.1113/jphysiol.2014.281204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Chen S., Wang Q., Christodoulou A., Mylonas N., Bakker D., Nederlof R., et al. Sodium glucose cotransporter-2 inhibitor empagliflozin reduces infarct size independently of sodium glucose cotransporter-2. Circulation. 2023;147:276–279. doi: 10.1161/CIRCULATIONAHA.122.061688. [DOI] [PubMed] [Google Scholar]
- 440.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Luongo T.S., Lambert J.P., Yuan A., Zhang X., Gross P., Song J., et al. The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Garcia-Rivas Gde J., Carvajal K., Correa F., Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br. J. Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Liu T., Takimoto E., Dimaano V.L., DeMazumder D., Kettlewell S., Smith G., et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Rezende F., Brandes R.P., Schröder K. Detection of hydrogen peroxide with fluorescent dyes. Antioxidants Redox Signal. 2018;29:585–602. doi: 10.1089/ars.2017.7401. [DOI] [PubMed] [Google Scholar]
- 446.Schiattarella G.G., Altamirano F., Tong D., French K.M., Villalobos E., Kim S.Y., et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. doi: 10.1038/s41586-019-1100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Weir E.K., Lopez-Barneo J., Buckler K.J., Archer S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Fernandez-Caggiano M., Kamynina A., Francois A.A., Prysyazhna O., Eykyn T.R., Krasemann S., et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat. Metab. 2020;2:1223–1231. doi: 10.1038/s42255-020-00276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Heusch G., Schulz R. A radical view on the contractile machinery in human heart failure. J. Am. Coll. Cardiol. 2011;57:310–312. doi: 10.1016/j.jacc.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 450.Nanadikar M.S., Vergel Leon A.M., Guo J., van Belle G.J., Jatho A., Philip E.S., et al. IDH3gamma functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart. Nat. Commun. 2023;14:2123. doi: 10.1038/s41467-023-37744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Heusch P., Canton M., Aker S., van de Sand A., Konietzka I., Rassaf T., et al. The contribution of reactive oxygen species and p38 mitogen activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br. J. Pharmacol. 2010;160:1408–1416. doi: 10.1111/j.1476-5381.2010.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Canton M., Skyschally A., Menabo R., Boengler K., Gres P., Schulz R., et al. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur. Heart J. 2006;27:875–881. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 453.Bolli R., Jeroudi M.O., Patel B.S., Aruoma O.I., Halliwell B., Lai E.K., McCay P.B. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial "stunning" is a manifestation of reperfusion injury. Circ. Res. 1989;65:607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- 454.Bolli R., Zughaib M., Li X.Y., Tang X.L., Sun J.Z., Triana J.F., McCay P.B. Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect of contractile function. J. Clin. Invest. 1995;96:1066–1084. doi: 10.1172/JCI118093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Schröder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 456.Xiao H., Jedrychowski M.P., Schweppe D.K., Huttlin E.L., Yu Q., Heppner D.E., et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. 2020;180:968–983. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Semenzato M., Kohr M.J., Quirin C., Menabo R., Alanova P., Alan L., et al. Oxidization of optic atrophy 1 cysteines occurs during heart ischemia-reperfusion and amplifies cell death by oxidative stress. Redox Biol. 2023;63 doi: 10.1016/j.redox.2023.102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Eaton P., Bell R.M., Cave A.C., Shattock M.J. Ischemic preconditioning: a potential role for protein S-thiolation? Antioxidants Redox Signal. 2005;7:882–888. doi: 10.1089/ars.2005.7.882. [DOI] [PubMed] [Google Scholar]
- 459.Kohr M.J., Sun J., Aponte A., Wang G., Gucek M., Murphy E., Steenbergen C. Simultaneous measurement of protein oxidation and s-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ. Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Cuello F., Eaton P. Cysteine-based redox sensing and its role in signaling by cyclic nucleotide-dependent kinases in the cardiovascular system. Annu. Rev. Physiol. 2019;81:63–87. doi: 10.1146/annurev-physiol-020518-114417. [DOI] [PubMed] [Google Scholar]
- 461.Charles R., Eaton P. Redox regulation of soluble epoxide hydrolase-implications for cardiovascular health and disease. Cells. 2022;11 doi: 10.3390/cells11121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Schaefer D., Cheng X. Recent advances in covalent drug discovery. Pharmaceuticals. 2023;16:663. doi: 10.3390/ph16050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Cohen M.S., Zhang C., Shokat K.M., Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Burgoyne J.R., Prysyazhna O., Richards D.A., Eaton P. Proof of principle for a novel class of antihypertensives that target the oxidative activation of PKG Ialpha (Protein Kinase G Ialpha) Hypertension. 2017;70:577–586. doi: 10.1161/HYPERTENSIONAHA.117.09670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Hu Q., Lukesh J.C. 3rd, H(2)S donors with cytoprotective effects in models of MI/R injury and chemotherapy-induced cardiotoxicity. Antioxidants (Basel) 2023;12:650. doi: 10.3390/antiox12030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Citi V., Piragine E., Testai L., Breschi M.C., Calderone V., Martelli A. The role of hydrogen sulfide and H2S-donors in myocardial protection against ischemia/reperfusion injury. Curr. Med. Chem. 2018;25:4380–4401. doi: 10.2174/0929867325666180212120504. [DOI] [PubMed] [Google Scholar]
- 467.Testai L., Marino A., Piano I., Brancaleone V., Tomita K., Di Cesare Mannelli L., et al. The novel H(2)S-donor 4-carboxyphenyl isothiocyanate promotes cardioprotective effects against ischemia/reperfusion injury through activation of mitoK(ATP) channels and reduction of oxidative stress. Pharmacol. Res. 2016;113:290–299. doi: 10.1016/j.phrs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 468.Ellmers L.J., Templeton E.M., Pilbrow A.P., Frampton C., Ishii I., Moore P.K., et al. Hydrogen sulfide treatment improves post-Infarct remodeling and long-term cardiac function in CSE knockout and wild-type mice. Int. J. Mol. Sci. 2020;21:4284. doi: 10.3390/ijms21124284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Salloum F.N. Hydrogen sulfide and cardioprotection - mechanistic insights and clinical translatability. Pharmacol. Ther. 2015;152:11–17. doi: 10.1016/j.pharmthera.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 470.Andreadou I., Schulz R., Papapetropoulos A., Turan B., Ytrehus K., Ferdinandy P., et al. The role of mitochondrial reactive oxygen species, NO and H2 S in ischaemia/reperfusion injury and cardioprotection. J. Cell Mol. Med. 2020;24:6510–6522. doi: 10.1111/jcmm.15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Luo Y., Liu L.M., Xie L., Zhao H.L., Lu Y.K., Wu B.Q., et al. Activation of the CaR-CSE/H2S pathway confers cardioprotection against ischemia-reperfusion injury. Exp. Cell Res. 2021;398 doi: 10.1016/j.yexcr.2020.112389. [DOI] [PubMed] [Google Scholar]
- 472.Wu D., Hu Q., Tan B., Rose P., Zhu D., Zhu Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca(2+)/calmodulin-dependent protein kinase II. Redox Biol. 2018;19:250–262. doi: 10.1016/j.redox.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Karwi Q.G., Bice J.S., Baxter G.F. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: a systematic review and meta-analysis. Basic Res. Cardiol. 2017;113:6. doi: 10.1007/s00395-017-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Shen Y., Shen Z., Luo S., Guo W., Zhu Y.Z. The cardioprotective effects of hydrogen sulfide in heart diseases: from molecular mechanisms to therapeutic potential. Oxid. Med. Cell Longev. 2015. 2015 doi: 10.1155/2015/925167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Chen Y., Zhang F., Yin J., Wu S., Zhou X. Protective mechanisms of hydrogen sulfide in myocardial ischemia. J. Cell. Physiol. 2020;235:9059–9070. doi: 10.1002/jcp.29761. [DOI] [PubMed] [Google Scholar]
- 476.Zhang P., Yu Y., Wang P., Shen H., Ling X., Xue X., et al. Role of hydrogen sulfide in myocardial ischemia-reperfusion injury. J. Cardiovasc. Pharmacol. 2020;77:130–141. doi: 10.1097/FJC.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 477.Sun J., Aponte A.M., Menazza S., Gucek M., Steenbergen C., Murphy E. Additive cardioprotection by pharmacological postconditioning with hydrogen sulfide and nitric oxide donors in mouse heart: S-sulfhydration vs. S-nitrosylation. Cardiovasc. Res. 2016;110:96–106. doi: 10.1093/cvr/cvw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Mahalakshmi A., Kurian G.A. Mitochondrial dysfunction plays a key role in the abrogation of cardioprotection by sodium hydrosulfide post-conditioning in diabetic cardiomyopathy rat heart. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:339–348. doi: 10.1007/s00210-019-01733-z. [DOI] [PubMed] [Google Scholar]
- 479.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H(2)S) donors: chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Chatzianastasiou A., Bibli S.I., Andreadou I., Efentakis P., Kaludercic N., Wood M.E., et al. Cardioprotection by H2S donors: nitric oxide-dependent and -independent mechanisms. J. Pharmacol. Exp. Therapeut. 2016;358:431–440. doi: 10.1124/jpet.116.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Bibli S.I., Andreadou I., Chatzianastasiou A., Tzimas C., Sanoudou D., Kranias E., et al. Cardioprotection by H2S engages a cGMP-dependent protein kinase G/phospholamban pathway. Cardiovasc. Res. 2015;106:432–442. doi: 10.1093/cvr/cvv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Andreadou I., Iliodromitis E.K., Rassaf T., Schulz R., Papapetropoulos A., Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015;172:1587–1606. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Karwi Q.G., Bornbaum J., Boengler K., Torregrossa R., Whiteman M., Wood M.E., et al. AP39, a mitochondria-targeting hydrogen sulfide (H2 S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017;174:287–301. doi: 10.1111/bph.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Yao H., Luo S., Liu J., Xie S., Liu Y., Xu J., et al. Controllable thioester-based hydrogen sulfide slow-releasing donors as cardioprotective agents. Chem. Commun. 2019;55:6193–6196. doi: 10.1039/c9cc02829c. [DOI] [PubMed] [Google Scholar]
- 485.Liang W., Chen J., Li L., Li M., Wei X., Tan B., et al. Conductive hydrogen sulfide-releasing hydrogel encapsulating ADSCs for myocardial infarction treatment. ACS Appl. Mater. Interfaces. 2019;11:14619–14629. doi: 10.1021/acsami.9b01886. [DOI] [PubMed] [Google Scholar]
- 486.Vass V., Szabo E., Bereczki I., Debreczeni N., Borbas A., Herczegh P., Tosaki A. Reperfusion-induced injury and the effects of the dithioacetate type hydrogen sulfide donor ibuprofen derivative, BM-88, in isolated rat hearts. Eur. J. Pharmaceut. Sci. 2023;185 doi: 10.1016/j.ejps.2023.106449. [DOI] [PubMed] [Google Scholar]
- 487.Pharoah B.M., Khodade V.S., Eremiev A., Bao E., Liu T., O'Rourke B., et al. Hydropersulfides (RSSH) outperform post-conditioning and other reactive sulfur species in limiting ischemia-reperfusion injury in the isolated mouse heart. Antioxidants (Basel) 2022;11:1010. doi: 10.3390/antiox11051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Lougiakis N., Papapetropoulos A., Gikas E., Toumpas S., Efentakis P., Wedmann R., et al. Synthesis and pharmacological evaluation of novel adenine-hydrogen sulfide slow release hybrids designed as multitarget cardioprotective agents. J. Med. Chem. 2016;59:1776–1790. doi: 10.1021/acs.jmedchem.5b01223. [DOI] [PubMed] [Google Scholar]
- 489.Wu D., Hu Q., Zhu D. An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid. Med. Cell Longev. 2018. 2018 doi: 10.1155/2018/4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Chang L., Wang Z., Ma F., Tran B., Zhong R., Xiong Y., et al. ZYZ-803 mitigates endoplasmic reticulum stress-related necroptosis after acute myocardial infarction through downregulating the RIP3-CaMKII signaling pathway. Oxid. Med. Cell Longev. 2019. 2019 doi: 10.1155/2019/6173685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Daiber A., Andreadou I., Oelze M., Davidson S.M., Hausenloy D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021;163:325–343. doi: 10.1016/j.freeradbiomed.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 492.Corvino A., Frecentese F., Magli E., Perissutti E., Santagada V., Scognamiglio A., et al. Trends in H(2)S-donors chemistry and their effects in cardiovascular diseases. Antioxidants (Basel) 2021;10:429. doi: 10.3390/antiox10030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Lecour S., Andreadou I., Bøtker H.E., Davidson S.M., Heusch G., Ruiz-Meana M., et al. IMproving preclinical assessment of cardioprotective therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST action. Basic Res. Cardiol. 2021;116:52. doi: 10.1007/s00395-021-00893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Osipov R.M., Robich M.P., Feng J., Liu Y., Clements R.T., Glazer H.P., et al. Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: comparison of different administration regimens and characterization of the cellular mechanisms of protection. J. Cardiovasc. Pharmacol. 2009;54:287–297. doi: 10.1097/FJC.0b013e3181b2b72b. [DOI] [PubMed] [Google Scholar]
- 495.Sodha N.R., Clements R.T., Feng J., Liu Y., Bianchi C., Horvath E.M., et al. Hydrogen sulfide therapy attenuates the inflammatory response in a porcine model of myocardial ischemia/reperfusion injury. J. Thorac. Cardiovasc. Surg. 2009;138:977–984. doi: 10.1016/j.jtcvs.2008.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Donnarumma E., Ali M.J., Rushing A.M., Scarborough A.L., Bradley J.M., Organ C.L., et al. Zofenopril protects against myocardial ischemia-reperfusion injury by increasing nitric oxide and hydrogen sulfide bioavailability. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Kar S., Kambis T.N., Mishra P.K. Hydrogen sulfide-mediated regulation of cell death signaling ameliorates adverse cardiac remodeling and diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1237–H1252. doi: 10.1152/ajpheart.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Ansari M., Kurian G.A. Mechanism of hydrogen sulfide preconditioning-associated protection against ischemia-reperfusion injury differs in diabetic heart that develops myopathy. Cardiovasc. Toxicol. 2020;20:155–167. doi: 10.1007/s12012-019-09542-9. [DOI] [PubMed] [Google Scholar]
- 499.Ansari M., Kurian G.A. Hydrogen sulfide preconditioning could ameliorate reperfusion associated injury in diabetic cardiomyopathy rat heart through preservation of mitochondria. Biochimie. 2019;158:208–216. doi: 10.1016/j.biochi.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 500.Ansari M., Prem P.N., Kurian G.A. Hydrogen sulfide postconditioning rendered cardioprotection against myocardial ischemia-reperfusion injury is compromised in rats with diabetic cardiomyopathy. Microvasc. Res. 2022;141 doi: 10.1016/j.mvr.2022.104322. [DOI] [PubMed] [Google Scholar]
- 501.Kar S., Shahshahan H.R., Hackfort B.T., Yadav S.K., Yadav R., Kambis T.N., et al. Exercise training promotes cardiac hydrogen sulfide biosynthesis and mitigates pyroptosis to prevent high-fat diet-induced diabetic cardiomyopathy. Antioxidants (Basel) 2019;8:638. doi: 10.3390/antiox8120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Chen J., Gao J., Sun W., Li L., Wang Y., Bai S., et al. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int. J. Cardiol. 2016;220:681–692. doi: 10.1016/j.ijcard.2016.06.200. [DOI] [PubMed] [Google Scholar]
- 503.Zhang Y., Gao J., Sun W., Wen X., Xi Y., Wang Y., et al. H(2)S restores the cardioprotective effects of ischemic post-conditioning by upregulating HB-EGF/EGFR signaling. Aging (Albany NY) 2019;11:1745–1758. doi: 10.18632/aging.101866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Polhemus D.J., Li Z., Pattillo C.B., Gojon G., Sr., Gojon G., Jr., Giordano T., Krum H. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 2015;33:216–226. doi: 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Zhang L., Wang Y., Li Y., Li L., Xu S., Feng X., Liu S. Hydrogen sulfide (H(2)S)-releasing compounds: therapeutic potential in cardiovascular diseases. Front. Pharmacol. 2018;9:1066. doi: 10.3389/fphar.2018.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.de Koning M.-S.L.Y., an Dorp P., Assa S., Pundziute-Do Prado G., Voskuil M., Anthonio R.L., et al. Sodium thiosulfate in acute myocardial infarction: A Randomized Clinical Trial, J. Am. Coll. Cardiol. Basic Trans. Sci. 2023 doi: 10.1016/j.jacbts.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Lassegue B., San M.A., Griendling K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Schröder K. NADPH oxidase-derived reactive oxygen species: dosis facit venenum. Exp. Physiol. 2019;104:447–452. doi: 10.1113/EP087125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Cipriano A., Viviano M., Feoli A., Milite C., Sarno G., Castellano S., Sbardella G. NADPH oxidases: from molecular mechanisms to current inhibitors. J. Med. Chem. 2023 doi: 10.1021/acs.jmedchem.3c00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Dinauer M.C., Orkin S.H., Brown R., Jesaitis A.J., Parkos C.A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327:717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- 511.Trevelin S.C., Shah A.M., Lombardi G. Beyond bacterial killing: NADPH oxidase 2 is an immunomodulator. Immunol. Lett. 2020;221:39–48. doi: 10.1016/j.imlet.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 512.Mortimer P.M., Mc Intyre S.A., Thomas D.C. Beyond the extra respiration of phagocytosis: NADPH oxidase 2 in adaptive immunity and inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Visnagri A., Oexner R.R., Kmiotek-Wasylewska K., Zhang M., Zoccarato A., Shah A.M. Nicotinamide adenosine dinucleotidephosphate oxidase-mediated signaling in cardiac remodeling. Antioxidants Redox Signal. 2023;38:371–387. doi: 10.1089/ars.2022.0176. [DOI] [PubMed] [Google Scholar]
- 514.Takac I., Schroder K., Zhang L., Lardy B., Anilkumar N., Lambeth J.D., et al. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J. Biol. Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Santos C.X., Hafstad A.D., Beretta M., Zhang M., Molenaar C., Kopec J., et al. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J. 2016;35:319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Nabeebaccus A.A., Reumiller C.M., Shen J., Zoccarato A., Santos C.X.C., Shah A.M. The regulation of cardiac intermediary metabolism by NADPH oxidases. Cardiovasc. Res. 2023;118:3305–3319. doi: 10.1093/cvr/cvac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 518.Zhang M., Prosser B.L., Bamboye M.A., Gondim A.N., Santos C.X., Martin D., et al. Contractile function during angiotensin-II activation: increased Nox2 activity modulates cardiac calcium handling via phospholamban phosphorylation. J. Am. Coll. Cardiol. 2015;66:261–272. doi: 10.1016/j.jacc.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Bell R.M., Cave A.C., Johar S., Hearse D.J., Shah A.M., Shattock M.J. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditoning. Faseb. J. 2005;19:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 520.Ma Q., Cavallin L.E., Yan B., Zhu S., Duran E.M., Wang H., et al. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Zhang M., Brewer A.C., Schroder K., Santos C.X., Grieve D.J., Wang M., et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Sciarretta S., Zhai P., Shao D., Zablocki D., Nagarajan N., Terada L.S., et al. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ. Res. 2013;113:1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Hecker L., Logsdon N.J., Kurundkar D., Kurundkar A., Bernard K., Hock T., et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Zhang M., Mongue-Din H., Martin D., Catibog N., Smyrnias I., Zhang X., et al. Both cardiomyocyte and endothelial cell Nox4 mediate protection against hemodynamic overload-induced remodelling. Cardiovasc. Res. 2018;114:401–408. doi: 10.1093/cvr/cvx204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Smyrnias I., Zhang X., Zhang M., Murray T.V., Brandes R.P., Schroder K., et al. Nicotinamide adenine dinucleotide phosphate oxidase-4-dependent upregulation of nuclear factor erythroid-derived 2-like 2 protects the heart during chronic pressure overload. Hypertension. 2015;65:547–553. doi: 10.1161/HYPERTENSIONAHA.114.04208. [DOI] [PubMed] [Google Scholar]
- 527.Hancock M., Hafstad A.D., Nabeebaccus A.A., Catibog N., Logan A., Smyrnias I., et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. Elife. 2018;7 doi: 10.7554/eLife.41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Nabeebaccus A.A., Zoccarato A., Hafstad A.D., Santos C.X., Aasum E., Brewer A.C., et al. Nox4 reprograms cardiac substrate metabolism via protein O-GlcNAcylation to enhance stress adaptation. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., et al. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 531.Beretta M., Santos C.X., Molenaar C., Hafstad A.D., Miller C.C., Revazian A., et al. Nox4 regulates InsP(3) receptor-dependent Ca(2+) release into mitochondria to promote cell survival. EMBO J. 2020;39 doi: 10.15252/embj.2019103530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 533.Kimura S., Zhang G., Nishiyama N., Shokoji T., Yao L., Fan Y., et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases. Comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818. [DOI] [PubMed] [Google Scholar]
- 534.Brandes R.P. Triggering mitochondrial radical release. A new function for NADPH oxidases. Hypertension. 2005;45:847–849. doi: 10.1161/01.HYP.0000165019.32059.b2. [DOI] [PubMed] [Google Scholar]
- 535.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxidants Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 537.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., et al. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M., Muller J., et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxidants Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 539.Wenzel P., Schuhmacher S., Kienhofer J., Muller J., Hortmann M., Oelze M., et al. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res. 2008;80:280–289. doi: 10.1093/cvr/cvn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Oelze M., Kröller-Schön S., Steven S., Lubos E., Doppler C., Hausding M., et al. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 541.Itani H.A., Dikalova A.E., McMaster W.G., Nazarewicz R.R., Bikineyeva A.T., Harrison D.G., Dikalov S.I. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension. 2016;67:1218–1227. doi: 10.1161/HYPERTENSIONAHA.115.07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Nguyen T.T., Stevens M.V., Kohr M., Steenbergen C., Sack M.N., Murphy E. Cysteine 203 of cyclophilin d is critical for cyclophilin d activation of the mitochondrial permeability transition pore. J. Biol. Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Bibli S.I., Papapetropoulos A., Iliodromitis E.K., Daiber A., Randriamboavonjy V., Steven S., et al. Nitroglycerine limits infarct size through S-nitrosation of cyclophilin D: a novel mechanism for an old drug. Cardiovasc. Res. 2019;115:625–636. doi: 10.1093/cvr/cvy222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Archer S.L., Gomberg-Maitland M., Maitland M.L., Rich S., Garcia J.G., Weir E.K. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1{alpha}-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 545.Giordano F.J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005;115:500–508. doi: 10.1172/JCI24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Powell C.S., Jackson R.M. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L189–L198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- 547.Rathore R., Zheng Y.M., Niu C.F., Liu Q.H., Korde A., Ho Y.S., Wang Y.X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic. Biol. Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.da Silva M.F., Natali A.J., da Silva E., Gomes G.J., Teodoro B.G., Cunha D.N., et al. Attenuation of Ca2+ homeostasis, oxidative stress, and mitochondrial dysfunctions in diabetic rat heart: insulin therapy or aerobic exercise? J. Appl. Physiol. (1985) 2015;119:148–156. doi: 10.1152/japplphysiol.00915.2014. [DOI] [PubMed] [Google Scholar]
- 550.Hempel N., Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Gorlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Sluijter J.P.G., Davidson S.M., Boulanger C.M., Buzas E.I., de Kleijn D.P.V., Engel F.B., et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: position paper from the working group on cellular biology of the heart of the European society of cardiology. Cardiovasc. Res. 2018;114:19–34. doi: 10.1093/cvr/cvx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Davidson S.M., Yellon D.M. Exosomes and cardioprotection - a critical analysis. Mol. Aspect. Med. 2018;60:104–114. doi: 10.1016/j.mam.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Lisi V., Senesi G., Bertola N., Pecoraro M., Bolis S., Gualerzi A., et al. Plasma-derived extracellular vesicles released after endurance exercise exert cardioprotective activity through the activation of antioxidant pathways. Redox Biol. 2023;63 doi: 10.1016/j.redox.2023.102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Bitirim C.V., Ozer Z.B., Aydos D., Genc K., Demirsoy S., Akcali K.C., Turan B. Cardioprotective effect of extracellular vesicles derived from ticagrelor-pretreated cardiomyocyte on hyperglycemic cardiomyocytes through alleviation of oxidative and endoplasmic reticulum stress. Sci. Rep. 2022;12:5651. doi: 10.1038/s41598-022-09627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Andreadou I., Daiber A., Baxter G.F., Brizzi M.F., Di Lisa F., Kaludercic N., et al. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: role of cardiac redox signaling. Free Radic. Biol. Med. 2021;166:33–52. doi: 10.1016/j.freeradbiomed.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 557.Wider J., Undyala V.V.R., Whittaker P., Woods J., Chen X., Przyklenk K. Remote ischemic preconditioning fails to reduce infarct size in the Zucker fatty rat model of type-2 diabetes: role of defective humoral communication. Basic Res. Cardiol. 2018;113:16. doi: 10.1007/s00395-018-0674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Davidson S.M., Riquelme J.A., Takov K., Vicencio J.M., Boi-Doku C., Khoo V., et al. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J. Cell Mol. Med. 2017;22:141–151. doi: 10.1111/jcmm.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Gambim M.H., do Carmo Ade O., Marti L., Verissimo-Filho S., Lopes L.R., Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Azevedo L.C., Janiszewski M., Pontieri V., Pedro Mde A., Bassi E., Tucci P.J., Laurindo F.R. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit. Care. 2007;11:R120. doi: 10.1186/cc6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Crewe C., Funcke J.B., Li S., Joffin N., Gliniak C.M., Ghaben A.L., et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metabol. 2021;33:1853–1868. doi: 10.1016/j.cmet.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Lai R.C., Arslan F., Lee M.M., Sze N.S., Choo A., Chen T.S., et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 563.Wang Y., Zhao R., Liu D., Deng W., Xu G., Liu W., et al. Exosomes derived from miR-214-enriched bone marrow-derived mesenchymal stem cells regulate oxidative damage in cardiac stem cells by targeting CaMKII. Oxid. Med. Cell Longev. 2018. 2018 doi: 10.1155/2018/4971261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Yadid M., Lind J.U., Ardona H.A.M., Sheehy S.P., Dickinson L.E., Eweje F., et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aax8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Chen G., Xu C., Gillette T.G., Huang T., Huang P., Li Q., et al. Cardiomyocyte-derived small extracellular vesicles can signal eNOS activation in cardiac microvascular endothelial cells to protect against Ischemia/Reperfusion injury. Theranostics. 2020;10:11754–11774. doi: 10.7150/thno.43163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Rolski F., Czepiel M., Tkacz K., Fryt K., Siedlar M., Kania G., Blyszczuk P. T lymphocyte-derived exosomes transport MEK1/2 and ERK1/2 and induce NOX4-dependent oxidative stress in cardiac microvascular endothelial cells. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/2457687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Herrera-Zelada N., Zuniga-Cuevas U., Ramirez-Reyes A., Norambuena-Soto I., Venegas-Zamora L., Troncoso M.F., et al. Endothelial activation impairs the function of small extracellular vesicles. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1143888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Makkos A., Agg B., Petrovich B., Varga Z.V., Gorbe A., Ferdinandy P. Systematic review and network analysis of microRNAs involved in cardioprotection against myocardial ischemia/reperfusion injury and infarction: involvement of redox signalling. Free Radic. Biol. Med. 2021;172:237–251. doi: 10.1016/j.freeradbiomed.2021.04.034. [DOI] [PubMed] [Google Scholar]
- 569.Bergmann O., Bhardwaj R.D., Bernard S., Zdunek S., Barnabe-Heider F., Walsh S., et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Senyo S.E., Steinhauser M.L., Pizzimenti C.L., Yang V.K., Cai L., Wang M., et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Soonpaa M.H., Field L.J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 572.Suzuki Y.J. Growth factor signaling for cardioprotection against oxidative stress-induced apoptosis. Antioxidants Redox Signal. 2003;5:741–749. doi: 10.1089/152308603770380043. [DOI] [PubMed] [Google Scholar]
- 573.Rassaf T., Weber C., Bernhagen J. Macrophage migration inhibitory factor in myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2014;102:321–328. doi: 10.1093/cvr/cvu071. [DOI] [PubMed] [Google Scholar]
- 574.Matsushita K., Iwanaga S., Oda T., Kimura K., Shimada M., Sano M., et al. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab. Invest. 2005;85:1210–1223. doi: 10.1038/labinvest.3700322. [DOI] [PubMed] [Google Scholar]
- 575.Smart N., Mojet M.H., Latchman D.S., Marber M.S., Duchen M.R., Heads R.J. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc. Res. 2006;69:164–177. doi: 10.1016/j.cardiores.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 576.Wang H., Zhao Y.T., Zhang S., Dubielecka P.M., Du J., Yano N., et al. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell. Physiol. 2017;232:3775–3785. doi: 10.1002/jcp.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Wang Z., Chen K., Han Y., Zhu H., Zhou X., Tan T., et al. Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol. 2018;72:259–269. doi: 10.1097/FJC.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Oshima Y., Ouchi N., Sato K., Izumiya Y., Pimentel D.R., Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Ogura Y., Ouchi N., Ohashi K., Shibata R., Kataoka Y., Kambara T., et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126:1728–1738. doi: 10.1161/CIRCULATIONAHA.112.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Szabo M.R., Pipicz M., Csont T., Csonka C. Modulatory effect of myokines on reactive oxygen species in ischemia/reperfusion. Int. J. Mol. Sci. 2020;21:9382. doi: 10.3390/ijms21249382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Korf-Klingebiel M., Reboll M.R., Klede S., Brod T., Pich A., Polten F., et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 2015;21:140–149. doi: 10.1038/nm.3778. [DOI] [PubMed] [Google Scholar]
- 582.Bortolotti F., Ruozi G., Falcione A., Doimo S., Dal Ferro M., Lesizza P., et al. In vivo functional selection identifies cardiotrophin-1 as a cardiac engraftment factor for mesenchymal stromal cells. Circulation. 2017;136:1509–1524. doi: 10.1161/CIRCULATIONAHA.117.029003. [DOI] [PubMed] [Google Scholar]
- 583.Ruozi G., Bortolotti F., Falcione A., Dal Ferro M., Ukovich L., Macedo A., et al. AAV-mediated in vivo functional selection of tissue-protective factors against ischaemia. Nat. Commun. 2015;6:7388. doi: 10.1038/ncomms8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Ruozi G., Bortolotti F., Mura A., Tomczyk M., Falcione A., Martinelli V., et al. Cardioprotective factors against myocardial infarction selected in vivo from an AAV secretome library. Sci. Transl. Med. 2022;14 doi: 10.1126/scitranslmed.abo0699. [DOI] [PubMed] [Google Scholar]
- 585.Nakayama N., Han C.E., Scully S., Nishinakamura R., He C., Zeni L., et al. A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Dev. Biol. 2001;232:372–387. doi: 10.1006/dbio.2001.0200. [DOI] [PubMed] [Google Scholar]
- 586.Bendre A., Buki K.G., Maatta J.A. Fam3c modulates osteogenic differentiation by down-regulating Runx2. Differentiation. 2017;93:50–57. doi: 10.1016/j.diff.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 587.Waerner T., Alacakaptan M., Tamir I., Oberauer R., Gal A., Brabletz T., et al. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227–239. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 588.Chen Z., Ding L., Yang W., Wang J., Chen L., Chang Y., et al. Hepatic activation of the FAM3C-HSF1-CaM pathway attenuates hyperglycemia of obese diabetic mice. Diabetes. 2017;66:1185–1197. doi: 10.2337/db16-0993. [DOI] [PubMed] [Google Scholar]
- 589.Robert-Cooperman C.E., Carnegie J.R., Wilson C.G., Yang J., Cook J.R., Wu J., et al. Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function. Diabetes. 2010;59:2209–2218. doi: 10.2337/db09-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Yang J., Robert C.E., Burkhardt B.R., Young R.A., Wu J., Gao Z., Wolf B.A. Mechanisms of glucose-induced secretion of pancreatic-derived factor (PANDER or FAM3B) in pancreatic beta-cells. Diabetes. 2005;54:3217–3228. doi: 10.2337/diabetes.54.11.3217. [DOI] [PubMed] [Google Scholar]
- 591.Cao X., Gao Z., Robert C.E., Greene S., Xu G., Xu W., et al. Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells. Diabetes. 2003;52:2296–2303. doi: 10.2337/diabetes.52.9.2296. [DOI] [PubMed] [Google Scholar]
- 592.Lee J.T., Ideker R.E., Reimer K.A. Myocardial infarct size and location in relation to the coronary vascular bed at risk in man. Circulation. 1981;64:526–534. doi: 10.1161/01.cir.64.3.526. [DOI] [PubMed] [Google Scholar]
- 593.Rochitte C.E., Azevedo C.F. The myocardial area at risk. Heart. 2012;98:348–350. doi: 10.1136/heartjnl-2011-301332. [DOI] [PubMed] [Google Scholar]
- 594.Heusch P., Nensa F., Heusch G. Is MRI really the gold standard for the quantification of salvage from myocardial infarction? Circ. Res. 2015;117:222–224. doi: 10.1161/CIRCRESAHA.117.306929. [DOI] [PubMed] [Google Scholar]
- 595.Matsui Y., Kyoi S., Takagi H., Hsu C.P., Hariharan N., Ago T., et al. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4:409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Kirshenbaum L.A. Regulation of autophagy in the heart in health and disease. J. Cardiovasc. Pharmacol. 2012;60:109. doi: 10.1097/FJC.0b013e31825f6faa. [DOI] [PubMed] [Google Scholar]
- 597.Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Kim I., Lemasters J.J. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxidants Redox Signal. 2011;14:1919–1928. doi: 10.1089/ars.2010.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Turkieh A., El Masri Y., Pinet F., Dubois-Deruy E. Mitophagy regulation following myocardial infarction. Cells. 2022;11:199. doi: 10.3390/cells11020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Scheffer D.D.L., Garcia A.A., Lee L., Mochly-Rosen D., Ferreira J.C.B. Mitochondrial fusion, fission, and mitophagy in cardiac diseases: challenges and therapeutic opportunities. Antioxidants Redox Signal. 2022;36:844–863. doi: 10.1089/ars.2021.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Luan Y., Luan Y., Feng Q., Chen X., Ren K.D., Yang Y. Emerging role of mitophagy in the heart: therapeutic potentials to modulate mitophagy in cardiac diseases. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/3259963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Stubbert D., Prysyazhna O., Rudyk O., Scotcher J., Burgoyne J.R., Eaton P. Protein kinase G Ialpha oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfide. Hypertension. 2014;64:1344–1351. doi: 10.1161/HYPERTENSIONAHA.114.04281. [DOI] [PubMed] [Google Scholar]
- 603.Prysyazhna O., Wolhuter K., Switzer C., Santos C., Yang X., Lynham S., et al. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation. 2019;140:126–137. doi: 10.1161/CIRCULATIONAHA.118.037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Xu M., Wang Y., Hirai K., Ayub A., Ashraf M. Calcium preconditioning inhibits mitochondrial permeability transition and apooptosis. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H899–H908. doi: 10.1152/ajpheart.2001.280.2.H899. [DOI] [PubMed] [Google Scholar]
- 605.Hausenloy D., Wynne A., Duchen M., Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 606.Song M., Chen Y., Gong G., Murphy E., Rabinovitch P.S., Dorn G.W. Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ. Res. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Elrod J.W., Wong R., Mishra S., Vagnozzi R.J., Sakthievel B., Goonasekera S.A., et al. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.